Abstract
Background
Surgical interventions are used for trigeminal neuralgia when drug treatment fails. Surgical treatments divide into two main categories, ablative (destructive) or non‐ablative. These treatments can be done at three different sites: peripherally, at the Gasserian ganglion level, and within the posterior fossa of the skull.
Objectives
To assess the efficacy of neurosurgical interventions for classical trigeminal neuralgia in terms of pain relief, quality of life and any harms. To determine if there are defined subgroups of patients more likely to benefit.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register, (13 May 2010), CENTRAL (Issue 2, 2010 part of the Cochrane Library), Health Technology Assessment (HTA) Database, NHS Economic Evaluation Database (NHSEED) and Database of Abstracts of Reviews of Effects (DARE) (Issue 4, 2010 (HTA, NHSEED and DARE are part of the Cochrane Library)), MEDLINE (January 1966 to May 2010) and EMBASE (January 1980 to May 2010) with no language exclusion.
Selection criteria
Randomised controlled trials and quasi‐randomised controlled trials of neurosurgical interventions used in the treatment of classical trigeminal neuralgia.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. We contacted authors for clarification and missing information whenever possible.
Main results
Eleven studies involving 496 participants met some of the inclusion criteria stated in the protocol. One hundred and eighty patients in five studies had peripheral interventions, 229 patients in five studies had percutaneous interventions applied to the Gasserian ganglion, and 87 patients in one study underwent two modalities of stereotactic radiosurgery (Gamma Knife) treatment. No studies addressing microvascular decompression (which is the only non‐ablative procedure) met the inclusion criteria. All but two of the identified studies had a high to medium risk of bias because of either missing data or methodological inconsistency. It was not possible to undertake meta‐analysis because of differences in the intervention modalities and variable outcome measures. Three studies had sufficient outcome data for analysis. One trial, which involved 40 participants, compared two techniques of radiofrequency thermocoagulation (RFT) of the Gasserian ganglion at six months. Pulsed RFT resulted in return of pain in all participants by three months. When this group were converted to conventional (continuous) treatment these participants achieved pain control comparable to the group that had received conventional treatment from the outset. Sensory changes were common in the continuous treatment group. In another trial, of 87 participants, investigators compared radiation treatment to the trigeminal nerve at one or two isocentres in the posterior fossa. There were insufficient data to determine if one technique was superior to another. Two isocentres increased the incidence of sensory loss. Increased age and prior surgery were predictors for poorer pain relief. Relapses were nonsignificantly reduced with two isocentres (risk ratio (RR) 0.72, 95% confidence intervaI (CI) 0.30 to 1.71). A third study compared two techniques for RFT in 54 participants for 10 to 54 months. Both techniques produced pain relief (not significantly in favour of neuronavigation (RR 0.70, 95% CI 0.46 to 1.04) but relief was more sustained and side effects fewer if a neuronavigation system was used. The remaining eight studies did not report outcomes as predetermined in our protocol.
Authors' conclusions
There is very low quality evidence for the efficacy of most neurosurgical procedures for trigeminal neuralgia because of the poor quality of the trials. All procedures produced variable pain relief, but many resulted in sensory side effects. There were no studies of microvascular decompression which observational data suggests gives the longest pain relief. There is little evidence to help comparative decision making about the best surgical procedure. Well designed studies are urgently needed.
Plain language summary
Neurosurgical interventions for the treatment of classical trigeminal neuralgia
Trigeminal neuralgia is defined as "sudden usually unilateral severe brief stabbing recurrent pains in the distribution of one or more branches of the fifth cranial nerve". It has an incidence rate of 12.6 per 100,000 person years and more commonly affects older age groups. The fifth cranial nerve is one of the largest in the head. The nerve is called trigeminal because it splits into three main branches. It provides sensation to the face. When neuralgia (nerve pain) occurs in the trigeminal nerve it causes severe and sudden face pain.
The causes of trigeminal neuralgia are unclear. Treatment of all people with classical trigeminal neuralgia begins with drug therapy, most frequently using one of several drugs also used to treat epilepsy, among which the gold standard remains carbamazepine. If drug therapy fails then surgical interventions may be used. Surgical treatments divide into two main categories: ablative (destroying the nerve) or non‐ablative (preserving nerve function and relieving the pressure on the nerve). These procedures result in pain relief for variable lengths of time.
For this review, we searched for all of the surgical procedures for trigeminal neuralgia. We found 11 studies, which included 496 patients, but only three had sufficient outcome data to report. These three studies, which involved a total of 181 participants, fulfilled the inclusion criteria and form the basis of this review. The primary aim of all three studies was to determine if one technique was better than the other. All three included studies evaluated destructive techniques. None of the three studies evaluated the non‐destructive procedure of microvascular decompression and this is a major drawback in the literature.
One study compared two different techniques of radiofrequency thermocoagulation, in 40 participants six months after the procedure. This technique involves heating the nerve by passing an electrical current through the tip of a special needle which has been introduced through the skin into a hole in the base of the skull and into the ganglion from which the three divisions of the trigeminal nerve branch out (Gasserian ganglion). If the radiofrequency was given as pulsed treatment (which causes the tip of the needle to heat up intermittently and not continuously) the original pain in all participants returned by three months. The continuous radiofrequency treatment then had to be applied, and these participants then achieved pain control comparable to those who had received continuous radiofrequency throughout. Changes in sensation ranging from mild to severe numbness were common in the conventional (continuous) radiofrequency treatment group.
A second trial, in 87 participants, looked at using one or two isocentres (specific points in the nerve) to deliver radiation to the trigeminal nerve just as it leaves the brainstem inside the skull. Use of medication afterwards was considered a surrogate measure for pain. Use of two isocentres increased the occurrence of sensory loss as a complication. Increased age and prior surgery were predictors for poorer pain relief. There were insufficient data given to judge the effectiveness of one procedure better than the other.
A third study compared two techniques for performing radiofrequency thermocoagulation of the Gasserian ganglion in 54 participants. The study compared two ways of introducing the needle and guiding it, using either X‐rays or a special neuronavigation system. Pain relief was measured by a questionnaire at three months. Both techniques provided pain relief (which did not differ significantly between the two arms) but it was more sustained if a neuronavigation system was used and this system also decreased side effects.
All the reviewed procedures resulted in pain relief and some participants were then able to stop taking medications. However, many procedures tended to result in sensory side effects. All the studies in this review had flaws in their methods and all but two showed considerable risk of bias. There is little evidence from these trials to guide the person with trigeminal neuralgia as to the most effective surgical procedure. There is now an urgent need to evaluate the surgical interventions used in trigeminal neuralgia and to design robust studies; either randomised controlled trials or long‐term prospective independently assessed cohort studies.
Background
The International Association for the Study of Pain (IASP) definition of trigeminal neuralgia is "sudden usually unilateral severe brief stabbing recurrent pains in the distribution of one or more branches of the fifth cranial nerve" (Merskey 1994).
Trigeminal neuralgia is an uncommon disease with point prevalence of 0.001% (Munoz 1988) and an annual incidence of 4.3 per 100,000 (Katusic 1990). It is a disease of older age groups and slightly more common in women (Rothman 1973). More recent data from general practice databases suggest an overall incidence rate of 12.6 per 100,000 person years with a mean age of 51.5 years and 66% female predominance (Koopman 2009). Trigeminal neuralgia was noted to occur in greater frequency in patients with multiple sclerosis (Katusic 1991).
The cause of trigeminal neuralgia remains speculative. Demyelination or other damage within the brainstem trigeminal circuitry is postulated for cases associated with multiple sclerosis or lacunar infarction, and demyelination of the root entry zone of the trigeminal nerve has clearly been demonstrated for 'idiopathic cases', possibly due to vascular compression. Numerous hypotheses have been put forward but evidence is still lacking. The ignition hypothesis suggests that trigeminal neuralgia pain results from light touch stimuli being interpreted as pain due to loss of myelin insulation between nerve fibres conveying pain and those conveying light touch (Devor 2002). Lay descriptions can be found in the books published by the Trigeminal Neuralgia Association, US (Weigel 2000; Zakrzewska 2006). The International Headache Society (IHS) has put forward the following diagnostic criteria for trigeminal neuralgia (Anon 2004). A. Paroxysmal attacks of pain lasting from a fraction of a second to two minutes, affecting one or more division of the trigeminal nerve and fulfilling criteria B and C. B. Pain has at least one of the following characteristics: (1) Intense, sharp, superficial or stabbing; (2) Precipitated from trigger areas or by trigger factors. C. Attacks are stereotyped in the individual patient. D. There is no clinically evident neurological deficit. E. Not attributed to another disorder.
These criteria were used in this review, with the exception of D as studies do not report these data. Neurological deficits in the trigeminal sensory distribution in trigeminal neuralgia can be present if the patient has had a prior surgical procedure, or if the condition has been present for a prolonged period (Barker 1997). When no secondary intrinsic brainstem or cerebello‐pontine angle structural cause can be found, such as tumour, multiple sclerosis, lacunar infarction, aneurysm or arterio‐venous malformation, the trigeminal neuralgia is called classical. Many studies also draw attention to what is being termed as atypical trigeminal neuralgia (Nurmikko 2001). These patients have additional features of burning and smarting which remain after the sharp shooting attacks for minutes to hours.The most common investigation carried out is magnetic resonance imaging to determine whether there is a symptomatic cause and to determine if there is vascular compression.
Due to the rarity of the condition and the difficulty of designing trials that take into account the natural pain remission periods that are common in patients with trigeminal neuralgia, there are few high quality randomised control trials in this area.
Treatment of all patients with classical trigeminal neuralgia begins with drug therapy, most frequently using anticonvulsants with the gold standard remaining carbamazepine. There are Cochrane systematic reviews on the use of anticonvulsant drugs in neuropathic pain (Wiffen 2011) and on the use of non‐anticonvulsant drugs for trigeminal neuralgia (Yang 2011).
When medical management fails, either due to breakthrough of pain or intolerable side effects from drug therapy, then surgery will be considered. There is now some evidence from cohort data to suggest that earlier surgical treatment may provide improved long‐term outcomes and improved patient satisfaction (Barker 1997; Zakrzewska 2005).
Surgical treatments divide into two main categories: ablative (destructive to the nerve) or non‐ablative (preserving nerve function and decompressing the nerve). All these operations have been described in jargon‐free language for patients in two publications by the Trigeminal Neuralgia Association US (Weigel 2000; Zakrzewska 2006) and a glossary is available on the website. It is generally accepted that the only non‐ablative technique is microvascular decompression, in which no damage to the trigeminal nerve is intended but it is the most invasive of all the procedures and necessitates on average a five‐day hospital stay. Ablative procedures can be done at three anatomical levels.
Peripheral, at the trigger zone area, for example cryotherapy (freezing of the nerve), neurectomy (cutting of the nerve) or alcohol injection.
Gasserian ganglion (point at which the three main branches of the trigeminal nerve meet), for example radiofrequency thermocoagulation (passing a current through to generate heat), glycerol rhizolysis (bathing the nerve in glycerol which destroys nerve fibres), balloon compression (applying pressure to the nerve) or stereotactic radiosurgery (the most common modality utilises the Gamma Knife);
Posterior fossa, for example partial sensory rhizotomy (cutting part of the nerve which transmits sensation) or stereotactic radiosurgery.
These procedures employ physical or chemical methods in order to damage the trigeminal nerve at specific sites. With the exception of partial sensory rhizotomy all the ablative techniques are minimally invasive, and require only short hospital stays. Most provide immediate relief from pain, but stereotactic radiosurgery (Gamma Knife, LINAC, Cyberknife system) generally requires six to eight weeks for maximal effect. It is a noninvasive method of delivering focused radiation to the trigeminal nerve, most commonly in the root entry zone but success with Gasserian ganglia targeting has also been reported (Regis 2006). Peripheral treatments are dependent on clear trigger points being identified by the patient. If there is more than one trigger site then multiple treatments are required. It has been shown that even if the original trigger site remains pain‐free, pain migrates in up to 60% of patients to another site and so overall pain relief is lost and medication restarted Zakrzewska 1986.
Life table analysis (survival graphs which indicate how quickly pain recurs) for all four ablative procedures indicates a constant rate of annual recurrence over time approaching approximately 10% each year (Lopez 2004). However, the major disadvantage of these procedures is the comparatively high rate of facial sensory loss which can become severe enough to be termed anaesthesia dolorosa. Its onset may be delayed in the case of stereotactic surgery. Trigeminal motor dysfunction (inability to use muscles of mastication) may occur and is often temporary.
Additional delivery systems for stereotactic radiosurgery are beginning to be explored including multiple Arc Linear Accelerator (e.g. X‐knife or LINAC), multiple port 'step and shoot' systems with either robotic delivery (Cyberknife), or multi‐leaf collimation (e.g. Novartis or Varian Trilogy). There are limited data available on the ability of these systems to reproduce Gamma Knife results when used with a similar isocentric targeting technique, and non‐isocentric targeting with the Cyberknife is still in the early experimental phase (Lim 2006).
The only non‐ablative procedure is microvascular decompression (MVD). This is a major neurosurgical procedure requiring access to the brain stem through the skull and carries with it 0.3% risk of death (Kalkanis 2003). In this procedure, once access to the posterior fossa has been established, a search is made for a blood vessel in contact with the nerve. The vessel is dissected free from the nerve and held apart by attaching it away from the nerve either by interposing a nonabsorbable material such as Teflon felt or other material. This operation preserves the anatomical integrity of the trigeminal nerve and in most cases also its function (Barker 1997). Up to 70% of patients may be pain free for up to 10 years (Zakrzewska 2005). No sensory loss is expected and the major complication is loss of hearing on the ipsilateral side, due either to direct trauma to the auditory nerve or mastoid air cells. If no blood vessel is found compressing the nerve, then some neurosurgeons will partially cut the trigeminal nerve. This results in good pain relief (the same as after microvascular decompression) but sensory loss occurs and this then reduces patient satisfaction (Zakrzewska 2005). Results of microvascular decompression have been shown to be superior when performed at hospitals with higher volumes (20 trigeminal neuralgia admissions per year) and by surgeons with high volumes (more than 29 cases per year) (Kalkanis 2003). There have been no randomised trials on this procedure reported but there are cohort studies (Tatli 2008).
The evidence for outcomes after surgical treatments are based on case series and only a few have used independent observers to assess outcome (Zakrzewska 2003). There are very few randomised controlled trials to evaluate efficacy and quality of life after all these procedures, the majority being peripheral treatments. The quality of reporting of surgical management of trigeminal neuralgia is extremely variable and recommendations for the reporting of outcomes have been suggested and endorsed by other neurosurgeons (Zakrzewska 2003). A recent systematic review Tatli 2008 using similar criteria found that up to early 2007 there were 412 publications, of which only 28 met their criteria. When preparing guidelines for diagnosis and management of trigeminal neuralgia Cruccu 2008 and Gronseth 2008 encountered the same problems and used only those studies that specified that independent observers assessed outcome.
There are no natural history studies to determine the prognosis of untreated trigeminal neuralgia and no studies comparing medical to surgical management.
Objectives
To assess the efficacy in terms of pain relief and quality of life of different neurosurgical interventions for the management of classical trigeminal neuralgia.
To assess the harms of these interventions.
To determine if there are any defined subgroups of patients more likely to benefit.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) or quasi‐RCTs involving neurosurgical interventions used in the treatment of classical trigeminal neuralgia. In the absence of RCTs we planned to mention in the Discussion section independently assessed, prospectively studied case series followed up for a mean/median time of five years and which use actuarial methodology (which has been agreed as the only way in which results can be reported (Dhople 2009)). Since the publication of the protocol this has been done by another group Tatli 2008.
Types of participants
Participants diagnosed with classical trigeminal neuralgia according to the International Headache Society (IHS) criteria (with the exception of the 'Absence of clinically evident neurological deficit' (IHS criterion 'D') as this not reported in trials) (Anon 2004).
Types of interventions
All neurosurgical procedures.
Peripheral at the trigger zone area, for example cryotherapy, neurectomy, alcohol injection, or streptomycin injections;
Gasserian ganglion, for example radiofrequency thermocoagulation, glycerol rhizolysis, balloon compression, or Gamma Knife;
Posterior fossa for example microvascular decompression, partial sensory rhizotomy, or Gamma Knife.
Types of outcome measures
Primary outcomes
Complete pain relief without medication at one year after randomisation, or in the case of observational studies, after the procedure.
Secondary outcomes
Surgical morbidity assessed after at least twelve months.
Quality of life assessed after at least twelve months.
Patient satisfaction assessed after at least twelve months.
Adverse events at any time including all causes of mortality.
Search methods for identification of studies
The search strategy was compared to others that had been developed for the production of guidelines Cruccu 2008 and for a review for Clinical Evidence Zakrzewska 2009.
We searched the Cochrane Neuromuscular Disease Group Specialized Register (13 May 2010), CENTRAL (Issue 2, 2010 part of the Cochrane Library), Health Technology Assessment (HTA) Database, NHS Economic Evaluation Database (NHSEED) and Database of Abstracts of Reviews of Effects (DARE) (Issue 4, 2010 (HTA, NHSEED and DARE are part of the Cochrane Library)), MEDLINE (January 1966 to May 2010) and EMBASE (January 1980 to May 2010). We searched the reference lists of all trials identified and contacted the authors for further information. We searched the references of published studies as well as trial registers in order to attempt to identify unpublished or ongoing studies.
There was no language restriction.
Electronic searches
For electronic searches please see Appendix 1, Appendix 2 and Appendix 3.
Searching other resources
We also searched reports by the National Institute for Health and Clinical Excellence (NICE) including http://guidance.nice.org.uk/IPG85.
Data collection and analysis
Selection of studies
Two review authors read the titles and abstracts (when available) of all reports independently identified through the searches. We obtained the full report of those studies appearing to meet the inclusion criteria, or for which there were insufficient data in the title or abstract, or both, to make a clear decision. We read the full reports and each author made an independent decision on whether the studies met the inclusion criteria or not. We resolved any disagreement by discussion and in the event of a difficulty consulted the editor. We assessed studies meeting the inclusion criteria for validity using the published recommendations of Zakrzewska (Zakrzewska 2003). Any studies rejected at this or a subsequent stage were recorded in the table of excluded studies and the reason for exclusion recorded. We did not include a discussion of costs and cost benefits drawing if necessary on nonrandomised studies as initially intended because of the lack of data.
Assessment of risk of bias in included studies
The authors independently assessed the quality of the individual trials during data extraction according to the Cochrane Handbook of Systematic Reviews of Interventions (Chapter 8.5) (Higgins 2008) using the domains: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias.
Data extraction
Two authors independently extracted data using specially designed data extraction forms. These have been piloted by Zakrzewska and Lopez (Zakrzewska 2003) and have been used subsequently in the preparation of practice guidelines for the American Academy of Neurologists and European Federation of Neurological Societies using their guidelines (Edlund 2004). We contacted authors for clarification and missing information whenever possible. We used translators where necessary.
For each trial we recorded the following data.
Year of publication, country of origin.
Type of study, randomisation, blinding, type of outcome measures used, use of independent assessor for outcomes.
Types of participants, including demographic characteristics, source of recruitment and criteria for inclusion.
Type of intervention, including number of patients in each arm, duration of procedure, setting (inpatient), length of follow‐up.
Risk of bias using the Cochrane Collaboration tool for assessing risk of bias.
Primary outcome: actuarial data at six months and then yearly for up to 10 years; number of failures compared to baseline assessments.
Secondary outcomes: complications classified into any or severe (life threatening); quality of life; and patient satisfaction.
Measures of treatment effect and data synthesis
For dichotomous outcomes, we planned to express the estimated relationships between the effects of compared interventions as risk ratios (RRs) together with 95% confidence intervals (CIs). For continuous outcomes, we planned to obtain mean differences between the estimated effects of the interventions together with 95% CIs. We used the Cochrane statistical package Review Manager (RevMan) to perform the analyses.
Assessment of heterogeneity
We planned to assess heterogeneity using the I² statistic and Chi² test provided by the RevMan analysis but this was not possible because of insufficient data.
Assessment of reporting biases
A funnel plot was not done to investigate the possibility of publication bias.
Results
Description of studies
Results of the search
The literature search yielded 528 citations (MEDLINE 396 studies, EMBASE 63 studies, the Cochrane Central Register of Controlled Trials (CENTRAL) 36 studies, the NMD register 33 studies, Health Technology Assessments 6 studies, Health Economic Evaluations Database 2 studies and DARE 4 studies), of which JZ and HA independently selected 11 for further scrutiny of the full report.
No ongoing trials were included in this review and there were no excluded studies.
Included studies
Details of the 11 studies are included in the Characteristics of included studies table but we excluded the majority of them from the analysis as they did not report our prespecified outcomes and showed high levels of bias and so provided low grade evidence. Three trials involving 181 patients had sufficient data and outcomes and are described below. Two further studies provided some data on the primary outcome. Shen 2006 used peripheral injections of either adriamycin or gentamicin into the trigger points and reported outcomes at one year but did not specify whether medication was continued. Stajcic 1990 used peripheral injections of lidocaine alone or lidocaine with streptomycin into the trigger points and reported data on outcomes at one year but did not mention whether medication was continued. See the Characteristics of included studies table for more details.
Trials comparing the use of neuronavigation in radiofrequency thermocoagulation with conventional intraoperative plain X‐rays
Xu 2006 was a randomised parallel group single centre trial from China involving 54 participants. This study compared two techniques for targeting radiofrequency thermocoagulation as a treatment for classical trigeminal neuralgia (the VectorVision system and Hartel's facial measurement technique). The participants fulfilled the International Association for the Study of Pain criteria and had not had prior surgical procedures for trigeminal neuralgia. The two groups appeared to have similar baseline clinical features. The participants were randomly divided into two groups and ultimately received percutaneous radiofrequency thermocoagulation of the Gasserian ganglion for the treatment of intractable trigeminal neuralgia (this part of the procedure was the same in both groups). One group of participants (n = 26) had the procedure done using a frameless neuronavigation system whilst the control group (n = 28) had the procedure done using conventional X‐ray guidance.
The study quality was poor with no details provided of the method of randomisation, blinding, and outcome assessment. The only outcome measure used was complete pain relief ascertained by a questionnaire at three months and it was not clear whether this was done by independent assessors. Patients were followed up for 13 to 58 months (mean 36 months, standard deviation (SD) 7 months) for the navigation group and 10 to 54 months (mean 34, SD 5 months) for the control group but no details were provided of the number lost to follow‐up. A power calculation was not done.
Trials comparing increasing the nerve length within the stereotactic radiosurgery (Gamma Knife) treatment volume with the standard nerve length treated and its effect on the treatment outcome
Flickinger 2001 was a comparative randomised controlled trial (RCT) using one or two isocentres (points) to deliver radiation to the trigeminal nerve. The investigators hypothesised that including a longer length of the trigeminal nerve within the radiosurgery treatment volume would increase the proportion of trigeminal neuralgia patients with complete pain relief from approximately 61% with one isocentre to 86% with two isocentres. A power calculation was done and it indicated that a total of 90 trigeminal neuralgia patients should be randomised for an 80% chance (power = 80) of demonstrating this degree of improvement with a 95% significance level (P < 0.05). A total of 88 patients with typical trigeminal neuralgia (24 had had prior surgery) were randomised to receive retrogasserian radiosurgical rhizotomy with 4 mm diameter collimators to either one isocentre (n = 44) or to two isocentres (n = 44) thus increasing the length of nerve treated. The patients were followed up for a median of 26 months with a range of 1 to 36 months. One patient randomised to two isocentres was lost to follow‐up the day after radiosurgery and was excluded. Numbers of patients, number of centres, and baseline characteristics were the same for both groups. Pain measurements using validated scales were not made either before or after surgery. Use of medication was considered a surrogate measure for pain. The study was stopped two patients below the calculated number needed because of the development of severe dysaesthesia in a patient receiving radiation to two isocentres.
Trials comparing the use of pulsed radiofrequency thermocoagulation (PRF) with conventional radiofrequency thermocoagulation (CRF) in the treatment of classical trigeminal neuralgia
Erdine 2007 compared two differing techniques of radiofrequency thermocoagulation in two comparable groups of 20 participants for six months in one centre. All participants had idiopathic trigeminal neuralgia, and all had a pain score of greater than five on the visual analogue scale. The groups were comparable at baseline. A power calculation was not done. One group of patients received conventional radiofrequency thermocoagulation (CRF) whilst the other group received pulsed radiofrequency thermocoagulation (PRF). Rather than the conventional continuous application of the current to the nerve, the pulsed technique was delivered in short blasts, so allowing the heat to be dissipated. The procedures were done under sedation using trial stimulation at first to confirm correct placement of the electrode, then either CRF with the needle tip temperature reaching 70 °C for 60 s or PRF with a needle tip temperature of 42 °C delivered in two bursts lasting 120 s each. Thermocoagulation was repeated during the procedure if necessary.
Excluded studies
There were no excluded studies.
Risk of bias in included studies
The details of quality assessment are in the Characteristics of included studies table. Figure 1 shows a summary of the review authors' judgements about each methodological quality item for each included study.
1.
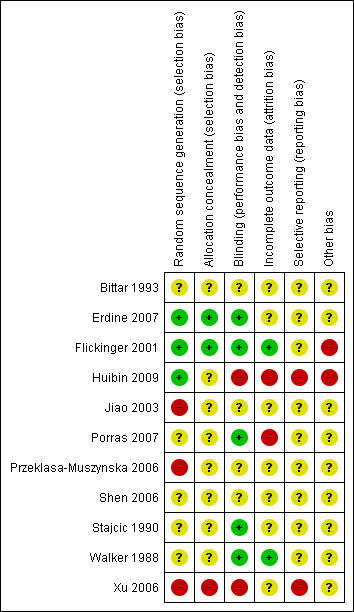
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
1. Selection bias (Randomisation and allocation concealment)
All included studies stated they were randomised but only two (Erdine 2007; Flickinger 2001) reported an adequate method of allocation concealment. The concealment of treatment allocation was unclear in Xu 2006. Huibin 2009 stated that the surgeons were independent of the rest of the trial and treatment allocation was done on the basis of a sealed envelope being opened in the operating theatre but this was not done for the first 10 patients. No mention of how this was achieved was stated in any of the other studies.
2. Performance bias (blinding of participants, researchers and outcome assessment)
Because of the nature of the studies, blinding of clinicians and participants was not practical for the neuronavigation trial (Xu 2006) owing to the obvious use of the frameless neuronavigation system. In the PRF versus CRF trial (Erdine 2007), the patients and the physicians who monitored the patients on monthly visits were blinded, while the study supervisor who monitored all the study data was not. The procedures were performed by an independent specialist who followed a randomisation chart. In the stereotactic radiosurgery trial (Flickinger 2001), participants were blinded to their treatment as were the physicians assessing outcome. Walker 1988 describes how the technicians were specifically hired for the project and did not have previous experience of this procedure and a third group performed the evaluations. In the Stajcic 1990 study the people who prepared the syringes with the drugs were independent and the syringes were covered with a sleeve to disguise the colour of the solution but no details are provided as to who performed the outcome assessments. Bittar 1993 performed a cross‐over study but provided no details of how this was done. Porras 2007 reported independent outcome assessors. Huibin 2009 did assessments immediately and then at three or five years but did not do assessments on individual treated nerves, which was important given that some patients had more than one nerve treated. The interventions and outcome assessments were performed by the same person in the Shen 2006 study. Jiao 2003 and Przeklasa‐Muszynska 2006 provide no details.
3. Attrition bias (loss of participants to follow‐up)
In the Gamma Knife nerve length trial (Flickinger 2001), “One patient randomized to two isocentres was completely lost to follow‐up the day after radiosurgery and was excluded, leaving 87 patients for this analysis”. There is no mention of participants lost to follow‐up in the other two trials (Erdine 2007; Xu 2006). The Erdine 2007 trial had a follow‐up period of six months. Follow‐up was very variable in the Xu 2006 study (10 to 58 months). Walker 1988 does report missing data, which suggests incomplete follow‐up. Jiao 2003 only assessed patients at 15 minutes but does not state if even this was complete. Stajcic 1990 provides outcomes but it is not clear whether the follow‐up time was the same for all patients. The results in the Porras 2007 study seem to suggest that all patients were followed up for four weeks. Huibin 2009, Shen 2006, Przeklasa‐Muszynska 2006 and Bittar 1993 did not report numbers lost to follow‐up and some studies do not even report length of study.
Effects of interventions
It was not possible to undertake meta‐analysis due to the differences in the intervention modalities and variable outcome measures. All the results described are from single trials. Please see individual tables for details.
1. Primary outcome pain relief at one year
1.1 The use of neuronavigation in radiofrequency thermocoagulation versus conventional intraoperative plain X‐rays (Xu 2006)
Overall improvement in pain was estimated and classified into complete pain relief, partial satisfactory pain relief, partial unsatisfactory pain relief, no change in symptoms and worsening of pain. Although definitions are provided, no baseline assessment of pain intensity is given.
Complete pain relief (pain relief survival) was shown using a Kaplan Meier graph with assessment conducted at 6 months, 12 months and then at yearly intervals for a maximum period of 45 months (Figure 2). It is not clear how many patients were still in the trial at 45 months, but 10% of patients remained unaccounted for after 36 months (44% relapsed (18 to 30 months) and 46% still pain free at 36 months).
2.
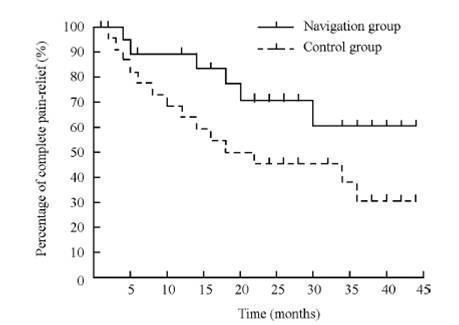
Pain relief survival in navigation (n = 26) and control (n = 28) groups Kaplan‐Meier. This is from Xu 2006 (with permission).
The neuronavigation group showed improved outcomes over the conventional X‐rays group. Seventy‐seven per cent achieved complete pain relief at 12 months without medications compared to 54% in the conventional X‐rays group (nonsignificant: risk ratio (RR) 0.70 (95% confidence interval (CI) 0.46 to 1.04)) (Analysis 1.1, Figure 3).
1.1. Analysis.
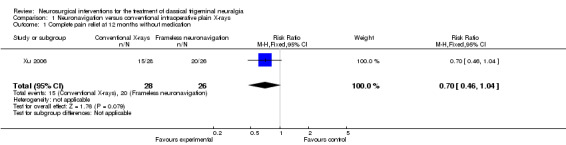
Comparison 1 Neuronavigation versus conventional intraoperative plain X‐rays, Outcome 1 Complete pain relief at 12 months without medication.
3.
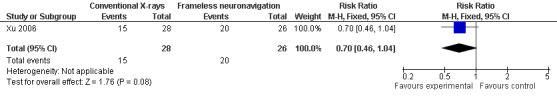
Forest plot of comparison: 1 Neuronavigation versus conventional intraoperative plain X‐rays, outcome: 1.1 Complete pain relief at 12 months without medication.
Table 1. Pain relief and relapse rates comparison table in the navigation group against the control group
| Navigation group n (%) | Control group n (%) | Total n (%) | |
| Total number | 26 (100) | 28 (100) | 54 (100) |
| Immediate pain relief | 26 (100) | 27 (95) | 53 (98) |
| Pain relief at 12 months | 20 (77) | 15 (54) | 35 (65) |
| Pain relief at 24 months | 18 (69) | 11 (40) | 29 (54) |
| Pain relief at 36 months | 15 (58) | 10 (35) | 25 (46) |
| Relapse | 10 (38) at 30 months | 14 (50) at 18 months | 24 (44) |
1.2. Increasing the nerve length within the stereotactic radiosurgery treatment volume versus treating the standard nerve length (Flickinger 2001)
Complete pain relief was achieved after a median of three months (1 week to 17 months) in 57 participants. Pain improved partially in 15 participants and another 15 had no benefit. Assessments took place at 6 months, 12 months and then at yearly intervals. A Kaplan‐Meier pain free survival graph was done; however, this was not published in the paper and no data were provided as to the statistical method for the comparison. Although relapse occurred slightly less often in the two isocentre patients the difference was not statistically significant, effects estimate 0.72 (CI 0.30 to 1.71) (Analysis 2.1).
2.1. Analysis.
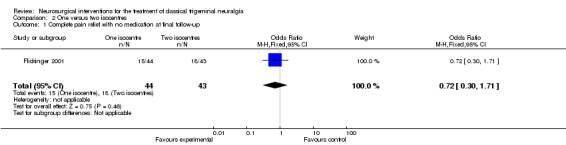
Comparison 2 One versus two isocentres, Outcome 1 Complete pain relief with no medication at final follow‐up.
Table 2. Pain relief and relapse rates in the two groups
| One isocentre (44 participants) | Two isocentres (43 participants) | Total n (%) | |||
| Maximum pain control n (%) | Pain control at final follow‐up n (%) | Maximum pain control n (%) | Pain control at final follow‐up n (%) | ||
| 87 (100) | |||||
| Complete pain relief, no drugs | 24 (54.5) | 15 (34.1) | 21 (48.8) | 18 (41.9) | 45 (51.7) |
| Complete pain relief with drugs | 5 (11.4) | 5 (11.4) | 7 (16.3) | 5 (11.6) | 12 (13.8) |
| Decreased pain relief with drugs | 8 (18.2) | 12 (27.3) | 7 (16.3) | 10 (23.3) | 15 (17.2) |
| No pain relief | 7 (15.9) | 12 (27.3) | 8 (18.6) | 10 (23.3) | 15 (17.2) |
| Relapse | 17 (38.6) | 13 (30.2) | 30 (34.5) | ||
1.3. Pulsed radiofrequency thermocoagulation (PRF) versus conventional radiofrequency thermocoagulation (CRF) in the treatment of classical TN (Erdine 2007)
a) Pain intensity of the attacks using a visual analogue scale (VAS) ('0' no pain to '10' worst possible pain).
b) Patient satisfaction using Patient Satisfaction Scale (PSS: '0' very dissatisfied to '10' very satisfied).
c) Additional pharmacological treatment: carbamazepine and/or gabapentin.
Pain relief was measured using a VAS. If complete pain relief was achieved the VAS score was 0. Some patients in the CRF group achieved complete pain relief as shown by the range but none in the PRF group achieved a score of 0. Data were not provided on how many had a VAS score of 0 after treatment. The results in the PRF group at six months showed a VAS score of 0 but this was because they underwent CRF. The timing of measurements was at six months, one year and at yearly intervals using a Kaplan‐Meier graph.
The time of measurement is not clear for the use of additional pharmacological treatment.
Table 3. VAS score: median (minimum ‐ maximum) for the whole group. No individual data
| CRF | PRF | ||
| Median VAS score | |||
| Pre‐procedure | 9 (7 to10) | 9 (7 to10) | |
| 1 day | 1 (0 to 5) | 8 (2 to 9) | |
| 3 months | 0.5 (0 to 2) | 8.5 (7 to10) | |
| 6 months | 0.5 (0 to 2) | 1 (0 to 2) | P < 0.001 |
| Median Patient Satisfaction Scale pre‐procedure | 1.5 (0 to 2) | 1 (0 to 2) | |
| Median Patient Satisfaction Scale post‐procedure | 8 (7 to 9) | 1 (1 to 8) | |
| Total n (%) | |||
| Use of medications n (%) | 1/20 (5) | 20/20 (100) | 21/40 (52.5) |
| Overall improvement n (%) | 20/20 (100) | 2/20 (10) | 22/40 (55) |
1.4 Peripheral blockade with adriamycin versus gentamicin (Shen 2006)
At 12 months 68% of those having adriamycin blocks were still pain free and 23% were still pain free at 30 months whereas only 31% of the gentamicin group were pain free and this dropped to 6% at 30 months.
1.5 Peripheral streptomycin versus lidocaine injections (Stajcic 1990)
Given the outcomes for all the patients it was possible to construct a Kaplain‐Meier graph and show that in the streptomycin group at one year 65% had had a recurrence whereas for the lidocaine group the figure was 75%. The log rank test was P = 0.56 showing that there was no difference between the two groups Figure 4.
4.
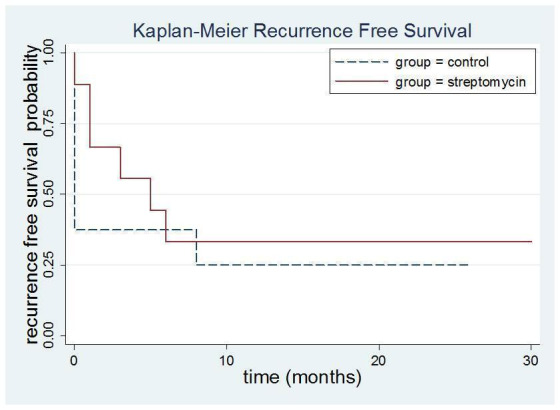
Kaplan‐Meier recurrence free survival streptomycin versus lignocaine. Log rank test P = 0.56. No evidence of a difference in recurrence between the two groups .
2. Surgical morbidity assessed after at least twelve months
This was combined with adverse events and is shown under outcome 5.
3. Quality of life assessed after at least twelve months
No study reported on this outcome.
4. Patient satisfaction assessed after at least twelve months
Erdine 2007 showed that patient satisfaction was improved after conventional RFT but not after pulsed as shown in Table 3. No other studies reported on this outcome.
5. Reported adverse events
A big discrepancy between the studies was found when describing adverse events. No clear definition of the degree of sensory disturbances was given.
Table 4. Reported adverse events in the three selected studies
| Study | Xu 2006 | Flickinger 2001 | Erdine 2007 | |||
| Number experiencing event n (%) | Number experiencing event n (%) | Number experiencing event n (%) | ||||
| Intervention (neuronavigation) | Control (X‐ray guided) | Intervention (2 isocentres GK) | Control (1 isocentre GK) | Intervention (PRF) | Control (CRF) | |
| Total number | 26 (100) | 28 (100) | 43 (100) | 44 (100) | 20 (100) | 20(100) |
| Mild and moderate paraesthesia or reduced sensation | 26 (100) | 28 (100) | 13 (30.2) | 7 (15.9) | 20 (100) | 0 |
| Dysaesthesia, severe paraesthaesia and anaesthesia dolorosa | 0 | 2 (7.1) | 1 (2.3) | 0 | 0 | 1 (5) |
| Reduced corneal reflex | 0 | 2 (7.1) | No data available | No data available | No data available | No data available |
| Corneal keratitis | 0 | 1 (3.5) | No data available | No data available | No data available | No data available |
| Masseter dysfunction | 0 | 1 (3.5) | No data available | No data available | No data available | No data available |
| Cerebrospinal fluid leak resolved | 0 | 1 (3.5) | No data available | No data available | No data available | No data available |
| Transient rise in blood pressure | 0 | 7 (24) | No data available | No data available | No data available | No data available |
| Severe adverse event (e.g. life‐threatening episodes, hospitalisation, death) | 0 | 0 | 0 | 0 | 0 | 0 |
| Adverse events leading to cessation of treatment | 0 | 0 | 0 | 0 | 20 (100) | 0 |
| Mortality | No mortality | No mortality | No mortality mentioned | No mortality mentioned | No mortality mentioned | No mortality mentioned |
Bittar 1993 reported that all participants having the streptomycin injection had marked facial swelling at the site of injection for three to seven days. However, neither Bittar 1993 nor Stajcic 1990 reported any sensory changes after the injections. Jiao 2003 reported two patients having a local haematoma. Przeklasa‐Muszynska 2006 reported 13/22 minor adverse events in the steroid group, 8/22 in the pentoxyphilline group and 11/22 in the control group. These ranged from cheek swelling (5), change in sensation (10) to pain (10) and were similar in each of the groups. Shen 2006 reported that in the adriamycin group 91% had facial anaesthesia lasting for a mean of 122 days compared to 17% for a mean of 7 days in the gentamycin group; 91% had local swelling lasting for mean of 66 days in the adriamycin group, whereas this affected 28% in the gentamicin group; and 14% had paraesthesia for 120 days, the same in both groups. Huibin 2009 reported that in the conventional group, 5 (25%) reported numbness whereas in the peripheral group it was 6 (20%) and in the conventional group they reported one case of keratitis and one of atrophy of the temporal muscles. Porras 2007 and Walker 1988 reported no adverse events.
2.3 Additional outcomes used in published papers but not specified in the protocol
Flickinger 2001 reported on the number of patients who required other treatments “22 went on to have additional procedures for control of their pain, including repeated radiosurgery in 9 patients, microvascular decompression in 3, glycerol rhizotomy in 7, radiofrequency rhizotomy in 1 and peripheral alcohol blocks in 2".
Pain relief was reported in all other studies but at varied timepoints. Bittar 1993 reported outcomes but it is not possible to determine the timepoint at which this occurred. Huibin 2009 reported at three years and five years with or without medication (see Table 5).
Table 5. Recurrence rates after conventional or peripheral radiofrequency (RFT) (Huibin 2009)
| Time | Conventional RFT (%) | Peripheral RFT (%) |
|
3 years recurrence without medication |
7 (35) | 11 (37) |
| 3 years recurrence with medication | 2 (25) | 8 (27) |
| 5 years recurrence without medication | 10 (50) | 16 (53) |
| 5 years with medication | 7 (35) | 12 (40) |
Jiao 2003 reported results 15 minutes after each procedure and the results were the same in both groups. Porras 2007 reported results up to four weeks and showed that those who had steroid injections returned to baseline levels of pain, whereas the glycerol rhizotomy group had statistically significantly improved outcomes when compared with baseline pain severity. Przeklasa‐Muszynska 2006 reported pain relief at intervals with longest one being 90 days. Nearly 30% had no pain relief and of those that did the pentoxyphilline group had better pain relief. Walker 1988 reported pain relief after laser therapy as an analysis of variance and suggested that patients with high level of pain did report some improvement at 10 weeks but the number of subjects was too small for this to be significant.
It should be noted that all of the studies in this section other than Flickinger 2001 were of very poor methodological quality and so the results should be regarded with caution.
Discussion
Trigeminal neuralgia is a rare form of neuropathic pain that in many instances responds to surgical therapies. A vast range of surgical options have been used and yet very few of them have ever been evaluated in a randomised controlled trial (RCT). This is a common finding in many surgical procedures as highlighted by Barkun 2009 who points out that not only is there a paucity of RCTs but those few RCTs that are published do not meet the current quality standards for optimal reporting. In this Cochrane systematic review not a single RCT met all the criteria proposed in the protocol and none got more than a moderate grading. There were 11 RCTs but eight were graded low and only five provided data on the primary outcome of pain relief at one year with any indication as to whether medication was used or reduced. Those that reported on peripheral treatments did not specify whether this was total pain relief or pain relief only at the trigger point. Quality of life was not reported by any study. As Ergina 2009 et al. point out, few of the surgical RCTs undertaken evaluate the procedure itself. Instead most focus on particular techniques as seen in this series. For example, the Flickinger 2001 trial looked at the use of one or two isocentres, Xu 2006 at the use of a navigation system, and Erdine 2007 at the use of pulsed radiofrequency thermocoagulation. Not a single RCT was identified on microvascular decompression which is currently the most frequently reported procedure.
No study provides details of how the participants were selected from all the potential patients being seen in the units and whether patient and surgeon preferences played a role in selection given that there are several different surgical procedures available for the management of trigeminal neuralgia. Pretreatment differences are impossible to ascertain and therefore it may well be that the studies start from different baselines, e.g. the duration of the condition varies from half a year to 55 years (Flickinger 2001), 1 year to 25 years (Erdine 2007) and 2.5 to 12 years (Xu 2006). The interventions used in trigeminal neuralgia have different long‐term effects and benefit‐to‐harm profiles and this can affect choice of treatments by all parties and lead to bias (Ergina 2009).
None of the studies stated clearly the inclusion and exclusion criteria for participant in the trials. The baseline characteristics are not well described: some participants may not have been accurately diagnosed, some may have secondary trigeminal neuralgia (Xu 2006 does not mention this) and others may have already undergone some form of surgical intervention (Flickinger 2001 included these). Erdine 2007 and Xu 2006 did not specify whether participants had had previous surgery, yet this affects outcomes (Flickinger 2001). Many people with trigeminal neuralgia are elderly and so some risk adjustment needs to be made for comorbidity. Comorbidities may also exclude some patients from the surgical procedures, for example microvascular decompression. In larger studies it may be possible to take these factors into consideration and do subgroup analysis, something that was not possible in this review. Erdine 2007 provides a pre‐operative assessment of pain and only includes participants with a visual analogue score (VAS) of at least 5 (scale 0 to 10) which is a measure often used in pain trials, whereas the other studies provide no baseline pain intensity score.
Method of randomisation was adequate in the Flickinger 2001 study, not stated in the Xu 2006 study and the Erdine 2007 study used a table of random numbers then put them in sealed envelopes given to the surgeon at the time of the operation. Methods of randomisation must be robust. Only the Flickinger 2001 study did a power calculation and was a multi‐centre design. All three studies provide demographics of the participants allocated to their treatment groups and there do not appear to be any major differences between the treatment groups within each study, which suggests that the randomisation was effective.
It will not always be possible to blind patients to the type of intervention they have undergone but before evaluations are done by independent observers, patients need to be reminded not to disclose this information. This is easier if outcomes are measured through questionnaires and without the need for an individual telephone or face to face interview. Blinding was adequate for the Flickinger 2001 and Erdine 2007 studies but it would have been impossible to blind patients in the Xu 2006 study as one arm of the study involved the use of a three pin skull fixation device along with a frameless neuronavigation system. Attempts were made to collect outcome measures in an unbiased way. No subgroup analysis was done in any of these studies because the number of participants was too small: Flickinger 2001 had 87 participants, Xu 2006 54 participants and Erdine 2007 40 participants.
Looking at the methodology of the included RCTs, all had varied objectives which led to different designs. Xu 2006 compared the use of a frameless neuronavigation system in guiding retrogasserian thermocoagulation with the use of conventional X‐rays. A VectorVision Navigation system (BrainLab) was used with computerised tomography (CT) guidance and this could have taken longer to set up then the conventional system but no data is provided. The extra time taken to set the system up could play a role in satisfaction as speed and comfort and not just accuracy are all important factors to consider when undertaking percutaneous ablative techniques. It was not clear whether the patients in the other group had uniplanar or biplanar fluoroscopy as the success rate in the latter can be higher. In the Xu 2006 study participants were given prophylactic antibiotics for the three or four days following the procedure but to our knowledge there is no existing evidence to support such practice. It is difficult to explain why the use of a neuronavigation system increases the chance of being pain free for longer as both destructive procedures are the same and the only difference is in more accurate placement of the needle, which should then be reflected in the immediate results which were not statistically significantly different.
The Flickinger 2001 study addressed the controversy about the best location of the radiotherapy therapy. Erdine 2007 and Xu 2006 used standard methods to determine whether adverse events could be reduced without compromising the primary outcome measure of pain relief if using a different technique.
In common with numerous surgical RCTs (Ergina 2009), the greatest problem with all the studies is the lack of standardised definitions for clinical outcomes which should include clinical, technical and patient related outcomes and, with resources increasingly limited, economic evaluations. Some complications need to be clinically assessed and reliance cannot just be placed on patient reports; however, any clinical assessment methods need to be carefully defined and agreed upon. If questionnaires are used both before and after the intervention they need to have been psychometrically tested and checked to ensure that they are sensitive to change. Some of these questionnaires can be generic but some should be disease specific. Lee 2010 have attempted to validate a frequently used instrument in pain studies, the Brief Pain Inventory, adding some disease specific questions. The Barrow Institute (Rogers 2000) have also attempted to develop such a measure and this was used by the Flickinger 2001 study. The measure has not been tested and does not measure outcomes in a valid way. Erdine 2007 used the widely accepted VAS with a scale of 0 to 10 both for pain intensity and patient satisfaction. All studies used pain relief as the primary outcome measure and as we all know, complete pain relief is easy to define but partial pain relief is extremely subjective and this was poorly assessed in all studies.
Xu 2006 used grades of partial satisfactory pain relief, partial unsatisfactory pain relief, no change and worse but did not provide pre‐operative measures to be able to determine what is meant by partial relief. Flickinger 2001 used the soft surrogate measure of drug use which is inadequate, as patients often take medication for reasons other than pain relief especially after stereotactic radiosurgery which is not expected to provide immediate pain relief. In Erdine 2007 the use of the VAS was satisfactory but they did not define what a clinically meaningful outcome would be and combined the scores to give a mean with no standard deviation, making the assumption that the scale is linear. Only Erdine 2007 attempted to measure patient satisfaction. Again this was done using a scale of 0 to 10 but the study provided no definition of what is a meaningful outcome and combined means with no standard deviation and only provided ranges, which in the conventional group are as wide as 1 to 8. Ergina 2009 stresses the need for the outcomes to be assessed by an independent observer who is masked to the treatment assignment. Xu 2006 used questionnaires at three months but did not make it clear who designed them and in whose name they were sent out, for example the surgeon performing the procedure or an independent observer. Erdine 2007 makes it clear that the outcomes were assessed on a monthly basis by an independent person. Flickinger 2001 states that follow‐up assessments were done by staff unaware of the procedure but it is not clear if these were done face to face or by a questionnaire.
Adverse events and complications were reported in all but three studies but another two only reported that no sensory loss was detected (Bittar 1993; Stajcic 1990). There is moderate grade evidence to show that all these ablative procedures do result in sensory loss which can be more significant than just a mild sensory deficit (Flickinger 2001; Erdine 2007; Huibin 2009) but again a lack of definitions makes it difficult to compare data. Even peripheral injections can result in marked sensory changes as reported by Shen 2006. It is essential that some negative data are reported, for example eye problems, as these can potentially occur with any ablative procedure and so should be reported as some of the poorer quality studies have done, for example Huibin 2009. Peripheral injections often result in marked local swelling at the site (Bittar 1993; Jiao 2003).
To ensure that all participants are accounted for and included in the analysis, a Kaplan‐Meier analysis has been proposed (Zakrzewska 2003) but this method was used in only two of the studies to assess pain relief. However, the actual graph is not shown in the Flickinger 2001 report and the median follow‐up time was 26 months with a range of 1 to 36 months. The time point for the analysis is not given in the Xu 2006 study (there was considerable variation in follow‐up time and so the data are probably only meaningful up to 36 months, the mean follow‐up time). The Stajcic 1990 study provides all the raw data so a Kaplan‐Meier analysis could be done. The Erdine 2007 data used the Wilcoxon signed rank test and reported outcomes at three and six months only. Only the Xu 2006 study provided data according to the primary outcome measure of pain relief at one year and could be graded good, whereas the other studies were graded as providing poor evidence. It is therefore impossible to compare outcomes between the three procedures due to the variation in both the measures used and the timing of the measures.
Evaluations of the interventions also need to take into account not just the surgery but also the surgical environment including pre‐ and postoperative care. This is important in trigeminal neuralgia interventions as some result in same day discharges (radiosurgery) whereas others require longer admissions (microvascular decompression) and all are carried out in the secondary care sector. Comorbidities will also determine type of procedure, for example patients on anticoagulants will not be offered surgery at the Gasserian ganglion level. None of the studies provided data on the effect that duration of condition could have on outcomes, a question increasingly being asked by patients.
Due to these methodological weaknesses the findings need to be interpreted with caution. The results reported, however, are similar to those found in case series and so suggest that surgical procedures do provide pain relief for some patients but that they are not free of adverse effects which are often long‐term and irreversible. Unfortunately, the procedure with probably the best outcomes, microvascular decompression, has not been the subject of an RCT and there are very few high quality longitudinal prospective cohort studies using independent observers and well validated outcome measures. Recent reviews using evidence based methodologies (Cruccu 2008; Gronseth 2008; Tatli 2008) suggest that microvascular decompression gives the longest pain free time. As microvascular decompression does not attempt to destroy the trigeminal nerve it is least likely to result in sensory loss, which is common in the other ablative procedures. Microvascular decompression has fewer long‐term irreversible adverse events with hearing loss being the most frequent long‐term adverse event.
Authors' conclusions
Implications for practice.
There is either no, or very low quality, evidence for most neurosurgical procedures for the treatment of trigeminal neuralgia because of the poor quality of the trials. All procedures result in some pain relief (with or without medications) and there is good evidence to show that ablative procedures result in sensory loss. There is no evidence to assess the effect of surgery on quality of life and no evidence of the economic costs. There are no RCTs on microvascular decompression which from observational data gives the longest pain relief periods. Thus there is little evidence to provide the patient with guidance as to the most effective surgical procedure for the management of trigeminal neuralgia and this is in line with the study by Spatz 2007 on decision making. Thus any future high quality trials in this area are likely to lead to a highly significant impact on practice.
Implications for research.
Although many of the surgical techniques used in trigeminal neuralgia have now entered into general use this review highlights the lack of high quality evidence to support this practice. In the area of ablative surgery there are a few trials but there is a complete lack of any high quality evidence for microvascular decompression. There are no comparisons between the different techniques nor between medical versus surgical management. As McCulloch 2002 suggests, not all studies need to be randomised control trials and there are alternative designs that can provide high quality data (McCulloch 2009). Relton 2010 has proposed that some procedures may led themselves to a design termed 'cohort multiple randomised controlled trial' (cmRCT). This may be of particular value in surgical trials as it would take into account learning curves and patient and surgeon preferences, and would also enable collection of outcome data on patients who do not wish to participate in trials. This methodology has been highly successful in a study of surgical versus nonsurgical treatment for lumber degenerative spondylolisthesis, the Spine Patient Outcomes Research Trial (SPORT) study (Birkmeyer 2002; Weinstein 2009). Trials on trigeminal neuralgia would led themselves to this design and would need to be multicentre given the rarity of the condition. There are no data on the optimal timing for surgery. There is an urgent need to gain high quality evidence in order to improve outcomes for patients with trigeminal neuralgia and there is now considerable methodological expertise available to design robust studies.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2011 | Amended | Corrected 'assessed as up to date' and 'next stage expected' dates. |
Acknowledgements
Ben Lopez, neurosurgeon, was a co‐author on our initial systematic review and helped to develop the protocol methodology and read all the studies up to 2005. Clare Aitkin, librarian at the British Medical Association helped to develop the search strategy which was in part based on the one done by Clinical Evidence (BMJ publishing). Mark Linskey assisted in drafting the protocol for this review and assisted in initial selection of studies in 2008. Yizhong Huang from Wuhan, China extracted data from the Chinese publications. Gonzalo Alvarez extracted data from the Spanish publication. The Cochrane Neuromuscular Disease Group editorial office provided invaluable support throughout. Joanna Zakrzewska undertook this study at UCL/UCLHT who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centre funding scheme. The Cochrane Neuromuscular Disease Group editorial base is supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. MEDLINE (OvidSP) search strategy
1 exp trigeminal neuralgia/ 2 (tic adj do?lo?re?ux?).ti,ab. 3 (trigemin$2 adj neuralg$).ti,ab. 4 or/1‐3 5 su.fs. 6 exp nerve block/ 7 exp rhizotomy/ 8 microvascular decompression.tw. 9 exp decompression surgical/ 10 exp radiosurgery/ 11 exp stereotaxic techniques/ 12 partial nerve section.tw. 13 neurectomy.mp. 14 exp denervation/ 15 neurectom$.tw. 16 rhizotom$.tw. 17 exp neurosurgical procedures/ or exp neurosurgery/ 18 neurosurg$ procedure$.tw. 19 radiofrequency.tw. 20 exp electrocoagulation/ 21 rhizolysis.tw. 22 gangliolysis.tw. 23 percutaneous.tw. 24 microcompression.tw. 25 exp balloon dilation/ 26 balloon compression.tw. 27 posterior fossa surgery.tw. 28 gamma knife.tw. 29 stereota?ic.mp. 30 radiation therapy.tw. 31 rt.fs. 32 exp radiotherapy/ 33 radiotherap$.tw. 34 radiation treatment.tw. 35 exp glycerol/ 36 glycerol.tw. 37 ablative surgery.mp. 38 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 39 4 and 38
Appendix 2. EMBASE (OvidSP) search strategy
1 exp trigeminal neuralgia/ 2 (tic adj do?lo?re?ux?).ti,ab. 3 (trigemin$2 adj neuralg$).ti,ab. 4 or/1‐3 5 su.fs. 6 exp nerve block/ 7 exp rhizotomy/ 8 microvascular decompression.tw. 9 exp decompression surgery/ 10 exp radiosurgery/ or exp radiofrequency ablation/ 11 exp stereotaxic surgery/ 12 partial nerve section.tw. 13 neurectomy.mp. 14 exp denervation/ 15 neurectom$.ti,ab. 16 rhizotom$.ti,ab. 17 neurosurgery/ or nerve surgery/ 18 neurosurg$ procedure$.ti,ab. 19 radiofrequency.tw. 20 radiofrequency/ 21 rhizolysis.ti,ab. 22 percutaneous.mp. 23 microcompression.ti,ab. 24 exp balloon dilatation/ 25 balloon compression.ti,ab. 26 posterior fossa surgery.ti,ab. 27 gamma knife.ti,ab. 28 stereota?ic.mp. 29 radiation therapy.ti,ab. 30 rt.fs. 31 exp radiotherapy/ 32 radiotherap$.ti,ab. 33 radiation treatment.ti,ab. 34 exp glycerol/ 35 glycerol.tw. 36 ablative surgery.mp. 37 adriamycin/dt [Drug Therapy] 38 (peripheral adj2 block).tw. 39 or/5‐38 40 4 and 39
Appendix 3. Cochrane Library search strategy
#1MeSH descriptor Trigeminal Neuralgia, this term only #2trigemin* near/2 neuralg* #3(tic dolo*) #4(#1 OR #2 OR #3) #5MeSH descriptor Nerve Block explode all trees #6MeSH descriptor Rhizotomy, this term only #7MeSH descriptor Decompression, Surgical, this term only #8MeSH descriptor Radiosurgery, this term only #9MeSH descriptor Stereotaxic Techniques explode all trees #10(microvascular decompression) #11(neurectomy) #12(partial nerve section) #13MeSH descriptor Denervation explode all trees #14neurectom* #15rhizotom* #16MeSH descriptor Neurosurgical Procedures explode all trees #17MeSH descriptor Neurosurgery explode all trees #18radiofrequency #19MeSH descriptor Electrocoagulation explode all trees #20(rhizolysis or gangliolysis or percutaneous or microcompression) #21MeSH descriptor Balloon Dilatation explode all trees #22balloon compression or posterior fossa surgery or gamma knife #23stereota* #24radiation therapy #25MeSH descriptor Radiotherapy explode all trees #26radiotherap* #27radiation treatment* #28MeSH descriptor Glycerol explode all trees #29glycerol #30ablative surgery #31(#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 ) #32(#4 AND #31)
#33(#32)
Data and analyses
Comparison 1. Neuronavigation versus conventional intraoperative plain X‐rays.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete pain relief at 12 months without medication | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.46, 1.04] |
Comparison 2. One versus two isocentres.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete pain relief with no medication at final follow‐up | 1 | 87 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.30, 1.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bittar 1993.
| Methods | A randomised, double‐blind, prospective study with cross over | |
| Participants |
Diagnostic criteria of patients included in the trial 13 patients with idiopathic TN defined using the International Headache Society criteria 7 patients with a traumatic aetiology (neuropathy) using local unit's criteria Age mean (range) 48.7, range 30 to 75 Gender 7 male, 13 female Severity 39 to 57 mm on a VAS Duration of condition mean (range) years Not available Number 20 |
|
| Interventions |
Intervention: streptomycin and lidocaine Type of intervention 1 g streptomycin + 3 ml 2% lidocaine injected into the trigger area weekly for 5 weeks. Standard medication was continued unaltered. Length of follow‐up mean (range, SD) month Not available Intervention: control lidocaine only Type of intervention 3 ml lidocaine injected locally into the trigger area weekly for 5 weeks. Standard medication was continued unaltered Length of follow‐up mean (range, SD) month Not available |
|
| Outcomes |
Primary outcome Pain relief (intensity measured as VAS). Not clear how this is reported, e.g was it the mean change from baseline, individual branches or overall pain relief. It was unclear what time frame was used and how much data taken from the daily diaries Secondary outcome Pain frequency from pain diaries Adverse events Nil reported although patients encouraged to report in diaries |
|
| Notes | This study included fewer than 30 patients; of these only 13 had idiopathic TN. Unequal groups and no randomisation provided, although the use of cross‐over design suggested there was a difference in outcome depending on order. Unknown how many patients were lost to follow‐up, what the length of the trial was, no power calculation, no estimate for what considered positive outcome. Not clear if pain relief overall or just of the treated nerve. No details as to whether pain medication was stopped or reduced as a result. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “ randomly assigned” A double‐blind cross‐over protocol was used. The wash‐out period for the cross‐over was 7 days. Typically the patients received 5 blocks of either streptomycin plus lidocaine or lidocaine alone for a period of 5 consecutive weeks. On the sixth week they crossed over. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details “double blind placebo controlled randomized design” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No way of determining if all patients provided outcome data and for how long |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No details of who did injections, who did assessments |
Erdine 2007.
| Methods | A randomised double‐blind, prospective, parallel group study. An independent observer was used. Patients treated in day stay unit and followed up monthly for 6 months and then for minimum 12 months. | |
| Participants |
Intervention: convential radiofrequency (CRF) Diagnostic criteria of patients included in the trial Idiopathic TN but no definition, VAS > 5 MRI Age mean (range, SD) 60 (42 to 87, 11.9) Sex 21 male, 19 female (total for both groups) Severity measured VAS 0 to 10 mean (range) : VAS 9 (7 to 10) Patient satisfaction with therapy prior to trial on scale 0 to 10 mean (range) Patient satisfaction 1.5 (0 to 2) Duration of condition months mean ( range, SD) 83.2 (12 to 196, 57.8) months Number 20 Intervention: pulsed radiofrequency (RF) Diagnostic criteria of patients included in the trial Idiopathic TN but no definition VAS > 5 MRI Age mean (range, SD) 64.3 (37 to 85, 12) Gender 21 male, 19 female (total for both groups) Severity measured on VAS 0 to 10 mean (range) VAS 9 (7 to 10) Patient satisfaction with therapy prior to trial on scale 0 to 10 mean (range) Patient satisfaction 1 (0 to 2) Duration of condition months mean (range, SD) 79.7 (12 to 300, 70) months Number 20 |
|
| Interventions |
Intervention: CRF Type of intervention Sedation propofol, midazolam and fentanyl done under fluoroscopic control in submentovertex projection. RFC‐3C RF generator with 100 mm needles with active tip of 5 mm Trial stimulation: 2 Hz with 0.1 to 1.5 V and then 50 Hz to localise affected branches CRF 70 °C for 60 s if required second round 60 s, if several branches, needle repositioned and repeated again Number patients > 30 20 Intervention: pulsed RF Type of intervention Sedation propofol, midazolam and fentanyl done under fluoroscopic control in submentovertex projection. RFC‐3C RF generator with 100 mm needles with active tip of 5 mm Trial stimulation: 2 Hz with 0.1 to 1.5 V and then 50 Hz to localise affected branches Pulsed RFT 42 °C, 2 bursts of 20 ms each then applied for 120 s output of 45 V Number patients 20 |
|
| Outcomes |
Primary outcomes a) Pain intensity of the attacks using VAS (“0” no pain to “10” worst possible pain). b) Patient satisfaction using Patient Satisfaction Scale (PSS: “0” very dissatisfied to “10” very satisfied. c) Additional pharmacological treatment: carbamazepine and/or gabapentin. d) Side effects and complications related to the technique (sensorial impairment, anaesthesia dolorosa or other). Outcome pain relief VAS median pre‐ and post‐treatment at 0, 3, 6 months Time of measurement six months, one year, yearly intervals Kaplan‐Meier Secondary outcomes Morbidity Medication use Patient satisfaction Adverse events |
|
| Notes | It is not clear if the included patients had surgical treatment in the past. The treatment groups may have been comparable at baseline. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The 40 patients were randomly assigned to one of the two treatment groups 120 patients in each group by using the table of random numbers” |
| Allocation concealment (selection bias) | Low risk | “The sealed envelope defining the group of the patient was opened in the operation room just before the application of the procedure and the choice of CRF or PRF was performed accordingly.” Procedure done by independent specialist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | “The patients and the specialist who monitored the patients using monthly visits were blinded while the study supervisor who monitored all the study data was not. The procedures were performed by an independent specialist who followed a randomizations chart.” Double‐blind, both patient and assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement as to whether all followed up for 6 months |
| Selective reporting (reporting bias) | Unclear risk | No evidence |
| Other bias | Unclear risk | No power calculation or indication of what constituted a good outcome |
Flickinger 2001.
| Methods | Multicentre randomised, prospective, double‐blind, parallel group study. Unclear whether an independent observer was present. Follow‐up median 26 months (1 to 36 months) | |
| Participants |
Intervention: one isocentre Diagnostic criteria of patients included in the trial Classical TN, 12 no prior surgical procedures Age mean (range) 68 (37 to 86) Gender 24 female, 19 male Severity (mean, SD) No details Duration of condition years median (range) Median 7 (1 to 31) years Number of patients 43 Intervention: two isocentres Diagnostic criteria of patients included in the trial Classical TN, 12 no prior surgical procedures Age mean (range) 69 (38 to 90) Gender 26 female, 18 male Severity (mean, SD) No details Duration of condition years median (range) 9 (0.6 to 55) years Number of patients 44 |
|
| Interventions |
Intervention: one isocentre Type of intervention 75 Gy 50% at the centre. Volume 5.4 ± 0.44 mm3 Number patients 43 Intervention: two isocentres Type of intervention 75 Gy 50% at the centre, separated by 3 to 5mm. Volume 8.7 ± 1.1 mm3 Number of patients 44 |
|
| Outcomes |
Primary outcome Pain relief Secondary outcome Time to relapse, other procedures Adverse events |
|
| Notes | Treatment groups comparable at baseline; however, the pain severity is not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer‐generated randomizations was blocked and stratified by institution. Enrollment was stopped after 43 of 45 intended patients were enrolled.” |
| Allocation concealment (selection bias) | Low risk | “Patients were unaware of the randomised treatment assignment to limit any placebo effect. We administered one isocenter radiosurgery in two equal portions, resetting the same treatment coordinates for the second portion to keep patients unaware of whether one or two different isocentres were treated. For two‐isocenter radiosurgery, the change in position between isocenters was imperceptible to patients.” |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Reset treatment co‐ordinates after one treatment so seemed everyone was getting two. “Physicians and staff unaware of the randomisation assignment conducted the follow‐up evaluations.” Double‐ blind, patient and assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “One patient randomized to two isocenters was completely lost to follow‐up the day after radiosurgery and was excluded, leaving 87 patients for this analysis” One dropout. |
| Selective reporting (reporting bias) | Unclear risk | “Follow‐up was 26 months (range 1 ‐ 36). The product limit method of Kaplan‐Meier was used to calculate the actuarial rates of complete pain relief". Did not show KM data, data collected at one time point. |
| Other bias | High risk | “Assignment conducted the follow‐up evaluations. The median follow‐up was 26 months (range 1 ‐ 36).” Follow‐up short and big range |
Huibin 2009.
| Methods | A prospective, double‐blind, partially randomised trial. Unclear whether an independent observer was present | |
| Participants |
Intervention: conventional radiofrequency thermocoagulation (CRFT) Diagnostic criteria of patients included in the trial All had sharp, episodic pain in Va and some in other divisions also. Provoked by light touch, initially responded to anticonvulsants Age mean (range) 64.5 (48 to 83) Gender 11 female, 9 male Severity VAS > 5 Duration of condition mean range) years 4.5 (0.1 to 10) years Number 20 Intervention: pulsed radiofrequency thermocoagulation (PRFT) Diagnostic criteria of patients included in the trial All had sharp, episodic pain in Va and some in other divisions also. Provoked by light touch, initially responded to anticonvulsants Age mean (range) 66.5 (38 to 87) Gender 17 female, 13 male Severity VAS > 5 Duration of condition mean (range) years 4.7 (0.2 to 30) years Number 30 |
|
| Interventions |
Intervention: conventional radiofrequency thermocoagulation (CRFT) Type of intervention CRFT was used with light sedation. The position of the needle tip was confirmed with fluoroscopy following entry through foramen ovale. A current of 0.1 to 1.5 V at 50 Hz frequency was then applied to reach a temperature at the tip of 75 °C. This was repeated 2 to 3 times for 60 s each time and a maximum application time of 180 s The intervention was repeated at 3 days if it failed to have an effect Length of follow‐up mean (range, SD) month 67 (63 to 74, 3.9) month Intervention: pulsed radiofrequency thermocoagulation (PRFT) Type of intervention PRFT under local anaesthesia was used. A CT scan was performed prior to the intervention to locate the supraorbital, infraorbital or mental foramen dependent on site of pain. A current of 0.1 to 1.5 V at 50 Hz frequency was then applied to reach a needle tip temperature of 80 °C. This was repeated 3 to 5 times for 60 s each and a mean application time of 240 s The intervention was repeated at 3 days if it failed to have an effect Length of follow‐up mean (range, SD) month 67 (63 to 74, 3.9) month |
|
| Outcomes |
Primary outcome Complete pain relief without medication measured at 6 months, 1 year and at yearly intervals thereafter. No Kaplan‐Meier graph available. No data at 3 and 5 years Secondary outcome Morbidity measured as above Adverse events |
|
| Notes | This study is described as a randomised controlled trial of 50 patients (10 of them were not randomly allocated) determining whether PRFT done at trigger points as compared to CRFT done at the level of the Gasserian ganglion while providing the same pain relief improves outcomes in terms of reduced sensory loss and eye complications. Pain relief was only assessed at 3 years and there are no details provided as to how this was done. There is insufficient evidence to determine if patients were blinded to the type of surgery they had. As trigger point treatment was used no details are provided as to whether overall pain relief was maintained or only on the treated branches. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation chart was used |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were opened in operating theatre but not for first 10 patients included in the study |
| Blinding (performance bias and detection bias) All outcomes | High risk | The surgeon was independent of the study but it was not clear who carried out the patients' assessments and how they were done. The patients would have known postoperatively which procedure they had as the CRFT group was sedated whereas the PRT group had only local anaesthesia |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The outcome was done on primary measure immediately and then 3 and 5 years. No details on dropout rate |
| Selective reporting (reporting bias) | High risk | The authors do not provide details of how many had PRFT on individual nerves, no details on outcomes between immediate and 3 years following intervention |
| Other bias | High risk | 10 patients in the PRFT group were not randomised, no mention of how many individual nerves were treated per patient in the PRFT group as some patients had pain in more than 1 nerve |
Jiao 2003.
| Methods | A randomised, prospective, parallel group study | |
| Participants |
Intervention: injection only Diagnostic criteria of patients included in the trial Intense, short pain in one or more trigeminal nerve distribution. Intractable or intolerant to medication Age mean (SD) 54 (14) Gender 9 male, 11 female Severity Not available Duration of condition mean (SD) years 5.5 (3) years Number 20 Intervention: injection and infrared laser Diagnostic criteria of patients included in the trial Intense, short pain in one or more trigeminal nerve distribution. Intractable or intolerable to medication Age mean (SD) 58 (8) years Gender 8 male, 10 female Severity Not available Duration of condition mean (SD) years 5.7 (2.3) years Number 18 |
|
| Interventions |
Intervention: injection only Type of intervention 5 ml lidocaine local injection, 0.5 mg vitamin B12 in saline once or twice every 5 days Length of follow‐up mean (range, SD) month 15 minutes after intervention Intervention: injection and infrared laser Type of intervention 5 ml lidocaine, 0.5 mg vitamin B12 in saline 1 or 2 times every 5 days Polarised infra red light 10 min at stellate ganglion Length of follow‐up mean 15 mins after intervention |
|
| Outcomes |
Primary outcome Improvement in pain VAS from baseline 15 min following intervention Secondary outcome Not available Adverse events None reported |
|
| Notes | This small poor quality RCT which aimed to determine if infrared irradiation of the stellate ganglion after multiple weekly lidocaine vitamin B injections improved outcomes. Both groups having injections reported pain relief in the short term but there was no additional benefit found from the addition of therapy applied to the stellate ganglion. However, no details provided as to overall versus triggered branch relief or whether medication was stopped or reduced. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Only described that patients were divided into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Selective reporting (reporting bias) | Unclear risk | Cannot find selective outcome reporting |
| Other bias | Unclear risk | Cannot find other sources of bias, e.g. detection bias, performance bias |
Porras 2007.
| Methods | A randomised double‐blind prospective study | |
| Participants |
Intervention: steroid rhizotomy Diagnostic criteria of patients included in the trial Idiopathic trigeminal neuralgia with no response to pharmacological treatment Age mean (SD) 60.5 (13.1) Gender 3 male, 7 female Severity 8 to 10 on a scale of 0 to 10 Duration of condition mean (range) years Not available Number 10 Intervention: glycerol rhizotomy Diagnostic criteria of patients included in the trial Idiopathic trigeminal neuralgia with no response to pharmacological treatment Age mean (SD) 59 (7.3) Gender 3 male, 7 female Severity 8 to 10 on a scale of 0 to 10 Duration of condition mean (range) years Not available Number 10 |
|
| Interventions |
Intervention: steroid rhizotomy Type of intervention Percutaneous Gasserian ganglion block with 40 mg of methyprednisolone acetate Length of follow‐up 4 weeks Intervention: glycerol rhizotomy Type of intervention Percutaneous Gasserian ganglion selective block with 0.4 cc of 100% glycerol Length of follow‐up 4 weeks |
|
| Outcomes |
Primary outcome Pain relief at 4 weeks |
|
| Notes | Lack of data. Randomisation said to have been done but with no details. No allocation concealment. Very short trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data given |
| Allocation concealment (selection bias) | Unclear risk | No data given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated that patients and doctors who did the VAS assessments were blind to the substance injected |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data |
| Selective reporting (reporting bias) | Unclear risk | incomplete data |
| Other bias | Unclear risk | No data given |
Przeklasa‐Muszynska 2006.
| Methods | A parallel group, randomised prospective study | |
| Participants |
Intervention: radiofrequency thermocoagulation (RFT) + steroid Diagnostic criteria of patients included in the trial Not available Age mean (range) 65 (49 to 83) Gender 14 female, 8 male Severity VAS 0 to 10, 8.4 McGill Pain Questionnaire 38.9 ± 11.5 Duration of condition mean (range) years 9.8 (0.8 to 40) years Number 22 Intervention: RFT + pentoxyphilline Diagnostic criteria of patients included in the trial Not available Age mean (range) 65 (49 to 83) Gender 13 female, 8 male Severity VAS 8.5 McGill Pain Questionnaire 43.4 ± 14.9 Duration of condition mean (range) years 9.8 (0.8 to 40) Number 21 Control: RFT only Diagnostic criteria of patients included in the trial Not available Age mean (range) 65 (49 to 83) Gender 14 female, 8 male Severity VAS 8.1 McGill Pain Questionnaire 41.1 ± 11.4 Duration of condition mean (range) years 9.8 (0.8 to 40) Number 22 |
|
| Interventions |
Intervention: RFT + steroid Type of intervention Radiofrequency thermocoagulation at Gasserian ganglion using 5 mm needle, 3 x 30 s 21 mV at 50 mA with 40 mg methylprednisolone Length of follow‐up mean 2 hrs, 7 days, 30 days, 90 days Intervention: RFT + pentoxyphilline Type of intervention Radiofrequency thermocoagulation at Gasserian ganglion using 5 mm needle, 3 x 30 s 21 mV at 50 mA with 10 mg pentoxyphilline Length of follow‐up mean 2 hrs, 7 days, 30 days, 90 days Control: RFT only Type of intervention Radiofrequency thermocoagulation at Gasserian ganglion using 5 mm needle, 3 x 30 s 21 mV at 50 mA with 2 ml normal saline Length of follow‐up mean 2 hrs, 7 days, 30 days, 90 days |
|
| Outcomes |
Primary outcome Pain relief on the VAS Time of measurement: 6 months, one year and at yearly intervals (Kaplan‐Meier) Secondary outcome VAS McGill Pain Questionnaire Adverse events |
|
| Notes | This RCT determines whether the injection of a steroid or pentoxyphilline ( agent to reduce inflammatory response) at the time of the radiofrequency thermocoagulation affects outcome at 90 days. Nearly 30% of patients received no benefit from RFT but this was reduced in the pentoxyphilline group at 90 days. There was some reduction in side effects in terms of swelling and sensory change when using pentoxyphilline. There are no details provided on randomisation, how bias was removed or of completeness of follow‐up. A power calculation was not done. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | In English abstract say “ randomly divided” in Polish abstract and text “ divided” but no mention of the word random |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of completeness of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Incomplete data |
| Other bias | Unclear risk | None mentioned |
Shen 2006.
| Methods | A randomised, parallel group, prospective study | |
| Participants |
Intervention: peripheral adriamycin Diagnostic criteria of patients included in the trial Paroxysmal facial pain, affecting one or more divisions of trigeminal nerve, unilateral, may be evoked pain. There is no clinically evident neurological deficit, pain not attributed to another disorder Age mean (SD) 65 (10) Gender 20 male, 15 female Severity Not available Duration of condition mean (SD) years 10.8 (12.4) Number 35 Intervention: peripheral gentamicin Diagnostic criteria of patients included in the trial Paroxysmal facial pain, affecting one or more division of trigeminal nerve, unilateral, may be evoked pain. There is no clinically evident neurological deficit, pain not attributed to another disorder Age mean (SD) 60 (9) Gender 22 male, 13 female Severity Not available Duration of condition mean (SD) years 5.7 (3.8) years Number 35 |
|
| Interventions |
Intervention: adriamycin Type of intervention Adriamycin 5 g/l into all trigeminal nerve exit foramina Once and then 1 or 2 weeks later Length of follow‐up mean month 30 months Intervention: gentamicin Type of intervention Gentamicin 8 x 104 U into all foramina once and then 1 or 2 weeks later Length of follow‐up mean month 30 months |
|
| Outcomes |
Primary outcome Relief VAS 0 to 10 Time of measurement: 6 months, 1 year and then at yearly intervals (Kaplan‐Meier) Adverse events |
|
| Notes | This is a poor quality randomised controlled trial to determine the effect of peripheral injections of gentamicin or adriamycin on pain relief at two and a half years. Although both treatments were initially effective, long term they were effective in only 23% of patients who received adriamycin and 6% of those who received gentamicin. The adriamycin group was more likely to have facial anaesthesia, which was permanent in up to 14% of patients. Not stated whether pain migrated to other branches and whether medication was reduced or stopped as a result of interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly, no description of how sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The first, second, third author designed the study, the first author performed all the intervention and assessed the result |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description |
| Selective reporting (reporting bias) | Unclear risk | No description |
| Other bias | Unclear risk | No description |
Stajcic 1990.
| Methods | A randomised, double‐blind, prospective, parallel group study | |
| Participants |
Intervention: peripheral streptomycin + lidocaine Diagnostic criteria of patients included in the trial Internation Headache Society criteria, excluded those with pain relief for over 24 hours after test doses of lidocaine No remissions for at least 1 year To locate correct nerve at first visit gave lidocaine block 9 had had previous local surgery Age range 49 to 85 Severity Not available Duration of condition range years 1.2 to 29 years Number 9 Control: peripheral lidocaine Diagnostic criteria of patients included in the trial International Headache Society criteria, excluded those with pain relief for over 24 hours after test doses of lidocaine No remissions for at least 1 year To locate correct nerve at first visit gave lidocaine block 9 had had previous local surgery Age range 49 to 85 years Severity Not available Duration of condition mean (range) years 1.2 to 29 years Number 8 |
|
| Interventions |
Intervention: peripheral streptomycin + lidocaine Type of intervention 1 g streptomycin + 3 ml 2% lidocaine into trigger area weekly for 5 weeks Usual medication not changed Length of follow‐up (mean in months) Shortest 6 months Control: peripheral lidocaine Type of intervention 3 ml lidocaine into trigger area weekly for 5 weeks Usual medication not changed Length of follow‐up (mean in months) Shortest 6 months |
|
| Outcomes |
Primary outcome Pain relief measured at 1 week and 30 months following intervention Adverse events Nil reported |
|
| Notes | Randomised double‐blind trial in 18 patients with TN of varying duration. There are insufficient details to determine whether there is bias, whether the groups were comparable, no details of how outcomes measured, no details of follow up time on all patients. Initially the results suggested some improvement in those having streptomycin but longer follow‐up showed that this was not maintained. However, no details provided as to overall versus triggered branch relief or whether medication was stopped or reduced | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly selected |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Fitted sleeve over injection barrel to decrease recognition independent person prepared injections in identical syringes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | May have been 1 dropout , shortest follow‐up time was 6 months not clear how many had final assessment at 30 months |
| Selective reporting (reporting bias) | Unclear risk | Table 3 provides outcome on all patients but no time frame |
| Other bias | Unclear risk | No power calculation, no details as to what classified as good outcome. No mention of whether pain relief gained was total or only for the treated branch |
Walker 1988.
| Methods | A randomised, double‐blind, prospective, parallel group study with an independent observer | |
| Participants |
Intervention: peripheral laser Diagnostic criteria of patients included in the trial 9 on medications 9 no medications “accepted criteria for TN”. "No previous surgical procedures" Age mean (range) 29 to 78 Gender 9 male, 9 female Severity VAS Duration of condition (range) years 1 to 18 yrs Number 18 Control: peripheral sham Diagnostic criteria of patients included in the trial 10 on medications 7 no medications “accepted criteria for TN”, "No previous surgical procedures" Age mean (range) 38 to 78 Gender 7 male, 10 female Severity VAS Duration of condition mean (range) years 2 to 20 yrs Number 17 |
|
| Interventions |
Intervention: peripheral laser Helium neon laser 632.5 nm,1 mW, 20 Hz Radial, median, ulnar, saphenous bilaterally, 3 painful facial area where exposure increased 30 s 1 week, 45 s second week, 60 s third to sixth week, 90s 7th to 10th week. Three times a week for 10 weeks Length of follow‐up mean (range, SD) month Not available Control: peripheral sham Sham machine Radial, median, ulnar, saphenous bilaterally, 3 painful facial area where exposure increased 30 s 1 week, 45 s second week, 60 s third to sixth week, 90 s 7th to 10th week. Three times a week for 10 weeks Length of follow‐up mean (range, SD) month Not available |
|
| Outcomes |
Primary outcome Pain relief VAS 0 to 100 and VRS 0 to 100. 1. low pain 0 to 25, 2, moderate pain 26 to 50, 3. moderate high pain 51 to 75, 4. high pain 76 to 100 reported weekly Time of measurement 6 months, 1 year, yearly intervals Kaplan‐Meier Adverse events not stated |
|
| Notes | Although this was described as a randomised controlled trial of laser therapy there are insufficient details provided to exclude bias. Care was taken to ensure everyone was blinded to the treatment and assessments were done independently. The outcome measures do not provide individual results but are reported as variances from initial baseline. Improvement was reported after 10 weeks in those with more severe pain. However, no details provided as to overall versus triggered branch relief or whether medication as stopped or reduced. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjects randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All subjects closed their eyes during the laser or placebo administration and thus could not see or feel whether they were receiving experimental or control treatment. Each technician administered both experimental and sham therapy and was allowed to participate for a maximum of 2 months before being rotated to another project. Recording of pain and of drug histories was done by a third team of researchers that was given only the coded name of each subject and did not know which condition was experimental and which was placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “missing scores were assigned the mean pain rating for that group for the rest of the week" |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Other bias | Unclear risk | Not possible to ascertain |
Xu 2006.
| Methods | A randomised, parallel group study comparing radiofrequency thermocoagulation (RFT) using the VectorVision navigation system to Hartel's technique of facial measurement. It is not clear if this study was double blind, and unclear if an independent observer was used. Day stay setting | |
| Participants |
Intervention: Gasserian RFT with navigation Diagnostic criteria of patients included in the trial TN fulfilling criteria of International Association for the Study of Pain, no prior surgery MRI Age mean (range, SD) 63 (48 to 79, 5) Gender 12 female, 14 male Severity (mean, SD) Not available Duration of condition years mean, range, SD) 5.3 (3 to 10, 1.2) years Number 26 Control: Gasserian RFT without navigation Diagnostic criteria of patients included in the trial TN fulfilling criteria of International Association for the Study of Pain, no prior surgery MRI Age mean (range, SD) 59 (45 to 73, 7) Gender 15 female, 13 male Severity (mean, SD) No indication Duration of condition years mean, ( range, SD) 4.9 (2.5 to 12, 1.5) years Number 28 |
|
| Interventions |
Intervention: Gasserian RFT with navigation Type of intervention VectorVision navigation system had frame attached 8 to 10 gauge needle, 1 mm exposed Test with 2 Hz , 50 Hz, temperature of 45 °C for 30 s, repeated with further lesion temperature 75 °C for 60 s Repeated 2 to 3 more times till blunt to touch Antibiotics 3 to 4 days, other medications stopped Length of follow‐up 13 to 58, mean 36 + 7 months Control: Gasserian RFT without navigation Type of intervention Hartel’s technique with measurement on the face 8 to 10 gauge needle, 1 mm exposed Test with 2 Hz , 50 Hz, temperature of 45 °C for 30 s, repeated with further lesion temperature 75 °C for 60 s Repeated 2 to 3 more times till blunt to touch Antibiotics 3 to 4 days, other medications stopped Length of follow‐up 10 to 54, mean 34 + 5 months |
|
| Outcomes |
Primary outcomes The patient's overall degree of improvement, that is: complete pain relief, partial satisfactory pain relief, partial unsatisfactory pain relief, no change and worse Outcome complete pain relief on Kaplan‐Meier Time of measurement 6 months, 1 year, yearly intervals Kaplan‐Meier Secondary outcome Morbidity Adverse events |
|
| Notes | A high risk of bias was identified in this study. This is a comparative study comparing 2 techniques for performing radiofrequency thermocoagulation. Few details are provided to determine the quality of the RCT, e.g. method of randomisation, blinding, outcome assessment, and the only outcome measure used was complete pain relief ascertained by a questionnaire at 3 months. Actuarial methodology shows that both techniques provide pain relief but this is more sustained if a neuronavigation system is used. This system also decreases side effects |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomly divided into two groups" no details provided |
| Allocation concealment (selection bias) | High risk | No details provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Immediate effectiveness of the surgery which was based on the condition of the participants was recorded in hospital. Three months after being discharged, all the participants were sent questionnaires addressing the absence or presence of facial pain, its characteristics, duration and time of recurrence, adverse effects, complications and the need for further treatment. The data available from these records were processed and stored in a designed computer database. Outcomes only measured once by questionnaire and timing not known. No clear data provided on blinding of either patient or assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown number lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Used questionnaires at 3 months but not other times and study continued beyond this time |
| Other bias | Unclear risk | Not clear |
TN: trigeminal neuralgia; VAS: visual analogue scale; SD: standard deviation; MRI: magnetic resonance imaging; RCT: randomised controlled trial; CT scan: computerised tomography scan.
Differences between protocol and review
In the secondary outcome measures all cause mortality was reported, not just mortality related to the procedure. We expanded the section on the amount of data we collected. A table of results of outcomes of independently assessed prospective surgical cases (none RCT) has not been provided as this has been published subsequent to the writing of this protocol and so references are provided to this study (Tatli 2008). Funnel plots and meta‐analysis were not done owing to lack of studies. No cost‐benefit studies were found.
Contributions of authors
Joanna Zakrzewska wrote the major draft of the protocol. Joanna Zakrzewska and Harith Akram independently went through all the studies identified by the search strategy and selected the studies. They independently extracted the data from the chosen studies. The results were then entered by Harith Akram and Joanna Zakrzewska wrote the first draft of the discussion and authors conclusions.
Sources of support
Internal sources
-
Joanna Zakrzewska, UK.
Undertook this work at UCL/UCLHT who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centre funding scheme.
External sources
No sources of support supplied
Declarations of interest
Joanna Zakrzewska had her travel expenses paid to a conference of the Leksell Gamma Knife Society to speak on trial design 2010.
Edited (no change to conclusions)
References
References to studies included in this review
Bittar 1993 {published data only}
- Bittar GT, Graff‐Radford SB. The effects of streptomycin/lidocaine block on trigeminal neuralgia: a double blind crossover placebo controlled study. Headache. 1993;33(3):155‐60. [PUBMED: 8486515] [DOI] [PubMed] [Google Scholar]
Erdine 2007 {published data only}
- Erdine S, Ozyalcin NS, Cimen A, Celik M, Talu GK, Disci R. Comparison of pulsed radiofrequency with conventional radiofrequency in the treatment of idiopathic trigeminal neuralgia. European Journal of Pain 2007;11(3):309‐13. [PUBMED: 16762570] [DOI] [PubMed] [Google Scholar]
Flickinger 2001 {published data only}
- Flickinger JC, Pollock BE, Kondziolka D, Phuong LK, Foote RL, Stafford SL, et al. Does increased nerve length within the treatment volume improve trigeminal neuralgia radiosurgery? A prospective double‐blind, randomized study. International Journal of Radiation Oncology, Biology, Physics 2001;51(2):449‐54. [PUBMED: 11567820] [DOI] [PubMed] [Google Scholar]
Huibin 2009 {published data only}
- Huibin Q, Jianxing L, Guangyu H, Dianen F. The treatment of first division idiopathic trigeminal neuralgia with radiofrequency thermocoagulation of the peripheral branches compared to conventional radiofrequency. Journal of Clinical Neuroscience 2009;16(1532‐2653 (Electronic), 0967‐5868 (Linking), 11):1425‐9. [DOI] [PubMed] [Google Scholar]
Jiao 2003 {published data only}
- Jiao XP, Gao M, Wang BG. Comparison of early outcomes between nerve block and nerve block combined with polarized infrared ray in treatment of trifacial neuralgia. Chinese Journal of Clinical Rehabilitation 2003;7(17):2479‐80. [Google Scholar]
Porras 2007 {published data only}
- Porras R, Tenopala S, Hernandez‐Santos JR, Torre Rivera G, Quiroga OJ, Castillejos VE, et al. Effectiveness of the intragasserian blockade with glicerol versus depot metilprednisolone acetate in the trigeminal neuralgia. Effects in the short term. Revista de la Sociedad Espanola del Dolor. 2007;14(8):574‐8. [EMBASE: 2008068362] [Google Scholar]
Przeklasa‐Muszynska 2006 {published data only}
- Przeklasa‐Muszynska A, Dobrogowski J, Wordliczek J. Estimation of therapeutic value of radiofrequency lesion of Gasserian ganglion with an addition of pentoxyphilline and methylprednisolone. Polska Medycyna Paliatywna 2006;5(1):14‐20. [EMBASE: 2006329235] [Google Scholar]
Shen 2006 {published data only}
- Shen Y, Zhao HL, Wang BG. Long‐term therapeutic efficacy of peripheral block by adriamycin versus gentamicin on treatment of trigeminal neuralgia. Chinese Journal of Clinical Rehabilitation 2006;10(48):42‐6. [EMBASE: 2007021499] [Google Scholar]
Stajcic 1990 {published data only}
- Stajcić Z, Juniper RP, Todorović L. Peripheral streptomycin/lidocaine injections versus lidocaine alone in the treatment of idiopathic trigeminal neuralgia. A double blind controlled trial. Journal of Cranio‐Maxillo‐Facial Surgery 1990;18(6):243‐6. [PUBMED: 2212020] [DOI] [PubMed] [Google Scholar]
Walker 1988 {published data only}
- Walker JB, Akhanjee LK, Cooney MM, Goldstein J, Tamayoshi S, Segal‐Gidan F. Laser therapy for pain of trigeminal neuralgia. The Clinical Journal of Pain 1988;3(4):183‐7. [EMBASE: 1988057399] [Google Scholar]
Xu 2006 {published data only}
- Xu SJ, Zhang WH, Chen T, Wu CY, Zhou MD. Neuronavigator‐guided percutaneous radiofrequency thermocoagulation in the treatment of intractable trigeminal neuralgia. Chinese Medical Journal 2006;119(18):1528‐35. [PUBMED: 16996006] [PubMed] [Google Scholar]
Additional references
Anon 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(Suppl 1):9‐160. [PUBMED: 14979299] [DOI] [PubMed] [Google Scholar]
Barker 1997
- Barker FG 2nd, Jannetta PJ, Bissonette DJ, Jho HD. Trigeminal numbness and tic relief after microvascular decompression for typical trigeminal neuralgia. Neurosurgery 1997;40(1):39‐45. [PUBMED: 8971822] [DOI] [PubMed] [Google Scholar]
Barkun 2009
- Barkun JS, Aronson JK, Feldman LS, Maddern GJ, Strasberg SM, Altman DG, et al. Evaluation and stages of surgical innovations. Lancet 2009;374(9695):1089‐96. [PUBMED: 19782874] [DOI] [PubMed] [Google Scholar]
Birkmeyer 2002
- Birkmeyer NJ, Weinstein JN, Tosteson AN, Tosteson TD, Skinner JS, Lurie JD, et al. Design of the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976.) 2002;27(1528‐1159 (Electronic), 0362‐2436 (Linking), 12):1361‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cruccu 2008
- Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K, et al. AAN‐EFNS guidelines on trigeminal neuralgia management. European Journal of Neurology 2008;15(10):1013‐28. [PUBMED: 18721143] [DOI] [PubMed] [Google Scholar]
Devor 2002
- Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clinical Journal of Pain 2002;18(1):4‐13. [PUBMED: 11803297] [DOI] [PubMed] [Google Scholar]
Dhople 2009
- Dhople AA, Adams JR, Maggio WW, Naqvi SA, Regine WF, Kwok Y. Long‐term outcomes of Gamma Knife radiosurgery for classic trigeminal neuralgia: implications of treatment and critical review of the literature. Clinical article. Journal of Neurosurgery 2009;111(2):351‐8. [PUBMED: 19326987] [DOI] [PubMed] [Google Scholar]
Edlund 2004
- Edlund W, Gronseth G, So Y, Franklin G. Clinical practice guideline process manual. 2004. American Academy of Neurology, 2005. [Google Scholar]
Ergina 2009
- Ergina PL, Cook JA, Blazeby JM, Boutron I, Clavien PA, Reeves BC, et al. Challenges in evaluating surgical innovation. Lancet 2009;374(9695):1097‐104. [PUBMED: 19782875] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gronseth 2008
- Gronseth G, Cruccu G, Alksne J, Argoff C, Brainin M, Burchiel K, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology 2008;71(15):1183‐90. [PUBMED: 18716236] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Kalkanis 2003
- Kalkanis SN, Eskandar EN, Carter BS, Barker FG 2nd. Microvascular decompression surgery in the United States, 1996 to 2000: mortality rates, morbidity rates, and the effects of hospital and surgeon volumes. Neurosurgery 2003;52(6):1251‐61. [PUBMED: 12762870] [DOI] [PubMed] [Google Scholar]
Katusic 1990
- Katusic S, Beard CM, Bergstralh E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945‐1984. Annals of Neurology 1990;27(1):89‐95. [PUBMED: 2301931] [DOI] [PubMed] [Google Scholar]
Katusic 1991
- Katusic S, Williams DB, Beard CM, Bergstralh EJ, Kurland LT. Epidemiology and clinical features of idiopathic trigeminal neuralgia and glossopharyngeal neuralgia: similarities and differences, Rochester, Minnesota, 1945‐1984. Neuroepidemiology 1991;10(5‐6):276‐81. [PUBMED: 1798430] [DOI] [PubMed] [Google Scholar]
Koopman 2009
- Koopman JS, Dieleman JP, Huygen FJ, de MM, Martin CG, Sturkenboom MC. Incidence of facial pain in the general population. Pain 2009;147:122‐7. [PUBMED: 19783099] [DOI] [PubMed] [Google Scholar]
Lee 2010
- Lee JY, Chen HI, Urban C, Hojat A, Church E, Xie SX, et al. Development of and psychometric testing for the Brief Pain Inventory‐Facial in patients with facial pain syndromes. Journal of Neurosurgery 2010;113(3):516‐23. [DOI: 10.3171/2010.1.JNS09669] [DOI] [PubMed] [Google Scholar]
Lim 2006
- Lim M, Cotrutz C, Romanelli P, Schaal D, Gibbs I, Chang SD, et al. Stereotactic radiosurgery using CT cisternography and non‐isocentric planning for the treatment of trigeminal neuralgia. Computer Aided Surgery 2006;11(1):11‐20. [PUBMED: 16531338] [DOI] [PubMed] [Google Scholar]
Lopez 2004
- Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery 2004;54(4):973‐82. [PUBMED: 15046666] [DOI] [PubMed] [Google Scholar]
McCulloch 2002
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324(7351):1448‐51. [PUBMED: 12065273] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCulloch 2009
- McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009;374(9695):1105‐12. [PUBMED: 19782876] [DOI] [PubMed] [Google Scholar]
Merskey 1994
- Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. 2nd Edition. Seattle: IASP Press, 1994. [Google Scholar]
Munoz 1988
- Munoz M, Dumas M, Boutros‐Toni F, Coquelle D, Vallat JM, Jauberteau MO, et al. A neuro‐epidemiologic survey in a Limousin town. Review Neurologique (Paris) 1988;144(4):266‐71. [PUBMED: 3262231] [PubMed] [Google Scholar]
Nurmikko 2001
- Nurmikko TJ, Eldridge PR. Trigeminal neuralgia‐‐pathophysiology, diagnosis and current treatment. British Journal of Anaesthesia 2001;87(1):117‐32. [PUBMED: 11460800] [DOI] [PubMed] [Google Scholar]
Regis 2006
- Régis J, Metellus P, Hayashi M, Roussel P, Donnet A, Bille‐Turc F. Prospective controlled trial of gamma knife surgery for essential trigeminal neuralgia. Journal of Neurosurgery 2006;104(6):913‐24. [PUBMED: 16776335] [DOI] [PubMed] [Google Scholar]
Relton 2010
- Relton C, Torgerson D, O'Cathain A, Nicholl J. Rethinking pragmatic randomised controlled trials: introducing the "cohort multiple randomised controlled trial" design. BMJ 2010;340:c1066. [PUBMED: 20304934] [DOI] [PubMed] [Google Scholar]
Rogers 2000
- Rogers CL, Shetter AG, Fiedler JA, Smith KA, Han PP, Speiser BL. Gamma knife radiosurgery for trigeminal neuralgia: the initial experience of The Barrow Neurological Institute. International Journal of Radiation Oncology, Biology, Physics 2000;47(4):1013‐9. [PUBMED: 10863073] [DOI] [PubMed] [Google Scholar]
Rothman 1973
- Rothman KJ, Monson RR. Epidemiology of trigeminal neuralgia. Journal of Chronic Diseases 1973;26(1):3‐12. [PUBMED: 4683548] [DOI] [PubMed] [Google Scholar]
Spatz 2007
- Spatz AL, Zakrzewska JM, Kay EJ. Decision analysis of medical and surgical treatments for trigeminal neuralgia: how patient evaluations of benefits and risks affect the utility of treatment decisions. Pain 2007;131(3):302‐10. [PUBMED: 17451880] [DOI] [PubMed] [Google Scholar]
Tatli 2008
- Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: literature study of respective long‐term outcomes. Acta Neurochirurgica 2008;150(3):243‐55. [PUBMED: 18193149] [DOI] [PubMed] [Google Scholar]
Weigel 2000
- Weigel G, Casey KF. Striking Back. The Trigeminal Neuralgia Handbook. Gainesville, Florida: The Trigeminal Neuralgia Association, 2000. [Google Scholar]
Weinstein 2009
- Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN, et al. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four‐year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. Journal of Bone and Joint Surgery. American Volume 2009;91(1535‐1386 (Electronic), 6):1295‐304. [PUBMED: 19487505] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2011
- Wiffen PJ, Derry S, Moore RA, McQuay HJ. Carbamazepine for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD005451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2011
- Yang M, Zhou M, He L, Chen N, Zakrzewska JM. Non‐antiepileptic drugs for trigeminal neuralgia. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD004029.pub3] [DOI] [PubMed] [Google Scholar]
Zakrzewska 1986
- Zakrzewska JM, Nally FF, Flint SR. Cryotherapy in the management of paroxysmal trigeminal neuralgia. Four year follow up of 39 patients. Journal of Maxillofacial Surgery 1986;14(1):5‐7. [PUBMED: 3456414] [DOI] [PubMed] [Google Scholar]
Zakrzewska 2003
- Zakrzewska JM, Lopez BC. Quality of reporting in evaluations of surgical treatment of trigeminal neuralgia: recommendations for future reports. Neurosurgery 2003;53(1):110‐22. [PUBMED: 12823880] [DOI] [PubMed] [Google Scholar]
Zakrzewska 2005
- Zakrzewska JM, Lopez BC, Kim SE, Coakham HB. Patient reports of satisfaction after microvascular decompression and partial sensory rhizotomy for trigeminal neuralgia. Neurosurgery 2005;56(6):1304‐11. [PUBMED: 15918947] [DOI] [PubMed] [Google Scholar]
Zakrzewska 2006
- Zakrzewska JM. Insights, facts and stories behind trigeminal neuralgia. Gainseville, Florida: Trigeminal Neuralgia Association, 2006. [Google Scholar]
Zakrzewska 2009
- Zakrzewska JM, Linskey ME. Trigeminal neuralgia. Clinical Evidence(Online) 2009;2009(1752‐8526 (Electronic), pii):1207. [Google Scholar]


