Abstract
Objectives
As part of the PIONEER Consortium objectives, we have explored which diagnostic and prognostic factors (DPFs) are available in relation to our previously defined clinician and patient-reported outcomes for prostate cancer (PCa).
Design
We performed a systematic review to identify validated and non-validated studies.
Data sources
MEDLINE, Embase and the Cochrane Library were searched on 21 January 2020.
Eligibility criteria
Only quantitative studies were included. Single studies with fewer than 50 participants, published before 2014 and looking at outcomes which are not prioritised in the PIONEER core outcome set were excluded.
Data extraction and synthesis
After initial screening, we extracted data following the Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of prognostic factor studies (CHARMS-PF) criteria and discussed the identified factors with a multidisciplinary expert group. The quality of the included papers was scored for applicability and risk of bias using validated tools such as PROBAST, Quality in Prognostic Studies and Quality Assessment of Diagnostic Accuracy Studies 2.
Results
The search identified 6604 studies, from which 489 DPFs were included. Sixty-four of those were internally or externally validated. However, only three studies on diagnostic and seven studies on prognostic factors had a low risk of bias and a low risk concerning applicability.
Conclusion
Most of the DPFs identified require additional evaluation and validation in properly designed studies before they can be recommended for use in clinical practice. The PIONEER online search tool for DPFs for PCa will enable researchers to understand the quality of the current research and help them design future studies.
Ethics and dissemination
There are no ethical implications.
Keywords: prostate disease, urological tumours, epidemiology
Strengths and limitations of this study.
A multidisciplinary team including patients, urologists, oncologists, radiation oncologists, methodological experts and pathologists were involved throughout the study.
The search was restricted from 2014 onwards, to maintain a pragmatic approach.
The main strength of this study is the extensive and comprehensive search and screening of the studies included.
Introduction
Prostate cancer (PCa) accounts for 15% of cancers diagnosed1 and is the second most common cancer in males worldwide.2 PCa is clinically and molecularly heterogeneous and is usually suspected based on the clinical findings of digital rectal examination and/or prostate-specific antigen (PSA) levels.1 However, which diagnostic or prognostic factors (DPFs) can be used to select patients for specific therapeutic options remains largely unclear.3 Specific biomarkers in urine or in blood are available on top of traditional PSA testing, such as PCA3, TMPRSS2-ERG fusion or kallikreins as incorporated in the Phi or 4Kscore test together with other parameters including family history.4–7 However, the European Association of Urology (EAU) guidelines (2021) currently do not provide general recommendations to implement these biomarkers into routine screening programmes due to limited data. As part of the American Society of Clinical Oncology (ASCO) guidelines, Eggener et al recommended commercially available biomarkers, which have been shown to provide prognostic significance and additional information beyond standard clinical models in patient selection in the localised context: Oncotype Dx Prostate, Prolaris, Decipher, and ProMark.8 However, no guidelines have recommended DPFs for other stages of PCa. The expert panel at the Advanced Prostate Cancer Consensus Conference (APCCC) consensus meeting of advanced PCa in Basel 2019, recommended AR-V7 for mCRPC as potentially useful, which ultimately led to the inclusion of AR-V7 testing in the NCCN guidelines.9
The PIONEER Consortium is an international collaboration coordinated by the EAU, which aims to establish the best evidence-based management and clinical practice of PCa across all disease stages using the power of big data analytics towards a more outcome-driven, value-based and patient-centric healthcare system.10 A key objective is to address one of the major challenges within the context of diagnostic or prognostic biomarkers/factors: the inability to incorporate DPFs into the management of PCa in terms of screening, diagnosis and treatment. It is therefore important to summarise and evaluate the evidence. Biomarkers can be classified into different types: diagnostic, prognostic, predictive and therapeutic—in this study we focus on the first two.11 A diagnostic biomarker or factor is useful when cancer is suspected and allows the early detection based on symptoms or tests.11 The overall aim of a diagnostic biomarker is to distinguish people with the diseases from people without the disease. A prognostic biomarker or factor is a clinical or biological characteristic which provides information on the likely course of the disease, that is, biochemical progression or disease recurrence.11 It enables clinicians to decide on the most suitable treatment depending on the likely course of the disease. In the sections below, we have used the terms biomarkers and factors interchangeably. Multiple DPFs can be measured in tissue, blood or urine. These come with different advantages and disadvantages and only a limited number of factors are currently available for PCa in standard clinical care.
We aimed to systematically review the evidence from 2014 onward to assess which DPFs are available in relation to previously defined outcomes for PCa.
Methods
The systematic review (SR) followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.12 A detailed protocol of the overall project was published elsewhere13 (please see the protocol attached as methods online supplemental appendix). Briefly, we followed the following four steps (figure 1):
Figure 1.
Overview of four stage process. CHARMS-PF, Checklist for Critical Appraisal and Data Extraction for Systematic Reviews of prognostic factor studies; DPFs, diagnostic and prognostic factors; PROBAST, Prediction model Risk Of Bias Assessment Tool; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies 2; QUIPS, Quality in Prognostic Studies; SR, systematic review.
bmjopen-2021-058267supp001.pdf (52.2KB, pdf)
Comprehensive systematic literature review of DPFs for all stages of PCa (localised, locally advanced, metastatic, and non-metastatic castration resistant) from 2014 onwards. DPFs developed before 2014 were not included, due to the significant changes influencing the staging of PCa (i.e., Consensus Conference on Gleason Grading of Prostatic Carcinoma [60]) that have taken place in diagnostic and prognostic practice and patient management since then.
Assessment and identification of final list of DPFs by a multidisciplinary expert panel.
Evaluation of quality of studies published using risk of bias (RoB) tools: Prediction model RoB Assessment Tool (PROBAST) if applicable; or Quality in Prognostic Studies (QUIPS) tool for prognostic and the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool for diagnostic factors.
Due to the heterogeneity of the studies identified no further formal quantitative assessments in the form of a meta-analyses could be performed. Hence, the findings of stages 1–3 have been reported here as the results of a SR.
Stage 1: comprehensive literature review
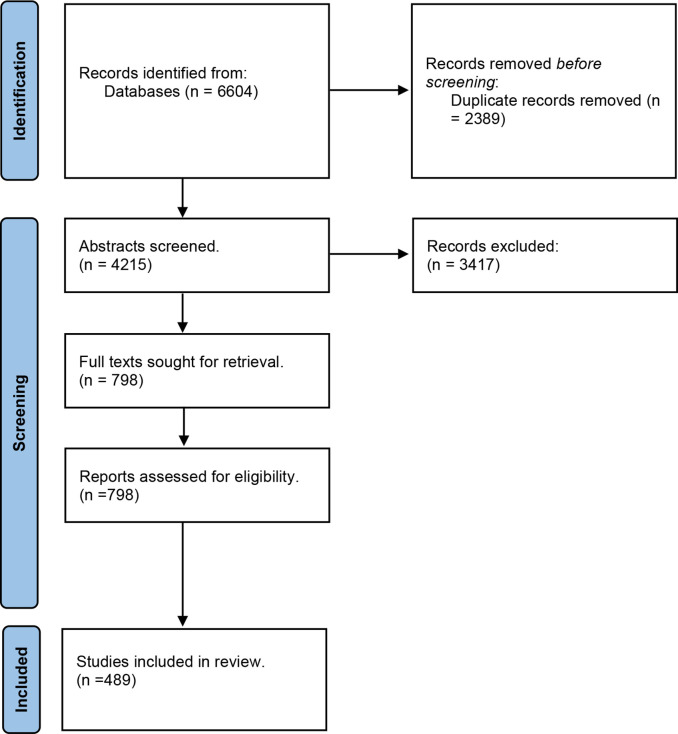
We developed the search criteria for the first search with an information scientist who specialises in SR for urology. MEDLINE, Embase and the Cochrane Library were searched on 21 January 2020. The second search was developed following a consultation with an independent information scientist group who excluded row 12, 14 and 16 of (see online supplemental table 1). We screened the EAU Guidelines reference list for PCa in our third search (see figure 2).
Figure 2.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; COS, Core outcome set.
bmjopen-2021-058267supp002.pdf (49KB, pdf)
Stage 2: multidisciplinary expert meeting
On the 20 March 2020, we invited a group of multidisciplinary participants to discuss the identified articles on DPFs (see online supplemental table 2). The participants were presented the search criteria and the extracted data. Data extraction followed the CHARMS-PF checklist and we added author and year of publication.
bmjopen-2021-058267supp003.pdf (36KB, pdf)
Stage 3: evaluation of quality of studies published using the RoB tools
Prior to the evaluation of the quality of studies, an initial pilot screening to prepare the raters for the use of PROBAST, QUADAS-2 and QUIPS was performed. This aimed to reach consensus on how to judge the domains of the assessments using the three RoB tools. Two urologists (FB and SS) and two epidemiologists (AH and KB) were involved in the pilot assessments. The group discussed any discrepancies. Articles which presented the development and validation either internal validation or external validation (i.e., the same data was used for both development and internal validation, such as bootstrapping or cross-validation; different populations were used for development and validation), of a diagnostic or prognostic model were assessed with PROBAST. Papers assessing single biomarkers or with/without validation were assessed with QUIPs for prognostic or QUADAS-2 for diagnostic biomarkers.
Evaluation of quality of studies published using QUADAS-2
The RoB of diagnostic factors without validation or single validated factors was evaluated using QUADAS-2. We assessed the following four domains: patient selection, index test, reference standards and flow and timing. The first three domains are assessed looking at applicability and all four domains were assessed in terms of RoB.14 We created a summative score after the diagnostic studies were assessed by two reviewers and in case of disagreement a third reviewer assessed the study.
Evaluation of quality of studies published using PROBAST (diagnostic)
The RoB of internal or external validated diagnostic models was assessed using the PROBAST RoB tool. PROBAST includes four domains assessing the RoB (i.e., participants, predictors, outcome, and analysis) and four domains assessing applicability (i.e., participants, predictors, and outcome) (see online supplemental table 3 for scoring information).
bmjopen-2021-058267supp004.pdf (37.5KB, pdf)
Evaluation of quality of studies published using QUIPS
To assess the articles which are single factors or were not internally or externally validated, we used the QUIPS rating procedure (see online supplemental table 4 for scoring information). To standardise the approach across raters, we used the QUIPS electronic spreadsheet (excel) from Hayden et al.15 There are no rules available for QUIPS on how to score the overall RoB of a paper. Due to the large number of papers and the need for synthesis, we followed the suggestions from Grooten et al., and categorised on the following criteria: (1) Paper was classified as low RoB if all domains were classified as having low RoB, or up to one moderate RoB; (2) Paper was classified as high RoB if one or more domains were classified as having high RoB, or ≥3 moderate RoB; (3) Paper was classified as having moderate RoB if all papers in between 1 or 2 (see online supplemental table 1). This assessment was based on the risk scores of individual assessments within the group. If the overall assessment was not possible due to differences in the individual category, a third assessor reviewed the assessments and the results were discussed.
bmjopen-2021-058267supp005.pdf (49.6KB, pdf)
Evaluation of quality of studies published using PROBAST (prognostic)
The RoB of prognostic validated models were assessed using PROBAST. As highlighted above, PROBAST includes four domains assessing the RoB (i.e., participants, predictors, outcome and analysis) and the domains assessing applicability (i.e., participants, predictors and outcome).
Results
Stage 1: comprehensive literature review
Stage 1 identified 6604 citations and contained three independent searches. After removing duplicates, we screened 4215 abstracts, from which 489 met the inclusion criteria.
Stage 2: multidisciplinary expert meeting
The group discussed the results and additional literature on DPFs was suggested to help the classification of the DPFs, such as the ASCO Guideline on Molecular Biomarkers in Localised Prostate Cancer.16
Stage 3: evaluation of quality of studies published using the RoB tools
The 489 articles were equally divided between six groups. The six groups received the guidance documents which were identified during the pilot phase.14 15 17–19 In addition, MvH and KB discussed questions with each individual group.
Evaluation of quality of studies published using QUADAS-2
The RoB of the 41 included studies was low for 10 studies, high for 23 studies and unclear for eight. RoB concerning applicability was low for 10 studies, high for 21 studies and unclear for 10 studies (see table 1). Table 2 shows the studies with an overall low RoB across both categories. Two studies were identified to have an overall low RoB.20 21
Table 1.
Overall judgement of RoB
| QUADAS-2, diagnostic | ||
| Overall judgement of RoB | RoB | Applicability |
| Low | 10 | 10 |
| High | 23 | 21 |
| Unclear | 8 | 10 |
| Total | 41 | |
| PROBAST, diagnostic | ||
| Overall judgement of RoB | RoB | Applicability |
| Low | 3 | 8 |
| High | 14 | 10 |
| Unclear | 3 | 2 |
| Total | 20 | |
| QUIPS | ||
| Overall judgement of RoB | RoB | |
| Low | 29 | |
| Moderate | 49 | |
| High | 307 | |
| Total | 385 | |
| PROBAST, prognostic | ||
| Overall judgement of RoB | RoB | Applicability |
| Low | 3 | 15 |
| High | 27 | 20 |
| Unclear | 13 | 8 |
| Total | 43 | |
PROBAST, Prediction model Risk Of Bias Assessment Tool; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies 2; QUIPS, Quality in Prognostic Studies; RoB, risk of bias.
Table 2.
Non-validated DPFs with overall low RoB: QUADAS-2
| Author | Year | Patient selection | Index test(s) | Reference standard | Flow and timing | Patient selection | Index test(s) | Reference standard | RoB | Applicability |
| Hagiwara20 | 2017 | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Kelly21 | 2015 | Low | Low | Low | Low | Low | Low | Low | Low | Low |
DPFs, diagnostic and prognostic factors; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies 2; RoB, risk Of bias.
Evaluation of quality of studies published using PROBAST (diagnostic)
We identified 20 papers to be assessed with PROBAST. The RoB of three papers was low, high for 14 and was unclear for three. The applicability of eight papers was high and was unclear for two (see table 1). Online supplemental table 1 shows the criteria on how to judge the RoB. One study had an overall low RoB across both domains. All categories except ‘predictors’ was scored to have a low RoB. There was little information available for the category predictors and therefore it was scored as ‘unclear’ (see table 3).
Table 3.
DPFs assessed with PROBAST
| Author | ROB | Applicability | Overall | ||||||
| Participants | Predictors | Outcome | Analysis | Participants | Predictors | Outcome | ROB | Applicability | |
| Diagnostic | |||||||||
| Guinney22 | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Joniau23 | Low | Low | Low | Low | Low | Low | Low | Low | Low |
| Prognostic | |||||||||
| Palsdottir24 | Low | Unclear | Low | Low | Low | Low | Low | Low | Low |
DPFs, diagnostic and prognostic factors; PROBAST, Prediction model Risk Of Bias Assessment Tool; RoB, risk of bias.
Evaluation of quality of studies published using QUIPS
The 12 assessors independently inserted the relevant information and assessed each domain such as participation, attrition, prognostic factor confounding and statistical analysis and reporting.
A total of 387 prognostic factors were assessed using QUIPs. A total of 307 papers were classified as high RoB. Forty-nine papers were classified as having a moderate RoB and 28 papers were scored as low RoB (see table 1). Out of the 28 papers with a low RoB, the most common moderate bias was linked to attrition (12 papers), followed by confounding (4 papers), participation (3 papers), outcome (1 paper), statistical analysis (1 paper) (see table 4).
Table 4.
Characteristics of DPFs with overall low RoB
| Author | Year | RoB | Population | Study design | Timing | Index | Outcomes |
| Palsdottir24 | 2019 | Diag. PROBAST | Localised PCa | Observational study | Pre treatment | S3M-MRI (Stockholm3 +PI RADS) | csPCa diagnosis |
| Guinney22 | 2017 | Prog. PROBAST | mCRPC | RCT | Post treatment | ePCR model | OS |
| Joniau23 | 2017 | Prog. PROBAST | Locally advanced PCa | Observational study | Post-treatment | Gleason score +PSA | Adverse pathological features at RP; LNI |
| Hagiwara20 | 2017 | QUADAS | Localised PCa | Observational study | Pre-treatment | WFA-reactive glycan-carrying PSA-Gi | PCa diagnosis, PSA-free survival |
| Kelly21 | 2015 | QUADAS | Localised PCa | Observational study | Pre-treatment | miR-141, –145, −155, let7a | PCa diagnosis |
| Aguilera25 | 2015 | QUIPS | High risk PCa | Observational study | Pre and post treatment | Age, rectal examination, PSA, biopsy Gleason score, uni/bilateral tumour, affected cylinder percentage) and postoperative | BCR |
| Alvim27 | 2019 | QUIPS | Metastatic PCa | Observational study | Post-treatment | PSA response (PSA reduction ≥50%) | OS, PFS |
| Bramhecha26 | 2019 | QUIPS | Localised PCa | Observational study | Post-treatment | PTEN deletion | BCR |
| Bruce28 | 2016 | QUIPS | Localised PCa | Observational study | Post-treatment | AZGP1 expression | BR-free survival, CR-free survival, PC-specific death |
| Francini29 | 2018 | QUIPS | mHSPC | Observational study | Post-treatment | Volume | OS, time to CRPC |
| Hamada30 | 2016 | QUIPS | High risk PCa | Observational study | Post-treatment | PSA, PSA density (PSAD), PSAD of the transition zone, percentage of positive cores (PPC), prostate volume, TZ volume, Gleason score, PPC from the dominant side | BCR |
| Hashimoto31 | 2020 | QUIPS | Localised PCa | Observational study | Post-treatment | Micro-lymphatic invasion, Gleason | BCR |
| Hung57 | 2017 | QUIPS | mCRPC | Observational study | Post-treatment | Neurovascular bundle preservation, blood loss, pT stage, pN stage, pGS, PNI, angiolymphatic invasion, tumour amount in specimen, ECE, PSM, SVI, Bladder neck invasion, Foley duration, post-op undetectable PSA | BCR |
| Kato32 | 2018 | QUIPS | High risk PCa | Observational study | Post-treatment | LC/IDC | PFS, CSS |
| Kluth33 | 2014 | QUIPS | Localised PCa | Observational study | Post-treatment | No of lymph nodes | BCR |
| Lara34 | 2014 | QUIPS Validated |
mCRPC | RCT | Post treatment | Bone resorption and formation | OS |
| Lee35 | 2016 | QUIPS | Localised PCa | Observational study | Post treatment | Positive surgical margin status and bilateral seminal vesicle invasion | BCR |
| Lévesque36 | 2019 | QUIPS | Localised PCa | Observational study | Post treatment | UGT2B17 expression | BCR |
| Lin37 | 2017 | QUIPS | Localised PCa | Observational study | Post treatment | Aberrant Promoter Methylation of Protocadherin8 (PCDH8) | BCR-free survival |
| Löffeler38 | 2015 | QUIPS | mCRPC | Observational study | Anytime | PSA doubling time, PSA nadir during ADT, haemoglobin and alkaline phosphatase levels at CRPC | OS |
| Narang39 | 2017 | QUIPS | Localised PCa | Observational study | Anytime | PSA: End-of-radiation PSA | BCR-free survival, MFS, CSS, OS |
| Ozden40 | 2017 | QUIPS | Localised PCa | Observational study | Post treatment | Age | RRP specimen, BCR, and BCR-free survival rates |
| Pei41 | 2016 | QUIPS | CRPC | Observational study | Pre and during treatment | Neutrophil-to-lymphocyte ratio | OS, PFS |
| Qu42 | 2016 | QUIPS | mPCa and CRPC | Observational study | Pre treatment | AR-V7 | Time to CRPC / CRPC: CSS |
| Qu43 | 2017 | QUIPS | PCa | Observational study | Pre and during treatment | AR-V7 | OS |
| Rüenauver44 | 2014 | QUIPS | Localised PCa | Observational study | Post treatment | YWHAZ | OS |
| Shimodaira45 | 2020 | QUIPS | Metastatic PCa | Observational study | Post treatment | Value of platelet counts | Disease specific survival |
| Strand46 | 2015 | QUIPS | Localised PCa | Observational study | Post treatment | 5-hydroxymethylcytosine score | BCR |
| Takagi47 | 2017 | QUIPS | Localised PCa | Observational study | Post treatment | Age, T stage, % of pos cores, Gleason score, PSA, Total ADT | BCR-free survival |
| Wang48 | 2016 | QUIPS | PCa | Observational study | Post treatment | Platelet to lymphocyte ratio (PLR) | PLR with PFS, CSS and OS n/a |
| Zacho49 | 2017 | QUIPS | Localised PCa | Observational study | Anytime | Bone scan index | Time to CRPC |
| Berg50 | 2014 | QUIPS validated | Under Active Surveillance | Observational study | ERG immunohisto-chemical staining | Overall AS progression, histopathologic progression |
ADT, androgen deprivation therapy; AS, Active Surveillance; BCR, biochemical recurrence; CSS, cancer-specific survival; DFPs, diagnostic and prognostic factors; mCRPC, metastatic castration resistant prostate cancer; n/a, not available; OS, overall survival; PCa, prostate cancer; PFS, progression-free survival; PI-RADS, Prostate Imaging Reporting and Data System; PROBAST, Prediction model Risk Of Bias Assessment Tool; PSA, Prostate Specific Antigen; PTEN, Phosphatase and tensin homolog; QUADAS, Quality Assessment of Diagnostic Accuracy Studies; QUIPS, Quality in Prognostic Studies; RCT, Randomised control trial; RoB, risk of bias; WFA, Wisteria floribunda agglutinin.
Evaluation of quality of studies published using PROBAST (prognostic)
The assessors identified 44 papers to be assessed with PROBAST, of those three scored a low RoB, 27 a high RoB and 13 were assessed as unclear (see table 1). In terms of applicability, 15 papers scored low, 20 high and eight unclear. Two papers were scored to have an overall low RoB22 23 (see table 3).
Characteristics of studies identified with low RoB
Details of the identified validated DPF models with an adequate quality are presented in table 5. We identified 32 studies with an overall low RoB (assessed with PROBAST, QUIPS, QUADAS-2). Out of these 32 studies, we identified one validated diagnostic model (assessed with PROBAST),24 two validated prognostic models (assessed with PROBAST),22 23 two non-validated diagnostic single factors (assessed with QUADAS-2)20 21 and 26 prognostic factors (assessed with QUIPS)20–50 which have not been validated and two single prognostic factors which have been validated (assessed with QUIPS).34 50 Prognostic factors assessed with QUIPS were identified with a low RoB for the localised PCa population. Sixty-seven per cent of the low RoB DPFs were intended to be measured after the treatment was performed. In addition, the most commonly measured outcome was biochemical recurrence followed by overall survival. However, it is important to take into consideration that even from the studies assessed with a low RoB, only 2 out of the 32 were of a non-observational study design.
Table 5.
DPFs with low risk of bias assessed with QUIPS
| Study | Time | Biases | Applicability | Overall score | ||||
| Participation | Attrition | Prognostic factor | Outcome | Confounding | Statistical analysis and reporting | |||
| Aguilera25 | 2015 | Low | Low | Low | Low | Moderate | Low | Low |
| Alvim27 | 2019 | Low | Low | Low | Low | Low | Low | Low |
| Bramhecha26 | 2019 | Low | Moderate | Low | Low | Low | Low | Low |
| Bruce28 | 2016 | Low | Moderate | Low | Low | Low | Low | Low |
| Francini29 | 2018 | Low | Low | Low | Low | Low | Moderate | Low |
| Hamada30 | 2016 | Low | Low | Low | Moderate | Low | Low | Low |
| Hashimoto31 | 2020 | Low | Low | Low | Low | Low | Low | Low |
| Hung57 | 2017 | Moderate | Low | Low | Low | Low | Low | Low |
| Kato32 | 2018 | Low | Moderate | Low | Low | Low | Low | Low |
| Kluth33 | 2014 | Low | Moderate | Low | Low | Low | Low | Low |
| Lara34 | 2014 | Low | Low | Low | Low | Moderate | Low | Low |
| Lee35 | 2016 | Low | Moderate | Low | Low | Low | Low | Low |
| Levesque36 | 2019 | Low | Moderate | Low | Low | Low | Low | Low |
| Lin37 | 2017 | Low | Moderate | Low | Low | Low | Low | Low |
| Loffeler38 | 2015 | Low | Low | Low | Low | Low | Low | Low |
| Narang39 | 2017 | Low | Moderate | Low | Low | Low | Low | Low |
| Ozden40 | 2017 | Moderate | Low | Low | Low | Low | Low | Low |
| Pei41 | 2016 | Low | Low | Moderate | Low | Low | Low | Low |
| Qu42 | 2016 | Low | Low | Low | Low | Low | Low | Low |
| Qu43 | 2017 | Low | Low | Low | Low | Low | Low | Low |
| Rizzardi58 | 2015 | Low | Low | Low | Low | Low | Low | Low |
| Ruenauver44 | 2014 | Low | Moderate | Moderate | Low | Low | Low | Low |
| Shimodaira45 | 2020 | Low | Moderate | Low | Low | Low | Low | Low |
| Strand46 | 2015 | Low | Moderate | Low | Low | Low | Low | Low |
| Takagi47 | 2017 | Low | Low | Low | Low | Moderate | Low | Low |
| Wang48 | 2016 | Low | Moderate | Low | Low | Low | Low | Low |
| Zacho49 | 2017 | Moderate | Low | Low | Low | Moderate | Low | Low |
| Berg50 | 2014 | Low | Low | Low | Low | Low | Low | Low |
DPFs, diagnostic and prognostic factors; QUIPS, Quality in Prognostic Studies.
As highlighted above, we identified three validated DPFs which were scored to have a low RoB and low risk concerning applicability. First, we identified the ‘Unified Prostate Cancer Risk Prediction Model Combining the Stockholm 3 Test and MRI’, a risk prediction model which combines clinical variables, genetic and protein biomarkers. Five hundred and thirty-two men were involved across three centres.24 Second, the DREAM challenge developed a set of five standardised raw event-level tables, using laboratory values, patients’ demographic information, medical history, lesion sites, previous treatments and vital signs of patients with mCRPC. These variables where combined by using data from four clinical trials.22 Third, Joniau et al., developed ‘Pretreatment Tables’ to predict the pathologic stage of locally advanced PCa after RP based on pretreatment PSA level and biopsy Gleason score.23
We identified two single factors which were validated and had low RoB. First, Lara et al., assessed and validated the serum biomarkers of bone metabolism (N-telopeptide and pyridinoline) and formation (C-terminal collagen propeptide and bone alkaline phosphatase)) in 778 CRPC patients as part of the randomised phase III SWOG trial (S0421) of docetaxel/prednisone with or without atrasentan.34 Second, Berg et al., showed that ERG expression can be used to estimate the risk of progression during AS including 265 patients at diagnosis and progression during AS.50
Discussion
Despite the large number of studies on DPFs which are published every year, there is a paucity of DPFs that are suitable to be incorporated into clinical practice. The majority of DPFs have not yet been validated and are identified in poor quality studies. Our analysis found that most identified studies had a high to moderate RoB due to poor design standards, conduct, reporting and/or analysis that is, generalisability and size of the population, poor model development (no testing or missing important confounders) or only correlation studies, missing data was rarely reported. However, we did identify a small number of validated DPFs with low RoB. We identified three validated models which combine: first, clinical variables, genetic and protein biomarkers, and improved clinical outcome performance of PCa diagnostics (The Unified Prostate Cancer Risk Prediction Model)24; second, laboratory values, patients’ demographic information, medical history, lesion sites, previous treatments and vital signs of patients with metastatic castration-resistant PCa (DREAM challenge)22; and third, pretreatment PSA level and biopsy Gleason score to predict the pathological stage of locally advanced PCa (‘Pretreatment Tables’).23
Two single factors have been validated: the serum biomarkers of bone metabolism in CRPC patients34 and the ERG expression, which can be used to estimate the risk of progression during AS,50 which has already been highlighted in the clinical guidelines.1
Aladawani et al., assessed prediction models for PCa to be used in primary care settings in their SR and identified five models which met their inclusion criteria. From these identified models only one model was externally validated and only one (the Lazzari model 251 had the potential to be implemented in primary care. Lazzari et al., had the lowest RoB (based on PROBAST); however, it must be externally validated before it can be implemented. Hence, Aladawani et al., also concluded that the existing models have limitations concerning study design and reporting performance.52
Tian et al., conducted a review on biomarkers for CRPC patients, however, their quality assessment was focused on study design (RCT vs. observational study), whereas we focused on biomarker specific tools.53 While Tian et al., and our review identified similar factors and quality scores, there were slight discrepancies between the overall RoB assessments. Tian et al., used an overall quality assessment scale from 1 to 6 instead of low, medium and high. In their assessment the validated prognostic study by Lara et al., 34 and the non-validated prognostic factor by Pei et al., 41 were scored on the quality scale as 4 (medium quality). We assessed Lara et al., 34 to have a low RoB with a moderate risk of confounding and Pei et al., 41 with a moderate RoB concerning the prognostic factor itself. This might explain the discrepancies between the two quality assessments. The reports by Alvim et al., Qu et al., were assessed to have the highest quality by Tian et al., 53 similar to our review. This illustrates that different quality assessment tools emphasise different criteria, which may result in small discrepancies. However, the overall conclusion for prognostic single factors was similar in our review and to the work of Tian et al.53
Similar issues have been identified for other urological cancers. For example, in kidney cancer, a large body of research was identified by Harrison et al., with very few validated studies and lots of heterogeneity.54 Schmitz-Dräger et al., published an International Consultation of Urologic Disease/WHO Consensus manuscript where they identified that in bladder cancer one of the main limitations for the lack of incorporation of modern bladder cancer tests into clinical practice decision making is linked to the scarcity of ‘good clinical practice guidelines’ for the evaluation of diagnostic markers.
There is a need for improved guidance on development and validation of diagnostic markers.55 To meet that need, we are developing the PIONEER DPF search tool, which will help researchers and clinicians to get a better understanding of the DPFs for PCa. The tool will not only summarise all relevant studies, but also provide information on the use and results of different RoB assessment tools, which will enable an understanding of the quality of published studies.
Future research should, therefore, focus on addressing the identified shortcomings such as heterogeneity, validation and poor RoB by designing more robust studies which consistently include RoB assessments such as PROBAST, QUIPS or QUADAS-2.
With the growing number of various therapeutic options, diagnosis and management of PCa requires an individualised approach to patient care. There is an unmet need for DPFs to guide decisions for optimal treatment and to predict which patients will benefit the most, from a particular management strategy. DPFs could potentially enhance the quality of patient counselling, but currently most need additional evaluation and validation in properly designed studies. Our SR highlights the need for well-designed Real-World Evidence studies, while the PIONEER online search tool can inform the design of new research studies, through providing a rigorous evaluation of the methodological quality of the studies.
The main strength of this study are the extensive and comprehensive search and screening of the studies included. In addition, we are developing an online search tool which showcases the identified and assessed studies. It provides an overview of the available DPFs and enables interested stakeholders to search for DPFs. To our knowledge, this is the first study which has been performed with this extensive amount of literature.
Patient and public involvement
This project has been overseen by a multistakeholder group part of the PIONEER Consortium. PIONEER brings together 35 key stakeholders from academic institutions, patient advocacy groups, European organisations, experts in legal data management, clinicians and pharmaceutical companies, as well as regulatory agencies, economics and ethics, and information and technology specialists. Patients and their family members are therefore involved and actively participate as an integral part of all research conducted by the PIONEER Consortium.
Limitations
Even though this review included three searches and assessments by a multidisciplinary group of fourteen researchers, we recognise potential limitations. Studies were only included from 2014 onwards and DPFs developed before 2014 were not included. However, significant changes which influence the staging of PCa (i.e., Consensus Conference on Gleason Grading of Prostatic Carcinoma56 have taken place in diagnostic and prognostic practice and patient management. This changed the staging of the patient population and therefore has an impact on DPFs.
In addition, there is a potential of subjectivity in the evaluation of the studies. Even though the studies have been assessed in duplicate, there might be variation across groups. However, given the overall moderate to high RoB, this does not influence the overall recommendation of the project.
Conclusion
At present DPFs that are capable of significantly improving diagnosis and prognosis in PCa are an unmet need as most of the DPFs identified require additional evaluation and validation in properly designed studies before they can be recommended for use in clinical practice. Well-designed real world evidence (RWE) studies can help to increase quality. Our SR aims to inform clinicians and patients about this rapidly evolving field, while the PIONEER online search tool for DPFs for PCa will enable researchers to perform future research, and to understand the quality of the current available studies.
Supplementary Material
Acknowledgments
PIONEER Consortium
Footnotes
Twitter: @beyer_katharina, @prostatePioneer, @Ric_Campi, @endourologist, @drimranomar, @alexhabs, @SajMacLennan
Contributors: KB, LM, ML, AH, FB, SS, MM, MIO, SM, MJR, BF, EV, ZD, AA, JZ, SJM, LC, JN; ABr, ABj and MVH conceptualised designed the review. Abstracts and full texts were reviewed and data extracted by KB, LM, ML, AH, FB, SS, MM, RH, AR, RC, IG, KS, TvdB and SA. Authors resolved disagreement by discussion where necessary. The risk of bias was assessed by KB, LM, ML, AH, FB, SS, MM, RH, AR, RC, IG, KS and SD. The manuscript was drafted by KB, LM, ML, ABr, MVH and reviewed by KB, LM, ML, AH, FB, SS, MM, RH, AR, RC, IG, KS, SD, TvdB, MG, GG, MIO, SM, MJR, BF, EV, ZD, AA, JZ, SJM, LC, JN, ABr, ABj and MVH. The whole project was supervised and guided by JZ, SJM, LC, JN, ABr, ABj and MVH. PIONEER Consotium acts as a gaurantor.
Funding: PIONEER is funded through the IMI2 Joint Undertaking and is listed under Grant Agreement No. 777492 and is part of the Big Data for Better Outcomes Programme (BD4BO). IMI2 receives support from the European Union’s Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). The views communicated within are those of PIONEER. Neither the IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained herein.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. not applicable.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study does not involve human participants.
References
- 1. Mottet NBJ, Briers E, Bolla M. Members of the EAU – ESTRO – ESUR –SIOG prostate cancer guidelines panel. EAU – ESTRO – ESUR – SIOG guidelines on prostate cancer. EAU annual Congress. Milan: EAU Guidelines Office, 2021. [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3. Filella X, Fernández-Galan E, Fernández Bonifacio R, et al. Emerging biomarkers in the diagnosis of prostate cancer. Pharmgenomics Pers Med 2018;11:83–94. 10.2147/PGPM.S136026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vedder MM, de Bekker-Grob EW, Lilja HG, et al. The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men. Eur Urol 2014;66:1109–15. 10.1016/j.eururo.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leyten GHJM, Hessels D, Jannink SA, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol 2014;65:534–42. 10.1016/j.eururo.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 6. Boegemann M, Stephan C, Cammann H, et al. The percentage of prostate-specific antigen (PSA) isoform [-2]proPSA and the Prostate Health Index improve the diagnostic accuracy for clinically relevant prostate cancer at initial and repeat biopsy compared with total PSA and percentage free PSA in men aged ≤65 years. BJU Int 2016;117:72–9. 10.1111/bju.13139 [DOI] [PubMed] [Google Scholar]
- 7. Bryant RJ, Sjoberg DD, Vickers AJ, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the protect study. J Natl Cancer Inst 2015;107. 10.1093/jnci/djv095. [Epub ahead of print: 11 04 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eggener SE, Rumble RB, Armstrong AJ, et al. Molecular biomarkers in localized prostate cancer: ASCO guideline. J Clin Oncol 2020;38:1474–94. 10.1200/JCO.19.02768 [DOI] [PubMed] [Google Scholar]
- 9. Gillessen S, Attard G, Beer TM, et al. Management of patients with advanced prostate cancer: report of the advanced prostate cancer consensus conference 2019. Eur Urol 2020;77:508–47. 10.1016/j.eururo.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 10. Omar MI, Roobol MJ, Ribal MJ, et al. Author correction: introducing pioneer: a project to harness big data in prostate cancer research. Nat Rev Urol 2020;17:482. 10.1038/s41585-020-0355-3 [DOI] [PubMed] [Google Scholar]
- 11. Carlomagno N, Incollingo P, Tammaro V, et al. Diagnostic, predictive, prognostic, and therapeutic molecular biomarkers in third millennium: a breakthrough in gastric cancer. Biomed Res Int 2017;2017:1–11. 10.1155/2017/7869802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beyer K, Moris L, Lardas M, et al. Diagnostic and prognostic factors in patients with prostate cancer: a systematic review protocol. BMJ Open 2021;11:e040531. 10.1136/bmjopen-2020-040531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 15. Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 16. Eggener SE, Rumble RB, Armstrong AJ, et al. Molecular biomarkers in localized prostate cancer: ASCO guideline. JCO 2020;38:1474–94. 10.1200/JCO.19.02768 [DOI] [PubMed] [Google Scholar]
- 17. Moons KGM, Wolff RF, Riley RD, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1–33. 10.7326/M18-1377 [DOI] [PubMed] [Google Scholar]
- 18. Riley RD, Moons KGM, Snell KIE, et al. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ 2019;364:k4597. 10.1136/bmj.k4597 [DOI] [PubMed] [Google Scholar]
- 19. Grooten WJA, Tseli E, Äng BO, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS-aspects of interrater agreement. Diagn Progn Res 2019;3:5. 10.1186/s41512-019-0050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagiwara K, Tobisawa Y, Kaya T, et al. Wisteria floribunda agglutinin and its reactive-glycan-carrying prostate-specific antigen as a novel diagnostic and prognostic marker of prostate cancer. Int J Mol Sci 2017;18. 10.3390/ijms18020261. [Epub ahead of print: 26 Jan 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelly BD, Miller N, Sweeney KJ, et al. A circulating microRNA signature as a biomarker for prostate cancer in a high risk group. J Clin Med 2015;4:1369–79. 10.3390/jcm4071369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guinney J, Wang T, Laajala TD, et al. Prediction of overall survival for patients with metastatic castration-resistant prostate cancer: development of a prognostic model through a crowdsourced challenge with open clinical trial data. Lancet Oncol 2017;18:132–42. 10.1016/S1470-2045(16)30560-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Joniau S, Spahn M, Briganti A, et al. Pretreatment tables predicting pathologic stage of locally advanced prostate cancer. Eur Urol 2015;67:319–25. 10.1016/j.eururo.2014.03.013 [DOI] [PubMed] [Google Scholar]
- 24. Palsdottir T, Nordström T, Aly M, et al. A unified prostate cancer risk prediction model combining the Stockholm3 test and magnetic resonance imaging. Eur Urol Oncol 2019;2:490–6. 10.1016/j.euo.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 25. Aguilera A, Bañuelos B, Díez J, et al. Biochemical recurrence risk factors in surgically treated high and very high-risk prostate tumors. Cent European J Urol 2015;68:302–7. 10.5173/ceju.2015.02.485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bramhecha YM, Rouzbeh S, Guérard K-P, et al. The combination of PTEN deletion and 16p13.3 gain in prostate cancer provides additional prognostic information in patients treated with radical prostatectomy. Mod Pathol 2019;32:128–38. 10.1038/s41379-018-0107-6 [DOI] [PubMed] [Google Scholar]
- 27. Alvim RG, Audenet F, Vertosick EA, et al. Performance prediction for surgical outcomes in partial nephrectomy using nephrometry scores: a comparison of arterial based complexity (ABC), renal, and Padua systems. Eur Urol Oncol 2018;1:428–34. 10.1016/j.euo.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bruce HM, Stricker PD, Gupta R, et al. Loss of AZGP1 as a superior predictor of relapse in margin-positive localized prostate cancer. Prostate 2016;76:1491–500. 10.1002/pros.23233 [DOI] [PubMed] [Google Scholar]
- 29. Francini E, Gray KP, Xie W, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). Prostate 2018;78:889–95. 10.1002/pros.23645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hamada R, Nakashima J, Ohori M, et al. Preoperative predictive factors and further risk stratification of biochemical recurrence in clinically localized high-risk prostate cancer. Int J Clin Oncol 2016;21:595–600. 10.1007/s10147-015-0923-3 [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto T, Nakashima J, Inoue R, et al. The significance of micro-lymphatic invasion and pathological Gleason score in prostate cancer patients with pathologically organ-confined disease and negative surgical margins after robot-assisted radical prostatectomy. Int J Clin Oncol 2020;25:377–83. 10.1007/s10147-019-01561-4 [DOI] [PubMed] [Google Scholar]
- 32. Kato M, Kimura K, Hirakawa A, et al. Prognostic parameter for high risk prostate cancer patients at initial presentation. Prostate 2018;78:11–16. 10.1002/pros.23438 [DOI] [PubMed] [Google Scholar]
- 33. Kluth LA, Xylinas E, Rieken M, et al. Does increasing the nodal yield improve outcomes in contemporary patients without nodal metastasis undergoing radical prostatectomy? Urol Oncol 2014;32:47.e1–47.e8. 10.1016/j.urolonc.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 34. Lara PN, Ely B, Quinn DI, et al. Serum biomarkers of bone metabolism in castration-resistant prostate cancer patients with skeletal metastases: results from SWOG 0421. J Natl Cancer Inst 2014;106:dju013. 10.1093/jnci/dju013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee S, Kim KB, Jo JK, et al. Prognostic value of focal positive surgical margins after radical prostatectomy. Clin Genitourin Cancer 2016;14:e313–9. 10.1016/j.clgc.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 36. Lévesque E, Caron P, Lacombe L, et al. A comprehensive analysis of steroid hormones and progression of localized high-risk prostate cancer. Cancer Epidemiol Biomarkers Prev 2019;28:701–6. 10.1158/1055-9965.EPI-18-1002 [DOI] [PubMed] [Google Scholar]
- 37. Lin Y-L, Li Y-L, Ma J-G. Aberrant promoter methylation of Protocadherin8 (PCDH8) in serum is a potential prognostic marker for low Gleason score prostate cancer. Med Sci Monit 2017;23:4895–900. 10.12659/MSM.904366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Löffeler S, Weedon-Fekjaer H, Wang-Hansen MS, et al. "Natural course" of disease in patients with metastatic castrate-resistant prostate cancer: Survival and prognostic factors without life-prolonging treatment. Scand J Urol 2015;49:440–5. 10.3109/21681805.2015.1059881 [DOI] [PubMed] [Google Scholar]
- 39. Narang AK, Trieu J, Radwan N, et al. End-of-radiation PSA as a novel prognostic factor in patients undergoing definitive radiation and androgen deprivation therapy for prostate cancer. Prostate Cancer Prostatic Dis 2017;20:203–9. 10.1038/pcan.2016.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ozden C, Aktas BK, Bulut S, et al. Effect of age on biochemical recurrence after radical prostatectomy. Kaohsiung J Med Sci 2017;33:91–5. 10.1016/j.kjms.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 41. Pei X-Q, He D-lin, Tian G, et al. Prognostic factors of first-line docetaxel treatment in castration-resistant prostate cancer: roles of neutrophil-to-lymphocyte ratio in patients from northwestern China. Int Urol Nephrol 2017;49:629–35. 10.1007/s11255-017-1524-z [DOI] [PubMed] [Google Scholar]
- 42. Qu Y, Zhang C, Du E, et al. Pim-3 is a critical risk factor in development and prognosis of prostate cancer. Med Sci Monit 2016;22:4254–60. 10.12659/MSM.898223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qu F, Xie W, Nakabayashi M, et al. Association of AR-V7 and prostate-specific antigen RNA levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin Cancer Res 2017;23:726–34. 10.1158/1078-0432.CCR-16-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rüenauver K, Menon R, Svensson MA, et al. Prognostic significance of YWHAZ expression in localized prostate cancer. Prostate Cancer Prostatic Dis 2014;17:310–4. 10.1038/pcan.2014.32 [DOI] [PubMed] [Google Scholar]
- 45. Shimodaira K, Nakashima J, Nakagami Y, et al. Prognostic value of platelet counts in patients with metastatic prostate cancer treated with endocrine therapy. Urol J 2020;17:42–9. 10.22037/uj.v0i0.4735 [DOI] [PubMed] [Google Scholar]
- 46. Strand SH, Hoyer S, Lynnerup A-S, et al. High levels of 5-hydroxymethylcytosine (5hmC) is an adverse predictor of biochemical recurrence after prostatectomy in ERG-negative prostate cancer. Clin Epigenetics 2015;7:111. 10.1186/s13148-015-0146-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takagi M, Demizu Y, Terashima K, et al. Long-term outcomes in patients treated with proton therapy for localized prostate cancer. Cancer Med 2017;6:2234–43. 10.1002/cam4.1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y, Xu F, Pan J, et al. Platelet to lymphocyte ratio as an independent prognostic indicator for prostate cancer patients receiving androgen deprivation therapy. BMC Cancer 2016;16:329. 10.1186/s12885-016-2363-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zacho HD, Gade M, Mortensen JC, et al. Bone scan index is an independent predictor of time to castration-resistant prostate cancer in newly diagnosed prostate cancer: a prospective study. Urology 2017;108:135–41. 10.1016/j.urology.2017.05.058 [DOI] [PubMed] [Google Scholar]
- 50. Berg KD, Vainer B, Thomsen FB, et al. ERG protein expression in diagnostic specimens is associated with increased risk of progression during active surveillance for prostate cancer. Eur Urol 2014;66:851–60. 10.1016/j.eururo.2014.02.058 [DOI] [PubMed] [Google Scholar]
- 51. Lazzeri M, Haese A, de la Taille A, et al. Serum isoform [−2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/ml: a multicentric European study. Eur Urol 2013;63:986–94. 10.1016/j.eururo.2013.01.011 [DOI] [PubMed] [Google Scholar]
- 52. Aladwani M, Lophatananon A, Ollier W, et al. Prediction models for prostate cancer to be used in the primary care setting: a systematic review. BMJ Open 2020;10:e034661. 10.1136/bmjopen-2019-034661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tian S, Lei Z, Gong Z, et al. Clinical implication of prognostic and predictive biomarkers for castration-resistant prostate cancer: a systematic review. Cancer Cell Int 2020;20:409. 10.1186/s12935-020-01508-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harrison H, Thompson RE, Lin Z, et al. Risk prediction models for kidney cancer: a systematic review. Eur Urol Focus 2021;7:1380-1390. 10.1016/j.euf.2020.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schmitz-Dräger BJ, Droller M, Lokeshwar VB, et al. Molecular markers for bladder cancer screening, early diagnosis, and surveillance: the WHO/ICUD consensus. Urol Int 2015;94:1–24. 10.1159/000369357 [DOI] [PubMed] [Google Scholar]
- 56. Epstein JI, Egevad L, Amin MB, et al. The 2014 international society of urological pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 2016;40:244–52. 10.1097/PAS.0000000000000530 [DOI] [PubMed] [Google Scholar]
- 57. Hung S-C, Yang C-K, Cheng C-L, et al. Long-term oncologic outcomes of robotic-assisted radical prostatectomy by a single surgeon. Anticancer Res 2017;37:4157–64. 10.21873/anticanres.11803 [DOI] [PubMed] [Google Scholar]
- 58. Rizzardi AE, Rosener NK, Koopmeiners JS, et al. Evaluation of protein biomarkers of prostate cancer aggressiveness. BMC Cancer 2014;14:244. 10.1186/1471-2407-14-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-058267supp001.pdf (52.2KB, pdf)
bmjopen-2021-058267supp002.pdf (49KB, pdf)
bmjopen-2021-058267supp003.pdf (36KB, pdf)
bmjopen-2021-058267supp004.pdf (37.5KB, pdf)
bmjopen-2021-058267supp005.pdf (49.6KB, pdf)
Data Availability Statement
No data are available. not applicable.