Abstract
Background
Infraorbital hollows can give a fatigued or aged appearance, which can be treated by volumizing the segmented transition from the tear trough to the cheek with hyaluronic acid filler. Due to thin skin and the complex anatomy of the infraorbital area, both short- and long-term side effects (SEs) from this treatment are very common. While some patients are clear surgical candidates vs filler candidates, in real-world practice, many, if not most, patients are on a continuum where either procedure is appropriate, and the treatment decision is individualized based on each person’s risk vs benefit profile.
Objectives
Common aesthetic SEs from hyaluronic acid filler treatment in the infraorbital area will be reviewed, including their etiology, prevention, detection, and treatment.
Methods
The author’s experience from injecting the infraorbital areas of more than 800 patients in private clinical practice and observations from both short- and long-term follow-ups over 8 years is leveraged to provide detailed guidance.
Results
Recommendations on injection techniques, patient selection, and patient education are presented along with algorithms for the prevention and management of bruising, short- and long-term swelling, bumps, and blue discoloration (which is usually secondary to swelling from the filler rather than just the filler alone placed or migrating too superficially).
Conclusions
For nearly all patients, complete dissolution of filler with hyaluronidase is not required to address the issue, and the guidelines provided here will assist clinicians in the management of SEs to increase patient satisfaction with their treatment and aesthetic outcome.
Level of Evidence: 5

The fatigued or sunken-in appearance and shadowing characteristic of infraorbital volume deficiency is a presenting concern for many patients in clinical practice. Due to the central role of the eyes in communication and perceptions of age and beauty and the predictable patterns of aging in this area (including skin laxity, reduced volume, and changes in the retaining ligaments and skeletal structures), this area is often the target of facial rejuvenation.1 In individuals seeking treatment for the infraorbital area, the transition between the lower eyelid and midface or cheeks is often segmented, and the resultant “hollowing” can require volumization of the tear trough and areas of the upper malar region. A nonsurgical approach to treating this area is generally aimed at improving skin quality and volumization with hyaluronic acid (HA) filler, neuromodulators, and/or energy-based devices. While the optimal intervention is surgery for some patients, nonsurgical intervention is needed to accommodate patient preference, fundamental objection to surgery, and ineligibility for surgery due to medical conditions, downtime, or financial considerations, even for those patients with more significant deficits such as prolapsed fat pads. Thus, an understanding of the appropriate use of fillers in the periorbital area, as well as knowledge of how to diagnose and manage common treatment side effects (SEs), is imperative for all cosmetic practitioners.
Given the delicacy of the skin around the eye, the dynamic nature of the resident musculature, and minimal subcutaneous fat, any topological irregularities resulting from injection are readily apparent. Furthermore, the compartmentalization of the area by ligaments (orbital retaining ligament above and zygomatico-cutaneous ligament [ZCL] below) and the presence of large important vessels (angular and infraorbital artery) makes the area one of the most complicated areas to treat with a different SE profile than the rest of the face.
In addition, the superficial lymphatic system in the infraorbital area is very delicate and often leads to malar edema. There is less drainage through the superficial lymphatics, possibly due to the presence of a malar septum as reported by Pessa and Garza,2 who showed a clear line of demarcation that limits the downward descent of subcutaneous dye, similar to a sharply demarcated black eye.3 This malar septum is described as originating from the periosteum along the orbital rim and inserting into the skin on the cheek 2.5 to 3 cm below the lateral canthus. It divides the suborbicularis oculi fat into superior and inferior sections. The inferior section connects with the cheek fat, and the superior section can contribute to a malar mound. This malar septum is thought to be a relatively impermeable barrier that allows tissue edema to accumulate above its cutaneous insertion.2 Therefore, it is vital to place most of the filler deep near the periosteum4 to prevent this SE.
For all these reasons above, it is recommended to only use dissolvable HA fillers so hyaluronidase may be used if prolonged or serious adverse events occur. In addition, knowledge of infraorbital anatomy5,6 and the skill set of an experienced injector is recommended. However, even with the above-listed criteria satisfied, SEs (swelling in particular) are relatively common, especially over time, and must be discussed with patients before treatment so that they can be managed appropriately. In fact, in a 7 year retrospective review by the author,7 the rate of patient-reported short-term swelling after infraorbital hollow (IOH) treatment with a 27-gauge cannula was 51% and long-term or delayed onset swelling, while less frequent, still occurred at a rate of approximately 19% as rated by trained evaluators counting even minimal swelling not noticeable to patients.7 Others using different techniques and patient populations have also published similar incidences of long-term swelling in this area from 6% to 24%.
In a study by Goldberg and Fiaschetti of 121 patients, 15% had edema following Restylane injection using a “layered feathering technique” in the suborbicularis plane with up to 0.5 cc each side placed in the orbital rim and zygomatic hollow.8 In another study of 303 treatments using Restylane in the preperiosteal plane using serial puncture with an average of 0.8 cc on each side, 6% had what the author called “persistent fullness”; 7 of the 18 resolved with hyaluronidase.9 In a study with 51 patients receiving Juvederm (Allergan, Irvine, CA) or Restylane (Galderma, Lausanne, Switzerland) with a linear threading or serial puncture technique 2 to 8 mm below the infraorbital rim extending along the length of the rim preperiosteally, 24% had prolonged edema lasting more than 1 month and 25% of these patients received hyaluronidase. None of the patients showed signs of inflammation, such as erythema, pain, or heat.10 In a longer retrospective study of 147 patients at least 5 years posttreatment, who received Restylane with a fanning technique in the suborbicularis plane, 11.5% had malar edema, 31.3% had bluish-gray dyschromia, and 30.5% had contour irregularities,11 all of which could be related to edema. Unfortunately, most patients and practitioners are not aware of the possibility of delayed-onset edema multiple years after treatment,12 so it is not uncommon for patients to receive extensive medical testing in search of a medical diagnosis when none exists. Here, guidance for the diagnosis and management of common short- and long-term nonischemic SEs is presented alongside practical tips for patient selection and education.
METHODS
The guidance presented here is based on the experience of the author in treating more than 800 patients (average age: 62.6 years, age range: 26-89, female/male ratio: 95%/5%) in private clinical practice from April 2013 to November 2021. Most patients had long-term follow-up appointments ranging from 1 to 7 years after their last treatment in addition to their short-term follow-up. Due to the location of the author’s practice, most patients are above 50 years of age, with a significant number above 65 years, and have characteristics that would normally preclude nonsurgical management due to severity of the volumetric deficit, presence of prolapsed fat pads, sun damage, wrinkled skin, and skin laxity. For these patients, in particular, the risk of SEs, especially short- and long-term swelling, is elevated. Thus, the guidance presented here is shaped by clinical experience managing a substantial number of patients with numerous risk factors for treatment SEs. However, for these same patients, the improvement to their appearance from the filler treatment is often dramatic (Video 1), and the patient’s desire to retain the filler despite SEs is great. This constellation of factors has led to a developed understanding of SE management for the treatment of the infraorbital hollow (IOH). All patients discussed here were treated in accordance with the principles outlined in the Declaration of Helsinki, and each patient consented to treatment and photography.
RESULTS
Patient Selection
As with any treatment, patient selection is an important part of preventing SEs. While some patients are clear and absolute surgical candidates vs filler candidates, in real-world practice, many, if not most, patients are on a continuum where either procedure is appropriate, and the treatment decision is individualized through the analysis of the patient’s risk vs benefit profile. Though the “ideal candidate” for infraorbital HA treatments is outlined in the first column of Table 1, in the author’s practice, few patients have these characteristics. Yet even patients with multiple “contraindications” (“Treat with caution,” Table 1) are often able to achieve dramatic improvement with fillers and are very satisfied as seen in Figure 1.
Table 1.
Criteria for Candidacy
| Ideal candidate | Treat with caution | Do not recommend treating |
|---|---|---|
| Good skin quality and elasticity | Prolapsed fat pads | Periocular erythema |
| Minimal volume loss (able to be corrected with 0.5 cc or less) | Skin laxity | History of prolonged periocular swelling |
| No superficial contour issues | Static rhytids | Double contours (visibility of both orbicularis retaining ligament and zygomatico-cutaneous ligament) |
| No visible veins | Need for more than 1.0 cc | Prominent swollen malar mounds |
| Visible veins | Severe skin laxity | |
| Thin skin | ||
| On blood thinners | ||
| Rosacea or eczema | ||
| History of allergies | ||
| Auto-immune disease | ||
| Mild malar edema | ||
| History of facial swelling |
Figure 1.
A 68-year-old female (A) before treatment and (B) 3 weeks later. The second photograph was taken 1 week after the second infraorbital hyaluronic acid filler treatment to camouflage her prolapsed fat pads.
Poor candidates for nonsurgical treatment have severe skin laxity that cannot be resolved with volume alone. These patients can be treated first with deep laser resurfacing with coagulation to tighten and smooth the skin to make them better filler candidates if surgery is not an option. Additionally, baseline chronic periocular edema, with or without double contours (tight ligaments binding both above and below in infraorbital region) as shown in Figure 2, often responds poorly to both filler and surgery.
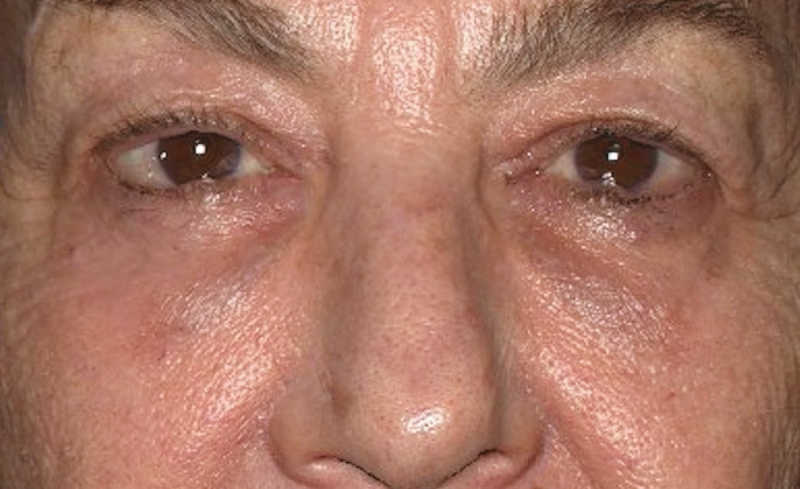
Figure 2.
A 62-year-old female with infraorbital edema before any cosmetic treatments with visible outlines of her both orbital retaining ligament and zygomatico-cutaneous ligament (double contours).
Patient Education and Informed Consent
Due to the increased risk of SEs associated with the treatment of IOH, a thorough discussion with the patient is necessary to ensure that the patient is fully informed and in agreement with the procedure. In the author’s practice, a specialized informed consent is reviewed with the patient. The following points are critical elements:
In clinical practice, the physician and patient can agree to use FDA-approved injectable fillers “off label” in locations not explicitly stated in the product label.
The areas around the eyes are more sensitive and more prone to reactions.
The SEs in this area are different than the rest of the face, with special attention paid to the potential for prolonged or delayed-onset swelling. The appearance of bumps or lumps and/or bluish-gray discoloration are most often due to distended vessels or swelling. The images included are shown in Figure 3. It is vital to educate the patients to return to the office if they have swelling in this area even years later before seeking alternative diagnoses to prevent unnecessary medical workups.
Plan for SEs. While patients do not have to pay for SE management directly (it is built into the cost of the treatment), no refunds are given if the filler needs to be dissolved as this is not due to the fault of the physician but instead a possible known reaction.
Though the risk of these events is higher in this area, the risk is taken together by the physician and patient. In the author’s clinical experience treating with HA fillers, none of these SEs are irreversible, and they can be managed nonsurgically.
Understanding the degree of improvement given the severity of the baseline condition to ensure realistic expectations (no change in the texture of the skin).
Figure 3.
Possible side effects from infraorbital hyaluronic acid filler injections shown in consent form: (A) a 68-year-old female with delayed-onset swelling and (B) a 78-year-old female with a linear vein appearing as a contour irregularity.
Treatment Planning and Prevention
Though a full review of resident anatomy is beyond the scope of this manuscript, it should be emphasized that the injector should have knowledge about the nerves, vasculature, lymphatics, muscles, ligaments, and fat compartments. Without this knowledge, the risk of SEs increases dramatically, and the likelihood of a good aesthetic result declines especially if an injector is unable to discern the plane into which they are injecting. Furthermore, while the SEs discussed here are nonischemic and centered on those affecting aesthetic outcomes, the proximity to the orbital rim and vessels connected to the ophthalmic artery warrant extreme caution; therefore, the depth and location of filler placement should be carefully selected.13-17
More broadly, the following considerations when injecting this area should be considered:
The volume of the injection is directly related to the risk of short-term swelling, and patients should be treated with the smallest possible volume, ideally ≤0.5 cc total.7
As much of the product as possible should be injected deep, underneath the muscle, with only the smallest amount placed superficially if needed to smooth out superficial contours.
In the author’s opinion, the product should be injected not only just above the ZCL but also below in the malar fat pads and lateral on the zygoma so that there is support and balance with less product needed in the IOH.
Only soft HA fillers with a low hydrophilicity should be used. In the author’s practice, treatment options are restricted to NASHA (Restylane, Galderma Laboratories, L.P. Fort Worth, TX), CPM gel (Belotero Balance, Merz North America, Inc., Raleigh. NC), VYC-15L (Juvéderm Volbella-XC, Allergan Inc., Irvine, CA), and RHA2 (TEOSYAL RHA 2, TEOXANE Laboratories, Geneva Switzerland).
Permanent or non-dissolvable fillers are not recommended in this area due to the increased risk of SEs.
Inject as much as possible with a cannula to maximize safety18 and reduce SEs such as bruising.19 A needle should be reserved for situations when the results would differ such as treating a depression inferior to the malar mound. The author uses a vertical needle injection at the lateral cutaneous insertion of the ZCL starting on the periosteum and continuing upward with a retrograde injection throughout each layer (not possible with a cannula). Deep needle injections should only be placed lateral to the lateral limbus for safety reasons.20
Inject retrograde with minimal force, while constantly moving the device with re-direction if resistance is encountered. This technique ensures that small amounts of filler are injected in any one location at low pressures. One possible theory of HA filler-induced blindness is that the force of the filler placed in the lumen of a vessel overcomes blood pressure and when there is sufficient volume, flows retrograde from vessels connected to the ophthalmic artery back to the bifurcation with the central retinal artery, though other theories are also described.
If using a cannula, the entry point should not be placed in the malar prominence (red outline in Figure 4A) because trauma from the cannula going in and out of this location is very likely to cause acute malar mounds (Figure 4B) due to the ligament/septum below binding this area and the poor superficial lymphatic flow above. The author starts with an injection port inferior and then adds a lateral entry point if needed.
Diligent before and after photography to assist in the diagnosis of SEs.
Figure 4.
(A) A 69-year-old female with the area outlined in red that is susceptible to swelling and, therefore, not recommended as an entry point and the recommended area circled in green. (B) A 42-year-old female with swollen malar mounds around the entry points 2 weeks after infraorbital hollow filler treatment, worse on her right (sleeping side).
Short-Term SEs
Postprocedure Edema
Edema is the most common SE of IOH treatment. There is significant inter-patient variability with regard to the risk of swelling, and it is often impossible to predict whether a patient will be prone to swelling following infraorbital injection.10 The swelling response comes from a cascade of events that leads to the leakage of fluid from the vessels in the area. The rate at which the area is filled with fluid is dependent upon the rate of fluid entering (intensity of the swelling response), the size of the area (confined by the ZCL and malar septum below and the orbicularis retaining ligament above),21 and how fast the fluid can leave the area (lymphatic system and septum permeability). Additional environmental factors may include blockage by the filler itself or reduced muscle contraction and movement in the area from treatment with neuromodulators.
The methods for the prevention and treatment of immediate swelling following HA filler placement in the IOH are presented in Table 2. Mixing 0.1 mL (1 mg) of 50 mg/5 mL of triamcinolone per 1 cc of HA filler dramatically reduces short-term swelling (Video 2). In a retrospective study from the author’s practice of more than 250 patients using this technique, there were no SEs reported among all skin types, and the rate of short-term swelling was reduced from 51% to 23%.7 In fact, multiple patients within the author’s practice who experienced swelling with treatment without triamcinolone have avoided this SE when triamcinolone is used for re-treatment.
Table 2.
Strategies for the Prevention and Treatment of Short-Term Swelling Following Injection of HA Filler in the Infraorbital Area
| Prevention of short-term swelling |
|---|
| Use the correct product (HA filler with low propensity for swelling) |
| Use lower volumes (ideally ≤0.5 cc per side per session) |
| Use antihistamines (for patients with a history of reactivity to injury, allergens, or history of hypersensitive foreign body response) |
| Sleep upright with several pillows |
| Decrease salt intake and aerobic activity (lower blood pressure) |
| Mix in triamcinolone (1 mg/mL of filler) |
| Silicate cream overnight after treatment for compression |
| Treatment of short-term swelling |
| Watchful waiting, firm massage (down and out with a lubricating cream or topical steroid) by injector, and reassurance |
| Topical cortisone cream to massage at home several times per day |
| Silicate cream during the day and overnight for compression |
| Diuretics or antihistamines(only effective in select cases, if allergic component is suspected) |
| Local triamcinolone injection (no more than 0.1 cc of 2.5 mg/cc) subdermal (avoid intradermal) |
| Oral steroids (Medrol dose pack or 10 to 40 mg prednisone ×5 days depending on patient weight and severity of swelling). |
HA, hyaluronic acid.
Compression, often with bandages, is a well-established modality for both the prevention and treatment of mild swelling in other areas of the body. Similarly, for the face, the author recommends the use of sodium or magnesium silicate tightening creams for several days after treatment to compress the tissue to prevent or mitigate swelling in the area (examples are Instant FirmX, Peter Thomas Roth Labs LLC, Saddle Brook, NJ or Rapid Reduction, True Earth Health Products, Farmingdale, NY). Most patients report worsening of swelling upon awakening, and thus the use of this cream before bed as overnight compression therapy is highly recommended to prevent swelling. While all brands with sodium silicate can cause a white residue if too much is used and not rubbed into the skin, the appearance or ability to mix with makeup or sunscreen is not a concern when using it overnight.
Treatment options for short-term swelling are outlined in Table 2. In many instances, only watchful waiting and reassurance with massage may be needed. Compression with silicate compression/tightening cream may also speed up the resolution of swelling dramatically, as shown in Figure 5. Topical steroids along with a strong massage are used to assist in moving the swelling out of the small confined under-eye area. Local steroid injections (2.5 mg/mL × 0.05-0.1 mL of TAC) can be injected with either a needle or a cannula. However, a cannula is recommended to prevent placing a bolus of the solution in the dermis, which could cause temporary atrophy and hypopigmentation (if this occurs, saline can be used to flush the area for quicker resolution).18,22,23 In addition, while extremely rare and with higher doses, steroid particles can cause vascular occlusion, so the same precautions taken with filler should be used with light force using a cannula and injecting small amounts while moving retrograde.24-26 Oral steroids can be used in cases when the swelling is affecting the patient’s quality of life and other measures are not effective.
Figure 5.
A 63-year-old female with mild swelling presenting 2 months after her first infraorbital hyaluronic acid filler treatment (A) before and (B) after application with sodium silicate for compression therapy.
Bruising
Bruising is another SE that is far more common and severe around the eye than other areas of the face. Prevention of bruising is important, as bruising can dramatically lengthen the amount of social downtime associated with the procedure. A summary of preventive measures and treatment for bruising is presented in Table 3. Like other SEs, prevention is key, and the use of a cannula, vein finder, and gentle injection technique is all vital. Immediate broad pressure following injection is recommended for 5 minutes. Pressure should not be restricted to the insertion point but should include the entire treated area. Other measures such as arnica, bromelain, and ice are also commonly used; however, evidence for these practices is limited.27 Because bruising is self-limiting, watchful waiting and reassurance are all that is required. However, for patients who must return to work or for whom bruising is mutually exclusive with social activity, laser or intense pulsed-light therapy can speed resolution. In addition, makeup concealers can completely hide bruising, especially if these are lightly patted on, so that additional layers can be applied to completely block the appearance.
Table 3.
Strategies for the Prevention and Treatment of Bruising From Injection of HA Filler in the Infraorbital Area
| Prevention of bruising |
|---|
| Use of a vein finder for entry point |
| Use of a cannula |
| Use of light force |
| Immediate postprocedure pressure |
| Use of arnica, bromelain, ice |
| Discontinue unnecessary medications that can increase the risk of bruising such as vitamin E and Ginkgo |
| Treatment of bruising |
| Watchful waiting and reassurance |
| Laser treatment (IPL/PDL/Nd:YAG) |
HA, hyaluronic acid; IPL, intense pulsed light; Nd:YAG, neodymium-doped yttrium aluminum garnet; PDF, pulsed dye laser.
Long-Term SEs
Prolonged or Late-Onset Edema
Swelling that persists longer than 4 weeks without improvement is considered prolonged edema. If the swelling is mild to moderate and the patient has a more severe baseline volumetric deficit and/or is not eligible for surgery, patients may not want treatment as often the swelling is not noticeable to them. For delayed-onset swelling, it is important to take a detailed medical history to identify that the possible underlying causes of the swelling, since trauma, infection, or exposure to an allergen can be a cause that resolves easily. There is some debate that filler migration and blockage of lymphatic flow through compression or exposure to the crosslinker (1,4-butanediol diglycidyl ether) as the HA filler is resorbed can also cause this condition.28 Regardless, in all cases of late-onset swelling, massage and silicate cream may be helpful. However, most other treatments are only effective when they address the underlying cause. For instance, oral antihistamines are recommended when there is a possible allergic component (eg, new antibiotic for infection) and this presents with deeper, broader facial swelling. Triamcinolone is recommended when the swelling is more severe from either trauma, an allergy not responding to antihistamines, or an acute inflammatory stimulus like a chalazion. Oral steroids should be reserved for severe cases.
If the inciting stimulus remains, patients will only respond to local triamcinolone for 2 to 6 weeks before the swelling returns. In the author’s practice, patients do not receive more than 2 injections of triamcinolone alone in the same area per year to prevent atrophy. Additionally, the injection is subdermal because the intradermal injection can cause hypopigmentation and dermal atrophy. Using a cannula can help to ensure that steroids are administered under the skin and decrease the likelihood that it is injected into a vessel, especially with larger-gauge cannulae.18,29 An additional option is camouflaging the swelling by adding additional volume in the cheek around it. If there is skin laxity, laser resurfacing reduces the lax area for fluid to accumulate. If swelling cannot be managed by the above methods in Table 4, or continues to return, the filler must be dissolved using hyaluronidase in part or in full. While studies have demonstrated the successful management of swelling using complete removal of the filler with hyaluronidase,30 this is the last resort for patients and in many cases is not necessary. If using hyaluronidase, it is important to warn the patient that the results can get worse for a few weeks while the skin re-adjusts to the new lower volume.
Table 4.
Treatment for Delayed-Onset or Long-Term (>4 weeks) Swelling
| Swelling treatment |
|---|
| Massage with cortisone cream |
| Application of silicate compression cream |
| Antihistamines or diuretics |
| Triamcinolone and/or hyaluronidase Mild long term or delayed onset Inject 0.1 mL of 2.5 mg/mLa triamcinoloneb with a cannula in each area. Moderate long term or delayed onset Inject 0.1-0.2 mL of 2.5 mg/mL triamcinolone with a few units of micro-dose hyaluronidase.c Severe long term or delayed onset Inject 0.2-0.4 mL of 2.5 mg/mL triamcinolone with 15 units of low-dose hyaluronidased in each location depending on the breadth of swelling. No resolution with triamcinolone or low-dose hyaluronidase Dilute the hyaluronidase by half (75 units/mL) and slowly increase the dosee with of each treatment from 15 to 75 units until full resolution. |
| Cheek filler Inject with a cannula under the ZCL to volumize and then with a needle in a vertical retrograde technique along the lateral ZCL to camouflage swelling above the ZCL. NEVER inject medial to the lateral limbus deep with a needle. |
| Laser resurfacing Tightens the skin, preventing the accumulation of fluid. |
| Complete reversal with hyaluronidase If either no change occurs or swelling improves but then relapses quickly without overall improvement with the above options, inject 150 units per 1 cc of under-eye filler to completely dissolve and return to baseline. Warn the patient that it may look worse than baseline for 1-2 weeks until the native HA rebuilds, and the skin re-adjusts to the new volume (recommend silicate compression cream while waiting). |
aAbout 2.5 mg/mL of triamcinolone can be obtained by mixing 0.1 mL of triamcinolone 50 mg/5 mL with 0.3 mL of bacteriostatic saline or a combination of 0.2 mL saline and 0.1 mL of lidocaine with epinephrinef;
bDO NOT REPEAT triamcinolone injections more than once within a month or more than twice within 6 months;
cMicro-dose hyaluronidase with 2.5 mg/mL of triamcinolone can be obtained by mixing 0.05 mL of hyaluronidase with 0.1 mL of triamcinolone (50 mg/5 mL) and 0.25 mL of saline (or 0.15 mL of saline and 0.1 mL of lidocaine with epinephrine)f;
dLow-dose hyaluronidase with 2.5 mg/mL of triamcinolone can be obtained by mixing 0.1 cc triamcinolone 50 mg/5 mL, 0.1 mL of hyaluronidase 150 u/mL (15U) with 0.2 mL of saline (or 0.1 mL of saline and 0.1 mL of lidocaine with epinephrine)f;
eIf using more than 0.1 mL of hyaluronidase, an intradermal test for allergy is recommended by placing 0.1 mL in the dermis to create a bleb and waiting 30 minutes to check for a reaction;
fIf using lidocaine with epinephrine, warn the patient that the area will turn white in color for a few hours and will feel numb. The change in color outlining the treated area assures the injector of proper placement, and the immediate improvement assures the patient that it is swelling and not filler causing the volume change. The temporary improvement from the epinephrine constricting the vasculature may wane after a few hours. Topical vasoconstrictors such as Mirvaso/Rhofade can also be used for this purpose but are more expensive and less efficacious than the silicate creams. ZCL, zygomatico-cutaneous ligament.
Bumps and Surface Irregularities
If a soft HA filler is placed with a cannula and the filler is not bolused too superficially, bumps and surface irregularities are almost never due to the lumpiness of the filler itself. While the filler is the origin of this side effect, it is NOT the direct cause. Most frequently, bumps are distended blood vessels (Figure 6A), which can be shown to the patient with a vein finder. Figure 7 illustrates how utilization of a vein finder can aid in the identification of the bump’s origins. When managing lumps and bumps, it is critical to identify the correct etiology, and baseline images are particularly helpful to inform a diagnosis. For example, in patients with some degree of fat pad prolapse, the highest point of the fat pad prolapse can often be seen after treatment, especially during eye movement with an upward gaze (Figure 8). In such an instance, not knowing the patient’s baseline features complicates the diagnosis of surface irregularities. In addition, granulomas are not within the scope of this paper but can present as bumps with surrounding erythema and should be treated with hyaluronidase immediately, either with or without anti-inflammatory antibiotics31 depending on symptoms such as pain or warmth.
Figure 6.
A 69-year-old female in 2014, one year after 2 infraorbital hollow treatments (A) presenting with a delayed-onset bump on her right that was a vein that became more prominent, and (B) after cheek injections to camouflage the protruding vein.
Figure 7.
A 66-year-old female presenting with (A) a bump 2 weeks after infraorbital filler was diagnosed with a vein finder (B) as a vein.
Figure 8.
A 64-year-old female (A) before treatment and (B) after 2 syringes of infraorbital hyaluronic acid filler to camouflage prolapsed fat pads with a small surface irregularity from the top of the fat pad.
Most often, bumps that arise at the time of filler placement are due to the patient’s own veins rising to the surface due to the increased volume in the area from the filler. An analogy of rising tides raising all ships may be used to explain this phenomenon to patients. An animation of before and after filler causing increased visualization of a vein is seen in Video 3. Bumps that surface after some time has passed are often due to a combination of both a vessel and increasing leakage of fluid from vessels causing late-onset swelling, as shown in Figure 6. In either case, injection with a low volume at initial treatment and reducing injection volume at subsequent injections to reduce the risk of protrusion are preventive measures. In many patients, surface irregularities from veins are much less bothersome than the original infraorbital depression and no treatment is needed. However, if the lump is bothersome, it can often be disguised through additional filler injections in the cheeks (Figure 6B) or, alternatively, an Nd:YAG laser and internal metal eye shields may be used to treat the veins outside the orbital rim. To eliminate some of the filler volume causing compression and to reduce the swelling that may be causing vein protrusion, 2.5 mg/mL triamcinolone and 7.5-15 U of hyaluronidase diluted in saline can lower the filler and swelling volume without creating a depression if evenly injected throughout the treated area with a cannula. Preventive measures and treatment for lumps and bumps are presented in Table 5.
Table 5.
Strategies to Prevent and Treat Bumps and Lumps From Injection of HA Filler in the Infraorbital Area
| Prevention of bumps and lumps |
|---|
| Inject lower volumes |
| Treatment of bumps and lumps |
| Watchful waiting and reassurance |
| Silicate compression cream to reduce swelling |
| Cheek filler to camouflage |
| Laser treatment (Nd:YAG) for veins outside the orbital rim |
| Injection of 7.5-15 U of micro-dose hyaluronidase to reduce the volume of HA filler ± 0.1 mL of 2.5 mg/cc triamcinolone if there is swelling |
HA, hyaluronic acid.
Blue-Gray Discoloration
The blue discoloration from HA filler in the superficial dermis is most often attributed to the Tyndall effect caused by the scattering of blue light by colloid particles.32 However, for this effect to occur, the particles should be around the same size as visible light (400-700 nm), which means smaller than 1 micron. As HA filler particles range from approximately 250 to 1000 microns in size, the Tyndall effect is unlikely the explanation. A publication by Rootman and colleagues33 also discusses how the Tyndall effect is not the likely cause for the blue color that HA filler sometimes creates, and they also published other possible theories related to the underlying vessels, though there are no definitive answers to date. The current author proposes the blue discoloration present after IOH filler may be due to the Rayleigh effect from swelling. The Rayleigh effect is caused by scattering of blue light by nano-colloids which measure 1/10 or less than the size of the wavelength of blue light (450-495 nm) which would be 45-50 nm or less. The hypothesis is that superficial edema contains nanoparticles (size ranging from 1 to 50 nm) which causes the Rayleigh effect. In fact, edematous fluid has proteins such as albumin that measures approximately 5-10 nm.34 It is most important to note that the blue color is not necessarily synonymous with visible superficial filler. In fact, in the infraorbital area, the author finds that late-onset blue-gray discoloration is nearly always synonymous with swelling (Figure 9). Frequent resolution of blue-gray discoloration with triamcinolone (Video 4) in the author’s practice further supports the hypothesis that blue-gray discoloration that does not occur immediately following injection is secondary to late-onset swelling and not simply significant amounts of HA filler migrating too superficially. Management is largely the same as late-onset swelling, as the removal of fluid will resolve the light-scattering effect.
Figure 9.
A 72-year-old female presenting 6 months after infraorbital hyaluronic acid treatment with bluish-gray swelling.
DISCUSSION
Though the infraorbital area is prone to more treatment SEs, there are many effective prevention and treatment measures. With the tools presented above, these common nonischemic SEs are nearly entirely manageable with quick, easy, and inexpensive solutions. Many late-onset SEs are secondary to swelling, including bumps from distended veins and accumulation of edematous fluid and resultant Rayleigh effect. Both early- and late-onset swelling are so common in the treatment of IOH that the narrative that swelling is due to poor injection technique is somewhat misleading and may prevent honest education of the patient. While poor injection technique can certainly worsen the risk of swelling, swelling is not necessarily indicative of poor technique especially when it occurs years later.
In addition, it makes little sense to automatically use hyaluronidase to dissolve all the filler in these instances. Swelling or increased water binding in this area is not inherently a “bad” outcome. In fact, it is likely that water binds around fillers in all areas35,36 but simply is more noticeable in this tight, small, thin-skinned area. Patients should be encouraged to weigh the impact of the revolumization against the impact of the swelling and resultant irregularities and participate in collaborative decision making. Before and after photographs should be referenced, and intermediate approaches should be considered. The impact of swelling on the patient’s impression of their appearance is highly personal, and treatment approaches to swelling should be determined with the patient’s wishes in mind. Camouflaging the SE with filler in the cheek, injecting triamcinolone, reducing laxity with laser resurfacing, and partial reduction of filler with evenly applied micro-dose hyaluronidase are all intermediate options that can achieve long-lasting natural-appearing results.
If considering triamcinolone, it is important to be educated about several rare case reports in which injection of triamcinolone alone directly into the eyelid or surrounding areas at high doses caused serious SEs.24-26,37,38 However, the doses in these case reports were much higher than our recommended dosage and a needle was used. One case report described iris depigmentation after an injection with a needle into the eyelid with 40 mg of triamcinolone and 8 mg of dexamethasone to treat an eyelid hemangioma.37 In another case, an injection of 4 mg/0.05 mL caused fat atrophy and hypopigmentation.38 The most serious case report describes 0.5 mL of methylprednisolone 80 mg/mL (40 mg) injected with a needle into a chalazion on the eyelid immediately after surgery which caused retinal occlusion.26
While these SEs need to be taken seriously, our technique recommends either 1 mg of triamcinolone mixed with the filler over a large area for prevention or 0.25 mg of triamcinolone placed in an area of swelling, both with a cannula and in an area distant and inferior to the eyelid. The recommended doses in this paper, therefore, range from 1/4th to 1/160th of the amounts used in the previous case reports. In addition, while the size of particles in triamcinolone varies from 1 to 1000 microns, it was reported that, in one analysis of triamcinolone 40 mg/mL, the majority (71%) of particles were 1 to 10 microns, with 88% of the particles 50 microns or less.39 Since the particle size of HA filler (250-1000 microns) is larger than the average particle size of triamcinolone, the addition of triamcinolone to HA filler7 should pose no additional risk for occlusion. Utilizing the recommended triamcinolone techniques in this paper, the author has treated over 250 patients with each technique safely without any serious or permanent SEs.
In most of the author’s IOH treatments which started in 2013, a 27-gauge cannula was used. Currently, when injecting the IOH area, the author continues to use the same blunt-end, flexible (low elastic modulus), 27-gauge cannula. Utilizing the author’s instrument and technique, as seen in another publication also utilizing a similar 27-gauge cannula,19 most patients report decrease pain, bruising, and swelling. In addition, after treating over 1600 IOH in over 800 patients, there have been no reports of vascular occlusion. This is likely due to (1) the specific type of 27-gauge cannula which allows the author to feel resistance and redirect; (2) the minimal force used during advancement of the cannula; (3) injecting only retrograde while moving the cannula with minimal force on the plunger; and (4) withdrawing the cannula most of the distance before wider re-direction to ensure that increased torque is not placed on fixed vessels. It is important to mention that not all 27-gauge cannulae are the same and a more thorough literature review and discussion on this topic will be published separately. While a more formal analysis in a prospective clinical trial of the percentages of each IOH SE with different cannulae and needles (differences could include tapering, length, elastic modulus, gauge, coating, size of opening and bevel) would be informative, this is outside the scope of this work. Thankfully, more information should be available shortly with the recent FDA approval of the IOH indication for VYC-15L.
CONCLUSIONS
Interestingly, patients treated with filler in the IOH may not need additional filler treatments for several years, if ever, due to the replacement of metabolized filler with increased amounts of bound water. Filler in this area is more durable in general, but for patients with swelling, the volume remains replenished for a very long period, and patients can be maintained with cheek filler. It matters little to the patient whether the volume is from their own fluid or the HA filler if the volume is in the correct place and appears natural. Together, the approaches in this paper outline preventive measures and treatment for SEs and how to manage them effectively and specifically. Paired with safe injection technique, the management of these SEs can support good long-term outcomes for patients and clinicians.
Disclosures
Dr Siperstein is a consultant, clinical trial investigator, speaker, and trainer for Galderma (Lausanne, Switzerland) and Allergan (Irvine, CA, USA).
Funding
Funding for this research was provided by Allergan, Galderma, and Merz (Raleigh, NC, USA).
REFERENCES
- 1.Farkas JP, Pessa JE, Hubbard B, Rohrich RJ.. The science and theory behind facial aging. Plast Reconstr Surg Glob Open. 2013;1(1):e8-e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pessa JE, Garza JR. The malar septum: the anatomic basis of malar mounds and malar edema. Aesthet Surg J. 1997; 17(1):11-17. [DOI] [PubMed] [Google Scholar]
- 3.Pessa JE, Zadoo VP, Adrian EK, Woodwards R, Garza JR. Anatomy of a “black eye”: a newly described fascial system of the lower eyelid. Clin Anat. 1998;11(3):157-161. [DOI] [PubMed] [Google Scholar]
- 4.Funt DK. Avoiding malar edema during midface/cheek augmentation with dermal fillers. J Clin Aesthet Dermatol. 2011;4(12):32-36. [PMC free article] [PubMed] [Google Scholar]
- 5.Naik MN. Hills and valleys: understanding the under-eye. J Cutan Aesthet Surg. 2016;9(2):61-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoukath S, Taylor GI, Mendelson BC, et al. The lymphatic anatomy of the lower eyelid and conjunctiva and correlation with postoperative chemosis and edema. Plast Reconstr Surg. 2017;139(3):628e-637e. [DOI] [PubMed] [Google Scholar]
- 7.Siperstein R, MJ, Speranza A. A retrospective review of the safety and efficacy of low-dose triamcinolone mixed with hyaluronic acid fillers to reduce post-injection infraorbital swelling. J Cutan Aesthet Dermatol (In press). [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg RA, Fiaschetti D. Filling the periorbital hollows with hyaluronic acid gel: initial experience with 244 injections. Ophthalmic Plast Reconstr Surg. 2006;22(5):335-341; discussion 341; discussion 341-343. 10.1097/01.iop.0000235820.00633.61 [DOI] [PubMed] [Google Scholar]
- 9.Steinsapir KD, Steinsapir SMG. Deep-fill hyaluronic acid for the temporary treatment of the naso-jugal groove: a report of 303 consecutive treatments. Ophthalmic Plast Reconstr Surg. 2006;22(5):344-348. [DOI] [PubMed] [Google Scholar]
- 10.Griepentrog GJ, Lucarelli MJ, Burkat CN, Lemke BN, Rose JG. Periorbital edema following hyaluronic acid gel injection: a retrospective review. Am J Cosm Surg. 2011;28(4):251-254. [Google Scholar]
- 11.Mustak H, Fiaschetti D, Goldberg RA. Filling the periorbital hollows with hyaluronic acid gel: long-term review of outcomes and complications. J Cosmet Dermatol. 2018;17(4):611-616. [DOI] [PubMed] [Google Scholar]
- 12.Lederhandler M, Belkin D, Anolik R, Geronemus RG.. The rise and fall of the pale puffy lower eyelid pillow. J Drugs Dermatol. 2021;20(4):475-476. [DOI] [PubMed] [Google Scholar]
- 13.Beleznay K, Carruthers JD, Humphrey S, Jones D. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41(10):1097-1117. [DOI] [PubMed] [Google Scholar]
- 14.Beleznay K, Carruthers JDA, Humphrey S, Carruthers A, Jones D. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39(6):662-674. [DOI] [PubMed] [Google Scholar]
- 15.Cotofana S, Schenck TL, Trevidic P, et al. Midface: clinical anatomy and regional approaches with injectable fillers. Plast Reconstr Surg. 2015;136(5 Suppl):219S-234S. [DOI] [PubMed] [Google Scholar]
- 16.Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125(2):699-708. [DOI] [PubMed] [Google Scholar]
- 17.Wollina U, Goldman A. Facial vascular danger zones for filler injections. Dermatol Ther. 2020;33(6):e14285. [DOI] [PubMed] [Google Scholar]
- 18.Alam M, Kakar R, Dover JS, et al. Rates of vascular occlusion associated with using needles vs cannulas for filler injection. JAMA Dermatol. 2021;157(2):174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beer KR. Safety and effectiveness of injection of calcium hydroxylapatite via blunt cannula compared to injection by needle for correction of nasolabial folds. J Cosmet Dermatol. 2014;13(4):288-296. [DOI] [PubMed] [Google Scholar]
- 20.Hufschmidt K, Bronsard N, Foissac R, et al. The infraorbital artery: clinical relevance in esthetic medicine and identification of danger zones of the midface. J Plast Reconstr Aesthet Surg. 2019;72(1):131-136. [DOI] [PubMed] [Google Scholar]
- 21.Alghoul M, Codner MA. Retaining ligaments of the face: review of anatomy and clinical applications. Aesthet Surg J. 2013;33(6):769-782. [DOI] [PubMed] [Google Scholar]
- 22.El-Amawy HS, Sarsik SM. Saline in dermatology: a literature review. J Cosmet Dermatol. 2020;20(7):2040-2051. [DOI] [PubMed] [Google Scholar]
- 23.Shiffman MA. Letter: Treatment of local, persistent cutaneous atrophy after corticosteroid injection with normal saline infiltration. Dermatol Surg. 2010;36(3):436. [DOI] [PubMed] [Google Scholar]
- 24.Li G, Xu D, Hu Z, Li H.. Embolic retinal and choroidal vascular occlusion after peribulbar triamcinolone injection: a case report. Medicine (Baltim). 2018;97(17):e0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edwards AO. Central retinal artery occlusion following forehead injection with a corticosteroid suspension. Pediatr Dermatol. 2008;25(4):460-461. [DOI] [PubMed] [Google Scholar]
- 26.Hosal BM, Zilelioglu G. Ocular complication of intralesional corticosteroid injection of a chalazion. Eur J Ophthalmol. 2003;13(9-10):798-799. [DOI] [PubMed] [Google Scholar]
- 27.Ho D, Jagdeo J, Waldorf HA. Is there a role for arnica and bromelain in prevention of post-procedure ecchymosis or edema? A systematic review of the literature. Dermatol Surg. 2016;42(4):445-463. [DOI] [PubMed] [Google Scholar]
- 28.De Boulle K, Glogau R, Kono T, et al. A review of the metabolism of 1,4-butanediol diglycidyl ether-crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39(12):1758-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugradar S, Hoenig J. Measurement of the force required by blunt-tipped microcannulas to perforate the facial artery. Ophthalmic Plast Reconstr Surg. 2019;35(5):441-446. [DOI] [PubMed] [Google Scholar]
- 30.Hilton S, Schrumpf H, Buhren BA, Bölke E, Gerber PA.. Hyaluronidase injection for the treatment of eyelid edema: a retrospective analysis of 20 patients. Eur J Med Res. 2014;19:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pradhan S, Madke B, Kabra P, Singh AL. Anti-inflammatory and immunomodulatory effects of antibiotics and their use in dermatology. Indian J Dermatol. 2016;61(5):469-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King M. Management of Tyndall effect. J Clin Aesthet Dermatol. 2016;9(11):E6-E8. [PMC free article] [PubMed] [Google Scholar]
- 33.Rootman DB, Lin JL. , Goldberg R. Does the Tyndall effect describe the blue hue periodically observed in subdermal hyaluronic acid gel placement? Ophthalmic Plast Reconstr Surg. 2014;30(6):524-527. [DOI] [PubMed] [Google Scholar]
- 34.Wiwanitkit V. Glomerular pore size corresponding to albumin molecular size, an explanation for underlying structural pathology leading to albuminuria at nanolevel. Ren Fail. 2006;28(1):101. [DOI] [PubMed] [Google Scholar]
- 35.Becker M, Balague N, Montet X, et al. Hyaluronic acid filler in HIV-associated facial lipoatrophy: evaluation of tissue distribution and morphology with MRI. Dermatology. 2015;230(4):367-374. [DOI] [PubMed] [Google Scholar]
- 36.Mundada P, Kohler R, Boudabbous S, et al. Injectable facial fillers: imaging features, complications, and diagnostic pitfalls at MRI and PET CT. Insights Imaging 2017;8(6):557-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Mahdi H. Iris depigmentation: an unusual complication of intralesional corticosteroid injection for capillary hemangioma. Middle East Afr J Ophthalmol 2010;17(1):100-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J, Chang M. Eyelid fat atrophy and depigmentation after an intralesional injection of triamcinolone acetonide to treat chalazion. J Craniofac Surg. 2017;28(3):e198-e199. [DOI] [PubMed] [Google Scholar]
- 39.Benzon HT, Chew T-L, McCarthy RJ, Benzon HA, Walega DR. Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology 2007;106(2):331-338. [DOI] [PubMed] [Google Scholar]