Abstract
The acyclic phosphonate analog adefovir is a potent inhibitor of retroviruses, including human immunodeficiency virus (HIV) type 1, and, unlike some antiviral nucleosides, does not require the initial phosphorylation step for its activity. Two oral dosages of the adefovir prodrug adefovir dipivoxil were evaluated in a phase I study with children with HIV infection. A total of 14 patients were stratified into age groups ranging from 6 months to 18 years of age. Eight patients received 1.5 mg of adefovir dipivoxil per kg of body weight, and six patients received 3.0 mg of adefovir dipivoxil per kg. Serum samples were obtained at intervals during the 8 h postdosing and were analyzed for adefovir concentrations. Patients were monitored for adverse effects. All samples collected resulted in quantifiable levels of adefovir (lower limit of quantitation, 25 ng/ml) from each patient. The areas under the concentration-versus-time curves (AUCs) were similar (P = 0.85) for the 1.5- and 3.0-mg/kg doses, while the apparent oral clearance (CL/F) was significantly higher (P = 0.05) for the 3-mg/kg dose. Pharmacokinetic parameters differed by patient age. In comparing those children older and younger than the median age of 5.1 years, AUC (P = 0.03), maximum concentration of drug in serum (P = 0.004), and the concentration at 8 h postdosing (P = 0.02) were significantly lower for the younger children. There were no significant differences for apparent volume of distribution and CL/F normalized to body surface area, but there was a suggestive difference in half-life (P = 0.07) among the subjects in the older and younger age groups. No significant adverse events were encountered. These data provide the basis for a multidose phase II study of adefovir dipivoxil in HIV-infected infants and children.
Nucleoside analogues remain essential for increasingly effective combination treatment regimens for human immunodeficiency virus (HIV) type 1 (HIV-1) infections. Unphosphorylated nucleoside analogues such as zidovudine, didanosine, zalcitabine, lamivudine, and stavudine rely on cellular nucleoside kinase for conversion to the active triphosphate form. The expression of these nucleoside kinases is cell type and cell cycle dependent, and resting lymphocytes and macrophages/monocytes do not express high levels of these enzymes. In contrast, adefovir dipivoxil, 9-[2-[[bis[(pivaloyloxy)methoxy]phosphinyl]methoxy]ethyl] adenine (bis-POM PMEA), contains a phosphonate moiety which has been stabilized by switching the oxygen in the phosphoester bond with the proximate carbon in the nucleotide. This class of compounds is not dependent on nucleoside kinase for its conversion to the active diphosphate form, phosphinylmethoxyethyl adenine (PMEA) diphosphate, and thus may possess greater activity than nucleoside analogs in a broader range of cell types. The active intracellular metabolite, PMEA diphosphate, is a potent inhibitor of retroviral reverse transcriptase (1). Therefore, PMEA may accumulate intracellularly in uninfected host cells, preventing infection and subsequent viral replication (2, 6). Synergistic effects with PMEA and zidovudine against HIV-1 replication have been reported (24).
Adefovir is poorly absorbed in a number of species, including rats (23), monkeys (3, 4, 12), and humans (10). The low oral bioavailability of adefovir appears to be in part a consequence of the limited intestinal permeability of the phosphonate (18). However, the oral bioavailability of adefovir has been substantially improved with the development of the prodrug adefovir dipivoxil. Studies of the prodrug in rats (22), dogs (13), cynomolgus monkeys (11, 19, 20, 21), and humans (8, 16) have demonstrated that plasma adefovir concentrations are sufficient for antiviral activity following oral administration. Some evidence suggests that adefovir dipivoxil has enhanced activity due to the increased cellular uptake and metabolism of the lipophilic prodrug (25), resulting in high intracellular concentrations of adefovir. Adefovir is eliminated from the serum exclusively by renal excretion of unchanged drug by a combination of glomerular filtration and tubular secretion.
Tablet and granule (for suspension) formulations of adefovir dipivoxil have been studied in HIV-infected adults (8, 16). Following oral administration of granules suspended in concentrated grape juice, adefovir dipivoxil was rapidly absorbed and cleaved to the parent compound, and no intact prodrug or monoester was detected in blood. The maximum concentration in serum (Cmax) for adefovir was dose proportional over the dose range of 200 to 500 mg, and the time to Cmax (Tmax) was approximately 1.5 h at all doses. Following the administration of a single dose of 125, 250, and 500 mg of adefovir dipivoxil as tablet formulations to adults, the observed Cmax values were 0.211 ± 0.131 (±1 standard deviation), 0.436 ± 0.073, and 0.831 ± 0.143 μg/ml, respectively. Recoveries in urine after administration of the first dose were 44.4% ± 15.6%, 32.8% ± 6.06%, and 29.6% ± 12.2%, respectively.
The rationale for pursuing a phase I study with HIV-infected infants and children at this juncture in the development of adefovir dipivoxil is multifold. The safety and pharmacokinetic parameters of drugs in infants and children often differ from those in adults, and the type and extent of variation are not predictable. Pediatric AIDS, like adult AIDS, is fatal, and no curative treatment is available. Unlike HIV-infected adults, HIV-infected infants commonly have a rapid downhill course and early death. Many of the antiretroviral agents in general use in adults have not undergone even phase I studies with children, and most lack U.S. Food and Drug Administration approval for use in individuals younger than 13 years of age. Ethically, infants with HIV infection deserve equal access to experimental drugs for this universally fatal disease. The present report describes the pharmacokinetic and safety data from a phase I single-dose study with HIV-infected infants and children.
MATERIALS AND METHODS
Study design and subjects.
The primary objectives of this phase I, open-label, single-dose study (AIDS Clinical Trials Group [ACTG] Protocol 310) were to determine the safety, tolerance, and pharmacokinetics of adefovir dipivoxil in infants and children. The study was designed to evaluate the pharmacokinetics, safety, and potential toxicity of single oral doses of 1.5 and 3.0 mg/kg of body weight in HIV-infected patients younger than 18 years of age stratified into two age cohorts (≤3 months and >3 months). The younger and older age groups were designed to include a minimum of four and eight patients in each dosage cohort, respectively. The older age group was further stratified to include two patients from each of the following age categories (3 months to <1 year, 1 year to <2 years, 2 to <10 years, and 10 to 18 years). This initial report includes data only for children older than 3 months of age for the 1.5-mg/kg cohort and 1 year of age or older for the 3.0-mg/kg cohort. Dose escalation began with the older age group and the lower dose. Each patient was studied only once. No other antiretroviral drugs were administered.
The study was conducted at four Pediatric AIDS Clinical Trial Group centers: St. Jude Children's Research Hospital, Memphis, Tenn.; The Johns Hopkins University Hospital, Baltimore, Md.; Children's Hospital of Philadelphia; and the University of California, San Francisco, according to ACTG Protocol 310. Parents or guardians of the participants agreed to the informed consent statements approved by the institutional review Boards of the respective institutions. Children were eligible for enrollment if they had a diagnosis of HIV infection by Centers for Disease Control and Prevention criteria (7) for category N (asymptomatic) or category A (mildly symptomatic); greater than 2,500-g birth weight; absence of any acute or chronic infections; negative pregnancy tests for girls of childbearing age; adequate renal function (less than grade 1 toxicity criteria for blood urea nitrogen and creatinine levels and urinalysis); and no greater than grade 1 toxicity criteria for complete blood count and differential platelets; reticulocytes, electrolytes, amylase, lipase, calcium, phosphorous, magnesium, glucose, bilirubin, aspartate transaminase (serum glutamic oxalacetic transaminase), alanine transaminase (serum glutamic pyruvic transaminase), uric acid, and electrocardiogram. The Division of AIDS Toxicity Tables for Grading of Pediatric Adverse Effects of the National Institute for Allergy and Infectious Diseases were used for grading of laboratory and clinical parameter values. Patients were deemed ineligible for enrollment if they were receiving (i) other investigational agents, (ii) drugs with theoretical or known adverse interactions with adefovir dipivoxil in vivo, (iii) drugs likely to interfere with the quantitation of adefovir, or (iv) drugs which may affect renal excretion (e.g., probenecid, acyclovir, ganciclovir, foscarnet, amphotericin B, and pentamidine).
Formulation and administration.
Adefovir dipivoxil granules were mixed with food prior to oral administration. The composition of the granule formulation included adefovir dipivoxil, 85.1%; pregelatinized starch, NF, 9.57%; croscarmellose sodium, NF, 4.26%; and magnesium stearate, NF, 1.06%. The dose for each patient, based on body weight at the time of study entry, was prepared by a pharmacist using an analytic balance at McKesson Biosciences Cooperation, under contract to the Division of AIDS, Pharmacy and Regulatory Affairs Branch, National Institute for Allergy and Infectious Diseases, and was shipped to each site. The preweighed granules were mixed in milk, formula, apple juice, grape syrup, vanilla ice cream, or water and were administered under the supervision of a research nurse.
Observations.
Prior to dosing and on day 6 or 7 postdosing, the following were measured, performed, or observed: physical examination, vital signs, medical history, complete blood cell count and differential, reticulocyte and platelet counts, urinalysis, serum electrolytes, blood urea nitrogen, creatinine, amylase, lipase, calcium, magnesium, glucose, bilirubin, aspartate transaminase, alanine transaminase, and uric acid. An electrocardiogram was performed prior to dosing and on day 1 after dosing.
All laboratory values except the white blood cell count were graded by using the standard toxicity tables. The following scheme, not included in the toxicity tables, was derived prospectively and was applied to the white blood cell count: 5,000 to 15,000/mm3 = grade 0; 4,000 to 5,000/mm3 = grade 1; 3,000 to 4,000/mm3 = grade 2; 1,000 to 3,000/mm3 = grade 3; and less than 1,000/mm3 = grade 4. Patients with signs, symptoms, and laboratory test values of grade 3 toxicity or greater were reviewed monthly by the protocol team.
One patient with a grade 3 neutropenia at the baseline when the drug was given was entered into the study, but the white cell count was grade 1 on the next day.
Pharmacokinetic design.
Blood samples (1.5 ml) were withdrawn from each patient at 0 h (predosing) and 0.5, 1.0, 1.5, 2, 3, 6, and 8 h postinitiation of dosing. Serum was separated, decanted, frozen, and stored at −20°C until it was analyzed. The concentrations of adefovir in serum samples were determined by a validated high-pressure liquid chromatography method (9). The method involves derivatization of adefovir with chloroacetaldehyde followed by reverse-phase ion-pair high-pressure liquid chromatography with fluorescence detection. The method was validated at Harris Laboratories (Lincoln, Nebr.) and was linear for both compounds over the range 25 to 1,000 ng/ml, with a limit of quantitation of 25 ng/ml. The intraday precision and accuracy for human serum were <4.1 and <1.8%, respectively; interday values were <4.8 and <1.5%, respectively. When necessary, samples with concentrations of adefovir beyond the linear range of the assay were diluted with pooled normal human serum prior to analysis.
A one-compartment first-order absorption model with a lag time was fit to each patient's concentration data by using maximum likelihood estimation (14). The model was parameterized with an apparent volume of distribution term (V/F) and first-order absorption (ka) and elimination (kel) rate constants. Each observation was weighted by assuming that the variance of the model estimate was inversely proportional to the value predicted by the model. Lag times estimated to be less than 0.1 h were assumed to be zero. Oral clearance (CL/F) was calculated as the product of kel and V/F. The area under the concentration-versus-time curve (AUC) was calculated by dividing the dose by CL/F, with the dose and the concentration referenced to those for PMEA.
RESULTS
A total of 14 patients (9 females and 5 males) were evaluated for pharmacokinetics, tolerance, and safety with one of the two doses (1.5 and 3.0 mg) of adefovir dipivoxil prodrug per kilogram of body weight (equivalent to 0.82 and 1.6 mg of adefovir per kg). Comparisons of dosing cohorts 1 (low dose) and 2 (high dose) for demographic and other characteristics are given in Table 1. There were no discernible differences among the subjects in the two dosage cohorts with the exception of age. The median age for the low-dose group was 3.8 years (range, 0.5 to 17.9 years), and that for the high-dose group was 5.3 years (range, 1.5 to 17.1 years). Two patients in the group ages 3 months to 1 year were enrolled in the low-dose cohort, but none of the patients in this age stratum was enrolled in the high-dose cohort. Two patients in each of the other three age strata received each dose.
TABLE 1.
Patient characteristics according to adefovir dipivoxil dose
| Parameter | 1.5 mg/kg | 3.0 mg/kg |
|---|---|---|
| No. of patients | 8 | 6 |
| Age stratum (no. of patients) 3 mo to 1 yr | 2 | |
| 1 to 2 yr | 2 | 2 |
| 2 to 10 yr | 2 | 2 |
| 10 to 18 yr | 2 | 2 |
| Gender (no. of patients) | ||
| Male | 3 | 2 |
| Female | 5 | 4 |
| Ethnicity (no. of patients) | ||
| Black | 7 | 5 |
| Hispanic | 1 | 1 |
| CDCa HIV disease category (no. of patients) | ||
| A | 5 | 5 |
| N | 3 | 1 |
| Body wt (kg) | ||
| Median | 15.9 | 18.2 |
| Range | 5.5–92.1 | 9.4–80 |
CDC, Centers for Disease Control and Prevention.
Adefovir pharmacokinetics.
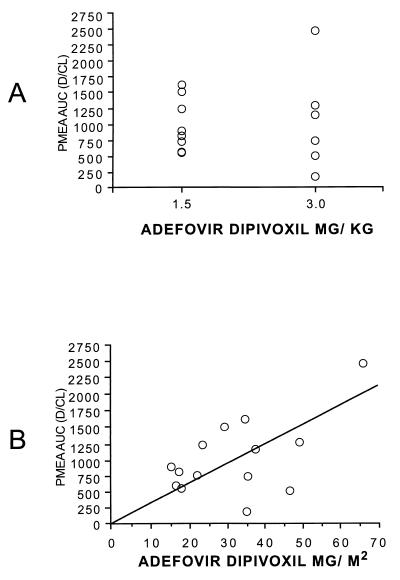
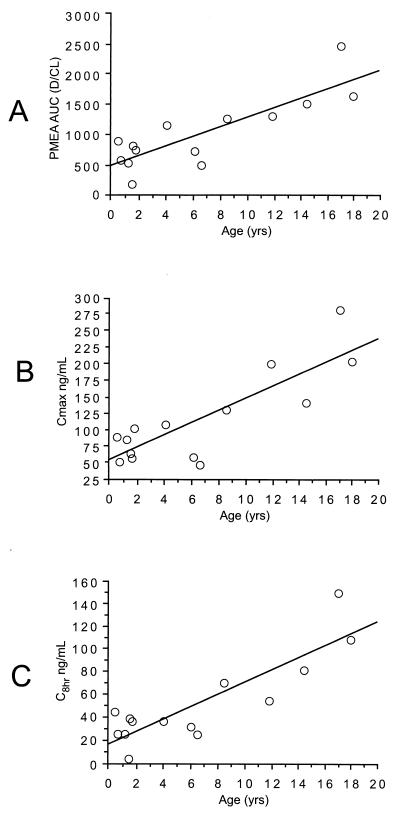
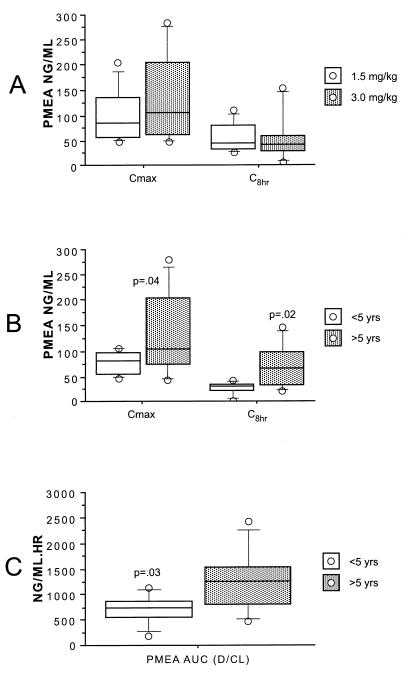
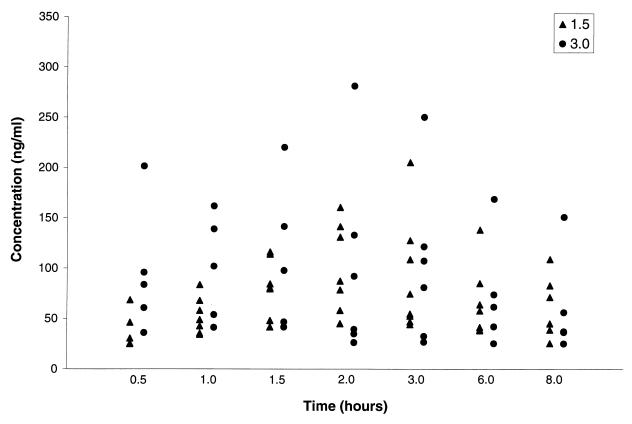
All patients ingested and retained the full dose of adefovir dipivoxil. Summary statistics for the pharmacokinetic parameters are provided in Table 2. Body surface area was used to normalize V/F and CL/F, as the correlations were similar but slightly higher than those for body weight. The AUC for PMEA was not significantly different (P = 0.85) for the 1.5- and 3.0-mg/kg doses (Fig. 1A). AUC did increase with dose, expressed as milligrams per square meter (r2 = 0.81; Fig. 1B). A univariate regression analysis suggested that age was a potentially significant covariate for pharmacokinetic parameters, with younger age associated with lower values of AUC, Cmax, and the concentration 8 h after drug administration (C8) (Fig. 2). As expected, given the similar AUCs for the low and high doses, CL/F was significantly higher (P = 0.05) for the 3-mg/kg dose than for the 1.5-mg/kg dose (value versus volume). The dosage cohort did not appear to significantly affect AUC (Fig. 1A), Cmax, or C8 (Fig. 3A). The measured concentrations for each dose (Fig. 4) indicate similar values, despite the twofold differences in doses. To further examine the effect of age on pharmacokinetic parameters, children were divided into two age groups on the basis of age above or below the median value, and data for these two groups were compared by a two-sample Student's t test. AUC (P = 0.03), Cmax (P = 0.04), and C8 (P = 0.02) were significantly lower for younger children (Fig. 3B and C). The half-life was suggestively shorter (P = 0.07) for the high-dose group (median, 4.4 h) than for the low-dose group (median, 6.9 h) (data not shown), but there was no significant difference for V/F (P = 0.23). When CL/F was normalized to body weight there was a weak (r2 = 0.14) relationship with age by linear regression analysis. However, there was no apparent relationship between age and CL/F normalized to body surface area by linear regression analysis.
TABLE 2.
Adefovir dipivoxil pharmacokinetic parametersa
| Dose (mg/kg) | Measure | Cmax (ng/ml) | C8 (ng/ml) | AUC (ng · h/ml) | t1/2 (h) | Tmax (h) | CL/F (liters/kg/h) | V/F (liters/kg) |
|---|---|---|---|---|---|---|---|---|
| 1.5 | Median | 86 | 42 | 854 | 6.9 | 2.0 | 1.0 | 8.1 |
| Mean | 101 | 54 | 993 | 6.5 | 1.8 | 1.0 | 9.0 | |
| Range | 48–205 | 26–109 | 551–1,622 | 2.3–8.9 | 1–3 | 0.5–1.5 | 1.7–16 | |
| Coef. var. (%) | 54 | 57 | 42 | 36 | 3,651 | 39 | 53 | |
| 3.0 | Median | 104 | 37 | 944 | 4.4 | 1.3 | 1.8 | 11.3 |
| Mean | 133 | 51 | 1056 | 4.0 | 1.4 | 2.9 | 14.1 | |
| Range | 46–280 | 4–150 | 191–2,465 | 0.9–7.6 | 0.5–3.0 | 0.7–8.6 | 1.9–34 | |
| Coef. var. (%) | 68 | 100 | 76 | 58 | 69 | 100 | 85 |
These pharmacokinetic parameters were generated after administration of a single dose and were not determined in the context of steady-state conditions. Coef. var., coefficient of variation.
FIG. 1.
Comparison of the dose of adefovir dipivoxil (bis-POM PMEA) and the AUC for adefovir (PMEA). (A) Dose based on body weight. (B) Dose based on body surface area (r2 = 0.81).
FIG. 2.
Comparison of age and AUC for PMEA or concentration in serum. (A) AUC compared with age (r2 = 0.71). (B) Cmax compared with age (r2 = 0.71). (C) C8 compared with age (r2 = 0.75).
FIG. 3.
Comparison of Cmax and C8 with dose (A) and age (B) and comparison of AUC with age (C). (A) Box plots showing the influence of adefovir dipivoxil dose on the Cmax and C8 of PMEA in serum. (B) Box plots showing the influence of patient age on the Cmax and C8 of PMEA in serum. (C) Box plots showing the influence of patient age on the AUC for PMEA in serum. D, range of AUC.
FIG. 4.
Measured concentrations of adefovir dipivoxil at nominal times after the administration of 1.5- and 3.0-mg/kg doses.
Adverse events.
No signs, symptoms, or laboratory test abnormalities of grade 3 or greater were attributed to the study drug in any of the patients. Among the eight patients in group 1 (1.5 mg/kg), one patient experienced two grade 3 events, namely, mild dehydration and mucus-like stools. However, these were determined to be associated with gastroenteritis due to a Salmonella sp. and were not drug related. With the observed adverse reaction rate of zero for this low-dose (1.5-mg/kg) group, the lower and upper 95% confidence limits were 0 and 37%, respectively. Thus, there is only a 2.5% chance that the true rate of adverse effects is greater than 37%. None of the six evaluable patients in the high-dose (3.0-mg/kg) group experienced an event of grade 3 or greater (95% confidence limits, 0 and 46%).
DISCUSSION
The purpose of drug evaluations with infants and children, in addition to adults, is to determine if differences in pharmacokinetics, safety, and efficacy occur due to age. Although this study was necessarily limited to a small number of patients, there is evidence for age-related differences in adefovir disposition. These differences in AUC, Cmax, and C8 values were clearly discernible for both dosages when the dosages were referenced to body weight. The overall variability in pharmacokinetic parameters makes it difficult to determine the respective influence of age, dose, or formulation on drug disposition; but these data suggest that younger children given the same dose on the basis of body weight will have substantially lower levels of systemic exposure. For example, for the 1.5-mg/kg dose, all four children younger than 5 years of age had an AUC of less than 1,000 mg · h/liter, while three of four children older than 5 years of age had AUC values greater than 1,250 mg · h/liter. The increase in serum adefovir concentrations with increasing age could represent either higher CL/F or lower bioavailability in the younger patients. However, a greater CL/F of adefovir in infants seems unlikely, because the drug is eliminated by a combination of glomerular filtration and tubular secretion, both of which should increase with age.
It is possible that the observed decrease in exposure to adefovir with younger patient age is related to the use of a nonoptimized formulation and the total amount of drug administered. The preweighed granules were mixed in formula, milk, water, baby food, juice, etc., prior to administration. While no obvious breach in the drug administration protocol was observed, the dosing was somewhat cumbersome. Recent data suggest that this strategy is not necessary when the formulation is used more uniformly in children (A. Wiznia, R. Nelson, R. Van Dyke, S. Bakshi, K. Cundy, K. Dawson, A. Kosloff, J. Esinhart, and D. Coakley, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. I-9, p. 365, 1998).
The pharmacokinetic data for adefovir are somewhat surprising in that the systemic exposure, as reflected by AUC, Cmax, and C8, was not significantly different for the two doses studied. Given the similar AUCs for each dose, there was a correspondingly higher estimate for apparent CL/F at the higher dose. Although adefovir is primarily eliminated by the kidney, there is no obvious reason to believe that renal function differences might exist between the two dose groups. The CL/F of adefovir in HIV-infected adults after administration of a 60-mg dose is 422 ± 82 ml/h/kg. Thus, the CL/F for children is greater than that for adults.
The possibility that there are dose-related differences in the absorption of adefovir dipivoxil warrants further scrutiny. The reason that no greater increase in AUC occurred when the dose of 3.0 mg/kg was given than when the 1.5-mg/kg dose was given is not apparent. Saturable absorption is one consideration. However, previous studies with adults have suggested dose-proportional increases in AUC (5, 15). The absorption of adefovir dipivoxil in adults is not saturated at doses of 60 to 500 mg (approximately 0.9 to 7.1 mg/kg). Limited intestinal permeability of the drug is suggested by studies with Caco-2 cells and other biological models (18). The additional variables of formulation and administration for younger children may further complicate absorption of a drug with variable absorption characteristics. The greater apparent CL/F at the lower dose in our study may have been the result of a prototype formulation that required dispensing of small amounts of the drug formulation. Larger numbers of patients of similar ages will need to be studied to clarify this issue. The proposed dosage of adefovir dipivoxil for adults is 60 or 120 mg per day in phase III clinical trials. Currently, the lower dose of 60 mg seems to be preferred because of the adequate antiviral effect and a lower incidence of proximal renal tubular dysfunction.
No drug-related adverse effects were observed in children when the doses of adefovir dipivoxil were adequate to achieve therapeutic concentrations in serum. In studies with adults who received adefovir dipivoxil, gastrointestinal adverse events, hyperbilirubinemia, and elevations in liver enzyme levels have been reported at frequencies higher than those in groups that received a placebo (5, 17, 25). Additionally, nephrotoxicity manifested as elevations in serum creatinine levels and hypophosphatemia has been reported in patients taking adefovir dipivoxil for longer than 20 to 24 weeks (17). In a pediatric multidose study of adefovir dipivoxil (1.5 or 3.0 mg/kg) administered in combination with nelfinavir and other reverse transcriptase inhibitors, gastrointestinal adverse effects were the most commonly reported events in these children administered the regimen for up to 20 weeks (Wiznia et al., 38th ICAAC).
This phase I single-dose study has provided data on the pharmacokinetics of adefovir dipivoxil in the pediatric population and a first estimation of the safety and tolerance of oral administration of a suspension formulation of adefovir dipivoxil to HIV-infected infants and children. The results of this study provide sufficient information to warrant proceeding to a multidose study to further evaluate the safety, pharmacokinetics, and efficacy of adefovir dipivoxil.
ACKNOWLEDGMENTS
This research was supported by the Pediatric AIDS Clinical Trials Group, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
The following individuals contributed to the research project: Christina Joy, Lorraine Wells, Sharon Huang, Nanna Howlett, Patricia M. Flynn, and Pearl Mildred Samson.
REFERENCES
- 1.Balzarini J, Naesens L, Herdewijn P, Rosenberg I, Holy A, Pauwels R, Baba M, Johns D G, DeClercq E. Marked in vivo antiretrovirus activity of 9-(2-phosphonylmethoxyethyl) adenine, a selective anti-human immunodeficiency virus agent. Proc Natl Acad Sci USA. 1989;86:332–336. doi: 10.1073/pnas.86.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini J, Zhang H, Herdewijn P, Johns D, DeClercq E. Intracellular metabolism and mechanism of antiretrovirus action of 9-(2-phosphosphonylmethoxy) adenine, a potent anti-human immunodeficiency virus compound. Proc Natl Acad Sci USA. 1991;88:1499–1503. doi: 10.1073/pnas.88.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzarini J, et al. Potent anti-simian immunodeficiency virus (SIV) activity and pharmacokinetics of 9-(2-phosphonylmethoxyethyl) adenine (PMEA) in rhesus monkeys. In: Schellekens H, Horzinek M C, editors. Animal models in AIDS. New York, N.Y: Elsevier Science Publications; 1990. pp. 131–138. [Google Scholar]
- 4.Balzarini J, Naesens L, Slachmuylders J, Niphuis H, Rosenberg L, Holy A, Schellekens H, DeClercq E. 9-(2-Phosphonylmethoxyethyl) adenine (PMEA) effectively inhibits retrovirus replication in vivo and Simian immunodeficiency virus (SIV) infection in rhesus monkeys. AIDS. 1991;5:21–28. doi: 10.1097/00002030-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Barditch-Crovo P, Toole J, Hendrix C W, Cundy K C, Ebling D, Jaffe H S, Lietman P S. Anti-human immunodeficiency virus (HIV) activity, safety, and pharmacokinetics of adefovir dipivoxil (9-[2-(bis-pivaloyloxymethyl)-phosphonylmethoxyethyl]adenine) in HIV-infected patients. J Infect Dis. 1997;176:406–413. doi: 10.1086/514057. [DOI] [PubMed] [Google Scholar]
- 6.Bronson J J, Ho H-T, DeBoeck H, Woods K, Ghazzouli I, Martin J C, Hitchcock M J M. Biochemical pharmacology of acyclic nucleotide analogues. Ann N Y Acad Sci. 1990;616:398. doi: 10.1111/j.1749-6632.1990.tb17859.x. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morbid Mortal Weekly Rep. 1994;43(RR-12):1–10. [Google Scholar]
- 8.Cundy K C. Oral bioavailability of PMEA (GS-0393) from the prodrug bis-POM PMEA (GS-0840) in HIV-infected patients. A report on data from protocol GS-93-401. Gilead Sciences Study Report DDM-GS-94-401. Foster City, Calif: Gilead Sciences, Inc.; 1994. [Google Scholar]
- 9.Cundy K C. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet. 1999;36:127–143. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- 10.Cundy K, Barditch-Crovo P, Walker R, Collier A, Ebeling D, Toole J, Jaffe H. Clinical pharmacokinetics of adefovir in human immunodeficiency virus type 1-infected patients. Antimicrob Agents Chemother. 1995;39:2401–2405. doi: 10.1128/aac.39.11.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cundy K C, Fishback J A, Shaw J-P, Lee M, Soike K F, Visor G C, Lee W A. Oral bioavailability of the antiretroviral agent 9-(2-phosphonylmethoxyethyl)adenine (PMEA) from three formulations of the prodrug bis(pivaloxyloxymethyl)-PMEA in fasted male cynomolgus monkeys. Pharm Res. 1994;11:839–843. doi: 10.1023/a:1018925723889. [DOI] [PubMed] [Google Scholar]
- 12.Cundy K C, Shaw J-P, Lee W A. Oral, subcutaneous and intramuscular bioavailability of the antiviral nucleotide analog 9-(2-phosphonylmethoxyethyl)adenine (PMEA) in cynomolgus monkeys. Antimicrob Agents Chemother. 1994;38:365–368. doi: 10.1128/aac.38.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cundy K C, Sue I-L, Visor G C, Mashburn J, Nakamun C, Lee W A, Shaw J-P. Oral formulations of adefovir dipivoxil: in vitro dissolution and in vivo bioavailability in dogs. J Pharm Sci. 1997;86:1334–1338. doi: 10.1021/js970264s. [DOI] [PubMed] [Google Scholar]
- 14.D'Argenio D Z, Schumitzky A. ADAPT II users guide: pharmacokinetics/pharmacodynamic systems analysis software. Los Angeles, Calif: Biomedical Simulations Resource; 1997. [Google Scholar]
- 15.Deeks S G, Collier A, Lalezari J, Pavia A, Rodrigue D, Drew W L, Toole J, Jaffe H S, Mulato A S, Lamy P D, Li W, Cherrington J M, Hellmann N, Kahn J. The safety and efficacy of adefovir dipivoxil, a novel anti-human immunodeficiency virus therapy, in HIV-infected adults: a randomized, double-blind, placebo-controlled trial. J Infect Dis. 1997;176:1517–1523. doi: 10.1086/514150. [DOI] [PubMed] [Google Scholar]
- 16.Gilead Sciences, Inc. A phase I/II study of the safety, tolerance, pharmacokinetics and anti-HIV activity of 9-[2-(bispivaloyloxymethyl)phosphonomethoxy)ethyl]adenine (bis-POM PMEA) and placebo in HIV-infected patients. Gilead Sciences Protocol GS-94-402. Foster City, Calif: Gilead Sciences, Inc.; 1994. [Google Scholar]
- 17.Kahn, J., S. Lagakos, M. Wulfsohn, D. Cherng, M. Miller, J. Cherrington, D. Hardy, G. Beall, R. Cooper, R. Murphy, N. Basgoz, E. Ng, S. Deeks, D. Winslow, J. Toole, and D. Coakley. Efficacy and safety of adefovir dipivoxil when added to antiretroviral therapy for the treatment of HIV-1 infected patients with CD4+ cells greater than 200 cells/mm3: a multi-center, randomized double blind, and placebo controlled study. JAMA, in press. [DOI] [PubMed]
- 18.Shaw J-P, Cundy K S. Biological screens of PMEA prodrugs. Pharm Res. 1993;10:S294. [Google Scholar]
- 19.Shaw J-P, Cundy K C. Oral bioavailability of PMEA from a prototype oral suspension formulation of the prodrug bis(POM)PMEA in fasted male cynomolgus monkeys. Gilead Sciences Study Report DDM-JPS-121593. Foster City, Calif: Gilead Sciences, Inc.; 1994. [Google Scholar]
- 20.Shaw J-P, Cundy K C. Oral bioavailability of PMEA from various formulations of PMEA prodrugs bis(POM)PMEA in fasted male cynomolgus monkeys. Gilead Sciences Study Report DDM-JPS-121593. Foster City, Calif: Gilead Sciences, Inc.; 1994. [Google Scholar]
- 21.Shaw J-P, Cundy K C. Oral bioavailability of PMEA from PMEA prodrugs in cynomolgus monkeys: salicylate esters, bis-POM PMEA sulfate and a clinical tablet formulation of bis-POM PMEA. Gilead Sciences Study Report 94-DDM-0840-001B. Foster City, Calif: Gilead Sciences, Inc.; 1994. [Google Scholar]
- 22.Shaw J-P, Cundy K C. Oral bioavailability of PMEA from PMEA prodrugs in Sprague-Dawley rats. Gilead Sciences Study Report DDM-JPS-121593. Foster City, Calif: Gilead Sciences, Inc.; 1994. [Google Scholar]
- 23.Shaw J R, Louie M S, Krishnamurthy K, Jones R J, Bidgood A M, Lee W A, Cundy K C. Pharmacokinetics and metabolism of selected prodrugs of PMEA in rats. Drug Metab Dispos. 1997;25:362–366. [PubMed] [Google Scholar]
- 24.Smith M S, Brian E L, DeClerq E, Pagano J G. Susceptibility of human immunodeficiency virus type-1 replication in vitro to acyclic adenosine analogues and synergy of the analogues with 3′-azido-3′-deoxythymidine. Antimicrob Agents Chemother. 1989;33:1482–1486. doi: 10.1128/aac.33.9.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srinivas R V, Robbins B L, Connelly M C, Gong Y F, Bischofberger N, Fridland A. Metabolism and in vitro antiretroviral activities of bis(pivaloyloxymethyl) prodrugs of acyclic nucleoside phosphonates. Antimicrob Agents Chemother. 1993;37:2247–2250. doi: 10.1128/aac.37.10.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]