Abstract
Impurity profiling of a pharmaceutical compound is now taking great attention during quality assessment of pharmaceuticals, as presence of small amount of impurities may affect safety and efficacy. In this work, a novel TLC chromatographic method coupled with densitometric detection was established for the simultaneous quantification of naphazoline HCl, pheniramine maleate and three of their official impurities, namely; naphazoline impurity B, pheniramine impurities; A & B. Chromatographic separation was carried out on TLC aluminum silica plates F254, as a stationary phase, using methanol: ethyl acetate: 33.0% ammonia (2.0: 8.0: 1.0, by volume), as a mobile phase. Plates were examined at 260.0 nm and International Council for Harmonisation (ICH) guidelines were followed for method’s validation. Important factors, such as; composition of mobile phase and detection wavelengths were optimized. Linearity was achieved over the ranges of 2.0–50.0 µg band−1 for naphazoline, 10.0–110.0 µg band−1 for pheniramine, 0.1–10.0 µg band−1 for naphazoline impurity B and 2.0–50.0 µg band−1 for both pheniramine impurities. The proposed method was assessed in terms of accuracy, precision and robustness where satisfactory results (recovery % ≈ 100% and RSD < 2) were obtained. The method was also applied for the simultaneous determination of naphazoline HCl and pheniramine maleate, in Naphcon-A® eye drops, with respective recoveries of 101.36% and 100.94%. Method greenness was evaluated and compared to the reported HPLC one via environmental, health and safety tool. The developed method has much potential over the reported one of being simple, selective, economic and time saving for the analysis of the five cited compounds.
Keywords: Naphazoline, Pheniramine, Impurities, TLC, EHS tool
Introduction
A great attention was given, by modern pharmaceutical analysis, to impurity profiling of the drug substances, as the presence of impurities, even in trace amounts, may affect the quality, potency and safety of the drug product [1]. In a different manner, Thin Layer Chromatography (TLC) is one of the most familiar and adaptable techniques used in detection and quantification of related impurities in the pharmaceutical filed. It has several advantages including; simplicity, cost-effectiveness, rapidness as well as good resolving power with accurate quantification of multicomponent mixtures [2].
Naphazoline HCl (NPZ) is chemically known as 2-(naphthalen-1-ylmethyl)-4, 5-dihydro-1H-imidazole; hydrochloride. It has a decongestant property through mimicking the sympathetic influence on alpha-adrenergic receptors. It plays an important role in managing allergic conjunctiva, as it can reduce eye swelling and edema by acting on those receptors in the conjunctiva arterioles [3]. NPZ is an authorized drug in the United States (USP) [4] and British (BP) [5] pharmacopeias where its determination was carried out by HPLC methods. Moreover, the BP states four specified official impurities; one of them is impurity B (NPZ impurity B). On reviewing literature, NPZ has been determined as a single drug or in combination using several techniques, namely; spectrophotometry [6–14], HPLC [15–27], TLC [28] and capillary electrophoresis [29–36].
Pheniramine maleate (PHN) is chemically designed as (Z)-but-2-enedioic acid; N, N-dimethyl-3-phenyl-3-pyridin-2-ylpropan-1-amine. It is widely available in eye drops due to its antihistaminic and anticholinergic effect [37]. PHN is official in USP and BP whereas HPLC technique was reported for its assay. Two impurities were stated in BP for PHN, namely; A & B [5]. The literature survey revealed different techniques for its quantification either in single or in combined form, such as titrimetric [38], spectrophotometric [39, 40], HPLC [17–19, 27, 41–48] and capillary electrophoretic ones [36, 49].
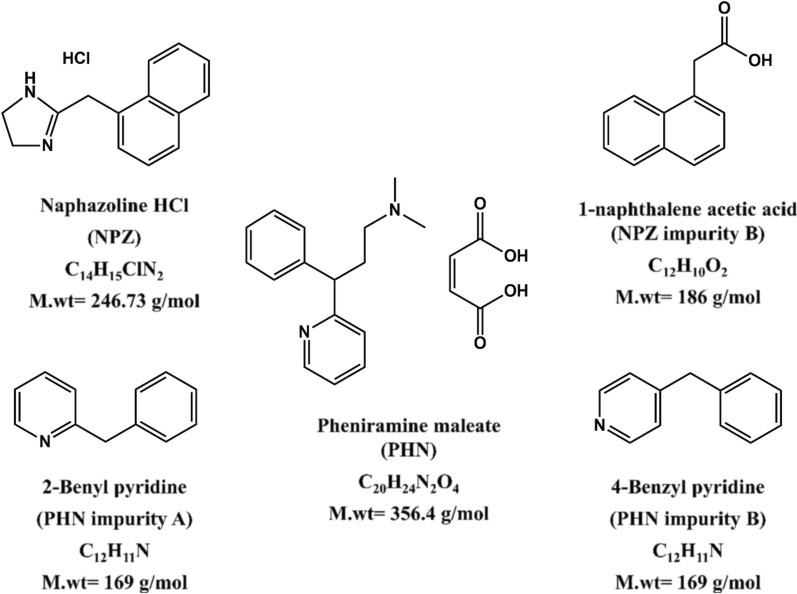
NPZ and PHN are usually co-formulated together in optic dosage forms used for eye inflammation treatment. The literature survey revealed some methods for their simultaneous quantification, such as HPLC [17–19, 27] and one capillary electrophoresis [36]. One of the reported HPLC methods described their determination in presence of three selected impurities [19]. As a result, the aim of this work was to develop a first validated, as per ICH guidelines [50], TLC densitometric method for detection and quantification of NPZ, PHN, NPZ impurity B, PHN impurities; A and B, (Fig. 1). The proposed method was successfully applied for their simultaneous determination in a quinary mixture as well as in pharmaceutical eye drops. Furthermore, the organic solvents used in this work were assessed and compared to that used in the reported HPLC one [19] by the aid of environmental, health and safety (EHS) tool [51].
Fig. 1.
Chemical structures of the five cited components
Methods/Experimental
Instruments
TLC system; a Camag Linomat autosampler (Muttenzl, Switzerland), a Camag micro syringe (100 µL), a Camag 35/N/30319 TLC scanner with win CATS software, UV lamp with a short wavelength at 260 nm (Desaga, Wiesloch, Germany) and TLC plates (10 × 20 cm) pre-coated with silica gel GF254 of 0.25 mm thickness (Merck, Darmstadt, Germany).
Materials and chemicals
Pure standard
NPZ and PHN were kindly provided by Eva pharma pharmaceutical company, Cairo, Egypt. Their purities were assessed to be 100.12 ± 0.102% and 99.58 ± 0.124%, respectively [5]. The impurities (NPZ impurity B, PHN impurities; A & B) were purchased from the German company Alfa Aesar Company. Their certified potency values were found to be 99.00%, 100.30%, and 99.70%, respectively.
Pharmaceutical dosage form
Naphcon-A® eye drops (Batch no.H13949-0615); manufactured by Alcon laboratories INC (Novartis Company), labeled to contain 0.25 & 3.0 mg mL−1 of NPZ and PHN, respectively, and has been purchased from Egyptian market.
Chemicals and reagents
Analytical-grade chemicals were used; methanol (Alpha, Egypt), ethyl acetate (Otsuka, Egypt), chloroform, acetone, 33.0% ammonia (El-Nasr, Egypt), hydrochloric acid (Sigma, Germany), 30.0% hydrogen peroxide solution (Adwic, Egypt) and sodium hydroxide pellets (Piochem, Egypt).
Solutions
Standard solutions
In 10-mL volumetric flasks, standard solutions of 20.0 mg mL−1, for NPZ, PHN and two PHN impurities, were separately prepared in methanol. For NPZ impurity B, 1.0 mg mL−1 standard solution was prepared.
Laboratory prepared mixtures
Different aliquots, from the five standard solutions, were transferred into separate 10-mL volumetric flasks to prepare laboratory prepared mixtures of various ratios. The volume of each flask was then completed to the mark using methanol.
Procedures
Construction of the calibration curves
Aliquots equivalent to 2.0–50.0 mg of NPZ, 10.0–110.0 mg of PHN, 0.1–10.0 mg of NPZ impurity B and 2.0–50.0 mg of two PHN impurities (A & B) were transferred from their corresponding solutions into five sets of 10-mL volumetric flasks. Volumes were then completed to the mark with methanol. 10.0 µL from each solution was applied as a band with 3.0 mm length onto TLC plates (10 × 20 cm). A mobile phase of methanol: ethyl acetate: 33.0% ammonia (2.0: 8.0: 1.0, by volume) was used for elution over 8.5 cm distance. The elution time was around 6.0 min. After that, the plates were removed, air dried and scanned at 260.0 nm. Calibration curves, for the five cited drugs, representing the polynomial relationship between peak area and corresponding concentration were constructed and regression parameters were computed.
Assay of laboratory prepared mixtures
Different mixtures of NPZ, PHN and their official impurities were prepared as mentioned under solution section and mixed with different ratios. The prepared mixtures were then analyzed by the proposed method as mentioned above.
Forced degradation study
Stability of the two cited drugs were studied under different conditions, namely; acidic, alkaline, oxidative, photolytic and thermal ones. For acidic and alkaline hydrolyses, a mass of 10 mg of each drug was separately dissolved in least amounts of methanol then refluxing in 1 M HCl or methanolic solutions of 1 M NaOH at 100 °C for 2 h. For oxidative studies, 0.5 mL of 5% H2O2 aqueous solution was separately added to 10 mL of 1.0 mg mL−1 solutions. The two drugs solutions were then kept at room temperature for 24 h. For photolytic study, thin layers of each powdered drug was uniformly spread in two Petri dishes, and exposed to UV light at 254 nm for 10 h at a distance of 15 cm. Thermal degradation was assessed through sealing each powdered drug in glass ampoules and heating in a thermostatic oven at 100 °C for 7 h. Finally, samples were periodically withdrawn for observing the forced degradation process.
Pharmaceutical application
The content of 10 Naphcon-A® eye drops were emptied. 20.0 mL aliquot was transferred into a 25-mL measuring flask. 3.0 mL methanol was added and the flask was then sonicated for 20.0 min. Volume was completed to the mark using methanol to obtain final concentration of 200.0 µg mL−1 NPZ and 2400.0 µg mL−1 PHN. 10.0 µLs from this solution were applied onto TLC plates. Finally, solutions were analyzed as mentioned before under construction of the calibration curves.
Results and discussion
The importance of impurity detection and determination evokes the requirement for developing simple, economical, rapid and accurate analytical techniques which can be utilized easily in quality control laboratories wherein cost and time are essential. Owing to simplicity, cost effectiveness, time saving, no need for tedious sample preparation and high sensitivity, TLC densitometry could be considered as one of the best options for that purpose [2, 52, 53] Here, we present a novel TLC densitometric method for the simultaneous determination of NPZ, PHN and three related official impurities (NPZ impurity B, PHN impurities; A & B) in their quinary mixture. Moreover, EHS tool is applied for greenness evaluation of this method in comparison to our previously reported HPLC one [19].
Development and optimization of TLC densitometric method
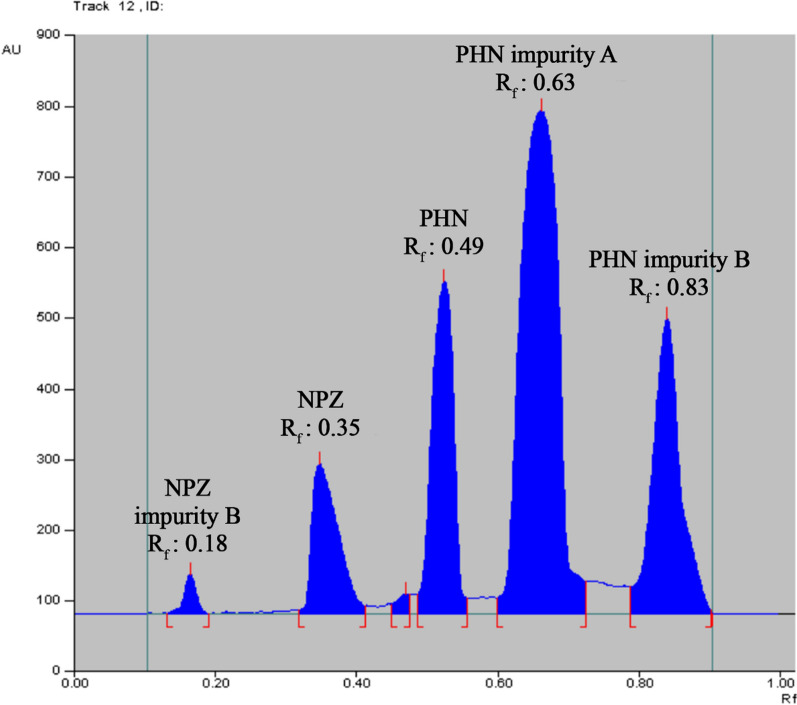
Various mobile phases were tried to get optimum separation and resolution between the five cited components. Firstly, mixtures with different ratios of methanol and ethyl acetate have been tried, but the separation between the five cited components was not achieved. Thus, different solvents were added individually to the previous mixture for improving the separation between the studied components such as chloroform, acetone and ammonia. Table 1 summarizes the obtained resolution values during this optimization phase. It was noticed that addition of ammonia to the conventional mixture enhanced separation and resolution between the studied drugs. Finally, a mixture of methanol–ethyl acetate–ammonia (2.0: 8.0: 1.0, by volume) was chosen for optimum suitability parameters. Moreover, different wavelengths were tried for evaluating the densitometric measurement as 260.0 nm and 280.0 nm. 260.0 nm was the wavelength of choice as it gave the highest sensitivity with minimum noise for measuring the five cited components. Retardation factor (Rf) values were sequentially at 0.18 ± 0.02, 0.35 ± 0.02, 0.49 ± 0.02, 0.63 ± 0.02 and 0.83 ± 0.02 for NPZ impurity B, NPZ, PHN, PHN impurity A and PHN impurity B (Fig. 2). Scanning profiles were obtained at 260.0 nm, and five calibration curves were then plotted.
Table 1.
The obtained resolution values during mobile phase optimization
| Experiment No. | Mobile phase composition | Rs1a | Rs2a | Rs3a | Rs4a |
|---|---|---|---|---|---|
| 1 | Methanol–ethyl acetate (3.5:6.5, v/v) | 1.32 | 1.24 | 1.34 | 0.57 |
| 2 | Methanol–ethyl acetate (3.0:7.0, v/v) | 1.35 | 1.33 | 1.37 | 0.62 |
| 3 | Methanol–ethyl acetate (2.5:7.5, v/v) | 1.42 | 1.37 | 1.40 | 0.70 |
| 4 | Methanol–ethyl acetate (2.0:8.0, v/v) | 1.47 | 1.39 | 1.45 | 0.83 |
| 5 | Methanol–ethyl acetate–chloroform (2.0:8.0:1.0, v/v/v) | 1.49 | 1.51 | 1.13 | 0.75 |
| 6 | Methanol–ethyl acetate–chloroform (2.0:8.0:0.8, v/v/v) | 1.52 | 1.53 | 1.22 | 0.78 |
| 7 | Methanol–ethyl acetate–chloroform (2.0:8.0:0.5, v/v/v) | 1.53 | 1.54 | 1.27 | 0.89 |
| 8 | Methanol–ethyl acetate–chloroform (2.0:8.0:0.2, v/v/v) | 1.55 | 1.57 | 1.35 | 0.94 |
| 9 | Methanol–ethyl acetate– acetone (2.0:8.0:1.0, v/v/v) | 1.46 | 1.44 | 1.21 | 1.13 |
| 10 | Methanol–ethyl acetate– acetone (2.0:8.0:0.8, v/v/v) | 1.47 | 1.47 | 1.23 | 1.17 |
| 11 | Methanol–ethyl acetate– acetone (2.0:8.0:0.5, v/v/v) | 1.49 | 1.48 | 1.27 | 1.22 |
| 12 | Methanol–ethyl acetate– acetone (2.0:8.0:0.2, v/v/v) | 1.51 | 1.49 | 1.36 | 1.28 |
aRs1, Rs2, Rs3 and Rs4 are the obtained resolutions between NPZ impurity B & NPZ, NPZ & PHN, PHN & PHN impurity A and PHN impurity A & PHN impurity B, respectively
Fig. 2.
TLC chromatogram of NPZ (30.0 µg band −1), PHN (90.0 µg band −1) and three of their official impurities as NPZ impurity B (10.0 µg band −1), PHN impurity A (40.0 µg band −1) and PHN impurity B (40.0 µg band −1) using a mobile phase of methanol: ethyl acetate: ammonia (2.0: 8.0: 1.0, by volume) and detection at 260.0 nm
System suitability parameters
To evaluate the performance of the proposed TLC method, system suitability parameters were calculated manually [54]. The results of retardation, resolution, capacity and tailing factors for the five components were obtained in Table 2.
Table 2.
Parameters required for system suitability tests of TLC densitometric method
| Parameter | NPZ impurity B | NPZ | PHN | PHN impurity A | PHN impurity B |
|---|---|---|---|---|---|
| Rf | 0.18 | 0.35 | 0.49 | 0.63 | 0.83 |
| Resolution (Rs) | NA | 1.50 | 1.58 | 1.40 | 1.64 |
| Tailing factor (T) | 1.50 | 0.80 | 1.30 | 1.20 | 1.20 |
| Retention factor (k')a | 4.56 | 1.86 | 1.04 | 0.59 | 0.20 |
| Selectivity factor (α)b | NA | 2.45 | 1.79 | 1.76 | 2.95 |
| Column efficiency (N)c | 262.44 | 196.00 | 635.04 | 425.11 | 737.86 |
| Height equivalent to theoretical plate (mm) | 0.034 | 0.046 | 0.014 | 0.021 | 0.012 |
aRetention factor (k') = (1 − Rf)/Rf
bCalculation of α = k'2/k'1
cColumn efficiency (N) = 16 (z/w)2, where z is the migration length of the spot, w is the spot width
Method validation
Method’s validation was conducted in agreement to ICH guidelines [50].
Linearity and range
Polynomial relationships were established between the integrated peak area and the corresponding concentration in the ranges of 2.0 − 50.0 µg band −1, 10.0–110.0 µg band −1, 0.1 − 10.0 µg band −1 and 2.0 − 50.0 µg band −1 for NPZ, PHN, NPZ impurity B and the two PHN related impurities, respectively.
Accuracy
Accuracy was assessed by applying the previously mentioned procedures on pure samples with various concentrations within the defined ranges. Satisfactory results regarding recovery % were computed in Table 3.
Table 3.
Regression parameters for determination of the studied drugs by the proposed TLC densitometric method
| Parameter | NPZ impurity B | NPZ | PHN | PHN impurity A | PHN impurity B |
|---|---|---|---|---|---|
| Range | 0.1–10.0 µg band−1 | 2.0–50.0 µg band−1 | 10.0–110.0 µg band−1 | 0.2–50.0 µg band−1 | 0.2–50.0 µg band−1 |
| Slope |
No. 1a = − 172.85 No. 2a = 3480.76 |
No. 1a = − 13.91 No. 2a = 1389.10 |
No. 1a = − 1.82 No. 2a = 584.57 |
No. 1a = − 9.78 No. 2a = 943.45 |
No. 1a = − 11.64 No. 2a = 962.46 |
| Intercept | 1343.80 | 3305.64 | 4730.10 | 14,708.36 | 11,166.09 |
| SE of the slope |
No. 1a = 11.13 No. 2a = 112.36 |
No. 1a = 0.54 No. 2a = 26.87 |
No. 1a = 0.10 No. 2a = 15.50 |
No. 1a = 0.45 No. 2a = 23.73 |
No. 1a = 0.59 No. 2a = 25.15 |
| SE of the Intercept | 182.40 | 249.72 | 492.06 | 232.59 | 202.55 |
| Specificity b (mean ± SD) | 99.11 ± 1.382 | 100.72 ± 0.221 | 100.25 ± 1.054 | 99.10 ± 1.152 | 98.89 ± 1.963 |
| Accuracy | 99.74 | 101.15 | 100.42 | 99.97 | 100.99 |
| Repeatability (RSD) | 1.28 | 1.47 | 1.29 | 0.74 | 1.03 |
| Intermediate precision (RSD) | 1.77 | 0.55 | 1.84 | 0.96 | 1.79 |
| Robustness | 0.98 | 0.78 | 0.84 | 1.07 | 1.45 |
| LOD (µg band−1) | 0.01 | 0.60 | 2.38 | 0.05 | 0.06 |
| LOQ (µg band−1) | 0.03 | 1.82 | 7.21 | 0.15 | 0.18 |
| Correlation coefficient (r) | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
aSlope 1 and 2 are the coefficients of a polynomial regression, A = ax2 + bx + c, where A is the integrated peak area, x is the concentration of the drug (μg band−1), a and b are coefficients 1 and 2, respectively, and c is the intercept
bAverage of determinations in seven laboratory-prepared mixtures
Precision
Repeatability
Three separate concentrations of NPZ (15.0, 25.0, 40.0 µg band−1), PHN (30.0, 50.0, 70.0 µg band−1), NPZ impurity B (3.0, 5.0, 8.0 µg band−1), PHN impurities; A & B (15.0, 25.0, 40.0 µg band−1) were analyzed intra-daily three times. Results were obtained preliminary to RSD calculation, Table 3.
Intermediate precision
Inter-daily analysis was also conducted for the formerly selected concentrations. Results are represented in Table 3.
Robustness
It was evaluated by studying the effect of deliberately changing the mobile phase composition; methanol (2.0 ± 0.2 mL), ethyl acetate (8.0 ± 0.2 mL) and ammonia (1.0 ± 0.1 mL). This study was conducted on three independent concentrations of NPZ (5.0, 20.0, 40.0 µg band−1), PHN (15.0, 40.0, 70.0 µg band−1), NPZ impurity B (1.0, 5.0, 8.0 µg band−1) and two PHN impurities (1.0, 20.0, 40.0 µg band−1 each). Satisfactory RSDs were obtained, Table 3.
Specificity
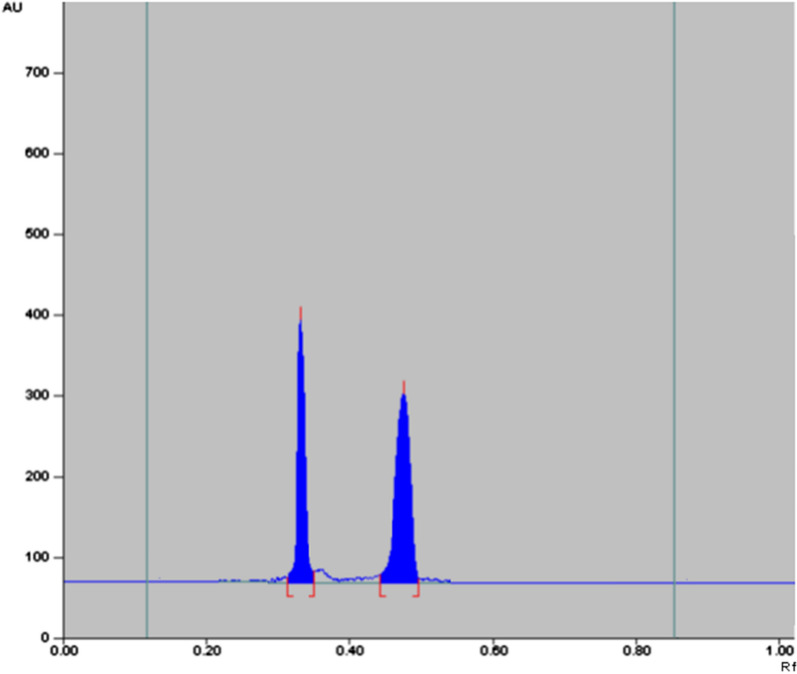
It was assessed by analysis of different laboratory prepared mixtures containing various ratios of the five studied components. Table 3 shows good recovery percentages and RSDs for analyzing those mixtures. Furthermore, forced degradation study was conducted whereas the two drugs were subjected to different stress conditions: (1) Acidic and alkaline hydrolysis via refluxing with 1 M HCl and 1 M NaOH for 2 h, respectively, (2) Oxidative degradation through treatment of each solution with 5% H2O2 then keeping at room temperature for 24 h, (3) Photostability on powdered drugs placed in petri dishes, using 254 nm UV light for 10 h, and finally (4) Dry heat by putting each drug powder in 100 °C oven for 7 h. The degradation study was monitored by the proposed TLC method. NPZ was only liable to alkaline hydrolysis giving its impurity B where its spot (Rf ≈ 0.35) disappeared accompanied by appearance of a new one (Rf ≈ 0.18) corresponding to this specified impurity. This outcome is consistent with what previously reported [20]. PHN was stable towards all conditions except for oxidation where ≈ 30.0% was degraded upon H2O2 treatment (Fig. 3).
Fig. 3.
TLC chromatogram of 100 µg band−1 PHN after H2O2 treatment; Rf ≈ 0.49 for PHN & ≈ 0.32 for its oxidative degradation
Analysis of pharmaceutical eye drops
The two active pharmaceutical ingredients (NPZ and PHN) were simultaneously quantified in their combined dosage form. Excipients did not have an impact on the obtained TLC chromatograms. In addition, method’s validity was proved using standard addition technique, Table 4.
Table 4.
Determination of NPZ, PHN in their dosage form and application of standard addition technique using the proposed TLC method
| Naphcon-A® eye drop | % found Meana ± SD |
Standard addition technique | ||
|---|---|---|---|---|
| Taken | Added | Recovery % | ||
| NPZ | 101.36 ± 1.51 | 10.0 µg band−1 | 5.0 µg band−1 | 101.30 |
| 10.0 µg band−1 | 101.75 | |||
| 20.0 µg band−1 | 99.99 | |||
| Mean ± SD | 101.01 ± 0.914 | |||
| PHN | 100.94 ± 1.73 | 20.0 µg band−1 | 10.0 µg band−1 | 100.95 |
| 20.0 µg band−1 | 99.02 | |||
| 40.0 µg band−1 | 100.84 | |||
| Mean ± SD | 100.27 ± 1.084 | |||
aAverage determinations of four eye drop dosage form solution
Statistical analysis
Statistical comparison between results of the suggested TLC method and that of official HPLC ones [5] were performed. The calculated values of student’s t-test and F-test indicated that there is no significant difference observed between those methods, Table 5.
Table 5.
Statistical comparison between the results obtained by the proposed method and the official BP method
| Parameter | TLC | Official BP method [5] | ||
|---|---|---|---|---|
| NPZ | PHN | NPZ | PHN | |
| Mean of recoveries | 101.15 | 100.42 | 99.63 | 99.71 |
| SD | 1.095 | 1.712 | 0.977 | 1.153 |
| Variance | 1.199 | 2.931 | 0.955 | 1.329 |
| n | 5 | 5 | 5 | 5 |
| Student’s t-test | 2.316 (2.306)a | 0.778 (2.306)a | NA | NA |
| F-test | 1.26 (6.39)a | 2.21 (6.39)a | NA | NA |
aThese values represent the corresponding tabulated values of t and F at p = 0.05
Greenness evaluation and methods comparison
In order to assess and compare this work with our previously reported HPLC one [19], EHS tool was applied. In this tool, nine categories representing safety, health and environmental hazards are utilized for organic solvents assessment whereas the lower the calculated score, the greener the solvent will be [51]. The calculated scores for methanol, ethyl acetate (used in this work) and acetonitrile (used in reported HPLC) revealed the dominance of the proposed method over our previously reported one in terms of environmental sustainability, Table 6. Finally, a comparative overview on those two methods along with a statistical F-test for their variances are shown in Table 7.
Table 6.
EHS assessment of the solvents used in this work (ethyl acetate & methanol) as well as the reported one (acetonitrile)
| Selected substance | Safety | Health | Environment | Totala | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Release potential | Fire/Explosion | Reaction/Decomposition | Acute toxicity | Irritation | Chronic toxicity | Persistency | Air Hazard | Water Hazard | ||
| Acetonitrile | 0.61 | 1.00 | 0.60 | 0.51 | 0.63 | 0.43 | 0.34 | 0.43 | 0.00 | 4.55 |
| Ethyl acetate | 0.62 | 1.00 | 0.00 | 0.28 | 0.63 | 0.17 | 0.03 | 0.17 | 0.00 | 2.89 |
| Methanol | 0.65 | 1.00 | 0.00 | 0.27 | 0.11 | 0.32 | 0.00 | 0.32 | 0.00 | 2.66 |
aObtained by summation of nine main categories scores
Table 7.
Comparative overview on reported HPLC and proposed TLC methods
| Ref. No | LOD | Elution time | EHS score | F-test | ||
|---|---|---|---|---|---|---|
| NPZ | PHN | NPZ | PHN | |||
| [19] | 1.29 µg mL−1 | 3.10 µg mL−1 | ≈ 30 min | 4.55 (acetonitrile) | 3.90 (6.39)a | 2.22 (6.39)a |
| This work | 0.60 µg band−1 | 2.38 µg band−1 | ≈ 6 min |
2.89 (ethyl acetate) 2.66 (methanol) |
||
aThis value represents the corresponding tabulated value of F at p = 0.05
Conclusion
A novel simple TLC densitometric method was established for the simultaneous detection and quantification of NPZ, PHN as well as three of their official impurities (NPZ impurity B, PHN impurities; A & B). The proposed method was validated in agreement to ICH guidelines. NPZ and PHN were successfully determined in their combined eye drops. EHS tool was utilized for greenness assessment of the organic solvents used in this work as well as the previously reported HPLC one. The proposed TLC densitometric method provides simplicity, low cost, fast analysis and environmental sustainability compared to the reported one. In addition, the capacity of the method to detect low concentrations of NPZ and PHN official impurities highlights it as a promising one for impurity profiling of those drugs.
Acknowledgements
The authors express their gratitude to Eva Pharma Pharmaceutical Company for donating us the pure NPZ and PHN sample.
Abbreviations
- BP
British pharmacopeia
- EHS
Environmental, Health and Safety tool
- ICH
International Council for Harmonisation
- NPZ
Naphazoline HCl
- PHN
Pheniramine maleate
- TLC
Thin Layer Chromatography
- USP
United States Pharmacopeia
Author contributions
KMK; Conceptualization, Methodology, Software, Validation, Visualization, Supervision, Project administration, Funding acquisition, Writing—original draft. MAH: Conceptualization, Methodology, Software, Formal analysis, Data curation, Visualization, Supervision, Project administration, Funding acquisition, Writing—review & editing. AMH; Methodology, Software, Validation, Formal analysis, Investigation, Funding acquisition, Project administration, Writing—original draft, Writing—review & editing. MAT; Methodology, Software, Validation, Formal analysis, Investigation, Funding acquisition, Project administration, Writing—original draft, Writing—review & editing. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tantawy MA, Weshahy SA, Wadie M, Rezk MR. A novel HPLC-DAD method for simultaneous determination of alfuzosin and solifenacin along with their official impurities induced: via a stress stability study; investigation of their degradation kinetics. Anal Methods. 2020;12:3368–3375. doi: 10.1039/d0ay00822b. [DOI] [PubMed] [Google Scholar]
- 2.Sherma J. Thin-layer chromatography. In: Meyers Robert A., editor. Encycl Anal Chem. Chichester: John Wiley & Sons; 2006. pp. 1–14. [Google Scholar]
- 3.Meloun M, Syrovy T, Vrana A. The thermodynamic dissociation constants of ambroxol, antazoline, naphazoline, oxymetazoline and ranitidine by the regression analysis of spectrophotometric data. Talanta. 2004;62:511–522. doi: 10.1016/j.talanta.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 4.USP 39 - NF 34. In: The United States Pharmacopeial. 2016.
- 5.BP. British Pharmacopeia, The Stationary Office, London. 2019.
- 6.Sayedel deen N, Hegazy M, Abdelkawy M, Abdelfatah R. Spectrophotometric, chemometric and chromatographic determination of naphazoline hydrochloride and chlorpheniramine maleate in the presence of naphazoline hydrochloride alkaline degradation product. Bull Fac Pharm Cairo Univ. 2013;51:57–68. [Google Scholar]
- 7.Korany MA, Bedair MM, El-Gindy A. Analysis of diphenhydramine hydrochloride and naphazoline hydrochloride in presence of methylene blue in eye drops by second derivative spectrophotometry. Drug Dev Ind Pharm. 1990;16:1555–1564. [Google Scholar]
- 8.Khalil S. Analytical application of atomic emission and atomic absorption spectrometry for the determination of imidazoline derivatives based on formation of ion-associates with sodium cobaltinitrite and potassium ferricyanide. Mikrochim Acta. 1999;130:181–184. [Google Scholar]
- 9.Souri E, Amanlou M, Farsam H, Afshari A. A rapid derivative spectrophotometric method for simultaneous determination of naphazoline and antazoline in eye drops. Chem Pharm Bull (Tokyo) 2006;54:119–122. doi: 10.1248/cpb.54.119. [DOI] [PubMed] [Google Scholar]
- 10.Casado-Terrones S, Fernandez-Sanchez JF, Canabate Diaz B, et al. A fluorescence optosensor for analyzing naphazoline in pharmaceutical preparations, comparison with other sensors. J Pharm Biomed Anal. 2005;38:785–789. doi: 10.1016/j.jpba.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Goicoechea HC, Collado MS, Satuf ML, Olivieri AC. Complementary use of partial least-squares and artificial neural networks for the non-linear spectrophotometric analysis of pharmaceutical samples. Anal Bioanal Chem. 2002;374:460–465. doi: 10.1007/s00216-002-1435-3. [DOI] [PubMed] [Google Scholar]
- 12.Hemmateenejad B, Ghavami R, Miri R, Shamsipur M. Net analyte signal-based simultaneous determination of antazoline and naphazoline using wavelength region selection by experimental design-neural networks. Talanta. 2006;68:1222–1229. doi: 10.1016/j.talanta.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 13.Chocholous P, Satinsky D, Solich P. Fast simultaneous spectrophotometric determination of naphazoline nitrate and methylparaben by sequential injection chromatography. Talanta. 2006;70:408–413. doi: 10.1016/j.talanta.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 14.Xia H, Wu HL, Gu HW, et al. Simultaneous determination of naphazoline and pyridoxine in eye drops using excitation-emission matrix fluorescence coupled with second-order calibration method based on alternating trilinear decomposition algorithm. Chinese Chem Lett. 2015;26:1446–1449. [Google Scholar]
- 15.Saito T, Morita S, Kishiyama I, et al. Simultaneous determination of dibucaine and naphazoline in human serum by monolithic silica spin column extraction and liquid chromatography–mass spectrometry. J Chromatogr B. 2008;872:186–190. doi: 10.1016/j.jchromb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Korodi T, Dulavova M, Urban E, et al. A stability indicating HPLC method for the determination of naphazoline and its degradation product and methyl parahydroxybenzoate in pharmaceutical preparations. J Liq Chromatogr Relat Technol. 2014;37:1321–1333. [Google Scholar]
- 17.Huang T, Chen N, Wang D, et al. A validated stability-indicating HPLC method for the simultaneous determination of pheniramine maleate and naphazoline hydrochloride in pharmaceutical formulations. Chem Cent J. 2014;8:7. doi: 10.1186/1752-153X-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira TC, Freitas JM, Munoz RAA, Richter EM. Development of a novel versatile method for determination of two antihistamines in association with naphazoline using cathodically pretreated boron-doped diamond electrode. Electroanalysis. 2018;30:868–876. [Google Scholar]
- 19.Kelani KM, Hegazy MA, Hassan AM, Tantawy MA. Determination of naphazoline HCl, pheniramine maleate and their official impurities in eye drops and biological fluid rabbit aqueous humor by a validated LC-DAD method. RSC Adv J. 2021;11:7051–7058. doi: 10.1039/d0ra10598h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoang VD, Hue NT, Tho NH, Nguyen HMT. Simultaneous determination of chloramphenicol, dexamethasone and naphazoline in ternary and quaternary mixtures by RP-HPLC, derivative and wavelet transforms of UV ratio spectra. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2015;139:20–27. doi: 10.1016/j.saa.2014.11.101. [DOI] [PubMed] [Google Scholar]
- 21.Bauer J, Krogh S. High-performance liquid chromatographic, stability indicating assay for disodium EDTA in ophthalmic preparations. J Chromatogr A. 1986;369:422–425. doi: 10.1016/s0021-9673(00)90151-1. [DOI] [PubMed] [Google Scholar]
- 22.Sasa SI, Al-momani IF, Jalal IM. Determination of naphazoline nitrate and antazoline sulphate in pharmaceutical combinations by reversed-phase HPLC. Anal Lett. 1990;23:953–971. [Google Scholar]
- 23.Ruckmick SC, Marsh DF, Duong ST. Synthesis and identification of the primary degradation product in a commercial ophthalmic formulation using NMR, MS, and a stability-indicating HPLC method for antazoline and naphazoline. J Pharm Sci. 1995;84:502–507. doi: 10.1002/jps.2600840422. [DOI] [PubMed] [Google Scholar]
- 24.Yesilada A, Gokhan N, Yilman MA, Ertan M. High performance liquid chromatographic identification of naphazoline and its degradation product in nasal preparations. Acta Pharm Turc. 1996;4:101–106. [Google Scholar]
- 25.Ali A, Ahmed M, Farooq U, et al. Stability indicating UHPLC-PDA assay for simultaneous determination of antazoline hydrochloride and naphazoline hydrochloride in ophthalmic formulations. Acta Chim Slov. 2017;64:332–341. doi: 10.17344/acsi.2017.3166. [DOI] [PubMed] [Google Scholar]
- 26.Chabenat C, Boucly P. Determination of naphazoline in rat plasma using column liquid chromatography with ultra violet detection. Biomed Chromatogr. 1992;6:241–243. doi: 10.1002/bmc.1130060508. [DOI] [PubMed] [Google Scholar]
- 27.Sidhu AS, Kennedy JM, Deeble S. General method for the analysis of pharmaceutical dosage forms by high-performance liquid chromatography. J Chromatogr A. 1987;391:233–242. doi: 10.1016/s0021-9673(01)94319-5. [DOI] [PubMed] [Google Scholar]
- 28.Marszall MP, Sroka WD, Balinowska A, et al. Ionic liquids as mobile phase additives for feasible assay of naphazoline in pharmaceutical formulation by HPTLC-UV-densitometric method. J Chromatogr Sci. 2013;51:560–565. doi: 10.1093/chromsci/bms168. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira TDC, Freitas JM, Abarza Munoz RA, Richter EM. A batch injection analysis system with square-wave voltammetric detection for fast and simultaneous determination of naphazoline and zinc. Talanta. 2016;152:308–313. doi: 10.1016/j.talanta.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 30.Nour El-Dien FA, Mohamed GG, Frag EYZ, Mohamed ME-B. Modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in pure and pharmaceutical preparations. Int J Electrochem Sci. 2012;7:10266–10281. [Google Scholar]
- 31.Marchesini A, Williner M, Mantovani V, et al. Simultaneous determination of naphazoline, diphenhydramine and phenylephrine in nasal solutions by capillary electrophoresis. J Pharm Biomed Anal. 2003;31:39–46. doi: 10.1016/s0731-7085(02)00600-3. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro MMAC, Oliveira TC, Batista AD, et al. A sub-minute electrophoretic method for simultaneous determination of naphazoline and zinc. J Chromatogr A. 2016;1472:134–137. doi: 10.1016/j.chroma.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Meloun M, Ferencikova Z, Netolicka L, Vrana A. The thermodynamic dissociation constant of naphazoline by the regression analysis of potentiometric data. Cent Eur J Chem. 2011;9:66–74. [Google Scholar]
- 34.Ghoreishi SM, Behpour M, Nabi M. A novel naphazoline-selective membrane sensor and its pharmaceutical applications. Sens Actuators B Chem. 2006;113:963–969. [Google Scholar]
- 35.Yesilada A, Tozkoparan B, Gökhan N, et al. Development and validation of a capillary electrophoretic method for the determination of degradation product in naphazoline HCl bulk drug substance. J Liq Chromatogr Relat Technol. 1998;21:2575–2588. [Google Scholar]
- 36.Koziol TR, Jacob JT, Achari RG. Ion-pair liquid chromatographic assay of decongestants and antihistamines. J Pharm Sci. 1979;68:1135–1138. doi: 10.1002/jps.2600680920. [DOI] [PubMed] [Google Scholar]
- 37.Parente G, Pazzaglia M, Vincenzi C, Tosti A. Contact dermatitis from pheniramine maleate in eyedrops. Contact Dermatitis. 1999;40:338. doi: 10.1111/j.1600-0536.1999.tb06097.x. [DOI] [PubMed] [Google Scholar]
- 38.Pandey AK, Dwivedi D. A validated titrimetric method for the determination oh pheniramine maleate in pure form and in their pharmaceutical formulation. Int Res J Pharm. 2017;8:138–141. [Google Scholar]
- 39.Pereira-rosario R, El-Gizawy S, Perrin JH, Riley CM. Analysis of nasal solutions containing pheniramine hydrochloride and pheniramine maleate by high performance liquid chromatography on a cyclodextrin bonded stationary phase and diod array spectrophotometry. Drug Dev Ind Pharm. 1986;12:2443–2465. [Google Scholar]
- 40.Abdel Fattah S, Kelany KO, El-zeany BA, El-tarras MF. Analysis of pheniramine maleate and colorpheniramine maleate via their Fe (III) Complexes. Anal Lett. 1987;20:1667–1678. [Google Scholar]
- 41.Das Gupta V, Ghanekar AG. Quantitative determinations of codeine phosphate, guaifenesin, pheniramine maleate, phenylpropanolamine hydrochloride, and pyrilamine maleate in an expectorant by high-PRESSURE liquid chromatography. J Pharm Sci. 1977;66:895–897. doi: 10.1002/jps.2600660649. [DOI] [PubMed] [Google Scholar]
- 42.Ali I, Al-othman ZA, Al-warthan A, et al. Enantiomeric separation and simulation studies of pheniramine, oxybutynin, cetirizine, and brinzolamide chiral drugs on amylose-based columns. Chirality. 2014;26:136–143. doi: 10.1002/chir.22276. [DOI] [PubMed] [Google Scholar]
- 43.Prava R, Seru G, Sama JR, Sidhhanadham AS. Chiral liquid chromatographic method development and validation for seperation of pheniramine enantiomers. Indo Am J Pharm Sci. 2016;3:1521–1533. [Google Scholar]
- 44.Caglar H, Buyuktuncel E. HPLC method development and validation: simultaneous determination of active ingredients in cough and cold pharmaceuticals. Int J Pharm Pharm Sci. 2014;6:421–428. [Google Scholar]
- 45.Pirol O, Sukuroglu M, Ozden T. Simultaneous determination of paracetamol, phenylephrine hydrochloride, oxolamine citrate and chlorpheniramine maleate by HPLC in pharmaceutical dosage forms. E-J Chem. 2011;8:1275–1279. [Google Scholar]
- 46.Jovanovic M, Stojanovic B, Rakic T, et al. Five different columns in the analysis of basic drugs in hydrophilic interaction liquid chromatography. Cent Eur J Chem. 2013;11:1150–1162. [Google Scholar]
- 47.Louhaichi MR, Jebali S, Loueslati MH, et al. Simultaneous determination of peudoephdrine, pheniramine, guaifesnisin, pyrilamine, chlorpheniramine and dextromethorphan in cough and cold medicines by high performance liquid chromatography. Talanta. 2009;78:991–997. doi: 10.1016/j.talanta.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 48.Rudolph D, Holkup L. HPLC determination and quantification of pheniramine and pyrilamine in over the counter cold medicines. Concordia Coll J Anal Chem. 2010;1(1):29–33. [Google Scholar]
- 49.Wu H-L, Huang C-H, Chen S-H, Wu S-M. Chiral quantitation of pheniramine, chlorpheniramine, and brompheniramine maleates by capillary zone electrophoresis. J Chromatogr Sci. 1999;37:24–30. [Google Scholar]
- 50.ICH Harmonised Tripartite Guideline. Validation of analytical procedures: text and methodology Q2 (R1). International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. 2014.
- 51.Koller G, Fischer U, Hungerbühler K. Assessing safety, health, and environmental impact early during process development. Ind Eng Chem Res. 2000 doi: 10.1021/ie990669i. [DOI] [Google Scholar]
- 52.Fekry RA, Kelani KM, Fayez YM, Tantawy MA. Comparative validated chromatographic methods for the simultaneous determination of caffeine, codeine, paracetamol along with the related compound “p-aminophenol” in tablets. J Planar Chromatogr - Mod TLC. 2022 doi: 10.1007/s00764-022-00150-y. [DOI] [Google Scholar]
- 53.Tantawy MA, Wahba IA, Saad SS, Ramadan NK. Two validated chromatographic methods for determination of ciprofloxacin HCl, one of its specified impurities and fluocinolone acetonide in newly approved otic solution. J Chromatogr Sci. 2021 doi: 10.1093/chromsci/bmab110. [DOI] [PubMed] [Google Scholar]
- 54.Variyar PS, Chatterjee S, Sharma A. Fundamentals and theory of HPTLC-based separation. In: Srivastava MM, editor. High-Performance Thin-Layer Chromatography (HPTLC) Berlin: Springer; 2011. pp. 27–39. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.