Abstract

Here we report the first total synthesis of several oligosaccharides resembling the capsular polysaccharide of swine pathogen S. suis serotype 18 repeating unit [→3)-d-GalNAc(α1-3)[d-Glc(β1-2)]-d-GalA4OAc(β1-3)-d-GalNAc(α1-3)-d-BacNAc4NAc(α1→]n. Access to the pentasaccharide repeating unit antigen proved to be very challenging due to the poor reactivity in the context of the trisaccharide. The challenge was overcome by the creation of a galacturonic acid in a late stage of the synthesis.
Streptococcus suis (S. suis) infections of farmed pigs cause serious economic losses, and humans have been increasingly infected by these antibiotic-resistant bacteria.1−3 Capsular polysaccharides (CPSs) surrounding Gram-negative bacteria are the basis of very successful glycoconjugate vaccines against the human pathogen Streptococcus pneumoniae. The vaccination of pigs and humans against S. suis to prevent rather than treat the disease would avoid the use of antibiotics and reduce the development of antibiotic resistance. Thirty-five S. suis serotypes can be distinguished based on their CPS structures. A glycoconjugate vaccine candidate against S. suis serotype 2 (SS2) based on isolated CPS4 and semisynthetic glycoconjugate vaccine candidates for SS2, SS3, SS9, and SS14 have been evaluated.4,5 The S. suis serotype 18 CPS pentasaccharide repeating unit provides an interesting challenge for synthetic chemists as a first step toward a glycoconjugate vaccine for this serotype. The SS18 pentasaccharide repeating unit made up of [→3)-d-GalNAc(α1-3)[d-Glc-(β1-2)]d-GalA4OAc(β1-3)-d-GalNAc(α1-3)-d-BacNAc4NAc(α1→]n (Figure 1a)6 requires the installation of a 1,2-cis linkage between d-bacillosamine and the reducing-end linker. The central galacturonic acid branching unit is a challenge concerning the poor reactivity and protecting group orthogonality.
Figure 1.

(a) Repeating unit of the S. suis serotype 18 CPS. (b) Retrosynthetic analysis of target oligosaccharides 1–5.
The SS18 pentasaccharide repeating unit (Figure 1a) contains the rare deoxy amino sugar d-bacillosamine in addition to d-galactosamine, d-galacturonic acid, d-glucose, and d-galactose. A linear approach will require the synthesis of the rare sugar d-bacillosamine derivative building block and the stereocontrolled 1,2-cis linkage between the d-bacillosamine derivative and the linker subsequently used for conjugation. The central galacturonic acid will have to be glycosylated twice at the C2 and C3 positions. The presence of C4-OAc at the d-galacturonic acid of pentasaccharide 1 complicates the synthesis by not allowing the use of most ester protecting groups. Target pentasaccharides 1 and oligosaccharides 2–5 resembling different portions of the CPS repeating unit can be prepared using a linear synthesis strategy with five building blocks 6–10 (Figure 1b).
d-Bacillosamine derivative 6 (Scheme 1) was synthesized starting from d-galactosamine building block77 and was used to glycosylate the protected reducing end linker 11 using NIS/TMSOTf as a promoter to afford the exclusively α-linked glycoside 12 in 68% yield. The bulky alkyl substituents of the 4,6-O-silylidene group prevent the attack of the nucleophile from the β-face of the donor, combined with through-space electron donation that stabilizes the oxocarbenium-like intermediate8 to ensure the complete stereoselectivity of the glycosylation. Silylidene removal using HF in pyridine9 yielded dihydroxy galactosamine derivative 13 (96%) followed by tosylation of the primary C6 hydroxyl10 to give 14 in 95% yield. C6-Deoxygenation was achieved via iodination with NaI in refluxing acetone (93% yield of 15), and subsequent dehalogenation/reduction with tributyltin hydride yielded fucosamine11 derivative 16 (76%). Selective acylation of the amine in 16 using trichloroacetyl choride12 afforded 17 in 85% yield. The triflation of 17 using triflic anhydride followed by C4 inversion with stoichiometric amounts of sodium azide provided d-bacillosamine derivative1318 in 68% yield over two steps. Oxidative cleavage of the naphthyl ether (Nap) by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) afforded d-bacillosamine derivative14 building block 6 in 90% yield.
Scheme 1. Synthesis of d-Bacillosamine Derivative Acceptor 6.
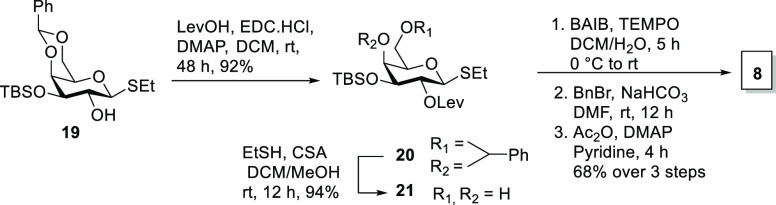
Galacturonic acid building block 8 was prepared from differentially protected galactose thioglycoside 19 (Scheme 2).15 Levulinoylation, followed by benzylidene acetal hydrolysis,16 gave dihydroxy galactose thioglycoside 21. Selective oxidation of the primary C6 alcohol to the carboxylic acid17 using TEMPO and subsequent benzylation followed by acetylation gave rise to d-galacturonic acid thioglycoside 8 in 68% yield over three steps. Glucose building block 9 was synthesized in one step from a known thioglycoside S1.18 (See the Supporting Information.)
Scheme 2. Synthesis of Differentially Protected Galacturonic Acid Building Block 8.
The oligosaccharide assembly commenced with the NIS/TMSOTf-promoted union of d-bacillosamine derivative 6 and d-galactosamine 7. Removal of the silylidene group by treatment with HF·Py followed by benzylation gave the differentially protected disaccharide 24 (Scheme 3). Oxidative removal of the naphthyl ether using DDQ furnished disaccharide acceptor 25 in 85% yield. The glycosylation of disaccharide 25 using galacturonic acid 8 afforded the protected trisaccharide. The subsequent cleavage of the silyl ether using HF·Py proved difficult and furnished a complex mixture of products. Desilylation using BF3·Et2O was successful and produced the desired acceptor1926 in 56% yield over two steps. Several attempts to synthesize tetrasaccharide 28 by the glycosylation of trisaccharide acceptor 26 using selenoglycoside 10 and the corresponding trichloroacetimidate 27(20,21) were not met with success. The poor nucleophilicity of the free hydroxyl group of 26 is a result of the electron-withdrawing groups at C4 and C6, which rendered glycosylations doomed to failure.
Scheme 3. Attempted Synthesis of Tetrasaccharide 28.

A less direct method using galactose in place of galacturonic acid had to be explored to overcome the reactivity problems associated with the low nucleophilicity of the central galacturonic acid unit. The glycosylation of disaccharide 25 with galactose building block 20 afforded trisaccharide 29 in 94% yield, which was liberated from the silyl ether protective group to furnish trisaccharide acceptor 30. The union of trisaccharide 30 and selenoglycoside 10 followed by the cleavage of the levulinoyl ester using hydrazine acetate afforded tetrasaccharide 32. The glycosylation of acceptor 32 with glucosamine building block 9 produced pentasaccharide 33 in 64% yield. The central galacturonic acid moiety was prepared by the camphorsulfonic acid (CSA)-mediated hydrolysis of the benzylidene acetal followed by BAIB/TEMPO oxidation, and the selective benzylation of carboxylic acid afforded pentasaccharide 35. The azide of pentasaccharide 35 was converted into the corresponding acetamide using zinc powder in a THF/Ac2O/AcOH mixture; subsequently, silylidene ether and the levulinoyl ester group were deprotected, and the hydrogenation reaction provided the S. suis serotype 18 CPS resembling the repeating unit pentasaccharide target 2 in 11% yield over four steps. In addition, pentasaccharide 35 was acetylated followed by azide conversion to acetamide, and the subsequent removal of silylidene and levulinoyl ester and the hydrogenation reaction provided the S. suis serotype 18 CPS repeating unit pentasaccharide target 1 in 15% yield over five steps22 (Scheme 4).
Scheme 4. Synthesis of Unprotected Pentasaccharides 1 and 2.

Three oligosaccharides (3, 4, and 5) that will be essential for subsequent immunological studies to identify the minimally protective glycan epitope were prepared using a divergent synthesis approach (Scheme 5). The benzylation of galactosamine diol 13 afforded 36 (see the Supporting Information), which was freed from the Nap ether to provide 37. The glycosylation of monosaccharide 37 with galacturonic acid building block 8 exclusively furnished the β-isomer of disaccharide 38 in 75% yield. Levulinoyl ester cleavage using hydrazine acetate afforded 39; then, glycosylation with 9 in the presence of TfOH and N-iodosuccinimide (NIS) at −20 °C produced trisaccharide 40 in 68% yield. The cleavage of levulinoyl ester and tert-butyldimethylsilyl (TBS) ether afforded diol 41. Conversion of the azide to the corresponding acetamide using Zn/AcOH/Ac2O followed by hydrogenation afforded trisaccharide 3 (46% yield over two steps). Trisaccharide 4 was prepared by the TBS removal of 38 in preparation for glycosylation with 10 to furnish trisaccharide 43. The global deprotection of 43 produced the desired trisaccharide 4. Disaccharide 5 was readily accessible by deprotection of disaccharide 39 in 46% yield over three steps.
Scheme 5. Synthesis of Oligosaccharides Resembling S. suis Serotype 18.
In conclusion, we report the total synthesis of several oligosaccharides resembling the CPS of swine pathogen S. suis serotype 18 that are the basis for immunological studies and the development of a glycoconjugate vaccine. The rare d-bacillosamine derivative was prepared from d-galactosamine using tin-mediated reduction and dehalogenation. Access to the pentasaccharide repeating unit antigen proved to be very challenging due to the poor reactivity of the trisaccharide intermediate. The challenge was overcome by the creation of galacturonic acid in a late stage of the synthesis. The conjugation-ready glycans prepared using the total synthesis approach will be used for immunological studies.
Acknowledgments
We acknowledge generous funding by the Max-Planck Society. Open access funding was enabled and organized by Project DEAL.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.2c00596.
Complete experimental procedures and NMR spectra of synthetic compounds (PDF)
Open access funded by Max Planck Society.
The authors declare no competing financial interest.
Supplementary Material
References
- Feng Y.; Zhang H.; Wu Z.; Wang S.; Cao M.; Hu D.; Wang C. Streptococcus suis infection. Virulence 2014, 5, 477–497. 10.4161/viru.28595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lun Z.-R.; Wang Q.-P.; Chen X.-G.; Li A.-X.; Zhu X.-Q. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect. Dis. 2007, 7, 201–209. 10.1016/S1473-3099(07)70001-4. [DOI] [PubMed] [Google Scholar]; b Oberli M. A.; Tamborrini M.; Tsai Y.-H.; Werz D. B.; Horlacher T.; Adibekian A.; Gauss D.; Möller H. M.; Pluschke G.; Seeberger P. H. Molecular Analysis of Carbohydrate–Antibody Interactions: Case Study Using a Bacillus anthracis Tetrasaccharide. J. Am. Chem. Soc. 2010, 132, 10239–10241. 10.1021/ja104027w. [DOI] [PubMed] [Google Scholar]
- Hernandez-Garcia J.; Wang J.; Restif O.; Holmes M. A.; Mather A. E.; Weinert L. A.; Wileman T. M.; Thomson J. R.; Langford P. R.; Wren B. W.; Rycroft A.; Maskell D. J.; Tucker A. W. Patterns of antimicrobial resistance in Streptococcus suis isolates from pigs with or without streptococcal disease in England between 2009 and 2014. Vet. Microbiol. 2017, 207, 117–124. 10.1016/j.vetmic.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arends J. P.; Zanen H. C. Meningitis Caused by Streptococcus suis in Humans. Rev. Infect. Dis. 1988, 10, 131–137. 10.1093/clinids/10.1.131. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Sella M.; Sianturi J.; Priegue P.; Shen D.; Seeberger P. H. Discovery of Oligosaccharide Antigens for Semi-Synthetic Glycoconjugate Vaccine Leads against Streptococcus suis Serotypes 2, 3, 9 and 14**. Angew. Chem., Int. Ed. 2021, 60, 14679–14692. 10.1002/anie.202103990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsaut L.; Martelet L.; Goyette-Desjardins G.; Beauchamp G.; Denicourt M.; Gottschalk M.; Segura M. Immunogenicity study of a Streptococcus suis autogenous vaccine in preparturient sows and evaluation of passive maternal immunity in piglets. BMC Vet. Res. 2021, 17, 72. 10.1186/s12917-021-02774-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Es D.; Groenia N. A.; Laverde D.; Overkleeft H. S.; Huebner J.; van der Marel G. A.; Codee J. D.C. Synthesis of E. faecium wall teichoic acid fragments. Bioorg. Med. Chem. 2016, 24, 3893–3907. 10.1016/j.bmc.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Jeanneret R. A.; Johnson S. E.; Galan M. C. Conformationally Constrained Glycosyl Donors as Tools to Control Glycosylation Outcomes. J. Org. Chem. 2020, 85, 15801. 10.1021/acs.joc.0c02045. [DOI] [PubMed] [Google Scholar]
- Hagen B.; van Dijk J. H. M.; Zhang Q.; Overkleeft H. S.; van der Marel G. A.; Codée J. D. C. Synthesis of the Staphylococcus aureus Strain M Capsular Polysaccharide Repeating Unit. Org. Lett. 2017, 19, 2514–2517. 10.1021/acs.orglett.7b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M.; Matsumoto H.; Goto K.; Hamasaki K. Synthesis of aminoglycoside derivatives on a Cbz-type heavy fluorous tag. Tetrahedron Lett. 2006, 47, 8831–8835. 10.1016/j.tetlet.2006.10.064. [DOI] [Google Scholar]
- Cheng J. M. H.; Dangerfield E. M.; Timmer M. S. M.; Stocker B. L. A divergent approach to the synthesis of iGb3 sugar and lipid analogues via a lactosyl 2-azido-sphingosine intermediate. Org. Biomol. Chem. 2014, 12, 2729–2736. 10.1039/C4OB00241E. [DOI] [PubMed] [Google Scholar]
- van Mechelen J.; Voorneveld J.; Overkleeft H. S.; Filippov D. V.; van der Marel G. A.; Codée J. D. C. Synthesis of orthogonally protected and functionalized bacillosamines. Org. Biomol. Chem. 2020, 18, 2834–2837. 10.1039/D0OB00256A. [DOI] [PubMed] [Google Scholar]
- Emmadi M.; Kulkarni S. S. Synthesis of orthogonally protected bacterial, rare-sugar and D -glycosamine building blocks. Nat. Protoc. 2013, 8, 1870–1889. 10.1038/nprot.2013.113. [DOI] [PubMed] [Google Scholar]
- Lloyd D.; Bylsma M.; Bright D. K.; Chen X.; Bennett C. S. Mild Method for 2-Naphthylmethyl Ether Protecting Group Removal Using a Combination of 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and β-Pinene. J. Org. Chem. 2017, 82, 3926–3934. 10.1021/acs.joc.7b00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T. K.; Matsumura F.; Wang P.; Yu S.; Chou C.-C.; Khoo K.-H.; Kitayama K.; Akama T. O.; Sugihara K.; Kanayama N.; Kojima-Aikawa K.; Seeberger P. H.; Fukuda M.; Suzuki A.; Aoki D.; Fukuda M. N. Identification of Mono- and Disulfated N-Acetyl-lactosaminyl Oligosaccharide Structures as Epitopes Specifically Recognized by Humanized Monoclonal Antibody HMOCC-1 Raised against Ovarian Cancer. J. Biol. Chem. 2012, 287, 6592–6602. 10.1074/jbc.M111.305334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan N. V.; Strebel T. R.; Boothello R. S.; Sheerin K.; Raghuraman A.; Sallas F.; Mosier P. D.; Watermeyer N. D.; Oscarson S.; Desai U. R. A Hexasaccharide Containing Rare 2-O-Sulfate-Glucuronic Acid Residues Selectively Activates Heparin Cofactor II. Angew. Chem., Int. Ed. 2017, 56, 2312–2317. 10.1002/anie.201609541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswarappa S. G.; Reppe K.; Geissner A.; Ménová P.; Govindan S.; Calow A. D. J.; Wahlbrink A.; Weishaupt M. W.; Monnanda B. P.; Bell R. L.; Pirofski L. A.; Suttorp N.; Sander L. E.; Witzenrath M.; Pereira C. L.; Anish C.; Seeberger P. H. A Semi-synthetic Oligosaccharide Conjugate Vaccine Candidate Confers Protection against Streptococcus pneumoniae Serotype 3 Infection. Cell Chem. Biol. 2016, 23, 1407–1416. 10.1016/j.chembiol.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R.; Elgland M.; Begum A.; Fyrner T.; Konradsson P.; Nystrom S.; Hammarstrom P. Impact of N-glycosylation site variants during human PrP aggregation and fibril nucleation. Biochim Biophys Acta Proteins Proteom. 2019, 1867, 909–921. 10.1016/j.bbapap.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Kelly D. R.; Roberts S. M.; Newton R. F. The Cleavage of t-Butyldimethylsilyl Ethers with Boron Trifluoride Etherate. Synth. Commun. 1979, 9, 295–299. 10.1080/00397917908064155. [DOI] [Google Scholar]
- Das R.; Mukhopadhyay B. Chemical O-Glycosylations: An Overview. ChemistryOpen 2016, 5, 401–433. 10.1002/open.201600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera A.; Rai D.; Kulkarni S. S. Total Syntheses of Conjugation-Ready Trisaccharide Repeating Units of Pseudomonas aeruginosa O11 and Staphylococcus aureus Type 5 Capsular Polysaccharide for Vaccine Development. J. Am. Chem. Soc. 2020, 142, 456–467. 10.1021/jacs.9b11309. [DOI] [PubMed] [Google Scholar]
- Qin C.; Schumann B.; Zou X.; Pereira C. L.; Tian G.; Hu J.; Seeberger P. H.; Yin J. Total Synthesis of a Densely Functionalized Plesiomonas shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen. J. Am. Chem. Soc. 2018, 140, 3120–3127. 10.1021/jacs.8b00148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.