Abstract
In patients with a small aortic annulus, the clinical benefits of aortic valve replacement depend on avoidance of patient-prosthesis mismatch as it is associated with reduced overall survival. Aortic root widening or enlargement is a useful technique to implant larger valve prosthesis to prevent patient-prosthesis mismatch. Posterior annular enlargement is the commonest technique used for aortic root enlargement. Consistent enlargement of the aortic root requires more extensive procedures like Manouguian or Konno-Rastan techniques. The patients commonly selected are younger patients with good life expectancy. However, caution is advised in applying this procedure in elderly patients, patients with heavily calcified annulus and when performing concomitant procedures. There is no definitive conclusion on the best material to use for the reconstruction of aortic annulus and aorta in aortic root enlargement procedures.
Keywords: Aortic root enlargement, Patient-prosthesis mismatch, Nicks, Manouguian, Aortic valve stenosis
Introduction
The natural history of aortic valve stenosis (AS) is unfavorable [1]. The average survival period of patients with symptomatic severe AS is 23 ± 5 months, and the 5-year survival is only 18% [1]. Aortic valve replacement (AVR) is recommended in severe symptomatic AS (class 1) or in asymptomatic severe AS (class IIB) to improve the life expectancy [2]. The main goal of AVR is the relief of left ventricular outflow tract obstruction and reducing the pressure overload of the left ventricle (LV), which results in symptomatic relief and improved survival [2].
Small aortic root
Many patients with AS have a small aortic root. The diameter of the aortic annulus is related to body surface area (BSA) or height, while the sino-tubular junction diameter, in addition, is also related to age, blood pressure, and gender [3]. However, there is no consensus on how to define a small aortic root. A small aortic root may be defined theoretically as an annulus or sino-tubular junction smaller than 1.0 cm/m2 [4]. Annulus exhibiting a mean and minimal diameter of < 23 mm and ≤ 21 mm, respectively, on 3D echocardiography or computed tomography (CT) scan during evaluation for transcatheter aortic valve implantation(TAVI), or any annulus where > 21 mm sized prosthesis cannot be implanted at the time of surgery are the common definitions used to describe small aortic root [5]. Sino-tubular junction diameter indexed for body height < 14 mm/m in women and < 15 mm/m in men is also a useful tool to identify patients with small aortic root [6]. A small aortic root is found in up to 33% of patients with AS and is associated with lower body height, smaller left ventricular dimensions, female sex, and Asian population [7]. Small aortic root with AS increases the risk of ischemic cardiac events, hemorrhagic strokes, and cardiovascular death [8].
Patient-prosthesis mismatch
In patients with a small aortic annulus, the clinical benefits of AVR depend on avoidance of patient-prosthesis mismatch (PPM) [9]. PPM is considered to be present when the effective prosthetic valve area, after insertion into the patient, is less than that of a normal human valve [10]. However, some recent reports have questioned the relevance of PPM [11, 12]; others have suggested that the true incidence of clinically relevant PPM is low in clinical practice [13]. The matter is further complicated by varying definitions of PPM and dispute over the more appropriate measure of orifice area (geometric or effective) [14].
Pibarot and Dumesnil defined PPM “as a prosthetic valve effective orifice area (EOA) indexed to a body surface area of less than 0.85 cm2/m2. It is considered to be moderate when the EOA is 0.85-0.65 cm2/m2 and severe when the EOA is <0.65 cm2/m2” [15]. The indexed EOA is the only parameter that correlates well with postoperative gradients and development of PPM. “Trans-valvar pressure gradient is directly related to the square of trans-valvar flow (cardiac output) and inversely related to the square of the valve EOA” [15]. Since cardiac output is related to body surface area, PPM occurs when the EOA of valve implanted is too small for body size [15]. PPM has been shown to be associated with worse hemodynamic performance, reduced durability of bioprosthetic valve, reduced left ventricular mass regression, increased reoperation rates, increased readmissions due to heart failure, and lower long-term survival [16]. Four meta-analyses address the long-term survival in patients with PPM [17–20]. The largest meta-analysis [16] included 58 studies with 40,381 patients (39,568 surgical AVRs and 813 TAVIs). The authors concluded that moderate PPM and severe PPM are associated with decreased survival. Analysis of the Society of Thoracic Surgery (STS) database of isolated AVRs from 2004 to 2014 was published in a recent review [21]. They found that the incidence of PPM declined from 13.8 to 6.2% and only 11% were severe; but the risk of mortality was 8% higher with moderate PPM and 32% higher with severe PPM at 10-year follow-up [21]. Several other studies had also shown reduced survival in patients with significant PPM [22, 23]. PPM was also found to increase early mortality especially in patients with depressed left ventricular function [24], where the increased afterload is poorly tolerated by the failing ventricle.
How to avoid PPM?
Indexed EOA can be calculated by dividing the in vivo EOA available with each size and make of the valve with BSA. If the calculated EOA value is below 0.85 cm2/m2, then PPM will occur [9]. If PPM is anticipated after sizing the annulus, the surgeon can (1) implant a prosthesis with a larger EOA (stentless bioprosthesis or mechanical prosthesis with larger in-vivo EOA), (2) use aortic homograft, (3) undertake ross procedure, or (4) perform aortic root enlargement (ARE) to implant a larger prosthesis [9, 25].
These points may be kept in mind when performing AVR in a small root:
Meticulous annular debridement and decalcification may allow implantation of a larger prosthesis.
In general, mechanical valves have better EOA than stented bioprosthetic valves.
Supra-annular valves have better EOA than intra-annular prosthesis [26].
Mattress sutures reduce the annulus by 2–4 mm; hence, simple interrupted or continuous sutures allow implantation of bigger sized valve.
Externally mounted leaflet design improves the hemodynamic performance in bioprosthetic valves [27].
Supra-annular implantation increases the risk of coronary orifice obstruction.
Stentless bioprosthetic implantation, Ross procedure, or homograft implantations are associated with threefold higher operative risk than conventional AVR [25].
Aortic root anatomy
Aortic root extends from the base of aortic valve leaflets to the level of sino-tubular junction [28]. “Approximately two thirds of the circumference of the aortic root is connected to the muscular ventricular septum, while the remaining one third is in fibrous continuity with anterior leaflet of the mitral valve. Aortic root includes the sinuses of Valsalva, interleaflet triangles, and the valvar leaflets” [29]. Aortic root can be represented as a truncated cone. Aortic annulus diameter is about 1.34 times the diameter of sino-tubular junction [30] or sino-tubular junction diameter is approximately 85% of aortic annulus diameter. Aortic root can be represented by 3 rings—basal ring, ventriculo-aortic junction, and the ring at the sino-tubular junction. Inside the root, the aortic valve is arranged like a crown and once it is excised, the “surgical annulus” is seen where the sutures are placed for valve implantation. The diameter at the base of the aortic root, the basal ring, is also called the “aortic annulus,” during surgical aortic valve sizing (Fig. 1a, b) (figures reproduced with permission [31]). The basal ring is not a true anatomical entity, but is defined as a virtual ring with three anchors at the nadir of each of the attachments of the aortic cusps. The basal ring is non-circular and calcification makes its shape more non-homogenous. During AVR, the native aortic leaflets are cut out along the line of the “surgical annulus,” but the sizing of the “annulus” is done under direct vision by selecting an aortic valve sizer that fits the size of the basal ring [30]. The sizers are unique to the prostheses make and are not interchangeable. The sewing ring for most valve prostheses is circular. However, the surgical annulus is not circular, but has semi-lunar shape in each sinus. When the sutures are tied for valve implantation, the base of the sinuses get pulled up and the commissures get pulled down; finally, the valve sits in a circular plane, corresponding to the ventricular-aortic junction [29]. The problems associated with prosthetic valve implantation in a small aortic root are highlighted in Fig. 2.
Fig. 1.

a, b Aortic root anatomy—reproduced with permission from reference 31. True ventricular- aortic junction is above the basal ring. Crown-shaped valves form the hemodynamic junction. When they are excised, as for AVR, the remnants form the surgical annulus, which is also crown-shaped
Fig. 2.
The valves are sized at the basal ring, while the sutures are placed at the crown shaped surgical annulus. Valve sizers are based on tissue annulus diameter. In intra-annular implantation, part of sewing ring also needs to be accommodated into the annulus which can result in valve not properly “fitting” in to the annulus. This can be avoided by supra-annular placement. Supra-annular valves run the risk of coronary ostia obstruction, especially in bicuspid valves, where para-commissural origin of coronaries is not uncommon. Since the sino-tubular junction is smaller than annulus, there can be considerable difficulty in lowering the valve to the annulus and for tying the sutures
The descriptions of ARE procedures are based in relation to the aortic sinuses and the commissures and the location of the coronary ostia. Structures in relation to the aortic root, which are of importance during root enlargement procedures, include the conduction system, the mitral valve, and the inter-ventricular septum [9]. The aortic valve is in fibrous continuity (aorto-mitral curtain) with the anterior leaflet of the mitral valve [9]. The line of attachment between the aortic and mitral valves covers one-third of the total aortic valve circumference. A line drawn through the middle of the anterior mitral valve leaflet normally will pass through the commissure between the left coronary and the non-coronary cusps. There can be age-dependent variation to this particular relationship. In individuals over 60 years of age, the midline corresponds to the commissural site, but, in most young adults, the line may cut through the non-coronary cusp [30]. The anterior commissure (between right and non-coronary cusps) is related to the inter-ventricular septum and the conduction system [9]. The first septal branch of the left anterior descending artery lies posterior to the posterior leaflet of the pulmonary valve [9, 32].
ARE
Over the last three decades, there have been over 20 described techniques of ARE. The terminology “ARE” may be a misnomer, as the procedure involves mostly the enlargement of the aortic annulus rather than the root per se [29]. Hence, the correct terminology should be aortic annular enlargement. These techniques were designed to allow the implantation of a larger size of the prosthetic valve, resulting in improved hemodynamic performance [9].
Preoperative considerations [9]
Adult patients need to be screened for concomitant coronary artery disease based on current guidelines [33].
Anomalous origin of coronary arteries is not uncommon in patients with bicuspid aortic valve.
The location of the first septal branch of the left anterior descending artery is important for planning the Ross-Konno operation.
Concomitant sub-aortic stenosis should be excluded as this may need septal myectomy.
The other important issues in the preoperative assessment are (1) presence or absence of ascending aortic aneurysm. (2) If the Ross operation is considered, echocardiography examination of the pulmonary valve is mandatory for identifying any insufficiency or abnormalities. (3) Presence of mitral valve regurgitation on the preoperative echocardiogram is vital as “Manouguian” technique can worsen the mitral valve insufficiency. (4) Any preoperative conduction abnormalities can increase the chance of permanent pacemaker implantation [9].
ARE operative steps [9]
Oblique incision or aortic transection above sino-tubular junction gives excellent exposure to the aortic valve. The aortic valve is excised. It is a wise idea to size the annulus with a Hegar dilator rather than the respective company made valve sizers. It gives a more accurate measurement of the size, which in turn aids in surgical decision making. If the surgeon decides to perform root enlargement, then the appropriate technique is used [9].
Root enlargement (Figs.3, 4, 5, 6, and 7)
Fig. 3.
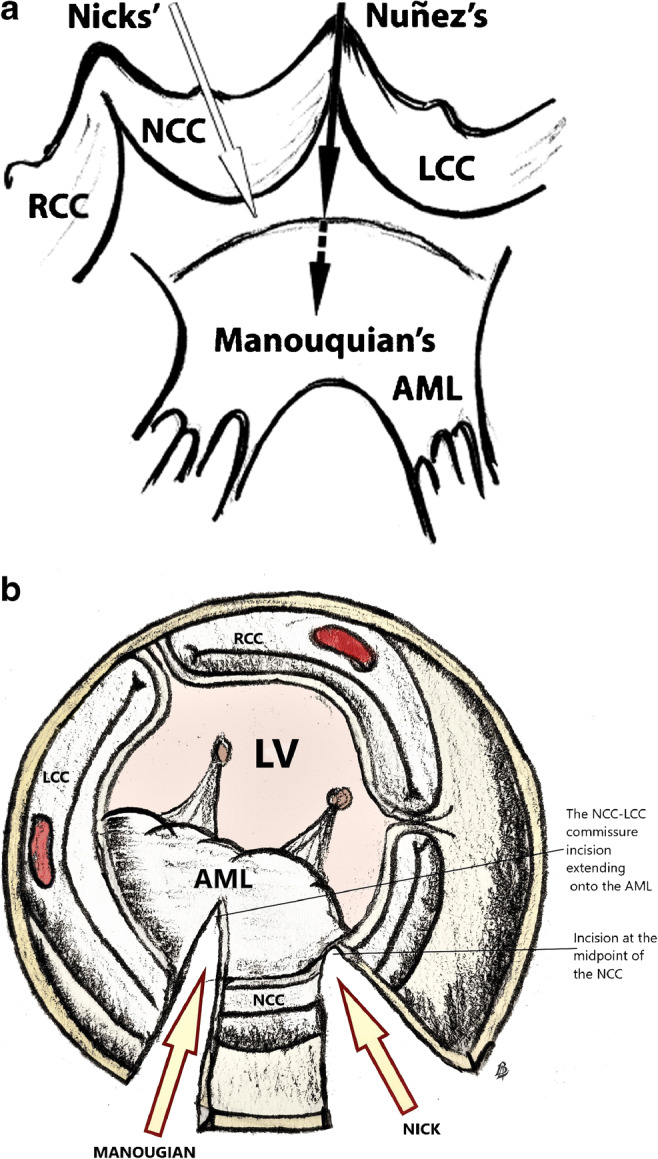
a, b The commonly performed posterior enlargement techniques. Antunes technique is similar to Nicks; however, it extends below the aortic annular ring to aorto-mitral curtain. a Reproduced with permission from reference [34]. RCC, right coronary cusp; NCC, non-coronary cusp; LCC, left coronary cusp; AML, anterior mitral leaflet; LV, left ventricle
Fig. 4.
a–c Steps in Nick’s procedure. The patch material can be pericardium (autologous or bovine) or prosthetic material like Dacron or coated graft. Sometimes, upsizing requires tilted positioning of the valve. RCC, right coronary cusp; NCC, non-coronary cusp; LCC, left coronary cusp; AML, anterior mitral leaflet; LV, left ventricle
Fig. 5.
a–c Steps in the Manouguian procedure. It is essential to make the incision on the center of anterior mitral leaflet in the clear zone. The center of anterior mitral leaflet does not necessarily correspond to the left and non-coronary commissure. Otherwise, it can induce mitral regurgitation. RCC, right coronary cusp; NCC, non-coronary cusp; LCC, left coronary cusp; AML, anterior mitral leaflet; LV, left ventricle; LA, left atrium
Fig. 6.
a–c Steps of the Konno–Rastan procedure. The aortotomy incision is extended to inter-ventricular septum, well leftward from the right coronary orifice. The inter-ventricular septal incision runs the risk of injury to first septal artery. RV, right ventricle; LV, left ventricle; IVS, inter-ventricular septum; RA, right atrium; Ao, aorta; PA, pulmonary artery
Fig. 7.
a, b. Right ventricular outflow patch is anchored to the left ventricular patch. The procedure is often talked about, but seldom performed. RVOT, right ventricle outflow tract; LVOT, left ventricle outflow tract; IVS, inter-ventricular septum; RA, right atrium; Ao, aorta; PA, pulmonary artery
Posterior enlargement—the Nicks-Nunez or Manouguian techniques are the most commonly performed posterior root enlargement techniques (Fig.3 a (reproduced with permission [34]) and b). “There is considerable confusion in the literature regarding the techniques proposed by the eponymous descriptors Nick’s and Manouguian’s enlargement” [14]. The Nicks method is a vertical incision through the mid-portion of the non-coronary sinus, extending to the annulus (Figs. 3 and 4) [35]. In the Nunez technique, the incision extends through the posterior commissure to aorto-mitral curtain [36]. These techniques can enlarge the root by 2 to 3 mm. If further enlargement is required, the Manouguian method is used. “This is a vertical incision through the commissure between the left and non-coronary sinus, through the aortic annulus and into the anterior leaflet of the mitral valve and roof of the left atrium” (Figs. 3 and 5) [9, 37]. The incision in the anterior leaflet of the mitral valve should be limited to the clear zone (Figs. 3 and 5). Mitral regurgitation can be induced by faulty mitral valve incision. “It is important to incise the center of the mitral valve ring and limiting the cut to the clear zone of the anterior mitral leaflet to avoid this complication. In the surgeon’s view, the incision line can shift to the antero-lateral side in the anterior mitral leaflet” [38].
Anterior enlargement (Figs. 6 and 7)—Anterior enlargement of the aortic root or aorto-ventriculoplasty was described by Konno and Rastan [39]. An anterior vertical aortotomy is performed into the right coronary sinus and the incision is then extended through the aortic annulus into the inter-ventricular septum. The incision is made well away from right coronary ostia. Deeper incisions in inter-ventricular septum can injure the first septal artery. The right ventricular free wall is incised sufficiently to enlarge the right ventricular outflow tract [9].
Root reconstruction [9]
Nicks/Nunez Technique—A diamond-shaped patch of autologous pericardium, bovine pericardium, prosthetic material, or composite material of pericardium and prosthetic material can be used for reconstruction. Aorto-mitral curtain lacks fibrous strength; hence, interrupted sutures are preferred to anchor the patch. At the level of the aortic annulus, interrupted sutures with pledgets are placed on the outside of the patch. The rest of the valve sutures are placed through the aortic annulus in a conventional fashion (Fig. 4).
In the Manouguian technique, a diamond-shaped patch is sutured into the deepest portion of the incision using interrupted or continuous sutures. It is easier to place the sutures in other areas of annulus before patch reconstruction. A second patch is fashioned to reconstruct the left atrial defect. The first patch is tailored to close the aortotomy incision (Fig. 5).
In Konno-Rastan aorto-ventriculoplasty, the reconstruction is begun by attaching a diamond-shaped prosthetic material patch to the ventricular defect using continuous sutures up to the level of the aortic annulus. “Interrupted sutures with pledgets are used to attach the base of the triangular right ventricular outflow tract patch to the left ventricular outflow tract patch at the level of the aortic annulus. The sutures are then passed through the sewing ring of the prosthetic valve” [9]. The rest of the valve sutures are taken in a conventional way. The right ventricular outflow tract patch is attached to the ventricular muscle using continuous suture. The left ventricular outflow tract patch is used to close the aortotomy incision [9] (Figs. 6 and 7).
Nicks procedure is the most commonly performed ARE because of its simplicity and low morbidity. However, some authors have raised concerns about the efficacy of this technique to prevent PPM. They point out that it “increased annular diameter by an average of 0.43 to 0.45 mm only [40] and allowed for the implantation of a larger prosthetic aortic valve in only about 20% of the study population.” On the contrary, others have described larger annular increase following the Nicks procedure [41]. “While it may be possible to implant a valve 1 size larger using a combination of the Nicks enlargement and oblique implantation of the prosthesis,” recent studies have suggested that tilting of the prosthesis reduced its hemodynamic performance [42]. ARE, as proposed by Manouguian (although controversy exists about who actually described the procedure first) [43–45], is technically more difficult than the Nicks procedure, but it allows for the implantation of a larger valve [40]. Authors from India reported that using the Manouguian technique in a group of 17 patients, annulus was enlarged by 4 to 6 mm [46]. In another study using the Manouguian procedure, the authors were able to enlarge the annulus by an average of 3.63 ± 0.95 mm. This was sufficient to allow for upsizing of the aortic valve by at least 1 size (70%, 1 size; 30%, 2 sizes) [40]. Reported annular enlargement is around 40 to 50% following the Konno-Rastan ARE [47]. Another study reported aortic annulus enlargement by an average of 6.08 ± 1.19 mm with this technique. This resulted in the implantation of a larger aortic valve prosthesis by 2 to 4 sizes (40%, 2 sizes; 55%, 3 sizes; 5%, 4 sizes) [40]. Because of its technical complexity, this procedure should probably be used only in patients who require extensive root widening [40] and in patients with associated hypoplasia of the left ventricular outflow tract. However, it is well worth to consider that consistent enlargement of aortic annulus requires more complex procedures like Konno or Manouguian ARE, where the operative risk is not negligible.
Another useful technique was described by Kinskey et al. [48] (Fig. 3). “The aortic incision is extended into the middle of the non-coronary sinus, through the aortic annulus and into the anterior margin of the fibrous mitro-aortic curtain, 10–15 mm below the aortic annulus.” Glutaraldehyde-treated bovine pericardium was used for root reconstruction. The authors were successful in upsizing the valve by two sizes with this technique. A septal myectomy was performed routinely to relieve the sub-valvular obstruction [25, 48].
Aorto-ventriculoplasty with pulmonary autograft replacement of the aortic valve (Ross-Konno) is a useful technique, when confronted by severe hypoplasia of the left ventricular outflow tract that sometimes occurs in young patients [49]. Enlargement of the aortic root can also be achieved in select circumstances using aortic allograft, along with the anterior leaflet of the mitral valve [50]. The indications for this kind of reconstruction are mostly in cases where a radical debridement was required for infective endocarditis [51]. Sub-aortic myectomy is performed in situations where there is significant hypertrophy of the subvalvar muscle [52].
ARE in double valve replacement
In patients with rheumatic heart disease, especially with mitral valve and AS, sometimes ARE is required. Posterior enlargement techniques like Nicks and Nunez can be employed; however, it is best to place the patch before mitral valve replacement is undertaken. In special situations, both aortic and mitral annulus need to be enlarged. The aortotomy incision is similar to the Manouguian technique, but the incision in the left atrial wall is extended superiorly, rather than to the right, along the direction of the superior vena cava. After excision of anterior mitral leaflet and aorto-mitral curtain, a suitable sized valve is sutured to the remnant of mitral annulus by interrupted pledgetted suture. About three-fourths of the circumference of the mitral prosthesis is secured to the native mitral ring. Aorto-mitral curtain is reconstituted by sewing the base of a triangle-shaped patch using interrupted pledgetted suture. The triangular patch is also used to close the aortotomy. Another triangular pericardial patch is used to close the opening of the left atrium. Pledgetted horizontal mattress stitches placed circumferentially around the true aortic annulus and the patch are used for seating the valve [43, 53–55]. “Perivalvular leakage, hemolysis, and dehiscence” are problems encountered with this technique. Bleeding complications can be lessened by the use of bovine pericardium, Dacron graft lined by autologous pericardium, or using a coated vascular graft. It is best to avoid large stented bioprosthesis in mitral position as it may lead to left ventricular outflow obstruction [55]. “Konno-Rastan procedure is an option in patients who have already undergone a mitral valve replacement and are no longer candidates for a Nicks or Manouguian procedure” [56].
Surgical results
A recently published meta-analysis showed that ARE did not increase operative mortality and bleeding or increased the incidence of pacemaker implantation [57]. However, it increased aortic cross-clamping time by 14 min and bypass time by 20 min. Another meta-analysis included 13,174 patients (AVR with ARE: 2819 patients; AVR without ARE: 10,355 patients) from studies published from 2002 to 2018. The incidence of ARE was 21.4% (5.7 to 26.3%). “Perioperative mortality was higher in the ‘AVR with ARE’ group (OR 1.506, 95% CI 1.209–1.875; p < 0.001), but not when adjusted for isolated AVR + ARE without any concomitant procedures (OR 1.625, 95% CI 0.968–2.726; p = 0.066 among 6 studies). The ‘AVR with ARE’ group showed lower risk of significant PPM (OR 0.472, 95% CI 0.295–0.756; p = 0.002)” [56]. It was also interesting to note that there was no report of the Konno-Rastan procedure in any studies. The reason is probably because it is technically very demanding. Other single-center studies have also reported excellent short- and long-term outcomes after ARE [14, 25, 58, 59]. A multi-center propensity-matched comparison between ARE vs. AVR was recently reported [60]. It showed that ARE was safe and was associated with good long-term follow-up. ARE with coronary bypass grafting showed increased bleeding and re-exploration rates for bleeding [61]. Overall, 8.8% of patients underwent ARE, and interestingly, half of them were operated in a single center (Toronto General Hospital). This shows that many surgeons are still reluctant to perform ARE.
In an editorial, Lazar mentions 3 most common reasons for surgeons to feel reluctant to perform ARE: (1) increased cross-clamp and bypass times, which can have deleterious effect in high-risk patients and in those patients requiring concomitant procedures; (2) concern about increased bleeding; and (3) lack of robust data that ARE actually improves short and long-term outcomes [16]. Surgeons from the University of Toronto reported 26% ARE in more than 7000 AVRs done from 1990 to 2014. Overall, ARE had increased operative mortality (4.3% vs. 3.0%; p = 0.008) but in those patients undergoing only isolated ARE, there was no difference in operative mortality, when compared to AVR (1.7% ARE vs. 1.1% AVR; p = 0.28) [60]. In their first study published in 1997, the operative mortality was higher in ARE compared to AVR (7.1% vs. 3.5%) [62]. However, recent reports from the same center showed decreased mortality and the authors felt that increased experience and comfort with the procedure led to better results [63]. They opined that ARE should be used “judiciously in selected patients most likely to derive maximal benefit.” Those patients will include younger patients and patients whose life expectancy is more than 10 years (class 1 B) [64]. Since many younger patients are choosing bioprosthetic valves, with the prospect of a future TAVI, implantation of a larger prosthesis is advantageous. Valve in valve TAVI in a smaller prosthesis (< 21 mm) showed inferior survival in short- and mid-term follow-up with higher transannular gradients [65]. However, ARE should be applied with caution when performing concomitant procedures and in patients with limited life expectancy and is hazardous in extremely calcified and fragile annulus [16]. Suture-less valve implantation and performing Ozaki procedure are alternatives in elderly patients with small annulus to prevent PPM [66]. In high-risk patients, where TAVI is also not an option due to anatomic factors, implantation of apico-aortic conduit may be considered. The advantages include avoidance of PPM, stroke, complete heart block, and sternotomy. However, it is contraindicated in the presence of any significant aortic insufficiency and may be the procedure of choice in porcelain aorta [67].
Complications of ARE and its prevention
Many complications are described in ARE. Sub-annular bleeding, even though rare, is the most catastrophic complication that can occur during the surgery and is associated with increased mortality [14]. The use of pliable materials like autologous pericardium, meticulous suturing techniques to anchor the patch at the sub-annular level, and use of double-layered suturing are some of the techniques described to prevent bleeding from the root [68]. When sub-annular bleeding is encountered, the traditional approach is to redo the entire procedure which can significantly increase the ischemic and bypass times. One technique describes the use of full-thickness double-armed sutures through the left atrial dome and then through the peri-adventitial, partial thickness of the neighboring aortic wall, thereby approximating the left atrial dome to the greater curvature of the aorta and reinforced with Teflon felt patch [69]. Peri-prosthetic leakage can often be prevented by the use of interrupted pledgetted sutures, placed from outside the patch, and also at the transition site between the patch and the annulus to anchor the valve. Late aneurysm formation is a possibility in patients when pericardium (both autologous and heterologous) is used as patch material. However, two long-term studies using autologous pericardium in ARE have shown that late aneurysm formation does not seem to occur in adult patients, unlike those reported in pediatric patients [66, 68]. The other described complications include aortic dissection, iatrogenic mitral regurgitation, and restricted leaflet motion, especially while using mechanical bi-leaflet valves, due to impingement of sub-valvular muscle shelf of left ventricular outflow tract on the leaflet [68].
Patch material for ARE
A variety of materials can be used for patch reconstruction in ARE. Synthetic patches like Dacron or polytetrafluoroethylene (PTFE), homografts (aortic or pulmonary artery), and autologous or heterologous (bovine/equine) pericardial patches [19, 35, 37, 70–75] are commonly used for reconstruction. However, all these materials have significant drawbacks. They include troublesome needle hole bleeding and difficulty of handling and, in the case of prosthetic material, lack of tensile strength and risk of late aneurysm formation with pericardial patches and risk of calcification in glutaraldehyde fixed pericardium. CorMatrix ECM (CorMatrix(™) Cardiovascular Inc., Roswell, GA) is an extracellular matrix derived from porcine small intestinal submucosa and has shown promising results, when used as a patch material in ARE. CorMatrix possesses adequate tensile strength, hemostatic ability, and does not evoke host immune response and is resistant to calcification. In addition, it promotes the growth of new population of host cells and can display normal aortic morphology at 30 weeks after the surgery. It is also easy to implant and is more resistant to infection [76, 77]. However, there are no published long-term results of the use of CorMatrix in ARE.
Conclusion
ARE is a useful technique to implant larger prosthesis to avoid PPM. Posterior annular enlargement is the commonest technique used for ARE. Consistent enlargement of the aortic root probably requires more extensive procedures like Manouguian or Konno-Rastan techniques. The patients commonly selected are younger patients with good life expectancy. However, caution is advised in applying this procedure in elderly patients, those with the heavily calcified annulus, and when performing concomitant procedures. There is no definitive conclusion on the best material to use for the reconstruction of aortic annulus and aorta in ARE.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not required, being a review article.
Informed consent
Not required.
Statement of human and animal rights
There was no infringement of human/animal rights.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Horstkotte D, Loogen F. The natural history of aortic valve stenosis. Eur Heart J. 1988;9:57–64. doi: 10.1093/eurheartj/9.suppl_e.57. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 3.Chambers J. Echocardiography and the small aortic root. J Heart Valve Dis. 1996;5:S264–S268. [PubMed] [Google Scholar]
- 4.Ghosh P, Kumar S, Pandey S, Kumar AS, Sinha N. Small aortic annulus: a functional definition. Ann Thorac Cardiovasc Surg. 1998;4:251–261. [PubMed] [Google Scholar]
- 5.Freitas-Ferraz AB, Tirado-Conte G, Dagenais F, et al. Aortic stenosis and small aortic annulus. Circulation. 2019;139:2685–2702. doi: 10.1161/CIRCULATIONAHA.118.038408. [DOI] [PubMed] [Google Scholar]
- 6.Muraru D, Maffessanti F, Kocabay G, et al. Ascending aorta diameters measured by echocardiography using both leading edge-to-leading edge and inner edge-to-inner edge conventions in healthy volunteers. Eur Heart J Cardiovasc Imaging. 2014;15:415–422. doi: 10.1093/ehjci/jet173. [DOI] [PubMed] [Google Scholar]
- 7.Bahlmann E, Cramariuc D, Minners J, et al. Small aortic root in aortic valve stenosis: clinical characteristics and prognostic implications. Eur Heart J Cardiovasc Imaging. 2017;18:404–412. doi: 10.1093/ehjci/jew159. [DOI] [PubMed] [Google Scholar]
- 8.Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 9.Doty JR, Raza S, Sabik JF III. Chapter 11-Aortic root enlargement techniques. In: Atlas of Cardiac Surgical Techniques: Elsevier; 2019. p. 156–76.
- 10.Rahimtoola SH. Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. 2010;55:2413–2426. doi: 10.1016/j.jacc.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 11.Koch CG, Khandwala F, Estafanous FG, Loop FD, Blackstone EH. Impact of prosthesis-patient size on functional recovery after aortic valve replacement. Circulation. 2005;111:3221–3229. doi: 10.1161/CIRCULATIONAHA.104.505248. [DOI] [PubMed] [Google Scholar]
- 12.Medalion B, Blackstone EH, Lytle BW, White J, Arnold JH, Cosgrove DM. Aortic valve replacement: is valve size important? J Thorac Cardiovasc Surg. 2000;119:963–974. doi: 10.1016/S0022-5223(00)70091-2. [DOI] [PubMed] [Google Scholar]
- 13.Cano Ó, Andrés A, Alonso P, et al. Incidence and predictors of clinically relevant cardiac perforation associated with systematic implantation of active-fixation pacing and defibrillation leads: a single-centre experience with over 3800 implanted leads. Europace. 2017;19:96–102. doi: 10.1093/europace/euv410. [DOI] [PubMed] [Google Scholar]
- 14.Dhareshwar J, Sundt TM, 3rd, Dearani JA, Schaff HV, Cook DJ, Orszulak TA. Aortic root enlargement: what are the operative risks? J Thorac Cardiovasc Surg. 2007;134:916–924. doi: 10.1016/j.jtcvs.2007.01.097. [DOI] [PubMed] [Google Scholar]
- 15.Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact, and prevention. Heart. 2006;92:1022–1029. doi: 10.1136/hrt.2005.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazar HL. Aortic root enlargement-is it a safe and effective approach to prevent patient-prosthesis mismatch and is it for everyone? Can J Cardiol. 2019;35:707–709. doi: 10.1016/j.cjca.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Dayan V, Vignolo G, Soca G, Paganini JJ, Brusich D, Pibarot P. Predictors and outcomes of prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging. 2016;9:924–933. doi: 10.1016/j.jcmg.2015.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Lin Y, Kang B, Wang Z. Indexed effective orifice area is a significant predictor of higher mid- and long-term mortality rates following aortic valve replacement in patients with prosthesis-patient mismatch. Eur J Cardiothorac Surg. 2014;45:234–240. doi: 10.1093/ejcts/ezt245. [DOI] [PubMed] [Google Scholar]
- 19.Head SJ, Mokhles MM, Osnabrugge RLJ, et al. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: a systematic review and meta-analysis of 34 observational studies comprising 27186 patients with 133141 patient-years. Eur Heart J. 2012;33:1518–1529. doi: 10.1093/eurheartj/ehs003. [DOI] [PubMed] [Google Scholar]
- 20.Takagi H, Yamamoto H, Iwata K, Goto SN, Umemoto T. A meta-analysis of effects of prosthesis-patient mismatch after aortic valve replacement on late mortality. Int J Cardiol. 2012;159:150–154. doi: 10.1016/j.ijcard.2012.04.084. [DOI] [PubMed] [Google Scholar]
- 21.Fallon JM, DeSimone JP, Brennan JM, et al. The incidence and consequence of prosthesis-patient mismatch aftersurgical aortic valve replacement. Ann Thorac Surg. 2018;106:14–22. doi: 10.1016/j.athoracsur.2018.01.090. [DOI] [PubMed] [Google Scholar]
- 22.Tasca G, Mhagna Z, Perotti S, et al. Impact of prosthesis-patient mismatch on cardiac events and midterm mortality after aortic valve replacement in patients with pure aortic stenosis. Circulation. 2006;113:570–576. doi: 10.1161/CIRCULATIONAHA.105.587022. [DOI] [PubMed] [Google Scholar]
- 23.Rao V, Jamieson WR, Ivanov J, Armstrong S, David TE. Prosthesis-patient mismatch affects survival after aortic valve replacement. Circulation. 2000;102:III5–III9. doi: 10.1161/01.cir.102.suppl_3.iii-5. [DOI] [PubMed] [Google Scholar]
- 24.Blais C, Dumesnil JG, Baillot R, Simard S, Doyle D, Pibarot P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation. 2003;108:983–988. doi: 10.1161/01.CIR.0000085167.67105.32. [DOI] [PubMed] [Google Scholar]
- 25.Coutinho GF, Correia PM, Paupério G, de Oliveira F, Antunes MJ. Aortic root enlargement does not increase the surgical risk and short-term patient outcome? Eur J Cardiothorac Surg. 2011;40:441–447. doi: 10.1016/j.ejcts.2010.11.064. [DOI] [PubMed] [Google Scholar]
- 26.Badano LP, Pavoni D, Musumeci S, et al. Stented bioprosthetic valve hemodynamics: is the supra-annular implant better than the intra-annular? J Heart Valve Dis. 2006;15:238–246. [PubMed] [Google Scholar]
- 27.Vriesendorp MD, de Lind van Wijngaarden RAF, Rao V, et al. An in vitro comparison of internally versus externally mounted leaflets in surgical aortic bioprostheses. Interact Cardiovasc Thorac Surg2020; 30:417–423. [DOI] [PubMed]
- 28.Anderson RH. Clinical anatomy of the aortic root. Heart. 2000;84:670–673. doi: 10.1136/heart.84.6.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piazza N, de Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH. Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circ Cardiovasc Interv. 2008;1:74–81. doi: 10.1161/CIRCINTERVENTIONS.108.780858. [DOI] [PubMed] [Google Scholar]
- 30.Becker AE. Surgical and pathological anatomy of the aortic valve and root. Oper Tech Cardiac Thorac Surg. 1996;1:3–14. [Google Scholar]
- 31.Hanneman K, Chan FP, Mitchell RS, Miller DC, Fleischmann D. Pre- and postoperative imaging of the aortic root. Radiographics. 2016;36:19–37. doi: 10.1148/rg.2016150053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hosseinpour AR, Anderson RH, Ho SY. The anatomy of the septal perforating arteries in normal and congenitally malformed hearts. J Thorac Cardiovasc Surg. 2001;121:1046–1052. doi: 10.1067/mtc.2001.113604. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner H, Falk V, Bax JJ, et al. ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 34.Nezić D, Knezević A, Borović S. Surgical techniques for posterior aortic root enlargement. J Thorac Cardiovasc Surg. 2008;135:1401–1402. doi: 10.1016/j.jtcvs.2007.11.055. [DOI] [PubMed] [Google Scholar]
- 35.Nicks R, Cartmill T, Bernstein L. Hypoplasia of the aortic root. The problem of aortic valve replacement. Thorax. 1970;25:339–346. doi: 10.1136/thx.25.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nuñez L, Aguado MG, Pinto AG, Larrea JL. Enlargement of the aortic annulus by resecting the commissure between the left and noncoronary cusps. Tex Heart Inst J. 1983;10:301–303. [PMC free article] [PubMed] [Google Scholar]
- 37.Manouguian S, Seybold-Epting W. Patch enlargement of the aortic valve ring by extending the aortic incision into the anterior mitral leaflet. New operative technique. J Thorac Cardiovasc Surg. 1979;78:402–412. [PubMed] [Google Scholar]
- 38.Kawachi Y, Tominaga R, Tokunaga K. Eleven-year follow-up study of aortic or aortic-mitral anulus-enlarging procedure by Manouguian's technique. J Thorac Cardiovasc Surg. 1992;104:1259–1263. [PubMed] [Google Scholar]
- 39.Konno S, Imai Y, Iida Y, Nakajima M, Tatsuno K. A new method for prosthetic valve replacement in congenital aortic stenosis associated with hypoplasia of the aortic valve ring. J Thorac Cardiovasc Surg. 1975;70:909–917. [PubMed] [Google Scholar]
- 40.Losenno KL, Gelinas JJ, Johnson M, Chu MWA. Defining the efficacy of aortic root enlargement procedures: a comparative analysis of surgical techniques. Can J Cardiol. 2013;29:434–440. doi: 10.1016/j.cjca.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Nakano S, Matsuda H, Shimazaki Y, et al. An appraisal of patch enlargement of the small aortic annulus in 33 patients undergoing aortic valve replacement. Eur J Cardiothorac Surg. 1992;6:347–349. doi: 10.1016/1010-7940(92)90170-3. [DOI] [PubMed] [Google Scholar]
- 42.Hartrumpf M, Albes JM, Krempl T, Rudolph V, Wahlers T. The hemodynamic performance of standard bileaflet valves is impaired by a tilted implantation position. Eur J Cardiothorac Surg. 2003;23:283–291. doi: 10.1016/s1010-7940(02)00804-7. [DOI] [PubMed] [Google Scholar]
- 43.Rastan H, Atai M, Hadi H, Yazdanyr A. Enlargement of mitral valvular ring. New technique for double valve replacement in children or adults with small mitral annulus. J Thorac Cardiovasc Surg. 1981;81:106–111. [PubMed] [Google Scholar]
- 44.Manouguian S, Kirchhoff PG. Aortic and aortic-mitral annular enlargement. J Thorac Cardiovasc Surg. 1996;112:207. doi: 10.1016/s0022-5223(96)70210-6. [DOI] [PubMed] [Google Scholar]
- 45.Rastan D. Aortic and aortic-mitral annular enlargement. J Thorac Cardiovasc Surg. 1995;109:818–819. doi: 10.1016/S0022-5223(95)70378-0. [DOI] [PubMed] [Google Scholar]
- 46.Sankar NM, Rajan S, Kalyan Singh RK, Cherian KM. Enlargement of small aortic annulus by modified Manouguian’stechnique. Asian Cardiovasc Thorac Ann. 1999;7:282–286. [Google Scholar]
- 47.Cobanoglu A, Thyagarajan GK, Dobbs J. Konno-aortoventriculoplasty with mechanical prosthesis in dealing with small aortic root: a good surgical option. Eur J Cardiothorac Surg. 1997;12:766–770. doi: 10.1016/s1010-7940(97)00221-2. [DOI] [PubMed] [Google Scholar]
- 48.Kinskey RH, Antune MJ, McKibbin JK. Enlargement of the narrow aortic root and oblique insertion of St Jude prosthesis. Br Heart J. 1983;50:330–332. doi: 10.1136/hrt.50.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddy VM, Rajasinghe HA, Teitel DF, Haas GS, Hanley FL. Aortoventriculoplasty with the pulmonary autograft: the “Ross-Konno” procedure. J Thorac Cardiovasc Surg. 1996;111:158–165. doi: 10.1016/S0022-5223(96)70412-9. [DOI] [PubMed] [Google Scholar]
- 50.Doty JR, Salazar JD, Liddicoat JR, Flores JH, Doty DB. Aortic valve replacement with cryopreserved aortic allograft: ten-year experience. J Thorac Cardiovasc Surg. 1998;115:371–379. doi: 10.1016/S0022-5223(98)70281-8. [DOI] [PubMed] [Google Scholar]
- 51.Pettersson G, Tingleff J, Joyce FS. Treatment of aortic valve endocarditis with the Ross operation. Eur J Cardiothorac Surg. 1998;13:678–684. doi: 10.1016/s1010-7940(98)00053-0. [DOI] [PubMed] [Google Scholar]
- 52.Kayalar N, Schaff HV, Daly RC, Dearani JA, Park SJ. Concomitant septal myectomy at the time of aortic valve replacement for severe aortic stenosis. Ann Thorac Surg. 2010;89:459–464. doi: 10.1016/j.athoracsur.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 53.Manouguian S, Abu-Aishah N, Neitzel J. Patch enlargement of the aortic and mitral valve rings with aortic and mitral double valve replacement. Experimental study. J Thorac Cardiovasc Surg. 1979;78:394–401. [PubMed] [Google Scholar]
- 54.Manouguian S, Kirchhoff PG. Patch enlargement of the aortic and the mitral valve rings with aortic-mitral double-valve replacement. Ann Thorac Surg. 1980;30:396–399. doi: 10.1016/s0003-4975(10)61281-7. [DOI] [PubMed] [Google Scholar]
- 55.Zhong Q, Xiao Y, Chen J, Ma R. Strategy of aortic root enlargement in patients undergoing aortic and mitral valve replacement. Ann Thorac Surg. 2010;90:782–787. doi: 10.1016/j.athoracsur.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 56.Sa´ MPBO, Carvalho MMB, Sobral Filho DC, et al. Impact of surgical aortic root enlargement on the outcomes of aortic valve replacement: a meta-analysis of 13174 patients. Interact Cardiovasc Thorac Surg. 2019;29:74–82. doi: 10.1093/icvts/ivy364. [DOI] [PubMed] [Google Scholar]
- 57.Yu W, Tam DY, Rocha RV, Makhdoum A, Ouzounian M, Fremes SE. Aortic root enlargement is safe and reduces the incidence of patient-prosthesis mismatch: ameta-analysis of early and late outcomes. Can J Cardiol. 2019;35:782–790. doi: 10.1016/j.cjca.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Correia PM, Coutinho GF, Branco C, Antunes MJ. Long-term follow-up of patients undergoing aortic root enlargement for insertion of a larger prosthesis. Eur J Cardiothorac Surg. 2016;50:82–88. doi: 10.1093/ejcts/ezv487. [DOI] [PubMed] [Google Scholar]
- 59.Kitamura M, Satoh M, Hachida M, Endo M, Hashimoto A, Koyanagi H. Aortic valve replacement in small aortic annulus with or without annular enlargement. J Heart Valve Dis. 1996;5:S289–S293. [PubMed] [Google Scholar]
- 60.Rocha RV, Manlhiot C, Feindel CM, et al. Surgical enlargement of the aortic root does not increase the operative risk of aortic valve replacement. Circulation. 2018;137:1585–1594. doi: 10.1161/CIRCULATIONAHA.117.030525. [DOI] [PubMed] [Google Scholar]
- 61.Tam DY, Dharma C, Rocha RV, et al. Early and late outcomes following aortic root enlargement: a multicentre propensity score-matched cohort analysis. J Thorac Cardiovasc Surg. 2020;160:908–919.e15. doi: 10.1016/j.jtcvs.2019.09.062. [DOI] [PubMed] [Google Scholar]
- 62.Sommers KE, David TE. Aortic valve replacement with patch enlargement of the aortic annulus. Ann Thorac Surg. 1997;63:1608–1612. doi: 10.1016/s0003-4975(97)00127-6. [DOI] [PubMed] [Google Scholar]
- 63.Peterson MD, Borger MA, Feindel CM, David TE. Aortic annular enlargement during aortic valve replacement: improving results with time. Ann Thorac Surg. 2007;83:2044–2049. doi: 10.1016/j.athoracsur.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 64.Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures. Ann Thorac Surg. 2013;95:S1–S66. doi: 10.1016/j.athoracsur.2013.01.083. [DOI] [PubMed] [Google Scholar]
- 65.Kilic T, Yilmaz I. Transcatheter aortic valve implantation: a revolution in the therapy of elderly and high-risk patients with severe aortic stenosis. J Geriatr Cardiol. 2017;14:204–217. doi: 10.11909/j.issn.1671-5411.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozaki S, Kawase I, Yamashita H, et al. Aortic valve reconstruction using autologous pericardium for aortic stenosis. Circ J. 2015;79:1504–1510. doi: 10.1253/circj.CJ-14-1092. [DOI] [PubMed] [Google Scholar]
- 67.Elmistekawy E, Lapierre H, Mesana T, Ruel M. Apico-aortic conduit for severe aortic stenosis: technique, applications, and systematic review. J Saudi Heart Assoc. 2010;22:187–194. doi: 10.1016/j.jsha.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chowdhury U, Singh S, George N, et al. Early evaluation of the aortic root after Nicks’ procedure. JTCVS Tech. 2020. 10.1016/j.xjtc.2020.08.017. [DOI] [PMC free article] [PubMed]
- 69.Chowdhury UK, Sankhyan LK, George N, et al. Posterior aortic root enlargement (Nick’s procedure), mechanical aortic valve replacement and patch closure of the sacciform proximal aortic arch aneurysm by “open” technique without circulatory arrest: a video presentation. Int Med. 2020;2:92–95. [Google Scholar]
- 70.Piehler JM, Danielson GK, Pluth JR, et al. Enlargement of the aortic root or annulus with autogenous pericardial patch during aortic valve replacement. Long-term follow-up. J Thorac Cardiovasc Surg. 1983;86:350–358. [PubMed] [Google Scholar]
- 71.Kinsley RH. The narrow aortic annulus. A technique for inserting a larger prosthesis. Am Heart J. 1977;93:759–761. doi: 10.1016/s0002-8703(77)80072-0. [DOI] [PubMed] [Google Scholar]
- 72.Brinster DR, Patel JA. The use of CorMatrix extracellular matrix for aortic root enlargement. J Cardiothorac Surg. 2014;9:178. doi: 10.1186/s13019-014-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dalichau H, Hannekum A, Niehues B, Irion A, Herse B. Hemodynamic and angiographic late results following enlargement of narrow aortic root using autologous pericardium in prosthetic aortic valve replacement. Thorac Cardiovasc Surg. 1985;33:288–295. doi: 10.1055/s-2007-1014143. [DOI] [PubMed] [Google Scholar]
- 74.Borracci RA, Rubio M, Camargo RLP, Archer M, Ingino C. Aortic root enlargement of a small annulus using the Nick’s technique during aortic valve replacement. Rev Argent Cardiol. 2014;82:504–507. [Google Scholar]
- 75.Morisaki A, Kato Y, Motoki M, Takahashi Y, Nishimura S, Shibata T. Rupture of equine pericardial aortic-root patch after aortic valve replacement with aortic annulus enlargement: a case report. J Cardiothorac Surg. 2014;9:109. doi: 10.1186/1749-8090-9-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quarti A, Nardone S, Colaneri M, Santoro G, Pozzi M. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interact Cardiovasc Thorac Surg. 2011;13:569–572. doi: 10.1510/icvts.2011.280016. [DOI] [PubMed] [Google Scholar]
- 77.Gerdisch MW, Akinwande AO, Matheny RG. Use of a novel acellular xenograft as a patch for aortic annular enlargement during aortic valve replacement. Innovations (Phila) 2010;5:60–62. doi: 10.1097/IMI.0b013e3181cbb421. [DOI] [PubMed] [Google Scholar]







