Key Points
Question
Are APOL1 high-risk genotypes observed in individuals with African ancestry associated with acute kidney injury (AKI) and death following hospitalization for COVID-19?
Findings
In this cohort of 990 veterans with African ancestry hospitalized with COVID-19, 1 in 8 had APOL1 high-risk genotypes. Of those with high-risk genotypes, 51.2% had AKI, and 19.2% died, suggesting that high-risk genotype may be associated with a 2-fold increase in the odds of severe AKI and death; this increased risk was observed even in patients with normal kidney function prior to COVID-19.
Meaning
APOL1 high-risk genotypes were associated with increased odds of AKI, AKI severity, and death in individuals with African ancestry hospitalized with COVID-19.
This cohort study of US veterans hospitalized with COVID-19 with genetic information and with African ancestry through the VA Million Veteran Program to evaluates the association of APOL1 variations with.acute kidney injury
Abstract
Importance
Coronavirus disease 2019 (COVID-19) confers significant risk of acute kidney injury (AKI). Patients with COVID-19 with AKI have high mortality rates.
Objective
Individuals with African ancestry with 2 copies of apolipoprotein L1 (APOL1) variants G1 or G2 (high-risk group) have significantly increased rates of kidney disease. We tested the hypothesis that the APOL1 high-risk group is associated with a higher-risk of COVID-19–associated AKI and death.
Design, Setting, and Participants
This retrospective cohort study included 990 participants with African ancestry enrolled in the Million Veteran Program who were hospitalized with COVID-19 between March 2020 and January 2021 with available genetic information.
Exposures
The primary exposure was having 2 APOL1 risk variants (RV) (APOL1 high-risk group), compared with having 1 or 0 risk variants (APOL1 low-risk group).
Main Outcomes and Measures
The primary outcome was AKI. The secondary outcomes were stages of AKI severity and death. Multivariable logistic regression analyses adjusted for preexisting comorbidities, medications, and inpatient AKI risk factors; 10 principal components of ancestry were performed to study these associations. We performed a subgroup analysis in individuals with normal kidney function prior to hospitalization (estimated glomerular filtration rate ≥60 mL/min/1.73 m2).
Results
Of the 990 participants with African ancestry, 905 (91.4%) were male with a median (IQR) age of 68 (60-73) years. Overall, 392 (39.6%) patients developed AKI, 141 (14%) developed stages 2 or 3 AKI, 28 (3%) required dialysis, and 122 (12.3%) died. One hundred twenty-five (12.6%) of the participants were in the APOL1 high-risk group. Patients categorized as APOL1 high-risk group had significantly higher odds of AKI (adjusted odds ratio [OR], 1.95; 95% CI, 1.27-3.02; P = .002), higher AKI severity stages (OR, 2.03; 95% CI, 1.37-2.99; P < .001), and death (OR, 2.15; 95% CI, 1.22-3.72; P = .007). The association with AKI persisted in the subgroup with normal kidney function (OR, 1.93; 95% CI, 1.15-3.26; P = .01). Data analysis was conducted between February 2021 and April 2021.
Conclusions and Relevance
In this cohort study of veterans with African ancestry hospitalized with COVID-19 infection, APOL1 kidney risk variants were associated with higher odds of AKI, AKI severity, and death, even among individuals with prior normal kidney function.
Introduction
A recent study of US veterans hospitalized with COVID-19 showed that acute kidney injury (AKI) was more common in non-Hispanic Black veterans compared with non-Hispanic White veterans.1 Importantly, AKI in non-Hispanic Black veterans was also associated with a higher risk of death.1 Studies suggest that African ancestry-specific kidney risk genetic variants may play a role in the risk of AKI in patients infected with COVID-19.2,3
Clinically relevant risk variants in the gene encoding for apolipoprotein L1 (APOL1) are common in individuals with African ancestry in the US, where approximately 45% carry at least 1 high-risk variant (RV) and 12% to 13% carry 2 RVs.4,5,6,7,8,9 The 2 RVs, termed G1 and G2, are common in individuals of West African descent owing to positive genetic selection. Individuals with at least 1 RV are resistant to lethal Trypanosoma brucei infections.9,10,11,12 However, having 2 RVs is associated with chronic kidney disease (CKD). The APOL1 RVs partially explain the disparities observed in the risk of end-stage kidney disease (ESKD) among individuals with African ancestry.13,14,15 In addition, APOL1 RVs are associated with several forms of nondiabetic kidney disease.16 Whether APOL1 RVs are associated with AKI in general has not been established.17,18,19
The pathophysiology of AKI in individuals with COVID-19 is poorly understood and likely multifactorial, particularly with respect to patients with APOL1 risk variants. Expression of APOL1 in kidney cells is upregulated by inflammation,3,10,20,21 which has been observed in several forms of kidney disease, including focal segmental glomerulosclerosis (FSGS), now well described in the COVID-19 literature.22,23,24,25 However, it appears that most cases are owing to acute tubular injury during critical illness, where the exaggerated inflammatory response and robust immune activation may be a second hit.3,20,26,27
In this study, we leveraged a national cohort of US veterans hospitalized with COVID-19 with genetic information and with African ancestry through the VA Million Veteran Program (MVP)28 to evaluate the association of APOL1 high-risk group (2 RVs) in African ancestry participants with AKI. Because AKI is a strong predictor of mortality in patients with COVID-19,1 our secondary goal was to evaluate the association of APOL1 genotypes with the risk of death.
Methods
Study Population and Design
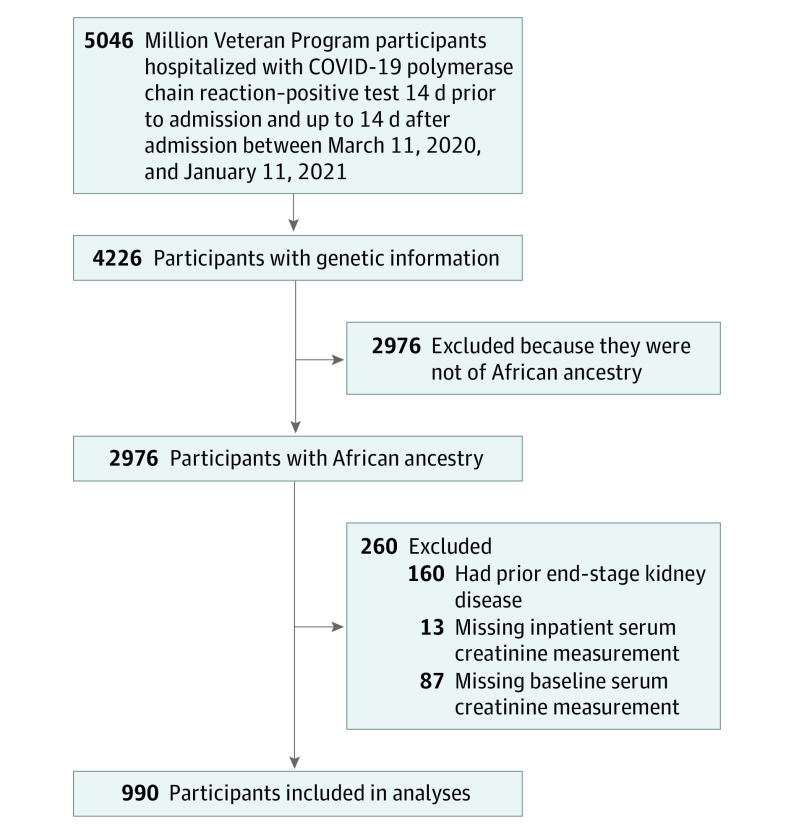
We identified 5046 MVP participants hospitalized with COVID-19 between March 11, 2020 and January 11, 2021. Of these, 4226 had genetic information. APOL1 RVs, our exposure of interest (individuals with 2 RVs were considered high-risk group), are found in individuals of recent West African ancestry.29,30 Consistent with this knowledge, we examined but found no participants without African ancestry who carried a high-risk APOL1 genotype. Hence, the cohort consisted of (1) MVP participants hospitalized with COVID-19, (2) those with genetic information available, and (3) those with African ancestry.28 After applying other exclusion criteria needed for AKI assessment as specified, our study sample size was 990 individuals (Figure 1).
Figure 1. Flowchart for Patient Eligibility.
Data were obtained from the VA Corporate Data Warehouse (CDW), the MVP Study Mart, the Observational Medical Outcomes Partnership version 5, and the COVID Share Data Resources.31,32,33 COVID-19 was diagnosed based on a positive polymerase chain reaction test result for SARS-CoV-2 from a nasopharyngeal specimen between March 1, 2020 and January 11, 2021 (eFigure 1 in Supplement 1). Participants entered the cohort if they had positive SARS-CoV-2 testing results and were hospitalized 14 days prior or 14 days after the positive test result. The distribution of the date of admission with regard to test positivity are presented in eFigure 2 in Supplement 1. Overall, 934 (95%) of the individuals had a positive test within the first 3 days of hospitalization or prior to the admission date. The MVP received approval by the VA Central institutional review board (IRB), the IRB of each site, and the MVP COVID-19 Scientific Steering Committee. Written informed consent was obtained from all participants. Data analysis was conducted between February 2021 and April 2021. This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement guidelines for observational research.34
Cohort Exclusion Criteria
Participants were excluded if they had a preadmission history of ESKD, defined as previous dialysis or kidney transplant and those with a baseline estimated glomerular filtration rate (eGFR) lower than 15 mL/min/1.73 m2 (n = 160) (eTable 1 in Supplement 1) or did not have serum creatinine levels measured during hospitalization (n = 13). We also excluded patients with no baseline outpatient serum creatinine level measurement used to characterize AKI, defined as the mean outpatient creatinine levels within the 7 to 365 days prior to hospitalization (n = 87)35 (Figure 1). Participant AKI assessment was restricted to the first qualifying hospitalization during the study period.
Outcomes and Follow-up
The primary outcome was AKI, defined using a modified Kidney Disease Improving Global Outcomes (KDIGO) creatinine-based criterion as a 0.3 mg/dL or 50% increase in serum creatinine levels from baseline using the peak serum creatinine during hospitalization (eTable 2 in Supplement 1).36
Our secondary outcomes included a modified KDIGO AKI severity stages result (stage 1, 0.3 mg/dL or 50% increase from baseline; stage 2, 100% increase from baseline; and stage 3, 200% increase from baseline or acute kidney replacement therapy [KRT]) or death. In this study, KRT was identified by diagnostic and procedural codes37 (eTable 3 in Supplement 1). Dates of death were obtained from the VA vital status files of the CDW.38,39
Because our goal was to capture AKI events that were proximally related to COVID-19 infection, participants were followed until discharge or up to 30 days because late AKI occurring after 30 days may be more likely owing to hospital complications. For the death outcome, participants were followed for up to 30 days after admission, consistent with other VA studies.39,40,41
Exploratory analysis included dipstick proteinuria and hematuria measurements at baseline and on admission,42 mechanical ventilation, and vasopressors.
APOL1 Genotype Exposure
APOL1 RVs G1 (rs73885319 p.S342G; rs60910145 p.I384M) and G2 (rs71785313, a 6-base pair deletion that removes amino acids N388 and Y389) were directly genotyped on the Affymetrix Axiom Biobank Array on DNA extracted from whole blood.4 Participants were defined as 2 RV carriers if they were homozygotes for G1/G1, homozygotes for G2/G2, or compound heterozygotes for G1/G2. The genotyping was completed by the MVP Genomic Core.43 The G1 and G2 genotypes were in Hardy-Weinberg equilibrium.
Subgroup Analysis
A subgroup analysis was planned for all outcomes, restricted to the population with baseline eGFR levels of 60 mL/min/1.73 m2 or higher, to evaluate if the effect of APOL1 high-risk genotypes in the risk of AKI in the context of COVID-19 was independent of CKD.
Covariates
Characteristics reported include demographics, comorbidities, hospitalization characteristics, laboratory tests, and medications. Determination of ancestry was based on a unified classification algorithm developed by MVP.44 This algorithm integrates genetically inferred ancestry based on the top 30 principal components of ancestry (PCs) with self-identified race/ethnicity (SIRE). The HARE (Harmonizing Genetic Ancestry and Self-identified Race/Ethnicity) algorithm classifies an MVP participant as “African” if their genetic ancestry is consistent with “African ancestry” and it informs ancestry in those with missing SIRE information.44 In MVP African ancestry assignment is population level and is derived from projecting the genetic PCs of MVP to those in the 1000 Genomes Project reference panel.43
Baseline comorbidities were obtained up to 730 days before the SARS-CoV-2 positive test. In-hospital risk factors for AKI were collected during the hospitalization prior to peak AKI. Drug exposures associated with high-risk of AKI were documented if they were administered prior to the peak creatinine using the bar-coded medication administration (BCMA) in the inpatient setting. Outpatient ACE inhibitors or ARB exposure was defined as a prescription fill within 180 days prior to admission by assessing outpatient VA pharmacy files. Diagnoses were defined using the International Classification of Diseases, Ninth Revision, Tenth Revision (ICD-9, ICD-10) procedures codes ICD-9/ICD-10 and Current Procedural Terminology (CPT) codes (eTable 3 in Supplement 1). Levels of eGFR were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.45
Statistical Analyses
The primary exposure variable was APOL1 variable high-risk group defined by the presence of 2 RVs (recessive model of inheritance). Logistic regression models were used to study the association of the exposure and the outcomes of interest. We also used a partial proportional odds (PPO) regression model to study the association of the APOL1 high-risk group and increasing severity stages of AKI.46 Nested multivariable models included (a) model 1, minimally adjusted (demographics, baseline eGFR and 10 PCs of ancestry) and (b) model 2, fully adjusted, also adjusted for preexisting comorbid conditions and inpatient risk factors. All logistic regression models passed the Goodness of fit test by the Hosmer-Lemeshow test (eTable 4 in Supplement 1). When conducting proportional odds regression analyses, proportional odds were assumed for all variables except for baseline eGFR, diastolic blood pressure, and remdesivir, as suggested by the Brant test and addressed by the PPO model46,47 (eTable 5 in Supplement 1). Nonlinear terms and secular effects were tested (eTable 6 and eTable 7 in Supplement 1). A subgroup analysis was done restricting the analytical file to individuals with a baseline eGFR level of 60 mL/min/1.73 m2 or higher. Sensitivity analyses restricted the outcome to AKI stages 2 and 3 (eTable 8 in Supplement 1). Exploratory analysis included other models of inheritance (codominant and dominant) (eTable 9 in Supplement 1) and dipstick proteinuria and hematuria. All statistical tests were 2-sided, where a P = .05 or a 95% CI that did not contain unity were considered statistically significant for the primary outcome. All analyses were conducted using R statistical software (version 3.6.1.; R Foundation, Inc).
Results
Baseline and Clinical Characteristics for the Participants
We studied 990 veterans enrolled in the MVP hospitalized with COVID-19 infection between March 2020 and January 2021, genotyped for APOL1 risk variants, who were of African ancestry and satisfied cohort entry criteria for AKI assessment (Figure 1). Baseline cohort characteristics by APOL1 genotype risk-group were summarized (Table 1). Clinical characteristics were similar across the different APOL1 risk groups, including baseline eGFR levels and the proportion of individuals with eGFR levels lower than 60 mL/min/1.73 m2, diabetes, and hypertension.
Table 1. Participants Clinical Characteristics According to APOL1 Genotype Risk Group.
| Characteristic | No. | APOL1, No. (%) | SMDa | |
|---|---|---|---|---|
| Low-risk group (1 or 0 risk variants) | High-risk group (2 risk variants) | |||
| No. | 865 | 125 | ||
| Baseline characteristics (outpatient) | ||||
| Sex | 0.13 | |||
| Female | 990 | 70 (8.0) | 15 (12.0) | |
| Male | 990 | 795 (91.9) | 110 (88.0) | |
| Age, median (IQR), y | 990 | 68.0 (60.0-73.0) | 68.0 (62.0-73.0) | 0.05 |
| Baseline | ||||
| eGFR, median (IQR), mL/min/1.73 m2b | 990 | 72.4 (56.5-88.3) | 70.9 (59.7-86.3) | 0.02 |
| eGFR <60 mL/min/1.73 m2 | 990 | 256 (29.6) | 33 (26.4) | 0.07 |
| Creatinine, median (IQR), mg/dL | 990 | 1.19 (1.00-1.43) | 1.18 (1.00-1.40) | 0.001 |
| BMI | 988 | 30.0 (25.6-34.8) | 28.7 (25.1-34.2) | 0.17 |
| Diabetes mellitus type 2 | 988 | 519 (60.1) | 73 (58.9) | 0.02 |
| Hypertension | 988 | 736 (85.2) | 104 (83.9) | 0.04 |
| Cardiovascular disease | 988 | 239 (27.7) | 42 (33.9) | 0.14 |
| Congestive heart failure | 988 | 165 (19.1) | 22 (17.7) | 0.04 |
| COPD | 988 | 236 (27.3) | 39 (31.5) | 0.09 |
| Liver disease | 988 | 90 (10.4) | 16 (12.9) | 0.08 |
| Human immunodeficiency virus | 990 | 4.3 (5) | 2 (0.2) | 0.19 |
| Outpatient dipstick proteinuria during the year prior | 614 | 0.06 | ||
| Negative or trace | 341 (63.9) | 49 (61.3) | ||
| 1+ | 96 (18.0) | 15 (18.8) | ||
| ≥2+ | 97 (18.2) | 16 (20.0) | ||
| Outpatient prescriptions | ||||
| RAAS inhibition the 180 d prior Inpatient characteristicsto hospitalization | 990 | 440 (50.9) | 65 (52.0) | 0.02 |
| Admission values, median (IQR) | ||||
| Serum albumin, g/dL | 821 | 3.60 (3.20-4.00) | 3.60 (3.20-3.90) | 0.15 |
| Serum creatinine, mg/dL | 922 | 1.30 (1.05-1.77) | 1.30 (0.98-1.80) | 0.03 |
| BP, mmHg | ||||
| Systolic | 976 | 132 (117-148) | 133 (119-148) | 0.02 |
| Diastolic | 974 | 78.0 (69.0-87.0) | 78.0 (70.0-88.0) | 0.10 |
| Hemoglobin, g/dL | 853 | 13.2 (11.8-14.4) | 13.3 (12.0-14.5) | 0.11 |
| Lymphocyte count, cells/mm3 | 835 | 5.65 (1.10-18.2) | 2.71 (0.90-17.9) | 0.02 |
| C reactive protein, g/L | 347 | 12.6 (5.40-68.5) | 20.6 (9.77-76.5) | 0.06 |
| Ferritin, ng/mL | 392 | 435 (226-833) | 454 (203-804) | 0.07 |
| Admission dipstick proteinuria | 357 | 0.4 | ||
| Negative or trace | 94 (31.2) | 13 (23.2) | ||
| 1+ | 79 (26.2) | 8 (14.3) | ||
| ≥2+ | 128 (42.5) | 35 (62.5) | ||
| Hospitalization characteristics | ||||
| Acute kidney injury | 990 | 328 (37.9) | 64 (51.2) | 0.3 |
| AKI severity stages | 990 | 0.3 | ||
| 0 | 537 (62.1) | 61 (48.8) | ||
| 1 | 217 (25.1) | 34 (27.2) | ||
| 2 | 46 (5.3) | 13 (10.4) | ||
| 3 | 65 (7.5) | 17 (13.6) | ||
| Length of stay | 990 | 6 (3-13) | 7 (3-13) | 0.05 |
| Vasopressors | 990 | 81 (9.4) | 17 (13.5) | 0.13 |
| Mechanical ventilation | 990 | 119 (13.7) | 22 (17.5) | 0.11 |
| Death | 990 | 98 (11.3) | 24 (19.2) | 0.22 |
Abbreviations: AKI, acute kidney injury; APOL1, apolipoprotein L1; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR estimated glomerular filtration rate; RAAS inhibition, renin angiotensin-aldosterone inhibition (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers); SMD, standard mean difference.
SMDs are the absolute difference in means or percentage divided by an evenly weighted pooled standard deviation, or the difference between groups in number of standard deviations.
Baseline eGFR is the mean outpatient baseline eGFR level −7 days to −365 days prior to the admission date.
Demographics and participant characteristics overall and by AKI status were summarized (Table 2). Overall, 905 (91.4%) were men, median (IQR) age was 68 (60-73) years, 701 (71%) had a GFR level of 60 mL/min/1.73 m2 or higher, with 493 (51%) of the participants hospitalized September 2020 or later (Table 2; eFigure 1 in Supplement 1).
Table 2. Participants Clinical Characteristics According to Acute Kidney Injury (AKI) Status.
| Characteristic | No. | No. (%) | SMD | |
|---|---|---|---|---|
| No AKI (n = 598) | AKI (n = 392) | |||
| Baseline characteristics (outpatient) | ||||
| Sex | .23 | |||
| Female | 990 | 66 (11.0) | 19.0 (5.0) | |
| Male | 990 | 532 (89.0) | 373.0 (95.2) | |
| Age, median (IQR), y | 990 | 66.0 (59.0-73.0) | 69.0 (63.0-74.0) | .25 |
| Baseline eGFR, median (IQR), mL/min/1.73 m2 | 990 | 77.0 (62.4-92.7) | 65.6 (48.8-80.8) | .52 |
| eGFR <60 mL/min/1.73 m2 | 990 | 132 (22.1) | 157 (40.1) | .40 |
| Body mass index, median (IQR) | 988 | 29.8 (25.6-34.2) | 29.9 (25.6-35.5) | .06 |
| Comorbidities | ||||
| Diabetes mellitus type 2 | 988 | 328 (54.9) | 264 (67.5) | .26 |
| Hypertension | 988 | 489 (81.9) | 351 (89.8) | .23 |
| Cardiovascular disease | 988 | 157 (26.3) | 124 (31.7) | .12 |
| Congestive heart failure | 988 | 104 (17.4) | 83 (21.2) | .10 |
| COPD | 988 | 158 (26.5) | 117 (29.9) | .08 |
| Cancer | 990 | 107(18.0) | 65(17.0) | .04 |
| Human immunodeficiency virus | 990 | 32 (5.0) | 13 (3.0) | .10 |
| Dipstick proteinuria, year prior | 614 | .41 | ||
| Negative or trace | 261 (70.4) | 129 (53.1) | ||
| 1+ | 63 (17.0) | 48 (19.8) | ||
| ≥2+ | 47 (12.7) | 66 (27.2) | ||
| Dipstick hematuria, year prior | 608 | .10 | ||
| Negative or trace | 273 (74.2) | 187 (77.9) | ||
| 1+ | 65 (17.7) | 34(14.1) | ||
| ≥2+ | 30 (8.1) | 19 (7.9) | ||
| Outpatient prescriptions | ||||
| RAAS inhibition | 990 | 269 (45.0) | 236 (60.2) | .31 |
| Inpatient characteristics | ||||
| Admission values, median (IQR) | ||||
| Serum creatinine, median (IQR), mg/dL | 922 | 1.1 (0.9-1.3) | 1.8 (1.4-2.6) | .99 |
| Blood pressure, median (IQR), mm Hg | ||||
| Systolic | 976 | 134.0 (121.0-150.0) | 128.0 (111.0-144.0) | .35 |
| Diastolic | 974 | 80.0 (72.0-89.0) | 74.0 (66.0-85.0) | .39 |
| Hemoglobin, median (IQR), g/dL | 853 | 13.1 (12.0-14.3) | 13.3 (11.7-14.7) | .04 |
| Lymphocyte count, median (IQR), cells/mm3 | 835 | 5.40 (1.20-19.8) | 5.95 (0.9-16.7) | .02 |
| C reactive protein, median (IQR), g/L | 347 | 11.4 (3.9-64.7) | 18.4 (7.8-77.8) | .15 |
| D Dimer, median (IQR), µg/mL FEU | 444 | 2.4 (0.9-346.0) | 2.3 (0.9-284.0) | .03 |
| Dipstick proteinuria on admission | 357 | .47 | ||
| Negative or trace | 74 (38.9) | 33 (19.8) | ||
| 1+ | 47 (24.7) | 40 (24.0) | ||
| ≥2+ | 69 (36.3) | 94 (56.3) | ||
| Dipstick hematuria on admission | 351 | .44 | ||
| Negative or trace | 117 62.9) | 72 (43.6) | ||
| 1+ | 47 (25.3) | 50 (30.3) | ||
| ≥2+ | 22 (11.8) | 43 (26.1) | ||
| Inpatient medicationsa | ||||
| RAAS inhibitors | 990 | 136 (22.7) | 57 (14.5) | .21 |
| NSAIDs | 990 | 49 (8.2) | 28 (7.1) | .04 |
| Vancomycin | 990 | 4 (0.7) | 15 (3.8) | .21 |
| Remdesivir | 990 | 182 (30.4) | 172 (43.9) | .28 |
| Dexamethasone, prior to peak creatinine | 990 | 131 (21.6) | 126 (32.1) | .23 |
| Steroids, anytime during hospitalization | 990 | 260 (43.5) | 237 (60.5) | .35 |
| Tocilizumab | 990 | 6 (1.0) | 24 (5.0) | .22 |
| Cefazolin | 990 | 47 (8.0) | 46 (12.4) | .19 |
| Beta-lactams | 990 | 8 (1.3) | 7 (1.9) | .12 |
| Inpatient characteristics | ||||
| Length of stay, median (IQR), d | 990 | 5 (2.0-10.0) | 10 (5.0-17.0) | .26 |
| Mechanical ventilation | 990 | 29 (4.9) | 112 (28.6) | .67 |
| Vasopressors | 990 | 10 (1.7) | 88 (22.0) | .67 |
| Death | 990 | 20 (3.3) | 102 (26.0) | .68 |
Abbreviations: COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; RAAS inhibition, renin angiotensin-aldosterone inhibition (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers): SMD, standard mean difference.
Unless otherwise specified, baseline eGFR level is the mean baseline eGFR −7 days to −365 days prior to the admission date.
The incidence of AKI was 392 (39.6%). Overall, AKI rates per month among study participants varied and are shown in eFigure 3 in Supplement 1. Of 392 individuals with AKI, 251 (25%) had stage 1, 59 (6%) stage 2, and 82 (8%) stage 3, including 28 (3%) who received dialysis due to AKI. Compared with individuals without AKI, individuals with AKI were older, and more likely to have eGFR levels lower than 60 mL/min, diabetes and hypertension, and positive baseline dipstick proteinuria.
Mechanical ventilation and vasopressors were required by 141 and 98 individuals, respectively. One-hundred twenty-two individuals died. Individuals with AKI were more likely than individuals without AKI to require mechanical ventilation (29% vs 5%, P < .001), vasopressors (22% vs 2%, P < .001), and to die (26% vs 3%, P < .001) (Table 2).
APOL1 Genotype
One-hundred twenty-five study participants (12.6%) carried 2 APOL1 risk alleles, consistent with frequencies reported in other studies of individuals with African ancestry in the US.4,5,6,7 However, 64 of 392 (16.3%) with AKI had 2 APOL1 RVs compared with 61 of 598 (10.2%) without AKI (P = .005). Furthermore, we observed a dose relationship with more severe forms of AKI, with stages 2 and stage 3 having 22.0% and 20.7% high-risk genotypes, respectively, with only 13.5% with stage 1 AKI having the high-risk genotype (eFigure 4 in Supplement 1).
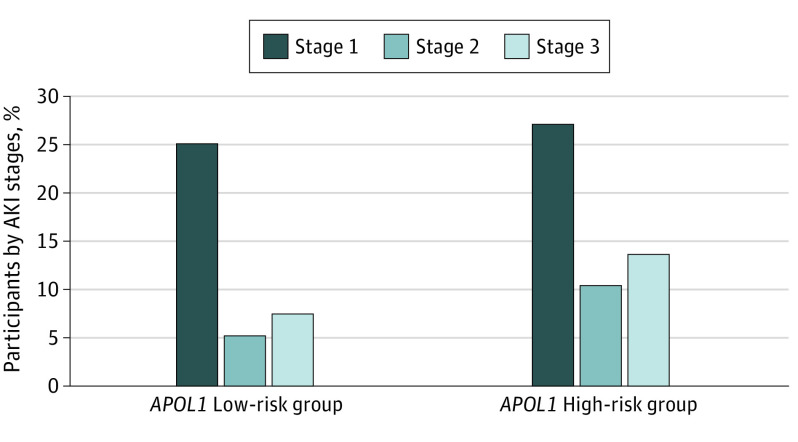
The APOL1 high-risk genotype doubled the risk of severe stages 2 and 3 AKI, with incidences of stages 2 and 3 AKI in the APOL1 high-risk group of 7 (13.6%) and 13 (10.4%), respectively, compared with 65 (7.5%) and 46 (5.3%) in the APOL1 low-risk group. For stage 1, the incidence was 27.2% for the APOL1 high-risk vs 25.1% for APOL1 low-risk group (Figure 2) (Table 1).
Figure 2. Incidence of Acute Kidney Injury (AKI) and AKI Stages by APOL1 Risk Group.
Association of APOL1 With Incident AKI and AKI Severity
In the minimally adjusted model (model 1), individuals with the APOL1 high-risk group had significantly increased odds of incident AKI (odds ratio [OR], 1.80; 95% CI, 1.21-2.69; P = .004). This association was also observed in the fully adjusted model (model 2) that included outpatient comorbidities and inpatient risk factors for incident AKI (OR, 1.95; 95% CI, 1.27-3.02; P = .002) (Table 3). For the association with AKI severity, we observed an increase of 88% in the odds of more severe stages of AKI (OR, 1.88; 95% CI, 1.30-2.71; P = .001) in model 1. The association was also observed in model 2 (OR, 2.03; 95% CI, 1.37-2.99; P < .001) (Table 3).
Table 3. Association of APOL1 High-Risk Group, AKI, AKI Stages, and Death in Veterans With African Ancestry Hospitalized With COVID-19a.
| Variable | No. | Primary outcome, acute kidney injury | Secondary outcomes | ||||
|---|---|---|---|---|---|---|---|
| AKI severity stages | Death | ||||||
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| All patients | |||||||
| Minimally adjusted | |||||||
| 2 Copies of APOL1 RVs | 125 | 1.80 (1.21-2.69) | .004 | 1.88 (1.30-2.71) | .001 | 1.92 (1.13-3.17) | .01 |
| 1 Or 0 copies of RVs | 865 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Fully adjusted model | |||||||
| 2 Copies of APOL1 RVs | 121 | 1.95 (1.27-3.02) | .002 | 2.03 (1.37-2.99) | <.001 | 2.15 (1.22– 3.72) | .007 |
| 1 Or 0 copies of RVs | 812 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Subgroup GFR ≥60 mL/min | |||||||
| Minimally adjusted | |||||||
| 2 Copies of APOL1 RVs | 92 | 1.88 (1.18-2.99) | .008 | 1.98 (1.28-3.06) | .002 | 2.54(1.32-4.72) | .004 |
| 1 Or 0 copies of RVs | 609 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Fully adjusted | |||||||
| 2 Copies of APOL1 RVs | 88 | 1.93 (1.15-3.26) | .01 | 2.11 (1.31-3.39) | .002 | 2.51(1.21-5.05) | .01 |
| 1 Or 0 copies of RVs | 569 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
Abbreviations: AKI, acute kidney injury; APOL1, apolipoprotein L1; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; RV, risk variants.
A recessive model of inheritance was used for these analyses. Logistic regression was used to evaluate the association of APOL1 high-risk group and AKI as a binary outcome. Partial proportional odds logistic regression was used to evaluate the association of APOL1 high-risk groups and AKI stages (controls, stage 1, stage 2 and stage 3). Model 1 or minimally adjusted: adjusted for age, sex, baseline eGFR and 10 PCs of ancestry. Model 2 or fully adjusted = model 1 + BMI, diabetes, hypertension, COPD, CHF, liver disease, smoking, systolic blood pressure, diastolic blood pressure, ACE inhibitor /ARBs outpatient (180 days prior to admission). Inpatient vancomycin, inpatient NSAID, ACE inhibitor/ARB inpatient, Remdesivir, dexamethasone (definitions included in eTable 3 in Supplement 1). All drug administration exposures were accounted for if they occurred prior to development peak creatinine.
Association of APOL1 With AKI and AKI Severity in the Subgroup of Individuals With Normal Baseline Kidney Function
Among individuals with baseline eGFR levels of 60 mL/min/1.73 m2 or higher (n = 701), participants in the APOL1 high-risk group had significantly increased odds of experiencing AKI in both model 1 (OR, 1.88; 95% CI, 1.18-2.99; P = .008) and in model 2 (OR, 1.93; 95% CI, 1.15-3.26; P = .01) (Table 3). There was also an increase in the odds of more severe stages of AKI in model 1 (OR, 1.98; 95% CI, 1.28-3.06; P = .002) and in model 2 (OR, 2.11; 95% CI, 1.31-3.39; P = .002) (Table 3).
Sensitivity Analysis
In the sensitivity analysis restricting the outcome to AKI stages 2 and 3, a 2-fold increase in the odds of AKI stages 2 and 3 in the APOL1 high-risk group was also noted, consistent with the primary analyses, both for the entire cohort and the subgroup with normal eGFR levels (eTable 8 in Supplement 1).
Association of APOL1 Risk Variants With Death, Mechanical Ventilation, and the Use of Vasopressors
There were 122 (12.3%) deaths overall; 24 (19.2%) in the APOL1 high-risk and 98 (11.3%) in the APOL1 low-risk group. The APOL1 high-risk group was associated with a 1.92 fold increase in the odds of death in model 1 (OR, 1.92; 95% CI, 1.13-3.17; P = .01) and in model 2 (OR, 2.15; 95% CI, 1.22-3.72; P = .007) (Table 3). This association was also present in the subgroup with normal eGFR levels, in model 1 (OR, 2.54; 95% CI, 1.32-4.72; P = .004) and model 2 (OR, 2.51; 95% CI, 1.21-5.05; P = .01). Overall, 102 (84%) individuals who died experienced AKI.
APOL1 high-risk genotype was not associated with mechanical ventilation (OR, 1.51; 95% CI, 0.86-2.55; P = .10) or a vasopressor requirement (OR, 1.61; 95% CI, 0.85-2.89; P = .10) in the fully adjusted models.
Exploratory Analyses
Association of APOL1 Risk Variants With Proteinuria and Hematuria
Urine dipstick proteinuria data were available in 614 participants at baseline. There was no association in the APOL1 high-risk group with outpatient baseline proteinuria in those with available measurements (OR, 1.12; 95% CI, 0.68-1.80; P = .60). Dipstick proteinuria data were available in 357 participants on admission. Ninety-six of 357 (27%) had new-onset proteinuria and 24 people had worsening dipstick proteinuria results. The APOL1 high-risk group was associated with proteinuria of 1+ or more on admission (n = 357) (OR, 2.20; 95% CI, 1.21-4.10; P = .01). There was no association of APOL1 high-risk group and hematuria defined as 1+ or more at baseline (n = 608) (OR, 0.95; 95% CI, 0.53-1.64; P = .90) or on admission (n = 351) (OR, 1.32; 95% CI, 0.74-2.31; P = .30).
Other Models of Inheritance
Other models of APOL1 inheritance were performed also as exploratory analyses. We observed significant associations with 2 RVs and AKI, AKI severity, and death in the codominant model, consistent with the recessive model. No associations were observed with a single RV (eTable 9 in Supplement 1).
Discussion
We report a robust association between individuals in the APOL1 high-risk group and AKI development and severity among participants with African ancestry hospitalized with COVID-19. Half of the participants with 2 APOL1 high-risk group variants developed AKI and 24 (19%) died. Because 1 in 8 individuals with African ancestry have 2 APOL1 kidney risk variants, this inherited genetic difference may in part explain the excess risk of AKI described in previous studies in veterans with African ancestry hospitalized owing to COVID-19.1 This increased risk of AKI was present even among individuals without preexisting CKD and followed a recessive model of inheritance, consistent with other kidney phenotypes.9,11,48 These associations were further accentuated among individuals with increasing AKI severity.
This study also identified an association of APOL1 high-risk group and death in participants of African ancestry hospitalized with COVID-19. Study participants baseline characteristics were similar between the APOL1 high-risk and low-risk groups. Recent studies report an association of APOL1 with endothelial cell defects, sepsis severity, and COVID-19 severity.3 Larger studies may further inform the association of APOL1 with death in the context of COVID-19.
In the general population, the association of APOL1 and AKI and APOL1 and death has been controversial.18,19,49,50 Potential explanations for the different findings include variation in the populations studied, outcome ascertainment, or the potential effects of a second hit in a given study setting.27 For example, not all individual carriers of 2 RVs will develop kidney disease and a second hit, including environmental or genetic modifiers, have been considered as playing a role.27,29 In the case of COVID-19, the heightened inflammatory response has been hypothesized to be a second hit. Similar hypotheses have been made in the contexts of uncontrolled HIV where the high levels of interferon are associated with the development of HIV-associated nephropathy and the risk of ESRD,51 or that observed with the therapeutic administration of interferon (IFNs).21
The pathophysiological mechanisms of APOL1 RVs remain incompletely characterized. Several studies have shown that APOL1 RVs represent gain of function variants with cytotoxic effects.52,53 When exposed to inflammatory triggers, podocytes and other specific cells overexpress APOL1, which compromises mitochondrial respiration, lysosome integrity, and autophagic flux resulting in cell death.54,55,56,57 Animal studies suggest that APOL1 variants impair mitophagy in endothelial cells, allowing the release of mitochondrial DNA that activates inflammasome.3 A second hit could also involve other genetic modifiers that can diminish cytotoxicity.48 The potential role of inflammation in the pathogenesis of AKI in the context of high-risk APOL1 and its relative contributions to different subtypes of AKI warrants further study.
The strengths of our study include the use of a large national data set with genetic data on participants with African ancestry and carefully curated creatinine-based definitions of AKI and KRT. The use of longitudinal phenotype data prior to COVID-19 infection enabled rigorous adjustment for baseline conditions and inpatient exposures that might influence the risk of AKI and severe COVID-19. Our estimates were robust and consistent across nested multivariable models, subgroup and sensitivity analyses that included known comorbidities associated with worse outcomes in COVID-19 and AKI. Further, these results were consistent among individuals without known CKD.
Limitations
Limitations of this study include reduced generalizability to women, the lack of quantitative proteinuria measurements, which precluded a subgroup analysis by proteinuria, or the detection of severe forms of nephrotic syndrome. The study lacked histologic data, with only 1 patient having a kidney biopsy during a COVID-19 hospitalization. Socioeconomic status (SES) indicators were not available, and some covariates were defined using diagnosis and procedure codes. However, covariate ascertaiment should not be differential by genotype. Another limitation of our study is that we could not identify a comparable replication cohort, which most likely will be available in the near future. Finally, our limited follow-up period reduced the ability to examine longer-term consequences of COVID-19–associated AKI.
Conclusions
In this cohort of participants with African ancestry hospitalized with COVID-19 infection, APOL1 RVs were associated with an increased risk of AKI, severe AKI, and death, independent of CKD. Our findings suggest that genetic risk assessment can inform COVID-19 kidney risk prognostication in individuals with African ancestry. Because APOL1 RVs are highly prevalent in the population with African ancestry, studies evaluating the role of existing and novel therapies are needed to reduce poor outcomes in this population.
eFigure 1. Proportion of cases admitted per month
eFigure 2. Days from COVID diagnosis to admission
eFigure 3. Rates of AKI in COVID-19 hospitalized participants with African ancestry enrolled in the Million Veteran Program
eFigure 4. Proportion of participants with 2 APOL1 risk variants by AKI status & AKI stages
eTable 1. ESRD definition (used for exclusion of prior ESRD)
eTable 2. Modified KDIGO AKI definition
eTable 3. Definitions and procedure codes for dialysis, comorbidities, and ventilator use
eTable 4. Goodness of Fit Test using Hosmer and Lemeshow test for the entire cohort (n=990)
eTable 5. Proportional odds assumption Brant test
eTable 6. Models with and without non-linear terms
eTable 7. Sensitivity analysis including month of admission in fully adjusted model
eTable 8. Association of APOL1 high risk group and AKI stages 2 & 3 in African ancestry veterans hospitalized with COVID-19
eTable 9. Association of APOL1 risk alleles and acute kidney injury and AKI stages and death in participants hospitalized with COVID-19 using co-dominant and dominant models
eTable 10. Participants clinical characteristics according to AKI stages
eTable 11. List of authors’ affiliations
eTable 12. MVP COVID-19 Science Program and MVP Core acknowledgement
List of investigators and staff in the VA Million Veteran Program
References
- 1.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2020;16(1):14-25. doi: 10.2215/CJN.09610620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.May RM, Cassol C, Hannoudi A, et al. A multi-center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 disease (COVID-19). Kidney Int. 2021;100(6):1303-1315. doi: 10.1016/j.kint.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Ma Z, Raman A, et al. APOL1 risk variants in individuals of African genetic ancestry drive endothelial cell defects that exacerbate sepsis. Immunity. 2021;54(11):2632-2649.e6. doi: 10.1016/j.immuni.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bick AG, Akwo E, Robinson-Cohen C, et al. ; VA Million Veteran Program . Association of APOL1 risk alleles with cardiovascular disease in blacks in the Million Veteran Program. Circulation. 2019;140(12):1031-1040. doi: 10.1161/CIRCULATIONAHA.118.036589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grams ME, Rebholz CM, Chen Y, et al. Race, APOL1 risk, and eGFR decline in the general population. J Am Soc Nephrol. 2016;27(9):2842-2850. doi: 10.1681/ASN.2015070763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22(11):2098-2105. doi: 10.1681/ASN.2011050519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen TK, Katz R, Estrella MM, et al. Association between APOL1 genotypes and risk of cardiovascular disease in MESA (multi-ethnic study of atherosclerosis). J Am Heart Assoc. 2017;6(12):6. doi: 10.1161/JAHA.117.007199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasser WG, Tzur S, Wolday D, et al. Population genetics of chronic kidney disease: the evolving story of APOL1. J Nephrol. 2012;25(5):603-618. doi: 10.5301/jn.5000179 [DOI] [PubMed] [Google Scholar]
- 9.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841-845. doi: 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Toole JF, Bruggeman LA, Madhavan S, Sedor JR. The cell biology of APOL1. Semin Nephrol. 2017;37(6):538-545. doi: 10.1016/j.semnephrol.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128(3):345-350. doi: 10.1007/s00439-010-0861-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipkowitz MS, Freedman BI, Langefeld CD, et al. ; SK Investigators . Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114-120. doi: 10.1038/ki.2012.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129-2137. doi: 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422-1426. doi: 10.1681/ASN.2010070730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsa A, Kao WH, Xie D, et al. ; AASK Study Investigators; CRIC Study Investigators . APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183-2196. doi: 10.1056/NEJMoa1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman BI, Kopp JB, Sampson MG, Susztak K. APOL1 at 10 years: progress and next steps. Kidney Int. 2021;99(6):1296-1302. doi: 10.1016/j.kint.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao B, Lu Q, Cheng Y, et al. ; TRIBE-AKI Consortium . A genome-wide association study to identify single-nucleotide polymorphisms for acute kidney injury. Am J Respir Crit Care Med. 2017;195(4):482-490. doi: 10.1164/rccm.201603-0518OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Privratsky JR, Li YJ, Haynes C, et al. Apolipoprotein L1 (APOL1) coding variants are associated with creatinine rise after cardiac surgery. J Cardiothorac Vasc Anesth. 2020;34(12):3314-3320. doi: 10.1053/j.jvca.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grams ME, Matsushita K, Sang Y, et al. Explaining the racial difference in AKI incidence. J Am Soc Nephrol. 2014;25(8):1834-1841. doi: 10.1681/ASN.2013080867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bird L. APOL1 variants contribute to racial disparity in sepsis. Nat Rev Immunol. 2021;21(12):759. doi: 10.1038/s41577-021-00647-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols B, Jog P, Lee JH, et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int. 2015;87(2):332-342. doi: 10.1038/ki.2014.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magoon S, Bichu P, Malhotra V, et al. COVID-19-related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020;2(4):488-492. doi: 10.1016/j.xkme.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoriello D, Khairallah P, Bomback AS, et al. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):2158-2167. doi: 10.1681/ASN.2020050744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudose S, Batal I, Santoriello D, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959-1968. doi: 10.1681/ASN.2020060802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velez JCQ, Caza T, Larsen CP. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. 2020;16(10):565-567. doi: 10.1038/s41581-020-0332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhary NS, Moore JX, Zakai NA, et al. APOL1 nephropathy risk alleles and risk of sepsis in blacks. Clin J Am Soc Nephrol. 2019;14(12):1733-1740. doi: 10.2215/CJN.04490419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruzel-Davila E, Wasser WG, Aviram S, Skorecki K. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant. 2016;31(3):349-358. doi: 10.1093/ndt/gfu391 [DOI] [PubMed] [Google Scholar]
- 28.Gaziano JM, Concato J, Brophy M, et al. Million Veteran Program: a mega-biobank to study genetic influences on health and disease. J Clin Epidemiol. 2016;70:214-223. doi: 10.1016/j.jclinepi.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 29.Friedman DJ, Pollak MR. APOL1 nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol. 2021;16(2):294-303. doi: 10.2215/CJN.15161219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman DJ, Pollak MR. Genetics of kidney failure and the evolving story of APOL1. J Clin Invest. 2011;121(9):3367-3374. doi: 10.1172/JCI46263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fihn SD, Francis J, Clancy C, et al. Insights from advanced analytics at the Veterans Health Administration. Health Aff (Millwood). 2014;33(7):1203-1211. doi: 10.1377/hlthaff.2014.0054 [DOI] [PubMed] [Google Scholar]
- 32.COVID-19: Shared Data Resource, 2020. Accessed October 15, 2021. https://vhacdwdwhweb100.vha.med.va.gov/phenotype/index.php/COVID-19:Shared_Data_Resource
- 33.Lynch KE, Deppen SA, DuVall SL, et al. Incrementally transforming electronic medical records into the observational medical outcomes partnership common data model: a multidimensional quality assurance approach. Appl Clin Inform. 2019;10(5):794-803. doi: 10.1055/s-0039-1697598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siew ED, Ikizler TA, Matheny ME, et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7(5):712-719. doi: 10.2215/CJN.10821011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Eckardt KU, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117-1129. doi: 10.1016/j.kint.2020.02.010 [DOI] [PubMed] [Google Scholar]
- 37.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694. doi: 10.1681/ASN.2006010073 [DOI] [PubMed] [Google Scholar]
- 38.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: The Veterans Health Administration COVID-19 (VACO) Index. PLoS One. 2020;15(11):e0241825. doi: 10.1371/journal.pone.0241825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gue YX, Tennyson M, Gao J, Ren S, Kanji R, Gorog DA. Development of a novel risk score to predict mortality in patients admitted to hospital with COVID-19. Sci Rep. 2020;10(1):21379. doi: 10.1038/s41598-020-78505-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemmelgarn BR, Manns BJ, Lloyd A, et al. ; Alberta Kidney Disease Network . Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423-429. doi: 10.1001/jama.2010.39 [DOI] [PubMed] [Google Scholar]
- 43.Hunter-Zinck H, Shi Y, Li M, et al. ; VA Million Veteran Program . Genotyping array design and data quality control in the Million Veteran Program. Am J Hum Genet. 2020;106(4):535-548. doi: 10.1016/j.ajhg.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang H, Hui Q, Lynch J, et al. ; VA Million Veteran Program . Harmonizing genetic ancestry and self-identified race/ethnicity in genome-wide association studies. Am J Hum Genet. 2019;105(4):763-772. doi: 10.1016/j.ajhg.2019.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson B, Harrell FE. Partial proportional odds models for ordinal response variables. Applied Stats. 1990;39:205-17. doi: 10.2307/2347760 [DOI] [Google Scholar]
- 47.Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990;46(4):1171-1178. doi: 10.2307/2532457 [DOI] [PubMed] [Google Scholar]
- 48.Limou S, Nelson GW, Kopp JB, Winkler CA. APOL1 kidney risk alleles: population genetics and disease associations. Adv Chronic Kidney Dis. 2014;21(5):426-433. doi: 10.1053/j.ackd.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukamal KJ, Tremaglio J, Friedman DJ, et al. APOL1 genotype, kidney and cardiovascular disease, and death in older adults. Arterioscler Thromb Vasc Biol. 2016;36(2):398-403. doi: 10.1161/ATVBAHA.115.305970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gutiérrez OM, Irvin MR, Zakai NA, et al. APOL1 nephropathy risk alleles and mortality in African American adults: a cohort study. Am J Kidney Dis. 2020;75(1):54-60. doi: 10.1053/j.ajkd.2019.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beckerman P, Susztak K. APOL1: the balance imposed by infection, selection, and kidney disease. Trends Mol Med. 2018;24(8):682-695. doi: 10.1016/j.molmed.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruggeman LA, O’Toole JF, Sedor JR. APOL1 polymorphisms and kidney disease: loss-of-function or gain-of-function? Am J Physiol Renal Physiol. 2019;316(1):F1-F8. doi: 10.1152/ajprenal.00426.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarthy GM, Blasio A, Donovan OG, et al. Recessive, gain-of-function toxicity in an APOL1 BAC transgenic mouse model mirrors human APOL1 kidney disease. Dis Model Mech. 2021;14(8):14. doi: 10.1242/dmm.048952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lan X, Jhaveri A, Cheng K, et al. APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol. 2014;307(3):F326-F336. doi: 10.1152/ajprenal.00647.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beckerman P, Bi-Karchin J, Park AS, et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med. 2017;23(4):429-438. doi: 10.1038/nm.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Granado D, Müller D, Krausel V, et al. Intracellular APOL1 risk variants cause cytotoxicity accompanied by energy depletion. J Am Soc Nephrol. 2017;28(11):3227-3238. doi: 10.1681/ASN.2016111220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olabisi OA, Zhang JY, VerPlank L, et al. APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci USA. 2016;113(4):830-837. doi: 10.1073/pnas.1522913113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Proportion of cases admitted per month
eFigure 2. Days from COVID diagnosis to admission
eFigure 3. Rates of AKI in COVID-19 hospitalized participants with African ancestry enrolled in the Million Veteran Program
eFigure 4. Proportion of participants with 2 APOL1 risk variants by AKI status & AKI stages
eTable 1. ESRD definition (used for exclusion of prior ESRD)
eTable 2. Modified KDIGO AKI definition
eTable 3. Definitions and procedure codes for dialysis, comorbidities, and ventilator use
eTable 4. Goodness of Fit Test using Hosmer and Lemeshow test for the entire cohort (n=990)
eTable 5. Proportional odds assumption Brant test
eTable 6. Models with and without non-linear terms
eTable 7. Sensitivity analysis including month of admission in fully adjusted model
eTable 8. Association of APOL1 high risk group and AKI stages 2 & 3 in African ancestry veterans hospitalized with COVID-19
eTable 9. Association of APOL1 risk alleles and acute kidney injury and AKI stages and death in participants hospitalized with COVID-19 using co-dominant and dominant models
eTable 10. Participants clinical characteristics according to AKI stages
eTable 11. List of authors’ affiliations
eTable 12. MVP COVID-19 Science Program and MVP Core acknowledgement
List of investigators and staff in the VA Million Veteran Program