Abstract
The in vitro activity of daptomycin was compared with those of vancomycin, linezolid, and quinupristin-dalfopristin against a variety (n = 203) of gram-positive bacteria, including methicillin-resistant Staphylococcus aureus and S. epidermidis (MRSA and MRSE, respectively), vancomycin-resistant enterococci (VRE), and vancomycin-intermediate S. aureus (VISA). Overall, daptomycin was more active against all organisms tested, except Enterococcus faecium and VISA, against which its activity was similar to that of quinupristin-dalfopristin. In time-kill studies with MRSA, MRSE, VRE, and VISA, daptomycin demonstrated greater bactericidal activity than all other drugs tested, killing ≥3 log CFU/ml by 8 h. Daptomycin may be a potential alternative drug therapy for multidrug-resistant gram-positive organisms and warrants further investigation.
Daptomycin is a cyclic polypeptide derived from Streptomyces roseosporus and representing a class of antimicrobial agents known as the peptolides (acid lipopeptide antibiotics) (3). The spectrum of activity is similar to those of vancomycin and teicoplanin, with activity against a wide variety of aerobic and anaerobic gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). Daptomycin is also active against vancomycin-resistant gram-positive bacteria, including enterococci (4, 5, 7). The mechanism of action differs from that of the glycopeptides, involving the disruption of amino acid transport by the cell membrane and alterations of the cytoplasmic membrane potential (1–3). The bactericidal activity of daptomycin is concentration dependent and is influenced by pH and ionized calcium concentrations (9). Like teicoplanin, daptomycin is highly protein bound (94%), and its in vitro activity is altered in the presence of serum or albumin. However, even in the presence of serum, the activity of daptomycin is superior to that of vancomycin or teicoplanin (6, 11). In early clinical trials, daptomycin was efficacious in patients with skin and skin structure infections and bacteremia at dosages of 2 to 3 mg/kg of body weight every 12 h (investigator's brochure, Eli Lilly & Co.). However, clinical trials were suspended when treatment failures were noted in patients with S. aureus endocarditis (Eli Lilly & Co., personal communication). Possible reasons for failure included the high degree of protein binding and the degree of penetration into cardiac vegetations (9). Experimental animal endocarditis studies replicating clinical trial dosages and achievable concentrations demonstrated that slightly higher dosage regimens of daptomycin were as effective as or more effective than vancomycin in reducing vegetative bacterial densities (8). Recently, renewed interest in daptomycin has occurred secondarily to the need for new agents with activity against vancomycin-resistant organisms, including enterococci. Our objectives were to reevaluate the bactericidal activity of daptomycin compared to those of vancomycin, the oxazolidinone linezolid, and the streptogramin quinupristin-dalfopristin against various gram-positive organisms.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy.)
Bacterial strains.
The 203 gram-positive organisms used consisted of the following: 100 S. aureus isolates, 50 methicillin-susceptible S. aureus (MSSA), 50 MRSA, and 3 vancomycin-intermediate-susceptible S. aureus (VISA). The 50 coagulase-negative staphylococci consisted of 25 methicillin-susceptible S. epidermidis (MSSE) isolates and 25 methicillin-resistant S. epidermidis (MRSE). The 50 enterococci consisted of 25 Enterococcus faecalis isolates and 25 E. faecium (20 of these isolates were resistant to vancomycin). MICs were also determined by use of a standard reference strain of S. aureus (ATCC 25923) with all antibiotics at baseline and during each susceptibility test for quality assurance purposes.
Antibiotics.
Daptomycin (Eli Lilly & Co. lot no. RS0113) was supplied by Cubist Pharmaceuticals, Inc., Cambridge, Mass. Vancomycin susceptibility-grade powder was purchased commercially (Sigma Chemical Co., St. Louis, Mo.). Linezolid susceptibility-grade powder (lot 5014-TJF-490 A) was supplied by Pharmacia and Upjohn, Inc., (Kalamazoo, Mich.), and quinupristin-dalfopristin susceptibility-grade powder (lot 9609410) was supplied by Rhone-Poulenc Rorer (Collegeville, Pa.).
MIC and MBC determinations.
The MICs and minimal bactericidal concentrations (MBCs) were determined for each isolate in duplicate by a microdilution technique with Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) supplemented with calcium (25 mg/liter) and magnesium (12.5 mg/liter) (SMHB) for vancomycin, linezolid, and quinupristin-dalfopristin. Mueller-Hinton broth supplemented with calcium at 75 mg/liter (physiological ionized Ca2+ concentration) and magnesium at 12.5 mg/liter (SMHB-PCA) was always used for microdilution susceptibility testing of daptomycin. The MIC and MBC of daptomycin were also determined with a subset of each organism (n = 22) and a 50:50 mixture of pooled human serum and SMHB-PCA. Susceptibility testing for each drug was performed in quadruplicate according to the guidelines of the National Committee for Clinical Laboratory Standards (10).
Killing curves.
The bactericidal activities of daptomycin, vancomycin, linezolid, and quinupristin-dalfopristin were compared by use of killing curve analyses with four representative clinical isolates (R499, an MRSA; MU50, a VISA; R227, an MRSE; and R588, a vancomycin-resistant enterococcus [VRE] [E. faecalis]). Three to five colonies from overnight growth on tryptic soy agar at 35°C were added to normal saline and adjusted as necessary to produce a 0.5 McFarland standard suspension of organisms. This suspension was diluted appropriately (1:10) with SMHB (SMHB-PCA for daptomycin) to achieve an inoculum of 107 CFU/ml. A 0.2-ml suspension of an organism was added to 1.7 ml of SMHB (final inoculum, 106 CFU/ml) with a 0.1-ml stock solution of each antibiotic at concentrations one and four times the MICs for the respective organism in a 24-well tissue culture plate (final volume, 2.0 ml per well). Culture wells were incubated at 35°C with constant shaking for 24 h. Samples (0.1 ml) were removed at 0, 8, and 24 h; appropriately diluted with cold 0.9% sodium chloride to reduce antibiotic carryover; plated onto tryptic soy agar (Difco) with an Autoplate Spiral Diluter (model 3000; Spiral Bioscience, Frederick, Md.); and incubated at 35°C for 24 h. The limit of detection for this method is 250 CFU/plate, corresponding to 2.5 log10 CFU/ml. Growth control wells for each organism were prepared without antibiotic and run in parallel to the antibiotic test wells. Killing curves were also determined with daptomycin at a concentration four times the MIC in SMHB-PCA and SMHB-PCA with pooled human serum (50:50) against the four representative isolates mentioned above. All time-kill curve experiments were performed in triplicate.
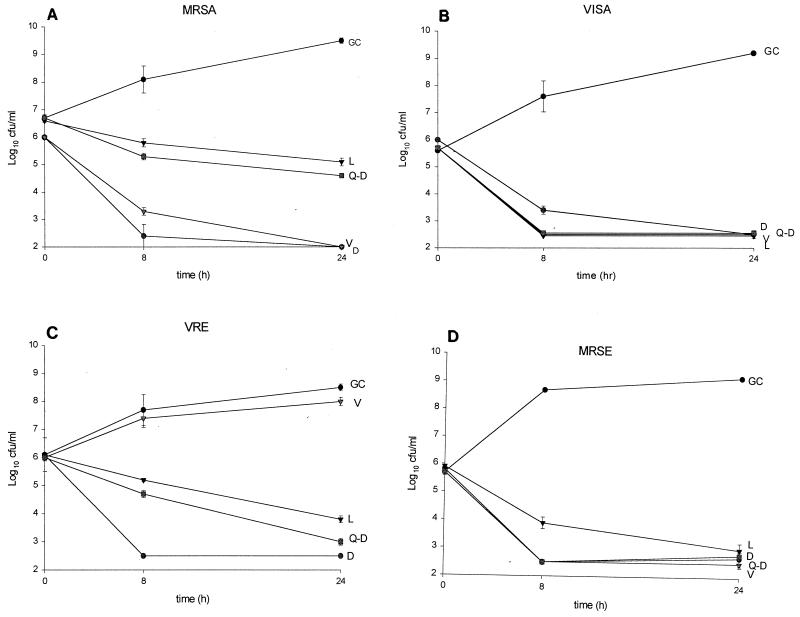
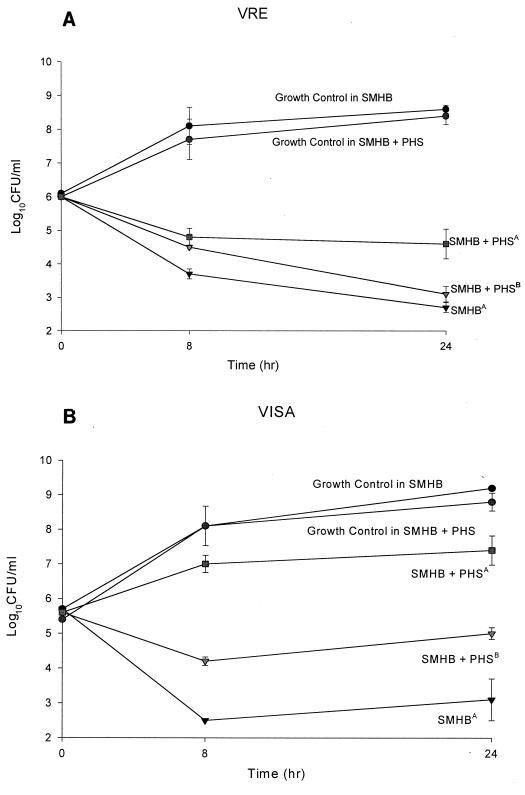
The drug activities are reported in Table 1. Against MSSA and MRSA, daptomycin activity was approximately 8- to 30-fold greater than those of all other agents. The activity of daptomycin against MSSE and MRSE was equal to that of quinupristin-dalfopristin and 2- to 16-fold greater than those of vancomycin and linezolid. Against VISA, quinupristin-dalfopristin was the most active agent, followed by daptomycin, linezolid, and vancomycin. The activity of daptomycin against both vancomycin-sensitive and -resistant E. faecalis was greater than those of all other agents tested. The activity of daptomycin against both vancomycin-sensitive and -resistant E. faecium was equal to that of quinupristin-dalfopristin and greater than those of all other agents tested. On average, the MICs of daptomycin in the presence of 50% serum against MSSA (n = 4), MRSA (n = 4), MSSE (n = 4), MRSE (n = 4), E. faecium (n = 5), and VISA (n = 1) increased one- to eightfold, while serum had no effect on the MICs of linezolid, quinupristin-dalfopristin, or vancomycin (data not shown). In time-kill studies, at the MIC, killing was minimal and regrowth occurred for all agents (data not shown). At concentrations four times the MIC, daptomycin and vancomycin achieved 99.9% killing of MRSA in 8 h, which was greater than the killing seen with linezolid and quinupristin-dalfopristin (P, <0.05) (Fig. 1). Against VRE at 8 and 24 h, daptomycin had greater activity than linezolid and quinupristin-dalfopristin (P, <0.05). Against MRSE, daptomycin, vancomycin, and quinupristin-dalfopristin had greater activity than linezolid early (P, <0.05); however, no differences were observed between the regimens at 24 h. All antibiotics tested achieved 99.9% killing of the VISA isolate by 24 h. The addition of pooled human serum to SMHB-PCA significantly (P, <0.05) decreased the activity of daptomycin. However, killing activity was significantly improved when the daptomycin concentration was adjusted according to the MIC obtained in SMHB-PCA plus pooled human serum; data are shown for two of the isolates (Fig. 2).
TABLE 1.
Activities of daptomycin (D), vancomycin (V), linezolid (L), and quinupristin-dalfopristin (Q-D) against various bacterial strains
| Organism (no. of isolates) | MICa (μg/ml) of the indicated drug:
|
MBC (μg/ml) of:
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50%
|
90%
|
Range
|
||||||||||||||
| D | V | L | Q-D | D | V | L | Q-D | D | V | L | Q-D | D | V | L | Q-D | |
| MSSA (50) | 0.13 | 0.50 | 4.0 | 0.5 | 0.13 | 1.0 | 4.0 | 1.0 | 0.06–0.5 | 0.25–2.0 | 0.5–8.0 | 0.13–2.0 | 0.6–1.0 | 0.25–4.0 | 4.0–64.0 | 0.25–8.0 |
| MRSA (50) | 0.13 | 0.50 | 2.0 | 0.25 | 0.13 | 1.0 | 4.0 | 1.0 | 0.06–0.5 | 0.25–2.0 | 1.0–8.0 | 0.03–1.0 | 0.6–1.0 | 0.5–8.0 | 1.0–64.0 | 0.25–16.0 |
| MSSE (25) | 0.13 | 1.0 | 2.0 | 0.13 | 0.50 | 1.0 | 4.0 | 0.50 | 0.06–1.0 | 0.50–2.0 | 1.0–4.0 | 0.03–2.0 | 0.06–1.0 | 0.50–8.0 | 8.0–64.0 | 0.06–8.0 |
| MRSE (25) | 0.13 | 1.0 | 2.0 | 0.06 | 0.25 | 1.0 | 4.0 | 0.25 | 0.13–2.0 | 0.50–2.0 | 2.0–4.0 | 0.06–8.0 | 0.06–2.0 | 0.50–64.0 | 2.0–64.0 | 0.05–32.0 |
| E. faecalisb (25) | 0.50 | 1.0 | 2.0 | 4.0 | 1.0 | 64.0 | 4.0 | 16.0 | 0.25–4.0 | 1.0–64.0 | 1.0–8.0 | 0.25–16.0 | 0.5–8.0 | 2.0–128.0 | 4.0–64.0 | 1.0–64.0 |
| E. faeciumb (25) | 2.0 | 64.0 | 4.0 | 0.50 | 4.0 | 64.0 | 4.0 | 4.0 | 0.50–1.0 | 4.0–8.0 | 1.0–2.0 | 0.25–0.25 | 0.50–4.0 | 4.0–128.0 | 2.0–128.0 | 0.25–64.0 |
| VISA (3) | 0.5–1.0 | 8.0–8.0 | 1.0–2.0 | 0.25–0.25 | 1.0–1.0 | 4.0–8.0 | 1.0–8.0 | 0.25–1.0 | ||||||||
| S. aureus ATCC 25923 | 0.13–0.25 | 1.0–2.0 | 4.0–8.0 | 0.25–0.5 | ||||||||||||
50% and 90%, MICs at which 50 and 90% of strains are inhibited, respectively.
Includes VRE.
FIG. 1.
Time-kill experiments performed at four times the MIC against MRSA R499 (A), VISA 992 (B), VRE R588 (E. faecalis) (C), and MRSE R227 (D). Results are means ± standard deviations. GC, growth control; D, daptomycin; V, vancomycin; L, linezolid; Q-D, quinupristin-dalfopristin. MICs of D, V, L, and Q-D, respectively, were as follows: for R499, 0.125, 0.5, 2.0, and 0.25; for 992, 0.5, 8.0, 2.0, and 0.25; for R588, 1.0, 128.0, 4.0, and 2.0; and for R277, 0.25, 1.0, 4.0, and 0.06.
FIG. 2.
(A) Time-kill experiments performed with daptomycin in broth and in broth plus pooled human serum (PHS) at four times the MIC against VRE 588. A superscript “A” indicates the MIC determined in SMHB-PCA (MIC, 1.0 μg/ml). A superscript “B” indicates the MIC determined in SMHB-PCA plus PHS (MIC, 2.0 μg/ml). (B) Time-kill experiments performed with daptomycin in broth and in broth plus PHS at four times the MIC against VISA 992. A superscript “A” indicates the MIC determined in SMHB-PCA (MIC, 0.5 μg/ml). A superscript “B” indicates the MIC determined in SMHB-PCA plus PHS (MIC, 1.0 μg/ml).
Daptomycin represents a unique semisynthetic antimicrobial agent with activity against a broad range of gram-positive pathogens, including VRE and vancomycin-resistant staphylococci. Unlike those of many other agents, the activity of daptomycin is bactericidal for staphylococci and enterococci and is concentration dependent. The activity of daptomycin in the presence of serum is decreased because of its high degree of protein binding; however, its activity is improved when concentrations are equal to or greater than four times the MIC (achieved clinically). This conclusion was supported by the killing curves determined in the presence of serum for VRE but to a lesser extent for the VISA isolate. Our susceptibility data are similar to those reported by others previously for gram-positive organisms, including more recent data against newer pathogens, such as VRE and VISA (N. V. Jacobus, B. Goldin, L. McDermott, and D. R. Snydman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. F-113, 1998; N. V. Jacobus, L. McDermott, J. R. Lonks, J. M. Boyce, and D. R. Snydman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., p. F-112, 1998). The primary mechanism of action of daptomycin relies heavily on ionized calcium content. The difference in the activities of daptomycin in SMHB (9) and in SMHB-PCA is notable and should be considered when one is determining MICs of this drug. Although early studies with daptomycin were not successful at low doses for patients with endocarditis, based upon the long serum half-life of 8.5 h and the dose-dependent postantibiotic effect of 1 to 6 h, it is likely that this drug could achieve success if given once daily. Newly proposed dosing for daptomycin at 4 and 6 mg/kg/day, administered once daily, should achieve serum concentrations necessary to improve the activity of the drug for a variety of gram-positive organisms.
Acknowledgments
This study was supported by a grant from Cubist Pharmaceuticals.
REFERENCES
- 1.Alborn W E, Jr, Allen N E, Preston D A. Daptomycin disrupts membrane potential in growing Staphylococcus aureus. Antimicrob Agents Chemother. 1991;35:2282–2287. doi: 10.1128/aac.35.11.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen N E, Alborn W E, Jr, Hobbs J N., Jr Inhibition of membrane potential-dependent amino acid transport by daptomycin. Antimicrob Agents Chemother. 1991;35:2639–2642. doi: 10.1128/aac.35.12.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen N E, Hobbs J N, Alborn W E. Inhibition of peptidoglycan biosynthesis in gram-positive bacteria by LY146032. Antimicrob Agents Chemother. 1987;31:1093–1099. doi: 10.1128/aac.31.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush L M, Boscia J A, Kaye D. Daptomycin ( LY146032) treatment of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1988;32:877–881. doi: 10.1128/aac.32.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eliopoulos G M, Willey S, Reiszner E, Spitzer P G, Caputo G, Moellering R C., Jr In vitro and in vivo activity of LY146032, a new cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1986;30:532–535. doi: 10.1128/aac.30.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrison M W, Vance-Bryan K, Larson T A, Toscano J P, Rotschafer J C. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 1990;34:1925–1931. doi: 10.1128/aac.34.10.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R N, Barry A L. Antimicrobial activity and spectrum of LY146032, a new lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob Agents Chemother. 1987;31:625–629. doi: 10.1128/aac.31.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaatz G W, Seo S M, Reddy V N, Bailey E M, Rybak M J. Daptomycin compared with teicoplanin and vancomycin for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1990;34:2081–2085. doi: 10.1128/aac.34.11.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamp K C, Rybak M J. Teicoplanin and daptomycin bactericidal activities in the presence of albumin or serum under controlled conditions of pH and ionized calcium. Antimicrob Agents Chemother. 1993;37:605–609. doi: 10.1128/aac.37.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 11.Rybak M J, Bailey E M, Lamp K C, Kaatz G W. Pharmacokinetics and bactericidal rates of daptomycin and vancomycin in intravenous drug abusers being treated for gram-positive endocarditis and bacteremia. Antimicrob Agents Chemother. 1992;36:1109–1114. doi: 10.1128/aac.36.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]