Abstract
Malperfusion syndrome (MPS) complicating acute type A aortic dissection (ATAAD) poses a continuing challenge and management dilemma for cardiovascular surgeons. MPS may involve any of the major arterial side branches resulting in myocardial, cerebral, spinal cord, visceral, and/or limb ischemia with varying frequency and severity. Despite the continuous improvement in diagnosis and management strategies for MPS with ATAAD, clinical outcomes remain poor and the optimal therapy is still debatable. The present review aimed to assess current evidence on ATAAD patients with MPS and how best to handle the challenge.
Keywords: Aortic dissection, Malperfusion syndrome, Open surgery, Endovascular repair
Introduction
Aortic dissection (AD) is defined as the disruption of the medial layer of the aortic wall leading to separation and formation of a true lumen (TL) and false lumen (FL) with or without communication. The incidence of AD is estimated at 6 per 100,000 persons per year [1, 2]. The International Registry of Aortic Dissection (IRAD) series revealed 67% of patients presented with type A AD and the remaining 33% type B. Two-thirds were men and the mean age was 63 years [3, 4]. Malperfusion syndrome (MPS) occurs anywhere from 16 to 34% and can happen with both acute type A and type B AD [3–6]. It is the second most common lethal complication of acute type A aortic dissection (ATAAD) after rupture. The early diagnosis and the extent of MPS are crucial in determining the initial treatment for the patient. Delay in diagnosing MPS or referring to an aortic center may increase fatality and reduce survival [7, 8]. Longer time intervals between the ATAAD and subsequent diagnosis with treatment will result in a higher probability of irreversible end-organ ischemia with death of the patient [9]. Patients who present with end-organ malfunction due to acute ischemia need immediate reperfusion with subsequent metabolic stabilization for the highest likelihood of survival [7–9]. In patients with MPS, the optimal therapeutic management is controversial, and several issues remain debatable [10, 11].
Definition and pathophysiology
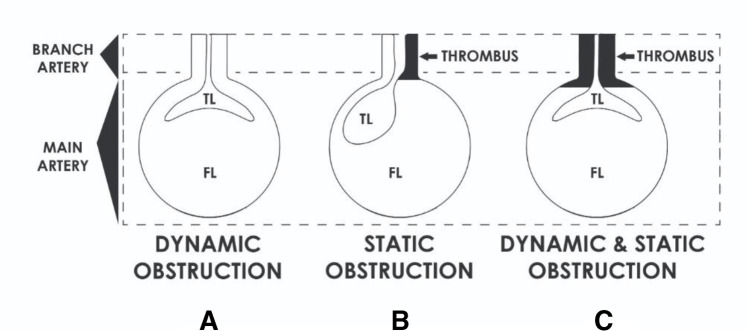
Malperfusion is defined as inadequate supply of oxygenated blood to a vital organ caused by branch arterial obstruction secondary to the dissection. Left uncorrected will lead to end-organ infarction, which is known as MPS. Understanding the pathogenesis of MPS is crucial and important to aid in the diagnosis and management. The mechanisms attributing to the obstruction of aortic branches can be classified as either dynamic, static, or both depending on the pattern of anatomic obstruction, which can result in intermittent or persistent malperfusion of the organs (Fig. 1) [11–13].
Fig. 1.
Drawings illustrating the pathophysiology and mechanism of malperfusion syndrome in branch artery resulting from aortic dissection: (A) dynamic obstruction, (B) static obstruction, (C) combined dynamic and static obstruction
Dynamic obstruction is a more common cause of MPS and is responsible for approximately 80% of cases [14]. There are two distinct aetiologies of dynamic malperfusion. First, insufficient flow through the TL may lead to hypoperfusion when branch vessel perfusion is maintained by the TL. The second mechanism of dynamic obstruction refers to the intimal flap rolling over and obstructing the orifice of a branch vessel to a vital organ [15]. This relationship between the mobile FL and branch vessel lumen is depicted in Fig. 1A. Dynamic obstruction is intermittent in nature and malperfusion may vary depending on changes in blood pressure and hemodynamic forces. Therefore, it can be adequately managed initially with medical therapy by controlling the blood pressure and hemodynamics. However, invasive interventions are needed if this fails [16, 17].
Static obstruction is characterized by narrowing or occlusion of branch vessels as results of protrusion of FL into the branch vessel with associated thrombosis (Fig. 1B). The combination of the hypercoagulable state and stasis within the blind end of the FL leads to thrombosis causing compression and obstruction of the TL [17]. This cannot be corrected with medical management and will require some form of invasive interventions [14, 16]. Patients can present with either dynamic (Fig. 1B), static, or a combination of both forms (Fig. 1C) of malperfusion and that is why it is essential to differentiate the status of malperfusion with angiogram prior to determining the modality of definitive therapy [17].
Incidence and diagnosis
The IRAD study cohort revealed clinical features of MPS were reported in 20–30% of patients and associated with poorer outcomes [3, 18]. Patients who died in hospital suffered from an increased rate of malperfusion complications, such as neurological deficits (24% vs. 15%), myocardial ischemia (15% vs. 9%), visceral ischemia (6% vs. 2%), renal failure (11% vs. 3%), and limb ischemia (14% vs. 7%), compared with survivors (p < 0.05) [18].
A total of 274 patients presented with ATAAD to the National Heart Institute of Malaysia (NHIM) between January 2000 and December 2020. There were 79 (28%) females and 195 (72%) males with average age of 52.0 years. Sixty-four (23.4%) patients presented with MPS (coronary MPS: 15 (23%); cerebral MPS: 14 (21%); spinal MPS: 2 (3%); mesenteric MPS: 6 (9%); renal MPS: 19 (30%); limb MPS: 8 (13%)). The incidence from our series correlates with the IRAD cohort study.
Combinations of patient history, physical examination, and investigations are crucial to make the diagnosis of malperfusion. The most common and classical presentation for ATAAD is excruciating chest pain radiating not only confined to the back but may also radiate to the neck, abdomen, pelvis, and the extremities. Persistent pain in any of these other body areas should alert the possibility of branch vessel occlusion with malperfusion and MPS.
Physical examination such as absence of peripheral pulses should alert the care provider of the likelihood of malperfusion. The presence of pulse deficits has long been recognized as a marker for malperfusion [19]. Pulse deficits were detected in nearly a third of IRAD patients and were found to be an independent predictor of early mortality [19]. Hospital mortality varied substantially according to the number of vessels involved; 24.7% in patients with no pulse deficits, and 36.2%, 48.9%, and 55.9% in patients who had decreased or absent pulsation in 1, 2, and 3 vessels, respectively (p < 0.001). Similarly, in-hospital adverse events occurred more frequently in the group with pulse deficits. Neurologic deficits (35% vs. 11%) and coma (27% vs. 9.1%) were threefold greater, renal failure 2 times higher (10% vs. 4.6%), and limb ischemia almost 14 times more frequent (29% vs. 2.1%) in patients presenting with pulse deficits than in those without.
Furthermore, patients with pulse deficits were more likely to have hypotension at presentation [19, 20]. The latter, defined as a systolic blood pressure ≤ 90 mmHg, was documented in > 25% of IRAD patients and was associated with a much higher rate of malperfusion complications and in-hospital mortality (55% vs. 10%, P < 0.001). In a series of 1073 patients with acute AD, Tsai et al. [20] reported incidence of neurologic deficits, myocardial ischemia, mesenteric ischemia, and limb ischemia as of 23%, 15%, 7%, and 15%, respectively, in patients with hypotension which were higher than compared to 12%, 7%, 3%, 7%, and 10%, respectively, in patients without hypotension (P < 0.001) [20]. Thus, IRAD data suggest that the occurrence of both pulse deficits and hypotension correlates with malperfusion complications and should move caregivers toward timely surgical or percutaneous interventions to re-establish blood flow to vital organs. Prolonged time intervals between the initial symptoms of ATAAD and confirmation of the diagnosis with subsequent treatment will affect in a greater likelihood of irreversible end-organ ischemia with poor patient prognosis. Time to operation was a predictive factor of survival in this high-risk category of patients.
In addition, abdominal tenderness, guarding and/or rebound tenderness, as well as flank tenderness should alert the examiner of potential mesenteric ischemia. Any new motor or sensory deficit of an extremity is also a characteristic of MPS of the extremities. Obviously, any new neurologic deficit should alert the examiner of the possibility of neurologic ischemia.
Laboratory result abnormalities are supportive and characteristic of MPS. A base deficit with metabolic acidosis, elevation of serum lactate levels, liver function tests, amylase and lipase levels, blood urea nitrogen and creatinine levels, myoglobin, total creatinine kinase, and clotting indicators (prothrombin time and international normalized ratio) are all evidence for end-organ malperfusion [21].
An imaging study such as computed tomographic angiography (CTA) scan has replaced conventional angiography in diagnosing AD and MPS, well-illustrated in Figs. 2, 3, 4, and 5. With the advent of three-dimensional reconstruction, topographic relationships of the TL and FL may be elucidated. Intraluminal thrombus is useful in identification of the FL although not completely specific. In over 90% of dissections, the FL diameter is larger than the TL [22]. Radiographic evidence of compression of the TL raises concern for MPS. With the presence of biochemical derangements suggestive of ischemia, CTA may provide additional evidence for revascularization.
Fig. 2.
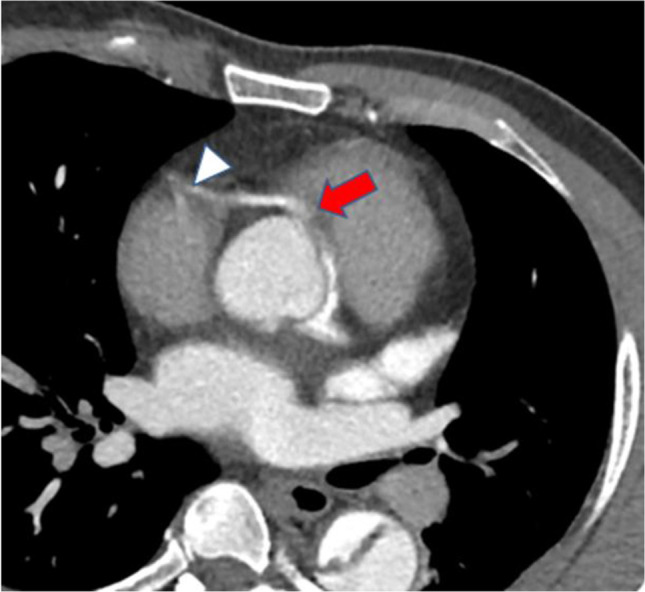
Axial CTA image at aortic root: type A dissection involving origin of the right coronary artery (red arrow), causing stenosis of its orifice and poor flow distally (white arrowhead). The left main coronary artery arises from the true lumen showing dense opacification. CTA, computed tomographic angiography
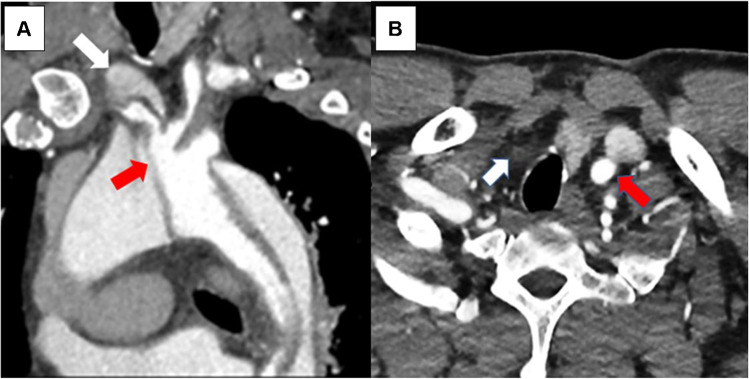
Fig. 3.
CTA images: type A aortic dissection extending into innominate, right common carotid, and right subclavian arteries. A Oblique multiplanar reconstruction of aortic arch showing intimal flap from arch extending into the innominate artery (red arrow). The flow in the right subclavian artery is mainly from false lumen appearing hypodense compared to true lumen (white arrow). B Axial section of the lower neck showing dense opacification of the left common carotid artery (red arrow) and non-opacification of the right common carotid artery (white arrow). CTA, computed tomographic angiography
Fig. 4.
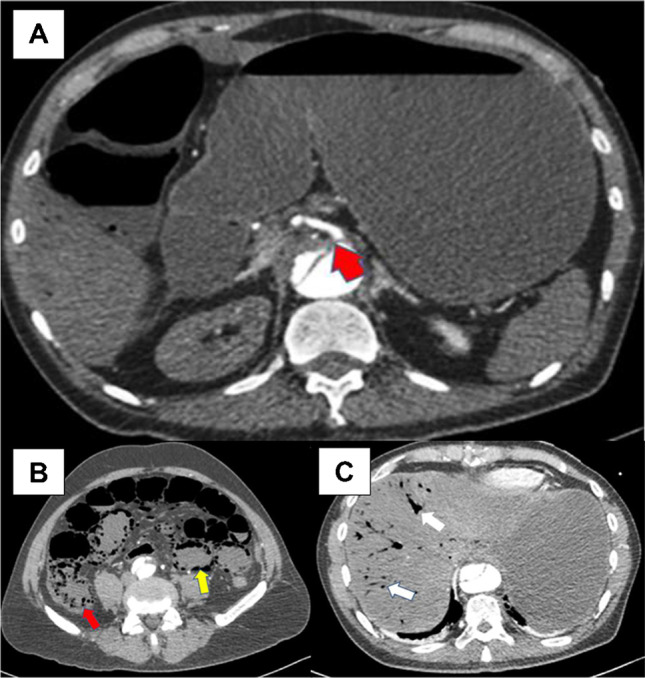
Axial CTA images: aortic dissection causing malperfusion of SMA resulting in small bowel and colonic infarction. A Malperfusion involving the SMA, causing severe stenosis of its origin (red arrow). B Small and large bowel infarction with intramural air in small bowel (yellow arrow) and ascending colon (red arrow). C Intrahepatic portal vein air (white arrows) due to small bowel infarction. CTA, computed tomographic angiography; SMA, superior mesenteric artery
Fig. 5.
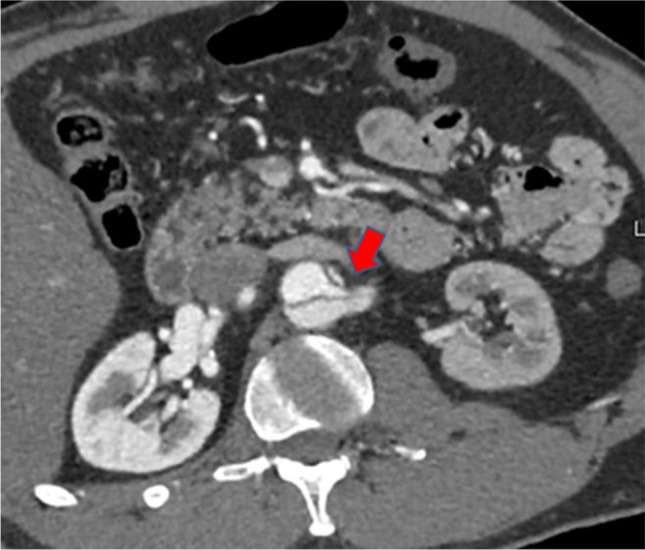
Axial CTA image: aortic dissection involving the left renal artery (red arrow), with malperfusion to the left kidney. The left kidney is hypodense compared to the normal right kidney. CTA, computed tomographic angiography
Definitive diagnosis of malperfusion can be made using angiography augmented with intravascular ultrasound and manometry [14, 16]. Although it is not readily available in many units, these modalities help in confirming the diagnosis and guiding us in choosing the right treatment modality.
Myocardial malperfusion
Coronary malperfusion complicates 10–15% of ATAAD cases [6, 23–25]. It may be due to static occlusion by extension of the AD into a coronary artery ostium and thrombosis, a dynamic flap occlusion at the level of the coronary sinuses covering the coronary ostia in diastole, pre-existing coronary disease, hypotension, or a combination of these [26]. It is nicely illustrated in CTA, with the malperfusion in the right coronary artery (RCA) (Fig. 2). In IRAD, ischemic electrocardiogram (ECG) abnormalities were observed in 17.3% of ATAAD, and findings of myocardial infarction (new Q waves or ST segments) in 7.1% [3]. The ECG diagnosis of non-transmural ischemia may be difficult in patients with concomitant left ventricular hypertrophy, which is found in 25% of patients with ATAAD. Troponin elevation may be found in also 25% of the patients with ATAAD [27].
The chest pain, ECG ischemic abnormalities, and elevated cardiac markers may mislead clinicians into considering a possible diagnosis of acute coronary syndrome and may expose the patient to inappropriate, and potentially harmful, coronary angiogram and treatments with antithrombotic agents [28]. In this setting, clinical laboratory findings and bed side echocardiography have been proposed to facilitate the timely and accurate diagnosis of ATAAD. An aortic dissection detection risk (ADDR) score for identification of acute AD at initial presentation was created on the basis of several clinical risk markers reported in the 2010 American Heart Association and American College of Cardiology guidelines [29]. The proposed ADDR score encompassed a risk assessment tool based on 3 groups of information—predisposing conditions, pain features, and clinical examination. The score ranges from 0 (none) to 3 (high probability) of the risk of ATAAD. In the IRAD study cohort, this diagnostic screening tool demonstrated satisfactory sensitivity (> 95%) to capture the vast majority of patients presenting with ATAAD [30]. Moreover, the IRAD substudy on Biomarkers (IRAD-Bio study) showed that d-dimer levels were markedly elevated in ATAAD and that useful in risk stratifying patients with suspected AD to rule out ATAAD if used within the first 24 h after symptom onset [30]. In ATAAD, the presence of cardiac malperfusion has been associated with poor surgical outcomes [31]. Rampoldi et al. [31] revealed that myocardial ischemia and infarction (odds ratio (OR) 1.76) and the necessity to perform coronary revascularization (OR 2.54) were independent preoperative predictors of mortality. Not surprisingly, preoperative left and/or right ventricular dysfunction were also strongly associated with high surgical mortality. However, while myocardial malperfusion carries an increased risk of operative mortality, timely intervention restoring coronary perfusion is the only viable treatment in these critically ill patients, and surgical aortic repair with or without revascularization still remains the treatment of choice. In very selected patients, emergency percutaneous coronary intervention (PCI) may be considered as a treatment option as a bridge to surgery . However, PCI can be technically challenging and potentially time consuming in ATAAD, and has no effect in treating the ongoing dissection process, may involve further injury of the aortic wall, and requires post-procedure antithrombotic medications that result in increased risk of bleeding, rupture, and/or tamponade.
In our NHIM study, 15 patients presented with coronary MPS and 8 patients survived with urgent repair (5 with revascularization; 3 without revascularization). Seven patients died (4 from urgent repair with revascularization; 2 from massive myocardial infarction while stabilization, and 1 from aortic rupture).
Based on the current evidence and our experience, ATAAD with coronary MPS should be attended immediately. Our recent, preferred cannulation is central aorta, using Seldinger method and bicaval venous cannulation. Myocardial protection will include retrograde cardioplegia. Based on Neri’s classification, we would do a direct repair for type A (ostial dissection) injury which includes trimming and reinforcing with Teflon strips before reimplanting [32]. Where-else for type B (dissection with coronary false channel) and type C (circumferential detachment with an inner cylinder intussusception), we would perform coronary artery bypass grafting using saphenous veins with the proximal anastomosed onto the graft.
Cerebral malperfusion
Cerebral malperfusion occurs in 6–14% of type A aortic dissection (TAAD) patients and results from partial or complete occlusion of the arch vessels by the intimal-medial flap, hypoxic encephalopathy secondary to shock or tamponade, and/or brain embolism from thrombus in the FL [3–6, 33]. Pacini et al. [34] defined cerebral malperfusion as stroke or transient ischemic attack, while Morimoto et al. [35] defined it as newly developed neurologic deficits along with evidence from CTA or carotid ultrasonography of decreased blood flow in carotid arteries. Image modality of CTA and computed tomography (CT) of the brain is crucial to assist in diagnosing cerebral MPS (Fig. 3). Clinical manifestations of stroke or coma were shown to be predictors for detrimental outcomes, and optimal management of ATAAD patients with cerebral MPS remains controversial. IRAD data showed that nearly 1 out of 10 ATAAD patients are complicated by major brain injury at the onset of dissection (cerebrovascular accident (CVA) 4.7%; coma 2.9%) [33]. Patients presenting with coma were more hemodynamically compromised with a greater incidence of hypotension, shock, and/or tamponade leading to end-organ dysfunction. Therefore, patient survival carrying a twofold or threefold higher mortality for patients with CVA and coma, respectively (CVA 40%; coma 60%; no brain injury 23%, p < 0.001) [21].
Major brain injury at presentation has long been considered a contraindication to emergent surgery with several authors proposing a delayed surgical approach after the neurologic status has improved [36, 37]. The concern is the risk of conversion to hemorrhagic infarction after restoration of blood flow and use of anticoagulation during cardiopulmonary bypass. At IRAD enrolling centers, presence and type of brain injury clearly influenced patient management. Surgery was not performed in 24% of patients with CVA and 33% of patients with coma, compared with 11% of patients without brain injury. However, when assessing hospital outcomes according to therapeutic management, the investigators showed that medical therapy was associated with dismal outcomes: 100% mortality in patients with coma and 76.2% in those with CVA. Conversely, surgery was found to be a protective factor against mortality (OR 0.058; p < 0.001), leading to a 50% survival benefit over medical management [3, 5, 21]. Nakamura et al. shared an excellent result of early operation in patients with cerebral MPS and ATAAD [38].
As the brain is sensitive to ischemic damage, minimizing the ischemic time is crucial to increase the chances of successful neurologic recovery. Over the last decade, a number of reports have documented the value of urgent aortic repair suggesting a cut-off value of 9–10 h for predicting lack of neurologic improvement [35, 37–39]. Nevertheless, IRAD data revealed that, despite longer interval times from symptoms to surgery (CVA 12.3 h, coma 13.8 h), CVA and coma resolved in 84% and 79% of patients. Moreover, evidence of reversal of brain injury was a protective factor against mortality, in the surgically managed population [33]. Therefore, the observations coming from IRAD indicate that ATAAD patients with neurologic injury should always be considered for intervention, especially if early surgery is feasible and there are no signs of neurologic devastation such as hemorrhagic stroke.
In our NHIM study, 14 patients presented with cerebral MPS (6 transient ischemic attack (TIA); 5 focal neurological deficits; 3 coma). Eleven patients survived (8 immediate operation; 3 delayed operation). All 3 patients with coma subjected to conservative management died.
As long as there is no hemorrhagic stroke and patient presenting within 10 h after the onset of the symptoms, we would perform immediate aortic repair followed by early rehabilitation. The choice of cannulation is the right axillary artery, using deep hypothermic circulatory arrest (22 °C) and selective antegrade cerebral perfusion for an established cerebral MPS. We would directly repair or reconstruct the proximally injured carotid artery, with hemiarch replacement. If the injury extends beyond one-third of the carotid artery, we would consider bypassing the carotid artery. In extensive intimal tear involving the arch and more than one neck vessel, we would consider combined root replacement plus total arch replacement with frozen elephant trunk with separate anastomosis of the neck vessels.
Spinal malperfusion
Isolated spinal malperfusion is a very rare entity, occurring about 1–3% of ATAAD [3–6, 40]. Spinal MPS is due to interruption of blood flow to the anterior spinal artery that is also supplied by the radicular arteries, especially in the T10–T12 region. The patient may present with acute paraparesis or paraplegia with presence of lower limb pulses. However, more often, spinal MPS is associated with mesenteric and lower limb ischemia masquerading the signs and symptoms. Sudden spinal cord injury is often incomplete and transient [13]. Spinal MPS is not a contraindication for surgery. Isolated spinal MPS requires urgent proximal aortic repair to establish the blood flow to the anterior spinal artery [13]. Persistent paraplegia after surgery may require higher perfusion pressure and cerebral-spinal fluid (CSF) drainage to improve circulation [41, 42].
In our NHIM study, 2 patients presented with paraparesis due to spinal MPS. Both survived with early operation. One had persistent postoperative paraparesis which recovered fully after 72 h of CSF drainage.
Mesenteric malperfusion
Mesenteric MPS is rare but among the most insidious and detrimental complication of ATAAD occurring in approximately 4–6%, from large multicentered registries [6, 43]. The perfusion disturbance can be intermittent, static, or mixed misleading to a spectrum of clinical pictures resulting in difficulty of diagnosing and managing mesenteric MPS. IRAD data [43] showed abdominal pain did not occur in more than 40% of patients with mesenteric ischemia, whereas about 20% of patients without mesenteric malperfusion had pain. Thus, abdominal pain, while important, is a non-specific symptom of acute mesenteric ischemia [44]. Consequently, the diagnosis is frequently too late to save the patient and the bowel as illustrated in CTA involving superior mesenteric artery (Fig. 4). Mesenteric malperfusion was frequently associated with clinical or imaging signs of other organ injuries, such as coma (10%), ischemic spinal cord damage (6.8%), acute renal failure (52.2%), and limb ischemia (38.5%), that may further complicate and delay the diagnosis [43].
ATAAD complicated by mesenteric malperfusion has been one of the strongest risk factors for early mortality (OR 2.5) and associated with extremely poor outcomes [44, 45]. While there is still ongoing debate, general consensus of early reperfusion is critical for mesenteric malperfusion. It is not clear whether it is best to perform initial central aortic repair or percutaneous and/or extra-anatomic reperfusion [45, 46]. In view of the unpredictable nature of ATAAD, some groups suggest immediate central aortic repair followed by investigation and treatment of residual malperfusion [7, 47]. Conversely, other authors recommend initial catheter-based end-organ reperfusion followed by delayed central aortic repair for selected patients with visceral ischemic dysfunction [8, 17, 48]. At IRAD centers, patients presenting with mesenteric malperfusion were less likely to undergo surgical/hybrid treatment (53% vs. 88%) and more likely to receive endovascular (16% vs. 1%) or medical (31% vs. 12%) management, compared to uncomplicated patients. These data undoubtedly reflect a resistance of surgeons to proceed with open surgery in such patients. At the same time, when assessing hospital mortality according to different therapeutic management, surgical/hybrid therapy was associated with superior clinical outcomes; in-hospital mortality was 41.7%, 72.7%, and 95.2%, in patients who underwent surgical/hybrid, endovascular, and medical treatment, respectively (P < 0.001). In addition, surgical/hybrid management emerged as a protective factor for early mortality in patients deemed operable by IRAD investigators. However, hybrid management (central aortic operation plus percutaneous treatment of mesenteric malperfusion) was performed in only a very few cases, and central aortic repair still represents the most common therapeutic approach, in this setting [43]. Yet, when visceral ischemia is clinically manifested and advanced, percutaneous fenestration with or without stenting to perfuse the ischemic organs, as an initial procedure and wait for resolution of organ failure before open central repair may be more likely to achieve patient survival in extremely high-risk individuals [45]. Contrariwise, in patients with malperfusion but no significant advanced end-organ dysfunction or peritonitis, proximal repair should occur first [17]. Thus, the complexities of management in such patients must require a prompt referral to dedicated team equipped with a full array of interventional, hybrid, and surgical techniques.
In our NHIM study, 6 patients presented with mesenteric MPS. Five died (2 had immediate repair with post operation bowel resection; 3 died from aortic rupture during stabilization). One patient survived with endovascular fenestration with delayed operation.
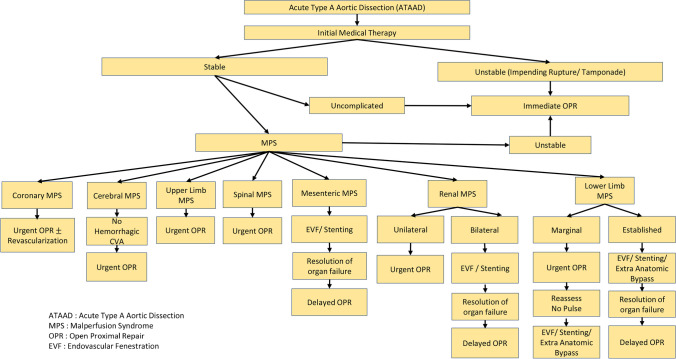
For established mesenteric MPS, we would recommend endovascular fenestration with or without stenting to resume mesenteric circulation and observe the patients to determine if an exploratory laparotomy is needed based on clinical features, increasing lactate and pneumatosis of the intestine on CT scan. If no laparotomy done or performed with or without bowel resection, we should wait for resolution of organ failure and normalization of lactate before central repair undertaken (Fig. 6).
Fig. 6.
Flow diagram showing how we will handle MPS with ATAAD
Renal malperfusion
The kidneys remain one of the most common organ systems affected by AD-mediated malperfusion [13]. Acute kidney injury (AKI) is common in the setting of ATAAD, mounting up to 20%, owing to multiple predisposing factors [3, 5, 6]. Acute tubular necrosis (ATN) may result from hypoperfusion perpetuated by an obstructive dissection flap, contrast-induced injury, and even relative hypotension from strict blood pressure control and may significantly limit kidney function. Though iodinated contrast agents are nephrotoxic, they are often required to correctly delineate both TL and FL as well as to identify organ perfusion as shown in Fig. 5. In this setting, contrast-mediated nephropathy is relatively common, especially when administered to poorly perfused kidneys. The wide differential makes renal malperfusion an elusive diagnosis; thus, providers must remain vigilant in attempting to identify ongoing renal malperfusion. Beyond its effect on long-term morbidity, AKI is an independent predictor of increased operative mortality and steps to detect renal malperfusion and mitigate its influence are crucial [18]. Serial testing of creatinine and monitoring of urine output are needed for early detection of renal MPS.
For isolated renal MPS, urgent open aortic repair with an aggressive renal replacement therapy is recommended, in view of a less life-threatening condition [49]. Bilateral renal MPS with deteriorating renal insufficiency poses an urgent need to relief the renal ischemia. Recommendation is urgent fenestration with or without stenting and medical stabilization followed by delayed open aortic repair [49].
In our NHIM study, 19 patients presented with renal MPS (15 had unilateral; 4 bilateral). Fifteen patients survived (11 urgent operation; 3 delayed operations after dialysis; 1 delayed operation after endovascular fenestration) and 4 patients died from aortic rupture.
Our strategy for renal MPS depends upon whether it is unilateral or bilateral involvement and is summarized in Fig. 6.
Limb malperfusion
Limb ischemia is an uncommon primary presentation of AD and was reported in 9.7% of IRAD cohort [1, 4, 7]. This complication was significantly associated with acute renal failure (OR, 2.78), acute mesenteric ischemia (OR, 6.9), and death (OR, 3.5). Femoral pulse deficit implies iliac artery obstruction and seems to be well tolerated in the short term. This is likely due to one or more ameliorating anatomic features of dissection, including the presence of a dynamic flap obstruction allowing intermittent flow, partial obstruction from a static dissection flap, or the presence of sufficient collaterals, including iliolumbar, internal iliac, deep circumflex, epigastric, and deep femoral arteries. Charlton-Ouw et al. [50] reported that extremity ischemia will resolve after proximal aortic repair in most cases. Only 21.6% of patients with lower limb ischemia required peripheral revascularization after proximal aortic repair. All patients with preoperative upper limb ischemia had postoperative resolution of their symptoms. By proceeding directly to aortic repair, they avoided unnecessary peripheral revascularization procedures and mortality during the period between peripheral revascularization and proximal aortic repair, which can be as high as 33% [17, 50]. They also recommend axillofemoral bypass for the uncommon cases of persistent bilateral lower limb ischemia and femoral-femoral crossover bypass for unilateral cases. Endovascular revascularization, including percutaneous aortic fenestration and branch artery stenting, has also been described as a bridge to central aortic repair to salvage the limb and correction of metabolic derangements [16, 49].
In our NHIM study, 8 patients presented with limb MPS (4 upper limb; 4 lower limb). Six patients survived. Urgent proximal aortic repair resolved all 4 upper limb MPS cases and 2 had aortic fenestration and femoral stenting. Two died from aortic rupture during stabilization.
Our approach in tackling the limb ischemia will depend upon whether the obstruction is marginal or established (Fig. 6). We would proceed with the open proximal repair for marginally threatened lower extremities and reassess the femoral pulses. Axillofemoral or axillobifemoral is indicated for patients with absence of femoral pulses even after central repair. This can be undertaken at the same setting. Apart from our usual central cannulation, we do canulate both femoral arteries and maintain partial cardiopulmonary bypass (CPB) for perfusing lower extremities especially when during circulatory arrest is a definite advantage in limiting ischemia. For established limb MPS, we would opt for endovascular fenestration and branch artery stenting, as a bridge to central aortic repair to salvage the limb and correction of metabolic derangements.
Conclusions
The presence of ATAAD with MPS is an important adverse factor for immediate and long-term survival, especially in the setting of mesenteric malperfusion. We believe it is important to distinguish between ongoing arterial obstruction and arterial obstruction with ischemic end-organ dysfunction. Those patients with malperfusion but no significant adverse end-organ effects (MPS) are best treated with immediate central surgical repair. An ATAAD is still a surgical emergency, and patients without end-organ ischemia, either with or without malperfusion, have an equal operative risk, which is significantly lower than patients with both malperfusion and end-organ dysfunction [17]. Patients with established MPS should undergo fenestration with or without stenting to re-perfuse the ischemic organs with stabilization prior to open surgical repair of the ATAAD. Figure 6 is a flow diagram of how we will handle the challenge of MPS with ATAAD, based on current evidence and institutional experience. However, the optimal management should be individualized for each patient based on presenting features, type of malperfusion, time to surgery, and available expertise.
Acknowledgements
The authors would like to thank Ms. Intan Fariza Bt Gaafar and Ms Aini Syakirin Binti Kepli from the Research Department of National Heart Institute, Kuala Lumpur, for their contribution in data analysis and preparing and processing the figures in this review.
Funding
None.
Declarations
Ethical statement
Not applicable.
Human and animal rights
Not applicable.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.LeMaire SA, Russel L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–113. doi: 10.1038/nrcardio.2010.187. [DOI] [PubMed] [Google Scholar]
- 2.Howard DP, Banerjee A, Fairhead JF, Perkins J, Silver LE, Rothwell PM. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10- year results from the Oxford Vascular Study. Circulation. 2013;127:2031–7. [DOI] [PMC free article] [PubMed]
- 3.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 4.Trimarchi S, Nienaber CA, Rampoldi V, et al. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129:112–122. doi: 10.1016/j.jtcvs.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection. A 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–1860. [DOI] [PubMed]
- 6.Czerny M, Schoenhoff F, Etz C, et al. The impact of preoperative malperfusion on outcome in acute type A aortic dissection: results from the GERAADA registry. J Am Coll Cardiol. 2015;65:2628–2635. doi: 10.1016/j.jacc.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 7.Geirsson A, Szeto WY, Pochettino A, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg. 2007;32:255–262. doi: 10.1016/j.ejcts.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Girdauskas E, Kuntze T, Borger MA, Falk V, Mohr F-W. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2009;138:1363–9. [DOI] [PubMed]
- 9.Narayan P, Rogers CA, Benedetto U, Caputo M, Angelini GD, Bryan AJ. Malperfusion rather than merely timing of operative repair determines early and late outcome in type A aortic dissection. J Thorac Cardiovasc Surg. 2017;154:81–6. [DOI] [PubMed]
- 10.Bonser RS, Ranasinghe AM, Loubani M, et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455–2474. doi: 10.1016/j.jacc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Patel HJ, Williams DM, Dasika NL, Deeb GM. Management of type A dissection with malperfusion. Ann Cardiothorac Surg. 2016;5:265–274. doi: 10.21037/acs.2016.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaji S. Acute medical management of aortic dissection. Gen Thorac Cardiovasc Surg. 2019;67:203–207. [DOI] [PubMed]
- 13.Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH., 3rd Malperfusion syndromes in aortic dissections. Vasc Med. 2016;21:264–273. doi: 10.1177/1358863X15625371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: percutaneous treatment of ischemic complications--principles and results. J Vasc Interv Radiol. 1997;8:605–625. [DOI] [PubMed]
- 15.Williams DM, Lee DY, Hamilton BH, et al. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch- vessel compromise. Radiology. 1997;203:37–44. [DOI] [PubMed]
- 16.Slonim SM, Miller DC, Mitchell RS, Semba CP, Razavi MK, Dake MD. Percutaneous balloon fenestration and stenting for life-threatening ischemic complications in patients with acute aortic dissection. J Thorac Cardiovasc Surg. 1999;117:1118–26. [DOI] [PubMed]
- 17.Deeb GM, Patel HJ, Williams DM. Treatment for malperfusion syndrome in acute type A and B aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2010;140:S98–S100. doi: 10.1016/j.jtcvs.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200–206. doi: 10.1161/hc0202.102246. [DOI] [PubMed] [Google Scholar]
- 19.Bossone E, Rampoldi V, Nienaber CA, et al. Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type A aortic dissection. Am J Cardiol. 2002;89:851–855. doi: 10.1016/S0002-9149(02)02198-7. [DOI] [PubMed] [Google Scholar]
- 20.Tsai TT, Bossone E, Isselbacher EM, et al. Clinical characteristics of hypotension in patients with acute aortic dissection. Am J Cardiol. 2005;95:48–52. doi: 10.1016/j.amjcard.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 21.Berretta P, Trimarchi S, Patel HJ, Gleason TG, Eagle KA, Eusanio MD. Malperfusion syndromes in type A aortic dissection: what we have learned from IRAD. J Vis Surg. 2018;4:65. doi: 10.21037/jovs.2018.03.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LePage MA, Quint LE, Sonnad SS, Deeb GM, Williams DM. Aortic dissection: CT features that distinguish true lumen from false lumen. AJR Am J Roentgenol. 2001;177:207–211. [DOI] [PubMed]
- 23.Neri E, Toscano T, Papalia U, et al. Proximal aortic dissection with coronary malperfusion: presentation, management, and outcome. J Thorac Cardiovasc Surg. 2001;121:552–560. doi: 10.1067/mtc.2001.112534. [DOI] [PubMed] [Google Scholar]
- 24.Imoto K, Uchida K, Karube N, et al. Risk analysis and improvement of strategies in patients who have acute type A aortic dissection with coronary artery dissection. Eur J Cardiothorac Surg. 2013;44:419–24. [DOI] [PubMed]
- 25.Immer FF, Grobéty V, Lauten A, Carrel TP. Does malperfusion syndrome affect early and mid-term outcome in patients suffering from acute type A aortic dissection? Interact Cardiovasc Thorac Surg. 2006;5:187–90. [DOI] [PubMed]
- 26.Janosi RA, Buck T, Erbel R. Mechanism of coronary malperfusion due to type A aortic dissection. Herz. 2009;34:478. doi: 10.1007/s00059-009-3272-z. [DOI] [PubMed] [Google Scholar]
- 27.Bonnefoy E, Godon P, Kirkorian G, Chabaud S, Touboul P. Significance of serum troponin I elevation in patients with acute aortic dissection of the ascending aorta. Acta Cardiol. 2005;60:165–170. doi: 10.2143/AC.60.2.2005027. [DOI] [PubMed] [Google Scholar]
- 28.Hansen MS, Nogareda GJ, Hutchison SJ. Frequency of and inappropriate treatment of misdiagnosis of acute aortic dissection. Am J Cardiol. 2007;99:852–856. doi: 10.1016/j.amjcard.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 29.Hiratzka LF, Bakris GL, Beckman JA, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/ SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–e369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 30.Rogers AM, Hermann LK, Booher AM, et al. Sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation: results from the international registry of acute aortic dissection. Circulation. 2011;123:2213–2218. doi: 10.1161/CIRCULATIONAHA.110.988568. [DOI] [PubMed] [Google Scholar]
- 31.Rampoldi V, Trimarchi S, Eagle KA, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Neri E, Toscano T, Papalia U, et al. Proximal aortic dissection with coronary malperfusion: presentation, management, and outcome. J Thorac Cardiovasc Surg. 2001;121:552–560. doi: 10.1067/mtc.2001.112534. [DOI] [PubMed] [Google Scholar]
- 33.Di Eusanio M, Patel HJ, Nienaber CA, et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145:s213–21.e1. doi: 10.1016/j.jtcvs.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 34.Pacini D, Leone A, Belotti LMB, et al. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg. 2013;43:820–6. doi: 10.1093/ejcts/ezs500. [DOI] [PubMed] [Google Scholar]
- 35.Morimoto N, Okada K, Okita Y. Lack of neurologic improvement after aortic repair for acute type A aortic dissection complicated by cerebral malperfusion: predictors and association with survival. J Thorac Cardiovasc Surg. 2011;142:1540–1544. doi: 10.1016/j.jtcvs.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Okada K, Yamashita T, Morimoto Y, Kawanishi Y, Okita Y. Surgical results of acute aortic dissection complicated with cerebral malperfusion. Ann Thorac Surg. 2005;80:72–6. doi: 10.1016/j.athoracsur.2004.12.049. [DOI] [PubMed] [Google Scholar]
- 37.Estrera AL, Garami Z, Miller CC, et al. Acute type A aortic dissection complicated by stroke: can immediate repair be performed safely? J Thorac Cardiovasc Surg. 2006;132:1404–1408. doi: 10.1016/j.jtcvs.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y, Tagusari O, Ichikawa Y, Morita A. Impact of immediate aortic repair on early and midterm neurologic status in patients with acute type A aortic dissection complicated by cerebral malperfusion. Ann Thorac Surg. 2011;92:336–338. doi: 10.1016/j.athoracsur.2010.12.065. [DOI] [PubMed] [Google Scholar]
- 39.Tsukube T, Hayashi T, Kawahira T, et al. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation. 2011;124:S163–S167. doi: 10.1161/CIRCULATIONAHA.110.011551. [DOI] [PubMed] [Google Scholar]
- 40.Lauterbach SR, Cambria RP, Brewster DC, et al. Contemporary management of aortic branch compromise resulting from acute aortic dissection. J Vasc Surg. 2001;33:1185–92. doi: 10.1067/mva.2001.115377. [DOI] [PubMed] [Google Scholar]
- 41.Medalion B, Bder O, Cohen AJ, Hauptman E, Schachner A. Delayed postoperative paraplegia complicating repair of type A dissection. Ann Thorac Surg. 2001;72:1389–1391. doi: 10.1016/S0003-4975(00)02590-X. [DOI] [PubMed] [Google Scholar]
- 42.Fleck T, Hutschala D, Weissl M, Wolner E, Grabenwoger M. Cerebrospinal fluid drainage as a useful treatment option to relieve paraplegia after stent-graft implantation for acute aortic dissection type B. J Thorac Cardiovasc Surg. 2002;123:1003–5. doi: 10.1067/mtc.2002.121500. [DOI] [PubMed] [Google Scholar]
- 43.Di Eusanio M, Trimarchi S, Patel HJ, et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2013;145:385–390.e1. doi: 10.1016/j.jtcvs.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 44.Howard TJ, Plaskon LA, Wiebke EA, Wilcox MG, Madura JA. Nonocclusive mesenteric ischemia remains a diagnostic dilemma. Am J Surg. 1996;171:405–8. doi: 10.1016/S0002-9610(97)89619-5. [DOI] [PubMed] [Google Scholar]
- 45.Perera NK, Galvin SD, Seevanayagam S, Matalanis G. Optimal management of acute type A aortic dissection with mesenteric malperfusion. Interact Cardiovasc Thorac Surg. 2014;19:290–4. doi: 10.1093/icvts/ivu127. [DOI] [PubMed] [Google Scholar]
- 46.Yamashiro S, Arakaki R, Kise Y, Inafuku H, Kuniyoshi Y. Management of visceral malperfusion complicated with acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2015;21:346–51. doi: 10.1093/icvts/ivv159. [DOI] [PubMed] [Google Scholar]
- 47.Estrera AL, Huynh TTT, Porat EE, Miller CC, 3rd, Smith JJ, Safi HJ. Is acute type A aortic dissection a true surgical emergency? Semin Vasc Surg. 2002;15:75–82. doi: 10.1053/svas.2002.33093. [DOI] [PubMed] [Google Scholar]
- 48.Yang B, Norton EL, Rosati CM, et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: 20-year experience. J Thorac Cardiovasc Surg. 2019;158:675–687.e4. doi: 10.1016/j.jtcvs.2018.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang B, Rosati CM, Norton EL, et al. Endovascular fenestration/stenting first followed by delayed open aortic repair for acute Type A aortic dissection with malperfusion syndrome. Circulation. 2018;138:2091–2103. doi: 10.1161/CIRCULATIONAHA.118.036328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charlton-Ouw KM, Sritharan K, Leake SS, et al. Management of limb ischemia in acute proximal aortic dissection. J Vasc Surg. 2013;57:1023–9. doi: 10.1016/j.jvs.2012.10.079. [DOI] [PubMed] [Google Scholar]