SUMMARY
Background
A phase 1 clinical trial was conducted in Mali, West Africa to assess the safety, immunogenicity and protective efficacy of a three-dose regimen of Sanaria® PfSPZ Vaccine, an attenuated, Plasmodium falciparum (Pf) sporozoite (SPZ) vaccine, administered via direct venous inoculation (DVI), against homologous controlled human malaria infection (CHMI) and natural Pf infection.
Methods
We recruited 18–50-year-old healthy, non-pregnant Malians for an open-label, dose-escalation (4·5×105, 9×105, 1·8×106 PfSPZ) pilot study (n=55) and thereafter a randomised, double-blind, placebo-controlled main trial of 1·8×106 PfSPZ or normal saline (NS) (n=120). Pilot dose-escalation and CHMI subjects enrolled on an as-available basis while main cohort participants were stratified by village and randomised (1:1) using permuted block design by the study statistician. Primary outcome was safety and tolerability and secondary outcome was vaccine efficacy (VE) against homologous PfSPZ CHMI or against naturally transmitted Pf infection. Artesunate/amodiaquine was administered to eliminate pre-existing parasitemia. Outcomes were analysed by mITT (safety, VE) and per-protocol (VE). Registration: ClinicalTrials.gov, number NCT02627456.
Findings
Adverse events and laboratory abnormalities post-vaccination in all dosing arms were few, mainly mild, and did not differ significantly between vaccine arms (all p>0·05). Unexpected, severe transaminitis, presumed due to artesunate/amodiaquine, occurred in four subjects (2 vaccinees, 2 controls). During PfSPZ CHMI, ~5 weeks after 3rd dose of 1·8×106 PfSPZ, 0/29 vaccinees and 1/15 controls became blood smear (BS)-positive; 0/29 vaccinees and 8/15 (53·3%) controls became PCR-positive (VE 1, 95%CI 0.73–1; p<0·001). In the main trial, 32/55 (58·2%) vaccinees and 42/54 (77·8%) controls became BS-positive during 24-week surveillance post-vaccination. VE (1-hazard ratio) was 0·51 per-protocol (95%CI 0·20–0·70; log-rank p=0·004) and 0·39 mITT (95%CI 0·04–0·62; p=0·033); VE (1-risk ratio) was 0·24 per-protocol (95%CI 0·02–0·41; p=0·031) and 0·22 mITT (95%CI 0·01–0·39; p=0·041). Antibody and Vδ2 T-cell responses were significantly higher in PfSPZ Vaccinees who remained uninfected.
Interpretation
A three-dose regimen of PfSPZ Vaccine was safe, well-tolerated, and conferred 51% VE against intense natural Pf transmission, similar to 52% VE reported for the five-dose regimen.
INTRODUCTION
The World Health Organization reported 229 million malaria cases and 409,000 deaths in 2019, with no meaningful reductions in case numbers since 2013 or deaths since 2016.1 A highly effective vaccine is urgently needed to stem resurgences, break the stalemate in progress, and ultimately, eliminate P. falciparum from highly endemic areas.
PfSPZ Vaccine is a malaria vaccine candidate, consisting of radiation-attenuated, cryopreserved whole P. falciparum (Pf) sporozoites (SPZ) that are metabolically active, motile, able to invade hepatocytes, but non-replicating and unable to progress to blood-stage infection. Attenuated sporozoites are thought to protect by eliciting CD8 T-cell responses targeting infected hepatocytes.2 Preventing blood-stage infection will prevent disease as well as onward transmission to support malaria elimination.
In our previous trial in Mali where Pf transmission is seasonally intense, five doses of 2·7×105 PfSPZ of PfSPZ Vaccine achieved vaccine efficacy (VE) of 52% by time-to-infection (1-hazard ratio) over the 24-week transmission season.3 However, three doses or fewer would facilitate vaccine implementation, particularly in mass vaccination programs for malaria elimination.4 In this trial, in the same village, we assessed three doses of 1·8×106 PfSPZ, increasing total dose 4-fold from 1·35×106 to 5·4×106 PfSPZ. The primary objective assessed safety and tolerability and the secondary assessed VE against naturally transmitted Pf infection in healthy Malian adults.
METHODS
Study design and participants
We conducted a two-part trial: first, an open-label pilot study of safety of two dose-escalations (4·5×105 to 9×105 to 1·8×106 PfSPZ) and VE of 1·8×106 PfSPZ against homologous (NF54) controlled human malaria infection (CHMI); then, a randomised, double-blind, placebo-controlled safety and efficacy trial (1·8×106 PfSPZ versus normal saline), where participants were followed for incident malaria infections by thick blood smear (BS) during the ensuing rainy season [Supplementary Appendix (SA), Figure S1, S2; page 18–19].
The trial involved a single centre in Donéguébougou, Mali, a rural community about 30km north of Bamako, Mali. Malaria transmission occurs from ~July-December.5 The trial was conducted according to Good Clinical Practices and ICH and institutional procedures and guidelines. The study was approved by the ethics review board in Mali (Faculté de Médecine de Pharmacie et d’OdontoStomatologie [FMPOS], Bamako, Mali), the US [National Institute of Allergy and Infectious Diseases (NIAID], National Institutes of Health [NIH], Bethesda, MD, USA) institutional review board, and the Mali national regulatory authority. NIAID was the study clinical sponsor and Sanaria Inc. was the IND sponsor, under a US Food and Drug Administration (FDA) IND allowance.
Participants
Eligible participants were healthy adult (18–50 years) non-pregnant women who used contraception during vaccination phase, or men who resided in Donéguébougou, Mali and surrounding villages (Banambani, Toubana, Torodo, Sirababougou, Zorokoro). Each participating village provided community permission; all participants provided individual written informed consent.6 Exclusion criteria included known allergies or contraindications to PfSPZ Vaccine or artesunate/amodiaquine (ASAQ), malaria vaccine within 5 years, abnormal laboratory findings, recent antimalarial medications, immunosuppressive medications, or blood products, a history of serious chronic illness, clinically significant electrocardiogram abnormalities, positive test for HIV, hepatitis B, hepatitis C, or known sickle cell disease (see full enrolment, SA, pages 6–8).
Randomisation and masking
For safety, we conducted the trial in a stepwise manner with two cohorts: the pilot dose-escalation open-label safety and CHMI cohort, and the follow-on main cohort.
Pilot dose-escalation subjects enrolled on an as-available basis. Main cohort participants were stratified by village (6) and randomised in double-blind manner using permuted block design. Participants were assigned (1:1) to three doses of 1·8×106 PfSPZ Vaccine or normal saline placebo. Randomisation code was provided directly by study statistician to site pharmacist via secure email before vaccinations started. Investigational product was labelled by the pharmacist with participant’s study identification number. PfSPZ Vaccine and placebo were clear, odorless, non-viscous solutions and could not be distinguished. Group assignments were unmasked at final study visit 24 weeks after third vaccination.
Procedures
PfSPZ Vaccine contains aseptic, purified, vialed, cryopreserved PfSPZ manufactured as described previously.7–9 Within 30 minutes of thawing, 0·5mL of PfSPZ Vaccine or PfSPZ Challenge or placebo [sterile isotonic normal saline (Hospira, Lake Forest, IL, USA)] was injected into an arm vein by DVI through 25-gauge needle over several seconds.
For safety, the pilot study (Figure 1) enrolled in a staggered manner the 3 PfSPZ Vaccine arms: one dose of 4·5×105 (January 2016), one dose of 9×105 (January 2016), 3 doses of 1·8×106 (January-May 2016) at ~8-week intervals. 1·8×106 PfSPZ Vaccine arm was randomised 1:1 prior to first vaccination to receive ASAQ prior to each vaccine dose or only prior to dose 3/CHMI alongside CHMI infectivity controls.
Figure 1. Trial profile.
Study completion was defined as staying in the study until the end of malaria transmission follow-up (study day 85 for 4·5×105 (n=5) or 9×105 (n=5) PfSPZ Vaccine; study day 30 for CHMI controls; study day 357 for Pilot 1·8×106 PfSPZ Vaccine; study day 281 for main 1·8×106 PfSPZ Vaccine and placebo). (A) One normal saline subject did not receive ASAQ dose between vaccine dose #2 and #3 given they were recently treated for malaria with artemether-lumefantrine. PfSPZ=Plasmodium falciparum sporozoite
Pilot 1·8×106 PfSPZ Vaccine arm underwent homologous CHMI ~5 weeks post-dose 3 (June-July 2016). CHMI infectivity controls enrolled April 2016 (Figure 1) and both PfSPZ Vaccine and controls were treated with ASAQ ~7 weeks before CHMI. All CHMI participants were followed for patent parasitemia 4 weeks post-challenge and treated with artemether/lumefantrine for parasitemia or at end of follow-up.
The main trial (March-August 2016) randomized participants to three doses of 1·8×106 PfSPZ or normal saline placebo at 1, 13, 19 weeks week intervals (Figure 1). The 1·8×106 PfSPZ pilot cohort received a fourth dose (13 weeks post-dose 3) contemporaneous with main cohort dose 3.
After each vaccination, participants were monitored at least 30 minutes for local and systemic AEs. Participants were assessed on-site immediately and then 3, 7, 14, 28, 42, 56 days post-vaccination, and study clinicians were always available for unscheduled visits. Solicited local and systemic AEs were recorded for seven days post-vaccination (SA, Table S1, page 21). Unsolicited AEs, including symptomatic malaria, serious AEs (SAEs), and new chronic conditions were recorded throughout follow-up. Protocol-specified laboratory assessments before and 3 and 7 days after each vaccination included complete blood count with differential, creatinine, and alanine aminotransferase. AE grading was based on FDA guidelines for vaccine trials10 adapted to local normal reference ranges (SA, Table S2, S3, pages 22–23).
We deemed participants to be enrolled upon ASAQ (Denk Pharma; Munich, Germany) treatment 1–2 weeks before first vaccination. Two tablets (100mg artesunate/270mg amodiaquine each) were given twice daily for three days (six doses total). All pilot 1.8×106 PfSPZ vaccinees and main trial participants received ASAQ again ~2 weeks before third vaccination.
We assessed co-infections before first vaccination. Gastrointestinal helminths/protozoa were detected in stool by modified qPCR11 at Laboratory of Parasitic Diseases, NIAID/NIH. Schistosoma haematobium eggs were quantified microscopically in fresh urine post-filtration and staining with 5% ninhydrin at College of American Pathologists (CAP)-certified Malaria Research and Training Centre (MRTC) clinical laboratory.3,11
BS were prepared before and multiple days after each vaccination, and during suspected malaria illness (SA, page 9). BS were examined by certified readers. Symptomatic malaria was defined as any Pf asexual parasitemia accompanied by temperature ≥37·5°C, clinical signs/symptoms of malaria, or both. Standard treatment with artemether/lumefantrine was provided for symptomatic malaria, but not asymptomatic parasitemia per Malian Ministry of Health guidelines (except during CHMI).
CHMI started 5 weeks post-dose 3 by inoculation of 3·2×103 PfSPZ of non-attenuated PfSPZ Challenge (NF54), the same parasite strain as PfSPZ Vaccine. BS were collected day 3, daily days 6–21, alternating days 23–27, and when clinically indicated. Paired qPCR samples were collected with each BS but assayed retrospectively.
For main trial follow-up, BS began 3, 7 and 14 days after third vaccination, then continued every two weeks for 11 additional scheduled assessments, and when clinically indicated, ending after 24 weeks.
Serum antibodies were measured by enzyme-linked immunosorbent assay (ELISA) to the major sporozoite surface protein (Pf circumsporozoite protein [PfCSP]), by automated immunofluorescence assay (aIFA) to air-dried PfSPZ, and by inhibition of sporozoite (PfSPZ) invasion (aISI) of HC-04 cells (hepatocytes) as previously described.12 PfCSP ELISA seroconversion was defined by net optical density (OD) 1·0 and OD 1·0 ratio, calculated by subtracting or dividing by (respectively) the pre-vaccination antibody OD 1·0, of ≥50 and ≥3·0, respectively.12 For aIFA, volunteers with net arbitrary fluorescence unit (AFU) 2·0×105 of ≥150 and a ratio of post- to pre- AFU 2·0×105 of ≥3·0 were considered to have responded positively. In the aISI, volunteers with a net ISI activity of ≥10% and ratio of post to pre-ISI activity of ≥3·0 were considered to have developed ISI activity.
We assessed T-cell responses using multi-parameter flow cytometry on fresh whole blood (details in SA, page 10; Table S13). Ex vivo measures were taken before and at 3, 7, 42, and 55 days after each vaccination, and every 4 weeks during follow-up.
Outcomes
The primary outcome was safety and tolerability of at least one vaccine dose, a modified intention to treat (mITT) analysis assessed as incidence and severity of local and systemic AEs occurring within 7 days of each vaccination and SAEs related to vaccination. Secondary outcomes were VE against CHMI (pilot study) measured by BS, and in main trial VE by time-to-infection analysis (1-hazard ratio) and by binary analysis (1-risk ratio) against naturally occurring Pf infection by BS (defined as at least two parasites identified by microscopic examination of 0·5μL blood). VE against symptomatic malaria and CHMI VE by qPCR were exploratory outcomes. CHMI VE was defined as 1-(proportion of infection under vaccine/proportion of infection under control). Humoral and cellular immune responses (described in SA, page 10), were exploratory endpoints. Planned outcomes were not changed during the trial, except for additional analysis of CHMI outcomes detailed below.
Statistical analysis
All participants who received at least one dose of investigational product (PfSPZ Vaccine or placebo) were included in safety analyses, including the pilot safety cohort.
For the pilot study, the pre-specified secondary outcome was time to first positive BS after CHMI, but as only 1 infectivity control was BS positive, time to first positive qPCR was also analysed with R version 3.3.1.
For the main cohort, 60 subjects/arm (PfSPZ Vaccine, placebo) provided 0·8 probability of observing serious or severe AEs that occurred with probability of 0·026/volunteer. With background malaria infection rate varying from 40% to 90%, we expected to detect a time-to-infection VE of at least 50% with 59–100% power (2-sided 0·05 conditional test) (SA, Table S8, page 32).
All randomised main cohort subjects were included or accounted for in mITT analysis whereas only those who received all three vaccinations were included in per-protocol analysis. The primary efficacy endpoint was time-to-first infection, with VE defined as 1-hazard ratio (HR). Imputation for missed visits for mITT and per-protocol analyses is described in more detail (see SA, page 12).
VE was assessed two ways: 1) Time-to-event analysis, in which significance was assessed by logrank test for interval-censored data. VE was evaluated from a parametric proportional hazards model assuming baseline hazard function as Weibull and allowing for interval censoring and stratification by village. 2) Proportion of subjects with at least one positive blood smear (binary endpoint) in each arm by Exact Cochran-Mantel-Haenszel test stratifying on village.
Participants that were censored before 160 days post-last vaccination were removed from the proportional analysis on efficacy, as pre-specified in the protocol.
For PfCSP antibody measurements, we analysed differences between vaccinees and controls, or between uninfected and infected vaccinees, using 2-tailed Barnard’s Test or Fisher’s Exact Test for seroconversion rates, and Wilcoxon Rank-Sum Test for net OD and OD ratios. We analysed changes in T-cell levels between the PfSPZ and placebo groups and by infection outcome within groups. T-cell levels or fold-changes were compared between groups at specific timepoints using Wilcoxon Rank-Sum Test. Repeated measures analysis was performed by fitting linear models using Generalized Estimating Equations (GEE) to assess differences between using log10 transformed fold change values as the outcome variable. For comparisons between vaccine groups study arm, day and an interaction term, study arm*day, were used as the predictor variables. For comparisons between infected and uninfected participants within vaccine groups, study day and infection outcome were used as the predictor variables. Age and sex of study participants were used as confounding variables in the full models, neither of which were significant. A gaussian (normal) distribution, identity link function and an autocorrelation covariance structure were specified. A robust (sandwich) variance estimator was applied to all models. Further details in the SA (SA, pages 13–14).
The study was monitored for safety by an independent Data and Safety Monitoring Board and a local medical monitor. This trial is registered at ClinicalTrials.gov, number NCT02627456.
Role of the funding source
The funders were involved in study design and management; data collection, analysis, and interpretation; and report writing. All authors had full access to all study data and had final responsibility for the decision to submit for publication.
RESULTS
Participants (pilot and main) were screened and enrolled from December 2015 to April 2016. 56 people enrolled into the pilot cohort with 40 receiving one dose of either 4·5×105 (n=5) or 9×105 (n=5) or three doses at 8-week intervals of 1·8×106 PfSPZ (n=30) of PfSPZ Vaccine (before the start of main cohort) (Figure 1). Fifteen subjects enrolled as CHMI controls received ASAQ, along with twenty-nine 1·8×106 PfSPZ vaccinees, 7 weeks before CHMI. Twenty-seven 1·8×106 PfSPZ vaccinees opted to receive a fourth dose (Figure 1).
120 participants enrolled into the main cohort and underwent randomisation: 60 received PfSPZ Vaccine and 60 placebo. First main cohort vaccinations occurred 1–8 April 2016, last vaccinations 4–13 August 2016 (after onset of malaria transmission season), and final study visits 19–26 January 2017. Main cohort vaccinations were originally scheduled for weeks 1, 9, 17 but were completed at weeks 1, 13, 19 weeks, with second and third vaccinations delayed due to an investigation of an out-of-specification phosphate buffered saline stability test result that was later determined to be invalid allowing resumption of immunization. All 120 participants received at least one vaccination and are included in the safety analyses. 112 participants (57 in the vaccine group and 55 in the placebo group) received all three vaccinations, of which 109 (55 in the vaccine group and 54 in the placebo group) completed follow-up through to last study visit (Figure 1). Baseline characteristics were well-balanced between vaccine and placebo groups (Table 1).
Table 1.
Baseline demographics of participants who received at least one vaccination, pilot and main cohorts and parasitaemia characteristics by blood smear.
| Pilot Safety Cohort + CHMI n=55 | Main Cohort n=120 | Total n=175 | |||||
|---|---|---|---|---|---|---|---|
| PfSPZ Vaccine | CHMI | PfSPZ Vaccine 1·8×106 (n=60) | Placebo (Normal Saline) (n=60) | ||||
| 4·5×105 (n=5) | 9×105 (n=5) | 1·8×106 (n=30) | Infectivity Controls (n=15) | ||||
| Sex | |||||||
| Female | 3 (60%) | 1 (20%) | 11 (36·7%) | 3 (20%) | 15 (25%) | 22 (36·7%) | 55 (31·4%) |
| Male | 2 (40%) | 4 (80%) | 19 (63·3%) | 12 (80%) | 45 (75%) | 38 (63·3%) | 120 (68·6%) |
| Age (years) 1 | |||||||
| Mean ± SD | 35.77 ± 8.93 | 34.26 ± 10.10 | 36.66 ± 9.02 | 30.05 ± 10.30 | 32.21 ± 8.85 | 32.27 ± 9.56 | 33 ± 9·4 |
| Min, Max | 22.57, 44.57 | 21.98, 49.60 | 18.16, 49.58 | 18.33, 49.88 | 8.24, 48.76 | 18.21, 47.78 | 18, 49 |
| Weight (kg) 2 | |||||||
| Mean ± SD | 54·4 ± 2·1 | 53 ± 1·9 | 61·4 ± 7·5 | 61 ± 10·6 | 62 ± 9·4 | 61·6 ± 8·9 | 61·2 ± 8·9 |
| Min, Max | 53, 58 | 51, 55 | 51, 77 | 46, 80 | 46, 99 | 46, 93 | 46, 99 |
| Village | |||||||
| Doneguebougou | 5 (100%) | 5 (100%) | 30 (100%) | 10 (66·7%) | 27 (45%) | 28 (46·7%) | 105 (60%) |
| Toubana | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13·3%) | 12 (20%) | 13 (21·7%) | 27 (15·4%) |
| Banambani | 0 (0%) | 0 (0%) | 0 (0%) | 2 (13·3%) | 8 (13·3%) | 8 (13·3%) | 18 (10·3%) |
| Torodo | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (11·7%) | 6 (10%) | 13 (7·4%) |
| Zorokoro | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6·7%) | 3 (5%) | 2 (3·3%) | 6 (3·4%) |
| Sirababougou | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (5%) | 3 (5%) | 6 (3·4%) |
| Hemoglobin typing 3 | |||||||
| HgbAA | 2 (40%) | 5 (100%) | 23 (76·7%) | 7 (46·7%) | 37 (61·7%) | 39 (65%) | 113 (64·6%) |
| HgbAS | 1 (20%) | 0 (0%) | 2 (6·7%) | 1 (6·7%) | 3 (5%) | 4 (6·7%) | 11 (6·3%) |
| HgbSS | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| HgbAC | 1 (20%) | 0 (0%) | 4 (13·3%) | 3 (20%) | 11 (18·3%) | 8 (13·3%) | 27 (15·4%) |
| HgbCC | 1 (20%) | 0 (0%) | 1 (3·3%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (1·1%) |
| Unknown | 0 (0%) | 0 (0%) | 0 (0%) | 4 (26·7%) | 9 (15%) | 9 (15%) | 22 (12·6%) |
| S. haematobium (urine) 4 | |||||||
| Positive | 0 (0%) | 0 (0%) | 2 (6·7%) | 4 (26·7%) | 7 (11·7%) | 5 (8·3%) | 18 (10·3%) |
| Helminth/Parasite qPCR (stool) 5 | |||||||
| Positive | 2 (40%) | 4 (80%) | 14 (46·7%) | 6 (40%) | 26 (43·3%) | 30 (50%) | 82 (46·9%) |
| Negative | 3 (60%) | 1 (20%) | 15 (50%) | 6 (40%) | 32 (53·3%) | 28 (46·7%) | 85 (48·6%) |
| NIC/Not Done | 0 (0%) | 0 (0%) | 1 (3·3%) | 3 (20%) | 2 (3·3%) | 2 (3·3%) | 8 (4·6%) |
| Pf parasitemia by blood smear 6 | |||||||
| Pre-vaccination (n=160) | 0 (0%) | 2 (40%) | 7 (23·3%) | 3 (20%) | 7 (11·7%) | 8 (13·3%) | 27 (16·9%) |
| During vaccination (n=160) | 0 (0%) | 0 (0%) | 6 (20%)7 | N/A | 1 (1·7%) | 3 (5%) | 10 (6·3%) |
| Post Vaccination #1 (n=160) | 0 (0%) | 0 (0%) | 5 (16·7%) | N/A | 0 (0%) | 0 (0%) | 5 (3·1%) |
| Post Vaccination #2 (n=146) | N/A | N/A | 4 (13·8%) | N/A | 1 (1·7%) | 3 (5·1%) | 8 (5·5%) |
| Post Vaccination #3 (n=138) | N/A | N/A | 0 (0%) | N/A | 32 (58·2%) | 42 (77·8%) | 74 (53·6%) |
| Post CHMI (n=44) | N/A | N/A | 0 (0%) | 1 (6·7%) | N/A | N/A | 1 (2·3%) |
| Post Vaccination #4 (n=27) | N/A | N/A | 18 (66·7%) | N/A | N/A | N/A | 18 (66·7%) |
Data are number of subjects (% of subjects) unless stated otherwise. PfSPZ=Plasmodium falciparum sporozoite. CHMI=controlled human malaria infection. SD=standard deviation. NIC=negative internal control, cannot rule out false negatives due to PCR inhibition.
Age is based on age at the time of enrollment (first dose of ASAQ).
Weight is based on weight measured at the time of screening.
Hemoglobin (Hgb) typing completed retrospectively.
Schistosomiasis testing completed at screening from 23 December 2015 to 18 April 2016.
Helminth testing was completed retrospectively and included testing for: Ascaris lumbricoides, Necator americanus, Ancylostoma duodenale, Giardia lamblia, Cryptosporidium spp, Entamoeba histolytica, Trichuris trichuria, Strongyloides stercoralis. Testing completed at screening from 23 December 2015 to 18 April 2016.
Post dose % are calculated based on ‘n’ at that time interval as outlined in Figure 1. Blood smear counts post dose 3 in the Main Cohort and post dose 4 in the Pilot Safety Cohort is only for the 24 week period post vaccination; per protocol proportional population is included as the ‘n’.
All positive BS during vaccination in Pilot 1·8×106 occurred in the arm randomised to no ASAQ until prior to dose 3 except for 1 participant who was BS positive post dose 2.
Of main cohort subjects who received at least one vaccination, 7/60 (11·7%) in the vaccine arm and 8/60 (13·3%) in the placebo arm were blood-smear positive for Pf at screening (Table 1). There were no significant differences between the groups in regard to helminths or protozoa in the stool, S. haematobium in the urine, or haemoglobin AA, AS, or AC (Tables 1; SA Table S15, page 48). All 120 participants completed ASAQ treatment prior to vaccination 1, with the last dose given to the vaccine group mean 9·4 days (SD 1·1) and to the placebo group mean 9·4 days (SD 1·4) before first vaccination. During vaccination, 1 (1·7%) PfSPZ vaccinee and 3 (5·1%) placebo subjects developed parasitemia, all occurring between vaccinations #2 and #3. All 114 participants remaining in the main cohort received ASAQ before their last vaccination, with last drug dose given to the vaccine group mean 7·8 days (SD 0·5) and to placebo group 7·8 days (SD 0·8) before last vaccination.
For pilot study, PfSPZ Vaccine was very well tolerated in those receiving 4·5×105 (n=5) and 9×105 (n=5) with few post vaccination AEs and no Grade 3 AEs or lab abnormalities reported. 1·8×106 PfSPZ vaccinations were also well tolerated by the majority of subjects however, one subject (randomised to ASAQ treatment prior to each vaccination) experienced an asymptomatic Grade 3 elevated ALT following Vaccination #1 (more details below and summarised in SA). Reported AEs post CHMI were few, with only one related AE (granulocyte decreased), deemed related to CHMI. Safety summaries for the pilot and CHMI cohorts are provided in more detail in the SA, Tables S16–17, pages 55–58 .
For the main trial, 349 injections were administered and were well-tolerated and safe. Most study participants reported no local or systemic AEs after vaccination (Table 2). Four (6·7%) of the 60 vaccine arm participants and five (8·3%) of the 60 placebo arm participants reported local injection site pain. Overall, three (5%) subjects in the vaccine arm and three (5%) subjects in the placebo group reported any systemic AE after vaccination (Table 2); the most common solicited systemic AE in the vaccine and placebo groups was headache (SA, Table S5, pages 25–26). Local or systemic AEs did not differ significantly between vaccine and placebo groups (all p values >0·05; Table 2; SA, Table S5, pages 25–26). Two SAEs were reported (snake bite, vaginal prolapse repair), both not related to vaccination and both occurring in placebo participants (Table 2).
Table 2.
Adverse events after vaccination.
| Main cohort n=120 | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1·8×106 PfSPZ Vaccine | Placebo (Normal Saline) | |||||||
| Dose 1 (n=60) | Dose 2 (n=58) | Dose 3 (n=57) | Total (n=60) | Dose 1 (n=60) | Dose 2 (n=59) | Dose 3 (n=55) | Total (n=60) | |
| Adverse Events (AEs) | ||||||||
| Local Reactogenicity1 | 2 (2) 3·3% | 2 (2) 3·4% | 0 (0) 0% | 4 (4) 6·7% | 1 (1) 1·7% | 4 (4) 6·8% | 1 (1) 1·8% | 6 (5) 8·3% |
| Systemic Reactogenicity | 1 (1) 1·7% | 5 (2) 3·4% | 1 (1) 1·8% | 7 (3) 5% | 3 (2) 3·3% | 1 (1) 1·7% | 0 (0) 0% | 4 (3) 5% |
| Laboratory Abnormalities2 | 1 (1) 1·7% | 5 (5) 8·6% | 4 (4) 7% | 10 (8) 13·3% | 1 (1) 1·7% | 2 (2) 3·4% | 2 (2) 3·6% | 5 (2) 3·3% |
| Related AEs3 | 4 (4) 6·7% | 11 (7) 12·1% | 5 (5) 8·8% | 20 (12) 20% | 5 (4) 6·7% | 7 (6) 10·2% | 3 (2) 3·6% | 15 (9) 15% |
| Unsolicited AEs4 | 12 (9) 15% | 19 (15) 25·9% | 61 (32) 56·1% | 92 (42) 70% | 20 (18) 30% | 30 (19) 32·2% | 66 (37) 67·3% | 116 (50) 83·3% |
| SAEs | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 1 (1) 1·7% | 0 (0) 0% | 1 (1) 1·8% | 2 (2) 3·3% |
| Symptomatic Malaria AEs 5 | ||||||||
| Total | 0 (0) 0% | 1 (1) 1·7% | 26 (24) 42·1% | 27 (25) 41·7% | 0 (0) 0% | 1 (1) 1·7% | 37 (31) 56·4% | 38 (31) 51·7% |
| Grade 1 | 0 (0) 0% | 0 (0) 0% | 19 (18) 31·6% | 19 (18) 30% | 0 (0) 0% | 1 (1) 1·7% | 24 (20) 36·4% | 25 (20) 33·3% |
| Grade 2 | 0 (0) 0% | 1 (1) 1·7% | 7 (7) 12·3% | 8 (8) 13·3% | 0 (0) 0% | 0 (0) 0% | 11 (11) 20% | 11 (11) 18·3% |
| Grade 3 | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 2 (2) 3·6% | 2 (2) 3·3% |
| Grade 4 | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% | 0 (0) 0% |
Data presented are number of AEs, (number of unique subjects), and % of unique subjects. Solicited local and systemic reactogenicity and laboratory adverse events were documented for 7 days after each vaccination and MedDRA coded and line listed below by preferred term. Unsolicited adverse events, including symptomatic malaria, serious AEs (SAEs), and new chronic medical conditions were recorded throughout the study. Each vaccine receipt is counted once at worst severity for any local and systemic parameter. Additional details regarding solicited local and systemic reactogenicity as well as laboratory adverse events are shown in the supplementary appendix (SA, Table S4, S5). Symptomatic malaria was defined as any Pf asexual parasitemia accompanied by an axillary temperature of at least 37·5°C, clinical signs and symptoms of malaria, or both. PfSPZ=Plasmodium falciparum sporozoite. AE=adverse events.
All local reactogenicity reported were injection site pain.
Laboratory abnormalities were included in this count if they occurred during the scheduled day 7 post vaccination visit and were within window for that visit (up to day 9).
Related AEs includes all AEs reported within 28 days of vaccination and determined as definitely, probably, or possibly related to vaccination; includes expected reactogencity as well as laboratory abnormalities.
Unsolicited AEs represented below does not include malaria AEs, but does include laboratory AEs occurring outside the predefined collection period post vaccination. All unsolicited AEs were determined not related to vaccination except for two transaminases increased in the placebo arm (onset 33, 39 days post dose 2, determined possibly related during the study, Grade 4 and Grade 3; further details provided in SA, Figure S3).
Though reported as malaria AEs, 2 malaria AEs are exclude for PfSPZ Vaccine given detection of P. malariae only, no Pf and 1 malaria AE is excluded in the placebo given detection of P. malariae + P. ovale only, no Pf.
Of significance, during the course of the pilot and main phase of the study, we noted multiple unanticipated significantly elevated, but asymptomatic, transaminases (Grade 3, 4) in four participants (2 PfSPZ Vaccine, 2 placebo) that occurred at varying timepoints after vaccination as well as ASAQ dosing (SA page 13; Table S6 pages 25–26; Figure S3). All four subjects were asymptomatic at presentation with no associated agranulocytosis. All laboratory abnormalities resolved without sequelae. Testing for potential other etiologies, through imaging, expanded laboratory testing, and serology, identified no other possible contributing causes, except a traditional medicine ingested by the first subject who presented with liver enzyme derangements (details in SA, page 14). The cause of elevated transaminases was judged to be most likely due to ASAQ treatment given equal involvement of PfSPZ vaccinees and controls.
Overall, laboratory abnormalities within 7 days post vaccination did not differ between the vaccine (8/60, 13·3%) and placebo (2/60, 3·3%; p=0·095, Fisher’s Exact test) arms (Table 2; SA, Table S6, pages 27–28). All laboratory abnormalities immediately post vaccination were Grade 1 except for the transaminase elevations described above.
For the pilot study CHMI, 0/29 (0%) vaccinees and 1/15 (6·7%) infectivity controls became BS positive. By qPCR 0/29 (0%) vaccinees and 8/15 (53 ·3%) infectivity controls became positive (Figure 3). By qPCR, VE was significant (p<0.001) by interval-censored log rank for time-to-infection, and was 1 (p<0·001, 95%CI 0.73–1) by proportional analysis. All 27 PfSPZ Vaccine recipients receiving a 4th dose were followed for naturally occurring infection with the main trial cohort; 18/27 (66· 7%) developed patent parasitemia over the 24-week follow up.
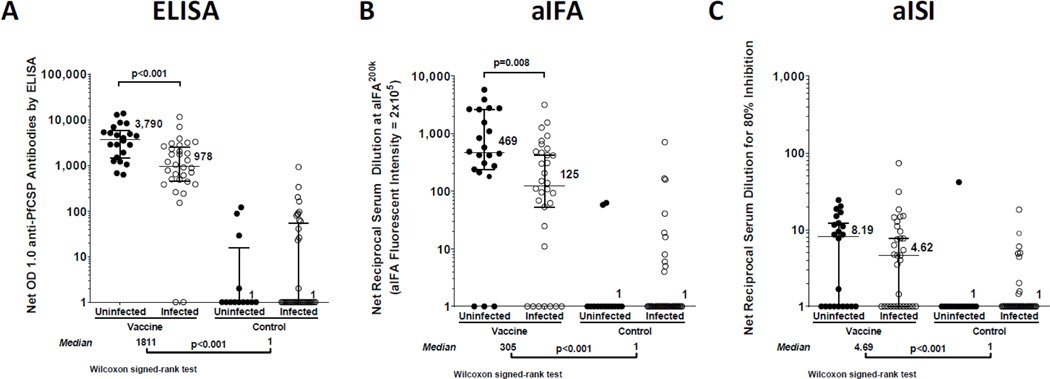
Figure 3. Antibody responses measured after vaccination.
Antibody results in Plasmodium falciparum circumsporozoite protein enzyme-linked immunosorbent assay (A), automated immunofluorescence assay (B), and automated inhibition of sporozoite invasion assay (C) or ELISA, aIFA, and aISI, respectively. Nets were obtained by subtracting pre-immune values from the values obtained from sera drawn 2 weeks after the third dose of 1·8×106 Plasmodium falciparum sporozoite. PfSPZ=Plasmodium falciparum sporozoite. PfCSP=Plasmodium falciparum circumsporozoite protein. OD=optical density.
For the main trial, VE analysis examined time to first Pf infection (TTE, [1-hazard ratio]) and occurrence of Pf infection (proportional, [1-risk ratio]) during a 24-week period up to the end of the malaria season, starting immediately after third vaccination (starting the week of 4 August 2016). Pf infection was defined as a positive BS. In the per-protocol population, follow up time was a median of 167 and mean 162.5 (CI: 156.0–169.0) days in the vaccine arm, and median of 167 and mean of 164.5 (CI:159.5, 169.6) days in the control arm. In the mITT population, follow up time was a median of 167 and mean of 162.5 (CI: 156.0–169.0) days in the vaccine arm, and median of 167 and mean of 164.6 (CI:159.7, 169.5) days in control arm.
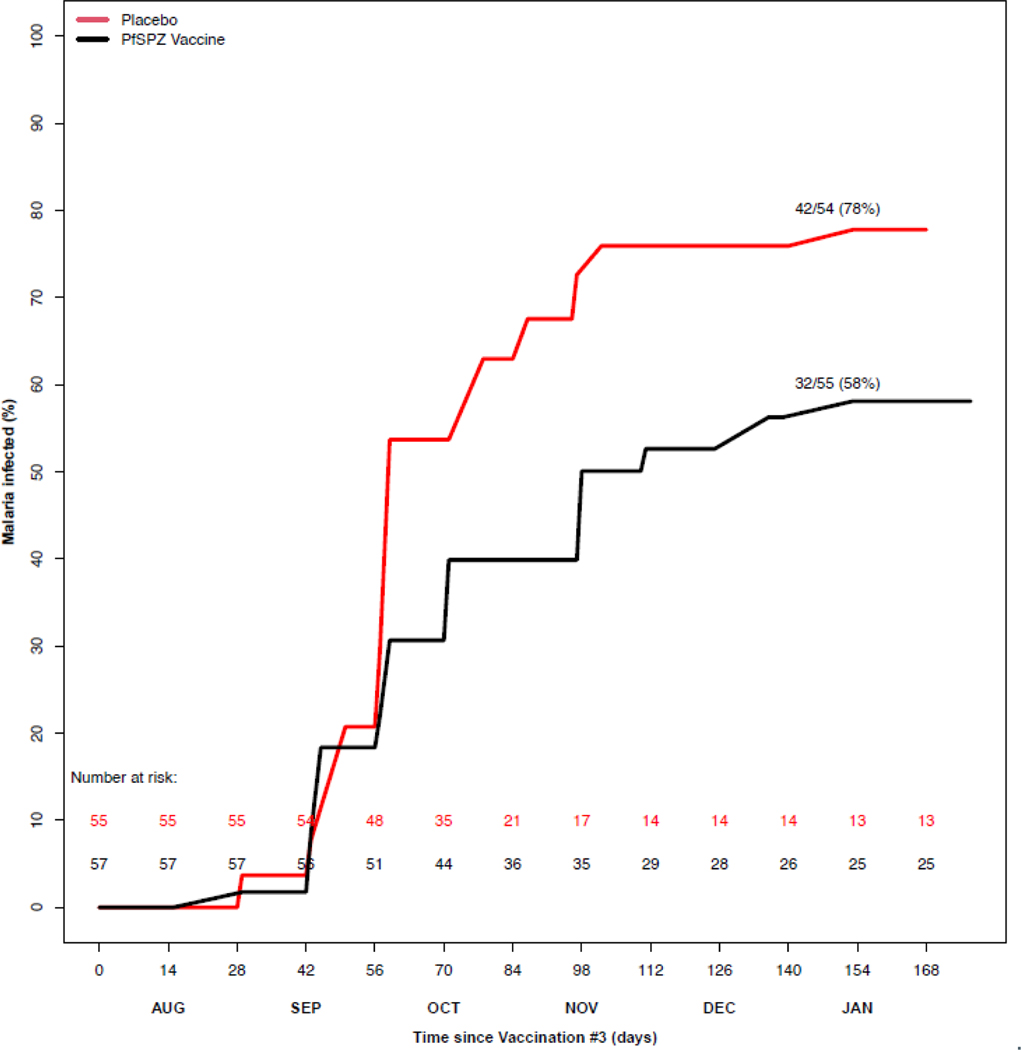
VE was assessed primarily from the per-protocol population, and efficacy is also reported for the mITT population. 112 subjects (57 vaccine; 55 placebo) had evaluable data for TTE per-protocol analysis, while 114 subjects (57 vaccine; 57 placebo) were evaluable for mITT analysis. PfSPZ Vaccine recipients had a significantly lower hazard of Pf infection, per-protocol, with VE (1- hazard ratio) of 0·51 (95%CI, 0·20–0·70; log-rank p=0·004). In mITT analyses, VE was 0·39 (95%CI, 0·04–0·62; log-rank p=0·033). We assessed the assumption of proportional hazards via Schoenfeld residuals, which indicated no violation of the proportional hazards assumption, with p values of 0·26 and 0·23, respectively, in the per-protocol and mITT populations (SA, Figure S7, page 54).
In VE proportional analysis, 109 subjects (55 vaccine; 54 placebo) were evaluable per-protocol, and 111 subjects (55 vaccine; 56 placebo) by mITT (Figure 2; SA, Table S10, page 36). In the placebo group, 42/54 (77·8%) participants became blood-smear positive versus 32/55 (58·2%) in the vaccine arm. VE (1-risk ratio) based on the binary outcome of infected or not during the season, using an exact Cochran-Mantel-Haenszel test stratified on village, was 0·24 (95%CI 0·02– 0·41; p=0·031) per-protocol, and 0·22 (95%CI, 0·01–0·39; log-rank p=0·041) by mITT analysis (SA, Table S10, page 36).
Figure 2. Protective efficacy of PfSPZ Vaccine against naturally occurring infection.
Protective efficacy was analysed by time to first positive blood smear, with day 0 starting immediately post receipt of third vaccination. The inverse survival curves include participants who received all three vaccinations and were evaluable for the primary efficacy endpoint (secondary trial outcome). PfSPZ=Plasmodium falciparum sporozoite.
In a proportional analysis that included all subjects, an empirical likelihood estimation was applied to the data (including censored subjects) to estimate the proportion of infection by the end of the 24-week follow up. For the per-protocol population, the proportion of infection was 0·76 [95%CI: 0·64–0·86] in the control arm, and 0·56 [95%CI:0·43–0·69] in the vaccine arm. The estimate of VE was 26%, slightly higher than the 24% based on proportional analysis that removed censored subjects.
Among 112 main cohort participants who received all three vaccinations, 25/57 (41·7%) in the vaccine arm and 31/55 (56·4%) in the placebo arm were treated for symptomatic malaria at least once (per-protocol TTE VE 0·23, 95%CI −0·30–0·54, p=0·33) (SA, Table S10, page 36). Median density of parasitemia at first positive blood smear did not differ significantly by group (SA, Table S9, page 34). To assess efficacy against repeated Pf infections, we used a Poisson regression that models the number of positive BS as a function of time and treatment arm. VE (1-relative risk) was 31% (95%CI 1–51, p=0·02) per-protocol, and 29% (95%CI −1–50, p=0·03) mITT.
By ELISA, aIFA, and aISI, seroconversions were significantly more frequent and antibody levels were significantly higher in PfSPZ Vaccinees than controls (Figure 3). Among PfSPZ Vaccinees, those who remained uninfected during follow up had significantly higher seroconversion rate, and significantly higher antibody levels, measured by ELISA and aIFA, but not by aISI; antibody levels and seroconversions are described in more detail in SA, pages 16–17. Serum antibody responses by ELISA, aIFA, and aISI are shown for individual subjects in SA, Tables S11–S12, pages 37–45 and as ratios of post-immunization (post) to pre-immunization (pre) values in SA, pages 16–17; Figure S4, page 46.
We assessed vaccine-induced T-cell responses using multi-parameter flow cytometry on fresh whole blood. Ex vivo measures were taken before and at 3, 7, 42, and 55 days after each vaccination, and every 4 weeks during follow-up (SA, page 10). PfSPZ Vaccine and placebo recipients did not differ in their V0b=δ2 T-cell levels, measured as the percentage of total CD3+ T-cells (%Vδ2), at baseline (2·61% versus 2·43%, p=0·88; Wilcoxon Rank-Sum Test); subsequent measures were calculated as fold-change from baseline. Repeated measures were analyzed by GEE, with a robust (sandwich) variance estimator applied to all models assuming autocorrelation structure (further details and GEE outputs in SA, pages 13–14 and Table S13).
During the immunization period in the dry season, %Vδ2 decreased in placebo recipients but not PfSPZ Vaccine recipients (p = 0·039, GEE) (Figure 4A). Among placebo recipients (Figure 4B), %Vδ2 declined, and the decline was greater in uninfected than infected subjects at end dry season (day 120 median fold-change: 0·69 vs 0·83; p= 0·015, GEE), and remained lower through transmission season (day 281 median fold-change: 0·83 vs 0·99; p = 0·008, GEE). Among PfSPZ Vaccine recipients (Figure 4C), %Vδ2 increased during vaccination in uninfected (protected) but not infected (non-protected) vaccinees (day 120 median fold-change: 1·08 vs 0·99; p= 0·045, GEE).
Figure 4. Vδ2 γδ T-cell dynamics during vaccination and follow up.
Comparison of Vδ2 T-cells in uninfected and infected vaccinees and controls. Fold-change from baseline of Vδ2 T-cells were compared between A) PfSPZ and placebo groups B) Uninfected and infected placebo volunteers and C) Uninfected and infected PfSPZ vaccinees. Data were analyzed during the dry (vaccination) and the transmission (follow up) seasons using Wilcoxon Rank-Sum Test at individual time points, and by generalized estimating equations (GEE) for repeated measures.
Overall, CD4 and CD8 T-cell values assessed as fold-change values from baseline did not differ between the placebo and PfSPZ Vaccine groups (SA, Figure S5, page 49). T-cell responses are described in additional detail in SA, page 17.
DISCUSSION
We tested PfSPZ Vaccine for protection against intensely transmitted Pf among Malian adults with lifelong exposure to malaria. The aim of the trial was to evaluate whether a three-dose regimen would be safe, immunogenic, and efficacious in this population. To compensate for fewer doses than our prior study 3, we administered 4-fold higher cumulative dosage (5·4×106 PfSPZ, compared to 1·35×106 PfSPZ). Our results demonstrated that three doses were equally safe and well-tolerated, induced higher anti-PfCSP antibody responses, and conferred similar protection across a 24-week transmission season.. These consistent findings give confidence that PfSPZ Vaccine is efficacious for protecting African adults from naturally occurring Pf infection and supports further PfSPZ Vaccine development and evaluation.
This is the first study to compare VE measured by CHMI with VE measured by natural exposure in the same population. In CHMI with parasites homologous to the vaccine strain (PfNF54), VE 5 weeks after last vaccine dose was 100% measured by PCR, while in the main study, VE against natural infection measured by BS during 24 weeks was much lower. Further, several pilot study subjects also developed natural infections, despite sterile immunity against homologous CHMI and receipt of a 4th vaccine dose. Thus, VE measured by homologous CHMI at 5 weeks overestimated field VE over 24 weeks. The low rate of patent parasitemia in Mali and other malaria-experienced populations during CHMI contrasts with that in malaria-naïve individuals, where PfSPZ Challenge (NF54) induces parasitemia in 100% of subjects.16
Despite the increased vaccine dosage in this trial, safety and reactogenicity remained excellent. As in our first trial, AE rates did not significantly differ from those in normal saline recipients. The safety record for PfSPZ Vaccine, including no evidence for product-associated fever, may warrant its testing in special populations such as pregnant women. The study also, unexpectedly, highlighted liver enzyme abnormalities in healthy adults after ASAQ, which may be underestimated if routine follow-up testing is not done. The finding is important, especially if pre-treatment is established as a component of the vaccine regimen, in which case safe and effective alternatives to ASAQ that optimize vaccine responses across multiple age groups and transmission settings require identification in future studies.
Our immunological studies identified non-mechanistic correlates of protection consistent with those we and others described in previous PfSPZ Vaccine trials.2,9,17 Antibody levels against PfCSP and PfSPZ were significantly increased after the third dose of PfSPZ Vaccine and were significantly higher among PfSPZ Vaccine recipients who remained uninfected throughout the transmission season. However, the higher antibody responses observed in this trial versus our prior trial were not associated with an increased level of VE. Further, antibody levels in Malian vaccinees were substantially lower than those observed in unprotected US vaccinees (SA, Figure S6, page 53), suggesting that PfCSP antibodies may not be a primary mediator of protection. 7Consistent with our last study,3 antibody responses after the same regimen in US subjects were 9·3 times higher than in Malian subjects (median OD 1·0 of 16795 vs 1811, see SA, Figure S6, page 53)15, possibly due to immune dysregulation. Antibody and T-cell responses to PfSPZ Vaccine are generally higher in older children than adults in Tanzania, raising the possibility that efficacy may prove higher in children than adults in Africa.18
In both US and Malian vaccinees, the Vδ2 subset of γδ T-cells has been associated with protection against patent infection after PfSPZ Vaccine administration.2,9,17 Here, we observed significant increases in Vδ2 T-cells among vaccine recipients who remained uninfected throughout the transmission season. While Vδ2 T-cells are associated with control of blood stage parasitemia,19,20 their expansion after PfSPZ vaccinations suggests a different role. In animal models of SPZ vaccination, γδ T-cells are required for induction of protective CD8+ T-cells, but are not required at infectious challenge to mediate sterile immunity.2 Our inability to associate total peripheral CD8 T-cell levels to PfSPZ Vaccine administration is consistent with the observation that liver-resident CD8 T-cells mediate activity.9,21
PfSPZ Vaccine is being developed for residents in endemic areas and for travelers to endemic areas.4,22 The focus is Africa, where >98% of deaths and >90% of infections occur,1 including most traveler infections. A practical regimen is critical to clinical development plans (CDPs) for both target groups. Our study, in addition to confirming protection against intensely transmitted, heterogeneous Pf parasites in Mali, as in our previous 5-dose study3, establishes that the same VE can be achieved with a practical 3-dose regimen. This result led immediately to Phase 2 assessments of three-dose regimens in Kenyan infants [NCT02687373] and Gabonese children [NCT03521973], and laid the foundation for planned Phase 3 trials of three-dose regimens in Equatoguinean adults and children and in malaria-naive US and EU adults.
For the travelers’ CDP, both efficacy studies in Mali were paired with studies of the same 5-dose and 3-dose regimens in U.S.13,15 with efficacy measured against CHMI using heterologous Pf. These comparisons establish heterologous CHMI as a rigorous test of VE in Africa, recognizing that immune responses were 5–30 times lower in Malian than U.S. subjects.3,13 These comparative studies have provided the foundation for a CDP that will use heterologous CHMI for pivotal Phase 3 trials in European and U.S. subjects. Studies in Africa are now directly comparing VE with 9×105 versus the 1·8×106 PfSPZ dosing used here [NCT 03989102].
This study had several limitations. Malaria varies widely by site in its presentation, transmission dynamics, response to control and treatment, and demographics of disease. Thus, any single study has potential limitations in reproducibility and generalizability. This was our second study at this site—same population, same season—hence we are confident in reproducibility, but caveat that generalizability remains limited and requires studies in other populations (perennial malaria transmission; less or more lifelong malaria exposure; children; immunocompromised or pregnant). We provided anti-malarial pre-treatment to avert potential suppression of vaccine responses, but other studies will be needed to confirm its usefulness. Another limitation is the length of follow up: we followed subjects through one transmission season. We need to determine how long VE lasts and if booster doses are required. These are just some of the limitations that we are currently addressing in ongoing and planned clinical trials.
No other vaccine has previously been shown to prevent Pf infection in African adults across an entire malaria season of 24 weeks. The most advanced pediatric malaria vaccine RTS,S/AS01 did not confer significant efficacy in Kenyan adults against Pf infection during 14 weeks follow-up24 (although an earlier RTS,S formulation in a different adjuvant protected Gambian adults during the first 9 weeks but not the last 6 weeks of follow-up25). Current studies of PfSPZ Vaccine are exploring condensed three-dose regimens administered over four weeks for flexibility in mass vaccination programs and for special populations such as pregnant women. PfSPZ Vaccine must be assessed in combination with other interventions to pursue elimination. Finally, the efficacy of boosting should be assessed, as giving a single booster dose following primary vaccination will simplify efforts to use PfSPZ Vaccine on a seasonal basis.
Supplementary Material
Research in context:
Evidence before this study
We searched PubMed, the Cochrane Library, and other relevant source data sources on January 22, 2021 for English-language articles on randomised controlled trials of malaria vaccines in adults published between January 1, 1980, and Feb 12, 2021. We searched using the following terms (“malaria vaccines”[MeSH Terms] OR “malaria”[All Fields] AND “vaccines”[All Fields]) OR “malaria vaccines”[All Fields] OR (“malaria”[All Fields] AND “vaccine”[All Fields]) OR “malaria vaccine”[All Fields]) AND (PfSPZ [All Fields] AND PfSPZ Vaccine [All Fields])) AND (“adults”[MeSH Terms] OR “adults”[All Fields]). For the Cochrane Library and other data sources, we used the key search terms “PfSPZ”, “malaria vaccines”, “adults”, AND “clinical trials”. Although PfSPZ Vaccine studies have been previously conducted in malaria endemic regions, only one trial of a whole malaria sporozoite vaccine in the field has reported efficacy against natural infection, using a 5-dose PfSPZ vaccine regimen.
Added value of this study
Our results build upon an earlier field trial with promising protective efficacy results, wherein 2.7·105 PfSPZ Vaccine given at 0, 28, 56, 84, and 140 days, conferred significant protection to Malian adults against naturally occurring P. falciparum infection across a transmission season. Here, three monthly doses of 1.8·106 PfSPZ Vaccine was well-tolerated and safe. There were no significant differences in local or systemic reactogenicity nor in laboratory abnormalities between PfSPZ Vaccine and placebo recipients nor any serious events reported. 78% of our adult controls developed P. falciparum infection. Vaccine efficacy was 51% by time to infection analysis (95% CI, 20–70%; log-rank p= 0·004), and 24% by proportional analysis (95% CI, 2–41; p=0·03) per-protocol. Anti-PfCSP antibody response and Vδ2 γδ T cell increase after vaccination were significantly related to infection risk during follow-up.
Implications of all the available evidence
Findings from our study confirm the protective efficacy of PfSPZ Vaccine against naturally occurring Pf infection in Mali, and establish an efficacious 3-dose regimen that is safe, well-tolerated, and practical for implementation.
Acknowledgments
This trial was supported mainly with federal funds from the US National Institute of Allergy Infectious Diseases, NIH Intramural Research Program. Sanaria manufactured the vaccine and supported the costs of transportation and syringe preparation. The manufacture of Sanaria PfSPZ Vaccine was supported in part by Small Business Innovation Research grant from the NIAID (5R44AI055229-11, “Plasmodium falciparum Whole Sporozoite Malaria Vaccine”). A number of patents on PfSPZ have been issued, allowed, or filed in the US and internationally.
We thank first and foremost the study volunteers for their participation in the study and the local communities of Donéguébougou, Banambani, Sirababougou, Torodo, Toubana, and Zorokoro for their support. We thank the Independent Medical Monitor for his expert advice and consultation during the course of the study and the members of the Data and Safety Monitoring Board for their insight, discussion, and recommendations. We also thank the FMPOS Ethics Committee, NIAID IRB, Malian Ministry of Health and Public Hygiene, and Malian Department of Pharmacy and Medicine. We are grateful to the USTTB Rector and his office and NIAID Division of Intramural Research for their constant support of our collaborative projects in Mali.
We thank our colleagues at the USTTB and University of Bamako for their collaboration as the emergency physicians and as the vaccinators during the course of the study. Additionally, we are grateful to Kati Health District and Kati CSCOM. We thank our MRTC colleagues within the clinical laboratory team and immunology core laboratory team and the Diakite laboratory for their assistance on this protocol. We are also thankful to our LMIV colleagues within the clinical trials team, project management team, vaccine development unit, immunology core unit, and logistics and sample management team. We also would like to thank J. Patrick Gorres for assisting in preparing and editing the manuscript. Additionally, this work could not have been completed without the support of the NIH team based in Bamako, Mali.
We also thank the Sanaria and Protein Potential Teams for the manufacturing of the investigational products, PfSPZ Vaccine and diluent components, preparation of vaccine at the clinical site, regulatory, quality and site activities, performance of antibody assays, and production of PfSPZ for T-cell studies. We thank OCRPRO and OCICB at the NIH for providing assistance in the development and finalisation of the case report forms and database and local support in Mali from MRTC for data entry and validation. We thank OCRPRO for providing external monitoring services for this study and ongoing support and guidance to our NIAID/MRTC programs. We gratefully acknowledge our colleagues at the Division of Pre-Clinical Innovation, National Center for Advancing Translation Sciences, National Institutes of Health (Rockville, MD, USA) for continuing scientific and technical support on use of the Acumen eX3 laser-scanning image cytometer for inhibition of sporozoite invasion and automated immunofluorescence assay assays with clinical sera.
The authors alone are responsible for the views expressed in this manuscript and they do not necessarily represent the decisions, policy, or views of the US Government. Mention of trade names, commercial products, or organisations does not imply endorsement by the US Government.
Funding
Intramural Research Program, National Institute of Allergy and Infectious Diseases (NIAID), U.S. National Institutes of Health (NIH); extramural NIAID/NIH grants including Small Business Innovation Research grant 5R44AI055229-11; and Sanaria Inc.
Footnotes
Conflicts of interests
YA, ERJ, TM, NK, BKLS, PFB, TLR, and SLH are salaried, full time employees of Sanaria Inc., the developer and sponsor of Sanaria PfSPZ Vaccine.
Data sharing
Individual level participant data will be made available with publication and upon execution of inter-institutional human data sharing agreement. Data can include all those described in the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mahamadou S. Sissoko, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Sara A. Healy, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Abdoulaye Katile, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Irfan Zaidi, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Zonghui Hu, Biostatistical Research Branch, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Bourama Kamate, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Yacouba Samake, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Kourane Sissoko, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Agnes Mwakingwe-Omari, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Jacquelyn Lane, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Alemush Imeru, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Rathy Mohan, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Ismaila Thera, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Cheick Oumar Guindo, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Amagana Dolo, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Karamoko Niare, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Fanta Koïta, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Amadou Niangaly, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Kelly M. Rausch, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Amatigue Zeguime, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Merepen A. Guindo, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Aissatou Bah, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA; Laboratory of Parasitic Diseases, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Yonas Abebe, Sanaria Inc, Rockville, Maryland, USA.
Eric R. James, Sanaria Inc, Rockville, Maryland, USA.
Anita Manoj, Sanaria Inc, Rockville, Maryland, USA.
Tooba Murshedkar, Sanaria Inc, Rockville, Maryland, USA.
Natasha KC, Sanaria Inc, Rockville, Maryland, USA; Protein Potential LLC, Rockville, Maryland, USA.
B. Kim Lee Sim, Sanaria Inc, Rockville, Maryland, USA; Protein Potential LLC, Rockville, Maryland, USA.
Peter F. Billingsley, Sanaria Inc, Rockville, Maryland, USA.
Thomas L. Richie, Sanaria Inc, Rockville, Maryland, USA.
Stephen L. Hoffman, Sanaria Inc, Rockville, Maryland, USA.
Prof Ogobara Doumbo, Malaria Research and Training Center, Mali-NIAID ICER, University of Science, Techniques and Technologies of Bamako, Mali.
Patrick E. Duffy, Laboratory of Malaria Immunology and Vaccinology, Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
REFERENCES
- 1.WHO. World Malaria Report 2020. Geneva: World Health Organization, 2020. [Google Scholar]
- 2.Zaidi I, Diallo H, Conteh S, et al. gammadelta T Cells Are Required for the Induction of Sterile Immunity during Irradiated Sporozoite Vaccinations. J Immunol 2017; 199(11): 3781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sissoko MS, Healy SA, Katile A, et al. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis 2017; 17(5): 498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richie TL, Billingsley PF, Sim BK, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine 2015; 33(52): 7452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dicko A, Sagara I, Diemert D, et al. Year-to-year variation in the age-specific incidence of clinical malaria in two potential vaccine testing sites in Mali with different levels of malaria transmission intensity. Am J Trop Med Hyg 2007; 77(6): 1028–33. [PubMed] [Google Scholar]
- 6.Diallo DA, Doumbo OK, Plowe CV, Wellems TE, Emanuel EJ, Hurst SA. Community permission for medical research in developing countries. Clin Infect Dis 2005; 41(2): 255–9. [DOI] [PubMed] [Google Scholar]
- 7.Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science 2011; 334(6055): 475–80. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman SL, Billingsley PF, James E, et al. Development of a metabolically active, non-replicating sporozoite vaccine to prevent Plasmodium falciparum malaria. Hum Vaccin 2010; 6(1): 97–106. [DOI] [PubMed] [Google Scholar]
- 9.Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 2013; 341(6152): 1359–65. [DOI] [PubMed] [Google Scholar]
- 10.Administration UFaD. Guidance for industry: Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. 2007. [DOI] [PubMed] [Google Scholar]
- 11.Easton AV, Oliveira RG, O’Connell EM, et al. Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming. Parasit Vectors 2016; 9: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mordmuller B, Surat G, Lagler H, et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017; 542(7642): 445–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epstein JE, Paolino KM, Richie TL, et al. Protection against Plasmodium falciparum malaria by PfSPZ Vaccine. JCI Insight 2017; 2(1): e89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyke KE, Ishizuka AS, Berry AA, et al. Attenuated PfSPZ Vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc Natl Acad Sci U S A 2017; 114(10): 2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyke KE, Singer A, Berry AA, et al. Multidose Priming and Delayed Boosting Improve PfSPZ Vaccine Efficacy against Heterologous P. falciparum Controlled Human Malaria Infection. Clin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 16.Mordmuller B, Supan C, Sim KL, et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malar J 2015; 14: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishizuka AS, Lyke KE, DeZure A, et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat Med 2016; 22(6): 614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jongo SA, Church LWP, Mtoro AT, et al. Safety and Differential Antibody and T-Cell Responses to the Plasmodium falciparum Sporozoite Malaria Vaccine, PfSPZ Vaccine, by Age in Tanzanian Adults, Adolescents, Children, and Infants. Am J Trop Med Hyg 2019; 100(6): 1433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costa G, Loizon S, Guenot M, et al. Control of Plasmodium falciparum erythrocytic cycle: gammadelta T cells target the red blood cell-invasive merozoites. Blood 2011; 118(26): 6952–62. [DOI] [PubMed] [Google Scholar]
- 20.Junqueira C, Polidoro RB, Castro G, et al. gammadelta T cells suppress Plasmodium falciparum blood-stage infection by direct killing and phagocytosis. Nat Immunol 2021; 22(3): 347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holz LE, Chua YC, de Menezes MN, et al. Glycolipid-peptide vaccination induces liver-resident memory CD8(+) T cells that protect against rodent malaria. Sci Immunol 2020; 5(48). [DOI] [PubMed] [Google Scholar]
- 22.Hoffman SL, Vekemans J, Richie TL, Duffy PE. The march toward malaria vaccines. Vaccine 2015; 33 Suppl 4: D13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rts SCTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386(9988): 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polhemus ME, Remich SA, Ogutu BR, et al. Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area. PLoS One 2009; 4(7): e6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bojang KA, Milligan PJ, Pinder M, et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 2001; 358(9297): 1927–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.