Abstract
Despite emotion regulation being altered in patients with obsessive-compulsive disorder (OCD), no studies have investigated its relation to multimodal amygdala connectivity. We compared cortico-limbic functional and structural connectivity between OCD patients and healthy controls (HC), and correlated this with the dispositional use of emotion regulation strategies and with OCD severity.
OCD patients (n=73) and HC (n=42) were assessed for suppression and reappraisal strategies using the Emotion Regulation Questionnaire (ERQ) and for OCD severity using the Y-BOCS. Resting-state functional magnetic resonance imaging (rs-fMRI) connectivity maps were generated using subject-specific left (LA) and right amygdala (RA) masks. We identified between-group differences in amygdala whole-brain connectivity, and evaluated the moderating effect of ERQ strategies. Significant regions and amygdala seeds were used as targets in probabilistic tractography analysis.
Patients scored higher in suppression and lower in reappraisal. We observed higher rs-fMRI RA – right post-central gyrus (PCG) connectivity in HC, and in patients this was correlated with symptom severity. Reappraisal scores were associated with higher negative LA – left insula connectivity in HC, and suppression scores were negatively associated with LA – precuneus and angular gyri connectivity in OCD. Structurally, patients showed higher mean diffusivity in tracts connecting the amygdala with the other targets.
RA – PCG connectivity is diminished in patients, while disrupted emotion regulation is related to altered amygdala connectivity with the insula and posterior brain regions. Our results are the first showing, from a multimodal perspective, the association between amygdala connectivity and specific emotional processing domains, emphasizing the importance of amygdala connectivity in OCD pathophysiology.
Introduction
Obsessive-compulsive disorder (OCD) is a chronic and disabling condition that affects 2–3% of the population (Ruscio et al. 2010), characterized by the presence of obsessions (i.e., anxiety-evoking intrusive thoughts, ideas or images or pathological urges) and compulsions, which are ritualistic, repetitive behaviours or mental acts carried out to alleviate anxiety. OCD is seen as being strongly linked to cortico-striatal circuitry abnormalities (Menzies et al. 2008), an idea that has received empirical support from functional and structural connectivity studies (Harrison et al. 2009; Fontenelle et al. 2011). However, the cortico-striatal model fails to account for some of the symptoms characterizing OCD patients (e.g., increased anxiety (Menzies et al. 2008)). Accordingly, more recent neurobiological models of the disorder place greater emphasis on brain regions outside the cortico-striatal loops that may be critically involved in the pathophysiology of OCD, such as limbic cortico-amygdalar circuitry (Milad and Rauch 2012).
The amygdala has a role in mediating fear and anxiety processes (LeDoux 2000). Its function has been well characterized in relation to trait and state anxiety (Baur et al. 2013), as well as in relation to anxiety disorders (Roy et al. 2013; Hahn et al. 2011). The amygdala is also a key neural component underlying emotion regulation processes (Banks et al. 2007; Lee et al. 2012) and strategies (Goldin, McRae, et al. 2009). For instance, insulo-amygdalar functional connectivity has been shown to be associated with the habitual use of cognitive reappraisal strategies (which consist of modifying the initial appraisal of a situation to alter its emotional significance, and which are considered to be adaptive), while connectivity between the amygdala and the supplementary motor area has been shown to be related with both cognitive reappraisal and expressive suppression (which consists of inhibiting ongoing emotion-expressive behaviour and which is considered to be maladaptive) (Picó-Pérez et al. 2017).
There is considerable evidence indicating that the amygdala is hyperactive in OCD patients when processing emotional information. This appears to be the case both with OCD-specific (Simon et al. 2010; Breiter et al. 1996; Mataix-Cols et al. 2004) and generic stimuli, such as emotional faces (Cardoner et al. 2011; Via et al. 2014). Moreover, studies have identified a general pattern of fronto-subcortical (including fronto-amygdalar) hyper-reactivity while anticipating and perceiving emotional stimuli; a pattern that is shared by OCD and social anxiety disorder (SAD) patients (Weidt et al. 2016). Likewise, OCD patients show diminished fronto-amygdala connectivity during emotion regulation (de Wit et al. 2015), although, by contrast, increased functional connectivity between the amygdala and dorso-lateral prefrontal cortex (dlPFC) has been shown to account for impaired working memory performance in OCD (de Vries et al. 2014). Overall, these findings have been interpreted as suggesting that greater amygdala activation in response to emotional stimuli in OCD may interfere with information processing in cognitive control networks.
Regarding white matter (WM) integrity, alterations in the uncinate fasciculus (a tract that connects the amygdala and the insula) have been reported in different studies with OCD samples, showing decreased fractional anisotropy (FA) (Admon et al. 2012; Benedetti et al. 2013) and increased mean (MD) and radial diffusivity (RD) in adult patients (Benedetti et al. 2013; Jayarajan et al. 2012), but increased FA in adolescent patients (Zarei et al. 2011). Moreover, differences in the inferior fronto-occipital fasciculus (IFOF), a tract that also passes through the amygdala, have been consistently found. In adult OCD patients decreased FA and increased MD have been reported (Benedetti et al. 2013; Garibotto et al. 2010), while in adolescent samples RD (Jayarajan et al. 2012) and FA increases (Gruner et al. 2012; Zarei et al. 2011) have been found. These changes in neural diffusion reflect neuronal impairment that likely contributes to structural connectivity alterations.
Despite the existence of both functional and structural amygdala connectivity alterations in OCD, and that the potential association between these may be informative about the nature of amygdala alterations in OCD, only two studies have investigated the link between these two measurements. Admon et al. (2012), using a gambling task, showed a deficit in fronto-limbic connectivity both at the functional and structural level, which was also associated with OCD symptom severity. On the other hand, Rus et al. (2017) used a negative affect task and found an increased functional connectivity between the amygdala and parieto-occipital regions, which was related to the structural connectivity estimates between these regions, which in turn were negatively associated with OCD symptom severity. To date, however, no studies have investigated multimodal connectivity alterations in OCD in relation with emotion regulation, although, as stated above, this may be underpinned by amygdala networks (Ochsner, Silvers, and Buhle 2012) and patients with OCD have shown decreased emotion regulation abilities, with difficulties in engaging in cognitive reappraisal strategies (Goldberg et al. 2016).
In this study, we aimed to determine whether there are differences in the resting-state functional magnetic resonance imaging (rs-fMRI) connectivity of the left (LA) and right amygdala (RA) in OCD patients versus healthy controls (HC). We also aimed to identify the extent to which abnormal amygdalar resting-state connectivity is associated with disruptions in WM fibre bundles, using diffusion-tension imaging (DTI) estimates. Given evidence that changes in the structural connectivity of the brain correspond to alterations in resting-state connectivity (van den Heuvel et al. 2009; Hermundstad et al. 2013), we hypothesized that functional connectivity differences would be related to alterations in the underlying WM microstructure. Finally, we also investigated the potential association of both functional and structural connectivity estimates with OCD symptom severity, as well as with anticipated group differences in the dispositional use of cognitive reappraisal and expressive suppression strategies, as assessed using the Emotion Regulation Questionnaire (ERQ).
Materials and Methods
Participants
134 subjects (86 OCD patients and 48 HC) participated in the study, of which 19 were excluded for different reasons, including excessive motion (2 patients and 2 HC) or preprocessing artifacts in functional data (4 patients and 3 HC), or problems with the DTI sequence acquisition (7 patients and 1 HC). The final sample was made up of 73 OCD patients (30 females, mean age ± SD = 37.74 ± 10.19 years) and 42 HC (20 females, mean age ± SD = 39.43 ± 9.79 years) (see Table 1 for further details). Details on inclusion and exclusion criteria are shown in the Supplementary Material.
Table 1.
Demographic and clinical characteristics of the sample.
| OCD (n=73), Mean (SD) | HC (n=42), Mean (SD) | Statisticc | p-value | |
|---|---|---|---|---|
| Age | 37.74 (10.19) | 39.43 (9.79) | -0.86 | .38 |
| Gender (male/female) | 43/30 | 22/20 | 0.462 | .56 |
| Handedness (R/L) a | 59/4 | 32/5 | 1.46 | .28 |
| ERQ Reappraisal b | 3.6 (1.49) | 4.76 (1.07) | -4.79 | <.0005 |
| ERQ Suppression b | 3.78 (1.58) | 2.96 (1.2) | 3.1 | <.005 |
| HDRS | 10.27 (5.65) | 2.04 (2.2) | 10.35 | <.0005 |
| HARS | 12.41 (7.16) | 2.56 (2.51) | 10.06 | <.0005 |
| Age at onset of OCD | 20.9 (8.43) | - | - | - |
| Y-BOCS Total | 22.14 (6.35) | - | - | - |
| Pharmacological treatment | N (%) | |||
| Medication free | 6 (8.21) | - | - | - |
| SSRIs | 24 (32.87) | - | - | - |
| Fluoxetine, 20-80 mg/d | 13 (17.8) | - | - | - |
| Escitalopram, 10-20 mg/d | 5 (6.84) | - | - | - |
| Sertraline, 200 mg/d | 3 (4.1) | - | - | - |
| Fluvoxamine, 200-300 mg/d | 2 (2.73) | - | - | - |
| Paroxetine, 40 mg/d | 2 (2.73) | - | - | - |
| Clomipramine, 75-300 mg/d | 8 (10.95) | - | - | - |
| SSRIs with Clomipramine | 12 (16.43) | - | - | - |
| SSRIs combinations | 1 (1.36) | - | - | - |
| Antipsychotic augmentations | 22 (30.13) | - | - | - |
| Comorbidities | N (%) | - | - | - |
| No | 45 (61.6) | - | - | - |
| Major Depressive Disorder | 10 (13.7) | - | - | - |
| General Anxiety Disorder | 3 (4.1) | - | - | - |
| Eating Disorder | 3 (4.1) | - | - | - |
| Tics | 3 (4.1) | - | - | - |
| Panic Disorder | 2 (2.7) | - | - | - |
| Dysthymia | 2 (2.7) | - | - | - |
| OCD Personality Disorder | 2 (2.7) | - | - | - |
| ADHD | 1 (1.4) | - | - | - |
| Agoraphobia | 1 (1.4) | - | - | - |
| Gambling Disorder | 1 (1.4) | - | - | - |
HC healthy controls, OCD obsessive-compulsive disorder patients, SD Standard Deviation, ERQ Emotion Regulation Questionnaire, HDRS Hamilton Depression Rating Scale, HARS Hamilton Anxiety Rating Scale, Y-BOCS Yale-Brown Obsessive-Compulsive Scale, ADHD Attention Deficit Hyperactivity Disorder.
Handedness had n=63 in the OCD group and n=37 in the HC group, the HDRS and the HARS n=25 in the HC group, and age at onset of OCD n=72.
ERQ scores were standardized by means of dividing the mean score of each subscale by the corresponding number of items (6 for reappraisal and 4 for suppression).
Student’s t-test for quantitative variables and χ² test for qualitative ones.
Behavioural assessment
Subjects were administered the Spanish version of the ERQ (Cabello et al. 2013) on the same day of the scan, which comprises the subscales of expressive suppression and cognitive reappraisal (Gross and John 2003). Further information on this scale can be found in the Supplementary Material. In addition, depressive symptoms were evaluated both in patients and controls by means of the 17-item Hamilton Depression Rating Scale (HDRS) (Hamilton 1960), and anxiety symptoms with the 14-item Hamilton Anxiety Rating Scale (HARS) (Hamilton 1959). Finally, in the patient group, global severity of OCD symptoms was assessed by means of the clinician-administered version of the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al. 1989).
Image acquisition and preprocessing
Image acquisition parameters are presented in the Supplementary Material. RS-fMRI BOLD data were preprocessed using afni_proc.py in AFNI v16.1.04 (Cox 1996) (http://afni.nimh.nih.gov/), after discarding the first 4 volumes to allow for stabilization of tissue magnetization. Diffusion-weighed imaging (DWI) data were visually inspected for motion and dropout slices, with individual volumes discarded (subjects were excluded when <14 volumes remained), and TORTOISE V2.5.2 (Pierpaoli et al. 2010) and the fat_proc* programs in AFNI were subsequently used for the preprocessing. Information regarding the specific preprocessing steps followed can be found in the Supplementary Material.
Resting-state data analyses
Seed extraction and first-level analyses
Subject-specific left (LA) and right amygdala (RA) masks were extracted from T1w anatomical images using the segmentation algorithm from FreeSurfer. These masks were subsequently mapped to the MNI152 template space and the voxel average time series of the preprocessed, resting-state BOLD time-series was calculated for each. Whole-brain connectivity maps were generated for each subject by correlating voxel-wise time-series with the mean signal inside each amygdala mask. To better approximate distributional properties upon which subsequent statistical tests are based, the Fisher-Z transform was subsequently applied to the correlation coefficients; however, to aid interpretability, all connectivity estimates are reported as Pearson’s correlation coefficients in this paper.
Second-level analyses and thresholding
To illustrate the regions functionally connected with the amygdala seeds, we computed group-specific one-sample t-tests using statistical parametric mapping software (SPM12 v6685) (http://www.fil.ion.ucl.ac.uk/spm/), adjusting for subject level estimates of motion. Whole-brain two-sample t-tests were conducted to compare the connectivity maps between HC and OCD groups. ERQ subscales were also included as covariates of interest, in interaction with group, to examine their potential relationship with amygdala connectivity.
Data were analysed at the whole-brain level, and the SPM cluster thresholding correction was used, requiring an uncorrected p-voxel of 0.001, and a family-wise error (FWE) corrected p-cluster of 0.05. Eigenvariates from regions with between-group connectivity differences were extracted and entered into an SPSS v21 (IBM Corporation, Armonk, NY) data matrix to assess correlations between connectivity and the severity of OCD symptoms. Pearson correlations were used, and assumptions of normality and homoscedascity were met in all analyses, unless otherwise noted.
DTI analyses
Target placement
Regions for which significant between-group differences in amygdalar functional connectivity were observed, or for which connectivity was associated with ERQ scores, were selected as targets together with amygdala seeds for the subsequent tractography analysis. Details about this placement are presented in the Supplementary Material.
Probabilistic tractography
WM regions of interest (referred to hereafter as WM-ROIs) connecting pairs of resting-state targets were identified using probabilistic tractography, as implemented in AFNI’s FATCAT utility toolkit (Taylor and Saad 2013). FATCAT efficiently finds connections within networks and provides quantitative measures for all identified WM-ROIs. DTI parameter (FA and first eigenvector) uncertainty maps for probabilistic tracking were calculated with FATCAT 3dDWUncert using 50 iterations (Taylor and Saad 2013). Parameters for probabilistic tracking were 5 seed points per voxel, 1000 Monte Carlo iterations, and a threshold fraction of 0.001, and, at each iteration, the algorithm found locations of tracts connecting pairs of targets. Standard propagation parameters for this algorithm were used: 60° maximum angle of propagation confined to voxels with FA>0.2.
Here we report FA and MD for each WM-ROI, as calculated by FATCAT, as well as the volume of the tracts. Tract volume was standardized with reference to the whole-brain volume (fractional volume of tract (fNV)). Only those WM-ROIs found between the same targets in >85% of subjects were selected for further analysis.
Results
Behavioral results
The mean ERQ sub-scores are presented in Table 1. OCD patients scored significantly higher than HC in suppression (t = 3.10, df = 104.20, p < .005), while HC scored significantly higher in reappraisal (t = −4.79, df = 107.36, p < .0005). Also, OCD patients scored significantly higher than HC both in the HDRS (t = 10.35, df = 94.3, p < .0005) and the HARS (t = 10.06, df = 95.88, p < .0005). HDRS and HARS scores were not, however, significantly associated with the ERQ.
Resting-state functional connectivity results
One-sample functional connectivity patterns
Similar whole-brain patterns of functional connectivity were evident for LA and RA seeds, as illustrated in Figure 1 in the Supplementary Material.
Fig. 1.
Placement of DTI targets based on resting-state functional connectivity results (in MNI space). LA and RA are shown in blue, and the rest of the targets in red. L=Left, R=Right.
Functional connectivity differences
Between-group connectivity comparisons revealed higher connectivity in HC between RA and the right post-central gyrus (PCG) (Table 2, Figure 2). Moreover, the connectivity between these two regions was significantly correlated with Y-BOCS scores in the patient group (r=.305, p=.009).
Table 2.
Resting-state functional connectivity results.
| Seed | ERQ scale | Contrast | Region | MNI coordinates (x, y, z) | Kea | t-statistic |
|---|---|---|---|---|---|---|
| RA | - | HC > OCD | Right post-central gyrus | 39, −27, 57 | 51 | 4.57 |
| LA | Reappraisal | HC < OCD | Left posterior insula | -45, −6, 9 | 90 | 5.77 |
| Precuneus | 9, −66, 39 | 400 | 5.16 | |||
| LA | Suppression | OCD - | Right angular gyrus | 30, -63, 57 | 123 | 4.65 |
| Left angular gyrus | -30, -69, 33 | 52 | 4.19 |
Regions showing between-group differences in amygdala connectivity, or in its association with ERQ scores (p < .05 FWE-cluster corrected).
LA left amygdala, RA right amygdala, HC healthy controls, OCD obsessive-compulsive disorder patients, ERQ Emotion Regulation Questionnaire, MNI Montreal Neurological Institute.
Fig. 2.
Between-group functional connectivity differences. RA – right PCG connectivity was significantly higher in HC than in OCD patients, and connectivity values were positively correlated with Y-BOCS scores in the OCD group.
Functional connectivity associations with the ERQ
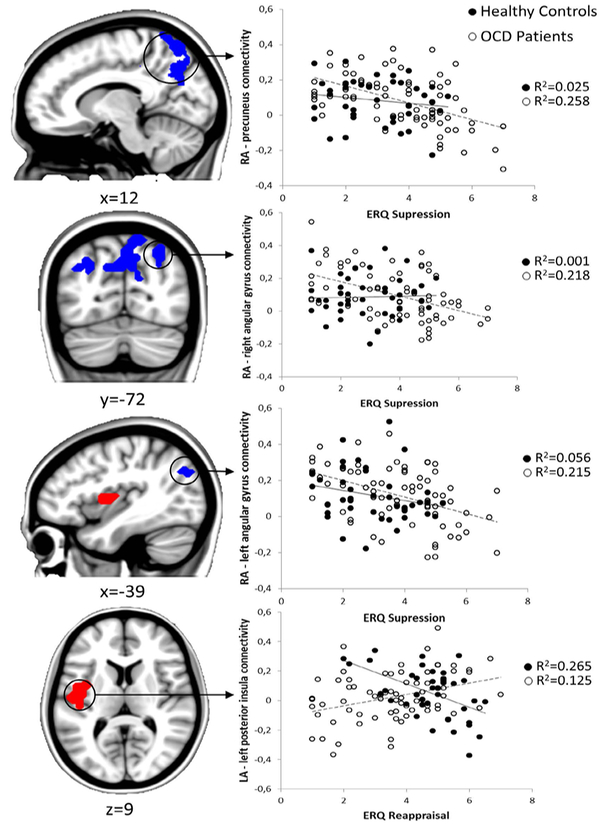
A group interaction effect was found in the association between reappraisal use and LA-left posterior insula connectivity, primarily due to a negative association between reappraisal and functional connectivity between these two regions in HCs. On the other hand, there was a negative association between suppression and functional connectivity between the LA and the precuneus and the bilateral angular gyri in the OCD group (Table 2, Figure 3).
Fig. 3.
Functional connectivity associations with the ERQ. Left: Regions showing an association with cognitive reappraisal (left posterior insula) are shown in red, while regions showing an association with suppression (precuneus and bilateral angular gyri) are shown in blue. Right: Scatter plots depicting the relationship between functional connectivity estimates and corresponding ERQ scores for both groups.
Structural connectivity results
Distribution of tracts
The following WM-ROIs connecting the amygdala and the resting-state clusters arising from the above analyses were identified in at least 85% of participants: (1) the left uncinate fasciculus, connecting LA and left insula targets; (2) the left IFOF, connecting LA and left angular gyrus targets; (3) the right IFOF, connecting RA and right angular gyrus targets; and (4) the right cortico-spinal tract, connecting RA with the right PCG. An example of the WM-ROIs from a representative subject can be found in Figure 4.
Fig. 4.
Example of the WM-ROIs consistently found between the probabilistic tractography targets from a representative subject (in subject DW space). The left uncinate fasciculus is shown in red, the left IFOF in purple, the right IFOF in light blue, and the right cortico-spinal tract in green. L=Left, R=Right.
Differences in DTI parameters
A multivariate analysis comparing FA, MD and fNV between HC and OCD patients was performed for each of the WM-ROIs, including age and sex as covariates. Groups significantly differed in the left uncinate fasciculus (OCD patients N = 69, HC N = 42, F(3, 105) = 3.26, p = .024), the right IFOF (OCD patients N = 67, HC N = 38, F(3, 99) = 3.78, p = .013) and the right cortico-spinal tract (OCD patients N = 66, HC N = 36, F(3, 96) = 3.3, p = .023), with a trend towards a significant difference observed for the left IFOF as well (OCD patients N = 69, HC N = 39, F(3, 102) = 2.5, p = .064). According to post-hoc tests, group differences were driven in all tracts by higher MD in the OCD group. Moreover, in patients, lower FA was found in the left uncinate fasciculus, and higher fNV in the right cortico-spinal tract (Table 3).
Table 3.
Structural connectivity differences.
| WM-ROI | DTI parameter | F-statistic | p-value | Direction of findings |
|---|---|---|---|---|
| FA | 4.09 | .046 | OCD < HC | |
| Left uncinate fasciculus | MD | 7.61 | .007 | OCD > HC |
| fNV | 0.14 | .703 | - | |
| FA | 0.79 | .374 | - | |
| Right IFOF | MD | 10.86 | .001 | OCD > HC |
| fNV | 0.04 | .829 | - | |
| FA | 0.04 | .834 | - | |
| Right cortico-spinal tract | MD | 6.75 | .011 | OCD > HC |
| fNV | 4.71 | .032 | OCD > HC |
Regions showing a between-group difference in the DTI parameters of the WM-ROIs, according to post-hoc analysis.
WM-ROI white-matter region of interest, DTI diffusion tensor image, IFOF inferior fronto-occipital fasciculus, FA fractional anisotropy, MD mean diffusivity, fNV fractional volume of tracts, HC healthy controls, OCD obsessive-compulsive disorder patients.
Associations between DTI parameters and the ERQ
No associations were observed between any of the DTI parameters and reappraisal or suppression scores, both within and across groups.
Associations with pharmacological, clinical and sociodemographic data
Use of medication did not influence the abovementioned results. Likewise, we did not observe any association between functional connectivity results and depression or anxiety scores (HDRS and HARS, respectively). Conversely, DTI estimates from the left uncinate fasciculus were correlated with depression and anxiety scores. Finally, greater age was significantly associated with lower FA across the entire sample as well as within each group for all WM-ROIs (further details about these analyses are presented in Supplementary Material).
Discussion
Our study showed a reduced functional connectivity between the RA and the right PCG in OCD patients, and the connectivity between these regions was found to correlate positively with symptom severity. Moreover, at the structural level, OCD patients displayed higher MD and fNV in the right cortico-spinal tract connecting these regions. Regarding correlations with ERQ scores, a group interaction effect was found in the association between reappraisal use and LA-left posterior insula connectivity, primarily due to a negative association between reappraisal use and functional connectivity between these regions in the HC group. On the other hand, we also observed a negative association between suppression scores and functional connectivity of the LA with the precuneus and bilateral angular gyri specifically in the OCD group. Finally, although we did not find associations between ERQ and DTI parameters within the tracts connecting these regions, we did observe between-group differences in MD and FA involving the left uncinate fasciculus and the right IFOF.
The reduced functional connectivity observed in OCD patients between the RA and the right PCG can reflect altered emotional processing. The PCG forms part of the somatosensory cortex, which serves to integrate somatosensory information with emotional input from the amygdala and, together with the amygdala and right visual cortices, has been found to be important for linking perception of emotional stimuli to motivation (Adolphs et al. 2000; Adolphs 2001). The right somatosensory cortex, for instance, has been shown to be active during the processing of incongruent somatosensory-emotional information (i.e., incongruent facial and voice expressions) (Klasen et al. 2011). Indeed, previous research in OCD patients has shown decreased functional connectivity in networks comprising the post-central gyrus (Moreira et al. 2017; Gürsel et al. 2018). In addition, the altered functional connectivity between the somatosensory cortex and the amygdala seems to be underpinned by an abnormal pattern of structural connectivity between these regions, since we observed increased MD in the right cortico-spinal tract, in agreement with previous reports in other OCD samples (Fontenelle et al. 2011). This finding, together with the greater volume of this tract observed in OCD, indicates that patients may have relatively more crossing fibers between the RA and the PCG, and, therefore, show less efficient connectivity between the regions. Anyhow, although functional connectivity between the RA and the right somatosensory cortex was decreased in OCD, such decreased connectivity correlated significantly with symptom severity, suggesting the presence of a compensatory mechanism aimed at ameliorating disrupted emotional processing.
In HCs, negative connectivity between the LA and the insula was associated with increased use of cognitive reappraisal strategies. This result is consistent with a previous study from our group where the anterior insula showed negative connectivity with the amygdala in healthy subjects with increasing use of reappraisal strategies (Picó-Pérez et al. 2017). Neural activity in the insula underlies the conscious representation of emotional bodily states (i.e., interoception) (Craig 2009), and, in the early phases of the emotion response, activity in the insula may promote the selection of the most appropriate reappraisal strategy in front of an aversive scenario via an ‘as-if’ representation of bodily states (Verdejo-Garcia, Clark, and Dunn 2012), which in turn may engage prefronto-parietal regulatory regions to finally dampen amygdala reactivity. Our data suggests that such mechanisms are altered in OCD patients. Moreover, patients showed higher MD and lower FA in the left uncinate fasciculus connecting these regions, which can be interpreted as decreased structural integrity of this tract. Previous studies have also found increased diffusivity (Jayarajan et al. 2012) and decreased FA (Admon et al. 2012) in this tract in patients with OCD. Although we did not find direct associations between ERQ scores and structural data, it may be speculated that structural integrity of the uncinatus is necessary to develop adaptive emotional responses in response to anxiety-invoking scenarios.
OCD patients also showed a negative association between the dispositional use of suppression strategies and functional connectivity between the LA and a set of parietal clusters involving the angular gyri and the precuneus. Such negative association between suppression and parieto-limbic connectivity may reflect the regulatory role of the parietal cortex on limbic activity during the deployment of suppression strategies, which were preferentially observed in patients. Indeed, activity in the angular gyri and the precuneus has previously been reported in the use of suppression strategies (Goldin, Manber, et al. 2009; Dörfel et al. 2014; Hayes et al. 2010). Previous studies in OCD samples, however, have shown disrupted functional and structural connectivity between the amygdala and posterior cortical regions (Rus et al. 2017). Therefore, an alternative explanation for our findings is that disrupted parieto-limbic connectivity may be associated with a preferential use of dysfunctional emotional regulation strategies (i.e., suppression). In agreement with these notions, we also observed increased MD in the IFOF of OCD patients, which concurs with other results showing abnormalities in parietal white matter (Kitamura et al. 2006) and decreased FA and increased MD in the bilateral IFOF connecting the amygdala with the parietal cortex in OCD (Benedetti et al. 2013; Garibotto et al. 2010).
Notably, previous research on the neural correlates of emotion regulation has identified significant activations in other (mainly prefrontal) regions (Buhle et al. 2013; Kohn et al. 2014) not reported here. For example, de Wit et al. (2015) showed decreased activity in the left dlPFC and increased activity in the dmPFC of OCD patients during emotion regulation. In this sense, it could be argued that we should have also identified alterations in amygdala connectivity with these other regions, but it is important to bear in mind that those findings stemmed from task-activation studies, in experimental contexts (i.e., during emotion induction), while we used a dispositional/trait measure of emotional regulation.
This research has several strengths. To date, this is the study evaluating the largest OCD dataset with a multimodal neuroimaging approach, and the first study to assess the neurobiological correlates of dispositional emotion regulation strategies in OCD. Combining multiple units of analysis to investigate the neurobiological correlates of brain disorders permits a deeper understanding of the mechanistic effects accounting for disrupted behaviors. Overall, when alterations in functional connectivity are paralleled by structural connectivity disruptions, brain pathologies may be associated with the direct links between pairs of structures. Conversely, if such structural connectivity alterations are not observed, functional network level alterations may be better suspected. Nevertheless, it is also important to note that variables such as developmental stage and spatial proximity might moderate these relationships (Bennett and Rypma 2013). Limitations of the current study should also be acknowledged. This includes the use of a 1.5T scanner, the assessment of a limited number of diffusion directions (N = 25), and the acquisition of non-isotropic voxels. Nevertheless, it is important to mention that previous DTI studies have shown reliability across different magnet strengths (i.e., 1.5T vs. 3T (Teipel et al. 2011; Grech-Sollars et al. 2015)). Moreover, in our study, DTI analyses were secondary in relation to functional assessments, which were performed with standard EPI sequences. Likewise, we observed some associations between the structural connectivity estimates from the left uncinate fasciculus and depressive and anxiety symptoms that may have partially contributed to our pattern of findings. Finally, most patients were on medication. Pharmacological treatment has been suggested to be a significant confounder in functional connectivity studies (Posner et al. 2014), although it is not wholly clear in what direction this might bias our results. In any case, it is important to note that treatment doses in our OCD sample were stable during the three months preceding the fMRI, and we did not find any relationship between our imaging findings and pharmacological treatment.
Conclusion
In conclusion, we have shown that functional and structural connectivity of the amygdala with the somatosensory cortex is disrupted in patients with OCD. While such alterations do not underpin the preferential use of suppression regulation strategies observed in these patients, the neural correlates of disrupted emotion regulation include both altered connectivity with posterior brain regions as well as between the amygdala and the insula. This study is the first taking specific emotional processing domains into account while using a multimodal perspective, and our findings emphasize the importance of integrating the role of amygdala connectivity in OCD pathophysiology.
Supplementary Material
Acknowledgements
Funding for this study was provided by the Instituto de Salud Carlos III (ISCIII) (PI13/01958, PI14/00413, PI16/00889), FEDER funds/European Regional Development Fund (ERDF) - a way to build Europe- and AGAUR (2017 SGR 1247). CIBERSAM is an initiative of the Carlos III Health Institute. MP-P is supported by a FI Grant from the Secretariat for Universities and Research of the Ministry of Business and Knowledge of the Government of Catalonia (2015 FI_B 00839), grant co-funded by the European Social Fund (ESF) “ESF, Investing in your future”. CS-M is supported by a ‘Miguel Servet’ contract from the ISCIII (CPII16/00048). This research was supported, in part, by the NIMH and NINDS Intramural Research Programs of the NIH (P.T.). Computations were performed using facilities provided by the University of Cape Town’s ICTS High Performance Computing team: http://hpc.uct.ac.za.
References
- Admon Roee, Bleich-Cohen Maya, Weizmant Ronit, Poyurovsky Michael, Faragian Sarit, and Hendler Talma. 2012. “Functional and Structural Neural Indices of Risk Aversion in Obsessive-Compulsive Disorder (OCD).” Psychiatry Research - Neuroimaging 203 (2–3): 207–13. 10.1016/j.pscychresns.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, and Damasio R. 2000. “A Role for Somatosensory Cortices in the Visual Recognition of Emotion as Revealed by Three-Dimensional Lesion Mapping.” The Journal of Neuroscience 20 (7): 2683–90. https://doi.org/123123123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs Ralph. 2001. “The Neurobiology of Social Cognition.” Current Opinion in Neurobiology 11: 213–39. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15667456. [DOI] [PubMed] [Google Scholar]
- Banks Sarah J., Eddy Kamryn T., Angstadt Mike, Nathan Pradeep J., and Phan K. Luan. 2007. “Amygdala-Frontal Connectivity during Emotion Regulation.” Social Cognitive and Affective Neuroscience 2 (4): 303–12. 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur Volker, Jürgen Hänggi Nicolas Langer, and Lutz Jäncke. 2013. “Resting-State Functional and Structural Connectivity within an Insula-Amygdala Route Specifically Index State and Trait Anxiety.” Biological Psychiatry 73 (1): 85–92. 10.1016/j.biopsych.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Benedetti Francesco, Giacosa Chiara, Radaelli Daniele, Poletti Sara, Pozzi Elena, Dallaspezia Sara, Falini Andrea, and Smeraldi Enrico. 2013. “Widespread Changes of White Matter Microstructure in Obsessive–compulsive Disorder: Effect of Drug Status.” European Neuropsychopharmacology 23 (7): 581–93. 10.1016/J.EURONEURO.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Breiter Hans C., Rauch Scott L., Kwong Kenneth K., Baker John R., Weisskoff Robert M., Kennedy David N., Kendrick Adair D., et al. 1996. “Functional Magnetic Resonance Imaging of Symptom Provocation in Obsessive-Compulsive Disorder.” Archives of General Psychiatry 53 (7): 595. 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Buhle Jason T, Silvers Jennifer a, Wager Tor D, Lopez Richard, Onyemekwu Chukwudi, Kober Hedy, Weber Jochen, and Ochsner Kevin N. 2013. “Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies.” Cerebral Cortex (New York, N.Y. : 1991), 1–10. 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello Rosario, Salguero José M., Fernández-Berrocal Pablo, and Gross James J.. 2013. “A Spanish Adaptation of the Emotion Regulation Questionnaire.” European Journal of Psychological Assessment 29 (4): 234–40. 10.1027/1015-5759/a000150. [DOI] [Google Scholar]
- Cardoner Narcís, Harrison Ben J., Pujol Jesús, Soriano-Mas Carles, Hernández-Ribas Rosa, López-Solá Marina, Real Eva, et al. 2011. “Enhanced Brain Responsiveness during Active Emotional Face Processing in Obsessive Compulsive Disorder.” The World Journal of Biological Psychiatry 12 (5): 349–63. 10.3109/15622975.2011.559268. [DOI] [PubMed] [Google Scholar]
- Cox RW 1996. “AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages.” Computers and Biomedical Research 29 (29): 162–73. 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD 2009. “How Do You Feel — Now? The Anterior Insula and Human Awareness.” Nature Reviews Neuroscience 10 (1): 59–70. 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dörfel Denise, Jan Peter Lamke, Falk Hummel, Wagner Ullrich, Erk Susanne, and Walter Henrik. 2014. “Common and Differential Neural Networks of Emotion Regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A Comparative FMRI Investigation.” NeuroImage 101: 298–309. 10.1016/j.neuroimage.2014.06.051. [DOI] [PubMed] [Google Scholar]
- Fontenelle Leonardo F, Bramati Ivanei E, Moll Jorge, Medlowicz Mauro V, de Oliveira-Souza Ricardo, and Tovar-Moll Fernanda. 2011. “White Matter Changes in OCD Revealed by Diffusion Tensor Imaging.” CNS Spectrums 16 (May): 101–9. 10.1017/S1092852912000. [DOI] [PubMed] [Google Scholar]
- Garibotto V, Scifo P, Gorini A, Alonso Clarke R., Brambati S, Bellodi L, and Perani D. 2010. “Disorganization of Anatomical Connectivity in Obsessive Compulsive Disorder: A Multi-Parameter Diffusion Tensor Imaging Study in a Subpopulation of Patients.” Neurobiology of Disease 37 (2): 468–76. 10.1016/J.NBD.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Goldberg X, Cardoner N, Alonso P, López-Solà C, Real E, Hernández-Ribas R, Jiménez-Murcia S, et al. 2016. “Inter-Individual Variability in Emotion Regulation: Pathways to Obsessive-Compulsive Symptoms.” Journal of Obsessive-Compulsive and Related Disorders 11 (October): 105–12. 10.1016/j.jocrd.2016.10.002. [DOI] [Google Scholar]
- Goldin Philippe, Manber Tali, Hakimi Shabnam, Canli Turhan, and Gross James J. 2009. “Neural Bases of Social Anxiety Disorder: Emotional Reactivity and Cognitive Regulation During Social and Physical Threat.” Arch Gen Psychiatry 66 (2): 170–80. 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin Philippe, Kateri McRae, Wiveka Ramel, and Gross James J. 2009. “The Neural Bases of Emotion Regulation: Reappraisal and Suppression of Negative Emotion.” Biological Psychiatry 63 (6): 577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Wayne K., Price Lawrence H., Rasmussen Steven A., Mazure Carolyn, Fleischmann Roberta L., Hill Candy L., Heninger George R., and Charney Dennis S.. 1989. “The Yale-Brown Obsessive Compulsive Scale.” Archives of General Psychiatry 46 (11): 1006. 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Grech-Sollars Matthew, Hales Patrick W, Miyazaki Keiko, Raschke Felix, Rodriguez Daniel, Wilson Martin, Gill Simrandip K, et al. 2015. “Multi-Centre Reproducibility of Diffusion MRI Parameters for Clinical Sequences in the Brain.” NMR in Biomedicine 28 (4): 468–85. 10.1002/nbm.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross James J, and Oliver P John. 2003. “Individual Differences in Two Emotion Regulation Processes: Implications for Affect, Relationships, and Well-Being.” Journal of Personality and Social Psychology 85 (2): 348–62. 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gruner Patricia, Vo An, Ikuta Toshikazu, Mahon Katie, Peters Bart D, Malhotra Anil K, Uluğ Aziz M, and Szeszko Philip R. 2012. “White Matter Abnormalities in Pediatric Obsessive-Compulsive Disorder.” Neuropsychopharmacology 37 (12): 2730–39. 10.1038/npp.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürsel Deniz A., Avram Mihai, Sorg Christian, Brandl Felix, and Koch Kathrin. 2018. “Frontoparietal Areas Link Impairments of Large-Scale Intrinsic Brain Networks with Aberrant Fronto-Striatal Interactions in OCD: A Meta-Analysis of Resting-State Functional Connectivity.” Neuroscience & Biobehavioral Reviews, February. 10.1016/j.neubiorev.2018.01.016. [DOI] [PubMed]
- Hahn Andreas, Stein Patrycja, Windischberger Christian, Weissenbacher Andreas, Spindelegger Christoph, Moser Ewald, Kasper Siegfried, and Lanzenberger Rupert. 2011. “Reduced Resting-State Functional Connectivity between Amygdala and Orbitofrontal Cortex in Social Anxiety Disorder.” NeuroImage 56 (3): 881–89. 10.1016/J.NEUROIMAGE.2011.02.064.. [DOI] [PubMed] [Google Scholar]
- Hamilton M 1959. “The Assessment of Anxiety States by Rating.” The British Journal of Medical Psychology 32 (1): 50–55. http://www.ncbi.nlm.nih.gov/pubmed/13638508. [DOI] [PubMed] [Google Scholar]
- Hamilton M 1960. “A Rating Scale for Depression.” Journal of Neurology, Neurosurgery, and Psychiatry 23 (1): 56–62. http://www.ncbi.nlm.nih.gov/pubmed/14399272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Ben J., Soriano-Mas Carles, Pujol Jesus, Ortiz Hector, López-Solà Marina, Hernández-Ribas Rosa, Deus Joan, et al. 2009. “Altered Corticostriatal Functional Connectivity in Obsessive-Compulsive Disorder.” Archives of General Psychiatry 66 (11): 1189. 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- Hayes Jasmeet Pannu, Morey Rajendra A, Petty Christopher M, Seth Srishti, Smoski Moria J, McCarthy Gregory, and Labar Kevin S. 2010. “Staying Cool When Things Get Hot: Emotion Regulation Modulates Neural Mechanisms of Memory Encoding.” Frontiers in Human Neuroscience 4: 230. 10.3389/fnhum.2010.00230.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermundstad Ann M, Bassett Danielle S, Brown Kevin S, Aminoff Elissa M, Clewett David, Freeman Scott, Frithsen Amy, et al. 2013. “Structural Foundations of Resting-State and Task-Based Functional Connectivity in the Human Brain.” Proceedings of the National Academy of Sciences of the United States of America 110 (15): 6169–74. 10.1073/pnas.1219562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuvel Martijn P van den, Mandl René C.W., Kahn René S., and Hulshoff Pol Hilleke E.. 2009. “Functionally Linked Resting-State Networks Reflect the Underlying Structural Connectivity Architecture of the Human Brain.” Human Brain Mapping 30 (10): 3127–41. 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayarajan Rajan Nishanth, Venkatasubramanian Ganesan, Viswanath Biju, Reddy Y.C. Janardhan, Srinath Shoba, Vasudev Mandapatti K., and Chandrashekar Channapatna R.. 2012. “White Matter Abnormalities in Children and Adolescents with Obsessive-Compulsive Disorder: A Diffusion Tensor Imaging Study.” Depression and Anxiety 29 (9): 780–88. 10.1002/da.21890. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Shioiri T, Kimura T, Ohkubo M, Nakada T, and Someya T. 2006. “Parietal White Matter Abnormalities in Obsessive-Compulsive Disorder: A Magnetic Resonance Spectroscopy Study at 3-Tesla.” Acta Psychiatrica Scandinavica 114 (2): 101–8. 10.1111/j.1600-0447.2006.00858.x.. [DOI] [PubMed] [Google Scholar]
- Klasen M, Kenworthy CA, Mathiak KA, Kircher TTJ, and Mathiak K. 2011. “Supramodal Representation of Emotions.” Journal of Neuroscience 31 (38): 13635–43. 10.1523/JNEUROSCI.2833-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, and Habel U. 2014. “Neural Network of Cognitive Emotion Regulation — An ALE Meta-Analysis and MACM Analysis.” NeuroImage 87: 345–55. 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE 2000. “Emotion Circuits in the Brain.” Annual Review of Neuroscience 23 (January): 155–84. 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee Hyejeen, Heller Aaron S, van Reekum Carien M, Nelson Brady, and Davidson. 2012. “Amygdala-Prefrontal Coupling Underlies Individual Differences in Emotion Regulation.” NeuroImage 62 (3): 1575–81. 10.1016/j.neuroimage.2012.05.044.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataix-Cols David, Wooderson Sarah, Lawrence Natalia, Brammer Michael J., Speckens Anne, and Phillips Mary L.. 2004. “Distinct Neural Correlates of Washing, Checking, and Hoarding SymptomDimensions in Obsessive-Compulsive Disorder.” Archives of General Psychiatry 61 (6): 564. 10.1001/archpsyc.61.6.564. [DOI] [PubMed] [Google Scholar]
- Menzies Lara, Chamberlain Samuel R., Laird Angela R., Thelen Sarah M., Sahakian Barbara J., and Bullmore Ed T.. 2008. “Integrating Evidence from Neuroimaging and Neuropsychological Studies of Obsessive-Compulsive Disorder: The Orbitofronto-Striatal Model Revisited.” Neuroscience and Biobehavioral Reviews 32 (3): 525–49. 10.1016/j.neubiorev.2007.09.005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad Mohammed R., and Rauch Scott L.. 2012. “Obsessive-Compulsive Disorder: Beyond Segregated Cortico-Striatal Pathways.” Trends in Cognitive Sciences 16 (1): 43–51. 10.1016/J.TICS.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira PS, Marques P, Soriano-Mas C, Magalhães R, Sousa N, Soares JM, and Morgado P. 2017. “The Neural Correlates of Obsessive-Compulsive Disorder: A Multimodal Perspective.” Translational Psychiatry 7 (8): e1224. 10.1038/tp.2017.189.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner Kevin, Silvers Jennifer, and Buhle Jason. 2012. “Functional Imaging Studies of Emotion Regulation: A Synthetic Review and Evolving Model of the Cognitive Control of Emotion.” Ann N Y Acad Sci 1252: 1–35. 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picó-Pérez Maria, Alonso Pino, Contreras-Rodríguez Oren, Martínez-Zalacaín Ignacio, López-Solà Clara, Jiménez-Murcia Susana, Verdejo-García Antonio, Menchón José M., and Soriano-Mas Carles. 2017. “Dispositional Use of Emotion Regulation Strategies and Resting-State Cortico-Limbic Functional Connectivity.” Brain Imaging and Behavior. 10.1007/s11682-017-9762-3. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu MO, Barnett A, Basser P, Chang L-C, Koay C, et al. 2010. “TORTOISE: An Integrated Software Package for Processing of Diffusion MRI Data.” ISMRM 18th Annual Meeting Stockholm (Sweden): #1597.
- Posner Jonathan, Marsh Rachel, Maia Tiago V, Peterson Bradley S, Gruber Allison, and Simpson H Blair. 2014. “Reduced Functional Connectivity within the Limbic Cortico-Striato-Thalamo-Cortical Loop in Unmedicated Adults with Obsessive-Compulsive Disorder.” Human Brain Mapping 35 (6): 2852–60. 10.1002/hbm.22371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Amy K, Julie L Fudge, Clare Kelly, Perry Justin S A, Daniele Teresa, Carlisi Christina, Benson Brenda, et al. 2013. “Intrinsic Functional Connectivity of Amygdala-Based Networks in Adolescent Generalized Anxiety Disorder.” Journal of the American Academy of Child and Adolescent Psychiatry 52 (3): 290–299. e2. 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus Oana Georgiana, Tim Jonas Reess, Wagner Gerd, Zimmer Claus, Zaudig Michael, and Koch Kathrin. 2017. “Functional and Structural Connectivity of the Amygdala in Obsessive-Compulsive Disorder.” NeuroImage Clinical 13: 246–55. 10.1016/j.nicl.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, and Kessler RC. 2010. “The Epidemiology of Obsessive-Compulsive Disorder in the National Comorbidity Survey Replication.” Molecular Psychiatry 15 (1): 53–63. 10.1038/mp.2008.94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon Daniela, Kaufmann Christian, Müsch Kathrin, Kischkel Eva, and Kathmann Norbert. 2010. “Fronto-Striato-Limbic Hyperactivation in Obsessive-Compulsive Disorder during Individually Tailored Symptom Provocation.” Psychophysiology 47 (4): 728–38. 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Taylor Paul A, and Saad Ziad S. 2013. “FATCAT: (An Efficient) Functional And Tractographic Connectivity Analysis Toolbox.” Brain Connectivity 3 (5): 523–35. 10.1089/brain.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel Stefan J., Reuter Sigrid, Stieltjes Bram, Acosta-Cabronero Julio, Ernemann Ulrike, Fellgiebel Andreas, Filippi Massimo, et al. 2011. “Multicenter Stability of Diffusion Tensor Imaging Measures: A European Clinical and Physical Phantom Study.” Psychiatry Research: Neuroimaging 194 (3): 363–71. 10.1016/J.PSCYCHRESNS.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia Antonio, Clark Luke, and Dunn Barnaby D.. 2012. “The Role of Interoception in Addiction: A Critical Review.” Neuroscience & Biobehavioral Reviews 36 (8): 1857–69. 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Via Esther, Cardoner Narcís, Pujol Jesús, Alonso Pino, López-Solà Marina, Real Eva, Contreras-Rodríguez Oren, et al. 2014. “Amygdala Activation and Symptom Dimensions in Obsessive-Compulsive Disorder.” The British Journal of Psychiatry : The Journal of Mental Science 204 (1): 61–68. 10.1192/bjp.bp.112.123364. [DOI] [PubMed] [Google Scholar]
- Vries Froukje E. de, de Wit Stella J., Cath Danielle C., van der Werf Ysbrand D., van der Borden Vionne, van Rossum Thomas B., van Balkom Anton J.L.M., van der Wee Nic J.A., Veltman Dick J., and van den Heuvel Odile A.. 2014. “Compensatory Frontoparietal Activity During Working Memory: An Endophenotype of Obsessive-Compulsive Disorder.” Biological Psychiatry 76 (11): 878–87. 10.1016/J.BIOPSYCH.2013.11.021.. [DOI] [PubMed] [Google Scholar]
- Weidt S, Lutz J, Rufer M, Delsignore A, Jakob NJ, Herwig U, and Bruehl AB. 2016. “Common and Differential Alterations of General Emotion Processing in Obsessive-Compulsive and Social Anxiety Disorder.” Psychological Medicine 46 (07): 1427–36. 10.1017/S0033291715002998. [DOI] [PubMed] [Google Scholar]
- Wit S. J. de, van der Werf YD, Mataix-Cols D, Trujillo JP, van Oppen P, Veltman DJ, and van den Heuvel OA. 2015. “Emotion Regulation before and after Transcranial Magnetic Stimulation in Obsessive Compulsive Disorder.” Psychological Medicine 45 (14): 3059–73. 10.1017/S0033291715001026. [DOI] [PubMed] [Google Scholar]
- Zarei Mojtaba, Mataix-Cols David, Isobel Heyman, Hough Morgan, Doherty Joanne, Burge Linda, Winmill Louise, Nijhawan Sunita, Matthews Paul M., and James Anthony. 2011. “Changes in Gray Matter Volume and White Matter Microstructure in Adolescents with Obsessive-Compulsive Disorder.” Biological Psychiatry 70 (11): 1083–90. 10.1016/J.BIOPSYCH.2011.06.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.