Abstract
Background
Allan-Herndon-Dudley syndrome (AHDS) is an X-linked recessive neurodegenerative disorder caused by mutations in the SLC16A2 gene that encodes thyroid hormone transporter. AHDS has been rarely reported in China.
Case presentation
This study reported a novel splicing mutation in the SLC16A2 gene in an 18-month-old male patient with AHDS. The patient was born to non-consanguineous, healthy parents of Chinese origin. He passed new-born screening for hypothyroidism, but failed to reach developmental milestones. He presented with hypotonia, severe mental retardation, dysarthria and ataxia. Genetic analysis identified a novel splicing mutation, NM_006517.4: c.431-2 A > G, in the SLC16A2 gene inherited from his mother. The patient received Triac treatment, (triiodothyroacetic acid), a thyroid hormone analogue for 3 months. Triac treatment effectively reduced serum TSH concentrations and normalized serum T3 concentrations in the patient.
Conclusions
This study reported the first case of AHDS treated by Triac in China. And the study expanded the mutational spectrum of the SLC16A2 gene in AHDS patients.
Supplementary information
The online version contains supplementary material available at 10.1186/s12887-022-03259-5.
Keywords: SLC16A2, Splicing variant, Allan-Herndon-Dudley syndrome, Thyroid hormone
Background
Allan-Herndon-Dudley syndrome (AHDS) is an X-linked neurodevelopmental disorder, first described at 1944 by Allan et al. [1]. This disorder was caused by the deficiency of monocarboxylate transporter 8 (MCT8), encoded by the SLC16A2 gene (Solute Carrier Family 16 Member 2) [2–4]. The SLC16A2 gene is located in Xq13.2, formerly called monocarboxylate transporters 8 (MCT8) [5]. As one of the thyroid hormone transporters, MCT8 expresses ubiquitously and facilitate the uptake of thyroid hormone into cells. Particularly, MCT8 mediates transport of active T3 and T4 across blood-brain-barrier and into central neurons [6]. Through binding to its nuclear receptors, thyroid hormone plays an important role in brain development and function. MCT8 deficiency due to SLC16A2 variant causes AHDS characterized by mental and motor developmental delay, and thyroid functional abnormalities of high serum T3, reduced T4, and normal or mildly increased Thyroid stimulating hormone (TSH) [7]. To date, a total of 159 variants have been reported, mostly missense/nonsense and insertion/deletion variants. Only few variants in splicing sites in the SLC16A2 gene were reported [5, 8–12]. The aim of the current study is to report a novel splicing variant in the SLC16A2 gene in a Chinese patient with AHDS and the hormonal effects of Triac in this patient.
Case presentation
The patient, an eighteen-month-old Chinese boy, was the first child born to healthy parents after uneventful pregnancy and delivery. His birth weight was 3 kg, birth height was 50 cm, and birth head circumstance was 34 cm. He passed hearing test and newborn screening for hypothyroidism after birth. He experienced feeding difficulty since his birth. At 6-month-old he presented with global hypotonia and developmental delay. He had never been able to hold up his head and could not sit. Physical examination revealed hypotonia in the trunk and hypertonia in the extremities. He had elongated face with bifrontal narrowing and flat nose, otherwise didn’t show dysplastic ears, pectus excavatum, scoliosis, flat feet, or lateral deviation of great toe etc. Thyroid function test in local medical center was performed as part of routine evaluation of global developmental delay, demonstrating increased free triiodothyronine (FT3) of 9.49 pg/ml (normal range 2.00-4.40 pg/ml), decreased free thyroxine (FT4) of 0.49 pg/dl (normal range 1.00-1.70 pg/dl) and mildly increased thyroid-stimulating hormone concentration (TSH) of 4.93 µIU/ml (normal range 0.27–4.20 µIU/ml) (Table 1). Similar symptoms were not observed in other family members. Further ultrasonography showed a normal thyroid gland. Brain magnetic resonance imaging (MRI) showed delayed myelination and mild cerebral atrophy. He was diagnosed as hypothyroidism and treated with levothyroxine sodium for 2 months. The patient discontinued levothyroxine sodium due to no improvement.
Table 1.
Clinical features of the AHDS patient compared with a patient with different splicing mutation
| Patients | The case in the current study | The case in the literature [8] | ||||
|---|---|---|---|---|---|---|
| Before Triac treatment | After Triac treatment | |||||
| Age | 6-month-old | 18-month-old | 24-month-old | 8-month-old | ||
| Mutation | c.431-2 A > G | c.431-1G > C | ||||
| Ethnicity | Chinese | Chinese | ||||
| Gender | Male | Male | ||||
| Age of diagnosis | 12-month-old | 8-month-old | ||||
| Family history | No | Yes | ||||
| Hypotonia | Yes | Yes | Yes | Yes | ||
| Dystonia | Yes | Yes | Yes | Yes | ||
| Head control | No | Improved | Improved | No | ||
| Ability to sit | No | No | No | No | ||
| Language development | No | No | No | No | ||
| Cognitive dysfunction | Yes | Mildly improved | improved | Yes | ||
| Hearing impairment | No | No | No | Not available | ||
| Seizure | No | No | No | Not available | ||
| Serum FT3 | 9.49 pg/ml | 8.12 pg/ml | 7.35pg/ml | 7.12 pg/ml | ||
| (normal range) | (2.00-4.40) | (2.66–4.82) | (4.10–7.42) | (2.41–5.50) | ||
| Serum FT4 | 0.49 pg/dl | 0.68 pg/dl | 0.07 pg/dl | 0.87 pg/dl | ||
| (normal range) | (1.00-1.70) | (1.12–1.77) | (0.19–0.29) | (0.96–1.77) | ||
| Serum TSH | 4.93 uIU/ml | 5.33 uIU/ml | 0.854 uIU/ml | 5.55 uIU/ml | ||
| (normal range) | (0.27–4.20) | (0.38–7.31) | (0.38–7.31) | (0.70–5.97) | ||
| Brain MRI | delayed myelination and mild cortical atrophy | delayed myelination and mild cortical atrophy | NA | delayed myelination and severe cortical atrophy | ||
When the patient was 12-month-old, he was not able to hold up his head, and could not sit and stand. He presented with severe global developmental delay, and generally could not reach any milestones. Physical examination revealed hypotonia in the trunk and hypertonia in the extremities. He had elongated face with bifrontal narrowing and flat nose. His height (75.4 cm) just reached − 2 SD (73 cm) and his weight (7 kg) was below − 2 SD (8.2 kg) on growth chart. The head circumference was 45 cm (-2 SD 44.2 cm) and chest circumference was 44 cm. Thyroid function test revealed increased FT3 of 8.86 pg/ml (normal range 2.66–4.82 pg/ml), decreased FT4 of 0.94 pg/dl (normal range 1.12–1.77 pg/dl), and normal TSH of 0.47 µIU/ml (normal range 0.38–7.31 µIU/ml). Euthyroxol was prescribed with methimazole to the patient for 3 months and then stopped due to no effect.
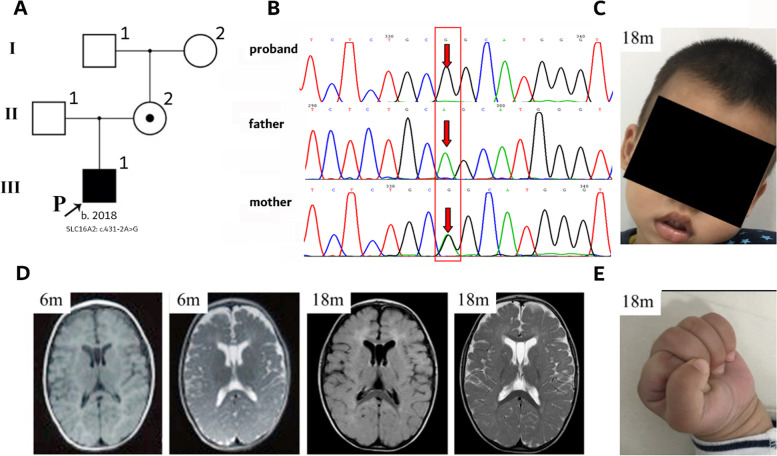
Direct sequencing of the SLC16A2 gene was applied to coding sequences of all exons of the SLC16A2 gene (NM_006517.4.) with exon/intron boundaries when the patient was 12-month-old. DNA was extracted using QIAamp DNA Blood Mini Kit (250) (QIAGEN, German). DNA as a template was amplified by PCR with primers designed by Primer 5.0 software program (Table S1). Sanger sequencing was performed in SLC16A2 gene. PCR amplified and sequenced using an ABI 3500 amplify instrument and a Genetic Analyzer 3130 (Applied Biosystems). Genetic analysis revealed the presence of a novel splicing variant (NM_006517.4: c.431-2 A > G). By using online programs FATHMM, NetGene2, Human Splicing Finder 3.1 and Mutation Taster, the pathogenicity of the variant c.431-2 A > G was predicted to be deleterious by causing splice acceptor site change. The variant c.431-2 A > G may result in splicing change at protein level. Pedigree analysis revealed that this variant was inherited from his heterozygous healthy mother, but was not found in his maternal grandparents (Fig. 1A/B). His heterozygous mother had normal thyroid function.
Fig. 1.
Characteristics of the 18-month-old male patient with AHDS. A Pedigrees of the AHDS patient. He harbored the variant from his healthy mother. Symbols and nomenclature follow standardized human pedigree nomenclature [13]. B Chromatograms of SLC16A2 variants identified in the patient. C Photograph of the patient at 18-month-old. He couldn’t hold his head steady. D MRI of the patient showed delayed myelination and mild cortical atrophy at 6-months old and worsening cortical atrophy at 18-months old. E, Dystonic posturing of the hands
At 18-month-old, the patient showed mild motor improvement. He had better head control and better eye to eye contact communication however, he was still not able to sit, walk and speak. He had mild feeding difficulty and drooling. He presented with hypotonia in the trunk and hypertonia in the extremities with clenched fists (Fig. 1C/E). Thyroid function test showed increased FT3 of 8.12 pg/ml (normal range 2.66–4.82 pg/ml), decreased FT4 of 0.68 pg/dl (normal range 1.12–1.77 pg/dl), and normal TSH of 5.33 µIU/ml (normal range 0.38–7.31 µIU/ml) (Table 1). Brain MRI showed thin corpus callosum, delayed myelination and cerebral atrophy (Fig. 1D).
Triac, a T3 analog, has been reported as a promising candidate to normalize serum T3 levels and thus alleviate the thyrotoxicosis in the patient with MCT8 deficiency. With a written and signed informed consent, Triac treatment has been initiated to treat the patient and maintained for 3 months. Triac dose started with 10 µg/kg (0.0875 mg) per day, doubling the dose (0.17 mg per day) after 2 weeks. After Triac treatment with the dose of 0.17 mg per day for 3 months, his height (82.4 cm) and weight (8.7 kg) were still mildly below − 2 SD (83 cm and 9.6 kg) on growth chart. The head circumference (48 cm) was in normal range and chest circumference reached 46.5 cm. Thyroid function test showed normal FT3 of 7.35 pmol/L (normal range 4.10–7.42 pmol/L), decreased FT4 of 5.19 pmol/L (normal range 14.45–22.74 pmol/L) and normal TSH of 0.854 µIU/ml (normal range 0.38–7.31 µIU/ml). And no abnormality was found in EEG (Electroencephalogram) and B-ultrasound of abdominal organs.
According to Human Gene Mutation Database (HGMD), a total of 5 splicing variants in the SLC16A2 gene have been reported, one of which carried a variant in the same intron with the case in this study [8]. To further characterize the novel splicing variant in this case, we compared the clinical and mutational features between these two cases (Table 1). Both cases presented with typical phenotype of AHDS.
Discussion and conclusions
We describe a Chinese patient diagnosed with AHDS, carrying a new splicing variant in the SLC16A2 gene never reported so far. The patient exhibited severe development delay, thyroid function abnormalities of elevated FT3 and decreased FT4, and delayed brain myelination, indicating the novel splicing variant c.431-2 A > G in the SLC16A2 gene is associated with severe phenotype of AHDS. To date, of 159 variants in the SLC16A2 gene, the splicing variants were reported in only few AHDS cases [5, 8, 9, 12]. Through literature review, one case with a splicing variant located close to c.431-2 A > G in the SLC16A2 gene has been reported [8]. An 8-month-old Chinese boy with AHDS carrying a splicing variant c.431-1G > C in the SLC16A2 gene presented with severe intellectual and motor developmental delay, delayed myelination of the white matter and elevated serum FT3 level. These results indicate the splicing site of c.430–431 in the SLC16A2 gene may be the key point for splice acceptor site. Further functional study is needed.
The T3 analog Triac has been reported as a promising candidate to normalize serum T3 levels and thus alleviate the thyrotoxicosis, and restore thyroid hormone signaling in the brain [3, 14]. There were no reports about the patients with MCT8 deficiency treated with Triac in China. Therefore, we refer to the literature published in 2014 by Dumitrescu, AM, et al., Triac therapy began at 0.0875 mg per day [3]. And we doubled the dose after 2 weeks. After 3 months of Triac treatment, the patient’s FT3 and TSH were back to normal.
Because of reduced FT4 and mildly increased TSH levels, some patients with AHDS have been initially suspected as hypothyroidism and treated with levothyroxine, just like the patient in the current study [15]. Although no beneficial effect on mental and motor function observed with levothyroxine, some improvements have been observed in body weight and heart rate due to amelioration of peripheral hyperthyroidism [15]. No significant improvement of mental and motor function were observed in our patient after 3-months Triac treatment.
The study supports that the novel splice acceptor site variation c.431-2 A > G in SLC16A2 gene is pathogenic, which is associated with typical phenotype of AHDS. Triac treatment effectively reduced serum TSH concentrations and normalized serum T3 concentrations in the patient. This is the first case of MCT8 deficiency treated by Triac in China.
Supplementary Information
Acknowledgements
The authors would like to thank the patient and his parents for their involvement in the present study.
Abbreviations
- AHDS
Allan-Herndon-Dudley syndrome
- MCT8
Monocarboxylate transporter 8
- MRI
Magnetic resonance imaging
- T3
Triiodothyronine
- T4
Thyroxine
- TSH
Thyroid stimulating hormone concentration
- MRI
magnetic resonance imaging
- HGMD
Human Gene Mutation Database
Authors’ contributions
CHZ and LL designed the study, XDC collected and analyzed the data, and CHZ and XDC wrote the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the Research Grants from Guangzhou Women and Children’s Medical Center (5001-4001003 and IP-2018-030) and Guangzhou Science and Technology Projects (202102080455). Funders were not involved in data analysis or interpretation.
Availability of data and materials
The variant data were deposited in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/ Submission ID: SUB11199328, NCBI tracking system #16,854,978, an SCV accession SCV002106318). Other data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee for Clinical Ethical Review, Guangzhou Women and Children’s Medical Center ([2020] No. 01501) and written informed consent was obtained from a parent of the patient. The study was conducted according to Declaration of Helsinki.
Consent for publication
The parent of the patient provided written informed consent for publication of medical data and images.
Competing interests
The authors did not have conflicts of interest to declare for this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li Liu, Email: liliuchina@qq.com.
Chunhua Zeng, Email: chunhuazeng@hotmail.com.
References
- 1.Allan W, Herndon CN, Dudley FC. Some examples of the inheritance of mental deficiency: apparently sex-linked idiocy and microcephaly. Am H Ment Defic. 1944;48:325–334. [Google Scholar]
- 2.Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet. 2004;364(9443):1435–7. doi: 10.1016/S0140-6736(04)17226-7. [DOI] [PubMed] [Google Scholar]
- 3.Dumitrescu AM, Liao X, Best TB, Brockmann K, Refetoff S. A Novel Syndrome Combining Thyroid and Neurological Abnormalities Is Associated with Mutations in a Monocarboxylate Transporter Gene. Am J Hum Genet. 2004;74(1):168–75. doi: 10.1086/380999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz CE, May MM, Carpenter NJ, Rogers RC, Martin J, Bialer MG, Ward J, Sanabria J, Marsa S, Lewis JA, et al. Allan-Herndon-Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet. 2005;77(1):41–53. doi: 10.1086/431313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafreniere RG, Carrel L, Willard HF. A novel transmembrane transporter encoded by the XPCT gene in Xq13.2. Hum Mol Genet. 1994;3(7):1133–9. doi: 10.1093/hmg/3.7.1133. [DOI] [PubMed] [Google Scholar]
- 6.Friesema EC, Jansen J, Heuer H, Trajkovic M, Bauer K, Visser TJ. Mechanisms of Disease: psychomotor retardation and high T3 levels caused by mutations in monocarboxylate transporter 8. Nat Clin Pract Endocrinol Metab. 2006;2(9):512–23. doi: 10.1038/ncpendmet0262. [DOI] [PubMed] [Google Scholar]
- 7.Groeneweg S, van Geest FS, Peeters RP, Heuer H, Visser WE. Thyroid hormone transporters. Endocr Rev. 2020;41(2):146–201. 10.1210/endrev/bnz008. [DOI] [PubMed]
- 8.Tang YL, Peng J, Xiong J, Pang N, Wu LW, Yang HY, Kessi M, Yin F. [A family with Allan-Herndon-Dudley syndrome due to SLC16A2 gene mutation] Zhonghua Er Ke Za Zhi. 2018;56(11):829–34. doi: 10.3760/cma.j.issn.0578-1310.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Gagliardi L, Nataren N, Feng J, Schreiber AW, Hahn CN, Conwell LS, Coman D, Scott HS. Allan-Herndon-Dudley syndrome with unusual profound sensorineural hearing loss. Am J Med Genet A. 2015;167A(8):1872–6. doi: 10.1002/ajmg.a.37075. [DOI] [PubMed] [Google Scholar]
- 10.Groeneweg S, van Geest FS, Abaci A, Alcantud A, Ambegaonkar GP, Armour CM, Bakhtiani P, Barca D, Bertini ES, van Beynum IM, et al. Disease characteristics of MCT8 deficiency: an international, retrospective, multicentre cohort study. Lancet Diabetes Endocrinol. 2020;8(7):594–605. doi: 10.1016/S2213-8587(20)30153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos HE, Morandini M, Carre A, Tron E, Floch C, Mandelbrot L, Neri N, De Sarcus B, Simon A, Bonnefont JP, et al. Pregnancy in women heterozygous for MCT8 mutations: risk of maternal hypothyroxinemia and fetal care. Eur J Endocrinol. 2011;164(2):309–14. doi: 10.1530/EJE-10-0679. [DOI] [PubMed] [Google Scholar]
- 12.Remerand G, Boespflug-Tanguy O, Tonduti D, Touraine R, Rodriguez D, Curie A, Perreton N, Des Portes V, Sarret C. Expanding the phenotypic spectrum of Allan-Herndon-Dudley syndrome in patients with SLC16A2 mutations. Dev Med Child Neurol. 2019 doi: 10.1111/dmcn.14332. [DOI] [PubMed] [Google Scholar]
- 13.Bennett RL, French KS, Resta RG, Doyle DL. Standardized Human Pedigree Nomenclature: Update and Assessment of the Recommendations of the National Society of Genetic Counselors. J Genet Couns. 2008;17:424. doi: 10.1007/s10897-008-9169-9. [DOI] [PubMed] [Google Scholar]
- 14.Grijota-Martínez C, Bárez-López S, Gómez-Andrés D, Guadaño-Ferraz A. MCT8 Deficiency: The Road to Therapies for a Rare Disease. Front Neurosci-Switz. 2020;14:380. 10.3389/fnins.2020.00380. [DOI] [PMC free article] [PubMed]
- 15.Groeneweg S, Visser WE, Visser TJ. Disorder of thyroid hormone transport into the tissues. Best Pract Res Clin Endocrinol Metab. 2017;31(2):241–53. doi: 10.1016/j.beem.2017.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The variant data were deposited in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/ Submission ID: SUB11199328, NCBI tracking system #16,854,978, an SCV accession SCV002106318). Other data that support the findings of this study are available from the corresponding author upon reasonable request.