Abstract
Introduction
Cervical cancer screening in general practice could be a routine moment to provide female smokers with stop smoking advice and support. The aim of this study is to assess the effect of a stop smoking strategy delivered by trained practice assistants after the cervical smear, and to evaluate the implementation process.
Methods and analysis
The study is a two-arm, pragmatic cluster randomised trial, in Dutch general practice. Randomisation takes place 1:1 at the level of the general practice. Practices either deliver the SUCCESS stop smoking strategy or the usual care condition. The strategy consists of brief stop smoking advice based on the Ask-Advise-Connect method and is conducted by trained practice assistants after routine cervical cancer screening. The primary outcome is the performance of a serious quit attempt in the 6 months after screening. Secondary outcomes are 7-day point prevalence abstinence, reduction in the number of cigarettes per day and transition in motivation to quit smoking. Follow-up for these measurements takes place after 6 months. Analysis on the primary outcome aims to detect a 10% difference between treatment arms (0.80 power, p=0.05, using a one-sided test), and will be performed according to the intention to treat principle. The process evaluation will assess feasibility, acceptability and barriers or enablers to the strategy’s implementation. For this purpose, both qualitative and quantitative data will be collected via questionnaires and in-depth interviews, respectively, in both individual study participants and involved staff.
Ethics and dissemination
The Dutch Ministry of Health, Welfare and Sport approved of the trial after an advisory report from the Health Council (Nr. 2018/17). A licence was provided to conduct the study under the Population Screening Act. Study results will be disseminated through publications in peer-reviewed journals and conference presentations.
Trial registration number
NL5052 (NTR7451).
Keywords: primary care, preventive medicine, protocols & guidelines, substance misuse
Strengths and limitations of this study.
Pragmatic cluster randomised trial in general practice, using a stop smoking strategy based on the Ask-Advise-Connect method in cervical cancer screening participants who smoke, performed by the practice assistant who also performs the cervical screening.
Inclusion of female smokers regardless of heaviness of smoking or motivation to stop smoking.
Elaborate process evaluation to assess the acceptability, feasibly and factors of influence on the implementation of the strategy.
Details in informed consent are omitted to avoid unbalanced participation, and will be provided after the study.
Introduction
Tobacco consumption remains the main preventable cause of mortality and morbidity rates in developed countries.1 2 Smoking causes up to 30% of cancer deaths.3 In women, 12%–19% of new cancer cases are attributable to smoking.4 5
To address preventable early death and disease related to smoking it is important to increase smoking cessation rates.2 6 7 This can be done by providing stop smoking advice and offering support to individuals when the prevalence of smoking-related disease is still low.2 8 9 Despite clear guidelines for clinicians,10 opportunities to provide advice or support are often underused.11 12 Less than one-third of smokers receives advice when consulting their general practitioner (GP). Especially those who do not (yet) have symptoms or disease related to smoking are more likely to be withheld from advice or support to quit.12–14
Cancer screening programmes can serve as a teachable moment for behaviour change in asymptomatic cancer screening participants.15–17 In the age category of invited women (30–60 years) approximately 1 in 5 to 6 are daily smokers.18 In 2019, around 413.000 women had their smear taken.19
Smoking is a risk factor for continued high-risk human papillomavirus (hrHPV) infection of the cervix, carcinoma in situ and cervical cancer.20–24 In addition, tobacco and nicotine might promote oncogenic mechanisms in cervical cells.25–29
Cervical cancer screening could be an opportunity to identify female smokers who still have a relatively low prevalence of tobacco-related disease, and routinely provide them with stop smoking advice and support in the general practice setting.15 30 31 A teachable moment could be created between participants’ own health behaviour (smoking) and the reason for their visit (cancer prevention). Such tailored advice has proven to be more effective compared with standardised information.15
We aim to study the effect of a stop smoking strategy delivered by practice assistants (PAs), consisting of brief stop smoking advice, conducted after cervical cancer screening in general practice by means of a pragmatic cluster randomised trial (primary objective). Due to the complex nature of the strategy and the heterogeneity of care delivered across general practices in the Netherlands a process evaluation (PE) is important to determine the (future) applicability of the strategy. This PE has the aim to obtain insight into the acceptability, feasibility and barriers and enablers to the strategy’s implementation among both individual study participants and involved staff, and will run in parallel to the main trial (secondary objective).32 33
Methods and analysis
Study design
This protocol was written following the recommendations of the Standard Protocol Items for Randomised Trials statement (online supplemental material S1)34 35 and the Template for Intervention Description and Replication Checklist (online supplemental material S2).36 The trial was designed based on previous research: studies on brief stop smoking advice in Dutch general practice and the Ask-Advise-Connect (AAC) method were used for intervention design, and a guide for smoking cessation research in Dutch was used to design baseline and follow-up questionnaires.37–41 For the PE design, we followed the recommendations from the Medical Research Council on complex interventions.33
bmjopen-2021-055812supp001.pdf (100.9KB, pdf)
bmjopen-2021-055812supp002.pdf (580.9KB, pdf)
A two-arm cluster randomised controlled trial (RCT) design was chosen to explore the primary objective. Randomisation will take place at the level of the general practice, for example, the clusters, and takes place after a practice consents with study participation. Practices are randomised 1:1 to receive either the ‘SUCCESS study stop smoking strategy’ or the usual care condition.
A cluster design reduces contamination at the level of individual study participants and PAs. Identification of eligible participants before randomisation is not possible. Also, sending an invitation for study participation together with the invitation for participation in the screening programme could alter the participation rate for the cancer screening programme.
This study pragmatically uses the routine appointment for cervical screening to implement the strategy. The setting and eligible participants are similar to usual care. The recruitment of individual participants and delivery of the strategy are expected to be brief and do not require extensive training of the involved healthcare professionals. Also, the primary outcome (performing a serious quit attempt 6 months after baseline) is directly relevant to trial participants. Lastly, the use of a practice plan enables intervention practices to link the strategy to referral for stop smoking counselling as organised within their practice.42 43
Study setting
This pragmatic cluster RCT takes place in general practices in the Netherlands. The strategy is linked to the visit for the cervical smear for the national cervical cancer screening programme. Typically, the cervical smear is delivered by PAs. The core team of a Dutch general practice consists of a GP, a PA and—in most practices—a qualified nurse. In countries with similar healthcare systems, PAs are known as ‘medical assistants’, ‘medical secretaries’, ‘healthcare assistants’ or ‘allied health personnel’. The GP is responsible for providing stop smoking advice or support in the practice. Nurses are regularly involved in disease management programmes and deliver stop smoking care in that context. PAs are currently not engaged in delivering stop smoking advice and will therefore be trained to deliver the strategy.
Patient and public involvement
Prior to the trial we interviewed female smokers aged 30–60 years to explore the prospective acceptability of the approach.44 Additionally, we held focus group discussions with PAs, nurses and GPs to prospectively identify potential barriers or enablers to the delivery and implementation of the strategy. The results were used for trial design, especially for the training of PAs delivering the strategy. Also, the results were used to select points of focus for the PE, namely: the role of PAs in stop smoking care, the interaction between female smoker and PA during the delivery of the strategy, and the availability and support of the PA’s direct colleagues in the practice. The study results will be shared with individual study participants and participating practices.
Methods effect study
Study participants
All women attending the cervical cancer screening programme, which consists of undergoing a cervical smear, at a practice enrolled in the study are eligible for participation. Women receive an invitation every 5 years, starting at 30 years. The last invitation is at 60 years. However, women who test positive for hrHPV at 60 years will receive their last invitation at 65 years.
All women attending cervical cancer screening are asked to participate in a questionnaire study about lifestyle, for the following reason: smoking status of cervical cancer participants is not known before they undergo a cervical smear. To obtain baseline smoking related variables while keeping eligible participants who smoke blinded to the true nature of the study both smokers and non-smokers are asked to participate.
We aim to investigate the effect of the stop smoking strategy on all type of smokers who participate in cervical screening, regardless of heaviness of smoking or motivation to stop smoking. For this purpose both daily and non-daily smokers are included.
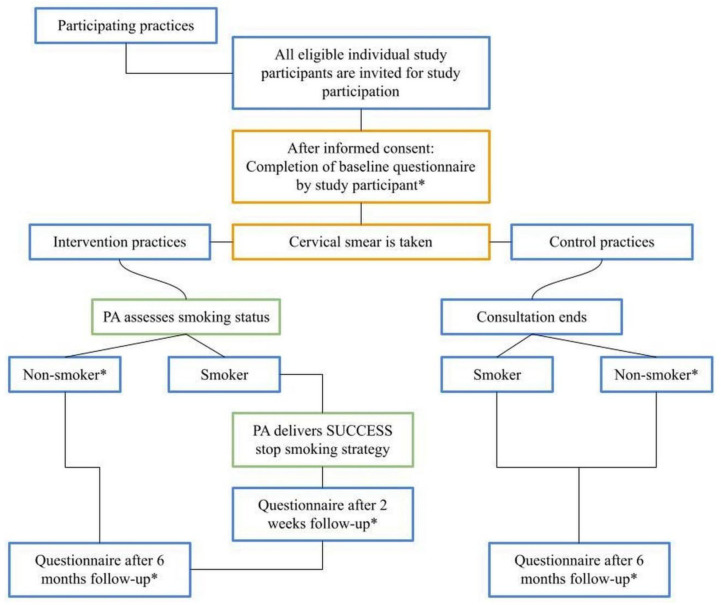
Smoking status is identified at baseline via the baseline questionnaire T1 (see figure 1 for a schematic overview of the effect study).
Figure 1.
Flow chart for study participants of the SUCCESS study. Practice(s) = participating general practice(s). *Baseline questionnaire for all study participants from both treatment arms (intervention and control practices) = Questionnaire T1; Questionnaire at 2 weeks follow-up for female smokers participating in the intervention arm = Questionnaire T1B-S-I (T1B-smoker-intervention); Questionnaire at 6 months follow-up: for non-smokers in both treatment arms = Questionnaire T2-NS (T2-non-smokers), for smokers in the intervention arm = Questionnaire T2-S-I (T2-smoker-intervention), for smokers in the control arm = Questionnaire T2-S-C (T2-smoker-control). PA, practice assistant.
Eligibility criteria
Participants are eligible for the study when all of the following inclusion criteria are met:
Written informed consent for participation in a questionnaire study about lifestyle.
Participating in the national cervical cancer screening programme, that is, undergoing a cervical smear at a practice participating in the study, on invitation from the national cervical screening programme organisation.
Participants are ineligible for the study if the following exclusion criterion is met:
Undergoing a cervical smear at a general practice enrolled in the study, NOT on invitation from the national cervical screening programme organisation.
The indication for a cervical smear not for cervical screening does not consists of a preventive action for cervical cancer by the individual patient. This could influence the use of the teachable moment for the strategy and the risk perception of participants at baseline.
Recruitment
Recruitment and eligibility criteria for clusters
Practices will be recruited consecutively via invitation by letter and/or by phone. During recruitment, practices are informed that they have a 50% change of being randomised into the control condition.
To deal with possible selection bias, control practices are offered the opportunity to attend the training and receive material developed for intervention practices, after the follow-up period has ended.
Recruitment of individual study participants
PAs will be responsible for recruiting participants. In both study arms, PAs are briefed on the study logistics, on how to recruit participants and ask informed consent, using a comprehensible protocol.
All women attending cervical cancer screening in participating practices will be asked to participate in the study. The PA informs them that their general practice is participating in a questionnaire study about lifestyle in cancer screening participants and provides verbal and written information about the purpose of the study. Written consent is asked to participate in a study about lifestyle in cancer screening participants, to complete a questionnaire at baseline, and to approach the participant for a follow-up questionnaire.
Informed consent procedure
Participants in both study arms will be blinded to the true nature of the study. They are informed about the fact that a study about lifestyle is currently running, but not about its true nature and the content of the intervention under study. Alongside questions regarding smoking, questions about physical activity and alcohol consumption have been added to create a broader baseline lifestyle questionnaire (questionnaire T1).
Details in informed consent are omitted to: (1) minimise selection bias and (2) not affect participants’ behaviour before they receive the intervention; both of which could occur if the nature of the intervention is revealed which could subsequently alter the true effect of the intervention. For a more elaborate description of the informed consent procedure, see box 1. For an example of the consent form in English, translated from the original version in Dutch, see online supplemental material S3.
Box 1. Details on informed consent procedure adopted in the SUCCESS study.
Selection bias
Selection bias at the patient level can occur if the patient does not give informed consent for participation or data collection, especially if this differs between treatment groups. This is a known disadvantage of asking informed consent after randomisation.84 If information is given to all participants, the participants from the reference group will be alerted and thus the contrast between study groups will be affected.80
Consent as partial intervention
Recruitment and consent where the true nature of the study is revealed to participants before randomisation or participation would be a partial intervention in this study. According to previous literature a postponed consent procedure seems preferable if information given during the informed consent procedure could be a part of the intervention and affect the outcome parameters.80–82
Participant information or the content of a baseline questionnaire could influence participant cognitions, emotions and behaviour. This can lead to biased estimates of the intervention effect.83
Enhanced applicability
We have considered the option to inform participants in advance (before informed consent) that information is withheld, but this could cause unnecessary anxiety.85 To our opinion, this approach enhances the applicability of the study to daily practice.86Under certain circumstances, the application of postponed informed consent in study designs is admissible and not in conflict with the Dutch law Medical Research Involving Human Subject Act.80 87
bmjopen-2021-055812supp003.pdf (60KB, pdf)
After study completion, all participants will be informed about the real purpose of the SUCCESS study. Participants will also be informed that data on other health behaviours collected at baseline (alcohol, physical activity) will anonymously be published in an online repository.
Randomisation and treatment allocation
Randomisation takes place at the level of the practice in a 1:1 ratio, and will be performed at the Amsterdam UMC. Individual study participants receive the type of care assigned to the participating practice they visit. Practices will remain in their allocated arm during the complete follow-up period.
Blinding
Blinding the recruiter is not possible as the PA will be responsible for recruiting participants. The researchers are not involved in subsequent follow-up appointments and will be blinded to treatment allocation of individual study participants until data analysis is complete. It is not possible to blind the researchers to the treatment allocation of the practices. However, the researchers will be blinded to treatment allocation of practices in the analysis stage, an independent research assistant will remove identifying information and group labels from the dataset.
Withdrawal
Study participants can leave the study at any time without giving a reason if they wish to do so without any consequences. After withdrawal individual subjects will not be replaced.
Intervention and procedures
At intervention practices, the stop smoking strategy will be conducted among all women who visit the practice for cervical screening, after the cervical smear has been taken. The PA verbally assesses the smoking status with all women, and delivers the subsequent steps of the stop smoking strategy to all smokers. The strategy is based on the AAC method.40 41 This method is a deviaton from the Ask-Advise-Refer method and based on the 5 A’s behaviour change model.45 The strategy consists of: (1) Ask about smoking status, (2) Provide brief stop smoking advice, and (3) Actively offer an appointment for support given by the nurse or GP. When providing brief stop smoking advice, the PA creates a teachable moment between the reason for the visit (namely, cervical cancer screening) and the health behaviour (namely, smoking).15 46 For a detailed description of the stop smoking strategy and the procedure, see box 2.
Box 2. Details of the stop smoking strategy and procedure following the Ask-Advise-Connect method.
1. Ask—Ask about smoking status
First, the PA asks smokers permission to talk about smoking and stopping smoking. If the smoker does consent, the PA provides brief stop smoking advice. If the smoker does not consent, the PA asks permission to address the subject another time (by the PA, the nurse, or the GP).
2. Advise—Provide brief stop smoking advice
The PA explains why (s)he asks about smoking after the smear and creates a teachable moment between the reason for the woman’s visit, for example, the cervical smear that aims at the prevention of cancer via early detection, and the woman’s own health behaviour, for example, smoking. Information is provided about the role of smoking in the development of cancer, but also on other tobacco related diseases. More specifically, an explanation is provided on the relationship between smoking and the development of cervical abnormalities and cervical cancer. Subsequently, The PA asks how the smoker thinks about smoking cessation. For this purpose the PA can ask two additional questions using a 0–10 scale: ‘How important is stopping smoking to you?’ and ‘How confident are you that you will succeed?’.
The PA uses a leaflet to illustrate the advice and explanation. This leaflet summarises the stop smoking advice in three key messages: (1) Stopping smoking is the most important you can do to lower your chance of developing cancer, (2) Your GP advices you to stop smoking, (3) Support is available to you for stopping smoking via your GP. Furthermore, the leaflet contains brief information on the impact of smoking on (cervical) cancer development and general health. An infographic illustrates the relationship between smoking and cancer development. A Dutch adaption from the infographic from Cancer Research UK4 88 is used. Also, information is provided on the advantages of stopping smoking and when to contact the GP for support. The provided information is based on existing literature and guidelines.10 23 40 Links are included to several stop smoking applications, to a patient information website developed by the Dutch Counsel for general practitioners (GPs) containing information about smoking and smoking cessation (thuisarts.nl) and to an information website on smoking cessation and support, developed by the Netherlands Institute for Mental Health and Addiction (Trimbos institute) (ikstopnu.nl, which also contains a quit line).
3. Connect—Actively offer an appointment for support given by the nurse or GP
Lastly, smokers are offered an appointment with a practice nurse or their GP for stop smoking counselling. All smokers are offered an appointment, independent of their motivation to quit smoking. The organisation of stop smoking counselling differs per practice. If smokers want an appointment, the PA arranges this according to within-practice agreements.
Smokers who have agreed to an appointment with the practice nurse or GP and subsequently follow a smoking cessation programme, do so according to guidelines for smoking cessation care in Dutch general practice.10 Smokers receive the type of counselling provided by their general practice. The researchers are not involved in the communication and content of these appointments.
Procedure
In intervention practice, the PA verbally assesses the smoking status of every woman visiting the practice for the smear for cervical cancer screening (Ask). This includes both women who did and who did not consent to participate in the SUCCESS study. If it concerns a smoker and the woman consents to talk about her smoking behaviour, the PA delivers the other steps of the strategy (Advise, Connect). This approach was chosen for two reasons: (1) it is not ethical to withhold stop smoking advice to women smokers only because they do not want to participate in the SUCCESS study, because addressing stop smoking behaviour falls under standard care for Dutch general practice10, (2) verbal assessment of the smoking status with every woman visiting the practice for cervical cancer screening is expected to facilitate the procedure for the PA.
Training PAs
PAs from intervention practices receive a one-time 2 hour training before starting the delivery of the stop smoking strategy in their practice. The training was developed using results from qualitative studies conducted among female smokers and involved staff,44 previous literature37 39 and in collaboration with experienced trainers. See table 1 for an overview of the training’s content. A certified trainer provides the training. PAs receive accreditation for participation in the study.
Table 1.
Overview of training for practice assistants (PAs)
| Step | Goal | Description | Duration |
| 1 | Enhance Knowledge | Background information on: smoking, impact of smoking on health and (cervical) cancer development, the aim and set-up of the SUCCESS study. | 20 min |
| 2 | Explore views and beliefs | An open group discussion of statements on smokers and smoking for the PAs to become aware of their own views and beliefs. | 15 min |
| 3 | Insight into motivational skills | A group exercise on motivation, to stimulate the PAs to think about what they (could) do or use when motivating others in daily life or during working routine. | 20 min |
| 4 | Practising interviewing technique based on motivational interviewing | An exercise for interviewing techniques on how to provide advice and support according to the motivational interviewing principles. For this purpose PAs practice in couples, using two contrasting coaching roles. One coaching role adopts a proactive approach from the PAs perspective aiming to convince the client what’s best for him/her, whereas in the other role the coach focuses on the client’s perspective and why he/she wants to change behaviour, asking questions instead of providing solutions. Each round, one PA acts as the coach and the other PA as a client who wants to change his/her lifestyle using something he/she is ambivalent about in daily life. Afterwards, experiences from the exercise are discussed in plenary. |
35 min |
| 5 | Practising the SUCCESS study stop smoking strategy | Practising with providing actual stopping smoking advice after the smear, based on the Ask-Advise-Connect method, going through example sentences developed for PAs to use when delivering the stop smoking strategy, and using the leaflet for smokers. Attention is paid to a clear provision of stop smoking advice, but with use of a respectful and inviting tone, and a non-judgemental attitude. | 20 min |
Control group
At control practices, the PA delivers the cervical smear. The smoking status is not assessed by the PA, but is assessed in the baseline questionnaire for comparison with the intervention group. The PA does not look at the questionnaire responses. No intervention is applied. Despite the fact that the stop smoking strategy is not applied in control practices, PAs can provide stop smoking advice or support during any other contact with these smokers as this is part of standard care in general practice.10
Data collection
In both study arms all participants who have given written consent are presented with a baseline questionnaire (T1), which consists of 10 questions.
Smokers from the intervention arm receive a first follow-up questionnaire 2 weeks after baseline (T1B-S-I), consisting of 37 questions for the PE. Smokers from both intervention and control arms receive a follow-up questionnaire 6 months after baseline respectively containing 44 questions (T2-S-I) and 42 questions (T2-S-C) assessing the primary, secondary and other outcomes measures. The T1B-S-I, T2-S-I and T2-S-C questionnaires (also) contain measurements for the PE, see the PE methods section.
A proportion (10%, randomly selected) of non-smokers from both study arms receive a follow-up questionnaire 6 months after baseline (T2-NS). The follow-up of non-smokers falls under the PE. For the outcomes measured in non-smokers, see the PE methods section. For an overview of the above, see figure 1.
Follow-up questionnaires are sent by research assistants via secured email or by mail. Non-responders identified as smoker at baseline receive a reminder after 2 weeks. In case of no reply, participants are contacted by telephone with an interval of 2 weeks. A short version of the T1B-S-I, T2-S-I and T2-S-C questionnaires (covering the primary and secondary outcomes and several questions as part of the PE, see tables 2–4) is offered to non-responding participants who have too little time or difficulties completing the whole questionnaire. Due to restricted availability of research assistants participants identified as non-smokers at baseline, receive a reminder via email or mail in case of non-response.
Table 2.
Details of follow-up effect study
| Description | Questionnaire T1* | Questionnaire T1B-S-I * |
Questionnaire T2-S-I and T2-S-C* |
|
| Baseline | Additional | Endpoint | ||
| Time | 0 | 2 weeks | 6 months | |
| General measurements | ||||
| Physical activity† | X | |||
| Alcohol consumption | X | |||
| Intention to change lifestyle within 6 months | X | |||
| Date of birth | X | |||
| Socioeconomic status‡ | X | |||
| Ethnic background | X | |||
| Children | X | |||
| Comorbidity | X | |||
| Marital status | X | |||
| Smoking related measurements | ||||
| Smoking status | X | X‡‡ | ||
| Heaviness of Smoking Index (HSI)§ | No of cigarettes (and/or cigars) per day | X | X‡‡ | |
| Time to first smoking in the morning | X | X‡‡ | ||
| E-cigarette use | X | X‡‡ | ||
| History of previous quit attempt | Number of quit attempts (>24 hours) in the past 6 months | X | ||
| Last quit attempt (<6 months, 6–12 months, >12 months ago) | X | |||
| Motivation to stop scale (MTSS)¶ | X | X‡‡ | ||
| Age starting smoking | X | |||
| Performing a serious quit attempt since baseline** | X‡‡ | |||
| Incidence of self-reported quit attempts since baseline | X‡‡ | |||
| 7-day point prevalence abstinence | X‡‡ | |||
| 24-hour point prevalence abstinence | X‡‡ | |||
| 6 months continuous abstinence | X‡‡ | |||
| Relapse(s) since quit attempt | X‡‡ | |||
| Reasons for performed quit attempt, reasons for relapse | X | |||
| Smoking status of partner | X | |||
| Constructs related to smoking | ||||
| Self-efficacy | X | |||
| Smoker and non-smoker identity | X | |||
| Perceived risk | X | |||
| Health perception†† | X | |||
Questionnaire T1: baseline questionnaire for all study participants from both treatment arms (intervention and control practices); Questionnaire T1B-S-I (T1B-smoker-intervention): questionnaire at 2 weeks follow-up for female smokers participating in the intervention arm. Questionnaire T2-S-I (T2-smoker-intervention) and Questionnaire T2-S-C (T2-smoker-control): questionnaire at 6 months follow-up, respectively, for smokers in the intervention arm and smokers in the control arm.
*Questionnaire items are self-reported measures.
†Assessed according to the Dutch Guideline for Healthy Exercise (Nederlandse Richtlijn Gezond Bewegen).
‡Educational level.
§HSI combined score: 0–2 low addiction, 3–4 moderate addiction, 5–6 high addiction (number of cigarettes per day: A. 10 or fewer (0 points) B. 11–20 (one point) C. 21–30 (two points) D. 31 or more (three points); Time to first smoking in the morning: A. Within 5 min (three points) B. 6–30 min (two points) C. 31–60 min (one point) D. After 60 min (0 points)).
¶MTSS score, answer to question ‘Which of the following describes you?’: 1. I don’t want to stop smoking, 2. I think I should stop smoking but don’t really want to, 3. I want to stop smoking but haven’t thought about when, 4. I really want to stop smoking but I don’t know when I will, 5. I want to stop smoking and hope to soon, 6. I really want to stop smoking and intend to in the next 3 months, 7. I really want to stop smoking and intend to in the next month.
**As a dichotomous outcome.
††Assessed with the General health question from the SF-12 Health Survey.
‡‡Measurements included in the short version of the T2-S-I and T2-S-C questionnaire.
Table 3.
Overview of process evaluation measurements by use of RE-AIM and CFIR
| RE-AIM | |||
| Outcome per domain | Population | Quantitative measures | Qualitative inquiry |
| Reach | |||
| Proportion of target population participating in the intervention | Potential target population |
|
|
| Characteristics of patients lost to follow-up | Study participants |
|
NA |
| Effectiveness | |||
| Impact of the intervention | Study participants |
|
|
| How different subpopulations responded to the intervention | Study participants |
|
|
| Adoption | |||
|
Proportion of practices that will adopt the intervention (eg, intention to use the intervention) |
Involved staff |
|
|
| The characteristics of the (non)participants | Involved staff |
|
|
| Implementation | |||
| To what extent the intervention is implemented as intended in practices | Involved staff, Study participants |
|
|
| Maintenance | |||
| To what extent the programme is sustained over time | Involved staff, Study participants |
|
|
| CFIR | |||
| Outcome per domain | Population | Qualitative inquiry | |
| Intervention characteristics | |||
| Intervention source and Evidence strength and quality and Relative advantage | Involved staff, Study participants |
|
|
| Adaptability | Involved staff, Study participants |
|
|
| Complexity: complexity to implement the SUCCESS study intervention in practice | Involved staff, Study participants |
|
|
| Design quality and packaging | Involved staff |
|
|
| Outer setting | |||
| Patient needs & resources | Involved staff, Study participants |
|
|
| Inner setting | |||
| Structural characteristics | Involved staff, Study participants |
|
|
| Networks and communications | Involved staff, Study participants |
|
|
| Culture | Involved staff |
|
|
| Implementation climate | Involved staff |
|
|
| Readiness for implementation | Involved staff |
|
|
| Characteristics of individuals | |||
| Knowledge and Beliefs about the Intervention | Involved staff, Study participants |
|
|
| Self-efficacy | Involved staff, Study participants |
|
|
| Individual stage of change | Involved staff, Study participants |
|
|
| Other personal attributes | Involved staff |
|
|
| Process | |||
| Planning | Involved staff |
|
|
| Engaging | Involved staff |
|
|
| Executing+reflecting and evaluating | Involved staff |
|
|
Questionnaire T1B-S-I (T1B-smoker-intervention): questionnaire at 2 weeks follow-up for female smokers participating in the intervention arm.
A-A-C, Ask-Advise-Connect; CFIR, Consolidated Framework for Implementation Research; GP, general practitioner; NA, not applicable; PA, practice assistant; RE-AIM, Reach, Effectiveness, Adoption, Implementation, Maintenance.
Table 4.
Quantitative measurements in smokers for the process evaluation
| Description | Questionnaire T1B-S-I* |
Questionnaire T2-S-I* |
Questionnaire T2-S-C* |
Questionnaire T2-NS* |
| Additional to baseline | Endpoint | Endpoint | Endpoint | |
| Time | 2 weeks | 6 months | 6 months | 6 months |
| Exposure | ||||
| Exposure to the stop smoking strategy, steps delivered | X§ | X | ||
| Exposure to smoking cessation advice (not after the smear) | X | X | ||
| Style of counselling received | X | X | ||
| Use of smoking cessation support | X¶ | X¶ | ||
| Use of medication for smoking cessation | X¶ | X¶ | ||
| Acceptability | ||||
| Acceptability of stop smoking advice after the smear | X* | X | ||
| Satisfaction with stop smoking counselling | X | X | ||
| Intention to consult general practice for support for a future quit attempt | X | X | ||
| Willingness to participate in cervical cancer screening on the next invitation by the national cervical cancer screening programme | X¶ | X¶ | X | |
| Willingness to receive advice about a healthy lifestyle after cervical cancer screening | X | X | X | |
| Willingness to receive advice about a healthy lifestyle after cervical cancer screening if the smear results necessitate further diagnostics. | X | X | X | |
| Willingness to participate in cervical cancer screening on the next invitation by the national cervical cancer screening programme, if healthy lifestyle advice would be a routine part of the consultation. | X | X | X | |
| Willingness to receive advice about a healthy lifestyle after cervical cancer screening about: healthy dietary intake/healthy weight/physical activity/smoking cessation/alcohol consumption. | X | X | X | |
| Constructs related to smoking | ||||
| Self-efficacy | X | |||
| Smoker and non-smoker identity | X | |||
| Locus of control | X | |||
| Goal ownership | X | |||
| Social support | X | |||
| Perceived risk | X | |||
| Health perception† | X | X | ||
| Psychological health‡ | X | |||
| Other | ||||
| Cervical smear test results from smear taken at baseline | X¶ | X¶ | X | |
| Reasons to participate in cervical cancer screening | X | X | X | |
| Lifestyle changes past 6 months | X | X | X | |
| Perception whether it is the general practice’s task to provide advice or support for a healthy lifestyle | X | X | X | |
| Comorbidity | X | |||
Questionnaire T1B-S-I (T1B-smoker-intervention): questionnaire at 2 weeks follow-up for female smokers participating in the intervention arm. Questionnaire T2-S-I (T2-smoker-intervention) and Questionnaire T2-S-C (T2-smoker-control): questionnaire at 6 months follow-up, respectively for smokers in the intervention arm and smokers in the control arm. Questionnaire T2-NS (T2-non-smokers): questionnaire at 6 months follow-up for non-smokers in both treatment arms.
*Questionnaire items are self-reported measures.
†Assessed with the General health question from the SF-12 Health Survey.
‡Assessed with the General Health Questionnaire.
§measurements included in the short version of the T1B-S-I questionnaire.
¶measurements included in the short version of the T2-S-I and T2-S-C questionnaire.
Outcomes and measurements
The primary, secondary and other outcomes are all self-reported outcomes. For details on all measurements in smokers for the effect study, including baseline, see table 2.
Primary and secondary outcomes
Both primary and secondary outcomes will be assessed 6 months after the intervention (baseline), following recommendations to assess smoking related outcomes 6 months after participants received the intervention.38 The primary outcome will be: performing a serious quit attempt, assessed as a dichotomous outcome.38 47 Secondary outcomes are: 7-day point prevalence abstinence (PPA), reduction in the number of cigarettes per day (measured as percentage in reduction and treated as a continuous outcome) and transition in motivation to quit smoking (motivation to stop scale) (coded as a binary outcome for improvement in motivational stage).38 48 49
Biochemical validation will not be used. The SUCCESS stop smoking strategy is a low demand intervention with one short face-to-face contact with the PA. Previous low demand smoking cessation trials reported little discrepancies between self-reported and biochemically validated abstinence,49–52 in these type of studies biochemical validation is not recommended.50 51 PPA and 6-month continuous abstinence fall under secondary and other outcomes, expected numbers are relatively low and do not outweigh the possible lack of acceptance50 and risk of selection bias that would arise if women are informed about a biochemical validation test before giving informed consent.
Other outcomes
Other outcomes, measured 6 months from baseline, in all participating female smokers are: (1) General measurements: children, comorbidity, marital status,38 (2) Smoking related measurements: Heaviness of Smoking Index (HSI), 24-hour PPA, 6 months continuous abstinence, relapse(s) since quit attempt, e-cigarette use, smoking status of partner38 53; (3) Constructs related to smoking: self-efficacy, smoker and non-smoker identity, perceived risk, health perception.38 54–56
Sample size
The sample size calculation has been adjusted for the design effect of clustered trials.57 58 The expected total number of participants of the national cervical cancer screening programme in the Netherlands for 2017 was 485.000. In 2017, there were 5068 general practices in the Netherlands. We estimated that each practice, on average, takes 96 women for the cervical cancer screening programme per year. The prevalence of smokers in Dutch women aged 30–60 years was 21.9% based on available data in 2016,59 on the basis of which we estimated 21 female smokers visit their GP for the cervical cancer screening programme each year. We expect circa 50% of the eligible population to participate in our study. Hence, we estimate it should be feasible to include 10 cancer screening participants who smoke per participating practice per year.
Among female smokers aged 30–60 years 34% performed a serious quit attempt in 2016.60 We aim for an absolute increase to 44% of smokers who performed a serious quit attempt after 6 months of follow-up, thus a 10% difference between the intervention and control arm, to obtain a clinically relevant effect. We assume an intracorrelation coefficient of 0.0130 based on previous research of smoking cessation interventions in general practice.37 61 This results in sample sizes of 330 in group one (intervention) and 330 in group two (control), which will be obtained by sampling 33 clusters with 10 subjects in both groups, to achieve 80% power to detect a difference between the group proportions of 0.1000. The group two proportion is 0.3400. The group one proportion is assumed to be 0.3400 under the null hypothesis and 0.4400 under the alternative hypothesis. The test statistic used is the one-sided Score test (Farrington and Manning), with a significance level of 0.0500.62 A one-sided test was chosen, because it is not likely that the strategy will cause fewer quit attempts.
Data management
Study data will be collected using electronic case report forms (Castor EDC) using audit trail and will be securely stored and regularly backed-up.
Analysis
For the main trial analysis will be performed in accordance with the intention to treat principle.38 49 To account for possible dependencies within the clusters a mixed logistic model will be used to analyse the effect of the stop smoking strategy on the percentage of smokers who performed a serious quit attempt over the past 6 months (primary outcome). The effect of the intervention on secondary outcomes will be analysed similarly. A one-sided p<0.05 will be considered to be statistically significant. Furthermore, in all appropriate cases 95% CIs will be given.
Methods for the PE
PE: study design and participants
For the PE, a mixed-methods sequential explanatory design is used.63 The PE runs in parallel to the effect study and addresses the following questions:
What are the reach, effectiveness, adoption, implementation and maintenance of the stop smoking strategy according to study participants and involved staff?
What barriers and facilitators influence the reach, effectiveness and implementation of the stop smoking strategy according to study participants and involved staff?
How do these barriers and facilitators explain differences in reach, effectiveness and implementation between practices?
First, quantitative data will be collected on the basis of the RE-AIM framework.64 65 In our PE, we use the RE-AIM constructs as follows: the reach and effectiveness centre around the study participants (eg, female smokers). The adoption, implementation and maintenance mainly centre around the delivery of the stop smoking strategy by the involved staff. For example: using qualitative data collected via questionnaires we will explore what the exposure to and acceptability of the strategy was among female smokers overall and in each practice (Reach), how many women started a quit attempt (Effectiveness), and to what extent involved staff started providing the stop smoking strategy (Adoption), delivered the strategy as intended (Implementation) and intend to proceed with the strategy after the trial has ended (Maintenance).
Subsequently, qualitative data will be collected to elaborate on the quantitative findings. For this purpose the Consolidated Framework for Implementation Research (CFIR)66 will be used, as a postimplementation evaluation approach that allows for a more elaborate evaluation of the RE-AIM domains ‘reach’, ‘effectiveness’ and ‘implementation’.66 67 CFIR consists of constructs associated with effective implementation. Constructs are categorised into five domains, such as ‘Intervention Characteristics’ (for example: the advantage of the strategy vs another or no strategy, the complexity of the intervention from the perspective of involved staff).
See table 3 for an overview of outcomes addressed with RE-AIM and CFIR frameworks.
As part of the research questions defined above, we aim to explore what active components were of the stop smoking strategy and the context that stimulated female smokers to perform a quit attempt. For this purpose we additionally use constructs of the Health Belief Model (HBM), this model has previously been used to study health behaviour in cancer screening participants.68
PE: measurements and data collection
Quantitative data
For the PE’s quantitative measurements in study participants and involved staff, see, respectively, tables 4 and 5. These measurements are all self-reported. The questionnaires for smokers at 2 weeks follow-up (T1B-S-I) and at 6 months follow-up (T2-S-I and T2-S-C), and the questionnaire for non-smokers at 6 months follow-up (T2-NS), contain questions to address exposure to the strategy and stop smoking advice or support (exposure will also be used as a quality check of the delivery of the strategy by PAs, also see under Reach in table 3), acceptability and several other constructs.38 54–56 69–73 Timing at 2 weeks follow-up was chosen to minimise recall bias of exposure to and acceptability of the strategy. The other measurements for the PE take place at 6 months follow-up, as they are linked to the measurements for the primary and secondary outcomes of the effect study.
Table 5.
Quantitative measurements in involved staff for the process evaluation
| Description | Questionnaire HCP_I_T1* | Questionnaire PP_I* |
Questionnaire HCP_I_T2* |
Questionnaire HCP_C_T2* |
| Before study commencement | Before study commencement | After study completion | After study completion | |
| Time | 0 | 0 | ±6–18 months | ±6–18 months |
| Practice characteristics | ||||
| Practice type and size | X | X | ||
| Type of EPR used | X | X | ||
| PA sociodemographics | ||||
| Age | X | X | ||
| Working experience | X | X | ||
| Smoking status | X | X | ||
| PA SC activities | ||||
| Moments used for SC activities (ask, advise, refer/connect) | X | X | ||
| Delivery of SC activities on a regular basis | X | X | X | |
| Within practice organisation SCC | ||||
| How is SCC organised | X | X | X | X |
| Who delivers SCC and which steps | X | X | ||
| Division of tasks and responsibilities for SCC | X | |||
| Type of SC support available | X | |||
| Availability of nurse/GP | X | |||
| Number of PAs performing smears | X | |||
| Number of PAs involved in intervention | X | |||
| Available time per smear | X | X | ||
| Number of smears per year | X | X | ||
| PA attitudes and experiences | ||||
| Expectations towards the SUCCESS strategy (including its effect, impact on patient interaction etc.) | X | X | X | X |
| Perceived role in SCC | X | X | X | |
| Self-efficacy | X | X | X | |
| Attitude towards smokers and SCC | X | X | X | |
| Risk perception | X | X | X | |
| Acceptability of the SUCCESS strategy | X | |||
| Feasibility of the SUCCESS strategy | X | |||
| Motivation to continue SC activities after trial (after routine cervical screening) | X | |||
| Intention to provide SC advice after routine cervical smear in future | X | |||
Questionnaire HCP_I_T1=baseline questionnaire for healthcare professionals from intervention practices; Questionnaire PP_I=practice plan for intervention practices; Questionnaire HCP_I_T2=follow up questionnaire for healthcare professionals from intervention practices, sent after study completion by the practice; Questionnaire HCP_C_T2=questionnaire for healthcare professionals from control practices, sent after study completion by the practice.
*Questionnaire items are self-reported measures.
EPR, electronic patient record; GP, general practitioner; PA, practice assistant; SC, smoking cessation; SCC, smoking cessation care.
Involved staff from practices in the intervention group complete a practice plan and baseline questionnaire before rolling out the strategy in their practice. A short follow-up questionnaire is sent to staff from intervention practices after study completion. Involved staff from practices in the control group receive a questionnaire after study completion. The purpose is to identify determinants at the practice level for stop smoking effectiveness (such as: practice demographics, PA attitudes and experiences with the strategy) and assess acceptability and feasibility. Measurements were selected based on previous literature.37 38 64 66
Qualitative data
A subset of study participants and involved staff will be invited to participate in an in-depth interview, after they have, respectively, completed follow-up and completed inclusion of study participants at their practice. Interview guides will be developed based on the CFIR. For the interviews with smokers interview guides will also be using HBM constructs (such as: perceived threat, susceptibility to illness, health motivation, perceived control or barriers).64–66 68 74
Semi structured in-depth interviews will be conducted with involved staff from 10 to 15 intervention practices. Practices will be sampled based on their effectiveness and delivery of the strategy, to obtain variation of these criteria.
Semi structured in-depth interviews will be conducted with 15–20 female smokers from interviewed intervention practices. Additionally we aim to invite a few female smokers from control practices. Purposive sampling strategy will be used to obtain a heterogeneous sample for the following criteria: age, educational level, HSI, performing a quit attempt, receiving stop smoking support after the smear, acceptability (reported in questionnaires), HPV status.
Interviews will either be conducted face-to-face, via telephone or videoconference, at the participant’s home or at the practice, depending on participants’ preference. Interviews will be audio recorded.
PE: analysis
Quantitative data
Descriptive statistics will be used for the quantitative PE measurements from questionnaires completed by involved staff and female smokers. Means, medians and proportions will be generated, overall and per practice. Between group comparisons and subgroup analysis will be conducted for intervention and control participants. Analysis will be performed with SPSS version 28.0.0.
Qualitative data
Audiorecordings will be transcribed verbatim, reviewed for accuracy, and imported into MAXQDA V.12 for analysis. For the interviews held with involved staff and female smokers an iterative process of data collection and data analysis will be adopted, in which thematic analysis is used. Both inductive and deductive coding will be used. The Framework Method will allow for a comprehensive use of thematic analysis: the coding tree will be charted into a matrix, enabling further data analysis and generation of themes.75 Two researchers will code the transcripts independently, three researcher will read all transcripts, discuss codes, categories and themes in detail until consensus is reached. Study results will be reported in accordance with the 32-item checklist of Consolidated Criteria for Reporting Qualitative Research.76
Combining quantitative and qualitative data
Quantitative and qualitative data will be analysed separately and will subsequently be integrated. Practices will be assigned into two groups based on their delivery of the intervention. First, the questionnaire data will be analysed, comparing the results of the two groups of practices. For the qualitative analysis of the in-depth interviews the two groups of practices will be compared as well. Subsequently, the quantitative and qualitative results will be integrated to draw final conclusions on the differences between the two groups and answer our research questions. The same approach is used for female smokers, comparing two groups of female smokers who did and who did not perform a quit attempt at 6 months follow-up.
Ethics and dissemination
The Dutch Ministry of Health, Welfare and Sport approved of the trial after an advisory report from the Health Council (Nr. 2018/17).77 A licence was provided to conduct the study under the Population Screening Act. Prior to the Health Council report, the study protocol was approved by the Medical Ethics Committee of the Academic Medical Centre, Amsterdam University Medical Centres, the Netherlands (2017_263). Findings will be disseminated by publication in peer-reviewed journals and scientific conference presentations, as well as in the media and to relevant patient organisations and authorities such as the organisation for the national cervical cancer screening programme and the association for Dutch PA.
Trial status
Recruitment of study participants started in September 2018 and is on-going. As of July 2021 70% of the intended 660 female smoker participants for the effect study have been included. Because of the COVID-19 pandemic, the Dutch national cervical cancer screening programme was temporarily stopped from March to July 2020, therefore recruitment of study participants was not possible during this period. Due to the adopted informed consent procedure, we chose to submit the study protocol at the end of recruitment of individual study participants in order to prevent information on the content of the intervention and trial design to be (publicly) available during recruitment and delivery of the intervention. Participant inclusion will end on 1 October 2021. We expect the collection of follow-up data for the effect study and PE to be complete by March 2022.
Discussion
Cervical cancer screening could serve as a routine moment to provide female smokers with brief stop smoking advice in the general practice setting. Three previous trials studied smoking cessation in cervical cancer screening participants.30 31 78 McBride et al found no difference in cessation rates after providing women with a self-help cessation kit after the smear test.78 A possible explanation could be that there was no interaction between caregiver and patient.15 16 46 The cluster RCT by Hall et al showed that a smoking cessation intervention delivered after the cervical smear is potentially effective on motivation to stop smoking, without deterring women from future screening participation.30 However, the intervention in this study was delivered by trained practice nurses, and not by PAs. Also, the intention for future screening participation was not compared with non-smokers or the general population.30 The most recent trial by Gorini et al used cervical cancer screening as an occasion for smoking cessation and physical activity counselling. Enhanced smoking cessation rates were observed in women in the preparation stage of the intervention arm.31 The intervention was delivered by midwifes, did not take place in the general practice setting and the study did not include a PE.31
Our trial takes place in general practice, the stop smoking strategy is delivered by PAs and includes an extensive PE. Furthermore, with the adoption of a strategy based on the AAC method,40 smokers are actively connected to follow-up within their practice. With complex interventions, it is important not to only address if an intervention is effective, but to get insight into what factors influence it’s delivery and implementation.33 79 These factors can exist at the level of the female smoker, the PA or factors at the practice level.64–66 Also, for this specific approach, it is important to take into consideration that the smear remains a delicate moment and it is important not to affect screening participation rates. For this purpose, we conduct the PE among involved participants and staff with the use of the Reach, Effectiveness, Adoption, Implementation and Maintenance (RE-AIM) and CFIR frameworks.64–66
This study design has several limitations. First, to avoid unbalanced participation (ie, to include smokers regardless of their motivation to stop smoking and heaviness of smoking) eligible participants are not informed about the true nature of the study at baseline. They will receive information about its true nature after the study. Second, smokers from the intervention group receive an additional questionnaire at 2 weeks follow-up to measure the exposure to and acceptability of the different steps of the stop smoking strategy as delivered by the PA (Questionnaire T1B-S-I). It may be that this questionnaire interferes with the strategy’s effect on the primary outcome (performing a quit attempt 6 months after baseline). To address this issue, in the data analysis phase participants who did complete this questionnaire will be compared with those who did not. Also, in the in-depth interviews with female smokers for the PE we will explore what effect Questionnaire T1B-S-I had on the performance of a quit attempt. Lastly, all outcomes measures are self-reported. For the primary outcome no biochemical validation will be used. However, as the strategy is a low demand intervention,50 51 and selection bias could occur if women are informed about a biochemical validation test before giving informed consent.80–83
Supplementary Material
Acknowledgments
We wish to thank the female smokers and healthcare professionals who participated in two previous qualitative studies that were used for this study’s design. We also wish to thank the Medical Ethics Committee from the Amsterdam University Medical Centres for their useful comments, the Health Council for reviewing and approving of our study proposal and the Ministry of Health, Welfare and Sport for the assignment of a licence to conduct the study under the Population Screening Act.
Footnotes
Contributors: MBLM, MC, ES, HCPMvW, NC and KMvA contributed to the conceptualisation and study design. MBLM and ES are responsible for day-to-day running of the trial and data collection. MBLM will undertake the analysis of quantitative data. MBLM, MC and KMvA will analyse the quantitative data. The first draft was written by MBLM. MBLM, MC, ES, HCPMvW, NC and KMvA reviewed and contributed to the article and approved the final manuscript.
Funding: This project was funded by a grant from the Dutch Cancer Society (UVA 2015-7853).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N Engl J Med 2014;370:60–8. 10.1056/NEJMra1308383 [DOI] [PubMed] [Google Scholar]
- 2.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med 2013;368:341–50. 10.1056/NEJMsa1211128 [DOI] [PubMed] [Google Scholar]
- 3.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst 2004;96:99–106. 10.1093/jnci/djh014 [DOI] [PubMed] [Google Scholar]
- 4.Brown KF, Rumgay H, Dunlop C, et al. The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015. Br J Cancer 2018;118:1130–41. 10.1038/s41416-018-0029-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanting C, de Vroome E, Elias S. De bijdrage van leefstijlfactoren AAN de incidentie van en de sterfte AAN kanker in Nederland: TNO; TNO-rapport, 2014. Available: https://www.kwf.nl/sites/default/files/2019-09/eindrapport-paf-studie.pdf
- 6.Jha P. Avoidable global cancer deaths and total deaths from smoking. Nat Rev Cancer 2009;9:655–64. 10.1038/nrc2703 [DOI] [PubMed] [Google Scholar]
- 7.Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013;381:133–41. 10.1016/S0140-6736(12)61720-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stead LF, Buitrago D, Preciado N. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013;31:CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice VH, Heath L, Livingstone-Banks J, et al. Nursing interventions for smoking cessation. Cochrane Database Syst Rev 2017;12:CD001188. 10.1002/14651858.CD001188.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chavannes NH, Meijer E, Wind LA. Herziene richtlijn ‘Behandeling van tabaksverslaving en stoppen met roken ondersteuning’. Ned Tijdschr Geneeskd 2017;161:D1394. [PubMed] [Google Scholar]
- 11.Borland R, Li L, Driezen P, et al. Cessation assistance reported by smokers in 15 countries participating in the International tobacco control (ITC) policy evaluation surveys. Addiction 2012;107:197–205. 10.1111/j.1360-0443.2011.03636.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stead M, Angus K, Holme I, et al. Factors influencing European GPs' engagement in smoking cessation: a multi-country literature review. Br J Gen Pract 2009;59:682–90. 10.3399/bjgp09X454007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleinjan MBJ, Verdurmen J, van Laar M. Facsheet Het bespreken van (stoppen met) roken door de huisarts en andere zorgverleners (tandarts, medisch specialisten en verloskundigen). Trimbos-instituut 2016. [Google Scholar]
- 14.Kotz D, Willemsen MC, Brown J, et al. Light smokers are less likely to receive advice to quit from their GP than moderate-to-heavy smokers: a comparison of national survey data from the Netherlands and England. Eur J Gen Pract 2013;19:99–105. 10.3109/13814788.2013.766792 [DOI] [PubMed] [Google Scholar]
- 15.Senore C, Giordano L, Bellisario C, et al. Population based cancer screening programmes as a teachable moment for primary prevention interventions. A review of the literature. Front Oncol 2012;2:45. 10.3389/fonc.2012.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emmons KM, Colditz GA. Realizing the potential of cancer prevention - the role of implementation science. N Engl J Med 2017;376:986–90. 10.1056/NEJMsb1609101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usher-Smith JA, Silarova B, Ward A, et al. Incorporating cancer risk information into general practice: a qualitative study using focus groups with health professionals. Br J Gen Pract 2017;67:e218–26. 10.3399/bjgp17X689401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gezondheidsenquete/Leefstijlmonitor [online], 2019. Available: https://www.rivm.nl/leefstijlmonitor/roken-onder-volwassenen
- 19.RIVM . Monitor bevolkingsonderzoek baarmoederhalskanker 2019, 2020. Available: file:///H:/Downloads/monitor-bevolkingsonderzoek-baarmoederhalskanker-2019%20(1).pdf
- 20.Castellsagué X, Muñoz N. Chapter 3: Cofactors in human papillomavirus carcinogenesis-role of parity, oral contraceptives, and tobacco smoking. J Natl Cancer Inst Monogr 2003;31:20–8. 10.1093/oxfordjournals.jncimonographs.a003477 [DOI] [PubMed] [Google Scholar]
- 21.Jensen KE, Schmiedel S, Frederiksen K, et al. Risk for cervical intraepithelial neoplasia grade 3 or worse in relation to smoking among women with persistent human papillomavirus infection. Cancer Epidemiol Biomarkers Prev 2012;21:1949–55. 10.1158/1055-9965.EPI-12-0663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Collaboration of Epidemiological Studies of Cervical Cancer, Appleby P, Beral V, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer 2006;118:1481–95. 10.1002/ijc.21493 [DOI] [PubMed] [Google Scholar]
- 23.Roura E, Castellsagué X, Pawlita M, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int J Cancer 2014;135:453–66. 10.1002/ijc.28666 [DOI] [PubMed] [Google Scholar]
- 24.Luhn P, Walker J, Schiffman M, et al. The role of co-factors in the progression from human papillomavirus infection to cervical cancer. Gynecol Oncol 2013;128:265–70. 10.1016/j.ygyno.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moktar A, Singh R, Vadhanam MV, et al. Cigarette smoke condensate-induced oxidative DNA damage and its removal in human cervical cancer cells. Int J Oncol 2011;39:941–7. 10.3892/ijo.2011.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campaner AB, Piato S, Galvão MAL, et al. Langerhans cells in cervical intraepithelial neoplasia related to smoking habits. J Low Genit Tract Dis 2006;10:223–8. 10.1097/01.lgt.0000225891.03613.f7 [DOI] [PubMed] [Google Scholar]
- 27.Holschneider CH, Baldwin RL, Tumber K, et al. The fragile histidine triad gene: a molecular link between cigarette smoking and cervical cancer. Clin Cancer Res 2005;11:5756–63. 10.1158/1078-0432.CCR-05-0234 [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Gu W, Zhang Y, et al. Nicotine promotes cervical carcinoma cell line HeLa migration and invasion by activating PI3k/Akt/NF-κB pathway in vitro. Exp Toxicol Pathol 2017;69:402–7. 10.1016/j.etp.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 29.Chatzistamatiou K, Moysiadis T, Vryzas D, et al. Cigarette smoking promotes infection of cervical cells by high-risk human papillomaviruses, but not subsequent E7 oncoprotein expression. Int J Mol Sci 2018;19:19020422. 10.3390/ijms19020422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall S, Reid E, Ukoumunne OC, et al. Brief smoking cessation advice from practice nurses during routine cervical smear tests appointments: a cluster randomised controlled trial assessing feasibility, acceptability and potential effectiveness. Br J Cancer 2007;96:1057–61. 10.1038/sj.bjc.6603684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorini G, Carreras G, Giordano L, et al. The Pap smear screening as an occasion for smoking cessation and physical activity counselling: effectiveness of the SPRINT randomized controlled trial. BMC Public Health 2012;12:740. 10.1186/1471-2458-12-740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell NC, Murray E, Darbyshire J, et al. Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455–9. 10.1136/bmj.39108.379965.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud 2013;50:587–92. 10.1016/j.ijnurstu.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 34.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 37.Verbiest MEA, Crone MR, Scharloo M, et al. One-hour training for general practitioners in reducing the implementation gap of smoking cessation care: a cluster-randomized controlled trial. Nicotine Tob Res 2014;16:1–10. 10.1093/ntr/ntt100 [DOI] [PubMed] [Google Scholar]
- 38.Mudde AN, Willemsen MC, Kremers S. Meetinstrument voor Onderzoek naar roken en stoppen Met roken. The Hague, the Netherlands: STIVORO voor een rookvrije toekomst, 2006. [Google Scholar]
- 39.Pieterse ME, Seydel ER, DeVries H, et al. Effectiveness of a minimal contact smoking cessation program for Dutch general practitioners: a randomized controlled trial. Prev Med 2001;32:182–90. 10.1006/pmed.2000.0791 [DOI] [PubMed] [Google Scholar]
- 40.Vidrine JI, Shete S, Cao Y, et al. Ask-advise-connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med 2013;173:458–64. 10.1001/jamainternmed.2013.3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vidrine JI, Shete S, Li Y, et al. The ask-advise-connect approach for smokers in a safety net healthcare system: a group-randomized trial. Am J Prev Med 2013;45:737–41. 10.1016/j.amepre.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 43.Ford I, Norrie J. Pragmatic trials. N Engl J Med 2016;375:454–63. 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 44.Mansour MB, Crone MR, van Weert HC, et al. Smoking cessation advice after cervical screening: a qualitative interview study of acceptability in Dutch primary care. Br J Gen Pract 2019;69:e15–23. 10.3399/bjgp18X700229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff . A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. public health service report. Am J Prev Med 2008;35:158–76. 10.1016/j.amepre.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res 2003;18:156–70. 10.1093/her/18.2.156 [DOI] [PubMed] [Google Scholar]
- 47.Hughes JR, Callas PW. Definition of a quit attempt: a replication test. Nicotine Tob Res 2010;12:1176–9. 10.1093/ntr/ntq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hummel K, Brown J, Willemsen MC, et al. External validation of the motivation to stop scale (MTSS): findings from the International tobacco control (ITC) Netherlands survey. Eur J Public Health 2017;27:129–34. 10.1093/eurpub/ckw105 [DOI] [PubMed] [Google Scholar]
- 49.West R, Hajek P, Stead L, et al. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299–303. 10.1111/j.1360-0443.2004.00995.x [DOI] [PubMed] [Google Scholar]
- 50.Velicer WF, Prochaska JO, Rossi JS, et al. Assessing outcome in smoking cessation studies. Psychol Bull 1992;111:23–41. 10.1037/0033-2909.111.1.23 [DOI] [PubMed] [Google Scholar]
- 51.SRNT Subcommittee on Biochemical Verification . Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4:149–59. 10.1080/14622200210123581 [DOI] [PubMed] [Google Scholar]
- 52.Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994;84:1086–93. 10.2105/AJPH.84.7.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borland R, Yong H-H, O'Connor RJ, et al. The reliability and predictive validity of the Heaviness of smoking index and its two components: findings from the International tobacco control four country study. Nicotine Tob Res 2010;12 Suppl:S45–50. 10.1093/ntr/ntq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shadel WG, Mermelstein R. Individual differences in self-concept among smokers attempting to quit: validation and predictive utility of measures of the smoker self-concept and abstainer self-concept. Ann Behav Med 1996;18:151–6. 10.1007/BF02883391 [DOI] [PubMed] [Google Scholar]
- 55.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–33. 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 56.Oncken C, McKee S, Krishnan-Sarin S, et al. Knowledge and perceived risk of smoking-related conditions: a survey of cigarette smokers. Prev Med 2005;40:779–84. 10.1016/j.ypmed.2004.09.024 [DOI] [PubMed] [Google Scholar]
- 57.Hewitt CE, Torgerson DJ, Miles JNV. Individual allocation had an advantage over cluster randomization in statistical efficiency in some circumstances. J Clin Epidemiol 2008;61:1004–8. 10.1016/j.jclinepi.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 58.Puffer S, Torgerson DJ, Watson J. Cluster randomized controlled trials. J Eval Clin Pract 2005;11:479–83. 10.1111/j.1365-2753.2005.00568.x [DOI] [PubMed] [Google Scholar]
- 59.Springvloet L, van der Pol P, van Dorsselaer S. Factsheet roken onder volwassenen en jongeren in Nederland. Kerncijfers 2015. Nationaal Expertisecentrum Tabaksontmoediging: Trimbos-instituut 2016. [Google Scholar]
- 60.Springvloet L, van Laar M. Roken onder volwassenen Kerncijfers 2016. Trimbos instituut 2017. [Google Scholar]
- 61.Lennox AS. Stages of change training for opportunistic smoking intervention by the primary health care team. Health Education J 1998. [Google Scholar]
- 62.Donner A, Klar N. Design and analysis of cluster randomization trials in health research. London.: Arnold, 2000. [Google Scholar]
- 63.Creswell JW, Plano Clark VL. Designing and conducting mixed methods research. SAGE Publications, 2017. [Google Scholar]
- 64.Glasgow RE, Harden SM, Gaglio B, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health 2019;7:64. 10.3389/fpubh.2019.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.RE-AIM . Available: www.RE-AIM.org
- 66.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Damschroder LJ, Lowery JC. Evaluation of a large-scale weight management program using the consolidated framework for implementation research (CFIR). Implement Sci 2013;8:51. 10.1186/1748-5908-8-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Champion VL, Skinner CS. The health belief model. In: Glanz K, Rimer BK, Viswanath K, eds. Health behavior and health education: theory, research, and practice: Jossey-Bass, 2008: 45–65. [Google Scholar]
- 69.Stevens C, Vrinten C, Smith SG, et al. Determinants of willingness to receive healthy lifestyle advice in the context of cancer screening. Br J Cancer 2018;119:251–7. 10.1038/s41416-018-0160-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevens C, Vrinten C, Smith SG, et al. Acceptability of receiving lifestyle advice at cervical, breast and bowel cancer screening. Prev Med 2019;120:19–25. 10.1016/j.ypmed.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leventhal H, Phillips LA, Burns E. The common-sense model of self-regulation (CSM): a dynamic framework for understanding illness self-management. J Behav Med 2016;39:935–46. 10.1007/s10865-016-9782-2 [DOI] [PubMed] [Google Scholar]
- 72.Maes S, Pomaki G, Joekes K. The goals and process inventory (GAPI). Leiden, Netherlands: Leiden University, Health Psychology Unit, 2001. [Google Scholar]
- 73.Hankins M. The reliability of the twelve-item general health questionnaire (GHQ-12) under realistic assumptions. BMC Public Health 2008;8:355. 10.1186/1471-2458-8-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conner M, Mcmillan B. The health belief model. In: Christensen AJ, Martin R, Morrison Smyth J, eds. Encyclopedia of health psychology. Kluwer Academic and Plenum Publishers, 2004: 126–8. [Google Scholar]
- 75.Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13:117. 10.1186/1471-2288-13-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 77.Gezondheidsraad . WBO: een stoppen-met-roken-strategie binnen het bevolkingsonderzoek baarmoederhalskanker 2018, 2018. Available: https://www.gezondheidsraad.nl/documenten/adviezen/2018/07/25/wbo-een-stoppen-met-roken-strategie-binnen-het-bevolkingsonderzoek-baarmoederhalskanker
- 78.McBride CM, Scholes D, Grothaus LC, et al. Evaluation of a minimal self-help smoking cessation intervention following cervical cancer screening. Prev Med 1999;29:133–8. 10.1006/pmed.1999.0514 [DOI] [PubMed] [Google Scholar]
- 79.Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350:h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schellings R, Kessels AG, ter Riet G, et al. Indications and requirements for the use of prerandomization. J Clin Epidemiol 2009;62:393–9. 10.1016/j.jclinepi.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 81.Boter H, HESTIA Study Group . Multicenter randomized controlled trial of an outreach nursing support program for recently discharged stroke patients. Stroke 2004;35:2867–72. 10.1161/01.STR.0000147717.57531.e5 [DOI] [PubMed] [Google Scholar]
- 82.Boter H, van Delden JJM, de Haan RJ, et al. Modified informed consent procedure: consent to postponed information. BMJ 2003;327:284–5. 10.1136/bmj.327.7409.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCambridge J, Kypri K, Elbourne D. In randomization we trust? There are overlooked problems in experimenting with people in behavioral intervention trials. J Clin Epidemiol 2014;67:247–53. 10.1016/j.jclinepi.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Puffer S, Torgerson DJ, Watson J. Cluster randomized controlled trials. J Eval Clin Pract 2005;11:479–83. 10.1111/j.1365-2753.2005.00568.x [DOI] [PubMed] [Google Scholar]
- 85.Dawson AJ. Methodological reasons for not gaining prior informed consent are sometimes justified. BMJ 2004;329:87.1. 10.1136/bmj.38112.692211.F7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suijker JJ, van Rijn M, Buurman BM, et al. Effects of nurse-led multifactorial care to prevent disability in Community-Living older people: cluster randomized trial. PLoS One 2016;11:e0158714. 10.1371/journal.pone.0158714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Staten-Generaal . Evaluatie Wet medischwetenschappelijk onderzoek met mensen [in Dutch]. Den Haag: Tweede kamer der Staten-Generaal; vergaderjaar 2006-2007, kamerstuk 29964 Nr. 4.
- 88.Cancer Research UK . Smoking and cancer types - Infographic. Available: https://publications.cancerresearchuk.org/publication/smoking-and-cancer-types-infographic
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-055812supp001.pdf (100.9KB, pdf)
bmjopen-2021-055812supp002.pdf (580.9KB, pdf)
bmjopen-2021-055812supp003.pdf (60KB, pdf)