Abstract
The management of type A aortic dissection presents a major therapeutic challenge in modern surgical practice. Whilst the traditional dictum, to provide timely surgical intervention with the minimum treatment needed to repair the ascending aorta as well as the primary tear, may be a reasonable strategy in older patients, a tailored approach is desired for younger patients to manage the immediate life-threatening condition, as well as for the management of lifelong complications of the residual dissected aorta. Endovascular technology continues to advance, providing an adjunctive role to open cardiac repair presently to manage downstream aortic pathology, with the aim of striving towards a complete endovascular solution for type A aortic dissections.
Keywords: Aortic dissection, Vascular surgery, Cardiac surgery, Endovascular surgery, Hybrid surgery, Treatment paradigm
Introduction
Type A aortic dissection (TAAD) continues to be a major therapeutic challenge in modern surgery. Despite significant advances in preventative medicine, it continues to be a significant cause of morbidity and mortality, with a mortality rate of greater than 50% in untreated TAAD within the first 48 h [1]. Globally, the incidence of aortic dissection varies between 2.9 and 4.3 cases per 100,000 persons per year [2], and often not established until autopsy [3]. Though advanced age is a significant aetiological factor, younger patients present more challenges in terms of immediate and longer-term management [4]. Genetics also contribute to disease presentation, with 8% of patients with aortic dissection reporting a family history of aortic dissection and a further 20% having a first degree relative with a dilated thoracic aorta in the Nordic Consortium for Acute Type A Aortic Dissection (NORCAAD) registry [4].
Classification
The DeBakey classification, first described in 1965, organised aortic dissection presentations into three subgroups: type 1 showing a diffuse dissection arising within the ascending aorta and extending beyond, with type 2 localised to the ascending aorta and type 3 arising distal to the left subclavian artery [4]. In 1970, the Stanford classification was developed to simplify the categorisation of aortic dissection presentations, into type A denoting aortic dissections involving the ascending aorta and type B for aortic dissections arising distal to the left subclavian artery [5]. In the era of hybrid operative intervention for aortic dissection, a further sub-categorisation has evolved from the initial Stanford classification, with the derivation of the “non-Type A, non-Type B” aortic dissection involving the aortic arch alone or arising as a result of retrograde propagation of a dissection originating distal to the left subclavian artery [6].
More recently, the Penn and DISSECT classification systems have been introduced to account for the complexity of aortic dissection presentations, as well as to assist with clinical decision making in the short and long term and determine operative mortality risk [7, 8]. The Penn classification was developed as a means of classifying clinical presentations on the basis of the presence or absence of branch vessel malperfusion or circulatory collapse [8, 9]. The DISSECT classification system, on the other hand, takes into account duration of dissection, site of primary intimal tear, maximum trans-aortic diameter of dissected aorta, segmental extent of aortic involvement, clinical complications related to dissection and thrombosis of the false lumen [7].
Epidemiology
Studies have shown that TAAD involving only the ascending aorta occurs in roughly 30% of all TAAD presentations. As such, a majority of TAADs are much more complex involving significant real estate of the aorta distal to the left subclavian artery requiring long-term follow-up and treatment [10].
Age is a significant factor in the aetiology and incidence of aortic dissections, with mean age at presentation being 63 years [10]. Several other risk factors are also known to be related to aortic dissection presentations, including hypertension, atherosclerosis, aortic aneurysms, cardiac surgery, Marfan syndrome and cocaine consumption [10].
In several ethnic groups, the mean age is lower, and this has implications in the initial modality of treatment and long-term follow-up and compounded by poor blood pressure control. In New Zealand, the incidence of aortic dissections is significantly higher in the Maori population and occurs at a much younger age [11–13]. This also appears to be the case in Asian countries like China [14] and India [15], where the mean age of aortic dissections is closer to 50 years. Following operative intervention, these ethnic groups unfortunately do not have good long-term follow-up, with poor blood pressure control and limited access to best medical therapy, leading to aortic dilatation and complex aneurysm formation of the arch and descending aorta in the long term [15].
Spectrum of treatment
The treatment paradigm for TAADs is to provide timely surgical repair with the minimum treatment needed to repair the ascending aorta as well as the primary tear [16]. Whilst this seems to have significant logic to it, this dictum needs to be tailored to the continual evolution of endovascular technology and of the availability of local technical expertise. Whilst the minimalist approach may be optimal for a TAAD dissection in an elderly patient, this would be considered grossly inadequate for a younger patient with a more complex dissection [16]. Nearly 70% of patients have diffuse involvement of most of the aorta and this leads to significant angulation and tortuosity, when the residual dissected aorta weakens and undergoes aneurysmal degeneration [17]. As such a sequential strategy should be considered for the surgical management of TAAD, initially addressing life-threatening conditions followed by the management of lifelong complications of residual dissection of the aorta [16].
Modern treatment options
Modern operative techniques for the management of TAAD extend from ascending aortic replacement, together with aortic valve re-suspension or replacement, to aortic arch replacement and reconstruction of arch branch vessels, as well as classical or frozen elephant trunk [16].
The introduction of the branch-first technique allowed for sequential reconstruction and reperfusion of arch branches, minimising total cerebral circulatory arrest, as well as shortening distal organ and cardiac ischaemic time, besides reducing air and particulate embolisation during proximal aortic repair [18]. Matalanis et al. reported favourable perioperative outcomes utilising the branch-first technique, with a 3.1% mortality rate and a low incidence of neurological, renal, cardiac and respiratory complications, thereby demonstrating the quality of vital organ perfusion and protection [19]. From a future proofing perspective, total debranching together with a classic or frozen elephant trunk offers an easier solution to treat downstream aortic dilatation with endovascular solutions in collaboration with vascular surgeons [19].
Whilst endovascular treatment of sequelae following TAAD repair for complex dissection is well described (Fig. 1), primary management of TAAD via a total endovascular approach utilising an endograft poses challenges with patient selection, vascular access, stent graft design and aortic valve involvement [20]. Case reports of highly selected patients have demonstrated modest success with endovascular management of acute, subacute and chronic TAAD confined to the ascending aorta [21, 22]. Endovascular management for TAAD has typically been reserved for patients considered high risk or frail, as an alternative for open surgical intervention, but with appropriate morphologic characteristics [20, 23–25].
Fig. 1.
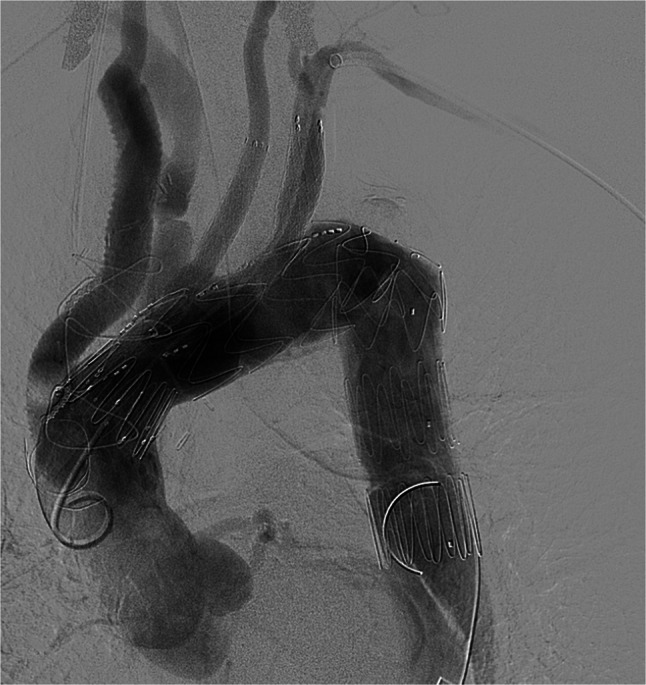
Completion of fenestrated aortic arch endograft with supra-aortic stenting following ascending aorta repair and innominate artery bypass for complex type A aortic dissection
Whilst trans-femoral, trans-apical, trans-carotid and trans-axillary approaches have made endovascular TAAD management feasible, it is limited by anatomical, technical and stent graft–related considerations [26]. From an anatomical perspective, considerations include the proximal and distal landing zones, the extent of the dissection distal to the sinotubular junction and proximal to the innominate artery and the absence of aortic regurgitation, as well as access vessel considerations [24, 27]. Whilst the absence of a proximal landing zone is the most common exclusion criterion for endovascular TAAD management, retrospective reviews of imaging of acute TAAD patients have demonstrated between a third to half of patients have suitable anatomy [24, 28]. Severe aortic regurgitation and dissection involving the aortic root are contraindications to endovascular management, whilst patients with connective tissue disorders could be considered for aortic endografting as a temporising measure until definitive open surgery [24, 28].
Advances in stent graft technology in the future will hopefully account for the distinct challenges of the ascending aorta, including the length of stent grafts and avoidance of multiple overlapping stents, as well as accounting for the haemodynamic force including significant changes in diameter and area throughout the cardiac cycle, to achieve an adequate landing length [20, 24]. Furthermore, an incorporated aortic valve and coronary artery solution, together with the ability to achieve a proximal seal to withstand displacement forces and prevent stent migration, adds extra complexity [20].
Whilst endovascular TAAD management avoids the need for median sternotomy, cardiopulmonary bypass and deep hypothermic circulatory arrest, it is not without risk of significant complications [29]. In particular, the fragility of the ascending aorta following TAAD can cause retrograde TAAD or aortic rupture during stent graft deployment [24]. Furthermore, the proximity of the coronary artery ostia and aortic valve to the proximal landing zone may cause inadvertent coronary artery coverage or aortic valve dysfunction [30]. The traversal of guidewires and sheaths across the aortic arch increases the risk of neuro-embolisation of thrombus [24], though the limited clinical evidence thus far has not demonstrated a higher incidence of stroke following endovascular treatment, when compared to open TAAD repair [20]. Finally, other potential complications include endoleaks leading to potential TAAD extension [26] and device migration, as well as supra-aortic vessel coverage [25].
Regarding the management of the sequalae following TAAD repair, a range of modern endovascular techniques and particularly the use of fenestrated or branched technology have been utilised to deal with downstream post-dissection aortic aneurysm (PDAA) presentations [31]. Whilst for fenestrated endografts, the connecting stents to the branch vessels can often be accomplished by groin access, it is different with branched endografts [32]. Branches to the visceral vessels are often downward facing and need access from the subclavian arteries to connect the branches, presenting a challenge with the branch-first technique of debranching all supra-aortic vessels [19, 31]. Advancement in endovascular armament has helped provide an innovative solution with the initial description of pre-curving available guide sheaths to development of steerable sheaths to allow for successful cannulation of these branched endografts from the groin rather than from proximal access [33, 34]. Complex endovascular repair of the PDAAs has been shown to be safe and durable, with the particularly vexed issue of stroke and spinal cord ischaemia reduced to under 5% with more contemporary results [31].
Understandably, the expertise to perform complex aortic endovascular interventions tends to be concentrated in larger centres with higher volumes [35]. However, lower volume centres also can set up an endovascular platform for management of future downstream thoracoabdominal aortic pathology following TAAD repair [36].
Our experience in hybrid open and endovascular intervention in a tertiary-level hospital in New Zealand (NZ) for a cohort of patients with complex TAADs was presented at the 2015 Charing Cross International Symposium [37]. Utilising a modified ascending repair with a single side branch, the ascending graft is planned to have at least 3 cm of landing zone distal to the side branch (Fig. 2). Once the ascending repair is completed, this side branch can be anastomosed to the innominate artery end to side, while the patient is being rewarmed. If the innominate artery is involved with the dissection, this graft is tunnelled to the base of the neck to be anastomosed to the common carotid artery on the right. This allows future proofing of the repair to endovascular solutions, but with the following limitations:
Endografts need at least 3 cm of proximal landing zone proximally within the prosthetic graft.
Native aortic valves and bioprosthetic valves can allow the nose cone of the endografts to be traversed to land the graft in the ascending aorta.
Debranching of the innominate or both the carotids then provides a simpler procedure of endovascular repair of the arch and proximal descending aorta.
Fig. 2.
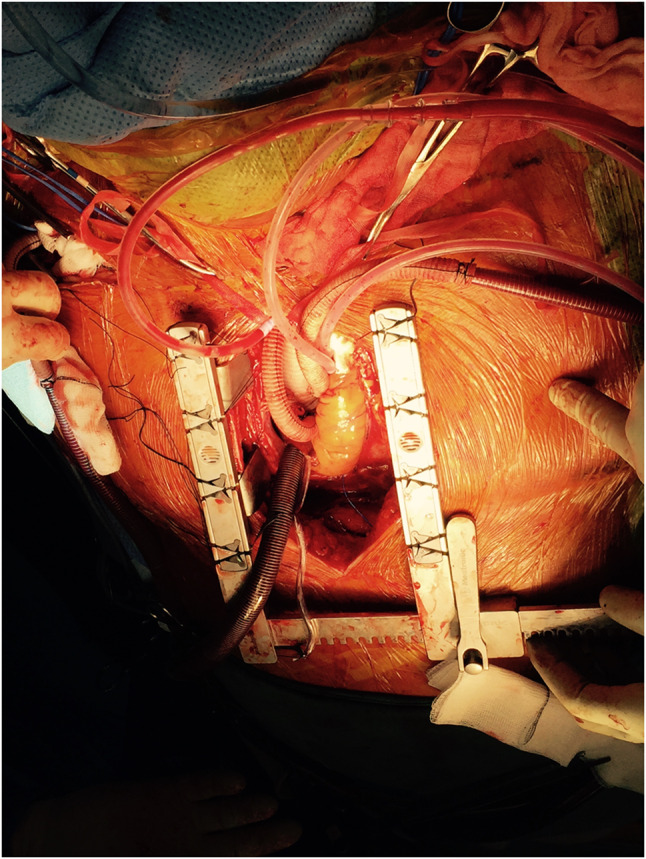
Intra-operative image of a type A aortic dissection repair with side branch anastomosed to the innominate artery
In our small cohort experience in NZ, nearly 60% of patients needed complex repair of the arch and descending aorta within 18 months [37].
Conclusions
The contemporary management of TAADs continues to evolve with the advancement of cardiac surgical operative techniques and endovascular technology. A collaborative approach with cardiac and vascular surgeons as well as interventional radiologists allows for a comprehensive manner of managing long-term sequelae of a diseased aorta.
The modern endovascular armamentarium has a range of tools to support this change in philosophy to manage complex dissections. While this loosely supports specific centres to take up the bulk of the treatment, smaller centres can make appropriate amendments to their treatment paradigm to optimise outcomes in the short term and provide a better platform for endovascular management in the future. A dedicated aortic team meeting pre-operatively for every patient presenting with a complex aortic dissection can lead to durable operative solution allowing for improved short-term outcomes with the ability to provide long-term care for the residual downstream pathology.
A useful point to conclude would be that in regard to TAADs, in younger patients, doing more upfront is less as opposed to the older patient, where doing less is more.
Funding
None.
Declarations
Informed consent
Obtained for the figures used in this review paper in line with the local health district’s consent process.
Human and animal rights statement
No research involving human participants and/or animals were undertaken for this review paper.
Ethics approval
Not applicable, being a review article.
Conflict of interest
The authors of this review paper have no conflicts of interest to declare.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Howard C, Ponnapalli A, Shaikh S, Idhrees M, Bashir M. Non-A non-B aortic dissection: A literature review. J Card Surg. 2021;36:1806–1813. doi: 10.1111/jocs.15349. [DOI] [PubMed] [Google Scholar]
- 2.LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8:103–113. doi: 10.1038/nrcardio.2010.187. [DOI] [PubMed] [Google Scholar]
- 3.Huynh N, Thordsen S, Thomas T, et al. Clinical and pathologic findings of aortic dissection at autopsy: Review of 336 cases over nearly 6 decades. Am Heart J. 2019;209:108–115. doi: 10.1016/j.ahj.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Gudbjartsson T, Ahlsson A, Geirsson A, et al. Acute type A aortic dissection - a review. Scand Cardiovasc J. 2020;54:1–13. doi: 10.1080/14017431.2019.1660401. [DOI] [PubMed] [Google Scholar]
- 5.Lempel JK, Frazier AA, Jeudy J, et al. Aortic arch dissection: a controversy of classification. Radiology. 2014;271:848–855. doi: 10.1148/radiol.14131457. [DOI] [PubMed] [Google Scholar]
- 6.Carino D, Singh M, Molardi A, et al. Non-A non-B aortic dissection: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2019;55:653–659. doi: 10.1093/ejcts/ezy337. [DOI] [PubMed] [Google Scholar]
- 7.Dake MD, Thompson M, van Sambeek M, Vermassen F, Morales JP. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg. 2013;46:175–190. doi: 10.1016/j.ejvs.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Augoustides JGT, Geirsson A, Szeto WY, et al. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med. 2009;6:140–146. doi: 10.1038/ncpcardio1417. [DOI] [PubMed] [Google Scholar]
- 9.Augoustides JGT, Szeto WY, Desai ND, et al. Classification of acute type A dissection: focus on clinical presentation and extent. Eur J Cardiothorac Surg. 2011;39:519–522. doi: 10.1016/j.ejcts.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 10.Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: A 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–1860. doi: 10.1161/CIRCULATIONAHA.117.031264. [DOI] [PubMed] [Google Scholar]
- 11.Wang TKM, Wei D, Evans T, Ramanathan T, Haydock D. Comparison of characteristics and outcomes for type A aortic dissection surgery by Maori, Pasifika or other ethnicities. N Z Med J. 2020;133:33–40. [PubMed] [Google Scholar]
- 12.Xu W, Mani K, Khashram M. Ethnic differences in incidence and outcomes of acute aortic syndromes in the Midland region of New Zealand. J Vasc Surg. 2021. 10.1016/j.jvs.2021.08.066. [DOI] [PubMed]
- 13.Khanafer A, Khashram M, Mann D. Recent changes in the management of aortic dissection. N Z Med J. 2015;128:9–11. [PubMed] [Google Scholar]
- 14.Wang W, Duan W, Xue Y, et al. Clinical features of acute aortic dissection from the Registry of Aortic Dissection in China. J Thorac Cardiovasc Surg. 2014;148:2995–3000. doi: 10.1016/j.jtcvs.2014.07.068. [DOI] [PubMed] [Google Scholar]
- 15.Nagaradona SR, Machiraju K, Kurapati SR, Boggula S, Setty SA, Azam S. Surgical management of acute Type A aortic dissection: An overview. Indian J Clin Cardiol. 2021;2:23–31. doi: 10.1177/2632463620978047. [DOI] [Google Scholar]
- 16.Malaisrie SC, Szeto WY, Halas M, et al. 2021 The American Association for Thoracic Surgery expert consensus document: Surgical treatment of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2021;162:735–758.e2. doi: 10.1016/j.jtcvs.2021.04.053. [DOI] [PubMed] [Google Scholar]
- 17.Rylski B, Milewski RK, Bavaria JE, et al. Outcomes of surgery for chronic type A aortic dissection. Ann Thorac Surg. 2015;99:88–93. doi: 10.1016/j.athoracsur.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 18.Kim M, Matalanis G. Technique and rationale for branch-first total aortic arch repair. JTCVS Tech. 2020;4:1–4. doi: 10.1016/j.xjtc.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matalanis G, Galvin SD. "Branch-first" continuous perfusion aortic arch replacement and its role in intra-operative cerebral protection. Ann Cardiothorac Surg. 2013;2:194–201. doi: 10.3978/j.issn.2225-319X.2013.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed Y, Houben IB, Figueroa CA, et al. Endovascular ascending aortic repair in type A dissection: A systematic review. J Card Surg. 2021;36:268–279. doi: 10.1111/jocs.15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felipe Gaia D, Bernal O, Castilho E, et al. First-in-human Endo-Bentall procedure for simultaneous treatment of the ascending aorta and aortic valve. JACC Case Rep. 2020;2:480–485. doi: 10.1016/j.jaccas.2019.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato N, Shimono T, Hirano T, Ishida M, Yada I, Takeda K. Transluminal placement of endovascular stent-grafts for the treatment of type A aortic dissection with an entry tear in the descending thoracic aorta. J Vasc Surg. 2001;34:1023–1028. doi: 10.1067/mva.2001.118808. [DOI] [PubMed] [Google Scholar]
- 23.Roselli EE, Hasan SM, Idrees JJ, et al. Inoperable patients with acute type A dissection: are they candidates for endovascular repair? Interact Cardiovasc Thorac Surg. 2017;25:582–588. doi: 10.1093/icvts/ivx193. [DOI] [PubMed] [Google Scholar]
- 24.Shah A, Khoynezhad A. Thoracic endovascular repair for acute type A aortic dissection: operative technique. Ann Cardiothorac Surg. 2016;5:389–396. doi: 10.21037/acs.2016.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petrov I, Stankov Z, Tasheva I, Stanilov P. Endovascular treatment of Acute Aortic Dissection Stanford Type A. JACC Cardiovasc Interv. 2018;11:218–219. doi: 10.1016/j.jcin.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 26.Harky A, Al-Adhami A. Stenting in type A aortic dissection: fantasy or reality? J Vis Surg. 2018;4:161. doi: 10.21037/jovs.2018.07.09. [DOI] [Google Scholar]
- 27.Khoynezhad A, Donayre CE, Walot I, Koopmann MC, Kopchok GE, White RA. Feasibility of endovascular repair of ascending aortic pathologies as part of an FDA-approved physician-sponsored investigational device exemption. J Vasc Surg. 2016;63:1483–1495. doi: 10.1016/j.jvs.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Tang H, Zhou JP, et al. The imaging assessment and specific endograft design for the endovascular repair of ascending aortic dissection. Clin Interv Aging. 2016;11:933–940. doi: 10.2147/CIA.S104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harky A, Chan J, MacCarthy-Ofosu B. The future of stenting in patients with type A aortic dissection: a systematic review. J Int Med Res. 2020;48:300060519871372. doi: 10.1177/0300060519871372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimpfer D, Czerny M, Kettenbach J, et al. Treatment of acute type a dissection by percutaneous endovascular stent-graft placement. Ann Thorac Surg. 2006;82:747–749. doi: 10.1016/j.athoracsur.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 31.Zeng Z, Zhao Y, Wu M, et al. Endovascular strategies for post-dissection aortic aneurysm (PDAA) J Cardiothorac Surg. 2020;15:287. doi: 10.1186/s13019-020-01331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amiot S, Haulon S, Becquemin J-P, et al. Fenestrated endovascular grafting: the French multicentre experience. Eur J Vasc Endovasc Surg. 2010;39:537–544. doi: 10.1016/j.ejvs.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Watkins AC, Avramenko A, Soler R, Fabre D, Haulon S. A novel all-retrograde approach for t-Branch implantation in ruptured thoracoabdominal aneurysm. J Vasc Surg Cases Innov Tech. 2018;4:301–304. doi: 10.1016/j.jvscit.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallitto E, Faggioli G, Bertoglio L, et al. Steerable sheath for cannulation and bridging stenting of challenging target visceral vessels in fenestrated and branched endografting. Ann Vasc Surg. 2020;67:26–34. doi: 10.1016/j.avsg.2019.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Locham S, Hussain F, Dakour-Aridi H, Barleben A, Lane JS, Malas M. Hospital volume impacts the outcomes of endovascular repair of thoracoabdominal aortic aneurysms. Ann Vasc Surg. 2020;67:232–241.e2. doi: 10.1016/j.avsg.2019.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Brescia AA, Patel HJ, Likosky DS, et al. Volume-outcome relationships in surgical and endovascular repair of aortic dissection. Ann Thorac Surg. 2019;108:1299–1306. doi: 10.1016/j.athoracsur.2019.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vasudevan T, El Gamel A. Pre-emptive bypass with type A repair: future proofing in complex dissections. In Charing Cross Symposium, R.M. Greenhalgh, Editor. 2015, BIBA Medical Charing Cross, London.


