Abstract
Unlike mammalian cells, malarial parasites are completely dependent on the de novo pyrimidine pathway and lack the enzymes to salvage preformed pyrimidines. In the present study, first, it is shown that 1843U89, even without polyglutamylation, is a potent folate-based inhibitor of purified malarial parasite thymidylate synthase. The binding was noncompetitive with respect to methylenetetrahydrofolate, and 1843U89 had a Ki of 1 nM. The compound also had potent antimalarial activity in vitro. Plasmodium falciparum cells in culture were inhibited by 1843U89, with a 50% inhibitory concentration of about 70 nM. The compound was effective against drug-sensitive as well as drug-resistant clones of P. falciparum. As predicted by the biochemistry of the parasite, the potent inhibition of parasite proliferation by 1843U89 could not be reversed with 10 μM thymidine. In contrast, in the presence of 10 μM thymidine, mammalian cells were unaffected by 1843U89 even at concentrations as high as 0.1 mM, thus offering a selectivity window of more than 1,000-fold. On this basis, folate-based thymidylate synthase inhibitors may represent a powerful additional tool that can be used to combat drug-resistant malaria.
Malaria is the cause of over 200 million infections and 2 million deaths per year (36). With the recent acceleration in malarial parasite drug resistance (27, 38), there is a strong need to identify new chemotherapeutic strategies. Malarial parasites are completely dependent on de novo pyrimidine biosynthesis and cannot salvage pyrimidines (30, 33). In sharp contrast, mammalian cells readily transport pyrimidine bases and nucleosides and convert them into nucleotides (24). Thus, a combination of an effective de novo pyrimidine biosynthesis inhibitor and a nucleoside (that only the host can use) should be efficacious (24).
Of all the de novo pyrimidine biosynthesis enzymes, thymidylate synthase (TS) is particularly attractive as a target for malaria chemotherapy. It is metabolically linked to dihydrofolate reductase (DHFR), a proven target of antimalarial agents such as pyrimethamine (6, 9, 39). DHFR and TS are part of a single bifunctional polypeptide (1, 3, 4). Even partial inhibition of TS leads to nucleotide imbalances and cell death (15, 16, 19, 40). The availability of many known nucleotide- and folate-based potent inhibitors of TS facilitates applications to treatments for malaria (2, 7, 17, 29). However, TS is one of the most conserved proteins in the cell. No inhibitors bind to TS from one species more tightly than they bind to TS from another species.
To inhibit malarial parasite TS selectively, one must exploit distant differences in metabolism between malarial and mammalian cells (24, 26, 28). For example, 5-fluoro-2′-deoxyuridylate (5-FdUMP) is a potent TS inhibitor (2, 20, 29), but it is 5-fluoroorotate, a metabolic precursor of 5-FdUMP, that causes inactivation of malarial parasite TS and inhibition of cell proliferation at nanomolar concentrations (24, 26). It takes high micromolar concentrations of 5-fluoroorotate to inhibit mammalian cells significantly (24). Furthermore, combining 5-fluoroorotate with uridine further improves the selective toxicity of 5-fluoroorotate (23, 24).
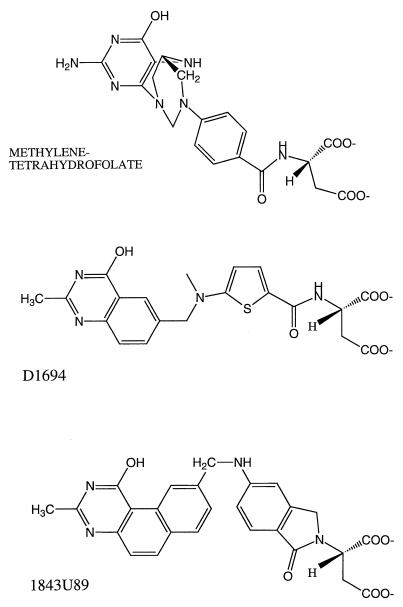
In an earlier study, the quinazoline folate-based mammalian TS inhibitor D1694 (Fig. 1) (17) was tested against malarial parasites in culture (28). As expected, the toxic effects of D1694 on mammalian cells could be reversed with 10 μM thymidine, but in a promising outcome, the toxic effects on malarial parasites remained unaltered (28). Unfortunately, D1694 had only modest potency against malarial parasites (50% inhibitory concentration [IC50], 20 μM). Kinetic studies revealed that D1694 was a relatively poor inhibitor of malarial parasite TS (Ki, 2.8 μM), but the polyglutamate form of D1694 was potent (Ki, 1.5 nM) (14). Thus, the poor activity of D1694 against parasite cells could be due, at least in part, to the poor polyglutamylation of D1694 (37).
FIG. 1.
Comparison of structures of the substrate methylenetetrahydrofolate to the folate-based TS inhibitors D1694 and 1843U89.
In the present study, we examined the antimalarial potential of 1843U89 (Fig. 1), a member of a new group of potent folate-based TS inhibitors that do not require polyglutamylation for tight binding to the target TS or for antiproliferative activity (7).
MATERIALS AND METHODS
Reagents.
1843U89 was kindly provided by Eric Furfine of Glaxo Wellcome, Research Triangle Park, N.C., and D1694 was provided by F. T. Boyle of Zeneca Pharmaceuticals, Cheshire, United Kingdom.
Inhibition of malarial parasite TS.
Recombinant malarial parasite DHFR-TS was purified to homogeneity as described previously (14, 32a). A discontinuous tritium release assay was used to measure enzyme activity under initial rate conditions (14, 31).
Cell culture.
Plasmodium falciparum clones D6 and W2 were kindly provided by D. Kyle and W. Milhous, Walter Reed Army Institute of Research (27), and FCR3, HB3, and 3D7 were provided by T. Wellems, National Institutes of Health (27). Parasites were propagated in vitro as described previously (35). Mouse lymphocytic leukemia (L1210) cells were obtained from the American Type Culture Collection, Manassas, Va.
Antiproliferation assays.
All cytotoxicity assays were performed in 96-well plates (5, 24, 28). P. falciparum-infected erythrocytes were set up with 0.5% initial parasitemia in 2% hematocrit and were exposed to various concentrations of 1843U89 for 48 h and then pulsed with 0.5 μCi of radioactive hypoxanthine for 24 h. Mouse L1210 cells were set up at an initial density of 4,000 cells per well in a 96-well plate with various concentrations of 1843U89. After 48 h, the cells were exposed to 0.5 μCi of hypoxanthine for 24 h. In all cases, incorporation of radioactivity into precipitable nucleic acids again served as a measure of cell proliferation.
RESULTS
Inhibition of malarial parasite TS by 1843U89.
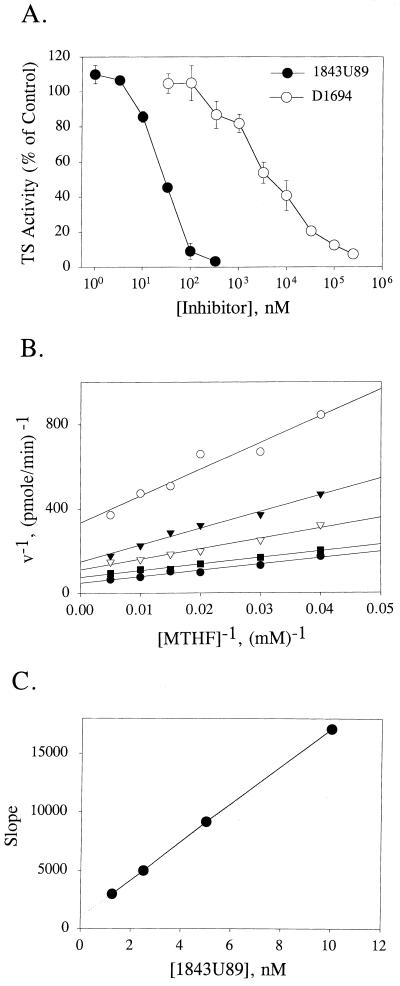
Exposure of purified malarial parasite TS to various concentrations of 1843U89 in the presence of subsaturating concentrations of substrates revealed that the compound was effective at nanomolar concentrations (Fig. 2A). It was not necessary to have 1843U89 in a polyglutamate form in order to see potent inhibition. In contrast, D1694 without polyglutamylation inhibited malarial parasite TS only at concentrations in the micromolar range (Fig. 2A).
FIG. 2.
Inhibition of malarial parasite TS by folate-based inhibitors. (A) Comparison of enzyme inhibition by 1843U89 and D1694. The substrates were used at a subsaturating concentration of 2 μM for dUMP and 35 μM for methylenetetrahydrofolate (MTHF). Each assay was performed with 20 U of TS activity. (B) Double-reciprocal plot to assess inhibition of malarial parasite TS by various amounts of 1843U89 (1.25, 2.5, 5, and 10 nM) in the presence of a fixed concentration of 50 μM dUMP and between 25 and 200 μM methylenetetrahydrofolate. (C) Secondary plot to estimate the strength of binding between 1843U89 and malarial parasite TS.
Tightness of 1843U89 binding.
A detailed kinetic analysis of the binding interactions between malarial parasite TS and 1843U89 showed that this compound was noncompetitive with respect to methylenetetrahydrofolate (Fig. 2B). From a secondary plot of the slopes of the inhibition lines from Fig. 2B, 1843U89 was found to have a Ki of 1 nM against malarial parasite TS (Fig. 2C).
Antiproliferative activity of 1843U89 against P. falciparum.
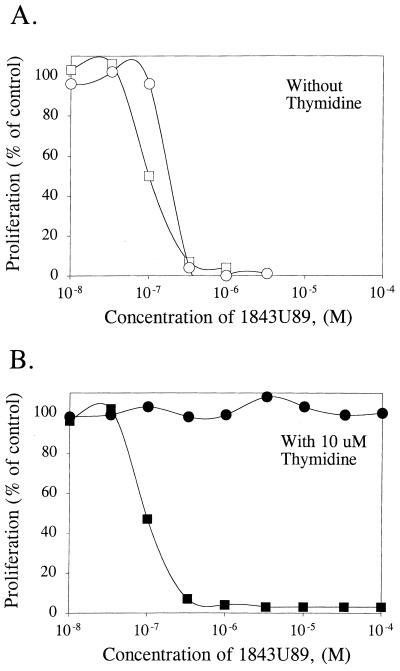
As with mammalian L1210 cells, nanomolar concentrations of 1843U89 were sufficient to inhibit proliferation of the human malarial parasite P. falciparum (Fig. 3A). Parasite clones that were derived from different parts of the world and that showed large differences in resistance to traditional antimalarial compounds (27) were approximately equally sensitive to 1843U89. For clones D6, HB3, 3D7, and W2 the 1843U89 IC50 was 70 nM both with and without 10 μM thymidine (data not shown). The IC50 for clone FCR3 was slightly higher (200 nM).
FIG. 3.
Inhibition of P. falciparum proliferation and mouse L1210 cell proliferation by 1843U89 in the presence of 10 μM thymidine. (A) Antiproliferative activity of P. falciparum (open squares) and mouse L1210 cells (open circles) in the absence of thymidine. (B) Antiproliferative activity of P. falciparum (closed squares) and mouse L1210 cells in the presence of 10 μM thymidine (closed circles).
Antimalarial activity of 1843U89 in the presence of thymidine.
P. falciparum-infected erythrocytes as well as mouse L1210 cells were tested for their sensitivities to 1843U89 in the presence of 10 μM thymidine. Unlike mammalian cells, all malarial parasite clones remained completely sensitive to 1843U89 in the presence of thymidine (Fig. 3B).
DISCUSSION
Given the absence of pyrimidine salvage pathways in P. falciparum, the whole de novo pyrimidine biosynthesis pathway can be considered a potential target for selective chemotherapy. Yet, with one exception (24), most inhibitors of de novo pyrimidine biosynthesis enzymes have unimpressive antiproliferative activity against intact malarial parasites (18, 22, 32). Atovaquone, which was originally thought to act by blocking the biosynthesis of orotate, inhibits parasite proliferation primarily by disrupting mitochondrial membrane potential (34).
The compound with a truly potent and selective antimalarial activity directed at pyrimidine metabolism is 5-fluoroorotate. This compound inhibits parasites with an IC50 of about 5 nM (24), is equally effective against drug-sensitive as well as drug-resistant P. falciparum (24), and can cure malaria in mice and is orally available (12, 23). However, in order to use this compound as a single agent without the possibility of drug resistance (11, 25, 27), it is necessary to achieve concentrations in the serum of the treated animal that approach 1 to 10 μM (23). Such concentrations are dangerously close to what is tolerated in mammalian cells (23).
The primary mechanism by which 5-fluoroorotate causes parasite death is probably quite different from the mechanism by which high doses of 5-fluoroorotate cause toxicity in the host. It is known that nanomolar levels of 5-fluoroorotate inhibit TS in malarial parasites (26). The toxicity of high levels of 5-fluoroorotate, in contrast, probably arises from incorporation of 5-fluoropyrimidine nucleotides into RNA and possibly DNA (8, 13, 20). This complicates the strategy of using nucleosides to rescue mammalian cells. Thymidine or uridine alone decreases the toxic effects of very high doses of 5-fluoroorotate but does not completely eliminate them (24).
In contrast to 5-fluoropyrimidine-based strategies, folate-based strategies have some distinct advantages. Folate analogs are not metabolically degraded and they cannot be incorporated into nucleic acids. If a folate analog inhibits TS with potency, selectivity will arise automatically if TS is the only target of the antifolate.
In the present study, we demonstrate that 1843U89 inhibits malarial parasites at midnanomolar concentrations. This is an approximately 1,000 times greater potency than that of the previous folate-based TS inhibitor tried against malarial parasites. Unlike folate-based DHFR inhibitors, which show as much as 1,000-fold differences in their antiproliferative activities against different clones of P. falciparum (6, 9, 10, 21, 39), 1843U89 was approximately equally effective against all parasite clones tested. 1843U89 seems to act through a mechanism which is independent of all currently used antimalarial drugs and which is independent of the common drug resistance mechanisms used by malarial parasites. The mechanism that underlies the slight resistance of clone FCR3 to 1843U89 is not yet clear. Consistent with the inability of malarial parasites to salvage pyrimidines, inhibition of parasite proliferation by 1843U89 persisted even in the presence of thymidine. In sharp contrast, in the presence of 10 μM thymidine, mouse L1210 cells were not susceptible to 1843U89, even when the limits of solubility of this compound were approached.
It is expected that small variation in the structure of 1843U89 might result in even more potent activity against malarial parasites, either because the compound binds to malarial parasite TS better or because it is a better substrate for transport or polyglutamylation in the parasite.
ACKNOWLEDGMENTS
P.K.R. thanks Eric Furfine and John Reardon of Glaxo Welcome and F. T. Boyle of Zeneca Pharmaceuticals for taking interest in malaria chemotherapy, for providing us with TS inhibitors, and for helpful discussions.
This work was supported by Public Health Service grants from the National Institute of Allergy and Infectious Diseases (grants AI26912 and AI40956). P.K.R. is a recipient of a Burroughs Wellcome Fund New Initiatives in Malaria Research Award.
REFERENCES
- 1.Bzik D J, Li W B, Horii T, Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci USA. 1987;84:8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carreras C W, Santi D V. The catalytic mechanism and structure of thymidylate synthase. Annu Rev Biochem. 1995;64:721–762. doi: 10.1146/annurev.bi.64.070195.003445. [DOI] [PubMed] [Google Scholar]
- 3.Chen G X, Zolg J W. Purification of the bifunctional thymidylate synthase-dihydrofolate reductase complex from the human malaria parasite Plasmodium falciparum. Mol Pharmacol. 1987;32:723–730. [PubMed] [Google Scholar]
- 4.Coderre J A, Beverley S M, Schimke R T, Santi D V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci USA. 1983;80:2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desjardins R E, Canfield C J, Haynes J D, Chulay J D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diggens S M, Gutteridge W E, Trigg P I. Altered dihydrofolate reductase associated with a pyrimethamine-resistant Plasmodium berghei berghei produced in a single step. Nature. 1970;228:579–580. doi: 10.1038/228579a0. [DOI] [PubMed] [Google Scholar]
- 7.Duch D S, Banks S, Dev I K, Dickerson S H, Ferone R, Heath L S, Humphreys J, Knick V, Pendergast W, Singer S, et al. Biochemical and cellular pharmacology of 1843U89, a novel benzoquinazoline inhibitor of thymidylate synthase. Cancer Res. 1993;53:810–818. [PubMed] [Google Scholar]
- 8.Evans R M, Laskin J D, Hakala M T. Assessment of growth-limiting events caused by 5-fluorouracil in mouse cells and in human cells. Cancer Res. 1980;40:4113–4122. [PubMed] [Google Scholar]
- 9.Ferone R. Dihydrofolate reductase from pyrimethamine-resistant Plasmodium berghei. J Biol Chem. 1970;245:850–854. [PubMed] [Google Scholar]
- 10.Foote S J, Galatis D, Cowman A F. Amino acids in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differ from those involved in pyrimethamine resistance. Proc Natl Acad Sci USA. 1990;87:3014–3017. doi: 10.1073/pnas.87.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gassis S, Rathod P K. Frequency of drug resistance in Plasmodium falciparum: a nonsynergistic combination of 5-fluoroorotate and atovaquone suppresses in vitro resistance. Antimicrob Agents Chemother. 1996;40:914–919. doi: 10.1128/aac.40.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez Z M, Rathod P K. Antimalarial activity of a combination of 5-fluoroorotate and uridine in mice. Antimicrob Agents Chemother. 1990;34:1371–1375. doi: 10.1128/aac.34.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidelberger C, Danenberg P V, Moran R G. Fluorinated pyrimidines and their nucleosides. Adv Enzymol Relat Areas Mol Biol. 1983;54:58–119. [PubMed] [Google Scholar]
- 14.Hekmat-Nejad M, Rathod P K. Kinetics of Plasmodium falciparum thymidylate synthase: interactions with high-affinity metabolites of 5-fluoroorotate and D1694. Antimicrob Agents Chemother. 1996;40:1628–1632. doi: 10.1128/aac.40.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houghton P J, Germain G S, Hazelton B J, Pennington J W, Houghton J A. Mutants of human colon adenocarcinoma, selected for thymidylate synthase deficiency. Proc Natl Acad Sci USA. 1989;86:1377–1381. doi: 10.1073/pnas.86.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingraham H A, Dickey L, Goulian M. DNA fragmentation and cytotoxicity from increased cellular deoxyuridylate. Biochemistry. 1986;25:3225–3230. doi: 10.1021/bi00359a022. [DOI] [PubMed] [Google Scholar]
- 17.Jackman A L, Taylor G A, Gibson W, Kimbell R, Brown M, Calvert A H, Judson I R, Hughes L R. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: a new agent for clinical study. Cancer Res. 1991;51:5579–5586. [PubMed] [Google Scholar]
- 18.Krungkrai J, Krungkrai S R, Phakanont K. Antimalarial activity of orotate analogs that inhibit dihydroorotase and dihydroorotate dehydrogenase. Biochem Pharmacol. 1992;43:1295–1301. doi: 10.1016/0006-2952(92)90506-e. [DOI] [PubMed] [Google Scholar]
- 19.Kunz B A, Kohalmi S E, Kunkel T A, Mathews C K, McIntosh E M, Reidy J A. International Commission for Protection Against Environmental Mutagens and Carcinogens. Deoxyribonucleoside triphosphate levels: a critical factor in the maintenance of genetic stability. Mutat Res. 1994;318:1–64. doi: 10.1016/0165-1110(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 20.Parker W B, Cheng Y C. Metabolism and mechanism of action of 5-fluorouracil. Pharmacol Ther. 1990;48:381–395. doi: 10.1016/0163-7258(90)90056-8. [DOI] [PubMed] [Google Scholar]
- 21.Peterson D S, Milhous W K, Wellems T E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Queen S A, VanderJagt D L, Reyes P. In vitro susceptibilities of Plasmodium falciparum to compounds which inhibit nucleotide metabolism. Antimicrob Agents Chemother. 1990;34:1393–1398. doi: 10.1128/aac.34.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathod P K, Gomez Z M. Plasmodium yoelii: oral delivery of 5-fluoroorotate to treat malaria in mice. Exp Parasitol. 1991;73:512–514. doi: 10.1016/0014-4894(91)90075-8. [DOI] [PubMed] [Google Scholar]
- 24.Rathod P K, Khatri A, Hubbert T, Milhous W K. Selective activity of 5-fluoroorotic acid against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1989;33:1090–1094. doi: 10.1128/aac.33.7.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rathod P K, Khosla M, Gassis S, Young R D, Lutz C. Selection and characterization of 5-fluoroorotate-resistant Plasmodium falciparum. Antimicrob Agents Chemother. 1994;38:2871–2876. doi: 10.1128/aac.38.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathod P K, Leffers N P, Young R D. Molecular targets of 5-fluoroorotate in the human malaria parasite, Plasmodium falciparum. Antimicrob Agents Chemother. 1992;36:704–711. doi: 10.1128/aac.36.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rathod P K, McErlean T, Lee P C. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rathod P K, Reshmi S. Susceptibility of Plasmodium falciparum to a combination of thymidine and ICI D1694, a quinazoline antifolate directed at thymidylate synthase. Antimicrob Agents Chemother. 1994;38:476–480. doi: 10.1128/aac.38.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyes P, Heidelberger C. Fluorinated pyrimidines. XXVI. Mammalian thymidylate synthetase: its mechanism of action and inhibition by fluorinated nucleotides. Mol Pharmacol. 1965;1:14–30. [PubMed] [Google Scholar]
- 30.Reyes P, Rathod P K, Sanchez D J, Mrema J E, Rieckmann K H, Heidrich H G. Enzymes of purine and pyrimidine metabolism from the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 1982;5:275–290. doi: 10.1016/0166-6851(82)90035-4. [DOI] [PubMed] [Google Scholar]
- 31.Roberts D. An isotopic assay for thymidylate synthetase. Biochemistry. 1966;5:3546–3548. doi: 10.1021/bi00875a022. [DOI] [PubMed] [Google Scholar]
- 32.Seymour K K, Lyons S D, Phillips L, Rieckmann K H, Christopherson R I. Cytotoxic effects of inhibitors of de novo pyrimidine biosynthesis upon Plasmodium falciparum. Biochemistry. 1994;33:5268–5274. doi: 10.1021/bi00183a033. [DOI] [PubMed] [Google Scholar]
- 32a.Shallom S L, Zhang K, Jiang L, Rathod P K. Essential protein-protein interactions between Plasmodium falciparum thymidylate synthase and dihydrofolate reductase domains. J Biol Chem. 1999;274:37781–37786. doi: 10.1074/jbc.274.53.37781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherman I W. Purine and pyrimidine metabolism of asexual stages. In: Sherman I W, editor. Malaria: parasite biology, pathogenesis, and protection. Washington, D.C.: ASM Press; 1998. pp. 177–184. [Google Scholar]
- 34.Srivastava I K, Vaidya A B. A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrob Agents Chemother. 1999;43:1334–1339. doi: 10.1128/aac.43.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 36.Trigg P I, Kondrachine A V. The current global malaria situation. In: Sherman I W, editor. Malaria: parasite biology, pathogenesis, and protection. Washington, D.C.: ASM Press; 1998. pp. 11–22. [Google Scholar]
- 37.Ward W H, Kimbell R, Jackman A L. Kinetic characteristics of ICI D1694: a quinazoline antifolate which inhibits thymidylate synthase. Biochem Pharmacol. 1992;43:2029–2031. doi: 10.1016/0006-2952(92)90646-z. [DOI] [PubMed] [Google Scholar]
- 38.White N J. Antimalarial drug resistance: the pace quickens. J Antimicrob Chemother. 1992;30:571–585. doi: 10.1093/jac/30.5.571. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y, Kirkman L A, Wellems T E. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc Natl Acad Sci USA. 1996;93:1130–1134. doi: 10.1073/pnas.93.3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshioka A, Tanaka S, Hiraoka O, Koyama Y, Hirota Y, Ayusawa D, Seno T, Garrett C, Wataya Y. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J Biol Chem. 1987;262:8235–8241. [PubMed] [Google Scholar]