Abstract
The treatment of complex aortic arch disease, in chronic or acute setting, has always represented a fascinating challenge for the heart surgeon also because, often, the involvement of the aortic arch is associated with a simultaneous involvement of the ascending aorta and of the proximal portion of the descending thoracic aorta. In recent years, there have been many surgical and/or endovascular techniques and approaches in a single step or multiple steps proposed with the aim of treating and simplifying these complex conditions. The first procedure available for this purpose was the conventional elephant trunk technique, proposed by the German surgeon Hans Borst, back in 1983. In the following years, the technique has undergone modifications, up to what is nowadays considered its most modern evolution, represented by the frozen elephant trunk which allows managing the proximal descending thoracic aorta using the antegrade release of a self-expandable stent graft. In this review article, we try to analyze the advantages and drawbacks of both techniques from clinical and practical points of view.
Keywords: Aortic arch, FET, Elephant trunk, Antegrade perfusion, Aortic surgery, Thoracic aorta
Introduction
Complex lesions of the thoracic aorta are traditionally treated in two surgical steps with the “elephant trunk technique” (Fig. 1). A relatively new approach is represented by the “frozen elephant trunk technique” (FET) which potentially allows treating the combined lesions of the thoracic aorta in a one-stage procedure combining endovascular treatment with conventional surgery using a hybrid prosthesis (Fig. 2). These are very complex and time-consuming operations, and good results can be obtained only if good strategies of myocardial, cerebral, and visceral protection are adopted. The aim of this review article is to analyze the benefits and pitfalls of the FET technique compared with the conventional elephant trunk (cET) technique in clinical practice.
Fig. 1.
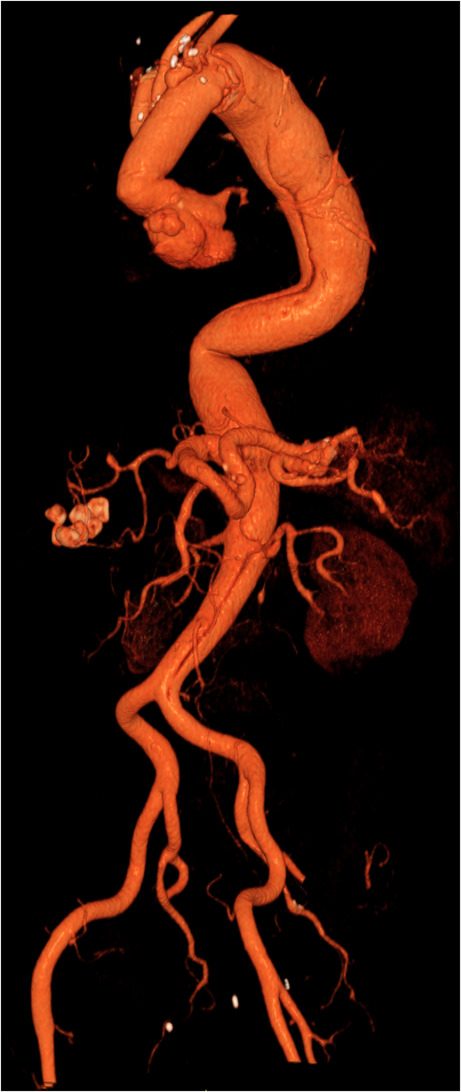
Post-operative CT scan of a cET technique
Fig. 2.
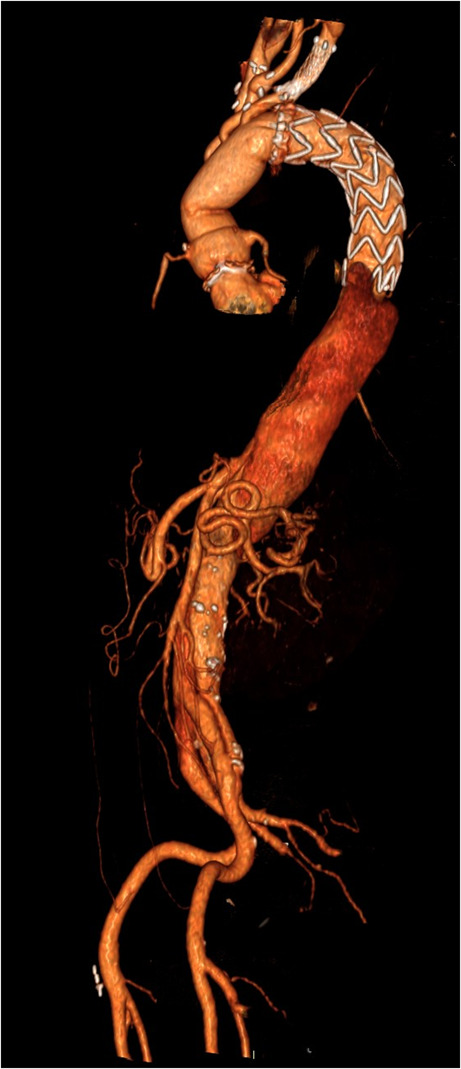
Post-operative CT scan of a FET technique
Differences between the elephant trunk procedure and FET technique
The classic 2-stage elephant trunk procedure proposed by Hans Borst in 1983, until about 10 years ago, represented the most frequently employed approach in case of extensive disease of the thoracic aorta. This technique is a two-step procedure: briefly, in the first operation, the aortic arch is replaced, and a free-floating extension of the arch prosthesis (elephant trunk) is left behind in the proximal descending aorta. In the second procedure, surgical or endovascular, the prosthetic trunk can be extended to the desired level through an open lateral thoracotomy or with the less-invasive release of an endovascular stent graft. According to Borst’s original suggestion, the length of the elephant trunk should not exceed 7–8 cm because a longer trunk is more likely to cause complications due to kinking and graft occlusion [1].
The main advantages of the elephant trunk procedure are mostly appreciated at stage two. In fact, the avoidance of dissection at the level of the distal aortic arch reduces the risk of nervous and bronchial structures’ injury, allows a facilitated and more expeditious graft-to-graft anastomosis, and avoids clamping proximal to the left subclavian artery that may reduce the risk of stroke and paraplegia after surgery. Furthermore, the elephant trunk may greatly act as a landing zone for following stent-graft deployments with an expected reduction of mortality and morbidity of the completing stage two.
A way to achieve one-stage repair of extensive aortic arch disease by combining the concepts of the cET principle and the endovascular stenting of descending aortic aneurysms was introduced in 2003 as the FET procedure [2].
Born as a hybrid procedure, the FET combines surgical and endovascular techniques, and it can be carried out with the availability of a hybrid prosthesis consisting of a self-expandable nitinol stent-graft distally and proximally by a vascular graft with zero porosity. This procedure contemplates, in a single stage, the endovascular repair of the descending thoracic aorta pathology and the conventional graft replacement of the aortic arch through a mid-sternotomy. The two commercially available hybrid prostheses available in Europe for FET interventions are represented by the E- vita Open Plus hybrid stent graft system manufactured by JOTEC (Hechingen, Germany) and by the Thoraflex Hybrid provided by Vascutek (Inchinnan, Scotland). Both hybrid prostheses are available in different sizes and with different delivery systems; they both have a sewing collar to facilitate distal anastomosis. The stented portion of these prostheses is available in different diameters (28–40 mm for the Thoraflex Hybrid and 20–40 mm for the E-vita Open Plus) and lengths (100 or 150 mm for the Thoraflex Hybrid and 130, 150, or 160 mm for the E-vita Open Plus). Between the two hybrid prostheses, the main difference is that the Thoraflex Hybrid graft has a quadrifurcated proximal vascular portion, which is tubular in the E-vita Open Plus, to facilitate single reimplantation of the epiaortic vessels and a more rapid reperfusion of the lower part of the body once the distal anastomosis is completed [3–5].
The FET procedure includes several surgical steps: (a) total resection of the aortic arch; (b) preparation of the distal aortic stump and, in case of aortic dissection, the obliteration of the false lumen (FL) using of 4 or 5 U-stitches with pledgets inside and a Teflon felt outside; (d) advancement of the hybrid system in the descending thoracic aorta over a guidewire that has been retrogradely positioned via the femoral artery into the true lumen under transesophageal control and after systemic heparinization; (e) deployment of the stent graft; (f) suture of the vascular collar to the distal aorta before (arch zone 2) or after the origin of the left subclavian artery (arch zone 3); (g) reimplantation of the left subclavian artery; (h) proximal anastomosis between the arch and ascending aorta prostheses; and (i) completion of the arch vessel reimplantation, left common carotid artery, and brachiocephalic trunk, preferably with a beating heart [6].
Alternatives to the E-vita Open Plus and to the Thoraflex Hybrid devices
In 2003, the Chinese surgeon Sun from Beijing developed a different hybrid device, the Cronus open stented graft (MircoPort, Shanghai, China), consisting of a regular Dacron vascular graft and interconnected Z-shaped stents. At the proximal and distal ends, there is an extra centimeter of Dacron sewing cuff. This hybrid prosthesis always needs an extravascular Dacron graft, straight or branched, in order to complete the reconstruction of the aortic arch. Sun’s procedure includes implantation of the hybrid graft into the descending aorta, followed by its anastomosis with a separate 4-branched vascular Dacron graft for total arch replacement. In order to minimize cerebral and cardiac ischemia, Sun’s procedure foresees a special anastomotic sequence for aortic reconstruction: proximal descending aorta–left carotid artery–ascending aorta–left subclavian artery–innominate artery [7, 8].
In 2009, Desai and Pochettino from the USA described the “Penn technique” for hybrid hemi-arch replacement with antegrade deployment of a standard 10- to 15-cm GORE-TAG thoracic aortic stent graft (W.L. Gore & Associates, Flagstaff, AZ) [9]. The rationale of this technique is to avoid the complexity of total arch replacement. In fact, once the stent graft is deployed and the balloon dilated into the descending thoracic aorta under direct vision, it is fixed to the native aorta. The hemi-arch anastomosis is then completed in a standard fashion with the TAG stent graft incorporated into the arch suture line. Some years later, in 2013, a slightly modification of the “Penn technique” was proposed by Roselli et al. [10]; in this case, the stent graft is released more proximally into the arch. Two of the flares on the proximal stent graft are resected in order to create a “fenestration” around the supra-aortic vessels. The stent graft at the base of the fenestration is sutured to the base of the branch artery. The distal aortic hemi-arch anastomosis is then performed in the usual fashion with a standard Dacron graft including the stent graft for 220 degrees of the circumference [11].
In 2014, a new device for aortic stent grafting was developed in Japan, the Frozenix–J Graft Open Stent (Japan Lifeline Co. Ltd., Tokyo, Japan), which consists of a distal stented portion that is made of a polyester tube with oval-shaped nitinol stents, and a proximal unstented graft. Before deployment, the stented portion is bent to conform to the curvature of the aorta in order to facilitate the insertion of the device. After deployment and distal anastomosis, the arch vessels are reconstructed with a separate branched graft in an end-to-end fashion [12].
A novel evolution of the E-vita stent graft is the E-vita Open NEO; it is available in three variants: the first one with a stent and an arch graft with a side branch for lower-body perfusion; the second one with a stent graft and the arch graft having individual branch grafts for selective anastomosis to the supra-aortic arch vessels; and third, the “Spielvogel type”: projected for a “no-arch-touch” technique with the suture line in zone 0. A new prototype is the E-Novia: this prosthesis will be suitable for acute type I aortic dissection; Penn B, C, and BC; and patients with severe concomitant disease. It will have a covered and non-covered stent-graft portion. The covered stent graft will be released in the descending thoracic aorta and non-covered stent-graft in the aortic arch (similarly the petticoat technique). The anastomosis would be then performed in zone 0, and hence, it could allow reducing ischemic times as well as hypothermic circulatory arrest time [13].
Tips and tricks for the positioning of the hybrid prostheses
Based on our experience, we identified several useful “tips and tricks” that could facilitate FET implantation:
The entire aorta has to be carefully investigated before operation through the angio computer tomography (CT) scan, especially in case of acute or chronic dissection, where it is mandatory to know the origin of the visceral arteries (true or false lumen) and the presence of the distal re-entry sites.
Place, under transesophageal control and after systemic heparinization, a guide wire in the thoracic aorta through the femoral artery, to correctly position the stent-graft portion, above all, in case of dissection, in order to be sure to put the prosthesis in the true lumen.
Drain the cerebrospinal fluid (spinal pressure < 12 mmHg) before and immediately after surgery.
Use crystalloid cardioplegia with long myocardial protection and protect the brain with antegrade cerebral perfusion;
Use the angioscopy to view inside the descending thoracic aorta before and immediately after release of the stent graft.
Restart systemic perfusion and “rewarming” immediately after completing the distal aortic arch anastomosis.
Avoid stent oversizing in acute aortic dissection and consider a 10–20% oversizing in the case of chronic aneurysm.
Consider using branched aortic arch grafts when epiaortic vessels are involved.
Avoid long stent-graft trunks (> 150 mm) to reduce the risk of spinal cord injuries (SCI).
Keep mean systemic pressure > 70–80 mmHg after device implantation to better perfuse the spinal cord.
Indications for the use of cET and FET
Chronic degenerative and post-dissection aortic aneurysms
In case of chronic degenerative aneurysm, the conventional surgical approach would include, as the first surgical step, the open replacement of the aortic arch using the cET technique, followed by a second-step open or endovascular repair of the aneurysmal descending or thoracoabdominal aorta [14, 15]. The rationale of this staged approach is fundamentally related to the advantage of eliminating the need of cross-clamping the aorta proximally to the left subclavian artery during the second operation. However, although this approach has allowed patients to be treated satisfactorily, its main limitation is represented by the fact that more than half of the patients did not arrive at the second surgical step, both because they died between the first and the second steps and because some patients refused another surgical operation. This drawback can be partially attenuated by the alternative FET technique which is extremely useful and effective in patients whose aneurysms are limited to the arch and proximal descending thoracic aorta and in patients who present saccular aneurysms of the mid-distal arch for which endovascular treatment is deemed technically unsuccessful or does not represent the best therapeutic option. However, as with the cET, the use of stent grafts in chronic aortic dissection is arguable because it does not constitute a definitive repair. In fact, the partially thrombosed and fibrotic arterial wall will not allow the stent graft to expand enough to obliterate the FL and, in the same time, distal re-entries often cause retrograde flow into the false lumen not allowing its depressurization and thrombization. This condition leaves the patients to be at risk for further reinterventions in the distal thoracic aorta. One of the main issues of cET and FET in both degenerative and chronic post-dissection aneurysms is the increased risk of permanent and transient SCI, with a reported incidence of between 0.4 and 3% [16, 17] in the case of cET and a significantly higher rate for FET, with studies reporting incidences greater than 10% [17, 18]. A possible explanation for this could lie in the prolonged times of hypothermic circulatory arrest or in the coverage of the proximal descending thoracic aorta with the occlusion of intercostal arteries along the stent-graft deployment [19].
Acute type A aortic dissection
Conventional treatment of acute type A aortic dissection (AAAD) is represented by emergent surgery of the ascending aorta with the resection of the primary intimal tear. Except in cases of dissection limited to the ascending aorta (De Backey type II), current consensus favors the open distal anastomosis in order to perform a more complete and radical repair with a more accurate re-approximation of the dissected aortic wall and a direct visualization of further arch intimal tears [15]. In fact, patent false lumen (PFL) rates are reported in more than 60% of the cases of thoracic aorta dissection and it is one of the most important risk factor for late dilatation and further reoperation [20, 21]. A direct association between the patency of the false lumen and reoperation on the remaining distal aorta has been also demonstrated [22]; so in light of this and in order to reduce the incidence of a patent FL and the consequent rate of reoperation, attempts to extend the aortic replacement have been performed. This more aggressive surgical approach for AAAD, i.e., a “prophylactic” total arch replacement as protocol in the case of a dissected aortic arch, irrespective of the location of the entry site, might improve long-term outcomes reducing the incidence of residual patent FL [23]. Complete thrombosis of the false lumen is one of the main objectives after surgery for aortic dissection, and when this does not occur, an increase of the pressurization of the false lumen will increase wall tension, elevating the risk of distal aortic enlargement and possible rupture. In this acute scenario, the cET and, more recently, the FET techniques are two surgical alternatives available despite their opposite approaches to the treatment of the dissected descending aorta.
The cET may be a valid option for total arch replacement. This technique facilitates interval repair of distal aortic aneurysms. However, in case of acute dissection, it can present some technical difficulties due to the small diameter of the true lumen. Moreover, if the false lumen is pressurized, the floating graft can result compressed and this phenomenon results in a little chance for late FL thrombosis.
The FET in acute type A aortic dissection (ATAAD) aims to depressurize and induce thrombosis of the FL, and moreover, it should be considered also as a valuable adjunct in patients with distal aortic malperfusion; re-entry tears involving the proximal descending thoracic aorta, distal arch, or descending thoracic aorta rupture; an aneurysmal distal arch; and a severely damaged aortic arch hindering safe distal aortic arch anastomosis [23]. In patients with malperfusion syndrome, the importance of the FET technique lies in its potential to fully open the compressed true lumen and to cover additional entry tears located in the proximal descending thoracic aorta, which maintain pressurization of the FL. Moreover, by inducing both coverage of secondary entry tears and obliteration of the peri-stent FL, the FET is assumed to further reduce the risk of distal aortic dilatation and therefore reduce late aortic-related events and the need for complex distal aortic reinterventions [24, 25]. A length of approximately 10 cm from the arch zone two or three (beyond the left common carotid artery or the left subclavian artery) usually seems to be enough to stabilize the dissected arterial wall and favors true lumen expansion downstream. The oversizing of the stent graft should be avoided to prevent formation of new intimal tears distal to the stent graft. However, data from previous studies showed positive aortic remodeling only at the level of the stent graft, with almost 20% of patients remaining at risk for secondary reintervention due to the negative remodeling in the distal aortic tracts. In this context, long-term clinical data are still needed to demonstrate a clear survival benefit from aggressive versus conservative management of the aortic arch in ATAAD.
Acute type B aortic dissection
The standard treatment for acute complicated type B aortic dissection (BAAD) is thoracic endovascular aortic repair (TEVAR). However, when endovascular treatment is not possible or contraindicated [26] because of unfavorable aortic anatomy, high risk of retrograde type A dissection, or connective tissue disorders, open surgery represents a viable alternative to primary TEVAR and its effectiveness has already been reported [27].
In fact, in the case of the proximal landing zone on a dilated aortic arch higher than 40 mm or excessively angulated, concomitant aneurysmal dilatation of the ascending aorta and/or aortic arch requiring surgical correction, and a dissected left subclavian artery with an additional intimal tear which could potentially maintain perfusion of the false lumen despite correct stent-graft deployment, open surgical treatment could be an optimal solution instead of the endovascular one. In these cases, FET has emerged as a safe, useful, and effective treatment option [26]. An expert consensus opinion from the Vascular Disease Domain of the European Association for Cardio-Thoracic Surgery (EACTS) recommended FET in type B acute aortic dissection when primary TEVAR is not feasible or the risk of retrograde type A-AAD is high [14]. Weiss et al. in 2014 published the first multicenter experience on FET in type BAAD showing an acceptable in-hospital mortality and SCI rates (14% and < 4%, respectively). The study also confirmed the efficacy of the hybrid prosthesis to induce obliteration of the peri-stent FL, with a complete FL thrombosis of 75% immediately after the operation increasing to 97% in the long term [27].
Results of cET vs FET
Several studies reported a comparison of cET and FET (Table 1): in a study in 2013, Leontyev et al. reported an in-hospital mortality of 8.7% for FET and 21.6% for cET and a higher incidence of SCI for FET (21.7% vs 4%) [17]. Similarly, Di Eusanio et al. reported higher in-hospital mortality incidence for patients who underwent cET (13.9% vs 4.8%) with a lower incidence of SCI in both groups (4.8% in the FET group vs 2.9% in the cET group) [28]. The group of Hannover reported a double incidence in terms of mortality for cET with a similar incidence of SCI and reintervention at follow-up (FU). A more recent study conducted on AAAD in Japan revealed similar in-hospital mortality for the two techniques (5% cET vs 6% FET) with no event of SCI [29]. In 2021, a multicenter Canadian study reported that FET repair is associated with lower in-hospital mortality as compared to cET, and results in similar risk of stroke and SCI [30].
Table 1.
Summary of studies comparing cET and FET
| Author | Technique | Hospital mortality | SCI | Follow-up survival | Reintervention |
|---|---|---|---|---|---|
| Leonntyev 2013 [17] | FET | 8.7 | 21.7 | 40 (5 years) | 10.9 |
| CET | 21.6 | 4.0 | 68 (5 years) | ||
| Di Eusanio 2014 [28] | FET | 4.8 | 4.8 | 72.8 (4 years) | |
| CET | 13.9 | 2.9 | 75.8 (4 years) | ||
| Shrestha 2015 [34] | FET | 12.4 | 4.8 | 62 (4 years) | 26.1 |
| CET | 24.7 | 5 | 44 (4 years) | 24 | |
| Inoue 2019 [29] | FET | 6 | 0 | – | – |
| CET | 5 | 0 | – | – | |
| Hage 2021 [30] | FET | 9 | 5 | – | – |
| CET | 13 | 2 | – | – |
Advantages and disadvantages of the “elephant trunks—classic and frozen—options” (Table 2).
Table 2.
Advantages and disadvantages of the cET and FET techniques
| cET | FET | ||
|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages |
| Simplifies distal aortic arch anastomosis | Needs a two-stage procedure | Allows single-stage treatment | Increased risk of SCI |
| Facilitates thoracoabdominal aortic interventions | Interval mortality | Facilitates thoracoabdominal aortic interventions | Technically demanding |
| Reduces the risk of additional distal aortic surgery | Cost of the device | ||
Classic elephant trunk
Advantages
Disadvantages
FET
Advantages
Disadvantages
Bologna experience with FET and cET
Bologna experience with the FET procedure started in 2007. From 2007 to nowadays, 340 patients were treated. The mean duration of antegrade selective cerebral perfusion (ASCP) was 95.5 ± 36.8 min with a mean cardiopulmonary bypass time of 230.2 ± 69.2 min. The myocardial ischemic times and the visceral ischemic times were 146.3 ± 52.4 min and 53.6 ± 9.8 min, respectively. Overall in-hospital mortality was assessed at 16.6% (including both elective and emergent surgeries). The stroke rate was 8.8% and the SCI rate was 10.3%. Renal failure (permanent dialysis) was reported in 3.3% of the cases. The mean hospital stay was 22.3 ± 19 days. Ninety patients (29%) needed a reintervention in the follow-up period: 86 pts received an endovascular treatment and 4 underwent open thoracoabdominal aorta repair.
With the introduction of the FET, the use of cET has undergone a significant reduction over the years.
In the last 20 years, 65 patients underwent cET in Bologna. The mean duration of ASCP was 91 ± 34.3 min with a mean cardiopulmonary bypass time of 219.8 ± 78 min. The myocardial ischemic times and the visceral ischemic times were 143.5 ± 50.2 min and 58.9 ± 26.7 min, respectively. The overall in-hospital mortality was 21.5%. The stroke rate was 9.2% and no SCI was reported. Renal failure (permanent dialysis) was reported in 4.6% of the cases. Fifteen patients (23.1%) underwent reintervention with TEVAR and 6 (9.2%) with open thoracoabdominal aorta repair.
Discussion
The treatment of complex lesions of the thoracic aorta, many of them extending from the ascending aorta to the descending thoracic aorta, has always been a fascinating “challenge” even for surgeons focused on aortic surgery.
In this sense, a propulsive push to this type of surgery was given in the early 1980s by the German surgeon Hans Borst, who developed the two-steps elephant trunk technique which, for many years, was undoubtedly the most widely used technique for the treatment of extensive pathologies of the thoracic aorta involving the ascending aorta and the aortic arch [1]. The ET technique had the great advantage of making the second procedure on the thoracic and/or thoracoabdominal aorta easier.
However, the main concern of the elephant trunk technique resided in the fact that the risks of two major surgical procedures and the risk during the time interval between the two interventions added up cumulatively. To reduce the cumulative risks of the staged approach, some surgeons have preferred a single-stage repair with a clamshell incision, a left lateral thoracotomy, or a combination with a median sternotomy and a left lateral thoracotomy. Along with the advent of transfemoral stent grafts for the treatment of descending aortic aneurysms, it became possible to securely anchor a stent graft in an elephant trunk prosthesis, previously placed during arch surgery. The idea of using an elephant trunk prosthesis, as a stent graft, has represented the antechamber to what can be considered the modern evolution of cET, and that has “revolutionized” the open surgery of the thoracic aorta, represented by the FET technique which is probably the latest introduced to the armamentarium of the cardiac surgeon to treat extensive lesions of the thoracic aorta. In fact, the chance to perform a simultaneous antegrade stenting of the descending aorta in only one step led most cardiovascular surgeons to switch to this treatment option.
Both procedures, the cET and the FET, as described above, present advantages and disadvantages, and a careful selection of which of the two techniques should be used should always be guided by etiology and anatomical indications. Undoubtedly, if on one hand the main advantage of the cET is that during the second-stage operation, the surgeon needs to anastomose only the descending thoracic aorta graft with the previously placed ET, instead of the distal aortic arch, simplifying the procedure considerably, from the other, one of the main disadvantages is the need of 2 operations, with the associated and cumulative risk of mortality and morbidity. In addition, death may occur in the interval between the first and the second step, owing to the rupture of the untreated segment of the aorta. Moreover, a large number of patients are lost to the second operation [33]. In fact, most patients—from 30 to 50% after the first procedure—did not reach the second operation because they did not show, or they refused, or more importantly, because there was no proper indication [43]. From this point of view, the main advantage of the FET is that it often does not necessitate a second procedure but, if required, it is not precluded.
Patients affected by aortic dissection with complex arch tears involving the distal arch and/or proximal descending thoracic aorta may benefit from treatment with FET [14, 15]. In this setting, a stent graft released in the descending thoracic aorta may enlarge the true lumen, obliterate secondary entry tears, and induce FL thrombosis and remodeling and hence improve freedom from distal redo and survival [36]. In an interesting manuscript, Dohle et al. studied the fate of the dissected aorta after FET: the aortic pathology is often transferred to the downstream aorta and a careful follow-up is mandatory: in 88% FL thrombosis at the stent graft compared with a 25% FL thrombosis down to the coeliac trunk. Within the first year, positive or stable aortic remodeling was found again in 90% of patients at the level of stent graft vs 58% at the origin of visceral arteries. In a recent multicenter Canadian study, the FET technique results superior in terms of in-hospital mortality, and neurological complications including SCI when used in AAAD [30].
The FET showed good results also for the treatment of chronic degenerative aneurysm [2, 34]. In the last report of the E-vita Open international registry of 2020 accounting for 1165 patients, the overall in-hospital mortality rate was 12% and permanent cerebral and spinal cord complications occurred in 5.2% and 3.9% respectively [36]. These results demonstrate that, even in the era of less-invasive approaches such as TEVAR after the conventional or the FET procedure, close surveillance is fundamental to reduce mortality at follow-up. Moreover, it should always be kept in mind that sudden death may occur whenever one is dealing with diffuse aortic diseases. Unlike with the cET, the main drawback of the FET is the increased risk of paraplegia, which ranges from 0 to 21.7% in the literature [44]. Likewise, in the multicenter study of Leontyev et al., the authors reported an overall rate of paraparesis and paraplegia of 7.5% with similar rates among different aortic pathologies: 6.5% in type A-AAD, 10% in type B-AAD, 10.9% in chronic type A aortic dissection, and 7.6% in thoracic aortic aneurysm [45]. A distal landing zone of T10 or more was identified as the only independent predictor for the occurrence of SCI [45].
Both the cET and the FET techniques are complex operations; however, the refinement of organ protection together with new technologies leads to a simplifying and increasing use of these surgical techniques. Only future research accompanied by longer follow-up data will clarify the actual benefit of the “new” over the “old” trunk procedure.
Conclusions
The cET and FET procedures represent the most significant technical improvements to the treatment of complex aortic arch pathologies in the last 40 years. The FET allows the durable exclusion of the proximal segment of an otherwise extensive thoracic/ thoracoabdominal aortic disease; furthermore, the stent graft in the mid-descending aortic segment facilitates a second open thoracoabdominal approach. Recent technical advances have given us the opportunity to reduce the surgical invasiveness. Facilitated graft implantation along with further improvement in the stented portion of the FET-hybrid prosthesis will probably be used more widely.
Author contribution
Dr. LDM and Dr. CM contributed equally to the manuscript. All of the authors read, reviewed, and approved the manuscript.
Data availability (data transparency)
Not applicable.
Code availability (software application or custom code)
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Statement of authorship: “These authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.”
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borst HG, Walterbusch G, Schaps D. Extensive aortic replacement using “elephant trunk” prosthesis. Thorac Cardiovasc Surg. 1983;31(1):37–40. doi: 10.1055/s-2007-1020290. [DOI] [PubMed] [Google Scholar]
- 2.Karck M, Chavan A, Hagl C, Friedrich H, Galanski M, Haverich A. The frozen elephant trunk technique: a new treatment for thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2003;125(6):1550–1553. doi: 10.1016/s0022-5223(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 3.Di Marco L, Pacini D, Murana G, Mariani C, Amodio C, Di Bartolomeo R. Total aortic arch replacement with frozen elephant trunk (Thoraflex). Ann Cardiothorac Surg. 2018;7(3). 10.21037/acs.2018.04.05 [DOI] [PMC free article] [PubMed]
- 4.Di Bartolomeo R, Pellicciari G, Cefarelli M, Di Eusanio M. Frozen elephant trunk surgery using the E-vita open plus prosthesis. Ann Cardiothorac Surg. 2013 doi: 10.3978/j.issn.2225-319X.2013.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariani C, Murana G, Leone A, Di Marco L, Pacini D. Frozen elephant trunk in aortic arch disease: different devices for different pathologies. Medicina (Kaunas). 2021;57(10). 10.3390/medicina57101090 [DOI] [PMC free article] [PubMed]
- 6.Di Marco L, Pantaleo A, Leone A, Murana G, Di Bartolomeo R, Pacini D. The frozen elephant trunk technique: European Association for Cardio-Thoracic Surgery position and Bologna experience. Korean J Thorac Cardiovasc Surg. 2017;50(1):1–7. doi: 10.5090/kjtcs.2017.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun L, Qi R, Zhu J, Liu Y, Zheng J. Total arch replacement combined with stented elephant trunk implantation: a new standard therapy for type a dissection involving repair of the aortic arch? Circulation. 2011;123(9):971–978. doi: 10.1161/CIRCULATIONAHA.110.015081. [DOI] [PubMed] [Google Scholar]
- 8.Ma W-G, Zhu J-M, Zheng J, et al. Sun’s procedure for complex aortic arch repair: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg. 2013;2(5):642–648. doi: 10.3978/j.issn.2225-319X.2013.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai ND, Pochettino A. Distal aortic remodeling using endovascular repair in acute DeBakey I aortic dissection. Semin Thorac Cardiovasc Surg. 2009;21(4):387–392. doi: 10.1053/j.semtcvs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Roselli EE, Rafael A, Soltesz EG, Canale L, Lytle BW. Simplified frozen elephant trunk repair for acute DeBakey type I dissection. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S197–201. doi: 10.1016/j.jtcvs.2012.11.068. [DOI] [PubMed] [Google Scholar]
- 11.Vallabhajosyula P, Szeto WY, Pulsipher A, et al. Antegrade thoracic stent grafting during repair of acute Debakey type I dissection promotes distal aortic remodeling and reduces late open distal reoperation rate. J Thorac Cardiovasc Surg. 2014;147(3):942–948. doi: 10.1016/j.jtcvs.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 12.Hata M, Akiyama K, Orime Y, Wakui S, Nakamura T, Shiono M. Less invasive quick open stenting using a J graft open stent for distal arch aneurysms. Thorac Cardiovasc Surg. 2016;64(4):330–332. doi: 10.1055/s-0035-1546299. [DOI] [PubMed] [Google Scholar]
- 13.Jakob H, Idhrees M, Bashir M. From E-VITA open plus to E-VITA NEO and E-NOVIA. J Card Surg. 2021;36(5):1814–1817. doi: 10.1111/jocs.15044. [DOI] [PubMed] [Google Scholar]
- 14.Shrestha M, Bachet J, Bavaria J, et al. Current status and recommendations for use of the frozen elephant trunk technique: a position paper by the Vascular Domain of EACTS. Eur J Cardio-thoracic Surg. 2015;47(5):759–769. doi: 10.1093/ejcts/ezv085. [DOI] [PubMed] [Google Scholar]
- 15.Czerny M, Schmidli J, Adler S, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery. (ES Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2019;55(1):133–62. 10.1093/ejcts/ezy313 [DOI] [PubMed]
- 16.Ma W-G, Zheng J, Sun L-Z, Elefteriades JA. Open stented grafts for frozen elephant trunk technique: technical aspects and current outcomes. Aorta (Stamford, Conn). 2015;3(4):122–135. doi: 10.12945/j.aorta.2015.14.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leontyev S, Borger MA, Etz CD, et al. Experience with the conventional and frozen elephant trunk techniques: a single-centre study. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2013;44(6):1076–1082. doi: 10.1093/ejcts/ezt252. [DOI] [PubMed] [Google Scholar]
- 18.Shrestha M, Martens A, Kaufeld T, et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years. Eur J Cardio-thoracic Surg. 2017;52(5):858–866. doi: 10.1093/ejcts/ezx218. [DOI] [PubMed] [Google Scholar]
- 19.Haldenwang PL, Häuser L, Prochnow N, et al. Low-flow lower body perfusion for spinal protection in a frozen elephant trunk simulation model. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2016;50(5):963–970. doi: 10.1093/ejcts/ezw146. [DOI] [PubMed] [Google Scholar]
- 20.Halstead JC, Meier M, Etz C, et al. The fate of the distal aorta after repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2007;133(1):127–135. doi: 10.1016/j.jtcvs.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 21.Rylski B, Hahn N, Beyersdorf F, et al. Fate of the dissected aortic arch after ascending replacement in type A aortic dissection†. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2017;51(6):1127–1134. doi: 10.1093/ejcts/ezx062. [DOI] [PubMed] [Google Scholar]
- 22.Berger T, Kreibich M, Morlock J, et al. True-lumen and false-lumen diameter changes in the downstream aorta after frozen elephant trunk implantation. Eur J Cardio-thoracic Surg. 2018;54(2):375–381. doi: 10.1093/ejcts/ezy031. [DOI] [PubMed] [Google Scholar]
- 23.Di Bartolomeo R, Leone A, Di Marco L, Pacini D. When and how to replace the aortic arch for type A dissection. Ann Cardiothorac Surg. 2016;5(4):383–388. doi: 10.21037/acs.2016.07.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsagakis K, Jánosi RA, Frey UH, et al. True lumen stabilization to overcome malperfusion in acute type I aortic dissection. Semin Thorac Cardiovasc Surg. 2019:1–9. 10.1053/j.semtcvs.2018.11.012 [DOI] [PubMed]
- 25.Mariani C, Botta L, Leone A, et al. Visceral malperfusion after Frozen Elephant Trunk in chronic aortic dissection: post-operative predictors and outcomes. Int J Cardiol. 2021;335:26–31. doi: 10.1016/j.ijcard.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Kreibich M, Berger T, Morlock J, et al. The frozen elephant trunk technique for the treatment of acute complicated type B aortic dissection. Eur J Cardio-thoracic Surg. 2018;53(3):525–530. doi: 10.1093/ejcts/ezx281. [DOI] [PubMed] [Google Scholar]
- 27.Weiss G, Tsagakis K, Jakob H, et al. The frozen elephant trunk technique for the treatment of complicated type B aortic dissection with involvement of the aortic arch: multicentre early experience. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2015;47(1):106–114. doi: 10.1093/ejcts/ezu067. [DOI] [PubMed] [Google Scholar]
- 28.Di Eusanio M, Borger M, Petridis FD, et al. Conventional versus frozen elephant trunk surgery for extensive disease of the thoracic aorta. J Cardiovasc Med (Hagerstown) 2014;15(11):803–809. doi: 10.2459/JCM.0b013e328364559c. [DOI] [PubMed] [Google Scholar]
- 29.Inoue Y, Matsuda H, Omura A, et al. Comparative study of the frozen elephant trunk and classical elephant trunk techniques to supplement total arch replacement for acute type A aortic dissection†. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2019;56(3):579–586. doi: 10.1093/ejcts/ezz104. [DOI] [PubMed] [Google Scholar]
- 30.Hage A, Hage F, Dagenais F, et al. Frozen elephant trunk for aortic arch reconstruction is associated with reduced mortality as compared to conventional techniques. Semin Thorac Cardiovasc Surg. 2021 doi: 10.1053/j.semtcvs.2021.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Svensson LG, Rushing GD, Valenzuela ES, et al. Modifications, classification, and outcomes of elephant-trunk procedures. Ann Thorac Surg. 2013;96(2):548–558. doi: 10.1016/j.athoracsur.2013.03.082. [DOI] [PubMed] [Google Scholar]
- 32.Idrees JJ, Roselli EE, Wojnarski CM, et al. Prophylactic stage 1 elephant trunk for moderately dilated descending aorta in patients with predominantly proximal disease. J Thorac Cardiovasc Surg. 2015;150(5):1150–1155. doi: 10.1016/j.jtcvs.2015.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castrovinci S, Murana G, De Maat GE, et al. The classic elephant trunk technique for staged thoracic and thoracoabdominal aortic repair: long-term results. J Thorac Cardiovasc Surg. 2015;149(2):416–422. doi: 10.1016/j.jtcvs.2014.09.078. [DOI] [PubMed] [Google Scholar]
- 34.Shrestha M, Beckmann E, Krueger H, et al. The elephant trunk is freezing: the Hannover experience. J Thorac Cardiovasc Surg. 2015;149(5):1286–1293. doi: 10.1016/j.jtcvs.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 35.Shrestha M, Martens A, Krüger H, et al. Total aortic arch replacement with the elephant trunk technique: single-centre 30-year results. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2014;45(2):286–289. doi: 10.1093/ejcts/ezt359. [DOI] [PubMed] [Google Scholar]
- 36.Tsagakis K, Pacini D, Grabenwöger M, et al. Results of frozen elephant trunk from the international E-vita Open registry. Ann Cardiothorac Surg. 2020;9(3):178–188. doi: 10.21037/acs-2020-fet-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shrestha M, Martens A, Kaufeld T, et al. Single-centre experience with the frozen elephant trunk technique in 251 patients over 15 years. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2017;52(5):858–866. doi: 10.1093/ejcts/ezx218. [DOI] [PubMed] [Google Scholar]
- 38.Di Eusanio M, Pantaleo A, Murana G, et al. Frozen elephant trunk surgery-the Bologna’s experience. Ann Cardiothorac Surg. 2013;2(5):597–605. doi: 10.3978/j.issn.2225-319X.2013.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsagakis K, Pacini D, Di Bartolomeo R, et al. Multicenter early experience with extended aortic repair in acute aortic dissection: is simultaneous descending stent grafting justified? J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S116–20. doi: 10.1016/j.jtcvs.2010.07.066. [DOI] [PubMed] [Google Scholar]
- 40.Dohle DS, Tsagakis K, Janosi RA, et al. Aortic remodelling in aortic dissection after frozen elephant trunk. Eur J Cardio-thoracic Surg. 2016 doi: 10.1093/ejcts/ezv045. [DOI] [PubMed] [Google Scholar]
- 41.Shrestha M, Kaufeld T, Beckmann E, et al. Total aortic arch replacement with a novel 4-branched frozen elephant trunk prosthesis: single-center results of the first 100 patients. J Thorac Cardiovasc Surg. 2016;152(1):148–159.e1. doi: 10.1016/j.jtcvs.2016.02.077. [DOI] [PubMed] [Google Scholar]
- 42.Tian DH, Wan B, Di Eusanio M, Black D, Yan TD. A systematic review and meta-analysis on the safety and efficacy of the frozen elephant trunk technique in aortic arch surgery. Ann Cardiothorac Surg. 2013;2(5):581–591. doi: 10.3978/j.issn.2225-319X.2013.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Bartolomeo R, Murana G, DiMarco L, et al. Frozen versus conventional elephant trunk technique: application in clinical practice. Eur J Cardio-thoracic Surg. 2017;51:i20–i28. doi: 10.1093/ejcts/ezw335. [DOI] [PubMed] [Google Scholar]
- 44.Preventza O, Liao JL, Olive JK, et al. Neurologic complications after the frozen elephant trunk procedure: a meta-analysis of more than 3000 patients. J Thorac Cardiovasc Surg. 2020;160(1):20–33.e4. doi: 10.1016/j.jtcvs.2019.10.031. [DOI] [PubMed] [Google Scholar]
- 45.Leontyev S, Tsagakis K, Pacini D, et al. Impact of clinical factors and surgical techniques on early outcome of patients treated with frozen elephant trunk technique by using EVITA open stent-graft: results of a multicentre study. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg. 2016;49(2):660–666. doi: 10.1093/ejcts/ezv150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.


