Abstract
An intuitive, clinically relevant index of microbial dysbiosis as a summary statistic of subgingival microbiome profiles is needed. Here, we describe a subgingival microbial dysbiosis index (SMDI) based on machine learning analysis of published periodontitis/health 16S microbiome data. The raw sequencing data, split into training and test sets, were quality filtered, taxonomically assigned to the species level, and centered log-ratio transformed. The training data set was subject to random forest analysis to identify discriminating species (DS) between periodontitis and health. DS lists, compiled by various “Gini” importance score cutoffs, were used to compute the SMDI for samples in the training and test data sets as the mean centered log-ratio abundance of periodontitis-associated species subtracted by that of health-associated ones. Diagnostic accuracy was assessed with receiver operating characteristic analysis. An SMDI based on 49 DS provided the highest accuracy with areas under the curve of 0.96 and 0.92 in the training and test data sets, respectively, and ranged from −6 (most normobiotic) to 5 (most dysbiotic) with a value around zero discriminating most of the periodontitis and healthy samples. The top periodontitis-associated DS were Treponema denticola, Mogibacterium timidum, Fretibacterium spp., and Tannerella forsythia, while Actinomyces naeslundii and Streptococcus sanguinis were the top health-associated DS. The index was highly reproducible by hypervariable region. Applying the index to additional test data sets in which nitrate had been used to modulate the microbiome demonstrated that nitrate has dysbiosis-lowering properties in vitro and in vivo. Finally, 3 genera (Treponema, Fretibacterium, and Actinomyces) were identified that could be used for calculation of a simplified SMDI with comparable accuracy. In conclusion, we have developed a nonbiased, reproducible, and easy-to-interpret index that can be used to identify patients/sites at risk of periodontitis, to assess the microbial response to treatment, and, importantly, as a quantitative tool in microbiome modulation studies.
Keywords: high-throughput nucleotide sequencing, microbiota, periodontitis, Biofilms, Fretibacterium fastidiosum, Treponema denticola
Introduction
It is now accepted that periodontitis is a result of an imbalance in the composition and function of the subgingival microbial community (dysbiosis) rather than an assault from a handful of pathogenic species (Curtis et al. 2020). This paradigm shift has been driven by the emergence of the concept of the microbiome—that is, all members of a microbial community in a particular habitat and their collective genomes (Turnbaugh et al. 2007) functioning collectively as a microbial community—with the advent of next-generation sequencing technologies that facilitated characterization of the oral microbial communities at unprecedented depth and taxonomic resolution. Comprehensive 16S rRNA gene-based microbiome analysis of subgingival biofilm samples has characterized a unique microbial signature associated with periodontitis (Griffen et al. 2012; Abusleme et al. 2013; Kirst et al. 2015) that is far more complex than that identified by earlier culture-based (Moore et al. 1982) and DNA probe–based (Socransky et al. 1998) studies.
Microbiome analysis has revolutionized our understanding of the microbiology of periodontitis and opened up new potential strategies for prevention and treatment of periodontitis—for example, by using microbiome modulators, such as prebiotics or probiotics, to reverse microbial dysbiosis (or reestablish normobiosis; Devine and Marsh 2009). However, the clinical utility of results from microbiome studies has been limited, primarily due to the technical nature of microbiome data. Results are usually presented in terms of diversity indexes, extensive microbial profiles, and long lists of differentially abundant genera and species—all of which are difficult to interpret or apply to individual samples in clinical settings (e.g., for risk stratification of patients or assessment of treatment response). Therefore, an intuitive, clinically relevant index of microbial dysbiosis as a summary statistic of microbial profiles of subgingival samples is required. Such an index will also be useful as a quantitative tool in microbiome modulation studies.
A dysbiosis ratio was recently described on the basis of the relative abundances of 5 health-associated and 4 periodontitis-associated genera in 618 subgingival samples pooled from several previous studies (Meuric et al. 2017). The resultant dysbiosis ratio differed significantly between the periodontitis and healthy groups and strongly correlated with pocket depth. However, the selection of genera for inclusion in the dysbiosis ratio was not based on formal statistical analysis (e.g., differential abundance or random forest). Furthermore, the data were not appropriately normalized to account for their compositionality as recently recommended (Gloor et al. 2017). Moreover, the diagnostic accuracy of the ratio was not statistically assessed.
In this study, we applied a machine learning approach to 6 previously published periodontitis microbiome data sets, taxonomically assigned to the species level and normalized by centered log-ratio (CLR) transformation, to develop and validate a new subgingival microbial dysbiosis index (SMDI) that can discriminate between health and periodontitis with high accuracy. We also applied the index to data sets in which nitrate was used to modulate the microbiome in vitro and in vivo to assess its utility in microbiome modulation studies.
Materials and Methods
Training and Test Data Sets
The data sets used in this study, including clinical characteristics and inclusion criteria, are listed in Appendix Table 1. Data from 3 landmark studies on the microbiome of periodontitis—namely, those by Abusleme et al. (2013), Griffen et al. (2012) and Kirst et al. (2015)—were combined and used as a training set: 96 healthy and 123 moderate to severe periodontitis samples, excluding the shallow pocket samples from the study by Griffen et al. Data from the studies by Pei et al. (2020) and Bizzarro et al. (2016; baseline samples) and the V1–V3 set of healthy subgingival samples from the Human Microbiome Project (HMP; NIH HMP Working Group et al. 2009) were combined and used as a test/validation set: 163 healthy and 67 moderate to severe periodontitis samples. The matching V3–V5 and V4 sets from the HMP and Griffen et al. studies, respectively, were used to assess reproducibility as a function of hypervariable region. The data sets from the studies by Rosier et al. (2020) and Jockel-Schneider et al. (2021) that involved assessing the microbiome-modulating properties of nitrate were used as additional test sets. An overview of the pipeline used for analysis of the data sets is presented in Figure 1, and the details are provided in turn.
Figure 1.

An overview of the pipeline used to analyze the study data sets. Raw data were trimmed, quality filtered, chimera checked, classified to the species level, and centered log-ratio transformed. The training data were then analyzed by random forest analysis to construct lists of discriminating species at different importance cutoffs, which in turn were used to calculate the subgingival microbial dysbiosis index (SMDI). The discriminating species list with the highest accuracy—based on receiver operating characteristic (ROC) analysis—was then used to compute the SMDI in the test data. NGS, next-generation sequencing.
Data Preprocessing and Taxonomy Assignment
The raw data in fastq format were downloaded from the Sequence Read Archive except for the HMP data, for which demultiplexed, trimmed fasta files were directly downloaded from https://www.hmpdacc.org/hmp/HM16STR/ (obtaining fastq files for a subset of samples from the HMP project is a daunting task). When applicable, paired-end reads were merged with PEAR (Zhang et al. 2014). Before primers were trimmed off, sequences were preprocessed with mothur (version 1.42; Schloss et al. 2009), which included removing sequences with ambiguous bases, primer mismatches, homopolymers >8 bases, average Q score <35 (30 for sets with low sequencing depth) over a sliding window of 50 nucleotides, or length below a cutoff value (this varied from one data set to another based on the hypervariable region targeted and chemistry used). Chimeras were detected with Uchime (Edgar et al. 2011) per the self-reference approach.
The high-quality, chimera-free sequences (Appendix Table 2) were assigned species-level taxonomies via our prioritized BLASTn-based algorithm as described elsewhere (Al-Hebshi et al. 2015; Al-Hebshi et al. 2017). In brief, the algorithm searches individual reads for matches at alignment coverage and percentage identity ≥98% in 4 well-curated reference 16S rRNA sequence databases, ranked by biological relevance. Reads are then assigned the species taxonomy of hits with the highest percentage identity and bit score belonging to the reference data set with the highest priority. In the case of ties, the reads are assigned multispecies taxonomy. Reads with no matches in the references set are clustered de novo into operational taxonomic units with USEARCH (Edgar 2010). Operational taxonomic units represented by <100 reads are filtered out, and the remaining are considered potentially novel species.
Read counts tables were generated for the species and genus levels and merged for the training and test data sets separately.The read counts were then normalized by CLR transformation with the “transform” function in R package “microbiome” (version 1.6.0; https://github.com/microbiome/microbiome/), which first transforms read counts to relative abundance and then applies a pseudocount (minimal relative abundance value / 2) to all zero count values. CLRs were then calculated as log(x) − mean(log[x]) for each sample.
Random Forest Analysis
CLR-transformed read counts were subject to random forest analysis (RFA; Breiman 2001) for predicting the health and disease classes of the samples by using the R “randomForest” package (version 4.6.14) with the parameter “ntree” (number of trees to grow) set to 1,000. Lists of discriminating species (DS) or discriminating genera (DG) were compiled at different cutoffs of mean decrease in Gini (MDG), which is a measure of importance of a taxon to the classification, with their mean CLR abundances in health and periodontitis. Each DS or DG item was determined as dysbiotic when its mean CLR abundance was greater in the periodontitis samples and normobiotic when its mean CLR abundance was greater in the healthy samples.
Calculation of SMDI
For each list of DS or DG (i.e., at the different MDG cutoffs), SMDI was calculated for individual samples in the training set as follows: SMDI = mean CLR abundance of dysbiotic DS/DG – mean CLR abundance of normobiotic DS/DG.
Receiver Operating Characteristic Analysis
To identify the DS or DG list that provides the highest discrimination between periodontitis and health, receiver operating characteristic (ROC) analysis was performed on SMDI calculated at the different MDG cutoffs. Accuracy was assessed in terms of area under curve (AUC). The list that resulted in the highest AUC was defined as the master DS/DG list and used to calculate SMDI in the test set as described earlier.
Results
SMDI Discriminates between Periodontitis and Health with High Accuracy
RFA of the training set identified 201 DS with MDG (importance) scores ≥0.1 (Appendix Data Set 1). Applying DS lists at different importance cutoffs to calculate the SMDI in the same set resulted in accuracy (or AUC) ≥0.918 with the highest (0.960; 95% CI, 0.937 to 0.984) obtained at an MDG cutoff of 0.4 (Fig. 2A; hereafter, master DS). The SMDI based on the master DS ranged from −4.19 (most normobiotic) to 5.06 (most dysbiotic) with 95% of the healthy samples and 85% of the periodontitis samples below and above zero, respectively (Fig. 2B). None of the healthy samples demonstrated high SMDI (maximum, 0.6) while several periodontitis samples presented with SMDI less than zero (as low as −4).
Figure 2.
Subgingival microbial dysbiosis index (SMDI) and receiver operating characteristic (ROC) curves. SMDI was computed by using discriminating species lists at different MDG cutoffs (mean decrease in Gini; identified by random forest analysis of the training data). ROC analysis was then performed to determine accuracy at each cutoff in terms of area under the curve (AUC). The ROC curves and AUC are shown for the (A) training data set and (C) test data set. Scatter plots of SMDI by sample type based on the master DS list (Fig. 3) are shown for the (B) training data set and (D) test data set. The x-axis represents the number of samples in the data set. The smaller panels represent the corresponding box plots (median, interquartile range, 95% CI, and outliers) and ROC curves. ***P < 0.001. Mann-Whitney test. Figure was produced with R package, SPSS, and Excel.
Using the master DS for calculation of the SMDI in the combined test sets resulted in an AUC of 0.919 (95% CI, 0.882 to 0.956), although ROC curve analysis revealed that a cutoff of 0.5 would provide a slightly higher accuracy (Fig. 2C). The resultant SMDI ranged from −6.1 to 4.0, again with the zero discriminating between most of the healthy and periodontitis samples (92% and 75%, respectively; Fig. 2D). Most samples with an SMDI <−4 were from the HMP data set. Unlike with the training set, there were a few healthy samples in the test set that presented with an SMDI higher than zero (as high as 3.8)
The master DS comprised 31 periodontitis-associated and 18 health-associated species (Fig. 3). The periodontitis-associated species had significantly higher importance scores, with 10 having an MDG ≥2 (Treponema denticola, Mogibacterium timidum, Fretibacterium sp. oral taxon 360, Fretibacterium fastidiosum, Tannerella forsythia, Treponema maltophilum, Eubacterium saphenum, Eubacterium nodatum, Filifactor alocis, and Desulfobulbus sp. oral taxon 041), as compared with only 1 health-associated species (Streptococcus sanguinis). Notably, most of these species were also identified as top DS in each of the 3 training data sets when analyzed individually with RFA (Appendix Data Set 2).
Figure 3.
Master discriminating species. Species identified with random forest analysis that provided the highest discrimination by subgingival microbial dysbiosis index (SMDI) between periodontitis and health in the training set (MDG ≥0.4). Left: periodontitis and health-associated species ranked by importance (MDG score). Right: mean centered log-ratio (CLR) abundances of discriminating species in the periodontitis and health samples. Figure was produced with Excel. MDG, mean decrease in Gini.
Healthy Sites in Periodontitis Have Varying Levels of Dysbiosis
When calculated for the healthy sites in periodontitis that were initially excluded from RFA (Griffen et al. 2012 data set), the SMDI ranged from −3.4 to 3.4, with the samples evenly distributed across the range—that is, from low to high SMDI (Appendix Fig. 1).
SMDI Highly Reproducible by Hypervariable Region
Analysis of matched V1–V3 and V3–V5 data sets from 126 healthy samples from the HMP revealed a very high correlation (r = 0.89, P < 0.001) in SMDI between the regions, although V3–V5 tended to have fewer outliers with high SMDI (Fig. 4A). Higher correlation (r = 0.95, P < 0.001) was found between the V1–V2 and V4 regions based on analysis of the Griffen et al. (2012) data set; however, the V4 shifted the SMDI values to the left by about 1 unit (Fig. 4B).
Figure 4.
Subgingival microbial dysbiosis index (SMDI) by hypervariable region. A scatterplot of SMDI for (A) 126 healthy samples from the Human Microbiome Project with V1–V3 and V3–V5 data and (B) 87 healthy and periodontitis samples from the Griffen et al. (2012) study with V1–V2 and V4 data. The index was calculated by using the master discriminating species list (Fig. 3). The x-axis represents the number of samples in the data set. The small panel represents the corresponding box plots (median, interquartile range, 95% CI, and outliers). NS, not significant; r, Spearman’s correlation coefficient. ***P < 0.001. Wilcoxon signed rank test. Figure was produced with R package and Excel.
SMDI as a Measure of Microbiome Modulation
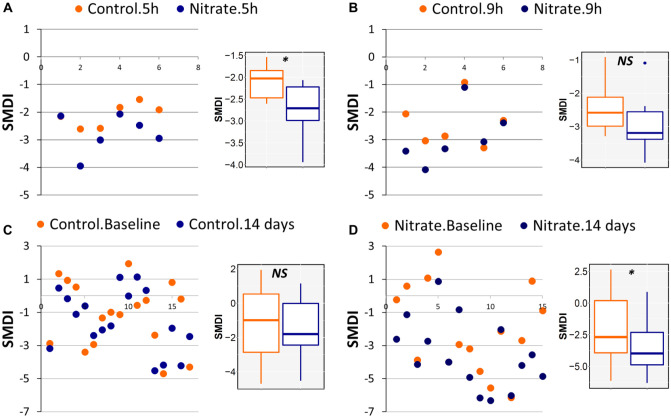
Applying the SMDI to data from the study by Rosier et al. (2020), in which clinical inocula had been grown in vitro for 5 and 9 h in the presence or absence of nitrate, showed that nitrate lowered SMDI scores at the 2 time points, although the difference was statistically significant at 5 h only (Fig. 5A, B). Similarly, applying the index to the data by Jockel-Schneider et al. (2021), which had involved periodontal recall patients with mild-moderate gingivitis, revealed that receiving a nitrate-rich diet for 14 d after treatment (mechanical debridement) resulted in a significant drop of SMDI scores, while treatment alone did not (Fig. 5C, D).
Figure 5.
Subgingival microbial dysbiosis index (SMDI) as a measure of microbiome modulation. Scatterplots of SMDI calculated for (A, B) microbiomes grown in vitro from clinical inocula for 5 and 9 h in the presence and absence of nitrate (data set from Rosier et al. 2020) and (C, D) subgingival plaque samples collected from periodontal recall group with mild-moderate gingivitis before treatment (baseline) and at the end of 14 d after treatment, during which patients received nitrate-rich or placebo diet. The index was calculated by using the master discriminating species list (Fig. 3). The x-axis represents the number of samples (in pairs) in the data set. The small panels represent the corresponding box plots (median, interquartile range, 95% CI, and outliers). *P < 0.05. Wilcoxon signed rank test. NS, not significant. Figure was produced with R package and Excel.
A Simplified SMDI Based on 3 Genera
Genus-level analysis identified 19 master DG at an MDG cutoff of 1.5 that provided the highest accuracy with the training data (AUC = 0.957; Appendix Fig. 2A). The top periodontitis-associated genera were Fretibacterium, Treponema, Mogibacterium, Peptostreptococcaceae genus 6, and Desulfobulbus, while Actinomyces and Streptococcus were the most important health-associated genera (Appendix Fig. 3). Applying the master DG to calculate the SMDI in the test data resulted in an AUC of 0.929 (Appendix Fig. 2C). The resultant SMDI ranged from −6.2 to 4.9 and −8.44 to 3.69 for the training and test data, respectively (Appendix Fig. 2B, D); however, in both cases, a value close to −2 rather than zero discriminated between the majority of periodontitis and healthy samples.
ROC curve analysis showed that while using DG with an MDG ≥4 improved the accuracy for the test data, it decreased it for the training data (compare Appendix Fig. 2A, C). Interestingly, at MDG ≥5, the DG included only Fretibacterium, Treponema, and Actinomyces; provided an accuracy of 0.937 and 0.930 in the training and test sets, respectively; and shifted the cutoff between health and periodontitis to zero again. The resultant SMDI (hereafter, simplified SMDI) ranged from −9.20 to 8.29 in the training set with the zero discriminating between 90% and 85% of the healthy and periodontitis samples; in the test set, the simplified SMDI ranged from −10.50 to 8.12 with the zero discriminating between 90% and 78% of the healthy and periodontitis samples (Appendix Fig. 4).
Discussion
The complexity of microbiome data sets makes them difficult to interpret, and consequently, they have limited clinical utility; therefore, a simple summary index is urgently needed to increase their applicability in translational studies. To the best of our knowledge, this is the first report dedicated to the development of a dysbiosis index, for the subgingival microbiome in particular and the oral microbiome in general, based on a machine learning approach. The index is also probably the first to be developed on the basis of species-level profiles. Using RFA coupled with ROC analysis, we present an SMDI based on a master list of 49 DS that achieved a diagnostic accuracy of 0.96 and 0.92 in the training and test data sets, respectively. Reassuringly, the master DS included the classical periodontal pathogens—that is, members of the red complex and Eubacterium spp. (Socransky et al. 1998)—as well as more recently identified taxa, including F. alocis, F. fastidiosum, and M. timidum (Perez-Chaparro et al. 2014), which confirmed the logic of the methodological approach. What was really interesting was the relative importance of these pathogens based on RFA. For example, M. timidum, F. fastidiosum, and Fretibacterium sp. oral taxon 360 were among the top periodontitis-associated species with an MDG >3. Porphyromonas gingivalis, in contrast, ranked 21st with an MDG <1. This pattern of relative importance seen in the combined data was also observed in the individual data sets (Appendix Data Set 2). Notably, Fretibacterium spp. either were not identified or were identified by other names (e.g., Synergistetes) in the original 3 studies, which reflects the importance of using updated reference databases and taxonomy annotations.
Although the SMDI discriminated between most of the periodontitis and healthy samples, there were still outliers, which is not surprising since the index is essentially a measure of dysbiosis and not merely a diagnostic tool of periodontitis, which has important clinical implications. In fact, we correlated the SMDI scores with probing depth and clinical attachment level values in the Bizzarro et al. (2016) data set and did not find the severity of disease to account for the outliers, such as low SMDI due to milder periodontitis (data not shown). Therefore, periodontitis samples with low SMDI could represent quiescent sites (Kinane et al. 2017) or patients who are hyperresponsive to the smallest amount of dysbiosis. Similarly, healthy samples with high SMDI could represent sites in patients who can tolerate higher dysbiosis or, more important, those at risk of developing periodontitis, who need to be monitored more closely. Indeed, when SMDI was calculated for samples from “apparently” healthy sites in patients with periodontitis (Griffen et al. 2012 data set), nearly half of the sites had an SMDI of less than zero, indicating that they are true healthy sites, while the other half had a high SMDI, suggesting that they are progressing sites or at risk of progression (Appendix Fig. 1). Overall, SMDI could provide a valuable tool to supplement clinical examination in assessing risk of progression and monitoring disease activity and response to treatment.
An important potential application of SMDI is to use it as a measure of microbiome modulation in in vitro, animal, and clinical studies. Microbiome modulation is emerging as an important strategy for the prevention and treatment of periodontitis—for example, the use of prebiotics or probiotics to promote normobiosis or restore dysbiosis. In vitro microbiome models can be used to screen thousands of potential microbiome modulators, and those with promising effects can be tested subsequently in animal models and then humans. However, there has been no quantitative and objective tool with which to measure microbiome modulation. In this article, by applying the SMDI to the data sets of Rosier et al. (2020) and Jockel-Schneider et al. (2021), we quantitatively measured, for the first time, the dysbiosis-lowering activity of nitrate in vitro and in vivo, demonstrating that the SMDI represents a potentially powerful tool in microbiome modulation studies.
At the genus level, an SMDI based on a master list of 19 DG (Appendix Fig. 3) resulted in accuracy comparable to the species-level index, but it had a cutoff of −2 rather than zero between periodontitis and health (or dysbiosis and normobiosis), which makes it less applicable. Also, it was less effective in demonstrating the dysbiosis-lowering properties of nitrate (data not shown). Nonetheless, we were still able to compute a simplified SMDI based on only 3 genera: Fretibacterium, Treponema, and Actinomyces, with the first having the highest importance score. This simple index could be used as an alternative to the species-level index for assessing clinical samples and perhaps as a basis for the development of a diagnostic assay; however, it is not suitable in microbiome modulation studies, simply because a modulator can have activity on other taxa.
Discrimination at the species level is very important in the context of oral health, since there are frequently species that belong to the same genus but are differentially associated with disease, such as P. gingivalis and Porphyromonas catoniae (Camelo-Castillo et al. 2015). Fortunately, the 16S rRNA sequence in oral bacteria is sufficiently variable to discriminate most of the species, especially based on the V1–V3 region (Dewhirst et al. 2010; Escapa et al. 2018). We previously developed a BLASTn-based algorithm that exploits the Human Oral Microbiome Database and other highly curated databases to reliably assign species-level taxonomies to 16S data from oral samples (Al-Hebshi et al. 2015). Critical to this analysis pipeline is to use highly stringent quality filters to minimize sequencing errors and thus misclassifications. Indeed, the DS species identified by RFA in this study are very consistent with the periodontitis and health-associated species described in the literature, despite the fact that some of the included studies used shorter regions of the 16S rRNA gene (e.g., V1–V2), thereby demonstrating the reliability of the algorithm. Nevertheless, and for the purpose of comparison, we still performed the analysis and computation of SMDI based on genus-level profiles.
More methodological considerations about the development of the index, including a comparison with the dysbiosis ratio previously described (Meuric et al. 2017), are described in the Appendix Discussion.
In conclusion, we have developed a reproducible, easy-to-interpret index that can be used for identification of patients/sites at risk of periodontitis as well as active sites, for assessment of microbial response to treatment, and, importantly, as a quantitative tool in microbiome modulation studies. However, applying it to new data sets requires some standardization of bioinformatic analysis of sequencing data, including taxonomy assignment and normalization.
Author Contributions
T. Chen, contributed to design, data analysis, and interpretation, critically revised the manuscript; P.D. Marsh, contributed to data interpretation, critically revised the manuscript; N.N. Al-Hebshi, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345211035775 for SMDI: An Index for Measuring Subgingival Microbial Dysbiosis by T. Chen, P.D. Marsh and N.N. Al-Hebshi in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Dental and Craniofacial Research (grant 1R03DE028379-01A1).
ORCID iDs: P.D. Marsh  https://orcid.org/0000-0002-1203-4457
https://orcid.org/0000-0002-1203-4457
N.N. Al-Hebshi  https://orcid.org/0000-0003-1841-9304
https://orcid.org/0000-0003-1841-9304
References
- Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. 2013. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 7(5):1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hebshi NN, Nasher AT, Idris AM, Chen T. 2015. Robust species taxonomy assignment algorithm for 16S rRNA NGS reads: application to oral carcinoma samples. J Oral Microbiol. 7:28934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hebshi NN, Nasher AT, Maryoud MY, Homeida HE, Chen T, Idris AM, Johnson NW. 2017. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci Rep. 7(1):1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzarro S, Laine ML, Buijs MJ, Brandt BW, Crielaard W, Loos BG, Zaura E. 2016. Microbial profiles at baseline and not the use of antibiotics determine the clinical outcome of the treatment of chronic periodontitis. Sci Rep. 6:20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L. 2001. Random forests. Mach Learn. 45:5–32. [Google Scholar]
- Camelo-Castillo AJ, Mira A, Pico A, Nibali L, Henderson B, Donos N, Tomas I. 2015. Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front Microbiol. 6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Diaz PI, Van Dyke TE. 2020. The role of the microbiota in periodontal disease. Periodontol 2000. 83(1):14–25. [DOI] [PubMed] [Google Scholar]
- Devine DA, Marsh PD. 2009. Prospects for the development of probiotics and prebiotics for oral applications. J Oral Microbiol [epub ahead of print 1 May 2009] in press. doi: 10.3402/jom.v1i0.1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG. 2010. The human oral microbiome. J Bacteriol. 192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. 2010. Search and clustering orders of magnitude faster than blast. Bioinformatics. 26(19):2460–2461. [DOI] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. 2011. Uchime improves sensitivity and speed of chimera detection. Bioinformatics. 27(16):2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. 2018. New insights into human nostril microbiome from the expanded Human Oral Microbiome Database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems. 3(6):e00187-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. 2017. Microbiome datasets are compositional: and this is not optional. Front Microbiol. 8:2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, Podar M, Leys EJ. 2012. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 6(6):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockel-Schneider Y, Schlagenhauf U, Stolzel P, Gossner S, Carle R, Ehmke B, Prior K, Hagenfeld D. 2021. Nitrate-rich diet alters the composition of the oral microbiota in periodontal recall patients. J Periodontol [epub ahead of print 20 Mar 2021] in press. doi: 10.1002/JPER.20-0778 [DOI] [PubMed] [Google Scholar]
- Kinane DF, Stathopoulou PG, Papapanou PN. 2017. Periodontal diseases. Nat Rev Dis Primers. 3:17038. [DOI] [PubMed] [Google Scholar]
- Kirst ME, Li EC, Alfant B, Chi YY, Walker C, Magnusson I, Wang GP. 2015. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl Environ Microbiol. 81(2):783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuric V, Le Gall-David S, Boyer E, Acuna-Amador L, Martin B, Fong SB, Barloy-Hubler F, Bonnaure-Mallet M. 2017. Signature of microbial dysbiosis in periodontitis. Appl Environ Microbiol. 83(14):e00462-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WE, Holdeman LV, Smibert RM, Hash DE, Burmeister JA, Ranney RR. 1982. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 38(3):1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH HMP Working Group; Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, et al. 2009. The NIH Human Microbiome Project. Genome Res. 19(12):2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei J, Li F, Xie Y, Liu J, Yu T, Feng X. 2020. Microbial and metabolomic analysis of gingival crevicular fluid in general chronic periodontitis patients: lessons for a predictive, preventive, and personalized medical approach. EPMA J. 11(2):197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Chaparro PJ, Goncalves C, Figueiredo LC, Faveri M, Lobao E, Tamashiro N, Duarte P, Feres M. 2014. Newly identified pathogens associated with periodontitis: a systematic review. J Dent Res. 93(9):846–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosier BT, Buetas E, Moya-Gonzalvez EM, Artacho A, Mira A. 2020. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep. 10(1):12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, et al. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 75(23):7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol. 25(2):134–144. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. 2007. The human microbiome project. Nature. 449(7164):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kobert K, Flouri T, Stamatakis A. 2014. PEAR: a fast and accurate Illumina paired-end read merger. Bioinformatics. 30(5):614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345211035775 for SMDI: An Index for Measuring Subgingival Microbial Dysbiosis by T. Chen, P.D. Marsh and N.N. Al-Hebshi in Journal of Dental Research