Abstract
The asymmetric total synthesis of four lignans, dimethylmatairesinol, matairesinol, (−)-niranthin, and (+)-niranthin has been achieved using reductive ring-opening of cyclopropanes. Moreover, we performed bioassays of the synthesized (+)- and (−)-niranthins using hepatitis B and influenza viruses, which revealed the relationship between the enantiomeric structure and the anti-viral activity of niranthin.
The total synthesis of four lignans including (−)- and (+)-niranthin has been achieved utilizing cyclopropanes. Based on bioassays of the (+)- and (−)-niranthins using HBV and IFV, we speculated the bioactive site of niranthin against HBV and IFV.
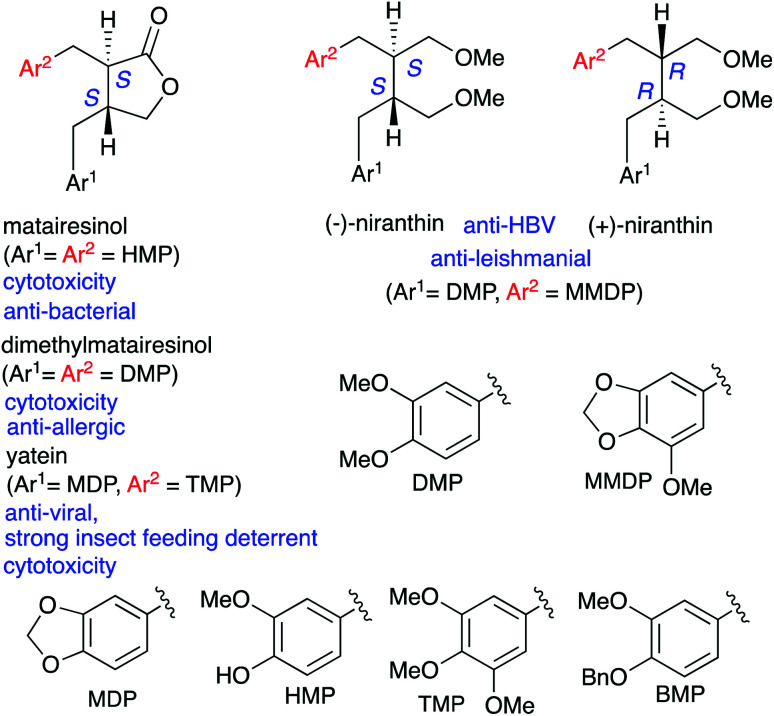
Lignans are attracting considerable attention due to their widespread distribution in plants and their varied bioactivity.1–5 For example, matairesinol,2 dimethylmatairesinol,3 yatein,4 and niranthin5 are found in nature and exhibit e.g., cytotoxicity,2b,d,3b,4b anti-bacterial,2c anti-allergic,3c anti-viral,4d,5b,e anti-leishmanial,5d and strong insect-feeding-deterrent activity.4c Among these compounds, anti-viral compounds have received significant attention owing to the worldwide pandemic of coronavirus disease 2019 (COVID-19). Although niranthin exhibits anti-viral activity toward the hepatitis B virus (HBV),5b,e the enantiomeric SAR (structure–activity relationship) for the anti-viral activity of niranthin has not been revealed so far. To examine the SAR for a pair of enantiomers, an independent asymmetric synthesis of both enantiomers is necessary. However, the alternative synthesis of (−)- or (+)-niranthin has not been reported.6 During our recent studies on the transformation of cyclopropanes,7 we have reported a reductive ring-opening of enantioenriched donor–acceptor (D–A) cyclopropanes and its application to an asymmetric total synthesis of yatein.7i As a further extension of this synthetic method, we disclose here the asymmetric total synthesis of (−)-dimethylmatairesinol, (−)-matairesinol, (+)-niranthin, and (−)-niranthin. Moreover, the results of bioassays using (+)-niranthin and (−)-niranthin against HVB and influenza virus (IFV) are described (Scheme 1).
Scheme 1. Some examples of bioactive dibenzyl lignans.
Scheme 2 outlines the enantioselective synthesis of optically active lactones 5a and 5b. Following our previous report,7i we attempted to synthesize the enantio-enriched bicyclic lactones 4a and 4b.
Scheme 2. Enantioselective synthesis of key intermediates 5a and 5b.
Initially, the cyclopropanation of enal 1 with dimethyl α-bromomalonate 2 using the Hayashi–Jørgensen catalyst afforded the desired optically active cyclopropylaldehydes 3a and 3b in good to high yield with high ee.7c,e,h–j,8,9 The reduction of the aldehydes to alcohols and subsequent lactonization with p-TsOH afforded lactones 4a and 4b in high yield with high ee. The optical purity of lactones 4a and 4b were determined using HPLC analyses on a chiral column, and the ee values of the enantioselective cyclopropanations were estimated based on these HPLC analyses. Next, treatment of bicyclic lactones 4a and 4b with hydrogen in the presence of a catalytic amount of Pd–C in AcOEt at 0 °C resulted in a regioselective reductive ring-opening to furnish benzyloxylactones 5a and 5b in good to high yield with high dr and high ee. In the hydrolysis step, debenzylation of the benzyloxyaryl group did not occur under these mild conditions, i.e., in AcOEt at 0 °C.7i
For the α-benzylation of 5a and 5b to afford 6a–c, the corresponding substituted benzylhalides were necessary. 3-Methoxy-4-benzyloxybenzylbromide and 3,4-dimethoxybenzylbromide were easily prepared by known methods (for details, see the ESI†); however, the preparation of 3,4-methylenedioxy-5-methoxybenzylbromide (16) required a modified procedure that involves the regioselective protection of the hydroxy group at the 3-position of 3,4,5-trihydroxybenzene (Scheme 3). The methylenedioxylation of gallic acid (10) during the first step resulted in a low yield of 11.10 Consequently, we successfully synthesized 12 using a cyclic boron-ester system.11 Arylmethylbromide 16 was derived from ester 12 in two steps in good to high yield.
Scheme 3. Preparation of substituted benzylbromide 16.
Next, enolates were generated from lactones 5a and 5b using K2CO3 in DMF, and successfully attacked the benzylhalides on the less-hindered side to afford α-benzyl lactones 6a–c with excellent dr values (Scheme 4).7i,12 The trans-α,β-disubstituted lactones 7a–c were obtained via the hydrolysis of the α-methoxycarbonyllactones 6a–c followed by decarboxylation. The transformation from the enol form to the keto form gave the thermodynamically favored trans products (7a–c) with excellent dr values.7i,12 Finally, the debenzylation of 7a using a catalytic amount of Pd–C in methanol under a hydrogen atmosphere afforded matairesinol (7d) in 96% yield. Thus, the total syntheses of dimethylmatairesinol (7b) and matairesinol (7d) were achieved, and spectral data of these natural products were consistent with reported data.2e,3d The absolute configuration of these compounds were determined using the known data of optical rotation values. The reduction of lactone 7c using LAH afforded diol 8 in 91% yield (Scheme 4). Subsequent dimethylation of the resulting diol 8 using NaH and MeI furnished (−)-niranthin in 89% yield with 95% ee.13 Spectral data of (−)-niranthin was also consistent with reported data.5b,e,6 Following the total synthesis of (−)-niranthin, we also achieved the total synthesis of (+)-niranthin via an alternative enantioselective cyclopropanation using a different enantiomeric Hayashi–Jørgensen catalyst derived from d-proline instead of l-proline (Scheme 5).14
Scheme 4. Alternative asymmetric total synthesis of dimethylmatairesinol, matairesinol, and (−)-niranthin.
Scheme 5. Alternative asymmetric total synthesis of (+)-niranthin.
(−)-Niranthin has been reported to exhibit anti-HBV activity.5b,e Aiming to shed light on the relationship between its enantiomeric structure and activity, we performed a bioassay on the synthesized (−)- and (+)-niranthin against not only HBV, but also the influenza virus (IFV). The anti-HBV activity results are summarized in Fig. 1 and 2, while the anti-IFV activity is summarized in Fig. 3 (for details, see the ESI†).
Fig. 1. HBV-infection assay using (−)- and (+)-niranthin.
Fig. 2. HBV-replication assay using (−)- and (+)-niranthin.
Fig. 3. Growth-inhibition assay of IFV using (−)- and (+)-niranthin.
Based on the assays using HBV-infected HepG2-hNTCP-C4 cells and HBV-replicating Hep38.7-tet cells, the amount of HBs antigen decreased in a concentration-dependent manner without apparent cytotoxicity. The 50% inhibition concentration (IC50) in the HBV-infected cells was calculated to be 14.3 ± 0.994 μM for (−)-niranthin and 9.11 ± 0.998 μM for (+)-niranthin (Fig. 1), while the IC50 in the HBV-replicating cells was calculated to be 16.2 ± 0.992 μM for (−)-niranthin and 24.2 ± 0.993 μM for (+)-niranthin (Fig. 2). These results show that (−)-niranthin and (+)-niranthin exhibit anti-HBV activity, and that there is no remarkable difference between the anti-HBV activity of both enantiomers. In contrast, based on the bioassay of (−)- and (+)-niranthins against IFV using MDCK cells, cytotoxicity of (−)-niranthin appears at >400 μM judging that cell viability without IFV is less than 80%, and (−)-niranthin inhibited IFV-infection to cells in a concentration-dependent manner on the concentration range of non-cytotoxicity, and exhibits anti-IFV activity at 200–400 μM judging that cell viability with IFV is over 50% (Fig. 3). However, (+)-niranthin does not exhibit anti-IFV activity, and similarly to (−)-niranthin, cytotoxicity appears at >400 μM. Thus, the anti-IFV activity between (−)- and (+)-niranthins is clearly different. Our findings suggest that the enantiomeric site in niranthin endows (−)-niranthin with more potent anti-IFV activity than (+)-niranthin. We speculated that the anti-HBV active site of niranthin might be a part of the molecular structure such as aromatic groups which are far from chiral centers. In contrast, anti-IFV active site of niranthin might be closer to the chiral centers (Scheme 6).
Scheme 6. A speculation for the bioactive site of niranthin against HBV and IFV.
Conclusions
We achieved the asymmetric total syntheses of four bioactive lignans: matairesinol, dimethylmatairesinol, (−)-niranthin, and (+)-niranthin. Key reactions include the Pd-catalyzed reductive ring-opening reaction of enantioenriched cyclopropanes under a hydrogen atmosphere and a highly stereoselective decarboxylation. Thus, we have achieved the first alternative total synthesis of (−)-niranthin and (+)-niranthin. Using the synthesized niranthin enantiomers, we investigated the relationship between the enantiomer structure and its anti-viral activity against the hepatitis B virus (HBV) and the influenza virus (IFV). The results indicate that although the anti-HBV activity does not differ significantly between these two enantiomers, the anti-IFV activity of (−)-niranthin is more potent than that of (+)-niranthin. This result may be interpreted in terms of a different recognition of the enantiomeric structure of a bioactive compound among different virus species.
Author contributions
R. Ota: investigation for the synthesis of lignans. D. Karasawa: investigation for the synthesis of lignans. M. Oshima: investigation for bioassay of niranthin using HBV. K. Watashi: investigation for bioassay and writing original draft of bioassay using HBV. N. Shimasaki: investigation for bioassay and writing original draft of bioassay using IFV. Y. Nishii: methodology, investigation and writing – original draft.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
This research was partially supported by Grants-in-Aid for Scientific Research on Basic Areas (A) ‘20H00288’ and (C) ‘18K05120’ from the JSPS. We thank Prof. Mutsumi Kimura (Shinshu University) for the HRMS measurements.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d2ra00499b
Notes and references
- For reviews, see: ; (a) Ward R. S. Nat. Prod. Rep. 1999;16:75. doi: 10.1039/A705992B. [DOI] [Google Scholar]; (b) Saleem M. Kim H. J. Ali M. S. Lee Y. S. Nat. Prod. Rep. 2005;22:696. doi: 10.1039/B514045P. [DOI] [PubMed] [Google Scholar]; (c) Pan J.-Y. Chen S.-L. Yang M.-H. Wu J. Shinkkonen J. Zou K. Nat. Prod. Rep. 2009;26:1251. doi: 10.1039/B910940D. [DOI] [PubMed] [Google Scholar]; (d1) Reynolds R. G. Nguyen H. Q. A. Reddel J. C. T. Thomson R. J. Nat. Prod. Rep. 2022 doi: 10.1039/D1NP00057H. [DOI] [PMC free article] [PubMed] [Google Scholar]; , and other recent references cited therein
- (a) Esterfield T. H. Bee J. J. Chem. Soc., Trans. 1910;97:1028. doi: 10.1039/CT9109701028. [DOI] [Google Scholar]; (b) Hirano T. Gotoh M. Oka K. Life Sci. 1994;55:1061. doi: 10.1016/0024-3205(94)00641-5. [DOI] [PubMed] [Google Scholar]; (c) Kumarasamy Y. Nahar L. Cox P. J. Dinan L. N. Ferguson C. A. Finnie D. A. Jaspars M. Sarker S. D. Pharm. Biol. 2003;41:203. doi: 10.1076/phbi.41.3.203.15099. [DOI] [Google Scholar]; (d1) Su S. Cheng X. Wink M. J. Pharm. Pharmacol. 2015;67:1316. doi: 10.1111/jphp.12426. [DOI] [PubMed] [Google Scholar]; , for a reference of synthesis of matairesinol, see:; (e) Eklund P. C. Sundell F. J. Smeds A. I. Sjöholm R. E. Org. Biomol. Chem. 2004;2:2229. doi: 10.1039/B402849J. [DOI] [PubMed] [Google Scholar]
- (a) Takaoka D. Takamatsu N. Saheki Y. Kono K. Nakaoka C. Hiroi M. Nippon Kagaku Kaishi. 1975;12:2192. doi: 10.1246/nikkashi.1975.2192. [DOI] [Google Scholar]; (b) Chang S.-T. Wang D. S.-Y. Wu C.-L. Shiah S.-G. Kuo Y.-H. Chang C.-J. Phytochemistry. 2000;55:227. doi: 10.1016/S0031-9422(00)00275-2. [DOI] [PubMed] [Google Scholar]; (c1) Tanabe H. Fukutomi R. Yasui K. Kaneko A. Imai S. Nakayama T. Isemura M. J. Health Sci. 2011;57:184. [Google Scholar]; , for a recent reference of synthesis of dimatairesinol, see:; (d) Yamauchi S. Nishimoto A. Nishiwaki H. Nishi K. Sugahara T. Bioorg. Med. Chem. Lett. 2020;30:127191. doi: 10.1016/j.bmcl.2020.127191. [DOI] [PubMed] [Google Scholar]
- (a) McDoniel P. B. Cole J. R. J. Pharm. Sci. 1972;61:1992. doi: 10.1002/jps.2600611224. [DOI] [PubMed] [Google Scholar]; (b) Novelo M. Cruz J. G. Hernández L. Pereda-Miranda R. Chai H. Mar W. Pezzuto J. M. J. Nat. Prod. 1993;56:1728. doi: 10.1021/np50100a011. [DOI] [PubMed] [Google Scholar]; (c) Harmatha J. Nawrot J. Entomol. Exp. Appl. 2002;104:51. doi: 10.1046/j.1570-7458.2002.00990.x. [DOI] [Google Scholar]; (d) Kuo Y.-C. Kuo Y.-H. Lin Y.-L. Tsai W.-J. Antiviral Res. 2006;70:112. doi: 10.1016/j.antiviral.2006.01.011. [DOI] [PubMed] [Google Scholar]
- For the reference regarding the isolation of natural (−)-niranthin, see: ; (a1) Anjaneyulu A. S. R. Rao K. J. Row L. R. Subrahmanyam C. Tetrahedron. 1973;29:1291. doi: 10.1016/S0040-4020(01)83146-X. [DOI] [Google Scholar]; , for references regarding bioassays using isolated natural (−)-niranthin, see:; (b) Huang R.-L. Huang Y.-L. Ou J.-C. Chen C.-C. Hsu F.-L. Chang C. Phytother. Res. 2003;17:449. doi: 10.1002/ptr.1167. [DOI] [PubMed] [Google Scholar]; (c1) Kassuya C. A. L. Silvestre A. Menezes-de-Lima O. Marotta D. M. Rehder V. L. G. Calixto J. B. Eur. J. Pharmacol. 2006;546:182. doi: 10.1016/j.ejphar.2006.07.025. [DOI] [PubMed] [Google Scholar]; , (anti-inflammatory); (d) Chowdhury S. Mukherjee T. Mukhopadhyay R. Mukherjee B. Sengupta S. Chattopadhyay S. Jaisankar P. Roy S. Majumder H. K. EMBO Mol. Med. 2012;4:1126. doi: 10.1002/emmm.201201316. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Liu S. Wei W. Shi K. Cao X. Zhou M. Liu Z. J. Ethnopharmacol. 2014;155:1061. doi: 10.1016/j.jep.2014.05.064. [DOI] [PubMed] [Google Scholar]
- For a reference regarding the total synthesis of (±)-niranthin, see: ; Schneiders G. E. Stevenson R. Org. Prep. Proced. Int. 1982;14:1. doi: 10.1080/00304948209354890. [DOI] [Google Scholar]
- For selected references in last decade, see: ; (a) Yoshida E. Nishida K. Toriyabe K. Taguchi R. Motoyoshiya J. Nishii Y. Chem. Lett. 2010;39:194. doi: 10.1246/cl.2010.194. [DOI] [Google Scholar]; (b) Sakuma D. Ito J. Sakai R. Taguchi R. Nishii Y. Chem. Lett. 2014;43:610. doi: 10.1246/cl.131179. [DOI] [Google Scholar]; (c) Ito J. Sakuma D. Nishii Y. Chem. Lett. 2015;44:297. doi: 10.1246/cl.141061. [DOI] [Google Scholar]; (d) Sakuma D. Yamada Y. Sasazawa K. Nishii Y. Chem. Lett. 2015;44:818. doi: 10.1246/cl.150085. [DOI] [Google Scholar]; (e) Takada S. Iwata K. Yubune T. Nishii Y. Tetrahedron Lett. 2016;57:2422. doi: 10.1016/j.tetlet.2016.04.076. [DOI] [Google Scholar]; (f) Takada S. Saito T. Iwata K. Nishii Y. Asian J. Org. Chem. 2016;5:1225. doi: 10.1002/ajoc.201600313. [DOI] [Google Scholar]; (g) Sasazawa K. Takada S. Yubune T. Takaki N. Ota R. Nishii Y. Chem. Lett. 2017;46:524. doi: 10.1246/cl.170081. [DOI] [Google Scholar]; (h) Takada S. Takaki N. Yamada K. Nishii Y. Org. Biomol. Chem. 2017;15:2443. doi: 10.1039/C7OB00278E. [DOI] [PubMed] [Google Scholar]; (i) Sone Y. Kimura Y. Ota R. Mochizuki T. Ito J. Nishii Y. Eur. J. Org. Chem. 2017:2842. doi: 10.1002/ejoc.201700345. [DOI] [Google Scholar]; (j) Kimura Y. Sone Y. Saito T. Mochizuki T. Nishii Y. Asian J. Org. Chem. 2017;6:977. doi: 10.1002/ajoc.201700222. [DOI] [Google Scholar]; (k) Saito T., Shimizu Y., Araki Y., Ohgami Y., Kitazawa Y. and Nishii Y., Eur. J. Org. Chem., and other references cited therein, 10.1002/ejoc.202101213 [DOI]
- For a reference regarding the asymmetric cyclopropanation using a Hayashi–Jørgensen catalyst, see: ; Xie H. Zu L. Li H. Wang J. Wang W. J. Am. Chem. Soc. 2007;129:10886. doi: 10.1021/ja073262a. [DOI] [PubMed] [Google Scholar]
- For references regarding the original Hayashi–Jørgensen catalyst, see: ; (a) Marigo M. Wabnitz T. C. Fielenbach D. Jørgensen K. A. Angew. Chem., Int. Ed. 2005;44:794. doi: 10.1002/anie.200462101. [DOI] [PubMed] [Google Scholar]; (b) Hayashi Y. Gotoh H. Hayashi T. Shoji M. Angew. Chem., Int. Ed. 2005;44:4212. doi: 10.1002/anie.200500599. [DOI] [PubMed] [Google Scholar]; (c1) Franzén J. Marigo M. Fielenbach D. Wabnitz T. C. Kjærsgaard A. Jørgensen K. A. J. Am. Chem. Soc. 2005;127:18296. doi: 10.1021/ja056120u. [DOI] [PubMed] [Google Scholar]; , for a recent review of total synthesis and patents using Hayashi–Jørgensen catalyst, see; (d) Reyes-Rodriguez G. J. Rezayee N. M. Vidal-Albalat A. Jørgensen K. A. Chem. Rev. 2019;119:4221. doi: 10.1021/acs.chemrev.8b00583. [DOI] [PubMed] [Google Scholar]
- (a) Song C. Zhao P. Hu Z. Shi S. Cui Y. Chang J. Bioorg. Med. Chem. Lett. 2010;20:2297. doi: 10.1016/j.bmcl.2010.01.166. [DOI] [PubMed] [Google Scholar]; (b) Takaoka S. Takaoka N. Minoshima Y. Huang J.-M. Kubo M. Harada K. Hioki H. Fukuyama Y. Tetrahedron. 2009;65:8354. doi: 10.1016/j.tet.2009.08.015. [DOI] [Google Scholar]
- (a) Cormier K. Curry R. D. Betsch M. P. Goguen J. A. Vogels C. M. Decken A. Turcotte S. Westcott S. A. J. Heterocycl. Chem. 2016;53:1807. doi: 10.1002/jhet.2490. [DOI] [Google Scholar]; (b) Pettit G. R. Singh S. B. Can. J. Chem. 1987;65:2390. doi: 10.1139/v87-399. [DOI] [Google Scholar]
- (a) Ferrié L. Bouyssi D. Balme G. Org. Lett. 2005;7:3143. doi: 10.1021/ol050690h. [DOI] [PubMed] [Google Scholar]; (b) Yamada K.-i. Konishi T. Nakano M. Fujii S. Cadou R. Yamamoto Y. Tomioka K. J. Org. Chem. 2012;77:5775. doi: 10.1021/jo300944f. [DOI] [PubMed] [Google Scholar]
- During the isolation process, a recrystallization of (−)-niranthin increased the ee value from 92% ee to 95% ee
- Similar to (−)-niranthin, a recrystallization of (+)-niranthin increased the ee value from 94% ee to 96% ee
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.