Abstract
At present, resilience refers to a highly heterogeneous concept with ill-defined determinants, mechanisms and outcomes. This call for action argues for the need to define resilience as a person-centered multi-dimensional metric, informed by a dynamic lifespan perspective and combining observational and interventional experimental studies to identify specific neural markers and correlated behavioral measures. The COVID-19 pandemic highlights the urgent need of such an effort with the ultimate goal of defining a new vital sign, an individual index of resilience, as a life-long metric with the capacity to predict an individual’s risk for disability in the face of a stressor, insult, injury or disease.
The Importance of Human Brain Resilience: The time is now!
As of the end of 2020, the coronavirus (severe acute respiratory syndrome-coronavirus 2 [SARS-CoV-2], coronavirus disease 2019 [COVID-19]) pandemic had resulted in over 88 million confirmed cases and nearly 2 million deaths worldwide. 1 In response to the pandemic, most countries issued physical-distancing directives, often including stay-at-home orders and strict lockdown measures. Thus, many of us have been faced with months of fears of infection and its potential health consequences, stress related to the impact of the pandemic on the health and economic well-being of ourselves and our loved ones, and concerns about long-lasting social consequences. Now, amid the hope of vaccination, we face the uncertainty of its timelines and effectiveness, and fear the threat of further pandemic waves and new virus strains.
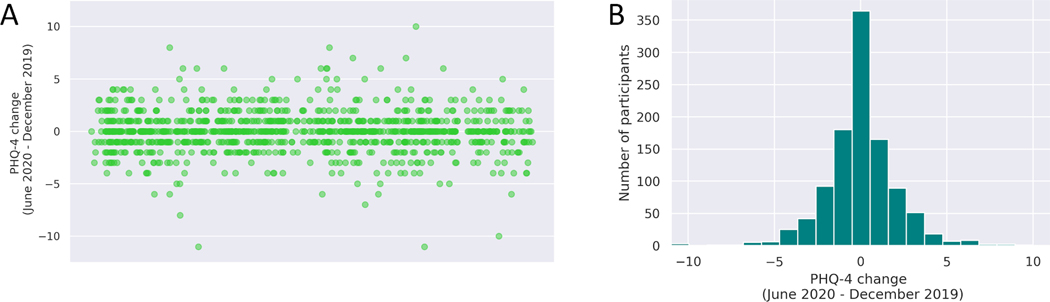
Deleterious mental and brain health consequences of such challenging circumstances are to be expected2 and yet, not everyone is or will be affected equally. Data from the Barcelona Brain Health Initiative (www.bbhi.cat/eng), a longitudinal cohort study of healthy adults assessing genetic, biological and lifestyle determinants of brain health, supports this notion (Figure 1). We have collected mental health information using validated web-based questionnaires at various time-points following the decree by the Spanish government of a national state of emergency, the strictly enforced lock-down mandate, and the phased re-opening of the country. From all participants we had the same information for two years prior to the COVID-19 pandemic,3,4 and are thus able to examine the impact the pandemic and the confinement have had on this well-characterized cohort of neurologically and psychiatrically intact middle-aged adults. As illustrated in Figure 1, while some individuals clearly have experienced negative mental health consequences, almost equal numbers seem to be doing better than they were before.
Figure 1.
Changes in mental health (as captured by the PHQ4 scale) from 2018 and 2019 to June 2020, following the onset of the COVID-19 pandemic and decree national state of emergency with strictly enforced lock-down mandate. Data from a subsample of 1026 participants of the Barcelona Brain Health Initiative (www.bbhi.cat/eng)3,4. A: Scattergram of change in PHQ4 score, where positive values indicate higher levels of psychological distress during the pandemic than at baseline over the preceding two years. B: Histograms of number of participants with a given change in PHQ-4. As can be seen, while some participants’ mental health and wellbeing was substantially worsened by the pandemic, others were doing better than before COVID-19.
What differentiates people who do well from those who do not when faced with the same circumstances and stressors?
Bzdok and Dunbar have highlighted the importance of social bonds.5 They argue that “in times of distress, crisis, or disaster, human resilience depends on the richness and strength of social connections, as well as active engagement in groups and communities.” There certainly is a rich literature on the negative impact of the subjective feeling of loneliness on an individual’s ability to cope with stressor, insult and injury, and sustain mental health6–9, and it is tempting to conclude that promoting social bonds and relations would enhance such resilience. However, the experimental evidence for that is still rather meager. The fact is, that to date we simply do not know what makes some people more resilient than others, and yet, the question is of fundamental importance well beyond the challenges of the COVID-19 pandemic. As Nassim Taleb argues in “The Black Swan” 10 human life is filled with events (‘black swans’) that are unpredictable, carry a massive impact, and for which after their occurrence we come up with an explanation to make them look less random and unexpected. Humans are notoriously bad at truly considering and estimating possibilities, and we fool ourselves into thinking we know more than we actually do and will be less affected than we end up being.11 Given such shortcomings, black swan events – like the COVID-19 pandemic - continue to occur: huge disasters for which we were utterly unprepared and have devastating health, social, and economic consequences. Taleb argues we can learn to navigate and even exploit a ‘black swan world’. For now, though, we should avoid trying to go ‘back to normal’ and instead learn from mistakes. The consequences of this and future unanticipated disasters (black swan events) will be less damaging if we gain a deeper understanding of what is brain resilience and how we can promote it. This manuscript is our call for such action and we identify four main needs: (1) the need to get conceptual clarity by defining terms on the basis of mechanistic and theoretical considerations; (2) the need for life-long, developmental perspectives; (3) the importance of experimental interventions and the utility of translatable, perturbation biomarkers; and finally, (4) the importance of an holistic mindset that considers biological and social determinants of health and spans individual experimentation and public health policy.
1. Defining Resilience: A call for theoretical precision anchored in mechanistic considerations.
The Oxford English Dictionary,12 defines resilience as “The quality or fact of being able to recover quickly or easily from, or resist being affected by, a misfortune, shock, illness, etc.”. Across different fields of study the term is a highly heterogeneous construct with distinct meanings. An effort of theoretical clarification is needed for the concept to be truly useful and guide neurobiological mechanistic investigation. A number of institutions and agencies have recognized the importance of such an effort. For example, the National Aging Institute is funding a collaboratory (see https://reserveandresilience.com) to promote an active exchange of ideas and develop consensus agreements leading to operational definitions.
In healthy individuals, resilience may correspond to a family of processes to resist the development of illness or distress. In individuals with established diseases, resilience may be associated with different degrees of pathological changes or variable degrees of symptomatology or disability in the face of equal pathological load. In clinical psychology and mental health, the concept of resilience has been historically linked to the study of individual differences (e.g., self-esteem, sense of control, perception of social support, etc.) that determine the capacity to cope with the impact of life traumas in order to maintain normal psychological and physical functioning and avoid serious mental illness. Rather than a capacity, resilience may represent a process of adapting well in the face of adversity, trauma, tragedy, threats or significant sources of stress (see American Psychological Association https://www.apa.org/topics/resilience). This underlies for example the ‘salutogenesis perspective’ 13, which encompasses the concept of a dynamic process related to a combination of individual characteristics (personality, motivational, self-efficacy, etc.), family, and social resources, that allow reacting positively or even thriving in the face of adversity14. This latter concept has been referred with the term Posttraumatic Growth (as opposite to post traumatic stress symptoms),15 referring to the positive personal changes that occur after experiencing a potentially traumatic event. Such changes can include development of new relations to others, embracing new interests, new appreciation of life values, or changed spiritual beliefs.16 Resilience may thus be an active process17 that not only involves the engagement of specific coping mechanisms but can promote successful adaptations and actual personal growth.18 Resilience may even include a proactive aspect related to the identification and prevention of risk.19
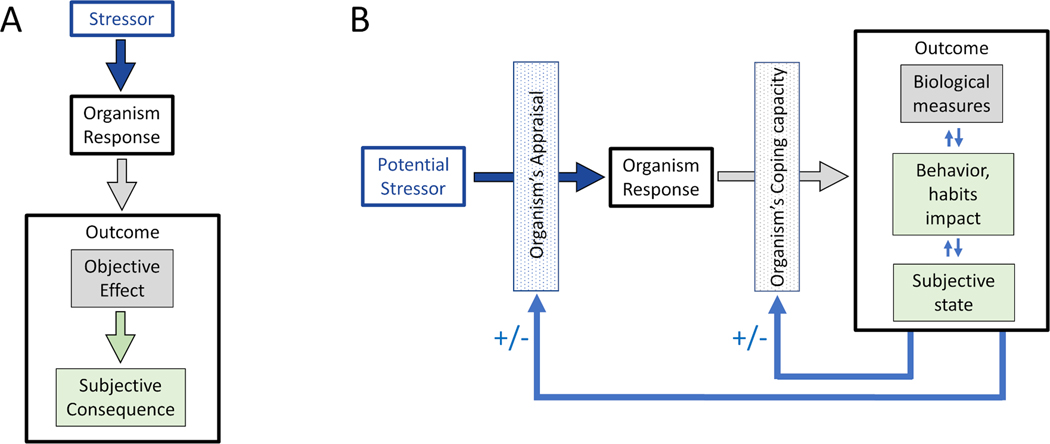
We propose resilience be conceptualized as a critical homeostatic mechanism. One can align Walter Cannon’s four general features of homeostasis to resilience (Table 1) and thus identify knowledge gaps and needed research. The stress-response paradigm, where the responses of a cell, biological system, entire individual or even communities or societies are evaluated after the exposure to stressors, offers a useful framework for the definition and study of resilience. It consists of three principal elements (Figure 2A): 1) a stressor; 2) an organism response; and 3) a given outcome. The stressor can be acute (e.g., viral infection or a vaccination) or chronic (e.g., a long-lasting disease or lasting consequences of an illness); psychological (e.g., life event or traumatic loss) or physical (e.g., injury or degree of pathological burden). Note also that the stressor does not need to be limited to an external agent, exposure, insult or influence, but could be the result of internal, organismic activity (e.g., gene expression). The organism’s response can be conceptualized as impact on brain structure or function and at any of its levels of analysis, including gene expression, molecular, metabolic, cellular, nuclei or network functions. Finally, the outcome could be objective – for example a metric of cognitive function or behavioral assessment – or subjective, i.e., related to wellbeing, and may include the change and adaptability responses related to personal or posttraumatic growth.
Table 1:
The four general features of homeostasis as defined by Walter Cannon and their adaptation to the concept of resilience.
| Homeostasis | Resilience | |
|---|---|---|
| General Feature | General Feature | Knowledge Gap |
| Constancy in an open system that requires mechanisms that act to maintain this system. | Resilience maximizes the likelihood to sustain brain and mental health across the lifespan and minimizes the risk of brain-related disability when faced with injury, insult or disease. | Is resilience-mediated brain and mental health a default state of human brain/behavior? If so, is lack of maintenance of brain and mental health a manifestation of pathology? |
| Steady-state conditions where any tendency toward change automatically meets with factors that resist change. | Resilience is the brain response to stressors that aims to (1) resist behavioral modification and (2) sustain wellbeing | What are all the cognitive and mental processes and their underlying brain mechanisms that contribute to define resilience? |
| The regulating system that determines the homeostatic state consists of many cooperating mechanisms acting simultaneously or successively. | Brain function is organized in dynamic brain networks defined by specific spatio-temporal characteristics | Is there a specific brain network or coupling patterns between networks that convey greater resilience? What is the oscillatory brain signature? How do the factors that contribute to resilience interact and relate to each other? |
| Homeostasis does not occur by chance but is the result of organized self-government. | Network reorganization and the mechanisms of neuroplasticity provide the substrate for resilience | What are adaptive plastic changes that promote resilience? How do changes in efficacy of mechanisms of plasticity relate to resilience? |
Figure 2.
Schematic representation of the stress-response paradigm (2A) and its modification to reflect resilience as an evolving, active process (2B).
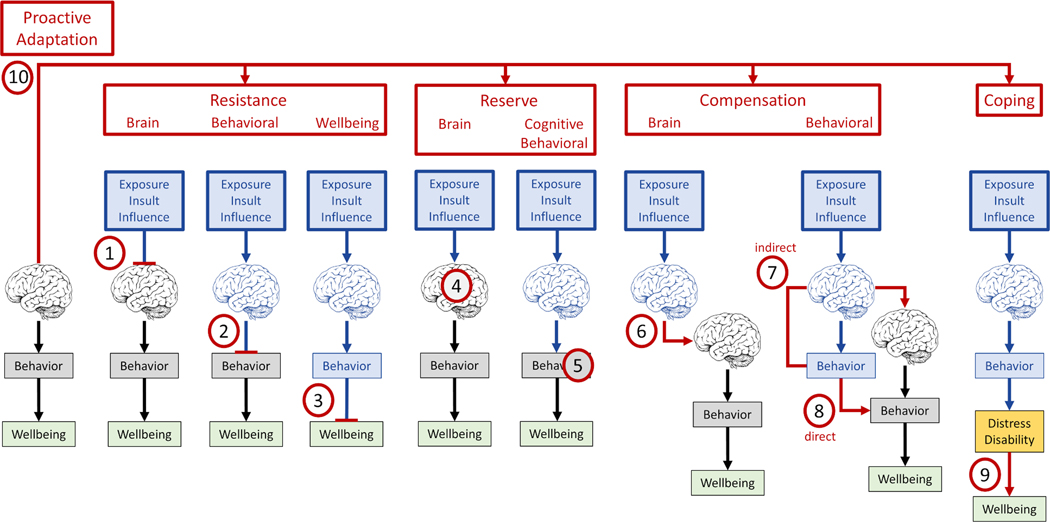
The term resilience offers a useful heuristic concept related to the capacity of the brain to resist, overcome or thrive in the face of adversity. However, there is substantial variation in how this general characteristic is expressed across different stages of development during the lifespan as well as in the face of distinct types of adversity (e.g., psychological stressors versus brain pathology), in healthy individuals versus those affected by distinct diseases or conditions. Thus, we argue there are different neurobiological mechanisms involved and often lumped together under the term resilience. In Figure 3 we schematically represent some of these various theoretical notions and offer some suggestions for possible terms to separate concepts under the umbrella of resilience. We suggest a distinction between Resistance, Reserve, Compensation, Coping and Proactive Adaptation with various subcategories depending on whether brain, behavior or wellbeing are primarily involved. We are cognizant that a clear separation of some of these theoretical mechanisms may not be possible and offer them to encourage debate and experimental exploration.
Figure 3.
Schematic representation of ten theoretical mechanisms conferring resilience and proposed terms to refer to them and differentiate them. Please see text for details. We are cognizant that a clear separation of some of these theoretical mechanisms may not be possible and offer them to encourage debate and experimental exploration. Changes in brain or behavior are marked in blue.
Resistance refers to mechanisms to resist or block the very impact of an external or internal exposure, insult or influence either onto the brain, the behavioral level or wellbeing. The notion of brain resistance (Figure 3-1) includes the definition of resistance offered by Arenaza-Urquijo and Vemuri 20 who in cognitive aging and dementia research have proposed ‘resistance’ to refer to the mechanism or capacity that enables some individuals to avoid or minimize the development of pathology. This would be distinct from functional and structural brain changes as well as psychological determinants that may minimize the consequences of AD pathology.21 We agree with that concept, but do not mean brain resistance as restricted to the case of dementia alone. Thus, for example, consider two individuals exposed to chronic stress which they asses as equally severe on the same validated scale and affects hypothalamic-pituitary-adrenal axis modifying cortisol secretion. One may show additional brain changes involving key areas such as the fronto-striatal or hippocampal systems, while the other may not show such brain changes because of brain resistance. In other words, brain resistance can refer to the prevention of any type of brain damage that can results from any kind of external or internal exposure, insult or influence. In behavioral resistance (Figure 3-2) brain structure or function may be affected (e.g., by a lesion, pathological change or exposure to adversity) but the impact on behavior would be blocked (i.e., no behavioral reorganization or adaptation is present), and thus behavior and in turn wellbeing will be unaffected. Finally, in wellbeing resistance (Figure 3-3) modification of brain and behavior would be blocked from impacting wellbeing (e.g., exposure to adversity and related behavioral changes do not lead to wellbeing readjustments for the subject). We realize that a lack of impact on behavior or well-being might involve brain changes, possibly by tapping into mechanisms of reserve or compensation.
Reserve refers to brain mechanisms, proprieties and capacities that allow for behavior, cognition and wellbeing to be better than expected given the severity of insult or disease. The notion is that an exposure, insult or influence alter some aspect of brain structure or function, but the impact is minimized thanks mechanisms that can leverage preexisting brain characteristics (e.g., thicker cortex, greater white regional matter integrity, etc.) in the case of brain reserve (Figure 3-4) or active cognitive (or affective) aspects (e.g., more efficient cognitive processing capacity) in the case of cognitive/behavioral reserve (Figure 3-5).
Compensation refers to a process where following the impact of an external or internal exposure, insult or influence and its resulting initial consequences, subsequent changes take place restoring brain activity (brain compensation, Figure 3-6) or modifying behavior and thus overcoming initial changes (behavioral compensation). The later can be direct when behavioral modification overcomes initial behavioral impact (Figure 3-8) or indirect, when behavioral impact leads to brain changes which in turn overcome initial behavioral consequences (Figure 3-7). Note that direct compensation (e.g., brain overactivations that compensate for brain damage to sustain cognition) may also be considered as a mechanism of reserve in some views.
In Coping-Personal Growth (Figure 3-9), an exposure, insult or influence alters some aspect of brain function, and consequently affects behavior and causes distress and disability, but the individual is able to sustain or restore wellbeing through improved coping strategies (e.g., behaviors, thoughts, attitudes, and emotions that one can use to modify stressors, such as humor, seeking support from loved ones, or engaging in relaxing activities) and environment adaptability.
Finally, with Proactive Adaptation (Figure 3-10) we refer to the fact that the brain may actively anticipate and counter potential exposures or engage specific resilience mechanisms as an evolving, active process.
2. Measuring Human Brain Resilience: A call for a lifespan approach.
The most common approximation to measure resilience is the use of scales and questionnaires, for example capturing personality-related dimensions (e.g. ‘feeling of control’), measures of social competence, and one’s perceptions of coping abilities and resources.22 However, the different ‘resilience scales’ share only moderate correlations between them (see for example23) and have limited utility to detect clinically meaningful changes over time.24 We believe the assessment of resilience should be approximated from the stress-response paradigm and the homeostasis framework (see Table 1 and Figure 2). Here, the concept of allostasis is useful, referring to the continuous adjustments made by the organism in the internal physiological milieu, to daily living experiences, as well as to risk behaviors or adverse conditions (e.g. loss of sleep, social isolation, income inequality, etc.). It should be noted that this approach requires longitudinal designs and repetitive assessments to be able to delineate a trajectory of change, for example in regard to the moment of stressor exposure.17
Behavioral and psychological assessments of resilience should be combined with biological markers. In fact, the model of allostatic load defined a panel of 10 markers, including indexes of cardiovascular, metabolic, hypothalamic-pituitary-adrenal (HPA)-axis activity and sympathetic nervous system activity, which reflect cumulative biological burden, that were found to be associated with future risk of cognitive, physical decline and mortality amongst older adults.25 Further studies linked the allostatic load index with measures of brain integrity, for example gray matter density in old age26 or reduced white matter integrity among overweight individuals.27
It is critical to consider cumulative influences and brain changes across the lifespan. So, for example, given the view of resilience as adaptability responses to psychological stress and vulnerability to psychopathology, the HPA-axis and the regulation of glucocorticoid release must play a major role. However, age-related changes in the reactivity of such neuroendocrine mechanisms have to be contemplated, as have concepts linked to endocrine ‘memory’, where a given neuroendocrine response leaves a trace that may affect function and response to new influences and stressors long term.28 The locus coeruleus’ noradrenaline and serotoninergic systems, the dopaminergic pathway (and their genetic variations29), and other systems linked to learning, extinction and reconsolidation memory processes are similarly important and change over time. Charney and colleagues30 review eleven possible neurochemical, neuropeptide, and hormonal mediators of the psychobiological response to stress and discuss their relation to resilience or vulnerability. For example, in the context of post-traumatic stress disorder, memories are reactivated by cues associated with the trauma and these effects are thought to be mediated by NMDA and β-adrenergic receptor activation. However, as reconsolidation processes put memories in a labile state, where they can be strengthened but also weakened, pharmacotherapeutic and other interventions that suppress the cascade of intracellular events involving these receptors may reduce the strength of the original traumatic memory, hence contributing to promote a resilient, adaptative response. Similarly, genetic variations in the gene coding for monoamine oxidase A (MAOA), the enzyme involved in the degradation of norepinephrine, serotonin and dopamine, can impact enzymatic activity, and influence the recruitment of brain regions involved in the processing of emotional (sad, angry) stimuli31 as well as result in abnormal recruitment of areas involved in inhibitory control.32 Targeted modulation of activity in such brain regions can modify individual resilience.
Resilience is linked to preservation of efficient mechanisms of plasticity33, i.e., the nervous systems’ ability to make rapid adaptations to changing internal (organismic) and external environmental demands34. The efficiency of the mechanisms of plasticity is known to change over the lifespan and be influenced by a number of inputs and conditions.35 Therefore, resilience is expected to be similarly influenced by age, chronic illnesses or lifestyles. For example, animal studies have shown that brain-derived neurotrophic factor (BDNF), a mediator of plasticity, influences the chronic stress-induced dendritic remodeling in the hippocampus and haplo-insufficient BDNF mice fail to show the expected decrease in the number of apical dendritic branches and spine densities in response to repeated restraint stress36. Relatedly, in humans,37 it was recently observed that women carriers of the BDNF Val66Met genotype Val allele were more resistant to intermittent pain-related stress associated with primary dysmenorrhea as compared to Met carriers, an effect that was associated with greater structural plasticity of the hippocampus.
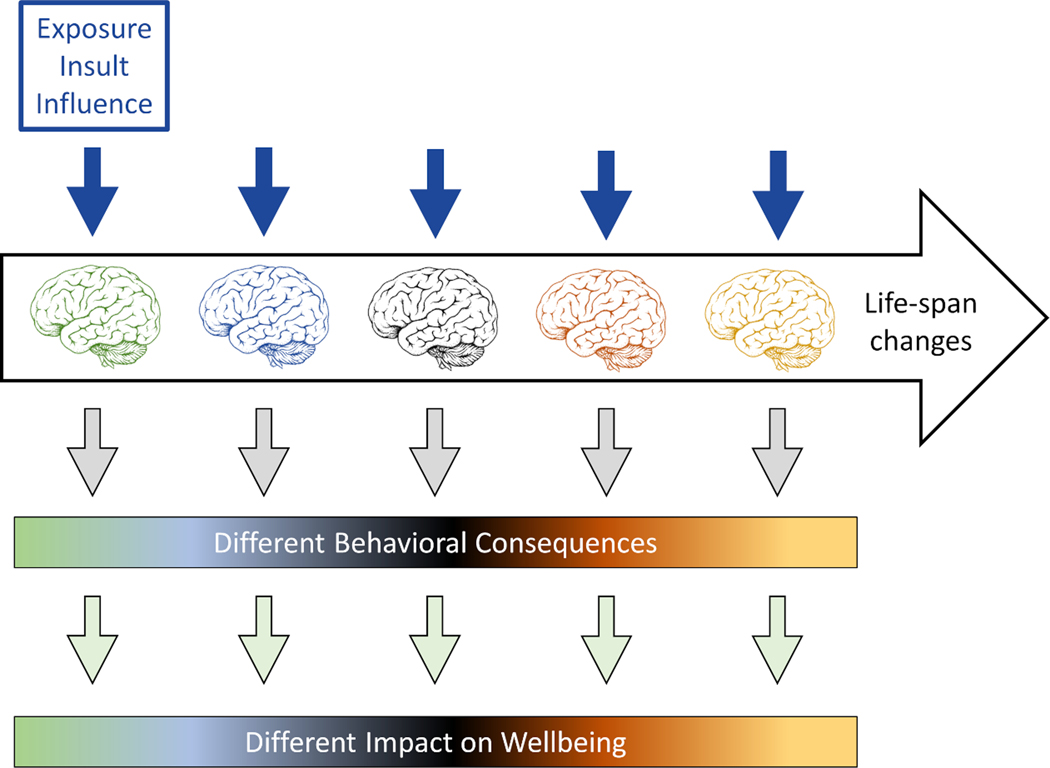
In summary, we argue for a multi-dimensional metric informed by a dynamic, life-long developmental conceptualization in which processes evolve and influence each other across the entire lifespan (Figure 4). Assessing resilience demands a deeper understanding of brain development from infancy (and even in utero) through childhood and then the continuing evolution of brain structure and function into adulthood and old age. It is important to understand the range of variability in organismic response and behavioral consequences to a given stressor that may be consequence of maturational and other developmental processes. This need is supported by compelling evidence that early life factors, including adverse childhood experiences in interaction with environmental aspects such as parental support, are critical to explain individual differences in brain and mental health. For example, cumulative exposure to residential segregation throughout young adulthood is associated with worse processing speed detected in midlife.38 Such early life factors interact with midlife and later life engagement in psychosocial factors, lifestyles and socioeconomic factors.39–41. For example, even though education during the early stages of life appears to be a critical factor that contributes to reduce the risk of dementia,42 Valenzuela et al.43 found, in a population of over 12.000 individuals followed during a 10 year period, that a global composite of cognitive activity that included education, occupational complexity, and social engagement across three stages of life (young adulthood, middle age and after retirement) was more meaningful than early (childhood) education alone and associated with greater protection for dementia. Further analyses indicated that occupation alone, and education plus cognitive activity engagement at late life were also protective factors. Other findings from the same research team,44 indicate protective effects of high level supervisory occupational experience occurring during midlife on late-life hippocampal atrophy. These findings, using outcomes that are relevant in the context of resilience (i.e. incident dementia and hippocampal atrophy) illustrate the need to consider the combined effects of psychosocial engagement at different life-course stages. Postulating resilience only makes sense after accounting for that full range of lifespan ‘developmental’ variability.
Figure 4.
Schematic illustration of the importance of lifespan considerations in the assessment of resilience. Throughout life, there is no period when the brain and its functions are static. The nature, extent and the rates of change vary by region and function and are influenced by genetic and well as environmental factors, both due to physiologic influences and by diseases-related pathological processes. Therefore, characterization and measurement of resilience needs to take a lifespan approach. Postulating ‘resilience’ only makes sense once one accounts for the full range of variability in organismic response and behavioral consequences that may be explainable by maturational and other developmental processes.
3. The Neural Substrate of Human Brain Resilience: A call for experimental approaches with translational perturbation biomarkers
Neuroimaging investigations have identified brain regions that show specific activity and connectivity patterns during exposure to stressful or violent stimuli and that may be correlated with scores in psychosocial scales of resilience or predict subsequent coping abilities. For example, Shina et al.45 found that activity in the ventromedial prefrontal cortex (VmPFC) predicts subsequent adaptative coping behaviors in real life conditions. Goldfarb et al.46 reported that hippocampal connectivity can predict individual variability in subsequent feelings of stress. Santarnecchi et al.47 observed that individual variability in the ratings of coping styles, a psychological construct associated with the capacity to tolerate stress and conflict, is related to spontaneous brain activity within and between areas belonging to the default-mode network (DMN, inferior parietal lobe) and the salience network (SN, anterior cingulate cortex). Similarly, Kong et al.48 observed that scores on a resilience scale were related with negative regional spontaneous activity within the orbitofrontal cortex (OFC) and that these associations mediated links between OFC activity and feelings of life satisfaction and hedonic balance.
Other studies have focused on identifying differences between subjects who have been exposed to different types of trauma with the underlying hypothesis that those who remain asymptomatic following trauma exposure are resilient (as compared with those who show symptoms of, for example, of post-traumatic stress disorder). These studies have revealed structural and functional differences in both cortical and subcortical structures implicated in stress processing and emotional regulation, including the amygdala, hippocampus, insular cortex and anterior and medial prefrontal cortices (see for review49) as well as functional differences in the coupling of mesocorticolimbic system reward areas (ventral striatum, ventral tegmental area) with the hippocampus.50 From a neurodevelopmental perspective a neural circuit involving the anterior cingulate (perigenual ACC) and other prefrontal areas, limbic structures such as the amygdala and hippocampus, and reward areas of the ventral striatum, has been proposed as a domain-general network for social resilience and risk factors, mediating the positive early life and in general, lifespan impact of environmental protective factors (e.g. maternal warmth, self-esteem) amongst at-risk individuals exposed to social stressors (e.g. ostracism, poverty, maternal depression).29 However, studies of children and adolescents at high risk for psychopathology due to socioeconomical disadvantage or threat-related adversity51 have also associated trait resilience measures with functional neural dynamics and interactions between neurocognitive networks, describing for example lower connectivity between the SN and the anterior DMN and central executive networks (CEN). Here, and comparing children and adolescents (some with and some without histories of adversity exposure) the authors51 found associations between trait-resilience (estimated using the Connor-Davidson Resilience Scale) and resting-state functional-connectivity MRI metrics of the mentioned networks, but only when the analysis included a ‘dynamic’ aspect (i.e. changing states of connectivity across fMRI acquisition time) but not with more classical ‘static’ resting-state network analysis. The finding of associations with ‘changing connectivity states’ suggests that measures of trait resilience may relate to the dynamic status of brain networks subtending cognitive processes, such as higher order control over internal stimuli.
Studies in older adults at risk for dementia have also addressed the neuroimaging substrate of resilience. In one study it was observed that carriers of the Apolipoprotein (ApoE) ε4 allele, the major risk factor for Alzheimer’s disease, exhibited positive associations between regional metabolism (FDG-PET) in frontal and temporal regions as a function of years of education. Brain metabolic patterns in these areas were also related to better episodic memory performance and were observed already in the group of ApoE ε4 bearers below 55 years of age.52 Such findings suggest that through reorganization in brain metabolic patterns, higher education may help counteract or remain resilient to the expected deleterious effect of pathology onto episodic memory. In other investigations, a ‘resilience signature’ has been described from a pattern of high regional glucose metabolism in anterior cingulate cortex and anterior temporal lobe areas characteristic of advanced age (80+) individuals who remain cognitively stable.53 Finally, some studies have reinforced the role of the frontal cortex, specifically the functional connectivity of the dorsolateral prefrontal cortex to the rest of the brain or to particular networks (DMN, SN), as a neural substrate of higher resilience, both in normal aging54 as well as against the adverse effect of early-stage entorhinal tau pathology on memory performance.55
All these are valuable approaches, and it is tempting to leverage such neuroimaging studies to try to identify a “human brain network of resilience”. However, animal work on the neural substrate of resilience illustrates the importance of interventional experimental designs that employ stimuli that can be precisely quantified and controlled, this is the case for example for a mild electric shock applied to the tail of mice or rats in stressor paradigms (tailshock paradigms). Studies have revealed that the experience of control protects the animal from the behavioral and health consequences of the stressor, and even induces a protection from the consequences of future uncontrollable stressors that is termed ‘behavioral immunization.’56 Novel neuroscience approaches that enable probing brain circuit function with cell-type and pathway specificity are providing unprecedented insights into the neural substrate of such stress-related resilience.57 For example, within the context of the tailshock paradigm, the use of techniques such as retrograde tracing with immediate gene expression analyses allows precise characterization of contributions of cortico-subcortical systems in the context of controllable versus uncontrollable stressor exposures, and a number of novel technologies, such as optogenetics, can enable experimental control over the inhibition or activation of selected cell-types, leading to separate behavioral effects. Dissecting the biological cascades in such a manner offers not only critical mechanistic insights but also suggests possible avenues to promote resilience with translational relevance to humans, where actual or perceived control over negative circumstances is a key coping element to stress.58,59 Thus, Limbachia et al.60 used an elegant moving-circles paradigm where two circles move around a screen sometime moving closer and at times moving away for each other and when they touch participants are delivered a mild electric stressor while their brain activity is monitored using functional MRI. When participants are given the possibility to control the duration of the stressor by pressing a button, stressor-related responses across threat-related brain regions decreased. Such experimental designs enable the study of the neural substrates of resilience in humans. More importantly, this illustrates the crucial elements to study human brain resilience: (1) controllable and precise experimental interventions, (2) detailed measures of physiologic impact, and (3) sensitive quantitative behavioral outcome measures (Figure 5A).Therefore, we believe that experimental perturbation approaches combining MRI-guided brain stimulation with functional connectivity MRI and high-density EEG are essential, as they allow to characterize individual brain dynamics of major brain networks with direct physiologic measures of high temporal resolution and in a highly reproducible manner.
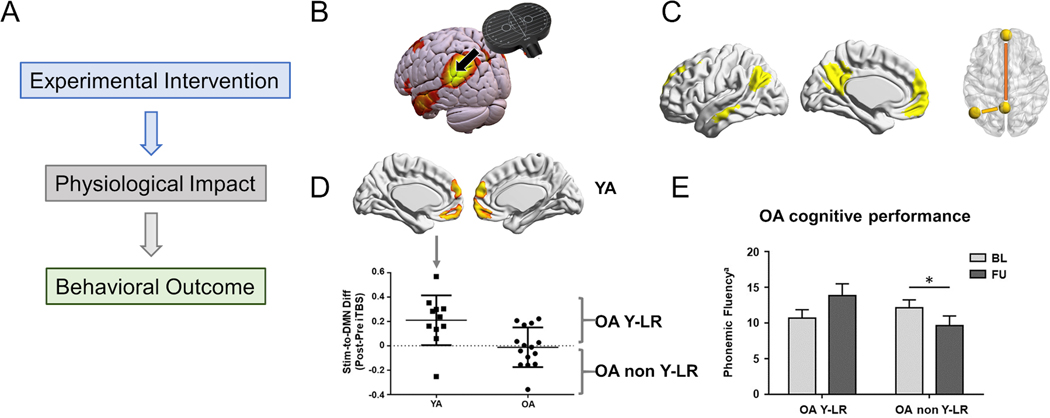
Figure 5.
A: Crucial elements for mechanistic insights into human brain resilience: (1) controllable and precise experimental interventions, (2) detailed measures of physiological impact, and (3) sensitive quantitative behavioral outcome measures. B-E: Modified from Abellaneda-Pérez et al.61: B: Schematic illustration of the study methodology using rs-fcMRI to define a target node of the default mode network (DMN) in the intra-parietal lobule for neuronavigated TMS. C: Core connectivity of the DMN studied. Left: Seed-to-DMN approach, Right: DMN-centered seed-to-seed approach, where the long-range DMN connectivity measure (mPFC-to-PCC) is shown in orange, and the local DMN connectivity measure (Stim-to-PCC) is shown in yellow. D: Seed-to-DMN analyses after iTBS. Top and bottom-left: Younger adults increased rs-FC within the anterior regions of the DMN (i.e., standard TMS response). Bottom-right: In the older adults, two distinct patterns of response were seen, some resembling the young (young-like responders, Y-LR) and others not (non Y-LR). E: Bar chart showing significant interactions and pairwise post-hoc comparisons between the older adults with ‘young-like’ and ‘non-young-like’ responses with regards to phonemic fluency at baseline and at three-years follow-up assessments. Phonemic fluency for letter M is displayed as mean with SEM. *Significant differences (p < 0.05). Abbreviations: Stim, stimulation site; DMN, default-mode network; Diff, difference; iTBS, intermittent theta-burst stimulation; FU, follow-up; BL, baseline; YA, younger adults; OA, older adults; OA YL-R, older adults with ‘young-like’ responses; OA non YL-R, older adults with ‘non-young-like’ responses.
A recent study illustrates the value of this perturbation approach. Abellaneda-Perez et al. 61 (Figure 5) compared the effects of an MRI-guided short train of intermittent theta burst stimulation (iTBS) to the intraparietal lobule on brain activity as measured by resting-state functional MRI (rs-fMRI). Among younger adults ages 20–27 years functional connectivity increased following iTBS in DMN nodes distant from the stimulation site. In contrast, older adults (age 60–79 years) on average exhibited increases in connectivity following iTBS only in DMN regions proximal to the stimulation site. Critically, older adults with functional responses to iTBS resembling those of the younger participants exhibited not only higher cognitive performance at baseline but also significantly less cognitive decline over the subsequent three years. Such ‘young-like’ functional responses to iTBS were related to the educational background attained by the older adults.
The blood oxygenation level-dependent (BOLD) signal of fMRI provides an indirect measure of neuronal firing and reflects slow-evolving hemodynamic activity that fails to capture the faster timescale of normal physiological function. However, similar perturbation approaches can be made combining brain stimulation with EEG thus gaining the added value of temporal information to characterize the ability of a given brain to cope with disruption and relate it to cognitive trajectories as an experimental metric predictive of resilience. Specifically, advances in high density EEG (hdEEG) recording and the analysis of the spatial distribution of the scalp electric field with source imaging methods can provide important insights into the temporal dynamics of neuronal activity in an individual’s brain.62,63 Such space-oriented analysis permits to disentangle frequency oscillations of different large-scale brain networks.64 Sophisticated spatial analysis tools allow sensing activity from deep brain structures, such as the hippocampus, the thalamus or the cerebellum, from scalp hdEEG recordings.65 Functional connectivity analysis in the source space can then reveal interactions between brain networks as well as between deep structures and cortical brain regions.66 For example, Ozdemir et al. 67 used fMRI-guided TMS and simultaneous hdEEG to characterize individual brain dynamics within discrete brain networks at high temporal resolution. TMS was used to induce controlled perturbations to individually defined nodes of the DMN or the DAN. Source-level EEG propagation patterns were shown to be network-specific and highly reproducible across repeated sessions one month apart. Additionally, individual differences in high-order cognitive abilities were significantly correlated with the specificity of TMS propagation patterns across DAN and DMN, but not with resting-state EEG dynamics. Such an experimental approach – using MRI-guided TMS for a controlled perturbation to model the effects of a given stressor, and hdEEG to characterize the brain response to said perturbation – can shed light to the study human brain resilience.
Consider admission to the hospital, anticipation of surgery, and anesthesia in the context of elective orthopedic procedures (e.g., hip or knee replacement) as a stressor, and the risk of post-surgical delirium as a manifestation of resilience to that stressor. This conceptual and experimental framework is being explored by Project 5 in the ongoing extension of The Successful Aging after Elective Surgery (SAGES) Study (NIH-supported P01 AG031720; Principal Investigator: Sharon Inouye; Project 5 Co-PI’s M. Shafi and A. Pascual-Leone)68 Shafi et al. 69 proposed that post-surgical delirium may result in susceptible individuals depending on their baseline brain state and pre-existing impairments in brain reactivity, connectivity and plasticity affecting the organism’s response when exposed to the stressor. Ongoing work is evaluating the validity of this conceptual model using the described fMRI-guided TMS-hdEEG perturbation approaches where EEG metrics of resting state activity and reactivity, combined with TMS-evoked potentials metrics of the local and distributed brain response to the TMS perturbation may allow prediction of delirium risk in individual patients. Such approaches may not only shed light onto the mechanisms of resilience but offer clinically meaningful physiologic biomarkers to guide the search for interventions to decrease the risk of post-operative delirium and facilitate recovery in patients during or after an episode of delirium.
Similarly, in patients with AD and different degrees of cognitive consequences of a given pathological load as quantified for example by amyloid and Tau-PET imaging, such fMRI-guided TMS-hdEEG perturbation studies may shed light into the neural substrate of individual resilience to the pathological process. In turn, it would be possible to examine the influence by lifestyle factors and modification as well as other interventions on such fMRI-guided TMS-hdEEG-derived spatio-temporal signature(s) of resilience(s). We therefore propose the systematic and longitudinal study with such perturbation biomarkers of large cohorts of individuals enriched for AD biomarkers information (including current plasma biomarkers), lifestyle assessments, and long-term follow up evaluations, to gain new knowledge of brain resilience processes in humans. Ultimately, it might even be possible to develop brain stimulation approaches to directly modulate the spatio-temporal signatures and thus enhance resilience and minimize the risk of brain-derived disability.
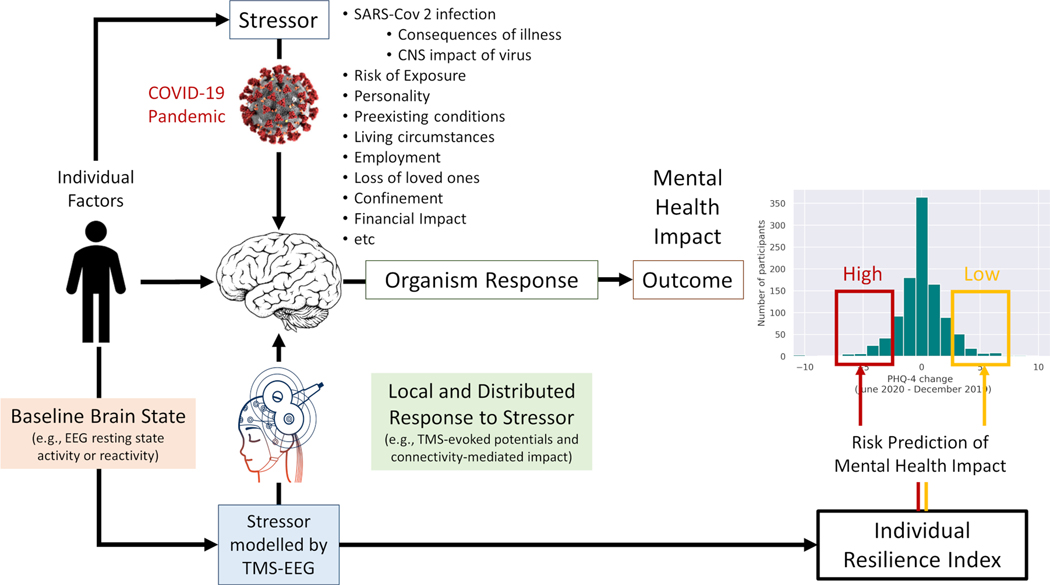
In conclusion, fMRI-guided TMS-hdEEG perturbation approaches can be applied to healthy individuals and patients alike, across the lifespan, in longitudinal studies with variable frequency and no loss of sensitivity and can be correlated with behavioral measures. Figure 6 summarizes the approach going back to the question of individual differences in the deleterious mental and brain health consequences the COVID pandemic (Figure 1). The severity of the stressor is not the same for all individuals as only some contracted SARS-CoV-2 infection and those who did had variable clinical courses with different severity of complications, possibly even with lasting CNS impact of the virus (e.g. anosmia or fatigue). In addition, there are individual differences in the risk of exposure, influenced for example by pre-existing conditions or affliction of friends and family. Further, there is the impact related to societal measures to contain the pandemic (e.g., quarantine and confinement regulations), which in turn affect people differently depending on individuals’ living conditions, socio-economic status, employment, etc. It is important to then consider the stressor-moderating influence of individual factors, such as personality traits or past experiences. In any case, experimentally, EEG can capture an individual’s baseline brain state and the local and distributed impact of the TMS pulses as metrics of the organism’s response to a stressor (with TMS perturbations modelling the stressor). Such physiologic TMS-hdEEG biomarkers can then be integrated with individual factors into an individual index of resilience to predict the individual risk of cognitive and mental health impact of the COVID-pandemic. Certainly, other methods of controlled perturbation and other metrics of brain and cognitive/behavioral outcomes can be considered, but it is important they fulfill the crucial elements mentioned above for deep mechanistic insights into human brain resilience: (1) controllable and precise experimental interventions, (2) detailed measures of physiologic impact, and (3) sensitive quantitative behavioral outcome measures. fMRI-guided TMS-hdEEG have the added benefit of being truly translatable biomarkers70 that can also be applied in animal models and provide experimental approaches to test computational models that predict resilience-related regions and resilience promoting brain organization based on modelled targeted attacks to brain connectivity.71,62
Figure 6.
Schematic illustration of the application of MRI-guided TMS-hdEEG in the study of resilience applied to the situation of the COVID-19 pandemic. The stressor is moderated by individual factors. The impact of the stressor can experimentally be modelled by TMS-EEG. The EEG resting state activity and reactivity, as well as the TMS-evoked potential and brain connectivity-mediated TMS impact on EEG provide biomarkers to integrate into an individual index of resilience to predict the individual risk of cognitive and mental health impact of the COVID-pandemic. Modelled after ongoing work supported by the Barcelona Brain Health Initiative (LCF/PR/PR16/11110004, La Caixa Foundation) and AGAUR – Generalitat Catalunya. TMS-EEG schematic modified from https://brainvision.com/applications/
4. The Determinants of Individual Human Brain Resilience: A call for a holistic mindset.
A one-size-fits-all approximation to the processes of resilience is likely inadequate. Instead, it seems advisable to adopt a person-centered approach, where all relevant contributing factors can ultimately be integrated into a unique, time-variant, individual index of resilience. Such an index would need to consider (1) resilience for what outcome?, e.g. maintaining or improving quality of life, reducing the risk of a neuropsychiatric diagnosis, etc.; and (2) resilience to what stressor?, e.g. defining the stressor in a quantifiable manner degree and duration, specific environmental circumstances, unfavorable lifestyles, brain pathological changes, etc.19 Ideally, such an ‘individual index of resilience’ would combine information of all possible sources of resilience for a given person and circumstance, and harbor predictive capacity. The value of such a “vital-sign” has been clearly illustrated in other medical specialties. For example, in the United States in the 40s the first cause of mortality was cardiovascular disease. Following the death of President Roosevelt due to heart attack in the setting of hypertension in 1945, the US government encouraged the proposal of a strong-impact study that could help address the challenge of cardiovascular diseases. Two years later, in 1947, a cohort longitudinal study was started in the city of Framingham, MA (and is currently still ongoing) that changed forever cardiovascular medicine and human life expectancy. One of the best-known results of this study is the Framingham risk score, a gender-specific algorithm that allows “measuring” the 10 years risk of cardio-vascular events and helps clinicians throughout the world prescribe specific and individualized treatments to prevent cardiovascular accidents in their patients. Now that brain diseases are the first cause of disability, exceeding cardiovascular disease and cancer, we need a similar “index” to measure people’s resilience and characterize their risk to develop, as well as their capacity to resist, brain pathologies and related disability. Thus, we argue for careful multi-dimensional and longitudinal (life-long) assessments of large numbers of individuals (i.e. many “N of 1 big-data experiments”) such that when sufficient data are available it might be possible to scale from individual to clusters or canonical profiles that apply to sub-cohorts of larger populations. However, it should be noted that as compared with the case of cardiovascular diseases, the scope and complexity of characterizing the processes of brain resilience and developing a meaningful individual index of it is many orders of magnitude greater.
The challenge is made even greater by the fact that focusing solely on the identification of the neural substrates of resilience – even leveraging perturbation biomarker approaches to extract intervention metrics – will not suffice to define an individual index of resilience. There is ample evidence of the importance of the genetic factors that seem to impact brain resilience even if their mechanisms of action remain unclear. Furthermore, according to some estimates, up to 80% of brain health is determined by modifiable factors and, therefore, they are likely to impact individual resilience. For example, there is abundant epidemiological evidence that adherence to certain lifestyle factors, including cognitive, physical, nutritional and sleep healthy habits can exert a positive effect on both objective (e.g. cognitive performance) and subjective (e.g. sense well-being) status. In addition, a growing literature reveals the positive influence of many of these same lifestyle factors, as well as the engagement of individuals in emotion-regulation practices such as meditation on brain structure and functional measures.73–76 Consequently, the effort towards an individual index of resilience needs to incorporate information about lifestyle, the psychological status of individuals, as well as an evaluation of general health risk factors (e.g. smoking, being overweight, or having abnormal states of hypertension, cholesterol or fasting glucose, see American Heart Association, American Stroke Association recommendations).77
In fact, we believe that such a ‘Lifestyle Medicine’ approach, needs to be expanded to consider the individual in a more holistic manner. For example, social determinants (where people live, learn, work, and play) also represent key factors of health, as highlighted by the Healthy People 2020 consortium (U.S. Department of Health & Human Services https://www.healthypeople.gov/2010/hp2020/default.asp.html). Supporting this claim, a recent meta-analysis78 reviewed all social and economic interventions conducted in the US since 1960, including programs of intensive educational, income maintenance, employment and housing/neighborhood change and health insurance coverage. Even though most of the studies reviewed were not originally designed to specifically improve health of the targeted populations, the meta-analysis revealed that almost 50% of adequately powered studies encountered positive effects in a wide range of health outcomes. Social determinants, which include the availability of daily resources, access to educational and health services, transportation, safety, exposure to segregation, violence or crime, and social support and opportunities for recreational and leisure-time activities, amongst others, ought to be considered in the evaluation of individual resilience.
Conclusion
On December 14, 2020, the United Nations announced the Decade of Healthy Aging (https://www.who.int/news/item/14–12-2020-decade-of-healthy-ageing-a-new-un-wide-initiative) as a global initiative to “add life to years” and “improve the lives of people, their families and communities, both during the COVID-19 pandemic, and beyond”. Life is unpredictable and a critical aspect to achieve such goals is a deeper understanding of human brain resilience to develop effective interventions to promote it. The time to act is now. First, conceptual clarity demand the definition of terms on the basis of careful theoretical consideration of possible mechanisms and would benefit from framing resilience as a fundamental homeostatic brain mechanism. Second, it is critical to adopt a life-long, developmental perspectives and embrace interventional experiments using translatable, perturbation biomarkers. The ultimate goal should be definition of an individual index of resilience, as a new vital sign, applicable across the lifespan with the capacity to predict an individual’s risk for disability in the face of a stressor, insult, injury or disease. This will be able to guide the development of new therapeutic approaches and public health policies to promote the human healthspan.
Acknowledgements
The authors are grateful to all Barcelona Brain Health Initiative participants, staff and researchers. We thank María Cabello-Toscano (MSc) and Dr. Kilian Abellaneda-Pérez for their help generating Figures 1 and 5, respectively. Funded by ‘la Caixa’ Foundation (LCF/PR/ PR16/11110004), the Guttmann Institute and Fundació Abertis. Dr. A. Pascual-Leone was partly supported by the National Institutes of Health (R24AG06142 and P01 AG031720). Dr. Bartrés-Faz was funded by an ICREA Academia 2019 Award and by a Spanish Ministry of Science, Innovation and Universities (RTI2018-095181-B-C21) research grant.
Footnotes
Potential Conflicts of Interest
Dr. A. Pascual-Leone is a co-founder of Linus Health and TI Solutions AG; serves on the scientific advisory boards for Starlab Neuroscience, Magstim Inc., and MedRhythms; and is listed as an inventor on several issued and pending patents on the real-time integration of noninvasive brain stimulation with electroencephalography and magnetic resonance imaging.
References
- 1.Worldometer. COVID-19 Coronavirus Pandemic. Worldometer, 2020. (https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1? ). [Google Scholar]
- 2.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020; 395(10227): 912–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cattaneo G, Bartrés-Faz D, Morris TP, et al. The Barcelona Brain Health Initiative: A Cohort Study to Define and Promote Determinants of Brain Health. Front Aging Neurosci. 2018. Oct 11;10:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattaneo G, Bartrés-Faz D, Morris TP, et al. The Barcelona Brain Health Initiative: Cohort description and first follow-up. PLoS One. 2020. Feb 11;15(2):e0228754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bzdok D, Dunbar RIM. The Neurobiology of Social Distance. Trends Cogn Sci. 2020;24:717–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakobson IS, Madsen LMR, Mau M. et al. The relationship between resilience and loneliness elucidated by a Danish version of the resilience scale for adults. BMC Psychol 2020;8: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein JY, Levin Y, Bachem R, Solomon Z. Growing Apart: A Longitudinal Assessment of the Relation Between Post-traumatic Growth and Loneliness Among Combat Veterans. Front Psychol. 2018. Jun 7;9:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerino E, Rollè L, Sechi C, Brustia P. Loneliness, Resilience, Mental Health, and Quality of Life in Old Age: A Structural Equation Model. Front Psychol. 2017. Nov 14;8:2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macià D D, Cattaneo G, Solana J, et al. Meaning in Life: A Major Predictive Factor for Loneliness Comparable to Health Status and Social Connectedness. Front Psychol. 2021. Feb 24;12:627547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taleb NN. The Black Swan. The impact of the highly improbable. (2nd Ed.) 2010. [Google Scholar]
- 11.Pascual-Leone A, Cattaneo G, Macià D, et al. Beware of Optimism Bias in the Context of the COVID-19 Pandemic. Ann Neurol. 2021. Mar;89(3):423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oxford English Dictionary. https://www.oed.com/ Copyright © 2021 Oxford University Press. All rights reserved. [Google Scholar]
- 13.Antonovsky A. The salutogenic model as a theory to guide health promotion 1. Health Promot Int. 1996;11:11–8. [Google Scholar]
- 14.Sisto A, Vicinanza F, Campanozzi LL, et al. Towards a Transversal Definition of Psychological Resilience: A Literature Review. Medicina (Kaunas). 2019;55:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph S, Linley PA. Growth following adversity: theoretical perspectives and implications for clinical practice. Clin Psychol Rev. 2006;26:1041–53. [DOI] [PubMed] [Google Scholar]
- 16.Tedeschi RG, Calhoun LG. The Posttraumatic Growth Inventory: measuring the positive legacy of trauma. J Trauma Stress. 1996;9:455–71. [DOI] [PubMed] [Google Scholar]
- 17.Kalisch R, Baker DG, Basten U, et al. The resilience framework as a strategy to combat stress-related disorders. Nat Hum Behav. 2017;1:784–790. [DOI] [PubMed] [Google Scholar]
- 18.Chmitorz A, Kunzler A, Helmreich I, et al. Intervention studies to foster resilience - A systematic review and proposal for a resilience framework in future intervention studies. Clin Psychol Rev. 2018;59:78–100. [DOI] [PubMed] [Google Scholar]
- 19.Wiig S, Aase K, Billett S, et al. Defining the boundaries and operational concepts of resilience in the resilience in healthcare research program. BMC Health Serv Res. 2020;20:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology. 2018;90:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arenaza-Urquijo EM, Przybelski SA, Machulda MM, et al. Better stress coping associated with lower tau in amyloid-positive cognitively unimpaired older adults. Neurology. 2020;94:e1571–e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoge EA, Austin ED, Pollack MH. Resilience: research evidence and conceptual considerations for posttraumatic stress disorder. Depress Anxiety. 2007;24:139–52. [DOI] [PubMed] [Google Scholar]
- 23.Nishimi K, Choi KW, Cerutti J, et al. Measures of adult psychological resilience following early-life adversity: how congruent are different measures? Psychol Med. 2020:1–10. doi: 10.1017/S0033291720001191. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Windle G, Bennett KM, Noyes J. A methodological review of resilience measurement scales. Health Qual Life Outcomes. 2011;9:8. doi: 10.1186/1477-7525-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zsoldos E, Filippini N, Mahmood A, et al. Allostatic load as a predictor of grey matter volume and white matter integrity in old age: The Whitehall II MRI study. Sci Rep. 2018;8:6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ottino-González J, Jurado MA, García-García I, et al. Allostatic load and disordered white matter microstructure in overweight adults. Sci Rep. 2018;8:15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Tissier P, Campos P, Lafont C, et al. An updated view of hypothalamic-vascular-pituitary unit function and plasticity. Nat Rev Endocrinol. 2017;5:257–267. [DOI] [PubMed] [Google Scholar]
- 29.Holz NE, Tost H, Meyer-Lindenberg A. Resilience and the brain: a key role for regulatory circuits linked to social stress and support. Mol Psychiatry. 2020;25:379–396. [DOI] [PubMed] [Google Scholar]
- 30.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. [DOI] [PubMed] [Google Scholar]
- 31.McEwen BS. In pursuit of resilience: stress, epigenetics, and brain plasticity. Ann N Y Acad Sci. 2016;1373:56–64. [DOI] [PubMed] [Google Scholar]
- 32.Lee BT, Ham BJ. Monoamine oxidase A-uVNTR genotype affects limbic brain activity in response to affective facial stimuli. Neuroreport. 2008. Mar 26;19:515–9. [DOI] [PubMed] [Google Scholar]
- 33.Passamonti L, Fera F, Magariello A, et al. Monoamine oxidase-a genetic variations influence brain activity associated with inhibitory control: new insight into the neural correlates of impulsivity. Biol Psychiatry. 2006. Feb 15;59:334–40. [DOI] [PubMed] [Google Scholar]
- 34.Pascual-Leone A, Amedi A, Fregni F, Merabet LB. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. [DOI] [PubMed] [Google Scholar]
- 35.Pascual-Leone A, Freitas C, Oberman L, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magariños AM, Li CJ, Gal Toth J, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011. Mar;21:253–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li WC, Chao HT, Lin MW, et al. Neuroprotective effect of Val variant of BDNF Val66Met polymorphism on hippocampus is modulated by the severity of menstrual pain. Neuroimage Clin. 2021. Jan 26;30:102576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caunca MR, Odden MC, Glymour MM, et al. Association of Racial Residential Segregation Throughout Young Adulthood and Cognitive Performance in Middle-aged Participants in the CARDIA Study. JAMA Neurol. 2020;77:1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caspi A, Houts RM, Ambler A, et al. Longitudinal Assessment of Mental Health Disorders and Comorbidities Across 4 Decades Among Participants in the Dunedin Birth Cohort Study. JAMA Netw Open. 2020;3:e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brain Health Across the Life Span. In ‘Brain health across the lifespan’. Chapt 7 Proceedings of a workshop. The National Academies Press. Copyright 2020 by the National Academy of Sciences [PubMed] [Google Scholar]
- 41.George KM, Lutsey PL, Kucharska-Newton A, et al. Life-Course Individual and Neighborhood Socioeconomic Status and Risk of Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol. 2020;189:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020. Aug 8;396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valenzuela M, Brayne C, Sachdev P, et al. Medical Research Council Cognitive Function and Ageing Study. Cognitive lifestyle and long-term risk of dementia and survival after diagnosis in a multicenter population-based cohort. Am J Epidemiol. 2011. May 1;173:1004–12. [DOI] [PubMed] [Google Scholar]
- 44.Suo C, Gates N, Fiatarone Singh M, et al. Midlife managerial experience is linked to late life hippocampal morphology and function. Brain Imaging Behav. 2017. Apr;11:333–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A. 2016;113:8837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldfarb EV, Rosenberg MD, Seo D, et al. Hippocampal seed connectome-based modeling predicts the feeling of stress. Nat Commun. 2020;11:2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santarnecchi E, Sprugnoli G, Tatti E, et al. Brain functional connectivity correlates of coping styles. Cogn Affect Behav Neurosci. 2018;18:495–508. [DOI] [PubMed] [Google Scholar]
- 48.Kong F, Wang X, Hu S, Liu J. Neural correlates of psychological resilience and their relation to life satisfaction in a sample of healthy young adults. Neuroimage. 2015;123:165–72. [DOI] [PubMed] [Google Scholar]
- 49.Bolsinger J, Seifritz E, Kleim B, Manoliu A. Neuroimaging Correlates of Resilience to Traumatic Events-A Comprehensive Review. Front Psychiatry. 2018. Dec 12;9:693. doi: 10.3389/fpsyt.2018.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richter A, Krämer B, Diekhof EK, Gruber O. Resilience to adversity is associated with increased activity and connectivity in the VTA and hippocampus. Neuroimage Clin. 2019;23:101920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iadipaolo AS, Marusak HA, Paulisin SM, et al. Distinct neural correlates of trait resilience within core neurocognitive networks in at-risk children and adolescents. Neuroimage Clin. 2018;20:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arenaza-Urquijo EM, Gonneaud J, Fouquet M, et al. Interaction between years of education and APOE ε4 status on frontal and temporal metabolism. Neurology. 2015;85:1392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arenaza-Urquijo EM, Przybelski SA, Lesnick TL, et al. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain. 2019;142:1134–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Franzmeier N, Hartmann J, Taylor ANW, et al. The left frontal cortex supports reserve in aging by enhancing functional network efficiency. Alzheimers Res Ther. 2018;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neitzel J, Franzmeier N, Rubinski A, Ewers M. Alzheimer’s Disease Neuroimaging Initiative (ADNI). Left frontal connectivity attenuates the adverse effect of entorhinal tau pathology on memory. Neurology. 2019;93:e347–e357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams JL, Maier SF, Transituational immunization and therapy of learned helplessness in the rat, J. Exp. Psychol. Anim. Behav. Process 3 (1977) 240–252. [Google Scholar]
- 57.Baratta M, Maier SF. New tools for understanding coping and resilience. Neuroscience Letters 2019;693:54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Southwick SM, Vythilingam M, Charney DS, The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu. Rev. Clin. Psychol. 2005;1:255–291. [DOI] [PubMed] [Google Scholar]
- 59.Diehl M, Hay EL, Risk and resilience factors in coping with daily stress in adulthood: the role of age, self-concept incoherence, and personal control, Dev. Psychol. 2010;46:1132–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Limbachia C, Morrow K, Khibovska A, et al. Controllability over stressor decreases responses in key threat-related brain areas. Commun Biol. 2021. Jan 5;4(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abellaneda-Pérez K, Vaqué-Alcázar L, Vidal-Piñeiro D, et al. Age-related differences in default-mode network connectivity in response to intermittent theta-burst stimulation and its relationships with maintained cognition and brain integrity in healthy aging. Neuroimage. 2019;188:794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michel CM, Murray MM. Towards the utilization of EEG as a brain imaging tool. Neuroimage 2012; 61:371–385. [DOI] [PubMed] [Google Scholar]
- 63.He B, Sohrabpour A, Brown E, Liu Z. Electrophysiological Source Imaging: A Noninvasive Window to Brain Dynamics. Annu Rev Biomed Eng 2018;20:171–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Michel CM, Pascual-Leone A. Predicting antidepressant response by electroencephalography. Nature Biotechnology 2020;38:417–419. [DOI] [PubMed] [Google Scholar]
- 65.Seeber M, Cantonas LM, Hoevels M, et al. Subcortical electrophysiological activity is detectable with high-density EEG source imaging. Nat Commun 2019;10:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He B, Astolfi L, Valdes-Sosa PA, et al. , Electrophysiological Brain Connectivity: Theory and Applications. IEEE Transactions on Biomedical Engineering 2019; May 7; 10.1109/TBME.2019.2913928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ozdemir RA, Tadayon E, Boucher P, et al. Individualized perturbation of the human connectome reveals reproducible biomarkers of network dynamics relevant to cognition. Proc Natl Acad Sci U S A. 2020;117:8115–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmitt Eva M., Jane S. et al. The Successful Aging after Elective Surgery (SAGES) Study: Cohort Description and Data Quality Procedures J Am Geriatr Soc. 2015; 63: 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shafi MM, Santarnecchi E, Fong TG, et al. Advancing the Neurophysiological Understanding of Delirium. J Am Geriatr Soc. 2017;65:1114–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diester I, Hefti F, Mansuy I, et al. Bridging the Gap Between Patients and Models. In Translational Neuroscience Toward New Therapies (pp. 209–244). Nikolich A, Hyman S. (eds). MIT Press, Cambridge, MA; 2015. [PubMed] [Google Scholar]
- 71.Albert R, Jeong H, Barabási AL. Error and attack tolerance of complex networks. Nature 2000;406:378–382. [DOI] [PubMed] [Google Scholar]
- 72.Santarnecchi E, Rossi S, Rossi A. The smarter, the stronger: intelligence level correlates with brain resilience to systematic insults. Cortex. 2015;64:293–309. [DOI] [PubMed] [Google Scholar]
- 73.Bartrés-Faz D, González-Escamilla G, Vaqué-Alcázar L, et al. Characterizing the Molecular Architecture of Cortical Regions Associated with High Educational Attainment in Older Individuals. J Neurosci. 2019;39:4566–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilckens KA, Stillman CM, Waiwood AM, et al. Exercise interventions preserve hippocampal volume: A meta-analysis. Hippocampus. 2020. doi: 10.1002/hipo.23292. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sala-Vila A, Valls-Pedret C, Rajaram S, et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: a randomized controlled trial. Am J Clin Nutr. 2020;111:590–600. [DOI] [PubMed] [Google Scholar]
- 76.Fjell AM, Sørensen Ø, Amlien IK, et al. Poor Self-Reported Sleep is Related to Regional Cortical Thinning in Aging but not Memory Decline-Results From the Lifebrain Consortium. Cereb Cortex. 2020. Nov 25:bhaa332. doi: 10.1093/cercor/bhaa332. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gorelick PB, Furie KL, Iadecola C, et al. Defining Optimal Brain Health in Adults: A Presidential Advisory From the American Heart Association/American Stroke Association. Stroke. 2017;48:e284–e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Courtin E, Kim S, Song S, et al. Can Social Policies Improve Health? A Systematic Review and Meta-Analysis of 38 Randomized Trials. Milbank Q. 2020;98:297–371. [DOI] [PMC free article] [PubMed] [Google Scholar]