Key Points
Questions
What is the association between amyloidogenic TTR gene variation (Val122Ile) and the risk of heart failure?
Findings
In this retrospective cohort study that included 7514 Black participants in the US with a median 11.1 years of follow-up, the incidence of heart failure was 15.6 per 1000 person-years among Val122Ile variant carriers compared with 7.2 per 1000 person-years among noncarriers, with an adjusted hazard ratio of 2.43.
Meaning
Being a carrier of the Val122Ile variant was significantly associated with an increased risk of heart failure among Black individuals living in the US.
Abstract
Importance
A genetic variant in the TTR gene (rs76992529; Val122Ile), present more commonly in individuals with African ancestry (population frequency: 3%-4%), causes misfolding of the tetrameric transthyretin protein complex that accumulates as extracellular amyloid fibrils and results in hereditary transthyretin amyloidosis.
Objective
To estimate the association of the amyloidogenic Val122Ile TTR variant with the risk of heart failure and mortality in a large, geographically diverse cohort of Black individuals.
Design, Setting, and Participants
Retrospective population-based cohort study of 7514 self-identified Black individuals living in the US participating in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study with genetic data available and without heart failure at baseline. The participants were enrolled at the baseline visit (2003-2007). The end of follow-up for the majority of outcomes was on December 31, 2018. All-cause mortality data were available through December 31, 2020.
Exposures
TTR Val122Ile (rs76992529) genotype.
Main Outcome and Measures
The primary outcome was incident heart failure (first hospitalization for heart failure or death due to heart failure). The secondary outcomes were heart failure mortality, cardiovascular mortality, and all-cause mortality. The multivariable Cox proportional hazards regression analyses were adjusted for genetic ancestry and demographic, clinical, and social factors.
Results
Among 7514 Black participants (median age, 64 years [IQR, 57-70 years]; 61% women), the population frequency of the TTR Val122Ile variant was 3.1% (232 variant carriers and 7282 noncarriers). During a median follow-up of 11.1 years (IQR, 5.9-13.5 years), incident heart failure occurred in 535 individuals (34 variant carriers and 501 noncarriers) and the incidence of heart failure was 15.64 per 1000 person-years among variant carriers vs 7.16 per 1000 person-years among noncarriers (adjusted hazard ratio [HR], 2.43 [95% CI, 1.71-3.46]; P < .001). Deaths due to heart failure occurred in 141 individuals (13 variant carriers and 128 noncarriers) and the incidence of heart failure mortality was 6.11 per 1000 person-years among variant carriers vs 1.85 per 1000 person-years among noncarriers (adjusted HR, 4.19 [95% CI, 2.33-7.54]; P < .001). Deaths due to cardiovascular causes occurred in 793 individuals (34 variant carriers and 759 noncarriers) and the incidence of cardiovascular death was 15.18 per 1000 person-years among variant carriers vs 10.61 per 1000 person-years among noncarriers (adjusted HR, 1.69 [95% CI, 1.19-2.39]; P = .003). Deaths due to any cause occurred in 2715 individuals (100 variant carriers and 2615 noncarriers) and the incidence of all-cause mortality was 41.46 per 1000 person-years among variant carriers vs 33.94 per 1000 person-years among noncarriers (adjusted HR, 1.46 [95% CI, 1.19-1.78]; P < .001). There was no significant interaction between TTR variant carrier status and sex on incident heart failure and the secondary outcomes.
Conclusions and Relevance
Among a cohort of Black individuals living in the US, being a carrier of the TTR Val122Ile variant was significantly associated with an increased risk of heart failure.
This retrospective population-based cohort study compares carriers of the amyloidogenic Val122Ile TTR variant vs noncarriers to assess the association of having the variant with risk of heart failure and mortality in self-identified Black individuals living in the US.
Introduction
Hereditary amyloidosis is an inherited condition characterized by extracellular deposition of amyloid protein in multiple tissues, such as the myocardium, nervous tissue, and the kidneys, resulting in their dysfunction.1,2,3 Hereditary transthyretin amyloidosis (hATTR) is characterized by misfolding of the tetrameric transthyretin protein complex that accumulates as extracellular amyloid fibrils and results in polyneuropathy, heart failure, and arrhythmias.1,2,3 A variation in the TTR gene results in a substitution from valine to isoleucine amino acid at position 122 (rs76992529; Val122Ile), leading to the misfolding of the transthyretin protein and its eventual extracellular deposition in the myocardium, causing hATTR cardiomyopathy (hATTR-CM).1,2,3,4,5
The amyloidogenic TTR Val122Ile variation is seen more commonly among Black individuals (population frequency in Black individuals is approximately 3%-4% and <0.1% in other populations) and may in part contribute to a higher risk for development of heart failure.4,5 Individuals carrying the TTR Val122Ile variation are more likely to have prevalent heart failure and have concentric left ventricular hypertrophy.6 Prior investigations indicated a modest association of this genetic variation with incident heart failure.7,8 However, this variant is not deemed clinically actionable by the American College of Medical Genetics and Genomics.9 With the advent of efficacious hATTR therapeutics,10 it is important to quantify the association of the TTR Val122Ile variant with incident heart failure and mortality.
This study evaluated self-identified Black participants from the ongoing Reasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study to examine the association of the TTR Val122Ile variant (rs76992529) with incident heart failure and mortality events in a large, geographically diverse cohort of Black individuals from the US, accounting for demographic, clinical, and social factors.
Methods
Study Population
In this investigation, data from the ongoing REGARDS cohort study,11 which recruited a total of 30 239 unrelated White and Black adults aged 45 years or older who had been living in the US at baseline, were used. Community-dwelling participants were randomly selected from a well-characterized, commercially available list to create a sample balance on race and ethnicity and sex across the stroke buckle (within the stroke belt along the coastal plains of Georgia, North Carolina, and South Carolina), the stroke belt (the remainder of Georgia, North Carolina, and South Carolina and Alabama, Arkansas, Louisiana, and Tennessee), and rest of the continental US.11 At baseline, information on demographics, health behaviors, and medical history was collected during a telephone interview.
During an in-home visit, investigators collected body measurements, a prescription inventory, electrocardiographic results, and blood and urine specimens. Participants or their proxies were contacted twice per year to record any hospitalizations, emergency department visits, or deaths during follow-up. Medical records and death certificates were obtained for possible cardiovascular events and adjudicated by clinical experts. All participants provided written informed consent and the institutional review boards of all the centers involved approved the study.
For the current analysis, only non-Hispanic Black adults for whom genotypic information on the TTR gene variant (rs76992529) was available were included. The self-reported race and ethnicity of participants were recorded using categorical, fixed options provided by the interviewer. Individuals identified as outliers in the principal component analysis for ancestry, participants with suspected heart failure at baseline,12 and those with information missing for covariates were excluded (eFigure 1 in the Supplement).
Genotypic Assessment
The exposure variable for this study was the TTR Val122Ile (rs76992529) genotype. Black participants who consented to genetic research were genotyped using Illumina Infinium Multi-Ethnic AMR/AFR BeadChip arrays (Illumina Inc).13 Quality control at the sample and variant level was performed. Samples were removed if they were internal duplicates, sex mismatches, or exhibited a high proportion of missing variants (missingness >5%). Principal component analysis was performed using SmartPCA version 7.2.1 software (Eigenstrat).14 Individuals were considered outliers and removed from subsequent analysis if they were outside the threshold of 6 SDs.
The global African ancestry percentage was calculated for each participant using Admixture version 1.3.0 software.15 Running an unsupervised method for k = 2 ancestral populations, variants were retained if they had a minor allele frequency greater than 0.01, were present in the study data set, and were present in the 1000 Genomes African reference population (n = 405) or the European reference population (n = 404).
The median African genetic ancestry percentage for the study population was 84% (IQR, 76%-91%). PLINK version 1.916 was used to code the genotypes as additive allele dosages for the effect allele of rs76992529. The TTR V122I variant did not deviate from the Hardy-Weinberg equilibrium (P > .05 using the Hardy-Weinberg test) in the study population.
Study Outcomes
The primary outcome was incident heart failure (first hospitalization for heart failure or death due to heart failure).17 The secondary outcomes were heart failure mortality, cardiovascular mortality, and all-cause mortality. Suspected hospitalizations for heart failure through December 31, 2018, were identified based on the biannual follow-up call to the participants or their proxies. Two clinician investigators adjudicated medical records using standardized forms, and a committee resolved any disagreements.18
Hospitalizations for heart failure were identified based on (1) signs and symptoms (cardiomegaly, jugular vein distension, central venous pressure >16 mm Hg, peripheral edema, pleural effusion, hepatomegaly, orthopnea, exertional dyspnea, paroxysmal nocturnal dyspnea, nocturnal cough, rales on the pulmonary examination, heart rate >120/min, or weight loss ≥4.5 kg within 5 days after diuresis); (2) biomarkers (eg, level of B-type natriuretic peptide, troponins, or creatine kinase-MB); and (3) imaging (eg, chest radiography, electrocardiography, echocardiography, or single-photon emission computed tomography).19
Mortality due to heart failure or cardiovascular causes (composite of death due to stroke, myocardial infarction, heart failure, sudden cardiac death, pulmonary embolism, or other cardiovascular causes) were adjudicated by 2 clinical experts using all available information (eg, medical records at hospitalization, baseline medical history, history of adjudicated cardiovascular events, death certificates, autopsy reports, the National Death Index, and interviews with proxies). The latest all-cause mortality data available (up to December 31, 2020) were used for the analysis.
Covariates
Participants self-reported their age, sex, region of residence, health insurance status, education level (≤high school degree vs college graduate or some college), income level (<$35 000 vs ≥$35 000), smoking status (current, past, or never smoker), alcohol use (number of drinks per week), and physical activity frequency (none, 1-3 times/week, and ≥4 times/week). Body mass index was calculated as weight in kilograms divided by height in meters squared. Clinical covariates included a history of coronary heart disease, a history of atrial fibrillation, diabetes status, dyslipidemia, left ventricular hypertrophy, estimated glomerular filtration rate, and systolic blood pressure.
At baseline, coronary heart disease was identified based on self-reported history of myocardial infarction or relevant procedures (eg, coronary angioplasty) or electrocardiographic evidence of myocardial infarction at baseline. Coronary heart disease events occurring during follow-up were adjudicated by clinician-investigators based on signs and symptoms, cardiac biomarkers, and electrocardiographic changes. The complete adjudication process has been previously described.20 Atrial fibrillation was either self-reported or was evident on the baseline electrocardiogram.
Glucose levels (fasting level ≥126 mg/dL or random level ≥200 mg/dL; to convert to mmol/L, multiply by 0.0555), use of diabetes medication, or both, were used to identify diabetes status at baseline. Dyslipidemia was based on cholesterol levels (total cholesterol level ≥240 mg/dL, low-density lipoprotein cholesterol level ≥160 mg/dL, or high-density lipoprotein cholesterol level ≤40 mg/dL; to convert to mmol/L, multiply by 0.0259), cholesterol-lowering medication use, or both. The estimated glomerular filtration rate was computed using the Chronic Kidney Disease Epidemiology Collaboration equation.21 Left ventricular hypertrophy on electrocardiogram was defined using the Sokolov-Lyon criteria. Blood pressure was calculated as a mean of 2 measurements after 5 minutes of rest.
The underlying blood pressure was computed after correcting for antihypertensive medication use (10 mm Hg was added for systolic blood pressure and 5 mm Hg was added for diastolic blood pressure) and was used in all analyses.22 To account for potential social factors that may confound the association, we adjusted for neighborhood characteristics using a neighborhood deprivation index as a summary measure. Based on the work of Diez Roux et al,23 the neighborhood deprivation index is a compilation of US Census–derived indicators of neighborhood socioeconomic status. The neighborhood deprivation index includes median household income; percentage of households with interest, dividend, or rental income; median value of housing units; percentage of persons aged 25 years or older who completed high school; percentage of persons aged 25 years or older who completed college; and percentage of persons in executive, managerial, or professional specialty occupations.24 The z score for each individual indicator is summed to provide an index of neighborhood-level deprivation. The first 10 principal components generated using SmartPCA were included in the models to account for African ancestry.13,14
Statistical Analysis
Baseline characteristics were summarized for participants with (carriers) vs without (noncarriers) the TTR gene variant (rs76992529) using median and IQR for continuous variables and counts and frequency for categorical variables. Poisson regression was used to estimate the incidence rate of all outcomes. An association between the gene variant (rs76992529) and incident heart failure was assessed using a multivariable-adjusted Cox proportional hazards regression model. The proportionality assumption assessed using Schoenfeld residuals was not violated.
We adjusted for confounders identified a priori in a sequential manner with model 1 adjusted for age, sex, body mass index, and the first 10 principal components of African ancestry (generated using SmartPCA) to account for population stratification. Model 2 included model 1 plus prevalent and incident coronary heart disease, which was considered as a time-varying covariate to account for the increase in heart failure risk after onset of coronary heart disease. Model 3 included model 2 plus systolic blood pressure, physical activity frequency, alcohol use, dyslipidemia, estimated glomerular filtration rate, diabetes status, smoking status, history of atrial fibrillation, history of stroke, and left ventricular hypertrophy.25,26,27,28,29 Systolic blood pressure was stratified into quartiles and was included as a categorical variable in the models to account for a potential nonlinear relationship.30 Model 4 included model 3 plus social factors that may influence the development of heart failure: region of residence, income level, health insurance status, and neighborhood deprivation index.31,32,33
In the sensitivity analyses, we used participant age on the time scale in the Cox proportional hazards regression models. We used a Fine-Gray subdistribution hazards model to account for non–heart failure–related death as a competing risk. We also assessed the association of the TTR variant with heart failure mortality, cardiovascular mortality, and all-cause mortality using a similar modeling approach as above. We examined for potential interaction of sex by the TTR variant status on the study outcomes using a multiplicative interaction term (sex × variant carrier status).
The primary analyses for this study were conducted in participants with all data available (exposure, outcome, and covariates). We also conducted sensitivity analyses for all study outcomes using multiple imputations for missing covariates under a missing at random assumption (eFigure 1 in the Supplement).
A 2-sided type I error of 0.05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for the analyses of the secondary outcomes should be interpreted as exploratory. The analyses were conducted using SAS version 9.4 (SAS Institute Inc) and Stata SE version 16.0 (StataCorp).
Results
Among 8916 Black participants who underwent genotyping, 247 were excluded during genomic quality control and 140 were excluded for heart failure at baseline or for missing follow-up data (eFigure 1 in the Supplement). The resulting 8527 participants constituted the multiple imputation data set for the sensitivity analyses. Participants with missing data on clinical covariates (11.9%) were excluded from the final analytic sample. In the final study sample of 7514 Black adults (median age, 64 years [IQR, 57-70 years]; 61% were women), 3.1% (n = 232) had the amyloidogenic TTR gene variant. The baseline characteristics of the study participants stratified by TTR gene variant status appear in the Table. The distribution of the variant carrier status stratified by demographic subgroups appears in eTables 1-2 in the Supplement.
Table. Baseline Characteristics of the Study Participants.
| Characteristics | TTR Val122Ile variant status | |
|---|---|---|
| Carriers (n = 232) | Noncarriers (n = 7282) | |
| Demographics | ||
| Age, median (IQR), y | 62.0 (57.0 to 69.0) | 63.0 (57.0 to 70.0) |
| Sex, No. (%) | ||
| Male | 89 (38.4) | 2845 (39.1) |
| Female | 143 (61.6) | 4437 (60.9) |
| Region of residence, No. (%) | ||
| Stroke belta | 112 (48.3) | 3571 (49.0) |
| Stroke buckleb | 41 (17.7) | 1259 (17.3) |
| Otherc | 79 (34.1) | 2452 (33.7) |
| Social factors | ||
| Income ≥$35 000, No. (%) | 91 (39.2) | 2704 (37.1) |
| No health insurance, No. (%) | 26 (11.2) | 717 (9.8) |
| College graduate or some college, No. (%) | 127 (54.7) | 3988 (54.8) |
| Neighborhood deprivation measure, median (IQR)d | −4.1 (−6.3 to −0.7) | −4.1 (−6.3 to −1.2) |
| Risk factors | ||
| Smoking status, No. (%) | ||
| Current smoker | 105 (45.3) | 3293 (45.2) |
| Past smoker | 77 (33.2) | 2716 (37.3) |
| Never smoker | 50 (21.6) | 1273 (17.5) |
| Alcohol use, median (IQR), No. of drinks/wk | 0 (0 to 0.3) | 0 (0 to 0.2) |
| Physical activity frequency, No. (%) | ||
| None | 78 (33.6) | 2632 (36.1) |
| 1-3 times/wk | 106 (45.7) | 2697 (37.0) |
| ≥4 times/wk | 48 (20.7) | 1953 (26.8) |
| Body mass index, median (IQR)e | 29.0 (25.5 to 34.1) | 29.8 (26.3 to 34.5) |
| Blood pressure, median (IQR), mm Hg | ||
| Systolic | 135.0 (122.0 to 147.0) | 136.0 (125.0 to 148.0) |
| Diastolic | 81.0 (74.0 to 88.0) | 82.0 (75.0 to 88.0) |
| Estimated glomerular filtration rate, median (IQR), mL/min/1.73 m2 | 93.5 (75.8 to 107.8) | 91.6 (74.3 to 106.8) |
| Comorbidities, No. (%) | ||
| Dyslipidemia | 137 (59.1) | 3923 (53.9) |
| Diabetes status | 65 (28.0) | 2088 (28.7) |
| Prevalent and incident coronary heart disease | 36 (15.5) | 962 (13.2) |
| Left ventricular hypertrophy | 28 (12.1) | 1068 (14.7) |
| History of atrial fibrillation | 13 (5.6) | 519 (7.1) |
Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee.
Located within the stroke belt along the coastal plains of Georgia, North Carolina, and South Carolina. Individuals living in the stoke buckle were not counted in the stroke belt category.
Rest of the continental US.
Compilation of US Census–derived indicators of neighborhood socioeconomic status (median household income; percentage of households with interest, dividend, or rental income; median value of housing units; percentage of persons aged ≥25 years who completed high school; percentage of persons aged ≥25 years who completed college; and percentage of persons in executive, managerial, or professional specialty occupations). The z score for each individual indicator is summed to provide an index of neighborhood-level deprivation.
Calculated as weight in kilograms divided by height in meters squared.
Primary Outcome
During a median follow-up of 11.1 years (IQR, 5.9-13.5 years), incident heart failure occurred in 535 individuals (34 variant carriers and 501 noncarriers) and the incidence rate of heart failure was 15.64 per 1000 person-years (95% CI, 11.27-21.70 per 1000 person-years) among variant carriers vs 7.16 per 1000 person-years (95% CI, 6.56-7.81 per 1000 person-years) among noncarriers. The distribution of incident heart failure cases stratified by age, sex, and region appears in eTable 3 in the Supplement.
The event frequency of the primary and secondary outcomes, stratified by TTR variant status, appears in eTable 4 in the Supplement. In the multivariable-adjusted model, TTR variant status was associated with a significantly higher risk of incident heart failure (hazard ratio [HR], 2.43 [95% CI, 1.71-3.46] for model 4; Figures 1 and 2). The parameter estimates for the fully adjusted model appear in eTable 5 in the Supplement. There was no significant interaction by sex on the risk of incident heart failure (P = .59 for interaction). The sex-stratified results appear in eTable 6 and eFigure 2 in the Supplement. The results were similar when age was considered on the time scale for the Cox proportional hazards regression analysis (HR, 2.47 [95% CI, 1.74-3.52] for model 4; Figure 2). In the competing risk analysis accounting for non–heart failure–related deaths as a competing event, the TTR variant carriers had a significantly higher risk of incident heart failure compared with noncarriers (HR, 2.31 [95% CI, 1.63-3.28] for model 4).
Figure 1. Risk of Incident Heart Failure Among Black Individuals Stratified by TTR Val122Ile Variant Status.
The median follow-up duration for the individuals who were TTR Val122Ile carriers was 11.1 years (IQR, 5.4-13.4 years) and for noncarriers was 11.1 years (IQR, 5.9-13.5 years). Among the 2 homozygous individuals, one had an incident heart failure event and the other died due to noncardiac causes. The x-axis represents the age at which the study participant was censored in the respective groups. The age at censoring is the sum of age at recruitment and the follow-up duration. The number at risk represents the participants at the given age who are at risk of developing a censoring event (outcome, death, or lost to follow-up).
Figure 2. Risk of Heart Failure Among TTR Val122Ile Variant Carriers.
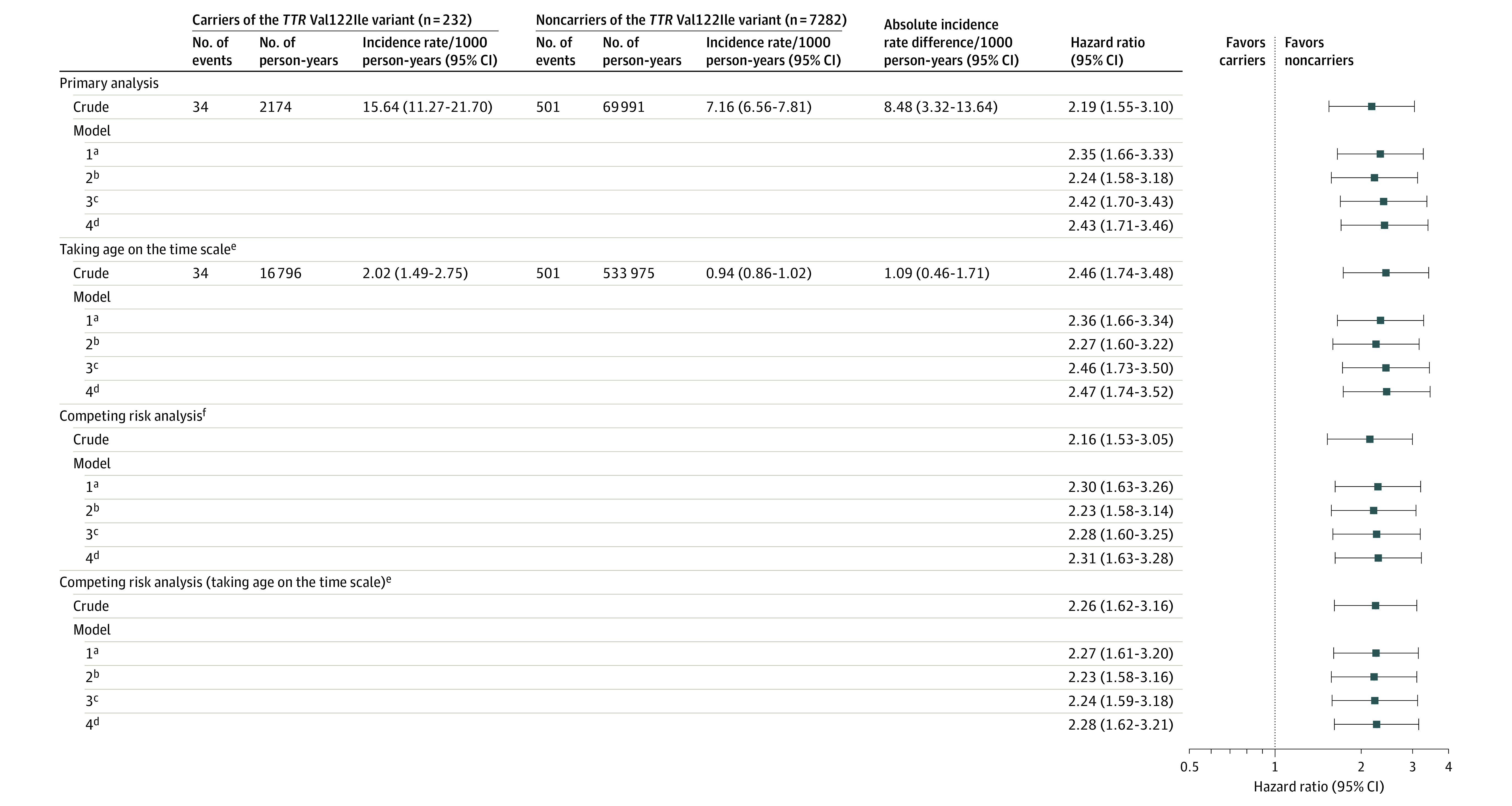
P<.001 for all model comparisons.
aIncludes age, sex, body mass index, and 10 principal components of African ancestry to account for population stratification. Systematic ancestral differences (population stratification) can contribute to differences in allele distribution between those developing and not developing the study outcomes. These differences may result in false genetic associations. The principal components capture this population stratification. Adjustment of the models for the principal components ensures that the reported associations are not resulting from the underlying population stratification. Age was not included when taken in the definition of time scale.
bIncludes model 1 plus prevalent and incident coronary heart disease (time varying).
cIncludes model 2 plus systolic blood pressure, smoking status, estimated glomerular filtration rate, alcohol use (number of drinks/week), history of atrial fibrillation, diabetes status, dyslipidemia, physical activity frequency, history of stroke, and left ventricular hypertrophy.
dIncludes model 3 plus region of residence, income level, education level, health insurance status, and the neighborhood deprivation measure.
eImplies that the time to event in the model was the age of the participant, with the participant being censored at the age of occurrence of the event. In this approach, the participants enter the observation period at the age when they were recruited in the study cohort and exit at the age at which they are censored (due to the occurrence of an event, death, or lost to follow-up).
fAccounts for non–heart failure–related deaths as a competing event.
Secondary Outcomes
During follow-up, deaths due to heart failure occurred in 141 individuals (13 variant carriers and 128 noncarriers). Heart failure death was adjudicated for 7414 individuals (226 variant carriers and 7188 noncarriers) and the incidence rate of heart failure death was 6.11 per 1000 person-years (95% CI, 3.58-10.43 per 1000 person-years) among variant carriers vs 1.85 per 1000 person-years (95% CI, 1.56-2.20 per 1000 person-years) among noncarriers. Compared with noncarriers, Black individuals with the TTR Val122Ile variant had a higher risk of heart failure death (HR, 4.19 [95% CI, 2.33-7.54] for model 4).
During follow-up, deaths due to cardiovascular causes occurred in 793 individuals (34 variant carriers and 759 noncarriers) and the incidence rate for cardiovascular mortality was 15.18 per 1000 person-years (95% CI, 10.99-20.96 per 1000 person-years) among variant carriers vs 10.61 per 1000 person-years (95% CI, 9.89-11.38 per 1000 person-years) among noncarriers. Compared with noncarriers, Black individuals with the TTR Val122Ile variant had a significantly higher risk of cardiovascular mortality (HR, 1.69 [95% CI, 1.19-2.39] for model 4).
During follow-up, deaths due to any cause occurred in 2715 individuals (100 variant carriers and 2615 noncarriers) and the incidence rate of all-cause mortality was 41.46 per 1000 person-years (95% CI, 34.56-49.74 per 1000 person-years) among variant carriers vs 33.94 per 1000 person-years (95% CI, 32.74-35.18 per 1000 person-years) among noncarriers. Compared with noncarriers, Black individuals with the TTR gene variant had a significantly higher hazard of all-cause mortality (HR, 1.46 [95% CI, 1.19-1.78] for model 4; Figures 3 and 4).
Figure 3. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among Black Individuals Stratified by TTR Val122Ile Variant Status.
For the individuals who were TTR Val122Ile carriers, the median follow-up duration was 11.1 years (IQR, 5.5-13.4 years) for heart failure mortality (panel A), 11.3 years (IQR, 5.7-13.5 years) for cardiovascular mortality (panel B), and 11.9 years (IQR, 5.8-14.7 years) for all-cause mortality (panel C). For the individuals who were TTR Val122Ile noncarriers, the median follow-up duration was 11.2 years (IQR, 6.0-13.5 years) for heart failure mortality (panel A), 11.3 years (IQR, 6.4-13.5 years) for cardiovascular mortality (panel B), and 11.7 years (IQR, 6.5-15.0 years) for all-cause mortality (panel C). Among the 2 homozygous individuals, one died due to noncardiovascular causes and the other participant is still alive. The x-axis represents the age at which the study participant was censored in the respective groups. The age at censoring is the sum of age at recruitment and the follow-up duration. The number at risk represents the participants at the given age who are at risk of developing a censoring event (outcome, death, or lost to follow-up).
Figure 4. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among TTR Val122Ile Variant Carriers.

P<.05 for all model comparisons.
aIncludes age, sex, body mass index, and 10 principal components of African ancestry to account for population stratification. Systematic ancestral differences (population stratification) can contribute to differences in allele distribution between those developing and not developing the study outcomes. These differences may result in false genetic associations. The principal components capture this population stratification. Adjustment of the models for the principal components ensures that the reported associations are not resulting from the underlying population stratification.
bIncludes model 1 plus prevalent coronary heart disease, systolic blood pressure, smoking status, estimated glomerular filtration rate, alcohol use (number of drinks/week), history of atrial fibrillation, diabetes status, dyslipidemia, physical activity frequency, history of stroke, and left ventricular hypertrophy.
cIncludes model 2 plus region of residence, income level, education level, health insurance status, and the neighborhood deprivation measure.
There was no significant interaction by sex on the risk of heart failure mortality (P = .86 for interaction), cardiovascular mortality (P = .70 for interaction), or all-cause mortality (P = .61 for interaction) (eTable 6 in the Supplement). The sex-stratified associations appear in eFigures 2-5 in the Supplement. In the sensitivity analyses, taking age on the time scale in the Cox proportional hazards regression model among TTR variant carriers, the HR was 4.26 (95% CI, 2.37-7.67) for heart failure mortality, 1.72 (95% CI, 1.22-2.44) for cardiovascular mortality, and 1.46 (95% CI, 1.19-1.79) for all-cause mortality (model 3 in eTable 7 in the Supplement).
The sensitivity analyses for all study outcomes using the multiple imputation data set (n = 8527) indicated similar results as the complete case analysis (eTables 8-10 in the Supplement). Compared with noncarriers, Black individuals with the TTR gene variant had a significantly higher hazard of incident heart failure (HR, 2.23 [95% CI, 1.55-3.22] in model 4), heart failure mortality (HR, 3.71 [95% CI, 2.12-6.55] in model 3), cardiovascular mortality (HR, 1.69 [95% CI, 1.23-2.36] in model 3), and all-cause mortality (HR, 1.42 [95% CI, 1.17-1.72] in model 3).
Discussion
In this retrospective cohort study of Black individuals living in the US, being a carrier of the TTR Val122Ile variant was significantly associated with an increased risk of heart failure. It also was associated with an increased risk of heart failure mortality, cardiovascular mortality, and all-cause mortality (eFigure 6 in the Supplement).
An autosomal-dominant disease, hATTR-CM has a median survival of nearly 2.5 years without treatment after receiving a diagnosis.34,35 Extrapolating the hATTR-CM–associated Val122Ile variant frequency to the population level suggests that approximately 1.4 million Black individuals carry this variant implicated in the development of heart failure and reduced overall survival. Despite the possible clinical implications, the Val122Ile TTR variant, which is seen relatively more commonly among individuals of African ancestry, is not included in the list of clinically actionable deleterious variants compiled by the American College of Medical Genetics and Genomics.9 Thus, this potentially deleterious variant may not be reported as clinically actionable, thereby reducing physician vigilance for hATTR-CM.
Recent data in younger Black individuals demonstrate that Val122Ile TTR variant carriers start exhibiting the subclinical structural and functional cardiac abnormalities during midlife, which is well before the traditional understanding of cardiac amyloidosis manifestation starting at approximately 65 years of age.36 Appropriate diagnostic imaging and testing combined with genetic screening, especially in those with a relevant family history, may allow for early detection of Black individuals at a higher risk of heart failure and death.37 Recently approved therapeutics targeting TTR may be more efficacious when used earlier in the disease course.10 Future investigations aimed at understanding the heterogeneity in phenotypic variability (ie, penetrance and expressivity) in large cohorts of TTR Val122Ile variant carriers may help elucidate the potential clinical yield of screening.
Prior investigations in the relatively smaller cohorts of Black individuals in the African Americans in the Arteriosclerosis Risk in Communities (ARIC) study (n = 3856) and the Cardiovascular Health Study (n = 805), which only accounted for age and sex in the analysis, indicated that older Black individuals with TTR Val122Ile might have an elevated risk of incident heart failure with a lack of a definitive signal for mortality.7,8 Even though all studies to date are consistent in the observed association of the TTR Val122Ile variant with incident heart failure, the investigation in the ARIC study did not demonstrate a signal for all-cause mortality among Black individuals carrying the variant.7,8 The relatively smaller sample size, the difference in the age distribution, the lack of accounting for African ancestry, and potential confounding by clinical and sociodemographic factors may have contributed to the relative difference in the results. Given the relatively higher prevalence of this variant in individuals with African ancestry, the findings support the greater inclusion of historically marginalized racial and ethnic groups in cardiovascular genetic and genomic research.38
Although the study findings indicate a robust association of the TTR Val122Ile variant with incident heart failure, it is unlikely to account for much of the excess heart failure risk experienced by Black individuals. The TTR Val122Ile variant may only partly contribute to the relatively higher prevalence of heart failure among Black individuals, which may predominantly result from adverse environmental factors (lifestyle and clinical factors, systemic racism, and social determinants of health).31,32,33
Limitations
This study has several limitations. First, the cohort did not include cardiac imaging of the study participants at baseline or follow-up visits. Hence, the study could not examine the association of the TTR variant with disease progression or the cardiac structure and functional status.
Second, the definitive diagnosis of hATTR-CM could not be established in the study population due to the lack of detailed imaging, endomyocardial biopsy, or autopsy specimens for examination in patients with heart failure. Third, a proportion of the study population was excluded due to incomplete data on the covariates. However, the sensitivity analyses using multiple imputations had similar results as the complete case analysis.
Fourth, the study sample size did not include a sufficient number of homozygous individuals to ascertain the association of the rs76992529 allele with the study outcomes in an additive model. Fifth, the study cohort prioritized recruitment in specific demographic subgroups that may not be nationally representative for the Black adult population of the US.
Sixth, the self-reported data for some demographic and clinical variables may potentially introduce measurement errors. Seventh, this is a retrospective cohort study that precluded ruling out potential residual confounding.
Eighth, individuals with suspected heart failure at baseline were excluded to better understand the association of the variant with heart failure incidence. This may have prevented identification of individuals with the TTR V122I variant who developed heart failure prior to enrollment. This approach may have contributed to the underestimation of the disease burden among TTR variant carriers.
Conclusions
Among a cohort of Black individuals living in the US, being a carrier of the TTR Val122Ile variant was significantly associated with an increased risk of heart failure.
eFigure 1. Derivation of the Study Population
eFigure 2. Sex-Stratified Association of TTR Val122Ile Variant with Incident Heart Failure
eFigure 3. Sex-Stratified Association of TTR Val122Ile Variant with All-Cause Mortality
eFigure 4. Sex-Stratified Association of TTR Val122Ile Variant with Cardiovascular Mortality
eFigure 5. Sex-Stratified Association of TTR Val122Ile Variant with Heart Failure Mortality
eFigure 6. Clinical Implications of TTR Val122Ile Variant Carrier Status in Black Individuals
eTable 1. Age, Sex, and Region-Stratified Distribution of TTR Variant Carriers in the Study Population
eTable 2. Age, Sex, and Region-Stratified Distribution of TTR Variant Carriers in the Study Population: Multiple Imputation Dataset
eTable 3. Age, Sex, and Region-Stratified Distribution of Study Outcomes by TTR Variant Carriers Status in the Study Population
eTable 4. Event Frequency of Study Outcomes Stratified by TTR Variant Carrier Status
eTable 5. Parameter Estimates of Full-Adjusted Model Assessing Association of TTR Variant Status with Incident Heart Failure
eTable 6. Sex-Stratified Association of TTR Val122Ile Variant with Incident Heart Failure, All-Cause Mortality, Cardiovascular Mortality, and Heart Failure Mortality
eTable 7. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among TTR Val122Ile Gene Variant Carriers: Taking Age on the Time Scale
eTable 8. Risk of Heart Failure Among TTR Val122Ile Gene Variant Carriers: Multiple Imputation Analysis
eTable 9. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among TTR Val122Ile Gene Variant Carriers: Multiple Imputation Analysis
eTable 10. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among TTR Val122Ile Gene Variant Carriers: Taking Age on the Time Scale (Multiple Imputation Analysis)
References
- 1.Rosenblum H, Castano A, Alvarez J, Goldsmith J, Helmke S, Maurer MS. TTR (transthyretin) stabilizers are associated with improved survival in patients with TTR cardiac amyloidosis. Circ Heart Fail. 2018;11(4):e004769. doi: 10.1161/CIRCHEARTFAILURE.117.004769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286-1300. doi: 10.1161/CIRCULATIONAHA.111.078915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7(7):398-408. doi: 10.1038/nrcardio.2010.67 [DOI] [PubMed] [Google Scholar]
- 4.Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African-Americans. Amyloid. 2015;22(3):171-174. doi: 10.3109/13506129.2015.1051219 [DOI] [PubMed] [Google Scholar]
- 5.Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19(7):733-742. doi: 10.1038/gim.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damrauer SM, Chaudhary K, Cho JH, et al. Association of the V122I hereditary transthyretin amyloidosis genetic variant with heart failure among individuals of African or Hispanic/Latino ancestry. JAMA. 2019;322(22):2191-2202. doi: 10.1001/jama.2019.17935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372(1):21-29. doi: 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) studies. Am Heart J. 2010;159(5):864-870. doi: 10.1016/j.ahj.2010.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DT, Lee K, Chung WK, et al. ; ACMG Secondary Findings Working Group . ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23(8):1381-1390. doi: 10.1038/s41436-021-01172-3 [DOI] [PubMed] [Google Scholar]
- 10.Maurer MS, Schwartz JH, Gundapaneni B, et al. ; ATTR-ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007-1016. doi: 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]
- 11.Howard VJ, Cushman M, Pulley L, et al. The Reasons for Geographic and Racial Differences in Stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135-143. doi: 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- 12.Goyal P, Mefford MT, Chen L, et al. Assembling and validating a heart failure-free cohort from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. BMC Med Res Methodol. 2020;20(1):53. doi: 10.1186/s12874-019-0890-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Geng X, Srinivasasainagendra V, et al. A PheWAS study of a large observational epidemiological cohort of African Americans from the REGARDS study. BMC Med Genomics. 2019;12(suppl 1):26. doi: 10.1186/s12920-018-0462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38(8):904-909. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 15.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655-1664. doi: 10.1101/gr.094052.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goyal P, Balkan L, Ringel JB, et al. The Dietary Approaches to Stop Hypertension (DASH) diet pattern and incident heart failure. J Card Fail. 2021;27(5):512-521. doi: 10.1016/j.cardfail.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heckbert SR, Kooperberg C, Safford MM, et al. Comparison of self-report, hospital discharge codes, and adjudication of cardiovascular events in the Women’s Health Initiative. Am J Epidemiol. 2004;160(12):1152-1158. doi: 10.1093/aje/kwh314 [DOI] [PubMed] [Google Scholar]
- 19.Bailey LN, Levitan EB, Judd SE, et al. Association of urine albumin excretion with incident heart failure hospitalization in community-dwelling adults. JACC Heart Fail. 2019;7(5):394-401. doi: 10.1016/j.jchf.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Safford MM, Brown TM, Muntner PM, et al. ; REGARDS Investigators . Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768-1774. doi: 10.1001/jama.2012.14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balakrishnan P, Beaty T, Young JH, Colantuoni E, Matsushita K. Methods to estimate underlying blood pressure: the Atherosclerosis Risk in Communities (ARIC) study. PLoS One. 2017;12(7):e0179234. doi: 10.1371/journal.pone.0179234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99-106. doi: 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 24.Keita AD, Judd SE, Howard VJ, Carson AP, Ard JD, Fernandez JR. Associations of neighborhood area level deprivation with the metabolic syndrome and inflammation among middle- and older- age adults. BMC Public Health. 2014;14:1319. doi: 10.1186/1471-2458-14-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parcha V, Patel N, Kalra R, et al. Obesity and serial NT-proBNP levels in guided medical therapy for heart failure with reduced ejection fraction: insights from the GUIDE-IT trial. J Am Heart Assoc. 2021;10(7):e018689. doi: 10.1161/JAHA.120.018689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parcha V, Patel N, Kalra R, et al. Racial differences in serial NT-proBNP levels in heart failure management: insights from the GUIDE-IT trial. Circulation. 2020;142(10):1018-1020. doi: 10.1161/CIRCULATIONAHA.120.046374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj NS, Gutiérrez OM, Arora G, et al. Racial differences in plasma levels of N-terminal pro-B-type natriuretic peptide and outcomes: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. JAMA Cardiol. 2018;3(1):11-17. doi: 10.1001/jamacardio.2017.4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel N, Cushman M, Gutiérrez OM, et al. Racial differences in the association of NT-proBNP with risk of incident heart failure in REGARDS. JCI Insight. Published online June 4, 2019. doi: 10.1172/jci.insight.129979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel N, Gutiérrez OM, Arora G, et al. Race-based demographic, anthropometric and clinical correlates of N-terminal-pro B-type natriuretic peptide. Int J Cardiol. 2019;286:145-151. doi: 10.1016/j.ijcard.2019.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381(3):243-251. doi: 10.1056/NEJMoa1803180 [DOI] [PubMed] [Google Scholar]
- 31.Parcha V, Kalra R, Best AF, et al. Geographic inequalities in cardiovascular mortality in the United States: 1999 to 2018. Mayo Clin Proc. 2021;96(5):1218-1228. doi: 10.1016/j.mayocp.2020.08.036 [DOI] [PubMed] [Google Scholar]
- 32.Parcha V, Kalra R, Suri SS, et al. Geographic variation in cardiovascular health among American adults. Mayo Clin Proc. 2021;96(7):1770-1781. doi: 10.1016/j.mayocp.2020.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parcha V, Malla G, Suri SS, et al. Geographic variation in racial disparities in health and coronavirus disease-2019 (COVID-19) mortality. Mayo Clin Proc Innov Qual Outcomes. 2020;4(6):703-716. doi: 10.1016/j.mayocpiqo.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng KY, Loungani RS, Rao VN, et al. Best practices for prognostic evaluation of a patient with transthyretin amyloid cardiomyopathy. JACC CardioOncol. 2019;1(2):273-279. doi: 10.1016/j.jaccao.2019.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(22):2872-2891. doi: 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinha A, Zheng Y, Nannini D, et al. Association of the V122I transthyretin amyloidosis genetic variant with cardiac structure and function in middle-aged Black adults: Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Cardiol. 2021;6(6):718-722. doi: 10.1001/jamacardio.2020.6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kittleson MM, Maurer MS, Ambardekar AV, et al. ; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology . Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142(1):e7-e22. doi: 10.1161/CIR.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 38.Mudd-Martin G, Cirino AL, Barcelona V, et al. ; American Heart Association Council on Genomic and Precision Medicine; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Considerations for cardiovascular genetic and genomic research with marginalized racial and ethnic groups and indigenous peoples: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2021;14(4):e000084. doi: 10.1161/HCG.0000000000000084 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Derivation of the Study Population
eFigure 2. Sex-Stratified Association of TTR Val122Ile Variant with Incident Heart Failure
eFigure 3. Sex-Stratified Association of TTR Val122Ile Variant with All-Cause Mortality
eFigure 4. Sex-Stratified Association of TTR Val122Ile Variant with Cardiovascular Mortality
eFigure 5. Sex-Stratified Association of TTR Val122Ile Variant with Heart Failure Mortality
eFigure 6. Clinical Implications of TTR Val122Ile Variant Carrier Status in Black Individuals
eTable 1. Age, Sex, and Region-Stratified Distribution of TTR Variant Carriers in the Study Population
eTable 2. Age, Sex, and Region-Stratified Distribution of TTR Variant Carriers in the Study Population: Multiple Imputation Dataset
eTable 3. Age, Sex, and Region-Stratified Distribution of Study Outcomes by TTR Variant Carriers Status in the Study Population
eTable 4. Event Frequency of Study Outcomes Stratified by TTR Variant Carrier Status
eTable 5. Parameter Estimates of Full-Adjusted Model Assessing Association of TTR Variant Status with Incident Heart Failure
eTable 6. Sex-Stratified Association of TTR Val122Ile Variant with Incident Heart Failure, All-Cause Mortality, Cardiovascular Mortality, and Heart Failure Mortality
eTable 7. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among TTR Val122Ile Gene Variant Carriers: Taking Age on the Time Scale
eTable 8. Risk of Heart Failure Among TTR Val122Ile Gene Variant Carriers: Multiple Imputation Analysis
eTable 9. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among TTR Val122Ile Gene Variant Carriers: Multiple Imputation Analysis
eTable 10. Risk of Heart Failure Mortality, Cardiovascular Mortality, and All-Cause Mortality Among TTR Val122Ile Gene Variant Carriers: Taking Age on the Time Scale (Multiple Imputation Analysis)




