Abstract
The risk of an aversive consequence occurring as the result of a reward-seeking action can have a profound effect on subsequent behavior. Such aversive events can be described as punishers, as they decrease the probability that the same action will be produced again in the future and increase the exploration of less risky alternatives. Punishment can involve the omission of an expected rewarding event (‘negative’ punishment) or the addition of an unpleasant event (‘positive’ punishment). Although many individuals adaptively navigate situations associated with the risk of negative or positive punishment, those suffering from substance use disorders or behavioral addictions tend to be less able to curtail addictive behaviors despite the aversive consequences associated with them. Here, we discuss the psychological processes underpinning reward seeking despite the risk of negative and positive punishment and consider how behavioral assays in animals have been employed to provide insights into the neural mechanisms underlying addictive disorders. We then review the critical contributions of dopamine signaling to punishment learning and risky reward seeking, and address the roles of interconnected ventral striatal, cortical, and amygdala regions to these processes. We conclude by discussing the ample opportunities for future study to clarify critical gaps in the literature, particularly as related to delineating neural contributions to distinct phases of the risky decision-making process.
Keywords: circuit, addiction, conflict, nucleus accumbens, prefrontal cortex, punishment
Graphical Abstract
Reward seeking can be potently modulated by the risk of an aversive event occurring: threat of reward omission (negative punishment) or concomitant delivery of an unpleasant stimulus (positive punishment) both cause animals to alter their behavior. In this review, we provide an overview of negative and positive punishment-based models of risky reward seeking, discussing the relevance of these approaches to understanding neuropsychiatric diseases such as substance use and alcohol use disorders. We then review literature establishing the necessity of nodes within a meso-cortico-limbic-striatal network that regulate these two types of risky reward seeking.
Legend: Pr (Probability), BLA (basolateral amygdala), D1R (dopamine D1-receptor), D2R (dopamine D2-receptor), IL (infralimbic cortex), LHb (lateral habenula), lOFC (lateral orbitofrontal cortex), mOFC (medial orbitofrontal cortex), mPFC (medial prefrontal cortex), NAcCore (nucleus accumbens core), NAcShell (nucleus accumbens shell), PAG (periaqueductal gray), PL (prelimbic cortex), RMTg (rostromedial tegmental nucleus), VTA (ventral tegmental area).
Introduction
Nearly all choices carry some degree of risk, defined as the possibility that an undesirable outcome might arise from a choice and subsequent course of action (Yates and Stone, 1992). Typically, the risk of an aversive event occurring following a particular action biases future behavior towards other, safer actions. However, this adaptive flexibility is compromised in a variety of neuropsychiatric conditions. Of particular note, alcohol use disorders (AUDs), substance use disorders (SUDs), and behavioral addictions are characterized by continued engagement in behaviors associated with the risk of both short- and long-term adverse consequences (Everitt et al., 2008; Feil et al., 2010; Figee et al., 2016).
Laboratory studies in humans and non-human animals have empirically assessed risk-taking by juxtaposing probabilistically delivered aversive events with reinforcement-seeking behavior or reward delivery (Bechara et al., 1994; Ersche et al., 2016; Jean-Richard-Dit-Bressel et al., 2018; Levy, 2017; Orsini et al., 2015). This general approach is designed to engender a state of internal conflict whereby actions associated with reward are tempered by the motivation to avoid unwanted outcomes (Miller, 1944). An organism’s response to a risky situation is dependent on multiple factors, including its subjective or interoceptive state, the probability of reward or punishment delivery, and the relative strength of these outcomes (and the salience of the stimuli that signal them) (Cardinal and Howes, 2005; Church, 1963; Simon et al., 2009). Studies examining these processes have begun to reveal an interconnected network of brain regions that regulate aspects of risky decision-making, with many of these same neural systems functioning aberrantly in SUDs and animal models of addiction (Everitt et al., 2008; Halladay et al., 2020; Marchant et al., 2013; Pascoli et al., 2015; Radke et al., 2015; Vanderschuren et al., 2017).
In this review, we first discuss approaches commonly employed to study risky reward seeking, focusing mostly on tasks that employ forms of punishment to alter behavior, and describe how these assays have been adapted to study addiction-relevant behavior (Fig. 1). We then review data from studies investigating the neural regulation of risky behaviors in laboratory animals and to a lesser extent, humans. Throughout, we detail pertinent data addressing the contributions of interconnected meso-cortico-limbic-striatal structures to risky decision-making (Fig. 2). This focus on structures interconnected with the midbrain dopamine (DA) system arises from the well-established link between DA dysfunction and psychiatric illnesses characterized by maladaptive risk-seeking behavior (e.g., SUDs) (Feil et al., 2010; Lubman et al., 2004; Volkow and Morales, 2015). We refer the reader to excellent recent reviews that address relevant regions and transmitter systems not discussed here (Jean-Richard-Dit-Bressel et al., 2018; Orsini et al., 2015).
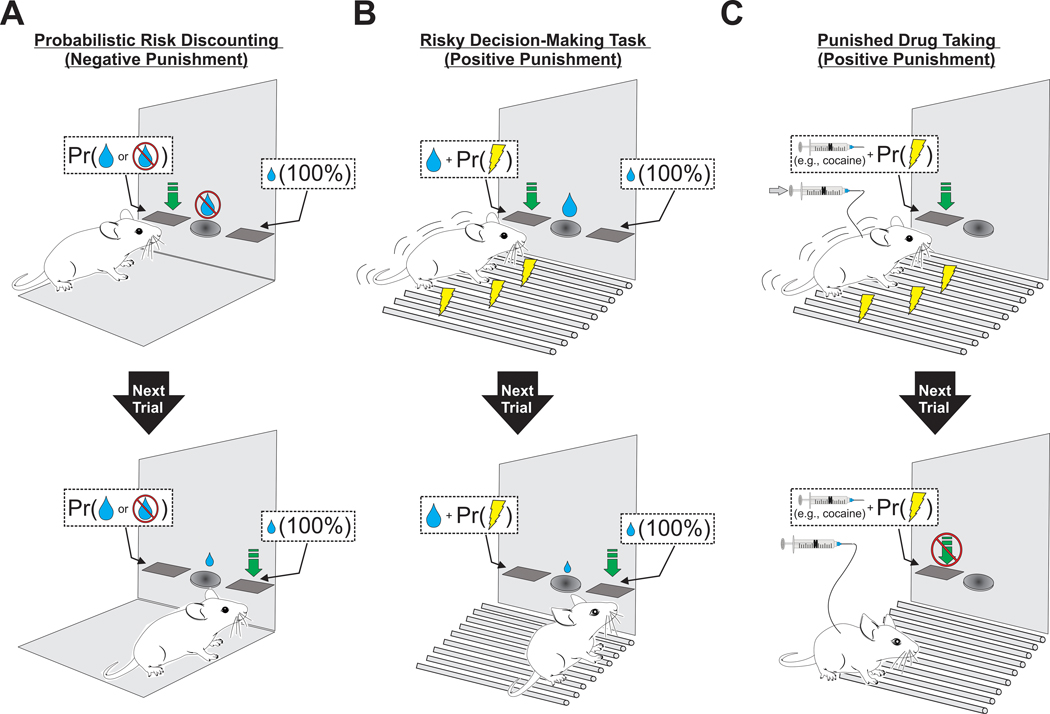
Figure 1.
Cartoon depictions of common methods for assessing risky reward seeking in rodents. (A) During performance of a negatively punished ‘probabilistic risk discounting’ task, animals learn that one lever is associated with a large amount of reward (depicted as a large liquid droplet) but is also associated with a particular probability (Pr) of reward omission (depicted as a droplet + ‘No’ symbol), while another lever guarantees delivery of a small amount of reward (depicted as a small liquid droplet). If the animal presses the large reward lever (green downward arrow) and reward is omitted, animals are more likely to switch away from the large reward option, and towards the small, certain option on the next trial (bottom panel). (B) As in A, an animal trained on a ‘risky decision-making’ task initially discriminates between two levers, one that delivers a large volume of liquid reward, and one that delivers a small volume. Selection of the large reward option is probabilistically (Pr) associated with footshock (yellow lightning bolts) punishment (top), which causes animals to flexibly shift their actions towards the guaranteed, safe option (bottom). (C) Positive punishment-based approaches have been used to examine the mechanisms contributing to ‘compulsive’ drug-related behavior. In this example, an animal self-administering cocaine (syringe) is subjected to probabilistically delivered footshocks concomitant with cocaine-seeking lever-presses (top). In animals sensitive to punishment, experience with footshock can cause animals to become more reticent, engaging in fewer bouts of drug-seeking (bottom). Pr (probability).
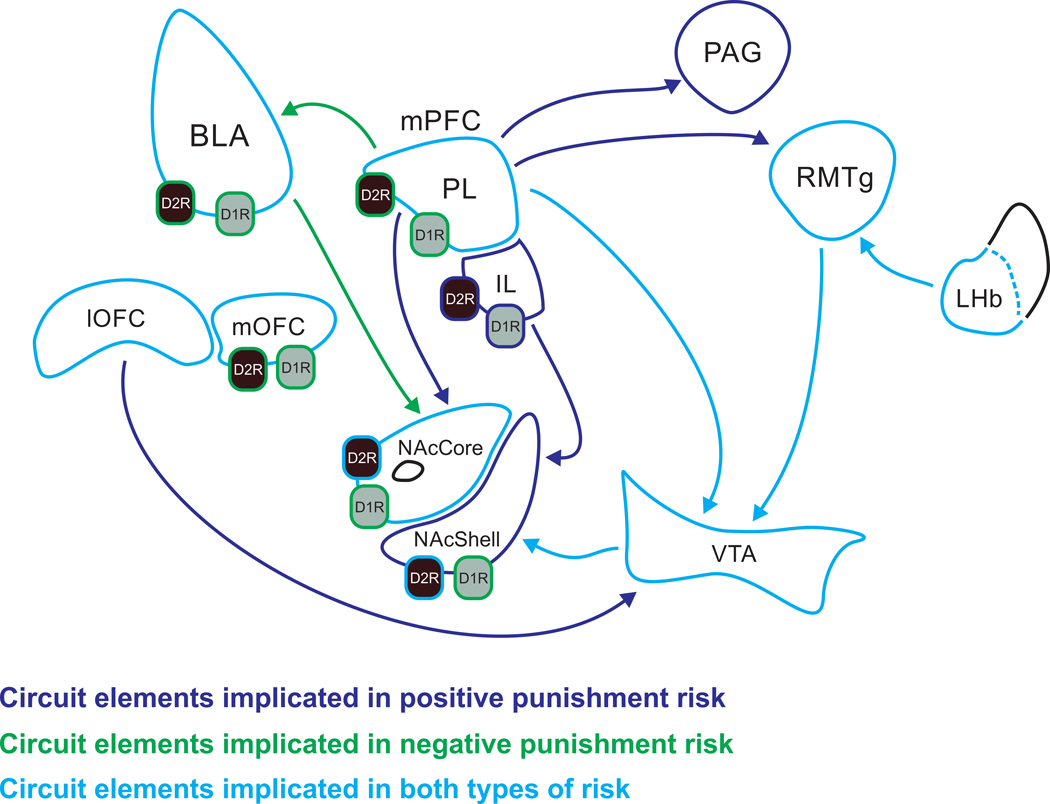
Figure 2.
Neural pathways implicated in risky reward-seeking behaviors. Regions, pathways, and receptors are outlined in different colors to depict their involvement in each form of ‘risk’: dark blue represents circuit elements that have been shown to be involved in positive punishment-based assays, green represents circuit elements involved in negative punishment-based assays, and light blue represents circuit elements that have been linked to both behaviors. Note, anatomical connections between regions that have not explicitly been implicated in risky reward seeking are not displayed. For simplicity, projections from VTA have been mostly eliminated. BLA (basolateral amygdala), D1R (dopamine D1-receptor), D2R (dopamine D2-receptor), IL (infralimbic cortex), LHb (lateral habenula), lOFC (lateral orbitofrontal cortex), mOFC (medial orbitofrontal cortex), mPFC (medial prefrontal cortex), NAcCore (nucleus accumbens core), NAcShell (nucleus accumbens shell), PAG (periaqueductal gray), PL (prelimbic cortex), RMTg (rostromedial tegmental nucleus), VTA (ventral tegmental area).
Assaying reward seeking in the face of risk
When operationalized by behavioral scientists, ‘risk’ is often introduced by associating some proportion of reward-seeking actions with punishment. Punishment is the response-contingent occurrence of an aversive event that causes a subsequent decrement in the behavior perceived to have caused the punishment (for review, see Jean-Richard-Dit-Bressel et al., 2018). Punishments can be thought as being either ‘positive’ or ‘negative,’ in the sense that positive punishment directly results in the addition of an unpleasant outcome (i.e., delivery of a stimulus that causes discomfort or pain), while negative punishment involves the omission of a reinforcing outcome (i.e., risk of loss and reinforcer uncertainty). These two forms of punishment reflect fundamental instrumental learning mechanisms that are apparent in everyday life. Speeding to avoid being late for work comes with the risk of positive punishment - a traffic citation or accident. The possibility of negative punishment also pervades decisions both small (i.e., buying a losing lottery ticket) and large (i.e., purchasing property in a ‘seller’s market’) where there is a risk of making the wrong choice and losing out. Indeed, influential neuroeconomic theories have been built around the power of punishment, particularly the fear of loss, to shape human behavior (Kahneman and Tversky, 1979; Loewenstein et al., 2007).
In laboratory animals (primarily rodents and non-human primates), reward seeking despite loss-related (negative) punishment can be assayed using probabilistic risk discounting or multi-armed bandit tasks (Cardinal and Howes, 2005; St. Onge and Floresco, 2009; Sutton and Barto, 1998). These tasks present subjects with a choice between multiple reward options that are each associated with distinct probabilities of reward omission. In the case of probabilistic risk discounting, rats can be trained to discriminate between two levers, one that delivers a guaranteed small amount of reward, and another that is associated with a large volume of reward, but also a certain probability of reward omission (Fig. 1A, top). Unexpected reward omission following selection of the large ‘risky’ option (i.e., a ‘loss’) normally decreases the probability of this action being performed on subsequent trials, while increasing the exploration of other opportunities to obtain reinforcement (Fig. 1A, bottom). On probabilistic discounting tasks, reward uncertainty increases over discrete blocks of choice trials such that most animals shift their behavior away from preferred ‘risky’ reward option as losses accumulate (Cardinal and Howes, 2005).
Behavioral performance on negative punishment tasks can be statistically modeled using reinforcement learning approaches, which can reveal latent variables, such as value, influencing performance (Constantinople et al., 2019b; Neftci and Averbeck, 2019). Microstructural, trial-by-trial analysis of behavior can also be employed to identify performance strategies adopted by the subject, including those associated with reward sensitivity (e.g., ‘win-stay’– selecting a risky option after ‘winning’ on the previous trial) or negative-feedback sensitivity (e.g., ‘lose-shift’ – switching to the safe or low risk option after ‘losing’ on the previous trial) (Estes, 1994; Orsini et al., 2015). Such computational analyses have provided insight into the psychological and biological processes that mediate negative punishment-based reward seeking.
‘Positive’ punishment tests can be thought of as ‘conflict’ tasks (as they were often originally described; Geller and Seifter, 1960; Vogel et al., 1971) due to the motivational competition produced by the desire to avoid noxious stimuli on one hand, and the prepotent drive to perform an instrumental reward-seeking action on the other. In a typical experimental preparation, a rodent (commonly in a state of food or water deprivation) can be asked to lever-press for reinforcement despite the possibility of footshock delivery concomitant with some proportion of presses (Estes, 1944; Geller and Seifter, 1960). Other noxious stimuli, such as pressurized air puffs (Spealman, 1978) or adulteration of liquid reinforcement with a bitter tastant (Wolffgramm, 1991; Wolffgramm and Heyne, 1991), have also be used as punishers in this kind of task.
The risk of positive punishment can profoundly affect behavior: reward seeking might become more infrequent, anxiety-related changes in posture and approach behavior can emerge, and the animal might begin to investigate other sources of reinforcement, if available (Geller et al., 1962; Geller and Seifter, 1960; Grant and Mackintosh, 1963; Halladay et al., 2020; Hunt and Brady, 1955; Millan and Brocco, 2003; Resstel et al., 2008; Van Der Poel, 1979; Vogel et al., 1971). Positive punishment-based decision-making has been assessed in rats (Simon et al., 2009) and mice (Glover et al., 2020) using a risky decision-making task, whereby animals learn to discriminate between a response option that delivers a guaranteed small reward, and another option delivers a large reward plus a probabilistically determined footshock (Fig. 1B, top). Like reward omission on the probabilistic risk discounting task, footshock punishment generally causes animals to shift their choice preference away from the preferred large reward and towards the explicitly safe small reward option over time (Fig. 1B, bottom).
A variety of experimenter-controlled factors contribute to the magnitude of effect that positive punishment can have on reward-related behaviors. To maintain a level of reinforcement seeking that allows for the detection of experimentally-induced increases in punishment sensitivity, punishment is usually delivered probabilistically (non-continuously), e.g., after a predetermined fraction of instrumental actions (Estes, 1944). In the case of discrete, temporally specific punishers like footshock, stimulus intensity can be titrated such that reinforcement-seeking behavior is not overly counteracted by opposing defensive reactions such as freezing (Estes, 1944; Hunt and Brady, 1955). Experiments must also be carefully designed to accommodate factors that vary due to individual differences. For example, positive punishment in the form of footshock has been shown to have greater inhibitory effects on food reward seeking in female, as compared to male, rodents (Chowdhury et al., 2019; Jacobs and Moghaddam, 2020; Orsini et al., 2016). This effect may not be due solely to sex-related differences in reactivity to the unconditioned stimulus (e.g., footshock) (Archer, 1975; Orsini et al., 2016), suggesting that the reported changes in punishment sensitivity reflect a cognitive phenomenon. Interestingly, despite a greater tendency to avoid risk in drug-free contexts, females are more likely than males to endure positive punishment when responding for drug rewards, including alcohol (Fulenwider et al., 2019; Radke et al., 2019a; Sneddon et al., 2020, but see Sneddon et al., 2019) and opioids (Monroe and Radke, 2020).
Susceptibility to the effect of positive punishment on reward seeking also appears to exist on a continuum, with some individuals being relatively more resistant to punishment-induced inhibition than others (Giuliano et al., 2018; Marchant et al., 2018; Spoelder et al., 2017, 2015). This spectrum of punishment sensitivity may be partially explained by genetic factors: for example, laboratory rats bred to prefer alcohol are often relatively resistant to positive punishment of alcohol drinking (in comparison to their non-preferring counterparts) (Giuliano et al., 2018; Houck et al., 2019; Timme et al., 2020), while various strains of commonly used laboratory mice exhibit marked differences in susceptibility to footshock or quinine-induced suppression of alcohol seeking (Halladay et al., 2017). Finally, it is important to recognize that differences in resistance to punishment can arise from differences in sensitivity to the reinforces or punishments used in the study, and thus controls are necessary to ensure that experimental manipulations do not alter factors such as reactivity to footshock or taste sensitivity.
Punishment-based models of risk seeking relevant to addictions
Abnormalities in risk processing are thought to contribute to the etiology of addictions (Crawford et al., 2003; Hawkins et al., 1992; Pilgrim et al., 2006) and the intractable patterns of compulsive reward seeking that characterize them (Feil et al., 2010; Figee et al., 2016; Koob and Volkow, 2010). One hypothesis is that AUDs, SUDs, and behavioral addictions arise from deficits in the ability to adjust behavior after feedback from ‘positive’ (e.g., adverse health) and ‘negative’ (e.g., monetary losses) punishment experience (Grant et al., 2010), and that these deficits may be triggered or exacerbated by chronic drug exposure. Performance on negative punishment-based tests of risky decision making such as the Iowa Gambling Task (IGT) are reliably impaired in SUD patients, with these individuals preferring options associated with large rewards despite the risk of large losses (Bechara et al., 2001; Bolla et al., 2005; Fishbein et al., 2005; Grant et al., 2000). Impairments in the ability of positive punishment to affect behavior have also been reported in cocaine-addicted individuals (Ersche et al., 2016). These findings have led to the development of animal models assessing the impact of self-administration of, or passive exposure to, abused substances on reward seeking despite punishment.
Consistent with a conceptual framework in which risk processing is impaired in addiction, a number of preclinical studies have shown that cocaine or alcohol exposure reduces sensitivity to losses in the context of negative punishment. For example, the preference for risky options on a rat gambling task was shown to be enhanced in a subset of animals by chronic cocaine self-administration (Cocker et al., 2020; Ferland and Winstanley, 2017), and relapse to cocaine use following abstinence was reported to be stronger in those rats that exhibited greater risk preference (Cocker et al., 2020). These observations are in line with human addiction studies showing that impaired decision-making is predictive of poor treatment outcomes (Darke et al., 2012; Stevens et al., 2013). In another clinical parallel, alcohol exposure during adolescence, which is a known risk factor for the development of AUDs (for review, see Crews et al., 2016), has also been shown to produce a long-lasting enhancement of risk preference as assessed by a loss-related (probabilistic discounting) negative punishment task in rats (Boutros et al., 2015; McMurray et al., 2016; Nasrallah et al., 2009; Schindler et al., 2016).
The ability of positive punishment to curtail drug-seeking behavior in preclinical models can also be altered by drug exposure (for review, see Hopf and Lesscher, 2014; Vanderschuren et al., 2017). Positive punishment-insensitive drug intake was first reported in the literature by Wolffgramm and colleagues (1991) who demonstrated that rats given nine months of alcohol access continued to drink more despite the adulteration of drinking liquid by the bitter tastant quinine, which had a profound punishing effect in alcohol-naïve control rats. Complementary data have also been provided by experiments in rodents whereby drug-seeking or -taking actions are paired with a more temporally restricted punishing stimulus like mild footshock (Fig. 1C). Early studies in monkeys demonstrated that electric shock punishment could inhibit stimulant self-administration, with parameters including stimulant dose and shock intensity modulating the magnitude of behavioral inhibition (Bergman and Johanson, 1981; Grove and Schuster, 1974; Johanson, 1977). More recent investigations in rodents have extended these findings, showing that long-term access to drugs of abuse results in punishment insensitivity in at least some animals (Deroche-Gamonet, 2004; Vanderschuren and Everitt, 2004), paralleling the clinical observation that SUDs develop in only a minority of individuals that partake in drug use (Anthony et al., 1994).
Punishment insensitivity following extended access or exposure to addictive drugs has been demonstrated for cocaine (Chen et al., 2013; Deroche-Gamonet, 2004; Kasanetz et al., 2010; Mitchell et al., 2014; Pelloux et al., 2007; Vanderschuren and Everitt, 2004) and alcohol (Barbier et al., 2017; Hopf et al., 2010; Houck et al., 2019; Kimbrough et al., 2017; Radke et al., 2017; Vendruscolo et al., 2012), as well as other addictive substances such as methamphetamine (Cadet et al., 2016; Torres et al., 2017) and fentanyl (Monroe and Radke, 2020). Extended cocaine access also causes cues associated with footshock to lose their ability to inhibit drug seeking, which is not true of extended exposure to natural rewards like sucrose (Vanderschuren and Everitt, 2004). Notably, the developmental trajectory of this compulsive-like phenotype often aligns with the progressive exacerbation of compulsive drug seeking that occurs in individuals with SUDs (for review, see Everitt and Robbins, 2005).
Positive punishment-based models have generated insights into the component psychological processes contributing to compulsive SUDs. For example, stress experience, which is a known etiological and precipitating factor in SUDs (for review, see Koob, 2009), has been shown to interact with factors such as genetics and sex to produce distinct trajectories of positive punishment-induced changes in reward seeking (Edwards et al., 2013; Radke et al., 2019a; Shaw et al., 2020). Chronic stress in mice (Edwards et al., 2013; Shaw et al., 2020) or early life adversity in rats (Radke et al., 2019a) each diminish the impact of quinine-based punishment on alcohol seeking, consistent with the idea that stressors increase compulsive drug-related behaviors.
Another parameter that can affect punisher efficacy in these models is whether punishment is applied to appetitive (drug-seeking) versus consummatory (drug-taking) actions (Everitt et al., 2018; Lüscher et al., 2020). It has been suggested that these two drug-related behaviors are mediated by separate psychological processes, and thus may be differentially susceptible to factors that contribute to the development of compulsivity, such as punishment sensitivity or habit formation (Everitt and Robbins, 2005; Lüscher et al., 2020). For example, Pelloux and colleagues (2015) behaviorally dissociated an instrumental cocaine-seeking action (e.g., press one lever for access to cocaine) from a separate, cocaine-taking action that became available after rats made a requisite number of seeking responses (e.g., press a second lever to receive cocaine infusion). They found that footshock punishment paired with the seeking response inhibited continued cocaine seeking, while the same punishment paired with the cocaine-taking action was less effective. These data further highlight how a myriad of variables moderate the effects of punishment on reward-seeking behavior, and imply there may be similarly complex factors, varying across individuals and situations, influencing how punishment affects compulsive drug-taking in AUDs and SUDs.
Armed with assays and models of punishment, researchers have worked to illuminate the neural underpinnings of risky decision-making (Fig. 2) – most often studied in drug-naïve animals working for natural rewards such as food – and the dysfunctions that arise in these neural systems following drug exposure. Studies employing the risk of loss (negative punishment) to affect behavior have provided valuable insights into the neural mechanisms that underlie reinforcement learning (Cohen et al., 2012; Fiorillo et al., 2013; Keiflin and Janak, 2015; Schultz, 2013; Schultz et al., 1997) as well as the circuits and neuromodulators that contribute to cost/benefit decision-making (Bercovici et al., 2018; Floresco et al., 2018; Floresco and Whelan, 2009; Jenni et al., 2017; Piantadosi et al., 2016; St. Onge et al., 2012b; Stopper and Floresco, 2011, 2014; Sugam et al., 2012; Zeeb et al., 2009, 2015). Many of the mechanisms uncovered thus far are shared with those contributing to reward seeking under risk of positive punishment, which encompass a network of meso-cortico-limbic-striatal regions and associated neuromodulatory systems critical to aversion and behavioral flexibility (Jean-Richard Dit Bressel and McNally, 2014; Jean-Richard-dit-Bressel and McNally, 2016; Jean-Richard-Dit-Bressel and McNally, 2015; Kim et al., 2011; Pascoli et al., 2015; Piantadosi et al., 2017; Simon et al., 2009; Vento et al., 2017).
Midbrain dopamine: encoding risk prediction, uncertainty, and punishment
Central among the known neural substrates underlying risky reward seeking are mesolimbic-projecting DA neurons of the midbrain ventral tegmental area (VTA). Alterations in DA function have been suggested to contribute to the abnormal risk-based neurocomputations evident in a variety of neuropsychiatric illnesses and a raft of studies have reported DA system dysregulation in AUDs and SUDs (for review, see Trifilieff and Martinez, 2014; Volkow et al., 2009). Behavioral addictions have also been linked to DA dysfunction: patients with restless-leg syndrome or Parkinson’s disease who receive treatment with DA-receptor agonists occasionally develop iatrogenic gambling addiction, suggesting that excessive risk-taking can be produced by DA receptor activation (Grall-Bronnec et al., 2018; Moore et al., 2014).
Dopamine, prediction error and risk
Based on the clinical evidence linking addiction with DA dysfunction and the fact that laboratory studies show that most drugs of abuse affect the function of mesolimbic-projecting DA neurons (Di Chiara and Imperato, 1988; Sulzer, 2011; Wise and Bozarth, 1998; Wise and Robble, 2020), work in rodents and non-human primates has attempted to identify the mechanisms (both psychological and cellular) by which the DA system, and perturbations of DA function, can affect risky reward seeking. This work has often been based around the observation that phasic signaling of mesolimbic DA neurons links cues or actions with rewards (Saddoris et al., 2015; Saunders et al., 2018) and encodes reward prediction error (RPE) – a discrepancy between an expected and received outcome that can be positive (outcome better than expected) or, as in the case of punishment, negative (outcome worse than expected) (Cohen et al., 2012; Fiorillo et al., 2013; Keiflin and Janak, 2015; Schultz, 2013; Schultz et al., 1997).
Reward learning models predict that these RPE signals are key to informing future actions or decisions by updating expectations based on knowledge accrued from prior experiences (Pearce and Hall, 1980; Rescorla and Wagner, 1972). Direct experimental evidence for this supposition comes from studies in rodents that have artificially instantiated RPEs via optogenetic manipulation of VTA DA neurons, such that stimulation of these neurons mimics positive RPEs and produces cue-related learning, while their inhibition can simulate negative RPEs and improve learning related to reward omissions (Chang et al., 2016; Steinberg et al., 2013). Of particular relevance to the current review, electrical stimulation of VTA neurons delivered during unexpected reward omissions has been shown to increase risky choices in rats performing a risk-discounting task, potentially by counteracting the ‘loss signal’ that would be expected to occur in VTA DA neurons as a result of negative RPE encoding (Stopper et al., 2014b). Thus, DA coding of RPEs appears central to the ability of feedback to adapt reward seeking in the face of negative punishment risk.
DA neurons can also contribute to risky reward seeking through certain non-RPE mechanisms. For example, DA neurons have been suggested to encode the degree of reward uncertainty associated with an action (Schultz, 2007). This takes the form of a ramping in neuronal activity prior to reward delivery that scales with the probability of reward omission, with maximal activity evident on the most uncertain trials (Fiorillo, 2003). This uncertainty-induced ramping of DA activity could reinforce risky actions, a computational process which may be maladaptively co-opted in disorders like addiction which are characterized by aberrant DA function (Di Chiara, 1999; Keiflin and Janak, 2015; Redish, 2004). Indeed, optogenetic self-stimulation of VTA DA neurons in mice produces compulsive reinforcement seeking that is insensitive to footshock punishment, further implying that DA hyperactivity is sufficient to produce excessively risky behavior (Pascoli et al., 2015).
Finally, the DA system may mediate aspects of risky reward seeking through the direct signaling of aversive events. While classic studies in monkeys and rats showed preferential DA firing to expectation violations for rewarding events (Mirenowicz and Schultz, 1996; Ungless et al., 2004), there is now compelling evidence from multiple species that subpopulations of DA neurons in the VTA and substantia nigra can be excited by aversive stimuli and cues that predict them (Badrinarayan et al., 2012; de Jong et al., 2019; Lammel et al., 2011, 2012; Matsumoto and Hikosaka, 2009a; McCutcheon et al., 2012). These aversion-encoding DA neurons are largely distinct from RPE-encoding DA neurons, as they tend to be localized more medially within the VTA (or laterally in the substantia nigra) and also express distinct molecular machinery (e.g., vesicular glutamate transporter 2) (Lerner et al., 2015; Verharen et al., 2020b). By encoding aversive or punishing outcomes, the activity of this subset of DA neurons could provide an important neural signal to multiple processes relevant to risk assessment (Oleson et al., 2012; Park and Moghaddam, 2017; Pultorak et al., 2018; Stelly et al., 2019; Verharen et al., 2020a; Wenzel et al., 2018).
Upstream DA modulators
DA neurons are positioned to affect risky reward seeking through multiple processes, from revising expectations and encoding uncertainty about specific courses of action, to signaling the aversive consequences of these actions. These functions are integrated across a wider network by brain regions upstream and downstream of midbrain DA neurons (Fig. 2). Particularly noteworthy in the context of risk is an upstream trisynaptic pathway connecting the glutamatergic lateral habenula (LHb) to the primarily gamma-aminobutyric acid (GABA)-expressing rostromedial tegmental nucleus (RMTg) and on to VTA DA neurons (Bromberg-Martin and Hikosaka, 2011; Hong et al., 2011; Matsumoto and Hikosaka, 2009b, 2007). A number of recent studies in rodents and non-human primates have suggested that aversive events activate the LHb to RMTg pathway, resulting in phasic decreases in DA neuron activation consistent with negative RPEs (Hong et al., 2011; Jhou et al., 2009; Li et al., 2019). In rats, pharmacological inactivation of the LHb or the RMTg decreased risk seeking in a probabilistically reinforced decision making context, suggesting that each structure is necessary for optimal decision-making in the face of loss-related feedback (Stopper and Floresco, 2014).
Direct evidence that phasic activation of these nuclei contribute to action selection in the face of negative punishment risk comes from a separate study utilizing the same behavioral assay, showing that pairing electrical stimulation of the LHb or RMTg with the delivery of risky wins (i.e., wins occurring despite a low expectation of reward, potentially causing positive RPEs in DA neurons) decreases risky actions, whereas stimulation of the LHb during the pre-choice deliberation period elevates response latencies and biases actions away from risky options (Stopper and Floresco, 2014). These findings suggest that outcome-related activity in the LHb may instantiate negative RPEs in DA neurons that are necessary for the adjustment of behavior following negative punishment.
Emerging evidence suggests that positively punished reward seeking engages communication between many of these same structures that ultimately converge on VTA DA neurons (Jhou and Vento, 2019). Initial studies in rats and monkeys suggested that the LHb contributes to the vigor of reward seeking, but was not directly involved in positive punishment-driven shifts in reward seeking (Jean-Richard Dit Bressel and McNally, 2014) or the ability of aversive conditioned stimuli and outcomes to affect DAergic RPE signals (Tian and Uchida, 2015). However, more recent findings in rats indicate that inputs from the LHb to the RMTg contribute to punished suppression of reward seeking when the punishment is maximally unpredictable (Li et al., 2019). The RMTg may be supported in performing these more nuanced computations by other regions that relay distinct types of information during punished decision-making. For example, optogenetically silencing projections from the prelimbic region of the medial prefrontal cortex and the parabrachial nucleus to the RMTg increases punishment resistance in rats when silencing occurs during choice deliberation and outcome evaluation, respectively (Li et al., 2019). In turn, optogenetically silencing RMTg terminals in the VTA has been shown to increase risky reward seeking when laser delivery was timed to occur either when rats received footshock punishment or when animals were deciding to engage in reward seeking that might incur punishment (Vento et al., 2017).
Other inhibitory afferents to the VTA can also regulate the ability of positive punishment to inhibit reward seeking. In mice, optogenetic excitation of an inhibitory input from lateral hypothalamus to the VTA, for example, has been reported to decrease compulsive-like sucrose seeking despite the threat of footshock (Nieh et al., 2015), while hyperexcitability of an orbitofrontal cortex excitatory input to the VTA can drive compulsive actions (Pascoli et al., 2015). Of note, unlike instrumental punishment, the ability of cues that predict potential punishment to suppress sucrose seeking may not depend on VTA DA neurons in some scenarios, as a recent investigation showed that chemogenetic activation of these neurons in rats does not affect cue-induced behavioral inhibition (Verharen et al., 2020a). Although there remain many unknowns regarding the specific contributions of VTA to risky reward seeking, these findings broadly support the idea that the regulation of VTA neuron excitability by multiple upstream structures is critical to punishment risk-induced changes in reward seeking.
Nucleus accumbens: utilizing dopamine to adjust risky behavior
Once inputs converge and are computed at the level of DA neurons, these neurons send signals to multiple projection targets to affect risky decision-making. Of the forebrain structures targeted by VTA neurons, mesolimbic projections to the nucleus accumbens (NAc) have long been recognized as integral to reward processing, learning, and incentive motivation (Berridge, 2012; de Jong et al., 2019; Radke et al., 2019b; Robbins and Everitt, 1992; Stern and Passingham, 1996; Sugam et al., 2012). In rodents, the activity of NAc neurons and DA release within this nucleus encodes outcomes and drives reward-seeking actions (Ambroggi et al., 2008, 2011; Ikemoto and Panksepp, 1999; Nicola, 2010; Saunders et al., 2018; Sugam et al., 2012, 2014). In a more explicit link to risky decision-making, phasic DA release in the NAc is correlated with reward preference and scales with outcomes in a manner consistent with RPE encoding in rats performing a loss-based punishment task (Sugam et al., 2012). DA levels in the NAc of rats also track parameters relevant to motivational state during performance on a negative-punishment based probabilistic discounting task, including reward rate and subjective preference (St. Onge et al., 2012a). Hence, a plausible hypothesis is that DA release in the NAc reflects changes in motivation related to the presence of risky or volatile patterns of reinforcement.
In line with this view, elevated stimulus-evoked DA signaling within the NAc is associated with increased (loss-based) risk preference and enhanced incentive motivation in adult rats that were exposed to alcohol during adolescence (Nasrallah et al., 2011; Spoelder et al., 2015). Furthermore, these behavioral abnormalities can be reversed by the normalization of VTA DA neuron activity produced by systemic administration of a GABA agonist, implying a causal contribution of this region to risk-based computations (Schindler et al., 2016). Pharmacological and optogenetic manipulations have directly demonstrated the necessity of the NAc and its DA innervation to updating actions based on changes in risk probability (Cocker et al., 2012; Floresco et al., 2018; Freels et al., 2020; Mai et al., 2015; St. Onge et al., 2012a; Stopper et al., 2013; Stopper and Floresco, 2011; Sugam et al., 2012; Zalocusky et al., 2016). For instance, inhibiting neural activity within the NAc of rats decreases risk-seeking behavior in loss-related punishment tasks (Cardinal and Howes, 2005; Stopper and Floresco, 2011). Dopamine itself can alter neural activity and plasticity within the NAc by affecting its cognate D1-receptor (D1R) and D2-receptor (D2R), receptors which are expressed in mostly non-overlapping populations medium spiny projections neurons in the striatum (Baimel et al., 2019; Gerfen et al., 1990; Scudder et al., 2018) and which induce opposite effects on cellular excitability, with D1R activation increasing excitability and D2R activation decreasing excitability (for review, see Tritsch and Sabatini, 2012).
Studies examining the role of these NAc DA receptors in risk-related actions have generally found that the D1R family of DA receptors influence loss-related decisions, while the D2R family can mediate negative feedback more generally across punishment types. In rats, intra-NAc microinfusion of the D1R/D2R antagonist flupenthixol or the D1R-specific antagonist SCH23390 decreases risk seeking, reducing the preference for reward options associated with losses (Mai et al., 2015; Stopper et al., 2013). On the other hand, pharmacological blockade (via eticlopride) or activation (via quinpirole) of NAc D2Rs was found to be without effect when tested on the same negative punishment-based task (Stopper et al., 2013). Interestingly, activation of D2Rs on NAc neurons in rats can promote risky behavior in some instances, possibly by preventing dynamic changes in D2R activation that signal unexpected reward omission and promote shifts in choice following negative feedback (Zalocusky et al., 2016). Indeed, measuring changes in calcium activity in D2R-expressing NAc neurons revealed that these neurons are active following losses, and their activity during subsequent pre-choice task phases biases rats towards less preferred, but safe (guaranteed) reward options (Zalocusky et al., 2016). In keeping with the idea that the activation of D2R-expressing NAc neurons opposes risk-seeking, this same study demonstrated that optogenetic activation of these neurons induces a bias away from risky actions, specifically in a subset of rats identified as risk-preferring.
Although less numerous, data from positive-punishment based tasks also generally support the idea that D2R-expressing NAc neurons signal negative feedback related to aversive events. For example, infusion of the D2R/D3R agonist quinpirole into the NAc of rats reduces risk-taking, and NAc D2R gene expression is inversely correlated with adolescent risk-taking in the same footshock-based punishment task (Mitchell et al., 2014). Blockade of NAc D2Rs with sulpiride on a conflict-based reward-seeking task resulted in rats expressing less avoidance of a cue associated with prior punishment (while D1R antagonism had the opposite effect) (Nguyen et al., 2018). These findings support prior observations that D2Rs in the NAc mediate the active avoidance of aversive and punishing stimuli (Blomeley et al., 2018; Boschen et al., 2011; Danjo et al., 2014; Zalocusky et al., 2016), as well as clinical data that D2R availability is reduced in the ventral striatum of patients with SUDs (for review, see Volkow et al., 2009).
Greater clarity into the relationship between the NAc and risky reward seeking will likely be provided by more detailed analyses of the two anatomically and neurochemically dissociable NAc subregions, the NAcCore and NAcShell (Floresco, 2015; Zahm, 2000), although extant data to this end are conflicting. For example, pharmacological inactivations of the rat NAcCore produce a generalized reduction in the vigor of behaviors associated with reward (sucrose) seeking or punishment avoidance, without directly affecting risk seeking per se (Floresco et al., 2018; Piantadosi et al., 2018, 2017). Yet, in rodent models of quinine-resistant alcohol drinking, both N-methyl-D-aspartate (NMDA)-receptor modulation and chemogenetic inhibition of medium spiny neurons in the NAcCore can selectively reduce punished drinking, without generally affecting motivation (Seif et al., 2015, 2013; Sneddon et al., 2021). This pattern of results suggests that, depending on the nature of the reward (e.g., drug vs. natural reinforcer) or the type of functional manipulation, the NAcCore can be shown to promote or suppress reward-seeking behaviors.
Regarding the function of the NAcShell on negative punishment-based tasks, pharmacological inactivation has been shown to either decrease (Stopper and Floresco, 2011) or increase (Floresco et al., 2018) risky choices in rats. In contrast, inhibiting neural activity in the NAcShell generally increases risky behavior in mice and rats performing positive punishment-based assays, resulting in continued to reward seeking despite the prospect of footshock, for example (Halladay et al., 2020; Piantadosi et al., 2018, 2017). In rats, phasic DA release in the NAcShell, but not NAcCore, increases in response to punishing stimuli (Badrinarayan et al., 2012), and evoked DA release is positively correlated with preference for large, risky rewards (those associated with footshock punishment) (Freels et al., 2020).
In sum, the NAc contributes to multiple processes relevant to risky reward seeking. Still, much work remains to be done to dissect the precise functions of the NAc, with present data regarding the direct contributions of this region to reward seeking despite negative and positive punishment risk appearing largely equivocal. An important component of future investigations will be to examine the contributions afferent inputs to the NAc, such as those arising from the prefrontal cortex (PFC) and amygdala, to these behaviors.
Cortical contributions to risky decision-making
Neuropsychological studies of patients with focal brain lesions have established the orbitofrontal cortex (OFC) and medial PFC (mPFC) as important arbiters of decision-making in gambling tasks where risk is characterized by the potential for diminished or omitted reward (Bechara et al., 1999, 1994; Clark et al., 2008, 2003; Fellows and Farah, 2005). Patients with damage to either region perform more poorly on these tasks, not adapting their reward-seeking strategies despite mounting ‘losses’ and displaying minimal physiological responsivity in anticipation of reward (or punishment) related feedback (Bechara et al., 1999, 1994). These observations have led to hypotheses ascribing a role for these cortical regions in conveying emotional content to actions that have the potential to be punished (Bechara, 2000). Functional neuroimaging experiments in humans have provided insight into specific risk-related processes that alter metabolic activity in the OFC and ventromedial PFC, including uncertainty and arousal (Blankenstein et al., 2017; Critchley et al., 2001; Hsu et al., 2005; Huettel et al., 2006; Knutson et al., 2001; Tobler et al., 2007), punishment and devaluation (Gottfried et al., 2002; O’Doherty et al., 2001; Valentin et al., 2007), task-feedback after losses (Steward et al., 2019), and guesses (Bolla et al., 2004; Elliott et al., 1997). While there are differences in prefrontal anatomy between the human, non-human primate, and rodent brain (Heilbronner et al., 2016; Laubach et al., 2018; Wise, 2008), studies in laboratory animals have been valuable in helping define the roles of cortical structures to risky decision-making.
Orbitofrontal cortex: subjective outcome valuation and re-valuation
As in the case of midbrain DA neurons, it is likely that distinct subpopulations of OFC neurons encode specific aspects of task performance during risk seeking, such as reward or punishment probability, and the updating of these contingencies across trials (Hikosaka and Watanabe, 2000; Murray and Izquierdo, 2007; O’Neill and Schultz, 2018; Rolls et al., 1996; Scott et al., 2005; Simmons and Richmond, 2008; Tremblay and Schultz, 2000, 1999). For example, in non-human primates performing a delayed reaction time task, a majority of recorded OFC neurons encoded reward outcome expectation, with a subset of these neurons doing so in a reward-type specific manner (Hikosaka and Watanabe, 2000). Similarly, in a memory guided saccade task, a greater proportion of OFC neurons encoded expected reward value than probability of reward or risk (O’Neill and Schultz, 2018). Consistent with this idea, neuronal recordings, again in non-human primates, have revealed separate populations of OFC neurons that encode reward and risk predictions as a function of experience (O’Neill and Schultz, 2013; Raghuraman and Padoa-Schioppa, 2014).
Two main anatomical subdivisions of the rodent OFC have been implicated in processes relevant to risky decision-making: the lateral OFC (lOFC) and medial OFC (mOFC) (Fig. 2). To study OFC function in the context of loss-related punishment, work in rodents has often made use of behavioral assays that model human/non-human primate gambling tasks. For example, in vivo recordings performed in a rat version of the two-arm bandit task found that lOFC neurons encode choice outcome (i.e., reward value) in a manner that influences encoding and performance on subsequent trials (Sul et al., 2010). This finding suggests a role of the lOFC in updating the value of rewards following violations in outcome expectation; a notion consistent with lesion/inactivation studies showing the lOFC is critical for updating behavior following reward devaluation (Gallagher et al., 1999; Graybeal et al., 2011; Pickens et al., 2005; but see Ostlund and Balleine, 2007) and changes in stimulus-reward contingency (Dalley et al., 2004; Dalton et al., 2016; Groman et al., 2019; Izquierdo, 2017; Mobini et al., 2002; Pais-Vieira et al., 2007; Piantadosi et al., 2019; Ragozzino, 2007). Neuronal recordings in rats have revealed that lOFC activity tracks outcome value and uncertainty in alternative choice tasks that employ spatial (Feierstein et al., 2006; Roesch et al., 2006) or odor (Kepecs et al., 2008; Schoenbaum et al., 1998) cues, as well as violations of outcome expectation (in the form of errors) (Kepecs et al., 2008).
However, when explicitly examining negative punishment in the form of probabilistic discounting, decreasing neural activity within lOFC fails to alter behavior in well-trained rats (St. Onge and Floresco, 2010; Zeeb and Winstanley, 2011). This finding suggests that, although the lOFC may contribute to establishing or updating subjective biases (Miller et al., 2018; Mobini et al., 2002), it is not required for their maintenance once established. This seems to echo evidence that lOFC encoding of risk and reward outcome differs in individuals based upon subjective reward evaluation; risk-preferring rats exhibit increased lOFC neuronal activity on receipt of large rewards when obtained in risky, as compared to risk-free, trials (Roitman and Roitman, 2010). Interestingly, adolescent exposure to alcohol, which elevates risk-seeking behavior in adulthood, paradoxically diminishes the activity of a population of reward-encoding neurons in the lOFC of rats (McMurray et al., 2016). lOFC neurons have also been shown to strongly encode reward history on a probabilistic reward choice task, yet silencing this region optogenetically during the task epoch associated with reward-history related lOFC activity does not affect reward choice in rats (Constantinople et al., 2019a). Instead, optogenetic lOFC inhibition prior to choices decreased the pro-risk bias that ‘wins’ on preceding trials had on subsequent action selection (Constantinople et al., 2019a). Although these data suggest that lOFC neurons may track reward value in a manner dependent on risk preference, whether these neural correlates relate directly to choice bias remains unclear.
Surprisingly, there is a relative paucity of data on the role of the lOFC in reward seeking despite positive punishment. Increased neuronal excitability in the lOFC has been shown to correlate with resistance to footshock punishment in mice compulsively self-stimulating DA neurons (Pascoli et al., 2015). Tamping down lOFC excitation via lesions or chemogenetic has been found to decrease reward seeking in the face of footshock risk in both rats and mice (Orsini et al., 2015; Pascoli et al., 2015). Pharmacological inhibition of this region in rats has also been reported to reduce engagement in a cue-guided punishment task, which may reflect an enhanced sensitivity to positive punishment-induced avoidance (Verharen et al., 2019). These effects are suggestive of an impairment in outcome encoding following a loss of lOFC function, which would be broadly consistent with the findings from negative punishment-based tasks discussed above.
However, other studies are seemingly inconsistent with this view: in rats, lOFC inactivation can disinhibit footshock-punished reward seeking (Jean-Richard-dit-Bressel and McNally, 2016), while lesions of this region do not produce increases in cocaine seeking despite footshock punishment (Pelloux et al., 2013). Aside from technical differences stemming from the precise location targeted and degree of functional inactivation or compensation, these discrepancies argue against a simple unitary role for lOFC in encoding aversive outcomes to guide risky behavior. Instead, it is likely that heterogeneity within ensembles of lOFC neurons (based upon distinct anatomical location, inputs and outputs, and/or molecular characteristics) allows this region to provide complex task or context-specific control over reward-seeking behavior (for review, see Izquierdo, 2017). Providing support for the supposition that divergent lOFC outputs dissociably affect behavior, a recent study in rats found distinct functional roles for OFC neurons that project to the NAcCore or amygdala in utilizing positive or negative feedback, respectively, following reward expectation violations (Groman et al., 2019). Another study showed that depotentiation of dorsomedial striatum-projecting lOFC neurons in mice was sufficient to reverse resistance to footshock punishment in mice self-stimulating DA neurons (Pascoli et al., 2018). Developing a more granular picture of the functional circuity through which lOFC acts to affect punished decision-making should prove to be a fruitful avenue for work in the coming years.
Until recently, the contribution of mOFC to risky reward seeking has not been an area of intense study. Damage to the mOFC in humans and monkeys impairs value-guided decisions, resulting in reward-seeking actions that are relatively haphazard and loosely linked to their expected value (Noonan et al., 2017, 2010). In rodents, limited data suggest that the mOFC may oppose negative punishment-based risk seeking by lessening the impact of low probability ‘wins’ (i.e., unexpected reward delivery) on subsequent actions (Stopper et al., 2014a), though a null effect has been reported in a comprehensive study of mOFC function in economic choice (Gardner et al., 2018). In the latter study, optogenetic inhibition was ineffective at altering behavior when delivered at the onset of trials during which rats could choose between visual cues that predicted rewards of distinct magnitude, which in well-trained animals does not involve ‘risk’ per se (Gardner et al., 2018). Thus, the mOFC may contribute to risk processing operationalized as negative punishment. In support of this, recent data from a rat probabilistic discounting task showed that blocking D1-receptors in the mOFC reduced risk-seeking by enhancing the effect of losses on upcoming trials, while D2-receptor blockade tended to shift choice towards riskier options (Jenni et al., 2021).
In the context of positive punishment risk, data generally support a role for the mOFC in opposing risk-seeking that is comparable to negative punishment-based tasks. The ability to use a conditioned stimulus to inhibit actions that could incur footshock punishment is impaired following lesioning (Ma et al., 2020) or inactivation (Verharen et al., 2019) of the rat mOFC. Compulsive (footshock resistant) alcohol seeking in mice has also been associated with alterations in the excitability of mOFC neurons and the expression of genes for specific NMDA-receptor subunits (Radke et al., 2017). While limited in scope, these data together suggest that mOFC may generally function to oppose risk-seeking across positive and negative punishment-based designs, though the effect of local neuromodulation via DA may recruit subpopulations of neurons to produce distinct effects.
Medial prefrontal cortex: top-down inhibition and updating biases
In humans, the anterior cingulate cortex (ACC) and dorsolateral PFC (dlPFC) are suggested to support decision-making via their contribution to enacting executive functions such as inhibitory control (Bembich et al., 2014; Manes et al., 2002). This view aligns well with the findings of human neuroimaging studies demonstrating activation of the dlPFC and ACC during probabilistic decision-making as assessed by the Iowa Gambling Task (IGT), which requires participants to select from decks of cards associated with hidden probabilities of reward and punishment, using feedback from previous selections to avoid losses and maximize gains (Bolla et al., 2005; Ernst et al., 2003, 2002; Li et al., 2010; Mohr et al., 2010). Neuropsychological studies support a causal role for dlPFC activity on the IGT and similar laboratory assays of negative punishment, with patients harboring dlPFC lesions making riskier choices (i.e., opting for low probability, high value options versus high probability, low value options) than non-lesioned participants (Clark et al., 2003; Fellows and Farah, 2005; Manes et al., 2002).
Bearing in mind the significant differences in cortical anatomy across species, many studies have demonstrated that the rodent mPFC mediates adaptive behavior following the introduction of probabilistically-delivered positive punishment (Halladay et al., 2020; Jacobs and Moghaddam, 2020; Orsini et al., 2018) or reward omission (Passecker et al., 2019; Rivalan et al., 2011; St. Onge and Floresco, 2010) on reward-seeking tasks (Fig. 2). In the context of loss-related negative punishment, disrupting neural transmission in the mPFC renders rats insensitive to reward contingency shifts (Balleine and Dickinson, 1998) and can impair the normal evaluation of risky actions (Jentsch et al., 2010; Paine et al., 2015, 2013; Piantadosi et al., 2016; St. Onge et al., 2012b; St. Onge and Floresco, 2010; van Holstein and Floresco, 2020; Zeeb et al., 2015). Inactivation of the mPFC prevents rats from updating their choice bias adaptively during risky negative punishment tasks (Jentsch et al., 2010; St. Onge and Floresco, 2010).
Performance on positive punishment-based tasks in rodents also appears to require mPFC activity. For instance, in vivo recordings of calcium activity indicate that mPFC neuron activity adapts as rats learn about the risk of a footshock punishment, such that the phasic inhibitory responses that occur during the execution of risky actions early in learning are diminished late in learning (Jacobs and Moghaddam, 2020). These correlative responses likely reflect a causal contribution of the mPFC, given that inhibiting neural activity in this region can result in rat reward-seeking behavior that is indifferent to the risk of positive punishment (Friedman et al., 2015; Orsini et al., 2018; Resstel et al., 2008; but see Jean-Richard-dit-Bressel and McNally, 2016).
This literature is generally in keeping with the commonly held view that mPFC is recruited to inhibit risky reward seeking, and that this function may be diminished in addiction (Hu et al., 2019; Lüscher et al., 2020; Smith and Laiks, 2018). For example, silencing rat mPFC neurons promotes, while activating this region attenuates, cocaine-seeking behavior in the face of potential footshock (Chen et al., 2013). It should be noted that the role of mPFC in regulating punished reward seeking is likely pathway dependent. In the context of alcohol seeking, silencing mPFC neurons projecting to the NAcShell increases alcohol seeking in mice that would otherwise be inhibited by a prior experience with response-contingent footshock punishment (Halladay et al., 2020). Similar optogenetic inhibition of a mPFC input to the midbrain periaqueductal gray (PAG) increases risky alcohol seeking in mice, possibly by preventing the aversive properties of the punishing stimulus from being encoded (whether footshock or the bitter taste of quinine) (Siciliano et al., 2019). In contrast, optogenetic inhibition of mPFC (or insular cortex) projections to the NAcCore has been shown to decrease risky alcohol seeking in rats (Seif et al., 2013).
Another factor that could contribute to heterogeneity in risk related mPFC function is the existence of prefrontal subregions, including the prelimbic (PL; functionally analogous to area 32 of the human ACC) and infralimbic (IL; akin to human ACC area 25) cortices, that are at least partially anatomically and functionally distinct (Passecker et al., 2019; van Holstein and Floresco, 2020; but see Zeeb et al., 2015; Moorman et al., 2015). In this context, while neurons in both the IL and PL are activated by a positive punishment-based risky decision-making task in mice, only IL activity correlated with lower levels of risk-taking (Glover et al., 2020). Likewise, subregion-specific recordings have found a population of neurons in the IL, but not PL, that encode alcohol-seeking behaviors in mice prior to footshock punishment, but this response disappears in favor of signaling behaviors associated with the punishment-induced inhibition of alcohol seeking (Halladay et al., 2020).
Alterations in synaptic plasticity and the excitability of PL pyramidal neurons have also been correlated with resistance to punishment in rats self-administering cocaine (Chen et al., 2013; Kasanetz et al., 2013). Within this same region, neurons encode outcomes associated with distinct probabilities of reinforcement, and guide upcoming actions based on prior feedback (Passecker et al., 2019). Optogenetic silencing of rat PL neurons (Passecker et al., 2019) or mouse NAcShell-projecting IL neurons (Halladay et al., 2020), has been found to increase risky actions, consistent with work demonstrating that pharmacological inactivation of either PL or IL produces insensitivity to cued punishment, perhaps by impairing the ability to use the learned cue-punish association to inhibit behavior (Verharen et al., 2019). These data argue for some degree of functional overlap between rodent mPFC subregions, which could be accomplished by distinct neuronal populations that project to separate downstream regions, but exist across the traditional mPFC subregion boundaries (Moorman et al., 2015).
Given the importance of DA to risky decision-making, it is noteworthy that the mPFC is reciprocally connected to the midbrain, and punishment or aversive events can induce changes in the dynamic communication between VTA cells and a dorsal mPFC region encompassing the PL in rats (Park and Moghaddam, 2017; Vander Weele et al., 2018). Furthermore, alcohol exposure causes dendritic abnormalities in the mouse mPFC (Jury et al., 2017) and alterations in markers for DA synthesis in the rat mPFC that are associated with increased risk preference (Boutros et al., 2015). Microinfusion of a D1R antagonist (SCH23390) or D2R agonist (quinpirole) into the IL of rats opposes footshock-punished reward seeking and promotes goal-directed behavior in the absence of punishment, suggesting prefrontal DA modulates adaptive behavior following changes in action-outcome contingency (Barker et al., 2013). This effect is similar to the blockade of mPFC D1Rs on a probabilistic discounting task in rats, which results in less risk seeking as a result of enhancing the impact of losses on future behavior (St. Onge et al., 2011).
DA can also affect risk seeking in distinct ways depending on the specific mPFC output pathway affected. For example, disrupting D2R-based modulation of mPFC neurons prevented rats from updating behavior following losses via output to the amygdala, while disrupting D1R-based mPFC modulation increased risky actions via projections to the NAc (Jenni et al., 2017). This dissociation ties in with growing evidence that the mPFC regulates footshock-punished reward seeking via discrete output pathways: mouse mPFC neurons projecting to the NAc, but not VTA, have been implicated in inhibition of punished reward seeking (Halladay et al., 2020; Kim et al., 2017). This raises a pertinent corollary question regarding what role, if any, mPFC inputs to the VTA play in regulating punished behaviors (Jhou and Vento, 2019; Verharen et al., 2020a). Overall, these data establish mPFC as a region that can regulate risky reward seeking largely via the top-down regulation of inhibitory control and behavioral flexibility. Identifying how specific subregions and their outputs contribute to distinct domains of risk seeking remains an area of active study.
Amygdala: encoding punished outcomes to guide future actions
The amygdala shares reciprocal projections with the OFC and mPFC, receives input from midbrain DA neurons, and innervates the NAc, factors which make this region particularly well positioned to regulate risky decision-making (Fig. 2). Indeed, amygdala interactions with cortical areas are known to mediate processes that are likely key to regulating behavior in the face of potential punishment across species, including the encoding of reward values (Malvaez et al., 2019; Rudebeck et al., 2013) and the updating of information about stimulus/response-outcome relationships following contingency shifts (Baxter et al., 2000; Burgos-Robles et al., 2017; Fiuzat et al., 2017). Studies in rodents have shown that the utilization of outcome-related information to guide action selection in risky contexts is critically dependent on the amygdala (Bercovici et al., 2018; Ghods-Sharifi et al., 2009; Jean-Richard-Dit-Bressel and McNally, 2015; Malvaez et al., 2019; Orsini et al., 2015; Piantadosi et al., 2017; Zeeb and Winstanley, 2011). Loss of these functions could explain the observation that humans with amygdala lesions exhibit suboptimal patterns of choice on gambling tasks and blunted autonomic responses to wins and losses indicative of impaired affective engagement (Bechara et al., 1999; Gupta et al., 2011).
Among the amygdala subnuclei pertinent to risky reward seeking, studies in rodents have consistently implicated the basolateral nucleus of the amygdala (BLA). Plasticity within this nucleus is critical to various forms of aversive learning, including Pavlovian fear and instrumental avoidance, both of which are impaired by manipulations that decrease BLA activity (Bravo-Rivera et al., 2014; Choi et al., 2010; Fanselow and LeDoux, 1999; Lázaro-Muñoz et al., 2010; Ramirez et al., 2015; Sengupta and Holmes, 2019). Given that positive punishment necessitates the integration of a primary aversive stimulus (e.g., footshock) with reward-seeking motivation, it is unsurprising that BLA activity is necessary for risk to inhibit reward seeking or induce choice flexibility (Ishikawa et al., 2020; Jean-Richard-Dit-Bressel and McNally, 2015; Orsini et al., 2015; Pelloux et al., 2013; Piantadosi et al., 2017). For example, pharmacological inactivation of BLA causes rats to continue to engage in reward-seeking behavior despite footshock punishment (Jean-Richard-Dit-Bressel and McNally, 2015; Piantadosi et al., 2017), and lesions of this nucleus prevent rats from shifting to reward options that are not associated with footshock risk (Orsini et al., 2015). This function extends to drug-seeking contexts, as BLA lesions have been shown to produce compulsive, footshock resistant cocaine-seeking in rats with extensive self-administration experience (Pelloux et al., 2013). Based on these results, BLA appears to be recruited to inhibit reward-seeking in the context of positive punishment.
In contrast to the risk-avoiding role of the BLA indicated by these studies, evidence suggests that the BLA typically promotes reward seeking in the face of negative punishment (Ghods-Sharifi et al., 2009; Larkin et al., 2016; Tremblay et al., 2014; but see Zeeb and Winstanley, 2011). Pharmacological inactivation of the rat BLA was shown to enhance loss-sensitivity, decreasing the selection of a preferred, but risky, action in favor of a less-preferred safe action (Ghods-Sharifi et al., 2009). Similarly, BLA lesions decreased the willingness of rats to ‘chase losses’, which refers to the preference to continue gambling to avoid a small loss at the risk of larger future losses (Tremblay et al., 2014). Blockade of D1Rs, receptors which natively function to increase the excitability of BLA neurons and promote reward seeking (Di Ciano and Everitt, 2004; Kröner et al., 2005), also decrease risky decisions in rats by diminishing the impact of wins (i.e., reward delivery despite low probability) on subsequent choices (Larkin et al., 2016). The BLA likely interacts with a distributed cortico-limbic-striatal network to modify behavior in the face of negative punishment risk. Pharmacological disconnections in rats performing a probabilistic discounting negative punishment task suggested that the BLA may interact with the NAc to promote risky actions despite loss, while top-down inhibition of this nucleus by the mPFC may be necessary to temper the drive towards preferred rewards mediated by the BLA (St. Onge et al., 2012b).
One apparent conclusion from these primarily pharmacological experiments is that the BLA fulfils opposing roles in modifying reward seeking depending on whether punishment is positive or negative: biasing mice away or towards risky options, respectively. Providing nuance to this generalization, recent optogenetic experiments have begun to reveal distinct contributions of BLA to particular phases of the risky decision-making process (Orsini et al., 2019). One such study optogenetically inhibited the BLA of rats during either the pre-choice deliberation phase or the outcome encoding phase of a footshock-punished decision-making task and found that post-choice silencing increased, while pre-choice silencing decreased risk-taking (Orsini et al., 2017). The post-choice silencing effect fits well with the view that the BLA encodes aversive outcomes to guide future actions; hence, the loss of BLA function during this epoch would make rats effectively ‘blind’ to the introduction of punishment and maintain reward seeking despite the potential harm (Jean-Richard-Dit-Bressel and McNally, 2015; Piantadosi et al., 2017). However, the finding that BLA inhibition timed to the pre-choice epoch decreased footshock-punished risk seeking is harder to reconcile with prior pharmacological experiments. One parsimonious explanation for this apparent disconnect is that BLA activity prior to choices promotes the selection of preferred rewards across domains of risky decision-making. Partial support for this hypothesis has been provided by a study that used pre-choice optogenetic silencing of rat BLA projections in the NAc to reduce responding for the preferred large (but uncertain) reward in a negative punishment task, although this bias was only present when the probability of reward was high (when the chance of reward was low, silencing instead increased risk-taking) (Bercovici et al., 2018).
Although the BLA is of clear importance to utilizing the experience of punished outcomes to adjust behavior, exactly how this region contributes to deliberations about risky actions remains a key question that is yet be fully parsed. Part of the answer likely lies in task-phase specific functional distinctions between BLA outputs to subregions of the NAc and other projection targets, including the central amygdala and ventral hippocampus, which likely represent valence in distinct ways (Beyeler et al., 2018, 2016). Dissecting these pathways in the context of risky decision-making should provide a rich seam for future studies to mine. Another key question relates to how BLA produces opposite patterns of behavior following reward omission or positive punishment. A recent investigation in rats provided compelling evidence that footshock induced suppression of cocaine seeking is disinhibited by BLA inactivation, while reward omission (extinction) induced suppression of cocaine seeking is inhibited by BLA inactivation (Pelloux et al., 2018). These data, in combination with the studies highlighted above, suggest a fundamental distinction in how BLA encodes positive versus negative punishment to affect reward seeking. Future experiments using projection-specific (e.g., calcium-imaging based) measurement of BLA neuron activity during risky reward-seeking may help disentangle the specific neural ensembles recruited by particular types of aversive outcomes.
Conclusions and key questions for the future
Here we have reviewed a host of data which establishes meso-cortico-limbic-striatal brain regions as integral nodes in various domains of risky reward-seeking behavior. While our functional understanding of these regions remains incomplete, a broad picture of their contributions has begun to emerge. The activity of DA neurons of the VTA are tightly regulated by upstream regions, enabling these neurons to signal aspects of incentive motivation and prediction error via output to targets including the NAc. Cortical regions also receive DA input, which likely influences the ability of subregions like the OFC to generate predictions about outcomes and the mPFC to maintain choice flexibility. Finally, the amygdala is necessary to update actions based on the risk inferred from the occurrence of salient outcomes, including wins and punishments.
Although this picture of circuit function is compelling, there are critical gaps in our understanding that have only begun to be addressed. One such question relates to the relevance of the recently appreciated diversity of VTA neuron composition and function (Morales and Margolis, 2017; Verharen et al., 2020b). For example, subtypes of VTA neurons can co-release DA and amino acid transmitters like glutamate within the NAc to mediate aversion (Qi et al., 2016; Yamaguchi et al., 2015; Zhang et al., 2015). Other non-canonical VTA neurons appear to release GABA and/or glutamate in structures known to regulate risk seeking such as the LHb and mPFC (Kabanova et al., 2015; Root et al., 2014; Stamatakis et al., 2013).
Even within ‘classical’ VTA DA neurons, DA release in terminal regions such as the mPFC and NAcCore and NAcShell is evoked by stimuli of distinct valence (de Jong et al., 2019; Kim et al., 2017; Lammel et al., 2011). Phasic DA release in the medial NAcShell is increased by aversive stimuli and the cues that predict them, whereas DA release in other regions of the NAc is generally decreased in response to the same stimuli (Badrinarayan et al., 2012; de Jong et al., 2019). Importantly, such heterogeneity may be the rule, rather than the exception, across the neural landscape. For example, molecularly distinct projection populations that contribute differently to positive and negative valence behaviors exist in the BLA (Beyeler et al., 2018, 2016; Kim et al., 2017; Shen et al., 2019) and other amygdala subregions (Hardaway et al., 2019; Janak and Tye, 2015; Miller et al., 2019). These data speak to functional diversity at the level of a given brain region, characteristics which have yet to be thoroughly investigated in the realm of risky reward seeking.
Related to such functional diversity, developing a map of the neural mechanisms contributing to risky actions necessitates an appreciation for the multiple convergent sub-processes that contribute to action selection (Orsini et al., 2019). Risky decisions can be conceptualized as being comprised of at least two distinct phases: the pre-choice deliberation phase and the outcome/feedback phase. Optogenetic techniques provide the ability to delineate the function of these epochs by enabling temporally precise control of neuronal excitability. Indeed, recent data have demonstrated that contributions to these distinct task epochs may be dissociable, even within a given brain region (Bercovici et al., 2018; Constantinople et al., 2019a; Friedman et al., 2015; Hernandez et al., 2019; Li et al., 2019; Orsini et al., 2017; Stopper et al., 2014b). This complex regulation may have important implications for the implementation of therapeutics designed to improve maladaptive decision-making. Treatments that monotonically affect regions or circuits may not be sufficient to reverse decision-making deficits unless they are delivered during particular phases of the decision-making process (Adams et al., 2017; Orsini et al., 2019). More detailed analyses in model systems using temporally precise manipulations to dissect component processes of decision-making will be useful in this regard.
In conjunction with epoch-specific analysis, there is a need to continue to move towards understanding the pathways and circuits that contribute to risky actions, rather than single brain regions. Many of the regions highlighted here are at most one or two synapses upstream or downstream from each other, leaving little doubt that functional connectivity between these nodes is critical. Techniques to identify inputs and outputs of discrete genetically-defined populations (e.g., Schwarz et al., 2015) and to trace and transsynaptically manipulate specific pathways (e.g., Zingg et al., 2020, 2017) provide avenues to investigate the connectomics of risky reward seeking. For example, in rats, the BLA contributes to aspects of risky decision-making via input to the NAc (Bercovici et al., 2018), yet it is unclear what influence this excitatory afferent has on NAc activity during discrete decision-making epochs to affect behavior. Additionally, BLA (like many cortico-limbic regions) sends projections to both the NAcShell and NAcCore, with these anatomically segregated pathways potentially contributing differentially to the inhibition and invigoration of behavior, respectively (Fig. 2; Cisneros-Franco and de Villers-Sidani, 2018; Folkes et al., 2020; Millan et al., 2017; Piantadosi et al., 2017; Stuber et al., 2012). Evaluating the function of these and other subregion-specific pathways may provide clarity to the sometimes-conflicting findings observed across positive and negative punishment tasks.
Finally, while compulsive behaviors in AUDs and SUDs incur consequences that can be considered punishers, such as job loss or health complications, these events are rarely experienced immediately on drug seeking or taking, rather, they are often delayed or accumulate on long timescales. This contrasts with the situation in a typical rodent behavioral assay, where the punishment is temporally proximal to the reward-seeking action(s) (Fig. 1C) and has led to reasonable criticisms of the translational significance of the pre-clinical paradigms (for expanded discussions, see Epstein and Kowalczyk, 2018; Vanderschuren et al., 2017). Perhaps many of the paradigms described in this review may be most relevant to individuals considering or seeking treatment for their addiction, given that in these cases there is a clear recognition of the threat of punishment, even if remote. Nonetheless, in recognition of the potential distinction between delayed and immediate punishment, assays of delayed punishment for use in animals have been developed. Delayed punishment has been shown to reduce cocaine self-administration in monkeys, albeit less effectively than immediate punishment (Woolverton et al., 2012). Similarly, recent work has shown that delayed punishment is less effective than immediate punishment at affecting subsequent risky choices, with this effect being more prominent in male rats than female rats (Liley et al., 2019; Rodríguez et al., 2018). This preliminary work sets the stage for future studies to further develop and characterize delayed punishment paradigms and assess the extent to which they may recruit neural mechanisms distinct from those involved in the effects of immediate punishment.
Ultimately, the data reviewed here provide evidence that a distributed set of interconnected brain regions contribute to risky reward seeking. The literature paints a picture of both shared and divergent neural regulation of risky reward-seeking behaviors, depending on the way in which risk is operationalized. Critically, dysfunction within many of these neural structures has been linked to disorders characterized by inappropriate behavior in the face of negative consequences, including AUDs, SUDs, and behavioral addictions. The use of cross-species assessments employing cutting-edge optical and viral techniques will continue to refine our understanding of the mechanisms contributing to reward seeking despite negative and positive punishment. These pre-clinical investigations have the potential to improve the lives of individuals suffering from these disorders by identifying mechanisms that may be exploited in the future to develop novel therapeutics.
Acknowledgements
Figure 1 utilized graphics from SciDraw.io, which are accessible under the Creative Commons 4.0 license. The authors would like to thank Ethan Tyler and Lex Kravitz for making a “Mouse” .svg available (doi.org/10.5281/zenodo.3925901), as well as Hassan Ghayas for making a “Syringe” .svg available (doi.org/10.5281/zenodo.4152947). P.T.P. and A.H. are supported by the NIAAA Intramural Research Program, including a Center on Compulsive Behaviors Fellowship to P.T.P. L.R.H. is supported by startup funds from Santa Clara University. A.K.R is supported by an R15 from NIH (AA027915).
Abbreviations
- ACC
anterior cingulate cortex
- AUD
alcohol use disorder
- BLA
basolateral amygdala
- D1R
dopamine D1-receptor
- D2R
dopamine D2-receptor
- DA
dopamine
- dlPFC
dorsolateral PFC
- GABA
gamma-aminobutyric acid
- IGT
Iowa Gambling Task
- IL
infralimbic cortex
- LHb
lateral habenula
- lOFC
lateral orbitofrontal cortex
- mOFC
medial orbitofrontal cortex
- mPFC
medial prefrontal cortex
- NAc
nucleus accumbens
- NAcCore
nucleus accumbens core
- NAcShell
nucleus accumbens shell
- NMDA
N-methyl-D-aspartate
- OFC
orbitofrontal cortex
- PAG
periaqueductal gray
- PFC
prefrontal cortex
- PL
prelimbic cortex
- RMTg
rostromedial tegmental nucleus
- RPE
reward prediction error
- SUD
substance use disorder
- VTA
ventral tegmental area
Footnotes
Conflicts of interest
The authors have no conflicts of interest to report.
References
- Adams WK, Haar CV, Tremblay M, Cocker PJ, Silveira MM, Kaur S, Baunez C, Winstanley CA, 2017. Deep-Brain Stimulation of the Subthalamic Nucleus Selectively Decreases Risky Choice in Risk-Preferring Rats. eNeuro 4. 10.1523/ENEURO.0094-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL, 2011. Roles of Nucleus Accumbens Core and Shell in Incentive-Cue Responding and Behavioral Inhibition. J. Neurosci. 31, 6820–6830. 10.1523/JNEUROSCI.6491-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM, 2008. Basolateral Amygdala Neurons Facilitate Reward-Seeking Behavior by Exciting Nucleus Accumbens Neurons. Neuron 59, 648–661. 10.1016/j.neuron.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, Kessler RC, 1994. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 2, 244–268. 10.1037/1064-1297.2.3.244 [DOI] [Google Scholar]
- Archer J, 1975. Rodent sex differences in emotional and related behavior. Behav. Biol. 14, 451–479. 10.1016/S0091-6773(75)90636-7 [DOI] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ, 2012. Aversive Stimuli Differentially Modulate Real-Time Dopamine Transmission Dynamics within the Nucleus Accumbens Core and Shell. J. Neurosci. 32, 15779–15790. 10.1523/JNEUROSCI.3557-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, McGarry LM, Carter AG, 2019. The Projection Targets of Medium Spiny Neurons Govern Cocaine-Evoked Synaptic Plasticity in the Nucleus Accumbens. Cell Rep. 28, 2256–2263.e3. 10.1016/j.celrep.2019.07.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A, 1998. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology 37, 407–419. 10.1016/s0028-3908(98)00033-1 [DOI] [PubMed] [Google Scholar]
- Barbier E, Johnstone AL, Khomtchouk BB, Tapocik JD, Pitcairn C, Rehman F, Augier E, Borich A, Schank JR, Rienas CA, Van Booven DJ, Sun H, Nätt D, Wahlestedt C, Heilig M, 2017. Dependence-induced increase of alcohol self-administration and compulsive drinking mediated by the histone methyltransferase PRDM2. Mol. Psychiatry 22, 1746–1758. 10.1038/mp.2016.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JM, Torregrossa MM, Taylor JR, 2013. Bidirectional modulation of infralimbic dopamine D1 and D2 receptor activity regulates flexible reward seeking. Front. Neurosci. 7. 10.3389/fnins.2013.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA, 2000. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J. Neurosci. Off. J. Soc. Neurosci. 20, 4311–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, 2000. Emotion, Decision Making and the Orbitofrontal Cortex. Cereb. Cortex 10, 295–307. 10.1093/cercor/10.3.295 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW, 1994. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50, 7–15. 10.1016/0010-0277(94)90018-3 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP, 1999. Different Contributions of the Human Amygdala and Ventromedial Prefrontal Cortex to Decision-Making. J. Neurosci. 19, 5473–5481. 10.1523/JNEUROSCI.19-13-05473.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE, 2001. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39, 376–389. 10.1016/S0028-3932(00)00136-6 [DOI] [PubMed] [Google Scholar]
- Bembich S, Clarici A, Vecchiet C, Baldassi G, Cont G, Demarini S, 2014. Differences in time course activation of dorsolateral prefrontal cortex associated with low or high risk choices in a gambling task. Front. Hum. Neurosci. 8. 10.3389/fnhum.2014.00464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercovici DA, Princz-Lebel O, Tse MT, Moorman DE, Floresco SB, 2018. Optogenetic Dissection of Temporal Dynamics of Amygdala-Striatal Interplay during Risk/Reward Decision Making. eneuro 5, ENEURO.0422–18.2018. 10.1523/ENEURO.0422-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Johanson CE, 1981. The effects of electric shock on responding maintained by cocaine in rhesus monkeys. Pharmacol. Biochem. Behav. 14, 423–426. 10.1016/0091-3057(81)90413-5 [DOI] [PubMed] [Google Scholar]
- Berridge KC, 2012. From prediction error to incentive salience: mesolimbic computation of reward motivation: From prediction error to incentive salience. Eur. J. Neurosci. 35, 1124–1143. 10.1111/j.1460-9568.2012.07990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Chang C-J, Silvestre M, Lévêque C, Namburi P, Wildes CP, Tye KM, 2018. Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala. Cell Rep. 22, 905–918. 10.1016/j.celrep.2017.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, Tye KM, 2016. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron 90, 348–361. 10.1016/j.neuron.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenstein NE, Peper JS, Crone EA, van Duijvenvoorde ACK, 2017. Neural Mechanisms Underlying Risk and Ambiguity Attitudes. J. Cogn. Neurosci. 29, 1845–1859. 10.1162/jocn_a_01162 [DOI] [PubMed] [Google Scholar]
- Blomeley C, Garau C, Burdakov D, 2018. Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat. Neurosci. 21, 29–32. 10.1038/s41593-017-0023-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL, 2005. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage 26, 480–492. 10.1016/j.neuroimage.2005.02.012 [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL, 2004. Sex-related differences in a gambling task and its neurological correlates. Cereb. Cortex N. Y. N 1991 14, 1226–1232. 10.1093/cercor/bhh083 [DOI] [PubMed] [Google Scholar]
- Boschen SL, Wietzikoski EC, Winn P, Cunha CD, 2011. The role of nucleus accumbens and dorsolateral striatal D2 receptors in active avoidance conditioning. Neurobiol. Learn. Mem. 96, 254–262. 10.1016/j.nlm.2011.05.002 [DOI] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Liu W, Crews FT, Markou A, 2015. Adolescent Intermittent Ethanol Exposure Is Associated with Increased Risky Choice and Decreased Dopaminergic and Cholinergic Neuron Markers in Adult Rats. Int. J. Neuropsychopharmacol. 18. 10.1093/ijnp/pyu003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk GJ, 2014. Neural Structures Mediating Expression and Extinction of Platform-Mediated Avoidance. J. Neurosci. 34, 9736–9742. 10.1523/JNEUROSCI.0191-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O, 2011. Lateral habenula neurons signal errors in the prediction of reward information. Nat. Neurosci. 14, 1209–1216. 10.1038/nn.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, Anandalingam KK, Pagan-Rivera PA, Anahtar M, Beyeler A, Tye KM, 2017. Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat. Neurosci. 20, 824–835. 10.1038/nn.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Walther D, Brannock C, Ladenheim B, McCoy MT, Collector D, Torres OV, Terry N, Jayanthi S, 2016. Increased expression of proenkephalin and prodynorphin mRNAs in the nucleus accumbens of compulsive methamphetamine taking rats. Sci. Rep. 6. 10.1038/srep37002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ, 2005. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neurosci. 6, 37. 10.1186/1471-2202-6-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Esber GR, Marrero-Garcia Y, Yau H-J, Bonci A, Schoenbaum G, 2016. Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat. Neurosci. 19, 111–116. 10.1038/nn.4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau H-J, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A, 2013. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362. 10.1038/nature12024 [DOI] [PubMed] [Google Scholar]
- Choi J-S, Cain CK, LeDoux JE, 2010. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn. Mem. 17, 139–147. 10.1101/lm.1676610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TG, Wallin-Miller KG, Rear AA, Park J, Diaz V, Simon NW, Moghaddam B, 2019. Sex differences in reward- and punishment-guided actions. Cogn. Affect. Behav. Neurosci. 19, 1404–1417. 10.3758/s13415-019-00736-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM, 1963. The varied effects of punishment on behavior. Psychol. Rev. 70, 369–402. 10.1037/h0046499 [DOI] [PubMed] [Google Scholar]
- Cisneros-Franco JM, de Villers-Sidani E, 2018. Bidirectional Control of Risk-Seeking Behavior by the Basolateral Amygdala. eneuro 5, ENEURO.0168–18.2018. 10.1523/ENEURO.0168-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, Robbins TW, 2008. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain 131, 1311–1322. 10.1093/brain/awn066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW, 2003. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia 41, 1474–1483. 10.1016/S0028-3932(03)00081-2 [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Dinelle K, Kornelson R, Sossi V, Winstanley CA, 2012. Irrational Choice under Uncertainty Correlates with Lower Striatal D2/3 Receptor Binding in Rats. J. Neurosci. 32, 15450–15457. 10.1523/JNEUROSCI.0626-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocker PJ, Rotge J-Y, Daniel M-L, Belin-Rauscent A, Belin D, 2020. Impaired decision making following escalation of cocaine self-administration predicts vulnerability to relapse in rats. Addict. Biol. 25, e12738. 10.1111/adb.12738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N, 2012. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature 482, 85–88. 10.1038/nature10754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Piet AT, Bibawi P, Akrami A, Kopec C, Brody CD, 2019a. Lateral orbitofrontal cortex promotes trial-by-trial learning of risky, but not spatial, biases. eLife 8, e49744. 10.7554/eLife.49744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Piet AT, Brody CD, 2019b. An Analysis of Decision under Risk in Rats. Curr. Biol. 29, 2066–2074.e5. 10.1016/j.cub.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AM, Pentz MA, Chou C-P, Li C, Dwyer JH, 2003. Parallel developmental trajectories of sensation seeking and regular substance use in adolescents. Psychol. Addict. Behav. J. Soc. Psychol. Addict. Behav. 17, 179–192. 10.1037/0893-164X.17.3.179 [DOI] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP, Broadwater MA, Robinson DL, 2016. Adolescent Alcohol Exposure Persistently Impacts Adult Neurobiology and Behavior. Pharmacol. Rev. 68, 1074–1109. 10.1124/pr.115.012138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ, 2001. Neural Activity in the Human Brain Relating to Uncertainty and Arousal during Anticipation. Neuron 29, 537–545. 10.1016/S0896-6273(01)00225-2 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW, 2004. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci. Biobehav. Rev. 28, 771–784. 10.1016/j.neubiorev.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wang NY, Phillips AG, Floresco SB, 2016. Multifaceted Contributions by Different Regions of the Orbitofrontal and Medial Prefrontal Cortex to Probabilistic Reversal Learning. J. Neurosci. 36, 1996–2006. 10.1523/JNEUROSCI.3366-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S, 2014. Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A. 111, 6455–6460. 10.1073/pnas.1404323111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Campbell G, Popple G, 2012. Retention, early dropout and treatment completion among therapeutic community admissions. Drug Alcohol Rev. 31, 64–71. 10.1111/j.1465-3362.2011.00298.x [DOI] [PubMed] [Google Scholar]
- de Jong JW, Afjei SA, Pollak Dorocic I, Peck JR, Liu C, Kim CK, Tian L, Deisseroth K, Lammel S, 2019. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 101, 133–151.e7. 10.1016/j.neuron.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche-Gamonet V, 2004. Evidence for Addiction-like Behavior in the Rat. Science 305, 1014–1017. 10.1126/science.1099020 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, 1999. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol. 375, 13–30. 10.1016/S0014-2999(99)00372-6 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A, 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. 85, 5274–5278. 10.1073/pnas.85.14.5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ, 2004. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. Off. J. Soc. Neurosci. 24, 7167–7173. 10.1523/JNEUROSCI.1581-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW, 2013. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl. Psychiatry 3, e296. 10.1038/tp.2013.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Frith CD, Dolan RJ, 1997. Differential neural response to positive and negative feedback in planning and guessing tasks. Neuropsychologia 35, 1395–1404. 10.1016/S0028-3932(97)00055-9 [DOI] [PubMed] [Google Scholar]
- Epstein DH, Kowalczyk WJ, 2018. Compulsive Seekers: Our take. Two Clinicians’ Perspective on a New Animal Model of Addiction. Neuropsychopharmacology 43, 677–679. 10.1038/npp.2017.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED, 2002. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 26, 682–691. 10.1016/S0893-133X(01)00414-6 [DOI] [PubMed] [Google Scholar]
- Ernst M, Kimes AS, London ED, Matochik JA, Eldreth D, Tata S, Contoreggi C, Leff M, Bolla K, 2003. Neural substrates of decision making in adults with attention deficit hyperactivity disorder. Am. J. Psychiatry 160, 1061–1070. 10.1176/appi.ajp.160.6.1061 [DOI] [PubMed] [Google Scholar]
- Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LHE, Luijten M, de Wit S, Sahakian BJ, Bullmore ET, Robbins TW, 2016. Carrots and sticks fail to change behavior in cocaine addiction. Science 352, 1468–1471. 10.1126/science.aaf3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK, 1994. Toward a statistical theory of learning. Psychol. Rev. 101, 282–289. 10.1037/0033-295X.101.2.282 [DOI] [Google Scholar]
- Estes WK, 1944. An experimental study of punishment. [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW, 2008. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. B Biol. Sci. 363, 3125–3135. 10.1098/rstb.2008.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Giuliano C, Belin D, 2018. Addictive behaviour in experimental animals: prospects for translation. Philos. Trans. R. Soc. B Biol. Sci. 373, 20170027. 10.1098/rstb.2017.0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW, 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489. 10.1038/nn1579 [DOI] [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE, 1999. Why We Think Plasticity Underlying Pavlovian Fear Conditioning Occurs in the Basolateral Amygdala. Neuron 23, 229–232. 10.1016/S0896-6273(00)80775-8 [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF, 2006. Representation of Spatial Goals in Rat Orbitofrontal Cortex. Neuron 51, 495–507. 10.1016/j.neuron.2006.06.032 [DOI] [PubMed] [Google Scholar]
- Feil J, Sheppard D, Fitzgerald PB, Yücel M, Lubman DI, Bradshaw JL, 2010. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci. Biobehav. Rev. 35, 248–275. 10.1016/j.neubiorev.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ, 2005. Different Underlying Impairments in Decision-making Following Ventromedial and Dorsolateral Frontal Lobe Damage in Humans. Cereb. Cortex 15, 58–63. 10.1093/cercor/bhh108 [DOI] [PubMed] [Google Scholar]
- Ferland J-MN, Winstanley CA, 2017. Risk-preferring rats make worse decisions and show increased incubation of craving after cocaine self-administration. Addict. Biol. 22, 991–1001. 10.1111/adb.12388 [DOI] [PubMed] [Google Scholar]
- Figee M, Pattij T, Willuhn I, Luigjes J, van den Brink W, Goudriaan A, Potenza MN, Robbins TW, Denys D, 2016. Compulsivity in obsessive–compulsive disorder and addictions. Eur. Neuropsychopharmacol. 26, 856–868. 10.1016/j.euroneuro.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, 2003. Discrete Coding of Reward Probability and Uncertainty by Dopamine Neurons. Science 299, 1898–1902. 10.1126/science.1077349 [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Song MR, Yun SR, 2013. Multiphasic Temporal Dynamics in Responses of Midbrain Dopamine Neurons to Appetitive and Aversive Stimuli. J. Neurosci. 33, 4710–4725. 10.1523/JNEUROSCI.3883-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S, 2005. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res. Cogn. Brain Res. 23, 119–136. 10.1016/j.cogbrainres.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Fiuzat EC, Rhodes SEV, Murray EA, 2017. The Role of Orbitofrontal-Amygdala Interactions in Updating Action-Outcome Valuations in Macaques. J. Neurosci. Off. J. Soc. Neurosci. 37, 2463–2470. 10.1523/JNEUROSCI.1839-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, 2015. The Nucleus Accumbens: An Interface Between Cognition, Emotion, and Action. Annu. Rev. Psychol. 66, 25–52. 10.1146/annurev-psych-010213-115159 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Montes DR, Tse MMT, van Holstein M, 2018. Differential Contributions of Nucleus Accumbens Subregions to Cue-Guided Risk/Reward Decision Making and Implementation of Conditional Rules. J. Neurosci. 38, 1901–1914. 10.1523/JNEUROSCI.3191-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Whelan JM, 2009. Perturbations in different forms of cost/benefit decision making induced by repeated amphetamine exposure. Psychopharmacology (Berl.) 205, 189–201. 10.1007/s00213-009-1529-0 [DOI] [PubMed] [Google Scholar]
- Folkes OM, Báldi R, Kondev V, Marcus DJ, Hartley ND, Turner BD, Ayers JK, Baechle JJ, Misra MP, Altemus M, Grueter CA, Grueter BA, Patel S, 2020. An endocannabinoid-regulated basolateral amygdala–nucleus accumbens circuit modulates sociability. J. Clin. Invest. 130, 1728–1742. 10.1172/JCI131752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freels TG, Gabriel DBK, Lester DB, Simon NW, 2020. Risky decision-making predicts dopamine release dynamics in nucleus accumbens shell. Neuropsychopharmacology 45, 266–275. 10.1038/s41386-019-0527-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A, Homma D, Gibb LG, Amemori K, Rubin SJ, Hood AS, Riad MH, Graybiel AM, 2015. A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict. Cell 161, 1320–1333. 10.1016/j.cell.2015.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulenwider HD, Nennig SE, Price ME, Hafeez H, Schank JR, 2019. Sex Differences in Aversion-Resistant Ethanol Intake in Mice. Alcohol Alcohol 54, 345–352. 10.1093/alcalc/agz022 [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G, 1999. Orbitofrontal Cortex and Representation of Incentive Value in Associative Learning. J. Neurosci. 19, 6610–6614. 10.1523/JNEUROSCI.19-15-06610.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MP, Conroy JC, Styer CV, Huynh T, Whitaker LR, Schoenbaum G, 2018. Medial orbitofrontal inactivation does not affect economic choice. eLife 7, e38963. 10.7554/eLife.38963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller I, Kulak JT, Seifter J, 1962. The effects of chlordiazepoxide and chlorpromazine on a punishment discrimination. Psychopharmacologia 3, 374–385. 10.1007/BF00408322 [DOI] [PubMed] [Google Scholar]
- Geller I, Seifter J, 1960. The effects of meprobamate, barbiturates, d-amphetamine and promazine on experimentally induced conflict in the rat. Psychopharmacologia 1, 482–492. 10.1007/BF00429273 [DOI] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Sibley DR, 1990. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. 10.1126/science.2147780 [DOI] [PubMed] [Google Scholar]
- Ghods-Sharifi S, St. Onge JR, Floresco SB, 2009. Fundamental Contribution by the Basolateral Amygdala to Different Forms of Decision Making. J. Neurosci. 29, 5251–5259. 10.1523/JNEUROSCI.0315-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano C, Peña-Oliver Y, Goodlett CR, Cardinal RN, Robbins TW, Bullmore ET, Belin D, Everitt BJ, 2018. Evidence for a Long-Lasting Compulsive Alcohol Seeking Phenotype in Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 43, 728–738. 10.1038/npp.2017.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover LR, Postle AF, Holmes A, 2020. Touchscreen-based assessment of risky-choice in mice. Behav. Brain Res. 393, 112748. 10.1016/j.bbr.2020.112748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ, 2002. Appetitive and Aversive Olfactory Learning in Humans Studied Using Event-Related Functional Magnetic Resonance Imaging. J. Neurosci. 22, 10829–10837. 10.1523/JNEUROSCI.22-24-10829.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall-Bronnec M, Victorri-Vigneau C, Donnio Y, Leboucher J, Rousselet M, Thiabaud E, Zreika N, Derkinderen P, Challet-Bouju G, 2018. Dopamine Agonists and Impulse Control Disorders: A Complex Association. Drug Saf. 41, 19–75. 10.1007/s40264-017-0590-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant EC, Mackintosh JH, 1963. A Comparison of the Social Postures of Some Common Laboratory Rodents. Behaviour 21, 246–259. [Google Scholar]
- Grant JE, Potenza MN, Weinstein A, Gorelick DA, 2010. Introduction to Behavioral Addictions. Am. J. Drug Alcohol Abuse 36, 233–241. 10.3109/00952990.2010.491884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED, 2000. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia 38, 1180–1187. 10.1016/S0028-3932(99)00158-X [DOI] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A, 2011. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat. Neurosci. 14, 1507–1509. 10.1038/nn.2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Keistler C, Keip AJ, Hammarlund E, DiLeone RJ, Pittenger C, Lee D, Taylor JR, 2019. Orbitofrontal Circuits Control Multiple Reinforcement-Learning Processes. Neuron 103, 734–746.e3. 10.1016/j.neuron.2019.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove RN, Schuster CR, 1974. Suppression of cocaine self-administration by extinction and punishment. Pharmacol. Biochem. Behav. 2, 199–208. 10.1016/0091-3057(74)90053-7 [DOI] [PubMed] [Google Scholar]
- Gupta R, Koscik TR, Bechara A, Tranel D, 2011. The amygdala and decision making. Neuropsychologia 49, 760–766. 10.1016/j.neuropsychologia.2010.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, Holmes A, 2017. Mouse strain differences in punished ethanol self-administration. Alcohol 58, 83–92. 10.1016/j.alcohol.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halladay LR, Kocharian A, Piantadosi PT, Authement ME, Lieberman AG, Spitz NA, Coden K, Glover LR, Costa VD, Alvarez VA, Holmes A, 2020. Prefrontal Regulation of Punished Ethanol Self-administration. Biol. Psychiatry 87, 967–978. 10.1016/j.biopsych.2019.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto JF, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A, Kash TL, 2019. Central Amygdala Prepronociceptin-Expressing Neurons Mediate Palatable Food Consumption and Reward. Neuron 102, 1037–1052.e7. 10.1016/j.neuron.2019.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JD, Catalano RE, Miller JY, 1992. Risk and Protective Factors for Alcohol and Other Drug Problems in Adolescence and Early Adulthood: Implications for Substance Abuse Prevention. Psychol. Bull. 112, 64–105. [DOI] [PubMed] [Google Scholar]
- Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN, 2016. Circuit-Based Corticostriatal Homologies Between Rat and Primate. Biol. Psychiatry 80, 509–521. 10.1016/j.biopsych.2016.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CM, Orsini CA, Labiste CC, Wheeler A-R, Ten Eyck TW, Bruner MM, Sahagian TJ, Harden SW, Frazier CJ, Setlow B, Bizon JL, 2019. Optogenetic dissection of basolateral amygdala contributions to intertemporal choice in young and aged rats. eLife 8, e46174. 10.7554/eLife.46174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K, Watanabe M, 2000. Delay Activity of Orbital and Lateral Prefrontal Neurons of the Monkey Varying with Different Rewards. Cereb. Cortex 10, 263–271. 10.1093/cercor/10.3.263 [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O, 2011. Negative Reward Signals from the Lateral Habenula to Dopamine Neurons Are Mediated by Rostromedial Tegmental Nucleus in Primates. J. Neurosci. 31, 11457–11471. 10.1523/JNEUROSCI.1384-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Chang S-J, Sparta DR, Bowers MS, Bonci A, 2010. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol. Clin. Exp. Res. 34, 1565–1573. 10.1111/j.1530-0277.2010.01241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Lesscher HMB, 2014. Rodent models for compulsive alcohol intake. Alcohol, Special Issue on Animal Models of Excessive Alcohol Consumption: Recent Advances and Future Challenges 48, 253–264. 10.1016/j.alcohol.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck CA, Carron CR, Millie LA, Grahame NJ, 2019. Innate and Acquired Quinine-Resistant Alcohol, but not Saccharin, Drinking in Crossed High-Alcohol-Preferring Mice [WWW Document]. Alcohol. Clin. Exp. Res. 10.1111/acer.14196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF, 2005. Neural Systems Responding to Degrees of Uncertainty in Human Decision-Making. Science 310, 1680–1683. 10.1126/science.1115327 [DOI] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Krasnova IN, Gu H, Lu H, Bonci A, Cadet JL, Stein EA, Yang Y, 2019. Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Proc. Natl. Acad. Sci. 116, 9066–9071. 10.1073/pnas.1819978116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML, 2006. Neural signatures of economic preferences for risk and ambiguity. Neuron 49, 765–775. 10.1016/j.neuron.2006.01.024 [DOI] [PubMed] [Google Scholar]
- Hunt HF, Brady JV, 1955. Some effects of punishment and intercurrent “anxiety” on a simple operant. J. Comp. Physiol. Psychol. 48, 305–310. 10.1037/h0042529 [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J, 1999. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Rev. 31, 6–41. 10.1016/S0165-0173(99)00023-5 [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Sakurai Y, Ishikawa A, Mitsushima D, 2020. Contribution of the prefrontal cortex and basolateral amygdala to behavioral decision-making under reward/punishment conflict. Psychopharmacology (Berl.) 237, 639–654. 10.1007/s00213-019-05398-7 [DOI] [PubMed] [Google Scholar]
- Izquierdo A, 2017. Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making. J. Neurosci. 37, 10529–10540. 10.1523/JNEUROSCI.1678-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DS, Moghaddam B, 2020. Prefrontal Cortex Representation of Learning of Punishment Probability During Reward-Motivated Actions. J. Neurosci. 40, 5063–5077. 10.1523/JNEUROSCI.0310-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard Dit Bressel P, McNally GP, 2014. The Role of the Lateral Habenula in Punishment. PLoS ONE 9, e111699. 10.1371/journal.pone.0111699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard-Dit-Bressel P, Killcross S, McNally GP, 2018. Behavioral and neurobiological mechanisms of punishment: implications for psychiatric disorders. Neuropsychopharmacology 43, 1639–1650. 10.1038/s41386-018-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard-dit-Bressel P, McNally GP, 2016. Lateral, not medial, prefrontal cortex contributes to punishment and aversive instrumental learning. Learn. Mem. 23, 607–617. 10.1101/lm.042820.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Richard-Dit-Bressel P, McNally GP, 2015. The role of the basolateral amygdala in punishment. Learn. Mem. 22, 128–137. 10.1101/lm.035907.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni NL, Larkin JD, Floresco SB, 2017. Prefrontal Dopamine D 1 and D 2 Receptors Regulate Dissociable Aspects of Decision Making via Distinct Ventral Striatal and Amygdalar Circuits. J. Neurosci. 37, 6200–6213. 10.1523/JNEUROSCI.0030-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni NL, Li YT, Floresco SB, 2021. Medial orbitofrontal cortex dopamine D1/D2 receptors differentially modulate distinct forms of probabilistic decision-making. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 10.1038/s41386-020-00931-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Woods JA, Groman SM, Seu E, 2010. Behavioral Characteristics and Neural Mechanisms Mediating Performance in a Rodent Version of the Balloon Analog Risk Task. Neuropsychopharmacology 35, 1797–1806. 10.1038/npp.2010.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC, 2009. The rostromedial tegmental nucleus (RMTg), a major GABAergic afferent to midbrain dopamine neurons, selectively encodes aversive stimuli and promotes behavioral inhibition. Neuron 61, 786–800. 10.1016/j.neuron.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhou TC, Vento PJ, 2019. Bidirectional regulation of reward, punishment, and arousal by dopamine, the lateral habenula and the rostromedial tegmentum (RMTg). Curr. Opin. Behav. Sci., Pain and Aversive Motivation 26, 90–96. 10.1016/j.cobeha.2018.11.001 [DOI] [Google Scholar]
- Johanson CE, 1977. The effects of electric shock on responding maintained by cocaine injections in a choice procedure in the rhesus monkey. Psychopharmacology (Berl.) 53, 277–282. 10.1007/BF00492364 [DOI] [PubMed] [Google Scholar]
- Jury NJ, Pollack GA, Ward MJ, Bezek JL, Ng AJ, Pinard CR, Bergstrom HC, Holmes A, 2017. Chronic Ethanol During Adolescence Impacts Corticolimbic Dendritic Spines and Behavior. Alcohol. Clin. Exp. Res. 41, 1298–1308. 10.1111/acer.13422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A, Pabst M, Lorkowski M, Braganza O, Boehlen A, Nikbakht N, Pothmann L, Vaswani AR, Musgrove R, Di Monte DA, Sauvage M, Beck H, Blaess S, 2015. Function and developmental origin of a mesocortical inhibitory circuit. Nat. Neurosci. 18, 872–882. 10.1038/nn.4020 [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A, 1979. Prospect Theory: An Analysis of Decision under Risk. Econometrica 47, 263–291. 10.2307/1914185 [DOI] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV, 2010. Transition to Addiction Is Associated with a Persistent Impairment in Synaptic Plasticity. Science 328, 1709–1712. 10.1126/science.1187801 [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest J-M, Berson N, Balado E, Fiancette J-F, Renault P, Piazza P-V, Manzoni OJ, 2013. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol. Psychiatry 18, 729–737. 10.1038/mp.2012.59 [DOI] [PubMed] [Google Scholar]
- Keiflin R, Janak PH, 2015. Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron 88, 247–263. 10.1016/j.neuron.2015.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Zariwala HA, Mainen ZF, 2008. Neural correlates, computation and behavioural impact of decision confidence. Nature 455, 227–231. 10.1038/nature07200 [DOI] [PubMed] [Google Scholar]
- Kim CK, Ye L, Jennings JH, Pichamoorthy N, Tang DD, Yoo A-CW, Ramakrishnan C, Deisseroth K, 2017. Molecular and Circuit-Dynamical Identification of Top-Down Neural Mechanisms for Restraint of Reward Seeking. Cell 170, 1013–1027.e14. 10.1016/j.cell.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Zhang X, Muralidhar S, LeBlanc SA, Tonegawa S, 2017. Basolateral to Central Amygdala Neural Circuits for Appetitive Behaviors. Neuron 93, 1464–1479.e5. 10.1016/j.neuron.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ, 2011. Anxiety Dissociates Dorsal and Ventral Medial Prefrontal Cortex Functional Connectivity with the Amygdala at Rest. Cereb. Cortex 21, 1667–1673. 10.1093/cercor/bhq237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough A, Kim S, Cole M, Brennan M, George O, 2017. Intermittent Access to Ethanol Drinking Facilitates the Transition to Excessive Drinking After Chronic Intermittent Ethanol Vapor Exposure. Alcohol. Clin. Exp. Res. 41, 1502–1509. 10.1111/acer.13434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D, 2001. Anticipation of Increasing Monetary Reward Selectively Recruits Nucleus Accumbens. J. Neurosci. 21, RC159–RC159. 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, 2009. Brain stress systems in the amygdala and addiction. Brain Res. 1293, 61–75. 10.1016/j.brainres.2009.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 35, 217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröner S, Rosenkranz JA, Grace AA, Barrionuevo G, 2005. Dopamine Modulates Excitability of Basolateral Amygdala Neurons In Vitro. J. Neurophysiol. 93, 1598–1610. 10.1152/jn.00843.2004 [DOI] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC, 2011. Projection-Specific Modulation of Dopamine Neuron Synapses by Aversive and Rewarding Stimuli. Neuron 70, 855–862. 10.1016/j.neuron.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC, 2012. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217. 10.1038/nature11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin JD, Jenni NL, Floresco SB, 2016. Modulation of risk/reward decision making by dopaminergic transmission within the basolateral amygdala. Psychopharmacology (Berl.) 233, 121–136. 10.1007/s00213-015-4094-8 [DOI] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR, 2018. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5. 10.1523/ENEURO.0315-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lázaro-Muñoz G, LeDoux JE, Cain CK, 2010. Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol. Psychiatry 67, 1120–1127. 10.1016/j.biopsych.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, Deisseroth K, 2015. Intact-Brain Analyses Reveal Distinct Information Carried by SNc Dopamine Subcircuits. Cell 162, 635–647. 10.1016/j.cell.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, 2017. Neuroanatomical substrates for risk behavior. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 23, 275–286. 10.1177/1073858416672414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Vento PJ, Parrilla-Carrero J, Pullmann D, Chao YS, Eid M, Jhou TC, 2019. Three Rostromedial Tegmental Afferents Drive Triply Dissociable Aspects of Punishment Learning and Aversive Valence Encoding. Neuron 104, 987–999.e4. 10.1016/j.neuron.2019.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lu Z-L, D’Argembeau A, Ng M, Bechara A, 2010. The Iowa Gambling Task in fMRI images. Hum. Brain Mapp. 31, 410–423. 10.1002/hbm.20875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley AE, Gabriel DBK, Sable HJ, Simon NW, 2019. Sex Differences and Effects of Predictive Cues on Delayed Punishment Discounting. eNeuro 6. 10.1523/ENEURO.0225-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein G, Rick S, Cohen JD, 2007. Neuroeconomics. Annu. Rev. Psychol. 59, 647–672. 10.1146/annurev.psych.59.103006.093710 [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Pantelis C, 2004. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction 99, 1491–1502. 10.1111/j.1360-0443.2004.00808.x [DOI] [PubMed] [Google Scholar]
- Lüscher C, Robbins TW, Everitt BJ, 2020. The transition to compulsion in addiction. Nat. Rev. Neurosci. 21, 247–263. 10.1038/s41583-020-0289-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Jean-Richard-dit-Bressel P, Roughley S, Vissel B, Balleine BW, Killcross S, Bradfield LA, 2020. Medial Orbitofrontal Cortex Regulates Instrumental Conditioned Punishment, but not Pavlovian Conditioned Fear. Cereb. Cortex Commun. 1. 10.1093/texcom/tgaa039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai B, Sommer S, Hauber W, 2015. Dopamine D1/D2 Receptor Activity in the Nucleus Accumbens Core But Not in the Nucleus Accumbens Shell and Orbitofrontal Cortex Modulates Risk-Based Decision Making. Int. J. Neuropsychopharmacol. 18, pyv043. 10.1093/ijnp/pyv043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Shieh C, Murphy MD, Greenfield VY, Wassum KM, 2019. Distinct cortical-amygdala projections drive reward value encoding and retrieval. Nat. Neurosci. 22, 762–769. 10.1038/s41593-019-0374-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T, 2002. Decision-making processes following damage to the prefrontal cortex. Brain J. Neurol. 125, 624–639. 10.1093/brain/awf049 [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Campbell EJ, Kaganovsky K, 2018. Punishment of alcohol-reinforced responding in alcohol preferring P rats reveals a bimodal population: Implications for models of compulsive drug seeking. Prog. Neuropsychopharmacol. Biol. Psychiatry 87, 68–77. 10.1016/j.pnpbp.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Li X, Shaham Y, 2013. Recent developments in animal models of drug relapse. Curr. Opin. Neurobiol. 23, 675–683. 10.1016/j.conb.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2009a. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841. 10.1038/nature08028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2009b. Representation of negative motivational value in the primate lateral habenula. Nat. Neurosci. 12, 77–84. 10.1038/nn.2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O, 2007. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447, 1111–1115. 10.1038/nature05860 [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF, 2012. Encoding of Aversion by Dopamine and the Nucleus Accumbens. Front. Neurosci. 6. 10.3389/fnins.2012.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MS, Amodeo LR, Roitman JD, 2016. Consequences of Adolescent Ethanol Consumption on Risk Preference and Orbitofrontal Cortex Encoding of Reward. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 41, 1366–1375. 10.1038/npp.2015.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Kim HA, Janak PH, 2017. Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience 360, 106–117. 10.1016/j.neuroscience.2017.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, 2003. The Vogel conflict test: procedural aspects, γ-aminobutyric acid, glutamate and monoamines. Eur. J. Pharmacol., Animal Models of Anxiety Disorders 463, 67–96. 10.1016/S0014-2999(03)01275-5 [DOI] [PubMed] [Google Scholar]
- Miller KJ, Botvinick MM, Brody CD, 2018. Value Representations in the Rodent Orbitofrontal Cortex Drive Learning, not Choice (preprint). Neuroscience. 10.1101/245720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NE, 1944. Experimental studies of conflict, in: Personality and The Behavior Disorders. Ronald, New York, NY, pp. 431–465. [Google Scholar]
- Miller SM, Marcotulli D, Shen A, Zweifel LS, 2019. Divergent medial amygdala projections regulate approach–avoidance conflict behavior. Nat. Neurosci. 22, 565–575. 10.1038/s41593-019-0337-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W, 1996. Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli. Nature 379, 449–451. 10.1038/379449a0 [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Weiss VG, Beas BS, Morgan D, Bizon JL, Setlow B, 2014. Adolescent risk taking, cocaine self-administration, and striatal dopamine signaling. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 39, 955–962. 10.1038/npp.2013.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho M-Y, Bradshaw CM, Szabadi E, Deakin JFW, Anderson IM, 2002. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl.) 160, 290–298. 10.1007/s00213-001-0983-0 [DOI] [PubMed] [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR, 2010. Neural Processing of Risk. J. Neurosci. 30, 6613–6619. 10.1523/JNEUROSCI.0003-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SC, Radke AK, 2020. Aversion-resistant fentanyl self-administration in mice. Psychopharmacology (Berl.). 10.1007/s00213-020-05722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TJ, Glenmullen J, Mattison DR, 2014. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern. Med. 174, 1930–1933. 10.1001/jamainternmed.2014.5262 [DOI] [PubMed] [Google Scholar]
- Moorman DE, James MH, McGlinchey EM, Aston-Jones G, 2015. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res., Role of corticostriatal circuits in addiction 1628, 130–146. 10.1016/j.brainres.2014.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Margolis EB, 2017. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 18, 73–85. 10.1038/nrn.2016.165 [DOI] [PubMed] [Google Scholar]
- Murray EA, Izquierdo A, 2007. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann. N. Y. Acad. Sci. 1121, 273–296. 10.1196/annals.1401.021 [DOI] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL, 2011. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc. Natl. Acad. Sci. U. S. A. 108, 5466–5471. 10.1073/pnas.1017732108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TWH, Bernstein IL, 2009. Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proc. Natl. Acad. Sci. U. S. A. 106, 17600–17604. 10.1073/pnas.0906629106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neftci EO, Averbeck BB, 2019. Reinforcement learning in artificial and biological systems. Nat. Mach. Intell. 1, 133–143. 10.1038/s42256-019-0025-4 [DOI] [Google Scholar]
- Nguyen D, Fugariu V, Erb S, Ito R, 2018. Dissociable roles of the nucleus accumbens D1 and D2 receptors in regulating cue-elicited approach-avoidance conflict decision-making. Psychopharmacology (Berl.) 235, 2233–2244. 10.1007/s00213-018-4919-3 [DOI] [PubMed] [Google Scholar]
- Nicola SM, 2010. The Flexible Approach Hypothesis: Unification of Effort and Cue-Responding Hypotheses for the Role of Nucleus Accumbens Dopamine in the Activation of Reward-Seeking Behavior. J. Neurosci. 30, 16585–16600. 10.1523/JNEUROSCI.3958-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM, 2015. Decoding Neural Circuits that Control Compulsive Sucrose Seeking. Cell 160, 528–541. 10.1016/j.cell.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Chau BKH, Rushworth MFS, Fellows LK, 2017. Contrasting Effects of Medial and Lateral Orbitofrontal Cortex Lesions on Credit Assignment and Decision-Making in Humans. J. Neurosci. 37, 7023–7035. 10.1523/JNEUROSCI.0692-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MP, Walton ME, Behrens TEJ, Sallet J, Buckley MJ, Rushworth MFS, 2010. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc. Natl. Acad. Sci. 107, 20547–20552. 10.1073/pnas.1012246107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C, 2001. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 4, 95–102. 10.1038/82959 [DOI] [PubMed] [Google Scholar]
- Oleson EB, Gentry RN, Chioma VC, Cheer JF, 2012. Subsecond Dopamine Release in the Nucleus Accumbens Predicts Conditioned Punishment and Its Successful Avoidance. J. Neurosci. 32, 14804–14808. 10.1523/JNEUROSCI.3087-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M, Schultz W, 2018. Predictive coding of the statistical parameters of uncertain rewards by orbitofrontal neurons. Behav. Brain Res. 355, 90–94. 10.1016/j.bbr.2018.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M, Schultz W, 2013. Risk prediction error coding in orbitofrontal neurons. J. Neurosci. Off. J. Soc. Neurosci. 33, 15810–15814. 10.1523/JNEUROSCI.4236-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Hernandez CM, Bizon JL, Setlow B, 2019. Deconstructing value-based decision making via temporally selective manipulation of neural activity: Insights from rodent models. Cogn. Affect. Behav. Neurosci. 19, 459–476. 10.3758/s13415-018-00649-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Hernandez CM, Singhal S, Kelly KB, Frazier CJ, Bizon JL, Setlow B, 2017. Optogenetic Inhibition Reveals Distinct Roles for Basolateral Amygdala Activity at Discrete Time Points during Risky Decision Making. J. Neurosci. 37, 11537–11548. 10.1523/JNEUROSCI.2344-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Heshmati SC, Garman TS, Wall SC, Bizon JL, Setlow B, 2018. Contributions of medial prefrontal cortex to decision making involving risk of punishment. Neuropharmacology 139, 205–216. 10.1016/j.neuropharm.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB, 2015. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci. Biobehav. Rev. 58, 147–167. 10.1016/j.neubiorev.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Trotta RT, Bizon JL, Setlow B, 2015. Dissociable Roles for the Basolateral Amygdala and Orbitofrontal Cortex in Decision-Making under Risk of Punishment. J. Neurosci. 35, 1368–1379. 10.1523/JNEUROSCI.3586-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B, 2016. Sex differences in a rat model of risky decision making. Behav. Neurosci. 130, 50–61. 10.1037/bne0000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW, 2007. Orbitofrontal Cortex Mediates Outcome Encoding in Pavlovian But Not Instrumental Conditioning. J. Neurosci. 27, 4819–4825. 10.1523/JNEUROSCI.5443-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Asinof SK, Diehl GW, Frackman A, Leffler J, 2013. Medial prefrontal cortex lesions impair decision-making on a rodent gambling task: reversal by D1 receptor antagonist administration. Behav. Brain Res. 243, 247–254. 10.1016/j.bbr.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, O’Hara A, Plaut B, Lowes DC, 2015. Effects of disrupting medial prefrontal cortex GABA transmission on decision-making in a rodent gambling task. Psychopharmacology (Berl.) 232, 1755–1765. 10.1007/s00213-014-3816-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais-Vieira M, Lima D, Galhardo V, 2007. Orbitofrontal cortex lesions disrupt risk assessment in a novel serial decision-making task for rats. Neuroscience 145, 225–231. 10.1016/j.neuroscience.2006.11.058 [DOI] [PubMed] [Google Scholar]
- Park J, Moghaddam B, 2017. Risk of punishment influences discrete and coordinated encoding of reward-guided actions by prefrontal cortex and VTA neurons. eLife 6, e30056. 10.7554/eLife.30056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, Flakowski J, Lüscher C, 2018. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature 564, 366–371. 10.1038/s41586-018-0789-4 [DOI] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Hiver A, Lüscher C, 2015. Sufficiency of Mesolimbic Dopamine Neuron Stimulation for the Progression to Addiction. Neuron 88, 1054–1066. 10.1016/j.neuron.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Passecker J, Mikus N, Malagon-Vina H, Anner P, Dimidschstein J, Fishell G, Dorffner G, Klausberger T, 2019. Activity of Prefrontal Neurons Predict Future Choices during Gambling. Neuron 101, 152–164.e7. 10.1016/j.neuron.2018.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Hall G, 1980. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol. Rev. 87, 532–552. 10.1037/0033-295X.87.6.532 [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A, 2007. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl.) 194, 127–137. 10.1007/s00213-007-0805-0 [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Minier-Toribio A, Hoots JK, Bossert JM, Shaham Y, 2018. Opposite Effects of Basolateral Amygdala Inactivation on Context-Induced Relapse to Cocaine Seeking after Extinction versus Punishment. J. Neurosci. 38, 51–59. 10.1523/JNEUROSCI.2521-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ, 2015. Differential vulnerability to the punishment of cocaine related behaviours: effects of locus of punishment, cocaine taking history and alternative reinforcer availability. Psychopharmacology (Berl.) 232, 125–134. 10.1007/s00213-014-3648-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Murray JE, Everitt BJ, 2013. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur. J. Neurosci. 38, 3018–3026. 10.1111/ejn.12289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi PT, Khayambashi S, Schluter MG, Kutarna A, Floresco SB, 2016. Perturbations in reward-related decision-making induced by reduced prefrontal cortical GABA transmission: Relevance for psychiatric disorders. Neuropharmacology 101, 279–290. 10.1016/j.neuropharm.2015.10.007 [DOI] [PubMed] [Google Scholar]
- Piantadosi PT, Lieberman AG, Pickens CL, Bergstrom HC, Holmes A, 2019. A novel multichoice touchscreen paradigm for assessing cognitive flexibility in mice. Learn. Mem. Cold Spring Harb. N 26, 24–30. 10.1101/lm.048264.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi PT, Yeates DCM, Floresco SB, 2018. Cooperative and dissociable involvement of the nucleus accumbens core and shell in the promotion and inhibition of actions during active and inhibitory avoidance. Neuropharmacology 138, 57–71. 10.1016/j.neuropharm.2018.05.028 [DOI] [PubMed] [Google Scholar]
- Piantadosi PT, Yeates DCM, Wilkins M, Floresco SB, 2017. Contributions of basolateral amygdala and nucleus accumbens subregions to mediating motivational conflict during punished reward-seeking. Neurobiol. Learn. Mem. 140, 92–105. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC, 2005. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav. Neurosci. 119, 317–322. 10.1037/0735-7044.119.1.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilgrim CC, Schulenberg JE, O’Malley PM, Bachman JG, Johnston LD, 2006. Mediators and Moderators of Parental Involvement on Substance Use: A National Study of Adolescents. Prev. Sci. 7, 75–89. 10.1007/s11121-005-0019-9 [DOI] [PubMed] [Google Scholar]
- Pultorak KJ, Schelp SA, Isaacs DP, Krzystyniak G, Oleson EB, 2018. A Transient Dopamine Signal Represents Avoidance Value and Causally Influences the Demand to Avoid. eNeuro 5. 10.1523/ENEURO.0058-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang H-L, Barker DJ, Miranda-Barrientos J, Morales M, 2016. VTA glutamatergic inputs to nucleus accumbens drive aversion by acting on GABAergic interneurons. Nat. Neurosci. 19, 725–733. 10.1038/nn.4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Held IT, Sneddon EA, Riddle CA, Quinn JJ, 2019a. Additive influences of acute early life stress and sex on vulnerability for aversion-resistant alcohol drinking. Addict. Biol. 25, e12829. 10.1111/adb.12829 [DOI] [PubMed] [Google Scholar]
- Radke AK, Jury NJ, Kocharian A, Marcinkiewcz CA, Lowery-Gionta EG, Pleil KE, McElligott ZA, McKlveen JM, Kash TL, Holmes A, 2017. Chronic EtOH effects on putative measures of compulsive behavior in mice. Addict. Biol. 22, 423–434. 10.1111/adb.12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Kocharian A, Covey DP, Lovinger DM, Cheer JF, Mateo Y, Holmes A, 2019b. Contributions of nucleus accumbens dopamine to cognitive flexibility. Eur. J. Neurosci. 50, 2023–2035. 10.1111/ejn.14152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Nakazawa K, Holmes A, 2015. Cortical GluN2B deletion attenuates punished suppression of food reward-seeking. Psychopharmacology (Berl.) 232, 3753–3761. 10.1007/s00213-015-4033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman AP, Padoa-Schioppa C, 2014. Integration of multiple determinants in the neuronal computation of economic values. J. Neurosci. Off. J. Soc. Neurosci. 34, 11583–11603. 10.1523/JNEUROSCI.1235-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, 2007. The Contribution of the Medial Prefrontal Cortex, Orbitofrontal Cortex, and Dorsomedial Striatum to Behavioral Flexibility. Ann. N. Y. Acad. Sci. 1121, 355–375. 10.1196/annals.1401.013 [DOI] [PubMed] [Google Scholar]
- Ramirez F, Moscarello JM, LeDoux JE, Sears RM, 2015. Active Avoidance Requires a Serial Basal Amygdala to Nucleus Accumbens Shell Circuit. J. Neurosci. 35, 3470–3477. 10.1523/JNEUROSCI.1331-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redish AD, 2004. Addiction as a Computational Process Gone Awry. Science 306, 1944–1947. 10.1126/science.1102384 [DOI] [PubMed] [Google Scholar]
- Rescorla R, Wagner A, 1972. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement, in: Classical Conditioning II: Current Research and Theory. [Google Scholar]
- Resstel LBM, Souza RF, Guimarães FS, 2008. Anxiolytic-like effects induced by medial prefrontal cortex inhibition in rats submitted to the Vogel conflict test. Physiol. Behav. 93, 200–205. 10.1016/j.physbeh.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedorn F, 2011. Inter-individual decision-making differences in the effects of cingulate, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Front. Behav. Neurosci. 5, 22. 10.3389/fnbeh.2011.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ, 1992. Functions of dopamine in the dorsal and ventral striatum. Semin. Neurosci., Milestones in Dopamine Research 4, 119–127. 10.1016/1044-5765(92)90010-Y [DOI] [Google Scholar]
- Rodríguez W, Bouzas A, Orduña V, 2018. Temporal discounting of aversive consequences in rats. Learn. Behav. 46, 38–48. 10.3758/s13420-017-0279-9 [DOI] [PubMed] [Google Scholar]
- Roesch MR, Taylor AR, Schoenbaum G, 2006. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron 51, 509–520. 10.1016/j.neuron.2006.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Roitman MF, 2010. Risk-preference differentiates orbitofrontal cortex responses to freely chosen reward outcomes. Eur. J. Neurosci. 31, 1492–1500. 10.1111/j.1460-9568.2010.07169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Critchley HD, Mason R, Wakeman EA, 1996. Orbitofrontal cortex neurons: role in olfactory and visual association learning. J. Neurophysiol. 75, 1970–1981. 10.1152/jn.1996.75.5.1970 [DOI] [PubMed] [Google Scholar]
- Root DH, Mejias-Aponte CA, Zhang S, Wang H-L, Hoffman AF, Lupica CR, Morales M, 2014. Single rodent mesohabenular axons release glutamate and GABA. Nat. Neurosci. 17, 1543–1551. 10.1038/nn.3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Mitz AR, Chacko RV, Murray EA, 2013. Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80, 1519–1531. 10.1016/j.neuron.2013.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM, 2015. Mesolimbic Dopamine Dynamically Tracks, and Is Causally Linked to, Discrete Aspects of Value-Based Decision Making. Biol. Psychiatry, Risk Phenotypes for Alcohol and Substance Abuse 77, 903–911. 10.1016/j.biopsych.2014.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Margolis EB, Janak PH, 2018. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 21, 1072–1083. 10.1038/s41593-018-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Soden ME, Zweifel LS, Clark JJ, 2016. Reversal of Alcohol-Induced Dysregulation in Dopamine Network Dynamics May Rescue Maladaptive Decision-making. J. Neurosci. Off. J. Soc. Neurosci. 36, 3698–3708. 10.1523/JNEUROSCI.4394-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M, 1998. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat. Neurosci. 1, 155–159. 10.1038/407 [DOI] [PubMed] [Google Scholar]
- Schultz W, 2013. Updating dopamine reward signals. Curr. Opin. Neurobiol. 23, 229–238. 10.1016/j.conb.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, 2007. Behavioral dopamine signals. Trends Neurosci. 30, 203–210. 10.1016/j.tins.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR, 1997. A Neural Substrate of Prediction and Reward. Science 275, 1593–1599. 10.1126/science.275.5306.1593 [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L, 2015. Viral-genetic tracing of the input–output organization of a central noradrenaline circuit. Nature 524, 88–92. 10.1038/nature14600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TR, Edwards EM, Smith CA, Hilgert KG, Schwartz GJ, Pritchard TC, 2005. Medial orbitofrontal cortex: its role in mediating satiety in the macaque. Chem. Senses 30 Suppl 1, i190. 10.1093/chemse/bjh178 [DOI] [PubMed] [Google Scholar]
- Scudder SL, Baimel C, Macdonald EE, Carter AG, 2018. Hippocampal-Evoked Feedforward Inhibition in the Nucleus Accumbens. J. Neurosci. 38, 9091–9104. 10.1523/JNEUROSCI.1971-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Chang S-J, Simms JA, Gibb SL, Dadgar J, Chen BT, Harvey BK, Ron D, Messing RO, Bonci A, Hopf FW, 2013. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci. 16, 1094–1100. 10.1038/nn.3445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif T, Simms JA, Lei K, Wegner S, Bonci A, Messing RO, Hopf FW, 2015. D-Serine and D-Cycloserine Reduce Compulsive Alcohol Intake in Rats. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 40, 2357–2367. 10.1038/npp.2015.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A, Holmes A, 2019. A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory. Neuron 103, 489–505.e7. 10.1016/j.neuron.2019.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw GA, Bent MAM, Council KR, Pais AC, Amstadter A, Wolstenholme JT, Miles MF, Neigh GN, 2020. Chronic repeated predatory stress induces resistance to quinine adulteration of ethanol in male mice. Behav. Brain Res. 382, 1–8. 10.1016/j.bbr.2020.112500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C-J, Zheng D, Li K-X, Yang J-M, Pan H-Q, Yu X-D, Fu J-Y, Zhu Y, Sun Q-X, Tang M-Y, Zhang Y, Sun P, Xie Y, Duan S, Hu H, Li X-M, 2019. Cannabinoid CB 1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 25, 337–349. 10.1038/s41591-018-0299-9 [DOI] [PubMed] [Google Scholar]
- Siciliano CA, Noamany H, Chang C-J, Brown AR, Chen X, Leible D, Lee JJ, Wang J, Vernon AN, Vander Weele CM, Kimchi EY, Heiman M, Tye KM, 2019. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012. 10.1126/science.aay1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JM, Richmond BJ, 2008. Dynamic changes in representations of preceding and upcoming reward in monkey orbitofrontal cortex. Cereb. Cortex N. Y. N 1991 18, 93–103. 10.1093/cercor/bhm034 [DOI] [PubMed] [Google Scholar]
- Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B, 2009. Balancing Risk and Reward: A Rat Model of Risky Decision Making. Neuropsychopharmacology 34, 2208–2217. 10.1038/npp.2009.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Laiks LS, 2018. Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog. Neuropsychopharmacol. Biol. Psychiatry, New directions in addiction 87, 11–21. 10.1016/j.pnpbp.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon EA, Ramsey OR, Thomas A, Radke AK, 2020. Increased Responding for Alcohol and Resistance to Aversion in Female Mice. Alcohol. Clin. Exp. Res. 44, 1400–1409. 10.1111/acer.14384 [DOI] [PubMed] [Google Scholar]
- Sneddon EA, Schuh KM, Frankel JW, Radke AK, 2021. The contribution of medium spiny neuron subtypes in the nucleus accumbens core to compulsive-like ethanol drinking. Neuropharmacology 187, 108497. 10.1016/j.neuropharm.2021.108497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon EA, White RD, Radke AK, 2019. Sex Differences in Binge-Like and Aversion-Resistant Alcohol Drinking in C57BL/6J Mice. Alcohol. Clin. Exp. Res. 43, 243–249. 10.1111/acer.13923 [DOI] [PubMed] [Google Scholar]
- Spealman RD, 1978. Pressurized air as a punisher. J. Exp. Anal. Behav. 29, 341–345. 10.1901/jeab.1978.29-341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelder M, Hesseling P, Baars AM, Klooster JGL ‘t, Rotte MD, Vanderschuren LJMJ, Lesscher HMB, 2015. Individual Variation in Alcohol Intake Predicts Reinforcement, Motivation, and Compulsive Alcohol Use in Rats. Alcohol. Clin. Exp. Res. 39, 2427–2437. 10.1111/acer.12891 [DOI] [PubMed] [Google Scholar]
- Spoelder M, Pol S, Janssen BSG, Baars AM, Vanderschuren LJMJ, Lesscher HMB, 2017. Loss of control over alcohol seeking in rats depends on individual vulnerability and duration of alcohol consumption experience. Behav. Pharmacol. 28, 334–344. 10.1097/FBP.0000000000000304 [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Abhari H, Floresco SB, 2011. Dissociable Contributions by Prefrontal D1 and D2 Receptors to Risk-Based Decision Making. J. Neurosci. 31, 8625–8633. 10.1523/JNEUROSCI.1020-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Onge JR, Ahn S, Phillips AG, Floresco SB, 2012a. Dynamic Fluctuations in Dopamine Efflux in the Prefrontal Cortex and Nucleus Accumbens during Risk-Based Decision Making. J. Neurosci. 32, 16880–16891. 10.1523/JNEUROSCI.3807-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Onge JR, Floresco SB, 2010. Prefrontal Cortical Contribution to Risk-Based Decision Making. Cereb. Cortex 20, 1816–1828. 10.1093/cercor/bhp250 [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Floresco SB, 2009. Dopaminergic Modulation of Risk-Based Decision Making. Neuropsychopharmacology 34, 681–697. 10.1038/npp.2008.121 [DOI] [PubMed] [Google Scholar]
- St. Onge JR, Stopper CM, Zahm DS, Floresco SB, 2012b. Separate Prefrontal-Subcortical Circuits Mediate Different Components of Risk-Based Decision Making. J. Neurosci. 32, 2886–2899. 10.1523/JNEUROSCI.5625-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Jennings JH, Ung RL, Blair GA, Weinberg RJ, Neve RL, Boyce F, Mattis J, Ramakrishnan C, Deisseroth K, Stuber GD, 2013. A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80, 1039–1053. 10.1016/j.neuron.2013.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH, 2013. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 16, 966–973. 10.1038/nn.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelly CE, Haug GC, Fonzi KM, Garcia MA, Tritley SC, Magnon AP, Ramos MAP, Wanat MJ, 2019. Pattern of dopamine signaling during aversive events predicts active avoidance learning. Proc. Natl. Acad. Sci. 116, 13641–13650. 10.1073/pnas.1904249116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Passingham RE, 1996. The nucleus accumbens in monkeys (Macaca fascicularis): II. Emotion and motivation. Behav. Brain Res. 75, 179–193. 10.1016/0166-4328(96)00169-6 [DOI] [PubMed] [Google Scholar]
- Stevens L, Betanzos-Espinosa P, Crunelle CL, Vergara-Moragues E, Roeyers H, Lozano O, Dom G, Gonzalez-Saiz F, Vanderplasschen W, Verdejo-García A, Pérez-García M, 2013. Disadvantageous Decision-Making as a Predictor of Drop-Out among Cocaine-Dependent Individuals in Long-Term Residential Treatment. Front. Psychiatry 4, 1–9. 10.3389/fpsyt.2013.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward T, Juaneda-Seguí A, Mestre-Bach G, Martínez-Zalacaín I, Vilarrasa N, Jiménez-Murcia S, Fernández-Formoso JA, Veciana de Las Heras M, Custal N, Virgili N, Lopez-Urdiales R, García-Ruiz-de-Gordejuela A, Menchón JM, Soriano-Mas C, Fernandez-Aranda F, 2019. What Difference Does it Make? Risk-Taking Behavior in Obesity after a Loss is Associated with Decreased Ventromedial Prefrontal Cortex Activity. J. Clin. Med. 8, 1–11. 10.3390/jcm8101551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB, 2014. What’s better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat. Neurosci. 17, 33–35. 10.1038/nn.3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB, 2011. Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cogn. Affect. Behav. Neurosci. 11, 97–112. 10.3758/s13415-010-0015-9 [DOI] [PubMed] [Google Scholar]
- Stopper CM, Green EB, Floresco SB, 2014a. Selective Involvement by the Medial Orbitofrontal Cortex in Biasing Risky, But Not Impulsive, Choice. Cereb. Cortex 24, 154–162. 10.1093/cercor/bhs297 [DOI] [PubMed] [Google Scholar]
- Stopper CM, Khayambashi S, Floresco SB, 2013. Receptor-Specific Modulation of Risk-Based Decision Making by Nucleus Accumbens Dopamine. Neuropsychopharmacology 38, 715–728. 10.1038/npp.2012.240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Tse MTL, Montes DR, Wiedman CR, Floresco SB, 2014b. Overriding phasic dopamine signals redirects action selection during risk/reward decision making. Neuron 84, 177–189. 10.1016/j.neuron.2014.08.033 [DOI] [PubMed] [Google Scholar]
- Stuber GD, Britt JP, Bonci A, 2012. Optogenetic Modulation of Neural Circuits that Underlie Reward Seeking. Biol. Psychiatry 71, 1061–1067. 10.1016/j.biopsych.2011.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM, 2012. Phasic Nucleus Accumbens Dopamine Encodes Risk-Based Decision-Making Behavior. Biol. Psychiatry 71, 199–205. 10.1016/j.biopsych.2011.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugam JA, Saddoris MP, Carelli RM, 2014. Nucleus accumbens neurons track behavioral preferences and reward outcomes during risky decision making. Biol. Psychiatry 75, 807–816. 10.1016/j.biopsych.2013.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sul JH, Kim H, Huh N, Lee D, Jung MW, 2010. Distinct roles of rodent orbitofrontal and medial prefrontal cortex in decision making. Neuron 66, 449–460. 10.1016/j.neuron.2010.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, 2011. How Addictive Drugs Disrupt Presynaptic Dopamine Neurotransmission. Neuron 69, 628–649. 10.1016/j.neuron.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG, 1998. Reinforcement learning: an introduction, Adaptive computation and machine learning. MIT Press, Cambridge, Mass. [Google Scholar]
- Tian J, Uchida N, 2015. Habenula Lesions Reveal that Multiple Mechanisms Underlie Dopamine Prediction Errors. Neuron 87, 1304–1316. 10.1016/j.neuron.2015.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme NM, Linsenbardt D, Timm M, Galbari T, Cornwell E, Lapish C, 2020. Alcohol-preferring P rats exhibit aversion-resistant drinking of alcohol adulterated with quinine. Alcohol Fayettev. N 83, 47–56. 10.1016/j.alcohol.2019.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, O’Doherty JP, Dolan RJ, Schultz W, 2007. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J. Neurophysiol. 97, 1621–1632. 10.1152/jn.00745.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, Cadet JL, 2017. Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav. Brain Res. 326, 265–271. 10.1016/j.bbr.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay L, Schultz W, 2000. Reward-related neuronal activity during go-nogo task performance in primate orbitofrontal cortex. J. Neurophysiol. 83, 1864–1876. 10.1152/jn.2000.83.4.1864 [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W, 1999. Relative reward preference in primate orbitofrontal cortex. Nature 398, 704–708. 10.1038/19525 [DOI] [PubMed] [Google Scholar]
- Tremblay M, Cocker PJ, Hosking JG, Zeeb FD, Rogers RD, Winstanley CA, 2014. Dissociable effects of basolateral amygdala lesions on decision making biases in rats when loss or gain is emphasized. Cogn. Affect. Behav. Neurosci. 14, 1184–1195. 10.3758/s13415-014-0271-1 [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D, 2014. Imaging addiction: D2 receptors and dopamine signaling in the striatum as biomarkers for impulsivity. Neuropharmacology 76, 498–509. 10.1016/j.neuropharm.2013.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL, 2012. Dopaminergic Modulation of Synaptic Transmission in Cortex and Striatum. Neuron 76, 33–50. 10.1016/j.neuron.2012.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP, 2004. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303, 2040–2042. 10.1126/science.1093360 [DOI] [PubMed] [Google Scholar]
- Valentin VV, Dickinson A, O’Doherty JP, 2007. Determining the Neural Substrates of Goal-Directed Learning in the Human Brain. J. Neurosci. 27, 4019–4026. 10.1523/JNEUROSCI.0564-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Poel AM, 1979. A note on ‘stretched attention’, a behavioural element indicative of an approach-avoidance conflict in rats. Anim. Behav. 27, 446–450. 10.1016/0003-3472(79)90181-7 [DOI] [Google Scholar]
- van Holstein M, Floresco SB, 2020. Dissociable roles for the ventral and dorsal medial prefrontal cortex in cue-guided risk/reward decision making. Neuropsychopharmacology 45, 683–693. 10.1038/s41386-019-0557-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Weele CM, Siciliano CA, Matthews GA, Namburi P, Izadmehr EM, Espinel IC, Nieh EH, Schut EHS, Padilla-Coreano N, Burgos-Robles A, Chang C-J, Kimchi EY, Beyeler A, Wichmann R, Wildes CP, Tye KM, 2018. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563, 397–401. 10.1038/s41586-018-0682-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Minnard AM, Smeets JA, Lesscher HM, 2017. Punishment models of addictive behavior. Curr. Opin. Behav. Sci. 8. [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ, 2004. Drug Seeking Becomes Compulsive After Prolonged Cocaine Self-Administration. Science 305, 1017–1019. 10.1126/science.1098975 [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, 2012. Corticosteroid-Dependent Plasticity Mediates Compulsive Alcohol Drinking in Rats. J. Neurosci. 32, 7563–7571. 10.1523/JNEUROSCI.0069-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento PJ, Burnham NW, Rowley CS, Jhou TC, 2017. Learning From One’s Mistakes: A Dual Role for the Rostromedial Tegmental Nucleus in the Encoding and Expression of Punished Reward Seeking. Biol. Psychiatry 81, 1041–1049. 10.1016/j.biopsych.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verharen JPH, Heuvel MW van den, Luijendijk M, Vanderschuren LJMJ, Adan RAH, 2019. Corticolimbic Mechanisms of Behavioral Inhibition under Threat of Punishment. J. Neurosci. 39, 4353–4364. 10.1523/JNEUROSCI.2814-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verharen JPH, Luijendijk MCM, Vanderschuren LJMJ, Adan RAH, 2020a. Dopaminergic contributions to behavioral control under threat of punishment in rats. Psychopharmacology (Berl.) 237, 1769–1782. 10.1007/s00213-020-05497-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verharen JPH, Zhu Y, Lammel S, 2020b. Aversion hot spots in the dopamine system. Curr. Opin. Neurobiol., Systems Neuroscience 64, 46–52. 10.1016/j.conb.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JR, Beer B, Clody DE, 1971. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacologia 21, 1–7. 10.1007/BF00403989 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F, 2009. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56, 3–8. 10.1016/j.neuropharm.2008.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Morales M, 2015. The Brain on Drugs: From Reward to Addiction. Cell 162, 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Wenzel JM, Oleson EB, Gove WN, Cole AB, Gyawali U, Dantrassy HM, Bluett RJ, Dryanovski DI, Stuber GD, Deisseroth K, Mathur BN, Patel S, Lupica CR, Cheer JF, 2018. Phasic Dopamine Signals in the Nucleus Accumbens that Cause Active Avoidance Require Endocannabinoid Mobilization in the Midbrain. Curr. Biol. 28, 1392–1404.e5. 10.1016/j.cub.2018.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA, 1998. A psychomotor stimulant theory of addiction. Psychol. Rev. 94, 469–492. 10.1037/0033-295X.94.4.469 [DOI] [PubMed] [Google Scholar]
- Wise RA, Robble MA, 2020. Dopamine and Addiction. Annu. Rev. Psychol. 71, 79–106. 10.1146/annurev-psych-010418-103337 [DOI] [PubMed] [Google Scholar]
- Wise SP, 2008. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 31, 599–608. 10.1016/j.tins.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J, 1991. An ethopharmacological approach to the development of drug addiction. Neurosci. Biobehav. Rev., Special Issue Advances in Ethopharmacology 15, 515–519. 10.1016/S0149-7634(05)80142-3 [DOI] [PubMed] [Google Scholar]
- Wolffgramm J, Heyne A, 1991. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol. Biochem. Behav. 38, 389–399. 10.1016/0091-3057(91)90297-F [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L, 2012. Suppression of cocaine self-administration in monkeys: effects of delayed punishment. Psychopharmacology (Berl.) 220, 509–517. 10.1007/s00213-011-2501-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Qi J, Wang H-L, Zhang S, Morales M, 2015. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur. J. Neurosci. 41, 760–772. 10.1111/ejn.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates F, Stone E, 1992. The risk construct. Risk-Tak. Behav. [Google Scholar]
- Zahm DS, 2000. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 24, 85–105. 10.1016/S0149-7634(99)00065-2 [DOI] [PubMed] [Google Scholar]
- Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K, 2016. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 531, 642–646. 10.1038/nature17400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Baarendse PJJ, Vanderschuren LJMJ, Winstanley CA, 2015. Inactivation of the prelimbic or infralimbic cortex impairs decision-making in the rat gambling task. Psychopharmacology (Berl.) 232, 4481–4491. 10.1007/s00213-015-4075-y [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA, 2009. Serotonergic and Dopaminergic Modulation of Gambling Behavior as Assessed Using a Novel Rat Gambling Task. Neuropsychopharmacology 34, 2329–2343. 10.1038/npp.2009.62 [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA, 2011. Lesions of the Basolateral Amygdala and Orbitofrontal Cortex Differentially Affect Acquisition and Performance of a Rodent Gambling Task. J. Neurosci. 31, 2197–2204. 10.1523/JNEUROSCI.5597-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Qi J, Li X, Wang H-L, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M, 2015. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat. Neurosci. 18, 386–392. 10.1038/nn.3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Chou X-L, Zhang Z-G, Mesik L, Liang F, Tao HW, Zhang LI, 2017. AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 93, 33–47. 10.1016/j.neuron.2016.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg B, Peng B, Huang J, Tao HW, Zhang LI, 2020. Synaptic Specificity and Application of Anterograde Transsynaptic AAV for Probing Neural Circuitry. J. Neurosci. 40, 3250–3267. 10.1523/JNEUROSCI.2158-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]