Abstract
Background:
Acquisition of HIV primary drug-resistant (PDR) infection can lead to poor virologic and clinical outcomes in individuals and hampers public health efforts in epidemic control. Monitoring PDR in HIV-positive blood donors can be used to inform nationwide trends in the spread of drug-resistant HIV strains.
Methods:
We conducted a cross-sectional study using genetic sequence analysis to assess HIV pol sequences, PDR, and risk factors for infection using audio computer-assisted structured interviews (ACASI) in four large blood centers in Brazil from 2007 to 2017.
Results:
Of 716 HIV-positive blood donors, 504 (70.4%) were successfully sequenced. HIV clade B (73.2%) was the most prevalent subtype, followed by a mix of non-B (21.2%) sub-types. A two-fold increase (from 4% to 8%) in recombinants prevalence was observed during the study period. Sixty-four (12.7%) presented PDR. Overall, HIV PDR prevalence remained stable during the study period. Drug resistance mutations for non-nucleoside reverse transcriptase inhibitors were found in 39 (7.7%) donors, while for nucleoside reverse transcriptase inhibitors were found in 26 (5.1%), and for protease inhibitors in 24 (4.8%) of HIV-infected donors. We did not find statistically significant differences in demographics, behavioral risk factors, or HIV genotypes when comparing volunteers with and without PDR.
Conclusion:
The HIV PDR rate among donors remained stable during the study period. HIV-positive blood donors can be an informative population to monitor primary HIV resistance and ultimately may help to increase the knowledge and awareness of HIV risk factors and PDR.
Keywords: HIV drug resistance, primary HIV drug resistance surveillance, transfusion safety, blood donors, Brazil
INTRODUCTION
The increasing prevalence of pretreatment drug resistance and high rates of resistance-associated mutations found in many countries primarily, among specific populations, is the price for the widespread availability of the HIV treatment programs (1,2).
HIV drug resistance (HIVDR) is due to changes in the genetic structure of the virus, affecting the ability of particular drugs or drug classes to block its replication effectively. There are two main categories: acquired HIV drug resistance, that occurs over time due to viral replication in individuals receiving antiretroviral therapy (ART), and transmitted HIV drug resistance or primary HIV drug resistance (PDR), that arises in people with no previous history of ART exposure, who were infected with an already drug-resistant strain HIV (3).
PDR may lead to suboptimal viral suppression, followed by immunologic failure, poor clinical outcomes in individual patients, adding risk for communities from viremic patients (4). Continued use of an already failed treatment will positively select for resistance mutations decreasing the efficacy of viral suppression by ART, favoring the risk of sexual and perinatal HIV transmission, and threaten the effectiveness of subsequent regimens.
The frequency and types of HIVDR are greatly dependent on the variety of drug classes currently proposed in each country, and according to the WHO, pretreatment HIVDR rates for non-nucleoside reverse transcriptase inhibitors (NNRTI) had increased worldwide from 11% to 29% since the global rollout of ART since 2001 (5). Also, the prevalence of NNRTI PDR is higher, notably in specific subpopulations, such as women, 11.8%, comparing to 7.8% among men initiating ART between 2014 and 2018 (3).
In Brazil, HIVDR mutations have evolved over the past 20 years, with an overall rate of 6.6% in 2001 (6), followed by 8.1% in a survey dated from 2007–2008 (7), and 16,3% was documented more recently in the year of 2017 (8). The prevalence of HIVDR varies across the country, ranging from 6.8%, in the Midwest, up to 11.2%, in the Southeast of Brazil (9). Most HIVDR studies address newly diagnosed and before ARV onset (9).
HIV infected blood donors are a readily accessible population that may, by proxy, act as a sentinel population for surveillance and monitoring drug-resistance trends. Although these donors may not represent the overall HIV population, we can infer that persons with a variable range of risk behaviors perform blood donation and can provide a picture of the currently circulating HIV strains, including genotypes, and the rates of PDR.
Our primary study aim was to evaluate the frequency of PDR among Brazilian blood donors from among a ten-year time frame (2007 to 2017), and secondarily to assess if PDR was associated with HIV different genotypes and donors behavioral risk factors.
MATERIALS AND METHODS
Study design and population
In this cross-sectional study, serologically confirmed HIV infection blood donors, during two phases of the NHLBI, NIH REDS program (Retrovirus Epidemiology Donor Study (II) and Recipient Epidemiology and Donor Evaluation Study (III) - International Component, Brazil REDS-II (2007–2012) and REDS-III (2012–2017) (10) were recruited to participate in molecular surveillance and behavioral risk factor assessments. Following the analysis of the work previously done, we extended that by describing in a more detailed and comprehensive narrative.
Settings
HIV infected blood donors from four large Brazilian blood centers were included in the study. Fundação Pró-Sangue (FPS) is located in the state of São Paulo, Fundação Hemominas in Belo Horizonte, Minas Gerais; Fundação Hemorio, Rio de Janeiro; all in the southeastern part of Brazil which is the most densely populated, and Fundação Hemope in the state of Pernambuco, in the northeastern part of the country (Figure 1). In 2016, 3,796,776 blood donations were collected in Brazil, and 593,949 (15%) of these blood units were collected in these participating blood centers.
Figure 1 -.
Locations of REDS-Brazil participating blood centers
In Brazil, HIV infected donors are asked to return to the blood center for notification of the routine donation testing results, and for the collection of additional samples for new confirmatory testing (11). In our study, all blood donors who returned to the blood bank were invited to participate.
A consecutive and sequentially convenience sample of HIV diagnosed blood donors enrolled in the research study. The participants collected blood samples for molecular surveillance and completed an audio computer-assisted structured interview (ACASI) (12). The ACASI instrument included questions on demographics, previous blood donation, motivational factors for donation, blood testing, HIV knowledge, and HIV behavior risk factors (occupational, non-occupational and sexual exposure). Details of the study recruitment, HIV behavior risk factor questionnaire, and HIV genotyping methods have been previously described (11,13).
Laboratory methods
HIV-1 Clade Typing and Drug Resistance Testing
Subtype and resistance analyses were performed at Fundação Pro-Sangue, São Paulo (14). In brief, an HIV genome fragment encompassing the protease gene and approximately 700 base pairs of the reverse transcriptase gene were amplified and sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems Foster City, CA) following the manufacturer’s protocol. Subsequently, the products of this reaction were analyzed by an ABI3500 automated sequencer (Applied Biosystems). Only partial HIV pol sequences were obtained. The calibrated population resistance tool version 5.0 beta (available through the Stanford University HIV Drug Resistance Database http://cpr.stanford.edu/cpr.cgi) and Bennett et al., 2009, were used to identify transmitted drug-resistant mutations (15). Mutations listed as causing or contributing to resistance are non-polymorphic in untreated persons and apply to all HIV-1 subtypes according to the World Health Organization (WHO) report (3). Sequences from this study were submitted to GenBank under the numbers JQ237931–JQ238236, and KC834581–KC834601.
HIV Drug Resistance definition
HIV drug resistance was defined as the presence of ≥1 surveillance drug resistance mutations (SDRM) (15) and classified for one or more of the following drugs: nevirapine, efavirenz, any NRTI, atazanavir, darunavir or lopinavir. NNRTI resistance was defined as resistance to nevirapine or efavirenz; NRTI resistance was defined as resistance to any nucleoside reverse transcriptase inhibitor drug; and protease inhibitor resistance was defined as resistance to atazanavir, darunavir or lopinavir (5,16).
Statistical analysis
Variables with continuous distributions were reported as median (interquartile range) or means (standard deviations), depending on the distribution, while categorical variables were summarized as absolute numbers and percentages. When appropriate, 95% confidence intervals were calculated. Differences between patients with and without PDR were assessed using chi-square, or Fisher’s exact test for categorical variables and T-test or Mann-Whitney U test, as appropriate, for continuous variables. All statistical analysis was performed using SAS 9.4, with a 2-sided p-value of < 0.05 considered to be significant. Missing data are shown in the tables for informative purposes but were excluded from the statistical comparisons. Statistical comparisons with HIV risk factors and PDR included all patients who reported each behavior risk. We did not classify behavior risk ordinarily, such as higher or lower risk exposures or calculate counts of total reported risks. HIV subtypes were treated as an outcome in the analysis alongside PDR.
Ethical issues
Study protocols were approved by the Federal Committee on Human Subjects (CONEP) of the Ministry of Health in Brazil as part of the REDS-II/III International Program, local ethics committees from each of the participating blood centers, IRB of record for Vitalant Research Institute and from the Research Triangle Institute. Written informed consent for all the procedures was obtained from each participant after recall to perform confirmatory HIV test and before the sample collection. As the finding can directly impact clinical care, all laboratory results were returned to the participants.
RESULTS
A total of 716 HIV infected blood donors were enrolled in the study: 341 from REDS-II (2007 to 2012) and 375 from REDS-III (2012 to 2017). Of these, 212 HIV donors were excluded from the analysis; 124 (17.3%) for reporting prior whatever ART use; 11 (1.5%) due to lack of information on ART use, and in 77 (10.7%) the HIV sequences were not successfully amplified due to low or undetectable viral load.
Thus, five hundred and four HIV infected donors (70.5% of enrolled cases) were included in the study, 304 from REDS II and 200 from REDS III. HIV case participation varied across the blood centers; 181 (35.9%) were from HEMOPE-Recife; 160 (31.7%) from HEMORIO-Rio de Janeiro; 95 (18.8%) from Fundação Pró-Sangue-São Paulo and 68 (13.5%) from HEMOMINAS-Belo Horizonte. There was a predominance of men 408 (81%), and most of the participants had more than 31 years old, 266 (52.8%).
Primary Drug Resistance (PDR)
Overall, 64 (12.7%) of the 504 HIV infected blood donors had PDR. The PDR prevalence varied across the blood centers. The highest PDR prevalence (17.9%) was observed at São Paulo, followed by Belo Horizonte (16.2%), Rio de Janeiro (13.1%), and the lowest (8.3%) was found in Recife. No demographic and regional differences were observed between HIV-positive participants with or without PDR (Table 1).
Table 1.
Demographic characteristics, sexual orientation and marital status among 504 blood donors from 2007 – 2017
| Demographic characteristics, sexual orientation, and marital status | REDS (2007 – 2017) | ||
|---|---|---|---|
| HIV infected blood donors n=504 | HIV infected donors with resistance n=64 | p-value | |
|
| |||
| n (%) | n (%) | ||
| Site | |||
| Hemope | 181 (35.9) | 15 (23.4) | 0.1 |
| Hemominas | 68 (13.5) | 11 (17.2) | |
| FPS | 95 (18.8) | 17 (26.6) | |
| Hemorio | 160 (31.7) | 21(32.8) | |
| Donor Status * | |||
| First Time | 220 (43.7) | 26 (40.6) | 0.7 |
| Repeat | 260 (51.6) | 33 (51.6) | |
| Unknown | 24 (4.8) | 5 (7.8) | |
| Donation Type * | |||
| Community | 303 (60.1) | 39 (60.9) | 0.5 |
| Replacement | 176 (34.9) | 20 (31.3) | |
| Unknown | 25 (5.0) | 5 (7.8) | |
| Gender | |||
| Male | 408 (81.0) | 53 (82.8) | 0.7 |
| Female | 96 (19.0) | 11 (17.2) | |
| Age (years) | |||
| 18 – 25 | 130 (25.8) | 19 (29.7) | 0.3 |
| 26 – 30 | 108 (21.4) | 14 (21.9) | |
| 31 – 39 | 149 (29.6) | 22 (34.4) | |
| 40 + | 117 (23.2) | 9 (14.1) | |
| Education level | |||
| Never been to school | 3 (0.6) | 1 (1.6) | 0.3 |
| Elementary school | 154 (30.6) | 18 (28.1) | |
| High/Technical school | 248 (49.2) | 29 (45.3) | |
| College or more | 97 (19.2) | 16 (25.0) | |
| Dońt know/ refused | 2 (0.4) | 0 (0.0) | |
| Education level B | |||
| ≤ Elementary School | 157 (31.2) | 19 (29.7) | 0.8 |
| > Elementary School | 345 (68.5) | 45 (70.3) | |
| Don‘t know/ refused | 2 (0.4) | 0 (0.0) | |
| Sexual Orientation * | |||
| Heterosexual | 286 (56.7) | 32 (50.0) | 0.3 |
| Bisexual | 96 (19.0) | 14 (21.9) | |
| Homosexual | 107 (21.2) | 17 (26.6) | |
| Refused/Don’t know | 15 (3.00) | 1 (1.5) | |
| Marital status | |||
| Single, never married | 276 (54.8) | 39 (60.9) | 0.4 |
| Living together, not married | 105 (20.8) | 14 (21.9) | |
| Married | 74 (14.7) | 5 (7.8) | |
| Divorced/Widowed/Separated | 49 (9.7) | 6 (9.4) | |
Self-reported
Primary Drug Resistance and HIV Behavior Risk Factors
PDR and sexual risk exposures
PDR frequency rates varied according to sexual exposure. Of 408 men, 195 (47.8%) reported sex with other men over the course of a lifetime; of these, 26 (13.3%) presented PDR. One hundred sixty-two (39.7%) men reported sex with other men in the last 12 months; of these, 27 (16.7%) had PDR.
Sex with a person who injects drugs or non-prescription substances (PWID) in the last 12 months was reported by 29 (5.8%) study participants; of these, four (13.8%) had PDR. Also, PDR was found in seven out of 51 (13.7%) blood donors that reported sex with a person under potential biological risk due to job exposure throughout the lifetime.
No significant statistical association was observed between reported sexual risk exposures, the number of sexual partners’ lifetime, nor in the last 12 months, and PDR.
PDR and Occupational and non-Occupational exposures
Overall, 67 (13.3%) of the HIV infected blood donors reported occupational exposure to needle or body fluid throughout a lifetime; of these, six (9.0%) had PDR. For non-occupational exposures, the highest PDR frequency was found among those reporting blood transfusions throughout a lifetime in which, out of 19 (3.8%) study participants, three (15.8%) presented PDR. Besides, 125 (24.8%) study participants reported tattoo, and of these, 19 (15.2%) had PDR. PWID ever was reported by 79 (15.7%); of these, 11 (13.9%) had PDR.
The remaining risk non-occupational exposures, such as body piercing, acupuncture, surgery, and endoscopic/colonoscopic corresponded to 12.6%, 12.5%, 13.4%, and 12.6% case of PDR, respectively. No subjects who had been an inmate, throughout a lifetime presented PDR. The prevalence of PDR by disclosed risk factors is represented in table 2.
Table 2.
General exposure, sexual and behavior risks among blood donors with evidence of HIV-1 primary antiretroviral resistance
| HIV REPORTED RISKS | Total | Primary HIV resistance† n=64 | p-value* |
|---|---|---|---|
|
| |||
| n | n (%) | ||
| Occupational Exposure | |||
| exposure to needle or body fluids, ever | 67 | 6 (9.0) | 0.4 |
| Non Occupational Exposure | |||
| Body piercing,ever | 111 | 14 (12.6) | 0.9 |
| Tattoos,ever | 125 | 19 (15.2) | 0.3 |
| Acunpuncture,ever | 32 | 4 (12.5) | 0.9 |
| Surgery procedure, ever | 328 | 44 (13.4) | 0.4 |
| Endoscopic/colonoscopic procedure, ever | 103 | 13 (12.6) | 0.9 |
| PWID‡ ever (injected any drugs or non-prescription substances) | 79 | 11 (13.9) | 0.7 |
| Blood transfusion, ever | 19 | 3 (15.8) | 0.7 |
| Inmate, ever | 11 | 0 (0.0) | 0.4 |
| Sexual exposure | |||
| SEX with | |||
| Inmate, ever | 31 | 4 (12.9) | 0.8 |
| blood transfusion recipient, ever | 24 | 2 (8.3) | 0.8 |
| HIV positive partner, ever | 42 | 4 (9.5) | 0.6 |
| HIV positive partner taking ART, ever | 42 | 4 (9.5) | 0.6 |
| PWID ever | 29 | 4 (13.8) | 0.7 |
| person with potential job exposure, ever | 51 | 7 (13.7) | 0.8 |
| Sexual partner of MSM§ - males* | 162 | 27 (16.7) | 0.1 |
| Sexual partner of MSM§ - females* | 3 | 0 (0.0) | 0.9 |
| MSM§, ever | 195 | 26 (13.3) | 0.8 |
| Lifetime number of sex partners | |||
| 0 | 5 | 1 (20.0) | 0.6 |
| 1 | 21 | 1 (4.8) | |
| 2 – 3 | 53 | 6 (11.3) | |
| 4 – 6 | 104 | 14 (13.5) | |
| 7 – 10 | 71 | 11 (15.5) | |
| 11 – 20 | 80 | 15 (18.8) | |
| 21+ | 69 | 6 (8.7) | |
| Number of sex partners, in the last 12 months | |||
| 0 | 20 | 4 (20.0) | 0.2 |
| 1 | 182 | 17 (9.3) | |
| 2 – 3 | 168 | 26 (15.5) | |
| 4 – 6 | 61 | 9 (14.8) | |
| 7 – 10 | 23 | 2 (8.7) | |
| 11+ | 16 | 0 (0.0) | |
Last 12 months
From the sequenced samples n=275 (71.2%)
Person who injected drugs or non-prescription substance (PWID)
Men who have sex with men (MSM)
Totals must be less due to missing values
Virologic HIV-1 characteristics during the period of donation
During the ten years study period, HIV subtype B was the most prevalent genotype, 369 (73.2%), followed by non-B sub-types 107 (21.2%), and 28 (5.6%) of recombinants. Also, small increases in subtype D (from 0.3% to 2%) and recombinant subtypes (from 4% to 8%) were observed. Subtype A was identified among one (0.3%) participant during REDS-II and was not detected during REDS-III. In the opposite direction, subtype G was not present among participants from REDS-II; however, it was detected in two (1%) participants during REDS-III. HIV subtype differences were not significant throughout the period studied. Table 3 shows the HIV genotype characteristic during the donation period.
Table 3.
Virologic HIV-1 characteristics by period of donation
| Characteristics | REDS-II (2007 – 2011) | REDS-III (2012 – 2017) | Total | p-value** | ||
|---|---|---|---|---|---|---|
|
| ||||||
| n (%) | 95% CI | n (%) | 95% CI | n | ||
| Genotypes | ||||||
| B | 229 (75.3) | (70.5 – 80.2) | 140 (70.0) | (63.6 – 76.4) | 369 | 0.13 |
| Non-B | 63 (20.7) | (16.2 – 25.3) | 44 (22.0) | (16.3 – 27.7) | 107 | |
| A | 1 (0.3) | (0.0 – 1.0) | - | - | ||
| C | 14 (4.6) | (2.3 – 7.0) | 8 (4.0) | (1.3 – 6.7) | ||
| D | 1 (0.3) | (0.0 – 1.0) | 4 (2.0) | (0.06 – 3.9) | ||
| F/F1 | 47 (15.5) | (11.4 – 19.5) | 30 (15.0) | (10.0 – 19.9) | ||
| G | - | - | 2 (1.0) | (0 – 2.4) | ||
| Recombinants | 12 (4.0) | (1.8 – 6.1) | 16 (8.0) | (4.2 – 11.8) | 28 | |
| Resistance | ||||||
| To NRTI* | 15 (4.9) | - | 11(5.5) | - | 26 | 0.8 |
| To NNRTI † | 23 (7.6) | - | 16 (8.0) | - | 39 | 0.8 |
| To PI ‡ | 18 (5.9) | - | 6 (3.0) | - | 24 | 0.1 |
| Any antiretroviral mutation | ||||||
| SDRMs § | 37 | 12.2% | 27 | 13.5% | 64 | 0.6 |
Nucleoside Reverse Transcriptase Inhibitor
Non-Nucleoside Reverse Transcriptase Inhibitor
Protease Inhibitors
Surveillance Drug Resistance Mutations
p-value for genotype B, non-B, recombinant by REDS-II/III
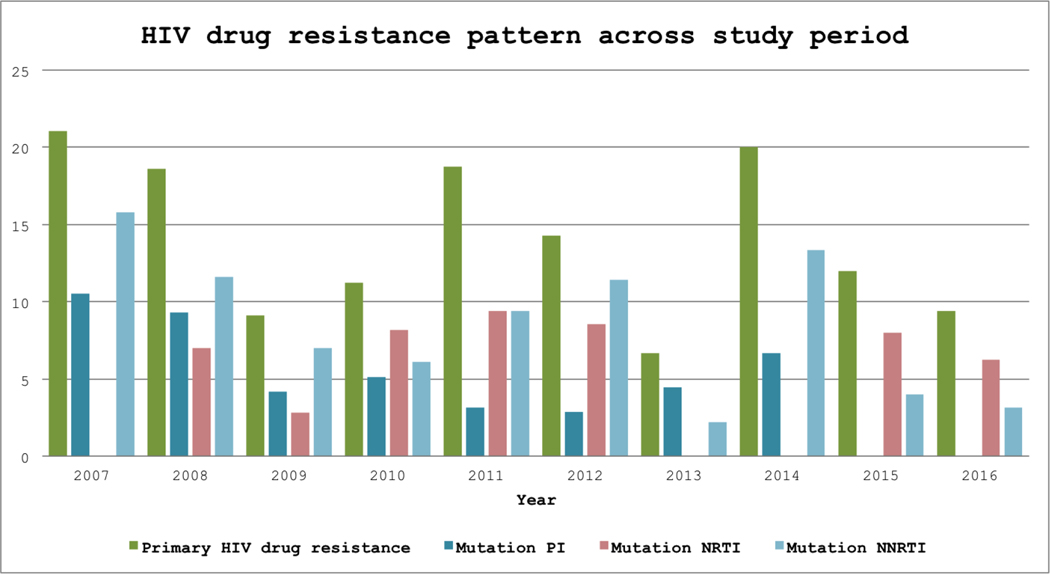
A decrease in the frequency of drug resistance mutation for protease inhibitor from 5.9% to 3%, followed by a modest increase for NRTI (4.9% to 5.5%) and NNRTI (7.6% to 8%) was observed. Considering drug class mutations, frequencies for PIs, NRTI, and NNRTI did not change between the different analyzed periods (Figure 2). NNRTI resistance was observed in 39 (7.7%) donors, while NRTI was found in 26 (5.1%) and protease inhibitors, 24 (4.7%) (Supplemental figure 1).
Figure 2 -.
Histograms of drug classes resistance by year
HIV subtype and association with HIV risk factors and behavior
The frequency of HIV subtypes varied according to the HIV risk factors and sexual behavior. For each subtype, more than half had surgery (ever), more than 20% had tattoos (ever), and more than 14% had body piercing (ever). Being a sexual partner of MSM during their lifetime was the more frequent sexual behavior reported, and was found among 41.5% participants with B subtype, 40.2% with Non-B subtypes, and 32.1% with recombinant subtypes. The majority of participants (69.5%) reported having had one to three sexual partners in the last 12 months independent of the subtype. There was no statistically significant difference among risk exposures, sexual behaviors, and HIV subtypes (Table S1).
DISCUSSION
We evaluated more than five hundred HIV-positive blood donors at four large Brazilian blood centers across a ten-year timeframe. The highest PDR was found in male donors who reported being sexual partners of MSM during the last 12 months. Our data suggest that the drug resistance mutations remained stable over the study period among the blood donation centers.
The overall PDR prevalence found in our study was consistent with PDR found in previous reports among recently diagnosed ART-naïve people living with HIV in Brazil between 2013 and 2015 (9), and among broader populations of HIV positive blood donors in Brazil, China and Spain (17,18).
We did not find significant differences in demographics, HIV behavior risk factors, or HIV genotypes when comparing persons with and without PDR. Additionally, the HIV PDR rate remained stable during the study period, when compared with the previous study report, in which 11.8% of PDR was found (13).
Blood centers located in the Southeast part of the country had accounted for a two-fold higher PDR prevalence compared to the Northeast (Recife). Currently, São Paulo state is responsible for more than 40% of patients receiving antiretroviral treatment in Brazil, and nearly a quarter of the people living with HIV on ART live in this city (9). The city of São Paulo, the capital of the most populous state in the country, was the first to introduce ART treatment in the 90s (9). Therefore, due to the sequential use of ART and broader use of unboosted protease inhibitors in the earliest years of the national HIV treatment program, it would be expected that a higher proportion of patients experiencing virological failure, and consequently transmitting HIV drug resistance would be found (9,19). However, the PDR rate was similar to 19.4% previously reported (13). Except for the Central-West region where the PDR prevalence in ART-naïve population was found to be lower (6.8%), the PDR rates found in our sample were somewhat similar to the overall 9.5% PDR prevalence observed in a nationwide observational study (9).
In line with previous Brazilian studies (9), genotype B was the most prevalent clade, reflecting the foundation of the HIV epidemic in the Brazilian population (20). We observed an increased frequency among the recombinant clades over the study period that potentially can reflect what is happening in the general population, as a result of lower ART adherence, mainly among young adults (19). In Brazil, there has been an increase in the genetic diversity of the epidemic when compared with other regions worldwide. In our country, unique recombinants were described in addition to the co-circulation of different HIV-1 clades (21).
Continuous surveillance of genotypes is needed to corroborate our findings (19). Overall, mutations leading to resistance appear to be similar among subtypes. Still, certain mutations seem to occur more frequently in non-B subtypes, in particular, consequently influencing first-line ART treatment choices (22).
Contemporary information on genotyping, drug resistance and the spread of HIVDR strains trends is difficult to obtain, particularly in low/middle-income countries, and the World Health Organization (WHO) suggested several monitoring activities to improve surveillance (2,23). Additionally, preventive measures such as post-exposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP) may have their efficacy jeopardized by drug resistance. As test-and-start ART strategies are considered, data to support optimal first-line therapy are needed worldwide (2). Supplementary resistance data extracted from variable samples and analysis of their associated HIV risk factors for PDR are lacking, although necessary to show a reliable picture of the current HIV epidemic status and strict public policies to mitigate HIV epidemics.
This study was completed before PrEP was offered as a Brazilian public health strategy in December 2017 and may serve as a baseline to understand the impact of this policy on drug resistance soon. In Brazil, PrEP is freely available for MSM and transgender women, and there are concerns that it may decrease the capacity of the tests used in the blood centers to identify infected individuals, and HIV breakthrough infection should occur (24).
We recognize some limitations. First, our convenience sample represents a population that came for blood donation, with an expected low HIV-risk self-perception, not entirely typical of the general population or high-risk key populations. However, given the diversity and relative proportions of the range of self-disclosed risk behaviors, the sample may be, by proxy, characteristic of the HIV infected population in the communities in Brazil where the hemocenters are located. Second, we did not successfully amplify one out of ten HIV-positives samples, representing a gap in our understanding of subtypes and drug resistance. Unamplifiable samples are likely to have lower viral loads, and we were not able to define the specific reasons why samples where unsuccessfully amplified. We did not run a multi-variate analysis to better approach associations. We considerer that our study did not present the right design, sample size, nor power for this purpose. We assessed previous ART exposure by a self-reported questionnaire. If persons stated there were on ART, we did not include their samples in the PDR group, but we could not substantiate the treatment status of any participant. Finally, we did not evaluate mutations of resistance for integrase inhibitor class among these naive HIV infected blood donors. However, this drug class, represented mainly by dolutegravir, was approved for experienced patients since the end of 2015, and as first-line therapy since 2017.
We conclude that ongoing efforts to conduct molecular surveillance on HIV infections in the blood donor population can contribute to an improved understanding of HIV genotype and drug resistance distributions in Brazil. HIV positive blood donors are an informative source of samples for drug resistance monitoring, and the diversity of risks suggests that donor data can be used to gain an impression of what is happening in the broader population as a whole (25). The role of blood donors for HIV surveillance will vary in different jurisdictions by epidemiology and donor criteria compliance. For public health, donor data merged with other surveillance national-wide databases can be employed for phylogenetic/phylodynamic analyses to understand better epidemics trends, dynamics of drug-resistance spread relative to current primary ART regimens and to provide insights into the success of policies to control HIV epidemics. This may be particularly important with the advent of the widespread availability of PEP and PrEP, and our data serve as a baseline in blood donors before the availability of PrEP. Blood center networks can share information about the national distribution of HIV infection through the different regions of Brazil, which may potentially improve the public health measures on HIV prevention and control.
Supplementary Material
Supplemental Table 1. Risk exposure, sexual and behavior risks among HIV+ blood donors by subtype
Supplemental figure 1 - Drug classes mutations by study year.
Supplemental figure 2. Distribution of mutations according to REDS study phase
Drug resistant mutation by REDS study period.
Acknowledgments:
We thanked the blood donors for providing consent and joined the present study and the health professional staff from the recruiter’s blood donor centers.
This study was supported by the NHLBI Recipient Epidemiology and Donor Evaluation Study (REDS-III) International Component – Brazil contracts HHSN 268201100001I and HHSN 268201100007I.
We gratefully acknowledge all of the research staff at each blood center in Brazil who have enrolled patients into the study and completed all study procedures. At each site the following specific people are recognized for their commitment and contribution to this project: Fundação Pró-Sangue (São Paulo) – Alfredo Mendrone Jr; ITACI – Instituto de Tratamento do Câncer Infantil (São Paulo) – Roberta Calcucci, Erivanda Bezerra; HEMOMINAS – Belo Horizonte (Minas Gerais) – Franciane Mendes de Oliveira, Valquíria Reis, Nayara Durte, Barbara Malta;. HEMOMINAS – Montes Claros (Minas Gerais) – José Wilson Sales, Maria Aparecida Souza, Rodrigo Ferreira; Fundação HEMOPE (Pernambuco) – Maria do Carmo Valgueir; Regina Gomes, Airly Goes Maciel, Rebeca Talamatu Dantas; HEMORIO (Rio de Janeiro) – Flavia Herculano, Ana Claudia Pereira, Ana Carla Alvarenga, Adriana Grilo, Fabiana Canedo; Instituto de Matemática e Estatística da Universidade de São Paulo – USP (São Paulo) – Pedro Losco Takecian, Mina Cintho Ozahata, Rodrigo Muller de Carvalho.
US Investigators: RTI – Research Triangle Institute, International – Christopher McClure; National Institutes of Health, National Heart, Lung, and Blood Institute – Simone A. Glynn.
We also acknowledge support from the University of California, San Francisco’s International Traineeships in AIDS Prevention Studies (ITAPS), US NIMH, and R25MH064712, especially 2018’s alumni and staff.
For the NHLBI Recipient Epidemiology and Donor Evaluation Study (REDS-III) International Component – Brazil, contracts HHSN 268201100001I and HHSN 268201100007I.
Abbreviations:
- ART
Antiretroviral therapy
- ACASI
Audio computer-assisted structured interview
- DRMs
Drug resistance mutations
- HAART
Highly active antiretroviral treatment
- HIVDR
HIV drug resistance
- PDR
Primary HIV drug resistance
- PI
protease inhibitors
- NRTI
nucleoside reverse transcriptase inhibitor
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- PEP
Post-exposure prophylaxis
- PrEP
Pre-exposure prophylaxis
Footnotes
The authors declare that they have no conflict of interest
REFERENCES
- 1.Gupta RK, Gregson J, Parkin N et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018;18:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Godfrey C, Thigpen MC, Crawford KW et al. Global HIV Antiretroviral Drug Resistance: A Perspective and Report of a National Institute of Allergy and Infectious Diseases Consultation. J Infect Dis 2017;216:S798–S800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. HIV Drug Resistance Report, 2019. (WHO/CDS/HIV/19.21). License: CC BY-NC-SA 3.0 IGO. ed, 2019. [Google Scholar]
- 4.Hamers RL, Schuurman R, Sigaloff KC et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: a multicentre cohort study. Lancet Infect Dis 2012;12:307–17. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH. HIV drug resistance report 2017. License: CC BY-NC-SA 3.0 IGO. ed. Geneva, 2017. [Google Scholar]
- 6.Brindeiro RM, Diaz RS, Sabino EC et al. Brazilian Network for HIV Drug Resistance Surveillance (HIV-BResNet): a survey of chronically infected individuals. AIDS 2003;17:1063–9. [DOI] [PubMed] [Google Scholar]
- 7.Inocencio LA, Pereira AA, Sucupira MC et al. Brazilian Network for HIV Drug Resistance Surveillance: a survey of individuals recently diagnosed with HIV. J Int AIDS Soc 2009;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira ACG, Coelho LE, Grinsztejn E et al. Transmitted drug resistance in patients with acute/recent HIV infection in Brazil. Braz J Infect Dis 2017;21:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arruda MB, Boullosa LT, Cardoso CC et al. Brazilian network for HIV Drug Resistance Surveillance (HIV-BresNet): a survey of treatment-naive individuals. J Int AIDS Soc 2018;21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleinman S, King MR, Busch MP et al. The National Heart, Lung, and Blood Institute retrovirus epidemiology donor studies (Retrovirus Epidemiology Donor Study and Retrovirus Epidemiology Donor Study-II): twenty years of research to advance blood product safety and availability. Transfus Med Rev 2012;26:281–304, 304.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Almeida-Neto C, Goncalez TT, Birch RJ et al. Risk factors for human immunodeficiency virus infection among Brazilian blood donors: a multicentre case-control study using audio computer-assisted structured interviews. Vox Sang 2013;105:91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blatyta PF, Custer B, Gonçalez TT et al. Undisclosed human immunodeficiency virus risk factors identified through a computer-based questionnaire program among blood donors in Brazil. Transfusion 2013;53:2734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alencar CS, Sabino EC, Carvalho SM et al. HIV genotypes and primary drug resistance among HIV-seropositive blood donors in Brazil: role of infected blood donors as sentinel populations for molecular surveillance of HIV. J Acquir Immune Defic Syndr 2013;63:387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barreto CC, Nishyia A, Araujo LV, Ferreira JE, Busch MP, Sabino EC. Trends in antiretroviral drug resistance and clade distributions among HIV-1--infected blood donors in Sao Paulo, Brazil. J Acquir Immune Defic Syndr 2006;41:338–41. [DOI] [PubMed] [Google Scholar]
- 15.Bennett DE, Camacho RJ, Otelea D et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PloS one 2009;4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006;42:1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bes M, Piron M, Casamitjana N et al. Epidemiological trends of HIV-1 infection in blood donors from Catalonia, Spain (2005–2014). Transfusion 2017;57:2164–2173. [DOI] [PubMed] [Google Scholar]
- 18.Zeng P, Liu Y, He M et al. The infection staging and profile of genotypic distribution and drug resistance mutation among the human immunodeficiency virus-1 infected blood donors from five Chinese blood centers, 2012–2014. PLoS One 2017;12:e0179328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz RS, Inocêncio LA, Sucupira MC et al. The Virological and Immunological Characteristics of the HIV-1-Infected Population in Brazil: From Initial Diagnosis to Impact of Antiretroviral Use. PLoS One 2015;10:e0139677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares MA, De Oliveira T, Brindeiro RM et al. A specific subtype C of human immunodeficiency virus type 1 circulates in Brazil. AIDS 2003;17:11–21. [DOI] [PubMed] [Google Scholar]
- 21.Sabino EC, Shpaer EG, Morgado MG et al. Identification of human immunodeficiency virus type 1 envelope genes recombinant between subtypes B and F in two epidemiologically linked individuals from Brazil. J Virol 1994;68:6340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor BS, Hammer SM. The challenge of HIV-1 subtype diversity. N Engl J Med 2008;359:1965–6. [DOI] [PubMed] [Google Scholar]
- 23.Jordan MR, Bennett DE, Wainberg MA et al. Update on World Health Organization HIV drug resistance prevention and assessment strategy: 2004–2011. Clin Infect Dis 2012;54 Suppl 4:S245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seed CR, Yang H, Lee JF. Blood safety implications of donors using HIV pre-exposure prophylaxis. Vox Sang 2017;112:473–476. [DOI] [PubMed] [Google Scholar]
- 25.Riemenschneider M, Hummel T, Heider D. SHIVA - a web application for drug resistance and tropism testing in HIV. BMC Bioinformatics 2016;17:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Risk exposure, sexual and behavior risks among HIV+ blood donors by subtype
Supplemental figure 1 - Drug classes mutations by study year.
Supplemental figure 2. Distribution of mutations according to REDS study phase
Drug resistant mutation by REDS study period.