Abstract
Significance:
Metformin has been proposed as a treatment for systemic lupus erythematosus (SLE). The primary target of metformin, the electron transport chain complex I in the mitochondria, is associated with redox homeostasis in immune cells, which plays a critical role in the pathogenesis of autoimmune diseases. This review addresses the evidence and knowledge gaps on whether a beneficial effect of metformin in lupus may be due to a restoration of a balanced redox state.
Recent Advances:
Clinical trials in SLE patients with mild-to-moderate disease activity and preclinical studies in mice have provided encouraging results for metformin. The mechanism by which this therapeutic effect was achieved is largely unknown. Metformin regulates redox homeostasis in a context-specific manner. Multiple cell types contribute to SLE, with evidence of increased mitochondrial oxidative stress in T cells and neutrophils.
Critical Issues:
The major knowledge gaps are whether the efficacy of metformin is linked to a restored redox homeostasis in the immune system, and if it does, in which cell types it occurs? We also need to know which patients may have a better response to metformin, and whether it corresponds to a specific mechanism? Finally, the identification of biomarkers to predict treatment outcomes would be of great value.
Future Directions:
Mechanistic studies must address the context-dependent pharmacological effects of metformin. Multiple cell types as well as a complex disease etiology should be considered. These studies must integrate the rapid advances made in understanding how metabolic programs direct the effector functions of immune cells. Antioxid. Redox Signal. 36, 462–479.
Keywords: metformin, lupus, mitochondria, ROS
Introduction
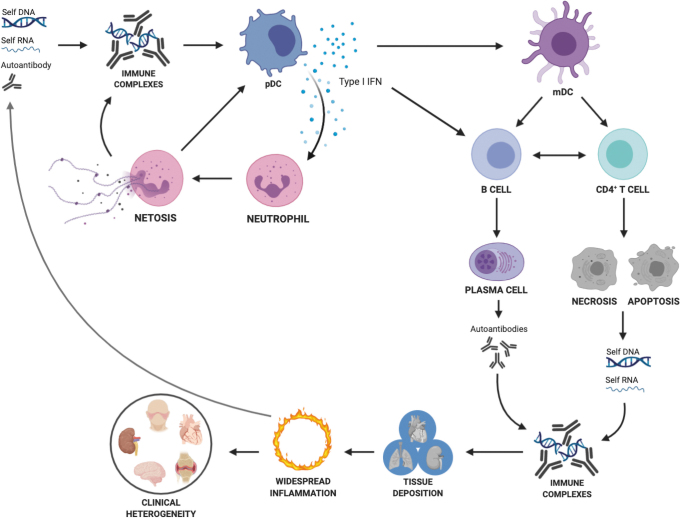
Systemic lupus erythematosus (SLE) is a clinically heterogeneous autoimmune disease in which tissue damage is caused by autoantibodies in the form of immune complexes (ICs), as well as inflammatory cytokines (Fig. 1) (136). Multiple immune cell types have been implicated in lupus pathogenesis, in addition to autoreactive B cells that differentiate into autoantibody producing cells. Autoreactive CD4+ T cells play a critical role by providing help to B cells to produce high-affinity class-switched autoantibodies and by producing inflammatory cytokines. Myeloid cells also provide critical contributions. Plasmacytoid dendritic cells (pDCs) secrete type I interferons (IFNs) in response to IC stimulation, and activated neutrophils produce neutrophil extracellular traps (NETs) that amplify the process. Immune cell activation is tightly linked to cellular metabolism in cell- and context-specific programs, which have been identified in all the major cell types involved in lupus pathogenesis. Accordingly, an activated cellular metabolism has been reported in SLE patients and in mouse models of the disease, so far largely in CD4+ T cells (86, 100).
FIG. 1.
Pathogenesis of SLE. Autoreactive B cells with the help of autoreactive CD4+ T cells lead to the production of autoantibodies, which form IC with autoantigens formed by nucleic acid–protein complexes. Increased autoantigen production from apoptotic cells and neutrophil extracellular traps as well as deregulated clearance amplify the pathogenic cascade. pDCs secrete type I IFN in response to IC stimulation. Type I IFN activate immune cells. Autoreactive CD4+ T cells also contribute to inflammation by producing inflammatory cytokines. ICs deposit in tissues and along with inflammatory cytokines, cause damage and further autoantigen release. IC, immune complexes; IFN, interferon; pDCs, plasmacytoid dendritic cells; SLE, systemic lupus erythematosus. Color images are available online.
The association between an overactive metabolism in immune cells and lupus pathogenesis has led to the proposition that metabolic inhibitors could have therapeutic benefits in lupus (103, 129). This hypothesis has been validated by a clinical trial reporting a reduction in disease activity in SLE patients treated with sirolimus, which inhibits the mammalian target of rapamycin (mTOR), a master regulator of cellular metabolism (67). Mitochondrial dysfunction, oxidative stress, and increased mitochondrial respiration feature preeminently among the alterations of CD4+ T cellular metabolism in lupus (36). Therefore, a normalization of mitochondrial functions would be expected to be beneficial in SLE.
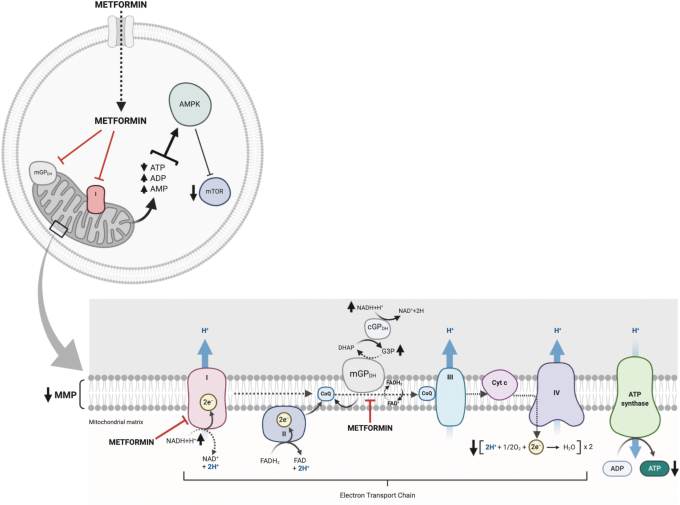
Metformin, or dimethyl biguanide, is a herbal remedy found in French lilac, which was used to treat the symptoms of the disease now known as diabetes as early as the 17th century. It was first synthesized in the 1920s, and was promoted as “Glucophage” in France in the 1950s. Metformin was approved for used in the United States in 1994, and it is now the drug recommended internationally as the first-line antidiabetic treatment. In addition to its affordability and ease of administration, its side effects are mild, with the primary concern being lactic acidosis, which occurs in <1:100,000 patients per year (132). There is a general agreement that the mitochondrion is the main target of metformin, where it inhibits the electron transport chain (ETC) complex I (nicotinamide adenine dinucleotide + hydrogen [NADH] dehydrogenase) (145) and mitochondrial glycerophosphate dehydrogenase (mGPDH) (77) (Fig. 2).
FIG. 2.
Mitochondrial mechanisms of action of metformin. Metformin is up taken into the cells mainly through the OCT1. Metformin inhibits the ETC complex I (NADH dehydrogenase) and mGPDH. By inhibiting ETC complex I, metformin suppresses oxidative phosphorylation and ATP production, which then activates AMPK and inhibits mTOR. The inhibition of mGPDH in the glycerol phosphate shuttle reduces the import of cytosolic NAD. AMPK, AMP-activated protein kinase; ATP, adenosine triphosphate; ETC, electron transport chain; mGPDH, mitochondrial glycerophosphate dehydrogenase; NAD, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide + hydrogen; OCT1, organic cation transporter 1; mTOR, mammalian target of rapamycin. Color images are available online.
By inhibiting ETC complex I, metformin can exert its regulatory effect through the suppression of oxidative phosphorylation (OXPHOS) as well as a reduction of adenosine triphosphate (ATP) production, which then activates adenosine monophosphate (AMP)-activated protein kinase (AMPK) and inhibits mTOR (140). Metformin can also increase AMP levels independently from OXPHOS by deactivating AMP deaminase (94), and it directly activates AMPK by promoting the formation of its trimeric complex (82). Moreover, metformin inhibits mTOR activation through AMPK-independent pathways (11, 56), and it can regulate non-mTOR pathways, such as the activation of p53 or NFκB (110).
The activity of ETC complex I, mGPDH, OXPHOS and the AMPK/mTOR pathway are all closely associated with redox homeostasis in immune cells, and redox homeostasis plays a critical role in the pathogenesis of autoimmune diseases (41, 44, 112, 119), including SLE (96, 98). Indeed, metformin has been proposed as a treatment for multiple autoimmune diseases based on positive results obtained in SLE (125, 141) and Behcet's disease (150) patients, as well as with results obtained from models of rheumatoid arthritis (21, 120), experimental autoimmune encephalomyelitis (126) and multiple sclerosis (89, 113), autoimmune insulitis (32), ankylosing spondylitis (108), and Sjögren's syndrome (61). The mode of action of metformin in autoimmune diseases is multifaceted and still far from being fully understood. Here, we review the effects of metformin on SLE pathogenesis from the viewpoint of redox homeostasis.
The effects of metformin on lupus pathogenesis
In a proof-of-concept trial in SLE patients with mild or moderate disease activity, adding metformin to standard therapy (mostly prednisone, a corticosteroid) reduced the risk of disease flares in half. In addition, prednisone exposure and therefore its adverse side effects were lower in the metformin add-on group than in the conventional treatment group (141). A follow-up randomized, double-blind, placebo-controlled trial was performed to test the efficacy of metformin to reduce disease flares as an add-on treatment to standard care in SLE patients with mild disease activity. Compared with placebo, metformin reduced the occurrence of major flares by 41% within the 12-month period of follow-up (125). A post hoc analysis of these two trials showed that patients with negative anti-DNA antibody and normal complement at baseline, as well as a concomitant use of hydroxychloroquine had a better response to metformin (124). These results suggest that metformin may be an effective tool to maintain SLE patients in remission with a potential steroid-sparing effect.
In the B6.Sle1.Sle2.Sle3 lupus-prone mice, metformin treatment reversed autoimmune pathogenesis when used in combination with a glucose inhibitor (149), and it delayed disease development when used as monotherapy in a preventive mode (148).
The beneficial effects of metformin were associated with a reduction of mitochondrial respiration in CD4+ T cells (149), indicating that it exerted the expected ETC inhibition, and that CD4+ T cells may be a functional target of the drug. Although T cell activation and acquisition of effector functions is marked by a sharp increase in glycolysis (76), IFN-γ production requires mitochondrial metabolism (116). The different ETC complexes have different functions in this process, with Complex I being necessary for the proliferation of Th1 cells, but not for their production on IFN-γ, while Complex III is required for both (6). Accordingly, metformin reduces the pool of IFN-γ producing T cells, while it increased interleukin-2 production in B6.Sle1.Sle2.Sle3 mice (149).
Metformin combined with a short course of treatment with CTLA4-Ig was also effective to reduce renal pathology in the NZB/WF1 model of lupus (24). This therapeutic effect was associated with a reduction in CD4+ T cell activation. In vitro, metformin altered gene expression in CD4+ T cells from SLE patients and healthy controls, with the top targeted pathways being mTOR, cMYC activation, and OXPHOS (133). As in T cells from lupus-prone mice, metformin decreased respiration in human CD4+ T cells in vitro (13, 133). Since cellular metabolism, including OXPHOS, glycolysis, and mTOR activation, is elevated in the CD4+ T cells from SLE patients and lupus-prone mice (149), this alone could account for the beneficial effect of metformin. Metformin also reduced disease severity in the Roquin san/san mouse model of lupus, at least in part through the AMPK/mTOR pathway, as well as a reduction of STAT3 activation in B and CD4+ T cells (69).
STAT3 activation is critical for the differentiation of follicular helper T (Tfh) cells, which are expanded in lupus (62). However, metformin had no effect on Tfh cells in the B6.Sle1.Sle2.Sle3 model of lupus (23). Metformin has also been shown to reduce the skewing to Th17 cells observed in CD4+ T cells of old healthy human donors (13), as well as in the Roquin san/san mouse model (69), again by inhibiting STAT3 activation. Since an expansion of Th17 cells has been associated with lupus pathogenesis (63), it is possible that a reduction of the frequency of Th17 cells could also account for the beneficial effect of metformin in lupus patients. The specific molecular mechanisms responsible for these effects are unknown.
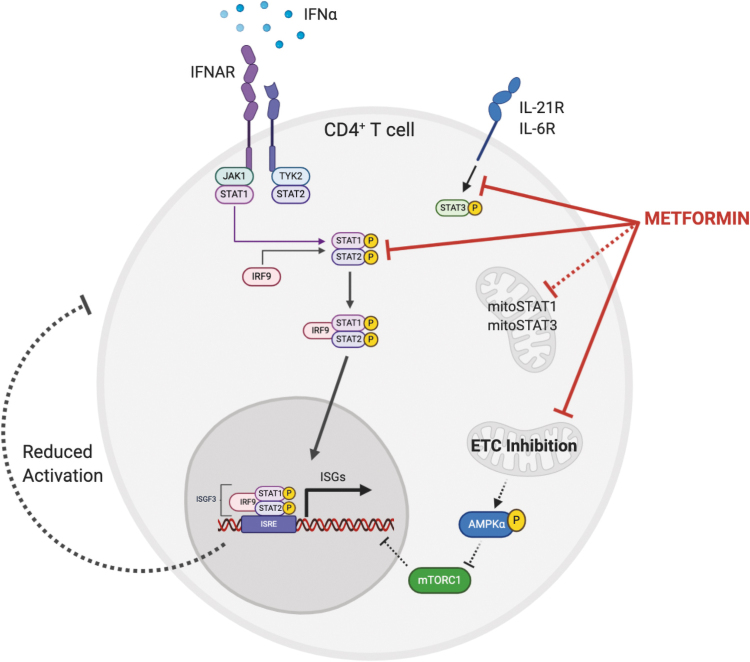
Metformin inhibited the transcription of IFN-stimulated genes (ISGs) after IFN-α treatment in CD4+ T cells from SLE patients and healthy controls (133). Since an increased activity of type I IFN is highly correlated by lupus pathogenesis, its inhibition by metformin could also be a part of its therapeutic effect. It was shown that STAT1 activation and function downstream on the type I IFN receptor were reduced by metformin. The inhibition of the ISG response was achieved with other ETC inhibitors, indicating that the effect of metformin is not unique, and that mitochondrial respiration plays a crucial role in this process (Fig. 3). However, the inhibition of ISG expression in human CD4+ T cells was independent of the AMPK/mTOR pathway (133), although this metabolic pathway is a well-recognized target of metformin (140). Therefore, the molecular link between decreased ETC activity and STAT1 activation is yet unknown.
FIG. 3.
Metformin inhibits the transcription of ISGs in IFN-α–stimulated human CD4+ T cells. STAT1 phosphorylation and function downstream on the type I IFN receptor were reduced by metformin. The inhibition of the ISG response was achieved with other ETC inhibitors, indicating that the effect of metformin is not unique, and that mitochondrial respiration plays a crucial role in this process. The inhibition of ISG expression was independent of the AMPK/mTOR pathway. Therefore, the molecular link between decreased ETC activity and STAT1 activation is unknown. It is possible that the same mechanism that is responsible for inhibiting STAT3 is also responsible for inhibiting STAT1 activation in IFN-stimulated T cells. This could involve noncanonical functions of STAT3, which in turn regulate mitochondrial metabolism. Noncanonical functions of STAT1 have also been linked to mitochondrial oxidative stress. ISG, interferon-stimulated gene. Color images are available online.
It is possible that the same mechanism that is responsible for inhibiting STAT3 activation in aging CD4+ T cells (13) and in the Roquin san/san mice (69) is also responsible for inhibiting STAT1 activation in IFN-stimulated T cells (133). Metformin directly inhibits STAT3 phosphorylation at canonical (e.g., as a nuclear transcription factor) Y705 (111), and at both canonical and noncanonical S727 (27), in which S727pSTAT3 is imported to the mitochondria where it increases ATP synthesis, calcium flux, and mitochondrial permeability transition pore (mPTP) opening while decreasing reactive oxygen species (ROS) release (109). Noncanonical functions of STAT3 have been shown to affect T cell effector functions (105). On the contrary, metformin increases canonical STAT1 phosphorylation in virally infected cells (135) and mesenchymal stem cells from lupus mice (52). Noncanonical mitochondrial functions of STAT1 have also been linked to mitochondrial oxidative stress (118). These pathways should be investigated specifically in inflammatory T cells, either due to aging or inflammatory diseases such as lupus.
A potential limitation is that metformin may increase glycolysis as a compensatory mechanism to decreased mitochondrial activity, and therefore promote T cell activation. In vitro, high, probably nonphysiological, concentrations of metformin increase glycolysis in activated T cells (149) and B cells (unpublished) from lupus mice. There was no effect however of metformin treatment in vivo on glycolysis in T cells (148), as well as no evidence for lactoacidosis. A recent study in a model of thyroiditis found that metformin decreased glycolysis in association with a reduction of mTOR activation (153). Therefore, there is no evidence supporting that a treatment with metformin could lead to a secondary activation of T cells through a compensatory increase in glycolysis.
In addition to immune cells, metformin may limit tissue injury through which lupus manifests. Oxidative stress and mitochondrial damage have been reported in the kidneys of patients with lupus nephritis as well as in animal models of the disease (12, 14) and in the liver of lupus-prone mice (99). It is likely that other end-organ targets of lupus pathogenesis also present similar oxidative stress. Their response to metformin warrants to be evaluated independently to the response of immune cells. Furthermore, it has been established that SLE patients have a higher prevalence of metabolic syndrome, which includes insulin resistance and high fasting blood glucose levels (3, 42, 84, 123). Some of these patients would be expected to be treated with metformin to lower hyperglycemia, which would potentially reduce the oxidative stress associated with metabolic syndrome, as well as lupus manifestations. However, very little, if any, data have been published on this topic (85), which should be addressed in future studies.
Although these results are encouraging to suggest that metformin may be a useful addition to the available treatments for SLE, they raise the question as to what kind of patients may have a better response to metformin. Further, if a differential response is identified among these patients, does it correspond to a specific mechanism by which metformin mitigates lupus pathogenesis? It should always be kept in mind that, contrary to immunosuppressive effects observed in autoimmune diseases such as SLE, metformin exerts immunostimulatory effects with antitumor activity (75). To account for this apparent discrepancy, it has been proposed that the effects of metformin on immune responses may vary with cell types, stimuli, and microenvironments, depending on the pathological context (80). The context-dependent pharmacological effects of metformin on the immune system need detailed investigations to address these issues. There is also a need to identify among the multitude of biomarkers that have been associated with lupus disease activity (131), or among markers of cellular metabolism, which ones may be associated with responsiveness to metformin treatment. This review focuses on the potential links between the therapeutic effects of metformin in SLE and redox homeostasis.
Redox homeostasis in lupus
Redox homeostasis is a critical factor to maintain normal cellular functions (43). Well-regulated oxidative stress triggers signaling pathways that initiate diverse cellular responses ranging from cell activation, protection from apoptosis, mitochondrial fission to autophagy (31). Both excess oxidative (40, 101, 102) and its opposite, reductive, stress in which electron acceptors are reduced, such as with defects in nicotine adenine dinucleotide phosphate (NADPH)-oxidase (NOX) (45, 93, 152), promote cellular and tissue injury (154). ROS-induced cell death exposes intracellular antigens to be targeted by the immune system, which is one of the key factors initiating and amplifying lupus pathogenesis. Redox homeostasis is attained by the sophisticated balance between the generation of active redox agents and their detoxification. The role of oxidative stress in SLE has been reviewed in detail (96, 98). Here, we summarize the mechanisms potentially targeted by metformin by which ROS are produced and detoxified, as well as the role of these mechanisms in the pathogenesis of lupus.
Mechanisms of mitochondrial ROS production
Across the inner mitochondrial membrane, a proton gradient from matrix to intermembrane space known as mitochondrial membrane potential (MMP) is maintained by a complex electron transport system. First, protons are translocated across the inner mitochondrial membrane by the ETC and mGPDH, creating a voltage gradient with negative charges inside the mitochondrial matrix. Importantly, both forward and reverse electron flows are involved, depending on specific microenvironments. Then at the expense of this electrochemical gradient, the F0F1–ATPase complex (ETC complex V) converts adenosine diphosphate (ADP) to ATP during OXPHOS. Of note, F0F1–ATPase, when inhibited, can also pump protons out of the mitochondrial matrix into the intermembrane space using energy from ATP, which increases the MMP. Physiologically, ∼2%–5% of the transported electrons uncouple from OXPHOS, forming endogenous ROS in the absence of ATP synthesis (68). Therefore, the factors regulating the rate of electron uncoupling from OXPHOS are crucial to control mitochondrial ROS (mROS) production.
Two distinct phases of mROS generation have been identified: (i) A progressive rise of ROS level (called “trigger ROS”) is usually detected after mitochondrial membrane hyperpolarization, which is strongly dependent on the MMP level above the phosphorylating membrane potential (Fig. 4A). It was reported that an 18% decrease in MMP value inhibits 90% of mROS production (65, 122). In normal conditions, MMP is sustained mainly by OXPHOS. Under certain conditions such as hypoxia or cell hyperactivity, when mitochondria are incapable of sustaining MMP driven by respiration, it is maintained at the expense of cytosolic ATP hydrolysis by mitochondrial ATPase (28). (ii) After the initial progressive rise of the ROS level, the subsequent ROS burst or oscillations associated with the MMP dissipation are named “ROS-induced ROS release” (RIRR).
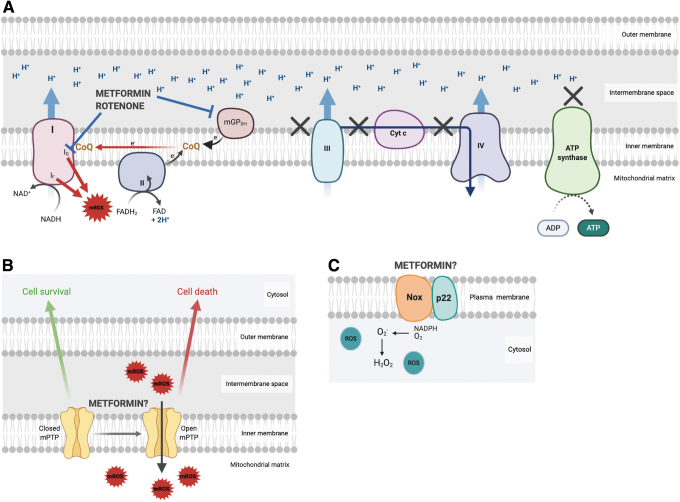
FIG. 4.
Proposed modes of action of metformin on the main sources of ROS production. (A) Metformin reduces mROS generated by electrons leaked from the ETC during reverse electron transfer by inhibiting the ETC complex I and mGPDH. (B) Mitochondrial permeability transition pore formation is triggered in part by mROS, which generates a second wave of mROS through ROS-induced ROS release. Whether metformin has any effect on this process is unknown. (C) NOX in the plasma membrane generates cytoplasmic ROS. Metformin has not been reported to interfere with this process. NOX, NADPH-oxidase; ROS, reactive oxygen species; mROS, mitochondrial ROS. Color images are available online.
Two modes of RIRR have been described: mPTP-associated RIRR (Fig. 4B) and inner membrane anion channel (IMAC)-associated RIRR. In mPTP-associated RIRR, “trigger ROS” lead the damage to the point of mPTP introduction and opening, which is accompanied by a sudden loss of MMP with the corresponding large ROS and nitric oxide (NO) burst. This mPTP-associated RIRR may induce conformational changes or loss of ETC complexes (142, 143). In IMAC-associated RIRR, oscillatory ROS are released in the cytosol through IMAC due to its permeability for superoxide produced by complex III (139). In summary, when hyperpolarization is initially induced, a higher MMP generates more ROS. To the contrary, the subsequent RIRR is associated with a sudden loss of MMP. How these two patterns linking mROS generation and MMP can be exploited for novel strategies targeting ETC complexes or mGPDH is discussed in detail below.
Mitochondrial membrane polarization and mROS production in immune cells
mROS are crucial in the early phase of T cell activation, proliferation, and selection of the cell death pathway through mitochondrial membrane hyperpolarization. Once activated through their T cell receptor (TCR), T cells present a transient and reversible mitochondrial hyperpolarization and ATP depletion. Several mechanisms contribute to this process. First, TCR signaling triggers the activation of ADP-dependent glucokinase (ADPGK). ADPGK is an alternative glycolytic enzyme that deviates glycolysis toward the mitochondrial glycerolphosphate shuttle (GPS), which downregulates respiration and ATP production. The activation of glycerol phosphate dehydrogenase (GPD2) in the GPS also increases the MMP and mROS production (7). In support of this mechanism, overexpression of ADPGK increases the generation of mROS, whereas downregulation of ADPGK or GPD2 levels inhibits this process (57).
Next, when mitochondrial ATP production is downregulated as a result of glycolysis being directed to the GPS, F0F1–ATPase uses energy to pump protons out of the mitochondrial matrix into the intermembrane space, thus causing MMP elevation (54). Finally, NO, acting as a competitive antagonist of oxygen, can also reversibly inhibit cytochrome c oxidase and cause mitochondrial hyperpolarization (104). All these mechanisms induce a transient and reversible mitochondrial hyperpolarization and mROS production leading to cell activation. Disruption of MMP has been proposed as the point of no return in apoptotic signaling (127). Mitochondrial hyperpolarization usually occurs before the disruption of MMP leading to apoptosis in response to different inducers, such as Fas (7), hydrogen peroxide (H2O2) (106), and NO (1).
In SLE patients, T cells present with persistent mitochondrial hyperpolarization, increased ROS production, and depleted ATP. Moreover, the ETC complex I has been identified as the main source of oxidative stress in these cells (30). Intracellular ATP concentration is a key switch in the decision of the cell to die by apoptosis or necrosis (70), as apoptosis is energy dependent. T cells from SLE patients undergo more spontaneous and less activation-induced apoptosis as a result of ATP depletion. Moreover, SLE T cells become more vulnerable to necrosis, which contributes to the exaggerated inflammatory cascade (40, 101, 102). Therefore, mitochondrial membrane hyperpolarization and associated mROS in T cells have been proposed as key mechanisms of SLE pathogenesis, and they represent premier targets for pharmacological intervention (98).
NETosis is a specific activation-induced cell death process by which neutrophils process pathogens. NETosis plays a critical role in lupus pathogenesis in which mROS has an essential contribution (73). Neutrophils stimulated with ribonucleoprotein IC found in SLE serum displayed increased levels of mROS, which enhanced the formation and release of NET that are enriched in oxidized mitochondrial DNA (mtDNA). Moreover, oxidized mtDNA induces the production of type I IFN through a stimulator of interferon response CGAMP interactor 1-dependent pathway. Accordingly, mROS inhibition reduced disease severity and type I IFN responses in a mouse model of lupus (73). Low-density granulocytes (LDGs), a distinct subset of proinflammatory NETosis-prone neutrophils found in the peripheral blood of SLE patients, present an enhanced mROS synthesis. NETosis in LDGs is, at least, in part dependent on mROS production, as treatment with the mROS scavenger MitoTempo abrogated the extrusion of NET structures in these cells (73).
Finally, peripheral blood lymphocytes (PBLs) of SLE patients showed a spontaneous mitochondrial antiviral signaling (MAVS) oligomerization, which was induced by oxidative stress independently of infection (18). MAVS oligomerization led to mitochondrial hyperpolarization, decreased ATP production, and further mitochondrial oxidative stress, which correlated with an increased secretion of type I IFN. Importantly, MAVS oligomerization and type I IFN production could be blocked with the mROS antioxidant, MitoQ (18).
MitoQ treatment of the MRL/lpr mouse model of lupus reversed ROS production, NETosis, and MAVS oligomerization in neutrophils, as well as reduced serum type I IFN and IC formation in kidneys, despite no change in serum autoantibody (38). Of note, the enhanced mROS in NETosis corresponds to mitochondrial membrane hypopolarization, contrary to mROS corresponding to mitochondria hyperpolarization in lupus T cells (102) and MAVS oligomerization induced in PBL (18). Therefore, mROS production in lupus neutrophils may be similar to RIRR, but this mechanism has not yet been defined for this cell type.
Overall, these results directly link mROS production to changes in MMP (hyperpolarization in T cells or MAVS oligomerization and hypopolarization in neutrophils) to lupus pathogenesis. mROS accumulation triggers inflammatory death (necrosis and NETosis) in lupus immune cells as well as type I IFN production, both of which contribute to pathogenesis. The cell types, source, and pathological context appear to be important for mROS production, but the detailed mechanisms leading to enhanced mROS production in SLE remain to be fully elucidated.
Low production of cellular ROS from NOX drives lupus pathogenesis
Superoxide radicals produced by the prototypic NOX are the other main sources of ROS, especially for phagocytes (Fig. 4C). Seven NOX isoforms (NOX1–5, DUOX1–2) have been reported with distinct catalytic domains. Among them, phagocytic NOX2 (encoded by GP91PHOX) is the best characterized as a multisubunit enzyme complex with both plasma membrane and cytosolic components. ROS generated by NOX2 through the incomplete oxidation of NADPH that produces superoxide anion contribute to NETosis (34, 47). A key role for NOX was also identified in the disposal of apoptotic cells in inflammatory macrophages by enhancing the maturation and acidification of efferosome (5).
Chronic granulomatous disease is a hereditary disease that exhibits some SLE-like features. Mutations in genes forming the NOX2 complex lead to a defective ROS production, which activates type I IFN production and increases the risk of autoimmune diseases (59). Further, a prominent type I IFN response signature was identified in humans lacking functional NOX2 (45). These observations challenge the idea that excessive ROS production is exclusively pathogenic, opening a new viewpoint to understand the pathogenesis of lupus.
Neutrophil cytosolic factor 1 (NCF1) encodes for p47phox, a key regulatory component of NOX2. Genetic associations have been reported between lupus and polymorphisms in NCF1 (138). A single amino acid substitution from arginine to histidine represents the strongest association between NCF1 and SLE (odds ratio >3 and allele frequency >10%), and this variant is associated with a lower ROS production (29, 152). In addition, decreased and increased copy numbers of NCF1 predispose to and protect against SLE, respectively (152). A protective role of NCF1 in lupus was validated in Ncf1-deficient SLE1.Yaa lupus-prone mice, which presented reduced ROS levels in pDCs that promoted the production of type I IFNs and accelerated the development of autoimmune symptoms (71).
Polymorphisms in the neutrophil cytosolic factor 2 (NCF2) gene, encoding for p67phox, another critical NOX subunit, have also been associated with lupus (4, 49, 50, 60, 151), and at least one amino acid substitution (H389Q) decreased NOX activity and cellular ROS production (71). Studies in the NZM2328 lupus-prone mice showed that Ncf2 deficiency and haploinsufficiency increased NETosis, even in the absence of NOX activity. This was associated with increased type 1 IFN expression and immune activation, which accelerated kidney disease (51). Additional support for a protective role of cellular ROS in lupus was shown in Nox2-deficient MRL/lpr mice, which also presented increased autoantibody production and exacerbated renal disease (19). The seeming discrepancy between the proinflammatory roles of reduced cellular ROS and increased mROS in the pathogenesis of lupus supports the concept that metabolic regulation of immune responses may vary between cell types, stimuli, microenvironments, and the pathogenic process.
ROS detoxification
ROS is detoxified by enzymatic and nonenzymatic antioxidants. Intracellular enzymatic antioxidants include manganese superoxide dismutase in the mitochondria and copper/zinc superoxide dismutase in the cytosol converting superoxide anions (O2–) to H2O2. Catalase then reduces H2O2 to H2O. Glutathione (GSH) combined with pyridine nucleotides (NADH/NAD and NADPH/NADP) represent the major nonenzymatic antioxidant and redox modulators in human cells. Oxidation of GSH to glutathione disulfide (GSSG) reduces H2O2 to H2O. Regeneration of GSH by GSH reductase from GSSG depends on NADPH produced by the pentose phosphate pathway (29). The reduction of GSSG to GSH is antagonized by acetyl-CoA (53), the pivotal molecule in the Krebs cycle and mitochondrial hemostasis.
Therefore, NADPH consumption to convert GSSG to GSH is a critical checkpoint of the antioxidative process. As mentioned above, incomplete NADPH oxidation during NETosis results in NOX2-mediated ROS formation in neutrophils. Thus, NADPH acts either anti- or pro-oxidant, maybe depending on cell type, source of ROS production, or pathological context. A comprehensive understanding of this context-dependent NADPH effect on redox homeostasis will require further studies.
GSH is depleted in the PBL of patients with SLE (36, 40, 117). N-acetylcysteine (NAC), the acetylated form of L-cysteine, has the advantage of being resistant to oxidation and permeable through cell membrane over other forms of cysteine supplementation (144). As such, NAC serves as a precursor of GSH synthesis and acts as an antioxidant by itself. Treatments with NAC inhibited mTOR activation in vitro (91) and improved clinical outcomes in murine lupus (128) as well as in SLE patients (66). Interestingly, NAC reduced the MMP in PBL from SLE patients by inhibiting the mitochondrial ETC complex I (30). Overall, these results suggest that an increased level of mROS has deleterious effects in lupus, which can be mitigated by antioxidants that reduce mitochondrial respiration.
As mentioned earlier, mitoQ, a mROS scavenger, showed beneficial effects in a preclinical model of lupus (38). It has been recently reported that SLE patients present lower levels of superoxide dismutase (SOD), an enzyme that neutralized superoxide radicals (147). Therefore, SOD supplementation could be beneficial in lupus, as it has been proposed for other autoimmune diseases (20). Finally, it has been proposed that the activation of the NRF2/Keap1 pathway, a major regulator of cellular oxidative stress, could be beneficial in lupus, including with drugs such as dimethyl-fumarate, which has been approved for the treatment of another T cell-mediated autoimmune disease, multiple sclerosis (92).
Metformin as a regulator of redox homeostasis
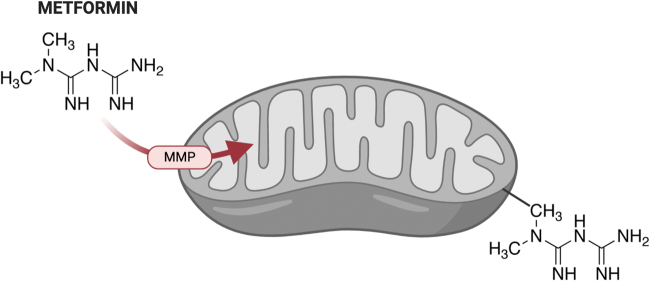
Since metformin regulates mitochondrial activity through its inhibition of complex I in the ETC (145), its effect on redox homeostasis should be considered in general, as well as its role as a modulator of lupus pathogenesis. Metformin tends to accumulate within the matrix of energized mitochondria, supporting the common notion that mitochondria are the primary target of metformin. Since no transporter has been identified to import metformin into mitochondria (140), it has been proposed that MMP drives positively charged metformin into the mitochondria, with the higher potential, the more metformin is imported across the membrane. In addition, the apolar hydrocarbon side-chain of metformin may help in the process by binding to hydrophobic structures, especially the phospholipids in the mitochondrial membrane (115) (Fig. 5).
FIG. 5.
Proposed mechanism of metformin import into mitochondria. The transporter by which metformin accumulates in mitochondria has not been identified. It has been proposed that negatively charged MMP drives positively charged metformin into the mitochondria. The two methyl groups may assist in the process by binding hydrophobic phospholipids in the mitochondrial membrane. The structure of metformin is indicated. MMP, mitochondrial membrane potential. Color images are available online.
Although there is a consensus that ETC complex I represents one of the major targets of metformin, several issues have been raised against considering this drug as an equivalent to rotenone, the reference complex I inhibitor. The maximal inhibitory effect of metformin on complex I activity is ∼40%, which is much lower than 80% inhibition by rotenone. In addition, it is still questionable whether metformin could reach a sufficient concentration level in the mitochondrial matrix to inhibit complex I. The reported IC50 for metformin on complex I activity is extremely high, in the 20 to 80 mmL/L range, while metformin concentration in cells or tissues has been reported in a 1–150 μM range (15, 33, 97). Given these discrepancies, it has been speculated that complex I inhibition is a downstream effect of metformin action on a yet unidentified target (140).
A beneficial effect of metformin has been documented in aging CD4+ T cells by reducing mitochondrial respiration leading to a decreased mROS production (13). By analogy, it is likely that it is also the case in lupus T cells, although it has not formally been shown. Interestingly, metformin has little to no effect on young T cells (13) or on the T cells of nonautoimmune mice (149). This suggests that metformin exerts a minimal inhibition on mitochondria in a noninflammatory homeostatic state. Given that metformin is most likely to require a high MMP to enter mitochondria and access complex I, one may speculate that metformin does not reach an effective concentration in cells with healthy mitochondria.
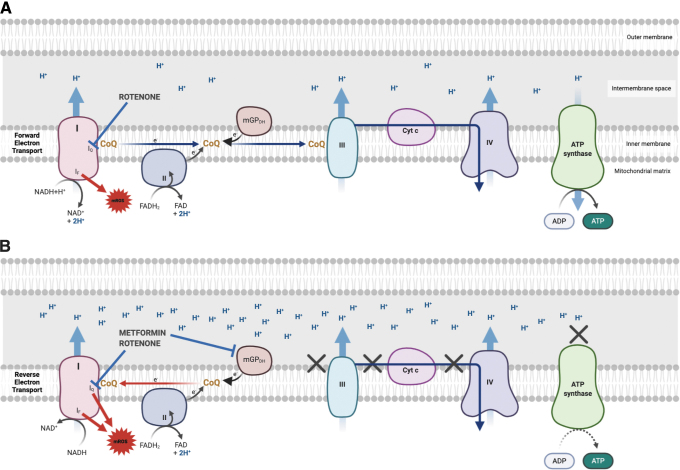
As mentioned above, mROS production is phase dependent, triggered by membrane hyperpolarization or RIRR, and it is therefore tightly associated with MMP and redox potential (NADH/NAD ratio). MMP produced by complex I depends on the forward (glutamate–malate) or reverse (succinate) electron flux (Fig. 6), and primary mROS production depends on higher MMP. Rotenone blocks both forward and reverse electron flows through the respiratory-chain complex I, causing both increased and decreased mROS production (Fig. 6A). However, most studies suggest that although metformin also inhibits complex I, it exerts only its inhibitory effect on mROS production by selectively blocking the reverse electron flow (9, 58).
FIG. 6.
ETC complex I produces ROS in both the forward and reverse directions. (A) During forward electron transfer, CoQ receives electrons from complexes I and II. During this process, electrons leak to produce superoxide from the IF site where NADH is oxidized to NAD+ in complex I. When the Q-binding site (IQ) is blocked, electrons cannot be transferred to CoQ, leak and generate ROS. (B) When the CoQ pool becomes over-reduced into ubiquinol, a high MMP favors the reverse transfer of electrons to complex I. During reverse electron transport, electrons leak from complex I, at either the IF or IQ sites, generating a large amount of superoxide. If the IQ site is blocked during reverse electron transport, CoQ is prevented from transferring electrons back to complex 1, which then reduces ROS production from the Iq site. Rotenone blocks both forward and reverse electron flows, causing both increased and decreased mROS production. Metformin exerts only its inhibitory effect on mROS production by selectively blocking the reverse electron flow. CoQ, coenzyme Q. Color images are available online.
A high rate of mROS production was induced by succinate as a complex II substrate, and it was fully suppressed by rotenone and substantially lowered by metformin pretreatment, suggesting that metformin inhibits the MMP produced by reverse electron flux (Figs. 4A and 6B). The presence of glutamate–malate plus succinate induced an amount of mROS that was intermediate between the succinate alone (highest) and the glutamate–malate alone (lowest)–induced mROS (9). Considering the documented mitochondrial hyperpolarization in SLE T cells and MAVS oligomerization in SLE PBL, it is reasonable to speculate that metformin may also reduce mROS production by selectively blocking the reverse electron flow through the respiratory-chain complex I in these cells. This has however never been investigated, and further studies with multiple immune cell types of human and mouse origins are needed to validate this hypothesis.
Complex I inhibition by metformin may also modulate RIRR-produced ROS (Fig. 4B), since rotenone decreased the magnitude of the ROS burst during mPTP-associated RIRR (155). Similarly, metformin inhibited mPTP opening and ROS generation in rat cardiac myoblasts during ischemia/reperfusion (22). However, the significance of metformin in RIRR and its relevance to immune cells remain questionable. Finally, metformin has been reported to induce mROS production in worms, a process that is essential to engage programs promoting longevity (26). This mROS burst is due to metformin enhancing several catabolic pathways, including the Krebs cycle and beta oxidation, which in turn increases respiration, which, combined with metformin-induced perturbation of the ETC, produces mROS. Whether this mitohormesis pathway, in which a modest production of mROS is beneficial (8), involves immune cells is unknown. However, the possible existence of mitohormesis in relationship with metformin should be investigated as part of a comprehensive evaluation of the effect of metformin on the inflamed immune system.
Metformin has also been identified as noncompetitive inhibitor of liver mGPDH (77). mGPDH is a flavin-linked respiratory-chain dehydrogenase that is encoded by the highly conserved GPD2 gene (87). Together with its cytosolic correspondent enzyme cGPDH, mGPDH constitutes the GPS. cGPDH catalyzes the conversion of dihydroxyacetone phosphate (DAP) to glycerol-3-phosphate (GP) coupled with the oxidation of cytosolic NADH. Consequently, GP is oxidized back to DAP by mGPDH. Concurrently, flavin adenine dinucleotide is reduced to FADH2, and the two electrons are transferred directly to coenzyme Q in complex II. GPS is a potential source of mROS, as it represents an alternative to the malate–aspartate shuttle (MAS) that transports NADH reducing equivalents into the mitochondria.
Unlike MAS that acts via complex I, GPS transports electrons directly to the coenzyme Q pool and bypasses complex I. The rate-limiting step for the flux through GPS is the mGPDH content, as cGPDH is present in excess in most tissues (87). In isolated mitochondria from guinea pig brain, GPDH-mediated mROS are produced through the reverse ETC flux (134), which could therefore be potentially inhibited by metformin. The effect of metformin on mGPDH and its related redox homeostasis has been addressed in an animal study. mGPDH activity was suppressed in the hepatocytes of mice treated with metformin, and the cytosolic redox state was elevated in contrast to mitochondrial redox (77, 78). Beside a direct inhibition of mGPDH function, metformin was proposed to downregulate mGPDH expression (130), although the mechanism by which this may occur is unknown.
Very little is known about the expression of mGPDH in immune cells, although it has been suggested to be expressed in macrophages (48). Consequently, the effect of metformin on mGPDH and its related redox hemostasis (Figs. 4A and 6B) has not yet been defined in immune cells and in lupus pathology. If mGPDH is functionally expressed in immune cells, it would be predicted that, based on the results obtained on hepatocytes, mGPDH inhibition could be one of the mechanisms by which metformin is beneficial by decreasing mROS and increasing cytosolic redox state.
The effect of metformin on ROS production mediated by NOX or other pathways
A strong suppression of NOX activity has been detected after metformin treatment, which decreased cytosolic ROS production in different cells and processes, such as ROS production induced by lithocholic acid in colorectal cancer cells (90), by fluctuating glucose levels (2) or by hyperglycemia (10) in human umbilical vein endothelial cells. The mechanisms underlying these observations remain unclear. One study suggested that metformin-mediated activation of AMPK may be responsible for inhibiting TGFβ-induced NOX4 expression (114). Another in vitro study showed that metformin blunted NETosis and calcium influx induced by phorbol myristate acetate, which may be at least partly due to the inhibitory effect of metformin on the protein kinase C (PKC)-NOX pathway (81). Since decrease in NOX-generated ROS enhanced lupus pathogenesis, it would be expected that the inhibition of NOX2 by metformin in SLE would enhance disease. However, this has not been generally observed. Therefore, it is unlikely that NOX2 is the target of metformin in this context (Fig. 4C). However, given the heterogeneous etiology and disease presentation in SLE, it is possible that metformin could have a worsening outcome in some patients due to this pathway, which should be dissected with mouse models.
Finally, the inhibitory effect of metformin on ROS production has been reported to be exerted through other pathways, such as the inhibition of PKC activity (79), or the upregulated expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway in endothelial cells (46). However, the significance of these pathways on redox hemostasis in immune cells remains questionable. Metformin also inhibits the activity of two enzymes, cyclooxygenase-2 (COX2) and inducible nitric oxide synthase (INOS), which generate reactive oxygen or nitrogen molecules (88). COX2 is overexpressed in human lupus T cells (146), and INOS is also overexpressed in the peripheral blood of lupus patients, although it is not clear if a specific cell type is responsible (95). It is therefore possible that the beneficial effect of metformin in lupus is medicated in part through these pathways, which should be directly evaluated in human studies as well as in preclinical models.
Conclusions
Metformin treatment has now been reported to have a beneficial effect when added to standard of care in SLE patients with mild disease activity (125). Studies in mouse models of lupus have also shown a beneficial effect for metformin, although its ability to revert disease on its own was shown in the monogenic Roquin san/san model (69), but not in the polygenic B6.Sle1.Sle2.Sle3 model (148, 149). The mechanism by which metformin exerts this disease-sparing effect is largely unknown, although increased AMPK activation has been reported in the B cells of Roquin san/san mice treated with metformin. Correlations between disease reduction and decreased mitochondrial respiration in CD4+ T cells have also been reported in B6.Sle1.Sle2.Sle3 mice (148, 149). Furthermore, the inhibition of the type I response in CD4+ T cells treated with metformin was achieved with other ETC inhibitors (133), implying that OXPHOS is most likely a critical target for metformin in SLE. Increased oxidative stress has been reported in lupus T cells (98), and its clinical significance has been validated by the therapeutic effect of NAC in murine lupus (128) as well as in SLE patients (66). Overall, a number of results suggest that elevated mROS is a validated target in lupus. Since the primary target of metformin is the ETC (145), which is the major source of mROS, it is logical to postulate that metformin reduces lupus activity by restoring mitochondrial redox homeostasis. This hypothesis has however never been formally tested. Another complicating factor is that defects in cellular ROS production through polymorphisms in NCF1 and NCF2 have been well documented in lupus (59), and the potential effect of metformin on this process has not been investigated in either murine or human lupus immune cells.
The relationship between redox and metformin has been investigated in a number of models in which it decreases immune inflammation. The effect of metformin on the levels of cytoplasmic or mROS has however been inconsistent. Metformin has been reported to decrease mROS levels in myeloid cells (72, 137) and cytoplasmic ROS in macrophages (16). On the contrary, metformin treatment increased mROS in T cells (25) and macrophages (55), as well as cytoplasmic ROS in macrophages (64). None of these studies has clearly established the molecular target(s) of metformin that was directly responsible for these changes in either cytoplasmic or mROS, except in one case where metformin has been shown to inhibit the expression of NOX2, although through a yet unknown mechanism (17). The disparity of these results clearly shows that it cannot be taken for granted that metformin exerts its beneficial effect in lupus by decreasing mROS levels in T cells.
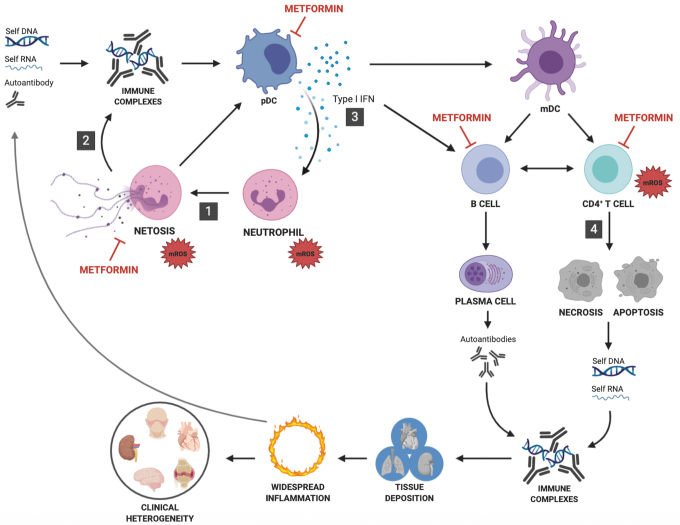
An objective examination of the literature shows a number of immune cells and processes in which redox alterations have been reported in SLE (Fig. 7). On the contrary, the beneficial effect of metformin in lupus has been associated with the normalization of the phenotypes of four cell types: neutrophils, pDCs, CD4+ T cells, and B cells (Fig. 7). Redox alterations have been reported only in neutrophils and CD4+ T cells in lupus. Therefore, it is unclear whether the beneficial effect of metformin on pDCs, CD4+ T cells, and B cells is due to redox regulation, at least intrinsically in these cells. It is possible that pDC normalization is secondary to the decrease of NETosis and production of oxidized mtDNA in neutrophils exposed to metformin. Similarly, normalization of B cells phenotypes may be secondary to the reduction of oxidative stress by metformin in CD4+ T cells.
FIG. 7.
Proposed model of the mechanisms by which metformin reduces lupus pathogenesis. In the oxidative state presented by immune cells in SLE, enhanced mROS synthesis in neutrophils leads to increased NETosis (1). Oxidized mtDNA produced by NETosis (2) induces pDCs to secrete type I IFN (3). Increased mROS production enhances TCR-induced T cell activation as well as T cell death (4). Metformin has been reported to inhibit the functions of neutrophils, CD4+ T cells, pDCs and B cells in either SLE patients or mouse models of the disease. Only neutrophils and CD4+ T cells present a direct involvement of mROS in their pathogenic phenotypes in SLE. The functions of pDCs and B cells may be inhibited by metformin through mROS-independent pathways, or as a consequence of neutrophils and T cell inhibition, respectively. Alternatively, mROS may contribute to pDC and B cell dysfunction in SLE, and metformin may function through a common mechanism in multiple pathogenic cell types in SLE. TCR, T cell receptor; mtDNA, mitochondrial DNA. Color images are available online.
It is also possible that both lupus pDCs and B cells are themselves subject to high levels of oxidative stress, a topic that has not been investigated. These questions stress the urgent need for careful mechanistic studies of metformin in lupus. It has to be acknowledged, however, that the combination of the ongoing discussions about metformin's molecular target(s) and the interdependence between immune cell types challenges the interpretation of the results these studies may bring. It has been proposed that immune cells, especially T cells, in lupus and in aging have some overlapping phenotypes (83, 99).
There is a large body of literature showing that metformin has beneficial effects in aging, including the normalization of mitochondrial functions (121). It has also been noted that metformin promotes autophagy, largely through AMPK activation, and that autophagy is defective in the lupus immune cells (107). Metformin could be one of the drugs that restores both autophagy and mitochondrial functions, improving outcomes on the immune system in aging and autoimmunity (39). The identification of other common targets for metformin in autoreactive and aging immune cells would be beneficial to both fields.
It could be argued that a drug does not require mechanistic studies to be beneficial to patients. Metformin is likely one of the best case-in-point for this argument, since it is widely prescribed for hyperglycemia in spite of persistent controversies on its mode of action. Two facts argue against this notion in SLE due to the extreme heterogeneity in etiology and clinical presentation of the disease. First, it is not known whether some patients respond better than others because their immune phenotypes correspond to a process, including oxidative stress, which can be “fixed” by metformin. Second, metformin is toxic in aging cells with damaged mitochondria and an impaired compensatory glycolytic response. As a consequence, aged worms or human fibroblasts treated with metformin die because they cannot generate ATP (35).
The aging immune system presents clear inflammatory overlaps with autoimmune diseases, including an expansion of Th17 cells (13). Therefore, one should be cautious in extrapolating the beneficial results obtained with metformin in SLE patients with mild disease activity to patients with active disease, whose immune cell response to metformin should be evaluated.
Finally, these mechanistic studies should be performed in the context of the rapidly evolving field of immunometabolism, in which exquisitely cell/context-dependent regulations have emerged even in a healthy immune system. A large number of metabolic alterations have been reported in lupus immune cells (129), which will have to be considered for a better understanding of the therapeutic potentials of metformin in SLE.
Abbreviations Used
- ADP
adenosine diphosphate
- ADPGK
ADP-dependent glucokinase
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- ATP
adenosine triphosphate
- CoQ
coenzyme Q
- COX2
cyclooxygenase-2
- DAP
dihydroxyacetone phosphate
- ETC
electron transport chain
- GP
glycerol-3-phosphate
- GPD2
glycerol phosphate dehydrogenase
- GPS
glycerophosphate shuttle
- GSH
glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- IC
immune complex
- IFN
interferon
- IMAC
inner membrane anion channel
- INOS
inducible nitric oxide synthase
- ISG
interferon-stimulated gene
- LDG
low-density granulocyte
- MAS
malate aspartate shuttle
- MAVS
mitochondrial antiviral signaling
- mGPDH
mitochondrial glycerophosphate dehydrogenase
- MMP
mitochondrial membrane potential
- mPTP
mitochondrial permeability transition pore
- mROS
mitochondrial ROS
- mtDNA
mitochondrial DNA
- mTOR
mammalian target of rapamycin
- NAC
N-acetylcysteine
- NAD
nicotinamide adenine dinucleotide
- NADH
nicotinamide adenine dinucleotide + hydrogen
- NADPH
nicotine adenine dinucleotide phosphate
- NCF
neutrophil cytosolic factor
- NETs
neutrophil extracellular traps
- NO
nitric oxide
- NOX
NADPH-oxidase
- OXPHOS
oxidative phosphorylation
- PBL
peripheral blood lymphocytes
- pDCs
plasmacytoid dendritic cells
- PKC
protein kinase C
- RIRR
ROS-induced ROS release
- ROS
reactive oxygen species
- SLE
systemic lupus erythematosus
- SOD
superoxide dismutase
- TCR
T cell receptor
- Tfh
follicular helper T
Authors' Contributions
All three authors contributed to the literature review and the writing of this article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This review was supported by a grant from the NIH (R01 AI128901) to L.M.
References
- 1. Almeida A, Almeida J, Bolaños JP, and Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A 98: 15294–15299, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. An H, Wei R, Ke J, Yang J, Liu Y, Wang X, Wang G, and Hong T. Metformin attenuates fluctuating glucose-induced endothelial dysfunction through enhancing GTPCH1-mediated eNOS recoupling and inhibiting NADPH oxidase. J Diabetes Complications 30: 1017–1024, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Apostolopoulos D, Vincent F, Hoi A, and Morand E. Associations of metabolic syndrome in SLE. Lupus Sci Med 7: e000436, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong DL, Eisenstein M, Zidovetzki R, and Jacob CO. Systemic lupus erythematosus-associated neutrophil cytosolic factor 2 mutation affects the structure of NADPH oxidase complex. J Biol Chem 290: 12595–12602, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bagaitkar J, Huang J, Zeng MY, Pech NK, Monlish DA, Perez-Zapata LJ, Miralda I, Schuettpelz LG, and Dinauer MC. NADPH oxidase activation regulates apoptotic neutrophil clearance by murine macrophages. Blood 131: 2367–2378, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailis W, Shyer JA, Zhao J, Canaveras JCG, Al Khazal FJ, Qu R, Steach HR, Bielecki P, Khan O, Jackson R, Kluger Y, Maher LJ, 3rd, Rabinowitz J, Craft J, and Flavell RA. Distinct modes of mitochondrial metabolism uncouple T cell differentiation and function. Nature 571: 403–407, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banki K, Hutter E, Gonchoroff NJ, and Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol 162: 1466–1479, 1999. [PMC free article] [PubMed] [Google Scholar]
- 8. Bárcena C, Mayoral P, and Quirós PM. Mitohormesis, an antiaging paradigm. Int Rev Cell Mol Biol 340: 35–77, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, and Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr 38: 33–42, 2006. [DOI] [PubMed] [Google Scholar]
- 10. Batchuluun B, Inoguchi T, Sonoda N, Sasaki S, Inoue T, Fujimura Y, Miura D, and Takayanagi R. Metformin and liraglutide ameliorate high glucose-induced oxidative stress via inhibition of PKC-NAD(P)H oxidase pathway in human aortic endothelial cells. Atherosclerosis 232: 156–164, 2014. [DOI] [PubMed] [Google Scholar]
- 11. Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, Tanti JF, Giorgetti-Peraldi S, and Bost F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 71: 4366–4372, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Bethunaickan R, Berthier CC, Zhang W, Kretzler M, and Davidson A. Comparative transcriptional profiling of 3 murine models of SLE nephritis reveals both unique and shared regulatory networks. PLoS One 8: e77489, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bharath LP, Agrawal M, McCambridge G, Nicholas DA, Hasturk H, Liu J, Jiang K, Liu R, Guo Z, Deeney J, Apovian CM, Snyder-Cappione J, Hawk GS, Fleeman RM, Pihl RMF, Thompson K, Belkina AC, Cui L, Proctor EA, Kern PA, and Nikolajczyk BS. Metformin enhances autophagy and normalizes mitochondrial function to alleviate aging-associated inflammation. Cell Metab 32: 44–55.e6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bona N, Pezzarini E, Balbi B, Daniele SM, Rossi MF, Monje AL, Basiglio CL, Pelusa HF, Arriaga SMM. Oxidative stress, inflammation and disease activity biomarkers in lupus nephropathy. Lupus 29: 311–323, 2020. [DOI] [PubMed] [Google Scholar]
- 15. Bridges HR, Jones AJ, Pollak MN, and Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462: 475–487, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bułdak Ł, Łabuzek K, Bułdak RJ, Kozłowski M, Machnik G, Liber S, Suchy D, Duława-Bułdak A, and Okopień B. Metformin affects macrophages' phenotype and improves the activity of glutathione peroxidase, superoxide dismutase, catalase and decreases malondialdehyde concentration in a partially AMPK-independent manner in LPS-stimulated human monocytes/macrophages. Pharmacol Rep 66: 418–429, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Bułdak Ł, Łabuzek K, Bułdak RJ, Machnik G, Bołdys A, Basiak M, and Bogusław O. Metformin reduces the expression of NADPH oxidase and increases the expression of antioxidative enzymes in human monocytes/macrophages cultured in vitro. Exp Ther Med 13: 794, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buskiewicz IA, Montgomery T, Yasewicz EC, Huber SA, Murphy MP, Hartley RC, Kelly R, Crow MK, Perl A, Budd RC, and Koenig A. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci Signal 9: ra115, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell AM, Kashgarian M, and Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med 4: 157ra141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chávez MD and Tse HM. Targeting mitochondrial-derived reactive oxygen species in T cell-mediated autoimmune diseases. Front Immunol 12: 703972, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen K, Lin ZW, He SM, Wang CQ, Yang JC, Lu Y, Xie XB, and Li Q. Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed Pharmacother 115: 108875, 2019. [DOI] [PubMed] [Google Scholar]
- 22. Chen X, Li X, Zhang W, He J, Xu B, Lei B, Wang Z, Cates C, Rousselle T, and Li J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 83: 256–270, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi SC, Titov AA, Abboud G, Seay HR, Brusko TM, Roopenian DC, Salek-Ardakani S, and Morel L. Inhibition of glucose metabolism selectively targets autoreactive follicular helper T cells. Nat Commun 9: 4369, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornaby C, Elshikha AS, Teng X, Choi SC, Scindia Y, Davidson A, and Morel L. Efficacy of the combination of metformin and CTLA4Ig in the (NZB × NZW)F1 mouse model of lupus nephritis. Immunohorizons 4: 319–331, 2020. [DOI] [PubMed] [Google Scholar]
- 25. Cui Y, Chang L, Wang C, Han X, Mu L, Hao Y, Liu C, Zhao J, Zhang T, Zhang H, Zhang Y, Liu Y, Zhao W, Wang J, Liu X, Sun B, Wang G, Kong Q, Han J, and Li H. Metformin attenuates autoimmune disease of the neuromotor system in animal models of myasthenia gravis. Int Immunopharmacol 75: 105822, 2019. [DOI] [PubMed] [Google Scholar]
- 26. De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, Braeckman BP, Schoofs L, and Temmerman L. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc Natl Acad Sci U S A 111: E2501–E2509, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deng XS, Wang S, Deng A, Liu B, Edgerton SM, Lind SE, Wahdan-Alaswad R, and Thor AD. Metformin targets Stat3 to inhibit cell growth and induce apoptosis in triple-negative breast cancers. Cell Cycle 11: 367–376, 2012. [DOI] [PubMed] [Google Scholar]
- 28. Di Lisa F, Silverman HS, and Hansford RG. Mitochondrial function and cell injury in single cardiac myocytes exposed to anoxia and reoxygenation. Transplant Proc 27: 2829–2830, 1995. [PubMed] [Google Scholar]
- 29. Dodson M, Darley-Usmar V, and Zhang J. Cellular metabolic and autophagic pathways: traffic control by redox signaling. Free Radic Biol Med 63: 207–221, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doherty E, Oaks Z, and Perl A. Increased mitochondrial electron transport chain activity at complex I is regulated by N-acetylcysteine in lymphocytes of patients with systemic lupus erythematosus. Antioxid Redox Signal 21: 56–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002. [DOI] [PubMed] [Google Scholar]
- 32. Duan W, Ding Y, Yu X, Ma D, Yang B, Li Y, Huang L, Chen Z, Zheng J, and Yang C. Metformin mitigates autoimmune insulitis by inhibiting Th1 and Th17 responses while promoting Treg production. Am J Transl Res 11: 2393–2402, 2019. [PMC free article] [PubMed] [Google Scholar]
- 33. Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, and Will Y. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol 233: 203–210, 2008. [DOI] [PubMed] [Google Scholar]
- 34. El-Benna J, Dang PM, and Gougerot-Pocidalo MA. Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 30: 279–289, 2008. [DOI] [PubMed] [Google Scholar]
- 35. Espada L, Dakhovnik A, Chaudhari P, Martirosyan A, Miek L, Poliezhaieva T, Schaub Y, Nair A, Döring N, Rahnis N, Werz O, Koeberle A, Kirkpatrick J, Ori A, and Ermolaeva MA. Loss of metabolic plasticity underlies metformin toxicity in aged Caenorhabditis elegans. Nat Metab 2: 1316–1331, 2020. [DOI] [PubMed] [Google Scholar]
- 36. Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, Phillips PE, Crow MK, Oess S, Muller-Esterl W, and Perl A. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol 182: 2063–2073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. This reference has been deleted.
- 38. Fortner KA, Blanco LP, Buskiewicz I, Huang N, Gibson PC, Cook DL, Pedersen HL, Yuen PST, Murphy MP, Perl A, Kaplan MJ, and Budd RC. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci Med 7: e000387, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gabandé-Rodríguez E, Gómez de Las Heras MM, and Mittelbrunn M. Control of inflammation by calorie restriction mimetics: on the crossroad of autophagy and mitochondria. Cells 9: 82, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gergely P Jr., Grossman C, Niland B, Puskas F, Neupane H, Allam F, Banki K, Phillips PE, and Perl A.. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum 46: 175–190, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glennon-Alty L, Hackett AP, Chapman EA, and Wright HL. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free Radic Biol Med 125: 25–35, 2018. [DOI] [PubMed] [Google Scholar]
- 42. Hallajzadeh J, Khoramdad M, Izadi N, Karamzad N, Almasi-Hashiani A, Ayubi E, Qorbani M, Pakzad R, Sullman MJM, and Safiri S. The association between metabolic syndrome and its components with systemic lupus erythematosus: a comprehensive systematic review and meta-analysis of observational studies. Lupus 27: 899–912, 2018. [DOI] [PubMed] [Google Scholar]
- 43. Hansen JM, Go YM, and Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol 46: 215–234, 2006. [DOI] [PubMed] [Google Scholar]
- 44. Hoffmann MH and Griffiths HR. The dual role of Reactive Oxygen Species in autoimmune and inflammatory diseases: evidence from preclinical models. Free Radic Biol Med 125: 62–71, 2018. [DOI] [PubMed] [Google Scholar]
- 45. Holmdahl R, Sareila O, Olsson LM, Bäckdahl L, and Wing K. Ncf1 polymorphism reveals oxidative regulation of autoimmune chronic inflammation. Immunol Rev 269: 228–247, 2016. [DOI] [PubMed] [Google Scholar]
- 46. Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, and Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun 396: 199–205, 2010. [DOI] [PubMed] [Google Scholar]
- 47. Hurtado-Nedelec M, Makni-Maalej K, Gougerot-Pocidalo MA, Dang PM, and El-Benna J. Assessment of priming of the human neutrophil respiratory burst. Methods Mol Biol 1124: 405–412, 2014. [DOI] [PubMed] [Google Scholar]
- 48. Ishizeki K, Takahashi N, and Nawa T. Induction of adipogenesis by the intrasplenic transplantation of chick serum clots. Arch Histol Cytol 67: 21–30, 2004. [DOI] [PubMed] [Google Scholar]
- 49. Jacob CO, Eisenstein M, Dinauer MC, Ming W, Liu Q, John S, Quismorio FP Jr., Reiff A, Myones BL, Kaufman KM, McCurdy D, Harley JB, Silverman E, Kimberly RP, Vyse TJ, Gaffney PM, Moser KL, Klein-Gitelman M, Wagner-Weiner L, Langefeld CD, Armstrong DL, and Zidovetzki R.. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci U S A 109: E59–E67, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jacob CO, Reiff A, Armstrong DL, Myones BL, Silverman E, Klein-Gitelman M, McCurdy D, Wagner-Weiner L, Nocton JJ, Solomon A, and Zidovetzki R. Identification of novel susceptibility genes in childhood-onset systemic lupus erythematosus using a uniquely designed candidate gene pathway platform. Arthritis Rheum 56: 4164–4173, 2007. [DOI] [PubMed] [Google Scholar]
- 51. Jacob CO, Yu N, Yoo DG, Perez-Zapata LJ, Barbu EA, Kaplan MJ, Purmalek M, Pingel JT, Idol RA, and Dinauer MC. Haploinsufficiency of NADPH Oxidase Subunit Neutrophil Cytosolic Factor 2 Is Sufficient to Accelerate Full-Blown Lupus in NZM 2328 Mice. Arthritis Rheumatol 69: 1647–1660, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jang SG, Lee J, Hong SM, Kwok SK, Cho ML, and Park SH. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatology (Oxford) 59: 1426–1438, 2020. [DOI] [PubMed] [Google Scholar]
- 53. Jeon SM, Chandel NS, and Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485: 661–665, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta 1604: 77–94, 2003. [DOI] [PubMed] [Google Scholar]
- 55. Kajiwara C, Kusaka Y, Kimura S, Yamaguchi T, Nanjo Y, Ishii Y, Udono H, Standiford TJ, and Tateda K. Metformin mediates protection against Legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen species. J Immunol 200: 623–631, 2018. [DOI] [PubMed] [Google Scholar]
- 56. Kalender A, Selvaraj A, Kim SY, Gulati P, Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, Marette A, Kozma SC, and Thomas G. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 11: 390–401, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kamiński MM, Sauer SW, Kamiński M, Opp S, Ruppert T, Grigaravičius P, Grudnik P, Gröne HJ, Krammer PH, and Gülow K. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep 2: 1300–1315, 2012. [DOI] [PubMed] [Google Scholar]
- 58. Kane DA, Anderson EJ, Price JW, 3rd, Woodlief TL, Lin CT, Bikman BT, Cortright RN, and Neufer PD. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med 49: 1082–1087, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kelkka T, Kienhöfer D, Hoffmann M, Linja M, Wing K, Sareila O, Hultqvist M, Laajala E, Chen Z, Vasconcelos J, Neves E, Guedes M, Marques L, Krönke G, Helminen M, Kainulainen L, Olofsson P, Jalkanen S, Lahesmaa R, Souto-Carneiro MM, and Holmdahl R. Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature. Antioxid Redox Signal 21: 2231–2245, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim-Howard X, Sun C, Molineros JE, Maiti AK, Chandru H, Adler A, Wiley GB, Kaufman KM, Kottyan L, Guthridge JM, Rasmussen A, Kelly J, Sánchez E, Raj P, Li QZ, Bang SY, Lee HS, Kim TH, Kang YM, Suh CH, Chung WT, Park YB, Choe JY, Shim SC, Lee SS, Han BG, Olsen NJ, Karp DR, Moser K, Pons-Estel BA, Wakeland EK, James JA, Harley JB, Bae SC, Gaffney PM, Alarcón-Riquelme M, Looger LL, and Nath SK. Allelic heterogeneity in NCF2 associated with systemic lupus erythematosus (SLE) susceptibility across four ethnic populations. Hum Mol Genet 23: 1656–1668, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim JW, Kim SM, Park JS, Hwang SH, Choi J, Jung KA, Ryu JG, Lee SY, Kwok SK, Cho ML, and Park SH. Metformin improves salivary gland inflammation and hypofunction in murine Sjögren's syndrome. Arthritis Res Ther 21: 136, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim SJ, Lee K, and Diamond B. Follicular helper T cells in systemic lupus erythematosus. Front Immunol 9: 1793, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koga T, Ichinose K, and Tsokos GC. T cells and IL-17 in lupus nephritis. Clin Immunol 185: 95–99, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Koren-Gluzer M, Aviram M, and Hayek T. Metformin inhibits macrophage cholesterol biosynthesis rate: possible role for metformin-induced oxidative stress. Biochem Biophys Res Commun 439: 396–400, 2013. [DOI] [PubMed] [Google Scholar]
- 65. Korshunov SS, Skulachev VP, and Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416: 15–18, 1997. [DOI] [PubMed] [Google Scholar]
- 66. Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, Francis L, Garcia R, Dawood M, Yu J, Ramos I, Coman I, Faraone SV, Phillips PE, and Perl A. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 64: 2937–2946, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lai ZW, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, Dawood M, Garcia R, Tily H, Francis L, Faraone SV, Phillips PE, and Perl A. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391: 1186–1196, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee HC and Wei YH. Mitochondrial role in life and death of the cell. J Biomed Sci 7: 2–15, 2000. [DOI] [PubMed] [Google Scholar]
- 69. Lee SY, Moon SJ, Kim EK, Seo HB, Yang EJ, Son HJ, Kim JK, Min JK, Park SH, and Cho ML. Metformin suppresses systemic autoimmunity in Roquin(san/san) mice through inhibiting B cell differentiation into plasma cells via regulation of AMPK/mTOR/STAT3. J Immunol 198: 2661–2670, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Leist M, Single B, Castoldi AF, Kühnle S, and Nicotera P. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 185: 1481–1486, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li J, Ding H, Meng Y, Li G, Fu Q, Guo Q, Yin Z, Ye Z, Zhou H, and Shen N. Taurine metabolism aggravates the progression of lupus by promoting the function of plasmacytoid dendritic cells. Arthritis Rheumatol 72: 2106–2117, 2020. [DOI] [PubMed] [Google Scholar]
- 72. Lin CF, Young KC, Bai CH, Yu BC, Ma CT, Chien YC, Su HC, Wang HY, Liao CS, Lai HW, and Tsao CW. Blockade of reactive oxygen species and Akt activation is critical for anti-inflammation and growth inhibition of metformin in phosphatase and tensin homolog-deficient RAW264.7 cells. Immunopharmacol Immunotoxicol 35: 669–677, 2013. [DOI] [PubMed] [Google Scholar]
- 73. Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, Ledbetter JA, Elkon KB, and Kaplan MJ. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med 22: 146–153, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. This reference has been deleted.
- 75. Ma R, Yi B, Riker AI, and Xi Y. Metformin and cancer immunity. Acta Pharmacol Sin 41: 1403–1409, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. MacIver NJ, Michalek RD, and Rathmell JC. Metabolic regulation of T lymphocytes. Ann Rev Immunol 31: 259–283, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Madiraju AK, Erion DM, Rahimi Y, Zhang XM, Braddock DT, Albright RA, Prigaro BJ, Wood JL, Bhanot S, MacDonald MJ, Jurczak MJ, Camporez JP, Lee HY, Cline GW, Samuel VT, Kibbey RG, and Shulman GI. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510: 542–546, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Madiraju AK, Qiu Y, Perry RJ, Rahimi Y, Zhang XM, Zhang D, Camporez JG, Cline GW, Butrico GM, Kemp BE, Casals G, Steinberg GR, Vatner DF, Petersen KF, and Shulman GI. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat Med 24: 1384–1394, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mahrouf M, Ouslimani N, Peynet J, Djelidi R, Couturier M, Therond P, Legrand A, and Beaudeux JL. Metformin reduces angiotensin-mediated intracellular production of reactive oxygen species in endothelial cells through the inhibition of protein kinase C. Biochem Pharmacol 72: 176–183, 2006. [DOI] [PubMed] [Google Scholar]
- 80. Marcucci F, Romeo E, Caserta CA, Rumio C, and Lefoulon F. Context-dependent pharmacological effects of metformin on the immune system. Trends Pharmacol Sci 41: 162–171, 2020. [DOI] [PubMed] [Google Scholar]
- 81. Menegazzo L, Scattolini V, Cappellari R, Bonora BM, Albiero M, Bortolozzi M, Romanato F, Ceolotto G, Vigili de Kreutzeberg S, Avogaro A, and Fadini GP. The antidiabetic drug metformin blunts NETosis in vitro and reduces circulating NETosis biomarkers in vivo. Acta Diabetol 55: 593–601, 2018. [DOI] [PubMed] [Google Scholar]
- 82. Meng S, Cao J, He Q, Xiong L, Chang E, Radovick S, Wondisford FE, and He L. Metformin activates AMP-activated protein kinase by promoting formation of the αβγ heterotrimeric complex. J Biol Chem 290: 3793–3802, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Minato N. T cell senescence and autoimmunity. In: Innovative Medicine: Basic Research and Development, edited by Nakao K, Minato N, and Uemoto S. Tokyo: Springer, 2015, pp. 119–128. [PubMed] [Google Scholar]
- 84. Mobini M, Niksolat F, Mohammadpour RA, Dashti Dargahloo S, and Marzban D. Metabolic syndrome in patients with systemic lupus erythematosus: association with disease activity, disease damage and age. Int J Rheum Dis 21: 1023–1030, 2018. [DOI] [PubMed] [Google Scholar]
- 85. Mok CC. Metabolic syndrome and systemic lupus erythematosus: the connection. Expert Rev Clin Immunol 15: 765–775, 2019. [DOI] [PubMed] [Google Scholar]
- 86. Morel L. Immunometabolism in systemic lupus erythematosus. Nat Rev Rheumatol 13: 280–290, 2017. [DOI] [PubMed] [Google Scholar]
- 87. Mráček T, Drahota Z, and Houštěk J. The function and the role of the mitochondrial glycerol-3-phosphate dehydrogenase in mammalian tissues. Biochim Biophys Acta 1827: 401–410, 2013. [DOI] [PubMed] [Google Scholar]
- 88. Najafi M, Cheki M, Rezapoor S, Geraily G, Motevaseli E, Carnovale C, Clementi E, and Shirazi A. Metformin: prevention of genomic instability and cancer: a review. Mutat Res Genet Toxicol Environ Mutagen 827: 1–8, 2018. [DOI] [PubMed] [Google Scholar]
- 89. Nath N, Khan M, Paintlia MK, Singh I, Hoda MN, and Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol 182: 8005–8014, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nguyen TT, Ung TT, Li S, Lian S, Xia Y, Park SY, and Do Jung Y. Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Sci Rep 9: 2003, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. O'Loghlen A, Pérez-Morgado MI, Salinas M, and Martín ME. N-acetyl-cysteine abolishes hydrogen peroxide-induced modification of eukaryotic initiation factor 4F activity via distinct signalling pathways. Cell Signal 18: 21–31, 2006. [DOI] [PubMed] [Google Scholar]
- 92. Ohl K and Tenbrock K. Oxidative stress in SLE T cells, is NRF2 really the target to treat? Front Immunol 12: 633845, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Olsson LM, Johansson ÅC, Gullstrand B, Jönsen A, Saevarsdottir S, Rönnblom L, Leonard D, Wetterö J, Sjöwall C, Svenungsson E, Gunnarsson I, Bengtsson AA, and Holmdahl R. A single nucleotide polymorphism in the NCF1 gene leading to reduced oxidative burst is associated with systemic lupus erythematosus. Ann Rheum Dis 76: 1607–1613, 2017. [DOI] [PubMed] [Google Scholar]
- 94. Ouyang J, Parakhia RA, and Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. J Biol Chem 286: 1–11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pan L, Yang S, Wang J, Xu M, Wang S, and Yi H. Inducible nitric oxide synthase and systemic lupus erythematosus: a systematic review and meta-analysis. BMC Immunol 21: 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Patel A and Perl A. Redox control of integrin-mediated hepatic inflammation in systemic autoimmunity. Antioxid Redox Signal 36: 367–388, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Pecinova A, Drahota Z, Kovalcikova J, Kovarova N, Pecina P, Alan L, Zima M, Houstek J, and Mracek T. Pleiotropic effects of biguanides on mitochondrial reactive oxygen species production. Oxid Med Cell Longev 2017: 7038603, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol 9: 674–686, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci 1346: 33–44, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Perl A. Review: metabolic control of immune system activation in rheumatic diseases. Arthritis Rheumatol 69: 2259–2270, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Perl A, Gergely P Jr., and Banki K. Mitochondrial dysfunction in T cells of patients with systemic lupus erythematosus. Int Rev Immunol 23: 293–313, 2004. [DOI] [PubMed] [Google Scholar]
- 102. Perl A, Gergely P Jr., Nagy G, Koncz A, and Banki K.. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol 25: 360–367, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Piranavan P, Bhamra M, and Perl A. Metabolic targets for treatment of autoimmune diseases. Immunometabolism 2: e200012, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Poderoso JJ, Helfenberger K, and Poderoso C. The effect of nitric oxide on mitochondrial respiration. Nitric Oxide 88: 61–72, 2019. [DOI] [PubMed] [Google Scholar]
- 105. Poholek CH, Raphael I, Wu D, Revu S, Rittenhouse N, Uche UU, Majumder S, Kane LP, Poholek AC, and McGeachy MJ. Noncanonical STAT3 activity sustains pathogenic Th17 proliferation and cytokine response to antigen. J Exp Med 217, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Puskas F, Gergely P Jr., Banki K, and Perl A.. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J 14: 1352–1361, 2000. [DOI] [PubMed] [Google Scholar]
- 107. Qi YY, Zhou XJ, and Zhang H. Autophagy and immunological aberrations in systemic lupus erythematosus. Eur J Immunol 49: 523–533, 2019. [DOI] [PubMed] [Google Scholar]
- 108. Qin X, Jiang T, Liu S, Tan J, Wu H, Zheng L, and Zhao J. Effect of metformin on ossification and inflammation of fibroblasts in ankylosing spondylitis: an in vitro study. J Cell Biochem 119: 1074–1082, 2018. [DOI] [PubMed] [Google Scholar]
- 109. Rincon M and Pereira FV. A new perspective: mitochondrial Stat3 as a regulator for lymphocyte function. Int J Mol Sci 19: 1656, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Rojas Quintero J, Chávez M, Torres W, Arraiz N, Cabrera de Bravo M, and Bermudez V. Metformin in cancer: chemical pathways for tumoral control independent of amp-dependent kinase. J Endocrinol Diabetes Obesity 2: 1036, 2014. [Google Scholar]
- 111. Saengboonmee C, Seubwai W, Cha'on U, Sawanyawisuth K, Wongkham S, and Wongkham C. Metformin exerts antiproliferative and anti-metastatic effects against cholangiocarcinoma cells by targeting STAT3 and NF-κB. Anticancer Res 37: 115–123, 2017. [DOI] [PubMed] [Google Scholar]
- 112. Saksida T, Jevtić B, Djedović N, Miljković Đ, and Stojanović I. Redox regulation of tolerogenic dendritic cells and regulatory T cells in the pathogenesis and therapy of autoimmunity. Antioxid Redox Signal 34: 364–382, 2020. [DOI] [PubMed] [Google Scholar]
- 113. Sanadgol N, Barati M, Houshmand F, Hassani S, Clarner T, Shahlaei M, and Golab F. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol Rep 72: 641–658, 2020. [DOI] [PubMed] [Google Scholar]
- 114. Sato N, Takasaka N, Yoshida M, Tsubouchi K, Minagawa S, Araya J, Saito N, Fujita Y, Kurita Y, Kobayashi K, Ito S, Hara H, Kadota T, Yanagisawa H, Hashimoto M, Utsumi H, Wakui H, Kojima J, Numata T, Kaneko Y, Odaka M, Morikawa T, Nakayama K, Kohrogi H, and Kuwano K. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res 17: 107, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Schäfer HJ, Mainka L, Rathgeber G, and Zimmer G. Photoaffinity cross-linking of oligomycin-sensitive ATPase from beef heart mitochondria by 3'-arylazido-8-azido ATP. Biochem Biophys Res Commun 111: 732–739, 1983. [DOI] [PubMed] [Google Scholar]
- 116. Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, and Chandel NS. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38: 225–236, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shah D, Aggarwal A, Bhatnagar A, Kiran R, and Wanchu A. Association between T lymphocyte sub-sets apoptosis and peripheral blood mononuclear cells oxidative stress in systemic lupus erythematosus. Free Radic Res 45: 559–567, 2011. [DOI] [PubMed] [Google Scholar]
- 118. Singh N, Lawana V, Luo J, Phong P, Abdalla A, Palanisamy B, Rokad D, Sarkar S, Jin H, Anantharam V, Kanthasamy AG, and Kanthasamy A. Organophosphate pesticide chlorpyrifos impairs STAT1 signaling to induce dopaminergic neurotoxicity: implications for mitochondria mediated oxidative stress signaling events. Neurobiol Dis 117: 82–113, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Smallwood MJ, Nissim A, Knight AR, Whiteman M, Haigh R, and Winyard PG. Oxidative stress in autoimmune rheumatic diseases. Free Radic Biol Med 125: 3–14, 2018. [DOI] [PubMed] [Google Scholar]
- 120. Son HJ, Lee J, Lee SY, Kim EK, Park MJ, Kim KW, Park SH, and Cho ML. Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis. Mediators Inflamm 2014: 973986, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Soukas AA, Hao H, and Wu L. Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol Metab 30: 745–755, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Starkov AA and Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 86: 1101–1107, 2003. [DOI] [PubMed] [Google Scholar]
- 123. Sun C, Qin W, Zhang YH, Wu Y, Li Q, Liu M, and He CD. Prevalence and risk of metabolic syndrome in patients with systemic lupus erythematosus: a meta-analysis. Int J Rheum Dis 20: 917–928, 2017. [DOI] [PubMed] [Google Scholar]
- 124. Sun F, Geng S, Wang H, Wang H, Liu Z, Wang X, Li T, Wan W, Lu L, Teng X, Morel L, and Ye S. Effects of metformin on disease flares in patients with systemic lupus erythematosus: post hoc analyses from two randomised trials. Lupus Sci Med 7: e000429, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sun F, Wang HJ, Liu Z, Geng S, Wang HT, Wang X, Li T, Morel L, Wan W, Lu L, Teng X, and Ye S. Safety and efficacy of metformin in systemic lupus erythematosus: a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Rheumatol 2: e210–e216, 2020. [DOI] [PubMed] [Google Scholar]
- 126. Sun Y, Tian T, Gao J, Liu X, Hou H, Cao R, Li B, Quan M, and Guo L. Metformin ameliorates the development of experimental autoimmune encephalomyelitis by regulating T helper 17 and regulatory T cells in mice. J Neuroimmunol 292: 58–67, 2016. [DOI] [PubMed] [Google Scholar]
- 127. Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, Fassy F, Reed JC, and Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med 186: 25–37, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Suwannaroj S, Lagoo A, Keisler D, and McMurray RW. Antioxidants suppress mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus (SLE). Lupus 10: 258–265, 2001. [DOI] [PubMed] [Google Scholar]
- 129. Teng X, Cornaby C, Li W, and Morel L. Metabolic regulation of pathogenic autoimmunity: therapeutic targeting. Curr Opin Immunol 61: 10–16, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Thakur S, Daley B, Gaskins K, Vasko VV, Boufraqech M, Patel D, Sourbier C, Reece J, Cheng SY, Kebebew E, Agarwal S, and Klubo-Gwiezdzinska J. Metformin targets mitochondrial glycerophosphate dehydrogenase to control rate of oxidative phosphorylation and growth of thyroid cancer in vitro and in vivo. Clin Cancer Res 24: 4030–4043, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Thanou A, Jupe E, Purushothaman M, Niewold TB, and Munroe ME. Clinical disease activity and flare in SLE: current concepts and novel biomarkers. J Autoimmun 119: 102615, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Thomas I and Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatr Diabetes 18: 10–16, 2017. [DOI] [PubMed] [Google Scholar]
- 133. Titov AA, Baker HV, Brusko TM, Sobel ES, and Morel L. Metformin inhibits the type 1 IFN response in human CD4(+) T cells. J Immunol 203: 338–348, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tretter L, Takacs K, Kövér K, and Adam-Vizi V. Stimulation of H(2)O(2) generation by calcium in brain mitochondria respiring on alpha-glycerophosphate. J Neurosci Res 85: 3471–3479, 2007. [DOI] [PubMed] [Google Scholar]
- 135. Tsai WL, Chang TH, Sun WC, Chan HH, Wu CC, Hsu PI, Cheng JS, and Yu ML. Metformin activates type I interferon signaling against HCV via activation of adenosine monophosphate-activated protein kinase. Oncotarget 8: 91928–91937, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Tsokos GC. Systemic lupus erythematosus. N Engl J Med 365: 2110–2121, 2011. [DOI] [PubMed] [Google Scholar]
- 137. Uehara T, Eikawa S, Nishida M, Kunisada Y, Yoshida A, Fujiwara T, Kunisada T, Ozaki T, and Udono H. Metformin induces CD11b+-cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and anti-tumor effects. Int Immunol 31: 187–198, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Urbonaviciute V, Luo H, Sjöwall C, Bengtsson A, and Holmdahl R. Low production of reactive oxygen species drives systemic lupus erythematosus. Trends Mol Med 25: 826–835, 2019. [DOI] [PubMed] [Google Scholar]
- 139. Vanden Hoek TL, Becker LB, Shao Z, Li C, and Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J Biol Chem 273: 18092–18098, 1998. [DOI] [PubMed] [Google Scholar]
- 140. Vial G, Detaille D, and Guigas B. Role of mitochondria in the mechanism(s) of action of metformin. Front Endocrinol (Lausanne) 10: 294, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang H, Li T, Chen S, Gu Y, and Ye S. NETs mitochondrial DNA and its autoantibody in Systemic Lupus Erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol 67: 3190–3200, 2015. [DOI] [PubMed] [Google Scholar]
- 142. Weber NE and Blair PV. Ultrastuctural studies of beef heart mitochondria. I. Effects of adenosine diphosphate on mitochondrial morphology. Biochem Biophys Res Commun 36: 987–993, 1969. [DOI] [PubMed] [Google Scholar]
- 143. Weber NE and Blair PV. Ultrastructural studies of beef heart mitochondria. II. Adenine nucleotide induced modifications of mitochondrial morphology. Biochem Biophys Res Commun 41: 821–829, 1970. [DOI] [PubMed] [Google Scholar]
- 144. Wernerman J and Hammarqvist F. Modulation of endogenous glutathione availability. Curr Opin Clin Nutr Metab Care 2: 487–492, 1999. [DOI] [PubMed] [Google Scholar]
- 145. Wheaton WW, Weinberg SE, Hamanaka RB, Soberanes S, Sullivan LB, Anso E, Glasauer A, Dufour E, Mutlu GM, Budigner GS, and Chandel NS. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife 3: e02242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Xu L, Zhang L, Yi Y, Kang HK, and Datta SK. Human lupus T cells resist inactivation and escape death by upregulating COX-2. Nat Med 10: 411–415, 2004. [DOI] [PubMed] [Google Scholar]
- 147. Yan L, Wang B, Chen S, Zhou H, Li P, Zhou L, Zhao Q, Wang B, and Chen W. The ratio of superoxide dismutase to standard deviation of erythrocyte distribution width as a predictor of systemic lupus erythematosus. J Clin Lab Anal 34: e23230, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yin Y, Choi S-C, Xu Z, Zeumer L, Kanda N, Croker BP, and Morel L. Glucose oxidation is critical for CD4+ T cell activation in a mouse model of systemic lupus erythematosus. J Immunol 196: 80–90, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, and Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med 7: 274ra18, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yong C, Dan L, Chenhong L, Yan S, Jianfei C, and Jianlong G. [Efficacy and safety of metformin for Behcet's disease and its effect on Treg/Th17 balance: a single-blinded, before-after study]. Nan Fang Yi Ke Da Xue Xue Bao 39: 127–133, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Yu B, Chen Y, Wu Q, Li P, Shao Y, Zhang J, Zhong Q, Peng X, Yang H, Hu X, Chen B, Guan M, Wan J, and Zhang W. The association between single-nucleotide polymorphisms of NCF2 and systemic lupus erythematosus in Chinese mainland population. Clin Rheumatol 30: 521–527, 2011. [DOI] [PubMed] [Google Scholar]
- 152. Zhao J, Ma J, Deng Y, Kelly JA, Kim K, Bang SY, Lee HS, Li QZ, Wakeland EK, Qiu R, Liu M, Guo J, Li Z, Tan W, Rasmussen A, Lessard CJ, Sivils KL, Hahn BH, Grossman JM, Kamen DL, Gilkeson GS, Bae SC, Gaffney PM, Shen N, and Tsao BP. A missense variant in NCF1 is associated with susceptibility to multiple autoimmune diseases. Nat Genet 49: 433–437, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhao L, Wu Q, Wang X, Wang S, Shi X, Shan Z, and Teng W. Reversal of abnormal CD4+ T cell metabolism alleviates thyroiditis by deactivating the mTOR/HIF1a/glycolysis pathway. Front Endocrinol 12: 659738, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zorov DB, Bannikova SY, Belousov VV, Vyssokikh MY, Zorova LD, Isaev NK, Krasnikov BF, and Plotnikov EY. Reactive oxygen and nitrogen species: friends or foes? Biochemistry (Mosc) 70: 215–221, 2005. [DOI] [PubMed] [Google Scholar]
- 155. Zorov DB, Filburn CR, Klotz LO, Zweier JL, and Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]