Abstract

The preparation of symmetrical (hetero)biaryls via arylazo sulfones has been successfully carried out upon visible light irradiation in the presence of PPh3AuCl as the catalyst. The present protocol led to the efficient synthesis of a wide range of target compounds in an organic-aqueous solvent under photocatalyst-free conditions.
Introduction
The symmetrical biaryl scaffold is a ubiquitous chemical motive in naturally occurring products1 as well as artificial bioactive species.2 In addition, the current growing interest for these biaryls is ascribable to their multifaced applications as (stereogenic) ligands,3 conducting and electroluminescent materials,4 and key constituents of molecular switches and devices.5 Thus, it is not surprising that starting from the early synthetic approaches based on the copper-catalyzed coupling of aryl halides under reductive conditions (the so-called Ullmann reaction),6 an impressive number of transition metal-catalyzed protocols was developed.7−12 In this context, the use of Au salts, complexes, or nanoparticles as the catalysts for homocoupling processes has been widely explored, and different substrates have been successfully employed, including, among the others, aryl boronic acids (in the presence of a stoichiometric amount of an inorganic base),13 haloarenes (mainly iodides, by following an Ullman-type coupling),14 and aromatics (via direct C–H bond functionalization under oxidative conditions).15 On the other hand, the opportunity to perform efficient and selective syntheses under mild conditions upon visible light irradiation allowed for the emergence of photochemistry as a sustainable synthetic approach. Currently, two strategies are mainly exploited to achieve these targets, namely, the use of a colored photocatalyst able to activate the substrates via electron16 or atom (hydrogen17 or halogen18) transfer and the use of commercially available or properly designed organic molecules that absorb in the visible region.19 However, although the synthesis of asymmetric biphenyls under either photochemical20 or photocatalyzed21 conditions has been largely documented, the related photoinduced homocoupling processes are still largely underdeveloped.22 These include the photocatalyzed Ullmann reaction of aryl halides using KNb3O8@AuNP,23 the [Au(I)]-photoredox-catalyzed coupling of aryl iodides developed by Barriault and co-workers24 (Scheme 1a) or the dual photoredox/nickel-catalyzed dimerization of aryl bromides.25 It should be noted that in most cases a high-energy-demanding UV radiation is required,26 and the use of visible light is relegated to the use of elaborated bimetallic (Ti/Pd) nanostructured composites as the photocatalyst at high temperatures.27
Scheme 1. Photoinduced Homocoupling for the Synthesis of Biaryls.
(a) [Au(I)]-catalyzed homocoupling of aryl iodides; (b) trace amount of biaryl byproducts from the [Au(I)] gold-catalyzed Suzuki-type coupling via arylazo sulfones; and (c) our proposal.
We recently focused on arylazo sulfones that incorporate a dyedauxiliary group (−N2SO2CH3) responsible for their color and their photoreactivity, useful precursors of reactive intermediates such as aryl, alkyl(aryl)sulfonyl, and aryldiazenyl radicals.28 These intermediates have been exploited in different synthetic protocols under photocatalyst-free conditions for the visible light-driven forging of C–C29 as well as C-heteroatom30 bonds.
In particular, the in situ generation of aryl radicals from arylazo sulfones has been recently merged with [Au(I)] catalysis by our groups for a Suzuki-type reaction.31 During this study, we were intrigued to detect in selected cases the corresponding homocoupling biaryl as a minor side product (2–5% yield in most cases; Scheme 1b).31 Stimulated by these early observations, we wondered if it was possible to design an unprecedented photocatalyst-free visible light-driven protocol for the synthesis of symmetrical (hetero)biaryls (Scheme 1c).
The present approach would represent an innovative procedure for the preparation of symmetrical biarenes via activation of an aromatic substrate upon direct visible light irradiation without the need for any sacrificial electron donors in stoichiometric amounts.
Results and Discussion
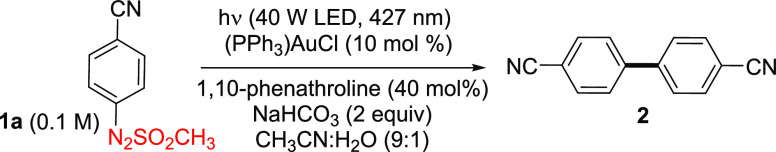
To test our proposal, we first repeated the conditions applied in the Suzuki coupling but by omitting the boronic acid by electing the p-cyanophenylazo sulfone 1a as the model substrate. However, the desired biaryl 2 was formed only in small amounts (see Table S1 in the Supporting Information for further information). We then carried out an extensive survey of reaction parameters (i.e., reaction media, light source, and the cocatalyst; see Table S1 for details) aiming to push the forging of the desired Ar–Ar bond. A representative list of control experiments is organized in Table 1. Upon this preliminary optimization stage, we were pleased to verify that by irradiating (Kessil lamp, 40 W, λem = 427 nm) an argon-equilibrated mixture of 1a (0.1 M in CH3CN/H2O 9:1), (PPh3)AuCl (10 mol %), 1,10-phenanthroline (40 mol %), and NaHCO3 (2 equiv) for 24 h led to biaryl 2 in a 75% yield (entry 1, Table 1).
Table 1. Optimization of the Reaction Conditions.
| entry | deviation from optimal conditions | 2 (% yield)a |
|---|---|---|
| 1 | 75b | |
| 2 | no (PPh3)AuCl | 0c |
| 3 | dark | 0 |
| 4 | no NaHCO3 | 0 |
| 5 | hν (390 nm) | 36 |
| 6 | 1,10-phenanthroline (20 mol %) | 60 |
| 7 | p-CNC6H4N2BF4 instead of 1a | <5 |
GC yield.
Isolated yield after flash chromatography.
Benzonitrile (88% yield) was found as the only product.
The presence of a buffering agent (NaHCO3) and the gold complex along with light irradiation is essential to the optimal outcome of the process (compare entry 1 vs entries 2–4). A lower 2 isolated yield was observed both by shifting to 390 nm (36%, entry 5) and by decreasing the loading of the 1,10-phenanthroline cocatalyst to 20 mol % (entry 6). Finally, the possible role of diazonium salts in the present methodology was excluded by replacing 1a with the p-cyanophenyl diazonium tetrafluoroborate salt; under optimal conditions, a trace amount of 2 was detected (entry 7).
With the optimized protocol in our hand, we explored the scope of the reaction by testing a broad range of diversely functionalized monosubstituted arylazo sulfones 1a–1ac (Figure S1 and Table 2). Gratifyingly, the corresponding biaryls were obtained in good to quantitative yields and with excellent functional group tolerance starting from 4- (2–15) and 3-substituted aromatic substrates (16–21), including the 4,4′-diacetyl derivative 12 (a precursor of antifungal N,N′-diaryl-bishydrazones)32 and the benzophenone dimer 15 (83% yield). In the case of 4, however, a higher amount of the catalyst was mandatory to achieve a 93% yield. In this series, however, the formation of 9 was not observed with 4-ethinylphenyl azosulfone 1h, and this is probably due to the competitive reactivity of [Au(I)] complexes with alkynes.33
Table 2. Synthesis of Symmetrical Biaryls from Monosubstituted Arylazo Sulfones.
(PPh3)AuCl (15 mol %) was employed.
1,2-Bis(4-nitrophenyl)diazene (11a, 7% yield) was isolated as the minor product.
Nitrobenzene was found as the only product.
The reaction proved slightly less efficient with ortho-substituted arylazo sulfones (1u–1ac). In most cases, a 15 mol % amount of (PPh3)AuCl to achieve satisfactory results (see dihaloderivatives 24–26 and 2,2′-diphenoxybiphenyl 29). As observed in previous works,30 the presence of a nitro group in the ortho-position in arylazo sulfones prevented the arylation as confirmed here, leading to the exclusive formation of nitrobenzene instead of the desired compound 28.
The protocol was next successfully extended to the synthesis of polysubstituted biaryls 31–38 (Table 3), such as the polychlorinated biaryls 31 and 32 known as alkoxyresorufin O-dealkylase inhibitors.34 The only exception is represented by the 2,2′-dinitro derivative 38, where again the hydrodeaminated meta-nitroanisole was formed instead. Gratifyingly, binaphthyls 39 and 40 and heteroarenes 41–43 were also isolated in up to quantitative yields. Interestingly, most protocols currently available for the synthesis of bipyridines present several limitations, including the low efficiency and limited scope.35 Furthermore, compound 42 found interesting application in the synthesis of binaphthyl–bipyridyl-based chiroptical switches.36
Table 3. Visible Light-Driven Preparation of (Hetero)biaryls 31–43.
(PPh3)AuCl (15 mol %) was employed.
3-Nitroanisole (70% yield) was found as the main product.
The mechanism proposed for the homocoupling is summarized in Scheme 2. Photolysis of arylazo sulfones to visible light causes the homolytic cleavage of the N–S bond, to release, after nitrogen loss from the first formed aryldiazenyl radical, an aryl (Ar•)/methanesulfonyl radical pair (Scheme 2, path a).31 Oxidative addition of Ar• onto the PPh3AuIL catalyst (path b) resulted in the formation of the PPh3AuIILAr species I, which, in turn, intercepts a further Ar• intermediate to afford the AuIII complex II (path c).37 The latter undergoes reductive elimination to release Ar–Ar while restoring the starting PPh3AuIL catalyst (path d).38 The intermediacy of an aryl radical was ascertained by an experiment carried out in the presence of TEMPO (0.05 M), showing a significant lowering of the biphenyl yield (from 75% to 29% in the case of 2). As for the role of the cocatalyst, bis-pyridyl and phenanthryl ligands have been frequently adopted as beneficial additives in Au-mediated photo- and electrochemical coupling reactions.39 Although a conclusive answer on the real role of pyridine-based additives in Au(I)-mediated processes was not completely ascertained to date, their role as stabilizing agents of the high-oxidation-state gold complexes has been postulated.
Scheme 2. Proposed Mechanism.
In the field of light-driven processes, the present Au-catalyzed homocoupling results competitive in terms of efficiency and feasibility, with the other approaches already reported in the literature.24−26 The use of an easily available Au complex and the absence of a redox agent characterize the present procedure.24
Conclusions
We presented herein the first visible light/Au(I)-catalyzed protocol for the preparation of symmetrical (hetero)biaryls by homocoupling of arylazo sulfones at room temperature in organic/aqueous media. The method exploits the properties of the N2SO2CH3 moiety as a dyedauxiliary group28 able to be activated directly with visible light without the intermediacy of a photocatalyst and exhibits an excellent functional group tolerance. The strategy has been exploited for the preparation of a wide range of symmetrical (hetero)biaryls in good to excellent yields with an easy setup.
Experimental Section
General
1H and 13C{1H} NMR spectra were recorded on a 300 MHz and 75 MHz spectrometer, respectively. The attributions were made on the basis of 1H and 13C NMR experiments; chemical shifts are reported in ppm downfield from TMS. GC analyses were performed using a HP SERIES 5890 II equipped with a fire ion detector (FID, temperature 350 °C). Analytes were separated using a Restek Rtx-5MS (30 m × 0.25 mm × 0.25 μm) capillary column with nitrogen as a carrier gas at 1 mL min–1. The injector temperature was 250 °C. The GC oven temperature was held at 80 °C for 2 min, increased to 250 °C by a temperature ramp of 10 °C min–1, and held for 10 min.
General Procedure for the Synthesis of Arylazo Sulfones 1a–1ap
Arylazo sulfones 1a–ap have been synthesized by following a known procedure starting from the corresponding aryl diazonium salts.29c,30b Diazonium salts were freshly prepared prior to use from the corresponding anilines and purified by dissolving in acetonitrile and precipitation by adding cold diethyl ether. To a cooled (0 °C) suspension of the chosen diazonium salt (1 equiv, 0.3 M) in CH2Cl2 was added sodium methanesulfinate (1.2 equiv) in one portion. The temperature was allowed to increase to room temperature, and the solution was stirred overnight. The resulting mixture was then filtered, and the obtained solution was evaporated affording the desired arylazo sulfone. The crude product was finally dissolved in CH2Cl2 and precipitated by adding cold n-hexane. Compounds 1a–1aa, 1ac–1al, 1an, and 1ao have been fully characterized in previous works.29c,30b
1-(Methylsulfonyl)-2-(2-phenoxyphenyl)diazene (1ab)
Orange solid, 56% yield, Tdec: 87–88 °C. 1H NMR (300 MHz, CDCl3) δ 7.82 (dd, J = 8.2, 1.7 Hz, 1H), 7.67–7.61 (m, 1H), 7.40–7.32 (m, 2H), 7.29–7.23 (m, 1H), 7.21–7.12 (m, 2H), 7.10–6.99 (m, 2H), 2.86 (s, 3H). 13C{1H} NMR (75 MHz, CDCl3) δ 157.9, 157.0, 139.8, 137.3, 130.2, 124.3, 124.0, 121.3, 118.5, 118.1, 34.1. HRMS (ESI) m/z: calcd for C13H12N2O3S+ ([M + H]+) 299.0442; found 299.0461.
3-Bromo-4-((methylsulfonyl)diazenyl)benzonitrile (1aj)
Orange solid, 69% yield, Tdec: 132–132.5 °C. 1H NMR (300 MHz, CDCl3) δ 8.36–7.93 (m, 1H), 7.82–7.76 (m, 2H), 3.27 (s, 3H). 13C{1H} NMR (75 MHz, CDCl3) δ 148.6, 138.1, 132.2, 128.0, 119.2, 119.1, 116.2, 35.1. HRMS (ESI) m/z: calcd for C8H6N3O2SBr+ ([M + H]+) 287.9442; found 287.9425.
1-(4-Methoxy-2-nitrophenyl)-2-(methylsulfonyl)diazene (1ak)
Orange solid, 54% yield, Tdec: 95–97 °C. 1H NMR (300 MHz, CDCl3) δ 7.85 (d, J = 9.1 Hz, 1H), 7.45 (d, J = 2.7 Hz, 1H), 7.34–7.13 (m, 2H), 4.03 (s, 3H), 3.16 (s, 3H). 13C{1H} NMR (75 MHz, CDCl3) δ 165.20, 134.47, 119.71, 119.06, 109.69, 56.89. HRMS (ESI) m/z: calcd for C8H6N3O2SBr+ ([M + H]+) 286.9364; found 286.9347.
1-(4-Chloronaphthalen-1-yl)-2-(methylsulfonyl)diazene (1am)
Orange solid, 34% yield, Tdec: 125–127 °C. 1H NMR (300 MHz, CDCl3) δ 8.76–8.49 (m, 1H), 8.42–8.18 (m, 1H), 7.83 (d, J = 8.2 Hz, 1H), 7.77–7.67 (m, 2H), 7.62 (d, J = 8.3 Hz, 1H), 3.33 (s, 3H). 13C{1H} NMR (75 MHz, CDCl3) δ 142.7, 140.7, 132.4, 131.5, 129.4, 128.5, 126.1, 125.0, 123.0, 114.4, 35.4. HRMS (ESI) m/z calcd for C11H9N2O2SCl+ ([M + H]+) 269.0152; found 269.0158.
Procedure for the Preparation of 2-((Methylsulfonyl)diazenyl)pyridine (1ap)
N′-(Pyridin-2-yl)methanesulfonohydrazide was initially prepared by mixing 2-hydrazinylpyridine (0.300 g, 2.7 mmol) and methanesulfonyl chloride (0.212 mL, 2.7 mmol) in 3 mL of pyridine (37.4 mmol), as previously described.40 The crude mixture containing the sulfonohydrazide was oxidized by treatment with N-bromosuccinimide (0.475 g, 2.7 mmol) following a known procedure41 to give 1ap as a yellow solid (134.7 mg, 0.72 mmol, 26% yield, Tdec: 72–73 °C).
1ap: 1H NMR (300 MHz, CDCl3) δ 8.91–8.64 (m, 1H), 8.01 (td, J = 7.6, 1.8 Hz, 1H), 7.94–7.85 (m, 1H), 7.59 (ddd, J = 7.4, 4.6, 1.3 Hz, 1H), 3.29 (s, 3H). 13C{1H} NMR (75 MHz, CDCl3) δ 150.3, 139.8, 139.3, 118.3, 117.7, 43.0. HRMS (EsI) m/z calcd for C6H7N3O2S+ ([M + H+]) 186.0337; found 186.0392.
General Procedure for the Photochemical Synthesis of Biaryls
A pyrex glass vessel was charged with the chosen arylazo sulfone (1a–ap, 0.5 mmol, 1.0 equiv, 0.1 M) and 40 mg of sodium bicarbonate (1.0 mmol, 0.2 M), and the solid was dissolved in degassed acetonitrile/water (9:1, 5.0 mL); then, triphenylphosphine gold(I) chloride (0.05 mmol, 10 mol %) and 1,10-phenanthroline (40 mol %, 0.04 M) were added and the obtained mixture was flushed with argon. Irradiation was carried out for 24 h by means of a 40 W Kessil lamp (emission at 427 nm; see Figure S2 for further information). The photolyzed solution was concentrated under reduced pressure and purified by silica gel column chromatography (cyclohexane–ethyl acetate mixture as eluant).
[1,1′-Biphenyl]-4,4′-dicarbonitrile (2)
From 104.5 mg (0.500 mmol) of 1a, 25.0 mg (0.05 mol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.1 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 38.3 mg of 2 (75% yield, white solid, mp = 233–234 °C). Spectroscopic data were in accordance with the literature data.42 When the reaction was carried out in the presence of TEMPO (0.1 M), product 2 was obtained in only 29% yield.
1H NMR (300 MHz, CDCl3) δ 7.80 (d, J = 8.4 Hz, 4H), 7.71 (d, J = 8.5 Hz, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 143.7, 133.1, 128.1, 118.5, 112.6.
4,4′-Difluoro-1,1′-biphenyl (3)
From 101.0 mg (0.500 mmol) of 1b, 25.0 mg (0.05 mol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 47.5 mg of 3 (>99% yield, white solid, mp = 93–95 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.49–7.40 (m, 2H), 7.39 (s, 2H), 7.32–7.25 (m, 2H), 7.14–7.05 (m, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 164.6 (d, J = 244.5 Hz), 137.0 (d, J = 3 Hz), 129.2 (d, J = 8.3 Hz), 116.4 (d, J = 21.8 Hz).
4,4′-Dichloro-1,1′-biphenyl (4)
From 109.7 mg (0.501 mmol) of 1c, 25.0 mg (0.05 mol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 26.8 mg of 4 (48% yield, white solid, mp = 145–146 °C). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) gave 4 in a 93% yield. Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.52–7.46 (m, 4H), 7.46–7.40 (m, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.3, 133.6, 128.9, 128.1.
4,4′-Dibromo-1,1′-biphenyl (5)
From 132.5 mg (0.502 mmol) of 1d, 25.0 mg (0.05 mol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 50.9 mg of 5 (65% yield, slightly orange solid, mp = 165–166 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.58 (d, J = 8.6 Hz, 4H), 7.43 (d, J = 8.6 Hz, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.8, 131.9, 128.4, 121.9.
4,4′-Diiodo-1,1′-biphenyl (6)
From 155.8 mg (0.502 mmol) of 1e, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 74.4 mg of 6 (73% yield, slightly yellow solid, mp = 202–203 °C). Spectroscopic data were in accordance with the literature data.431H NMR (300 MHz, CDCl3) δ 7.61 (d, J = 8.6 Hz, 4H), 7.13 (d, J = 8.6 Hz, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 139.5, 138.2, 128.8, 93.6.
4,4′-Dimethyl-1,1′-biphenyl (7)
From 90.0 mg (0.502 mmol) of 1f, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 28.2 mg of 7 (71% yield, white solid, mp = 118–120 °C). Spectroscopic data were in accordance with the literature data.441H NMR (300 MHz, CDCl3) δ 7.52 (d, J = 8.1 Hz, 4H), 7.28 (d, J = 7.8 Hz, 4H), 2.43 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.2, 136.6, 129.3, 126.7, 21.0.
4,4′-Di-tert-butyl-1,1′-biphenyl (8)
From 120.0 mg (0.500 mmol) of 1g, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 66.5 mg of 8 (>99% yield, white solid, mp = 126–127 °C). Spectroscopic data were in accordance with the literature data.451H NMR (300 MHz, CDCl3) δ 7.57–7.50 (m, 4H), 7.49–7.43 (m, 4H), 1.37 (s, 18H). 13C{1H} NMR (75 MHz, CDCl3) δ 150.1, 138.3, 126.8, 125.8, 34.6, 31.5.
4,4′-Dimethoxy-1,1′-biphenyl (10)
From 119.5 mg (0.504 mmol) of 1i, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 95:5) to afford 38.3 mg of 10 (71% yield, white solid, mp = 178–180 °C). Spectroscopic data were in accordance with the literature data.441H NMR (300 MHz, CDCl3) δ 7.49 (d, J = 8.8 Hz, 4H), 6.97 (d, J = 8.8 Hz, 4H), 3.85 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 158.6, 133.4, 127.6, 114.1, 55.2.
4,4′-Dinitro-1,1′-biphenyl (11)
From 115.5 mg (0.502 mmol) of 1j, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 43.4 mg of 11 (71% yield, white solid, mp 225–226 °C) and 4.8 mg of 1,2-bis(4-nitrophenyl)diazene 11a (7% yield, red oil). Spectroscopic data were in accordance with the literature data.441H NMR (300 MHz, CDCl3) δ 8.37 (d, J = 8.8 Hz, 4H), 7.79 (d, J = 8.8 Hz, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 148.2, 145.1, 128.5, 124.5.
1,1′-([1,1′-Biphenyl]-4,4′-diyl)bis(ethan-1-one) (12)
From 113.0 mg (0.500 mmol) of 1k, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 92:8) to afford 60.3 mg of 12 (>99% yield, white solid, mp 187–189 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 8.05 (d, J = 8.4 Hz, 4H), 7.71 (d, J = 8.5 Hz, 4H), 2.64 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 197.5, 144.2, 136.5, 128.9, 127.3, 26.6.
4,4′-Bis(trifluoromethyl)-1,1′-biphenyl (13)
From 127.3 mg (0.500 mmol) of 1l, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane) to afford 45.0 mg of 13 (62% yield, white solid, mp 85–87 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.83–7.58 (m, 8H). 13C{1H} NMR (75 MHz, CDCl3) δ 143.4, 130.7 (q, J = 32.3 Hz), 127.8, 126.2 (q, J = 3.8 Hz), 123.5 (q, J = 270 Hz).
Dimethyl [1,1′-Biphenyl]-4,4′-dicarboxylate (14)
From 121.7 mg (0.500 mmol) of 1m, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 9:1) to afford 57.8 mg of 14 (85% yield, white solid, mp 215–216 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.3 Hz, 4H), 7.69 (d, J = 8.3 Hz, 4H), 3.95 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 167.4, 144.9, 130.8, 130.3, 127.8, 52.8.
[1,1′-Biphenyl]-4,4′-diylbis(phenylmethanone) (15)
From 144.0 mg (0.500 mmol) of 1n, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 75.2 mg of 15 (83% yield, slightly yellow solid, mp 215–216 °C). Spectroscopic data were in accordance with the literature data.461H NMR (300 MHz, CDCl3) δ 7.93 (d, J = 8.3 Hz, 4H), 7.87–7.82 (m, 4H), 7.77 (d, J = 8.3 Hz, 4H), 7.65–7.59 (m, 2H), 7.52 (dd, J = 8.2, 6.8 Hz, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 196.3, 144.0, 137.7, 137.2, 132.7, 130.9, 130.2, 128.5, 127.3.
[1,1′-Biphenyl]-3,3′-dicarbonitrile (16)
From 104.5 mg (0.500 mmol) of 1o, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 95:5) to afford 40.8 mg of 16 (80% yield, white solid, mp = 190–192 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.90–7.75 (m, 4H), 7.71 (d, J = 7.7 Hz, 2H), 7.61 (t, J = 7.7 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 140.3, 131.9, 131.6, 130.8, 130.2, 118.5, 113.7.
3,3′-Difluoro-1,1′-biphenyl (17)
From 101.0 mg (0.500 mmol) of 1p, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 29.8 mg of 17 (63% yield, colorless oil). Spectroscopic data were in accordance with literature data.471H NMR (300 MHz, CDCl3) δ 7.49–7.34 (m, 4H), 7.29 (dt, J = 10.1, 2.1 Hz, 2H), 7.19–6.92 (m, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 164.5 (d, J = 244.5 Hz), 142.0 (q, J = 3.8 Hz), 130.2 (d, J = 8.3 Hz), 122.5 (d, J = 3 Hz), 114.5 (d, J = 21 Hz), 113.9 (d, J = 22.5 Hz).
3,3′-Dichloro-1,1′-biphenyl (18)
From 108.5 mg (0.500 mmol) of 1q, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 35.8 mg of 18 (65% yield, slightly orange oil). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 1.7 Hz, 2H), 7.45–7.41 (m, 2H), 7.38–7.33 (m, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 141.8, 135.0, 130.3, 128.0, 127.4, 125.4.
3,3′-Dibromo-1,1′-biphenyl (19)
From 132.3 mg (0.501 mmol) of 1r, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 56.6 mg of 19 (73% yield, white solid, mp = 52–54 °C). Spectroscopic data were in accordance with literature data.481H NMR (300 MHz, CDCl3) δ 7.72 (t, J = 1.9 Hz, 1H), 7.51 (ddt, J = 9.7, 7.9, 1.1 Hz, 2H), 7.35 (d, J = 7.9 Hz, 1H). 13C{1H} NMR (75 MHz, CDCl3) δ 141.9, 131.0, 130.5, 130.3, 125.9, 123.1.
3,3′-Diiodo-1,1′-biphenyl (20)
From 155.4 mg (0.501 mmol) of 1s, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 54.8 mg of 20 (54% yield, white solid, mp 73–74 °C). Spectroscopic data were in accordance with the literature data.491H NMR (300 MHz, CDCl3) δ 8.00 (d, J = 54.4 Hz, 1H), 7.73–7.64 (m, 1H), 7.59–7.47 (m, 1H), 7.19 (t, J = 7.9 Hz, 1H). 13C{1H} NMR (75 MHz, CDCl3) δ 135.7, 131.3, 130.2, 126.1, 94.5.
1,1′-([1,1′-Biphenyl]-3,3′-diyl)bis(ethan-1-one) (21)
From 113.0 mg (0.500 mmol) of 1t, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 92:8) to afford 59.5 mg of 21 (>99% yield, white solid, mp 125–126 °C). Spectroscopic data were in accordance with the literature data.481H NMR (300 MHz, CDCl3) δ 8.19 (t, J = 1.8 Hz, 2H), 7.96 (m, J = 7.7, 1.8, 1.1 Hz, 2H), 7.81 (m, J = 7.7, 1.9, 1.1 Hz, 2H), 7.56 (t, J = 7.7 Hz, 2H), 2.66 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 198.0, 140.8, 137.9, 131.9, 129.3, 127.9, 127.0, 26.9.
[1,1′-Biphenyl]-2,2′-dicarbonitrile (22)
From 104.5 mg (0.500 mmol) of 1u, 25.0 mg (0.05 mmol, 10 mmol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 95:5) to afford 37.7 mg of 22 (74% yield, white solid, mp = 171–173 °C). Spectroscopic data were in accordance with the literature data.501H NMR (300 MHz, CDCl3) δ 7.87–7.80 (m, 2H), 7.75–7.69 (m, 2H), 7.62–7.55 (m, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 141.3, 133.3, 132.6, 130.3, 128.9, 117.2, 112.1.
2,2′-Difluoro-1,1′-biphenyl (23)
From 102.0 mg (0.502 mmol) of 1v, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 40.6 mg of 23 (85% yield, white solid, mp = 116–118 °C). Spectroscopic data were in accordance with the literature data.471H NMR (300 MHz, CDCl3) δ 7.42 (dddd, J = 10.6, 7.9, 4.9, 2.3 Hz, 4H), 7.31–7.16 (m, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 161.2, 157.9, 131.3 (t, J = 2.3 Hz), 129.5 (t, J = 4.5 Hz), 123.8 (t, J = 2.3 Hz), 115.6 (q, J = 7.5 Hz).
2,2′-Dichloro-1,1′-biphenyl (24)
From 108.5 mg (0.500 mmol) of 1w, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 24.8 mg of 24 (45% yield, white solid, mp = 60–61 °C). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) afforded 24 in an 83% yield. Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.58–7.46 (m, 2H), 7.40–7.33 (m, 4H), 7.31–7.26 (m, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 139.0, 134.1, 131.8, 130.0, 129.8, 127.1.
2,2′-Dibromo-1,1′-biphenyl (25)
From 130.8 mg (0.500 mmol) of 1x, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 36.5 mg of 25 (45% yield, white solid, mp = 77–79 °C). The same reaction performed with 34.9 mg of (PPh3)AuCl (15 mol %) gave 25 in a 57% yield. Spectroscopic data were in accordance with the literature data.511H NMR (300 MHz, CDCl3) δ 7.72–7.67 (m, 2H), 7.42–7.37 (m, 2H), 7.31–7.26 (m, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 142.6, 133.1, 131.5, 129.9, 127.7, 124.1.
2,2′-Diiodo-1,1′-biphenyl (26)
From 155.0 mg (0.501 mmol) of 1y, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 35.6 mg of 26 (35% yield, white solid, mp = 109–112 °C). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) afforded 26 in a 79% yield. Spectroscopic data were in accordance with the literature data.511H NMR (300 MHz, CDCl3) δ 7.95 (dd, J = 8.0, 1.2 Hz, 2H), 7.41 (dd, J = 7.5, 1.2 Hz, 2H), 7.20 (dd, J = 7.6, 1.7 Hz, 2H), 7.09 (td, J = 7.7, 1.7 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 149.1, 139.0, 130.0, 129.5, 128.2, 99.8.
2,2′-Dimethoxy-1,1′-biphenyl (27)
From 118.0 mg (0.500 mmol) of 1z, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 28.8 mg of 27 (54% yield, white solid, mp = 154–156 °C). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) gave 27 in a 97% yield. Spectroscopic data were in accordance with the literature data.451H NMR (300 MHz, CDCl3) δ 7.37 (ddd, J = 8.2, 7.4, 1.8 Hz, 2H), 7.29 (dd, J = 7.4, 1.9 Hz, 2H), 7.11–6.92 (m, 4H), 3.81 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 157.2, 131.6, 128.7, 128.0, 120.5, 111.2, 55.8.
Irradiation of 1-(Methylsulfonyl)-2-(2-nitrophenyl)diazene (1aa)
From 114.5 mg (0.500 mmol) of 1aa, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 61.5 mg of nitrobenzene (yellow oil, quantitative yield).
2,2′-Diphenoxy-1,1′-biphenyl (29)
From 139.0 mg (0.500 mmol) of 1ab, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane) to afford 19.6 mg of 29 (24% yield, white solid, mp 100–102 °C). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) gave 29 in a 58% yield. Spectroscopic data were in accordance with the literature data.521H NMR (300 MHz, CDCl3) δ 7.47 (dd, J = 7.6, 1.8 Hz, 2H), 7.34–7.12 (m, 9H), 7.06–7.00 (m, 2H), 6.96–6.87 (m, 5H). 13C{1H} NMR (75 MHz, CDCl3) δ 157.6, 154.8, 132.1, 129.9, 129.5, 128.9, 123.2, 122.8, 118.9, 118.8.
2,2′-Bis(methylthio)-1,1′-biphenyl (30)
From 115.0 mg (0.500 mmol) of 1ac, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane) to afford 32.6 mg of 30 (53% yield, white solid, mp 45–46 °C). Spectroscopic data were in accordance with the literature data.481H NMR (300 MHz, CDCl3) δ 7.48–7.39 (m, 2H), 7.33 (d, J = 7.2 Hz, 2H), 7.28–7.15 (m, 4H), 2.41 (s, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.5, 137.8, 129.7, 128.2, 124.7, 124.2, 15.4.
2,2′,5,5′-Tetrachloro-1,1′-biphenyl (31)
From 126.0 mg (0.500 mmol) of 1ad, 25.0 mg (0.05 mol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 69.6 mg of 31 (96% yield, white solid, mp 65–66 °C). Spectroscopic data were in accordance with the literature data.481H NMR (300 MHz, CDCl3) δ 7.43 (s, 2H), 7.36 (dd, J = 8.6, 2.5 Hz, 2H), 7.28 (d, J = 2.4 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.2, 132.2, 131.5, 130.6, 130.4, 129.4.
3,3′,4,4′-Tetrachloro-1,1′-biphenyl (32)
From 126.0 mg (0.500 mmol) of 1ae, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 42.8 mg of 32 (59% yield, white solid, mp 172–173 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.62 (d, J = 2.2 Hz, 2H), 7.51 (d, J = 8.3 Hz, 2H), 7.36 (dd, J = 8.4, 2.2 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.9, 133.4, 132.6, 131.1, 128.9, 126.3.
3,3′-Dichloro-4,4′-difluoro-1,1′-biphenyl (33)
From 119.2 mg (0.500 mmol) of 1af, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 54.2 mg of 33 (83% yield, white solid, mp 139–141 °C). Spectroscopic data were in accordance with literature data.531H NMR (300 MHz, CDCl3) δ 7.56 (dd, J = 6.9, 2.4 Hz, 2H), 7.38 (ddd, J = 8.5, 4.5, 2.4 Hz, 2H), 7.23 (dd, J = 9.5, 7.7 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 159.7 (d, J = 249 Hz), 136.4 (d, J = 3.8 Hz), 129.3, 126.9 (d, J = 7.5 Hz), 121.8 (d, J = 18 Hz), 117.3 (d, J = 21 Hz). 19F NMR (376 MHz, CDCl3): δ −112.0.
.2,2′-Dichloro-4,4′-difluoro-1,1′-biphenyl (34)
From 118.9 mg (0.500 mmol) of 1ag, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 20.5 mg of 34 (31% yield, colorless liquid). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) gave 34 in a 58% yield. Spectroscopic data were in accordance with the literature data.541H NMR (300 MHz, CDCl3) δ 7.29–7.21 (m, 4H), 7.08 (td, J = 8.3, 2.6 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 164.0 (d, J = 249 Hz), 134.8 (d, J = 9.8 Hz), 133.7 (d, J = 3.0 Hz), 132.6 (d, J = 9.0 Hz), 114.3 (d, J = 21 Hz). 19F NMR (376 MHz, CDCl3): δ −117.3.
3,3′,5,5′-Tetrakis(trifluoromethyl)-1,1′-biphenyl (35)
From 171.0 mg (0.501 mmol) of 1ah, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mmol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 37.6 mg of 35 (32% yield, white solid, mp 79–81 °C). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) gave 35 in a 58% yield. Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 8.01 (d, J = 12.4 Hz, 6H). 13C{1H} NMR (75 MHz, CDCl3) δ 140.6, 133.8 (q, J = 33.8 Hz), 128.6 (q, J = 272 Hz), 127.7 (d, J = 3.0 Hz), 122.9 (m, J = 3.8 Hz). 19F NMR (376 MHz, CDCl3): δ −63.3.
2,2′-Dibromo-5,5′-bis(trifluoromethyl)-1,1′-biphenyl (36)
From 177.0 mg (0.504 mmol) of 1ai, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 36.9 mg of 36 (30% yield, pale yellow solid, mp 98–100 °C). The same reaction performed with 37.5 mg of (PPh3)AuCl (15 mol %) gave 36 in a 90% yield. Spectroscopic data were in accordance with the literature data.551H NMR (300 MHz, CDCl3) δ 7.88–7.76 (m, 4H), 7.57 (dd, J = 8.3, 2.3 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.3, 135.9, 131.8 (q, J = 31.5 Hz), 128.3 (q, J = 272 Hz), 126.5 (q, J = 6.8 Hz), 120.3 (q, J = 1.5 Hz). 19F NMR (376 MHz, CDCl3): δ −63.2.
2,2′-Dibromo-[1,1′-biphenyl]-4,4′-dicarbonitrile (37)
From 144.5 mg (0.500 mmol) of 1aj, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 9:1) to afford 40.8 mg of 37 (56% yield, pale orange solid, mp 180–183 °C). 1H NMR (300 MHz, CDCl3) δ 8.00 (d, J = 1.5 Hz, 2H), 7.74–7.70 (m, 2H), 7.34 (d, J = 7.9 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 145.2, 136.3, 131.2, 123.7, 117.3, 116.9, 114.5. HRMS (ESI) m/z calcd for C14H6N2Br2+ ([M + H]+) 360.8970; found 360.8947.
Irradiation of 1-(4-Methoxy-2-nitrophenyl)-2-(methylsulfonyl)diazene (1ak)
From 129.6 mg (0.5 mmol) of 1ak, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 53.6 mg of 3-nitroanisole (70% yield).
1,1′-Binaphthalene (39)
From 117.1 mg (0.500 mmol) of 1al, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 62.6 mg of 39 (99% yield, white solid, mp 159–161 °C). Spectroscopic data were in accordance with the literature data.421H NMR (300 MHz, CDCl3) δ 7.98 (ddd, J = 8.3, 3.1, 1.4 Hz, 2H), 7.63 (dd, J = 8.2, 7.0 Hz, 1H), 7.52 (ddt, J = 8.2, 6.8, 3.1 Hz, 2H), 7.43 (dd, J = 8.6, 1.2 Hz, 1H), 7.35–7.27 (m, 1H). 13C{1H} NMR (75 MHz, CDCl3) δ 138.3, 133.4, 132.7, 128.0, 127.8, 127.7, 126.4, 125.9, 125.7, 125.3.
4,4′-Dichloro-1,1′-binaphthalene (40)
From 135.6 mg (0.501 mmol) of 1am, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: neat cyclohexane) to afford 81.4 mg of 40 (99% yield, red solid, mp 217–218 °C). Spectroscopic data were in accordance with the literature data.561H NMR (300 MHz, CDCl3) δ 8.39 (d, J = 8.5 Hz, 2H), 7.69 (d, J = 7.6 Hz, 2H), 7.67–7.52 (m, 4H), 7.38–7.35 (m, 4H). 13C{1H} NMR (75 MHz, CDCl3) δ 137.1, 134.0, 132.2, 130.9, 127.9, 127.2, 127.0, 125.8, 124.9.
3,3′-Bipyridine (41)
From 92.51 mg (0.500 mmol) of 1an, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 3:7) to afford 74.1 mg of 41 (94% yield, slightly yellow solid, mp 64–66 °C). Spectroscopic data were in accordance with the literature data.571H NMR (300 MHz, CDCl3) δ 8.86 (d, J = 2.4 Hz, 2H), 8.67 (dd, J = 4.9, 1.6 Hz, 2H), 7.91 (dt, J = 7.9, 2.0 Hz, 2H), 7.44 (dd, J = 7.9, 4.8 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 149.3, 148.1, 134.8, 133.7, 124.0.
2,2′-Dichloro-3,3′-bipyridine (42)
From 109.5 mg (0.500 mmol) of 1ao, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 7:3) to afford 56.0 mg of 42 (>99% yield, slightly yellow solid, mp 202–204 °C). Spectroscopic data were in accordance with the literature data.581H NMR (300 MHz, CDCl3) δ 8.50 (dd, J = 4.8, 1.9 Hz, 2H), 7.67 (dd, J = 7.6, 1.9 Hz, 2H), 7.47–7.36 (m, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 150.4, 149.9, 140.0, 133.0, 122.5.
2,2′-Bipyridine (43)
From 92.50 mg (0.500 mmol) of 1ap, 25.0 mg (0.05 mmol, 10 mol %) of (PPh3)AuCl, 40.0 mg of NaHCO3 (1.0 mmol), and 40.0 mg of 1,10-phenanthroline (0.2 mmol, 40 mol %) in 5 mL of degassed acetonitrile/water (9:1). Purification was carried out by silica gel chromatographic column (eluant: cyclohexane/ethyl acetate 3:7) to afford 38.6 mg of 43 (49% yield, slightly yellow solid, mp 70–71 °C). The same reaction was performed with 37.5 mg of (PPh3)AuCl (15 mol %) and afforded 43 in an 86% yield. Spectroscopic data were in accordance with the literature data.591H NMR (300 MHz, CDCl3) δ 8.69–8.65 (m, 2H), 8.40 (dd, J = 8.0, 1.2 Hz, 2H), 7.81 (td, J = 7.8, 1.8 Hz, 2H), 7.29 (ddd, J = 7.5, 4.8, 1.2 Hz, 2H). 13C{1H} NMR (75 MHz, CDCl3) δ 156.0, 149.2, 137.1, 123.9, 121.3.
Procedure for the Photochemical Synthesis of Biaryls 2 on a Larger Scale
A pyrex glass vessel was charged with the arylazo sulfone 1a (2.36 mmol, 1.0 equiv, 0.1 M) and 400 mg of sodium bicarbonate (4.72 mmol, 2 equiv, 0.2 M), and the solid was dissolved in degassed acetonitrile/water (9:1, 24.0 mL); then, 118.2 mg of triphenylphosphine gold(I) chloride (0.24 mmol, 10 mol %) and 187 mg of 1,10-phenanthroline (40 mol %) were added and the obtained mixture was flushed with argon. Irradiation was carried out for 24 h by means of a 40 W Kessil lamp (emission at 427 nm; see Figure S3). The photolyzed solution was concentrated under reduced pressure and purified by silica gel column chromatography (cyclohexane–ethyl acetate 95:5 mixture as an eluant). Product 2 was obtained as a pale yellow solid in a 70% yield (337 mg, 1.66 mmol).
Acknowledgments
The authors thank Deborah Fabris (University of Pavia) for fruitful discussion and Prof. Mariella Mella and Prof. Enrico Monzani (University of Pavia) for NMR analyses.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.2c00225.
Experimental details and copy of 1H and 13C NMR spectra of the prepared compounds (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Kozlowski M. C.; Dugan E. C.; DiVirgilio E. S.; Maksimenka K.; Bringmann G. Asymmetric Total Synthesis of Nigerone and ent-Nigerone: Enantioselective Oxidative Biaryl Coupling of Highly Hindered Naphthols. Adv. Synth. Catal. 2007, 349, 583–594. 10.1002/adsc.200600570. [DOI] [Google Scholar]; b DiVirgilio E. S.; Dugan E. C.; Mulrooney C. A.; Kozlowski M. C. Asymmetric Total Synthesis of Nigerone. Org. Lett. 2007, 9, 385–388. 10.1021/ol062468y. [DOI] [PubMed] [Google Scholar]
- See for instance:; Mahajan B. T.; Mujawar S.; Ghosh S.; Pabbaraja A. K.; Singh Micro-electro-flow reactor (μ-EFR) system for ultra-fast arene synthesis and manufacture of daclatasvir. Chem. Commun. 2019, 55, 11852–11855. 10.1039/C9CC06127D. [DOI] [PubMed] [Google Scholar]
- Bringmann G.; Breuning M.; Tasler S. The lactone concept: an efficient pathway to axially chiral natural products and useful reagents. Synthesis 1999, 1999, 525–558. 10.1055/s-1999-3435. [DOI] [Google Scholar]
- Marín A.; Telo J. P.; Collado D.; Nàjera F.; Pérez- Inestrosa E.; Pischel U. Bis (dioxaborine) Dyes with Variable π-Bridges: Towards Two-Photon Absorbing Fluorophores with Very High Brightness. Chem. - Eur. J. 2018, 24, 2929–2935. 10.1002/chem.201704544. [DOI] [PubMed] [Google Scholar]
- Erbas-Cakmak S.; Leigh D. A.; McTernan C. T.; Nussbaumer A. L. Artificial molecular machines. Chem. Rev. 2015, 115, 10081–10206. 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ullmann F.; Bielecki J. Ueber Synthesen in der Biphenylreihe. Chem. Ber. 1901, 34, 2174–2185. 10.1002/cber.190103402141. [DOI] [Google Scholar]; b Fanta P. E. The Ullmann synthesis of biaryls, 1945-1963. Chem. Rev. 1964, 64, 613–632. 10.1021/cr60232a002. [DOI] [PubMed] [Google Scholar]
- a Vasconcelos S. N. S.; Reis J. S.; de Oliveira I. M.; Balfour M. N.; Stefani H. A. Synthesis of symmetrical biaryl compounds by homocoupling reaction. Tetrahedron 2019, 75, 1865–1959. 10.1016/j.tet.2019.02.001. [DOI] [Google Scholar]; b Nelson T. D.; Crouch R. D.. Cu, Ni, and Pd Mediated Homocoupling Reactions in Biaryl Syntheses: The Ullmann Reaction. In Organic Reactions; John Wiley & Sons, 2004; Vol. 34. [Google Scholar]; c Hassan J.; Sévignon M.; Gozzi C.; Schulz E.; Lemaire M. Aryl– aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1470. 10.1021/cr000664r. [DOI] [PubMed] [Google Scholar]; d Kramer S. Homogeneous Gold-Catalyzed Aryl–Aryl Coupling Reactions. Synthesis 2020, 52, 2017–2030. 10.1055/s-0039-1690882. [DOI] [Google Scholar]
- a Lv L.; Qiu Z.; Li J.; Liu M.; Li C.-J. N2H4 as traceless mediator for homo- and cross- aryl coupling. Nat. Commun. 2018, 9, 4739 10.1038/s41467-018-07198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jutand A.; Mosleh A. Nickel-and palladium-catalyzed homocoupling of aryl triflates. scope, limitation, and mechanistic aspects. J. Org. Chem. 1997, 62, 261–274. 10.1021/jo961464b. [DOI] [PubMed] [Google Scholar]
- See for recent examples:; a Budiman Y. P.; Jayaraman A.; Friedrich A.; Kerner F.; Radius U.; Marder T. B. Palladium-Catalyzed Homocoupling of Highly Fluorinated Arylboronates: Studies of the Influence of Strongly vs Weakly Coordinating Solvents on the Reductive Elimination Process. J. Am. Chem. Soc. 2020, 142, 6036–6050. 10.1021/jacs.9b11871. [DOI] [PubMed] [Google Scholar]; b Appa R. M.; Lakshmidevi J.; Naidu B. R.; Venkateswarlu K. Pd-catalyzed oxidative homocoupling of arylboronic acids in WEPA: A sustainable access to symmetrical biaryls under added base and ligand-free ambient conditions. Mol. Catal. 2021, 501, 111366 10.1016/j.mcat.2020.111366. [DOI] [Google Scholar]
- a Nakatani J.; Nozoe T. Homocoupling of a Grignard Reagent toward the Synthesis of 2, 2′-Bis (trifluoromethyl)-4, 4′-diaminobiphenyl. Org. Process Res. Dev. 2021, 25, 2442–2446. 10.1021/acs.oprd.1c00227. [DOI] [Google Scholar]; b Liu Y.; Berges J.; Zaid Y.; Chahdi F. O.; Van Der Lee A.; Harakat D.; Clot E.; Jaroschik F.; Taillefer M. Aerobic and Ligand-Free Manganese-Catalyzed Homocoupling of Arenes or Aryl Halides via in Situ Formation of Aryllithiums. J. Org. Chem. 2019, 84, 4413–4420. 10.1021/acs.joc.8b02834. [DOI] [PubMed] [Google Scholar]
- a Pei X.; Zhou G.; Li X.; Xu Y.; Panicker R. C.; Srinivasan R. Sterically controlled C–H/C–H homocoupling of arenes via C–H borylation. Org. Biomol. Chem. 2019, 17, 5703–5707. 10.1039/C9OB00995G. [DOI] [PubMed] [Google Scholar]; b Cho S. H.; Kim J. Y.; Kwak J.; Chang S. Recent advances in the transition metal-catalyzed twofold oxidative C–H bond activation strategy for C–C and C–N bond formation. Chem. Soc. Rev. 2011, 40, 5068–5083. 10.1039/c1cs15082k. [DOI] [PubMed] [Google Scholar]
- Xie W.-W.; Liu Y.; Yuan R.; Zhao D.; Yu T.-Z.; Zhang J.; Da C.-S. Transition Metal-Free Homocoupling of Unactivated Electron-Deficient Azaarenes. Adv. Synth. Catal. 2016, 358, 994–1002. 10.1002/adsc.201500445. [DOI] [Google Scholar]
- a Matsuda T.; Asai R.; Shiose S.; Kato K. Homocoupling of arylboronic acids catalyzed by simple gold salts. Tetrahedron Lett. 2011, 52, 4779–4781. 10.1016/j.tetlet.2011.07.030. [DOI] [Google Scholar]; b Parmentier T. E.; Dawson S. R.; Malta G.; Lu L.; Davies T. E.; Kondrat S. A.; Freakley S. J.; Kiely C. J.; Hutchings G. J. Homocoupling of phenylboronic acid using atomically dispersed gold on carbon catalysts: catalyst evolution before reaction. ChemCatChem 2018, 10, 1853–1859. 10.1002/cctc.201701840. [DOI] [Google Scholar]
- For the homocoupling of aryl iodides see for instance:; a Dabiri M.; Kashi B. S. R.; Lehi N. F.; Bashiribo S. Synthesis of gold nanoparticles decorated on sulfonated three-dimensional graphene nanocomposite and application as a highly efficient and recyclable heterogeneous catalyst for Ullmann homocoupling of aryl iodides and reduction of p-nitrophenol. Appl. Organometal. Chem. 2018, 32, e4189 10.1002/aoc.4189. [DOI] [Google Scholar]; b Chen T.; Chen B.-T.; Bukhryakov K. V.; Rodionov V. O. Thiols make for better catalysts: Au nanoparticles supported on functional SBA-15 for catalysis of Ullmann-type homocouplings. Chem. Commun. 2017, 53, 11638–11641. 10.1039/C7CC06146C. [DOI] [PubMed] [Google Scholar]; aryl bromides:; c Wang J.; Xu A.; Jia M.; Bai S.; Cheng X.; Zhaorigetu B. Hydrotalcite-supported Pd–Au nanocatalysts for Ullmann homocoupling reactions at low temperature. New J. Chem. 2017, 41, 1905–1908. 10.1039/C6NJ03541H. [DOI] [Google Scholar]; aryl chlorides:; d Ehara M.; Priyakumar U. D. Gold-Palladium Nanocluster Catalysts for Homocoupling: Electronic Structure and Interface Dynamics. Chem. Rec. 2019, 19, 947–959. 10.1002/tcr.201800177. [DOI] [PubMed] [Google Scholar]
- Ishida T.; Aikawa S.; Mise Y.; Akebi R.; Hamasaki A.; Honma T.; Ohashi H.; Tsuji T.; Yamamoto Y.; Miyasaka M.; Yokoyama T.; Tokunaga M. Direct C-H Arene Homocoupling over Gold Nanoparticles Supported on Metal Oxides. ChemSusChem 2015, 8, 695–701. 10.1002/cssc.201402822. [DOI] [PubMed] [Google Scholar]
- Chemical Photocatalysis, 2nd ed.; König B., Ed.; De Gruyter, 2020. [Google Scholar]
- Capaldo L.; Ravelli D.; Fagnoni M. Direct Photocatalyzed Hydrogen Atom Transfer (HAT) for Aliphatic C-H Bonds Elaboration. Chem. Rev. 2022, 122, 1875–1924. 10.1021/acs.chemrev.1c00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Campbell M. W.; Polites V. C.; Patel S.; Lipson J. E.; Majhi J.; Molander G. A. Photochemical C–F Activation Enables Defluorinative Alkylation of Trifluoroacetates and-Acetamides. J. Am. Chem. Soc. 2021, 143, 19648–19654. 10.1021/jacs.1c11059. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhao H.; McMillan A. J.; Constantin T.; Mykura R. C.; Juliá F.; Leonori D. Merging Halogen-Atom Transfer (XAT) and Cobalt Catalysis to Override E2-Selectivity in the Elimination of Alkyl Halides: A Mild Route toward contra-Thermodynamic Olefins. J. Am. Chem. Soc. 2021, 143, 14806–14813. 10.1021/jacs.1c06768. [DOI] [PubMed] [Google Scholar]
- Fagnoni M.Sustainable Organic Synthesis: Tools and Strategies; Protti S.; Palmieri A., Eds.; The Royal Society of Chemistry, 2022; pp 150–180. [Google Scholar]
- a Lee J.; Hong B.; Lee A. Visible-light-promoted, catalyst-free Gomberg–Bachmann reaction: synthesis of biaryls. J. Org. Chem. 2019, 84, 9297–9306. 10.1021/acs.joc.9b00557. [DOI] [PubMed] [Google Scholar]; b Bergami M.; Protti S.; Ravelli D.; Fagnoni M. Flow Metal-Free Ar-C Bond Formation via Photogenerated Phenyl Cations. Adv. Synth. Catal. 2016, 358, 1164–1172. 10.1002/adsc.201600019. [DOI] [Google Scholar]; c Kloss F.; Neuwirth T.; Haensch V. G.; Hertweck C. Metal-Free Synthesis of Pharmaceutically Important Biaryls by Photosplicing. Angew. Chem., Int. Ed. 2018, 57, 14476–14481. 10.1002/anie.201805961. [DOI] [PubMed] [Google Scholar]
- a Babu S. S.; Muthuraja P.; Yadav P.; Gopinath P. Aryldiazonium Salts in Photoredox Catalysis–Recent Trends. Adv. Synth. Catal. 2021, 363, 1782–1809. 10.1002/adsc.202100136. [DOI] [Google Scholar]; b König B. Photocatalysis in organic synthesis–past, present, and future. Eur. J. Org. Chem. 2017, 2017, 1979–1981. 10.1002/ejoc.201700420. [DOI] [Google Scholar]; c Madasu J.; Shinde S.; Das R.; Patel S.; Shard A. Potassium tert-butoxide mediated C–C, C–N, C–O and C–S bond forming reactions. Org. Biomol. Chem. 2020, 18, 8346–8365. 10.1039/D0OB01382J. [DOI] [PubMed] [Google Scholar]; d Kvasovs N.; Gevorgyan V. Contemporary methods for generation of aryl radicals. Chem. Soc. Rev. 2021, 50, 2244–2259. 10.1039/D0CS00589D. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Akram M. O.; Banerjee S.; Saswade S. S.; Bedi V.; Pati N. T. Oxidant-free oxidative gold catalysis: the new paradigm in cross-coupling reactions. Chem. Commun. 2018, 54, 11069–11083. 10.1039/C8CC05601C. [DOI] [PubMed] [Google Scholar]; f Witzel S.; Hoffmann M.; Rudolph M.; Rominger F.; Dreuw A.; Hashmi A. S. K. A Radical Chain: Mononuclear “Gold Only” Photocatalysis. Adv. Synth. Catal. 2022, 364, 581–592. 10.1002/adsc.202101113. [DOI] [Google Scholar]
- de Gracia Retamosa M.; Döndaş H. A.; Sobhani S.; Nájera C.; Yus M. A.; Sansano J. M. Photocatalytic Homocoupling Transformations. Synthesis 2021, 53, 3653–3672. 10.1055/a-1517-7329. [DOI] [Google Scholar]
- Crabbe B. W.; Kuehm O. P.; Bennett J. C.; Hallett-Tapley G. L. Light-activated Ullmann homocoupling of aryl halides catalyzed using gold nanoparticle-functionalized potassium niobium oxides. Catal. Sci. Technol. 2018, 8, 4907–4915. 10.1039/C8CY00996A. [DOI] [Google Scholar]
- Tran H.; McCallum T.; Morin M.; Barriault L. Homocoupling of iodoarenes and bromoalkanes using photoredox gold catalysis: A light enabled Au(III) reductive elimination. Org. Lett. 2016, 18, 4308–4311. 10.1021/acs.orglett.6b02021. [DOI] [PubMed] [Google Scholar]
- Masuda Y.; Ishida N.; Murakami M. Aryl Ketones as Single-Electron-Transfer Photoredox Catalysts in the Nickel-Catalyzed Homocoupling of Aryl Halides. Eur. J. Org. Chem. 2016, 2016, 5822–5825. 10.1002/ejoc.201601352. [DOI] [Google Scholar]
- Crabbe B. W.; Kuehm O. P.; Bennett J. C.; Hallett-Tapley G. L. Light-activated Ullmann homocoupling of aryl halides catalyzed using gold nanoparticle-functionalized potassium niobium oxides. Catal. Sci. Technol. 2018, 8, 4907–4915. 10.1039/C8CY00996A. [DOI] [Google Scholar]
- Feizpour F.; Jafarpour M.; Rezaeifard A. Band Gap Modification of TiO2 Nanoparticles by Ascorbic Acid-Stabilized Pd Nanoparticles for Photocatalytic Suzuki–Miyaura and Ullmann Coupling Reactions. Catal. Lett. 2019, 149, 1595–1610. 10.1007/s10562-019-02749-z. [DOI] [Google Scholar]
- a Qiu D.; Lian C.; Mao J.; Fagnoni M.; Protti S. Dyedauxiliary Groups, an Emerging Approach in Organic Chemistry. The Case of Arylazo Sulfones. J. Org. Chem. 2020, 85, 12813–12822. 10.1021/acs.joc.0c01895. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Meng N.; Liu Q.; Liu R.-S.; Lüa Y.; Zhao X.; Wei W. Recent Advances in Arylations and Sulfonylations of Arylazo Sulfones. Chin. J. Org. Chem. 2021, 41, 4639–4650. 10.6023/cjoc202107022. [DOI] [Google Scholar]; c Chu X.-Q.; Ge D.; Cui Y.-Y.; Shen Z.-L.; Li C.-J. Desulfonylation via Radical Process: Recent Developments in Organic Synthesis. Chem. Rev. 2021, 121, 12548–12680. 10.1021/acs.chemrev.1c00084. [DOI] [PubMed] [Google Scholar]
- See for instance:; a Dossena A.; Sampaolesi S.; Palmieri A.; Protti S.; Fagnoni M. Visible light promoted metal-and photocatalyst-free synthesis of allylarenes. J. Org. Chem. 2017, 82, 10687–10692. 10.1021/acs.joc.7b01532. [DOI] [PubMed] [Google Scholar]; b Terlizzi L. D.; Cola I.; Raviola C.; Fagnoni M.; Protti S. Dyedauxiliary Group Strategy for the α-Functionalization of Ketones and Esters. ACS Org. Inorg. Au 2021, 1, 68–71. 10.1021/acsorginorgau.1c00020. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Malacarne M.; Protti S.; Fagnoni M. A Visible-Light-Driven, Metal-free Route to Aromatic Amides via Radical Arylation of Isonitriles. Adv. Synth. Catal. 2017, 359, 3826–3830. 10.1002/adsc.201700619. [DOI] [Google Scholar]
- a Lian C.; Yue G.; Mao J.; Liu D.; Ding Y.; Liu Z.; Qiu D.; Zhao X.; Lu K.; Fagnoni M.; Protti S. Visible-light-driven synthesis of arylstannanes from arylazo sulfones. Org. Lett. 2019, 21, 5187–5191. 10.1021/acs.orglett.9b01788. [DOI] [PubMed] [Google Scholar]; b Li A.; Li Y.; Liu J.; Chen J.; Lu K.; Qiu D.; Fagnoni M.; Protti S.; Zhao X. Metal-Free Trifluoromethylthiolation of Arylazo Sulfones. J. Org. Chem. 2021, 86, 1292–1299. 10.1021/acs.joc.0c02669. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Xu Y.; Yang X.; Fang H. Additive-and photocatalyst-free borylation of arylazo sulfones under visible light. J. Org. Chem. 2018, 83, 12831–1283. 10.1021/acs.joc.8b01662. [DOI] [PubMed] [Google Scholar]; d Chawla R.; Jaiswal S.; Dutta P. K.; Yadav L. D. S. Photocatalyst-free visible light driven synthesis of (E)-vinyl sulfones from cinnamic acids and arylazo sulfones. Tetrahedron Lett. 2020, 61, 151898 10.1016/j.tetlet.2020.151898. [DOI] [Google Scholar]
- Sauer C.; Liu Y.; De Nisi A.; Protti S.; Fagnoni M.; Bandini M. Photocatalyst-free, Visible Light Driven, Gold Promoted Suzuki Synthesis of (Hetero) biaryls. ChemCatChem 2017, 9, 4456–4459. 10.1002/cctc.201701436. [DOI] [Google Scholar]
- Thamban Chandrika N.; Dennis E. K.; Shrestha S. K.; Ngo H. X.; Green K. D.; Kwiatkowski S.; Deaciuc A. G.; Dwoskin L. P.; Watt D. S.; Garneau-Tsodikov S. N, N′-diaryl-bishydrazones in a biphenyl platform: broad spectrum antifungal agents. Eur. J. Med. Chem. 2019, 164, 273–281. 10.1016/j.ejmech.2018.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dorel R.; Echavarren A. M. Gold (I)-catalyzed activation of alkynes for the construction of molecular complexity. Chem. Rev. 2015, 115, 9028–9072. 10.1021/cr500691k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b de Haro T.; Nevado C. Gold-Catalyzed Ethynylation of Arenes. J. Am. Chem. Soc. 2010, 132, 1512–1513. 10.1021/ja909726h. [DOI] [PubMed] [Google Scholar]; c Asiri A. M.; Hashmi A. S. K. Gold-catalysed reactions of diynes. Chem. Soc. Rev. 2016, 45, 4471–4503. 10.1039/C6CS00023A. [DOI] [PubMed] [Google Scholar]; d Hashmi A. S. K.; Wieteck M.; Braun I.; Nçsel P.; Jongbloed L.; Rudolph M.; Rominger F. Gold-catalyzed synthesis of dibenzopentalenes–evidence for gold vinylidenes. Adv. Synth. Catal. 2012, 354, 555–562. 10.1002/adsc.201200086. [DOI] [Google Scholar]
- Edwards P. R.; Hrycay E. G.; Bandiera S. M. Differential inhibition of hepatic microsomal alkoxyresorufin O-dealkylation activities by tetrachlorobiphenyls. Chem.-Biol. Interact. 2007, 169, 42–52. 10.1016/j.cbi.2007.05.004. [DOI] [PubMed] [Google Scholar]
- a Blakemore D.Synthetic Methods in Drug Discovery; The Royal Society of Chemistry, 2016; Vol. 1, pp 1–69. [Google Scholar]; b Markovic T.; Rocke B. N.; Blakemore D. C.; Mascitti V.; Willis M. C. Pyridine sulfinates as general nucleophilic coupling partners in palladium-catalyzed cross-coupling reactions with aryl halides. Chem. Sci. 2017, 8, 4437–4442. 10.1039/C7SC00675F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K.; Yasui M.; Ema T. Binaphthyl–bipyridyl cyclic dyads as a chiroptical switch. J. Am. Chem. Soc. 2018, 140, 5334–5338. 10.1021/jacs.8b01860. [DOI] [PubMed] [Google Scholar]
- a Hopkinson M. N.; Tlahuext-Aca A.; Glorius F. Merging visible light photoredox and gold catalysis. Acc. Chem. Res. 2016, 49, 2261–2272. 10.1021/acs.accounts.6b00351. [DOI] [PubMed] [Google Scholar]; b Witzel S.; Hashmi A. S. K.; Xie J. Light in Gold Catalysis. Chem. Rev. 2021, 121, 8868–8925. 10.1021/acs.chemrev.0c00841. [DOI] [PubMed] [Google Scholar]; c Zidan M.; Rohe S.; McCallum T.; Barriault L. Recent advances in mono and binuclear gold photoredox catalysis. Catal. Sci. Technol. 2018, 8, 6019–6028. 10.1039/C8CY01765D. [DOI] [Google Scholar]
- a Nijamudheen A.; Datta A. Gold-catalyzed cross-coupling reactions: an overview of design strategies, mechanistic studies, and applications. Chem.–Eur. J. 2020, 26, 1442–1487. 10.1002/chem.201903377. [DOI] [PubMed] [Google Scholar]; b Chan A. Y.; Perry I. B.; Bissonnette N. B.; Buksh B. F.; Edwards G. A.; Frye L. I.; Garry O. L.; Lavagnino M. N.; Li B. X.; Liang Y.; Mao E.; Millet A.; Oakley J. V.; Reed N. L.; Holt Sakai A.; Ciaran Seath P.; MacMillan D. W. C. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 2022, 122, 1485–1522. 10.1021/acs.chemrev.1c00383. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Font P.; Ribas X. Fundamental Basis for Implementing Oxidant-Free Au(I)/Au(III) Catalysis. Eur. J. Inorg. Chem. 2021, 2021, 2556–2569. 10.1002/ejic.202100301. [DOI] [Google Scholar]
- a Ye X.; Zhao P.; Zhang S.; Zhang Y.; Wang Q.; Shan C.; Wojtas L.; Guo H.; Chen H.; Shi X. Facilitating Gold Redox Catalysis with Electrochemistry: An Efficient Chemical-Oxidant-Free Approach. Angew. Chem., Int. Ed. 2019, 58, 17226–17230. 10.1002/anie.201909082. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Xia Z.; Corcé V.; Zhao F.; Przybylski C.; Espagne A.; Jullien L.; Le Saux T.; Gimbert Y.; Dossmann H.; Mouriès-Mansuy V.; Ollivier C.; Fensterbank L. Photosensitized oxidative addition to gold(I) enables alkynylative cyclization of o-alkylnylphenols with iodoalkynes. Nat. Chem. 2019, 11, 797–805. 10.1038/s41557-019-0295-9. [DOI] [PubMed] [Google Scholar]
- Perugine A. M.; Abbott M. B.; Soares Da Costa T. P. World Patent WO2019/241850A12019.
- Russell Bowman W.; Forshaw; Hall J. A.; Kitchin K. P. J. P.; Mott A. W. Reduction of Ag (I) by 1-acyl-2-arylhydrazines: Mechanism of photographic infectious development. Tetrahedron 1996, 52, 3961–3972. 10.1016/S0040-4020(96)00050-6. [DOI] [Google Scholar]
- Chen D.-L.; Sun Y.; Chen M.; Li X.; Zhang L.; Huang X.; Bai Y.; Luo F.; Peng B. Desulfurization of diaryl (heteroaryl) sulfoxides with benzyne. Org. Lett. 2019, 21, 3986–3989. 10.1021/acs.orglett.9b01144. [DOI] [PubMed] [Google Scholar]
- Minus M. B.; Moor S. R.; Pari F. F.; Nirmani L. P. T.; Chwatko M.; Okoke B.; Singleton J. E.; Nelson T. L.; Lynd N. A.; Anslyn E. V. “Benchtop” biaryl coupling using Pd/Cu cocatalysis: application to the synthesis of conjugated polymers. Org. Lett. 2021, 23, 2873–2877. 10.1021/acs.orglett.1c00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi B.; Barzegar H.; Vali H. Au–Pd bimetallic nanoparticles supported on a high nitrogen-rich ordered mesoporous carbon as an efficient catalyst for room temperature Ullmann coupling of aryl chlorides in aqueous media. Chem. Commun. 2018, 54, 7155–7158. 10.1039/C8CC00475G. [DOI] [PubMed] [Google Scholar]
- Lakshimidevi J.; Appa R. M.; Naidu B. R.; Prasad S. S.; Sarma L. S.; Venkateswarlu K. Au–Pd bimetallic nanoparticles supported on a high nitrogen-rich ordered mesoporous carbon as an efficient catalyst for room temperature Ullmann coupling of aryl chlorides in aqueous media. Chem. Commun. 2018, 54, 12333–12336. 10.1039/C8CC06940A. [DOI] [PubMed] [Google Scholar]
- Cho S. K.; Song S. H.; Hahn J. T.; Jung D. Biaryl Diketone Synthesis via Palladium-catalyzed Carbonylative Coupling with Carbon Monoxide or Various Metal Carbonyls. Bull. Korean Chem. Soc. 2016, 37, 1567–1570. 10.1002/bkcs.10904. [DOI] [Google Scholar]
- Li M.-X.; Tang Y.-L.; Gao H.; Mao Z.-W. Efficient Pd-catalyzed oxidative homocoupling of arylboronic acids in aqueous NaClO. Tetrahedron Lett. 2020, 61, 151784 10.1016/j.tetlet.2020.151784. [DOI] [Google Scholar]
- Zhao Q.; Chen L.; Lang H.; Wu S.; Wang L. Pd(OAc)2/PPh3-Catalyzed Desulfonylative Homocoupling of Arylsulfonyl Chlorides. Chin. J. Chem. 2015, 33, 535–538. 10.1002/cjoc.201400868. [DOI] [Google Scholar]
- Goldstein J. H.; Tarpley A. R. Nuclear magnetic resonance spectra and substituent effects for symmetrically substituted dihalobiphenyls. J. Phys. Chem. A 1971, 75, 421–430. 10.1021/j100673a022. [DOI] [Google Scholar]
- Zhu J.; Chen P.-H.; Lu G.; Liu P.; Dong G. Ruthenium-catalyzed reductive cleavage of unstrained aryl–aryl bonds: reaction development and mechanistic study. J. Am. Chem. Soc. 2019, 141, 18630–18640. 10.1021/jacs.9b11605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems S.; Touplas G.; Reisenbauer J. C.; Morandi B. A site-selective and stereospecific cascade Suzuki–Miyaura annulation of alkyl 1,2-bisboronic esters and 2,2′-dihalo 1,1′-biaryls. Chem. Commun. 2021, 57, 3909–3912. 10.1039/D1CC00648G. [DOI] [PubMed] [Google Scholar]
- Qu X.; Li T.; Zhu Y.; Sun P.; Yang H.; Mao J. Ligand-free highly effective iron/copper co-catalyzed formation of dimeric aryl ethers or sulfides. Org. Biomol. Chem. 2011, 9, 5043–5046. 10.1039/c1ob05155e. [DOI] [PubMed] [Google Scholar]
- Puthiaraj P.; Sureshab P.; Pitchumani K. Aerobic homocoupling of arylboronic acids catalysed by copper terephthalate metal–organic frameworks. Green Chem. 2014, 16, 2865–2875. 10.1039/c4gc00056k. [DOI] [Google Scholar]
- Wang G.; Wu Z.; Liang Y.; Liu W.; Zhan H.; Song M.; Sun Y. Exploring the coordination confinement effect of divalent palladium/zero palladium doped polyaniline-networking: As an excellent-performance nanocomposite catalyst for C-C coupling reactions. J. Catal. 2020, 384, 177–188. 10.1016/j.jcat.2020.02.021. [DOI] [Google Scholar]
- van Kalkeren H. A.; Leenders S. H. A. M.; Hommersom C. R. A.; Rutjes F. P. J. T.; van Delft F. L. In situ phosphine oxide reduction: a catalytic Appel reaction. Chem.–Eur. J. 2011, 17, 11290–11295. 10.1002/chem.201101563. [DOI] [PubMed] [Google Scholar]
- Budniak A. K.; Masny M.; Prezelj K.; Grzeszkiewicz M.; Gawraczyński J.; Dobrzycki L.; Cyrański M. K.; Koźmiński W.; Mazej Z.; Fijałkowski K. J.; Grochala W.; Leszczyński P. J. Reconnaissance of reactivity of an Ag(II)SO4 one-electron oxidizer towards naphthalene derivatives. New J. Chem. 2017, 41, 10742–10749. 10.1039/C7NJ02299A. [DOI] [Google Scholar]
- Tran R. Q.; Dinh L. P.; Jacoby S. A.; Harris N. W.; Swann W. A.; Williamson S. N.; Semseya R. Y.; Yet L. Synthesis of 3-aryl-1-phosphinoimidazo [1, 5-a] pyridine ligands for use in Suzuki–Miyaura cross-coupling reactions. RSC Adv. 2021, 11, 28347–28351. 10.1039/D1RA05417A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel A. J.; Hedidi M.; Bentabed-Ababsa G. M.; Roisnel T.; Mongin F.; Wheatley A. E. H. Extending motifs in lithiocuprate chemistry: unexpected structural diversity in thiocyanate complexes. Dalton Trans. 2016, 45, 6094–6104. 10.1039/C5DT03882K. [DOI] [PubMed] [Google Scholar]
- Cook X. A. F.; Pantaine L. R. E.; Blakemore D. C.; Moses I. B.; Sach N. W.; Shavnya A.; Willis M. C. Base-Activated Latent Heteroaromatic Sulfinates as Nucleophilic Coupling Partners in Palladium-Catalyzed Cross-Coupling Reactions. Angew. Chem., Int. Ed. 2021, 60, 22461–22468. 10.1002/anie.202109146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.