Abstract
Aim
To investigate the directional and selective disconnection of the sensorimotor cortex (SMC) subregions in chronic stroke patients with hand dysfunction.
Methods
We mapped the resting‐state fMRI effective connectivity (EC) patterns for seven SMC subregions in each hemisphere of 65 chronic stroke patients and 40 healthy participants and correlated these patterns with paretic hand performance.
Results
Compared with controls, patients demonstrated disrupted EC in the ipsilesional primary motor cortex_4p, ipsilesional primary somatosensory cortex_2 (PSC_2), and contralesional PSC_3a. Moreover, we found that EC values of the contralesional PSC_1 to contralesional precuneus, the ipsilesional inferior temporal gyrus to ipsilesional PSC_1, and the ipsilesional PSC_1 to contralesional postcentral gyrus were correlated with paretic hand performance across all patients. We further divided patients into partially (PPH) and completely (CPH) paretic hand subgroups. Compared with CPH patients, PPH patients demonstrated decreased EC in the ipsilesional premotor_6 and ipsilesional PSC_1. Interestingly, we found that paretic hand performance was positively correlated with seven sensorimotor circuits in PPH patients, while it was negatively correlated with five sensorimotor circuits in CPH patients.
Conclusion
SMC neurocircuitry was selectively disrupted after chronic stroke and associated with diverse hand outcomes, which deepens the understanding of SMC reorganization.
Keywords: effective connectivity, functional reorganization, Granger causality analysis, resting‐state functional magnetic resonance imaging, stroke
Stroke remains the primary reason for adult disability, and hand function recovery is vital for survivors to regain quality of life. Here, we found two functional reorganization patterns in chronic stroke patients with different hand outcomes. For stroke patients with a partially paretic hand (PPH), all motor‐related sensorimotor circuits were positive, while for stroke patients with a completely paretic hand (CPH), this relationship was reversed. Meanwhile, the effective couplings among the PSC_1, inferior temporal gyrus, precuneus, and postcentral gyrus were correlated with hand performance in all stroke patients. PMC, primary motor cortex; PSC, primary somatosensory cortex.
1. INTRODUCTION
Stroke remains the primary reason for adult disability, 1 and hand function recovery is vital for survivors to regain functional independence. 2 Motor outcomes following stroke have been found to be associated with infarction size, 3 lesion topography, 4 , 5 gray matter plasticity, 6 corticospinal tract integrity, 7 functional network connectivity, 8 , 9 and frequency‐specific local oscillations. 10 Benefiting from these neuroimaging discoveries in stroke populations, recent studies have suggested that modulating key sensorimotor nodes by noninvasive brain stimulation 11 , 12 , 13 , 14 , 15 could promote motor recovery after stroke. Hence, neuroimaging opens the door for understanding the pathophysiology of motor deficits following stroke and may inspire progress in personalized, neurobiologically informed neuromodulation. 16 , 17
Resting‐state functional magnetic resonance imaging (fMRI) can non‐invasively explore intrinsic human brain activity, 18 and connectivity analysis provides an effective framework for understanding information interactions among brain regions after stroke. 19 In well‐recovered stroke patients, although activation patterns are close to those in healthy controls, the network connectivity is aberrant. 20 Plastic changes in functional connectivity throughout the sensorimotor regions have been demonstrated to be associated with upper extremity dysfunction and recovery following stroke. 16 , 21 In cross‐sectional studies, disrupted interhemispheric sensorimotor connectivity was positively correlated with motor dysfunction, 22 while it was inversely affected by the lesion load of the corticospinal tract. 23 , 24 In longitudinal studies, connectivity between the ipsilesional primary motor cortex (PMC) and contralesional supplementary motor area (SMA) in the early days could predict long‐term motor outcomes after stroke, 25 and dynamic connectivity changes in the cerebrocerebellar circuits were accompanied by spontaneous recovery in stroke patients with pontine 26 or subcortical 27 infarcts. In neurorehabilitation studies, regulating the bilateral PMC through bihemispheric transcranial direct current stimulation, 15 priming the ipsilesional PMC through intermittent theta burst stimulation, 11 and inhibiting the contralesional PMC through repetitive transcranial magnetic stimulation 13 could facilitate motor recovery following stroke. However, the direction of functional interactions between the sensorimotor cortex (SMC) and whole brain following stroke is less clear.
Effective connectivity (EC) is mostly employed for task‐evoked fMRI data, including dynamic causal modeling, psychophysiological interactions, structural equation modeling, and Granger causality analysis. 28 In contrast to the non‐directional characteristic of functional connectivity, EC analysis can delineate the causal influences among brain regions. Using dynamic causal modeling, 13 , 29 , 30 , 31 several milestone studies have investigated EC among key sensorimotor areas following stroke. Rehme et al. reported that the interhemispheric coupling between the bilateral PMC was associated with illness duration 29 and the severity of deficits. 30 Grefkes et al. found that inhibiting the contralesional PMC by transcranial magnetic 13 or noradrenergic 31 stimulation increased the improvement‐associated couplings from the ipsilesional SMA to the PMC. Using structural equation modeling, Sharma et al. found that influence from the contralesional prefrontal cortex to the SMA was correlated with better motor performance during motor imagery in well‐recovered survivors. 20 Another study investigating resting‐state EC among the frontoparietal area and sensorimotor system found that stroke patients showed decreased influences from the superior parietal lobule to both the PMC and SMA in the lesioned hemisphere. 32 However, as a data‐driven exploratory method, Granger causality analysis has rarely been used in stroke studies. The SMC involves a wide spectrum of integrated motor functions and can be divided into seven subregions in each hemisphere. 33 Given the different functions of SMC subregions and their associations with motor deficits after stroke, we investigated whether the resting‐state EC patterns of the SMC subregions suffer selective disruption in chronic subcortical stroke patients with hand dysfunction.
Here, we first defined the seven SMC subregions on the basis of the probabilistic cytoarchitectonic atlases for each hemisphere and then calculated the whole‐brain resting‐state fMRI EC patterns for each SMC subregion in each participant. Next, we examined EC differences between all stroke patients and healthy participants and between stroke subgroups with different hand outcomes. Finally, brain‐behavior correlations between EC patterns and hand performance were also explored.
2. METHODS
2.1. Recruitment of participants
Our project was approved by the Hospital Ethics Committee (2014 Interim Review No. 279) and was conducted in line with the Helsinki requirements. Before enrollment, each subject was notified and signed an informed consent form. In this study, we collected 65 chronic stroke patients with left subcortical infarction/hemorrhage and 40 healthy controls. Patients satisfying the following criteria were recruited: (a) first‐episode stroke with a lesion mainly involved in the left subcortical nuclei (eg, basal ganglia and thalamus); (b) aged 30–80 years; (c) course of disease ≥3 months; (d) motor impairments of the upper limb and hand as evaluated by the Fugl‐Meyer scale; and (e) dextromanuality as evaluated by the Edinburgh Handedness Inventory. We excluded those patients from this study who had MRI contraindications, severe cognitive impairment/aphasia/neglect, and unstable illness states (eg, serious atrial fibrillation and multiple organ failure). Healthy participants who had no neuropsychiatric history or cognitive impairments were recruited from the local community.
As per previous studies, 8 , 10 we used the Paretic Hand Scale (see Supporting Materials) to divide stroke patients into the partially (PPH) and completely (CPH) paretic hand subgroups. This scale was specifically designed to evaluate the practical function of the hand in everyday life. Stroke patients who could finish one or more tasks were categorized as having PPH, while those could not finish any task were classified as having CPH.
2.2. Behavioral assessment
The Hand and Wrist subscale of the Fugl‐Meyer Assessment (FMA‐HW) was used to evaluate paretic hand performance in all stroke patients before fMRI scanning. 8 The FMA‐HW subscale, which was regarded as the primary measurement, consists of a wrist section (five items) and a hand section (seven items), with a possible score ranging from 0 to 24.
2.3. Collection of imaging data
Imaging data were acquired using a 3‐Tesla scanner (SIEMENS Trio, Germany). T1‐weighted images were collected using an MPRAGE sequence: 192 sagittal slices, 1 mm slice thickness, 0.5 mm gap, 1900/3.42/900 ms repetition time/echo time/inversion time, 240 × 240 field of view, 9° flip angle, and 256 × 256 matrix size. T2‐weighted images were collected using a TSE sequence: 30 axial slices, 5 mm slice thickness, without gap, 6000/93 ms repetition time/echo time, 220 × 220 field of view, 120° flip angle, and 320 × 320 matrix size. Resting‐state functional imaging data were acquired using an EPI sequence: 30 axial slices, 4 mm slice thickness, 0.8 mm gap, 2000/30 ms repetition time/echo time, 220 × 220 field of view, 90° flip angle, 64 × 64 matrix size, 240 volumes, and scanning time 8:06 (m:ss). During scanning, each participant was asked to keep their eyes closed and mind relaxed and to not move to the greatest extent.
2.4. Mapping lesion overlap
We first used MRIcron software (https://people.cas.sc.edu/rorden/mricron/install.html) to delineate the lesion profiles of each stroke patient on T2‐weighted images (see Supporting Materials). Then, the T2‐weighted lesion masks of all stroke patients were standardized to the MNI space. Finally, we summed each resampled lesion mask with a resolution of 1 × 1 × 1 mm3 to establish the lesion map (Figure 1).
FIGURE 1.
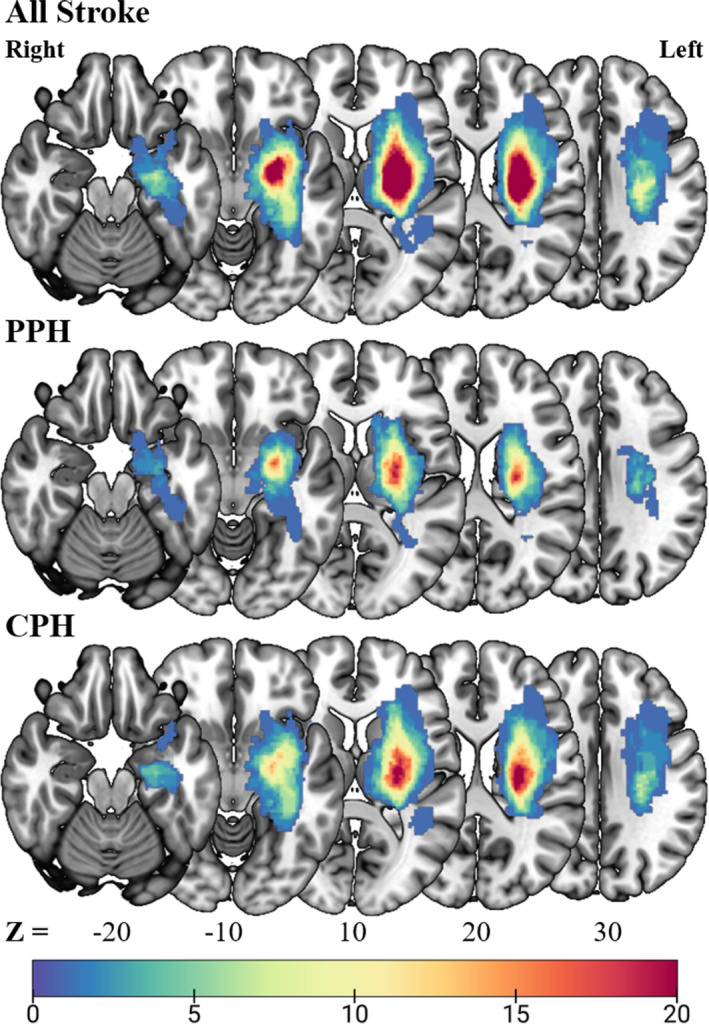
Lesion overlap map for all stroke, PPH, and CPH patients. The color bar indicates the frequency of patients having lesions in each voxel in the left (ipsilesional) hemisphere. CPH, completely paretic hand; PPH, partially paretic hand
2.5. Imaging data preprocessing
We employed DPABI software (http://rfmri.org/DPABI) to preprocess the resting‐state functional imaging data. 34 The processing steps involved (a) deletion of the first ten volumes, (b) correction of slice timing, (c) realignment of head motion, (d) standardization to the MNI space using the unified segmentation of structural images, (e) spatial smoothing (FWHM = 6 mm), (f) linear detrending, and (g) bandpass filtering (0.01–0.1 Hz). Finally, we regressed out the six head motion parameters, global mean signal, cerebrospinal fluid signal, and white matter signal. During image preprocessing, no participants were discarded based on the predefined criteria of head motion (exceeding 2 mm/degree). To eliminate the influences of head motion on the EC results, 35 we regressed out the framewise displacement in all subsequent between‐group statistical analyses.
2.6. Definition of the SMC subregions
We defined seven SMC subregions in the ipsilesional and contralesional hemispheres on the basis of the probabilistic cytoarchitectonic atlas, as integrated in SPM12 software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). 33 For each hemisphere, the seven SMC subregions included premotor_6, PMC_4a, PMC_4p, primary somatosensory cortex_1 (PSC_1), PSC_2, PSC_3a, and PSC_3b (Figure 2).
FIGURE 2.
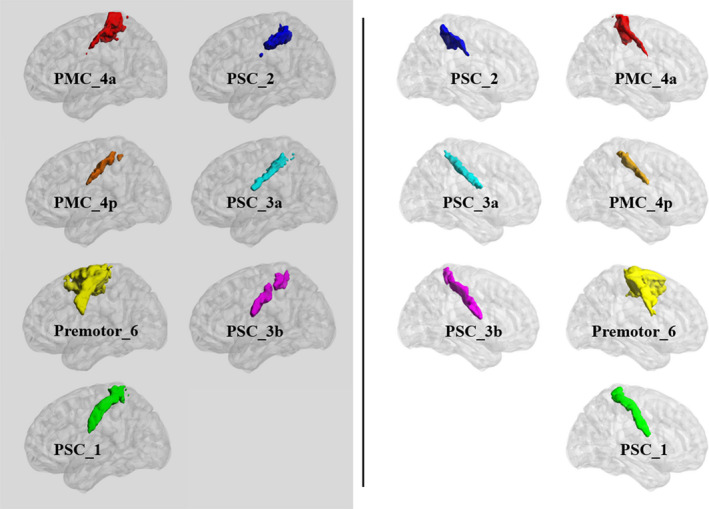
In total, fourteen seeds were defined by sensorimotor subregions based on the cytoarchitectonic atlas. The left panel shows seven ipsilesional (left) hemisphere seeds, and the right panel shows seven contralesional (right) hemisphere seeds
2.7. EC analysis of the SMC subregions
The Granger causality analysis module within REST software (http://restfmri.net/forum/REST_V1.8) was used to generate voxelwise seed‐based EC maps. 36 We employed the vector autoregression model to perform the Granger causality analysis. First, we performed a bivariate coefficient Granger causality analysis to obtain the EC maps for all participants and then converted these maps to z‐values using Fisher's conversion. Next, we employed one‐sample t‐tests to obtain the group EC patterns of each of the seven SMC subregions in each hemisphere.
2.8. Statistical analysis
To analyze the baseline data of all participants, SPSS software (version 25.0, IBM Inc.) was used. We first performed the Shapiro–Wilk test to assess the normality of all continuous variables (age, duration of illness, lesion volumes, FMA‐HW score, and framewise displacement). Then, we found that only age was distributed normally and thus was analyzed by the two independent samples t‐test, while the other variables were distributed abnormally and were analyzed by the Mann–Whitney test. For the sex ratio and stroke type, we employed the chi‐square test to analyze the differences between groups. To infer the EC differences between groups, we used two‐sample t‐tests to explore disrupted EC between all stroke patients and healthy participants as well as between CPH patients and PPH patients with sex, age and framewise displacement as covariates. The AlphaSim method with a p < 0.0001 was adopted to perform multiple comparisons (voxel p = 0.001, cluster ≥ 43, FWHM = 7.9 mm, with a gray matter mask). Finally, we used multiple linear regression analysis within SPM12 software (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) to explore associations between the EC patterns of each subregion and the FMA‐HW scores in all stroke patients with sex, age, and framewise displacement as covariates. We also performed the same analysis in both the PPH and CPH subgroups. The AlphaSim method with a p < 0.01 was adopted to perform multiple comparisons (voxel p = 0.001, cluster ≥ 19, FWHM = 7.0 mm, with a gray matter mask). For surviving brain regions from the multiple linear regression analysis, we first extracted the EC values within these regions and then correlated the EC values of each surviving region with the FMA‐HW scores in all stroke patients, PPH patients, and CPH patients by using Pearson correlation analysis. For the visualization of all results, we used the 3D surface of BrainNet Viewer software (www.nitrc.org/projects/bnv). 37
3. RESULTS
3.1. Basic demographic and clinical data
Sixty‐five patients with left subcortical chronic stroke (32 PPH vs. 33 CPH) and forty healthy controls were recruited. We found that there were significant differences in the sex ratio (p = 0.009) and no significant differences in age (p = 0.671) or framewise displacement (p = 0.089) between stroke patients and healthy controls. Furthermore, we found that there were no significant differences in age (p = 0.811), sex ratio (p = 0.110), stroke type (p = 0.540), duration of illness (p = 0.305) or framewise displacement (p = 0.823) between the PPH and CPH subgroups. However, the lesion volumes of the CPH patients were significantly larger than those of the PPH patients (p = 0.006), and the FMA‐HW scores of the PPH patients were significantly higher than those of the CPH patients (p < 0.001) (Table 1).
TABLE 1.
Demographic and clinical data of participants recruited in this study
| Baseline characteristics | Study 1 | p value | Study2 | p value | ||
|---|---|---|---|---|---|---|
| All stroke | Controls | PPH | CPH | |||
| Age (years)b | 55.89 ± 9.71 | 55.12 ± 7.57 | 0.671 | 56.19 ± 10.53 | 55.60 ± 9.00 | 0.811 |
| Sex (male:female)a | 54:11 | 24:16 | 0.009 | 29:3 | 25:8 | 0.110 |
| Hand dominance | Right | Right | — | Right | Right | — |
| Stroke type (ischemic:hemorrhagic)a | 30:35 | — | — | 16:16 | 14:19 | 0.540 |
| Duration of illness (months) | 14.70 ± 16.07 | — | — | 15.31 ± 14.87 | 14.12 ± 17.36 | 0.305 |
| Lesion hemisphere (left:right) | Left | — | — | Left | Left | — |
| Lesion location | Subcortical | — | — | Subcortical | Subcortical | — |
| Lesion volume (ml) | 12.78 ± 9.50 | — | — | 9.45 ± 5.57 | 16.00 ± 11.33 | 0.006 |
| FMA‐HW score | 6.17 ± 6.67 | — | — | 11.25 ± 6.15 | 1.24 ± 1.22 | <10−9 |
| Framewise displacement (mm) | 0.14 ± 0.08 | 0.11 ± 0.05 | 0.089 | 0.13 ± 0.07 | 0.14 ± 0.10 | 0.823 |
Values expressed as the mean ± SD; the superscript a indicates the chi‐square test, b indicates two independent sample t‐test, and all others are Mann–Whitney tests.
Abbreviations: CPH, completely paretic hand; FMA‐HW, Fugl‐Meyer Assessment of Hand and Wrist; PPH, partially paretic hand.
The bold value used to highlight the significant p values.
3.2. Differences in EC between stroke patients and controls
Compared with controls, stroke patients demonstrated increased EC from the ipsilesional PMC_4p to the ipsilesional precentral gyrus, contralesional postcentral gyrus, and ipsilesional middle occipital cortex, from the bilateral precuneus, contralesional middle temporal gyrus, contralesional hippocampus, and ipsilesional middle frontal gyrus to the ipsilesional PSC_2, and from the bilateral hippocampus and ipsilesional precuneus to the contralesional PSC_3a. Additionally, compared with controls, stroke patients demonstrated decreased EC from the ipsilesional PSC_3a to the bilateral superior temporal gyrus (Table 2, Figure 3).
TABLE 2.
Comparison of EC between stroke patients and healthy controls and between stroke subgroups
| Disrupted effective connectivity | MNI | Cluster | t value | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Stroke > Control | |||||
| Ipsilesional PMC_4p to ipsilesional precentral gyrus | −36 | −18 | 36 | 730 | 5.78 |
| Ipsilesional PMC_4p to contralesional postcentral gyrus | 42 | −36 | 54 | 61 | 3.96 |
| Ipsilesional PMC_4p to ipsilesional middle occipital cortex | −24 | −78 | 36 | 222 | 5.00 |
| Ipsilesional precuneus to ipsilesional PSC_2 | −3 | −54 | 48 | 219 | 5.37 |
| Contralesional precuneus to ipsilesional PSC_2 | 12 | −54 | 48 | 127 | 4.38 |
| Ipsilesional middle frontal gyrus to ipsilesional PSC_2 | −36 | 36 | 18 | 137 | 4.52 |
| Contralesional middle temporal gyrus to ipsilesional PSC_2 | 54 | −30 | −15 | 77 | 5.26 |
| Contralesional hippocampus to ipsilesional PSC_2 | 21 | −33 | −9 | 145 | 5.09 |
| Ipsilesional hippocampus to contralesional PSC_3a | −36 | 0 | −15 | 83 | 6.82 |
| Contralesional hippocampus to contralesional PSC_3a | 30 | −6 | −15 | 116 | 4.68 |
| Ipsilesional precuneus to contralesional PSC_3a | −9 | −51 | 60 | 103 | 5.42 |
| Stroke < Control | |||||
| Ipsilesional PSC_3a to ipsilesional superior temporal gyrus | −63 | −24 | 3 | 65 | −5.80 |
| Ipsilesional PSC_3a to contralesional superior temporal gyrus | 42 | −3 | −12 | 144 | −4.81 |
| PPH < CPH | |||||
| Ipsilesional inferior parietal lobe to ipsilesional premotor_6 | −36 | −60 | 42 | 34 | −4.37 |
| Ipsilesional inferior parietal lobe to ipsilesional PSC_1 | −36 | −57 | 39 | 55 | −4.32 |
| Ipsilesional inferior temporal gyrus to ipsilesional PSC_1 | −57 | −30 | −24 | 47 | −5.22 |
| Contralesional middle frontal cortex to ipsilesional PSC_1 | 42 | 42 | 15 | 30 | −4.17 |
Abbreviations: CPH, completely paretic hand; PMC, primary motor cortex; PPH, partially paretic hand; PSC, primary somatosensory cortex.
FIGURE 3.
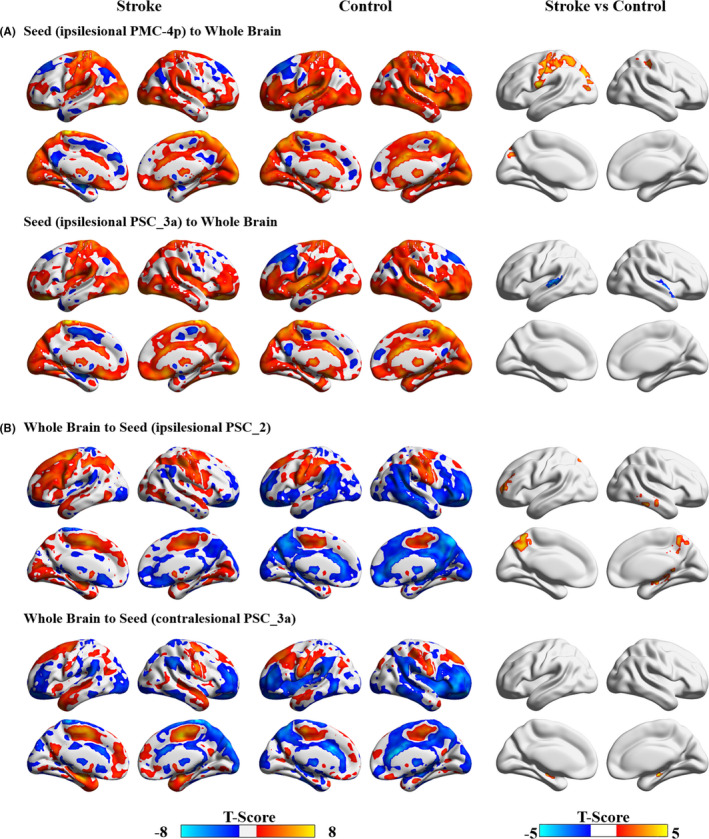
Disrupted effective connectivity in stroke patients compared with healthy controls. (A) Group differences in effective connectivity from seeds to the whole brain. (B) Group differences in effective connectivity from the whole brain to seeds. AlphaSim corrected: p < 0.0001. PMC, primary motor cortex; PSC, primary somatosensory cortex
3.3. Brain‐behavior correlations including all stroke patients
Specifically, the FMA‐HW scores were negatively related to the EC values of the contralesional PSC_1 to the contralesional precuneus (r = −0.558, p < 0.001) and the ipsilesional inferior temporal gyrus to the ipsilesional PSC_1 (r = −0.455, p < 0.001). However, the FMA‐HW scores were positively related to the EC values of the ipsilesional PSC_1 to the contralesional postcentral gyrus (r = 0.517, p < 0.001) (Figure 4).
FIGURE 4.
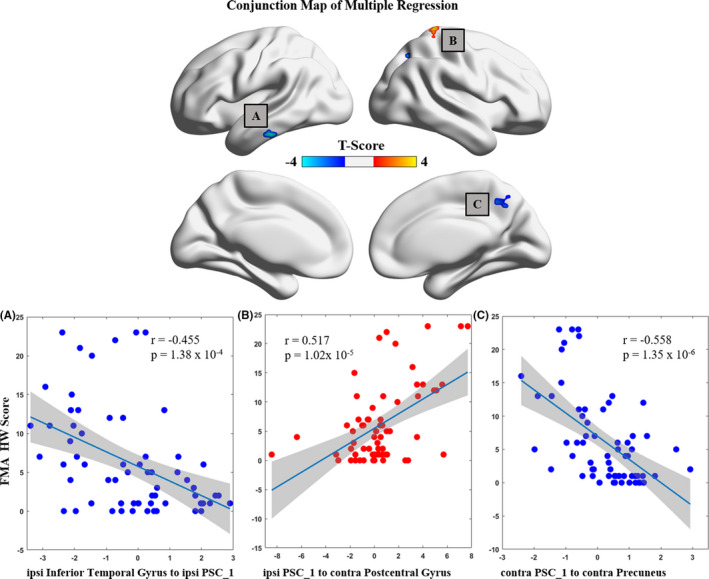
Correlations between connectivity patterns and paretic hand performance for all stroke patients. AlphaSim corrected: p < 0.01. Contra, contralesional; FMA‐HW, Fugl‐Meyer Assessment of Hand and Wrist; ipsi, ipsilesional; PSC, primary somatosensory cortex
3.4. Distinct EC patterns between stroke patients with CPH and PPH
Compared with the CPH patients, the stroke patients with PPH demonstrated decreased EC from the ipsilesional inferior parietal lobe to the ipsilesional premotor_6 and ipsilesional PSC_1 and from the ipsilesional inferior temporal gyrus and contralesional middle frontal cortex to the ipsilesional PSC_1 (Table 2, Figure 5).
FIGURE 5.
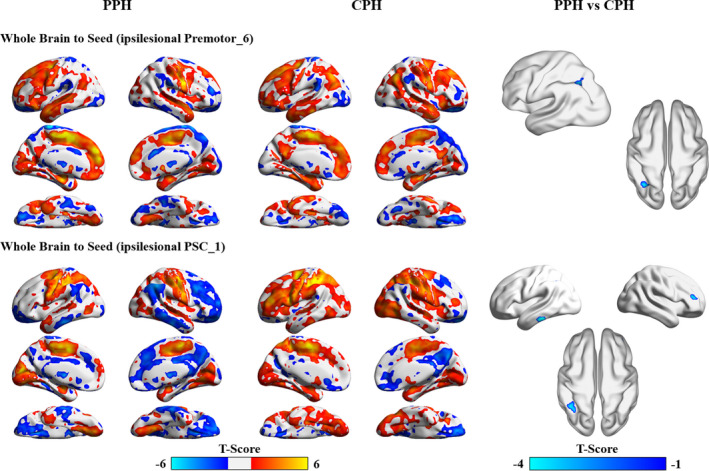
Disrupted effective connectivity between stroke patients with partially and completely paretic hands. AlphaSim corrected: p < 0.0001. CPH, completely paretic hand; PPH, partially paretic hand; PSC, primary somatosensory cortex
3.5. Brain‐behavior correlations across stroke subgroups
The stroke patients with PPH showed only positive brain‐behavior correlations. Specifically, the FMA‐HW scores were positively related to the EC values of the ipsilesional PSC_1 to the contralesional precentral gyrus (r = 0.516, p = 0.002), the contralesional precuneus (r = 0.507, p = 0.003), contralesional putamen (r = 0.555, p < 0.001), contralesional middle temporal gyrus (r = 0.609, p < 0.001), ipsilesional superior temporal gyrus (r = 0.732, p < 0.001), and ipsilesional cerebellum posterior lobe (r = 0.605, p < 0.001) to the contralesional PSC_1, and the ipsilesional PSC_2 to the contralesional postcentral gyrus (r = 0.612, p < 0.001). In contrast, the stroke patients with CPH showed only negative brain‐behavior correlations. Specifically, the FMA‐HW scores were negatively related to the EC values of the ipsilesional PMC_4a to the ipsilesional superior parietal lobe (r = −0.665, p < 0.001), the contralesional PMC_4a to the ipsilesional postcentral gyrus (r = −0.663, p < 0.001), the ipsilesional PMC_4p to the contralesional calcarine (r = −0.691, p < 0.001), the contralesional PMC_4p to the ipsilesional precuneus (r = −0.469, p = 0.005), and the ipsilesional PSC_3b to the contralesional lingual gyrus (r = −0.610, p < 0.001) (Figure 6).
FIGURE 6.
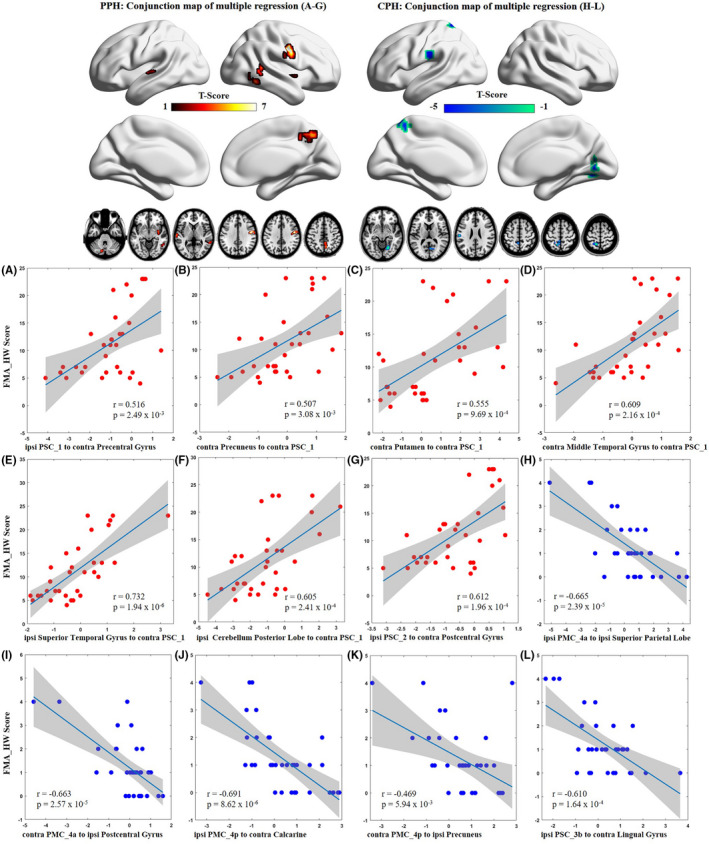
Dissociated correlations between connectivity patterns and different hand outcomes in stroke subgroups. AlphaSim corrected: p < 0.01. Contra, contralesional; CPH, completely paretic hand; FMA‐HW, Fugl‐Meyer Assessment of Hand and Wrist; ipsi, ipsilesional; PMC, primary motor cortex; PPH, partially paretic hand; PSC, primary somatosensory cortex
4. DISCUSSION
The challenge in post‐stroke neuroimaging studies is to identify the intervention targets 38 and predict the long‐term outcomes. 39 Using Granger causality analysis of resting‐state fMRI data in chronic stroke patients, we found that the EC patterns of SMC subregions were selectively disrupted and correlated with hand dysfunction. Most importantly, the correlations between EC patterns and hand performance in stroke patients with PPH were positive, while those in stroke patients with CPH were negative. These findings indicate that injury of the subcortical motor pathway results in specific disruption of sensorimotor circuits, which may inspire the development of neuroimaging biomarkers 40 and stimulation targets 41 in neurorehabilitation practice after chronic stroke.
4.1. Disrupted sensorimotor circuits following chronic stroke
Usually, stroke patients with severe motor impairments exhibit hyperactivation of the sensorimotor system. 42 , 43 , 44 In fact, activation in the ipsilesional premotor and primary motor areas 45 without recruitment of contralesional activity 46 is related to good motor outcomes. However, chronic stroke patients who receive bilateral arm training demonstrate a recovery‐associated increase in activation in the contralesional SMC. 47 Recent connectivity studies have suggested that recovery of motor function is driven by decreased interhemispheric influences from the contralesional PMC to the ipsilesional PMC following stroke. 13 , 48 Additionally, a previous electrophysiological study also revealed that overactivation of the contralesional PMC might inhibit the motor output of the ipsilesional PMC in chronic stroke patients. 49 Contrary to these findings, 13 , 48 , 49 we demonstrated here that stroke patients show increased EC from the ipsilesional PMC_4p to the ipsilesional precentral gyrus and contralesional postcentral gyrus, and paretic hand performance is positively related to the EC strength of the ipsilesional PSC_1 to the contralesional postcentral gyrus. Our results verify but also extend previous findings, highlighting the importance of interhemispheric disturbances among sensorimotor regions for hand dysfunction observed in chronic stroke patients. 9 , 22 Furthermore, these data suggest a functional relevance of the disrupted influences from the ipsilesional to contralesional somatosensory cortices due to the strong brain‐behavior correlations.
Stroke patients with motor deficits typically show recruitment of non‐motor regions (eg, the prefrontal, parietal, and temporal lobes), with the consensus that greater activation of non‐motor areas leads to poorer functional recovery. 50 The anterior precuneus is closely related to sensorimotor processing. 51 One longitudinal study indicated that hyperactivation in the precuneus is correlated with slower motor recovery following stroke. 52 Here, we demonstrated that not only task‐related activation of the precuneus but also EC strength from the bilateral precuneus to the ipsilesional PSC_2 is increased in chronic stroke patients. Furthermore, EC strength from the contralesional PSC_1 to the contralesional precuneus is negatively correlated with paretic hand performance. The precuneus has extensive connections with the SMC system, which plays important roles in visual goal‐directed hand movements. 53 Thus, our data suggest that the exchange of information between the somatosensory cortex and precuneus is needed for chronic stroke patients to support visual processing during affected hand movements. Complex motor tasks, for example, novel and skilled sequential hand movements, often require audiomotor processing support from the bilateral temporal gyrus. 54 A previous study found that increased spontaneous activity in the contralesional superior temporal gyrus is negatively correlated with motor dysfunction after chronic stroke. 10 Interestingly, in this study, we demonstrated that stroke patients display decreased EC from the ipsilesional PSC_3a to the bilateral superior temporal gyrus, and paretic hand performance is negatively related to EC strength from the ipsilesional inferior temporal gyrus to the ipsilesional PSC_1. Considering the supporting role of the temporal gyrus in complex sensorimotor processing, our data suggest that disrupted interactions between temporal and sensory cortices might impair audiomotor coordination during affected hand movements following chronic stroke. The hippocampus is involved in motor learning consolidation, which could optimize subsequent behavior. 55 After rehabilitation, chronic stroke patients show increased gray matter volume within the bilateral hippocampus 56 and strengthened functional coupling between the ipsilesional inferior parietal lobe and bilateral parahippocampal gyrus, 57 and these neuroplastic changes are positively correlated with motor improvements. Here, we demonstrated that EC strength from the bilateral hippocampus to the contralesional PSC_3a is increased in chronic stroke patients. Our data suggest that the hippocampus might learn and store the missing sensory input originating from the paretic hand to compensate for the information processing of the somatosensory cortex. Collectively, from the perspective of functional integration, our study expands the previous findings that disrupted causal interactions among the SMC, precuneus, temporal cortex, and hippocampus might underlie the pathophysiological mechanisms of hand dysfunction after chronic stroke.
4.2. Differently disrupted sensorimotor circuits between PPH and CPH patients
It is well known that the premotor cortex gives rise to the cortico‐reticulospinal tract and is specifically related to proximal movement, 58 which is a good substitution for hand function recovery after mild to moderate stroke. 50 The premotor cortex involves transferring sensory stimuli into motor programs, 59 and their structural pathways are connected to the parietal lobe associated with motor output. 60 In chronic stroke patients, activation of the contralesional dorsal premotor cortex is increased, and interference of their activity with transcranial magnetic stimulation can deteriorate motor performance. 61 , 62 Furthermore, strengthened EC between the anterior intraparietal sulcus and PMC within the ipsilesional hemisphere is evident in the well‐recovered subgroup. 63 These findings emphasize the important roles of the premotor cortex and its interactions with the parietal lobe in motor dysfunction after chronic stroke. We extended earlier findings by revealing that stroke patients with CPH show increased EC from the ipsilesional inferior parietal lobe to the ipsilesional premotor_6 and PSC_1. Therefore, we speculate that the facilitatory influences from the frontoparietal areas to the SMC system in the lesioned hemisphere might represent greater top‐down control over the SMC to assist motor execution after chronic stroke. 32 The prefrontal cortex and temporal gyrus play crucial roles in higher‐order planning as well as audiomotor coordination during complex hand movement. 20 For stroke patients with acute striatal infarcts, increased gray matter thickness has been found in the frontal and temporal cortices but not the motor cortex. 64 Another longitudinal task fMRI study also demonstrated that reduced activation in the prefrontal and temporal cortices over time is related to motor recovery following stroke. 65 Furthermore, a recent functional connectivity study found that increased coupling between the ipsilesional PMC and the contralesional middle frontal gyrus could predict long‐term motor outcomes after stroke. 25 Well in line with these findings, we found that stroke patients with CPH demonstrate increased EC from the contralesional middle frontal cortex and ipsilesional inferior temporal gyrus to the ipsilesional PSC_1. Recent evidence has revealed that persistent recruitment of remote cortices during spontaneous recovery is associated with poor motor outcomes. 66 Thus, the convergently increased EC from these non‐motor regions to the ipsilesional SMC subregions might contribute to maintaining the final hand outcomes, while it indicates an unoptimizable process of recovery‐associated neuroplasticity in severely stroke patients.
4.3. Dissociated sensorimotor circuits correlated with different hand outcomes
Two different functional reorganizations have been reported after rehabilitation in chronic stroke patients. 67 Specifically, patients with intact or damaged PMCs and their descending motor pathway show decreased or increased activation in the ipsilesional SMC. Interestingly, we also found two brain‐behavior correlations in which paretic hand performance is positively correlated with EC patterns in stroke patients with PPH but negatively correlated with EC patterns in CPH patients. In stroke patients with PPH, convergence of positive correlations to the contralesional PSC_1 indicates that this region holds a highly important function within the sensorimotor network configurations (eg, brain hub) to drive motor recovery. 68 However, in stroke patients with CPH, distributed negative correlations from non‐motor regions (eg, the visual cortex) may represent a compensatory cognitive strategy, for example, visuospatial processing, to sustain poor hand outcomes. Except for physiological processes (eg, decreased GABAergic inhibition and increased NMDA facilitation), 66 excessive activation within the SMC system during paretic hand movement is primarily determined by the structural integrity of the corticospinal tract in stroke patients. 42 , 43 Therefore, we speculate that the different injury loads of the corticospinal tract between CPH and PPH patients may be the important reason for these dissociated positive and negative correlations. 69
4.4. Limitations and future considerations
First, stroke patients show a heavy male predominance because endogenous estrogen can exert neuroprotective effects for premenopausal women away from a higher risk for stroke. 70 To address this bias, we regressed out sex in the statistical analyses. Second, this was not a longitudinal/interventional study, making it difficult to infer the dynamic evolution of EC patterns in SMC subregions during the process of motor recovery. Third, considering the enormous values of injured corticospinal tracts for predicting motor recovery in stroke patients, 7 , 71 it will be promising to combine structural and functional biomarkers for the prediction of treatment responses. Finally, although modulating bilateral PMC targets has been shown to be beneficial for stroke patients with hand dysfunction, 11 , 13 , 14 , 15 , 72 future studies might consider additional targets found in this study (eg, the postcentral gyrus) to design neuromodulation experiments. 41 , 73
5. CONCLUSION
In this study, we systematically investigated EC patterns between sensorimotor subregions and the whole brain after chronic stroke. We found that large‐scale sensorimotor circuits are selectively disrupted and that dissociated motor‐related neurocircuitry is associated with different hand outcomes in chronic stroke patients, which has rarely been reported in previous studies. Our findings indicate that these disrupted sensorimotor circuits might be considered potential neuroimaging biomarkers and stimulation targets to repair lesion‐induced abnormal motor networks, 74 which in turn, facilitate hand rehabilitation after chronic stroke.
CONFLICTS OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
C.T. and Z.Z. developed and designed the study concept; C.T. coordinated and collected the data; F.L. conducted the data analyses under supervision from C.T. and Z.Z.; F.L. and C.T. wrote the initial draft; and all authors revised and confirmed the final version.
Supporting information
Supplementary Material
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Natural Science Foundation of China (82002378), Zhejiang Provincial Natural Science Foundation (LGF19H270001), China Postdoctoral Science Foundation (2020M671726), and Shanghai Sailing Program (20YF1445100).
Liu F, Chen C, Hong W, et al. Selectively disrupted sensorimotor circuits in chronic stroke with hand dysfunction. CNS Neurosci Ther. 2022;28:677–689. doi: 10.1111/cns.13799
DATA AVAILABILITY STATEMENT
Data are available upon request from the corresponding authors.
REFERENCES
- 1. Stinear CM. Prediction of motor recovery after stroke: advances in biomarkers. Lancet Neurol. 2017;16(10):826‐836. [DOI] [PubMed] [Google Scholar]
- 2. Ramos‐Murguialday A, Broetz D, Rea M, et al. Brain‐machine interface in chronic stroke rehabilitation: a controlled study. Ann Neurol. 2013;74(1):100‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui LL, Zhang Y, Chen ZY, Su YY, Liu Y, Boltze J. Early neutrophil count relates to infarct size and fatal outcome after large hemispheric infarction. CNS Neurosci Ther. 2020;26(8):829‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel JS, Ramsey LE, Snyder AZ, et al. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci USA. 2016;113(30):E4367‐E4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corbetta M, Ramsey L, Callejas A, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85(5):927‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dang C, Liu G, Xing S, et al. Longitudinal cortical volume changes correlate with motor recovery in patients after acute local subcortical infarction. Stroke. 2013;44(10):2795‐2801. [DOI] [PubMed] [Google Scholar]
- 7. Feng W, Wang J, Chhatbar PY, et al. Corticospinal tract lesion load: an imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78(6):860‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hong W, Lin Q, Cui Z, Liu F, Xu R, Tang C. Diverse functional connectivity patterns of resting‐state brain networks associated with good and poor hand outcomes following stroke. Neuroimage‐Clinical. 2019;24:102065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang C, Zhao Z, Chen C, et al. Decreased functional connectivity of homotopic brain regions in chronic stroke patients: a resting state fMRI study. PLoS ONE. 2016;11(4):e0152875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao Z, Tang C, Yin D, et al. Frequency‐specific alterations of regional homogeneity in subcortical stroke patients with different outcomes in hand function. Hum Brain Mapp. 2018;39(11):4373‐4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Volz LJ, Rehme AK, Michely J, et al. Shaping early reorganization of neural networks promotes motor function after stroke. Cereb Cortex. 2016;26(6):2882‐2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ameli M, Grefkes C, Kemper F, et al. Differential effects of high‐frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66(3):298‐309. [DOI] [PubMed] [Google Scholar]
- 13. Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. NeuroImage. 2010;50(1):233‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allman C, Amadi U, Winkler AM, et al. Ipsilesional anodal tDCS enhances the functional benefits of rehabilitation in patients after stroke. Sci Transl Med. 2016;8(330):330re1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176‐2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grefkes C, Fink GR. Connectivity‐based approaches in stroke and recovery of function. Lancet Neurol. 2014;13(2):206‐216. [DOI] [PubMed] [Google Scholar]
- 17. Grefkes C, Ward NS. Cortical reorganization after stroke: how much and how functional? Neuroscientist. 2014;20(1):56‐70. [DOI] [PubMed] [Google Scholar]
- 18. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700‐711. [DOI] [PubMed] [Google Scholar]
- 19. Carter AR, Shulman GL, Corbetta M. Why use a connectivity‐based approach to study stroke and recovery of function? NeuroImage. 2012;62(4):2271‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: relating outcome to motor network connectivity. Ann Neurol. 2009;66(5):604‐616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grefkes C, Fink GR. Reorganization of cerebral networks after stroke: new insights from neuroimaging with connectivity approaches. Brain. 2011;134(Pt 5):1264‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carter AR, Astafiev SV, Lang CE, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Qin W, Zhang J, Zhang X, Yu C. Enhanced interhemispheric functional connectivity compensates for anatomical connection damages in subcortical stroke. Stroke. 2015;46(4):1045‐1051. [DOI] [PubMed] [Google Scholar]
- 24. Carter AR, Patel KR, Astafiev SV, et al. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012;26(1):7‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park C‐H, Chang WH, Ohn SH, et al. Longitudinal changes of resting‐state functional connectivity during motor recovery after stroke. Stroke. 2011;42(5):1357‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu J, Liu H, Zhang M, et al. Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci. 2011;31(42):15065‐15071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133(Pt 4):1224‐1238. [DOI] [PubMed] [Google Scholar]
- 28. Rehme AK, Grefkes C. Cerebral network disorders after stroke: evidence from imaging‐based connectivity analyses of active and resting brain states in humans. J Physiol. 2013;591(1):17‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage. 2011;55(3):1147‐1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol. 2008;63(2):236‐246. [DOI] [PubMed] [Google Scholar]
- 31. Wang LE, Fink GR, Diekhoff S, Rehme AK, Eickhoff SB, Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann Neurol. 2011;69(2):375‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inman CS, James GA, Hamann S, Rajendra JK, Pagnoni G, Butler AJ. Altered resting‐state effective connectivity of fronto‐parietal motor control systems on the primary motor network following stroke. NeuroImage. 2012;59(1):227‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325‐1335. [DOI] [PubMed] [Google Scholar]
- 34. Yan CG, Wang XD, Zuo XN, Zang YF. DPABI: data processing & analysis for (resting‐state) brain imaging. Neuroinformatics. 2016;14(3):339‐351. [DOI] [PubMed] [Google Scholar]
- 35. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song X‐W, Dong Z‐Y, Long X‐Y, et al. REST: a toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6(9):e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS ONE. 2013;8(7):e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37(6):1552‐1555. [DOI] [PubMed] [Google Scholar]
- 39. Ktena SI, Schirmer MD, Etherton MR, et al. Brain connectivity measures improve modeling of functional outcome after acute ischemic stroke. Stroke. 2019;50(10):2761‐2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rehme AK, Volz LJ, Feis D‐L, et al. Identifying neuroimaging markers of motor disability in acute stroke by machine learning techniques. Cereb Cortex. 2015;25(9):3046‐3056. [DOI] [PubMed] [Google Scholar]
- 41. Koch PJ, Hummel FC. Toward precision medicine: tailoring interventional strategies based on noninvasive brain stimulation for motor recovery after stroke. Curr Opin Neurol. 2017;30(4):388‐397. [DOI] [PubMed] [Google Scholar]
- 42. Ward NS, Newton JM, Swayne OBC, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129(Pt 3):809‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170‐180. [DOI] [PubMed] [Google Scholar]
- 44. Rehme AK, Fink GR, von Cramon DY, Grefkes C. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal FMRI. Cereb Cortex. 2011;21(4):756‐768. [DOI] [PubMed] [Google Scholar]
- 45. Favre I, Zeffiro TA, Detante O, Krainik A, Hommel M, Jaillard A. Upper limb recovery after stroke is associated with ipsilesional primary motor cortical activity: a meta‐analysis. Stroke. 2014;45(4):1077‐1083. [DOI] [PubMed] [Google Scholar]
- 46. Rehme AK, Eickhoff SB, Rottschy C, Fink GR, Grefkes C. Activation likelihood estimation meta‐analysis of motor‐related neural activity after stroke. NeuroImage. 2012;59(3):2771‐2782. [DOI] [PubMed] [Google Scholar]
- 47. Luft AR, McCombe‐Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292(15):1853‐1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. 2020;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400‐409. [DOI] [PubMed] [Google Scholar]
- 50. Calautti C, Baron JC. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke. 2003;34(6):1553‐1566. [DOI] [PubMed] [Google Scholar]
- 51. Margulies DS, Vincent JL, Kelly C, et al. Precuneus shares intrinsic functional architecture in humans and monkeys. Proc Natl Acad Sci USA. 2009;106(47):20069‐20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. NeuroImage. 2003;20(4):2166‐2180. [DOI] [PubMed] [Google Scholar]
- 53. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564‐583. [DOI] [PubMed] [Google Scholar]
- 54. Lacourse MG, Orr EL, Cramer SC, Cohen MJ. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. NeuroImage. 2005;27(3):505‐519. [DOI] [PubMed] [Google Scholar]
- 55. Albouy G, Sterpenich V, Balteau E, et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58(2):261‐272. [DOI] [PubMed] [Google Scholar]
- 56. Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39(5):1520‐1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang XU, Wang H, Xiong X, et al. Motor imagery training after stroke increases slow‐5 oscillations and functional connectivity in the ipsilesional inferior parietal lobule. Neurorehabil Neural Repair. 2020;34(4):321‐332. [DOI] [PubMed] [Google Scholar]
- 58. Liu J, Wang C, Qin W, et al. Corticospinal fibers with different origins impact motor outcome and brain after subcortical stroke. Stroke. 2020;51(7):2170‐2178. [DOI] [PubMed] [Google Scholar]
- 59. Grefkes C, Eickhoff SB, Nowak DA, Dafotakis M, Fink GR. Dynamic intra‐ and interhemispheric interactions during unilateral and bilateral hand movements assessed with fMRI and DCM. NeuroImage. 2008;41(4):1382‐1394. [DOI] [PubMed] [Google Scholar]
- 60. Schulz R, Koch P, Zimerman M, et al. Parietofrontal motor pathways and their association with motor function after stroke. Brain. 2015;138(Pt 7):1949‐1960. [DOI] [PubMed] [Google Scholar]
- 61. Bestmann S, Swayne O, Blankenburg F, et al. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS‐fMRI. J Neurosci. 2010;30(36):11926‐11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Johansen‐Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99(22):14518‐14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schulz R, Buchholz A, Frey BM, et al. Enhanced effective connectivity between primary motor cortex and intraparietal sulcus in well‐recovered stroke patients. Stroke. 2016;47(2):482‐489. [DOI] [PubMed] [Google Scholar]
- 64. Liu H, Peng X, Dahmani L, et al. Patterns of motor recovery and structural neuroplasticity after basal ganglia infarcts. Neurology. 2020;95(9):e1174‐e1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(Pt 11):2476‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dancause N. Vicarious function of remote cortex following stroke: recent evidence from human and animal studies. Neuroscientist. 2006;12(6):489‐499. [DOI] [PubMed] [Google Scholar]
- 67. Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. NeuroImage. 2006;31(2):710‐720. [DOI] [PubMed] [Google Scholar]
- 68. Warren DE, Power JD, Bruss J, et al. Network measures predict neuropsychological outcome after brain injury. Proc Natl Acad Sci USA. 2014;111(39):14247‐14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7(10):915‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Riley JD, Le VU, Der‐Yeghiaian L, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42(2):421‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nowak DA, Grefkes C, Dafotakis M, et al. Effects of low‐frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65(6):741‐747. [DOI] [PubMed] [Google Scholar]
- 73. Grefkes C, Fink GR. Disruption of motor network connectivity post‐stroke and its noninvasive neuromodulation. Curr Opin Neurol. 2012;25(6):670‐675. [DOI] [PubMed] [Google Scholar]
- 74. Grefkes C, Fink GR. Noninvasive brain stimulation after stroke: it is time for large randomized controlled trials! Curr Opin Neurol. 2016;29(6):714‐720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Data Availability Statement
Data are available upon request from the corresponding authors.


