Abstract
Objective
To understand intensivist perceptions of the appropriateness of time-limited trials (TLTs)—a strategy to align life-sustaining care with patient goals and values in the midst of clinical uncertainty.
Design
We conducted a mixed-methods sequential explanatory study of intensive care unit (ICU) intensivists regarding appropriateness of utilising TLTs in three vignettes centred on invasive mechanical ventilation (IMV); continuous renal replacement therapy (CRRT); and heated high-flow nasal cannula (HHFNC). Semistructured interviews were conducted using the Tailored Implementation of Chronic Diseases framework. Data were analysed using thematic and matrix analysis.
Setting
Two academic medical centres in the USA participated in the randomised surveys and one centre participated in the semistructured interviews.
Participants
Pulmonary and critical care intensivists and fellows.
Primary and secondary outcomes
To understand intensivists perceptions of the appropriateness in using TLTs.
Results
Of 115 physicians surveyed, 71 initiated the survey and 44 completed the entire survey with a response rate of 38% (N=44/115) and a completion rate of 62% (N=44/71). While 35% (N=23/66) of intensivists had never heard of a TLT, of the intensivists who had heard of a TLT, 77% (N=33/43) had participated in one. In response to the vignettes, appropriateness of using a TLT varied (IMV: 74% (N=46/62); CRRT 78% (N=49/63); HHFNC 92% (N=56/61) as did the durations of the TLT. Semistructured interviews with 11 intensivists revealed having clarity about patient goals and clinical endpoints facilitated successful TLTs while lack of an evidenced-based framework was a barrier.
Conclusion
More than half of the physicians who responded had conducted or participated in a TLT. To increase the use of TLTs in the ICU, clinicians desire a more robust, evidence-based framework on how to conduct TLTs.
Keywords: Adult intensive & critical care, MEDICAL ETHICS, Protocols & guidelines, Quality in health care
Strengths and limitations of this study.
Our work begins to explore why intensivists use time-limited trials (TLTs) with patients and surrogate decision makers through a mixed-methods approach.
A sequential explanatory mixed-methods approach was used to examine perceptions and practices of TLTs and to deeply understand barriers and facilitators to the use of TLTs.
Intensivists from two academic medical centres were included in the survey. One academic centre participated in the semistructured interviews, and their practices may not generalise to other hospital and clinical settings.
The intensive care unit (ICU) is filled with complex high-stakes decision-making where time is scarce and outcomes hard to foresee. For some patients and surrogate decision makers, initial decisions to pursue life-sustaining medical treatments must be made emergently. As a result, understanding of the consequences of these decisions may be unclear initially, due to insufficient information about the patient’s prognosis and an inability to incorporate a patient’s values or preferences.1–6 This misalignment of life-sustaining care with patient’s goals and values may result in moral distress for patients, surrogate decision-makers and healthcare providers.7 8
Care in the ICU is often balanced between aggressive, life-sustaining care, goals and values of patients, and clinical uncertainty. Time-limited trials (TLTs) have been proposed as a strategy to better align care with patient preferences and has demonstrated reductions in invasive treatments among patients unlikely to survive their hospitalisation as reported in a subgroup analysis of one study.9 For example, a patient’s clinical trajectory might be unknown, making it difficult for his or her healthcare team, as well as a surrogate decision maker, to decide whether and what forms of life support to implement, and for how long. TLTs are an agreement between patients, surrogate decision-makers and the healthcare team to provide a life-sustaining therapy for a defined period of time with specific target endpoints to be reached during this time period (eg, ventilator liberation).1 At the end of the TLT, a family meeting is held to evaluate the patient’s progress and discuss the next steps in the patient’s care.10
Despite TLTs being described in the literature and offered as a promising strategy to align life-sustaining care with patient’s values and goals,11 few studies have explored clinician knowledge or implementation of TLTs in the ICU.9 In light of this gap, we sought to understand whether and how intensivists use TLTs to guide clinical decision making.
Methods
We conducted a cross-sectional cohort study of pulmonary critical care intensivists and fellows intentionally using a sequential explanatory mixed-methods design, wherein the quantitative survey data informed the qualitative semistructured interviews12 (online supplemental appendix A).
bmjopen-2021-059325supp001.pdf (196.3KB, pdf)
Patient and public involvement
The study did not involve patients or the public in the design, conduct, or reporting.
Study setting
All pulmonary critical care intensivists and fellows at two academic medical centres (University of Michigan and University of Texas Southwestern) were asked once to anonymously complete an online survey describing three clinical vignettes in October 2019. Subsequently, a convenience sample of intensivists and fellows were invited to participate in semistructured interviews from the University of Michigan between October 2019 and September 2020. The intensivists who participated in the semistructured interviews were from one academic medical centre because of the limitations placed on clinical research during the coronavirus pandemic. Additionally, the intensivists participating in the semistructured interviews were not asked to disclose if they had participated in the anonymous surveys and they were not provided access to the survey vignettes.
Surveys
The vignettes focused on three common life-sustaining medical treatments provided in the ICU: invasive mechanical ventilation (IMV), continuous renal replacement therapy (CRRT), and heated-high flow nasal cannula (HHFNC; online supplemental appendix B). The vignettes were generated by two critical care intensivists with the objective to probe physicians on their clinical decision-making on the utilisation of a TLT in clinical vignettes where a TLT may be appropriate. A definition of a TLT was provided and the participants were subsequently asked about their knowledge and utilisation of a TLT. The vignettes were presented to the participants in a randomised order via Qualtrics (Qualtrics 2019, Provo, Utah, USA). Intensivists were asked whether a TLT was appropriate for each scenario and if the participant selected yes, they were asked to rank what clinical endpoints they would use to assess if the intervention was successful. If the participant selected no, they were asked to explain their answer. Lastly, they were asked to quantify the duration of the TLT. Demographic information was provided by the participants.
Intensivist characteristics are reported as counts (percentages), means (SDs), or medians (IQR) as appropriate. We used two-sided significance testing and considered a p<0.05 to be significant.
An exploratory post hoc analysis was conducted to generate hypothesis and identify if intensivist demographics (age, race, gender, institution) and prior participation in a TLT would be associated with the decision to use a TLT for each intervention using logistic regression. We also performed an exploratory analysis using linear regressions to identify whether intensivist demographics (age, race, gender, institution) and prior participation in a TLT would be associated with the recommended duration (days) of the TLT. As this was an exploratory analysis, we did not adjust for multiple comparisons.
All quantitative analyses were conducted with Stata software V.15.1 (StataCorp).
Semi-structured interviews
In light of intensivists reporting via the survey that TLTs may be appropriate in certain hypothetical clinical scenarios, semistructured interviews were conducted to better understand barriers and facilitators to the use of TLTs (online supplemental appendix A). One investigator (JNE) conducted all the interviews as she had no existing relationships with the participants. JNE has extensive experience conducting semi-structured interviews.
Interviews were guided by the Tailored Implementation in Chronic Diseases (TICD) framework (online supplemental appendix C). The TICD is a comprehensive, integrated checklist that is intended to be used as a screening tool to identify factors which are likely to be important in fostering change in healthcare practices by identifying barriers and facilitators.13 The TICD encompasses seven domains—guideline factors, individual health professional factors, patient factors, professional interactions, incentives and resources, capacity for organisational change, social, political and legal factors—and 57 determinants of practice.13 The TICD framework was used to identify facilitators and barriers to TLT use by converting the checklist into a series of questions.14
The interviews were conducted over the phone or face-to-face. All interviews were audiotaped and transcribed verbatim. The interview transcripts were coded by JNE and an experienced research assistant, who coded 20% of the data, using the TICD in MAXQDA (VERBI Software, Berlin, Germany). The two coders dual coded 20% of the transcripts to refine the coding scheme. Disagreements were infrequent and were resolved with discussion among the two coders and the reconciled transcripts produced from those discussions were included in the analysis. The remaining transcripts were coded by JNE. Participants were sampled until thematic saturation was reached, such that no new information was being conveyed. Three members of the research team (JNE, EMV, TSV) then used matrix analysis to display, analyse and interpret the coded data.15 The themes which emerged were subsequently organised using the 5Ws: who, what, when, where, and why.
Results
Survey data
Of 115 intensivists surveyed, 71 initiated the survey and 44 completed the entire survey with a response rate of 38% (N=44/115) and a completion rate of 62% (N=44/71). Of those who responded, the majority were male and younger physicians (table 1). After reading the definition, 35% (N=23/66) of intensivists had never heard of a TLT, of the intensivists who had heard of a TLT (N=43), 77% (N=33/43) had participated in one.
Table 1.
Demographics of participants who participated in the survey
| Variable | N=71 |
| Site | |
| 1: N (%) | 45 (63) |
| 2: N (%) | 15 (21) |
| Unknown | 11 (15.5) |
| Race | |
| White: N (%) | 41 (58) |
| Black or African American: N (%) | 0 (0) |
| Hispanic or Latinx: N (%) | 3 (4) |
| Asian: N (%) | 13 (18) |
| Other: N (%) | 1 (1) |
| Declined to answer: N (%) | 13 (18) |
| Sex | |
| Male: N (%) | 42 (60) |
| Female: N (%) | 15 (21) |
| Declined to answer: N (%) | 14 (19) |
| Age | |
| ≤30: N (%) | 4 (5) |
| 31–40: N (%) | 34 (48) |
| 41–50: N (%) | 13 (18) |
| 51–60: N (%) | 4 (6) |
| ≥61: N (%) | 3 (4) |
| Declined to answer: N (%) | 13 (18) |
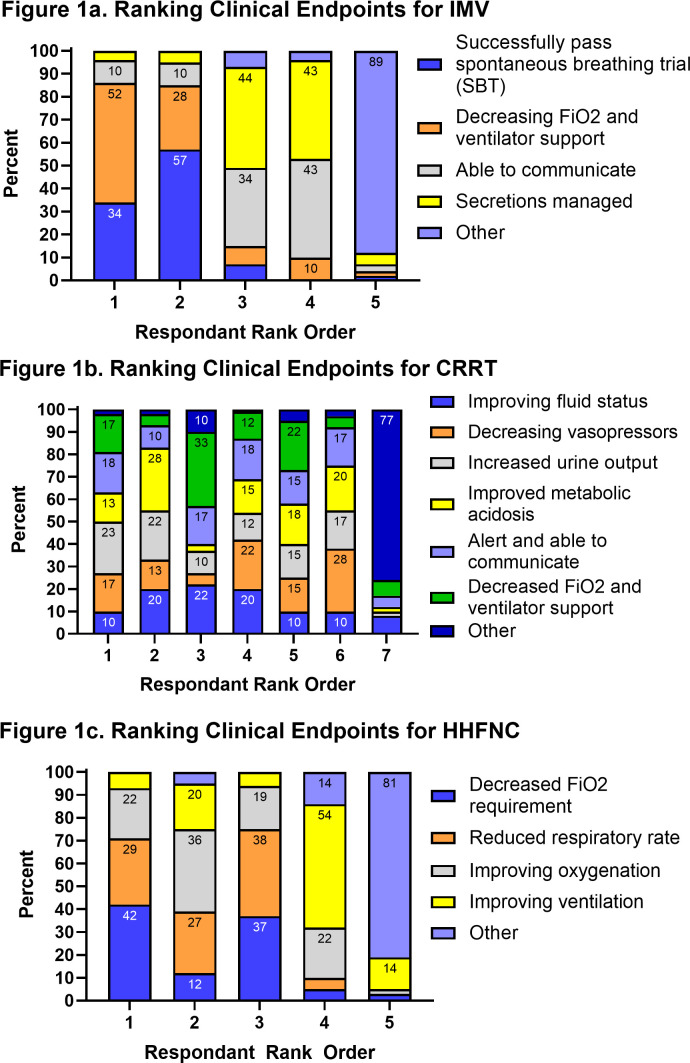
When presented with three hypothetical clinical vignettes, all intensivists believed TLTs were appropriate in at least one scenario. However, intensivist reports of appropriateness varied based on the life-sustaining treatment: 74% (N=46/62) for IMV, 78% (N=49/63) for CRRT and 92% (N=56/61) for HHFNC (table 2). The suggested duration of TLTs also varied by treatment: IMV 4 days (IQR: 3–7 days); CRRT 5.5 days (IQR: 4–7 days); HHFNC 5 days (IQR: 3–7 days) (table 2). The suggested duration of the TLT for IMV and HHFNC was not statistically different based on the intensivist’s belief of the appropriateness of the TLT (IMV: No vs Yes difference of −2.2 days 95% CI −5.67 to 1.24, p=0.21; HHFNC: No vs Yes difference 2.1 days 95% CI −3.79 to 8.09, p=0.47) but was different for CRRT (CRRT: No vs Yes difference of 6.4 days 95% CI 0.51 to 12.2 p=0.03). Intensivists also varied in how they ranked the importance of different clinical endpoints for each hypothetical scenario (figure 1). Decreasing FiO2 was ranked as the most important clinical endpoint by 52% of participants in the IMV vignette and 42% of participants in the HHFNC vignettes. However, there was no clear agreement in the clinical endpoints for CRRT.
Table 2.
Appropriateness of using time-limited trials
| Clinical vignette | Time-limited trial treatment | N (%) Appropriate | Average duration (days) |
| A 79-year-old man with severe idiopathic pulmonary fibrosis was admitted to the medical ICU for acute hypoxic respiratory failure 3 days ago. On arrival to the ICU, he was intubated for worsening hypoxaemia. His ventilator settings are currently: Tidal volume 450 mL, respiratory rate 18, FiO2 70%, PEEP 14 cm H2O. Over 3 days, his condition has neither improved nor worsened while on appropriate treatment. He has not tolerated any spontaneous breathing trials. | IMV | 46 (74%) | 4 (IQR: 3,7) |
| A 56-year-old woman with alcoholic cirrhosis was admitted to the medical ICU 4 days ago for septic shock from spontaneous bacterial peritonitis. Her last drink was 4 months ago. She is not currently a transplant candidate but may be in the future. Over 4 days, her renal function has worsened, and she was started on continuous renal replacement therapy (CRRT) yesterday. | CRRT | 49 (78%) | 5.5 (IQR: 4,7) |
| A 77-year-old woman with acute myeloid leukaemia was admitted to the medical ICU with hypoxic respiratory failure 3 days ago. She is on HHFNC with an FiO2 of 85%. Her pulse oximetry is 92% at rest and drops to the mid 80s with any activity. She has stated that she does not want to be intubated. Over past 3 days, she has not improved despite appropriate treatment. During this time, her condition has neither improved nor worsened. | HHFNC | 56 (92%) | 5 (IQR: 3,7) |
HHFNC, heated high flow nasal cannula; ICU, intensive care unit; IMV, invasive mechanical ventilation.
Figure 1.
Variation exists in ranking which clinical endpoint physicians would use to define if an intervention in a TLT was successful. Physicians were asked to RANK clinical endpoints from most important (ranked 1) to least important in helping them decide if the patient was clinically improving during the TLT. (A) Ranking clinical endpoints in IMV; (B) ranking clinical endpoints for CRRT; (C) ranking clinical endpoints for HHFNC. CRRT, continuous renal replacement therapy; HHFNC, heated high-flow nasal cannula; IMV, invasive mechanical ventilation.
In the exploratory analyses, neither intensivist demographic factors or prior experience with TLTs were associated with ratings of appropriateness of TLTs or with duration of TLTs (online supplemental appendix D, online supplemental tables 1,2).
Semistructured interviews
There were 11 interviews conducted of which 8 were with attending physicians and three were with pulmonary critical care fellows. We organised our qualitative findings using the 5 Ws (table 3).
Table 3.
Characteristics of TLTs mentioned by ICU physicians
| Characteristic | Description | Representative quotes |
| Who | Patient factors: TLTs tend to be used for older patients with reversible disease processes whose clinical trajectories are unknown, who tend to have serious underlying conditions and/or ‘want everything’. | ‘It should be a bridge to something. And if we're coming in with things that aren't potentially bridgeable, then it doesn't make really sense, what we're doing.’ ‘I remember one lady she knew her oncologist like 10 or 15 years and when he came in [to the ICU] and was like, ‘Hey, listen, I really recommend you do that.’ I mean, you can't replace a 15-year relationship.’ |
| Family factors: TLTs help provide families time to make decisions and come to an agreement about the care of their loved ones. | ||
| Clinician factors: TLTs should be able to be initiated by anyone on the care team, which sometimes might be consultants who have an established relationship with the patient. | ||
| Unit factors: ICU physicians felt that patient and family preferences are best elicited elsewhere, prior to a critical event. | ||
| What | TLTs give ICU clinicians the ability to try out life-sustaining therapies over a defined period of time and watch for defined clinical endpoints. No change in a patient’s status by the end of the agreed on time period is often viewed as a poor outcome. | ‘Rarely do I hear people talk about it formally, and I think that a very, very small amount has ever heard of a time-limited trial, they probably have experienced it and just not known that’s what it was called. So, I think the majority probably know what these things are in practice, but don't think about them formally.’ ‘Usually, you think about these for your patients that are coming in with severe medical comorbidities that may or may not benefit from ICU-level care. And so in those settings, I generally use it when I expect that things are going to go poorly, to define an endpoint essentially ahead of time.’ |
| Despite the lack of formal guidelines, ICU physicians are generally familiar with TLTs. However, they do not necessarily call them TLTs. | ||
| TLTs tend to be personalised based on patients’ comorbidities and severity of illness. | ||
| When | Some ICU physicians might consider a TLT immediately after a patient is admitted to the ICU, whereas others prefer to provide 48 hours of aggressive care first. | ‘My thought is that the time-limited trial has be within the timeframe of what I think is a natural course of the disease process. So I can't offer a time-limited trial for three days if the natural course of something is going to be more on the order of weeks.’ ‘If I establish rapport and we're initiating some sort of time-limited trial with a family and then I’m coming off service and handing it off, I think is always hard. It’s always hard to not see something through. But at the same time, I realize that’s the nature of our practice.’ |
| TLTs can last anywhere from 48 hours to 2 weeks. | ||
| There might be multiple TLTs across the ICU admission; one TLT can sometimes lead to another. | ||
| ICU physicians prefer that the same team that initiates a TLT complete it, but recognise that the academic staffing model makes this challenging. | ||
| Where | TLTs are often planned and/or discussed by clinicians with the healthcare team during ICU rounds. | ‘I can see how it’s challenging during rounds to have a discussion like this, but I also think that if it’s the right thing to do for patients and family, then it should be done at the bedside. I guess the answer to [when a TLT should be initiated] for me would be wherever and whenever is the right moment and time that this needs to happen… Getting people and family all in the same room, it’s more ideal. But if that’s going to take three hours from now, four hours from now, then we should just do it right then and there.’ ‘Probably a mix of both. So we might have that discussion on rounds, with the team, to decide how we want to approach this. And then certainly in family discussions, when we're talking about prognosis and next steps in management or goals of care or anything like that. So definitely there (in family meetings), but I think (during) rounds, we talked about that too.’ |
| TLTs are often formalised and/or agreed on with surrogate decision makers during family meetings. | ||
| Patient values, even if elicited and documented outside of the ICU, will be taken into consideration when creating a TLT. | ||
| Why | Patients: TLTs help ensure that care addresses the patients’ immediate needs while being concordant with values and preferences. | ‘If we're talking about a CRRT patient who has multiorgan failure from septic shock or something like that, and in my discussions with the family it was clear that this patient is in the dying process. Escalating care, more lifesaving therapies was not what they would want, then I wouldn't even consider a time-limited trial then.’ ‘It also allows a little bit of time, if things are truly going poorly, to declare themselves. You can then make a firmer recommendation and essentially remove some of that decision-making burden from that patient, from the patient’s family. … I think it’s helpful from a stewardship standpoint.’ |
| Families: TLTs help convey the seriousness of the situation while alleviating some of the decision-making burden. | ||
| Clinicians: TLTs should not be used to delay death. It is important to balance the need to buy time in the face of uncertainty while not placing patients in ‘ICU purgatory’. | ||
| Unit: TLTs help physicians be effective stewards of ICU resources by ensuring that the provision of life support is in concordance with the patients’ current health status, and the patient and families’ preferences and values. |
ICU, intensive care unit; TLTs, time-limited trials.
Who
Specific patient, family, physician, and hospital factors were identified when intensivists were deciding whether and when a TLT should be used. For some clinicians interviewed, the patient needed a potentially reversible condition that would benefit from ICU-level care.
If I'm doing a time-limited trial, my expectation or the expectation that I'm giving a family is that their condition can improve. So if I don't think that the interventions that I'm offering can even potentially improve a patient, then I try not to offer it.
For other intensivists, they felt that TLTs are ideal for end-of-life decision-making. For example, families can sometimes struggle with medical decision-making for the patient and a TLT can be used as a tool to help provide time for these families.
My experience is that it’s often a negotiation tactic between providers and patients’ families, when basically the families are more optimistic and maybe unrealistically optimistic about their loved one’s trajectory. And so the physician uses time-limited trials to create a boundary with clear expectations and metrics that can be followed and assessed. And that gives an element of control for the families to feel like, you know, they advocated for their family members the way they think best. And at the end of the day, whatever the outcome is, they feel more at peace with the decisions.
Furthermore, using TLTs may help families feel that all options have been explored and the burden of the decision is not theirs alone.
Both the family and medical team are happy with (the TLT) and both parties sort of feel like it was an agreed on trial…it would provide the family with a sense of assurance that they ‘did everything’ and no rock was left unturned. And then if the patient did transition over to a more palliative approach that they could do so without this fear or lingering thought in the back of their mind of what is right.
Intensivists had differing opinions as to who on the healthcare team should initiate the discussion of a TLT with the patient or family member. Some felt only the attending intensivist should have these conversations while others felt that anyone on the healthcare team could initiate these conversations. However, the intensivists interviewed felt that the patients’ goals, values, and wishes should ideally be elicited and documented before a patient is admitted to an ICU.
Realistically the elicitation of values probably should be happening in the outpatient setting before this [ICU admission], which, you know, is the longstanding issue with a lot of these things. But yeah, that’s one thing that is helpful, when you can see those notes in there, when it’s done.
Another intensivist went on to say that:
I think we do it far too late. Oftentimes it’s some catastrophic event where they need to be intubated or something like that. And it’s just flung on the family. And it just adds actual layer of stress and complexity to it. I think in an ideal world, these conversations would be had on the floor, preceding a transfer to the ICU.
What
Despite the lack of formal guidelines, interviewed intensivists reported knowing what TLTs were but noted that they did not always use that terminology. Many of them identified learning about TLTs through observation.
I don't know if people call them time-limited trials. I don't use that terminology very frequently, but I do frequently say we're going to meet again in 72 hours to discuss where we are and the progress…
There seemed to be general agreement that a TLTs is a way to trial life-sustaining therapies over a defined period of time and watch for clinical endpoints.
We are going to use an intervention to see if there’s any response or improvement. And it’s a way to also help frame things to the family or surrogates of the patient to help them understand what we're trying to accomplish, to think about things in terms of time-limited trials.
Intensivists felt that no change in a patient’s clinical status by the end of the agreed on time period was a poor outcome.
Life support itself does not fix the problem. It really just buys time. And we're going to reassess, typically in three days. I will also say that like failure to improve is also a loss.
When
The timing of when and for how long a TLT should be initiated varied. However, most intensivists reported that life-sustaining care should be provided for those who prefer it for the first 48 hours on admission to the ICU.
I'm going to give them everything I've got for 48 hours before I even consider anything. And then once that 48 hours is up, then I'm going to start thinking about a time-limited trial.
Another intensivist explained:
I try to do 48 hours of aggressive critical care and then reevaluate at that point. Obviously if things change dramatically in those first 48 hours that will affect it. But I don't really start a time-limited trial as soon as they are admitted to the ICU. I think there’s too many factors to go into creating a time-limited trial. Unless the patient was imminently suffering, or the patient had and totally uncurable reason for being in the ICU.
Additionally, the staffing models of the ICU were not a barrier to TLT use. Intensivists were cognizant that when leaving a TLT in place when coming off of rotation, the new clinical team may view the patient’s prognosis differently. They would also feel comfortable rethinking someone else’s TLT.
It’s just my own personality and a bit of skepticism with regard to, ‘Well, if it’s me, what else can I be doing differently?’ As opposed to, ‘Oh, okay, yeah, that sounds great. Let’s just kind of continue that.’ I don't think or feel like I'm doing good patient care and adequate justice when it comes to patients if I don't kind of go through everything and [see if] there’s something else that I can do.
In fact, their clinical rotation had little to do with their decisions to initiate TLTs.
I think whenever the time [-limited trial] is appropriate, that’s when you do it…I wouldn't hesitate just because I'm about to go off of service.
Where
TLTs were often discussed among the healthcare team informally during morning rounds where opinions from nurses and respiratory therapists could be elicited. However, TLTs were formalised in family meetings when the healthcare team and specifically the intensivists met with surrogate decision makers to discuss prognosis, timing of the TLT, and clinical milestones.
I think a majority of the time they might happen in a family meeting, where we formalize it and we say this is what you see as the clinicians. What do you see as the family, what’s important for the patients and what are we going to do going forward and how long are we going to try it for?
Why
Intensivists reported two primary reasons for implementing: to provide time to reduce clinical uncertainty and to guide decision-making to ensure that care provided aligns with the patients’ values and goals.
It’s very easy in this specialty, especially when your resident hasn’t even started presenting [during rounds] and it’s a paragraph worth of past medical history and you're like, “What are we doing?” … Just think about what does the patient, himself or herself, find most valuable. And see if you can get them there while maintaining dignity.
For intensivists, TLTs can be used to balance the need for additional time to understand the patient’s trajectory with the need to avoid keeping patients in the ICU without a clear clinical benefit while also practising being a good steward of ICU resources.
It again gives families time to process. It gives some sort of timetable for medical improvement to show itself and, you know, then it also provides at least some sort of kind of finality or decision point. So in situations where …things are never going to be better, it does probably shorten overall stays, and I would imagine potentially with less conflict.
We organised our barriers and facilitators of TLT use in the ICU using the TICD.
Barriers
In concordance with the quantitative findings, interviewees disagreed on when to use TLTs and why. The intensivists emphasised the lack of empirical evidence; documentation issues and lack of visible place for a TLT in the electronic medical record; consultants who disagree with the ICU team and provide families mixed messages; and a lack of established/measurable clinical endpoints as other factors that make it challenging to initiate and maintain TLTs.
Facilitators
Interviewees felt that a TLT checklist would be helpful but were not sure what elements that checklist might contain. Quality communication and information sharing during handoffs were also viewed as necessary when TLTs were incomplete at the end of their service rotation. Lastly, TLTs were easier to establish and maintain when the patient had clear directives (eg, ‘I do not want to be mechanically ventilated for more than 3 days.’) and had clearly communicated his or her values and preferences with their surrogate decision makers.
Discussion
Key findings
Among intensivists from two academic medicine centres, nearly 40% of intensivists never heard of a TLT, but of the intensivists who had heard of a TLT, more than half reported having participated in a TLT. This finding contrasted with the interviews, in which intensivists understood the concept of a TLT but did not always use the term ‘TLT’. Variation in physician decision making exists in deciding when, why and for how long a TLT should continue.
Relationship to previous studies
Previous work on TLTs highlighted how infrequently physicians discuss TLTs with surrogate decision-makers, especially in patients at high risk of dying.4 9 16 17 More recently, Chang et al implemented a TLT in a broader cohort of patients—patients in which providers felt ICU treatment would be non-beneficial, but patients or surrogate decision makers were in favour of pursuing ICU care—and demonstrated that TLTS could be used to mitigate conflicts among the healthcare providers and surrogate decision-makers.9 Despite the increasing attention on TLTs In the ICU, it is unknown why intensivists consider them in practice. Our work begins to explore why intensivists use TLTs with patients and surrogate decision-makers. One theme that emerged is that TLTs should not be used to prolong death. Additionally, intensivists were uncertain of the utility of TLTs because of the lack of evidence-based studies demonstrating improved patient and surrogate decision maker outcomes with the use of TLTs.
The optimal duration for a TLT has been the focus of prior work. In their editorial, Quill and Holloway had suggested various durations for TLTs, but these suggestions were not evidenced based.10 A more recent study on patients with solid tumours admitted to the ICU suggested a TLT of 1–4 days may be appropriate.18 Our work extends this work by showing that physician opinions on the preferred duration of a TLT varied within and across all ICU interventions.
Our work also sheds more light on the timing of when a TLT should be implemented. We found that intensivists varied regarding when they would consider initiating a TLT. Some intensivists felt that a TLT should never be considered on ICU admission, while others felt TLTs should be considered on ICU admission. A common factor which all intensivists considered in their decision making when considering a TLT was the need to ensure proper ICU utilisation. There was a strong acknowledgement on the constraints of ICU resources and need to ensure that the ICU was being used in an optimal manner. The intensivists’ decision-making centres on providing individualised care for patients and their families by balancing the ethical principles of medicine (autonomy, respect and beneficence) against the need for ensuring the ICU remains available for other patients who may benefit from ICU care. TLTs are aligned with the three ethical principles by strategically using a specified amount of time to reduce clinical uncertainty. This ideally results in an alignment of medical management with the patients’ values and goals and reducing non-beneficial ICU care.
Study implications
Despite professional organisations calling for physicians to use shared decision making in the ICU, our work demonstrates there are significant barriers that need to be addressed before TLTs can be fully implemented.19 The lack of clear evidence to support the use of TLTs makes providing desired guidelines challenging. Without this clear evidence, physicians may not engage in these conversations with surrogate-decision makers.20 Additionally, physicians in states without clear legislative guidance on treatment futility may not feel comfortable implementing TLTs for fear of legal ramifications.21 22
As evidenced in both the quantitative and qualitative findings, there is the possibility that different intensivists may have different views on purpose of the TLT and what clinical factors should be considered to define a successful TLT, thereby undermining the TLT altogether.23 This would be particularly relevant for patients who are handed off to other intensivists or when consultants have differing views. In the era of frequent patient handoffs, breakdowns in communication may hinder the routine implementation of TLTs.
Strengths and limitations
Our study has several strengths. To our knowledge, this is the first study to specifically probe intensivist knowledge and understanding of TLTs using clinical vignettes. Additionally, by using an evidenced-based implementation framework, the TICD, we identified ICU- and physician-level barriers and facilitators to the use of TLTs in the ICU. Lastly, we included a wide variety of intensivists (fellows to senior faculty) from two different academic medical centres in our surveys. Our study also has a few notable limitations. First, we only included intensivists from two academic medical centres, and their practices may not generalise to other hospital and clinical settings. Additionally, invitations to participate in the study were sent to potential participants only once. Second, the intensivists who participated were predominately white men which may limit generalisability. Third, intensivists who participated in the semi-structured interviews were from one academic medical centre because of the limitations placed on clinical research during the coronavirus pandemic. However, we did receive a wide variety of responses and achieved thematic saturation. Fourth, the interview transcripts were coded predominantly by one researcher. However, these codes were used for a matrix analysis that allowed three authors to review the themes and the quotes from the transcripts. Fifth, a survey was used to assess intensivist views on TLTs which may lead to certain forms of survey bias. Lastly, this research was initiated prior to the coronavirus pandemic and how the pandemic has influenced the implementation and utilisation of TLTs in the setting of resource constraints is unknown.
Conclusion
TLTs are generally acceptable but infrequently used in practice. To increase the use of TLTs in the ICU, clinicians desire a more robust, evidence-based framework on how to conduct TLTs.
Supplementary Material
Acknowledgments
We would like to acknowledge the contributions to data analysis from Lewis Miles.
Footnotes
Twitter: @L_VigliantiMD
Contributors: EMV and JNE designed the study, performed the statistical analyses, interpreted the results and compiled the manuscript. CAN provided critical revisions for the manuscript. TI and JK provided critical revisions of the manuscript. TV refined the analyses, assisted in interpreting the findings and provided critical revisions for the manuscript. EMV accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish.
Funding: This work was supported by grants K12 HL138039 (EMV, JNE, TI), K23 HL157364 (EMV), K23 HL140165 (TV), K23 HL148498 (CAN) and K23 HL146890 (JK) from the National Heart, Lung and Blood Institute at the NIH and R01 HS 028038 (TV) from the Agency for Healthcare Research and Quality.
Competing interests: CAN receives consulting fees from Boehringer Ingelheim. None of the other authors have conflicts of interests to disclose. This work does not represent the official views of the US Government or Department of Veterans Affairs.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Requests for deidentified and transcribed data would be evaluated.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was deemed exempt by the institutional review board of the University of Michigan Medical School (No: HUM00169627).
References
- 1.VanKerkhoff TD, Viglianti EM, Detsky ME, et al. Time-Limited trials in the intensive care unit to promote Goal-Concordant patient care. Clin Pulm Med 2019;26:141–5. 10.1097/CPM.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruser JM, Cox CE, Schwarze ML. Clinical momentum in the intensive care unit. A latent contributor to unwanted care. Ann Am Thorac Soc 2017;14:426–31. 10.1513/AnnalsATS.201611-931OI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med 2017;43:1847–9. 10.1007/s00134-017-4873-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenker Y, Tiver GA, Hong SY, et al. Discussion of treatment trials in intensive care. J Crit Care 2013;28:862–9. 10.1016/j.jcrc.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheunemann LP, Cunningham TV, Arnold RM, et al. How clinicians discuss critically ill patients' preferences and values with surrogates: an empirical analysis. Crit Care Med 2015;43:757–64. 10.1097/CCM.0000000000000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White DB, Curtis JR. Care near the end-of-life in critically ill patients: a North American perspective. Curr Opin Crit Care 2005;11:610–5. 10.1097/01.ccx.0000184301.76007.70 [DOI] [PubMed] [Google Scholar]
- 7.Azoulay E, Pochard F, Kentish-Barnes N, et al. Risk of post-traumatic stress symptoms in family members of intensive care unit patients. Am J Respir Crit Care Med 2005;171:987–94. 10.1164/rccm.200409-1295OC [DOI] [PubMed] [Google Scholar]
- 8.St Ledger U, Reid J, Begley A, et al. Moral distress in end-of-life decisions: a qualitative study of intensive care physicians. J Crit Care 2021;62:185–9. 10.1016/j.jcrc.2020.12.019 [DOI] [PubMed] [Google Scholar]
- 9.Chang DW, Neville TH, Parrish J, et al. Evaluation of time-limited trials among critically ill patients with advanced medical illnesses and reduction of Nonbeneficial ICU treatments. JAMA Intern Med 2021;181:786. 10.1001/jamainternmed.2021.1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quill TE, Holloway R. Time-Limited trials near the end of life. JAMA 2011;306:1483–4. 10.1001/jama.2011.1413 [DOI] [PubMed] [Google Scholar]
- 11.Dzau VJ, McClellan MB, McGinnis JM, et al. Vital directions for health and health care: priorities from a national Academy of medicine initiative. JAMA 2017;317:1461–70. 10.1001/jama.2017.1964 [DOI] [PubMed] [Google Scholar]
- 12.Ivankova NV, Creswell JW, Stick SL. Using mixed-methods sequential explanatory design: from theory to practice. Field methods 2006;18:3–20. 10.1177/1525822X05282260 [DOI] [Google Scholar]
- 13.Wensing M, Oxman A, Baker R, et al. Tailored implementation for chronic diseases (TICD): a project protocol. Implement Sci 2011;6:103. 10.1186/1748-5908-6-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flottorp SA, Oxman AD, Krause J, et al. A checklist for identifying determinants of practice: a systematic review and synthesis of frameworks and taxonomies of factors that prevent or enable improvements in healthcare professional practice. Implement Sci 2013;8:35. 10.1186/1748-5908-8-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braun V, Clarke V, Hayfield N. Handbook of research methods in health social sciences. Singapore: Springer, 2019. [Google Scholar]
- 16.White DB, Engelberg RA, Wenrich MD, et al. Prognostication during physician-family discussions about limiting life support in intensive care units. Crit Care Med 2007;35:442–8. 10.1097/01.CCM.0000254723.28270.14 [DOI] [PubMed] [Google Scholar]
- 17.Cunningham TV, Scheunemann LP, Arnold RM, et al. How do clinicians prepare family members for the role of surrogate decision-maker? J Med Ethics 2018;44:21–6. 10.1136/medethics-2016-103808 [DOI] [PubMed] [Google Scholar]
- 18.Shrime MG, Ferket BS, Scott DJ, et al. Time-Limited trials of intensive care for critically ill patients with cancer: how long is long enough? JAMA Oncol 2016;2:76–83. 10.1001/jamaoncol.2015.3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kon AA, Davidson JE, Morrison W, et al. Shared decision making in ICUs: an American College of critical care medicine and American thoracic Society policy statement. Crit Care Med 2016;44:188–201. 10.1097/CCM.0000000000001396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White DB, Engelberg RA, Wenrich MD, et al. The language of prognostication in intensive care units. Med Decis Making 2010;30:76–83. 10.1177/0272989X08317012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantor MD, Braddock CH, Derse AR, et al. Do-Not-Resuscitate orders and medical futility. Arch Intern Med 2003;163:2689–94. 10.1001/archinte.163.22.2689 [DOI] [PubMed] [Google Scholar]
- 22.McCabe MS, Storm C. When doctors and patients disagree about medical futility. J Oncol Pract 2008;4:207–9. 10.1200/JOP.0848503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenker Y, Tiver GA, Hong SY, et al. Association between physicians' beliefs and the option of comfort care for critically ill patients. Intensive Care Med 2012;38:1607–15. 10.1007/s00134-012-2671-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-059325supp001.pdf (196.3KB, pdf)
Data Availability Statement
Data are available on reasonable request. Requests for deidentified and transcribed data would be evaluated.