Abstract.
This study aimed to analyze the depressive and anxiety states of adolescent girls with polycystic ovary syndrome (PCOS). This was a cross-sectional, multicenter, case–control study. A total of 100 participants (PCOS group, 51; control group, 49) aged 13–18 yr were included in the study. Body mass index was higher in patients with PCOS (P = 0.002). In the PCOS group, 28.5% of the patients had moderate-to-severe depressive symptoms, whereas the incidence was lower in controls (8.3%, P = 0.021). The State-Trait Anxiety Inventory (STAI)-State, STAI-Trait, and physical, psychosocial, and total Pediatric Quality of Life Inventory PedsQL scores were higher in the PCOS group, suggesting that anxiety was more common and the quality of life was worse in patients with PCOS than in healthy participants (P = 0.01, P = 0.03, P = 0.02, P = 0.046, and P = 0.047, respectively). The serum free testosterone (fT) levels were positively correlated with the depression and anxiety scores and negatively correlated with the psychosocial PedsQL scores. In conclusion, adolescent girls diagnosed with PCOS demonstrated higher depressive and anxiety symptoms and lower psychosocial quality of life scores than their healthy counterparts. A relationship was found between the fT level and all psychological measures.
Keywords: Polycystic ovary syndrome, adolescent, psychological well-being, depression, hyperandrogenism
Highlights
● The incidence of emotional disturbance was high among adolescents with PCOS.
● The incidence of moderate-to-severe depression was 28.5% in adolescent girls with PCOS.
● Serum free testosterone level correlated with all psychological measures.
Introduction
Polycystic ovary syndrome (PCOS), a common endocrine disorder in women, is characterized by heterogeneous complications (1). The prevalence of PCOS has increased in the general population, with reported rates of 6–10% in adult women and 6–18% in adolescent girls (2,3,4). PCOS is also common among female adolescents and is characterized by ovulatory dysfunction and hyperandrogenism. The World Health Organization defines adolescents as young persons aged between 10 and 19 yr who show critical growth, development, and pubertal changes (5). Menstrual irregularity, sonographic polycystic ovarian morphology, and acne are normal pubertal physiological changes during adolescence. For this reason, the PCOS diagnostic criteria used in adults are not applied in adolescents. International guidelines aiming to optimize the accuracy of diagnosis and to improve health care for adolescents have been published (5,6,7). According to the guidelines, evidence of menstrual irregularity in adolescents according to time after menarche and the presence of clinical or laboratory evidence of hyperandrogenism are sufficient for the diagnosis of PCOS. Pelvic investigation with ultrasonography for diagnosing PCOS is not recommended because of the physiological multifollicular appearance of the ovaries in adolescents. The follicle size and number change with age, and the greatest number of small follicles is observed during adolescence and young adulthood, with a significant decrease in follicle count with age. The ovarian volume also increases during puberty and reaches the adult volume in the years after menarche (7, 8).
The prevalence of moderate-to-severe depressive and anxiety symptoms is high in patients with PCOS (9). This may be due to various reasons, including high body mass index (BMI) and the demoralization experienced by patients with PCOS (10). The symptoms of PCOS, when severe, may lead to social withdrawal of patients. Increased BMI, hyperandrogenism, infertility, and insulin resistance (IR) have been reported to contribute to the exacerbation or development of depressive and anxiety symptoms in adult women with PCOS (11). The emotional well-being of adolescent girls with PCOS has not been evaluated in detail, and few studies in this age group have investigated the components of PCOS associated with depression.
In this study, we aimed to analyze the depressive and anxiety states of adolescent girls with PCOS and to investigate the relationship between psychological state and health-related quality of life (HRQoL) and the clinical/biochemical characteristics of this population.
Materials and Methods
Study design
This was a cross-sectional, multicenter, case-control study. A total of 100 participants, aged 13–18 yr, were included in the study. Of the participants, 51 were categorized into the PCOS group. This group comprised adolescent girls who were diagnosed with PCOS and admitted to the pediatric endocrinology clinic of Çiğli Regional Training Hospital and Akdeniz University Hospital. The control group consisted of 49 age-matched healthy volunteers who visited our hospitals for primary health-care services (e.g., vaccination). Participants in the control group had no menstrual irregularity or psychiatric disease and no clinical evidence of hyperandrogenism. All participants (control and PCOS groups) were living in the city center and were secondary or high school students. The previous clinical evaluations, health reports, and prescriptions of the participants in the control group were examined through a national data system called “e-nabiz,” which is supported by the Turkish Ministry of Health. Adolescent girls who had previously visited the psychiatry clinic and who had been diagnosed with any psychiatric disease and were using psychiatric medications were excluded from the control group. In cases in which the existence of a psychiatric problem was noticed for the first time after the questionnaire survey, the patients were referred to the psychiatry clinic for a detailed evaluation. The other exclusion criteria for both the PCOS and control groups were as follows: hyperprolactinemia, thyroid dysfunction, Cushing’s syndrome, androgen-secreting tumors, late-onset congenital adrenal hyperplasia, cognitive disability, and presence of any other coexisting chronic illness. Participants who were using any medication that may affect the psychological evaluation, such as antidepressants, melatonin, or stimulant drugs, were also excluded from the study. Participants who were born small for gestational age or premature were also excluded because they may have several other accompanying problems. The psychological features of the PCOS group were compared with those of the control group. The relationship between clinical/biochemical parameters and psychiatric scores was also investigated in the PCOS group.
Definitions and diagnosis of PCOS
We determined the diagnosis of PCOS according to the guidelines for the diagnosis and treatment of PCOS in adolescents (7). The diagnosis was made in patients who had at least 2 yr since menarche and were admitted to the endocrine clinic with menstrual irregularity (oligomenorrhea: menstrual cycle > 45 d) and hyperandrogenism (biochemical: free testosterone [fT] level higher than the reference levels of our laboratory; clinical: physical examination findings of hyperandrogenism, such as a modified Ferriman–Gallwey [mFG] score of > 8). Pelvic ultrasound was not used for the diagnosis of PCOS in adolescent patients who had < 8 yr since menarche because of the psychological multifollicular appearance of the ovaries during this period (7). Although ultrasonographic evaluation was not used to diagnose PCOS in adolescents, it was performed to investigate possible coexisting abnormalities in the uterus and ovaries.
Clinical and laboratory investigations
Data on sociodemographic characteristics (age, age at menarche, and menstrual irregularities) and clinical characteristics (anthropometric measures and presence of acanthosis nigricans, hirsutism, or acne) were collected using a questionnaire. An electronic scale was used to measure body weight with the participants wearing light clothes. A wall-mounted stadiometer was used to measure height in the upright standing position and during deep inspiration. BMI was calculated as weight divided by height squared (kg/m2). The standard deviation scores (SDS) for height, weight, and BMI were measured according to the reference values for Turkish children (12). Patients with a BMI higher than the 95th percentile for age and sex according to Turkish children’s reference values were defined as obese (12). The mFG scoring system was used to evaluate the severity of hirsutism (13). Hirsutism was defined as an mFG score of ≥ 8. Bioelectrical impedance analysis (Tanita BC-418; Tanita Corp., Tokyo, Japan) was used to determine the percentage body fat (PBF).
Fasting glucose levels were measured using the hexokinase assay. Thyroid-stimulating hormone, luteinizing hormone, follicle-stimulating hormone, dehydroepiandrosterone sulfate, androstenedione, and prolactin were measured using chemiluminescence immunoassay. Fasting insulin, fT, and 17-hydroxyprogesterone levels were measured using the radioimmunoassay method. A spectrophotometric method was used to measure the alanine aminotransferase and aspartate aminotransferase levels. Peripheral blood samples were obtained in the morning (08.00–09.00 a.m.) after a 10-hr fasting period between the 3rd and 5th days of menstrual bleeding. The homeostatic model assessment of IR (HOMA-IR) was calculated using the following formula: fasting insulin (µU/mL) × fasting glucose (mg/L) / 405, and a value > 4 was considered to indicate IR in participants in the pubertal stage (14).
Psychiatric evaluation
A specialized child and adolescent psychiatrist performed the psychiatric evaluation. The following inventories were administered to participants in both the case and control groups.
Beck Depression Inventory (BDI): BDI is a self-assessment measure consisting of 21 items that evaluate the severity of somatic, emotional, mental, and motivational symptoms of depression. In this inventory, each item is scored between 0 and 3. High test scores indicate an increase in depressive symptoms (15). Total scores of 0–9 indicate no or minimal depressive symptoms; 10–18, mild depressive symptoms; 19–29, moderate depressive symptoms; and 30–63, severe depressive symptoms. The validity and reliability coefficients of the Turkish version of the scale are similar to those of the original form (16).
State–Trait Anxiety Inventory (STAI): This 40-item self-report scale assesses separate dimensions of anxiety: “state” (STAI-S) and “trait” (STAI-T). Items are rated on a four-point Likert-type scale. Scores range from 20 to 80 for each subtest, with higher scores indicating higher anxiety levels (17). The Turkish version of this inventory has been proven valid and reliable in the adolescent age group (18).
Pediatric Quality of Life Inventory (PedsQL): This inventory was designed by Varni et al. (19) to assess the HRQoL of children and adolescents. The 23-item PedsQL scale assesses physical and psychosocial functioning. Items are rated on a five-point Likert-type scale. The reliability and validity of the scale has been confirmed by Cakin Memik et al. (20).
Ethics
Ethics committee approval was obtained for this study. Before the study, informed consent was obtained from both the adolescent participants and their parents. The study was conducted in accordance with the Declaration of Helsinki and Ethical Guidelines For Medical and Health Research Involving Human Subjects.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS for Windows, version 23.0; SPSS Inc., Chicago, IL, USA). Continuous values are expressed as median (interquartile range or mean ± standart deviation) whereas categorical values are expressed as numbers and percentages. Categorical variables were compared using Pearson’s chi-square and Fisher’s exact tests. The normality of distribution was evaluated using the Shapiro–Wilk test. The distribution was also controlled when continuous measurements were compared. Normally distributed variables were compared using the t-test, and variables that did not show a normal distribution were compared using the Mann-Whitney U test. Pearson’s correlation test was used to investigate the relationships between the variables. Statistical significance was set at P < 0.05. Multivariate linear regression (backward) models were used to assess independent determinants of psychiatric parameters.
Results
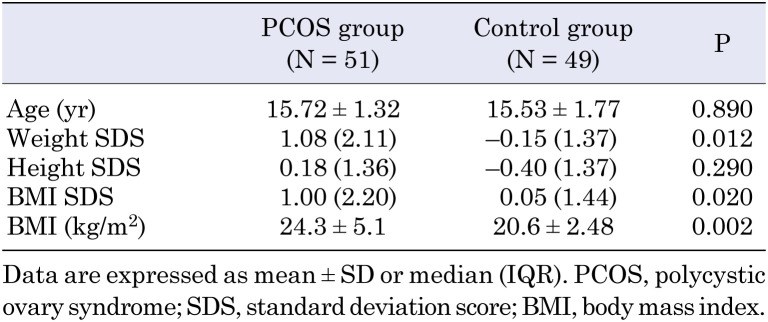
A total of 51 patients with PCOS and 49 controls completed the questionnaires and were included in the study. The mean age of the adolescents was similar between the PCOS and control groups (15.72 ± 1.32 vs. 15.53 ± 1.77 yr, P = 0.890; Table 1). The height SDS was similar between the groups, whereas the weight SDS, BMI, and BMI SDS were significantly higher in the PCOS group (P = 0.290, P = 0.012, P = 0.002, and P = 0.020, respectively; Table 1).
Table 1. Age and anthropometric measures of the participants.
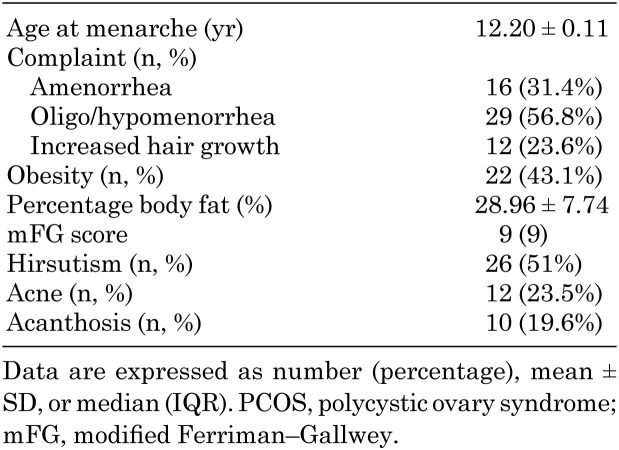
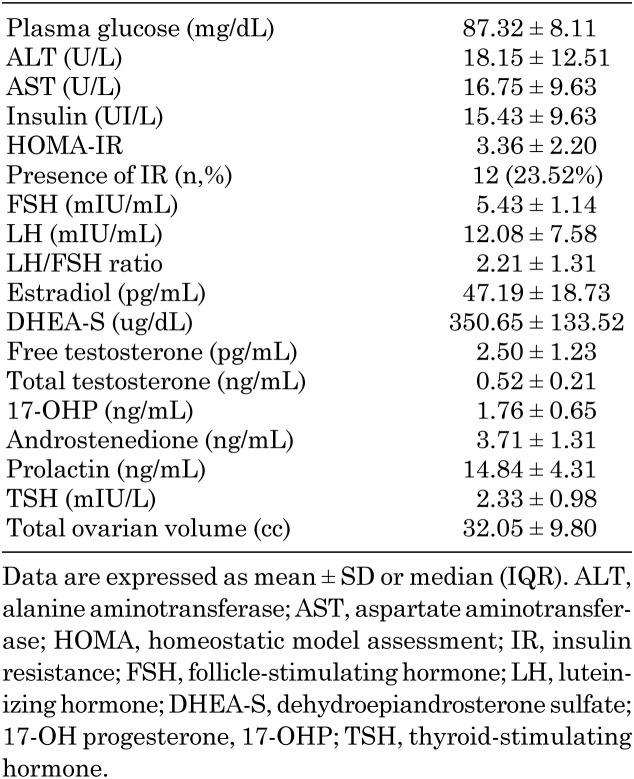
The clinical characteristics of patients diagnosed with PCOS are summarized in Table 2. Oligomenorrhea was the most common complaint (56.8%) on admission. The obesity rate was 43.1% among patients with PCOS, and the mean PBF was 28.96 ± 7.74%. Hirsutism was present in approximately one of every two patients, whereas the incidence of acne and acanthosis was 23.5 and 19.6%, respectively. The laboratory characteristics of the PCOS group are shown in Table 3. The mean fasting plasma glucose level was 87.32 ± 8.11 mg/dL, and the mean insulin level was 15.43 ± 9.63 UI/L. By calculating the HOMA-IR, the incidence of IR was found to be 23.52%. In addition, an oral glucose tolerance test was performed in 12 patients who had impaired fasting or postprandial glucose levels, and four of these patients were diagnosed with type 2 diabetes mellitus (T2DM). The incidence of T2DM in our study group was 7.8% (4/51).
Table 2. Clinical characteristics of patients with PCOS (N = 51).
Table 3. Laboratory characteristics of patients with PCOS (N= 51).
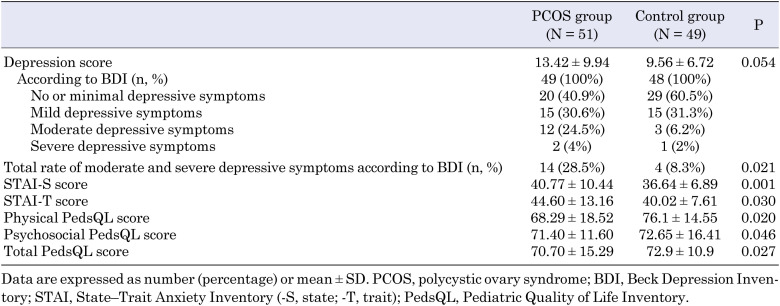
The psychological scores of patients with PCOS are shown in Table 4. Although the mean of the total BDI scores of adolescents with PCOS (mean, 13.42 ± 9.94) was higher than the BDI scores of controls (mean, 9.56 ± 6.72), the difference was not significant (P = 0.054, Table 4). Conversely, 28.5% of the patients in the PCOS group had moderate-to-severe depressive symptoms, whereas the incidence was significantly lower (8.3%) in the control group (P = 0.021, Table 4). The STAI-S score was 40.77 ± 10.44 and the STAI-T score was 44.60 ± 13.16 in the PCOS group. Both scores were higher than those in the control group, which indicates that adolescent girls with PCOS were more likely to have anxiety (P = 0.010 and P = 0.030, respectively; Table 4). The physical PedsQL, psychosocial PedsQL, and total PedsQL scores were lower in the PCOS group (P = 0.020, P = 0.046, and P = 0.047, respectively; Table 4).
Table 4. Psychological scores of patients with PCOS.
Correlational tests showed that the BDI score was significantly positively correlated with the STAI-T and STAI-S scores but negatively correlated with all PedsQL scores (physical, psychosocial, and total scores), indicating that higher levels of depression were associated with worse HRQoL (R = 0.683, P < 0.001; R = 0.885, P < 0.001; R = –0.405, P < 0.001; R = –0.861, P < 0.001; and R = –0.756, P < 0.001, respectively). A significant negative correlation was found between the STAI-S and PedsQL scores (physical, psychosocial, and total scores), indicating that higher anxiety levels were associated with worse HRQoL (R = –0.388, P = 0.012; R = –0.515, P = 0.001; and R = –0.502, P = 0.001, respectively). A significant negative correlation was also found between the STAI-T score and all PedsQL scores (R = –0.449, P = 0.003; R = –0.809, P < 0.001; and R = –0.750, P < 0.001, respectively).
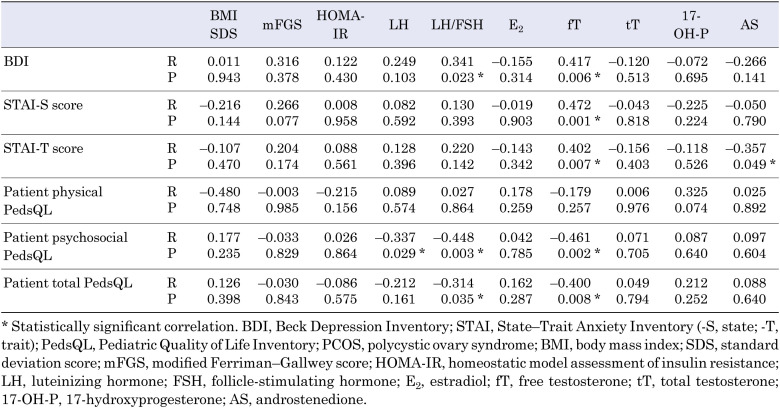
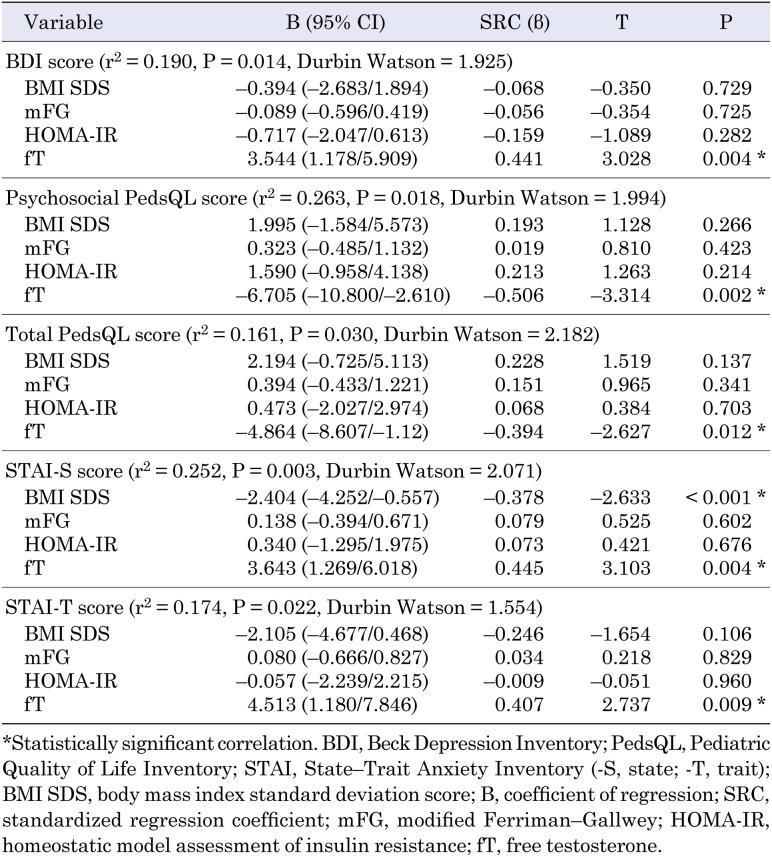
The serum fT levels were positively correlated with the BDI, STAI-T, and STAI-S scores and negatively correlated with the psychosocial PedsQL and total PedsQL scores (R = 0.417, P = 0.006; R = 0.402, P = 0.007; R = 0.472, P = 0.001; R = –0.461, P = 0.002; and R = –0.400, P = 0.008, respectively; Table 5). No significant correlations were observed between the levels of other androgens and the psychiatric scores. Multivariate regression analysis revealed that the fT level was significantly associated with the BDI score (r2 = 0.190, adjusted r2 = 0.149, P = 0.014; β-coefficient = 3.544, P = 0.014), psychosocial PedsQL score (r2 = 0.263, adjusted r2 = 0.185, P = 0.018; β-coefficient = –6.705, P = 0.002), total PedsQL score (r2 = 0.161, adjusted r2 = 0.119, P = 0.030; β-coefficient = –4.864, P = 0.030), STAI-S score (r2 = 0.252, adjusted r2 = 0.214, P = 0.003; β-coefficient = 3.643, P = 0.004), and STAI-T score (r2 = 0.174, adjusted r2 = 0.133, P = 0.022; β-coefficient = 4.513, P = 0.009) (Table 6).
Table 5. Correlations between BDI, STAI-S, STAI-T, physical PedsQL psychosocial PedsQL, total PedsQL scores, and clinical and laboratory parameters in patients with PCOS.
Table 6. Multivariate backward linear regression analysis.
Discussion
In this study, we aimed to analyze the psychological state of adolescent girls with PCOS. To provide a multidisciplinary approach to adolescents with PCOS, health professionals should be aware that this patient population may have psychological problems. Revealing any accompanying psychiatric problems and providing necessary support will also be beneficial for both the adolescent patients and their families in coping with the disease.
In this study, more depressive and anxiety symptoms were observed in adolescent girls diagnosed with PCOS than in healthy participants. Some studies have also shown a higher incidence of emotional disturbance among adolescents with PCOS than among healthy adolescents (3, 21, 22). Sari et al. (22) reported that the most common psychiatric disorder in adolescents with PCOS was major depression, with an incidence of 30%. In our study, the incidence of moderate-to-severe depression was 28.5% in the PCOS group, which is consistent with the literature.
The reasons for the high incidence of depression in patients with PCOS are not fully understood (23). One possible reason may be that adolescent girls are highly concerned about their physical features (24). A high BMI and unpleasant body features can lead to demoralization and social withdrawal of adolescents. Previous studies have reported obesity as a major factor causing depressive symptoms in adolescents with PCOS (25, 26). Depression may be independently associated with BMI, as weight gain and obesity are distressing symptoms (27). Simon et al. (28) reported that obesity was associated with an approximately 25% higher odds of mood disorder in the general population. Although the obesity rate was higher (43.1%) in our study, no significant correlation was found between BMI and psychiatric scores. The small sample size may be the reason for this result, although some other studies from Turkey have reported similar results to ours (21, 22, 29). In those studies, patients with PCOS were also notably more overweight/obese (obesity rate, 41–52%) than controls, consistent with our results (22, 29). One of the studies reported no difference in psychiatric scores between subgroups when patients with PCOS were divided into the overweight/obese, normal weight, and underweight subgroups (29).
Several studies have also investigated the effects of IR on the psychological profiles of patients with PCOS (30, 31). In a study in women diagnosed with PCOS, anxiety was found to be associated with an increased incidence of IR (30). In a recent meta-analysis, moderate-to-severe depression and anxiety symptoms were weakly associated with IR (31). Contrary to the findings in the adult population, we found no significant relationship between depressive/anxiety symptoms and IR among adolescents with PCOS. Besenek and Gurlek (29) and Sari et al. (22) also did not find an association between IR and psychiatric scores in adolescent patients with PCOS. A possible reason for this result might be that the patients were recently diagnosed with PCOS and have not been exposed to IR for a long time. Most of the previous studies were conducted in the adult age group; thereby, such patients have been exposed to the effects of IR for several years.
Hirsutism has been reported to be a distressing feature among adult women with PCOS (31, 32). In contrast, a study conducted in an adolescent population reported no significant correlation between hirsutism and psychiatric scores (22). Similarly, Çoban et al. (21) found no significant relationship between self-esteem scores, PedsQL scores, and hirsutism in adolescent patients with PCOS (33). In the present study, we found no association between psychiatric scores and hirsutism. Another study found a notable inconsistency in the perception of hirsutism between patients and clinicians: patients perceived their hirsutism as more severe than clinicians did (34). The authors also reported that the patient’s own assessments of the degree of hirsutism seemed more strongly associated with specific impacts on the quality of life (QoL) and with the depression risk (34). This may also explain our results. We did not find a relationship between physical aspects of hyperandrogenism and psychiatric scores, whereas the serum fT level, which is an objective indicator of hyperandrogenism, was positively correlated with the depression scores. Besenek and Gurlek (29) examined the effects of PCOS on psychological well-being and reported that the biochemical parameters of hyperandrogenism might be as effective as physical manifestations. However, the relationship between androgen levels and psychiatric disorders in women remains controversial. As psychiatric disorders are more prevalent during the reproductive period in a woman’s life, gonadal hormone levels have been hypothesized to contribute to mood disorders through activational effects on the brain (35). Although some previous studies suggested that androgens influence mood (33, 35), others found no association between androgens and mood iugn patients with PCOS (36, 37). In terms of anxiety, some literature reports have shown the anxiogenic effects of testosterone (38), whereas others have demonstrated its anxiolytic effect regardless of PCOS (39). A previous study in adults demonstrated that the anxiety scores were higher in patients with PCOS with hirsutism than in patients with PCOS without hirsutism (40). The study did not report whether the higher anxiety level was related to social anxiety or to state–trait anxiety. Emeksiz et al. (3) found that adolescents with PCOS have greater anxiety, especially social and generalized anxiety. We also found a relationship between the serum fT level and the state–trait anxiety scores, consistent with the literature.
HRQoL is a multidimensional concept used to describe the physical, emotional, and social aspects of diseases (19). PCOS symptoms may negatively affect the patients’ psychological and social well-being and sexuality, leading to a worsened QoL (41). A recent review showed that adolescent girls with PCOS have a reduced QoL relative to healthy girls, similar to our finding (42). In one study, BMI and hirsutism scores were reported to be associated with the physical aspects of QoL. However, the roles of biochemical, endocrine, and metabolic parameters were reported to be less important (43). In the current study, serum fT levels were negatively correlated with the psychosocial and total PedsQL scores.
Study limitations
Our study had some limitations. First, the control participants were not BMI-matched to the patients with PCOS. This topic is controversial in the literature, with some studies including a BMI-matched control group (3) and others including a BMI-unmatched control group (22, 29, 44). This situation is contradictory because obesity may be associated with depression or anxiety regardless of PCOS. The psychiatric scores of healthy participants may be higher in studies with BMI-matched groups. In contrast, obesity may also affect the higher psychiatric scores in the PCOS group compared to control participants in BMI-unmatched studies. Second, we did have not access to detailed sociodemographic characteristics of the patient and control groups, such as family income and number of people living at home, which may be related to psychiatric scores. Third, psychological scores were obtained using self-rating scales, and semistructured psychiatric interviews were not performed. However, our main objective was not to identify clinical psychiatric conditions. Therefore, self-rating scales for depressive and anxiety symptoms were used to evaluate the association between psychiatric scores and clinical, anthropometric, and biochemical characteristics. Fourth, we did not evaluate PCOS-related QoL, which might have affected the results. In a study examining the fertility concerns of adolescent girls with PCOS, concerns about future fertility were found to be associated with significant reductions in QoL (45). Finally, the fT level was not investigated in the control group because the mFG score of all control participants was < 8 and there were no other findings suggestive of hyperandrogenism (e.g., acne).
Conclusion
In the present study, we investigated the relationships among the severity of depression and anxiety; HRQoL; and clinical, anthropometric, and biochemical characteristics in adolescents with PCOS. More symptoms of depression and anxiety were observed in adolescent girls diagnosed with PCOS than in healthy participants. The psychosocial QoL scores were also lower in patients with PCOS. Our results showed that 28.5% of the patients with PCOS had moderate-to-severe depressive symptoms. We also found a positive correlation between serum fT levels and depression and state–trait anxiety scores and negative correlations between serum fT levels and psychosocial and total QoL scores. Further studies are needed to verify these observations and to clarify the potential mechanisms underlying the observed relationships.
Conflict of interests
The authors declare no conflicts of interest.
References
- 1.Zangeneh FZ, Jafarabadi M, Naghizadeh MM, Abedinia N, Haghollahi F. Psychological distress in women with polycystic ovary syndrome from Imam Khomeini Hospital, Tehran. J Reprod Infertil 2012;13: 111–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Hickey M, Doherty DA, Atkinson H, Sloboda DM, Franks S, Norman RJ, et al. Clinical, ultrasound and biochemical features of polycystic ovary syndrome in adolescents: implications for diagnosis. Hum Reprod 2011;26: 1469–77. doi: 10.1093/humrep/der102 [DOI] [PubMed] [Google Scholar]
- 3.Emeksiz HC, Bideci A, Nalbantoğlu B, Nalbantoğlu A, Çelik C, Yulaf Y, et al. Anxiety and depression states of adolescents with polycystic ovary syndrome. Turk J Med Sci 2018;48: 531–6. [DOI] [PubMed] [Google Scholar]
- 4.Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, et al. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril 2013;100: 470–7. doi: 10.1016/j.fertnstert.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peña AS, Witchel SF, Hoeger KM, Oberfield SE, Vogiatzi MG, Misso M, et al. Adolescent polycystic ovary syndrome according to the international evidence-based guideline. BMC Med 2020;18: 72. doi: 10.1186/s12916-020-01516-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibáñez L, et al. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr 2015. doi: 10.1159/000375530 [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, et al. An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Horm Res Paediatr 2017;88: 371–95. doi: 10.1159/000479371 [DOI] [PubMed] [Google Scholar]
- 8.Kelsey TW, Dodwell SK, Wilkinson AG, Greve T, Andersen CY, Anderson RA, et al. Ovarian volume throughout life: a validated normative model. PLoS One 2013;8: e71465. doi: 10.1371/journal.pone.0071465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooney LG, Dokras A. Depression and anxiety in polycystic ovary syndrome: etiology and treatment. Curr Psychiatry Rep 2017;19: 83. doi: 10.1007/s11920-017-0834-2 [DOI] [PubMed] [Google Scholar]
- 10.Veldhuis JD, Pincus SM, Garcia-Rudaz MC, Ropelato MG, Escobar ME, Barontini M. Disruption of the joint synchrony of luteinizing hormone, testosterone, and androstenedione secretion in adolescents with polycystic ovarian syndrome. J Clin Endocrinol Metab 2001;86: 72–9. [DOI] [PubMed] [Google Scholar]
- 11.Jones GL, Balen AH, Ledger WL. Health-related quality of life in PCOS and related infertility: how can we assess this? Hum Fertil (Camb) 2008;11: 173–85. doi: 10.1080/14647270802078179 [DOI] [PubMed] [Google Scholar]
- 12.Neyzi O, Bundak R, Gökçay G, Günöz H, Furman A, Darendeliler F, et al. Reference values for weight, height, head circumference, and body mass index in Turkish children. J Clin Res Pediatr Endocrinol 2015;7: 280–93. doi: 10.4274/jcrpe.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferriman D, Purdie AW. The aetiology of oligomenorrhoea and/or hirsuties: a study of 467 patients. Postgrad Med J 1983;59: 17–20. doi: 10.1136/pgmj.59.687.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28: 412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 15.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4: 561–71. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 16.Hisli N. Beck depresyon envanterinin universite ogrencileri icin gecerliligi gAravsoBDIiaussJP.
- 17.Spielberger CD. State‐Trait anxiety inventory. The Corsini Encyclopedia of Psychology. 2010:1-. [Google Scholar]
- 18.Öner N, LeCompte WA. Durumluk-sürekli kaygı envanteri el kitabı. Boğaziçi Üniversitesi Yayınları; 1985. [Google Scholar]
- 19.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care 1999;37: 126–39. doi: 10.1097/00005650-199902000-00003 [DOI] [PubMed] [Google Scholar]
- 20.Cakin Memik N, Ağaoğlu B, Coşkun A, Uneri OS, Karakaya I. The validity and reliability of the Turkish pediatric quality of life inventory for children 13–18 years old. Turk Psikiyatr Derg 2007;18: 353–63 (in Turkish). [PubMed] [Google Scholar]
- 21.Çoban ÖG, Tulacı ÖD, Adanır AS, Önder A. Psychiatric disorders, self-esteem, and quality of life in adolescents with polycystic ovary syndrome. J Pediatr Adolesc Gynecol 2019;32: 600–4. doi: 10.1016/j.jpag.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 22.Sari SA, Celik N, Uzun Cicek A. Body perception, self-esteem, and comorbid psychiatric disorders in adolescents diagnosed with polycystic ovary syndrome. J Pediatr Adolesc Gynecol 2020;33: 691–6. doi: 10.1016/j.jpag.2020.08.018 [DOI] [PubMed] [Google Scholar]
- 23.Dokras A, Clifton S, Futterweit W, Wild R. Increased risk for abnormal depression scores in women with polycystic ovary syndrome: a systematic review and meta-analysis. Obstet Gynecol 2011;117: 145–52. doi: 10.1097/AOG.0b013e318202b0a4 [DOI] [PubMed] [Google Scholar]
- 24.Sadeeqa S, Mustafa T, Latif S. Polycystic ovarian syndrome-related depression in adolescent girls: a review. J Pharm Bioallied Sci 2018;10: 55–9. doi: 10.4103/JPBS.JPBS_1_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 2009;92: 1966–82. doi: 10.1016/j.fertnstert.2008.09.018 [DOI] [PubMed] [Google Scholar]
- 26.Rasgon NL, Rao RC, Hwang S, Altshuler LL, Elman S, Zuckerbrow-Miller J, et al. Depression in women with polycystic ovary syndrome: clinical and biochemical correlates. J Affect Disord 2003;74: 299–304. doi: 10.1016/S0165-0327(02)00117-9 [DOI] [PubMed] [Google Scholar]
- 27.Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord 1992;16: 999–1003. [PubMed] [Google Scholar]
- 28.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry 2006;63: 824–30. doi: 10.1001/archpsyc.63.7.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besenek M, Gurlek B. Hyperandrogenism in polycystic ovary syndrome affects psychological well-being of adolescents. J Obstet Gynaecol Res 2021;47: 137–46. doi: 10.1111/jog.14444 [DOI] [PubMed] [Google Scholar]
- 30.Livadas S, Chaskou S, Kandaraki AA, Skourletos G, Economou F, Christou M, et al. Anxiety is associated with hormonal and metabolic profile in women with polycystic ovarian syndrome. Clin Endocrinol (Oxf) 2011;75: 698–703. doi: 10.1111/j.1365-2265.2011.04122.x [DOI] [PubMed] [Google Scholar]
- 31.Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2017;32: 1075–91. doi: 10.1093/humrep/dex044 [DOI] [PubMed] [Google Scholar]
- 32.Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod 2007;22: 2279–86. doi: 10.1093/humrep/dem108 [DOI] [PubMed] [Google Scholar]
- 33.Jedel E, Gustafson D, Waern M, Sverrisdottir YB, Landén M, Janson PO, et al. Sex steroids, insulin sensitivity and sympathetic nerve activity in relation to affective symptoms in women with polycystic ovary syndrome. Psychoneuroendocrinology 2011;36: 1470–9. doi: 10.1016/j.psyneuen.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 34.Pasch L, He SY, Huddleston H, Cedars MI, Beshay A, Zane LT, et al. Clinician vs self-ratings of hirsutism in patients with polycystic ovarian syndrome: associations with quality of life and depression. JAMA Dermatol 2016;152: 783–8. doi: 10.1001/jamadermatol.2016.0358 [DOI] [PubMed] [Google Scholar]
- 35.Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med 2004;66: 356–62. [DOI] [PubMed] [Google Scholar]
- 36.Hollinrake E, Abreu A, Maifeld M, Van Voorhis BJ, Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil Steril 2007;87: 1369–76. doi: 10.1016/j.fertnstert.2006.11.039 [DOI] [PubMed] [Google Scholar]
- 37.Rasgon NL, Rao RC, Hwang S, Altshuler LL, Elman S, Zuckerbrow-Miller J, et al. Depression in women with polycystic ovary syndrome: clinical and biochemical correlates. J Affect Disord 2003;74: 299–304. doi: 10.1016/S0165-0327(02)00117-9 [DOI] [PubMed] [Google Scholar]
- 38.Stanikova D, Luck T, Pabst A, Bae YJ, Hinz A, Glaesmer H, et al. Associations between anxiety, BMI and sex hormones in women. Front Psychiatry 2019;10: 479. doi: 10.3389/fpsyt.2019.00479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol 2014;35: 42–57. doi: 10.1016/j.yfrne.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benson S, Janssen OE, Hahn S, Tan S, Dietz T, Mann K, et al. Obesity, depression, and chronic low-grade inflammation in women with polycystic ovary syndrome. Brain Behav Immun 2008;22: 177–84. doi: 10.1016/j.bbi.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 41.Behboodi Moghadam Z, Fereidooni B, Saffari M, Montazeri A. Measures of health-related quality of life in PCOS women: a systematic review. Int J Womens Health 2018;10: 397–408. doi: 10.2147/IJWH.S165794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson NA, Peña AS. Quality of life in adolescent girls with polycystic ovary syndrome. J Paediatr Child Health 2020;56: 1351–7. doi: 10.1111/jpc.15097 [DOI] [PubMed] [Google Scholar]
- 43.Hahn S, Janssen OE, Tan S, Pleger K, Mann K, Schedlowski M, et al. Clinical and psychological correlates of quality-of-life in polycystic ovary syndrome. Eur J Endocrinol 2005;153: 853–60. doi: 10.1530/eje.1.02024 [DOI] [PubMed] [Google Scholar]
- 44.Ghazeeri G, Fakih A, Abbas HA, Harajly S, Awwad J. Anxiety, cognitive, and depressive assessment in adolescents with polycystic ovarian syndrome: a pilot study. J Pediatr Adolesc Gynecol 2013;26: 269–73. doi: 10.1016/j.jpag.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 45.Trent ME, Rich M, Austin SB, Gordon CM. Fertility concerns and sexual behavior in adolescent girls with polycystic ovary syndrome: implications for quality of life. J Pediatr Adolesc Gynecol 2003;16: 33–7. doi: 10.1016/S1083-3188(02)00205-X [DOI] [PubMed] [Google Scholar]