Abstract
Significance:
Nonhealing wounds are an ever-growing global pandemic, with mortality rates and management costs exceeding many common cancers. Although our understanding of the molecular and cellular factors driving wound healing continues to grow, standards for diagnosing and evaluating wounds remain largely subjective and experiential, whereas therapeutic strategies fail to consistently achieve closure and clinicians are challenged to deliver individualized care protocols. There is a need to apply precision medicine practices to wound care by developing evidence-based approaches, which are predictive, prescriptive, and personalized.
Recent Advances:
Recent developments in “advanced” wound diagnostics, namely biomarkers (proteases, acute phase reactants, volatile emissions, and more) and imaging systems (ultrasound, autofluorescence, spectral imaging, and optical coherence tomography), have begun to revolutionize our understanding of the molecular wound landscape and usher in a modern age of therapeutic strategies. Herein, biomarkers and imaging systems with the greatest evidence to support their potential clinical utility are reviewed.
Critical Issues:
Although many potential biomarkers have been identified and several imaging systems have been or are being developed, more high-quality randomized controlled trials are necessary to elucidate the currently questionable role that these tools are playing in altering healing dynamics or predicting wound closure within the clinical setting.
Future Directions:
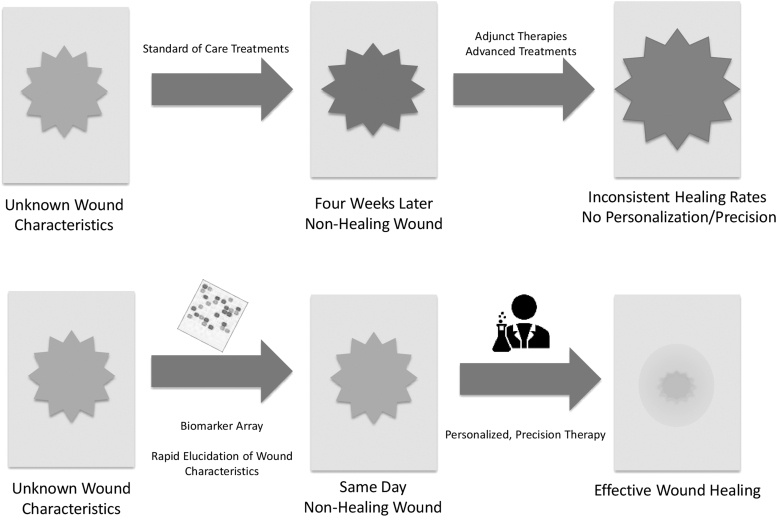
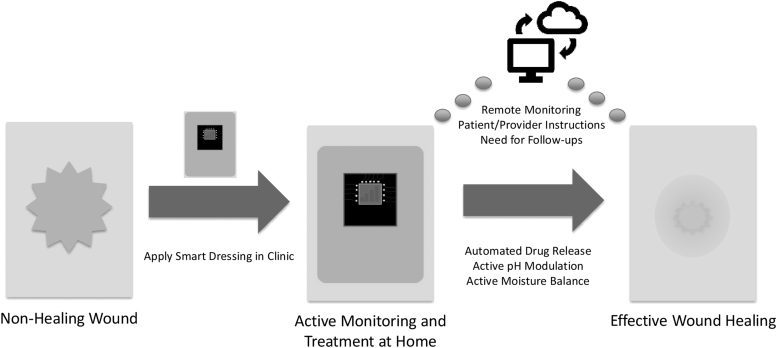
The literature supports the need for the development of effective point-of-care wound assessment tools, such as a platform diagnostic array that is capable of measuring multiple biomarkers at once. These, along with advances in telemedicine, synthetic biology, and “smart” wearables, will pave the way for the transformation of wound care into a precision medicine.
Clinical Trial Registration number: NCT03148977.
Keywords: wound healing, diagnostics, biomarkers, imaging, smart dressings, synthetic biology

Maximillian A. Weigelt, MD
SCOPE AND SIGNIFICANCE
Nonhealing wounds are a global pandemic, with management costs and mortality rates exceeding those of many common cancers. Existing standards for the diagnosis and management of nonhealing wounds remain subjective and experiential, whereas available treatments fail to consistently achieve wound closure. There is a great need for advanced wound diagnostics capable of elucidating the nebulous microenvironment of nonhealing wounds; such tools would transform wound healing into a precision medicine by allowing clinicians to deliver personalized therapeutic regimens. The most promising biomarkers and imaging modalities with the potential to achieve this vision are reviewed here.
TRANSLATIONAL RELEVANCE
Although many possible wound-healing biomarkers have been identified, their translation into simple, cost-effective clinical assays remains challenging. Many biomarkers of interest, such as genes and transcription factors, are not easily quantifiable at the point-of-care. This highlights the need for a diagnostic platform tool that can quantitatively and simultaneously measure many biomarkers, for example, proteases, other small molecules, genes, and beyond. In addition, imaging systems for wounds are promising but their utility remains uncertain. Continued research on the cellular and biochemical mechanisms underlying wound healing will be invaluable to coalesce the role of imaging and biomarkers in the future of wound healing.
CLINICAL RELEVANCE
Nonhealing wounds are challenging for clinicians, owing to the high degree of difficulty, cost, and time required to adequately assess wound status. Such wounds often fail to respond to established standards of care and advanced therapies. Currently, available advanced wound diagnostic tools may be challenging to implement in a clinical setting due to unclear utility or incomplete validation. By herein outlining the strengths and limitations of currently available biomarkers and imaging systems, the authors provide clinicians with guidance for their clinical implementation while also highlighting important areas for future clinical research.
DISCUSSION
Nonhealing wounds: a clinical conundrum and call to action
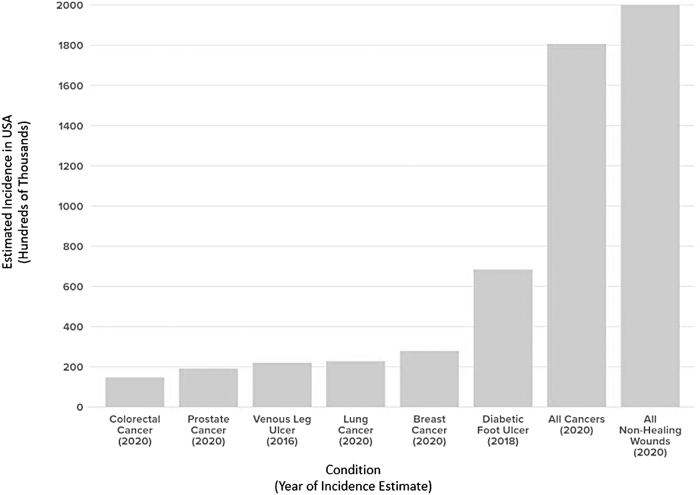
Nonhealing wounds represent an immense and ever-growing global pandemic, with incidence and mortality rates exceeding those of many common cancers (Fig. 1).1–10 Despite their staggering international cost burden (Table 1),4,11–13 the “silent epidemic” of nonhealing wounds has historically suffered from lack of public awareness and policy support due to being overshadowed by other medical comorbidities such as diabetes and obesity.1 Lack of formal wound care training in U.S. medical schools and the fact that no single medical specialty is responsible for wounds have also contributed to the underappreciation of nonhealing wounds as an international pandemic.4
Figure 1.
Estimated incidence of various subtypes of cancer versus nonhealing wounds in the United States. Some types of nonhealing wounds have higher incidence than some of the most common cancers, and nonhealing wounds overall are estimated to occur at a higher rate than cancer in general.2,5–10
Table 1.
Approximate annual cost of wound care by region
Despite the fact that modern advances in wound research have increased our understanding of the molecular and cellular factors driving impaired wound healing, very little has been successfully translated into the clinical setting.14 Standards for the diagnosis and stratification of wounds remain surprisingly subjective and experiential, sometimes lacking rigor or precision. Algorithms using clinical signs to detect wound characteristics, for example, “NERDS” for superficial infection (Nonhealing, Exudative, Red/bleeding surface, Debris [yellow/black necrotic tissue], Smell) and “STONEES” for deep infection (Size increase, Temperature increase, Os probe to/probe to bone, New or satellite areas of breakdown, Exudate/Erythema/Edema, Smell),15 have been developed; whether or not such tools are accurate predictors of the characteristics they attempt to measure remains controversial,16,17 and accordingly none have been widely adopted.
Available therapeutic strategies fail to consistently convert nonhealing wounds into those capable of closure, whereas practitioners remain unable to deliver individualized care.14 The U.S. Wound Registry (USWR) suggests that healing rates exceeding 40% are not currently possible for nonhealing wounds in real-world settings.18 Such phenomena are apparent across the continuum of wound care, whether in civilian settings or on the front lines of battle. It has been estimated that nearly 24% of modern combat deaths could be preventable with smart diagnostics for triage or to help guide the timing of wound closure.19
There remains a tremendous and ever-growing need to change subjective, qualitative, and often ineffective wound care into quantitative, evidence-based approaches that are predictive, prescriptive, and personalized. The establishment of objective global standards for wound assessment and the development of algorithms to determine which wounds will benefit from specific interventions will allow for the invention of individualized treatment strategies for those in need.14
Nonhealing wounds remain a significant management challenge owing to the high degree of difficulty, cost, and time required to adequately assess wound status, which is typically nonquantitative or merely experiential. Accurate appraisal of wounds requires years of specialized training and even so is limited by subjectivity and significant intra- and interobserver variability. Some indicators of wound status such as temperature, perfusion, and bacterial colonization are not readily appreciable to the naked eye. The slow delivery of lab reports and monitoring that requires multiple follow-up visits hinder the delivery of appropriate treatment.
Although there is no universally agreed-upon definition of a nonhealing wound, it is typically considered to be any wound which “fail[s] to proceed through an orderly and timely process to produce anatomic and functional integrity.”20 Wounds classified as recalcitrant, complex, chronic, hard-to-heal, or that result in amputation are included under the umbrella of nonhealing wounds.21 Nonhealing wounds as discussed within the scope of this article will include primarily venous leg ulcers (VLUs), diabetic foot ulcers (DFUs), and pressure ulcers (PUs). Other acute wounds, for example, combat wounds will also be considered. Note that although these wounds are commonly considered together within the category of nonhealing wounds, their pathophysiology is different and this may affect the diagnostic and therapeutic modalities that are best suited for each.
Nonhealing wounds suffer from a lack of consistent standards of care, with varying guidelines for classification, diagnosis, and management, and there are a few tools to aid practitioners with these tasks. There is no single objective system for wound evaluation or prognostic factors, and technical approaches vary from provider to provider.14 Nonhealing wounds often fail to respond appropriately to established standards of care, and there is a paucity of reliable metrics to predict this response.14 A multitude of advanced therapies are available (Table 2),22–29 but these also achieve inconsistent results and there are no algorithms to determine which patients will benefit from which interventions or to aid in the development of personalized, hierarchical treatment schedules for a given patient.14
Table 2.
Overview of current approaches to major chronic wound subtypes
| Wound Type | Healing Status Metric | Standard of Care | Select Advanced Treatments + Level of Evidence (or FDA Approval?) |
|---|---|---|---|
| Venous leg ulcers | 30% size reduction in 4 weeks5 | • Compression5 • Leg elevation5 |
• Oasis24 • Apligraf24 • Epifix24 • Dermagraft (FDA)26 |
| Diabetic foot ulcers | 50% size reduction in 4 weeks22 | • Offloading25 • Vascular optimization25 • Infection control25 |
• HBOTa • Becaplermin (FDA)29 • Oasis24 • Integra24 • Apligraf24 • Epifix24 • Grafix24 • Omnigraft (FDA)26 • Dermagraft (FDA)26 • EZ Derm (FDA)26 • Allopatch (FDA)26 |
| Pressure ulcers | PUSH Tool27: • Size • Exudate amount • Tissue type |
• Off-loading22 • Nutritional optimization22 • Reduce moisture, shear, friction22 |
• EZ Derm (FDA)26 |
Tools for evaluating wound healing status remain subjective and experiential, and often contended. Despite established standards of care, and the existence of many advanced treatments for most wound subtypes, clinicians remain unable to consistently achieve wound closure. FDA—Product is FDA-approved for the stated indication.
Evidence supporting the efficacy of HBOT in improving healing for diabetic foot ulcers remains conflicting. Alloderm—donated acellular human dermal allograft; Allopatch—donated acellular human dermal allograft; Apligraf—Bilayered living cellular construct; Dermagraft—cryopreserved cellular human fibroblast-derived dermal substitute; Epifix—dehydrated human amnion/chorion membrane; EZ Derm—porcine-derived dermal xenograft; Grafix—cryopreserved placental membrane; Oasis—porcine-derived acellular dermal matrix.
FDA, Food and Drug Administration; HBOT, hyperbaric oxygen therapy; PUSH, Pressure Ulcer Scale for Healing.
Food and Drug Administration (FDA)-approved treatments for nonhealing wounds have been underwhelming; for example, Becaplermin (recombinant human platelet-derived growth factor) was approved for the treatment of DFUs in 1997.30 Despite the FDA approval, Becaplermin's effectiveness has not translated well into the clinical setting, which, along with its high cost, has led to its use being limited.31 The same is true for other modalities such as hyperbaric oxygen therapy and the various novel cell- and tissue-based products (CTPs). This may be due to a lack of generalizability of many of the randomized-controlled trials (RCTs) that were carried out to get FDA approval for these products. Indeed, it has been suggested that many pivotal trials for CTPs were carried out on patients with less severe wounds than average and excluded many common and serious comorbidities shared by more than 50% of patients with nonhealing wounds.32
The global need to transform wound care into a precision medicine is clear and has been answered by worldwide efforts to bring together outstanding practitioners and experts in wound healing (e.g., Symposium on Advanced Wound Care, Wound Healing Society, Alliance of Wound Care Stakeholders, U.S. Wound Registry, Diabetic Foot Consortium). Biomarkers for wound healing hold promise for the standardization of wound diagnosis, monitoring, and treatment by providing a “molecular barcode” for wounds.3,14,33,34 The continued development of imaging systems and other sensing devices (e.g., smart bandages or “wearables”) to provide real-time measurement of various wound milieu characteristics has been major advances toward the development of smart point-of-care tools for the clinician's arsenal in the war on wounds.14 There is a need for a platform diagnostic that can monitor biomarkers associated with healing. Such a platform would revolutionize the diagnosis of nonhealing wounds, catapult our understanding of the molecular and cellular mechanisms behind impaired wound healing to new heights, and usher in a modern age of therapeutic strategies for wound care.
This article will review the advanced wound diagnostic modalities and burgeoning technologies (biomarkers and analysis of such with advanced computational models, and sensing devices such as imaging systems and electronic noses) with the most robust evidence to support their potential to transform the wound care landscape; their suggested roles, underlying physiology, and possible therapeutic strategies to target them.
Biomarkers and the wound microenvironment: molecular barcoding of wounds
Whether healing appropriately, infected, or otherwise complicated, the wound microenvironment is characterized by a lush landscape of biomarkers with functional, and potentially diagnostic and therapeutic, significance. Biomarkers are quantitatively measurable substances that signal the presence of an underlying physiologic or pathologic process, or they allow for the determination of treatment response.3 Processes of interest for wounds include stage of healing, bacterial colonization/infection, degree and character of inflammation, fibroblast senescence, and keratinocyte activation, to name a few.3,20,30
Biomarkers for wound healing continue to garner increasing attention within the wound care community, as the prevalence of Pubmed articles related to “wound biomarkers” has increased by 558% from the years 2000 to 2017. Modern high-throughput “omics” approaches (e.g., genomics, proteomics, lipidomics, metabolomics) have allowed for an increasingly detailed evaluation of the wound-healing environment at a molecular level.3,33 Analysis of human wound biomaterials has led to the identification of hundreds of compounds with potential diagnostic, prognostic, or indicative value.3,33,35–37 The potential benefits of biomarkers for wound healing include the development of novel and personalized treatment modalities, earlier identification of wounds requiring intervention, and their use as measurable endpoints or inclusion/exclusion criteria for clinical trials.3,33 We suggest that each wound has a unique biochemical footprint, and that each patient would benefit from receiving treatment tailored to their wound's individual genetic, proteomic, and metabolomic makeup—this is the same principle on which today's advanced cancer treatments are based.30
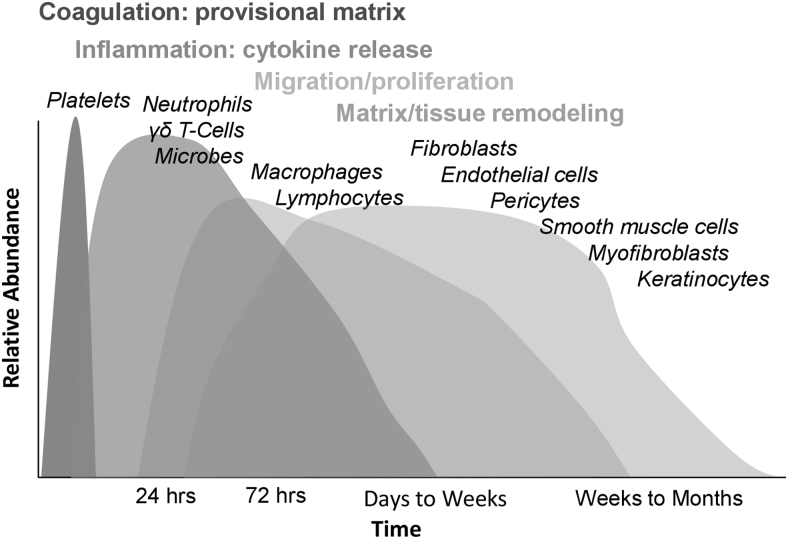
The cellular and molecular mechanisms underlying tissue repair and impaired wound healing remain incompletely understood.38 Normal acute wound healing progresses through four distinct yet overlapping phases requiring intricate conversation between cellular constituents and extracellular matrix (ECM) components: hemostasis, inflammation, proliferation, and maturation (Fig. 2).38 A derangement in any of these phases or the processes that comprise them can result in impaired wound healing (Fig. 3).38 This may be further exacerbated by systemic factors such as age, obesity, diabetes, smoking, nutritional status, immune status, and others.38,39
Figure 2.
Acute wound-healing responses—phases and cellular effectors. Normal wound healing proceeds through multiple distinct yet overlapping phases: coagulation, inflammation, migration/proliferation, and remodeling.38
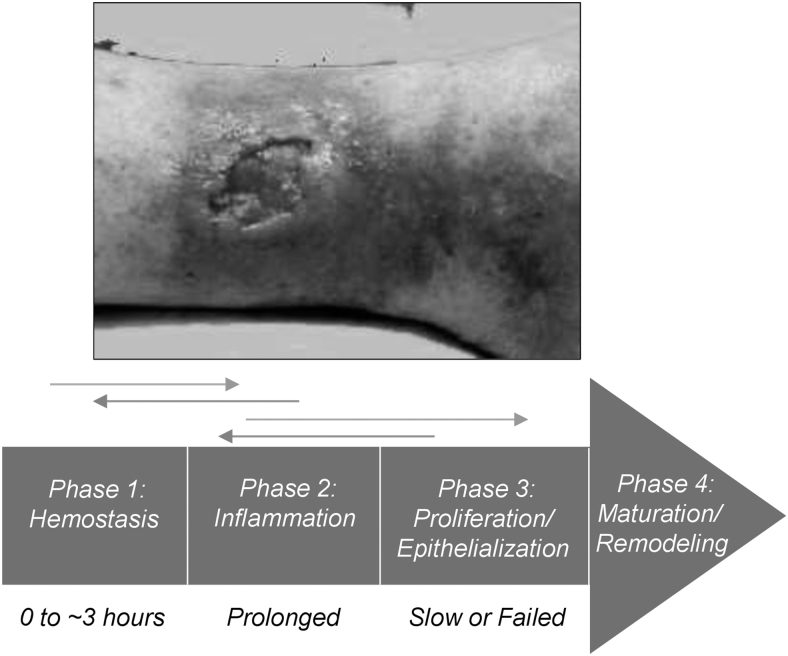
Figure 3.
Nonsequential progression toward wound closure; stalled, chronically inflamed wound bed in a venous ulcer. A derangement in any of the normal phases of wound healing can result in a nonhealing wound. Nonhealing wounds are frequently characterized by prolonged, deleterious inflammation, dysfunctional proliferation, and/or failed epithelialization.38
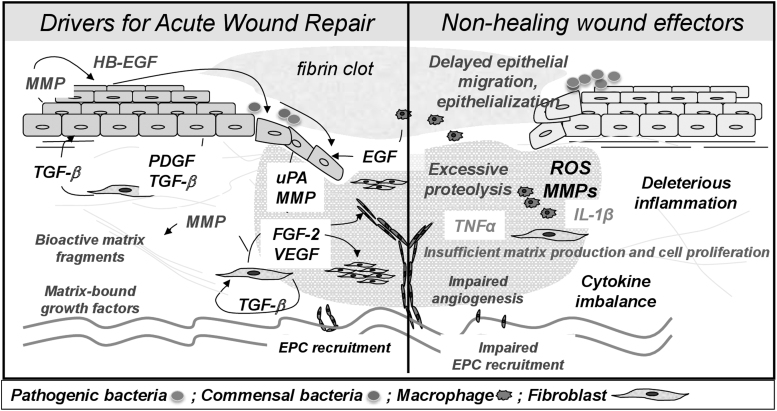
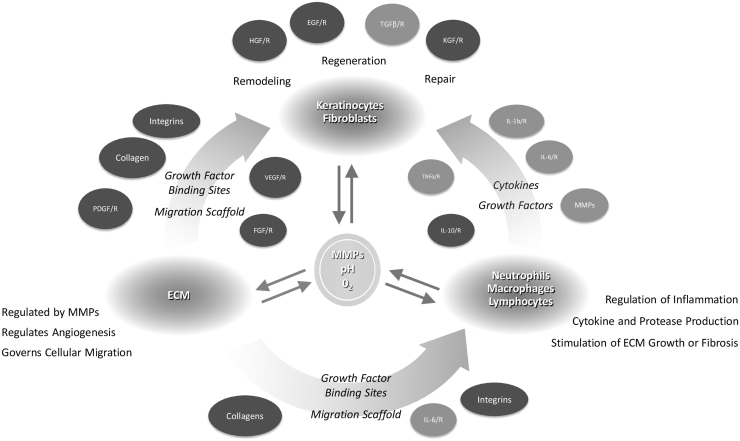
Inflammation is a key player in the pathogenesis of wounds—the inflammatory profiles of acute and nonhealing wounds are markedly different, and nonhealing wounds exhibit markedly altered genomic and transcriptomic profiles, especially at the wound edge (Fig. 4).14,40–47 In regular wound healing, pro-inflammatory cytokine release by the clot and surrounding wound tissue leads to the sequential infiltration of neutrophils, macrophages, and lymphocytes.39 Under normal conditions, this inflammation is a self-limiting process; on the other hand, nonhealing wounds are characterized by a persistent inflammation that is less effective to support progression of healing.40 For example, neutrophils are typically absent by 72 h in acute wounds, but persist in nonhealing wounds,40 whereas recent observations show overall deregulated recruitment of inflammatory cells in DFUs.45 Accordingly, nonhealing wounds are characterized by a persistence of both pro-inflammatory mediators, such as tumor necrosis factor alpha and interleukin one beta (IL-1β),38,48–50 and anti-inflammatory signals48–50; such dysregulation and mixed signals that lack spatiotemporal control result in ineffective inflammation. In addition, these factors lead to elevations in matrix metalloproteases (MMPs), which degrade ECM and growth factors, thus inhibiting cell migration, angiogenesis, collagen synthesis, mitogenic activity, and other processes that are instrumental for repair.38,40 A break in the skin also allows bacteria to access the underlying tissue, which further increases inflammation and MMP levels via endotoxin release and host production of antimicrobial factors.39 Other factors known to play a role in impaired wound healing include depletion of local stem cell populations and increases in cellular senescence.40,51,52
Figure 4.
The chronic wound microenvironment. Chronic wounds are characterized by a prolonged, deleterious inflammatory state. Imbalance in pro-inflammatory cytokines, excessive proteolysis by MMPs, and various other cellular derangements prevent the timely and orderly restoration of the skin barrier. ECM, extracellular matrix; EGF, epidermal growth factor; EPC, endothelial progenitor cell; FGF, fibroblast growth factor; IL-1β, interleukin one beta; MMPs, matrix metalloproteases; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; TNFα, tumor necrosis factor alpha; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor.14,41–43
Thus, the wound-healing system comprised many highly organized layers of scale, with a delicate interplay between the various cell types, intercellular messengers, synthetic products, and enzymes.40 This interplay may broadly be affected by variations in immune response, metabolism, neuroendocrine signaling, oxygenation, pH, and other factors, all of which are linked to complex changes in genome expression.39,40 In nonhealing wounds, the cellular processes necessary for repair are inhibited in ways that are likely unique to each wound for each given patient. This explains, at least in part, why available treatment modalities for nonhealing wounds are unable to achieve a consistent response for a particular wound type and underscores challenges in standardizing patients for clinical trials.14
Despite the fact that many possible biomarkers for wound healing have been identified, their translation into simple, cost-effective, and predictive clinical assays remains challenging.3,35 No biomarkers have yet been widely adopted for diagnostic or monitoring purposes. Recently, National Institutes of Health (NIH), under the leadership of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), formed Diabetic Foot Consortium, a research network that aims at testing and validating multiple predictive biomarkers (tissue based or transepidermal water loss measurements) that can predict either the clinical outcomes or recurrence of previously healed chronic wounds. The establishment of standardized molecular analyses for determining wound healing status will allow for more effective identification and stratification of nonhealing wounds based on their predicted response to various treatment modalities, and it will also aid in the quest to uncover novel therapeutic strategies for individualized wound care.
Many potential biomarkers that have been identified are genes, transcription factors, micro-RNAs, and other molecules requiring either cumbersome techniques (e.g., polymerase chain reaction [PCR], immunohistochemistry) to evaluate or need to undergo wide clinical validation, which requires time and resources. As such, we have chosen to focus on the few biomarkers with the most robust evidence to support their ability to modulate and/or provide insight into the wound microenvironment, and to help transform wound care into a personalized, predictive, and precision medicine.
Proteases
Proteases are the currently the best-studied biomarkers. Elevated protease activity (EPA) in nonhealing wounds has been known for some time to be associated with impaired wound healing.53,54 MMPs, human neutrophil elastase (HNE), and cathepsin G (CG) are the best studied.3,55 MMPs are a family of zinc-dependent endopeptidases with critical importance for normal physiological wound healing.3 By modulating the release of cytokines, growth factors, and other biologically active molecules, MMPs regulate key wound-healing processes, including cell–cell and cell–matrix interactions, cell migration and death, ECM remodeling, and angiogenesis.54,56–58
Dysregulation of MMPs and their inhibitors (tissue inhibitors of metalloproteases [TIMPs]) is a significant contributor to the failure of wound healing; in chronic wounds, MMP expression is altered at the mRNA and protein levels.58,59 Deleteriously elevated levels of MMPs in chronic wounds result in unintended cleavage of essential growth factors and cytokines, tipping the balance of ECM turnover to favor net destruction.58 This increased proteolytic activity is chiefly due to the significant influx of inflammatory cells (i.e., neutrophils and macrophages) as well as modulation by invading bacteria—for example, Pseudomonas aeruginosa secretes thermolysin, which activates MMPs 1, 8 and 9.58
More than 24 MMPs have been identified with diverse functions.58 Factors affecting the activity of MMPs are extremely complex; they can exert opposite effects on the same biological process by virtue of nuances in the wound microenvironment.56 Overexpression of various MMPs, most significantly MMP-2 and MMP-9, and increased MMP/TIMP ratios have been demonstrated in wound fluid, wound tissue, and serum of patients with nonhealing wounds3,54,59,60 Shifting pH down to six reduces the activity of most chronic wound-associated MMPs by 40–90%, which is part of the rationale linking wound acidification to improved healing.61 Wound fluid MMP expression profile varies depending on the stage of healing as well as the age of the wound.54 MMP expression may also be useful in assessing the infection status of a wound; for example, as previously stated, P. aeruginosa is known to activate MMPs 1, 2, 8, and 9.58,62 On the other hand, a case-control study of 129 patients with VLUs found that infected ulcers demonstrated high levels of MMPs 1 and 8, whereas noninfected ulcers were MMP-2 and -9 predominant.63 This highlights the complexity inherent in the relationship between MMPs, wound healing trajectory, and infection status, as well as the limited clinical utility of a single measurement of protease activity.
Further research is needed to develop the utility of protease levels as an effective biomarker for nonhealing wounds. MMP overexpression varies by wound etiology and within subgroups of similar wounds due to variations between patients, making the establishment of global threshold values a challenging prospect.64 Various studies have been carried out to this end, all resulting in different suggested cut-off values to indicate impaired healing in populations with DFUs or wounds of varied aetiologies.53,59,60,64
For example, MMP-9 levels >0.38 pg/ug predicted identified nonhealing DFUs with sensitivity of 81.8% and specificity of 64.6%.59 Another team determined that wound fluid total MMP levels greater than 962.2 pg/mL accurately discriminated between healing and nonhealing VLUs with sensitivity of 92% and specificity of 61%.60 Finally, a study of 290 wounds of varied etiologies suggested values of 5.5 mU/μL for HNE (sensitivity 67%, specificity 86%) and 13 mU/μL for total MMP (sensitivity 81%, specificity 71%) to distinguish healing status.53 Widely accepted, quantitative cut-off values that allow for the consistent determination of wound healing status have yet to be discovered.53
WoundChek Protease (WCP; WoundChek™ Laboratories)is a qualitative, immunochromatographic test for the assessment of EPA in wounds currently available for purchase in the United Kingdom.65 The WCP system measures the combined activity of proteases, primarily MMP-8, MMP-9, and HNE, and provides a binary measure of protease activity—elevated (EPA) versus nonelevated (NEPA).66 This assay was found to have a sensitivity and specificity of 28% and 90%, respectively, for identifying nonhealing wounds.64,67 Limiting factors of this study included small sample size and retrospectivity.
WCP may also be a useful tool to study how EPA affects wound healing. One study used WCP to assess EPA in 35 patients with DFUs who underwent dermal grafting with Integra® or Hyalomatrix®. Graft integration rate at 1 month was found to be significantly lower in the group with EPA compared with the group without it; every patient in whom the graft failed was found to have EPA before graft application, whereas 36% of patients with EPA experienced successful grafting.66 Although the sample size was small, this study highlights the potential usefulness of EPA in predicting clinical response to treatment. Further research could shed light on whether graft success can be improved with protease-modulating therapies. Indeed, a clinical trial is ongoing to determine the utility of a quantitative, point-of-care MMP assay to predict graft success in patients undergoing cutaneous autografting for acute burn injury.
Some therapies with protease modulation capabilities exist, and others continue to be developed. Doxycycline is a broad-spectrum MMP inhibitor (MMPI) with well-characterized anti-inflammatory properties, and it has been observed to reduce protease activity in DFUs.68 A recent study found microneedling to be effective in achieving intradermal delivery of doxycycline in an in vitro human skin model, with a resulting decrease in MMP activity.69 Novel collagen-based dressings (e.g., oxidized regenerated cellulose/collagen; Promogran®) have the ability to absorb, retain and inactivate inflammatory proteases, which has been demonstrated both in vitro and in human chronic wounds.70 The potency of these effects is related to the amount of intact collagen fiber in the dressing.70 The mechanism of protease modulation by collagen dressings may be due to acting as a competitive inhibitor or “sacrificial substrate” for aberrantly expressed proteases.70 Negative-pressure wound therapy (NPWT) may also be an effective treatment for wounds with elevated proteases, as chronic wounds treated with NPWT exhibit lower levels of pro-MMP-9 and decreased MMP-9/TIMP-1 ratios.71
Significant efforts have been made to develop pharmaceutical MMPIs for applications, such as wound healing and cancer.72 No broad-range MMPIs were successful in cancer clinical trials due to the fact that MMPs as a broad group exert opposing effects on various pathophysiological processes, for example, tumorigenesis.72 To our knowledge, MMPIs have not been tested in wound-healing trials. Increasingly high-resolution elucidation of MMP structure based on modern protein engineering techniques has spurred renewed efforts to design MMPIs.72 Several new protein-based inhibitors, customized TIMPs, and anti-MMP antibodies are currently being tested.72
pH
pH may be the overarching taskmaster of the wound microenvironment and the biomarkers therein. Skin is acidic, with normal pH ranging from 4 to 6.61 On wounding, a temporary physiologic acidosis occurs due to local production of organic acids and increased pCO2 due to stasis of tissue perfusion.61 In unimpeded acute wound healing, wound pH progresses from neutral to acidic as epithelialization occurs.73 Alkaline acute wounds have been noted to heal more slowly than those with neutral pH.73,74 Similarly, chronic wounds are characterized by a persistent alkaline wound environment.73,74 Further, most pathogenic bacteria require a pH above 6, and bacterial growth is inhibited at values lower than this.61
Thus, it is generally accepted that wound healing occurs best at low pH.73 The pH of chronic wounds has been observed to be generally between 7.15 and 8.9, with values as high as 9.25 being reported.74 Studies of VLUs have suggested pH ranges of 7.3–8.9 or 7.5–7.9, with decreases in wound surface pH occurring concurrently with healing and re-epithelialization.61,75 One group found that the pH of PUs increases with worsening ulcer grade, with average pH values of 5.7, 6.9, and 7.6 for stage I, II, and III PUs, respectively.76
A plethora of cellular processes depend on pH, such as cell cycle progression and enzymatic activity.73 Figure 5 demonstrates the dynamic relationship between pH, proteases, and wound-healing physiology.77,78 MMP-2, neutrophil elastase, and CG have highest activity at pH 7–8, which fits in with our understanding of the increased protease activity seen in chronic wounds.74,79 Acidification of wounds promotes proliferation of fibroblasts, keratinocytes, and blood vessels and increases oxygen dissociation from hemoglobin in tissues.74,80 Low pH increases the antimicrobial activity of silver dressings, and hypochlorite kills bacteria twice as quickly at pH 6 compared with pH 8.74,81,82
Figure 5.
Relationship of biomarkers to physiologic wound healing. Overview of the role of pH and MMPs within the complex wound microenvironment. Some, but not all, important wound-healing cytokines and substrates are shown—those in orange, although necessary for physiologic healing, are commonly overexpressed in nonhealing wounds. MMPs and pH interplay intricately within the wound microenvironment in a phenomenon known as “dynamic reciprocity.” pH affects ECM synthesis, enzyme activity, cell cycle progression, oxygen delivery, fibroblast activity, and bacterial growth. The effects of MMPs are also manifold, as they are crucial for ECM remodeling and angiogenesis, cell migration, and cytokine regulation, though their overexpression results in poor wound healing. FGFR, fibroblast growth factor; HGF, hepatocyte growth factor; IL-X, interleukin X; KGF, keratinocyte growth factor; /R, receptor.77,78
Existing therapies can acidify the environment of wounds: for example, manuka honey,83 occlusive dressings (due to retention of acidic wound exudate),61 and even a pH-switchable antimicrobial nanofiber dressing.84 These dressings have other properties that improve wound healing and there are not yet any good randomized controlled trials to suggest that acidifying therapies are superior to the rest of the field, though they are commonly employed by wound care practitioners.
The role of measuring pH directly in chronic wounds remains unclear. Dissemond and team measured wound fluid pH in 39 patients with various wound types for 12 months and found that pH can vary by up to 1.73 over time in a given patient.85 Although pH increases with bacterial colonization, this further complicates interpretation since acidic or basic pH could be considered normal based on the infection status and healing stage of the wound. Thus, pH measurements would be best considered not in a vacuum but rather in concert with clinical signs and other biomarkers.
The understanding of wound fluid pH has until recently been limited by measurement techniques. Litmus paper has low resolution and is subject to inaccurate interpretation by the measurer.74 Glass pH electrodes have issues with sterility and discomfort for the patient, and both litmus paper and glass electrodes require the wound dressing to be removed.74 The recent invention of smart dressings with continuous pH-monitoring capabilities opens the door to further characterizing pH as a biomarker of interest.86 The ability to observe granular pH trends over time will shed light on the potential ability of pH, either alone or in tandem with other markers, to predict or detect infection, or to determine whether the wound is responding appropriately to treatment.
In addition to standing on its own as a potential biomarker, pH modulates and interacts with other biomarkers that exert effects on the wound microenvironment, such as nitric oxide (NO) and uric acid (UA). NO plays a key role in various processes that are necessary for normal tissue repair, including angiogenesis, granulation tissue formation, keratinocyte migration, collagen production, and the activation and upregulation of growth factors.87 The utility of wound fluid NO as a biomarker for human wounds remains unclear, with the few existing studies being limited by small sample sizes and cumbersome methods of detection such as spectrophotometry.87 Nonetheless, wound fluid NO measurements were found to discriminate between healing and nonhealing wounds of various etiologies with an area under the curve (AUC) of 0.81.87 The development of more elegant, high-fidelity NO measurement techniques (e.g., field effect transistors)88 may aid in future research and is ongoing.88
UA is another biomarker whose production is linked to pH. The hypoxic environment of VLUs results in the depletion of ATP and an accumulation of purine metabolites such as adenosine and inosine, which are then broken down into UA in a process that occurs more abundantly at alkaline pH.89 UA is believed to inhibit healing by directly promoting inflammation,90 whereas its synthesis contributes to tissue damage via production of damaging superoxide radicals.89 Several tools have been developed for point-of-care monitoring of UA in wound fluid, namely: paper-based smart bandages,91,92 inkjet-printed carbon nanotubes,93 gauze with embroidered electrochemical sensors,94 and a wearable enzymatic biosensor.95 However, it remains unclear as to how the elucidation of UA levels will guide management decisions for nonhealing wounds. Further research could make use of novel UA biosensing technologies to further characterize the relationship between UA, wound-healing outcomes, and response to treatment.
Biomarkers for infection detection
The prevention and identification of wound infections remains a clinical conundrum.34 Cardinal signs of infection (increased pain, erythema, edema, heat, and purulent exudate) may be diminished by physiological phenomena commonly seen in patients with chronic wounds, namely: neuropathy, vasculopathy, venous disease, and impaired leukocyte function secondary to diabetes.34 Clinical signs are still seen as controversial in diagnosing wound infection,34 having consistently exhibited poor correlation with the current gold standard of microbiological tissue culture.16,96 For example, in the case of DFUs, it is generally agreed upon that infection should be diagnosed based upon clinical signs as set forth by the Infectious Diseases Society of America (IDSA) (purulent exudate OR two of warmth, tenderness, pain, induration, or erythema being diagnostic of infection).97,98
However, research suggests that IDSA signs alone or together are not significant predictors of wound infection, and in fact performed no better than chance in identifying tissue-culture-positive infections in a cohort of 64 patients with DFUs.99 It may be that so-called “secondary” signs of infection, unique to wound healing by secondary intention (serous exudate + inflammation, delayed healing, discoloration, friable granulation tissue, foul odor, and wound breakdown), are more effective at identifying infection than the classic signs.96 Superficial wound swabbing is an alternative that suffers from lack of technique standardization and poor efficacy; a systematic review of eight randomized controlled trials found superficial swabs to be only 49% sensitive and 62% specific when compared with deep tissue cultures.100
Although tissue culture is considered the gold standard for wound infection detection, it is far from a holy grail. It is invasive, requires anesthesia, and is often foregone over concerns about harming the patient or worsening the wound bed.16,34 It may be contraindicated in certain cases, such as severe peripheral arterial disease.16 Qualitative cultures, although inexpensive, have low sensitivity and require 1–3 days turnaround for results.101 Quantitative cultures, although more accurate, are expensive, labor intensive and require specialized facilities.101 These delays allow the bacterial burden to change character by the time results are received.102 In addition, false negative results are often seen in patients already taking antibiotics, a situation commonly encountered in clinical practice.98
Most importantly, recent advances in microbiological metagenomics have changed the way we look at the wound microbiome and revealed significant limitations in conventional culture techniques. The advent of next-generation sequencing techniques, such as 16S RNA pyrosequencing, allows for the identification of the complete bacterial genome of a wound.101 In doing so, we have realized that the chronic wound microbiome is much more complex than was previously appreciated101; indeed, less than 2% of known bacterial species can routinely grown in culture, most notably anaerobes that are known to be present in most chronic wounds.103 Metagenomic sequencing may be difficult to interpret, as it also identifies dead or commensal organisms that might be a source of confusion.102 In addition, these techniques are highly expensive, require specialized facilities and personnel, and are largely used thus far in research settings, placing them far from regular bedside application.101
Further, in recent years, it has become increasingly recognized that most bacteria are found in wound biofilms.104 Biofilms are three-dimensional (3D) collections of bacteria composed of 80–85% extracellular polymer and 15–20% organism, by mass.104 These biofilms protect bacteria from antimicrobials and the host immune response.98 They inhibit wound healing by creating a physical barrier to re-epithelialization and opsonization, and they promote a chronic inflammatory environment through the constant release of waste products.105
It has been observed that biofilms complicate 60% of chronic wounds, compared with only 6% of acute wounds.106 Repeating patterns of co-aggregated species termed “functional equivalent pathogroups” have been observed to synergistically form pathological biofilms, further highlighting the importance considering the entire wound microenvironment when characterizing the nature of infections.104 Biofilms can develop in chronic wounds as quickly as within 10 h, and they tend to recur even 2 days after being removed by surgical debridement.104 They are invisible to the naked eye, detectable only by scanning electron microscopy. Hence the importance of developing strategies to easily identify and treat biofilms cannot be overstated, and many wound care products have been developed to target them (Table 3).107–130
Table 3.
Anti-biofilm agents for wound care
| Product | Mechanism of Anti-Biofilm Action | Anti-biofilm Evidence | Healing Outcomes Evidence |
|---|---|---|---|
| Polyhexanide (PHMB) | Disruption and increased permeability of bacterial cell membranes | Level VI103 | Level IV104 |
| Poloxamer-based surfactants (Plurogel®; Medline Industries, Inc., Northfield, IL) | Inhibition of bacterial surface adherence Reduces cohesion of constituent biofilm molecules |
Level VI105 | Level IV106 Level IV107 |
| Benzalkonium chloride-based surfactant (BlastX™; Next Science, St. Paul, MN) | Dissolves the extracellular polysaccharide matrix, exposing encapsulated bacteria for removal Osmotic lysis of cell wall |
Level VI108 | Level I109 Level I110 |
| Cadexomer iodine (IODOSORB™; Smith and Nephew, London, United Kingdom) | Directly destroys biofilms Collapses bacterial glycocalyx Traps bacteria within beads. |
Level VI111 Level VI112 Level IV113 |
Level I114 |
| Honey | High osmolarity Low pH Peroxide produced by breakdown of glucose |
Level VI115 | Level I116 |
| Hypochlorous acid | Chemical inactivation of various cellular processes, including amino acid modification and protein synthesis | Level VI117 | Low-to-strong based on wound type118 |
| Lasers and phototherapy | Induction of oxidative stress Impaired polysaccharide production |
Level VI119 | None |
| Low-frequency ultrasound | Microstreaming and cavitational effects | Level VI120 | Varies121 |
| Electroceuticals | Disruption of electrostatic adhesion forces Superoxide production Bacterial membrane enzyme disruption |
Level VI122 Level VI123 Level VI124 |
Level VI124 Level III125 |
A list of available anti-biofilm wound care products currently available on the market, their mechanisms of action, and levels of evidence to support their ability to destroy biofilms and improve wound-healing outcomes. Adapted with permission from Weigelt et al.107
PHMB, polyhexamethylene biguanide.
To further complicate matters, recent research has demonstrated that bacteria that classically occupy the extracellular space (such as Staphylococcus aureus) can hide and persist intracellularly in skin cells, including within professional (macrophages, dendritic cells, B cells) and nonprofessional (keratinocytes, fibroblasts, endothelial cells) antigen-presenting cells, thus contributing to the recalcitrant nature of skin wounds.131,132 In the case of S. aureus, this is achieved by phenotype switching of the bacteria into a “small-colony-variant” (SCV) that exhibits slower growth and metabolism, downregulated virulence genes, low cytotoxicity, and increased antibiotic resistance.132 By virtue of these traits, SCV bacteria can evade the host immune response and may remain dormant intracellularly for years; this may be why wound infections are sometimes observed to re-appear after apparently successful antimicrobial treatment.132 The SCV phenotype is upregulated by the acidic environment of phagolysosomes, can hijack them, and can revert into the classical or “aggressive” cytotoxic phenotype on exposure to neutral pH. Targeting intracellular pathogens is difficult, as conventional antibiotics tend to remain in the extracellular space, or do not remain long enough or maintain high enough concentrations intracellularly for effective bacterial killing.132
With this in mind, efforts are ongoing to imagine novel therapeutic strategies for these stealthy pathogens, including: lysosomal alkalizing agents (e.g., chloroquine combined with other antibiotics)132; peptide antibiotics that can cross eukaryotic cell membranes132; thymol-loaded wound dressings133; and hyaluronan-based nanogels,134 to name a few. Further, the critical role of Perforin-2, a membrane-bound protein present in endosomal vesicles, in cells' innate immune response against intracellular pathogens has recently come to light.131 This represents a valuable weapon in the battle against intracellular bacteria as efforts are ongoing to leverage activation of this protein to fight persistent wound infections.
Overall, the development of novel biomarkers for the assessment of wound infection status would aid in untangling the complex web of the wound microbiome. It is clear that three facets of wound microbiota should be considered: total bacterial load, diversity, and the presence of biofilms.3 Recent research on acute phase reactants, neutrophilic enzymes, and volatile organic compounds (VOCs) may improve our ability to robustly characterize the microbial status of wounds.
Acute phase reactants
Inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and procalcitonin may aid in the diagnosis of infection in nonhealing wounds. These biomarkers have been studied predominantly in DFUs, with conflicting results. The utility of inflammatory markers in differentiating infected DFU (IDFU) from noninfected DFU (NIDFU) remains unclear. Some authors found CRP to be significantly elevated in IDFUs, with varying sensitivity and specificity,135–137 whereas others found no association.138 Similar results have been described for ESR138–141 and procalcitonin.135–140 In general, it is agreed that the measurement of multiple markers provides the most utility135,136,138–140; for example, a composite algorithm using CRP and procalcitonin was developed with an AUC of 0.947 to identify IDFUs.135
There are limitations of these studies that make interpretation of the results challenging. Some studies looked at hospitalized patients whereas some took place in an outpatient setting. Pretest probabilities of infection are higher in hospitalized patients, which upwardly skews the positive-predictive value of a test. Further, some authors included patients with osteomyelitis and other high-grade IDFUs whereas others looked only at mildly infected (IDSA grade II) versus uninfected (IDSA grade I). This is relevant as it affects the levels of inflammatory markers. For example, procalcitonin is higher in higher-grade IDFUs139 and has been observed to increase very little in localized infections without systemic manifestations.135 CRP, on the other hand, has been shown to increase significantly in response to local infection.135 We suggest that the most utility would be gained by studying mildly IDFU versus NIDFU in the outpatient setting, as did Ingram et al., since these patients frequently pose the most significant clinical challenge when considering whether or not to prescribe antibiotics.136
Most notably, the use of clinical grading as the comparator in these studies is a significant limitation due to its uncertain validity. The IDSA recommends that the diagnosis of infected DFUs be based primarily on clinical assessment, namely: the presence of either purulent secretion or two of warmth, tenderness, pain, induration, or erythema.97 However, clinical signs of infection are often absent in patients with diabetes.138 Future research should use tissue biopsy as a reference standard, although as previously mentioned microbiological results can also be misleading. This highlights the need for highly sensitive tools to identify infection in patients lacking clinical signs. Inflammatory markers do not currently offer this ideal, objective information; as such, they are best interpreted by experienced providers in the context of both clinical signs and microbiological analysis.
Despite this, CRP may be an effective tool to monitor treatment response in chronic wounds as has previously been demonstrated with CRP and ESR in osteomyelitis.142 Goodfield found that hospitalized VLU patients with elevated CRP demonstrated improved wound-healing outcomes and an according decrease in CRP when treated with antibiotics, whereas patients without elevations in CRP did not respond to antibiotic therapy.143 Further, Liu et al. found decreasing wound fluid CRP levels in correlation to positive wound outcomes in a cohort of 20 patients with chronic trauma-related wounds who received treatment with debridement, antibiotics, and NPWT.144 Efforts are ongoing to develop devices that are capable of high-resolution measurement of CRP in wound fluid,80 which stands to further characterize the utility of CRP as a wound-healing biomarker.
Myeloperoxidase and lysozyme
Myeloperoxidase (MPO) and lysozyme (LYS) are enzymes secreted by host neutrophils in response to infection.145 Spectrophotometric analysis of MPO and LYS activities in wound fluid has revealed them to be significantly elevated in clinically infected wounds of various etiologies when compared with noninfected wounds.146–148 These studies also served as proof-of-viability for various enzyme detection systems: chitosan-148 and peptidoglycan146-based colorimetric assays for LYS and a similar concept for MPO.147 Schiffer et al. developed a test strip with covalently immobilized MPO substrate, which demonstrated a significant change in color when exposed to fluid from clinically infected wounds, as compared with noninfected wound fluid.149 These studies were all limited by their use of clinical judgment as the comparator for determination of infection.
Others have studied the correlation between wound fluid MPO/LYS and quantitative superficial wound culture. Blockhuis et al. developed a 30-min colorimetric assay to measure the activity of four enzymes in wound fluid (HNE, CatG, MPO, and LYS) and compared the results with clinical judgment and quantitative superficial wound culture.145 They found that three models of interpretation for their test were superior to clinical judgement in predicting infection as diagnosed by wound culture (AUC ranging from 0.67 to 0.74).145 Although the model requiring only one positive enzyme was the most sensitive, models involving multiple enzymes were more specific, highlighting the importance of measuring multiple enzymes.
Schiffer et al. measured HNE, MPO, and LYS levels spectrophotometrically and colorimetrically, and they compared them with clinical judgment and quantitative culture results.150 Measured HNE and LYS levels were significantly elevated in wounds determined to be infected or critical by microbiologic analysis, whereas MPO failed to reach statistical significance on its own.150 The authors indicate that combined assessment of all three enzyme activities by both color changes and spectrophotometric analysis could have successfully identified 85% of culture-positive samples that were judged to be uninfected clinically.150
A screen-printed amperomatric sensor for fast detection of MPO in wound fluid has been developed by Hajnsek and team.151 They found a significant difference in MPO activity in infected and critical wounds compared with uninfected wounds based on quantitative wound fluid culture.151 Such technology could set the stage for continued research by allowing immediate high-resolution measurement of enzyme levels in wound fluid.
Although MPO and LYS have some promise as biomarkers for wound infection, existing research has been limited by the use of clinical judgment and superficial swab/fluid cultures as standards of comparison. Future studies should use the gold standard deep tissue culture as the comparator.
Volatile organic compounds
VOC is a generic term referring to a wide range of molecules with a boiling point less than 300°C, for example, alcohols, aldehydes, ketones, isocyanates, sulfides, and hydrocarbons.152 The human body contains and emits many such molecules as part of normal physiologic processes, and they can thus be detected from tissue (such as skin) and bodily fluids.152 VOCs change in their relationship to metabolic state, hormonal changes, and ingestion of certain foods, and they are also emitted by bacteria present on the skin.152 VOCs have been identified as possible biomarkers for various malignancies and other medical conditions such as asthma, inflammatory bowel disease, and diabetes.153 Recent research has highlighted the potential to use VOCs to discriminate between infected and noninfected wounds and even to identify specific bacterial strains based on unique VOC profiles. The VOC profile of a wound reflects the compounds carried to and from the wound by the blood, metabolites produced by underlying cells and bacterial flora, and VOCs from the environment.152
The most common methods to evaluate VOCs in wounds are mass spectrometry and “electronic noses” (e-noses). E-noses are sensors or sensor arrays that can detect, identify, and discriminate between chemicals and compounds from a given sample of air (“headspace”).154 Such sensors swell upon adsorption of a gas, thereby increasing their electrical resistance. This pattern of resistance is transmitted to a computer for analysis to identify the compound.153,154
One of the earliest applications of e-nose technology for wounds was by Greenwood at al., who in 1997 used a 20-strong array of polymer sensors to evaluate VOC profiles in 15 patients with chronic ulcers of venous, arterial, or mixed etiology. Thirteen out of 15 patients demonstrated a definite change in the computed aroma profile as the ulcers progressed to healing.155 More recently, Tian and team used an e-nose comprising seven gas sensors (six metal oxide and one electrochemical) coupled with a probabilistic neural network computational model to accurately identify 100% of monospecies and 94.9% of two-species bacterial cultures in vitro.156 Moving into the clinical domain, a study of 10 patients with severe burns revealed different VOC profiles between infected and uninfected wounds as determined by cultures of wound swabs and dressings.157
Although e-noses are powerful tools that are capable of providing rapid results, they are subject to some limitations in their current form. Many factors can have a bearing on the VOC readings, including wound etiology and duration, types of dressings, and topical treatment agents used.158 Electrical VOC sensors are quite susceptible to temperature, humidity, and external interference from environmental compounds; the strict control of experimental conditions and procedures required to mitigate this phenomenon are difficult to achieve in a clinical setting.158
Elucidation of which specific VOCs should be targeted by e-nose systems will allow for the continued development of more advanced tools, since e-noses can only detect VOC patterns they have been preprogrammed to detect.153 Mass spectrometry has been used to this end due to its high sensitivity. Ashrafi and team recently used gas chromatography mass spectrometry to identify VOC profiles for both planktonic and biofilm bacteria, in in vitro and ex vivo cutaneous human wound models. VOC production was found to be affected by biofilm development and by the skin model used. Further, they identified VOCs specific for either planktonic or biofilm growth and others with significantly different relative abundances between the two bacterial states. Finally, some VOCs were observed to correlate directly with biofilm metabolic activity and mass.159
In a subsequent experiment, they used electrical stimulation, ciprofloxacin, or both to disrupt biofilms (measured by fluorescence staining, enumeration, metabolic assays, and biomass quantification). They observed a significant variation in VOCs after biofilm disruption, for example butanedione and acetic acid ethyl ester were identified as possible breakdown products of methicillin-resistant Staphylococcus Aureus biofilms.160 This highlights the possibility of using VOCs to track treatment response as part of a biofilm-based wound care approach. Though a valuable tool for research, direct clinical application of mass spectrometry is limited by significant time requirements and high cost.152,153
In general, the analysis of sensor output data from complex mixtures of molecules is difficult but can theoretically be achieved by using modern data analysis methods.158 Optimization and maturation of sensor equipment, guided by further identification of VOCs of interest, will be instrumental in bringing this technology into the clinic.152 VOC sampling technology has the potential to provide painless, rapid, noninvasive diagnostic information for nonhealing wounds.153 VOC analysis may allow for the assessment of the wound-healing trajectory as well as the determination of treatment bioavailability and bacterial antibiotic susceptibility.152 Valuable directions for future research have been suggested, including: developing a VOC library to develop fingerprints for given bacteria or wound states; determining the change in VOC profile that occurs over time based on the wound-healing trajectory; and the effects of ingested medications, if any, on VOC profiles.152
Wounded warriors: biomarkers for combat wound triage
War has historically been a significant driver of medical practice and innovation.161 Blast injuries make up more than half of all combat wounds in modern warfare162; this, along with the advent of body armor, has led to a shift in combat wound patterns from central (trunk, head) to the periphery (extremities).161 These blast-related injuries have massive zones of effect that can result in severe multisystem trauma affecting soft tissue, bone, and neurovascular structures with significant potential for gross bacterial colonization.163,164 Although the proportion of survivable combat traumas has increased, up to 24% of combat deaths may be preventable—most of these deaths are due to hemorrhage and occur before reaching surgical care, highlighting the need for markers to guide triage.19
Large wound beds due to penetrating blast injuries are difficult to manage.165 Standard of care is to leave these wounds open and treat them with negative pressure dressings alongside serial debridements and washouts with eventual primary closure.161 However, the question of when to initiate primary closure is a challenging conundrum for even the most experienced military surgeons.166 Premature closure of a wound is more likely to lead to dehiscence, resulting in unnecessary procedures and increased morbidity for the patient.166 On the other hand, late closure of wounds results in prolonged hospital stays, delayed rehabilitation, increased risk of hospital-acquired infections, and other complications.166 The fact remains that up to 25% of war wounds dehisce fully or partially after closure.167
Conventional visual assessment (i.e., the “4 Cs”: color, consistency, contractility when stimulated, capacity to bleed when incised) has not been found to correlate reliably with wound outcomes.166 Factors that may affect a surgeon's decision to initiate primary closure may include wound appearance, location, perfusion, presence of local or systemic infection, nutritional status, white blood cell count, ESR, and CRP; however, clinical algorithms based on these factors have also been poorly performed.167 A wound that one surgeon might deem ready for closure, another would disagree with—as such, there is a need for objective biomarkers and clinical decision-making algorithms to guide appropriate timing of closure for both combat casualties and complex civilian trauma.161
As such, much research has been conducted to shed light on the molecular landscape of combat wounds. Much like chronic wounds, failed (dehisced) combat wounds are characterized by a dysregulated immune response, leading to an aberrant, prolonged inflammatory state that promotes cellular infiltration and tissue breakdown.163 It is suspected that the immune dysregulation in the local wound environment is affected by the systemic pro-inflammatory response to massive injury.168 Various prospective studies on service members with penetrating blast injuries to the extremities highlighted possible serum, tissue, and wound effluent biomarkers of wound failure, heterotopic ossification, and critical colonization using Luminex bead array assays.163,165,168–170
Other methods have been used to characterize prognostic biomarkers for combat casualties. Luczek et al. used NMR spectroscopy to study plasma metabolites in 78 injured fighters with blast injuries and gunshot wounds. They found that hypoxanthine and the heme precursor 5-aminolevulinic acid outperformed lactate as markers of traumatic injury. Further, succinate outperformed lactate as a marker of mortality.19 Thus, these molecules may represent biomarkers to improve triaging of combat wounds, although the development of effective point-of-care tests is paramount as NMR spectroscopy is not an agile modality.
Janak and team sought to determine the relationship between urinary biomarkers and injury severity according to the Injury Severity Score (ISS). They found four biomarkers that were significantly higher in severe (ISS >24) versus nonsevere combat injuries: kidney injury molecule 1 (Kim-1), cystatin C, neutrophil gelatinase-associated lipocalin, and liver-type fatty acid binding protein (LFABP). Notably, Kim-1 plays a role in renal recovery and LFABP is a marker for renal hypoxia and vulnerability, thus the elevation of these markers may suggest the need to optimize the patient for renal recovery. Interestingly, LFABP levels drawn at admission were found to predict a subsequent diagnosis of severe abdominal injury with 80% sensitivity and 75% specificity.171 The authors used a 22-patient cohort of civilian burn patients as surrogates to test generalizability and found good concordance in biomarker trends between both groups.
Modern computational techniques have allowed for even greater predictive insight. Forsberg et al. used machine learning to develop highly predictive algorithms to help surgeons decide when to close wounds. They prospectively enrolled four patients with a minimum 75 cm2 combat wound and 18 civilian trauma patients to assess validity and reliability. Serum, tissue, and wound effluent biomarkers were measured by using Luminex bead assay, whereas quantitative transcriptomic PCR was used to characterized gene expression. A Random Forest (a type of machine-learning algorithm) model designed to select the 10 most important decision criteria was found to have an AUC of 0.79 for predicting wound failure, and these results were generalizable to the civilian population.166 Using the same cohort of patients, Dente et al. created a predictive model for blood stream infections (BSI) and pneumonia (PNA) in critically injured people by using Bayesian belief networks (a probabilistic model). A model incorporating volume of blood products received, presence of critical colonization in the wound, elevated serum IL-2 receptor, and serum Monokine Induced by Gamma Interferon was found to have an AUC of 0.832 (sensitivity of 0.5, specificity of 0.912) for predicting BSI. For PNA, a model using serum IL-7, head ISS, chest ISS, and critical colonization achieved an AUC of 0.856 (sensitivity 0.556, specificity 0.953) for predicting PNA in the same cohort.172 Small samples sizes in these analyses may have increased the risk of overfitting the models and overestimating their accuracies, although computational steps were taken to minimize these risks.166,172
Although much light has been shed on the ability of biomarkers to guide the care of wounded service members, the relationships between these biomarkers and wound outcomes would benefit from further validation in larger patient cohorts. In addition, generalizability comes into question when considering the relatively homogenous population of service members, that is, predominantly young, otherwise healthy males.166 Global efforts continue to improve these diagnostic tools and invent new ones; for example, the Combat Wound Initiative Program is a multidisciplinary, collaborative, interservice translational research program seeking to provide state-of-the-art tailored wound care tools.167 Continued research efforts in this field will hopefully provide objective measures for guiding combat wound closure as well as for aiding in the management of complex civilian trauma.168
Illuminating the chronic wound microenvironment
There is a great need for diagnostic tools that can provide objective measurement criteria and thus guide evidence-based treatment decisions to the benefit of patients with wounds.173 Imaging systems have the potential to fulfill this need and revolutionize wound care.173 Many imaging modalities and commercial devices have emerged with the ability to measure wound parameters, including tissue oxygenation, burn depth, and bioburden. Such technologies are advantageous due to their noninvasive nature, and most function in the optical range of the electromagnetic spectrum, which presents minimal risk to the patient.174 The ideal imaging system should be able to be handled by users with varying levels of education and experience as well as fit into challenging clinical environments.174 Here, we present the technologies with the greatest clinical evidence for their potential to help transform wound care into a precision medicine.
Ultrasound
Ultrasound (US) is a frequently used diagnostic tool in medicine due to its low cost, low risk, and ability to provide feedback in real time.175 It involves the use of a probe to propagate sound waves through the skin, wherein they are reflected, scattered, or absorbed by the underlying tissues and create an image dependent on the amplitude of the returning echoes.176 The US waves with higher frequencies have lower tissue penetrance but produce higher-resolution images; the opposite is true for lower frequencies.176 When it comes to skin imaging, high-frequency ultrasound (HFUS) scanners of 15 mHz or greater are generally considered optimal to provide sufficient detail.176 Such devices have been used to measure skin characteristics in a wide range of dermatological conditions, including skin tumors, fibrosing disorders, psoriasis, and wound healing.176
It has been suggested that wound epithelialization alone is not an absolute indicator of wound healing status: The new epithelium may lack barrier function or the underlying dermis may still exhibit derangements in molecular healing processes.177 Indeed, nonhealing wounds are characterized by functional healing deficiencies that are likely unique to each wound. The US has demonstrated the ability to measure tissue properties, which may shed light on the true functional status of wounded skin tissue. HFUS has demonstrated comparable ability to histology in evaluating key characteristics of the wound-healing process, such as depth, collagen accumulation, and granulation tissue formation in porcine wound models and human cadaveric tissue sections.178 The same characteristics have been measured via US in human DFUs.179
Mukai et al. used three-dimensionally reconstructed HFUS images to track microvascular growth in a murine full-thickness wound healing model.180 HFUS has also been used to quantitatively assess the healing of artificially induced PUs on guinea pigs by monitoring structural changes in deep tissue layers,181 as well as to characterize the sonographic morphology of various cellulose-derived artificial skin products.182 Gnyawali et al. used US elastography, which measures tissue stiffness by quantifying distortion in response to mechanical stress,177 to demonstrate that diabetic wounds in mice are characterized by a persistent early inflammatory phase with delayed recovery of elasticity.183
The ability of HFUS to study wound healing has been further examined in clinical settings. Kuhn and Agern used HFUS on 20 patients with 22 nonhealing leg ulcers of varying etiology (predominately VLUs), 18 of whom were treated with grafting of a BLCC, and compared US assessment with conventional visual assessment for the determination of healing. They found that most patients exhibited significant derangements in elastic and collagen fibers in the underlying dermis at the time of “clinical healing” as determined by visual assessment of complete re-epithelialization.184 The follow-up period was not long enough to determine whether these findings were associated with an increased risk of wound recurrence. The authors suggested that such wounds, if identified, could be intervened upon with adjunctive therapies such as extracorporeal shockwave therapy. Even so, it is not certain that this phenomenon is an abnormal wound-healing process.
Dyson et al. performed HFUS analysis of human punch biopsies and found significant variation in wound diameter over time at the point halfway between the base of the wound and the surface; they theorized that this was due to narrowing of the wound base from myofibroblast contraction and of the wound surface due to scab formation and epithelialization.185 Thus, the fact that epithelialization occurs before the underlying tissue is fully repaired is not necessarily a deleterious process, and it certainly makes sense from an evolutionary perspective as restoration of the epidermis is perhaps the most time-sensitive process required to prevent further insults, for example, pathogen invasion.
With all this in mind, it remains unclear what actionable interventions are made possible by US assessment: What specific therapies should be employed to target the US-measurable tissue characteristics discussed thus far, if intervention is even necessary, and the resulting effects on wound healing outcomes. This represents a potential area for future research. At the very least, HFUS could be used to compare treatment modalities in their ability to improve the speed and strength of dermal healing.184
The most promising clinical application for US in the wound healing space is the early detection and evaluation of deep-tissue pressure injuries (DTPIs) and PUs. The National Pressure Injury Advisory Panel defines a DTPI as a localized area of nonblanchable deep red, maroon, or purple discoloration, or epidermal separation that reveals a dark wound bed or blood-filled blister.186 Many PUs begin at the skin surface due to friction and shearing forces, whereas some PUs begin as DTPIs that form at the bone–muscle interface far below the skin surface.186
The prevalence of DTPIs is believed to be underestimated, as they may remain invisible until they reach advanced stages; deep tissue damage at the bone level can take up to a week to appear on the skin.186,187 There is no way to visually assess the degree of skin damage below the surface of stage 1 PUs, pressure-related intact discolored areas of skin, or even normal-appearing skin; as such, patients who present to hospitals for care may already have incurred significant and irreversible vascular or skin damage, and underlying tissue may already be dead or dying.187 Early detection of such lesions would facilitate prompt intervention to benefit the patients. Further, if these patients later develop a DTI or PU, it may be labeled as hospital-acquired despite having been present yet undetectable or in early stages of progression on admission, which has significant legal and cost implications for the hospital.187
To this end, Scheiner et al. used US (2.5 to 12 MHz) to scan 13 common PU sites in 33 patients at high risk for PUs who were admitted to the emergency department at an academic hospital center (23 patients completed the study). The US scans were able to identify pressure necrosis at the levels of the subcutaneous fat and in the deep muscularis at the level of bone. Patients were examined for PUs every day for 7 days after the initial scan, which was conducted on admission. Twenty percent of patients were found to have subsurface DTIs that later appeared as PUs on the skin. The US identified these sites with a sensitivity of 100% and a negative-predictive value of 100%.187 Another group used HFUS to scan the heels of 130 patients at high risk for PUs who were admitted to the hospital for a period of 1 year. They noted that 89.8% of patients had abnormal scans (fluid accumulation beneath the dermis at the bony prominence) of at least one heel, but too few patients developed PUs to determine any predictive pattern or capability of the HFUS scans.188
Other groups have suggested US patterns that may predict progression of DTIs into PUs. Matsumoto et al. evaluated 11 patients with visible DTPIs at a Japanese university hospital by using 18 MHz US and found that those exhibiting a “cloud-like” echogenicity pattern exhibited progression of injury, whereas those exhibiting a “cobblestone-like” pattern did not get worse.186 Another small study using 10 MHz US identified key ultrasonographic signs unique to DTPI as well as some specific characteristics that may predict progression. Both of these studies were limited by small sample size and a short follow-up period.189 Future studies using US to detect subclinical PUs/DTPIs and to determine algorithms predicting progression in larger cohorts will be valuable.
Autofluorescence imaging
Autofluorescence (AF) occurs when biological substrates emit light in the UV-visible to near-infrared spectral range (∼200–1100 nm) when excited by a specific wavelength of incumbent light.190 Subcellular labeling techniques and computer-based image composition software have allowed for the characterization and study of endogenous chromophores in human cells.190 The AF signals from the cell cytoplasm arise mainly from the reduced form of NADPH and from flavins—the spectral emissions of these compounds are directly related to their redox state, which is a reflection of energy metabolism, oxidative defense, biosynthesis, and signal transduction in the cell.190
Other compounds with discernible spectral signals that can be evaluated by using AF include collagen, elastin, free fatty acids, vitamin A, lipofuscin-like pigments, and porphyrins.190 Indeed, laser-induced AF has been used to quantify endogenous collagen in burn wound biopsies.191 Mehrvar et al. used AF to quantify NADH/FAD (redox ratio) in wounds of diabetic and nondiabetic mice and found the ratio to be decreased in diabetic wounds, reflecting higher production of reactive oxygen species and greater oxidative stress.192 This is an example of how AF allows for real-time determination of the metabolic state of wounds.
The ability of AF to noninvasively characterize various tissue compounds has been exploited to the benefit of patients with wounds. As previously mentioned, clinical signs and symptoms and classic swabbing techniques have significant limitations for the characterization of wound bioburden. One AF imaging device (LID, MolecuLight i:X, MolecuLight Inc., Toronto, ON, Canada) has been developed for fast characterization of wound bioburden. The device allows for the visualization of bacteria at loads >104 CFUs/g by using violet light to target bacteria-specific porphyrins that fluoresce red (S. aureus porphyrins) or cyan (Pseudomonal pyoverdine) in a composite image generated on the high-resolution LCD display, whereas background endogenous tissues (e.g., collagen) show up as green.193 By facilitating easy visualization of bacteria within the wound, LID has the potential to allow for more efficient targeted debridement and evaluation of debridement success.193
The LID has demonstrated promising results in clinical trials. A study on 33 patients with wounds of varying etiology found LID to have a positive predictive value (PPV) and negative predictive value (NPV) of 95.4% and 100%, respectively, compared with microbiological swab for the identification of clinically significant bacterial load in wounds.194 The LID allowed for targeted sampling of the wound bed with swabs, which may have otherwise resulted in false-negative readings.194 A nonrandomized single blind trial of 60 patients with chronic wounds found a PPV of 100% compared with both quantitative PCR and semi-quantitative curettage culture for the identification of clinically significant bioburden.195
Another trial of 31 patients with DFUs found that LID more accurately identified the presence of moderate or heavy bacterial loads compared with the standard Levine swabbing technique (78% accuracy for AF vs. 52% with swabbing), and furthermore maximized the effectiveness of bacterial load swab sampling with no detrimental impact on clinical workflow.196 Similar results were noted in other small cohorts of chronic wounds (sensitivity 72%, accuracy 74% when combined with clinical signs and symptoms) and pediatric burns.197,198 Finally, the utility of LID to guide targeted debridement was observed in a study of 22 DFUs in which fluorescence imaging demonstrated significant remaining bioburden after initial debridement in each wound.173
Conversely, one group did not observe a clear benefit of LID in 14 patients with burn wounds, in which they observed a sensitivity and specificity of 78% and 64%, respectively, for AF-guided swabs compared with standard swabs. They did, however, note 100% sensitivity and NPV for Pseudomonas, highlighting the potential strength of LID in ruling out Pseudomonas infection in burns.199 Their study may have been limited by their definition of a positive bacterial swab, as they considered any bacterial load to be positive whereas the LID does not detect loads below 104 CFUs/g. The exact level of bacterial bioburden that inhibits wound healing remains controversial.
DaCosta et al. used a different AF imaging device, operating on the same mechanism as LID, to assess 28 patients with wounds—primarily DFUs with various other etiologies. They found that AF identified clinically significant bacterial load in 85% of wound peripheries that were missed by conventional swab methods. Conventional visual assessment missed 50% of wounds with high bacterial load as identified by AF. During the longitudinal follow-up phase of 12 patients, it was observed that 90% of patients would have been sent home at least once with false-negative high bacterial loads that were detected successfully by AF. This represents missed opportunities to treat the wound early, resulting in increased chronicity and morbidity for the patient.194,200
The most promising utility of AF imaging for wound care appears to be the mitigation of false-negative results, that is, the identification of bacterial load that has yet to manifest clinical signs and symptoms or that may be missed by using conventional swabbing techniques.200 Limitations of using AF for wound evaluation include the need for a dark room during imaging, which is not always easy or feasible. However, a disposable “DarkDrape” is available to create a portable dark environment in settings where lights can't be turned off. Some authors consider active bleeding or visual vascularized tissue to be a relative contraindication for AF modalities, as these characteristics show up black and may confound image interpretation.194 Silver dressings have been noted to impart a similar effect.194 In addition, the 405 nm wavelength blue light used only penetrates to a depth of 1–1.5 mm, meaning that deeper bacteria evade quantification.200
AF cannot differentiate between many commensal and pathogenic bacteria due to the similarities in endogenous chromophores across species.200 Finally, since visualization of bacteria depends on their metabolic activity, bacteria in biofilm may not be visualized due to the decreased metabolic activity associated with the sessile bacterial phenotype.193 Nonetheless, AF imaging is a promising modality to improve the care of patients with wounds by providing fast, simple, and effective feedback on bacterial bioburden, which merits further exploration in large clinical trials with homogenous wound etiologies.
Hyperspectral and multispectral imaging
Both multispectral imaging and hyperspectral imaging (HSI) (spectral imaging [SI]) are spectrophotometric approaches wherein data are collected at multiple points simultaneously from a wound area.174 They involve the reconstruction of a reflectance spectrum of light in the visible to near-infrared wavelengths (∼400–1100 nm) for every pixel in image by recording serial images of biologic tissue in multiple narrow spectral bands.201 These data are processed into a 3D “data cube” from which two-dimensional (2D) maps of tissue parameters, most commonly oxygenation, are generated.202 Although these modalities are quite similar, HSI typically has more spectral channels and better spectral resolution, with the trade-off being slower image acquisition times.
SI has most commonly been used to quantify tissue oxygenation of DFUs via the measurement of oxy- and deoxy-hemoglobin.203 Two studies have observed an association between oxygenation of peri-wound skin and healing of DFUs, where increased peri-wound oxygenation was associated with an increased probability of healing at 6 months.203,204 These studies then retrospectively derived “healing indices” based on SI oxygenation measurements to predict DFU healing, which offered higher NPV and PPV than the commonly used metric of wound size change in 4 weeks. The sensitivity and specificity for predicting healing were found to be 93% and 86%, respectively, in the first study, and 80% and 74%, respectively, in the second study.203,204 Thus, SI may allow for the early identification of at-risk DFUs and spur more expeditious preventative measures.204 These studies were limited by small sample size and poorly defined healing indices, and their results have not been confirmed in prospective studies.205
Building on these data, a commercial SI device (DeepView®; SpectralMD Inc., Dallas, TX) designed for advanced burn and wound care management demonstrated a sensitivity and specificity of 98.8% and 93.9%, respectively, when combined with a machine-learning algorithm in predicting which areas of a DFU would heal at 28 days.206 Conversely, a more recent study using novel computational software for SI analysis of 43 patients with DFUs found a negative association between baseline oxygenation and healing at 12 weeks.205 The authors attribute this to a possible inverse relationship between oxygenation of hemoglobin and extravascular tissue oxygenation in patients with microvascular disease or neuropathy (i.e., microvascular shunting).205 Further prospective studies are necessary to establish the utility of HSI for predicting DFU healing.
Yudovsky et al. retrospectively analyzed HSI data collected from 21 patients with DFUs and compared them with those from ulcer-free diabetics to try and predict the risk of ulcer formation.207 Using images that were collected an average of 58 days before ulceration was clinically apparent, they found that SI predicted DFU formation with a sensitivity and specificity of 95% and 80%, respectively.207 These claims have not yet been confirmed in prospective studies.
Spectral imaging techniques have historically been limited by their application in darker skin types, where melanin has been found to affect accurate determination of hypervascularization and metabolism, and to under-estimate hemoglobin oxygenation; however, these limitations may be diminished by utilization of more advanced SI techniques such as polarization-sensitive HSI.208
Optical coherence tomography
Optical coherence tomography (OCT) uses low coherence inferometry from optical laser beam scattering to provide structural information about the skin.209 After projecting a focused laser beam into the skin, backscattered light from subsurface cutaneous structures enters the scanning system and interacts with a reference laser, providing measurements of the scattering intensity at each tissue depth.210 This allows for visualization of skin microstructures to a depth of ∼2 mm at a resolution of 3–5 μm.209 It has been likened to “optical US” as it provides 2D cross-sectional images but uses IR light instead of sound waves.211 Functional extensions of OCT have become increasingly popular and allow for the determination of a wide variety of parameters (e.g., OCT angiography or doppler OCT for quantification of blood flow, spectroscopic OCT for molecular properties).212 For these reasons, OCT is becoming increasingly accepted as a tool to diagnose basal cell carcinoma and other skin conditions.210
The OCT holds promise as a tool to allow for more effective wound research. Li et al. used phase-variance OCT to track regeneration of the vascular network in diabetic mice who received treatment with a pro-angiogenic ointment, demonstrating the ointment's efficacy.213 OCT has also demonstrated significant value as a tool for translational research, as one group used it to evaluate epithelialization in an ex vivo human skin wound model. OCT accurately identified epidermis, papillary and reticular dermis, and migrating keratinocytes in the wound bed. It was, thus, able to demonstrate the efficacy of allogeneic adipose stem cells in increasing re-epithelialization speed. The advantages of OCT compared with histopathological analysis for such research include faster turnaround, avoidance of the deleterious effects of biopsy, and the ability to visualize the entire wound at once instead of small biopsy sections.214
OCT offers a way to noninvasively assess cutaneous wound-healing stages without the deleterious effects of biopsy. Studies have found OCT assessment to correlate well with histopathologically defined stages of healing in acute wounds in human subjects.215 Some groups have used OCT to analyze chronic wounds. OCT was able to detect partial epidermal loss, vasoconstriction, vasodilation, and epithelialization in six patients with VLUs, pyoderma gangrenosum, and vasculitis.216 Assessment of 15 patients with chronic lower extremity wounds (VLUs and mixed arteriovenous ulcers) revealed a unique abnormal peri-wound vessel morphology (glomerulus-like, believed to be associated with tissue growth) that differs from more distant peri-wound skin, or skin from the contralateral extremity.210 Thus, OCT has the potential to aid in the determination of wound etiology and assessment of wound healing, though its exact clinical utility has yet to be proven. A potential limitation of OCT is difficulty in image interpretation, although this may be assuaged by the continued development of machine-learning-based image interpretation approaches.
Future directions
Synthetic biology
Given the incredible complexity of the wound microenvironment and the delicate interplay of many processes required for normal tissue repair, it is a significant challenge to create diagnostics capable of evaluating the entire wound landscape and providing accurate spatiotemporal analytics to better inform treatment decisions. Synthetic biology is an exciting field with great potential to meet this challenge.
Synthetic biology seeks to engineer delicate control over cellular behavior and biological processes.217 Synthetic biologists have developed tools that allow for rational engineering of biologic systems with specific, customizable functions.218,219 This holds great promise for molecular diagnostics and has been used to develop portable, point-of-care biosensors.220 One example, the “toehold switch,” allows for cell-free translational expression of a target gene (encoding a reporter protein) to be activated by specific target molecules, providing a colorimetric readout.220 Initially created with the ability to detect RNAs, recent technical refinements allow for a single toehold-switch sensor to respond to multiple targets, including RNA, DNA, proteins, and other small molecules.220 Assays can be freeze-dried on paper or other porous material to facilitate transport and storage at room temperature.219
Paper-based synthetic biology platforms based on the toehold switch principle have been devised with the capability of accurately identifying and quantifying species-specific mRNA and clinically relevant host biomarkers (e.g., calcitonin) from the gut.221 One team recently combined cell-free biosensors with a nanostructural electrochemical interface to achieve expanded, multiplexed characterization of multiple antibiotic resistance genes in parallel.219 Such coupling of gene-circuit based sensors with electrochemical systems allows for data to be shared easily with computational tools.219 Limitations of the toehold switch include its very high cost and laboriousness, as time-heavy sequence optimization is required for each target of interest and subsequently the risk of design failure is high.220 Efforts to mitigate the latter point using a multi-step transduction process are ongoing.220
The potential applications of diagnostics rooted in synthetic biology for wound care are limited only by the imagination. Combining the measurement of biofilm markers, polymicrobial metabolites, and antibiotic resistance genes could facilitate rapid and reliable infection detection as well as appropriate and prompt treatment choice. Other systems might combine intrinsic wound factors such as metabolic activity, inflammatory profile, and protease activity to further guide the delivery of therapy and allow for high-resolution tracking of healing response with subsequent fine-tuning of the treatment strategy. Synthetic networks designed to evaluate dysfunctional signaling pathways would provide insight into propensity of the wound to respond to a given treatment, for example, angiogenic growth factors for wounds defective in angiogenesis.222 Multiplexing many such assays onto a single strip of paper as described earlier holds great promise for the evolving landscape of wound healing by providing instant feedback on wound status and the characteristics of the wound microenvironment (Fig. 6).
Figure 6.
A theoretical diagnostic platform for wound biomarkers. The current approach to wound appraisal and treatment is limited by the time cost of determining healing status, as well as the inability of available treatments to achieve consistent wound closure and provide personalized/precision therapies. A diagnostic platform with an array of wound biomarkers would theoretically allow for instant determination of wound status and facilitate tailoring of specific, individualized wound therapies.14
In addition, as care shifts to increasingly take place in the home and/or via telemedicine (TM) such a diagnostic platform could be shipped directly to patients or included in the tool kit of visiting care providers, helping to guide therapy and determine the need for in-person clinic visits. In the future, skin grafts or existing CTPs used for wound care might be genetically modified in such a manner so as to provide information about the microenvironment of the wound—for example, markers of “take,” infection, or other factors. Genetically altered bacteria, that is, synthetic probiotics could also be created to respond to specific components of the wound microenvironment.218
Synthetic biology has the potential to create systems that are capable of intelligent and autonomous therapy delivery. Toehold-switch-based hydrogel templates have been engineered to allow for active burst release of chemotherapeutic drugs in response to cancer-specific DNA sequences.223 Current gene and engineered-cell therapies, which involve modifying a cell's genetic programming to induce therapeutically beneficial behavior, have been limited by lack of fine control over the timing, strength, and cellular context of therapeutic delivery.217 Building complex synthetic gene circuits into existing gene and engineered-cell therapies has the potential to refine their therapeutic execution by programming the cells to respond to microenvironmental queues in a sophisticated manner.217 For wounds, this could be a genetically engineered skin substitute whose cells can detect antimicrobial resistance genes and respond by producing appropriate antimicrobial molecules or releasing pro-angiogenic factors in response to a deficiency of the same. Fibroblasts could be engineered to produce certain ECM molecules in specific amounts in response to the ECM composition of the wound, or cells might alter their contractility in response to the stiffness of the ECM. Biofilm-responsive elements could produce biofilm-degrading enzymes. These capabilities could be combined into a single “smart” engineered tissue that is capable of measuring and treating multiple aspects of wound chronicity. A chief limitation of such an approach is the fact that cell-based approaches are quite laborious in nature, as they require that all processes are encoded inside a living organism.219
Although promising, difficulties inherent in synthetic biology approaches currently limit their development and clinical application. It remains difficult to engineer gene circuits that behave exactly as intended, often requiring many iterations to be built and tested at no small cost before a given goal is achieved.217 A common reason for this is the fact that engineered circuits may fail to accurately capture the dynamic, heterogenous in vivo environment in which the circuit must operate—this is evident when considering, for example, the differences between cultured cell lines and primary cells. Two seemingly identical circuits often behave quite differently in vitro than in vivo, owing to often unaccounted-for contextual effects such as resource competition and other dynamic qualities of the cellular microenvironment.
Continued development of advanced biological circuit simulation software, as well as “organs-on-a-chip” or organ buds grown three-dimensionally in vitro may be helpful to overcome these challenges. Finally, as with any medical therapy the issue of ensuring safe and effective delivery to the patient's body is a potential hurdle. Immunogenicity is a potential complicating factor as either the delivery vector or delivered molecule may cause T cell sensitization or produce anti-“drug” antibodies.217
Nonetheless, as we continue to identify pathways that are deficient or altered in nonhealing wounds, our ability to engineer synthetic biologic networks as advanced diagnostic and therapeutic tools targeting the factors of wound chronicity will continue to grow. As such, the realm of synthetic biology holds significant promise for transforming wound care into a precision medicine.
The changing landscape of care: telemedecine and “smart” wearables
The increasing popularity of TM stands to change the clinical practice of wound care, especially for patients located in remote geographical areas.224 The implementation of TM, optimally via real-time synchronous video conferencing, may enable better distribution of health services, promote equality of care, save patients' time and travel costs, and save room space for health care organizations.225 Studies evaluating the clinical outcomes of TM are scarce, and those pertaining specifically to wound care are rarer still.226 The results of these studies are mixed, and RCTs and comparative studies are lacking.227 One RCT demonstrated greater size reductions for PUs at 12 weeks for TM compared with face-to-face visits,228 whereas another RCT failed to reveal any changes in clinical outcomes due to TM.229 One study showed that TM resulted in improved healing times and decreased amputation rates for DFUs,230 whereas a different RCT on DFUs failed to recapitulate these results.231 Nonetheless, these studies and studies on populations with nonhealing wounds of various etiologies have generally demonstrated noninferiority of TM approaches in achieving beneficial wound-healing outcomes.225–227
TM may reduce costs to patients and the health care system; studies have suggested savings of €4,583 euros per patient over 9 months for wounds of varying etiologies,226 and savings of €2,039 euros per patient over 6 months in those with DFUs.231 These results suggest that TM is a safe option to remotely manage wounds that may reduce time and money costs for patients and providers.227 TM is limited by providers' inability to physically sense the wound and the inability to carry out manual procedures such as debridement, which require the physical presence of an experienced wound care specialist.225 Further studies evaluating the quality of life follow-up, satisfaction of patients and staff, success in treatment implementation, cost reduction, and causes of hospitalization and mortality are necessary to coalesce the role of TM in the future of wound care.225
The use of TM for wound care will continue to increase. Even today, for most patients the management and treatment of wounds is conducted largely in the community.232 This highlights the need for tools to facilitate the rapid, accurate, point-of-care evaluation of wound conditions by practitioners with various levels of training, expertise, and experience.232 Given that certain clinical criteria or scoring algorithms (e.g., NERDS/STONEES15) cannot be used in TM settings given the constraints of existing technologies, the ability to quantitatively monitor wound status at a distance and without removing the dressing would be of immense value.232 The development of intelligent, centralized technologies is necessary to fulfill this need and would stand to reduce treatment times, minimize complications, and reduce costs.232 “Smart” systems (e.g., smart dressings, smart bandages) with the ability to measure, report, and even respond to wound conditions hold great potential to solve many of the aforementioned challenges (Fig. 7).224 Indeed, smart dressings have been engineered to measure wound parameters, including pH, temperature, oxygen, moisture, enzyme levels, and mechanical and electrical properties of skin.224 Others have active drug delivery systems that release therapy in response to a specific external stimulus. For example, hydrogel-based dressings can release antibiotics in response to supposed markers of wound infection. These dressings respond to temperature or pH by using heat or light (UV or near-infrared) to release drugs from the hydrogel carrier into the wound.233,234
Figure 7.
Connected “smart” dressings for wound care. As the wound care landscape continues to shift toward telemedicine, smart dressings have great potential to facilitate remote wound status monitoring. This could guide at-home wound care by providing instructions to patients or visiting clinicians, and determine the need for follow-up appointments. Smart dressings are also being engineered to deliver therapy in an automated fashion, which might include drug delivery (e.g., antimicrobials), pH modulation, and moisture balance.14,224,233,234
The key challenge with designing these dressings is identifying which parameters to measure and which pathophysiological processes to target.224 Indeed, temperature and pH have not yet been characterized as reliable markers of wound infection. As such, there is a gap between the advanced platforms developed by researchers and the products that are used in current clinical practice.224 There is a need for dressings that can detect more specific markers and perhaps release multiple drugs, and the development of such dressings will be facilitated by the ongoing refinement and characterization of accurate biomarkers for wound healing.224
CONCLUSION
The literature supports both the need for and the potential clinical utility of, a platform diagnostic that can monitor biomarkers associated with the healing process. Such a platform would stand to revolutionize the diagnosis of nonhealing wounds, catapult our understanding of the molecular and cellular mechanisms behind impaired wound healing to new heights, and usher in a modern age of therapeutic strategies for wound care. There is still much we do not know about the cellular and molecular mechanisms underlying impaired wound healing, and we still struggle to translate our basic science knowledge into effective point-of-care tools. As described in this review, the discovery and translation of many biomarkers and imaging systems, at various stages of their characterization and many with yet uncoalesced significance, is ongoing. Difficulty in designing high-quality randomized controlled trials is a key factor hindering the clinical implementation of these technologies Further work is necessary to ascertain the exact significance of these biomarkers as well as to engineer treatments that target them, thus transforming wound care into a precision medicine. Synthetic biology may offer an attractive solution to these challenges by allowing for the creation of advanced cell-based materials with the ability to dynamically respond to changes in the wound environment. The increasing popularity of TM and inception of smart “wearables” that are capable of remotely monitoring and even responding to wound conditions stand to improve global access to care and reduce time and money costs for patients and practitioners.
SUMMARY
Nonhealing wounds are a global pandemic with mortality rates and management costs exceeding many common cancers. Although our understanding of the molecular and cellular factors driving wound healing has grown, clinicians remain unable to consistently achieve wound closure. Standards for the diagnosis and evaluation of wounds remain subjective and imprecise, and nonhealing wounds often resist treatment with available therapies. There is a need to transform wound healing into a precision medicine; advanced wound diagnostic modalities (namely biomarkers and imaging systems) have the potential to achieve this goal by granting clinicians the ability to tailor individualized therapeutic regimens based on the unique molecular signature of a given wound.
Although many potential biomarkers have been identified, their translation into fast, effective, cost-efficient point-of-care tools remains challenging. MMPs and pH are the biomarkers with the best evidence to support their potential utility as prognostic indicators, and both have a multitude of ways by which they can be modulated. Other promising modalities include acute phase reactants, volatile emissions, and the use of artificial intelligence algorithms. A variety of other biomarkers are still in the early stages of investigation, and all require further translational and clinical research before they earn a spot in the clinician's arsenal. This highlights the need for a platform diagnostic that can quantify multiple diverse biomarkers simultaneously, which would be a valuable tool for both clinicians and researchers alike.
Imaging systems are similarly positioned; promising applications for AF, spectral imaging, and OCT in the realm of wound infection detection, wound healing research, and more have been identified, though further clinical validation of these systems is necessary. Finally, the future of wound healing as a precision medicine lies within the realms of synthetic biology, TM, and smart dressings as care delivery continues to shift into the home rather than the clinic.
Take-Home Messages
Nonhealing wounds represent an immense and ever-growing global pandemic, with incidence and mortality rates exceeding those of many common cancers.
A nonhealing wound is typically considered to be any wound that “fail[s] to proceed through an orderly and timely process to produce anatomic and functional integrity.”
Existing standards for diagnosis and evaluation of nonhealing wounds are subjective and experiential, and such wounds frequently resist treatment with available therapies, making them a significant challenge for clinicians.
There is a need to transform wound healing into a precision medicine wherein clinicians can deliver personalized therapeutic strategies based on the molecular characteristics of a given wound. Advanced wound diagnostics, specifically biomarkers and imaging systems, have the potential to achieve this vision.
Although many possible biomarkers for wound healing have been identified, their translation into simple, cost-effective, and predictive clinical assays remains challenging. MMPs and pH have the best evidence to support their potential utility; however, these and the many other biomarkers thus far identified require further translational research and clinical validation before their role in the future of wound healing becomes clear.
This highlights the need for a diagnostic platform tool that is capable of quantifying multiple, diverse biomarkers simultaneously in an array to facilitate high-quality studies across the research continuum.
Similarly, advanced imaging systems may be useful in various domains of wound healing, such as infection quantification, early detection, and burn evaluation, but these would also benefit from further validation in large RCTs before they become a fixture in the clinician's arsenal.
The future of wound care as a precision medicine lies in synthetic biology, TM, and smart dressings as the landscape of care continues to shift toward the home.
Abbreviations and Acronyms
- 2D
two-dimensional
- 3D
three-dimensional
- AF
autofluorescence
- AUC
area under the curve
- BSI
blood stream infections
- CG
cathepsin G
- CRP
C-reactive protein
- CTPs
cell- and tissue-based products
- DFU
diabetic foot ulcer
- DTPIs
deep-tissue pressure injuries
- ECM
extracellular matrix
- e-nose
electronic nose
- EPA
elevated protease activity
- ESR
erythrocyte sedimentation rate
- FDA
Food and Drug Administration
- HBOT
hyperbaric oxygen therapy
- HFUS
high frequency ultrasound
- HNE
human neutrophil elastase
- HSI
hyperspectral imaging
- IDFU
infected DFU
- IDSA
Infectious Diseases Society of America
- IL
interleukin
- ISS
Injury Severity Score
- Kim-1
kidney injury molecule 1
- LFABP
liver-type fatty acid binding protein
- LID
autofluorescence imaging device (MolecuLight i:X, MolecuLight Inc., Toronto, ON, Canada)
- LYS
lysozyme
- MMP
matrix metalloprotease
- MMPI
matrix metalloprotease inhibitor
- MPO
myeloperoxidase
- MSI
multispectral imaging
- NEPA
nonelevated protease activity
- NERDS
Nonhealing, Exudative, Red/bleeding surface, Debris [yellow/black necrotic tissue], Smell
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- NIDFU
noninfected DFU
- NIH
National Institutes of Health
- NO
nitric oxide
- NPV
negative predictive value
- NPWT
negative-pressure wound therapy
- OCT
optical coherence tomography
- PCR
polymerase chain reaction
- PNA
pneumonia
- PPV
positive predictive value
- PU
pressure ulcer
- RCT
randomized-controlled trial
- ROS
reactive oxygen species
- SCV
small-colony-variant
- SI
spectral imaging
- STONEES
Size increase, Temperature increase, Os probe to/probe to bone, New or satellite areas of breakdown, Exudate/Erythema/Edema, Smell
- TIMP
tissue inhibitors of metalloprotease
- TM
telemedicine
- TNFα
tumor necrosis factor alpha
- UA
uric acid
- US
ultrasound
- USWR
U.S. Wound Registry
- VLU
venous leg ulcer
- VOC
volatile organic compound
- WCP
WoundChek Protease
ACKNOWLEDGMENTS AND FUNDING SOURCES
None. This work is supported by the National Institute of Health grants (U01DK119085 to R.S.K., H.A.L.-T., and M.T.-C.; R01NR015649, RC1DK086364, R01NR01388, and R01AR073614 to M.T.-C.) and the Precision Healing research grant to the University of Miami (to R.S.K.).
AUTHOR CONTRIBUTIONS
All authors made significant contributions to the conception, writing, and reviewing of the article, revision of intellectual and technical content; they gave final approval of the version to be published. All authors assume responsibility and accountability for the information contained herein.
AUTHOR DISCLOSURE AND GHOSTWRITING
M.A.W., H.A.L.-T., M.T. -C., and R.S.K.: None to disclose. W.D.L. is a founding member and current Chief Scientific Officer of Precision Healing Inc., as well as Chief Executive Officer of Precision Healing, Inc. R.W. is the Director of Research and Development for both Precision Healing Inc., and Precision Healing, Inc. D.S. is currently Vice President of Research and Innovation for both Precision Healing Inc., and Precision Healing, Inc. I.M.H. is currently the Senior Director of Biological Sciences at Precision Healing, Inc. No ghostwriters were employed in the writing of this article.
ABOUT THE AUTHORS
Dr. Maximillian A. Weigelt, MD, completed a research fellowship in Wound Healing at University of Miami Department of Dermatology. He is now a PGY1 resident at Cleveland Clinic Foundation. Dr. Hadar A. Lev-Tov, MD, MAS, is Assistant Professor of Dermatology and Director of the Wound Healing Fellowship at University of Miami Department of Dermatology. Dr. Marjana Tomic-Canic, PhD, is Professor of Dermatology, Vice Chair of Research and Director of the Wound Healing and Regenerative Medicine Research Program at the University of Miami Department of Dermatology. W. David Lee, MAS, is a founding member and current Chief Scientific Officer of Precision Healing Inc., as well as Chief Executive Officer of PrecisionHealing Inc. Dr. Ryan Williams, PhD, is the Director of Research and Development for Precision Healing Inc. Dr. David Strasfeld, PhD, is Vice President of Research and Innovation for Precision Healing Inc. Dr. Robert S. Kirsner, MD, PhD, is Chairman and Harvey Blank Professor of Dermatology in the Department of Dermatology at University of Miami Dr. Ira M. Herman, PhD, is Professor and Director, Emeritus of the Center for Innovations in Wound Healing at the Tufts University School of Medicine as well as Senior Director of Biological Sciences at Precision Healing, Inc.
REFERENCES
- 1. Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009;17:763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Escandon J, Vivas A, Tang J, Rowland K, Kirsner R. High mortality in patients with chronic wounds. Wound Repair Regen 2011;19:526–528. [DOI] [PubMed] [Google Scholar]
- 3. Lindley LE, Stojadinovic O, Pastar I, Tomic-Canic M. Biology and biomarkers for wound healing. Plast Reconstr Surg 2016;138:18S–28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value Health 2018;21:27–32. [DOI] [PubMed] [Google Scholar]
- 5. Alavi A, Sibbald RG, Phillips TJ, et al. What's new: management of venous leg ulcers: treating venous leg ulcers. J Am Acad Dermatol 2016;74:643–664; quiz 665–646. [DOI] [PubMed] [Google Scholar]
- 6. Nelzém O. Prevalence of venous leg ulcer: the importance of the data collection method. Phlebolymphology 2008;15:143–150. [Google Scholar]
- 7. Boulton AJM, Armstrong DG, Kirsner RS, et al. Diagnosis and management of diabetic foot complications. 2018. https://professional.diabetes.org/sites/professional.diabetes.org/files/media/foot_complications_monograph.pdf (last accessed November 1, 2020). [PubMed]
- 8. Statistics About Diabetes. American Diabetes Association. https://www.diabetes.org/resources/statistics/statistics-about-diabetes#:~:text=Overall%20numbers,of%20the%20population%2C%20had%20diabetes.&text=Undiagnosed%3A%20Of%20the%2034.2%20million,and%207.3%20million%20were%20undiagnosed. Published 2020 (last accessed August 26, 2020).
- 9. Agale SV. Chronic leg ulcers: epidemiology, aetiopathogenesis, and management. Ulcers 2013;2013:1–9. [Google Scholar]
- 10. Cancer Facts & Figures 2020. American Cancer Society. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf. Published 2020 (last accessed September 10, 2020).
- 11. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open 2015;5:e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapp S, Santamaria N. The financial and quality-of-life cost to patients living with a chronic wound in the community. Int Wound J 2017;14:1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hjort A, Gottrup F. Cost of wound treatment to increase significantly in Dernmark over the next decade. J Wound Care 2010;19:173–178. [DOI] [PubMed] [Google Scholar]
- 14. Sheets A, Hwang C, Herman I. Developing “smart” point-of-care diagnostic tools for “next-generation” wound care. In: Jeffrey L, ed. Translating Regenerative Medicine To The Clinic. London: Elsevier, Inc., 2016:251–261. [Google Scholar]
- 15. Sibbald RG, Woo K, Ayello E. Increased bacerial burden and infection: NERDS and STONES. Wounds 2007;3:25–46. [DOI] [PubMed] [Google Scholar]
- 16. Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA. Does this patient have an infection of a chronic wound. JAMA 2012;307:605–611. [DOI] [PubMed] [Google Scholar]
- 17. Woo KY, Sibbald RG. A cross-sectional validation study of using NERDS and STONEES to assess bacterial burden. Ostomy Wound Manage 2009;55:40–48. [PubMed] [Google Scholar]
- 18. Fife CE, Eckert KA, Carter MJ. Publicly reported wound healing rates: the fantasy and the reality. Adv Wound Care (New Rochelle) 2018;7:77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lusczek ER, Muratore SL, Dubick MA, Beilman GJ. Assessment of key plasma metabolites in combat casualties. J Trauma Acute Care Surg 2017;82:309–316. [DOI] [PubMed] [Google Scholar]
- 20. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4:560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olsson M, Jarbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen 2019;27:114–125. [DOI] [PubMed] [Google Scholar]
- 22. Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and management of lower-extremity ulcers. N Engl J Med 2017;377:1559–1567. [DOI] [PubMed] [Google Scholar]
- 23. Alavi A, Sibbald RG, Phillips TJ, et al. What's new: management of venous leg ulcers: approach to venous leg ulcers. J Am Acad Dermatol 2016;74:627–640; quiz 641–622. [DOI] [PubMed] [Google Scholar]
- 24. Kallis P, Friedman A, Lev-Tov H. A guide to tissue-engineered skin substitutes. J Drugs Derm 2018;17:57–63. [PubMed] [Google Scholar]
- 25. Alavi A, Sibbald RG, Mayer D, et al. Diabetic foot ulcers: part II. Management. J Am Acad Dermatol 2014;70:21..e21–e24; quiz 45–26. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Panayi AC, Bayer LR, Orgill DP. Current available cellular and tissue-based products for treatment of skin defects. Adv Skin Wound Care 2019;32:19–25. [DOI] [PubMed] [Google Scholar]
- 27. Stotts NA, Roedeheaver GT, Thomas DR, et al. An instrument to measure healing in pressure ulcers: development and validation of the Pressure Ulcer Scale for Healing (PUSH). J Gerontology 2001;56A:M795–M799. [DOI] [PubMed] [Google Scholar]
- 28. Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: review and advancements. Crit Care 2015;19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waycaster C, Gilligan A, Motley T. Cost-effectiveness of becaplermin gel on diabetic foot ulcer healing. J Am Podiat Med Assn 2016;106:273–282. [DOI] [PubMed] [Google Scholar]
- 30. Darwin E, Tomic-Canic M. Healing chronic wounds: current challenges and potential solutions. Curr Dermatol Rep 2018;7:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papanas S, Maltezos E. Becaplermin gel in the treatment of diabetic foot ulcers. Clin Interv Aging 2008;3:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Serena TE, Fife CE, Eckert KA, Yaakov RA, Carter MJ. A new approach to clinical research: integrating clinical care, quality reporting, and research using a wound care network-based learning healthcare system. Wound Repair Regen 2017;25:354–365. [DOI] [PubMed] [Google Scholar]
- 33. Broadbent J, Walsh T, Upton Z. Proteomics in chronic wound research: potentials in healing and health. Proteomics Clin Appl 2010;4:204–214. [DOI] [PubMed] [Google Scholar]
- 34. Tegl G, Schiffer D, Sigl E, Heinzle A, Guebitz GM. Biomarkers for infection: enzymes, microbes, and metabolites. Appl Microbiol Biotechnol 2015;99:4595–4614. [DOI] [PubMed] [Google Scholar]
- 35. Broszczak DA, Sydes ER, Wallace D, Parker TJ. Molecular aspects of wound healing and the rise of venous leg ulceration: omics approaches to enhance knowledge and aid diagnostic discovery. Clin Biochem Rev 2017;38:35–55. [PMC free article] [PubMed] [Google Scholar]
- 36. Tomic-Canic M, Ayello EA, Stojadinovic O, Golinko M, Brem H. Using gene transcription patterns (bar coding scans) to guide wound debridement and healing. Adv Skin Wound Care 2008;21:493–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of B-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol 2005;167:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014;6:265sr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010;89:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007;25:19–25. [DOI] [PubMed] [Google Scholar]
- 41. Geevarghese A, Herman IM. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res 2014;163:296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Demidova-Rice TN, Durham JT, Herman IM. Wound healing angiogenesis: innovations and challenges in acute and chronic wound healing. Adv Wound Care (New Rochelle) 2012;1:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Demidova-Rice TN, Geevarghese A, Herman IM. Bioactive peptides derived from vascular endothelial cell extracellular matrices promote microvascular morphogenesis and wound healing in vitro. Wound Repair Regen 2011;19:59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stone RC, Stojadinovic O, Rosa AM, et al. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Sci Transl Med 2017;4:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sawaya AP, Stone RC, Brooks SR, et al. Deregulated immune cell recruitment orchestrated by FOXM1 impairs human diabetic wound healing. Nat Commun 2020;11:4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stojadinovic O, Pastar I, Vukelic S, et al. Deregulation of keratinocyte differentiation and activation: a hallmark of venous ulcers. J Cell Mol Med 2008;12:2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brem H, Stojadinovic O, Diegelmann RF, et al. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol Med 2007;13:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vukelic S, Stojadinovic O, Pastar I, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem 2011;286:10265–10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jozic I, Vukelic S, Stojadinovic O, et al. Stress signals, mediated by membranous glucocorticoid receptor, activate PLC/PKC/GSK-3beta/beta-catenin pathway to inhibit wound closure. J Invest Dermatol 2017;137:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sawaya AP, Jozic I, Stone RC, et al. Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling. JCI Insight 2019;4:e129320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liang L, Stone RC, Stojadinovic O, et al. Integrative analysis of miRNA and mRNA paired expression profiling of primary fibroblast derived from diabetic foot ulcers reveals multiple impaired cellular functions. Wound Repair Regen 2016;24:943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stojadinovic O, Pastar I, Nusbaum AG, Vukelic S, Krzyzanowska A, Tomic-Canic M. Deregulation of epidermal stem cell niche contributes to pathogenesis of nonhealing venous ulcers. Wound Repair Regen 2014;22:220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Serena TE, Cullen BM, Bayliff SW, et al. Defining a new diagnostic assessment parameter for wound care: elevated protease activity, an indicator of nonhealing, for targeted protease-modulating treatment. Wound Repair Regen 2016;24:589–595. [DOI] [PubMed] [Google Scholar]
- 54. Ligi D, Mosti G, Croce L, Raffetto JD, Mannello F. Chronic venous disease—part II: proteolytic biomarkers in wound healing. Biochim Biophys Acta 2016;1862:1900–1908. [DOI] [PubMed] [Google Scholar]
- 55. Hasmann A, Gewessler U, Hulla E, et al. Sensor materials for the detection of human neutrophil elastase and cathepsin G activity in wound fluid. Exp Dermatol 2011;20:508–513. [DOI] [PubMed] [Google Scholar]
- 56. Hingorani DV, Lippert CN, Crisp JL, et al. Impact of MMP-2 and MMP-9 enzyme activity on wound healing, tumor growth and RACPP cleavage. PLoS One 2018;13:e0198464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol 2015;44–46:113–121. [DOI] [PubMed] [Google Scholar]
- 58. Krishnaswamy VR, Mintz D, Sagi I. Matrix metalloproteinases: the sculptors of chronic cutaneous wounds. Biochim Biophys Acta Mol Cell Res 2017;1864(11 Pt B):2220–2227. [DOI] [PubMed] [Google Scholar]
- 59. Jindatanmanusan P, Luanraksa S, Boonsiri T, Nimmanon T, Arnutti P. Wound fluid matrix metalloproteinase-9 as a potential predictive marker for the poor healing outcome in diabetic foot ulcers. Patholog Res Int 2018;2018:1631325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stacey MC, Phillips SA, Farrokhyar F, Swaine JM. Evaluation of wound fluid biomarkers to determine healing in adults with venous leg ulcers: a prospective study. Wound Repair Regen 2019;27:509–518. [DOI] [PubMed] [Google Scholar]
- 61. Schneider LA, Korber A, Grabbe S, Dissemond J. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res 2007;298:413–420. [DOI] [PubMed] [Google Scholar]
- 62. Okamoto T, Akaike T, Suga M, et al. Activation of human matrix metalloproteinases by various bacterial proteinases. J Biol Chem 1996;272:6059–6066. [DOI] [PubMed] [Google Scholar]
- 63. Serra R, Grande R, Buffone G, et al. Extracellular matrix assessment of infected chronic venous leg ulcers: role of metalloproteinases and inflammatory cytokines. Int Wound J 2016;13:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lazaro JL, Izzo V, Davies AH, Lobmann R. Elevated levels of matrix metalloproteinases and chronic wound healing: an updated review of clinical evidence. J wound Care 2016;25:277–287. [DOI] [PubMed] [Google Scholar]
- 65. Serena TE. Development of a novel technique to collect proteases from chronic wounds. Adv Wound Care (New Rochelle) 2014;3:729–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Izzo V, Meloni M, Vainieri E, Giurato L, Ruotolo V, Uccioli L. High matrix metalloproteinase levels are associated with dermal graft failure in diabetic foot ulcers. Int J Low Extrem Wounds 2014;13:191–196. [DOI] [PubMed] [Google Scholar]
- 67. Serena T, Cullen B, Bayliff S, et al. Protease Activity Levels Associated with Healing Status of Chronic Wounds. In: Systagenix, ed. Torrance, California: WoundChek Laboratories, 2011. [Google Scholar]
- 68. Stechmiller J, Cowan L, Schultz G. The role of doxycycline as a matrix metalloproteinase inhibitor for the treatment of chronic wounds. Biol Res Nurs 2010;11:336–344. [DOI] [PubMed] [Google Scholar]
- 69. Omolu A, Bailly M, Day RM. Assessment of solid microneedle rollers to enhance transmembrane delivery of doxycycline and inhibition of MMP activity. Drug Deliv 2017;24:942–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tati R, Nordin S, Abdillahi S, Mörgelin M. Biological wound matrices with native dermis-like collagen efficiently modulate protease activity. J Wound Care 2018;27:199–209. [DOI] [PubMed] [Google Scholar]
- 71. Moues CM, van Toorenenbergen AW, Heule F, Hop WC, Hovius SE. The role of topical negative pressure in wound repair: expression of biochemical markers in wound fluid during wound healing. Wound Repair Regen 2008;16:488–494. [DOI] [PubMed] [Google Scholar]
- 72. Levin M, Udi Y, Solomonov I, Sagi I. Next generation matrix metalloproteinase inhibitors—novel strategies bring new prospects. Biochim Biophys Acta Mol Cell Res 2017;1864(11 Pt A):1927–1939. [DOI] [PubMed] [Google Scholar]
- 73. Percival SL, McCarty S, Hunt JA, Woods EJ. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen 2014;22:174–186. [DOI] [PubMed] [Google Scholar]
- 74. Wallace L, Gwynne L, Jenkins T. Challenges and opportunities of pH in chronic wounds. Ther Deliv 2019;10:719–735. [DOI] [PubMed] [Google Scholar]
- 75. Roberts G, Hammad L, Collins C, Shearman C, Mani R. Some effects of sustained compression on ulcerated tissues. Angiology 2002;53:451–456. [DOI] [PubMed] [Google Scholar]
- 76. Tsukada K, Tokunaga K, Iwama T, Mishima Y. The pH changes of pressure ulcers related to the healing process of wounds. Wounds 1992;4:16–20. [Google Scholar]
- 77. Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen 2011;19:134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep 2018;7:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Greener B, Hughes AA, Bannister NP, Douglass J. Proteases and pH in chronic wounds. J Wound Care 2013;14:59–61. [DOI] [PubMed] [Google Scholar]
- 80. Salvo P, Dini V, Kirchhain A, et al. Sensors and biosensors for C-reactive protein, temperature and pH, and their applications for monitoring wound healing: a review. Sensors (Basel) 2017;17:2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lawton AS, Robson MC. The influence of pH on chronic wound healing and the antimicrobial activity of chlorine. Wound Manage Prev 2018;64:8–10. [Google Scholar]
- 82. Percival SL, Thomas J, Linton S, Okel T, Corum L, Slone W. The antimicrobial efficacy of silver on antibiotic-resistant bacteria isolated from burn wounds. Int Wound J 2011;9:488–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Oryan A, Alemzadeh E, Moshiri A. Biological properties and therapeutic activities of honey in wound healing: a narrative review and meta-analysis. J Tissue Viability 2016;25:98–118. [DOI] [PubMed] [Google Scholar]
- 84. Wang J, Chen XY, Zhao Y, et al. pH-Switchable antimicrobial nanofiber networks of hydrogel eradicate biofilm and rescue stalled healing in chronic wounds. ACS Nano 2019;13:11686–11697. [DOI] [PubMed] [Google Scholar]
- 85. Dissemond J, Witthoff M, Brauns TC, Haberer D, Goos M. [pH values in chronic wounds. Evaluation during modern wound therapy]. Hautarzt 2003;54:959–965. [DOI] [PubMed] [Google Scholar]
- 86. Kassal P, Zubak M, Scheipl G, Mohr GJ, Steinberg MD, Murković Steinberg I. Smart bandage with wireless connectivity for optical monitoring of pH. Sens Actuators B Chem 2017;246:455–460. [Google Scholar]
- 87. Bernatchez SF, Menon V, Stoffel J, et al. Nitric oxide levels in wound fluid may reflect the healing trajectory. Wound Repair Regen 2013;21:410–417. [DOI] [PubMed] [Google Scholar]
- 88. Jiang S, Cheng R, Wang X, et al. Real-time electrical detection of nitric oxide in biological systems with sub-nanomolar sensitivity. Nat Commun 2013;4:2225. [DOI] [PubMed] [Google Scholar]
- 89. Fernandez ML, Upton Z, Shooter GK. Uric acid and xanthine oxidoreductase in wound healing. Curr Rheumatol Rep 2014;16:396. [DOI] [PubMed] [Google Scholar]
- 90. Fernandez MLU, Z., Edwards H, Finlayson K, Shooter GK.. Elevated uric acid correlates with wound severity. Int Wound J 2011;9:139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens Bioelectron 2018;117:696–705. [DOI] [PubMed] [Google Scholar]
- 92. Kassal P, Kim J, Kumar R, et al. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem Commun 2015;56:6–10. [Google Scholar]
- 93. Jarosova R, McClure SE, Gajda M, et al. Inkjet-printed carbon nanotube electrodes for measuring pyocyanin and uric acid in a wound fluid simulant and culture media. Anal Chem 2019;91:8835–8844. [DOI] [PubMed] [Google Scholar]
- 94. Liu X, Lillehoj PB. Embroidered electrochemical sensors on gauze for rapid quantification of wound biomarkers. Biosens Bioelectron 2017;98:189–194. [DOI] [PubMed] [Google Scholar]
- 95. RoyChoudhury S, Umasankar Y, Jaller J, et al. Continuous monitoring of wound healing using a wearable enzymatic uric acid biosensor. J Electrochem Soc 2018;165:B3168–B3175. [Google Scholar]
- 96. Gardner S, Frantz R, Doebbeling B. The validity of clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair Regen 2001;9:178–186. [DOI] [PubMed] [Google Scholar]
- 97. Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132–e173. [DOI] [PubMed] [Google Scholar]
- 98. Glaudemans AW, Uckay I, Lipsky BA. Challenges in diagnosing infection in the diabetic foot. Diabet Med 2015;32:748–759. [DOI] [PubMed] [Google Scholar]
- 99. Gardner SE, Hillis SL, Frantz RA. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs 2009;11:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chakraborti C, Le C, Yanofsky A. Sensitivity of superficial cultures in lower extremity wounds. J Hosp Med 2010;5:415–420. [DOI] [PubMed] [Google Scholar]
- 101. Martin JM, Zenilman JM, Lazarus GS. Molecular microbiology: new dimensions for cutaneous biology and wound healing. J Invest Dermatol 2010;130:38–48. [DOI] [PubMed] [Google Scholar]
- 102. Kallstrom G. Are quantitative bacterial wound cultures useful? J Clin Microbiol 2014;52:2753–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tatum OL, Dowd SE. Wound healing finally enters the age of molecular diagnostic medicine. Adv Wound Care (New Rochelle) 2012;1:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Omar A, Wright JB, Schultz G, Burrell R, Nadworny P. Microbial biofilms and chronic wounds. Microorganisms 2017;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gompelman M, van Asten SA, Peters EJ. Update on the role of infection and biofilms in wound healing: pathophysiology and treatment. Plast Reconstr Surg 2016;138(3 Suppl):61S–70S. [DOI] [PubMed] [Google Scholar]
- 106. James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 107. Weigelt MA, McNamara SA, Sanchez PA, Kirsner RS. Evidence-based review of antibiofilm agents for wound care. Adv Wound Care 2020;10:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Davis SC, Harding A, Gil J, et al. Effectiveness of a polyhexanide irrigation solution on methicillin-resistant Staphylococcus aureus biofilms in a porcine wound model. Int Wound J 2017;14:937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lenselink E, Andriessen A. A cohort study on the efficacy of a polyhexanide-containing biocellulose dressing in the treatment of biofilms in wounds. J Wound Care 2011;20:534., 536–539. [DOI] [PubMed] [Google Scholar]
- 110. Yang Q, Larose C, Della Porta AC, Schultz GS, Gibson DJ. A surfactant-based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int Wound J 2017;14:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zölß C, Cech JD. Efficacy of a new multifunctional surfactant-based biomaterial dressing with 1% silver sulphadiazine in chronic wounds. Int Wound J 2016;13:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Palumbo F, Harding KG, Abbritti F, et al. New surfactant-based dressing product to improve wound closure rates of nonhealing wounds: a European multicenter study including 1036 patients. Wounds 2016;28:233–240. [PubMed] [Google Scholar]
- 113. Miller KG, Tran PL, Haley CL, et al. Next science wound gel technology, a novel agent that inhibits biofilm development by gram-positive and gram-negative wound pathogens. Antimicrob Agents Chemother 2014;58:3060–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wolcott R. Disrupting the biofilm matrix improves wound healing outcomes. J Wound Care 2015;24:366–371. [DOI] [PubMed] [Google Scholar]
- 115. Kim D, Namen Ii W, Moore J, et al. Clinical assessment of a biofilm-disrupting agent for the management of chronic wounds compared with standard of care: a therapeutic approach. Wounds 2018;30:120–130. [PubMed] [Google Scholar]
- 116. Akiyama H, Oono T, Saito M, Iwatsuki K. Assessment of cadexomer iodine against Staphylococcus aureus biofilm in vivo and in vitro using confocal laser scanning microscopy. J Dermatol 2004;31:529–534. [DOI] [PubMed] [Google Scholar]
- 117. Roche ED, Woodmansey EJ, Yang Q, Gibson DJ, Zhang H, Schultz GS. Cadexomer iodine effectively reduces bacterial biofilm in porcine wounds ex vivo and in vivo. Int Wound J 2019;16:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Malone M, Johani K, Jensen SO, et al. Effect of cadexomer iodine on the microbial load and diversity of chronic non-healing diabetic foot ulcers complicated by biofilm in vivo. J Antimicrob Chemother 2017;72:2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. O'Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database Syst Rev 2014:CD003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lu J, Cokcetin NN, Burke CM, et al. Honey can inhibit and eliminate biofilms produced by Pseudomonas aeruginosa. Sci Rep 2019;9:18160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Clark M, Adcock L. Honey for Wound Management: a Review of Clinical Effectiveness and Guidelines. Canadian Agency for Drugs and Technologies in Health. https://www.ncbi.nlm.nih.gov/books/NBK538361/. Published 2018 (last accessed April 9, 2020). [PubMed]
- 122. Sakarya S, Gunay N, Karakulak M, Ozturk B, Ertugrul B. Hypochlorous Acid: an ideal wound care agent with powerful microbicidal, antibiofilm, and wound healing potency. Wounds 2014;26:342–350. [PubMed] [Google Scholar]
- 123. Armstrong DG, Bohn G, Glat P, et al. Expert recommendations for the use of hypochlorous solution: science and clinical application. Ostomy Wound Manage 2015;61:S2–S19. [PubMed] [Google Scholar]
- 124. Rupel K, Zupin L, Ottaviani G, et al. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. NPJ Biofilms Microbiomes 2019;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Crone S, Garde C, Bjarnsholt T, Alhede M. A novel in vitro wound biofilm model used to evaluate low-frequency ultrasonic-assisted wound debridement. J Wound Care 2015;24:64., 66–69, 72. [DOI] [PubMed] [Google Scholar]
- 126. Chang YR, Perry J, Cross K. Low-frequency ultrasound debridement in chronic wound healing: a systematic review of current evidence. Plast Surg (Oakv) 2017;25:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kim H, Park S, Housler G, Marcel V, Cross S, Izadjoo M. An overview of the efficacy of a next generation electroceutical wound care device. Mil Med 2016;181(5 Suppl):184–190. [DOI] [PubMed] [Google Scholar]
- 128. Banerjee J, Das Ghatak P, Roy S, et al. Silver-zinc redox-coupled electroceutical wound dressing disrupts bacterial biofilm. PLoS One 2015;10:e0119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Barki KG, Das A, Dixith S, et al. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann Surg 2019;269:756–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Whitcomb E, Monroe N, Hope-Higman J, Campbell P. Demonstration of a microcurrent-generating wound care device for wound healing within a rehabilitation center patient population. J Am Coll Clin Wound Spec 2012;4:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tomic-Canic M, Burgess JL, O'Neill KE, Strbo N, Pastar I. Skin microbiota and its interplay with wound healing. Am J Clin Dermatol 2020;21(Suppl 1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Huitema L, Phillips T, Alexeev V, Tomic-Canic M, Pastar I, Igoucheva O. Intracellular escape strategies of Staphylococcus aureus in persistent cutaneous infections. Exp Dermatol 2020 [Epub ahead of print]; DOI: 10.1111/exd.14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Garcia-Salinas S, Gámez E, Landa G, Arruebo M, Irusta S, Mendoza G. Antimicrobial wound dressings against fluorescent and methicillin-sensitive intracellular pathogenic bacteria. ACS APpl Mater Interfaces 2020;12:51302–51313. [DOI] [PubMed] [Google Scholar]
- 134. Montanari E, Mancini P, Galli F, et al. Biodistribution and intracellular localization of hyaluronan and its nanogels. A strategy to target intracellular S. aureus in persistent skin infections. J Control Release 2020;326:1–12. [DOI] [PubMed] [Google Scholar]
- 135. Jeandrot A, Richard JL, Combescure C, et al. Serum procalcitonin and C-reactive protein concentrations to distinguish mildly infected from non-infected diabetic foot ulcers: a pilot study. Diabetologia 2008;51:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ingram JR, Cawley S, Coulman E, et al. Levels of wound calprotectin and other inflammatory biomarkers aid in deciding which patients with a diabetic foot ulcer need antibiotic therapy (INDUCE study). Diabet Med 2018;35:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Umapathy D, Dornadula S, Rajagopalan A, et al. Potential of circulatory procalcitonin as a biomarker reflecting inflammation among South Indian diabetic foot ulcers. J Vasc Surg 2018;67:1283–1291.e1282. [DOI] [PubMed] [Google Scholar]
- 138. Uzun G, Solmazgul E, Curuksulu H, et al. Procalcitonin as a diagnostic aid in diabetic foot infections. J Exp Med 2007;213:305–312. [DOI] [PubMed] [Google Scholar]
- 139. Jonaidi Jafari N, Safaee Firouzabadi M, Izadi M, Safaee Firouzabadi MS, Saburi A. Can procalcitonin be an accurate diagnostic marker for the classification of diabetic foot ulcers? Int J Endocrinol Metab 2014;12:e13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Massara M, De Caridi G, Serra R, et al. The role of procalcitonin as a marker of diabetic foot ulcer infection. Int Wound J 2017;14:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ong E, Farran S, Salloum M, et al. Does everything that's counted count? Value of inflammatory markers for following therapy and predicting outcome in diabetic foot infection. Int J Low Extrem Wounds 2017;16:104–107. [DOI] [PubMed] [Google Scholar]
- 142. Fritz JM, McDonald JR. Osteomyelitis: approach to diagnosis and treatment. Phys Sportsmed 2008;36:nihpa116823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Goodfield MJD. C-reactive protein levels in venous ulceration: an indication of infection? JAAD 1988;18:1048–1052. [DOI] [PubMed] [Google Scholar]
- 144. Liu T, Yang F, Li Z, Yi S, Bai X. A Prospective pilot study to evaluate wound outcomes and levels of serum C-reactive protein and interleukin-6 in the wound fluid of patients with trauma-related chronic wounds. Ostomy Wound Manage 2014;60:30–37. [PubMed] [Google Scholar]
- 145. Blokhuis-Arkes MH, Haalboom M, van der Palen J, et al. Rapid enzyme analysis as a diagnostic tool for wound infection: comparison between clinical judgment, microbiological analysis, and enzyme analysis. Wound Repair Regen 2015;23:345–352. [DOI] [PubMed] [Google Scholar]
- 146. Hasmann A, Wehrschuetz-Sigl E, Kanzler G, et al. Novel peptidoglycan-based diagnostic devices for detection of wound infection. Diagn Microbiol Infect Dis 2011;71:12–23. [DOI] [PubMed] [Google Scholar]
- 147. Hasmann A, Wehrschuetz-Sigl E, Marold A, et al. Analysis of myeloperoxidase activity in wound fluids as a marker of infection. Ann Clin Biochem 2013;50(Pt 3):245–254. [DOI] [PubMed] [Google Scholar]
- 148. Tegl G, Rollett A, Dopplinger J, Gamerith C, Guebitz GM. Chitosan based substrates for wound infection detection based on increased lysozyme activity. Carbohydr Polym 2016;151:260–267. [DOI] [PubMed] [Google Scholar]
- 149. Schiffer D, Tegl G, Vielnascher R, et al. Myeloperoxidase-responsive materials for infection detection based on immobilized aminomethoxyphenol. Biotechnol Bioeng 2016;113:2553–2560. [DOI] [PubMed] [Google Scholar]
- 150. Schiffer D, Blokhuis-Arkes M, van der Palen J, Sigl E, Heinzle A, Guebitz GM. Assessment of infection in chronic wounds based on the activities of elastase, lysozyme and myeloperoxidase. Br J Dermatol 2015;173:1529–1531. [DOI] [PubMed] [Google Scholar]
- 151. Hajnsek M, Schiffer D, Harrich D, et al. An electrochemical sensor for fast detection of wound infection based on myeloperoxidase activity. Sens Actuators B Chem 2015;209:265–274. [Google Scholar]
- 152. Thomas AN, Riazanskaia S, Cheung W, et al. Novel noninvasive identification of biomarkers by analytical profiling of chronic wounds using volatile organic compounds. Wound Repair Regen 2010;18:391–400. [DOI] [PubMed] [Google Scholar]
- 153. Ashrafi M, Bates M, Baguneid M, Alonso-Rasgado T, Rautemaa-Richardson R, Bayat A. Volatile organic compound detection as a potential means of diagnosing cutaneous wound infections. Wound Repair Regen 2017;25:574–590. [DOI] [PubMed] [Google Scholar]
- 154. Wilson AD, Baietto M. Advances in electronic-nose technologies developed for biomedical applications. Sensors (Basel) 2011;11:1105–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Greenwood JE, Crawley BA, Clark SL, et al. Monitoring wound healing by odour. J Wound Care 1997;6:219–221. [DOI] [PubMed] [Google Scholar]
- 156. Tian F, Xu X, Shen Y, et al. Detection of wound pathogen by an intelligent electronic nose. Sens Mater 2009;21:155–166. [Google Scholar]
- 157. Byun H-G, Persaud KC, Pisanelli AM. Wound-state monitoring for burn patients using E-nose/SPME system. ETRI J 2010;32:440–446. [Google Scholar]
- 158. Šetkus A, Galdikas A-J, Kancleris Ž-A, et al. Featuring of bacterial contamination of wounds by dynamic response of SnO2 gas sensor array. Sens Actuators B Chem 2006;115:412–420. [Google Scholar]
- 159. Ashrafi M, Novak-Frazer L, Bates M, et al. Validation of biofilm formation on human skin wound models and demonstration of clinically translatable bacteria-specific volatile signatures. Sci Rep 2018;8:9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ashrafi M, Novak-Frazer L, Morris J, Baguneid M, Rautemaa-Richardson R, Bayat A. Electrical stimulation disrupts biofilms in a human wound model and reveals the potential for monitoring treatment response with volatile biomarkers. Wound Repair Regen 2019;27:5–18. [DOI] [PubMed] [Google Scholar]
- 161. Hahm G, Glaser JJ, Elster EA. Biomarkers to predict wound healing: the future of complex war wound management. Plast Reconstr Surg 2011;127 Suppl 1:21S–26S. [DOI] [PubMed] [Google Scholar]
- 162. Chromy BA, Eldridge A, Forsberg JA, et al. Wound outcome in combat injuries is associated with a unique set of protein biomarkers. J Transl Med 2013;11:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Brown TS, Hawksworth JS, Sheppard FR, Tadaki DK, Elster E. Inflammatory response is associated with critical colonization in combat wounds. Surg Infect 2011;12:351–357. [DOI] [PubMed] [Google Scholar]
- 164. Brown TS, Safford S, Caramanica J, Elster EA. Biomarker use in tailored combat casualty care. Biomark Med 2010;4:465–473. [DOI] [PubMed] [Google Scholar]
- 165. Utz ER, Elster EA, Tadaki DK, et al. Metalloproteinase expression is associated with traumatic wound failure. J Surg Res 2010;159:633–639. [DOI] [PubMed] [Google Scholar]
- 166. Forsberg JA, Potter BK, Wagner MB, et al. Lessons of war: turning data into decisions. EBioMedicine 2015;2:1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Stojadinovic A, Elster E, Potter BK, et al. Combat wound initiative program. Mil Med 2010;175:18–24. [DOI] [PubMed] [Google Scholar]
- 168. Hawksworth JS, Stojadinovic A, Gage FA, et al. Inflammatory biomarkers in combat wound healing. Ann Surg 2009;250:1002–1007. [DOI] [PubMed] [Google Scholar]
- 169. Forsberg JA, Elster EA, Andersen RC, et al. Correlation of procalcitonin and cytokine expression with dehiscence of wartime extremity wounds. J Bone Joint Surg Am 2008;90:580–588. [DOI] [PubMed] [Google Scholar]
- 170. Forsberg JA, Potter BK, Polfer EM, Safford SD, Elster EA. Do inflammatory markers portend heterotopic ossification and wound failure in combat wounds? Clin Orthop Relat Res 2014;472:2845–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Janak JC, Stewart IJ, Sosnov JA, et al. Urinary biomarkers are associated with severity and mechanism of injury. Shock 2017;47:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Dente CJ, Bradley M, Schobel S, et al. Towards precision medicine: accurate predictive modeling of infectious complications in combat casualties. J Trauma Acute Care Surg 2017;83:609–616. [DOI] [PubMed] [Google Scholar]
- 173. Raizmann R, Dunham D, Lindvere-Teene L, et al. Use of a bacterial fluorescence imaging device: wound measurement, bacterial detection and targeted debridement. J Wound Care 2019;28:824–834. [DOI] [PubMed] [Google Scholar]
- 174. Thatcher JE, Squiers JJ, Kanick SC, et al. Imaging techniques for clinical burn assessment with a focus on multispectral imaging. Adv Wound Care (New Rochelle) 2016;5:360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Lucas VS, Burk RS, Creehan S, Grap MJ. Utility of high-frequency ultrasound: moving beyond the surface to detect changes in skin integrity. Plast Surg Nurs 2014;34:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zmudzinska M, Czarnecka-Operacz M, Silny W. Principles of dermatologic ultrasound diagnostics. Acta Dermatovenerol Croat 2008;16:126–129. [PubMed] [Google Scholar]
- 177. Sen CK, Ghatak S, Gnyawali SC, Roy S, Gordillo GM. Cutaneous imaging technologies in acute burn and chronic wound care. Plast Reconstr Surg 2016;138(3 Suppl):119S–128S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhang Y, Zhao R, Cao Y, et al. Ultrasonography superior over visual assessment in evaluation of wound healing after dermabrasion. J Surg Res 2019;234:202–209. [DOI] [PubMed] [Google Scholar]
- 179. Mohafez H, Ahmad SA, Hadizadeh M, et al. Quantitative assessment of wound healing using high-frequency ultrasound image analysis. Skin Res Technol 2018;24:45–53. [DOI] [PubMed] [Google Scholar]
- 180. Mukai K, Zhu W, Nakajima Y, Kobayashi M, Nakatani T. Non-invasive longitudinal monitoring of angiogenesis in a murine full-thickness cutaneous wound healing model using high-resolution three-dimensional ultrasound imaging. Skin Res Technol 2017;23:581–587. [DOI] [PubMed] [Google Scholar]
- 181. Moghimi S, Miran Baygi MH, Torkaman G, Mahloojifar A. Quantitative assessment of pressure sore generation and healing through numerical analysis of high-frequency ultrasound images. J Rehabil Res Dev 2010;47:99–108. [DOI] [PubMed] [Google Scholar]
- 182. Wortsman X, Navarrete N. Two-dimensional and three-dimensional ultrasound of artificial skin. J Ultrasound Med 2017;36:225–230. [DOI] [PubMed] [Google Scholar]
- 183. Gnyawali SC, Sinha M, El Masry MS, et al. High resolution ultrasound imaging for repeated measure of wound tissue morphometry, biomechanics and hemodynamics under fetal, adult and diabetic conditions. PLoS One 2020;15:e0241831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Kuhn C, Angehrn F. Use of high-resolution utlrasound to monitor the healing of leg ulcers: a prospective single-center study. Skin Res Technol 2009;15:161–167. [DOI] [PubMed] [Google Scholar]
- 185. Dyson M, Moodley S, Verjee L, Verling W, Weinman J, Wilson P. Wound healing assessment using 20 MHz ultrasound and photography. Skin Res Technol 2003;9:116–121. [DOI] [PubMed] [Google Scholar]
- 186. Matsumoto M, Nakagami G, Kitamura A, et al. Ultrasound assessment of deep tissue on the wound bed and periwound skin: a classification system using ultrasound images. J Tissue Viability 2021;30:28–35. [DOI] [PubMed] [Google Scholar]
- 187. Scheiner J, Farid K, Raden M, Demisse S. Ultrasound to detect pressure-related deep tissue injuries in adults admitted via the emergency department: a prospective, descriptive, pilot study. Wound Manage Prev 2017;63:36–46. [PubMed] [Google Scholar]
- 188. Helvig EI, Nichols LW. Use of high-frequency ultrasound to detect heel pressure injury in elders. J Wound Ostomy Continence Nurs 2012;39:500–508. [DOI] [PubMed] [Google Scholar]
- 189. Aoi N, Yoshimura K, Kadono T, et al. Ultrasound assessment of deep tissue injury in pressure ulcers: possible prediction of pressure ulcer progression. Plast Reconstr Surg 2009;124:540–550. [DOI] [PubMed] [Google Scholar]
- 190. Croce AC, Bottiroli G. Autofluorescence spectroscopy and imaging: a tool for biomedical research and diagnosis. Eur J Histochem 2014;58:2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Prabhu V, Acharya A, Satish Rao BS, et al. Probing endogenous collagen by laser-induced autofluorescence in burn wound biopsies: a pilot study. J Biophotonics 2018;11:e201700394. [DOI] [PubMed] [Google Scholar]
- 192. Mehrvar S, Rymut KT, Foomani FH, et al. Fluorescence imaging of mitochondrial redox state to assess diabetic wounds. IEEE J Transl Eng Health Med 2019;7:1800809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Blumenthal E, Jeffery S. Autofluorescence imaging for evaluating debridement in military and trauma wounds. Mil Med 2018;183(suppl_1):429–432. [DOI] [PubMed] [Google Scholar]
- 194. Hurley C, McClusky P, Sugrue R, Clover J, Kelly J. Efficacy of a bacterial fluorescence imaging device in an oupatient wound care clinic: a pilot study. J Wound Care 2019;28:438–443. [DOI] [PubMed] [Google Scholar]
- 195. Rennie M, Lindvere-Teene L, Tapang K, Linden R. Point-of-care fluorescence imaging predicts the presence of pathogenic bacteria in wounds: a clinical study. J Wound Care 2017;26:452–460. [DOI] [PubMed] [Google Scholar]
- 196. Ottolino-Perry K, Chamma E, Blackmore KM, et al. Improved detection of clinically relevant wound bacteria using autofluorescence image-guided sampling in diabetic foot ulcers. Int Wound J 2017;14:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Farhan N, Jeffery S. Utility of MolecuLight i:X for managing bacterial burden in pediatric burns. J Burn Care Res 2020;41:328–338. [DOI] [PubMed] [Google Scholar]
- 198. Serena T, Harrell K, Serena L, Yaakov R. Real-time bacterial fluorescence imaging accurately identifies wounds with moderate-to-heavy bacterial burden. J Wound Care 2019;28:346–357. [DOI] [PubMed] [Google Scholar]
- 199. Pijpie A, Ozdemir Y, Sinnige J, Kwa K, Middelkoop E, Meij-de Vries A. Detection of bacteria in burn wounds with a novel handheld autofluorescence wound imaging device: a pilot study. J Wound Care 2019;28:548–554. [DOI] [PubMed] [Google Scholar]
- 200. DaCosta RS, Kulbatski I, Lindvere-Teene L, et al. Point-of-care autofluorescence imaging for real-time sampling and treatment guidance of bioburden in chronic wounds: first-in-human results. PLoS One 2015;10:e0116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Calin MA, Parasca SV, Savastru R, Manea D. Characterization of burns using hyperspectral imaging technique—a preliminary study. Burns 2015;41:118–124. [DOI] [PubMed] [Google Scholar]
- 202. Jayachandran M, Rodriguez S, Solis E, Lei J, Godavarty A. Critical review of noninvasive optical technologies for wound imaging. Adv Wound Care (New Rochelle) 2016;5:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Khaodhiar L, Dinh T, Schomacker KT, et al. The use of medical hyperspectral technology to evaluate microcirculatory changes in diabetic foot ulcers and to predict clinical outcomes. Diabetes Care 2007;30:903–910. [DOI] [PubMed] [Google Scholar]
- 204. Nouvong A, Hoogwerf B, Mohler E, Davis B, Tajaddini A, Medenilla E. Evaluation of diabetic foot ulcer healing with hyperspectral imaging of oxyhemoglobin and deoxyhemoglobin. Diabetes Care 2009;32:2056–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Jeffcoate WJ, Clark DJ, Savic N, et al. Use of HSI to measure oxygen saturation in the lower limb and its correlation with healing of foot ulcers in diabetes. Diabet Med 2015;32:798–802. [DOI] [PubMed] [Google Scholar]
- 206. Baxter R, Thatcher J, Plant K, et al. Development of a non-invasive imaging device for prediction of diabetic foot ulcer healing potential. Paper presented at: Symposium on Advanced Wound Care Spring; May 7–11, 2019; San Antonio, TX. [Google Scholar]
- 207. Yudovsky D, Nouvong A, Schomacker K, Pilon L. Assessing diabetic foot ulcer development risk with hyperspectral tissue oximetry. J Biomed Opt 2011;16:026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Vasefi F, MacKinnon N, Saager RB, et al. Polarization-sensitive hyperspectral imaging in vivo: a multimode dermoscope for skin analysis. Sci Rep 2014;4:4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Lindert J, Tafazzoli-Lari K, Tushaus L, et al. Optical coherence tomography provides an optical biopsy of burn wounds in children-a pilot study. J Biomed Opt 2018;23:1–6. [DOI] [PubMed] [Google Scholar]
- 210. Holmes J, Schuh S, Bowling FL, Mani R, Welzel J. Dynamic optical coherence tomography is a new technique for imaging skin around lower extremity wounds. Int J Low Extrem Wounds 2019;18:65–74. [DOI] [PubMed] [Google Scholar]
- 211. Greaves NS, Iqbal SA, Hodgkinson T, et al. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair Regen 2015;23:483–494. [DOI] [PubMed] [Google Scholar]
- 212. Park KS, Choi WJ, Song S, Xu J, Wang RK. Multifunctional in vivo imaging for monitoring wound healing using swept-source polarization-sensitive optical coherence tomography. Lasers Surg Med 2018;50:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Li J, Bower AJ, Arp Z, et al. Investigating the healing mechanisms of an angiogenesis-promoting topical treatment for diabetic wounds using multimodal microscopy. J Biophotonics 2018;11(3):10..1002/jbio.201700195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Glinos GD, Verne SH, Aldahan AS, et al. Optical coherence tomography for assessment of epithelialization in a human ex vivo wound model. Wound Repair Regen 2017;25:1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Greaves NS, Benatar B, Whiteside S, Alonso-Rasgado T, Baguneid M, Bayat A. Optical coherence tomography: a reliable alternative to invasive histological assessment of acute wound healing in human skin? Br J Dermatol 2014;170:840–850. [DOI] [PubMed] [Google Scholar]
- 216. Kuck M, Strese H, Alawi SA, et al. Evaluation of optical coherence tomography as a non-invasive diagnostic tool in cutaneous wound healing. Skin Res Technol 2014;20:1–7. [DOI] [PubMed] [Google Scholar]
- 217. Kitada T, DiAndreth B, Teague B, Weiss R. Programming gene and engineered-cell therapies with synthetic biology. Science 2018;359:eaad1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Braff D, Shis D, Collins JJ. Synthetic biology platform technologies for antimicrobial applications. Adv Drug Deliv Rev 2016;105(Pt A):35–43. [DOI] [PubMed] [Google Scholar]
- 219. Sadat Mousavi P, Smith SJ, Chen JB, et al. A multiplexed, electrochemical interface for gene-circuit-based sensors. Nat Chem 2020;12:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Li H, Tang Y, Li B. Homogeneous and universal detection of various targets with a dual-step transduced toehold switch sensor. Chembiochem 2020;21:1418–1422. [DOI] [PubMed] [Google Scholar]
- 221. Takahashi MK, Tan X, Dy AJ, et al. A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers. Nat Commun 2018;9:3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Chin LY, Carroll C, Raigani S, et al. Ex vivo perfusion-based engraftment of genetically engineered cell sensors into transplantable organs. PLoS One 2019;14:e0225222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Xiao M, Lai W, Wang F, Li L, Fan C, Pei H. Programming drug delivery kinetics for active burst release with DNA toehold switches. J Am Chem Soc 2019;141:20354–20364. [DOI] [PubMed] [Google Scholar]
- 224. Derakhshandeh H, Kashaf SS, Aghabaglou F, Ghanavati IO, Tamayol A. Smart bandages: the future of wound care. Trends Biotechnol 2018;36:1259–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Gamus A, Keren E, Kaufman H, Chodick G. Synchronous video telemedecine in lower extremities ulcers treatment: a real-world data study. Int J Med Inf 2019;124:31–36. [DOI] [PubMed] [Google Scholar]
- 226. Le Goff-Pronost M, Mourgeon B, Blanchere JP, Teot L, Benateau H, Dompmartin A. Real-world clinical evaluation and costs of telemedicine for chronic wound management. Int J Technol Assess Health Care 2018;34:567–575. [DOI] [PubMed] [Google Scholar]
- 227. Teot L, Geri C, Lano J, Cabrol M, Linet C, Mercier G. Complex wound healing outcomes for outpatients receiving care via telemedicine, home health, or wound clinic: a randomized controlled trial. Int J Low Extrem Wounds 2020;19:197–204. [DOI] [PubMed] [Google Scholar]
- 228. Arora M, Harvey LA, Glinsky JV, et al. Cost-effectiveness analysis of telephone-based support for the management of pressure ulcers in people with spinal cord injury in India and Bangladesh. Spinal Cord 2017;55:1071–1078. [DOI] [PubMed] [Google Scholar]
- 229. Stern A, Mitsakakis N, Paulden M, et al. Pressure ulcer multidisciplinary teams via telemedicine: a pragmatic cluster randomized stepped wedge trial in long term care. BMC Health Serv Res 2014;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Bolton L. Telemedicine improves chronic ulcer outcomes. Wounds 2019;31:114–116. [PubMed] [Google Scholar]
- 231. Fasterholdt I, Gerstrom M, Rasmussen BSB, Yderstraede KB, Kidholm K, Pedersen KM. Cost-effectiveness of telemonitoring of diabetic foot ulcer patients. Health Informatics J 2018;24:245–258. [DOI] [PubMed] [Google Scholar]
- 232. McLister A, McHugh J, Cundell J, Davis J. New developments in smart bandage technologies for wound diagnostics. Adv Mater 2016;28:5732–5737. [DOI] [PubMed] [Google Scholar]
- 233. Gianino E, Miller C, Gilmore J. Smart wound dressings for diabetic chronic wounds. Bioengineering (Basel) 2018;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Qiao B, Pang Q, Yuan P, Luo Y, Ma L. Smart wound dressing for infection monitoring and NIR-triggered antibacterial treatment. Biomater Sci 2020;8:1649–1657. [DOI] [PubMed] [Google Scholar]