Abstract
BACKGROUND:
Newborn screening identifies individuals affected by a specific disorder within an apparently healthy population prior to the appearance of symptoms so that appropriate interventions can be initiated in time to minimize the harmful effects. Data on population based cut-off values, disease ranges for true positive cases, false positive rates, true positive rates, cut-off verification and comparisons with international cut-off ranges have not been done for Saudi Arabia.
OBJECTIVE:
Establish population-based cut-off values and analyte ratios for newborn screening assays and clinically validate the values.
DESIGN:
Population-based screening.
SETTING:
Tertiary care hospitals and laboratories.
METHODS:
After method verification, initial cut-off values were established by analyzing 400-500 dry blood spot (DBS) samples which were further evaluated after one year. About 74 000 patient results were reviewed to establish cut-off ranges from DBS samples received from five different hospitals during 2013-2020. Analysis was performed by tandem mass spectrometry (TMS) and a genetic screening processor. Confirmation of initial positive newborn screening results for different analytes were carried out using gas chromatography-mass spectrometry, high performance liquid chromatography and TMS.
MAIN OUTCOME MEASURES:
Cut-off values, ratios, positive predictive values, false positive rate, true positive rate and disease range.
SAMPLE SIZE:
74 000 samples.
RESULTS:
Population based cut-off values were calculated at different percentiles. These values were compared with 156 true positive samples and 80 proficiency samples. The false positive rate was less than 0.04 for all the analytes, except for valine, leucine, isovalerylcarnitine (C5), biotinidase (BTD), 17-hydroxyprogesterone and thyroid stimulating hormone. The highest false positive rate was 0.14 for BTD which was due to pre-analytical errors. The analytical positive predictive values were greater than 80% throughout the eight years.
CONCLUSION:
We have established clinical disease ranges for most of the analytes tested in our lab and several ratios which gives excellent screening specificity and sensitivity for early detection. The samples were representative of the local populations.
LIMITATIONS:
Need for wider, population-based studies.
CONFLICT OF INTEREST:
None.
INTRODUCTION
Newborn screening (NBS) of infants shortly after birth (24-72 hours) for conditions recommended by the national Newborn Screening Committee in Saudi Arabia can prevent disabilities and possibly death.1,2 The objective of the NBS program is to diagnose infants born with certain genetic, metabolic and functional disorders.3 With the increasing number of disorders for which screening methods are available, there is a greater need for a clinical and differential diagnosis of genetic metabolic disorders. Tandem mass spectrometry (TMS) has the capacity to screen for a wide range of inborn errors of metabolism (IEM) in a single test with a dry blood spot (DBS) sample.4 The IEM disorder profile includes aminoacidemias, fatty acid oxidation disorders, and organic acidemias, as well as endocrine and enzymatic disorders. Early screening and diagnosis may decrease the mortality and morbidity rates in children with IEM. More than 50 different disorders can be screened with TMS, which has many advantages such as rapid testing, high sensitivity, high specificity, high throughput, low sample volume, as well as low maintenance and operational cost per sample.5,6
The genetic screening processor (commercial name: GSP Instrument) from Perkin Elmer can be used to screen for endocrine disorders such as congenital adrenal hyperplasia and congenital hypothyroidism and enzymatic disorders by measuring biotinidase (BTD) and galactosemia (GALT). The GSP instrument is designed to provide a rapid diagnosis and to overcome most of the disadvantages due to changes in chemistry and the handling process.7,8 NBS is not diagnostic, but determines whether the baby has a high or low risk of having an inherited metabolic disease. As in many scientific tests, cut-off values are used to determine which levels are normal and abnormal. NBS searches for markers of disease and the cut-off values inform the assessment of a high or low risk of disease. It is important to determine precise cut-off values for the local population screened, and the values are re-evaluated when required.9–12
In this study, we describe how to determine initial cut-off values, and then evaluate, validate and monitor the values through the data analysis and perform external quality control of samples and confirm positive samples. The DBS samples were referred to our laboratory from several hospitals located in major cities including Riyadh, Jeddah, Dammam, Al-Ahsa and Madina under the Ministry of National Guard Health Affairs. We also received and analyzed NBS samples from other hospitals and laboratories. This study focused on the analytical procedure useful for all newborn screening laboratories to assess high risk, but clinically asymptomatic, newborns. It is also helpful for clinicians to evaluate the laboratory NBS results with population based cutoff values and ratios. The parents and other siblings of the affected child were also screened upon the request of the NBS committee, but NBS was not routinely performed for all newborns.
We know of 7-8 hospitals and laboratories performing newborn screening in Saudi Arabia. No one has published data on population based cut-off values, disease ranges for true positive cases, false positive rates, true positive rates, cut-off verification and comparisons with international cut-off ranges, which indicates the clinical importance of our work. This study is one of the largest to determine NBS cut-off ranges and the results will be helpful for all laboratories and physicians across the country in the detection of IEM. We followed approved guidelines for newborn screening by TMS from the Clinical And Laboratory Standard Institute (CLSI) for cut-off determination and methods of performance evaluation.13
METHODS
This study was a population-based screening study to establish cut off ranges for all newborn screening analytes including amino acids, acylcarnitines, analyte ratios, 17-hydroxyprogesterone (17-OHP), thyroid stimulating hormone (TSH), biotinidase activity (BTD) and galactose-1-phosphate uridyltransferase deficiency (GALT). The initial cut-off values were established with 400-500 DBS samples followed by a yearly review. The whole data sets of about 74 000 normal patient samples were reviewed over the 8 years from 2013 to 2020, and the statistical parameters and disease ranges were calculated. All DBS samples that passed preanalytical and analytical requirements for clinical analysis (i.e. sample collection, storage, transportation, pass quality control) were included. Samples were excluded if the wrong sample was collected, samples were not filed properly in filter cards, samples were hemolyzed, or had no identifier or did not meet all other acceptable criteria established for routine clinical DBS.
The non-derivatized amino acid and acylcarnitine reagent kit was purchased from Chromsystem Germany (Part #57000) for the tandem mass spectrometry analysis. The reagents for 17-OHP, TSH, BTD and GALT for the genetic screening processor were obtained from Perkin Elmer. Whatman 903 filter paper was used for the blood collection. Screening for IEMs was done with the Waters TQD mass spectrometer, integrated with an ACQUITY UPLC system (Waters, MA, USA), the GSP Instrument from Perkin Elmer (GSP Instrument, https://www.perkinelmer.com/product/genetic-screening-processor-2021-2021-0010 and the Panthera DBS puncher.
The DBS samples were collected by the heel prick method from 74 000 infants in five hospitals during 2013 to 2020. The barcoded filter paper card (Whatman 903), with instructions for collection, were distributed from the Biochemical Metabolic Laboratory (BML) to all the participating hospitals, located in Riyadh, Jeddah, Dammam, Al-Ahsa and Madinah. A few drops of blood were collected on the filter paper, and allowed to thoroughly saturate the five circles on the card, followed by air-drying for 4-5 hours. All the samples were transferred to our lab within 3 days of collection, stored in a polystyrene box packed with ice to keep the samples cold during transportation. All samples were collected within 24 to 72 hours of birth.
DBS were checked for acceptability against the standard protocol before processing. Five 96-well plates were punched with Panthera. Four plates were loaded into the GSP Instrument. The other plate was extracted with an internal standard and loaded into the TMS. Mass spectrometry detection was performed in the multiple reaction monitoring mode. Each compound was quantified by calculating the signal intensity ratio of the compound to its internal standard. The parameters of the GSP are built in, and it performs the analysis without any external setting.
All positive results were reported to the hospital newborn screening committee, which consisted of several molecular geneticists and physicians. All abnormal results were communicated to the healthcare provider who requested additional diagnostic testing to determine if the newborn had the disorder in question. The newborn samples were screened for 22 types of treatable IEM by tandem MS and the GSP, using the DBS as recommended by the Ministry of Health. There is no benefit for screening of untreatable IEMs. For confirmation, urinary organic acids were determined by gas chromatography-mass spectrometry, and the plasma amino acids by ion exchange chromatography on the Biochrom 30 (http://www.biochrom.co.uk/). The serum level of 17-hydroxyprogesterone and the BTD activity were also measured in our laboratory. As a final confirmatory testing, positive samples were sent to an international specialized laboratory performing molecular and enzymatic analysis.
RESULTS
Initial cut-off ranges were determined by analyzing 400-500 DBS samples with the tandem MS and GSP. The positive predictive values, negative predictive values, sensitivity and accuracy were determined by using proficiency testing samples provided from the United States Centers for Disease Control (CDC) (Table 1). After one year, six percentiles (95%, 97%, 98%, 99%, 99.5%, 99.9%) were calculated from the normal patient samples to re-evaluate cut off ranges. The values at 99% were very close to initial cut-off values with slightly higher false positive rates for some analytes (data not shown). To establish new ranges based on a larger sample size, NBS results of about 74 000 patients samples (from 2013 to 2020) were reviewed and different percentiles (95%, 97%, 98%, 99%, 99.5%, 99.9%) were calculated for all analytes except the C0 (low), methionine (low), BTD and GALT, where lower percentiles (0.1%, 0.25%, 0.50%, 0.75%, 1.0%) were calculated (Table 2). The percentile disorder ranges of different analytes were calculated from the results of 156 confirmed positive cases for amino acids, amino acid ratios, acylcarnitines, acylcarnitines ratios, 17-OHP, TSH, BTD and GALT (Table 3). High and low target ranges of specific analytes for different diseases and their ratios were determined from true positive cases (Table 4). The number of true positives, the true positive prevalence rates (disease prevalence per 100 000), the false positives and false positive rates are shown in Table 5. True positive rates (sensitivity) with 95% confidence interval limit are shown in Table 6. The data are shown in Figure 1. Initial and the new cut-off values for some analytes are shown in Figure 2.
Table 1.
Validation of initial cut-off values by CDC proficiency samples.
| Analytes | Cut-off (μmol/L) | Positive samples (n) | Negative samples (n) | Analytical PPV (%) | NPV (%) | Sensitivity (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| Valine | <174 | 5 | 25 | 100 | 100 | 100 | 100 |
| Leucine/Isoleucine | <281 | 5 | 25 | 100 | 100 | 100 | 100 |
| Methionine | <70 | 5 | 25 | 100 | 100 | 100 | 100 |
| Phenylalanine | <165 | 5 | 25 | 100 | 100 | 100 | 100 |
| Citrulline | <49 | 5 | 25 | 100 | 100 | 100 | 100 |
| Tyrosine | <217 | 5 | 25 | 100 | 100 | 100 | 100 |
| Arginine | <99 | 5 | 25 | 100 | 100 | 100 | 100 |
| C0 | <57 | 5 | 25 | 100 | 96 | 80 | 96 |
| C3 | <6.0 | 5 | 25 | 100 | 100 | 100 | 100 |
| C4 | <0.95 | 5 | 25 | 100 | 100 | 100 | 100 |
| C5 | <0.56 | 5 | 25 | 100 | 100 | 100 | 100 |
| C5DC | <0.47 | 5 | 25 | 100 | 100 | 100 | 100 |
| C5OH | <0.5 | 5 | 25 | 100 | 100 | 100 | 100 |
| C6 | <0.13 | 5 | 25 | 100 | 100 | 100 | 100 |
| C8 | <0.14 | 5 | 25 | 100 | 100 | 100 | 100 |
| C10 | <0.17 | 5 | 25 | 100 | 100 | 100 | 100 |
| C14 | <0.6 | 5 | 25 | 100 | 100 | 100 | 100 |
| C14:1 | <0.39 | 5 | 25 | 100 | 100 | 100 | 100 |
| C16 | <9.30 | 5 | 25 | 100 | 100 | 100 | 100 |
| C18 | <2.00 | 5 | 25 | 83 | 100 | 100 | 96 |
For abbreviations, see footnote Table 2. PPV: positive predictive value, NPV: negative predictive value.
Table 2.
Percentiles and cut-off selection (μmol/L) and comparison of Region for the Stork Study (R4S) and Centers for Disease Control cutoff values.14
| Analyte | 95% | 97% | 98% | 99% | 99.5% | 99.9% | Cut off | R4S Cut-off | CDC Cut-off |
|---|---|---|---|---|---|---|---|---|---|
| GLY | 465 | 502 | 532 | 585 | 635 | 733 | <733 | 767 | NA |
| ALA | 327 | 357 | 381 | 419 | 463 | 536 | <536 | 507 | NA |
| VAL | 113 | 124 | 133 | 148 | 166 | 208 | <208 | 212 | 300 |
| LEU/ILEU | 152 | 166 | 178 | 199 | 221 | 258 | <258 | 235 | 290 |
| ORN | 119 | 130 | 139 | 153 | 164 | 177 | <177 | NA | NA |
| ASP | 83 | 95 | 104 | 119 | 131 | 138 | <138 | NA | NA |
| MET (high) | 31 | 35 | 37 | 43 | 50 | 82 | <82 | 44 | 75 |
| PHE | 71 | 84 | 89 | 99 | 107 | 143 | <107 | 97 | 150 |
| CIT | 25 | 28 | 31 | 36 | 40 | 73 | <73 | 28 | 55 |
| TYR | 140 | 184 | 200 | 227 | 241 | 269 | <241 | 207 | 350 |
| GLU | 484 | 521 | 525 | 530 | 533 | 536 | <536 | 551 | NA |
| ARG | 25 | 30 | 33 | 36 | 38 | 42 | <42 | 32 | 70 |
| C0 (high) | 25 | 32 | 35 | 39 | 41 | 44 | <44 | 59 | NA |
| C2 | 28 | 36 | 39 | 42 | 45 | 49 | <49 | 52 | NA |
| C3 | 2.8 | 3.7 | 4.0 | 4.6 | 5.3 | 5.9 | <5.9 | 4.74 | 5.65 |
| C4 | 0.4 | 0.6 | 0.6 | 0.8 | 0.9 | 1.0 | <1.0 | 0.75 | 1.30 |
| C5 | 0.16 | 0.25 | 0.30 | 0.37 | 0.46 | 0.70 | <0.70 | 0.39 | 0.7 |
| C6 | 0.06 | 0.08 | 0.09 | 0.10 | 0.10 | 0.11 | <0.10 | 0.18 | 0.4 |
| C5DC | 0.15 | 0.20 | 0.22 | 0.25 | 0.28 | 0.38 | <0.38 | 0.17 | 0.35 |
| C8 | 0.06 | 0.08 | 0.09 | 0.11 | 0.14 | 0.27 | <0.27 | 0.21 | 0.45 |
| C10 | 0.09 | 0.12 | 0.13 | 0.15 | 0.17 | 0.55 | <0.17 | 0.26 | 0.45 |
| C12 | 0.12 | 0.16 | 0.18 | 0.20 | 0.24 | 0.33 | <0.33 | 0.41 | NA |
| C14 | 0.22 | 0.28 | 0.30 | 0.34 | 0.38 | 0.56 | <0.38 | 0.50 | 0.75 |
| C16 | 3.54 | 4.34 | 4.62 | 5.09 | 5.62 | 6.57 | <6.57 | 6.0 | 7.50 |
| C18 | 1.03 | 1.30 | 1.40 | 1.58 | 1.79 | 2.51 | <1.79 | 1.7 | 2.3 |
| C5:1 | 0 | 0 | 0.01 | 0.02 | 0.03 | 0.05 | <0.25 | 0.08 | 0.25 |
| C5OH | 0.25 | 0.32 | 0.36 | 0.42 | 0.50 | 0.52 | <0.50 | 0.38 | 0.80 |
| C8:1 | 0.07 | 0.11 | 0.12 | 0.16 | 0.21 | 0.32 | <0.21 | NA | NA |
| C10:1 | 0.05 | 0.06 | 0.07 | 0.08 | 0.09 | 0.10 | <0.10 | 0.18 | 0.30 |
| C14:1 | 0.13 | 0.18 | 0.20 | 0.25 | 0.33 | 0.37 | <0.37 | 0.37 | 0.60 |
| C14OH | 0.01 | 0.02 | 0.02 | 0.02 | 0.04 | 0.04 | <0.04 | NA | NA |
| C16:1 | 0.24 | 0.30 | 0.33 | 0.40 | 0.46 | 0.51 | <0.53 | NA | NA |
| C16:1OH | 0.05 | 0.06 | 0.06 | 0.07 | 0.08 | 0.12 | <0.12 | 0.13 | NA |
| C16OH | 0.02 | 0.03 | 0.04 | 0.06 | 0.08 | 0.11 | <0.10 | 0.08 | 0.13 |
| C18:1 | 1.75 | 2.25 | 2.47 | 2.90 | 3.38 | 3.76 | <3.76 | 2.5 | 3.5 |
| C18:1OH | 0.02 | 0.03 | 0.03 | 0.05 | 0.08 | 0.12 | <0.12 | 0.07 | NA |
| C18OH | 0.02 | 0.03 | 0.03 | 0.04 | 0.04 | 0.09 | <0.09 | 0.06 | 0.10 |
| PHE/TYR | 1.04 | 1.35 | 1.46 | 1.70 | 1.96 | 2.98 | <1.96 | NA | NA |
| C3/C2 | 0.15 | 0.19 | 0.20 | 0.23 | 0.25 | 0.41 | <0.25 | NA | NA |
| C5/C0 | 0.01 | 0.02 | 0.02 | 0.03 | 0.03 | 0.06 | <0.03 | NA | NA |
| C8/C10 | 1.00 | 1.50 | 1.50 | 2.00 | 2.50 | 2.5 | <2.05 | NA | NA |
| TSH (μU/mL) | 9 | 10 | 12 | 15 | 21 | 38 | <21 | NA | 13.6 |
| 17OHP (nmol/L) | 22 | 28 | 33 | 44 | 57 | 93 | <93 | NA | 47.6 |
| Analyte | 0.1% | 0.25% | 0.50% | 0.75% | 1.00% | Cut off | ----- | ----- | |
| C0 (Low) | 0.0 | 2.4 | 4.50 | 5.20 | 5.6 | >4.50 | NA | NA | 8.0 |
| Meth (Low) | 5.71 | 6.05 | 6.45 | 6.78 | 6.97 | >6.05 | NA | NA | NA |
| GALT (U/dL) | 3.3 | 4.6 | 5.5 | 6.1 | 6.5 | >3.3 | NA | NA | NA |
| BTD (U/dL) | 18.5 | 28.2 | 37.0 | 43.3 | 49 | >50 | NA | NA | NA |
GLY: glycine, ALA: alanine, VAL: valine, LEU/ILEU: leucine/isoleucine, ORN: ornithine, ASP: aspartic acid, MET: methionine, PHE: phenylalanine, CIT: citrulline, TYR: tyrosine, GLU: glutamic acid, ARG: arginine, C0: free carnitine, C2: acetylcarnitine, C3: propionylcarnitine, C4: butyryl-/isobutyrylcarnitine, C5: isovaleryl-/2-methylbutyrylcarnitine, C6: hexanoylcarnitine, C5DC: glutarylcarnitine, C8: octanoylcarnitine, C10:decanoylcarnitine, C12: dodecanoylcarnitine, C14: tetradecanolycarnitine, C16: pamitoylcarnitine, C18: stearylcarnitine, C5:1: tiglylcarnitine, C5OH: hydroxyl-isovalerylcarnitine, C8:1: otenylcarnitine, C10:1: dodecenylcarnitine, C14:1: tetradecenylcarnitine, C14OH: hydroxyl tetradecanoylcarnitine, C16:1: hexadecenoylcarnitine, C16:1OH: hydroxyl-hexadecenoylcarnitine, C16OH: hydroxypalmitoylcarnitine, C18:1: oleylcarnitine, C18:1OH: hydroxyl-oleylcarnitine, C18OH: hydroxyl-stearylcarnitine: TSH: thyroid stimulating harmone, 17-OHP: 17-hydroxy progesterone, GALT: galactosemia: BTD: biotinidase deficiency
Table 3.
Percentile disorder ranges (μmol/L).
| Analyte | Conditions | n | 1% | 5% | 10% | 25% | 50% | 75% | 90% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|
| Valine | MSUD | 7 | 352 | 357 | 362 | 383 | 401 | 428 | 459 | 492 |
| Leucine/Isoleucine | MSUD | 567 | 633 | 714 | 801 | 847 | 1130 | 1165 | 1292 | |
| Meth | HCY | 7 | 122 | 129 | 137 | 154 | 164 | 239 | 660 | 1020 |
| Citrulline | CIT-1/ASA | 13 | 95 | 110 | 134 | 158 | 201 | 394 | 1462 | 2323.6 |
| Tyrosine | TYT-1 | 1 | 713 | 713 | 713 | 713 | 713 | 713 | 713 | 713 |
| Phenylalanine | PKU | 1 | 274 | 274 | 274 | 274 | 274 | 274 | 274 | 274 |
| C3 | MMA/PA | 16 | 7.16 | 7.50 | 7.73 | 10.35 | 13.30 | 21.15 | 36.20 | 44.82 |
| C3/C2 | MMA/PA | 0.64 | 0.68 | 0.81 | 1.17 | 1.48 | 3.47 | 4.15 | 7.97 | |
| C5 | IVA | 5 | 1.12 | 1.14 | 1.21 | 1.61 | 2.01 | 2.96 | 7.62 | 8.77 |
| C5/C0 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 0.09 | 1.16 | 2.12 | ||
| C5/C2 | 0.13 | 0.13 | 0.14 | 0.14 | 0.14 | 0.16 | 0.50 | 0.81 | ||
| C5OH | 3MCC/HMG Co Lyase | 14 | 0.82 | 0.83 | 0.90 | 1.48 | 2.60 | 6.02 | 8.72 | 25.24 |
| C5DC | GA-1 | 3 | 0.88 | 0.90 | 0.93 | 1.02 | 1.17 | 2.94 | 4.00 | 4.64 |
| C8 | MCAD | 5 | 0.83 | 0.90 | 1.00 | 1.22 | 3.35 | 9.51 | 15.00 | 18.69 |
| C10 | 0.06 | 0.07 | 0.07 | 0.09 | 0.19 | 0.55 | 0.93 | 1.19 | ||
| C8/C10 | 13.50 | 13.50 | 13.50 | 13.81 | 15.21 | 16.69 | 18.40 | 19.63 | ||
| C14:1 | VLCAD | 9 | 0.57 | 0.68 | 0.82 | 1.45 | 2.07 | 2.97 | 3.13 | 3.52 |
| 17-OHP | CAH | 15 | 88 | 104 | 121 | 219 | 300 | 300 | 318 | 338 |
| TSH | CH | 25 | 29 | 29 | 30 | 58 | 133 | 226 | 300 | 300 |
| BTD | BTD | 16 | 8 | 11 | 12 | 21 | 30 | 40 | 45 | 46 |
| GALT | GALT | 19 | 0.7 | 1.1 | 1.1 | 1.5 | 2.2 | 2.5 | 2.6 | 2.9 |
For abbreviations, see footnote Table 2.
Table 4.
Target range calculations (μmol/L).
| Analyte | Conditions | No. of cases | High target ranges | |
|---|---|---|---|---|
| Low (99th)a | High (5th)b | |||
| Valine | MSUD | 7 | 256 | 356 |
| Leu/Isoleucine | 263 | 633 | ||
| Meth | HCY | 7 | 84 | 117.6 |
| Citrulline | CIT-1 | 13 | 72 | 110 |
| Phenylalanine | PKU | 7 | 104 | 274 |
| Tyrosine | TYT-1 | 8 | 238 | 631 |
| C3 | MMA/PA | 16 | 5.1 | 7.5 |
| C3/C2 | 0.24 | 0.68 | ||
| C5 | IVA | 5 | 0.80 | 1.14 |
| C5/C0 | 0.03 | 0.09 | ||
| C5/C2 | 0.04 | 0.13 | ||
| C5OH | 3-HMG CoA/3MCC | 14 | 0.50 | 0.84 |
| C5DC | GA-1 | 3 | 0.29 | 0.61 |
| C8 | MCAD | 5 | 0.27 | 0.93 |
| C10 | 0.07 | 0.17 | ||
| C8/C10 | 2.05 | 13.5 | ||
| C14:1 | VLCAD | 9 | 0.39 | 0.63 |
| 17OHP | CAH | 15 | 80 | 103.9 |
| TSH | CH | 25 | 21 | 29 |
Calculated from normal population
Calculated from true positive cases. For abbreviations, see footnote Table 2. MSUD: Maple syrup urine disease.
Table 6.
True positive rates (sensitivity) for analytes.
| Analyte | Proportion | 95% CI |
|---|---|---|
| CIT | 0.4545 | 0.2465–0.6626 |
| VAL, LEU, ILEU | 0.0533 | 0.0025–0.1042 |
| METH | 0.2414 | 0.0857–0.3971 |
| TYR | 0.4000 | 0.0960–0.7036 |
| PHE | 1.00 | 1.000 |
| C3, C3/C2 | 0.6190 | 0.4113–0.8267 |
| C5 | 0.0538 | 0.0079–0.0996 |
| C5OH | 0.5000 | 0.3268–0.6732 |
| C5DC | 0.1818 | 0.0502–0.3134 |
| C8 & C10 | 0.6667 | 0.3999–0.9334 |
| C14:1 | 0.2727 | 0.1411–0.4043 |
| 17-OHP | 0.2239 | 0.1241–0.3422 |
| TSH | 0.1838 | 0.1187–0.2489 |
| GALT | 0.3220 | 0.2028–0.4413 |
| BTD | 0.0232 | 0.0521–0.1432 |
For abbreviations, see footnote Table 2
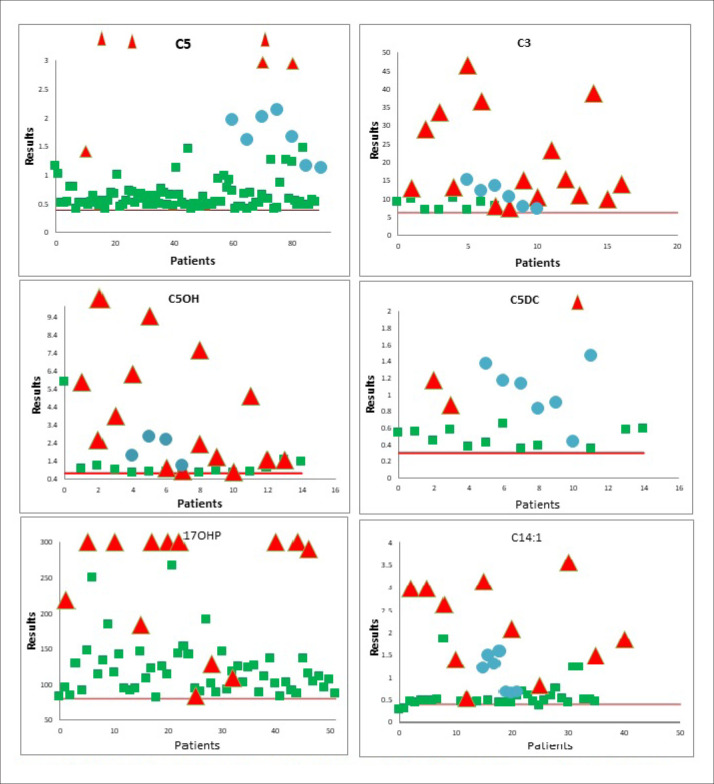
Figure 1. Distribution of true positive (red), false positive (green) and true positive proficiency (blue) samples around the cut-off (red line).
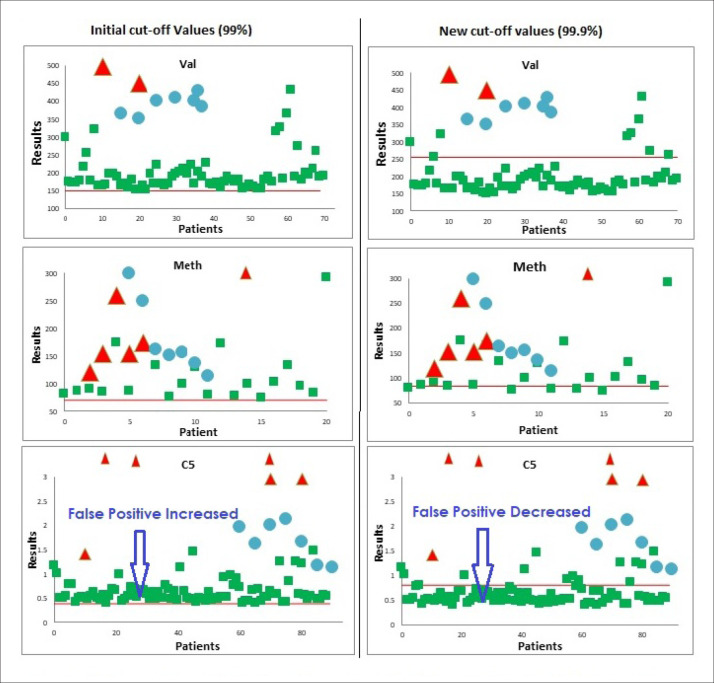
Figure 2. Initial and new cut-off values (true positive red, false positive green, true positive proficiency blue, cut-off red line).
DISCUSSION
After the verification of the method of performance specification (linearity, precision, comparison and sensitivity), the initial cut-off was determined by analyzing 400-500 DBS samples with the tandem MS and GSP. For dicarboxylic, unsaturated and hydroxylated forms of carnitines, the cut-off values were obtained from the literature because most of the results were very low. Validation of initial cut-off values was performed by participating in the international proficiency testing program from the CDC for newborn screening. After analyzing 5 positive and 25 negative proficiency-testing samples, the results were submitted to the CDC. Our performance was 100% satisfactory, except for a few false positive results for valine and oleoylcarnintine (C18:1). The positive predictive values, negative predictive values, sensitivity and accuracy were calculated. TSH, 17 OH-progesterone, GALT and BTD were not included in the proficiency testing program (Table 1).
After one year, the results of normal patient samples were reviewed to evaluate the initial cut-off ranges. As the patient data did not have a Gaussian distribution, the cut-off ranges were estimated by calculating different percentiles. Typically, six percentiles (95%, 97%, 98%, 99%, 99.5%, 99.8%) were determined and compared with initial ranges. The values at 99% were very close to initial cut-off values with slightly higher false positive rates for some analytes.
After 8 years, the newborn screening results of about 74 000 normal patient samples were reviewed. These samples were received from five different hospitals located in Riyadh, Jeddah, Dammam, Al-Ahsa and Madinah under the Ministry of National Guard during 2013 to 2020. To determine population-based cut-off values, six percentiles (95%, 97%, 98%, 99%, 99.5%, 99.9%) were calculated for all the analytes in our NBS panel except the C0(low), Methionine (low), BTD and GALT, where lower percentiles (0.1%, 0.25%, 0.50%, 0.75%, 1.0%) were calculated. These percentiles were estimated because the data were not of a Gaussian distribution as recommended by mass spectrometry guidelines from the National Committee for Clinical Laboratory Standards. The newly established initial cut-off values were compared with initial cut off values and necessary changes were made. For most of the analytes, the 99% and 99.9% percentile distribution were selected as the new cut-off values for the normal population (Table 2). The selection of the new values was assessed by reviewing the results of the 156 true positive cases, identified and confirmed in our laboratory. The selection is also based on the satisfactory performance evaluation from the CDC proficiency-testing programs. The results of the C5:1 in most of the samples were very low, and the percentile calculation was not possible. Based on the literature, we used 0.25 μmol/L as the cut-off value, which was verified by analyzing the true positive proficiency samples from the CDC.
The values corresponding to the 0.5% and 0.1% percentile (C0=4.5 μmol/L and GALT=3.3 U/dL) were selected for the C0 (low) and GALT, respectively, because these cut-off values were used for the evaluation of the CDC proficiency testing results, which gave 100% satisfactory performance for both analytes. For BTD, four true positive patients were identified and confirmed with results ranging between 40 – 50 U/dL. The calculated cut-off value with the 0.75% percentile was 43.3 U/dL. All the proficiency sample results were much lower, from 5–15 U/dL, which did not help to differentiate between a false positive from a true positive patient. As a result, we used >50 U/dL (normal) as the cut-off value for BTD, which caused an increased number of false positive results per year.
The newly established cut-off values were compared with the cut-off values determined by Region for the Stork Study (R4S) and CDC.14 A comparison of the three values (our lab, CDC and R4S) is presented in Table 2. The established cut-off values are greater than the R4S and lower than the CDC cut-off values for more than half of the analytes of amino acids and acylcarnitine. The cut off of citrulline (<73 μmol/L), methionine (<82 μmol/L), C4 (<1.0 μmol/L) and C18:1 (3.76 μmol/L) are higher than R4S and CDC values. For the TSH and 17-hydroxyprogesterone, our cut-off values are higher than the CDC values. These variations are due to age, health status of the newborn at the time of collection, environmental conditions during transport, DBS collection procedures, methods used, instrument platform and stability of the analyte measured.
The results of the 156 true positive cases and 80 proficiency-testing samples were evaluated against new cut off ranges and no false negative results were obtained, verifying the accuracy of these ranges.
The percentile disorder ranges were calculated from confirmed positive cases for amino acids, amino acid ratios, acylcarnitines, acylcarnitines ratios, 17-OHP, TSH, BTD and GALT (Table 3). The high target disorder ranges were obtained from the 99th percentile of the normal population low range was taken from 5th percentile of all the disorder ranges of the same analyte. We included positive proficiency sample results in the calculation of the target range for MSUD, tyrosinemia and PKU (Table 4) due to the low number of true positive samples. Most of the false positive cases were within this high target range. We observed that most of the positive proficiency samples were near the lowest 5th percentile of the disorder ranges, and the false positive patient samples were near the 99th percentile of the normal population, but not close to the lowest 5th percentile of the disorder ranges.
From the number of true positive, true positive prevalence (disease prevalence) and false positive rates, the disease prevalence was calculated per 100 000. TSH had the highest prevalence (27.2) and PKU had lowest prevalence (1.0). The disease prevalence related to BTD deficiency, 17-OHP, GALT, C14:1, C5OH and C3 were all greater than 14. The false positive rate was less than 0.04 for all the analytes, except for valine, leucine, C5, BTD, 17-OHP and TSH (Table 5). The highest false positive rate of BTD (0.144) was connected to a pre-analytical error where the DBS samples were packed in biohazard bags and sent to the laboratory without drying for 3-4 hours. We observed that the BTD activity decreased 10% to 20% if the DBS were not completely dried. Decreased enzyme activity can also be caused by insufficient cooling of the samples in case of high external temperatures. Valine (0.068) and C5 (0.086) also had a high false positive rate, which was due to lower cut-off values. The high positive rate for TSH (0.107) and 17-OHP (0.049) was due to early sampling. In terms of leucine (0.068), we could not find any pre-analytical or analytical reason for the high false positive rate. It was not related to the lower cut-off values, as some of the true positive proficiency testing samples were just above the initially set cut-off value. In the 8 years, no questions were raised by physicians from any of the five tertiary care hospitals about the cut-off values except the higher false positive results for BTD. We also did not observe any false negative results since the start of the NBS program.
Table 5.
Analytical false positive rates and true positive prevalence.
| Parameters | True positives (TP) (n) | TP prevalence (Per 100 000) (n) | False positives (n) | False positive rate (%) |
|---|---|---|---|---|
| VAL | 4 | 5.7 | 70 | 0.068 |
| LEU | 4 | 5.7 | 70 | 0.068 |
| MET | 7 | 10 | 20 | 0.019 |
| PHE | 5 | 7.1 | 1 | 0.001 |
| CIT | 10 | 14.3 | 13 | 0.013 |
| TYR | 4 | 5.7 | 5 | 0.005 |
| C8 | 6 | 8.6 | 4 | 0.004 |
| ARG | 3 | 4.3 | 4 | 0.004 |
| C10 | 6 | 8.6 | 4 | 0.004 |
| C8/C10 | 6 | 8.6 | 4 | 0.004 |
| C14:1 | 11 | 15.7 | 21 | 0.020 |
| C3 | 17 | 24.3 | 8 | 0.008 |
| C3/C2 | 17 | 24.3 | 8 | 0.008 |
| C5 | 6 | 8.6 | 89 | 0.086 |
| C5/C0 | 6 | 8.6 | 89 | 0.086 |
| C5/C2 | 6 | 8.6 | 89 | 0.086 |
| C5OH | 15 | 21.4 | 16 | 0.016 |
| C5DC | 3 | 4.3 | 27 | 0.026 |
| BTD | 18 | 27.7 | 148 | 0.144 |
| GALT | 20 | 28.6 | 39 | 0.038 |
| 17OHP | 17 | 24.3 | 50 | 0.049 |
| TSH | 28 | 40 | 110 | 0.107 |
For abbreviations, see footnote Table 2.
The true positive rate (sensitivity) with 95% confidence intervals were calculated. During the last 8 years, we had no false negative results after reporting as normal. There were no recalls from our hospital newborn screening committee, which is responsible for treatment and management of all true positive cases. Therefore, we were unable to make receiver operating characteristic curves and calculate area under the curve; however, true positive rates (sensitivity) were calculated for all analytes having positive results with 95% confidence intervals (Table 6).
About 80 unknown proficiency-testing samples were analyzed and evaluated against new cut-off values during 2013 to 2020. A few false positive results were reported, but there were no false negative results. The overall performance was satisfactory with 100% accuracy. Distribution of true positive (red), false positive (green) and true positive proficiency (blue) samples around the cut-off (red Line) are shown in Figure 1 for few analytes. The true positive samples were far from the cut-off ranges for most of the analytes. The comparison of initial and the new cut-off values for some analytes was also prepared. Number of false positives was significantly reduced for valine, pentanylcarnitine (C5) and methionine (Figure 2). The new cut-off values for C3 were lower than the initial values, which did not result in an increase in the false positive rate. Similarly, many changes were made in the initial cut-off values for the amino acids, acylcarnitines and ratios to obtain accurate normal ranges, representing the local population.
We used to collect dry blood spot samples early during 24-72 hours after birth for several reasons including family demands to discharge early and hospital capacity. However, some metabolites may not always be sufficiently high/low for a reliable diagnosis at this early age. In most NBS programs blood sampling between 48-72 hours is preferred. While this study is the largest study in the region, a wider population-based study is still desirable with the different types of reagent kits used in the country. Therefore, we considered our established cut-off ranges as representative of the local populations, but a wider population-based study would still be desirable.
In conclusion, establishing specific and population-based cut-off values and analyte ratios are imperative for quick and accurate diagnoses of IEM. Established cut-off values also support a reduction in false positive rates, increases the positive predictive values, and prevents unnecessary testing as well as family anxiety. Using population-based data with true positive samples, we established cut-off values for each analyte tested in our laboratory. We calculated several ratios for various analytes, which provides excellent screening specificity and sensitivity.
ACKNOWLEDGMENTS
The authors would like to thank all the staff of the newborn screening section of the biochemical metabolic laboratory in the Department of Pathology and Laboratory Medicine at the Ministry of National Guard-Health Affairs, King Abdulaziz Medical City, Riyadh, Kingdom of Saudi Arabia.
Funding Statement
None.
ETHICAL APPROVAL
This study was approved by the Institutional Research Board of King Abdullah International Medical Research Center IRBC/0250/20 according to principles of the Helsinki Declaration.
REFERENCES
- 1.Van Karnebeek CDM, Stockler S. Treatable inborn errors of metabolism causing intellectual disability: a systematic literature review. Mol Genet Metab. 2012;105:368–81. [DOI] [PubMed] [Google Scholar]
- 2.Howell R, Terry S, Tait VF, Olney R, Hilton CF, Grosse S.et al. CDC grand rounds: newborn screening and improved outcomes. MMWR Morb Mortal Wkly Rep. 2012;61:390–3. [PubMed] [Google Scholar]
- 3.March of Dimes. Newborn screening tests for your baby. 2012. https://www.marchofdimes.org/baby/newborn-screening-tests-for-your-baby.aspx. Accessed 2016, 26 May.
- 4.Wilcken B, Wiley V, Hammond J, Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–12. 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- 5.Chace DH, Kalas TA. A biochemical perspective on the use of tandem mass spectrometry for newborn screening and clinical testing. Clin Biochem. 2005;38:296–309. 10.1016/j.Clinbio-chem.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Copeland S. A review of newborn screening in the era of tandem mass spectrometry: what's new for the pediatric neurologist? Semin Pediatr Neuro. 2008;15:110–6. [DOI] [PubMed] [Google Scholar]
- 7.Fingerhut, R, Torresani, T. Evaluation of the genetic screening processor (GSP™) for newborn screening. Anal Methods. 2013;5(1):4769–4776. [Google Scholar]
- 8.Pettersson, K, Siitari, H, Hemmilä, I. Time-resolved fluoroimmunoassay of human choriogonadotropin. Clin Chem. 1983;29(1):60–64. [PubMed] [Google Scholar]
- 9.Jiang Xiang, Tang Fang, . The adjustment of 17-hydroxyprogesterone cut-off values for congenital adrenal hyperplasia neonatal screening by GSP according to gestational age and age at sampling, J Pediatr Endocrinol Metab 2019. Nov 26;32(11):1253–1258. [DOI] [PubMed] [Google Scholar]
- 10.Skrinska Victor, Khneisser Issam, . Introducing and Expanding Newborn Screening in the MENA Region, Int J Neonatal Screen. 2020. Mar;6(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Chi-Ju, Wei Na, . Diagnosis and ther-apeutic monitoring of inborn errors of metabolism in 100,077 newborns from Jining city in China, BMC Pediatr. 2018. Mar 13;18(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welling Lindsey, Boelen Anita, . Nine years of newborn screening for classical galactosemia in the Netherlands: Effectiveness of screening methods, and identification of patients with previously unreported phenotypes, Mol Genet Metab. 2017. Mar;120(3):223–228. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and laboratory standard institute; Newborn screening by tandem mass spectrometry approved guideline, 2011; I/LA32-A;30:16. [[AUTHOR: Is this the document? (Date of Publication: May 26, 2017: https://clsi.org/standards/products/newborn-screening/documents/nbs04/]]
- 14.McHugh DMS, Cameron CA, Abdenur JE, Abdulrahman M, Adair O, Al Nuaimi SA, et al.. Clinical validation of cutoff target ranges in newborn screening of metabolic disorders by tandem mass spectrometry: A worldwide collaborative project, Genetic in Medicine. 2011;13:3. [DOI] [PubMed] [Google Scholar]