Abstract
The “Branch-First total arch replacement” technique has been used extensively in both elective and acute situations, including in type A aortic dissection. The focus of the Branch-First technique is to reduce the risk of neurological and end-organ dysfunction associated with arch replacement by optimising neuroprotection, distal organ perfusion and myocardial protection. The Branch-First technique is a valuable alternative to the frozen elephant trunk (FET) technique in type A aortic dissection, providing a stable landing zone for subsequent interventions on the distal aorta should they be required. Combining the Branch-First technique with FET in appropriate cases can further improve outcomes. We discuss the merits of the Branch-First technique, and contrast them to those of FET techniques for repair of type A aortic dissection.
Keywords: Total aortic arch repair, Aortic arch replacement, Antegrade cerebral perfusion, Branch-First, Frozen elephant trunk, Acute aortic dissection
Introduction
Operations for acute type A aortic dissection are performed in the emergency setting, and thus the presenting clinical status, patients’ premorbid condition and the extent of disease are highly variable. Until recent times, the standard emergency operation has been the “hemiarch” procedure to simplify and shorten the procedure, with the underlying presumption of reducing operative mortality and the deferment of more complex interventions, if needed, to an elective procedure in “expert” hands [1]. However, experience with these limited aortic resections has demonstrated unsatisfactory in both short- and long-term outcomes, as a consequence of continued false lumen (FL) pressurisation, resulting in aneurysmal degeneration, rupture, malperfusion and the need for further high-risk interventions [1–3].
Increasingly greater lengths of aortic resections, such as total arch replacement, are being advocated at the index operation with the aim of removing more of the large proximal entry tears, reducing pressure in FL and leading to FL healing with better early and late outcomes [4]. Nonetheless, aortic arch replacement is a technically challenging surgical procedure that may be associated with increased rates of morbidity and mortality in inexperienced centres [5].
We have previously described our “Branch-First total arch replacement” technique which we have extensively utilised in both elective and acute situations, including in type A aortic dissection, with excellent outcomes [6–8]. The technique has now been adopted in an increasing number of centres in Australia and New Zealand, as well as some in the UK and Germany. In addition, senior cardiac trainees are often first operators in many of these cases, without any worsening of results. The focus of the Branch-First technique is to reduce the risk of neurological and end-organ dysfunction associated with arch replacement by optimising neuroprotection, distal organ perfusion and myocardial protection. An additional side benefit of the confidence in cerebral and organ preservation is the ability to perform the procedure in an unhurried and thus more exacting fashion.
In appropriate cases, we have performed the Branch-First total arch replacement technique in concert with the frozen elephant trunk (FET) technique, with improved outcomes compared to standard arch replacement and FET. We will elaborate on the merits of the Branch-First technique alone or with FET, versus standard arch replacement and FET techniques for repair of type A aortic dissection.
Summary of operative technique
The full details of the branch-first technique have previously been described [8–12]. In summary, it involves serial debranching and reperfusion of the supra-aortic vessels using a trifurcation graft with a side perfusion port (TAPP graft). It allows perfusion to the heart and distal organs during the period of debranching, minimising myocardial and distal ischaemia. Antegrade cerebral perfusion then continues via the TAPP graft while arch reconstruction occurs, avoiding excessive hypothermia for cerebral protection. Intraoperative cerebral monitoring is performed by a combination of electroencephalography (EEG) bispectral index monitoring, near infrared spectroscopy (NIRS) and bilateral transcranial Doppler (TCD).
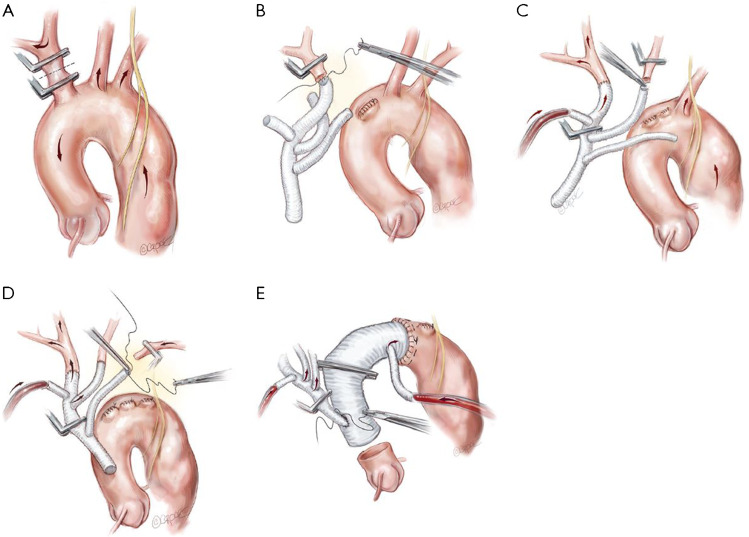
For cardiopulmonary bypass, the arterial cannula is generally placed in the femoral artery. In cases where clinical or radiographic point to high risk of retrograde malperfusion, the axillary artery or direct aortic cannulation is used. Whichever cannulation site is used, great care in exercised in detecting cerebral malperfusion. If this is at all suspected, additional inflow cannulas are added. The fact that arch branches are the very first steps in the reconstruction ensures that the risk of malperfusion is kept at minimum. A dedicated head circuit is used for antegrade cerebral perfusion to maintain cerebral flow independent of the rest of the body. Each of the supra-aortic vessels is carefully mobilised and, once bypass is established, is sequentially anastomosed to the TAPP graft (Fig. 1A–D).
Fig. 1.
Branch-First total arch replacement technique. (A) The innominate artery is clamp isolated. Right hemispheric cerebral perfusion is maintained through the left common carotid and subclavian arteries via rich collateral networks. (B) The innominate artery is anastomosed to the first limb of the trifurcation graft and the proximal stump is ligated. (C) Clamps are rearranged and antegrade cerebral perfusion is commenced via the side port of the trifurcation graft to the innominate artery. The left common carotid artery is clamp isolated and anastomosed to the second limb of the trifurcation graft. (D) Clamps are rearranged and antegrade cerebral perfusion continues via the side port of the trifurcation graft to the innominate and left common carotid arteries. The left subclavian artery is clamp isolated and anastomosed to the third limb of the trifurcation graft. Clamps are again rearranged for continuous antegrade perfusion via the trifurcation graft to all three supra-aortic vessels. (E) The distal anastomosis is completed and a cross-clamp placed across the aortic graft proximal to the perfusion side port to commence antegrade distal perfusion. The trifurcation graft is anastomosed to the new ascending aorta sufficiently distant from the distal aortic anastomosis to allow an adequate landing zone in case of future endovascular procedures. Copyright Beth Croce, Bioperspective.com
Note that debranching progresses from the most proximal branch towards the left subclavian artery. This provides better exposure to the deeply lying left subclavian artery as each branch is divided and retracted out of the way. If the left subclavian artery remains difficult to access, a short extension of the skin incision along the anterior border of the left sternocleidomastoid muscle can improve exposure; or alternatively, the debranching of the left subclavian artery can be delayed until distal circulatory arrest is instituted. The aortic arch can then be resected to create more space for the left subclavian artery anastomosis.
As each vessel is attached and de-aired, antegrade cerebral perfusion is commenced to achieve a mean arterial pressure of 50 to 60 mmHg (Fig. 1C–E). This process takes advantage of rich collateral networks around the head and neck to provide ipsilateral cerebral perfusion during the brief period that each single arch branch is in isolation. Moderate hypothermia is used with a target temperature of 25 °C in cases where distal circulatory arrest is anticipated.
Although distal aortic control can be achieved by clamping, or endoluminal balloon occlusion, distal circulatory arrest is preferred in acute dissection to reduce trauma to the distal aorta. Myocardial arrest is also initiated at this time and maintained by antegrade and retrograde cold blood cardioplegia. A Dacron graft with a pre-attached side port (Ante-Flo graft, Vascutek Ltd, Renfrewshire, Scotland, UK) is used for the distal anastomosis. The aortic clamp is repositioned on the graft proximal to the side port to allow for antegrade distal perfusion via the side port (Fig. 1E).
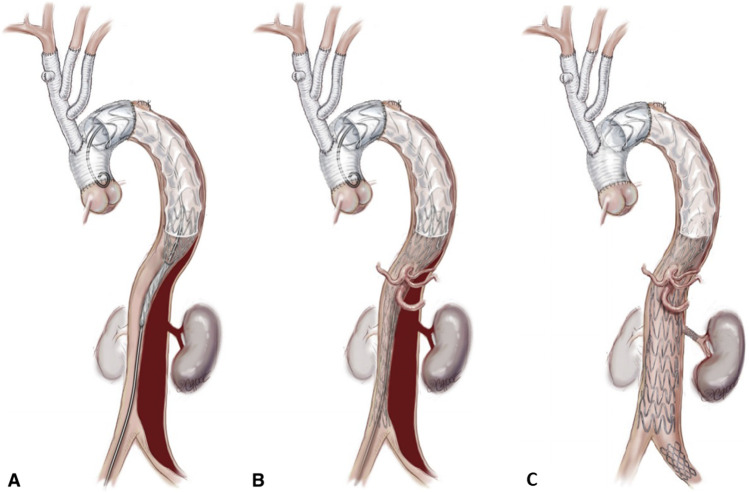
On occasions where there is a large entry tear beyond the reach of exclusion, and particularly in cases of retrograde type A dissection, we use the FET graft, either the Thoraflex hybrid graft (Vascutek Terumo, Inchinnan, Scotland, UK) or the E-vita hybrid graft (Jotec GmbH, Hechingen, Germany) (Fig. 2).
Fig. 2.
The Branch-First technique can be readily used in combination with a frozen elephant trunk graft in selected patients. Either the Thoraflex hybrid graft (Vascutek Terumo, Inchinnan, Scotland, UK) or the E-vita hybrid graft (Jotec GmbH, Hechingen, Germany) is used. Copyright Beth Croce, Bioperspective.com
The proximal extent of the repair varies with aortic pathology. In cases where a complex root replacement is required, a separate graft is used for the root reconstruction and a graft-to-graft anastomosis is performed with distal aortic graft. Otherwise, the arch graft is anastomosed directly to the sino-tubular junction.
Finally, the common stem of the TAPP graft is anastomosed to the new ascending aorta. A key point here is that the trifurcation graft anastomosis to the new ascending graft is situated sufficiently distant from the distal aortic anastomosis to allow more than adequate landing zone in case of future endovascular procedures (Fig. 1E).
At our institution to date, we have performed Branch-First arch replacement surgery for a total of 156 patients, of which 41 (26%) were performed emergently for acute type A aortic dissection. In this cohort of type A dissection patients, 59% were male and the median age was 73 years. The Dacron Ante-Flo graft was used in the majority of cases (n = 32, 78%) and the FET graft was used in the rest of the cases (n = 9, 22%). In terms of distal extent, 24 patients (59%) had debranching of all three supra-aortic vessels, with the remainder requiring only two-vessel debranching for complete correction of pathology. In regard to proximal extent, 30 patients (73%) had the aorta replaced to the level of the sino-tubular junction, 8 patients (20%) underwent Bentall procedures and 3 patients (7%) underwent David procedures. Median clamp times for the innominate, left carotid and left subclavian arteries were 14, 10 and 14 min, respectively. The median recorded lowest core temperature was 25 °C. There were five in-hospital mortalities, which occurred in higher risk cases with preoperative organ malperfusion or rupture. Postoperative morbidity included return to theatre for bleeding (n = 6), tracheostomy (n = 4) and new permanent cerebrovascular event (n = 2). Three patients had preoperative limb ischaemia secondary to malperfusion from aortic dissection; however, there were no new cases of limb ischaemia postoperatively.
Advantages of Branch-First total arch replacement
One of the core advantages of the Branch-First technique is the debranching of the supra-aortic vessels in the first stage of the procedure, prior to any myocardial or distal circulatory arrest. This prioritises and secures antegrade cerebral perfusion from dependable undissected branches of the trifurcation graft which in itself is connected to a dedicated pump such that cerebral flows and pressures are under complete control. Clearly, this is a major bonus in acute type A dissection, where irrespective of the arterial inflow cannula choice, there is a risk of arch branch malperfusion.
While it may be argued that isolated branch vessel clamping may result in ipsilateral hemispheric ischaemia, we have not observed a pattern of ipsilateral watershed infarction, nor any persistent cerebral oximetry associated with this. In fact, we frequently observe vigorous arch vessel back bleeding during the graft de-airing. This is not surprising given the very rich collateral network across the midline of the head and neck, which greatly augments that provided by the circle of Willis. Furthermore, the short clamp times, typically less than 10 min, are a major reduction of reliance on collaterals compared to standard unilateral antegrade perfusion techniques, where one depends on such collaterals for significantly longer times. Even so, studies have failed to demonstrate inferiority of the latter technique compared to bilateral antegrade perfusion.
There is little modification required to our technique for anomalous or aberrant anatomy of the supra-aortic vessels. Each vessel can still be debranched in the usual manner from proximal to distal arch, since they are clamped individually for a very short period of time. In bovine arch, the proximal innominate artery and left common carotid artery can be clamped separately in sequence, just distal to their take-off from the common trunk. Regardless of anomalous anatomy, the vessels “come online” sequentially, taking advantage of the rich collateral networks in the same fashion to provide cerebral supply.
Another major advantage of the Branch-First technique is a major reduction in myocardial and distal ischaemic times. Suboptimal myocardial protection during such longer and complex operations is a significant contributor to postoperative morbidity and mortality. No matter how good a myocardial preservation technique is utilised, the longer the period of ischaemia, the more likely for its imperfections to show. While distal circulatory arrest is used for the distal anastomosis, the combination of reducing its duration by doing “Branch-First”, and the added distal organ collateral flow provided by all three arch vessel continuous perfusion, significantly reduces vital organ and spinal cord ischaemia.
Further advantages of the Branch-First technique include total arch replacement with the ability to further reduce the number of large proximal entry tears compared to standard hemiarch techniques, greatly reducing input into FL pressurisation. While distal anastomosis is often conveniently placed in zones 1 or 2, zone 3 and even upper descending distal anastomoses can readily be performed in cases where a large entry tear is present beyond the reach of “hemiarch” resection. As all arch branches have secure blood supply from undissected Dacron trifurcation graft, the risk subsequent malperfusion from unfavourable true lumen (TL) perfusion in native arch is obviated. Again, physical isolation of the arch branches from remaining dissected distal aorta prevents branch FL perfusion from pressurising the distal aortic FL. These factors, together with appropriate root management at the initial operation, should greatly reduce the need for future difficult and risky re-sternotomy. There is also greater facility in applying standard thoracic endovascular (TEVAR) extensions if required in the future, rather than the need for complex branched or fenestrated stent grafts. The reason is not only the absence of dependence of arch branch supply from residual dissected aorta, but also the proximalisation of the origin from the ascending aorta compared to the site of distal anastomosis, providing a comfortably long landing zone for a TEVAR to achieve a good seal against endoleaks (Fig. 1E).
The FET graft
The use of a FET graft is an excellent option when dissection extends beyond the arch. FET goals include the expansion of the collapsed TL, promotion of thrombosis of the FL and coverage of any proximal descending re-entry tears to reduce the risk of re-pressurisation of the FL [13–15]. Thus, it aims to treat or prevent concomitant malperfusion syndrome and avoid future dilatation of the FL in the distal aorta [16, 17]. In cases of particularly fragile tissue, FET provides extra buttressing at the level of the distal aortic anastomosis. In cases of future progression of distal aortic pathology, FET provides an excellent proximal landing zone for a second-stage TEVAR [14]. If open thoraco-abdominal aortic replacement is required, the FET eliminates the need for high thoracotomy access and the requirement for extensive dissection in the region of the previously operated distal arch, reducing the risk of injury to surrounding structures such as the recurrent laryngeal nerve and pulmonary artery [7].
Nonetheless, routine use of FET does have some disadvantages. An infrequent but alarming complication of standard dissection repair with FET is the development of paraparesis or paraplegia (0–21%) [18]. Of course the risk of spinal cord injury (SCI) may be reduced with shorter coverage of the descending aorta, cerebrospinal fluid (CSF) drainage and shorter period of distal circulatory arrest [16, 19, 20].
Other risks include the phenomenon of stent-induced new intimal tear (SINE), which would greatly increase FL pressurisation, continued FL growth and even stent collapse with TL compromise. Inadvertent placement of FET in FL can lead to further compromise of TL flow and accelerate FL growth [21]. Graft infection and aorto-oesophageal fistula are also risks as is the case for any aortic stent graft.
It is important to recognise that while FL thrombosis in the “peri-graft” descending aorta is very common, it is relatively uncommon in the thoraco-abdominal aorta [22]. While the rate of progression of FL dilatation may be slower in those more distal segments, the subsequent open or closed repairs required are not necessarily any less complex or morbid.
The concern about the hazards of routine use of FET is further heightened by the appreciation that a significant but not always predictable proportion of acute dissection patients have obliteration of their FL by total arch replacement alone [23, 24]. These patients will have undergone the risks of FET unnecessarily.
Other legitimate objections to the routine use of FET in aortic dissection include expense, availability and specific training.
FET as an adjunct to the Branch-First technique
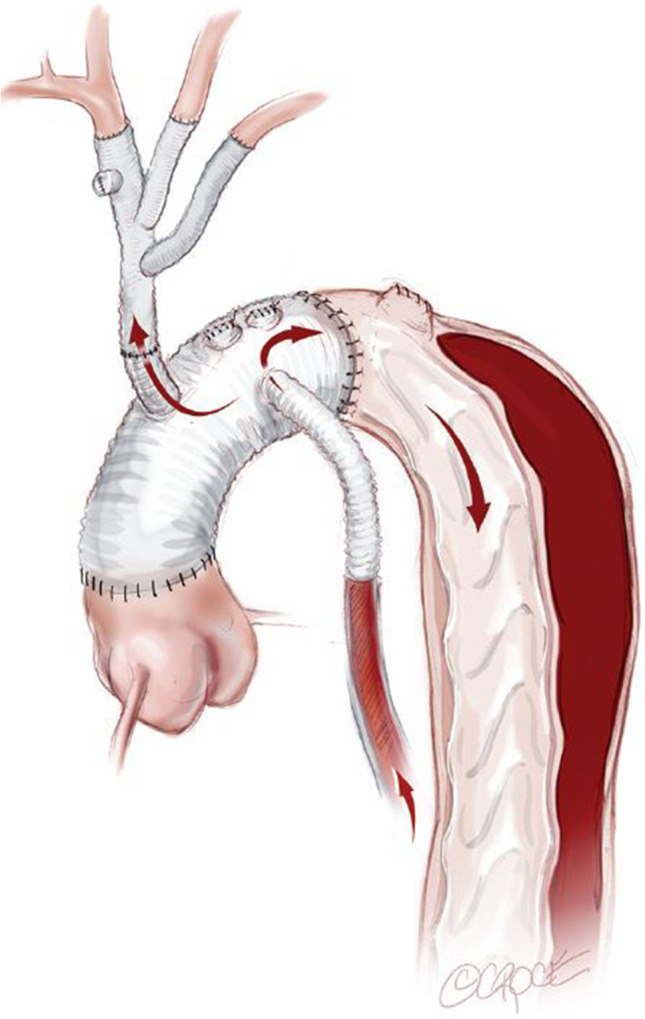
The Branch-First technique can be readily used in combination with a FET graft (Fig. 2). We do use FET in selected patients. This includes those with severely compromised TL associated with preoperative distal malperfusion symptoms. However, in the absence of such features, our preference is to perform Branch-First total arch replacement and carefully observe the patient clinically and with computed tomography (CT) imaging postoperatively. If there are any features of persistent/recurrent clinical ischaemia or TL compromise, we proceed to TEVAR in the radiology suite. In this way, the more extensive interventions are saved for those in need. The TEVAR implant can also be more accurately sized and placed under stable haemodynamics to further reduce the risks inherent in FET. Finally, we will often combine the covered TEVAR with the balloon fenestration and uncovered stent graft thoraco-abdominal as per the “Stent-Assisted Balloon-Induced Intimal Disruption and Relamination in Aortic Dissection Repair (STABILISE)” technique to effect a total aortic repair [25, 26] (Fig. 3).
Fig. 3.
A covered TEVAR has been placed with an overlap proximally to the surgically created landing zone and extending distally to the level of the mid descending aorta. The remaining (A) thoracic and (B) abdominal aorta down to the aortic bifurcation is lined with bare metal uncovered stent grafts (Zenith Dissection Endovascular Stent, Cook Medical Inc). The stents are balloon dilated to rupture the septum between TL and FL to effectively create a single aortic channel. (C) Full deployment of bare metal uncovered stent graft after balloon rupture of intimal septum and complete obliteration of TL. Copyright Beth Croce, Bioperspective.com
It is interesting that SCI in our series of Branch-First with FET had an extremely low paraplegia risk [7]. It is our belief that this is due to the reduction of the duration of distal circulatory arrest and by using uninterrupted three-vessel antegrade cerebral perfusion maximising collateral supply to the spinal cord via the vertebral arteries.
Conclusion
The Branch-First total arch replacement technique, with the adjunct of a FET in suitably selected cases, is a valuable alternative in the setting of acute type A aortic dissection. Our outcomes in terms of operative morbidity and mortality, as well as interval distal aortic complications, have been superior to those of “hemiarch” technique. We believe that this is due to the more secure cerebral and vital organ preservation, and reduced bleeding and malperfusion complications. We believe that selective rather than routine use of FET reduces risks of paraplegia and other stent-related complications. Further distal interventions can be performed only in those who need it and in a controlled haemodynamic and interventional environment. The long landing zone and absence of dissected aorta-dependent arch branches greatly facilitate future open or endovascular procedures.
Funding
None.
Declarations
This manuscript was prepared in accordance with ethical standards. No patient data is presented in this manuscript and informed consent is not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Di Bartolomeo R, Pantaleo A, Berretta P, et al. Frozen elephant trunk surgery in acute aortic dissection. J Thorac Cardiovasc Surg. 2015;149:S105–9. [DOI] [PubMed]
- 2.Evangelista A, Maldonado G, Gruosso D, Teixido G, Rodríguez-Palomares J, Eagle K. Insights from the International registry of acute aortic dissection. Glob Cardiol Sci Pract. 2016;2016:e201608. [DOI] [PMC free article] [PubMed]
- 3.Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD):new insights into an old disease. JAMA. 2000;283:897–903. [DOI] [PubMed]
- 4.Ogino H. Acute type A aortic dissection: the role of frozen elephant trunk. Ann Cardiothorac Surg. 2020;9:233–5. [DOI] [PMC free article] [PubMed]
- 5.Evangelista A, Isselbacher EM, Bossone E, et al. Insights from the International Registry of Acute Aortic Dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137:1846–60. [DOI] [PubMed]
- 6.Galvin SD, Perera NK, Matalanis G. Surgical management of acute Type A aortic dissection: branch-first arch replacement with total aortic repair. Ann Cardiothorac Surg. 2016;5:236–44. [DOI] [PMC free article] [PubMed]
- 7.Kim M, Matalanis G. Aortic arch replacement using the branch-first and frozen elephant trunk techniques. Ann Cardiothorac Surg. 2020;9:259–61. [DOI] [PMC free article] [PubMed]
- 8.Matalanis G, Perera NK, Galvin SD. Aortic arch replacement without circulatory arrest or deep hypothermia: The branch-first technique. J Thorac Cardiovasc Surg. 2015;149:S76–82. [DOI] [PubMed]
- 9.Galvin SD, Matalanis G. Continuous perfusion “Branch-first” aortic arch replacement: a technical perspective. Ann Cardiothorac Surg. 2013;2:229–34. [DOI] [PMC free article] [PubMed]
- 10.Kim M, Matalanis G. Technique and rationale for branch-first total aortic arch repair. JTCVS Tech. 2020;4:1–4. [DOI] [PMC free article] [PubMed]
- 11.Matalanis G, Galvin SD. “Branch-first” continuous perfusion aortic arch replacement and its role in intra-operative cerebral protection. Ann Cardiothorac Surg. 2013;2:194–201. [DOI] [PMC free article] [PubMed]
- 12.Matalanis G, Koirala RS, Shi WY, Hayward PA, McCall PR. Branch-first aortic arch replacement with no circulatory arrest or deep hypothermia. J Thorac Cardiovasc Surg. 2011;142:809–15. [DOI] [PubMed]
- 13.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg. 2015;47:759–69. [DOI] [PubMed]
- 14.Tian DH, Ha H, Joshi Y, Yan TD. Long-term outcomes of the frozen elephant trunk procedure: a systematic review. Ann Cardiothorac Surg. 2020;9:144–51. [DOI] [PMC free article] [PubMed]
- 15.De Paulis R, Di Bartolomeo R, Murana G, et al. Frozen versus conventional elephant trunk technique: application in clinical practice. Eur J Cardiothorac Surg. 2017;51:i20–8. [DOI] [PubMed]
- 16.Di Marco L, Pantaleo A, Leone A, Murana G, Di Bartolomeo R, Pacini D. The frozen elephant trunk technique: European Association for Cardio-Thoracic Surgery position and Bologna experience. Korean J Thorac Cardiovasc Surg. 2017;50:1–7. [DOI] [PMC free article] [PubMed]
- 17.Katayama A, Uchida N, Katayama K, Arakawa M, Sueda T. The frozen elephant trunk technique for acute type A aortic dissection: results from 15 years of experience†. Eur J Cardiothorac Surg. 2015;47:355–60. [DOI] [PubMed]
- 18.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardiothorac Surg. 2015;47:759–69. [DOI] [PubMed]
- 19.Di Marco L, Murana G, Fiorentino M, et al. The frozen elephant trunk surgery: a systematic review analysis. Indian J Thorac Cardiovasc Surg. 2019;35:118–26. [DOI] [PMC free article] [PubMed]
- 20.Preventza O, Liao JL, Olive JK, et al. Neurologic complications after the frozen elephant trunk procedure: A meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg. 2020;160:20–33.e4. [DOI] [PubMed]
- 21.Damberg A, Schälte G, Autschbach R, Hoffman A. Safety and pitfalls in frozen elephant trunk implantation. Ann Cardiothorac Surg. 2013;2:669–76. [DOI] [PMC free article] [PubMed]
- 22.Dohle D-S, Jakob H, Schucht R, et al. The impact of entries and exits on false lumen thrombosis and aortic remodelling. Eur J Cardiothorac Surg. 2017;52:508–15. [DOI] [PubMed]
- 23.Takahara Y, Sudo Y, Mogi K, Nakayama M, Sakurai M. Total aortic arch grafting for acute type A dissection: analysis of residual false lumen. Ann Thorac Surg. 2002;73:450–4. [DOI] [PubMed]
- 24.Di Eusanio M, Berretta P, Cefarelli M, et al. Total arch replacement versus more conservative management in Type A acute aortic dissection. Ann Thorac Surg. 2015;100:88–94. [DOI] [PubMed]
- 25.Hofferberth SC, Nixon IK, Boston RC, McLachlan CS, Mossop PJ. Stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair: the STABILISE concept. J Thorac Cardiovasc Surg. 2014;147:1240–5. [DOI] [PubMed]
- 26.Matalanis G, Ip S. A new paradigm in the management of acute type A aortic dissection: Total aortic repair. J Thorac Cardiovasc Surg. 2019;157:3–11. [DOI] [PubMed]