Abstract
Background
Potentially inappropriate medications (PIMs) and polypharmacy in older adults lead to increase the risk of adverse drug events. This study aimed to evaluate the effectiveness of pharmacist intervention combining the criteria for detecting PIMs with the deprescribing algorithm on correcting PIMs, reducing the number of medications, and readmissions.
Methods
A prospective observational study was conducted at a Japanese University Hospital enrolling new inpatients aged ≥65 years prescribed ≥1 daily medication. Pharmacists detected PIMs based on the criteria combined the screening tool of older persons’ potentially inappropriate prescriptions criteria version 2 with the screening tool for older persons’ appropriate prescriptions for Japanese, examined changes using the deprescribing algorithm, and suggested changes to the physician. The proportion of patients whose number of medications was reduced at discharge and the rate of readmissions within 30 and 90 days were compared between patients without PIMs (without PIMs group), patients who were not suggested to change PIMs (no suggestions group), and patients who were suggested to change PIMs (suggested group).
Results
The study enrolled 544 patients (median age 75.0 years, 54.4% males, median number of medications 6.0/patient). The number of patients with PIMs was 240 (44.1%), and 304 patients had no PIMs (without PIMs group). Among the patients with PIMs, 125 (52.1%) patients received pharmacist suggestions to change ≥1 PIMs (suggested group), and 115 patients received no suggestions for change (no suggestions group). The total number of PIMs was 432, of which changes were suggested for 189 (43.8%). Of these 189 cases, 172 (91.0%) were changed. The proportion of patients whose number of medications was reduced was significantly higher in the suggested group than in the without PIMs group and the no suggestions group [56.8% (71/125) vs. 26.6% (81/304) and 19.1% (22/115), respectively; P < 0.001 in both comparisons]. There were no significant differences in the rates of readmissions within 30 and 90 days among the three groups.
Conclusions
Pharmacist intervention combining the criteria for detecting PIMs with the deprescribing algorithm was effective for correcting PIMs and may be associated with a reduction in the number of medications.
Keywords: Deprescribing, Potentially inappropriate medications, Polypharmacy, STOPP-J, STOPP criteria version 2
Introduction
The global population has been aging, and this trend is particularly remarkable in developed countries [1]. Older adults often have multimorbidity, resulting in a state of polypharmacy. Inappropriate prescriptions and polypharmacy in older adults are associated with an increase in adverse drug events, drug-drug interactions, hospitalizations, medical resource utilization, healthcare costs, and mortality [2–8], which have been targeted for correction.
Cochrane review suggested that it is uncertain whether intervention to polypharmacy for older adults reduces the potentially inappropriate medications (PIMs) or patients’ clinical outcome [9]. Meanwhile, several randomized clinical trials have demonstrated the efficacy of pharmacist intervention on correcting PIMs [10], long-term discontinuations of PIMs [11], and reducing the number of medications prescribed [12]. As explicit criteria to screen for PIMs, the effectiveness of the screening tool of older persons’ potentially inappropriate prescriptions (STOPP)/screening tool to alert doctors to right treatment (START) criteria has been reported [13, 14]. As implicit criteria to reduce inappropriate polypharmacy, the deprescribing algorithm has been proposed [15]. Since the prevalence and type of PIMs vary by country and health care setting [16], corrective measures based on these variations should be developed in each country.
In Japan, polypharmacy in older adults has been a social problem as well [17–19], and incentives in medical fees have been paid for interventions to reduce the number of drugs in patients with polypharmacy. However, few studies have evaluated the effectiveness of intervention for PIMs in Japan [20, 21]. The screening tool for older persons’ appropriate prescriptions for Japanese (STOPP-J) has been published in 2016 by the Japan Geriatrics Society [22], and “List of drugs to be prescribed with special caution” in STOPP-J includes 29 criteria indicating PIMs [22]. We previously reported the effectiveness of pharmacist intervention using STOPP criteria version 2 (STOPP-v2) or STOPP-J in Japanese clinical settings [23, 24]. STOPP-J detected significantly more patients with PIMs than STOPP-v2 because of its wide applicability, although the number of changes in PIMs was comparable for both criteria [24]. STOPP-v2 and STOPP-J includes multiple criteria which targeted drugs are overlapped. Therefore, we hypothesized that the combination of STOPP-v2 and STOPP-J could detect PIMs in accordance with the prevalence of PIMs in Japan, and correct PIMs more efficiently. Moreover, previous our studies showed that there were many PIMs that could not change due to the risk of withdrawal symptoms or disease exacerbation [23, 24]. Combining the deprescribing algorithm [15] with the criteria for screening PIMs may assist in determining whether PIMs can be safely changed. In addition, it is necessary to evaluate whether pharmacist intervention for PIMs is effective in reducing the number of medications and improving clinical outcomes in order to resolve the problems of polypharmacy in Japan.
The objective of this study was to evaluate the effectiveness of pharmacist intervention combining the criteria for detecting PIMs with the deprescribing algorithm on reducing the number of medications and the rate of readmissions in a Japanese clinical setting.
Methods
Study design and settings
A prospective observational study was conducted from January to August 2018 at five medical units of Kobe University Hospital, Japan. The main departments in these units were General Internal Medicine, Neurology, Rheumatology and Clinical Immunology, Neurosurgery, Gastrointestinal Surgery, Cardiovascular Surgery, Cardiovascular Internal Medicine, Orthopaedic Surgery, and Breast Surgery. The study protocol was approved by the Ethical Committee of Kobe University Hospital (No. 1758), and the study was performed in accordance with the Declaration of Helsinki and its amendments. Informed consent was obtained in the form of opt-out on the website of the hospital. This report was followed the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) statement [25].
Detecting and changing PIMs by pharmacist intervention
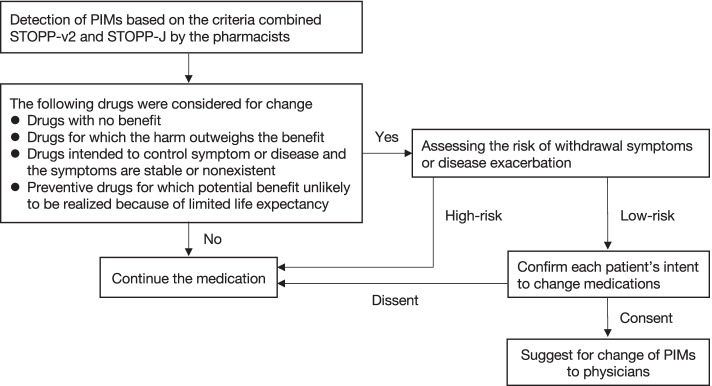
Fifteen clinical pharmacists with 1 to 16 years of experience were participated in the study. Before participating in the study, they were trained to detect and change PIMs based on STOPP-v2 and STOPP-J. One or two of them worked in each subject unit on weekdays. Only oral medications were included for detecting and changing of PIMs, and external medications were not included in the study. The scheme for detecting and changing PIMs is shown Fig. 1. Namely, pharmacists conducted medication reconciliation, confirmed medical history and laboratory data, and detected PIMs at the time of patient admission. For the detection of PIMs, we used the criteria combined STOPP-v2 with STOPP-J. The pharmacists assessed whether the detected PIMs could be changed according to the deprescribing algorithm [12, 15]. The following drugs were considered for dose reduction, discontinuation, or change to other drugs if those risk of withdrawal symptoms or disease exacerbation was judged to be low: drugs with no benefit, drugs for which the harm outweighs the benefit, drugs intended to control symptom or disease and the symptoms are stable or nonexistent, and preventive drugs for which potential benefit unlikely to be realized because of limited life expectancy [15]. The pharmacists also confirmed each patient’s intent to change medications, and based on those intents and their assessments, suggested for discontinuation/change of PIMs to physicians. The pharmacists and the physicians discussed and arrived at a consensus regarding any changes.
Fig. 1.
The scheme for detecting and changing PIMs. Abbreviations: PIMs, potentially inappropriate medications; STOPP-v2, Screening Tool of Older Persons’ potentially inappropriate Prescriptions criteria version 2; STOPP-J, Screening Tool for Older Persons’ appropriate Prescriptions for Japanese
STOPP-v2 consists of 80 criteria, each of which contains the drug class and indication [13]. Of the 29 criteria in STOPP-J, the drugs for 21 criteria overlap with STOPP-v2, and the drugs for other 8 criteria do not include in STOPP-v2. Accordingly, we combined the 8 criteria in STOPP-J with all 80 criteria in STOPP-v2, and used these combined 88 criteria in this study. In addition, we provided specific examples of the criterion for “any drug prescribed without an evidence-based clinical indication” in STOPP-v2. These examples of PIMs were detected in our previous studies [23, 24] and referred in the deprescribing algorithm [15], and are as follows: “concomitant use of proton-pump inhibitor (PPI) or H2 receptor antagonists and mucosal protective agent without a clinical indication,” “use of symptom control drug (such as antitussive agents or antiemetic drugs) when symptoms have already resolved,” and “use of vitamins in patients who have no clinical indication and can eat an adequate diet.”
Sample size and study subjects
This study was conducted for a fixed period (8 months), and we assumed that 400–500 samples would be included during the study period. Patients aged ≥65 years who were newly admitted, had been in the hospital for ≥7 days, and were prescribed at least one daily medication were included. The main subjects of STOPP-J were individuals aged ≥75 years and older individuals who were not yet aged 75 years, but who were frail or in need of special care [22]. The targeted participants of this study were hospitalized patients, and based on the targeted age of STOPP-v2 [13], the subjects of this study were integrated to individuals aged ≥65 years. Since it was difficult to change medications in patients with short hospitalization in our previous studies [23, 24], we included patients who were hospitalized for ≥7 days in this study.
In order to assess the effectiveness of pharmacist intervention on deprescribing, we compared the proportion of patients whose total number of medications was reduced more than one at discharge compared with that at admission and the changes in the number of medications during the hospitalization between the following three groups: patients without PIMs (without PIMs group), patients who were not suggested to change PIMs by the pharmacists based on the deprescribing algorithm or patients’ dissent (no suggestions group), and patients who were suggested to change one or more PIMs by the pharmacists (suggested group). In addition, the rate of readmissions within 30 and 90 days in the three groups were evaluated as a clinical outcome for the patients. Readmissions included only unscheduled readmissions, and excluded scheduled admissions, such as for clinical examination, surgery, or chemotherapy. Patient characteristics (age, number of medications, and length of hospitalization) were also compared between the three groups. The number of PIMs, PIMs suggested changes, and PIMs changed before discharge were evaluated.
Statistical analysis
The chi-square test or Fisher’s exact test followed by Bonferroni correction was used to compare the proportions of categorical variables between three groups (the proportion of patients whose total number of medications was reduced at discharge and the rate of readmissions within 30 and 90 days), and P values < 0.017 (0.05/3) were considered to indicate statistical significance. The statistical significance of the difference in median values between the three groups was analyzed by the Kruskal-Wallis test, followed by Dunn’s multiple comparisons test (age, number of medications, length of hospitalization, and changes in the number of medications during the hospitalization), and P values < 0.05 were considered to indicate statistical significance. All statistical analyses were performed with GraphPad Prism 6 (La Jolla, CA, USA).
Results
Patient characteristics
The characteristics of the study population are shown in Table 1. A total of 544 patients were included (median age 75.0 years, 296 [54.4%] males, median number of medications 6.0, median length of hospitalization 19.0 days). The number of patients with PIMs based on the criteria combined STOPP-v2 with STOPP-J was 240 (44.1%), and 304 patients had no PIMs (without PIMs group). Of the 240 patients with PIMs, 125 (52.1%) patients received pharmacist suggestions to change one or more PIMs (suggested group), and 115 patients received no suggestions for change (no suggestions group). The patients in the suggested group were significantly older than those in the without PIMs group (P = 0.029). Both patients in the suggested group and the no suggestions group had a higher number of medications than those in the without PIMs group (P < 0.001 and < 0.001). The patients in the suggested group had a longer length of hospitalization than those in the without PIMs group and the no suggestions group (P = 0.0029 and < 0.001). The most common department of all the study patients was Cardiovascular Surgery, followed by Orthopaedic Surgery.
Table 1.
Characteristics of study population
| Total (n = 544) | Without PIMs group (n = 304) | No suggestions group (n = 115) | Suggested group (n = 125) | ||
|---|---|---|---|---|---|
| Male | n (%) | 296 (54.4) | 167 (54.9) | 62 (53.9) | 67 (53.6) |
| Age (years) | Median (IQR) | 75.0 (70.0–80.0) | 74.0 (69.0–79.0) | 75.0 (69.0–80.0) | 77.0 (71.0–81.0)* |
| Number of medications | Median (IQR) | 6.0 (4.0–9.0) | 5.0 (3.0–7.0) | 7.0 (5.0–10.0) ‡‡‡ | 8.0 (6.0–11.0)*** |
| Length of hospitalization (days) | Median (IQR) | 19.0 (14.0–30.0) | 19.0 (13.0–29.0) | 18.0 (11.0–23.0) | 25.0 (16.0–37.5)**††† |
| Departments | |||||
| Cardiovascular Surgery | n | 185 | 115 | 32 | 38 |
| Orthopaedic Surgery | n | 107 | 52 | 31 | 24 |
| Gastrointestinal Surgery | n | 61 | 43 | 10 | 8 |
| Neurology | n | 55 | 29 | 10 | 16 |
| Breast Surgery | n | 47 | 22 | 12 | 13 |
| Neurosurgery | n | 46 | 22 | 7 | 17 |
| Rheumatology and Clinical Immunology | n | 31 | 14 | 9 | 8 |
| Cardiovascular Internal Medicine | n | 9 | 5 | 4 | 0 |
| General Internal Medicine | n | 3 | 2 | 0 | 1 |
Abbreviations: IQR interquartile range, PIMs potentially inappropriate medications
*P < 0.05
**P < 0.01
***P < 0.001 compared with the without PIMs group
†††P < 0.001 compared with the no suggestions group
‡‡‡P < 0.001 compared with the without PIMs group (Kruskal-Wallis test, followed by Dunn’s multiple comparisons test)
Detected and corrected PIMs by pharmacist intervention
The number of each PIM and those changed after pharmacist intervention are shown in Table 2. The total number of PIMs based on the criteria combined STOPP-v2 with STOPP-J was 432. Of these, 189 (43.8%) were suggested for change by the pharmacists based on the deprescribing algorithm and patients’ consent, and 172 (91.0%) of whom were discontinued or changed after the pharmacist intervention. The most frequent PIMs identified was “Benzodiazepines for ≥ 4 weeks,” with 108 detected, 20 change suggestions, and 16 executed changes. The second most frequently identified PIMs was “Any drug prescribed without an evidence-based clinical indication,” with 84 detected, 75 change suggestions, and 67 executed changes. PIMs detected frequently in this criterion were “concomitant use of proton-pump inhibitor (PPI) or H2 receptor antagonists and mucosal protective agent without indication” and “use of vitamins in patients who have no indication and can eat an adequate diet,” and the number of detected, change suggestions, and executed changes were 26, 24, 18 and 29, 24, 20, respectively.
Table 2.
Number of PIMs and those changed after pharmacist suggestion
| Criteriaa | Detected (n = 432) | Suggested (n = 189) | Changed (n = 172) |
|---|---|---|---|
| STOPP-v2 | 358 | 168 | 151 |
| Drug indication criteria | |||
| Any drug prescribed without an evidence-based clinical indication | 84 | 75 | 67 |
| Any duplicate drug class prescription | 10 | 5 | 5 |
| Cardiovascular System criteria | |||
| Beta-blocker in combination with verapamil or diltiazem | 1 | 0 | 0 |
| Thiazide diuretic with current significant hypokalaemia, hyponatraemia, hypercalcaemia or with a history of gout | 1 | 1 | 1 |
| ACE inhibitors or Angiotensin Receptor Blockers in patients with hyperkalaemia | 7 | 3 | 3 |
| Coagulation System criteria | |||
| Aspirin, clopidogrel, dipyridamole, vitamin K antagonists, direct thrombin inhibitors or factor Xa inhibitors with concurrent significant bleeding risk | 1 | 1 | 1 |
| Ticlopidine in any circumstances | 4 | 3 | 3 |
| NSAID and vitamin K antagonist, direct thrombin inhibitor or factor Xa inhibitors in combination | 1 | 1 | 1 |
| NSAID with concurrent antiplatelet agents without PPI prophylaxis | 1 | 0 | 0 |
| Central Nervous System criteria | |||
| Benzodiazepines for ≥4 weeksb | 108 | 20 | 16 |
| Antipsychotics in those with parkinsonism or Lewy Body Disease | 3 | 1 | 1 |
| Anticholinergics/antimuscarinics in patients with delirium or dementia | 5 | 4 | 3 |
| First-generation antihistamines | 3 | 3 | 3 |
| Renal System criteria | |||
| NSAIDs if eGFR < 50 mL/min/1.73m2 | 5 | 3 | 3 |
| Gastrointestinal System criteria | |||
| PPI for uncomplicated peptic ulcer disease or erosive peptic oesophagitis at full therapeutic dosage for > 8 weeks | 28 | 12 | 11 |
| Drugs likely to cause constipation in patients with chronic constipation where non-constipating alternatives are appropriate | 3 | 1 | 0 |
| Respiratory System criteria | |||
| Benzodiazepines with acute or chronic respiratory failure | 1 | 0 | 0 |
| Musculoskeletal System criteria | |||
| NSAID with established hypertension or heart failure | 7 | 6 | 6 |
| Long-term use of NSAID for symptom relief of osteoarthritis pain where paracetamol has not been tried | 1 | 1 | 1 |
| Long-term corticosteroids as monotherapy for rheumatoid arthritis | 3 | 0 | 0 |
| COX-2 selective NSAIDs with concurrent cardiovascular disease | 3 | 2 | 2 |
| NSAID with concurrent corticosteroids without PPI prophylaxis | 1 | 0 | 0 |
| Oral bisphosphonates in patients with a history of upper gastrointestinal disease | 1 | 1 | 1 |
| Urogenital System criteria | |||
| Antimuscarinic drugs for overactive bladder syndrome with concurrent dementia or chronic cognitive impairment or narrow-angle glaucoma, or chronic prostatism | 3 | 3 | 2 |
| Endocrine System criteria | |||
| Sulphonylureas with a long duration of action with type 2 diabetes mellitus | 16 | 4 | 4 |
| Beta-blockers in diabetes mellitus with frequent hypoglycaemic episodes | 1 | 0 | 0 |
| Drugs that predictably increase the risk of falls in older people | |||
| Benzodiazepines | 23 | 8 | 7 |
| Vasodilator drugs with persistent postural hypotension | 22 | 7 | 7 |
| Hypnotic Z-drugs | 9 | 2 | 2 |
| Antimuscarinic/anticholinergic drug burden | |||
| Concomitant use of two or more drugs with antimuscarinic/anticholinergic properties | 2 | 1 | 1 |
| STOPP-J | 74 | 21 | 21 |
| Sulpiride | 3 | 1 | 1 |
| H2 receptor antagonists | 32 | 12 | 12 |
| Laxative magnesium oxide (decreased kidney function) | 23 | 8 | 8 |
| α-glucosidase inhibitors | 15 | 0 | 0 |
| SGLT2 inhibitors | 1 | 0 | 0 |
Abbreviations: ACE angiotensin-converting enzyme, COX-2 cyclooxygenase-2, eGFR estimated glomerular filtration rate, NSAID non-steroidal anti-inflammatory drug, PPI proton-pump inhibitors, SGLT2 sodium-glucose transporter 2, STOPP-v2 Screening Tool of Older Persons’ potentially inappropriate Prescriptions criteria version 2, STOPP-J Screening Tool for Older Persons’ Appropriate Prescriptions for Japanese
aList of drugs includes only PIMs detected during the study period
bThe criterion of “Benzodiazepines for ≥4 weeks” included both benzodiazepines and hypnotic Z-drugs
Effectiveness of pharmacist intervention on reducing the total number of medications
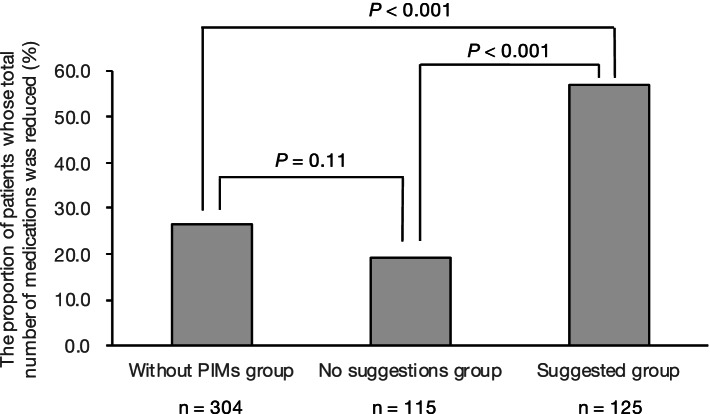
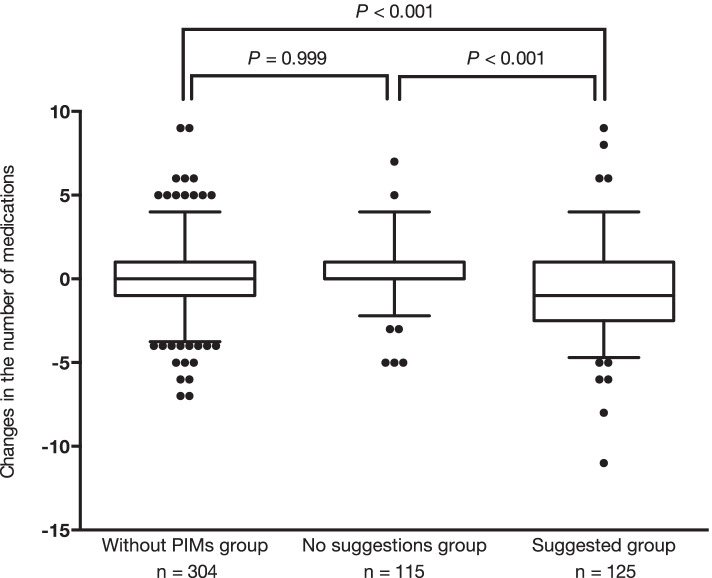
The proportion of patients whose total number of medications was reduced at discharge was 26.6% (81/304) in the without PIMs group, 19.1% (22/115) in the no suggestions group, and 56.8% (71/125) in the suggested group; the differences between the suggested group and the other groups were significant (P < 0.001 in both comparisons) (Fig. 2). The changes in the number of medications during the hospitalization decreased significantly in the suggested group than in the without PIMs group or the no suggestions group: median (interquartile range), − 1.0 (− 2.0 to 1.0) vs. 0.0 (− 1.0 to 1.0) and 0.0 (0.0 to 1.0); P < 0.001 in both comparisons (Fig. 3). The number of patients whose total number of medications was reduced more than two during the hospitalization were 43 (14.1%) in the without PIMs group, 11 (9.6%) in the no suggestions group, and 42 (33.6%) in the suggested group.
Fig. 2.
Proportion of patients whose total number of medications at discharge was reduced by more than one. The chi-square test followed by Bonferroni correction was used to compare the proportions of categorical variables between three groups, and P values < 0.017 were considered to indicate statistical significance. Abbreviations: PIMs, potentially inappropriate medications
Fig. 3.
Changes in the number of medications during the hospitalization. Boxes represent interquartile ranges; whiskers, the 5th and 95th percentile in each category; dots mark outliers. Abbreviations: PIMs, potentially inappropriate medications
There were no significant differences in the rates of readmissions within 30 and 90 days among the three groups (Table 3).
Table 3.
The rate of readmissions within 30 and 90 days
| Without PIMs group (n = 304) | No suggestions group (n = 115) | Suggested group (n = 125) | P values | ||||
|---|---|---|---|---|---|---|---|
| Without PIMs group vs Suggested group | No suggestions group vs Suggested group | Without PIMs group vs No suggestions group | |||||
| Readmissions within 30 days | n (%) | 10 (3.3) | 1 (0.9) | 7 (5.6) | 0.28 | 0.068 | 0.30 |
| Readmissions within 90 days | n (%) | 24 (7.9) | 2 (1.7) | 9 (7.2) | 1.00 | 0.062 | 0.021 |
The Fisher’s exact test followed by Bonferroni correction was used to compare the proportions of categorical variables between three groups, and P values < 0.017 were considered to indicate statistical significance
Abbreviations: PIMs potentially inappropriate medications
Discussion
In the present study, we used the criteria combined STOPP-v2 with STOPP-J, and suggested for a prescription change according to the deprescribing algorithm in patients with detected PIMs. Out of a total of 432 detected PIMs, 189 were suggested by pharmacists for change, and 91.0% of whom were changed. Furthermore, the suggested group showed a significantly higher proportion of patients whose total number of medications was reduced at discharge than the groups without PIMs or received no suggestions. No significant differences were observed in the rates of readmissions between the suggested group and the other two groups.
Our study suggested that pharmacist intervention was effective in correcting PIMs and may be associated with a reduction in the number of medications during the hospitalizations. No statistical sample size calculations were conducted before the start of the study. However, the power of the post-hoc analysis of the comparison between the suggested group and the other two groups for the proportion of patients whose total number of medications was reduced at discharge was 1.0 for both comparisons, thus we considered that the sample size of this study was sufficient. In Japan, medical fees began to be provided in 2016 for interventions to reduce the number of two or more drugs in patients with polypharmacy. More than 30% of patients in the suggested group had a reduction of two or more drugs in this study. A retrospective observational study using nationwide health insurance reimbursement claims data in Japan suggested that a 7.3% reduction in nationwide polypharmacy after the implementation of this health policy in 2016 [26]. On the other hand, there are few studies which evaluated the intervention for PIMs in Japan [20, 21]. Several randomized clinical trials in other countries have demonstrated the efficacy of pharmacist intervention on correcting PIMs [10–12], and our results were in line with these previous studies [10–12]. Meanwhile, this study did not show a reduction in readmissions by the pharmacist intervention for PIMs. Although the no suggestions group had a lower rate of readmissions than the other two groups, the differences were not statistically significant. In some cases, correcting PIMs was not suggested for patients with shorter hospitalization who were scheduled for readmission, thus the no suggestions group included more scheduled readmissions. Patients scheduled for readmission were less likely to be unscheduled readmission, which may have resulted in a lower rate of unscheduled readmissions in the no suggestions group. Cochrane review suggested that it is uncertain whether interventions for polypharmacy and medication reviews reduces hospital admissions, quality of life, or mortality [9, 27], and subsequent randomized clinical trial also did not demonstrate that clinical pharmacist intervention can reduce adverse drug-related incidents or clinically important medication errors during the posthospitalization [28]. Whereas, the other randomized clinical trial suggested that clinical pharmacist intervention including follow-up after discharge can reduce the number of emergency department visits and hospital readmissions [29]. In addition to intervention during hospitalization, post-discharge follow-up may be necessary to improve clinical outcome such as readmissions.
In this study, PIMs corresponded to “any drug prescribed without an evidence-based clinical indication” were most frequently changed. We described mucosal protective agents or vitamins as examples for this criterion, and these drugs were frequently detected as PIMs. Previous studies in Japan [18, 19, 23, 24] or outside of Japan [30, 31] have not been frequently detected these PIMs. However, our study suggested that these drugs may be abundantly prescribed in Japan. Although discontinuation of these drugs may not entirely contribute to improve clinical outcomes, it may lead to reduce unnecessary drug costs. PIMs related to benzodiazepines and PPI were frequently detected in this study, nevertheless the proportion of change of these drugs were low. Inappropriate prescribing of these drugs needs to be corrected in order to prevent adverse drug events in older patients. In this study, the pharmacists assessed whether PIMs could be safely changed according to the deprescribing algorithm, and suggested for those change based on the patients’ intents as well. Reasons for not suggesting changes of PIMs included that prescribed medications were needed for disease control and those benefits were high, the change was difficult due to the high risk of withdrawal symptoms, and lack of patient’s consent. The deprescribing algorithm was useful for pharmaceutical assessment. However, the combination of the other deprescribing algorithms by the Bruyère Research Institute [32, 33] may be effective for more specific assessment of prescription changes in each type of drug. In some cases where the scheduled duration of hospitalization was short, the pharmacists judged it too difficult to change PIMs before discharge and did not suggest those changes; thus, the length of hospitalization was longer in the suggested change group than the no suggestions group. Discontinuation of drugs with risk of withdrawal symptoms, such as benzodiazepines, is difficult in short hospitalization. Consequently, collaboration with community pharmacies, as in previous study [11], would be necessary for long-term changes of those PIMs.
Among the eight criteria unique to STOPP-J, H2 receptor antagonists and laxative magnesium oxide were frequently detected as PIMs. These PIMs were also detected in previous study in Japan [18, 24], and should be corrected, especially in patients with chronic kidney disease or those who have suffered an adverse event. Meanwhile, out of a total of 88 criteria combining the STOPP-v2 and STOPP-J in this study, PIMs were actually detected in 35 criteria. The contents of PIMs were similar in our previous studies [23, 24]. In order to detect PIMs more efficiently, it may be better to narrow down the contents of the criteria for the detection of PIMs.
This study has several limitations. First, it was an observational study, and we could not compare the changes in the number of medications during hospitalization between patients whose pharmacist suggested changes in PIMs and the entire population of patients with PIMs for whom no pharmacist intervention. Although there may be an association between pharmacist intervention for patients with PIMs and a reduction in their number of medications, a design that accurately evaluates the effect of pharmacist intervention is needed. Second, this study conducted at a single Japanese university hospital. Thus, our results may not be generalizable to other clinical settings or countries. However, our results may be useful in an aging population like Japan or clinical settings where prescribing trends are similar.
Conclusion
The present study suggested the effectiveness of pharmacist intervention combining the criteria for detecting PIMs with the deprescribing algorithm on correcting PIMs and those association with a reduction the total number of medications at a discharge. Pharmacist intervention could correct most of the PIMs that were judged to be needed for change, and may be effective in reducing the number of medications during the hospitalizations, whereas did not demonstrate a reduction in readmissions. Pharmacist intervention could be useful in Japanese clinical settings, where PIMs and polypharmacy has been an urgent problem.
Acknowledgements
We would like to thank Ms. Moeko Kubo, Ms. Keiko Yamaoka, Mr. Daichi Enomoto, Ms. Yui Mishima, Ms. Asuka Toda, Ms. Tomoko Kurimura, Ms. Yoko Kunimitsu, Ms. Haruna Hirayama, Ms. Ai Takeda, Ms. Yuka Nakatani, and Ms. Naho Marugami for their contribution to this study.
Abbreviations
- ACE
Angiotensin-converting enzyme
- COX-2
Cyclooxygenase-2
- eGFR
Estimated glomerular filtration rate
- NSAID
Non-steroidal anti-inflammatory drug
- PIMs
Potentially inappropriate medications
- PPI
Proton-pump inhibitor
- SGLT2
Sodium-glucose transporter 2
- START
Screening Tool to Alert doctors to Right Treatment
- STOPP
Screening Tool of Older Persons’ potentially inappropriate Prescriptions
- STOPP-J
Screening Tool for Older Persons’ Appropriate Prescriptions for Japanese
- STOPP-v2
Screening Tool of Older Persons’ potentially inappropriate Prescriptions criteria version 2
- STROBE
Strengthening the Reporting of Observational studies in Epidemiology
Authors’ contributions
TK and IY conceptualized and designed the study. MF, MS, KS and SB contributed the pharmacist intervention. TK, KY and TO analyzed the data. TK wrote the manuscript. KY, TO and IY supervised the study and revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by JSPS KAKENHI Grant Number JP17H00562.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participants
The study protocol was approved by the Ethical Committee of Kobe University Hospital (No. 1758), and the study was performed in accordance with the Declaration of Helsinki and its amendments. Informed consent was obtained in the form of opt-out on the website of the hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations . World Population Ageing. 2019. [Google Scholar]
- 2.Tosato M, Landi F, Martone AM, Cherubini A, Corsonello A, Volpato S, et al. Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing. 2014;43(6):767–773. doi: 10.1093/ageing/afu029. [DOI] [PubMed] [Google Scholar]
- 3.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 4.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5(4):345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Campbell SE, Seymour DG, Primrose WR, ACMEPLUS Project A systematic literature review of factors affecting outcome in older medical patients admitted to hospital. Age Ageing. 2004;33(2):110–115. doi: 10.1093/ageing/afh036. [DOI] [PubMed] [Google Scholar]
- 6.Espino DV, Bazaldua OV, Palmer RF, Mouton CP, Parchman ML, Miles TP, et al. Suboptimal medication use and mortality in an older adult community-based cohort: results from the Hispanic EPESE study. J Gerontol A Biol Sci Med Sci. 2006;61(2):170–175. doi: 10.1093/gerona/61.2.170. [DOI] [PubMed] [Google Scholar]
- 7.Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69(5):543–552. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350:h949. doi: 10.1136/bmj.h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rankin A, Cadogan CA, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018;9(9):CD008165. doi: 10.1002/14651858.CD008165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankenthal D, Lerman Y, Kalendaryev E, Lerman Y. Intervention with the screening tool of older persons potentially inappropriate prescriptions/screening tool to alert doctors to right treatment criteria in elderly residents of a chronic geriatric facility: a randomized clinical trial. J Am Geriatr Soc. 2014;62(9):1658–1665. doi: 10.1111/jgs.12993. [DOI] [PubMed] [Google Scholar]
- 11.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889–1898. doi: 10.1001/jama.2018.16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balsom C, Pittman N, King R, Kelly D. Impact of a pharmacist-administered deprescribing intervention on nursing home residents: a randomized controlled trial. Int J Clin Pharm. 2020;42(4):1153–1167. doi: 10.1007/s11096-020-01073-6. [DOI] [PubMed] [Google Scholar]
- 13.O'Mahony D, O'Sullivan D, Byrne S, O'Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–218. doi: 10.1093/ageing/afu145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill-Taylor B, Walsh KA, Stewart S, Hayden J, Byrne S, Sketris IS. Effectiveness of the STOPP/START (screening tool of older Persons' potentially inappropriate prescriptions/screening tool to alert doctors to the right treatment) criteria: systematic review and meta-analysis of randomized controlled studies. J Clin Pharm Ther. 2016;41(2):158–169. doi: 10.1111/jcpt.12372. [DOI] [PubMed] [Google Scholar]
- 15.Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–834. doi: 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher P, Lang PO, Cherubini A, Topinková E, Cruz-Jentoft A, Montero Errasquín B, et al. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol. 2011;67(11):1175–1188. doi: 10.1007/s00228-011-1061-0. [DOI] [PubMed] [Google Scholar]
- 17.Onda M, Imai H, Takada Y, Fujii S, Shono T, Nanaumi Y. Identification and prevalence of adverse drug events caused by potentially inappropriate medication in homebound elderly patients: a retrospective study using a nationwide survey in Japan. BMJ Open. 2015;5(8):e007581. doi: 10.1136/bmjopen-2015-007581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang CH, Umegaki H, Watanabe Y, Kamitani H, Asai A, Kanda S, et al. Potentially inappropriate medications according to STOPP-J criteria and risks of hospitalization and mortality in elderly patients receiving home-based medical services. PLoS One. 2019;14(2):e0211947. doi: 10.1371/journal.pone.0211947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masumoto S, Sato M, Maeno T, Ichinohe Y, Maeno T. Potentially inappropriate medications with polypharmacy increase the risk of falls in older Japanese patients: 1-year prospective cohort study. Geriatr Gerontol Int. 2018;18(7):1064–1070. doi: 10.1111/ggi.13307. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto R, Fujii K, Shimoji S, Utsumi A, Hosokawa K, Tochino H, et al. Study of pharmacist intervention in polypharmacy among older patients: non-randomized, controlled trial. Geriatr Gerontol Int. 2020;20(3):229–237. doi: 10.1111/ggi.13850. [DOI] [PubMed] [Google Scholar]
- 21.Komagamine J, Hagane K. Intervention to improve the appropriate use of polypharmacy for older patients with hip fractures: an observational study. BMC Geriatr. 2017;17(1):288. doi: 10.1186/s12877-017-0681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima T, Mizukami K, Tomita N, Arai H, Ohrui T, Eto M, et al. Screening tool for older Persons' appropriate prescriptions for Japanese: report of the Japan geriatrics society working group on "guidelines for medical treatment and its safety in the elderly". Geriatr Gerontol Int. 2016;16(9):983–1001. doi: 10.1111/ggi.12890. [DOI] [PubMed] [Google Scholar]
- 23.Kimura T, Ogura F, Yamamoto K, Uda A, Nishioka T, Kume M, et al. Potentially inappropriate medications in elderly Japanese patients: effects of pharmacists' assessment and intervention based on screening tool of older Persons' potentially inappropriate prescriptions criteria ver.2. J Clin Pharm Ther. 2017;42(2):209–214. doi: 10.1111/jcpt.12496. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Ogura F, Kukita Y, Takahashi T, Yamamoto K, Ioroi T, et al. Efficacy of pharmacists’ assessment and intervention based on screening tool for older persons’ appropriate prescriptions for Japanese compared with screening tool of older Persons' potentially inappropriate prescriptions criteria version 2 in older patients with cardiovascular disease. Geriatr Gerontol Int. 2019;19(11):1101–1107. doi: 10.1111/ggi.13773. [DOI] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Ishida T, Yamaoka K, Suzuki A, Nakata Y. Effectiveness of polypharmacy reduction policy in Japan: nationwide retrospective observational study. Int J Clin Pharm. 2021. 10.1007/s11096-021-01347-7 Epub ahead of print. [DOI] [PubMed]
- 27.Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2(2):CD008986. doi: 10.1002/14651858.CD008986.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurwitz JH, Kapoor A, Garber L, Mazor KM, Wagner J, Cutrona SL, et al. Effect of a multifaceted clinical pharmacist intervention on medication safety after hospitalization in persons prescribed high-risk medications: a randomized clinical trial. JAMA Intern Med. 2021;181(5):610–618. doi: 10.1001/jamainternmed.2020.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med. 2018;178(3):375–382. doi: 10.1001/jamainternmed.2017.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bo M, Gibello M, Brunetti E, Boietti E, Sappa M, Falcone Y, et al. Prevalence and predictors of inappropriate prescribing according to the screening tool of older People's prescriptions and screening tool to alert to right treatment version 2 criteria in older patients discharged from geriatric and internal medicine wards: a prospective observational multicenter study. Geriatr Gerontol Int. 2019;19(1):5–11. doi: 10.1111/ggi.13542. [DOI] [PubMed] [Google Scholar]
- 31.Pérez T, Moriarty F, Wallace E, McDowell R, Redmond P, Fahey T. Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. BMJ. 2018;363:k4524. doi: 10.1136/bmj.k4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pottie K, Thompson W, Davies S, Grenier J, Sadowski CA, Welch V, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(5):339–351. [PMC free article] [PubMed] [Google Scholar]
- 33.Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(5):354–364. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.