Abstract
Objective.
Microelectrode arrays are standard tools for conducting chronic electrophysiological experiments, allowing researchers to simultaneously record from large numbers of neurons. Specifically, Utah electrode arrays (UEAs) have been utilized by scientists in many species, including rodents, rhesus macaques, marmosets, and human participants. The field of clinical human brain-computer interfaces currently relies on the UEA as a number of research groups have FDA clearance for this device through the investigational device exemption pathway. Despite its widespread usage in systems neuroscience, few studies have comprehensively evaluated the reliability and signal quality of the Utah array over long periods of time in a large dataset.
Approach.
We collected and analyzed over 6000 recorded datasets from various cortical areas spanning almost 9 years of experiments, totaling 17 rhesus macaques (Macaca Mulatta) and 2 human subjects, and 55 separate microelectrode Utah arrays. The scale of this dataset allowed us to evaluate the average life of these arrays, based primarily on the signal-to-noise ratio of each electrode over time.
Main Results.
Using implants in primary motor, premotor, prefrontal, and somatosensory cortices, we found that the average lifespan of available recordings from UEAs was 622 days, although we provide several examples of these UEAs lasting over 1000 days and one up to 9 years; human implants were also shown to last longer than non-human primate implants. We also found that electrode length did not affect longevity and quality, but iridium oxide metallization on the electrode tip exhibited superior yield as compared to platinum metallization.
Significance.
Understanding longevity and reliability of microelectrode array recordings allows researchers to set expectations and plan experiments accordingly and maximize the amount of high-quality data gathered. Our results suggest that one can expect chronic unit recordings to last at least two years, with the possibility for arrays to last the better part of a decade.
Keywords: electrode array reliability, longevity, chronic signal quality, cortex, non-human primate electrophysiology, human electrophysiology
1. Introduction
First developed in 1992, a microelectrode array recording platform now called the “Utah Electrode Array” (UEA) has provided the opportunity for researchers to record from populations of neurons simultaneously, in-vivo, in a chronic preparation (1). Today, UEAs are a standard data collection method for systems neuroscience and represent a common choice for chronic multi-electrode array recordings, particularly in animal models such as rodents and non-human primates (NHPs), including macaques and marmosets (2-4). With the popularity of such a tool comes increased scrutiny on the reliability and failure modes of the technology. UEAs, like all chronic neural implants that interface with brain tissue, usually degrade in their recording capabilities over time (4); in some cases, these implants can experience more acute issues, either during surgical implantation or shortly thereafter, whereas in many other cases these implants can reliably record unit activity for several years (4). These implants have been in widespread use for many years, yet there have been few attempts to quantify the reliability of this technology in-vivo (4). Most previous efforts to quantify the signal quality of UEAs over time, while laudable, have focused heavily on the consistency of neuronal characteristics in a small sample size of implants and subjects (5-9). Other studies have examined the longevity and reliability of other chronic electrophysiological recording technologies, to help researchers manage expectations and select the right tool for the job (10-14). However, one previous study has examined the failure modes and reliability of UEAs from a large sample size, albeit with UEAs undergoing significant changes in manufacturing processes (4). Additionally, other studies evaluate the differences and capabilities of various recording arrays, but not with non-human primate subjects, making cross-species comparison difficult (14-17). The current work aims to extend this previous study by considering more recent UEAs whose manufacturing process has become relatively stable and considers UEAs with iridium oxide metallization optimized for stimulation as well as platinum metallization on the electrode tips.
UEAs currently remain the only FDA-cleared intracortical implant for chronic human neuroscientific studies under Investigational Device Exemptions (IDE), an allowance which is held by several groups across the United States. There have been at least 18 human subjects who have received a chronic UEA implant for brain computer interface (BCI) studies reported in the literature as of 2019 (18), and we estimate that at least another 7 people have been implanted since then (19). Human studies require ongoing consideration of the risk-benefit ratio of implanting human subjects, which will be informed by more information on the expected reliability and longevity of these implants over time. A majority of human studies expect participants to remain in the study for at least 1 year, which is supported by a significant body of evidence demonstrating the safety and efficacy of these devices for long term recording (2-16). Here we extend this earlier work by examining a large number of array implants over extended durations up to 9 years in both humans and non-human primate subjects.
It is likely that the UEA will continue to be the dominant neural recording platform for human intracortical BCI studies in the near future. FDA clearance for investigational approval requires the collection of significant longitudinal safety and efficacy data along with the institutional knowledge for a successful clinical trial design (20). A number of efforts are currently underway to develop more advanced tools for chronic implants across non-human and human primates alike (21-25), but given the prevalence of the UEA for chronic recording and stimulation, it is imperative to characterize the reliability of these arrays across and within species, to take full advantage of their capabilities.
Here, using data from over 6000 recording sessions in 55 array implants across human (n=2) and non-human primate (n=17) subjects, we assess the reliability of long-term unit recordings in primary motor, dorsal and ventral premotor, prefrontal, and primary somatosensory cortices. We show that nearly fifty percent of implants exhibit year-long recordings with a yield greater than 40% of the total available electrodes displaying satisfactory quality (SNR>1.5). Moreover, long-term recordings from ~1000 days and up to nine years are shown to be possible in some cases. We also examine the influence of electrode length and electrode-tip metallization on the longevity and quality of recordings.
2. Methods
2.1. Non-Human Primate Subjects
Recordings collected during the period of 2003-2020 from a total of 55 UEAs implanted in 17 rhesus macaques (Macaca Mulatta) (10 female, 7 male) were analyzed. This project analyzed data from the maximum possible number of recordings throughout the history of our research group. All animals were implanted with at least one (often two or more) UEAs (Blackrock Microsystems, Inc. Salt Lake City, UT). The earliest implants were manufactured by Cyberkinetics Neurotechnology Systems, Inc., Foxboro MA whose research business was sold to Blackrock Microsystems, Inc. in 2008. The metallization, number of electrodes, and electrode length of these arrays varied depending on brain area, scientific need, and implantation year, and individual array details are included in Table 1. Briefly, arrays were either implanted as an 8x8 or 10x10 grid, with either 1.5 mm or 1.0 mm length electrodes (although never mixed within a given array), with electrode tips metallized with either platinum or iridium-oxide. Since our research group focuses primarily on upper limb motor tasks, UEAs were most often implanted in primary motor (M1), dorsal premotor (PMd), or ventral premotor cortices (PMv). Other implanted areas included somatosensory (S1), orofacial primary motor (M1o), and prefrontal cortex (PFC). We saw no appreciable difference in signal longevity or quality between the two areas which had large enough numbers of implants to compare (dorsal/ventral premotor versus primary motor areas). All implants were connected to CerePort connectors with analog headstages produced by Blackrock Microsystems. All arrays were wired in a consistent manner; none were hand soldered.
Table 1.
List of microelectrode arrays included in the current study. Column A indicates the abbreviation used for the identity of the animal and brain area. Many animals were implanted with multiple arrays. Column B indicates the sex of the animal in which the array was implanted. Column C indicates the brain area in which the array was implanted. Column D indicates the date of initial array implantation in YYMMDD format. The rightmost column is reserved for reasons for explant. “Medical” encompasses infections, skin retraction, or other medical issues which required the implant to be removed, unrelated to signal quality. “Signal Quality” refers to poor signal quality or yield. “Study End” indicates that the arrays were explanted not due to poor signal quality, but due to the end of a given experiment. “Hardware failure” indicates non-array components failing such that recording ability was compromised. The final four rows of Table 1 are reserved for the human participant arrays which were included in the study.
| Name | Sex | Brain Area |
Implant Date | Electrode Length (mm) |
Size | Metallization | Number of Recordings |
Reason for Termination |
|---|---|---|---|---|---|---|---|---|
| AtPmv | F | PMv | 080123 | 1.0 | 96 | Platinum | 47 | Signal Quality |
| AtM1b | F | M1 | 130305 | 1.0 | 128 | Iridium Oxide | 40 | Signal Quality |
| AtM1o | F | M1o | 080123 | 1.0 | 96 | Platinum | 13 | Medical |
| BiM1 | M | M1o | 100824 | 1.0 | 96 | Iridium Oxide | 10 | Signal Quality |
| BoPMda | M | PMd | 030128 | 1.0 | 96 | Platinum | 317 | Signal Quality |
| BoM1b | M | M1 | 080408 | 1.5 | 96 | Iridium Oxide | 68 | Signal Quality |
| BoPMdb | M | PMd | 080408 | 1.0 | 96 | Platinum | 68 | Signal Quality |
| BoM1a | M | M1 | 030128 | 1.0 | 96 | Platinum | 6 | Signal Quality |
| CoPMd | F | PMd | 090413 | 1.0 | 96 | Platinum | 82 | Medical |
| CoM1 | F | M1 | 090413 | 1.5 | 96 | Platinum | 68 | Medical |
| CoPMv | F | PMv | 090413 | 1.5 | 96 | Platinum | 54 | Medical |
| JaPMv | M | PMv | 121002 | 1.5 | 96 | Iridium Oxide | 27 | Signal Quality |
| JaPMd | M | PMd | 121002 | 1.0 | 96 | Iridium Oxide | 17 | Signal Quality |
| KiM1ips | F | M1ipsi | 120604 | 1.0 | 96 | Iridium Oxide | 327 | Study End |
| KiM1con | F | M1con | 120604 | 1.0 | 96 | Iridium Oxide | 51 | Study End |
| LeM1c | M | M1l | 150720 | 1.0 | 128 | Iridium Oxide | 202 | Signal Quality |
| LeM1b | M | M1m | 150720 | 1.0 | 128 | Iridium Oxide | 63 | Signal Quality |
| LeM1a | M | M1 | 110711 | 1.5 | 96 | Iridium Oxide | 38 | Signal Quality |
| LePMd | M | PMd | 110711 | 1.5 | 96 | Platinum | 32 | Signal Quality |
| LePMv | M | PMv | 110711 | 1.5 | 96 | Platinum | 25 | Signal Quality |
| MkM1c | M | M1m | 071128 | 1.0 | 96 | Iridium Oxide | 501 | Hardware Failure |
| MkM1b | M | M1l | 060906 | 1.0 | 96 | Platinum | 32 | Hardware Failure |
| MkM1d | M | M1l | 071128 | 1.0 | 96 | Platinum | 32 | Hardware Failure |
| MkM1a | M | M1m | 060906 | 1.0 | 96 | Platinum | 28 | Hardware Failure |
| MkPMd | M | PMd | 060906 | 1.0 | 96 | Platinum | 14 | Hardware Failure |
| NkM1b | F | M1contra | 140303 | 1.0 | 96 | Iridium Oxide | 42 | Study End |
| NkPFC | F | PFCcontra | 140303 | 1.0 | 96 | Iridium Oxide | 13 | Study End |
| NkM1a | F | M1ipsi | 140303 | 1.0 | 96 | Iridium Oxide | 12 | Study End |
| NiPMv | F | PMv | 041103 | 1.0 | 96 | Platinum | 91 | Signal Quality |
| NiM1 | F | M1 | 041103 | 1.0 | 96 | Platinum | 86 | Signal Quality |
| NiPMd | F | PMd | 041103 | 1.0 | 96 | Platinum | 21 | Signal Quality |
| OrM1a | F | M1 | 070501 | 1.5 | 96 | Platinum | 52 | Signal Quality |
| OrM1b | F | M1o | 070501 | 1.0 | 96 | Platinum | 52 | Signal Quality |
| OrPmv | F | PMv | 070501 | 1.0 | 96 | Platinum | 18 | Signal Quality |
| RjPMd | M | PMd | 031027 | 1.0 | 96 | Platinum | 156 | Signal Quality |
| RjM1a | M | M1 | 031027 | 1.0 | 64 | Platinum | 81 | Signal Quality |
| RjM1b | M | M1 | 050804 | 1.0 | 96 | Platinum | 21 | Hardware Failure |
| RjPMv | M | PMv | 050804 | 1.0 | 96 | Platinum | 10 | Hardware Failure |
| RoM1a | M | M1 | 040602 | 1.0 | 96 | Platinum | 211 | Signal Quality |
| RoPMd | M | PMd | 040602 | 1.0 | 96 | Platinum | 34 | Signal Quality |
| RxM1a | F | M1 | 050328 | 1.0 | 96 | Platinum | 97 | Signal Quality |
| RxPMva | F | PMv | 050328 | 1.0 | 96 | Platinum | 80 | Signal Quality |
| RxM1b | F | M1 | 060712 | 1.5 | 96 | Platinum | 55 | Signal Quality |
| RxPMdb | F | PMd | 060712 | 1.0 | 96 | Platinum | 42 | Signal Quality |
| RxM1c | F | M1 | 090729 | 1.5 | 96 | Iridium Oxide | 33 | Signal Quality |
| RxPMvb | F | PMv | 060712 | 1.0 | 96 | Platinum | 27 | Signal Quality |
| RxPMda | F | PMd | 050328 | 1.0 | 96 | Platinum | 10 | Signal Quality |
| RxS1a | F | S1 | 090729 | 1.5 | 96 | Iridium Oxide | 6 | Signal Quality |
| VePMv | F | PMv | 050809 | 1.0 | 32 | Platinum | 132 | Medical |
| VeM1a | F | M1 | 050809 | 1.0 | 96 | Platinum | 110 | Medical |
| VePMd | F | PMd | 050809 | 1.0 | 96 | Platinum | 48 | Medical |
| ZiM1a | F | M1contra | 120730 | 1.0 | 96 | Iridium Oxide | 384 | Study End |
| ZiM1c | F | M1ipsi | 120730 | 1.0 | 96 | Iridium Oxide | 5 | Study End |
| P1_A | F | M1 | 120210 | 1.5 | 96 | Platinum | 350 | Medical |
| P1_P | F | M1 | 120210 | 1.5 | 96 | Platinum | 335 | Medical |
| P2_A | M | M1 | 150504 | 1.5 | 88 | Platinum | 722 | N/A |
| P2_P | M | M1 | 150504 | 1.5 | 88 | Platinum | 718 | N/A |
Data collection was conducted using Blackrock’s Cerebus data acquisition system. Neural data recorded as analog signals were amplified with a gain of 5000, bandpass filtered using the built-in hardware filter between 0.3 Hz and 7.5 kHz and digitized at 30 kHz. Spike waveforms were detected and saved into the “.nev” file format at 46 samples per waveform. Spike events were detected using a global root mean squared signal energy (RMS) threshold set by the experimenter; occasionally the experimenter manually adjusted individual electrodes’ thresholds.
Due to the broad date range of these recordings, training protocols and behavioral tasks varied widely across our subjects. Most subjects were trained to perform upper limb motor tasks, either involving two-dimensional planar reaching tasks using a two-link exoskeletal robot (Kinarm, BKIN Technologies, Ltd., Kingston, Ontario), unconstrained three-dimensional reaching, grasping, and reach-to-grasp tasks, or brain-computer interface (BCI) tasks. One animal also engaged in orofacial behavior.
2.2. Human Subjects
Two 96-channel arrays were implanted in participant P1, and two 88-channel arrays were implanted in participant P2. Arrays were implanted in the hand knob area of motor cortex (precentral gyrus). P2 also had two 32 channel arrays implanted in somatosensory cortex for the purposes of electrical stimulation; recording and stimulation performance for these arrays has been previously described (26). Participant P1 contributed to 350 recording sessions over 2.7 years. Participant P2 contributed to 721 recordings sessions over 5.3 years (8). Data collection with participant P2 is ongoing. Neural data were recorded at the beginning of each BCI testing session while the participants were at rest or speaking with experimenters.
2.3. Array Insertion and Recording Procedure
High-speed insertion (150-285 microseconds) of electrode arrays was performed with the pneumatic Blackrock Electrode Inserter System which consists of a wand with a hammer at the end that translates either 1mm or 1.5 mm depending on the electrode length of the arrays. The inserter pressure was always set to 20 PSI. In all cases, the inserter wand was firmly attached to a micromanipulator that was affixed to a stereotaxic frame or surgical table. The surface of the wand’s hammer was positioned on and parallel to the back end of the array before inserting. All connector pedestals used in this study consisted of 128-channel Cereport-style connectors that were attached to the skull with 8 bone screws per pedestal. Data acquisition was performed with cables attached to the Cereports without wireless transmission.
2.4. Neural Data Processing
Each “.nev” file was processed to evaluate the number of electrodes that displayed spike events over the entire recording. A channel’s spikes were included in an signal-to-noise ratio (SNR) calculation if at least fourteen spike events were detected throughout the entire recording. Each electrode’s SNR was calculated by measuring the average peak-to-trough amplitude of the electrode’s waveforms and dividing it by two times the average standard deviation of the voltage of the waveforms. The average standard deviation was computed by taking the mean of standard deviation values over all 46 samples of the waveform. If the electrode’s SNR exceeded 1.5, the electrode was designated as “good” and counted towards that recording’s array yield. This threshold guarantees that a channel’s signal is 50% higher than the noise of the channel. This threshold may be seen as too high by some, but we preferred to have confidence in a “good” channel, rather than include channels of nebulous quality. It should be noted that neural signals with an SNR of less than 1.5 can still carry information that may be useful for BCI or scientific applications. Moreover, each recording was manually inspected and erroneous “good” channels, often having inaccurate SNR measurements due to artifacts, were removed from the “good” electrode count. No spike sorting was performed so our SNR values were conservative and were lower than would be expected from individually sorted units. Also, due to the lack of spike sorting, we could not address the degree to which array recordings change in their capability to detect separable units within a given electrode’s signal. All statistics and figures displaying SNR were calculated across all electrodes of the array in which spikes were detected
After manual inspection, our search resulted in data from 19 subjects (17 NHPs and 2 humans) and 55 Utah microelectrode arrays, totaling 6132 recording sessions. Due to different array sizes (8x8, 10x10), array yield of arrays was not presented as the absolute number of electrodes but rather as the percentage of total recordable electrodes, thus allowing for comparisons across different electrode configurations. SNR was also calculated as an average across all electrodes within a given recording session.
It is important to note that the number of arrays we could examine decreased with time due to loss of signals, hardware failure, infections surrounding the percutaneous connector, and experiment termination (see Table 1). Some analyses took loss of arrays into account to represent the number of arrays that survived past a certain point in time (see Figure 3). Other analyses prioritized calculating the reliability and yield of functioning arrays, considering only arrays from which viable recordings were possible (see Figure 5).
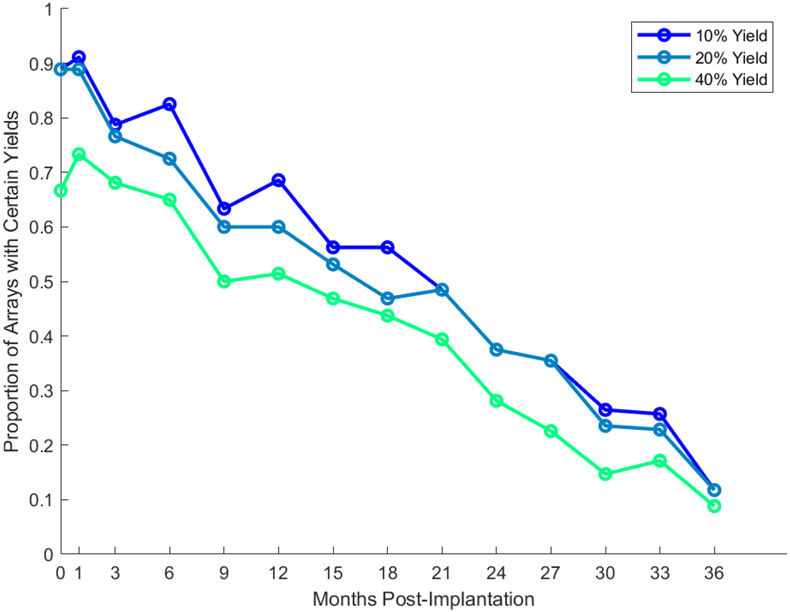
Figure 3.
Proportion of arrays exceeding a certain yield at month-to-month intervals post-implantation. Each line delineates a different percentage yield threshold. If an array is not recorded from in a given time window, it is not counted in the “proportion” estimate. After their last available recording date, arrays are counted in the proportion measurement, to accurately depict the degradation of arrays on average. However, arrays which ceased recording due to medical, hardware failure, or study end reasons (see Table 1) were not included in the proportion measurement after their last available recording date.
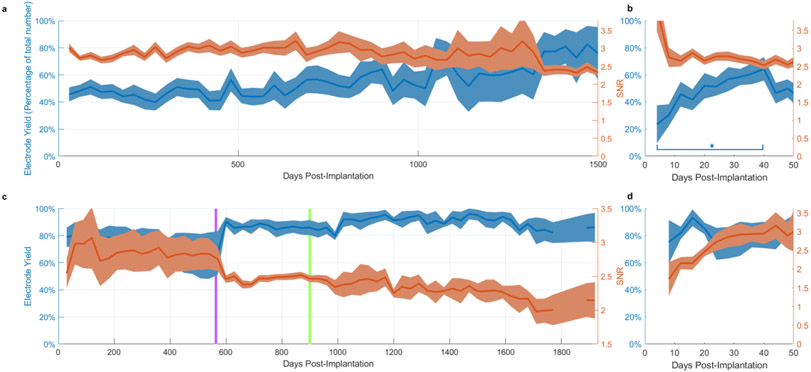
Figure 5.
Reliability of viable chronic recordings over time. Shaded regions denote standard error of the mean. a. Average yield (blue) and SNR (red) over arrays with viable recordings (i.e. array recordings that were not terminated) versus number of days post-implantation for NHP implants. Full time range not shown (maximum lifetime is in excess of 3000 days). b. Close-up of average yield and SNR over the first 40 days post-implantation. A significant difference was observed between the first week and fourth week of recordings (p = 0.0122, 2-sample t-test, 2-tailed). c,d. equivalent statistics for human implants. Further details can be found in Downey et al., 2018 (8). The purple vertical line indicates the date at which the R.M.S. spike threshold was set to a different value during data collection (see methods for details). The change in the figure is due to a larger number of recordings becoming possible to average across, reducing variance in the estimate of yield and SNR. The green line indicates the date data collection with P1 ended.
3. Results
3.1. Lifetime of chronic recordings
A total of over 6000 recording sessions over 55 arrays in 17 animals and two human subjects were analyzed by computing SNR values of unsorted waveforms for each electrode on the array (Figure 1). A subset of electrodes was then selected as “good” if their SNR exceeded 1.5 and passed our manual examination from which overall yield was assessed over time. Over the 55 array implants, there was a large variance in lifetime of recordings from 44 days to over 3000 days (Figure 2). Recordings from UEAs were terminated for several reasons including loss of signals, electrode assembly hardware failure (i.e. the titanium connector pedestal detached from the skull), infections surrounding the connector pedestal, or completion of an experiment (Table 1).
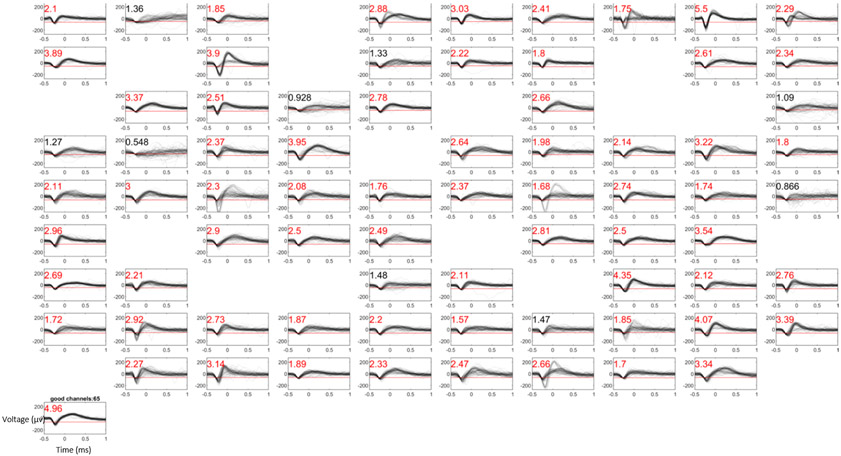
Figure 1.
Example waveforms and signal-to-noise ratio (SNR) from a UEA recording session (Subject Mk, array MkM1c). SNR values are presented in the top left corner of each panel. Red SNR values indicate electrodes which exceeded the requisite SNR threshold of 1.5 to be considered as “good” electrodes included in subsequent analyses of array yield. The red line in each panel indicates the threshold across which a waveform must cross to be considered an action potential. Empty panels indicate electrodes in which less than fourteen spikes were detected within the given recording session, and therefore were not included in analysis.
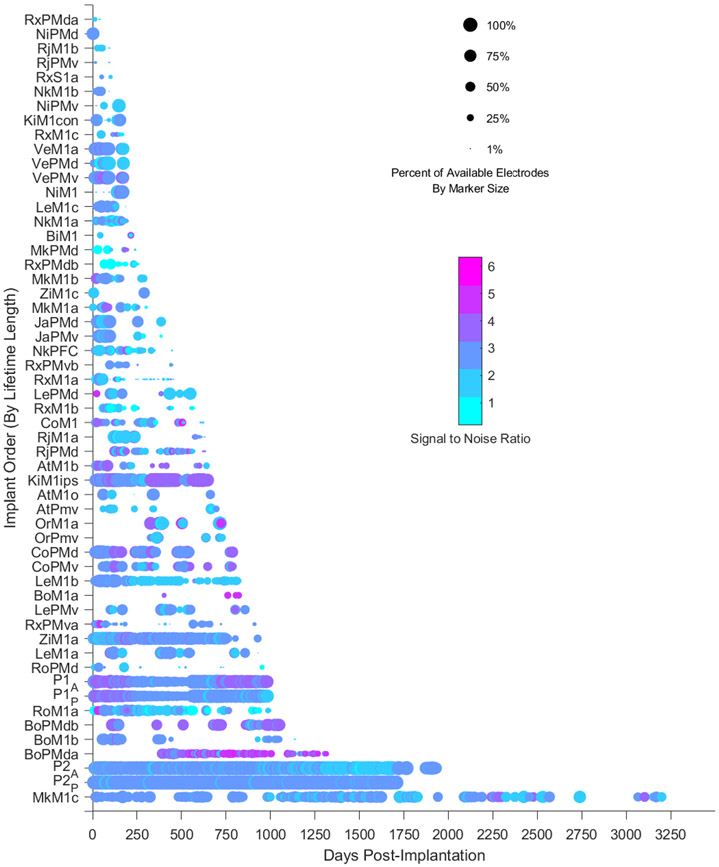
Figure 2.
Summary heat map of signal-to-noise ratio (circle color) and array yield (circle size) over time for all array implants analyzed. Each colored circle denotes a single recording session. The color of the circle denotes the average SNR of the array for that given recording session. The size of the circle denotes the percentage of electrodes in that array that demonstrated a signal-to-noise ratio above a threshold of 1.5.
To gauge the likelihood that a UEA would provide high quality signals over time, we examined the proportion of arrays that exceeded a fixed percentage yield at month-to-month intervals (Figure 3). We observed a slight increase in the proportion of arrays exceeding a given yield in the first month of recording post-implantation followed by a steady decrease over 36 months. Nearly seventy percent of arrays displayed at least a 40% yield in the first three months of recordings and fifty percent of arrays exceeded the same yield threshold after one year of recording. A long tail of reliability was observed with nearly ten percent of arrays exceeding the same yield threshold out to at least 36 months post-implantation. Based on a linear regression fit to the 40% threshold data in Figure 3, arrays typically experience a ~2% drop in yield every 30 days (slope = −0.00058 per day).
3.2. Extended long-term recordings
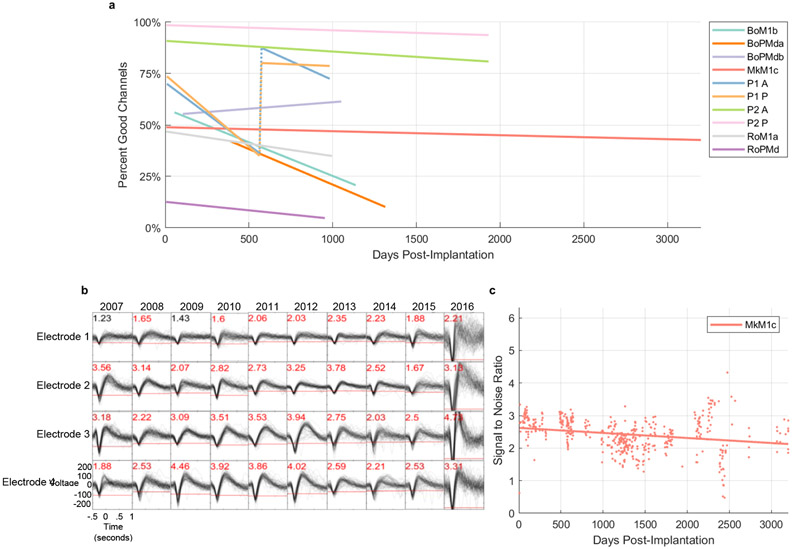
To examine the day-to-day variability in signal quality among very long-term recordings, we focused on a subset of arrays that continued to record good signals beyond 950 days (Figure 4a). Sixteen out of 55 arrays displayed longevity well over 800 days of which three lasted well into five years of service (two of the three arrays were implanted in human subject P2). All linear regressions are statistically significant (p < .05), save for “BoPMdb”, the only array whose regression displayed an upward trend. One array (Mk) exhibited extended recording capabilities to nearly nine years, only needing to be explanted due to an infection near the connector. This subset of arrays generally displayed a gradual decrease in signal quality over time with variations from recording to recording presumably due to several factors such as micromovements of the array that may have occurred in the home cage such as sudden head accelerations (excluding our human participants) (27), headstage malfunctions, and sources of electrical noise of unknown origin that could not be eliminated. The two arrays implanted in a human subject (P2) are still implanted as of October 2021 with good recording quality.
Figure 4. Extended long-term performance for a subset of arrays.
a. Yield for long-term array implants as a function of days post-implantation. Lines indicate best fit linear regressions to the data for each array. All linear regressions are statistically significant (p < .05), save for “BoPMdb”, the only array whose regression displayed an upward trend. The recording quality metrics exhibit a discontinuity at day 565 for subject P1, due to a change in the spike threshold from −5.25 to −4.5 RMS, indicated by the vertical dashed lines. b. Example spike waveforms from one array implant (MkM1c) at regular intervals over nearly 9 years. SNR values are presented at the top left corner of each panel (red font denotes electrodes that exceeded an SNR threshold of 1.5). c. SNR of Monkey Mk’s recordings, over the lifetime of the implant.
3.3. Reliability among arrays possessing viable recordings
We next examined the temporal evolution of average yield and SNR among arrays from which recordings continued to be available and did not include arrays from which recordings were terminated (Figure 5). Therefore, the number of arrays contributing to the average decreased with time, and standard error values increased. However, with this measure, we were able to confirm that arrays implanted in NHPs typically maintained their electrode yield and SNR over most of their lifetime (Figure 5a). In contrast to long-term yield, short-term yield displayed a rapid increase over the first 40 days post-implantation in NHP implants (Figure 5b). A significant difference was observed between the first week and fourth week of recordings (p = 0.0122, 2-sample t-test, 2-tailed).
Using the same methods used for NHP analyses, we also examined the signal quality over time for UEAs implanted in human participants as part of a brain-machine interface study (28). We found that electrode yield increased slightly and SNR steadily increased in the shortterm, saturating to some overall maximum value after approximately four weeks (Figure 5c,d). Due to the consistent and long-term nature of the human BCI study, signal quality metrics were available for human arrays for almost 1000 and 2000 days for subject P1 and P2, respectively. The electrode yield from these two human participants was generally higher and more consistent over time as compared to our NHP results though a slow decline in SNR was noted over time as in the NHP arrays.
3.4. Performance effects of electrode tip metallization and length
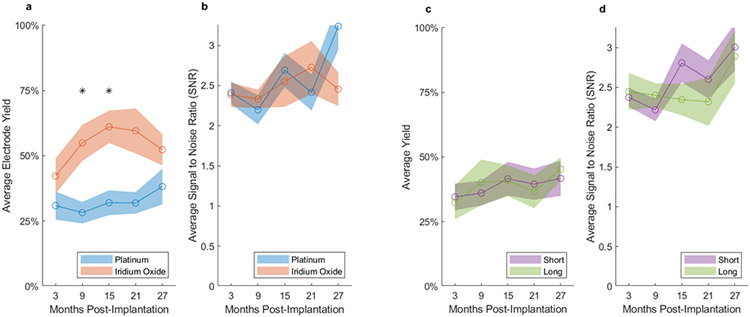
Platinum metallization of the electrode tips was adopted in the early fabrication of UEAs but was not well suited for microstimulation of the cortex. The option to use iridium oxide metallization was made available in 2009 which allowed for chronic stimulation due to its improved charge injection capacity, as well as recording (iridium oxide arrays were used for acute stimulation in our lab but were not used for chronic stimulation) (29). We directly compared recording yield and SNR between these two metallization options and found that average array yield was significantly higher for iridium oxide as compared to platinum tips during chronic recordings in the intermediate time range although there was no difference in the short and long term (Figure 6a). In contrast, there was no significant difference in mean SNR between the two metallization materials at any time (Figure 6b).
Figure 6. Effects of electrode tip metallization and electrode length on array performance in NHP implants.
a, c. The average yield for each six-month date window was calculated for each array; an average was then taken across all arrays with the same metallization or electrode length (short-1.0 mm, long-1.5 mm), respectively. Shaded regions for all plots indicate the standard error of the mean, across arrays. b, d. Average SNR based on metallization and electrode length, respectively. Statistical tests were corrected for multiple comparisons using Bonferroni correction. Stars indicate statistically significant differences between groups, Bonferroni corrected. Averages were calculated over recordings and arrays for which data was available for each time window. Only NHP data were used for this figure.
We also compared recording performance between short (1.0 mm) and long (1.5 mm) electrodes. Given our research interests in motor and premotor cortices, our initial bias was to use long electrodes to target as close to layer 5 as possible in the thicker motor cortex. However, we were also concerned about the known observation that UEAs sink over the long term by compressing superficial layers, and so we also used short electrodes in some cases in order to prevent the tips from sinking into white matter particularly in the thinner premotor cortex (unpublished histology). We found no difference in average yield or SNR between short and long electrodes (Figure 6c,d).
During our analysis, questions arose about the potential impact of manufacturer changes or surgeon experience over the entire range of dates during which implants were placed in subjects. To address these concerns, we analyzed the reliability of all NHP implants, based on their implantation date. Despite having been implanted by different surgeons, and having undergone largely minor changes in manufacturing process, we observed no significant relationship between array longevity and date of implant (r = 0.11, p = 0.43).
3.5. Maximum performance over array lifetime
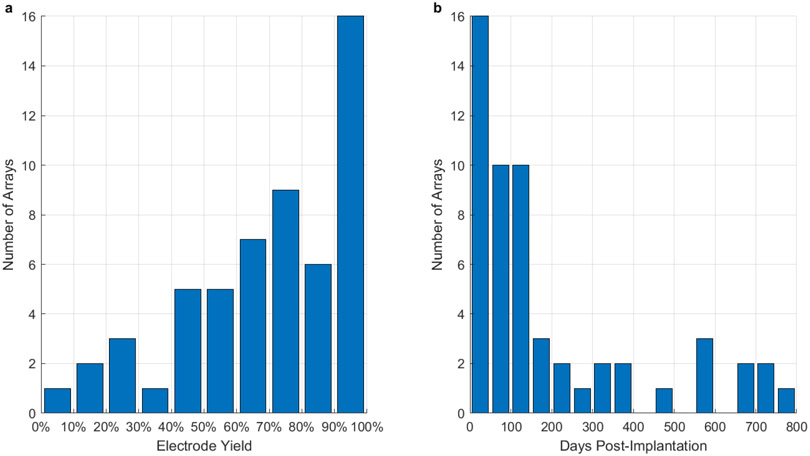
We next addressed the maximum possible performance for these arrays over their lifetimes. We calculated the distribution of maximum yield over arrays and the time post-implantation when the maximum yield occurred. Despite the large variance in maximum yield across arrays, we found that 16 (out of 55) arrays exhibited a maximum yield exceeding 90% (Figure 7a). The maximum yield typically occurred within the first 150 days of a given array’s recording life (Figure 7b). The results in Figure 7b reject a chi-squared test of uniformity at a p-value of (p = .002)
Figure 7. Maximum possible yield.
a. Distribution of the maximum recorded electrode yield over all arrays, from 0% to 100% of possible electrodes. b. Distribution of dates when the maximum number of recorded channels occurred. Most maximum recording days occurred within the first 150 days post-implantation. This data rejects a chi-squared test of uniformity at a p-value of (p = .002)
4. Discussion
UEAs are now a standard recording device for chronic electrophysiology in animals and is the only chronic intracortical recording technology that is FDA-cleared for investigational purposes in humans. Assessing the longevity and reliability of these devices is important for long-term experiments in NHPs and is of particular significance for chronic use in human BCIs. Most previous publications have examined the signal quality of Utah arrays using data from a limited number of subjects or explicitly focused on physical failure modes of the arrays (however, see (4) for one exception); other papers have examined the longevity and/or reliability of electrophysiological recording techniques, while not specifically focusing on the Utah Array (5-13,18). We found that nearly sixty percent of array implants exceeded 40% electrode yield after 6 months post implantation and nearly fifty percent of array implants exceeded 40% yield at one year. We also observed a subset of arrays (n=12) that exhibited moderate yield and SNR beyond 900 days post-implantation and one implant lasted nearly 9 years. We also characterized an important aspect of this recording technology, namely, a rapid increase in electrode yield within the first 40 days post-implantation in the non-human primate implants, most likely because of acute inflammation and subsequent recovery after surgery (8). Early recovery of signal quality post-implantation is likely due to alleviation of acute inflammation and potential small hematomas as swelling and bleeding reduce. The acute immune response to a foreign body is associated with activated microglia and astrocytes which begins at the moment of insertion and lasts approximately 6-8 weeks post-implantation followed by a chronic response associated with glial encapsulation of the foreign body (30). The improved yield in the first 40 days post-implantation may be related to the termination of the acute immune response whereas the long-term decrease in yield may be in part the result of gliotic encapsulation.
We also observed that the yield of human implants was higher than NHP implants. There are many potential reasons for this difference, despite the hardware itself being identical: Utah arrays approved for human implant undergo additional quality control checks prior to implant; surgical procedures are executed by neurosurgeons as opposed to an investigator with a scientific or engineering background; most importantly, human study participants and their caregivers take care of the connector and surrounding skin whereas monkeys can damage their own connector or the skin around the percutaneous connector pedestal leading to infection or failure of the implant. The results directly reported here are limited to two human participants, but successful long term human implants have been commonly reported in the literature (18). It should be noted that, as with the NHPs, the human patients had multiple array implants per subject even in the same cortical area so it seems unlikely that the difference in yield is due to the number of array implants. However, in some NHP cases, arrays were placed very close to one another such that the edge of one array was nearly touching another which was not true of the human implants. That may have played some role given that our best NHP yield (see Figure 4a) were among implants that were more than 2 mm from each other.
Our findings also suggest that UEAs with iridium oxide metallized tips result in higher recording yield as compared to platinum metallization (in non-human primates). Iridium oxide also has the added benefit that chronic electrical stimulation is possible. However, these results should be interpreted with caution given the fact that our platinum implants were done earlier than the iridium oxide implants over the 17 years of implants examined in this study. The improved yield of the iridium oxide implants may have been due to improvements in surgical techniques as we learned better implantation methods. There have been a few improvements in the UEA manufacturing process over the 17-year period most of which were relatively minor with the exception of transitioning from array to wafer scale manufacturing in 2009-10 that may explain the improved yield of the iridium oxide implants (31). Nevertheless, when considering all our implants regardless of metallization, we found no correlation between array longevity and date of implant.
4.1. Species-specific differences in signal quality
Data from our human subjects tended to display higher array yields and SNR than the NHP data. However, the number of human datasets was vastly smaller (only 4 arrays and 2 subjects) than the datasets in the NHPs, and so it is difficult to draw any strong conclusions regarding differences in signal quality between humans and NHPs. One possibility may be that the surgical ability with which microelectrode arrays were implanted may have had a significant impact on the eventual efficacy and quality of long-term recordings in each subject. With each human implant, trained neurosurgeons and a team of surgical staff supported the careful placement of UEAs; with each NHP implant, the process was conducted by PIs, postdocs, and graduate students. Although veterinarian and animal clinic personnel are sometimes available for NHP implantation procedures in some research institutions, the availability of these resources is far from guaranteed. An added factor that may have contributed to the human implants’ higher signal quality and lifespan was more diligent care. NHPs have a higher likelihood of implant damage due to their activity and behavior, while human participants are generally more careful with their implants. Moreover, the wound margins surrounding the percutaneous connectors tend to become dirtier and more prone to infections in NHPs versus humans.
Although our ability to comment on the consistency of human implant reliability is limited due to our small sample size, previous work indicates that the duration of human implants can also vary (18). Across 18 chronic human implants (including the two participants included here), at least 9 remained implanted for at least 1 year and 7 of these for more than 2 years. Importantly, the authors noted that some of these experiments remain ongoing and that the total reported duration does not indicate that the array failed.
4.2. Study Limitations
One of our unique advantages in investigating questions concerning multi-electrode array signal quality is the massive amount of data available to us over the past 17 years. However, our intentions for implanting these arrays were to address scientific questions regarding cortical function and not to specifically address array longevity and reliability. Therefore, one limitation of this study is that we did not sample recordings evenly in time, and we sometimes terminated recording when we were finished with experiments instead of due to array failure. An additional limitation is the difficulty of cross-species comparisons of results. This challenge is present in any cross-species study, born out here by the substantial differences in anatomy (cortical thickness and relative size of the gyrus) and anatomical landmarks, which may have had an additional effect on the differences in longevity and reliability demonstrated between human and non-human primate results presented here.
Another limitation due to the size and scale of the datasets with which we analyzed was the lack of spike sorting to distinguish individual single units within a given electrode. As a result, all our metrics and analyses are on the scale of individual electrodes and arrays, instead of individual single units. Our decision to forgo spike sorting, therefore, underestimates unit yield and SNR because two high SNR single units of different amplitudes on a single electrode, for example, would result in a lower average SNR. It should be reiterated that meaningful information about the brain may be derived from channels which exhibit poorly isolated units.
The Utah Array continues to be a reliable and valuable tool for systems neuroscience and human brain-computer interfaces. We have demonstrated that most non-human primate implants will reliably last over a year, with the potential for arrays to last for the better part of a decade; human implants also display impressive longevity, lasting multiple years. While the Utah array may be acceptable for current animal research, more research and development must be done to create chronic implants that will reliably last into years and decades of use, for human applications and more advanced animal studies.
Acknowledgements:
We would like to thank Dawn Paulsen, Josh Coles, Rebecca Junod, and Carrie Anne Balcer for assistance in surgical implants, animal care and training.
Funding:
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (Grants R01 NS045853 and R01 NS111982 to N.G.H.). The human data was collected with support from the National Institute Of Neurological Disorders And Stroke of the National Institutes of Health under Award Numbers UH3NS107714 and U01NS108922 and Defense Advanced Research Projects Agency (DARPA) and Space and Naval Warfare Systems Center Pacific (SSC Pacific) under Contracts N66001-16-C4051 and N66001-10-C-4056. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, DARPA, or SSC Pacific.
Footnotes
Conflicts of Interest
N.G.H. serves as a consultant for Blackrock Microsystems, Inc., the company that sells the multi-electrode arrays and acquisition system used in this study.
Ethics Statement: All animals were treated according to the University of Chicago Institutional Animal Care and Use Committee. The surgical and behavioral procedures involved in this study were approved by the University of Chicago Institutional Animal Care and Use Committee and conform to the principles outlined in the Guide for the Care and Use of Laboratory Animals. Two human participants were implanted with UEAs during a study conducted under Investigational Device Exemptions (IDE) granted by the US Food and Drug Administration and with approval from the University of Pittsburgh Institutional Review Board (NCT01364480 and NCT01894802).
References
- 1.Jones KE, Campbell PK, Normann RA. A glass/silicon composite intracortical electrode array. Ann Biomed Eng. 1992;20(4):423–37. [DOI] [PubMed] [Google Scholar]
- 2.Walker J, MacLean J, Hatsopoulos NG. The marmoset as a model system for studying voluntary motor control. Dev Neurobiol. 2017;77(3):273–85. [DOI] [PubMed] [Google Scholar]
- 3.Black BJ, Kanneganti A, Joshi-Imre A, Rihani R, Chakraborty B, Abbott J, et al. Chronic recording and electrochemical performance of utah microelectrode arrays implanted in rat motor cortex. J Neurophysiol. 2018;120(4):2083–90. [DOI] [PubMed] [Google Scholar]
- 4.Barrese JC, Rao N, Paroo K, Triebwasser C, Vargas-Irwin C, Franquemont L, et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J Neural Eng. 2013;10(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousche PJ, Normann RA. Chronic recording capability of the utah intracortical electrode array in cat sensory cortex. J Neurosci Methods. 1998;82(1):1–15. [DOI] [PubMed] [Google Scholar]
- 6.Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Neural Syst Rehabil Eng. 2005;13(4):524–41. [DOI] [PubMed] [Google Scholar]
- 7.Chestek CA, Gilja V, Nuyujukian P, Foster JD, Fan JM, Kaufman MT, et al. Long-term stability of neural prosthetic control signals from silicon cortical arrays in rhesus macaque motor cortex. J Neural Eng. 2011;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downey JE, Schwed N, Chase SM, Schwartz AB, Collinger JL. Intracortical recording stability in human brain-computer interface users. J Neural Eng. 2018;15(4). [DOI] [PubMed] [Google Scholar]
- 9.Fraser GW, Schwartz AB. Recording from the same neurons chronically in motor cortex. J Neurophysiol. 2012;107(7):1970–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozai TDY, Catt K, Li X, Gugel Z V., Olafsson VT, Vazquez AL, et al. Mechanical failure modes of chronically implanted planar silicon-based neural probes for laminar recording. Biomaterials [Internet]. 2015;37:25–39. Available from: 10.1016/j.biomaterials.2014.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salatino JW, Ludwig KA, Kozai TDY, Purcell EK. Glial responses to implanted electrodes in the brain. Nat Biomed Eng [Internet]. 2017;1(11):862–77. Available from: 10.1038/s41551-017-0154-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. [DOI] [PubMed] [Google Scholar]
- 13.Buzsáki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7(5):446–51. [DOI] [PubMed] [Google Scholar]
- 14.Ward MP, Rajdev P, Ellison C, Irazoqui PP. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res [Internet]. 2009;1282:183–200. Available from: 10.1016/j.brainres.2009.05.052 [DOI] [PubMed] [Google Scholar]
- 15.McConnell GC, Rees HD, Levey AI, Gutekunst CA, Gross RE, Bellamkonda RV. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J Neural Eng. 2009;6(5). [DOI] [PubMed] [Google Scholar]
- 16.Vetter RJ, Williams JC, Hetke JF, Nunamaker EA, Kipke DR. Chronic neural recording using siliconsubstrate microelectrode arrays implanted in cerebral cortex. IEEE Trans Biomed Eng. 2004;51(6):896–904. [DOI] [PubMed] [Google Scholar]
- 17.Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, et al. Brain responses to micro-machined silicon devices. Brain Res. 2003;983(1–2):23–35. [DOI] [PubMed] [Google Scholar]
- 18.Bullard AJ, Hutchison BC, Lee J, Chestek CA, Patil PG. Estimating Risk for Future Intracranial, Fully Implanted, Modular Neuroprosthetic Systems: A Systematic Review of Hardware Complications in Clinical Deep Brain Stimulation and Experimental Human Intracortical Arrays. Neuromodulation. 2020;23(4):411–26. [DOI] [PubMed] [Google Scholar]
- 19.Collinger JL. Personal Communication. 2021. [Google Scholar]
- 20.Center for Devices and Radiological Health. Implanted Brain-Computer Interface (BCI) Devices for Patients with Paralysis or Amputation - Non-clinical Testing and Clinical Considerations [Internet]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/implanted-brain-computer-interface-bci-devices-patients-paralysis-or-amputation-non-clinical-testing
- 21.Jun JJ, Steinmetz NA, Siegle JH, Denman DJ, Bauza M, Barbarits B, et al. Fully integrated silicon probes for high-density recording of neural activity. Nature. 2017;551(7679):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musk E. An integrated brain-machine interface platform with thousands of channels. bioRxiv [Internet]. 2019. Jan 1;703801. Available from: http://biorxiv.org/content/early/2019/07/18/703801.1.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piech DK, Johnson BC, Shen K, Ghanbari MM, Li KY, Neely RM, et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat Biomed Eng [Internet]. 2020;4(2):207–22. Available from: 10.1038/s41551-020-0518-9 [DOI] [PubMed] [Google Scholar]
- 24.Colachis SC, Dunlap CF, Annetta NV., Tamrakar SM, Bockbrader MA, Friedenberg DA. Long-term intracortical microelectrode array performance in a human: A 5 year retrospective analysis. J Neural Eng. 2021;18(4). [DOI] [PubMed] [Google Scholar]
- 25.Dunlap CF, Colachis SC, Meyers EC, Bockbrader MA, Friedenberg DA. Classifying Intracortical Brain-Machine Interface Signal Disruptions Based on System Performance and Applicable Compensatory Strategies: A Review. Front Neurorobot. 2020;14(October). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes CL, Flesher SN, Weiss JM, Downey JE, Collinger JL, Gaunt RA. Neural stimulation and recording performance in human somatosensory cortex over 1500 days. medRxiv. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santhanam G, Linderman MD, Gilja V, Afshar A, Ryu SI, Meng TH, et al. HermesB: A continuous neural recording system for freely behaving primates. IEEE Trans Biomed Eng. 2007; [DOI] [PubMed] [Google Scholar]
- 28.Collinger JL, Wodlinger B, Downey JE, Wang W, Tyler-Kabara EC, Weber DJ, et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet. 2013;381(9866):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Negi S, Bhandari R, Rieth L, Solzbacher F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed Mater. 2010;5(1):15007. [DOI] [PubMed] [Google Scholar]
- 30.Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. Journal of Neuroscience Methods. 2005. [DOI] [PubMed] [Google Scholar]
- 31.Blackrock Microsystems. Personal Communication.