Abstract
The ascending aorta has a unique microstructure and biomechanical properties that allow it to absorb energy during systole and return energy during diastole (Windkessel effect). Derangements in aortic architecture can result in changes to biomechanics and inefficiencies in function. Ultimately biomechanical failure may occur resulting in aortic dissection or rupture. By measuring aortic biomechanics with either in vivo or ex vivo methods, one may be able to predict tissue failure in patients with aortic disease such as aneurysms. An understanding of the biomechanical changes that lead to these tissue-level failures may help guide therapy, disease surveillance, surgical intervention, and aid in the development of new treatments for this deadly condition.
Keywords: Aortic disease, Aortic structure, Ascending aortic aneurysms, Biomechanical properties, Valves
Aortic structure and function
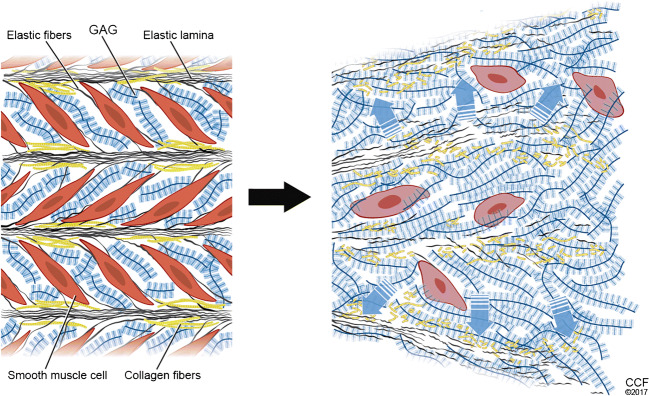
The ascending aorta has unique biomechanical properties that distinguish it from other vessels. The structure of the ascending aorta is intimately related to its tissue mechanics. The aortic wall is composed of 3 layers, including an intima composed of a single layer of endothelial cells that abuts a basal lamina and subendothelial connective tissue consisting of interlacing networks of collagen and elastic fibers [1]. Small bundles of longitudinally oriented smooth muscle cells and scant fibroblasts also populate this region [1]. The internal elastic lamina marks the innermost point of the media. The media is 0.5 to 2 mm thick and contains concentric elastic laminae that alternate with circumferentially arranged smooth muscle cells [1]. The tunica media is the thickest layer and is composed of more than 50 alternating layers of elastic lamellae and smooth muscle cells [2]. Elastin fibers consist of a core of elastin surrounded by microfibrils, which connect to smooth muscle cells through dense plaques on the cell surface [2]. Interspersed between these elastic lamellae and smooth muscle cells are collagen fibrils that provide the aortic media with its stiffness [2]. These medial components are embedded in ground substance consisting mainly of chondroitin sulfate proteoglycans [1]. The external elastic lamina defines the external boundary of the media and start of the adventitia. This segment is rich in loose connective tissue (type I collagen) and vasa vasorum, nerve fibers, lymphatics, and adipocytes [1]. Aneurysmal histopathology, often termed medial degeneration, is characterized by disruption and loss of medial elastin, remodeling of fibrillar collagens, increased deposition of proteoglycans, and loss of smooth muscle cells [3]. The structure of the ascending aorta endows it with compliance and resilience. The compliance of the aorta is afforded by its numerous elastic lamellae, which decrease in number along its length [4]. This compliance allows the ascending aorta to efficiently store energy during systole and return this energy during diastole to propel blood to more distal aortic segments (Windkessel effect) [4]. The resilience of the aorta comes from the collagen fibers embedded in the aortic media (mainly type III collagen), and the adventitia (type I collagen) [4]. This protective sheath of collagen fibers prevents the aorta from over-distending and damaging elastic fibers and microfibril-cell connections in the tunica media [4]. A diverse array of proteoglycans are embedded in the extracellular matrix, where they serve roles in regulating residual stress across the aortic wall, collagen fibril assembly, and sequestration of growth factors [4, 5]. In the normal state, these components of the aortic wall work in an elegant fashion to deliver blood to tissues throughout the body. Failure of these components, such as in medial degeneration (Fig. 1) [6], can lead to dilation of the ascending aorta and may lead to tissue failure, namely in the form of aortic dissection or rupture. An understanding of the biomechanical changes that lead to these tissue-level failures can help guide therapy, disease surveillance, and surgical intervention, and aid in the development of new treatments for this deadly condition.
Fig. 1.
Medial degeneration is the histologic hallmark of aortic aneurysms, the consequence of which may lead to aortic structural weakness and ultimately dissection. The 4 major components of these lesions include elastic fiber fragmentation/loss, smooth muscle cell loss, collagen fiber disorganization, and abundant proteoglycan accumulation. Reproduced from Cikach FS, Koch CD, Mead TJ, et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018;3: pii:97167 [6] with permission
Biomechanical investigations in aortic disease
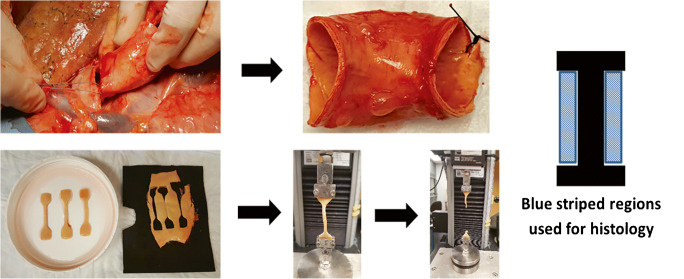
Numerous investigations into the biomechanical properties of ascending aortic aneurysms and normal aortic tissue have been done [7–21]. Some studies focus on computational models that seek to understand the location of stresses on the aorta [18, 22, 23]. Others have performed mechanical tensile testing on explanted aortic tissues to understand the compliance, stiffness, efficiency, and strength of aneurysmal and normal aorta (Fig. 2). The latter studies typically involve either uniaxial or biaxial tensile testing of fresh aortic specimens obtained at the time of prophylactic aortic replacement. Uniaxial testing allows for measurement of tissue stiffness (elastic modulus) in either circumferential or axial directions, and also importantly allows for assessment of tissue failure properties. Alternatively, biaxial tensile testing allows for bidirectional assessment of tissue mechanics that can mimic in vivo behavior, thus providing a more physiologic estimate of aortic tissue mechanics (Fig. 3). While ex vivo tensile testing is recognized as the gold standard for understanding tissue mechanics, more contemporary research has focused on correlating ex vivo tensile testing to in vivo biomechanics obtained from ultrasound or magnetic resonance angiography in an attempt to aid in risk stratification for patients with aneurysms who may need prophylactic aortic repair [24, 25].
Fig. 2.
Sequence of biomechanical testing includes tissue harvest, sectioning of specimens, uniaxial or biaxial tensile testing, and adjacent histologic analysis
Fig. 3.

Specimen for biaxial tensile testing. This testing can proceed equi-biaxial (specimen equally displaced in two directions) or unequi-biaxial fashion (specimen displaced in one direction while other is held constant)
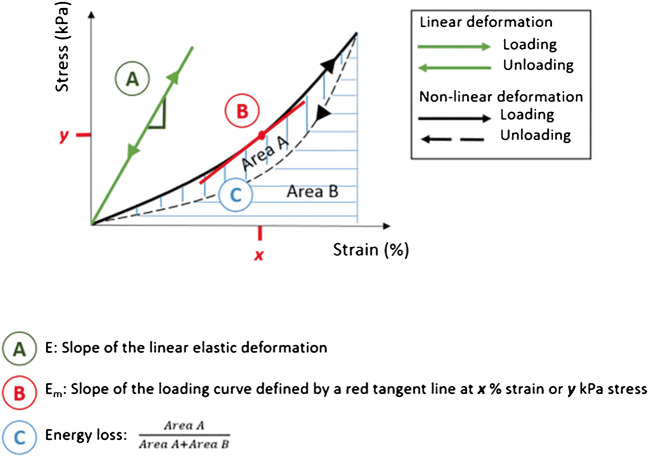
Variations in tissue processing, testing protocols, and definitions of stress and strain make comparisons between ex vivo biomechanical studies difficult [9]. Taking these shortcomings into consideration, general trends in tensile testing data can be observed in dilated ascending tissue versus control tissue. Tissue stiffness, taken as incremental elastic modulus (stiffness) or the slope of the stress versus strain curve (Fig. 4) [9], is greater in ascending aneurysm tissue compared to healthy ascending aorta and implies that aneurysmal tissue is stiffer than healthy tissue [26].
Fig. 4.
Typical stress-strain curve for vascular tissue demonstrating non-linear, anisotropic behavior. From this curve, one can derive several metrics including elastic modulus and energy loss. Reproduced from Avanzini A, Battini D, Bagozzi L, Bisleri G. Biomechanical evaluation of ascending aortic aneurysms. Biomed Res Int. 2014; 10.1155/2014/820385 [9] with permission
An area that has seen an abundance of interest in the past several years involves utilizing in vivo analysis of tissue stiffness and comparing these data to ex vivo mechanical properties [26]. These comparisons can help validate in vivo biomechanical testing as a tool that may one day be used to supplement aortic diameter in determining timing of prophylactic aortic aneurysm repair. A variety of imaging modalities have been used to characterize the biomechanics of the ascending aorta in aneurysmal and normal tissue [26]. Two-dimensional echo and M-mode measurements with aortic pressure data allow for determination of pressure-diameter relationships, which provide reasonable assumptions of tissue distensibility and an estimation of stiffness. Indeed, one group utilized these parameters to estimate tissue stiffness by two-dimensional surface echocardiography in a group of Marfan patients and showed that patients with Marfan syndrome had significantly stiffer aortas compared to control subjects [27]. Recently, two-dimensional speckle tracking echocardiography has been adapted from its typical application of assessing left ventricular strain to assessing strain of the ascending aorta. This method has a significant advantage over tissue Doppler due to its angle independence and its determination of aortic wall deformation in all vessel segments [28]. It was recently utilized by Teixeira et al. to determine circumferential ascending aortic strain in a population of patients with either low-flow or normal-flow aortic stenosis [28]. Circumferential ascending aortic strain, distensibility, and stiffness index characterize the in vivo mechanical properties of the ascending aorta and serve to supplement ex vivo mechanics obtained from tensile testing of normal and diseased tissues. Alreshidan et al. investigated the correlation between in vivo and ex vivo biomechanics by utilizing two-dimensional speckle tracking from transesophageal echocardiography images of 19 patients with ascending aneurysm undergoing prophylactic aortic repair [24]. They obtained aortic tissue from four regions of the ascending aorta (anterior, posterior, inner curvature, outer curvature) for ex vivo equi-biaxial tensile testing to determine if the ex vivo stiffness was similar to in vivo stiffness [24]. The main goal of this study was to determine if the mechanics of the ascending aorta could be measured with echocardiography to provide an estimate of aneurysmal degeneration and lend more robust evidence (rather than size alone) to the determination of appropriate timing of surgical intervention. These authors discovered that stiffness was similar between the in vivo and ex vivo measurements [24]. Almost simultaneously, another group led by Bieseviciene also sought to characterize the in vivo biomechanics of the ascending aorta in patients with aneurysm and normal controls [25]. The authors used transthoracic echocardiography and two-dimensional speckle tracking echocardiography to determine longitudinal deformations [25]. Similar to the previous authors, they were hoping to identify better predictors for aortic intervention that take into account biomechanics in addition to size. The authors found subtle, but significant, differences in aortic wall stiffness between aneurysm patients and controls, with a trend towards increased stiffness in aneurysms greater than 4.5 cm in diameter [25]. A potential issue with these studies is whether the in vivo mechanical assessment of aortic stiffness in aneurysmal patients is relevant to consequences of aneurysmal deterioration such as dissection. The biomechanics that govern aortic dissection have not been described well, and recent computational models and theories regarding its initiation and progression are just beginning to gain traction [2, 4, 6, 29–32]. Leading theory suggests that radial stress and strain across lamellar units is likely a significant contributor to dissection initiation, which is not detectable utilizing the above methods [29].
Aortic dimension in aortopathy
The use of aortic size in estimating individual risk for aortic dissection or rupture relies on an understanding of the Law of Laplace. Aortic diameter is itself not a mechanical property but acts as a substitute for circumferential wall stress. The mechanical understanding of the law states that circumferential stress in a vessel with a fixed wall thickness is a function of aortic radius and the pressure exerted on the vessel wall (Fig. 5) [9]. While this equation and interpretation allow for an easier understanding of how aortic growth and wall stress are related, they unfortunately provide an over-simplification of a complex anatomic, biologic, and biomechanical relationship. The Law of Laplace makes significant assumptions, most importantly that the vessel in question behaves in a linear elastic, isotropic fashion. However, we know from the aforementioned ex vivo biomechanical studies that the stress versus strain curve of vascular tissue is non-linear and has directional dependency (circumferential and longitudinal strains). In addition, the Law of Laplace fails to take into account the underlying tissue biology that may result in two aortas of identical size having a very different risk of dissection/rupture.
Fig. 5.
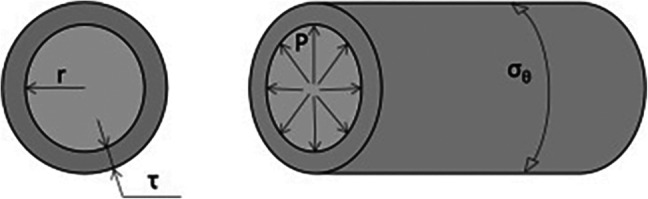
Determinants and equation for the Law of Laplace: σθ = Pr/τ. Reproduced from Avanzini A, Battini D, Bagozzi L, Bisleri G. Biomechanical evaluation of ascending aortic aneurysms. Biomed Res Int. 2014; 10.1155/2014/820385 [9] with permission
Despite the shortcomings of aortic diameter, it does provide a starting point for deciding which patient should undergo prophylactic aortic repair, and when this should happen. We know from early work that once the ascending aorta reaches 6 cm, the likelihood of an acute aortic dissection is 31% [33]. Thus, to avoid an acute aortic event, operations on ascending aortic aneurysms of degenerative etiology (absence of genetic or syndromic aortopathy) are usually suggested when they reach 5.5 cm or a documented growth rate > 0.5 cm/year [3, 34]. Additionally, in patients who are undergoing concomitant cardiac surgery, prophylactic aortic replacement is recommended if the ascending aorta is > 4.5 cm. This threshold changes dramatically for those individuals with connective tissue disorders such as Marfan syndrome or Loeys-Dietz syndrome (> 5.0 cm for Marfan, 4.4–4.6 cm for Loeys-Dietz syndrome) [3, 34].
An area of controversy includes those individuals with bicuspid aortic valve aortopathy. The data on aortic events in this group suggests that risk of acute aortic syndrome is overall low, yet still greater than the general population [35–37]. This prompted changes to the 2014 American College of Cardiology Foundation/American Heart Association guidelines [3] on valvular heart disease, suggesting a surgical threshold of ≥ 5.5 cm in the absence of significant valve disease or family history of aortic dissection at smaller diameter aortas. A more recent study of dissection risk in patients with bicuspid aortic valve aortopathy by our group found a significant increase in risk of dissection at diameters greater than 5.3 cm, and a gradual increase in risk at aortic root diameters greater than 5.0 cm [38]. In addition, a near constant 3–4% risk of dissection was present at aortic diameters ranging from 4.7 to 5.0 cm, revealing that watchful waiting may place these patients at risk [38]. In addition, institutions that have broad surgical experience with this population tend to have low hospital mortality and risk of stroke from aortic surgery; our own experience with these outcomes in this group demonstrates a hospital mortality and risk of stroke of 0.25% and 0.75%, respectively [38]. A statement of clarification in the American College of Cardiology/American Heart Association guidelines was published in 2015, recommending surgery for patients with aortic diameter ≥ 5.0 cm if the patient is low risk and the surgery is performed by an experienced surgical team at a center with established surgical expertise in this condition [39].
Work from our group utilized a machine learning approach to classify patterns of bicuspid aortopathy based on valve morphology and patient characteristics, such as age [40]. Several clear associations became apparent from this work. First, younger patients with left-right fusion tended to have aneurysms of the root phenotype that were more often associated with severe aortic regurgitation, and likely represent a genetically triggered aortopathy. On the other side of the spectrum, older individuals tended to have aortic arch aneurysm associated with right-noncoronary cusp fusion and aortic stenosis; these arch aneurysms may be more associated with longstanding hemodynamic perturbations due to the valve morphology and stenotic lesion (Fig. 6) [40].
Fig. 6.
The three aortic aneurysm phenotypes associated with bicuspid aortopathy. Each phenotype is associated with particular patient characteristics and associated pathology such as aortic regurgitation in the root phenotype and stenosis in the arch phenotype. Reproduced from Wojnarski CM, Roselli EE, Idrees JJ, et al. Machine-learning phenotypic classification of bicuspid aortopathy. J Thorac Cardiovasc Surg. 2018;155:461–469.e4 [40] with permission
Issues with aortic size criteria have been brought to light from the International Registry of Aortic Dissection, which revealed that 59% of patients suffered aortic dissection at diameters < 5.5 cm, and a significant number of patients suffered dissections at < 5 cm [41–43]. To help combat the issues with aortic diameter, aortic size indices, such as aortic cross-sectional area indexed to height, have been implemented into guidelines for certain patient populations (10 cm2/m in Marfan syndrome), which provide better risk stratification than size cutoffs alone [3, 44]. These indices have been applied to patients with bicuspid valve–associated aortopathy and to those with tricuspid valves [45, 46]. A ratio greater than 10 cm2/m has been associated with aortic dissection in these groups and improves stratification for prediction of death compared to traditional size metrics [28, 29].
Future directions and applications
An understanding of ascending aortic biomechanics will be critical to advancing diagnostics and treatment approaches for those with ascending aortopathy. Basic and translational work focused on imaging modalities and biomarkers to monitor disease progression will improve our ability to predict aortic events and risk stratify this complex population. Magnetic resonance imaging and computed tomography techniques combined with machine learning algorithms may allow for assessment of aortic microstructure and allow us to move beyond aortic size for risk assessment.
An understanding of biomechanics can also advance therapeutics, including device design for aortic stent grafts. The compliance of the device must match the compliance of the ascending aorta for adequate seal and fixation; this is especially true when attempting to land a device in zone 0. To combat this challenge, our group at the Cleveland Clinic established a study termed MATADORS (Multidisciplinary study of Ascending Tissue characteristics And hemodynamics for the Development of novel aORtic Stentgrafts) Dissection Collaboration Project, consisting of surgeons, clinicians, biomedical engineers, basic scientists, and industry collaborators with a shared goal of improving our understanding of aortic biomechanics and pathophysiology in patients with ascending aneurysms and type A dissections. The MATADORS study has enrolled over 150 subjects to date and includes patients with a wide spectrum of degenerative aneurysms all the way to syndromic connective tissue disorders. Our work has resulted in new discoveries related to aortic dissection pathophysiology [6], in vivo imaging of aortic microstructure to aid in risk stratification, and input into stent graft device design, all demonstrating the importance of biomechanics regarding aortic disease initiation, progression, management, and treatment.
Funding
Authors have nothing to disclose with regard to commercial support.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ovalle W, Nahirney P. Cardiovascular system. In: Netter’s essential histology, 2nd ed. Philadelphia: Elsevier/Saunders; 2013. pp. 173-94.
- 2.Karimi A, Milewicz DM. Structure of the elastin-contractile units in the thoracic aorta and how genes that cause thoracic aortic aneurysms and dissections disrupt this structure. Can J Cardiol. 2016;32:26–34. doi: 10.1016/j.cjca.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv. 2010;76:E43–86. [DOI] [PubMed]
- 4.Humphrey JD, Schwartz MA, Tellides G, Milewicz DM. Role of mechanotransduction in vascular biology: focus on thoracic aortic aneurysms and dissections. Circ Res. 2015;116:1448–1461. doi: 10.1161/CIRCRESAHA.114.304936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azeloglu EU, Albro MB, Thimmappa VA, Ateshian GA, Costa KD. Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am J Physiol Heart Circ Physiol. 2008;294:H1197–H1205. doi: 10.1152/ajpheart.01027.2007. [DOI] [PubMed] [Google Scholar]
- 6.Cikach FS, Koch CD, Mead TJ, et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. JCI Insight. 2018;3:pii:97167. [DOI] [PMC free article] [PubMed]
- 7.Sokolis DP, Kefaloyannis EM, Kouloukoussa M, Marinos E, Boudoulas H, Karayannacos PE. A structural basis for the aortic stress-strain relation in uniaxial tension. J Biomech. 2006;39:1651–1662. doi: 10.1016/j.jbiomech.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Pham T, Martin C, Elefteriades J, Sun W. Biomechanical characterization of ascending aortic aneurysm with concomitant bicuspid aortic valve and bovine aortic arch. Acta Biomater. 2013;9:7927–7936. doi: 10.1016/j.actbio.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avanzini A, Battini D, Bagozzi L, Bisleri G. Biomechanical evaluation of ascending aortic aneurysms. Biomed Res Int. 2014. 10.1155/2014/820385. [DOI] [PMC free article] [PubMed]
- 10.Azadani AN, Chitsaz S, Mannion A, Mookhoek A, Wisneski A, Guccione JM, Hope MD, Ge L, Tseng EE. Biomechanical properties of human ascending thoracic aortic aneurysms. Ann Thorac Surg. 2013;96:50–58. doi: 10.1016/j.athoracsur.2013.03.094. [DOI] [PubMed] [Google Scholar]
- 11.Khanafer K, Duprey A, Zainal M, Schlicht WD, Berguer R. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. J Thorac Cardiovasc Surg. 2011;142:682–686. doi: 10.1016/j.jtcvs.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara A, Morganti S, Totaro P, Mazzola A, Auricchio F. Human dilated ascending aorta: mechanical characterization via uniaxial tensile tests. J Mech Behav Biomed Mater. 2016;53:257–271. doi: 10.1016/j.jmbbm.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 13.Duprey A, Khanafer K, Schlicht M, Avril S, Williams D, Berguer R. In vitro characterisation of physiological and maximum elastic modulus of ascending thoracic aortic aneurysms using uniaxial tensile testing. Eur J Vasc Endovasc Surg. 2010;39:700–707. doi: 10.1016/j.ejvs.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Choudhury N, Bouchot O, Rouleau L, et al. Local mechanical and structural properties of healthy and diseased human ascending aorta tissue. Cardiovasc Pathol. 2009;18:83–91. [DOI] [PubMed]
- 15.Tong J, Cheng Y, Holzapfel GA. Mechanical assessment of arterial dissection in health and disease: advancements and challenges. J Biomech. 2016;49:2366–2373. doi: 10.1016/j.jbiomech.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 16.García-Herrera CM, Atienza JM, Rojo FJ, et al. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med Biol Eng Comput. 2012;50:559–66. [DOI] [PubMed]
- 17.Sommer G, Sherifova S, Oberwalder PJ, et al. Mechanical strength of aneurysmatic and dissected human thoracic aortas at different shear loading modes. J Biomech. 2016;49:2374–82. [DOI] [PMC free article] [PubMed]
- 18.Martin C, Sun W, Pham T, Elefteriades J. Predictive biomechanical analysis of ascending aortic aneurysm rupture potential. Acta Biomater. 2013;9:9392–9400. doi: 10.1016/j.actbio.2013.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iliopoulos DC, Deveja RP, Kritharis EP, et al. Regional and directional variations in the mechanical properties of ascending thoracic aortic aneurysms. Med Eng Phys. 2009;31:1–9. [DOI] [PubMed]
- 20.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann Thorac Surg. 2003;75:1210–1214. doi: 10.1016/S0003-4975(02)04711-2. [DOI] [PubMed] [Google Scholar]
- 22.Martufi G, Forneris A, Appoo JJ, Di Martino ES. Is there a role for biomechanical engineering in helping to elucidate the risk profile of the thoracic aorta? Ann Thorac Surg. 2016;101:390–398. doi: 10.1016/j.athoracsur.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 23.Trabelsi O, Duprey A, Favre J-P, Avril S. Predictive models with patient specific material properties for the biomechanical behavior of ascending thoracic aneurysms. Ann Biomed Eng. 2016;44:84–98. doi: 10.1007/s10439-015-1374-8. [DOI] [PubMed] [Google Scholar]
- 24.Alreshidan M, Shahmansouri N, Chung J, et al. Obtaining the biomechanical behavior of ascending aortic aneurysm via the use of novel speckle tracking echocardiography. J Thorac Cardiovasc Surg. 2017;153:781–8. [DOI] [PubMed]
- 25.Bieseviciene M, Vaskelyte JJ, Mizariene V, Karaliute R, Lesauskaite V, Verseckaite R. Two-dimensional speckle-tracking echocardiography for evaluation of dilative ascending aorta biomechanics. BMC Cardiovasc Disord. 2017;17:27. doi: 10.1186/s12872-016-0434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emmott A, Garcia J, Chung J, et al. Biomechanics of the ascending thoracic aorta: a clinical perspective on engineering data. Can J Cardiol. 2016;32:35–47. [DOI] [PubMed]
- 27.Baumgartner D, Baumgartner C, Matyas G, et al. Diagnostic power of aortic elastic properties in young patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2005;129:730–9. [DOI] [PubMed]
- 28.Teixeira R, Moreira N, Baptisa R, et al. Circumferential ascending aortic strain and aortic stenosis. Eur Heart J Cardiovasc Imaging. 2013;14:631–41. [DOI] [PubMed]
- 29.Roccabianca S, Bellini C, Humphrey JD. Computational modelling suggests good, bad and ugly roles of glycosaminoglycans in arterial wall mechanics and mechanobiology. J R Soc Interface. 2014;11:20140397. doi: 10.1098/rsif.2014.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roccabianca S, Ateshian GA, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol. 2014;13:13–25. doi: 10.1007/s10237-013-0482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphrey JD. Possible mechanical roles of glycosaminoglycans in thoracic aortic dissection and associations with dysregulated transforming growth factor-ß. J Vasc Res. 2013;50:198–199. doi: 10.1159/000342436. [DOI] [PubMed] [Google Scholar]
- 32.Humphrey JD, Milewicz DM, Tellides G, Schwartz MA. Cell biology. Dysfunctional mechanosensing in aneurysms. Science. 2014;344:477–79. [DOI] [PMC free article] [PubMed]
- 33.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–1880. doi: 10.1016/S0003-4975(02)04147-4. [DOI] [PubMed] [Google Scholar]
- 34.Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;151:959–66. [DOI] [PubMed]
- 35.Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104–12. [DOI] [PubMed]
- 36.Tzemos N, Therrien J, Yip J, et al. Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300:1317–25. [DOI] [PubMed]
- 37.Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27. [DOI] [PubMed]
- 38.Wojnarski CM, Svensson LG, Roselli EE, et al. Aortic dissection in patients with bicuspid aortic valve-associated aneurysms. Ann Thorac Surg. 2015;100:1666–1673. doi: 10.1016/j.athoracsur.2015.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiratzka LF, Creager MA, Isselbacher EM, et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;133:680–6. [DOI] [PubMed]
- 40.Wojnarski CM, Roselli EE, Idrees JJ, et al. Machine-learning phenotypic classification of bicuspid aortopathy. J Thorac Cardiovasc Surg. 2018;155:461–9 e4. [DOI] [PubMed]
- 41.Pape LA, Tsai TT, Isselbacher EM, et al. Aortic diameter ≥ 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116:1120–7. [DOI] [PubMed]
- 42.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98. [DOI] [PubMed]
- 43.Guo D-C, Pannu H, Tran-Fadulu V, et al. Mutations in smooth muscle α-actin(ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39:1488–93. [DOI] [PubMed]
- 44.Svensson LG, Khitin L. Aortic cross-sectional area/height ratio timing of aortic surgery in asymptomatic patients with Marfan syndrome. J Thorac Cardiovasc Surg. 2002;123:360–1. [DOI] [PubMed]
- 45.Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126:892–893. doi: 10.1016/S0022-5223(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 46.Masri A, Kalahasti V, Svensson LG, et al. Aortic cross-sectional area/height ratio and outcomes in patients with a trileaflet aortic valve and a dilated aorta. Circulation. 2016;134:1724–37. [DOI] [PubMed]