Abstract
Arterial cannulation for cardiopulmonary bypass (CPB) is an important determinant of outcome in aortic surgery. Unlike traditional cardiac operations, aortic pathology may preclude the cannulation of the distal ascending aorta. In other cases, special need of the pathology/operation may demand an alternative cannulation site. Choosing the right cannulation site, especially in type A aortic dissection, is the most crucial initial step. The decision about cannulation sites should be individualized and patient-specific. Various cannulation techniques include femoral, right axillary, innominate, carotid, central aortic, direct true lumen, transapical, and trans-atrial left ventricle cannulation. The ideal cannulation should be easy, quick, and suitable for all clinical scenarios. It should allow smooth conduct of CPB without malperfusion or cerebral embolization. The cannulation strategy should also provide an option for selective antegrade cerebral perfusion and it should be free from neurovascular and local site complications. There is no ideal cannulation technique. Each technique has its pros and cons. Excellent results and drawbacks have been reported with each technique. Final selection of the cannulation site is dependent upon several factors. However, a surgeon’s familiarity with a particular technique plays a major role in selection. Despite this, there is a definite shift in surgeons’ preference from femoral to central cannulation (axillary, carotid, innominate, aortic) over the last few decades. The aim of this review is to give a brief overview of the cannulation techniques in aortic surgery and discuss the decision-making process.
Keywords: Arterial cannulation, Aortic surgery, Aortic dissection, Aortic aneurysm, Type A aortic dissection
Introduction
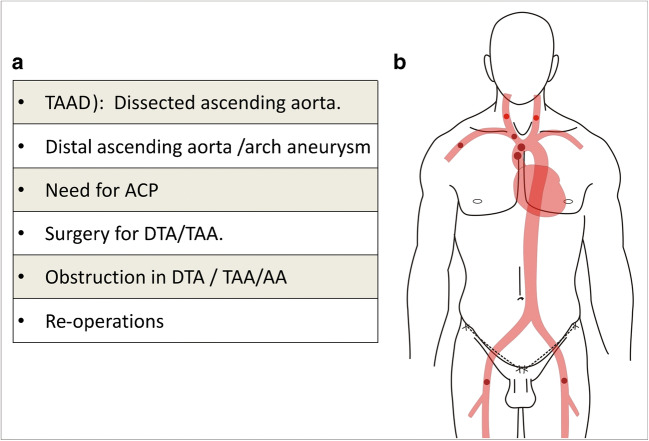
Surgical intervention on the aorta may be needed in aortic aneurysms; acute aortic syndromes like dissection, intramural hematoma, penetrating atherosclerotic ulcer, traumatic aortic rupture and pseudoaneurysm; and obstructive lesions like interruption/coarctation, and aorto-arteritis. Cannulation for arterial inflow during cardiopulmonary bypass (CPB) is an important determinant of outcome of surgical intervention in aortic surgery. Unlike traditional cardiac operations, aortic pathology may preclude distal ascending aorta for cannulation and an alternative cannulation site is needed. In other cases, special need of the pathology/operation may demand an alternative cannulation site (Fig. 1a). The cannulation site and strategy need to be individualized to avoid complications like malperfusion, retrograde cerebral athero-embolism, and propagation of dissection. In addition, there should be a provision for cerebral perfusion or another organ perfusion, if needed. At the same time, the arterial cannulation technique should be easy, quick, and reproducible. Over half a century, several cannulation techniques have been used by surgeons (Fig. 1b). Each technique has its pros and cons. No strategy has been found to be perfect or free from complications. Excellent results and drawbacks have been reported with each technique. Surgeon’s familiarity with a particular technique is the principal determinant of cannulation strategy. Despite this, there is a definite shift in surgeons’ preference from femoral to central cannulation (axillary, innominate, aortic) over the last few decades. The aim of this review is to give a brief overview of all the cannulation strategies in aortic surgery and discuss the decision-making in the selection of the optimal cannulation for a particular clinical scenario.
Fig. 1.
Need and possible arterial cannulation sites in aortic surgeries. a Various reasons for alternate cannulation site in aortic surgeries. (AA, abdominal aorta; ACP, antegrade cerebral perfusion; DTA, descending thoracic aorta; TAA, thoraco-abdominal aorta; TAAD, type A aortic dissection). b Possible arterial cannulation sites in aortic surgeries
Femoral cannulation
Since the 1950s, one of the common femoral arteries (FAs) was the preferred site of cannulation for aortic operations involving the distal ascending aorta and/or arch. However, in the last two decades, there has been a paradigm shift towards antegrade perfusion. Despite decreasing popularity, few established centers still choose femoral arterial cannulation and provide comparable outcomes [1–4]. The greatest advantage of femoral arterial cannulation is familiarity of the anatomy to cardiovascular surgeons. Femoral arterial cannulation is relatively easy, quick, and reproducible. It is especially suited in emergency situation for an unstable patient [5]. The potential disadvantages include retrograde perfusion-induced disruption of atheromatous plaques in abdominal/thoracic aorta and subsequent athero-embolization to the visceral or cerebral arteries. This is a possibility in elderly patients with heavy atheromatous burden. In type A aortic dissection (TAAD), the retrograde flow can pressurize the false lumen, especially if distal reentry tear is present and aortic cross-clamp excludes the primary tear, leading to malperfusion of cerebral and visceral vessels. Pressurizing of false lumen can also lead to new intimal tears and propagation of dissection [6, 7]. Post-repair, the distal anastomosis may bleed because of false lumen pressurization [8]. Another disadvantage of femoral cannulation is its inability to provide cerebral perfusion through the same cannulation during arch reconstruction or open distal anastomosis. FA may not be suitable in presence of aortoiliac aneurysmal or obstructive disease, and if involved, in the dissection process. In presence of severe coarctation or interruption of the aorta, femoral cannulation alone will not be sufficient. The theoretical advantages and disadvantages are summarized in Table 1.
Table 1.
Advantages and disadvantages of common cannulation techniques
| Cannulation technique | Advantages | Disadvantages |
|---|---|---|
| Femoral |
♦ Familiar ♦ Quick and easy ♦ Suitable in hemodynamically unstable patient |
♦ Possibility of malperfusion in dissection ♦ Athero-embolization in elderly. ♦ Need for additional cerebral protection strategy ♦ Ischemia of lower limb ♦ Not suitable in the presence of aorto-iliac obstruction/aneurysm, and coarctation. ♦ Wound problems are relatively common |
| Axillary |
♦ Antegrade flow ♦ Provides antegrade cerebral perfusion ♦ Decreased embolic risk ♦ Least involved by atherosclerosis or dissection |
♦ Time-consuming and may need graft ♦ Technically more demanding especially in obese ♦ Chance of arterial injury ♦ Additional incision ♦ Chance of brachial plexus injury |
| Carotid cannulation |
♦ Quick and easy ♦ Antegrade flow ♦ Provides antegrade cerebral perfusion |
♦ Athero-embolic risk ♦ Possible cerebral hyperperfusion (??) |
| Central aortic cannulation (Seldinger technique) |
♦ No additional incision required ♦ Quick, ♦ Can be used in hemodynamically unstable patients. ♦ It also ensures antegrade perfusion of the true lumen |
♦ Technically more demanding. Difficult to puncture true lumen in presence of circumferential dissection. ♦ Echocardiography dependent. Need expertise. ♦ Chances of aortic rupture, false lumen cannulation ♦ Unable to provide antegrade cerebral protection ♦ Cerebral embolism and stroke if there is thrombus in false lumen at cannulation site. ♦ Need to rely on deep hypothermia as cerebral protection strategy. Prolonged CPB time. Bleeding and pulmonary dysfunction. ♦ Not suitable in presence of aortic rupture and cardiac tamponade. |
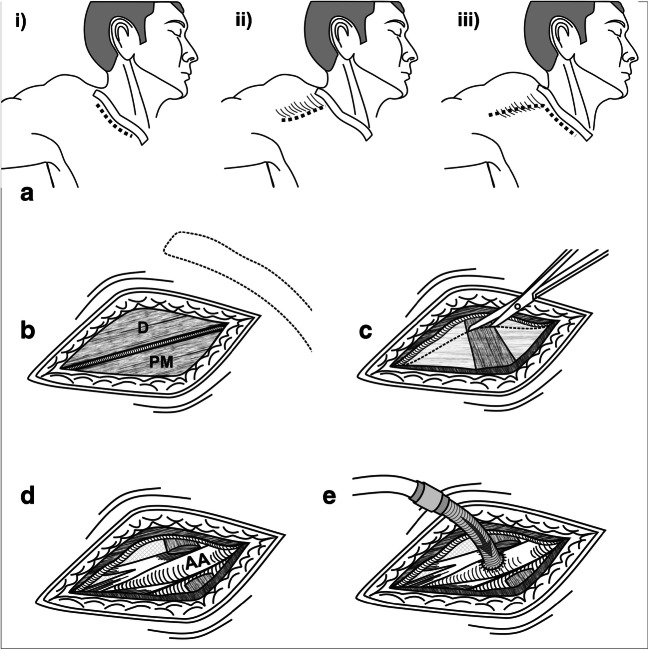
The common FA can be cannulated percutaneously using the Seldinger technique or in an open manner. The commonly used technique combines features of both. In an open manner, the common FA is exposed just below the inguinal ligament using either a transverse or a vertical incision. After exposure, the common FA is cannulated either by using the Seldinger technique (most common), or by direct cannulation using a transverse arteriotomy (uncommon). Surgical steps of the open Seldinger technique are shown in Fig. 2a–f. Open Seldinger technique for femoral arterial cannulation is preferred as it is simple and quick, requires minimal dissection, and maintains distal limb perfusion [4].
Fig. 2.
Common femoral artery cannulation. a Common femoral artery cannulation using the open Seldinger technique. The common femoral artery is exposed by giving a vertical or transversely oblique incision at mid-inguinal point, just below inguinal crease. b The incision is deepened up to the femoral sheath. The femoral sheath is opened in a limited manner to expose about 3 cm of common femoral artery (CF, common femoral artery; PF, profunda femoral artery; SF, superficial femoral artery). Excessive dissection of the artery should be avoided, and it should not be bared off its adventitia. c A superficial U-shaped purse-string suture is placed in the adventitia. One should avoid a circular purse-string suture. d Puncture is made through the U-suture. A guidewire is passed. e The cannula, mounted on the dilator, is threaded over the guidewire. f At the end, the cannula is removed and arteriotomy is temporarily controlled with the purse-string suture. Proximal and distal clamps are applied, and the original purse-string suture is removed. The arteriotomy is closed transversely. We do not tie the original purse-string suture as it may pucker the artery and compromise the lumen. g When the artery is small, an 8-mm vascular graft is sutured to the common femoral artery in an end-to-side fashion. The graft is connected to the arterial line. h Prevention of lower limb ischemia. An 8-mm sheath is inserted in the superficial femoral artery and directed distally. The sheath is connected to proximal hub of the main arterial cannula using a luer-lock connector
Rarely, especially if the common FA is relatively small, an 8-mm vascular graft is sutured to the common FA in an end-to-side fashion, and this graft is connected to the arterial circuit (Fig. 2g). Alternatively, a distal perfusion cannula may be inserted in superficial FA to perfuse the lower limb (Fig. 2h).
Axillary cannulation
Axillary artery cannulation was first described by Villard et al. in 1976 and became popular when Sabik et al. described it in 1995 [9, 10]. With growing evidence of advantages of antegrade perfusion, more and more surgeons prefer right axillary artery cannulation for CPB, especially in arch and TAAD surgeries [11–14].. The axillary artery can be approached by deltopectoral, infraclavicular, or a combined approach (Fig. 3a). We prefer the deltopectoral approach and the operative steps are illustrated in (Fig. 3b–e).
Fig. 3.
Technique of axillary artery cannulation. a Various approaches for axillary artery: infraclavicular (i), deltopectoral (ii), or a combined approach (iii). b Deltopectoral approach for second and third parts of axillary artery. A 5-cm incision is given over the deltopectoral groove. The plane between the deltoid and pectoralis major muscle is identified, split, and cephalic vein retracted laterally (D, deltoid muscle; PM, Pectoralis major muscle). c Clavipectoral fascia is opened and Pectoralis minor muscle is divided. d The axillary artery (AA) is dissected free. The axillary artery is supero-posterior and lateral to the axillary vein. It is surrounded medially, laterally, and posteriorly by the respective cords of the brachial plexus. e An 8-mm vascular graft is sutured to the axillary artery in an end-to-side fashion and connected to the arterial line via a 3/8–1/4 straight connector
Most commonly, a graft is sutured to the axillary artery for cannulation. Less frequently, direct cannulation of the artery is performed using an angled cannula after a transverse arteriotomy, and snaring the proximal part of artery over the cannula. One should be careful as not to insert the cannula for more than 2 cm into the artery. There is a potential danger of occlusion of right common carotid or vertebral artery, if the cannula is inserted too much [2]. Even though direct cannulation is less time-consuming, it has some potential concerns. If the artery is fragile and small, especially in TAAD, there are high chances of iatrogenic dissection, injury to the axillary artery, and distal limb ischemia when the cannula, along with the artery, is snugged proximally. If the artery is small, as in patients with low body surface area, there may be difficulty in establishing full CPB flows. Closure of the arteriotomy is slightly difficult and may narrow the artery in direct cannulation subset. In graft cannulation subset, simple transection and oversewing the graft will suffice [15]. Sabik et al. showed a definite lower incidence of complications with graft cannulation technique (2% vs 7%). [16]
Infraclavicular approach gives good exposure of the first part of axillary artery and a wider caliber artery is available for cannulation. A transverse incision is given 2 cm below the clavicle, starting from the medial end of the clavicle to the junction of mid- and lateral third of the clavicle. The incision is deepened, pectoralis major muscle fibers are split, anterior layer of clavipectoral fascia is divided, subclavius is split, and posterior layer of clavipectoral fascia is divided. Following this, neurovascular bundle is exposed, artery is dissected, and cannulation is done as described above.
Right axillary artery cannulation provides antegrade perfusion. Hence, there are lesser chances of false lumen pressurization and malperfusion. There is less risk of retrograde athero-embolization and wound complications. It also provides an option for antegrade cerebral perfusion (ACP) (Table 1). On the other hand, it is time-consuming and technically more difficult, especially in obese patients. It may not be a good choice for initial cannulation in hemodynamically unstable patients. Uncommonly, in a small-caliber vessel, it may have high resistance to flows. Less-frequent complications include brachial plexus injury, and a new dissection of axillary or innominate artery (IA) [17]. Right axillary artery cannulation is avoided if it is involved in atherosclerosis, or if there is subclavian artery stenosis or dissection. Involvement of IA is not a contraindication for right axillary cannulation unless there is a separate intimal tear in the IA, which could lead to false lumen pressurization and high resistance to arterial inflow.
Comparison of femoral vs right axillary cannulation: evidence
Even though there are only a handful of studies showing right axillary artery cannulation to be superior to femoral cannulation in aortic aneurysm and TAAD in terms of mortality and neuroprotection, there is no standard universal protocol to recommend it as a first choice. Most of these studies are retrospective in nature and there are no randomized controlled trials. However, there are 2 meta-analyses [18, 19] based on well-chosen retrospective studies. Ren et al. [18] concluded that right axillary cannulation in TAAD repair reduces mortality and neurological dysfunction significantly with a relative risk reduction of 58% and 28% respectively. Benedetto et al. did a meta-analysis of 14 studies to evaluate the impact of arterial cannulation on operative outcomes in patients undergoing proximal aortic and arch surgeries [19]. The included studies compared peripheral (femoral) cannulation vs central (ascending aortic, right axillary, or IA) cannulation. This meta-analysis concluded that central cannulation was superior to femoral cannulation in proximal aortic and arch surgery with absolute risk reduction of 41% and 29% in terms of in-hospital mortality and permanent neurologic dysfunction. This risk reduction of in-hospital mortality and neurologic dysfunction was more pronounced in TAAD (52% and 40% respectively). Among the central cannulation, axillary artery was superior to others in reducing the in-hospital mortality and neurologic dysfunction by 65% and 39% respectively. A review paper by Gulbins et al. concluded that axillary artery cannulation with side graft was safer in aortic arch and TAAD surgeries compared to femoral cannulation, and decreased the incidence of in-hospital mortality and stroke [20].
On the other hand, Di Eusanio et al., retrospectively, studied the impact of cannulation strategy on aortic arch surgery, both in aneurysm and in dissection in 473 patients [21]. In central cannulation, ascending aorta, right axillary, and IA cannulation were performed. To avoid the treatment bias, propensity score matching and multivariate regression analysis were done to match the cohorts. They concluded that both the central and peripheral (femoral) cannulation in arch surgery had a similar incidence of in-hospital mortality and neurologic dysfunction in unmatched and matched cohorts. By subgroup analysis, they demonstrated that there was no superiority of central over peripheral cannulation in TAAD. The authors believed that it was due to their patient-specific decision-making on optimal cannulation site, for example, choosing axillary cannulation in heavily atherosclerotic descending aorta and femoral cannulation in critical hemodynamically unstable TAAD. Etz et al. retrospectively studied 401 patients, who underwent surgery for TAAD, by either central (axillary/ central aortic) or femoral cannulation and found no statistically significant difference in incidence of in-hospital mortality, neurological dysfunction, and postoperative malperfusion [22]. It was probably due to their strategy of individualized approach on the selection of cannulation. However, patients undergoing antegrade perfusion had a better long-term survival, 71% versus 51% at 10 years (p = 0.025). From the retrospective analysis of published literature, it was clear that FA cannulation was more often used in hemodynamically unstable patients. In addition, ACP, as a cerebral protection strategy, was more often used with axillary artery cannulation. The authors of both the studies were aware of two possible sources of bias. One was the preoperative condition of the patients; the other was the difference in the brain protection strategies [19]. May be these two biases or patient selection strategies tilted the balance in favor of axillary artery cannulation.
IA
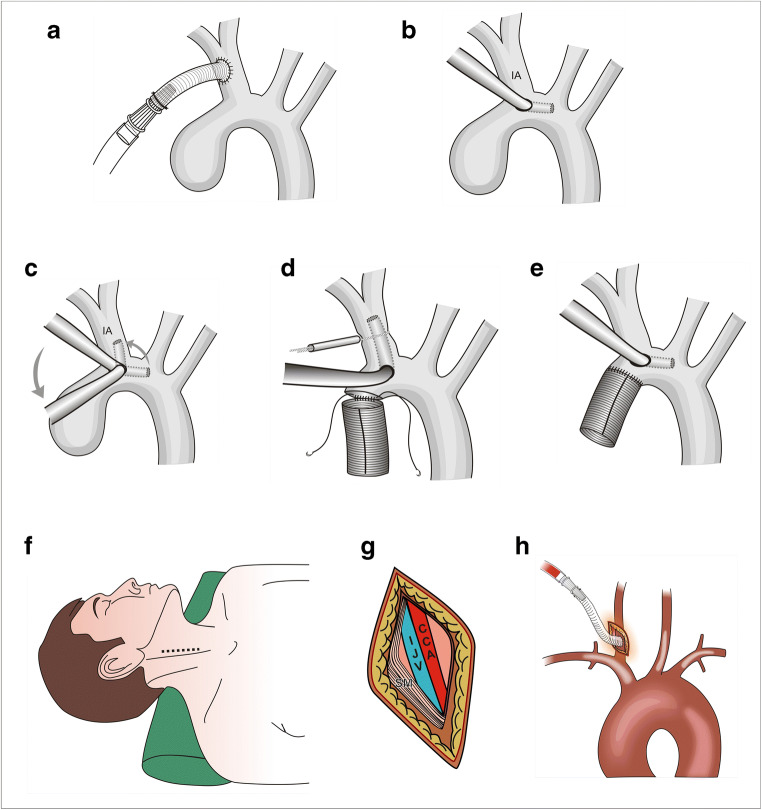
IA, as arterial inflow for redo, TAAD, and aneurysm repair, was first described by Banbury and Cosgrove in 2000 [23]. Although not as widely used as axillary cannulation, it is an emerging modality for antegrade perfusion in aortic surgeries. It can be used in both elective and emergency surgeries unless involved by atherosclerosis or dissection [24]. IA can be cannulated directly or using a Dacron graft. It can be exposed by slightly extending the sternotomy incision cranially above the suprasternal notch. IA is dissected from origin till bifurcation. While dissecting, the surgeon should be careful not to injure the right recurrent laryngeal nerve at right subclavian artery take-off. One can do direct cannulation after applying two purse strings, with the cannula tip directed towards the arch. Alternately, and more commonly, a vascular graft is sutured to the IA in an end-to-side fashion and is used for arterial inflow (Fig. 4a). Usually, direct cannulation with 10–12-Fr cannula is performed for isolated ACP, and a graft is used for total CPB. Theoretically, graft cannulation is preferred to avoid cannulation site dissection and high flow jet by direct cannulation dislodging the atheroma [25]. In patients undergoing hemiarch replacement, without thrombus or atheroma in the vicinity of IA origin, we use a “cannula translocation” technique (Fig. 4b–e). Advantages of IA cannulation include (i) no additional incision, (ii) technically easy, especially in obese patients, (iii) larger caliber vessel, (iv) no risk of brachial plexus injury and limb ischemia, (v) less chances of cerebral malperfusion, and (vi) availability of ACP [26]. IA cannulation may be limited by occasional involvement of proximal IA in dissection process or atherosclerosis.
Fig. 4.
Technique of Innominate Artery And Common Carotid Artery Cannulation. a An 8-mm vascular graft is sutured to the innominate artery midway between the origin and the bifurcation in an end-to-side fashion and connected to the arterial line via a 3/8–1/4 straight connector. b Cannula translocation technique of innominate artery cannulation. The distal ascending aorta is cannulated very close to the origin of the innominate artery (IA). Initially, the tip of the cannula is in the transverse arch. c Just before the arch is opened, the bypass flow is reduced, purse string around the cannula is loosened, the cannula tip is rotated, and 3–4 cm of tip is pushed in the innominate artery. d The snare around the innominate artery is tightened and the aortic clamp removed. Under surface of the arch is resected and the distal anastomosis is performed in an open fashion. e After completion of the anastomosis, the arch and the grafts are deaired, clamp is applied on the graft, innominate snare and cannula purse-strings are loosened, and the cannula is pulled and directed towards the transverse arch. f Technique of common carotid artery cannulation. The neck is kept extended with chin turned towards the opposite side. A 5-cm-long skin incision is given in the mid-third of the neck along the anterior border of sternocleidomastoid. g Sternomastoid muscle is retracted posteriorly. The carotid sheath is opened, and middle part of common carotid artery is dissected circumferentially. h After heparinization, an 8-mm vascular graft is sutured to the common carotid artery and connected to the arterial line via a 3/8–1/4 straight connector
Unlike axillary or femoral cannulation, studies involving IA are limited. There is no study comparing exclusively IA and FA. A review by Harky et al. [27] included 5 studies involving 1338 patients of which 722 underwent right axillary artery cannulation and 616 underwent IA cannulation in proximal aortic and arch procedures for aneurysms or TAAD. Pooled data showed no statistically significant difference in incidence of in-hospital mortality, stroke, or temporary neurologic dysfunction [22]. In summary, IA is emerging as a potential alternative to right axillary artery cannulation as an antegrade perfusion modality, provided anatomically suitable.
Carotid cannulation
Common carotid artery (CCA) cannulation was first described by Urbanski in 2006 [28]. Even though technically easy, initially, there was a fear of possible iatrogenic damage to the artery resulting in irreversible cerebral injury, increased chance of embolic load, or cerebral bleed due to direct high-velocity jet [29]. However, these were only theoretical risks and not yet proven. CCA cannulation is gradually gaining popularity. In our center, right common carotid cannulation is the preferred option in case of arch interventions. The technique of CCA cannulation is shown in Fig. 4f–h.
Usually, the middle segment of the right CCA is used for cannulation. If the right side is involved in the dissection process, the left CCA may be used. It is important to evaluate the CCA preoperatively clinically and by ultrasound or computerized tomographic angiography (CTA) and exclude any dissection or advanced atherosclerosis. Once CCA is exposed, after full heparinization, an 8-mm Dacron graft is sutured in end to side fashion. This graft is connected directly to the arterial inflow line. It is important to maintain mean arterial pressure of 80 to 90 mmHg during anastomosis to ensure good cerebral flow through the contralateral carotid artery.
Advantages of the CCA cannulation include technical ease, quick, and availability of large-caliber vessel. It provides antegrade perfusion and good CPB flows even in obese patients. It is also available for ACP. In addition, good access to internal jugular vein gives an option of going onto emergency CPB, if needed. Graft sutured to the CCA can be used for arch vessel reimplantation. There is almost no wound complication or nerve injury [30]. Disadvantages of CCA cannulation include the possibility of cerebral athero-embolization. There is a concern, though unproven, about cerebral hyperperfusion-induced edema and hemorrhage. Because of the lack of comparative studies or randomized controlled trials, the CCA cannulation is not universally acclaimed. Yet, it is surely a potential alternative to right axillary artery cannulation. In our personal experience of more than one decade, the CCA is our first choice for arterial cannulation.
Central aortic cannulation (Seldinger technique)
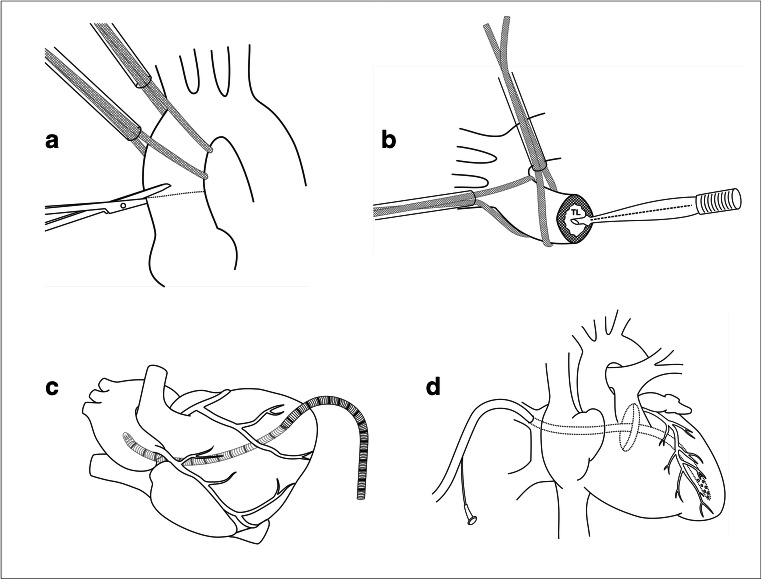
This technique of cannulating the true lumen of ascending aorta is used in patients with acute TAAD. It was first described by Lijoi et al. [31] in 1998. Based on the imaging studies, the true lumen is located and cannulated using the Seldinger technique under echocardiographic guidance [32] (Fig. 5). After cannulation, CPB is initiated and under deep hypothermic circulatory arrest (DHCA), the distal/arch repair is performed first, and CPB is re-established through the sidearm of the graft. On the positive side, it does not require an additional incision, it is quick, and, thus, preferable in hemodynamically unstable patients. It also ensures antegrade perfusion of the true lumen. Table 1 summarizes the advantages and disadvantages of central aortic cannulation.
Fig. 5.
Technique of central aortic cannulation (Seldinger technique). a Under echocardiographic guidance, true lumen is punctured using a thick bore puncture needle (FL, false lumen; TL, true lumen). A guidewire is passed. b The dilator is passed over the guidewire. c The cannula is threaded over the guidewire. After confirming the position of the tip of cannula in the true lumen, the cannula is connected to the arterial line. d After achieving deep hypothermic circulatory arrest, the aortic cannula is removed. Arch is cut opened at desired level. Graft is anastomosed to the cut end of arch. e After the arch anastomosis is completed, arterial line is connected to the sidearm of the graft and perfusion is started. After deairing of the arch and the graft, a clamp is applied to the graft, and one proceeds for aortic root procedure
Evidence has shown that the central aortic cannulation is safe and effective [32–36], though a mild, statistically insignificant, increase in stroke incidence has been seen in central cannulation subset. When compared to the femoral cannulation, significant benefit of mortality and stroke incidence has been seen in central aortic cannulation group [35–37]. However, Sabashnikov et al. found a significant decrease in long-term survival in this group, compared to axillary cannulation, and attributed it to possible undetected subtle malperfusion due to cannula malposition during surgery [38]. Since these studies are retrospective with a limited sample size, the results should be interpreted carefully.
Direct true lumen cannulation (Samurai cannulation)
First described by Jakob et al. in 2007 [39], it is an uncommonly used cannulation modality in TAAD for antegrade flow. Central aortic cannulation (see above) is easier in a partially dissected aorta but technically very difficult in a circumferentially dissected aorta. In patients with the circumferentially dissected aorta, direct true lumen cannulation is an option for antegrade flow. Some surgeons have used this technique in routine [40]. The technique of direct true lumen cannulation is summarized in Fig. 6a, b. Preoperatively, the relationship of true and false lumen is clearly defined by imaging. Intraoperatively, after opening the pericardium, the ascending aorta is circumferentially dissected and looped with umbilical tapes. After heparinization, the right atrial venous cannula and the left heart vent are introduced. When lines are ready, head-end is lowered, venous blood is drained, and the left ventricle vent is put on till the mean blood pressure drops below 30 mmHg. The ascending aorta is transected, and blood pool is sucked. The true lumen is identified, and the arterial cannula is introduced into the true lumen after deairing the aorta. Finally, the aorta is snugged gently around the cannula and CPB started. During this process, a normothermic circulatory arrest period of 40 to 120 s, depending on the expertise, is unavoidable [40]. Similar to direct central aortic cannulation (see above), the arch is repaired under DHCA and CPB is re-established through the sidearm of the graft. Although some routinely use this modality, it is not universally accepted as the primary cannulation strategy due to its radical nature. But its role as a “bail out,” when patient has flat line hemodynamics, cannot be rejected. Potential concerns include chances of cerebral embolism, aortic rupture due to snugging, normothermic circulatory arrest, DHCA, prolonged CPB, and inability to provide ACP. Studies available are retrospective and of limited sample size. More studies are needed to assess the long-term outcome [41].
Fig. 6.
Less-frequently performed cannulation techniques. a Technique of direct true lumen cannulation (Samurai cannulation). After opening pericardium, ascending aorta is circumferentially dissected and looped with umbilical tapes. After heparinization, right atrial venous cannula and left heart vent are introduced. Venous blood drained and left ventricle vent put on till the mean blood pressure drops below 30 mmHg. The ascending aorta is transected, and blood pool is sucked. b The true lumen is identified, and the arterial cannula is introduced into the true lumen after deairing the aorta. The aorta is snared gently around the cannula and cardiopulmonary bypass started. The remaining steps are similar to Fig. 5d and Fig. 5e. c Technique of transapical cannulation. After venous cannulation, the apex of the heart is gently lifted. Under purse-string suture control, a small (1 cm) ventriculotomy is made near the apex. The pre-selected, long, flexible cannula is passed through the ventriculotomy and negotiated across the left ventricle and the aortic valve under echocardiographic guidance. d Technique of trans-atrial left ventricular cannulation. A purse-string suture is placed at the right superior pulmonary vein – left atrial junction. Through the purse-string suture, a long, flexible perfusion cannula is inserted across the mitral valve in the left ventricle, under echocardiographic guidance to ensure the position
Transapical cannulation
Transapical cannulation was first described by Wada et al. in 1976 for mitral valve surgery through left thoracotomy [42]. Even though few prefer this for aortic surgeries in TAAD, it is not accepted as the first-line modality. It is rather used as a secondary option to avoid retrograde perfusion. It is preferred when peripheral arteries are unsuitable, and the central cannulation is difficult with a chinked out true lumen in a hemodynamically unstable patient. Technically, after pericardiotomy and heparinization, venous cannulation is done, and the left ventricle is lifted. A purse-string suture is placed at the apex, and a 1-cm long incision is given. Under echocardiographic guidance, a standard sized flexible cannula is inserted through the ventriculotomy and negotiated through the aortic valve up to the sinotubular junction (Fig. 6c). Cannula is not advanced too much to avoid false lumen perfusion [43, 44]. Once the circulatory arrest is attained, the cannula is removed, and the distal arch anastomosis is performed. Antegrade perfusion is re-established through the sidearm of the graft. Potential concerns with transapical cannulation include unfamiliarity, bleeding at the apex, and possibility of malperfusion, and aortic regurgitation. It is not opted in aortic stenosis as it may be difficult to negotiate the cannula across a stenosed valve. In redo cases, and in patients with significant left ventricular enlargement, it may be difficult to access the left ventricular apex [ 45].
Trans-atrial left ventricular cannulation
This is an alternative antegrade cannulation method described by Schoeneich et al. in 2015 [46]. Technically, after pericardiotomy and heparinization, two-stage venous cannula is inserted. A purse-string suture is placed at the right superior pulmonary vein—left atrial junction. Through the purse-string suture, a long, flexible perfusion cannula is inserted across the mitral valve in the left ventricle, under echocardiographic guidance (Fig. 6d). CPB is initiated and the arterial cannula is removed once DHCA is attained. After completion of the distal anastomosis, graft cannulation is done for antegrade flow. This technique is easy and quick and can be used in hemodynamically unstable patients. It ensures true lumen perfusion and avoids manipulation of the dissected aorta. Detailed assessment of preoperative images is not necessary for this technique. Disadvantages include the need for DHCA, mitral regurgitation, chances of pulmonary hyperperfusion, and edema due to cannula malposition [47].
Decision-making
The characteristics of an ideal cannulation technique are listed in Table 2.
Table 2.
Characteristics of an ideal cannulation technique
| SN | Characteristics |
|---|---|
| 1. | Quick, familiar |
| 2. | Technically easy, |
| 3 | Should allow smooth conduct of CPB, Minimum bypass time. |
| 4. | Suitable for all clinical scenarios |
| 5 | No malperfusion |
| 6 | Should provide option for antegrade cerebral perfusion |
| 7 | No retrograde athero-embolization. |
| 8 | Should not propagate dissection further. |
| 9 | No local site complication |
| 10 | No vascular/nerve injury |
It is obvious from the table that no single cannulation strategy fulfills all the above criteria. Appropriate cannulation technique is just like a piece of a jigsaw puzzle. The suitableness of a piece is decided by the shape of adjacent pieces (Fig. 7). Similarly, the cannulation strategy is decided by several factors (Table 3).
Fig. 7.
Appropriate cannulation technique is just like a piece of jigsaw puzzle. Suitableness of a piece is decided by the shape of adjacent pieces
Table 3.
Factors determining the arterial cannulation strategy
| SN | Group | Factors |
|---|---|---|
| A | Patient-related factors | Age: young vs elderly |
| Clinical scenario: hemodynamic instability, aortic rupture, tamponade. | ||
| Re-operation | ||
| Atheromatous burden | ||
| Comorbidities: renal dysfunction, pulmonary dysfunction, stroke | ||
| Accompanying aorto-iliac disease or coarctation | ||
| B | Disease-related factors | Pathology: aneurysm vs dissection |
| Extent of disease: ascending aorta, arch (proximal), total arch, descending thoracic aorta | ||
| Involvement of branch vessels: innominate, carotids, subclavian, axillary, femoral | ||
| C. | Surgery-/surgeon-related factors | Extent of procedure: ascending aorta, hemi-arch, total arch |
| CPB strategy: deep hypothermia vs moderate hypothermia | ||
| Cerebral protection strategy: deep hypothermia/ retrograde cerebral perfusion/antegrade cerebral perfusion | ||
| Surgeon related: familiarity, training | ||
| Institutional protocol | ||
| D | Infrastructure-related factors | Availability of transesophageal echocardiography, epi-aortic scanning, cerebral oximetry. |
| Availability of branched grafts, long perfusion cannula, antegrade cerebral perfusion cannula. |
Choice of cannulation in aneurysmal disease
The algorithm for aneurysmal diseases is pretty straightforward. If the disease is limited to the aortic root or the proximal ascending aorta, the distal ascending aortic cannulation is performed routinely. However, if an arch intervention is required, optimal cannulation strategy is decided on the basis of age, comorbidities, condition of aortic branch vessels, cerebral protection strategy, institutional protocols, and surgeon’s training. In all possible scenarios, the axillary/carotid/innominate cannulation can be used if the chosen artery is unobstructed and free of the atherosclerotic process. In young patients without atheromatous debris in the descending aorta, the common FA can be used for cannulation. In the presence of significant aorto-iliac obstruction or coarctation, the common FA should not be used as a sole arterial inflow.
Choice of cannulation in TAAD
In contrast to a routine cardiac case, arterial cannulation strategy plays an important role in the management of acute type A dissection. Maintaining adequate arterial inflow is the crux of surgery for TAAD, and arterial cannulation is the Achilles’ heel of the whole operation. Apart from bleeding, cannulation-related problems are the major cause of mortality and morbidity. Thus, arterial cannulation requires a very careful planning. In TAAD, conventional ascending aortic cannulation is not possible, and an alternative cannulation strategy is required for arterial inflow. However, there are certain concerns with alternative cannulation strategies. First is the cannulation of true lumen. False lumen has very limited run off and supplies only a small part of the body. Thus, false lumen cannulation leads to pressurization of false lumen, compression of true lumen, and end-organ ischemia. Secondly, there is a complex pattern of intimal tears in descending aorta and distal arch. These tears can act as one-way flutter valve. Even if the true lumen is cannulated, this flutter valve mechanism can cause pressurization of the false lumen leading to malperfusion. It is more commonly seen with retrograde perfusion via FA. Though rare, it is a distinct possibility and needs prevention and immediate remedy. The third concern is that of brain protection. As open distal anastomosis is the standard of care in current times, there remains a need for additional brain protection strategy. In summary, by choosing an optimal arterial cannulation strategy, we aim at perfusing the true lumen, prevention/correction of malperfusion, and availability of ACP. Over half a century, several cannulation techniques have been used by surgeons. Each technique has its pros and cons. No strategy is perfect or free from complications. Excellent results and drawbacks have been reported with each technique. Surgeon’s familiarity with a particular technique is the principal determinant of cannulation strategy. As seen in Table 3, the optimal cannulation strategy is dependent upon multiple factors. Some of these will be discussed here in detail.
A. Hemodynamic status
If the patient is hemodynamically unstable, CPB initiation at the earliest should be the priority to improve the overall outcome [48]. In such a scenario, easy and quick to perform cannulation strategy should be preferred. Femoral cannulation is the most preferred cannulation in hemodynamically unstable patients as it is technically easy and quick to perform. If poor hemodynamics are due to tamponade or aortic rupture, femoro-femoral bypass is lifesaving. If the poor hemodynamics are due to coronary malperfusion or cardiac failure, one can opt from the femoral, central aortic, or transapical cannulation, depending upon familiarity and institutional practice. If the hemodynamics are extremely poor or the patients require cardio-pulmonary resuscitation (CPR) before sternotomy, one can institute femoro-femoral bypass immediately. If sternotomy has already been done in such scenario, central aortic cannulation would be a wise option. If the dissection is circumferential and echocardiographic assistance is not available, transapical or direct true lumen cannulation would be quicker than femoral cannulation.
If the patient is hemodynamically stable, antegrade flow is the preferred option to avoid retrograde cerebral embolism and malperfusion. Right axillary artery cannulation is the widely accepted option, if anatomically suitable and surgeon’s expertise is good. Carotid artery cannulation is also an equally good option, if anatomically suitable. We routinely use the right CCA. If the right CCA is compromised due to dissection, the left CCA/femoral/central cannulation is used. Some institutions practice IA cannulation. However, one should be cautious as the proximal IA may be involved in the dissection process. If the surgeon is confident and the above options are unavailable, or as per institutional protocol, one can opt for central aortic or transapical cannulation.
Though some centers use femoral cannulation routinely, it has the risk of malperfusion and retrograde athero-embolization in elderly patients. Hence, one may avoid femoral cannulation in elderly patients, if other options are available. Because of its familiarity, when a junior surgeon is operating in the middle of the night, femoral cannulation is not a bad option, even though not the best option. Antegrade flow can be established through graft cannulation in such patients, once the distal anastomosis is done.
Malperfusion
Malperfusion of one or more organs has been reported in 15 to 30% of patients [49] in TAAD. Malperfusion is of two types: preoperative malperfusion and new malperfusion developing after initiation of CPB. Malperfusion is the major cause of death in TAAD after rupture and tamponade. In the presence of visceral or limb malperfusion, an antegrade perfusion strategy is used. Retrograde perfusion through FA could increase the chances of malperfusion by pressurizing the false lumen. Antegrade flow via the right axillary, innominate, or carotid artery decreases the chances of malperfusion significantly but does not eliminate it totally [50]. Central aortic and transapical cannulation could be tried if the previously mentioned antegrade cannulation methods are not available for anatomic reasons. In cases of dissection flap involving carotid arteries with cerebral malperfusion, carotid cannulation ensures better cerebral perfusion compared to other cannulation options. If antegrade perfusion via axillary/carotid/innominate/aortic cannulation fails to resolve pre-operative visceral/limb malperfusion, additional femoral cannulation is recommended.
Newly developed malperfusion during CPB should be diagnosed and tackled immediately. Intra-operative malperfusion is associated with high mortality. Although there is no standard algorithm to deal with intraoperative malperfusion, the change or addition of arterial cannulation to different sites is usually beneficial [50]. If CPB has been initiated with femoral cannulation, addition or change with antegrade cannulation is helpful. Similarly, if malperfusion develops with antegrade cannulation, additional femoral cannulation may resolve the malperfusion. Occasionally, when malperfusion is noticed with the axillary or the carotid cannulation, additional central aortic cannulation is remedial.
CPB and cerebral protection strategy
Various neuroprotection strategies include deep hypothermic circulatory arrest, retrograde cerebral perfusion, and antegrade cerebral protection. Among the three, ACP ensures continuous cerebral blood flow and does not require deep hypothermia. This reduces complications related to deep hypothermia and prolonged CPB. Currently, ACP is becoming increasingly popular as a brain protection strategy. Cannulation of the right axillary artery, the carotid artery, and the IA ensures continuous antegrade cerebral flow even at moderate hypothermia. FA cannulation and central aortic cannulation require deep hypothermia for cerebral protection. Even if ACP is planned with femoral or central aortic cannulation, it will need an interruption in cerebral perfusion before placing ostial cannula for ACP. Thus, CPB (deep hypothermia vs moderate hypothermia) and cerebral protection strategy also contribute to the choice of arterial cannulation. Cannulation of the right axillary artery, the carotid artery, or the IA facilitates moderately hypothermic CPB and ACP.
Condition of the aorta, branch vessels, and cannulation site
Stroke is the major cause of morbidity/mortality in postoperative aortic surgical patients especially in TAAD, and its incidence may vary between 3 and 30% [51]. Calcific or atherosclerotic aorta, ostial atherosclerosis of the carotid vessels, and retrograde flow through the FA leading to sandblasting effect can also contribute to cerebral thromboembolism. One could minimize this complication by detailed preoperative assessment. If the thoracoabdominal aorta is severely atherosclerotic, femoral cannulation should be avoided. If the innominate or the left carotid artery ostium is diseased, manipulation and ostial perfusion should be avoided. In such a condition, right axillary or carotid cannulation is preferred. If the ascending aorta is heavily calcified, aortic cross-clamp should be avoided and CPB/cannulation strategy is tailored accordingly. In the presence of heavy thrombus load or circumferential dissection, central aortic cannulation should be avoided.
The presence of dissection or advanced atheromatous disease may exclude the axillary, carotid, innominate, and femoral arteries as possible cannulation site. Hence, a careful evaluation of these arteries should be performed pre-operatively. Delineation of the tears in peripheral arteries may not be very clear in CTA. If suspected, one can avoid or add an alternate cannulation [52]. Preoperatively, one should be vigilant to assess the possibility of aberrant right subclavian artery. If unnoticed, it may lead to cerebral ischemia and catastrophic neurologic damage during ACP [53]. Cannulation site infection and lymphatic leak are not uncommon after femoral cannulation. Hence, in the presence of active skin infection or large lymph nodes, femoral cannulation should be avoided.
AIIMS Protocol: Our current practice is summarized underneath:
Moderately hypothermic CPB with ACP is our preferred strategy. Antegrade arterial inflow is our preferred choice.
As routine CTA may not include the axillary, carotid, and femoral arteries, detailed pre-operative evaluation of the carotid, brachial, and femoral pulses is performed clinically. If any of these pulses is weak, a duplex ultrasound scan of arteries is performed.
For almost a decade, the right CCA is the preferred site for arterial cannulation. If the right CCA is having dissection, the left CCA, central aortic, or femoral cannulation is used in the same order. In the presence of advance atherosclerotic disease in the right CCA, the right axillary artery is used.
In elderly patients, femoral cannulation is usually avoided.
In case of hemodynamic instability, aortic rupture, tamponade, or ongoing cardio-pulmonary resuscitation, a femoro-femoral bypass is the first choice.
In case of femoral cannulation, after the arch repair, antegrade perfusion is established through the sidearm of the graft or direct cannulation of the graft.
Double cannulation: Along with primary right CCA cannulation, a femoral cannulation is performed, if (i) there is an intimal tear in the IA, (ii) occlusive disease of descending thoracic/abdominal aorta, (iii) radiological/clinical evidence of lower body/visceral malperfusion, and (iv) new-onset malperfusion at the initiation of CPB.
In case of new-onset malperfusion with femoral cannulation, central aortic cannulation is performed.
Summary
Choosing the right cannulation site especially in TAAD is the most crucial initial step. Selecting the optimal arterial cannulation is essentially a preoperative decision by careful clinical examination and detailed imaging assessment by the operating surgeon himself [54]. The decision of cannulation site selection should be individualized and patient-specific. Various available arterial cannulation methods include femoral, right axillary, innominate, carotid, central aortic, direct true lumen, transapical, and trans-atrial left ventricle cannulation. The ideal cannulation should be easy, quick, and suitable for all clinical scenarios. It should allow smooth conduct of CPB without malperfusion or cerebral embolization. The cannulation strategy should also provide an option for selective ACP. In addition, the cannulation should not cause vascular or neurological compromise and should be free from local site complications. It is difficult to get all these characteristics in a particular cannulation strategy. Each technique has its pros and cons. Excellent results and drawbacks have been reported with each technique. The final selection of the cannulation site is dependent upon a myriad of factors. However, a surgeon’s familiarity with a particular technique plays a major role in selection. Even when a carefully planned cannulation site is selected, one should ensure the adequacy of perfusion during CPB, and if malperfusion is suspected, immediate remedial measures should be taken.
Acknowledgements
The authors would like to express their heartfelt gratitude to Mr. Ramchandra B. Pokale, Chief Artist, Centre for Community Medicine; Dr. P Rajshekar, Additional Professor, Department of Cardiothoracic and Vascular Surgery; and Dr. Chaitanya Chittimuri, Senior Resident, Department of Cardiothoracic and Vascular Surgery, All India Institute of Medical Sciences, New Delhi, for preparation of digital drawings of this article.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contribution
Not applicable.
Funding
None.
Declarations
Ethical approval for research involving human participants and/ or animals
Not applicable as it is an invited review article.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neri E, Massetti M, Capannini G, Carone E, Tucci E, Diciolla F, Prifti E, Sassi C. Axillary artery cannulation in type A aortic dissection operations. J Thorac Cardiovasc Surg. 1999;118:324–329. doi: 10.1016/S0022-5223(99)70223-0. [DOI] [PubMed] [Google Scholar]
- 2.Yavuz S, Goncu MT, Turk T, et al. Axillary artery cannulation for arterial inflow in patients with acute dissection of the ascending aorta. Eur J Cardiothorac Surg. 2002;22:313–315. doi: 10.1016/s1010-7940(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 3.Fusco DS, Shaw RK, Tranquilli M, Kopf GS, Elefteriades JA. Femoral cannulation is safe for type a dissection repair. Ann Thorac Surg. 2004;78:1285–1289. doi: 10.1016/j.athoracsur.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 4.Tsiouris A, Elkinany S, Ziganshin BA, Elefteriades JA. Open seldinger-guided femoral artery cannulation technique for thoracic aortic surgery. Ann Thorac Surg. 2016;101:2231–2235. doi: 10.1016/j.athoracsur.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 5.David TE. Surgery for acute type A aortic dissection. J Thorac Cardiovasc Surg. 2015;150:279–83. [DOI] [PubMed]
- 6.Abe T, Usui A. The cannulation strategy in surgery for acute type A dissection. Gen Thorac Cardiovasc Surg. 2016;65:1–9. doi: 10.1007/s11748-016-0711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David TE, Armstrong S, Ivanov J, Barnard S. Surgery for acute type A aortic dissection. Ann Thorac Surg. 1999;67:1999–2001. doi: 10.1016/s0003-4975(99)00353-7. [DOI] [PubMed] [Google Scholar]
- 8.Moizumi Y, Motoyoshi N, Sakuma K, Yoshida S. Axillary artery cannulation improves operative results for acute type A aortic dissection. Ann Thorac Surg. 2005;80:77–83. doi: 10.1016/j.athoracsur.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Villard J, Froment JC, Milleret R, Dureau G, Amouroux C, Boivin J, Seffert P, Morel JJ. Type I, complete, acute aortic dissection. Value of arterial perfusion by the axillary route. Ann Chir Thorac Cardiovasc. 1976;15:133–135. [PubMed] [Google Scholar]
- 10.Sabik JF, Lytle BW, McCarthy PM, Cosgrove DM. Axillary artery: an alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J Thorac Cardiovasc Surg. 1995;109:885–890. doi: 10.1016/S0022-5223(95)70312-8. [DOI] [PubMed] [Google Scholar]
- 11.Wong DR, Coselli JS, Palmero L, Bozinovski J, Carter SA, Murariu D, LeMaire SA. Axillary artery cannulation in surgery for acute or subacute ascending aortic dissections. Ann Thorac Surg. 2010;90:731–737. doi: 10.1016/j.athoracsur.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 12.Van Arsdell GS, David TE, Butany J. Autopsies in acute type A aortic dissection. Surgical implications. Circulation. 1998;98:II299–302. [PubMed]
- 13.Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 14.Pasic M, Schubel J, Bauer M, Yankah C, Kuppe H, Weng YG, Hetzer R. Cannulation of the right axillary artery for surgery of acute type A aortic dissection. Eur J Cardiothorac Surg. 2003;24:231–235. doi: 10.1016/s1010-7940(03)00307-5. [DOI] [PubMed] [Google Scholar]
- 15.Baribeau YR, Westbrook BM, Charlesworth DC, Maloney CT. Arterial inflow via an axillary artery graft for the severely atheromatous aorta. Ann Thorac Surg. 1998;66:33–37. doi: 10.1016/s0003-4975(98)00397-x. [DOI] [PubMed] [Google Scholar]
- 16.Sabik JF, Nemeh H, Lytle BW, Blackstone EH, Gillinov AM, Rajeswaran J, Cosgrove DM. Cannulation of the axillary artery with a side graft reduces morbidity. Ann Thorac Surg. 2004;77:1315–1320. doi: 10.1016/j.athoracsur.2003.08.056. [DOI] [PubMed] [Google Scholar]
- 17.Imanaka K, Kyo S, Tanabe H, Ohuchi H, Asano H, Yokote Y. Fatal intraoperative dissection of the innominate artery due to perfusion through the right axillary artery. J Thorac Cardiovasc Surg. 2000;120:405–406. doi: 10.1067/mtc.2000.107206. [DOI] [PubMed] [Google Scholar]
- 18.Ren Z, Wang Z, Hu R, Wu H, Deng H, Zhou Z, Hu X, Jiang W. Which cannulation (axillary cannulation or femoral cannulation) is better for acute type A aortic dissection repair? A meta-analysis of nine clinical studies. Eur J Cardiothorac Surg. 2015;47:408–415. doi: 10.1093/ejcts/ezu268. [DOI] [PubMed] [Google Scholar]
- 19.Benedetto U, Mohamed H, Vitulli P, Petrou M. Axillary versus femoral arterial cannulation in type A acute aortic dissection: evidence from a meta-analysis of comparative studies and adjusted risk estimates. Eur J Cardiothorac Surg. 2015; 48:953–9. [DOI] [PubMed]
- 20.Gulbins H, Pritisanac A, Ennker J. Axillary versus femoral cannulation for aortic surgery: enough evidence for a general recommendation? Ann Thorac Surg. 2007;83:1219–1224. doi: 10.1016/j.athoracsur.2006.10.068. [DOI] [PubMed] [Google Scholar]
- 21.Di Eusanio M, Pantaleo A, Petridis FD, et al. Impact of different cannulation strategies on in-hospital outcomes of aortic arch surgery: a propensity-score analysis. Ann Thorac Surg. 2013;96:1656–1663. doi: 10.1016/j.athoracsur.2013.06.081. [DOI] [PubMed] [Google Scholar]
- 22.Etz CD, von Aspern K, da Rocha ESJ, et al. Impact of perfusion strategy on outcome after repair for acute type A aortic dissection. Ann Thorac Surg. 2014;97:78–85. doi: 10.1016/j.athoracsur.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 23.Banbury MK, Cosgrove DM. Arterial cannulation of the innominate artery. Ann Thorac Surg. 2000;69:957. doi: 10.1016/s0003-4975(99)01519-2. [DOI] [PubMed] [Google Scholar]
- 24.Eldeiry M, Ghincea C, Aftab M, Cleveland JC, Fullerton D, Reece TB. Innominate versus axillary artery cannulation for the hemiarch repair. J Surg Res. 2018;232:234–239. doi: 10.1016/j.jss.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Preventza O, Garcia A, Tuluca A, et al. Innominate artery cannulation for proximal aortic surgery: outcomes and neurological events in 263 patients. Eur J Cardiothorac Surg. 2015;48:937–942. doi: 10.1093/ejcts/ezu534. [DOI] [PubMed] [Google Scholar]
- 26.Preventza O, Bakaeen FG, Stephens EH, Trocciola SM, de la Cruz KI, Coselli JS. Innominate artery cannulation: an alternative to femoral or axillary cannulation for arterial inflow in proximal aortic surgery. J Thorac Cardiovasc Surg. 2013;145:S191–S196. doi: 10.1016/j.jtcvs.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 27.Harky A, Grafton-Clarke C, Hadlett M, Shuttleworth E. In thoracic aortic surgery, is innominate artery cannulation a safe and effective alternative to axillary artery cannulation? Interact Cardiovasc Thorac Surg. 2019;29:604–607. doi: 10.1093/icvts/ivz130. [DOI] [PubMed] [Google Scholar]
- 28.Urbanski PP. Carotid artery cannulation in acute aortic dissection with malperfusion. J Thorac Cardiovasc Surg. 2006;131:1398–1399. doi: 10.1016/j.jtcvs.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 29.Bachet J. Carotid artery cannulation in aortic surgery: why not? Multimed Man Cardiothorac Surg. 2015. 10.1093/mmcts/mmv028. [DOI] [PubMed]
- 30.Urbanski PP, Sabik JF, Bachet JE. Cannulation of an arch artery for hostile aorta. Eur J Cardiothorac Surg. 2017;51:2–9. doi: 10.1093/ejcts/ezw325. [DOI] [PubMed] [Google Scholar]
- 31.Lijoi A, Scarano F, Dottori V, Parodi E, Casali G, Bartolozzi F. Stanford type A aortic dissection. A new surgical approach. Tex Heart Inst J. 1998;25:65–67. [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki T, Asai T, Matsubhayashi K, et al. Safety and efficacy of central cannulation through ascending aorta for type A aortic dissection. Interact Cardiovasc Thorac Surg. 2010;11:34–37. doi: 10.1510/icvts.2009.231852. [DOI] [PubMed] [Google Scholar]
- 33.Inoue Y, Ueda T, Taguchi S, at al. Ascending aorta cannulation in acute type A aortic dissection. Eur J Cardiothorac Surg. 2007;31:976–9. [DOI] [PubMed]
- 34.Frederick JR, Yang E, Trubelja A, Desai ND, Szeto WY, Pochettino A, Bavaria JE, Woo YJ. Ascending aortic cannulation in acute type A dissection repair. Ann Thorac Surg. 2013;95:1808–1811. doi: 10.1016/j.athoracsur.2012.10.086. [DOI] [PubMed] [Google Scholar]
- 35.Rosinski BF, Idrees JJ, Roselli EE, et al. Cannulation strategies in acute type A dissection repair: a systematic axillary artery approach. J Thorac Cardiovasc Surg. 2019;158:647–659.e5. doi: 10.1016/j.jtcvs.2018.11.137. [DOI] [PubMed] [Google Scholar]
- 36.Reece TB, Tribble CG, Smith RL, Singh RR, Stiles BM, Peeler BB, Kern JA, Kron IL. Central cannulation is safe in acute aortic dissection repair. J Thorac Cardiovasc Surg. 2007;133:428–434. doi: 10.1016/j.jtcvs.2006.09.059. [DOI] [PubMed] [Google Scholar]
- 37.Klotz S, Heuermann K, Hanke T, Petersen M, Sievers HH. Outcome with peripheral versus central cannulation in acute type A dissection. Interact Cardiovasc Thorac Surg. 2015;20:749–753. doi: 10.1093/icvts/ivv041. [DOI] [PubMed] [Google Scholar]
- 38.Sabashnikov A, Heinen S, Deppe AC, Zeriouh M, Weymann A, Slottosch I, Eghbalzadeh K, Popov AF, Liakopoulos OJ, Rahmanian PB, Madershahian N, Kroener A, Choi YH, Kuhn-Régnier F, Simon AR, Wahlers T, Wippermann J. Axillar or aortic cannulation for aortic repair in patients with Stanford a dissection? Ann Thorac Surg. 2016;102:787–794. doi: 10.1016/j.athoracsur.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Jakob H, Tsagakis K, Szabo A, Wiese I, Thielmann M, Herold U. Rapid and safe direct cannulation of the true lumen of the ascending aorta in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007;134:244–245. doi: 10.1016/j.jtcvs.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 40.Kitamura T, Torii S, Kobayashi K, Tanaka Y, Sasahara A, Ohtomo Y, Horikoshi R, Miyaji K. Samurai cannulation (direct true-lumen cannulation) for acute Stanford type A aortic dissection. Eur J Cardiothorac Surg. 2018;54:498–503. doi: 10.1093/ejcts/ezy066. [DOI] [PubMed] [Google Scholar]
- 41.Conzelmann LO, Kayhan N, Mehlhorn U, Weigang E, Dahm M, Vahl CF. Reevaluation of direct true lumen cannulation in surgery for acute type A aortic dissection. Ann Thorac Surg. 2009;87:1182–1186. doi: 10.1016/j.athoracsur.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 42.Wada J, Komatsu S, Nakae S, Kazui T. A new cannulation method for isolated mitral valve surgery “apicoaortic-pa” cannulation. Thoraxchir Vask Chir. 1976;24:204–212. doi: 10.1055/s-0028-1095905. [DOI] [PubMed] [Google Scholar]
- 43.Wada H, Matsumura H, Minematsu N, Amako M, Nishimi M, Tashiro T. Direct and transapical central cannulation in acute type A aortic dissection. Ann Vasc Dis. 2014;7:286–291. [DOI] [PMC free article] [PubMed]
- 44.Matsushita A, Manabe S, Tabata M, Fukui T, Shimokawa T, Takanashi S. Efficacy and pitfalls of transapical cannulation for the repair of acute type A aortic dissection. Ann Thorac Surg. 2012;93:1905–1909. doi: 10.1016/j.athoracsur.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Wada S, Yamamoto S, Honda J, Hiramoto A, Wada H, Hosoda Y. Transapical aortic cannulation for cardiopulmonary bypass in type A aortic dissection operations. J Thorac Cardiovasc Surg. 2006;132:369–372. doi: 10.1016/j.jtcvs.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Schoeneich F, Rahimi-Barfeh A, Grothusen C, Cremer J. Transatrial left-ventricular cannulation in acute aortic dissection type A: a novel cannulation technique. Eur J Cardiothorac Surg. 2015;48:e51–e52. doi: 10.1093/ejcts/ezv247. [DOI] [PubMed] [Google Scholar]
- 47.Rahimi-Barfeh A, Grothusen C, Haneya A, Schöttler J, Eide AM, Erdmann M, Friedrich C, Hoffmann G, Cremer J, Schoeneich F. Transatrial Cannulation of the left ventricle for acute type A aortic dissection: a 5-year experience. Ann Thorac Surg. 2016;101:1753–1758. doi: 10.1016/j.athoracsur.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 48.Bossone E, Pyeritz RE, Braverman AC, Peterson MD, Ehrlich M, O'Gara P, Suzuki T, Trimarchi S, Gilon D, Greason K, Desai ND, Montgomery DG, Isselbacher EM, Nienaber CA, Eagle KA. Shock complicating type A acute aortic dissection: clinical correlates, management, and outcomes. Am Heart J. 2016;176:93–99. doi: 10.1016/j.ahj.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 49.Deeb GM, Williams DM, Bolling SF, et al. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg. 1997;64:1669–75. [DOI] [PubMed]
- 50.Orihashi K. Malperfusion in acute type A aortic dissection: unsolved problem. Ann Thorac Surg. 2013;95:1570–6. [DOI] [PubMed]
- 51.Bossone E, Corteville DC, Harris KM, et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation. 2013;128:S175–179. [DOI] [PubMed]
- 52.Rylski B, Czerny M, Beyersdorf F, et al. Is right axillary artery cannulation safe in type A aortic dissection with involvement of the innominate artery? J Thorac Cardiovasc Surg. 2016;152:801–7.e1. doi: 10.1016/j.jtcvs.2016.04.092. [DOI] [PubMed] [Google Scholar]
- 53.Battaloglu B, Secici S, Colak C, Disli OM, Erdil N, Kutlu R. Aberrant right subclavian artery and axillary artery cannulation in type A aortic dissection repair. Ann Thorac Surg. 2013;96:e1–2. [DOI] [PubMed]
- 54.Rajiah P, Schoenhagen P. The role of computed tomography in pre-procedural planning of cardiovascular surgery and intervention. Insights Imaging. 2013;4:671–689. doi: 10.1007/s13244-013-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.