Abstract
Significance:
Systemic autoimmunity affects 3%–5% of the population worldwide. Systemic lupus erythematosus (SLE) is a prototypical form of such condition, which affects 20–150 of 100,000 people globally. Liver dysfunction, defined by increased immune cell infiltration into the hepatic parenchyma, is an understudied manifestation that affects up to 20% of SLE patients. Autoimmunity in SLE involves proinflammatory lineage specification in the immune system that occurs with oxidative stress and profound changes in cellular metabolism. As the primary metabolic organ of the body, the liver is uniquely capable to encounter oxidative stress through first-pass derivatization and filtering of waste products.
Recent Advances:
The traffic of immune cells from their development through recirculation in the liver is guided by cell adhesion molecules (CAMs) and integrins, cell surface proteins that tightly anchor cells together. The surface expression of CAMs and integrins is regulated via endocytic traffic that is sensitive to oxidative stress. Reactive oxygen species (ROS) that elicit oxidative stress in the liver may originate from the mitochondria, the cytosol, or the cell membrane.
Critical Issues:
While hepatic ROS production is a source of vulnerability, it also modulates the development and function of the immune system. In turn, the liver employs antioxidant defense mechanisms to protect itself from damage that can be harnessed to serve as therapeutic mechanisms against autoimmunity, inflammation, and development of hepatocellular carcinoma.
Future Directions:
This review is aimed at delineating redox control of integrin signaling in the liver and checkpoints of regulatory impact that can be targeted for treatment of inflammation in systemic autoimmunity. Antioxid. Redox Signal. 36, 367–388.
Keywords: integrins, immune synapse, endosomes, systemic lupus erythematosus, liver, hepatic injury, T cell signaling, Rab GTPases, immunity, reactive oxygen species, oxidative stress, autoimmunity, cell adhesion molecules
Introduction
The immune system is the body's defense against pathogenic attack, and is governed by a dynamic interplay among cell adhesion molecules (CAMs), integrins, chemokines, reactive oxygen species (ROS), and endosomal trafficking. Dysfunction in these factors can lead to systemic autoimmunity, which is a loss of tolerance for self-antigens (131, 132).
Affecting 3%–5% of the global population, systemic autoimmunity represents diseases in which the immune system attacks its own cells, tissues, and organs (107). Immune cells circulate through the body's organs using cell surface proteins such as integrins and CAMs. These proteins are present both on the immune cells themselves and on the endothelial cells within the organs, and act to localize immune cells into compartments where they can promote immune attack or immunosuppression (111, 115). The integrins and CAMs that govern immune cell infiltration into and out of an organ are regulated by intracellular signaling processes, including ROS.
The integrins and CAMs, which mediate immune cell margination into the liver, are modified through the signaling of ROS, through endosomal trafficking, or both. The molecular “switches” that regulate endosomal trafficking include Ras-like protein from rat brain (Rab) GTPases, some of which contain the redox-sensitive motifs GXXXGK(S/T)C and/or NKCD (65). These motifs contain a redox-sensitive cysteine to promote Rab GTPase function, controlling endosomal trafficking and cell surface expression of integrins and CAMs (Table 1).
Table 1.
Redox-Sensitive Rab GTPases Control Cell Surface Expression of Receptors That Mediate Signal Transduction and Adhesion
| Rab GTPase | Relevant function |
|---|---|
| Rab1A/B | Integrin β1 recycling (191) |
| Rab4A | CD4 recycling (120) |
| Rab4B | MHC II recycling (86) |
| Rab4 | Recycling of αvβ3 integrin from early endosomes to the plasma membrane (142) |
| Rab14 | Membrane trafficking between the golgi complex and endosomes (78) |
| Rab15 | Receptor recycling from the endocytic recycling compartment (167) |
| Rab8A | Integrin β1 recycling on basolateral surface (44) |
| Rab8B | Required for Wnt/β-catenin signaling (35) |
| Rab13 | Activity-dependent β1-integrin recycling (146) |
| Rab3C | Regulation of IL6-STAT3 pathway (18) |
| Rab3D | EMT through AKT signaling pathway (190) |
| Rab22A | Clathrin-independent endocytosed cargoes including integrin β1 and TfR (54) |
The redox-sensitive Rab GTPases from Figure 2 control the recycling of cell surface proteins and signaling molecules that are implicated in immune functions and hepatic dysfunction.
CD, cluster of differentiation; EMT, epithelial to mesenchymal transition; MHC II, major histocompatibility complex II; Rab, Ras-like protein from rat brain; STAT3, signal transducer and activator of transcription 3; TfR, transferrin receptor; CD71.
The liver is an organ that serves both an immunomodulatory role and an antioxidant role, dampening the immune response from first-pass metabolism and reducing oxidative stress both within the liver and throughout the body. Immune dysfunction and inflammation are associated with states of increased oxidative stress (138) and altered expression of CAMs and integrins (53). Taken together, these represent critical targets of autoimmune regulation and therapeutic interventions. In this review, we explore the mechanisms that govern immune cell infiltration, which promote liver dysfunction in the setting of inflammation and autoimmunity. Driven by pathologic inflammation and oxidative stress, autoimmune-mediated liver disease can be treated either by addressing the underlying inflammatory state driving liver dysfunction or by enhancing our body's own antioxidant systems to reduce oxidative stress.
Integrin- and CAM-Based Communications Between the Liver and the Innate and Adaptive Immune Systems Shape Autoimmunity
Our immune system consists of an innate response and an adaptive response. The Innate Immune System is composed of barrier surfaces, such as the skin, which physically prevent pathogens and foreign substances from entering our bodies. Neutrophils, dendritic cells, and macrophages can phagocytose pathogens resulting in a nonspecific, generalized immunity to pathogens. The Adaptive Immune System picks up where the innate immune response leaves off to mount a specific attack against pathogens that break through the barriers of innate immunity. Immune cells circulate throughout the body, continuously surveilling different organs by using cell surface markers such as integrins, CAMs, and chemokines to enter and exit organs, including the liver (19) (Fig. 1).
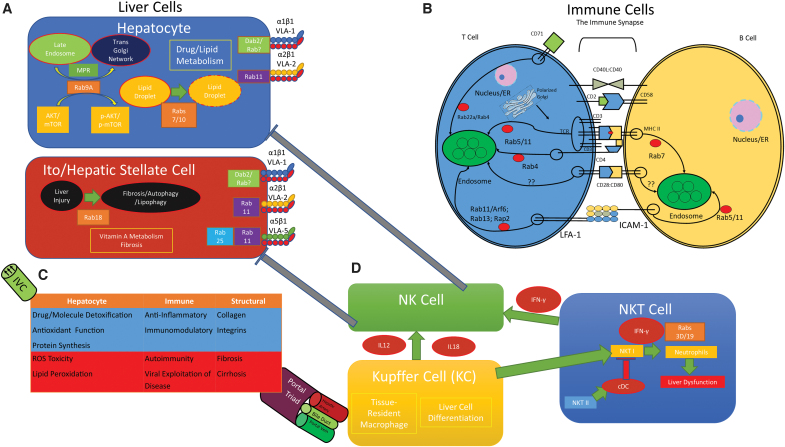
FIG. 1.
Effectors and targets of autoimmunity in the liver. (A) Rab GTPases regulate lipid droplet maturation, MPR trafficking to the trans Golgi network, and activate mTOR in hepatocytes. Ito/HSCs are responsible for vitamin A metabolism and fibrosis, which is regulated by Rab18. Rab18 promotes fibrosis/autophagy/lipophagy in response to liver injury. α1β1 (VLA-1) expression on hepatocyte and HSC surface is regulated by clathrin-dependent endocytosis and Dab2, but the involvement of Rab GTPases is unknown. α2β1 (VLA-2) expression on hepatocyte and HSC surface is regulated by Rab11. α5β1 (VLA-5) expression on HSC surface is regulated by Rab11 and the ROS-sensitive Rab25. (B) The interaction between a T cell and its cognate APC is tightly regulated. The MHC II/TCR interaction, CD2/CD58, and CD28/CD80 is the c-SMAC, while the interactions flanking this is the p-SMAC. Rab GTPases recycle the transmembrane proteins involved in the immune synapse. (C) A simple diagram of liver function within its context as a “filter” of sorts. The liver acts as a filter, detoxifier, and reducer of oxidative stress. Nutrients, processed foodstuffs, and bile pass through the portal triad, composed of the hepatic artery, the bile duct, and the portal vein. The major cells of the liver—hepatocyte, immune, and structural—are presented. Physiological functions are represented in blue, while disease pathologies are represented in red. After modification by biochemical mechanisms in the liver, processed materials are sent out to the rest of the body into the inferior vena cava, which drains into the right atrium of the heart. (D) NK cells, NKT cells, and Kupffer cells all act on each other and with other cells of the liver. NKT cells can promote hepatic inflammation through IFN-γ signaling when they are NKT I. NKT II reduces inflammation by activating cDCs, which suppress NKT I activity, thus reducing inflammatory liver dysfunction. NKT cells also promote NK cell signaling through the release of IFN-γ. NK cells can inhibit Ito/HSC signaling and hepatocyte signaling. APC, antigen presenting cell; CD, cluster of differentiation; cDC, conventional dendritic cell; c-SMAC, central supramolecular activation cluster; Dab2, disabled homolog 2; ER, endoplasmic reticulum; HSC, hepatic stellate cell; ICAM-1, intracellular adhesion molecule-1; IFN-γ, interferon gamma; IL-12/IL-18, interleukin 12/18; LFA-1, lymphocyte function-associated antigen 1; MHC II, major histocompatibility complex II; MPR, mannose-6-phosphate receptor; mTOR, mechanistic target of rapamycin; NK, natural killer; NKT, natural killer T; NKT I, type I NKT; NKT II, type II NKT; p-SMAC, peripheral supramolecular activation cluster; Rab, Ras-like protein from rat brain; ROS, reactive oxygen species; TCR, T cell receptor; VLA-1, very late antigen-1; integrin α1β1; VLA-2, very late antigen-2; integrin α2β1; VLA-5, very late antigen-5; integrin α5β1; fibronectin receptor. Color images are available online.
When a pathogen breaches the barriers of the innate immune response, antigen presenting cells (APCs), such as dendritic cells, phagocytose the pathogen and migrate to the nearest lymph node. Cell surface markers, such as integrins, mediate APC migration to the nearest afferent lymphatic channel, while also processing the pathogen onto major histocompatibility complex II (MHC II) for presentation onto its cell surface (173).
The matured APC interacts with integrins located near high endothelial venules (HEVs) at the nearest lymph node to maximize contact with circulating lymphocytes of the adaptive immune response. Specifically, CD11c+ dendritic cells control the surface expression of lymphocyte-binding surface proteins, such as L-Selectin, ICAM-1 (intracellular adhesion molecule-1), and chemokines CCL19 [chemokine (C-C motif) ligand 19], CCL21 [chemokine (C-C motif) ligand 21], CXCL12 [chemokine (C-X-C) ligand 12], and CXCL13 [chemokine (C-X-C) ligand 13] (111). Upregulation of these proteins forces entry of circulating naïve lymphocytes to promote the formation of the immune synapse (IS), which primes the adaptive immune response to facilitate a less generalized and more specific attack against the foreign pathogen (115).
The handoff from innate to adaptive immunity requires the formation of the IS between APCs and T cells. In the IS, integrins act to latch an APC together with a T cell to facilitate a long-term binding between both cells. The engagement of the T cell receptor (TCR) by the MHC II/antigen complex facilitates an interaction between lymphocyte function-associated antigen 1 (LFA-1) and ICAM-1 by promoting the movement of LFA-1 (αLβ2; CD11a/CD18) on T cells and ICAM-1 on APCs toward the TCR/MHC II/antigen complex (39).
As the TCR is crosslinked by MHC I or MHC II, the adhesiveness of the cell–cell anchoring molecule LFA-1, an integrin important in the migration and differentiation of T cells in the immune response, is increased (39, 84). The adhesiveness of an integrin is regulated by both conformation (the affinity of the integrin to bind a ligand) and valency (clustering of integrins on the cell surface) (84).
The central region of the IS, known as the c-SMAC, or central supramolecular activation cluster, is composed of the TCR, CD2 [important as a checkpoint for IS formation and has both adhesion and costimulatory roles (34)], CD4 (on helper T cells) or CD8 (on cytotoxic T cells), and CD28 [a costimulatory protein (1)]. The peripheral region of the IS, called the p-SMAC, or peripheral supramolecular activation cluster, is composed of LFA-1, which binds to ICAM on APCs to strengthen the IS (38).
Central to the cell–cell interactions discussed above are the integrins (like LFA-1), which are transmembrane heterodimeric cell surface proteins, consisting of an α or β chain (32). Integrins act as molecular “hooks and loops,” which mediate intercellular synaptic communication. Extracellular domains of integrins are important in facilitating cell adhesion both to neighboring cells and to the extracellular matrix (ECM) (76). Intracellular domains of integrins are important in converting external stimuli into intracellular signaling cascades (75).
ECM proteins share an integrin-binding motif, Arg-Gly-Asp (RGD), which allows for integrin-expressing cells to bind to the ECM with great strength. Those integrins that mediate cell–cell interactions bind counter-receptors, such as ICAMs (important in cell–cell synapse formation), and vascular cell adhesion molecules (VCAMs; expressed on vascular cells to promote cell adhesion to the vascular endothelium) (75). The tight interaction between extracellular and intracellular domains allows for two types of signaling, referred to by Danen as inside-out and outside-in signaling (32).
Inside-out signaling describes signaling that occurs from intracellular processes, which impact integrin conformation and affinity by altering integrin cytoplasmic tails (32). These changes in integrin strength and specificity are mediated by talins and kindlins, which bind to and separate integrin tails that lead to integrin activation (114).
Outside-in signaling describes the binding of ligand to an integrin and the effects of ligand binding on integrin clustering. Amplification of signal transduction is efficiently mediated by the formation of a growing adhesion plaque (32): Ligand binding to the extracellular domains of integrins recruits adapter proteins to the intracellular domains of the integrins. As a result, intracellular signal transduction is enhanced by the presence of more linkage to the cytoskeleton. These interactions occur as a result of cell–ECM interactions, however, and not necessarily through interactions with soluble signaling molecules (50). These signaling events are the hallmarks of the adaptive immune response.
A combination of inside-out and outside-in signaling takes place within T cell activation. After exposure of an innate immune cell to pathogen, homeostatic chemokines, such as CXCR4 [chemokine (C-X-C motif) receptor type 4], CXCR5 [chemokine (C-X-C motif) receptor type 5], CCR6 [chemokine (C-C motif) receptor type 6], and CCR7 [chemokine (C-C motif) receptor type 7], help in APC and lymphocyte migration into a lymph node (41). The interaction between pathogen and APC mediates the leukocyte adhesion cascade (84), which facilitates movement of primed innate immune cells out of the general circulation and into lymphatics. This process involves selectin-dependent rolling, chemokine-triggered activation, and integrin-dependent adhesion to the endothelial cells of the vasculature.
Inflammatory chemokines mediate the transition of neutrophils and monocytes from rolling to arrest adhesion. Upon binding to chemokine receptors, chemokines increase the adhesiveness of integrins for their ligands on the endothelium. Chemokines generated by the endothelial cells induce arrest of rolling leukocytes through the binding of leukocyte integrins to immunoglobulin superfamily of proteins, such as ICAM-1 and VCAM-1 (84). These chemokines are not soluble—they are immobilized onto the endothelium (156).
Study of the liver integrin and CAM landscape challenged previously hypothesized theories about hepatic immunity. As the liver has a dual blood supply (portal vein and hepatic artery), the blood converges into sinusoids located within the hepatic architecture (10). Sinusoids lack tight junctions and do not express “typical” endothelial markers such as PECAM-1 (platelet endothelial cell adhesion molecule-1), CD34, VE-cadherin, P-selectin, or E-selectin (92, 155, 165). Based on the lack of expression of these markers, it was hypothesized that invading leukocytes were physically trapped in the hepatic circulation.
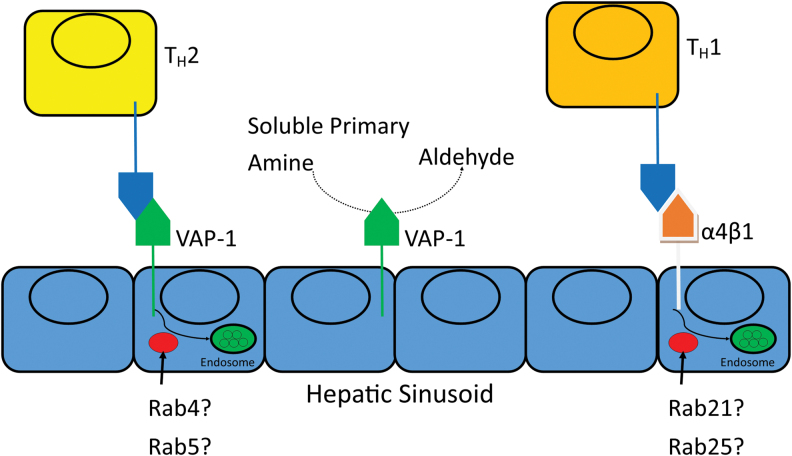
Human liver sinusoids upregulate vascular adhesion protein-1 (VAP-1) during an immune response (10). VAP-1 is typically found in the cytoplasmic vesicles of endothelial cells, and is a cell surface-mounted CAM with enzymatic/catalytic activity. It catalyzes the conversion of soluble primary amines into their aldehyde counterparts (148). VAP-1 is found in hepatic sinusoidal endothelial cells, and it acts to mediate T helper type 2 (TH2) adhesion (while α4β1 [very late antigen-4; VLA-4] mediates T helper type 1 [TH1] adhesion), suggesting a role in liver inflammation (91) (Fig. 3).
FIG. 3.
VAP-1 recruits leukocytes and oxidizes primary amines in the liver during inflammatory states. VAP-1 is a cell surface protein with enzymatic activity, which binds to CD11b to recruit macrophages that may mediate liver damage in autoimmunity. Studies using VAP-1-GFP fusion proteins localized VAP-1 to early endosomes, suggesting that Rab GTPases such as Rab4 and Rab5 may be involved in the cell surface expression of VAP-1. VAP-1, vascular adhesion protein. Color images are available online.
Patients with liver disease have increased levels of VAP-1 (89), and increases in soluble VAP-1 correlate with Non Alcoholic Fatty Liver Disease severity in humans (184). VAP-1 mediates T cell recruitment, and acts as an amine oxidase to produce oxidants and as an adhesion molecule for circulating lymphocytes in the liver (10). Our understanding of liver integrin expression is limited, but the CAM VAP-1 has been theorized as a potential therapeutic target (147). Treatment with semicarbazide-sensitive amine oxidase (SSAO) inhibitors, which interfere with the catalytic activity of VAP-1, results in an antitumor effect on liver cancer through the downregulation of CD11b+ Gr-1+ myeloid-derived suppressor cells (94).
In addition, glucocorticoids have been used to manage liver inflammation for decades and are classically used for treatment of autoimmune hepatitis (AIH) (195). Whether steroids may be involved in regulating VAP-1 expression is unknown. The classic model of glucocorticoid-mediated anti-inflammation involves the suppression of the proinflammatory transcription factor (nuclear factor kappa B) NF-κB (97). Glucocorticoids preferentially decrease the expression of ICAM-1, the cell adhesion ligand for the LFA-1 integrin found on lymphocytes. The treatment of patients with glucocorticoids causes a transient leukocytosis, as the inhibition of integrins causes a demargination of leukocytes that are bound to vascular endothelium (29). Since integrin expression on cell surface can also be regulated by endosomal trafficking enzymes, this remains an open area of research interest.
Redox Mechanisms Control the Expression of CAMs and Integrins via Endosomal Traffic
The integrins, CAMs, etc. which facilitate the immune response are all regulated by endosomal trafficking. The endosome is a complex structure responsible for the targeting of endocytosed materials to a variety of different areas within the cell. The endosome and its associated recycling pathways act to return many of the proteins, lipids, and membranes that were endocytosed back to the surface of the plasma membrane (54).
Endocytosis can occur via clathrin-dependent or clathrin-independent pathways. Clathrin-dependent endocytosis involves adapter protein recognition of the cytoplasmic domains of the plasma membrane proteins of interest. These adapter proteins package the internalized proteins into clathrin-coated vesicles (27). Clathrin-independent endocytosis is not as well studied and is an open area of investigation (151).
After either clathrin-dependent or clathrin-independent endocytosis, the internalized material finds its way to the early endosome, which is where sorting takes place. Sorting determines the fate of these endocytosed materials. The efficient regulation of this sorting and recycling represents targets for physiological activity and for diseases, which may have dysfunctional endosomal sorting pathologies.
Rab GTPases are critical to the endocytic recycling necessary to maintaining cellular homeostasis. In brief, these small guanosine triphosphate (GTP)-binding proteins function as molecular switches to regulate endocytic recycling. They are important in vesicle carrier formation, intracellular vesicle movement, and fusion of vesicles with target membranes. When bound to GTP, they are in an “on” state, where they carry out their biochemical functions. This is carried out by guanine nucleotide exchange factors, which replace guanosine diphosphate (GDP) with GTP. The autohydrolysis of GTP to GDP leads to switching “off” of enzyme function (54).
The cell surface expression of the transferrin receptor (TfR; CD71), important in iron homeostasis, is regulated by endosomal trafficking through the dynamic interplay of a variety of Rab GTPases. After binding to its ligand transferrin, CD71 is rapidly internalized to early endosomes (59). Studies of CD71 in a Chinese hamster ovary cell line found that CD71 is trapped in Rab22a-containing vesicles, and overexpression of Rab22a prevented its recycling (105).
HTLV-I-related endogenous retroviral sequence (HRES-1)/Rab4, a GTPase homologous to the Rab4 family of GTPases, regulates the expression of CD71, in addition to CD4. Curiously, the overexpression of Rab4 increases CD71 expression on the surface of cells, an observation that is opposite to the activity of Rab22a (120). HRES-1/Rab4 is also involved in the maintenance of mitochondrial homeostasis involving the mitophagy regulating protein Drp1, and it is responsible for reducing the expression of the zeta chain of CD3 by promoting endocytic recycling for degradation in the lysosome (45, 120), making this an enzyme of unique and varying function (16). HRES-1/Rab4 is also overexpressed in human lupus T cells, and its activity in lupus pathogenesis is an open area of investigation (45).
Trafficking of integrins is mediated by the small GTPases Rab and ADP ribosylation factor (Arf) (33). Rab GTPases have endosomal compartmentalization, which suggests unique functions for each GTPase (162). Rab5 and Rab21 are known to endocytose integrins from the plasma membrane into early endosomes (130). Arfs, on the contrary, are important in integrin recycling back to the plasma membrane and focal adhesion dynamics (137).
T cell activation causes endosomal recycling of the TCR to increase or decrease TCR expression on the T cell (140). Once the TCR binds to antigen and MHC, the centrosome and Golgi apparatus reorganize to shift protein production and microtubule assembly toward the intracellular side of the newly formed IS (150). While the endocytosis and recycling occur through both clathrin-dependent and clathrin-independent mechanisms, which response predominates is unknown.
A variety of proteins are known to recycle the TCR, such as flotillins, which are not necessary for TCR internalization, but are required for recycling (26). T cell activation causes the internalization of the zeta subunit of the TCR complex in a clathrin-independent manner. The vesicles containing TCRζ are positive for flotillin-2, a protein that is necessary to target certain membrane proteins to their final locations and in regulating Rab11a-dependent recycling of integrins such as α5- and β1-integrins (73). Deficiency of flotillin-2 is associated with decreased lung metastases in mouse breast cancer animal models (7). The sorting of the endocytosed TCRs from early endosomes back to the cell surface is regulated by GTPases, such as Rab4 and Rab5 (199).
MHC II expression in B cells is regulated by Rab7, a GTPase involved in early to late endosomal fusion (8). While the binding of CD28 on T cells to CD80 on APCs is critical to immune activation, precise mechanisms involving their recycling and cellular trafficking are unclear. Mechanisms governing CD2 recycling, along with its ligand, CD58, are also unclear.
In the p-SMAC, LFA-1 and ICAM-1 are also recycled by GTPases. LFA-1 is recycled (129) through Rab11/Arf6 (42), Rab13 (123), and Rap2 (164) endosomal trafficking. ICAM-1 recycling to the early endosome is regulated by Rab5 in circulating T cells and both Rab5/Rab11 in neutrophils (42, 169).
Unlike cell surface proteins, cytokines are synthesized, loaded into vesicles/endosomes, and exocytosed into the extracellular space. Cytokine release via recycling mechanisms is not well understood. The Golgi apparatus in T cells is polarized toward the APC, helping to secrete both cell surface proteins and secreted cytokines out of the cell and directly into the synapse (150). Two pathways exist for cytokine secretion: synapse-directed and nonspecific multidirectional export. The Rab GTPases Rab3d and Rab19 are expressed along with the secretory vesicles containing interleukin 2 (IL-2) and interferon gamma (IFN-γ). Some compartments containing interleukin (IL-4) also localize with Rab37 (74).
Endosomal aberrations and deficiencies in intracellular trafficking can promote the pathogenesis of systemic lupus erythematosus (SLE), a systemic autoimmune disease. In SLE patients, plasmacytoid dendritic cells (pDCs) have increased IFNα production in response to toll-like receptor 7 (TLR7) activation but decreased IFNα production in response to toll-like receptor 9 (TLR9) activation. This stems from the “trapping” of TLR7 in the late endosome and lysosomal compartments of pDCs, and is strongly associated with an SLE phenotype in humans (118).
In particular, TLR7 directly associates with LAMP1 and Rab7 in the lysosomal and late endosomal compartments, respectively. This study also found that SLE disease activity index (SLEDAI) positively correlated with IFNα levels generated by pDCs. These results imply that deficient trafficking and trapping of TLR7 in the late endosome elicited greater IFNα levels and increased disease activity, as detected by SLEDAI (118). The coordination of intercellular and intracellular signaling is tightly regulated. One mechanism of such regulation is through redox signaling within the cell. Oxidative stress occurs because of an overproduction of oxidative species and an underproduction of reducing species, generating ROS.
ROS are formed through normal physiological and metabolic processes. Five classical ROS are hydrogen peroxide (H2O2), superoxide anion (O2−), hypochlorous acid (HOCl), singlet oxygen (1O2), and hydroxyl radical (•OH) (22). The single valence electrons present on superoxide, 1O2, and hydroxyl radicals can compromise cellular stability, nucleic acid integrity, and organelle function.
In a normally functioning, healthy cellular environment, these damaging ROS are neutralized by enzymatic reactions, which prevent further cellular damage, specifically superoxide dismutase (SOD), catalase, and glutathione peroxidase (62). In addition, a basal amount of ROS maintains cellular structure and function. For example, ROS are necessary signaling requirements for adaptive T cell responses (48).
ROS signaling modifies integrin and CAM expression, and, by extension, organ-specific margination of circulating cells. In the case of α2bβ3 integrin, ROS can change integrin shape through redox-sensitive cysteine residues, which ultimately alter binding affinity (56). ROS are critical signaling intermediates in promoting intracellular signaling. External stimuli lead to upregulation of enzyme systems such as Cytochrome P450 and myeloperoxidase. Combining this with the activity of the mitochondrial respiratory chain, ROS generation upregulates phosphatases, Ras/Rho GTPases, and other enzyme systems responsible for altering cell shape and migration/attachment. This ultimately leads to changes in cell adhesion, migration, proliferation, differentiation, and survival (51).
Indeed, we see a classic overlap between liver pathologies that overwhelm redox reactions with the invasion of inflammatory cells, such as dendritic cells and eosinophils (98). Migration of leukocytes through VAP-1 is dependent on ROS generation. Inhibition of the catalytic activity of VAP-1 or removal of the H2O2 from its environment eliminated the enzymatic role of sVAP-1, the soluble form of VAP-1. This reduced the migrating cells to the liver by ∼50%, suggesting that the outside-in signaling of ROS through VAP-1 enzymatic activity plays an important role in leukocyte margination in the liver (184).
The enzymatically active VAP-1 localizes to early endosomes and is involved in lysosomal trafficking (183). Whether the recycling of VAP-1 occurs by GTPases, enzymes involved in membrane and endosomal trafficking, is poorly understood. However, since it is localized to early endosomes, it is reasonable to hypothesize that Rab GTPases associated with the early endosome, such as Rab4 (180) and Rab5 (54, 162), may mediate this recycling. While VAP-1 is implicated in T cell, B cell, monocyte, and granulocyte binding in HEVs, its high upregulation in liver sinusoids makes it a critical target of hepatitis investigation (53).
ROS itself can modulate integrin expression and modification through the regulation of phosphatases, as all protein tyrosine phosphatases also contain a redox-sensitive cysteine residue—when oxidized, the phosphatase is inactive. Protein tyrosine phosphatase (PTP) with PEST (proline-, glutamic-acid-, serine-, and threonine-rich) domains (PTP-PEST), SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2), and low molecular weight protein tyrosine phosphatase are all phosphatases that regulate integrin expression, which are inactivated by oxidation (170).
Catalase directly binds to the SHP-2 phosphatase and the adapter protein growth factor receptor-bound protein 2 (Grb2) upon integrin binding to protect these proteins from ROS, specifically H2O2-mediated oxidation (192, 193). Hydrogen peroxide-inducible clone-5 (Hic-5), a protein involved in the binding of integrin with the actin cytoskeleton, has a ROS-sensing domain on its N-terminus (170). Under conditions of profound oxidative stress, Hic-5's ROS-sensing domain forces release of Hic-5 and signals its movement into the nucleus (158).
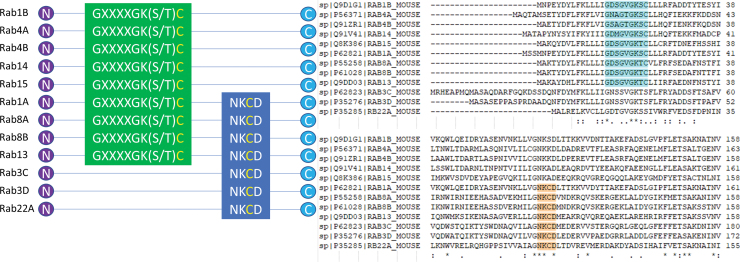
The GTPases responsible for intracellular trafficking are also sensitive to ROS. Clustal Omega sequence alignment of 12 members of the murine Rab GTPase family shows the existence of either the GXXXGK(S/T)C motif, the NKCD motif, or both (65, 160). These motifs contain a redox-sensitive cysteine that, under conditions of oxidative stress, becomes oxidized and activates the GTPase activity of the Rab enzymes (Fig. 2). A redox-sensitive cysteine also exists in the Ras homolog enriched in brain (Rheb) GTPase, which controls complex 1 of the mechanistic target of rapamycin (mTORC1) activation. That Rab GTPases can be activated by ROS alone and that Rab GTPases control integrin signaling suggest that ROS has the potential to directly alter the intracellular and extracellular integrin milieu through the activities of GTPases (Fig. 4).
FIG. 2.
Redox-sensitive motifs in Rab GTPases can control cell surface protein expression. GTP, redox agents, or both can allosterically activate GTPases depending on binding motifs. Representative schematics of 12 mouse Rab GTPases, which contain either the GXXXGK(S/T)C motif, the NKCD motif, or both. A Clustal Omega alignment is provided for reference, with the GXXXGK(S/T)C motif in blue and the NKCD motif in orange. Aligned Rab GTPases, sensitive to ROS, can mediate integrin and cell surface protein expression relevant to SLE and autoimmunity. GTP, guanosine triphosphate; SLE, systemic lupus erythematosus. Color images are available online.
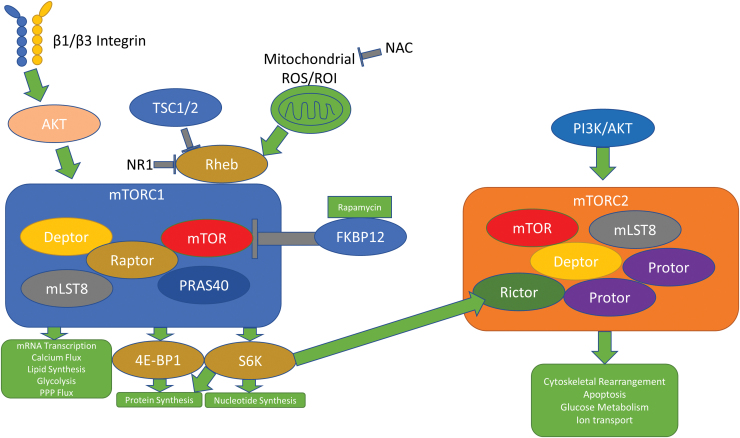
FIG. 4.
The mTOR pathway responds to oxidative stress and integrin signals. While both mTORC1 and mTORC2 respond to rapamycin in an inhibitory manner, mTORC2 promotes downstream cellular growth effects through upstream PI3K/AKT signaling. mTORC1 responds to Rheb through ROS, TSC1/2, and the newly discovered drug NR1 to mediate downstream effects. AKT also promotes mTORC1 activity through β1 and β3 signaling. mTORC1, complex 1 of the mechanistic target of rapamycin; PI3K, phosphatidylinositol 3-kinase; Rheb, Ras homolog enriched in brain; ROI, reactive oxygen intermediate; TSC, tuberous sclerosis complex. Color images are available online.
Abnormal CAM and Integrin Expression Lead to Immune Dysfunction
The transition from innate to adaptive immune response is complex and tightly regulated. Defects, either in this transition or in general immunity, can lead to autoimmunity, which affects 3%–5% of people globally (107). One such example is SLE, an autoimmune disease of unknown etiology; its prevalence ranges from 20 to 150 per 100,000 people worldwide. Our current knowledge of SLE pathogenesis involves the dynamic interplay of ultraviolet light exposure, genetic predisposition, hormonal dysregulation, and other factors, which lead to disease manifestation in susceptible individuals. This ultimately leads to immune cell dysregulation and the generation of immune complexes, cytokines, antibodies, and autoreactive immune cells (176).
SLE affects the liver (98), kidney, lung, heart, brain, and skin (176). The spectrum of disease varies among patients, with different patients having different organ involvement. For example, one of the most common causes of death in SLE is kidney failure, secondary to immune complex deposition in kidney glomeruli (172). Yet other patients suffer from diffuse alveolar hemorrhage, a rare complication that affects <10% of SLE patients but is fatal in up to 50% of such cases (81). In addition, up to 20% of patients suffer from liver inflammation marked by elevated liver function tests (LFTs) and pathologic liver histology (98).
Genome-wide association studies have found that variation in the ITGAM gene, which codes for CD11b (a β2 integrin family member), is one of the strongest risk factors for SLE (43). A proposed mechanism for its pathogenesis is through defects in clearing apoptotic debris and self-reactive immune cells (141). As a whole, CD11b variants can reduce ligand binding and phagocytosis, increase cytokine production, elevate anti-dsDNA (double-stranded DNA) antibodies, and promote the autoreactivity of B cells (83).
ITGAM gene mutations correlate with increased skin, kidney, and neuropsychiatric manifestations of SLE. However, in mouse models of lupus, the absence of CD11b (downstream of ITGAM) has a protective effect. Using a pristane-induced mouse model of lupus, Zhuang et al. found that CD18−/− mice had little to no evidence of lung injury (200). Whether this observation holds true in humans is unknown.
Integrins and CAMs are also implicated in neuropsychiatric manifestations of SLE. Anti-SLE and anti-RA (rheumatoid arthritis) medications impact circulating CAMs, which correlate with neuropsychiatric manifestations of SLE (30). SLE patients generally have higher CAM levels than healthy controls, and circulating CAM levels correlate with disease severity (31).
Specifically, prednisolone and cyclophosphamide significantly decreased P-selectin and VCAM-1 expression in patients presenting with neuropsychiatric lupus (104). Functionally, P-selectin serves to bind and capture a circulating lymphocyte (100), while VCAM-1, recently proposed as a biomarker for lupus nephritis (112), acts as an adhesion molecule, helping to traffic lymphocytes and inflammatory cells (28). These CAMs may represent specific targets for treating neuropsychiatric lupus, potentially through preventing leukocyte migration into the central nervous system.
Liver disease in autoimmunity is also linked to biomarkers of SLE, such as autoantibodies and SLEDAI. Conventional treatment of SLE includes treatment with anti-inflammatory drugs, such as corticosteroids (176). Liu et al. found that treatment with the corticosteroid prednisone decreases both SLEDAI and LFTs (98). This suggests that liver dysfunction in the setting of SLE may originate autoimmunity and downstream inflammation. The liver is a complex organ, and its role in SLE further complicates its study. SLE-associated liver diseases can be classified as either immunological comorbidities (overlap syndromes), nonimmunological comorbidities related to SLE, and SLE-mediated direct hepatic injury (9).
Rabs, CAMs, and Integrins Modulate Liver Inflammation
The liver contains a mix of different cells, each of which integrates signals through Rabs and cell surface proteins (Fig. 1). While hepatocytes promote detoxification, antioxidation, and protein synthesis, immune cells are primarily anti-inflammatory and immunomodulatory. Collagen, integrins, and CAMs exist to anchor immune cells together. In states of pathology, dysfunctional liver cells lead to ROS toxicity, lipid peroxidation, autoimmunity, fibrosis, and cirrhosis. The heterogeneous population of liver cells use integrins, CAMs, and endosomal trafficking to accomplish their physiological tasks; in states of pathology, or inflammation, these pathways are dysfunctional and lead to hepatic inflammation.
Responsible for drug and lipid metabolism, hepatocytes are the primary functional units of the liver. The antioxidant pathways outlined above take place in hepatocytes and are subject to integrin-mediated sequestration of immune cells. Rab GTPases are implicated in a variety of hepatocellular functions outside of immunity. For example, Rab10 interacts with Dynamin 2 to promote the maturation of lipophagic organelles (96). Rab7 has also been established as a “central regulator” of lipophagy in the hepatocytes, promoting lipid droplet breakdown (153). Rab9A mediates carcinogenesis in Hep3b and HepG2 human liver carcinoma cell lines, potentially through the AKT signaling pathway (168). Male Wistar rats fed a Lieber-DeCarli diet, which is a model of experimental alcoholic liver disease, show decreases in Rab3d and Rab18 in whole liver sections, while also showing a decrease in Rab2, Rab5, Rab7, and Rab18 in isolated lipid droplet fractions (139).
Hepatic stellate cells (HSCs; Ito cells) are the primary mediators of hepatic fibrosis in response to liver injury and are important in their storage of vitamin A. These cells differentiate into cells that resemble myofibroblasts in response to liver injury to promote fibrosis downstream of transforming growth factor beta (TGFβ) signaling (60). Rab18 is implicated as a driver of autophagy and fibrosis in human HSC cell lines, and has been hypothesized as potential target of therapies against liver fibrosis (6). Rab18 is a “retinoid responsive mediator” required for the activation of HSCs in mouse models of liver fibrosis (124).
Kupffer cells (KCs) are resident tissue macrophages that are unique to the liver and are critical to the liver's response to toxicity. They are important in autoimmunity for their ability to regulate their neighboring cells. They can directly impact immune tolerance by secreting interleukin 10 (IL-10) to activate regulatory T cells. They can also activate HSCs to promote fibrosis, and have been shown to respond to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections by secreting interleukin 18 (IL-18) and interleukin 1 (IL-1) to stimulate natural killer (NK) cells in liver pathologies (179).
Natural killer T (NKT) cells are lymphocytes with innate-like properties. In the liver, they exist to recognize both self and pathogenic lipid antigens, and promote immune tolerance. KC signaling can prime NKT cells to activate HSCs, kill hepatocytes, and activate neutrophils under pathologic states. However, NKT Type II can serve tolerance roles through conventional dendritic cell (cDC) signaling to inhibit Type I NKT cells. NKT Type I-mediated hepatic destruction is mediated by IFN-γ signaling, (5) which is regulated by Rab3D and Rab19 (74). Cholangiocytes are a rare population in the liver, but have recently come to the forefront of immunobiology. While the mechanism defining their ability to present antigens to T cells is unclear, cholangiocytes can present lipid antigens and derivatives of riboflavin to NKT cells, specifically through a CD1-iTCR (invariant T cell receptor) interaction (4).
NK cells comprise up to 40% of intrahepatic lymphocytes in humans and up to 20% of intrahepatic lymphocytes in mouse. Here, they facilitate tolerance to the diverse repertoire of pathogens that flow through the general circulation from the gut. Like NKT cells, NK cells integrate responses from other liver cells to mediate hepatic injury. HSCs can inhibit NK cell activity through TGF-b, and regulatory T cells can inhibit NK cell activity through IL-10 and TGF-b. NK cells can be activated by IFN-γ from NKT cells and dendritic cells, or through interleukin 12 (IL-12) and IL-18 from KCs to directly damage hepatocytes to promote liver injury and inhibit both liver regeneration and liver fibrosis through HSC inhibition (174).
Hepatocytes and HSCs both express α1β1 (very late antigen-1; VLA-1) and α2β1 (very late antigen-2; VLA-2) (67). α1β1 retains monocytes and circulating T cells under physiological conditions (49), while α2β1, which is upregulated during inflammatory states (58), mediates monocyte adhesion and NK cell proliferation in the liver (166). HSCs also express α5β1 (very late antigen-5; VLA-5), also known as the fibronectin receptor, in times of inflammation (67). VLA-5 has been shown to promote monocyte localization to infarcted myocardial tissue (175). Whether this interaction takes place in the liver is unknown.
While integrins are composed of heterodimers, the individual components are also trafficked through the activities of Rab GTPases. α1 is regulated by Rab21 and functions in cell migration. β1 is not only trafficked by Rab21, but also by Rab5, myosin X (Myo10), disabled homolog 2 (Dab2), and Numb, and functions in cell migration and cancer cell invasion. α2β1 (VLA-1) is trafficked by Rab11, and α5β1 (VLA-5) is trafficked by Rab11, Rab25, receptor component protein (RCP), chloride intracellular channel protein 3 (CLIC3), GIPC PDZ domain-containing family, member 1 (GIPC1), protein kinase D1 (PRKD1), syntaxin-6 (STX6), and vesicle-associated membrane protein 3 (VAMP3) (33).
Rab11 mediates the surface translocation of the TGFβ receptor in HSCs, suggesting a role for Rab11 in mediating hepatic fibrosis, as that is driven by TGFβ signaling (177). In addition, Rab25 regulates autophagy and lipid droplet degradation in HSCs in a ROS-dependent manner (198), suggesting that α5β1 (VLA-5) may also be involved in ROS-dependent surface expression. α1β1 is endocytosed by Dab2 in a clathrin-dependent manner, but the involvement of Rab GTPases in this regulation is unknown (11) (Fig. 1).
Mechanistic Target of Rapamycin Acts as a Sensor of Oxidative Stress and Effector of Inflammation in the Liver
The mechanistic target of rapamycin (mTOR) is a target of immunomodulation in autoimmune diseases, including SLE (188). mTORC1 and mTORC2, two different mTOR complexes, control distinct regulatory pathways in the cell. When acted upon by rapamycin, a macrolide synthesized by soil microbes first discovered on Easter Island, the mTOR/AKT/phosphatidylinositol 3-kinase (PI3K) pathway is inhibited, ultimately leading to decreased cell growth, proliferation, and survival. mTORC1 acts downstream of amino acids, glucose, immune cytokines, hormones, and mitochondrial ROS to promote messenger RNA (mRNA) transcription, calcium flux, nucleotide synthesis, protein synthesis, lipid synthesis, glycolysis, and flux through the pentose phosphate pathway (PPP).
mTORC1's ability to phosphorylate and activate eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) and S6 kinase (S6K) leads to increases in protein synthesis and nucleotide synthesis. mTORC2 acts downstream of the PI3K/AKT pathway to promote cytoskeleton rearrangement, apoptosis, glucose metabolism, and ion transport (152).
mTORC1 reacts to oxidative stress through its interaction with the Rheb protein, a member of the Ras superfamily of G proteins (127). The small molecule inhibitor NR1 preferentially binds the switch II domain of Rheb, halting mTORC1 downstream activities, but leaving mTORC2 signaling unaffected (106). HEK293T cells treated with phenylarsine oxide (PAO), a cell-permeable reagent that causes crosslinkage of vicinal thiol groups and oxidizes cysteine residues, result in activation of mTORC1 and not mTORC2, suggesting that ROS promote mTORC1 activity through the activation of the tuberous sclerosis complex (TSC) 2/Rheb pathway (197) (Fig. 4).
In times of increased stress, this pathway is responsible for generating antioxidant molecules. Cancer cells experience immense oxidative stress, and signaling through PI3K/AKT pathways mediates the upregulation of the classical antioxidant signaling molecule nuclear factor erythroid 2-related factor 2 (Nrf2) in addition to the metabolism of glutathione and the production of nicotinamide adenine dinucleotide phosphate (NADPH). Nrf2, when activated, acts as a transcription factor, upregulating the genes responsible for generating glutathione-S-transferase, SOD, and PPP enzymes, which generate NADPH (85).
Studies in mouse breast cancer models show crosstalk between the AKT/mTORC1 pathway and integrin expression/ECM remodeling. Mammary tissue-specific deletion of the β1 and β3 integrin subunits decreases mTORC1 activity (13). Since β1 and β3 integrins facilitate outside-in signaling, it is reasonable to conclude that integrin signaling modulates ROS responses in an mTOR-dependent manner. β1 integrins are critical to liver regeneration (163), and it is worth noting that mTOR signaling mediates physiological autophagy in the liver, which, when dysfunctional, can lead to liver fibrosis, predisposition to HBV infection, nonalcoholic fatty liver disease, liver ischemia and reperfusion injury, and liver cancer (181). Dysfunctional β1 integrin signaling has also been shown to promote hepatocellular carcinoma through the activation of AKT (2).
Paradoxically, long-term usage of rapamycin leads to increased ROS. Rapamycin administration in mouse models of liver dysfunction shows that while, in the short term, rapamycin can decrease ROS generation and lipid droplet formation (thus decreasing the incidence of nonalcoholic steatohepatitis and hepatocellular carcinoma), chronic, long-term administration can increase interleukin 6 (IL-6) and signal transducer and activator of transcription 3 (STAT3) signaling, which ultimately overcomes the beneficial effects of rapamycin (178). Case reports have even suggested that long-term rapamycin usage can reactivate latent HBV, and in vitro studies found that this may be linked to inducing hepatocellular autophagy (72).
Antioxidant Mitigation of Liver Dysfunction
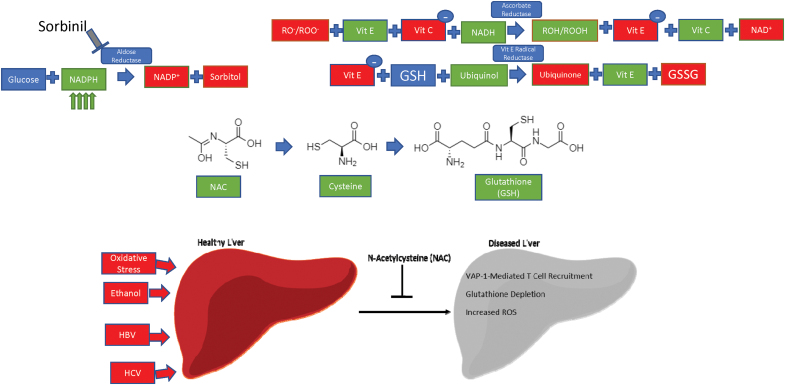
While ROS play a key role in regulating integrin and CAM expression, both dependently and independently of endosomal trafficking, ROS are also harmful to cellular components. The liver has more phagocytic cells than anywhere else in the body, and is uniquely positioned to mount an anti-inflammatory and redox response due to its continuous encounter with foreign food antigens (87). For example, liver damage caused by increased oxidative stress from chronic alcohol exposure predisposes to liver inflammation and hepatitis (116). Oxidative stress is implicated in the pathology and treatment of SLE—mitigation of oxidative stress by N-acetylcysteine (NAC) improves disease activity in mouse models of SLE (133).
In addition, ethanol-mediated ROS generation exacerbates the liver-damaging effects of HCV infection, which can cause liver fibrosis, hepatocellular carcinomas, and chronic, long-standing hepatitis (136). Mammalian redox systems exist to neutralize the damaging effects of ROS. The key players in these systems are NADPH and reduced glutathione (GSH), which act as electron carriers—most of the following pathways, in one way or another, exploit either NADPH [and its nonphosphorylated counterpart nicotinamide adenine dinucleotide (NADH) (196)] or glutathione to reduce the damaging effects of ROS on the cell (Table 2).
Table 2.
Implication of Antioxidant Enzymes in Human Disease Pathogenesis
| Enzyme | Disease implications |
|---|---|
| TAL | Autoimmunity (multiple sclerosis, rheumatoid arthritis, SLE) (24, 149), acetaminophen-induced hepatotoxicity (125) |
| SOD | ALS (36), HCC (40), hypertension (99), ARDS (52) |
| Catalase | Diabetes mellitus, acatalasemia, Alzheimer's disease, Parkinson's disease, vitiligo (121) |
| Glutathione peroxidase | Cancer (12), diabetes mellitus (108), cardiovascular disease (108) |
| Glutathione reductase | Hemolytic anemia, cardiovascular disease (3) |
| AR | Diabetes mellitus (185), cardiovascular disease (154), reperfusion injury (159) |
| IDH2 | Carcinogenesis (gliomas and AML, predominantly) (113) |
| NNT | Heart failure (157), familial glucocorticoid deficiency 1 (110), primary adrenal insufficiency (143) |
| G6PD | Hemolysis and favism in response to oxidative stress (47) |
Enzymes responsible for catalyzing antioxidant reactions in the human body are also implicated in human disease.
ALS, amyotrophic lateral sclerosis; AML, acute myeloid leukemia; AR, aldose reductase; ARDS, acute respiratory distress syndrome; G6PD, glucose-6-phosphate dehydrogenase; HCC, hepatocellular carcinoma; IDH2, isocitrate dehydrogenase 2; NNT, nicotinamide nucleotide transhydrogenase; SLE, systemic lupus erythematosus; SOD, superoxide dismutase; TAL, transaldolase.
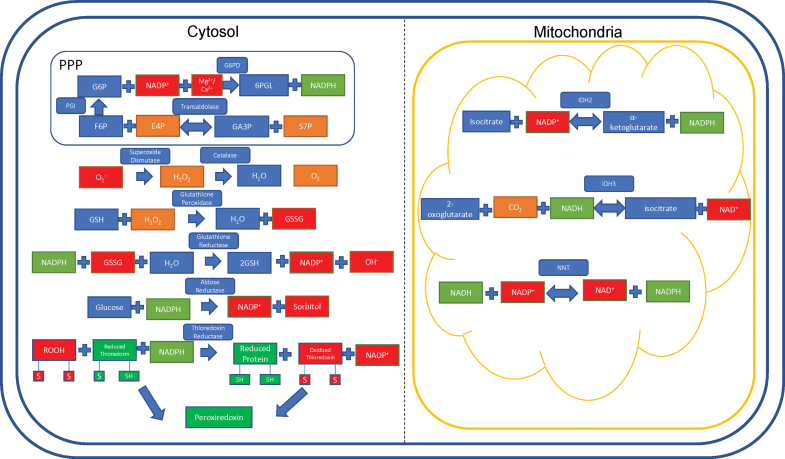
The PPP, also known as the hexose monophosphate shunt, generates intermediates for protein synthesis and nucleotide synthesis. The PPP is responsible for 5-carbon sugar metabolism and the generation of NADPH, crucial for recharging the antioxidant systems in response to oxidative stress (64). The PPP contains two arms: a nonoxidative branch and an oxidative branch, so named after their effects on the cell. The nonoxidative branch is regulated by the rate-limiting, reversible enzyme transaldolase (TAL), which catalyzes glyceraldehyde-3-phosphate and sedoheptulose-7-phosphate to erythrose 4-phosphate and fructose-6-phosphate (135). Haploinsufficiency of TAL has been reported in 4 of 27 patients with acetaminophen-induced liver failure, who were then responsive to NAC therapy (125). As of 2019, there have been 34 patients reported with complete TAL deficiency (187).
Glucose-6-phosphate dehydrogenase (G6PD) is the rate-limiting enzyme of the PPP, and is the most common enzyme deficiency present in ∼400 million people worldwide (15). In physiological states, G6PD converts glucose-6-phosphate into 6-phosphogluconate, reducing NADP+ into NADPH in the process. Deficiencies in this enzyme lead to a reduced capacity of erythrocytes to clear oxidative stress, which can lead to hemolysis and favism in response to oxidative drugs such as primaquine (malaria drug) and sulfamethoxazole (sulfa drug/antibiotic) (47).
Glutathione exists in two forms: oxidized (GSSG) and GSH. Glutathione acts as an electron carrier, preventing free electrons from binding to oxygen (O2) and wreaking havoc on the cell. In this case, NADPH harnesses the electrons from GSSG and is converted into NADP+. GSSG is then converted to GSH, along with a •OH (103).
Superoxide is generated as a by-product of O2 metabolism through natural processes and is a potent free radical (61). SOD acts to convert the highly active superoxide radical into molecular O2 and H2O2 (171). The remaining H2O2 is acted upon by the enzyme catalase to produce pure O2 and water (H2O) (21).
Aldose reductase (AR) is an enzyme located in the cytosol, which preferentially uses NADPH to promote its enzymatic activity. Using NADPH, AR converts glucose into sorbitol (88), a compound that is known to cause cataracts and visual disturbances through changes in osmotic pressure. Sorbinil is a pharmacological compound being investigated for use as a therapeutic against these diseases by acting as an inhibitor of AR (Fig. 5) (71).
FIG. 5.
Current antioxidant therapies and their potential in reversing liver dysfunction. Sorbinil inhibits aldose reductase to prevent the catalysis of NADPH, resulting in increased NADPH concentrations. Vitamins C and E act through ascorbate reductase and Vit E radical reductase to transfer electrons from ROS-damaged proteins to glutathione. NAC, a potent antioxidant, is a precursor to GSH through a cysteine intermediate. NAC reduces glutathione depletion, ROS generation, and potentially VAP-1 mediated T cell recruitment as a result of liver damage that can occur from oxidative stress, ethanol intoxication, HBV or HCV infection. Metadoxine and Silymarin/Silybin regenerate glutathione peroxidase to facilitate ROS clearance through a glutathione-dependent mechanism. GSH, reduced glutathione; HBV, hepatitis B virus; HCV, hepatitis C virus; IV, intravenous; NAC, N-acetylcysteine; NADPH, nicotinamide adenine dinucleotide phosphate; NAI-ALF, nonacetaminophen-induced acute liver failure; Vit C, vitamin C; Vit E, vitamin E. Color images are available online.
Thioredoxin (TRX) represents a family of proteins that are important in reducing oxidized proteins into reduced proteins by acting as molecular intermediates between oxidized proteins and NADPH (23).
Isocitrate dehydrogenase 2 (IDH2) is a reversible mitochondrial enzyme. IDH2 either goes in the forward direction to generate α-ketoglutarate and NADPH from isocitrate, or goes in the reverse direction, generating isocitrate and NADP+ from α-ketoglutarate. IDH2 can also convert 2-oxoglutarate into D-threo-isocitrate (101). In parallel, CO2 and NADPH are used up and generate NADP+ in the process. Preferentially targeting IDH2 can serve to dramatically decrease NADPH utilization, thus increasing antioxidant stores in the cell. IDH2 mutations are implicated in the progression of liquid and solid tumor cancers. Enasidinib, an FDA-approved glioblastoma multiforme therapy, preferentially inhibits the mutant R140Q IDH2 enzyme to kill cancer cells in mouse and human models (194). The incidence of IDH2 mutations in leukemia is up to 19% depending on the subtype of leukemia (109).
Nicotinamide nucleotide transhydrogenase (NNT) is a nuclear-encoded gene located in the inner mitochondrial membrane (70). Hoek and Rydström describe NNT as a physiological buffer system, serving to transfer electrons to NADPH from NADH and vice versa (70). The forward and reverse reactions are catalyzed by the proton motive force in the mitochondria, which is hypothesized to be due, in part, to a conformational change in the enzyme itself (145).
In the forward reaction, the buildup of the proton motive force from the cytoplasm into the mitochondria pushes the reaction forward, reducing NADP+ into NADPH. Conversely, the reverse reaction takes place under pathologic conditions (69), and is catalyzed by protons moving across the inner membrane from the mitochondrial compartment into the cytoplasmic compartment (70). The incidence of NNT mutations is ∼10% among patients with familial glucocorticoid deficiency, although prevalence information is unavailable at this time (143) (Fig. 6).
FIG. 6.
Antioxidant pathways in the liver. The redox pathways present in the cytosol and the mitochondria act to reduce oxidative stress by generating NADPH, glutathione, or directly reducing harmful ROS into their neutral counterparts. The box around the transaldolase and G6PD reactions represents the interaction between the oxidative and nonoxidative branches of the pentose phosphate pathway linked by PGI. The thioredoxin proteins converge to facilitate Prx activity; this is detailed in Figure 7. 6PGL, 6-phosphoglucono-δ-lactone; E4P, erythrose 4-phosphate; F6P, fructose 6-phosphate; G6P, glucose 6-phosphate; G6PD, glucose-6-phosphate dehydrogenase; GA3P, glyceraldehyde 3-phosphate; GSSG, oxidized glutathione; IDH2, isocitrate dehydrogenase 2; NADH, nicotinamide adenine dinucleotide; NNT, nicotinamide nucleotide transhydrogenase; PGI, phosphoglucoisomerase; Prx, peroxiredoxin; S7P, sedoheptulose 7-phosphate. Color images are available online.
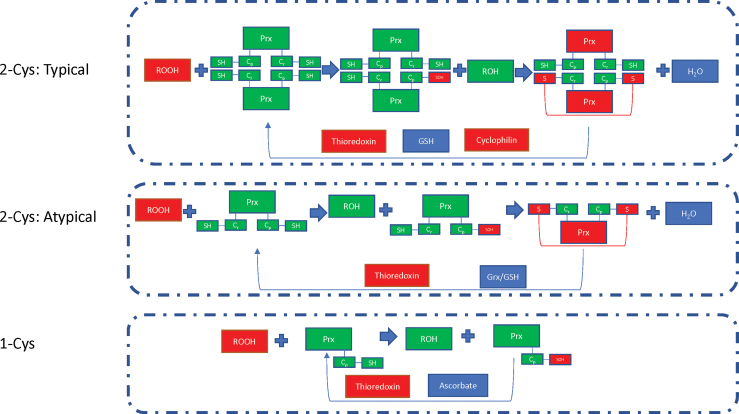
Peroxiredoxins (Prxs) are thiol-dependent peroxidases, which act to remove ROS and come in three subtypes. In typical 2-Cys Prxs, the main peroxidative cysteine residue (Cp) reacts with the resolving cysteine residue (Cr) that is contained in the second subunit of the Prx dimer. In atypical 2-Cys Prxs, the oxidized Cp reacts with the Cr residue on the same subunit molecule. In 1-Cys Prxs, the Cp residue generates sulfenic acid that is then regenerated directly through the transfer of an electron to the thiol group when ascorbate is present (122).
In states of acute liver damage, Prxs have been shown to translocate to the mitochondria and become hyperoxidized, eliminating their antioxidant function in the cell. Interestingly, Prxs can protect against ROS by reducing the expression of adhesion molecules in vascular endothelial cells and inhibit inflammatory response (102), two processes that, when dysfunctional, can directly lead to liver dysfunction. Autoantibodies against Prxs have also been shown to be upregulated in patients with autoimmune diseases, such as SLE and RA (80) (Fig. 7).
FIG. 7.
The Prxs, which reduce oxidative stress, operated via three variant mechanisms based on the number of cysteines involved and their localization within two (typical 2-Cys formation) or a single subunit molecules (atypical 2-Cys formation) or reliance of a single cysteine (1-Cys formation). Cys, cysteine; Grx, glutaredoxin. Color images are available online.
Targeting mTOR, ROS, and Integrins Has Therapeutic Potential in Autoimmune Liver Disease
We are only just starting to understand the importance of hepatic injury in the context of SLE. About 20% of SLE patients suffer from liver dysfunction (98). Lifestyle factors and interactions with infectious agents also predispose to inflammatory states in the liver, which involves central lymphocyte migration into the hepatic parenchyma (116).
While the liver is an organ of immense immune activity, pathologic inflammation almost always involves migration of peripherally circulating leukocytes and lymphocytes into the liver. For example, AIH, an autoimmune condition in which the immune system attacks its own hepatocytes, is characterized by CD4+ and CD8+ effector T cell responses (182). Primary biliary cirrhosis, a disease marked by inflammatory destruction of biliary epithelial cells, is also predominated by inflammatory infiltrates of CD4+ and CD8+ T cells, B cells, macrophages, eosinophils, and NK cells (77).
That mTOR inhibitors, such as rapamycin (sirolimus), everolimus, and mTOR kinase, are used to prevent immune rejection of transplanted livers and are used as therapies for hepatocellular carcinoma also suggest mTOR's important role in mediating hepatic inflammation, and that targeting mTOR has therapeutic benefits against liver inflammation (46). Indeed, rapamycin is used in the treatment of SLE, and considering liver dysfunction is present alongside SLE and can be reduced with rapamycin (either alone or with other immunosuppressants) (98) generates interesting questions in its role as an inhibitor of autoimmune-mediated hepatic dysfunction. Rapamycin has also been shown to treat refractory and post-transplant AIH (20, 82).
Reducing oxidative stress through NAC administration has beneficial effects outside of its classical role as a reversal agent of acetaminophen overdose (Table 3). Due to the impact of oxidative stress on integrin signaling, the effect of antioxidant therapy on integrins and abrogating hepatic dysfunction remains an outstanding question. Aberrant ROS production is hypothesized to be a driver of T cell dysfunction, which then leads to autoimmunity. We have reported that NAC blocks ROS production by complex I of the mitochondrial electron transport chain in lupus T cells (37).
Table 3.
Therapeutic Benefit of N-Acetylcysteine in Patients with Liver Failure
| Disease model | Therapeutic indication | Study type | Route of administration | Year of study |
|---|---|---|---|---|
| NAI-ALF (119) | “Recommend the use of NAC along with conventional treatments in patients with NAI-ALF in nontransplant centers while awaiting referrals and conclude the use of NAC as safe.” | Prospective randomized control trial | IV | 2017 |
| Acute liver failure without clinical or historical evidence of acetaminophen overdose (93) | “Intravenous NAC improves transplant-free survival in patients with early stage non-acetaminophen-related acute liver failure.” | Prospective, double-blind trial | IV | 2011 |
| NAI-ALF; no liver transplant center (117) | “The use of NAC causes reduction in NAI-ALF mortality and its use was safe.” | Prospective study | Oral | 2009 |
| Heat injury-mediated ischemic hepatitis (186) | “The use of NAC therapy in heat illness with ischemic hepatitis may prove most beneficial to those whose transition to a higher level of care is delayed or impossible.” | Case report | IV | 2019 |
NAC and other antioxidant therapies may improve liver disease and inflammation in other organs of patients with systemic autoimmunity.
IV, intravenous; NAC, N-acetylcysteine; NAI-ALF, nonacetaminophen-induced acute liver failure.
While hepatic dysfunction in SLE and autoimmunity occurs as a result of KC, NK cell, and NKT cell dysfunction (Fig. 1), we posit that targeting the T cells that mediate systemic autoimmunity may also inhibit liver dysfunction, which may lead to a decrease of autoreactive intrahepatic inflammation in the setting of SLE. The mechanisms that define the interactions between T cells and hepatocytes are poorly understood, and this is an open area for investigation.
Our laboratory has defined hepatic manifestations of SLE in mouse models (128). This squares well with clinical manifestations of SLE, which show dysfunctional LFTs correlating with SLEDAI scores. Treatment of SLE patients with corticosteroids not only reduced SLEDAI but also decreased liver disease severity as shown by decreased LFTs (98).
In a study of 85 patients with liver disease (49 patients with autoimmune cholestatic disease and 36 patients with AIH), patients with either disease condition had increased lipid and protein oxidative injury products, suggesting that either of these autoimmune hepatic conditions is associated with increased oxidative stress. Whether the oxidative stress leads to the disease or the disease leads to increased oxidative stress is unknown, but the study concluded that the increase of oxidative stress corresponded to “liver inflammatory activity and fibrosis” in both diseases (79).
In the treatment of inflammatory bowel disease (IBD), anti-integrin therapies, such as Natalizumab (α4 integrin), vedolizumab (α4β7 integrin), and ertolizumab (β7-integrin) (126), decrease disease flares by preventing the interactions between circulating immune cells and endothelial CAMs. While these therapies are effective for treating IBD, anti-integrin therapies have been shown to have adverse side effects, including hepatocellular injury and liver failure in <5% of patients receiving therapy (though this is still less than the ∼29% of IBD patients who develop abnormal liver tests without therapy) (68). Mouse studies have found that loss of αvβ5 promotes liver regeneration through inhibition of TGFβ signaling, though no studies have confirmed this in humans (55).
Targeting integrin expression via endosomal trafficking remains an open area of investigation. As of now, no therapeutics exist to reduce endosome-mediated disease burden in liver dysfunction. However, a completed clinical trial sought to exploit the endosomes of cancer cells using fimaporfin, a light-activated compound that incorporates into tumor cell endosomes (14).
Since Rab GTPases can regulate endosomal trafficking and sorting, Rabs have been an active area of research regarding what diseases they can cause. Overexpression of a variety of Rab isoforms is implicated in cancers, neurodegenerative diseases, and immune disorders (57). While nonspecific Rab inhibitors exist, such as 3-PEHPC (which inhibits many different Rab isoforms) (16), few, if any, inhibitors exist to directly target a single Rab GTPase, perhaps due to the high homology among members of the Rab family.
Antioxidants have also been used as disease therapeutics. Vitamins E and C work through vitamin E radical reductase and ascorbate reductase, respectively, to reduce redox agent burden (17). Metadoxine is an antioxidant that has been shown to improve survival in patients with alcoholic hepatitis when combined with the standard treatment regimen of prednisone and pentoxifylline due to its ability to synthesize glutathione (66). Alcoholic hepatitis involves immune cell margination into the liver, which may be dependent on ROS-mediated trafficking of cell surface receptors. Silymarin/Silybin, active extract derivatives from milk thistle, act to enhance both glutathione peroxidase activity and SOD, and have been found to improve clinical outcomes in patients with inflammatory liver diseases (25, 161).
Two dietary supplements, Resveratrol and Quercetin, have been under extensive study as antioxidant molecules. Resveratrol is a natural polyphenol, which has antioxidant, anti-inflammatory, and anticancer properties. It stimulates expression of sirtuins, which then promote antioxidant enzyme production (144). Quercetin is a flavonoid found in fruits and vegetables that has been shown to increase catalase, SOD, and glutathione peroxidase activities to reduce ROS in the cell (189). Relevant for our studies of immunity, quercetin can block TNFα-mediated NF-κB signaling in vitro and ultimately decreases cytokine release in vivo (95). NAC regenerates glutathione stores, which are depleted in acute acetaminophen toxicity (63).
In the context of autoimmunity, our group found that NAC provides a therapeutic effect when administered to SLE patients by blocking the mTOR pathway (90). Our group is currently expanding on this finding in a phase 2 clinical trial (134) (Fig. 5).
Conclusions
The tightly controlled immune response to a pathogen, when dysfunctional or retargeted to a self-antigen, can lead to systemic autoimmunity. SLE, a prototypical systemic autoimmune disease, is characterized by potentially life-threatening involvement of all organs of the body, underlain by ROS generation, dysregulated endosomal trafficking, and abnormal CAM and integrin expression. Changes in CAMs and integrins facilitate liver invasion by immune cells—the redox control of integrin expression is crucial to controlling hepatic dysfunction in the setting of systemic autoimmunity. Through the direct modification of integrins or the endosomal trafficking of integrins via redox-sensitive Rab GTPases, ROS can modulate immune attack in states of autoimmunity. Targeting ROS-mediated signaling and pathways that exploit ROS generation and neutralization can serve as potentially powerful therapeutics to help alter immune-mediated liver injury.
Acknowledgment
The authors thank Brandon Wyman for his careful reading and editing of the article.
Abbreviations Used
- •OH
hydroxyl radical
- 1O2
singlet oxygen
- 6PGL
6-phosphoglucono-δ-lactone
- AIH
autoimmune hepatitis
- ALS
amyotrophic lateral sclerosis
- AML
acute myeloid leukemia
- APC
antigen presenting cell
- AR
aldose reductase
- ARDS
acute respiratory distress syndrome
- Arf
ADP ribosylation factor
- CAM
cell adhesion molecule
- CD
cluster of differentiation
- cDC
conventional dendritic cell
- Cp
peroxidative cysteine residue
- Cr
resolving cysteine residue
- c-SMAC
central supramolecular activation cluster
- Cys
cysteine
- Dab2
disabled homolog 2
- E4P
erythrose 4-phosphate
- ECM
extracellular matrix
- EMT
epithelial to mesenchymal transition
- ER
endoplasmic reticulum
- F6P
fructose 6-phosphate
- G6P
glucose 6-phosphate
- G6PD
glucose-6-phosphate dehydrogenase
- GA3P
glyceraldehyde 3-phosphate
- GDP
guanosine diphosphate
- Grx
glutaredoxin
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GTP
guanosine triphosphate
- H2O2
hydrogen peroxide
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HEV
high endothelial venule
- Hic-5
hydrogen peroxide-inducible clone-5
- HRES-1
HTLV-I-related endogenous retroviral sequence
- HSC
hepatic stellate cell/Ito cell
- IBD
inflammatory bowel disease
- ICAM-1
intracellular adhesion molecule-1
- IDH2
isocitrate dehydrogenase 2
- IFN-γ
interferon gamma
- IL-10
interleukin 10
- IL-12
interleukin 12
- IL-18
interleukin 18
- IS
immune synapse
- IV
intravenous
- KC
Kupffer cell
- LFA-1
lymphocyte function-associated antigen 1
- LFT
liver function test
- MHC
major histocompatibility complex
- MPR
mannose-6-phosphate receptor
- mTOR
mechanistic target of rapamycin
- mTORC1
complex 1 of the mechanistic target of rapamycin
- mTORC2
mechanistic target of rapamycin complex 2
- NAC
N-acetylcysteine
- NADH
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAI-ALF
nonacetaminophen-induced acute liver failure
- NF-κB
nuclear factor kappa B
- NK
natural killer
- NKT
natural killer T
- NKT I
type I NKT
- NKT II
type II NKT
- NNT
nicotinamide nucleotide transhydrogenase
- Nrf2
nuclear factor erythroid 2-related factor 2
- O2
oxygen
- pDC
plasmacytoid dendritic cell
- PGI
phosphoglucoisomerase
- PI3K
phosphatidylinositol 3-kinase
- PPP
pentose phosphate pathway
- Prx
peroxiredoxin
- p-SMAC
peripheral supramolecular activation cluster
- RA
rheumatoid arthritis
- Rab
ras-like protein from rat brain
- Rheb
ras homolog enriched in brain
- ROI
reactive oxygen intermediate
- ROS
reactive oxygen species
- S7P
sedoheptulose 7-phosphate
- SHP-2
SH2 domain-containing protein tyrosine phosphatase-2
- SLE
systemic lupus erythematosus
- SLEDAI
systemic lupus erythematosus disease activity index
- SOD
superoxide dismutase
- STAT3
signal transducer and activator of transcription 3
- TAL
transaldolase
- TCR
T cell receptor
- TfR
transferrin receptor; CD71
- TGFβ
transforming growth factor beta
- TLR7
toll-like receptor 7
- TSC
tuberous sclerosis complex
- VAP-1
vascular adhesion protein-1
- VCAM
vascular cell adhesion molecule
- Vit C
vitamin C
- Vit E
vitamin E
- VLA-1
very late antigen-1; integrin α1β1
- VLA-2
very late antigen-2; integrin α2β1
- VLA-5
very late antigen-5; integrin α5β1; fibronectin receptor
Authors' Contributions
All the authors conceived and wrote this review in its entirety.
Author Disclosure Statement
The authors do not have any competing financial interests to disclose.
Funding Information
This work was supported by grants R01-AI072648 and R01-AI122176 from the National Institutes of Health and the Central New York Community Foundation.
References
- 1. Andres PG, Howland KC, Dresnek D, Edmondson S, Abbas AK, and Krummel MF. CD28 signals in the immature immunological synapse. J Immunol 172: 5880–5886, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Bai X, Wang J, Guo Y, Pan J, Yang Q, Zhang M, Li H, Zhang L, Ma J, Shi F, Shu W, Wang Y, and Leng J. Prostaglandin E2 stimulates β1-integrin expression in hepatocellular carcinoma through the EP1 receptor/PKC/NF-κB pathway. Sci Rep 4: 6538, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, and Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390: 191–214, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banales JM, Huebert RC, Karlsen T, Strazzabosco M, LaRusso NF, and Gores GJ. Cholangiocyte pathobiology. Nat Rev Gastroenterol Hepatol 16: 269–281, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bandyopadhyay K, Marrero I, and Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell Mol Immunol 13: 337–346, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. BasuRay S. RAB18 modulates autophagy in human stellate cells. J Clin Lipidol 13: 832–838, 2019. [DOI] [PubMed] [Google Scholar]
- 7. Berger T, Ueda T, Arpaia E, Chio, II, Shirdel EA, Jurisica I, Hamada K, You-Ten A, Haight J, Wakeham A, Cheung CC, and Mak TW. Flotillin-2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Oncogene 32: 4989–4994, 2013. [DOI] [PubMed] [Google Scholar]
- 8. Bertram EM, Hawley RG, and Watts TH. Overexpression of rab7 enhances the kinetics of antigen processing and presentation with MHC class II molecules in B cells. Int Immunol 14: 309–318, 2002. [DOI] [PubMed] [Google Scholar]
- 9. Bessone F, Poles N, and Roma MG. Challenge of liver disease in systemic lupus erythematosus: clues for diagnosis and hints for pathogenesis. World J Hepatol 6: 394–409, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonder CS, Norman MU, Swain MG, Zbytnuik LD, Yamanouchi J, Santamaria P, Ajuebor M, Salmi M, Jalkanen S, and Kubes P. Rules of recruitment for Th1 and Th2 lymphocytes in inflamed liver: a role for alpha-4 integrin and vascular adhesion protein-1. Immunity 23: 153–163, 2005. [DOI] [PubMed] [Google Scholar]
- 11. Bridgewater RE, Norman JC, and Caswell PT. Integrin trafficking at a glance. J Cell Sci 125: 3695–3701, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brigelius-Flohé R and Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta 1790: 1555–1568, 2009. [DOI] [PubMed] [Google Scholar]
- 13. Bui T, Rennhack J, Mok S, Ling C, Perez M, Roccamo J, Andrechek ER, Moraes C, and Muller WJ. Functional redundancy between β1 and β3 integrin in activating the IR/Akt/mTORC1 signaling axis to promote ErbB2-driven breast cancer. Cell Rep 29: 589.e6–602.e6, 2019. [DOI] [PubMed] [Google Scholar]
- 14. Bush J. Study to Assess Safety, Tolerability and Immune Response of Fimaporfin-induced Photochemical Internalisation of Antigen/Adjuvant. 2016. https://ClinicalTrials.gov/show/NCT02947854 (Accessed on June 16, 2021).
- 15. Cappellini MD and Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 371: 64–74, 2008. [DOI] [PubMed] [Google Scholar]
- 16. Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, Lai ZW, Miklossy G, Singh RR, Chudakov DM, Malorni W, Middleton F, Banki K, and Perl A. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis 73: 1888–1897, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol 71: 725–731, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Chang Y-C, Su C-Y, Chen M-H, Chen W-S, Chen C-L, and Hsiao M. Secretory RAB GTPase 3C modulates IL6-STAT3 pathway to promote colon cancer metastasis and is associated with poor prognosis. Mol Cancer 16: 135, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol 125: S3–S23, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chatrath H, Allen L, and Boyer TD. Use of sirolimus in the treatment of refractory autoimmune hepatitis. Am J Med 127: 1128–1131, 2014. [DOI] [PubMed] [Google Scholar]
- 21. Chelikani P, Fita I, and Loewen PC. Diversity of structures and properties among catalases. Cell Mol Life Sci 61: 192–208, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chio IIC and Tuveson DA. ROS in cancer: the burning question. Trends Mol Med 23: 411–429, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collet JF and Messens J. Structure, function, and mechanism of thioredoxin proteins. Antioxid Redox Signal 13: 1205–1216, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Colombo E, Banki K, Tatum AH, Daucher J, Ferrante P, Murray RS, Phillips PE, and Perl A. Comparative analysis of antibody and cell-mediated autoimmunity to transaldolase and myelin basic protein in patients with multiple sclerosis. J Clin Invest 99: 1238–1250, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Comelli MC, Mengs U, Schneider C, and Prosdocimi M. Toward the definition of the mechanism of action of silymarin: activities related to cellular protection from toxic damage induced by chemotherapy. Integr Cancer Ther 6: 120–129, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Compeer EB, Kraus F, Ecker M, Redpath G, Amiezer M, Rother N, Nicovich PR, Kapoor-Kaushik N, Deng Q, Samson GPB, Yang Z, Lou J, Carnell M, Vartoukian H, Gaus K, and Rossy J. A mobile endocytic network connects clathrin-independent receptor endocytosis to recycling and promotes T cell activation. Nat Commun 9: 1597, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Conner SD and Schmid SL. Regulated portals of entry into the cell. Nature 422: 37–44, 2003. [DOI] [PubMed] [Google Scholar]
- 28. Cook-Mills JM. VCAM-1 signals during lymphocyte migration: role of reactive oxygen species. Mol Immunol 39: 499–508, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, and Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A 89: 9991–9995, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. da Rosa Franchi Santos LF, Costa NT, Maes M, Simão ANC, and Dichi I. Influence of treatments on cell adhesion molecules in patients with systemic lupus erythematosus and rheumatoid arthritis: a review. Inflammopharmacology 28: 363–384, 2020. [DOI] [PubMed] [Google Scholar]
- 31. da Rosa Franchi Santos LF, Stadtlober NP, Costa Dall'Aqua LG, Scavuzzi BM, Guimarães PM, Flauzino T, Batisti Lozovoy MA, Mayumi Iriyoda TV, Vissoci Reiche EM, Dichi I, Maes M, and Colado Simão A. Increased adhesion molecule levels in systemic lupus erythematosus: relationships with severity of illness, autoimmunity, metabolic syndrome and cortisol levels. Lupus 27: 380–388, 2018. [DOI] [PubMed] [Google Scholar]
- 32. Danen EHJ. Integrin signaling as a cancer drug target. ISRN Cell Biol 2013: 135164, 2013. [Google Scholar]
- 33. De Franceschi N, Hamidi H, Alanko J, Sahgal P, and Ivaska J. Integrin traffic—the update. J Cell Sci 128: 839–852, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Demetriou P, Abu-Shah E, McCuaig S, Mayya V, Valvo S, Korobchevskaya K, Friedrich M, Mann E, Lee LY, Starkey T, Kutuzov MA, Afrose J, Siokis A, Meyer-Hermann M, Depoil D, and Dustin ML. CD2 expression acts as a quantitative checkpoint for immunological synapse structure and T-cell activation. bioRxiv 589440, 2019. [Google Scholar]
- 35. Demir K, Kirsch N, Beretta Carlo A, Erdmann G, Ingelfinger D, Moro E, Argenton F, Carl M, Niehrs C, and Boutros M. RAB8B is required for activity and caveolar endocytosis of LRP6. Cell Rep 4: 1224–1234, 2013. [DOI] [PubMed] [Google Scholar]
- 36. Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, Warner C, Deng G, Soriano E, Smyth C, Parge HE, Ahmed A, Roses AD, Hallewell RA, Pericak-Vance MA, and Siddique T. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science 261: 1047–1051, 1993. [DOI] [PubMed] [Google Scholar]
- 37. Doherty E, Oaks Z, and Perl A. Increased mitochondrial electron transport chain activity at complex I is regulated by N-acetylcysteine in lymphocytes of patients with systemic lupus erythematosus. Antioxid Redox Signal 21: 56–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dustin ML, Chakraborty AK, and Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol 2: a002311, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dustin ML and Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341: 619–624, 1989. [DOI] [PubMed] [Google Scholar]
- 40. Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, and Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene 24: 367–380, 2005. [DOI] [PubMed] [Google Scholar]
- 41. Esche C, Stellato C, and Beck LA. Chemokines: key players in innate and adaptive immunity. J Invest Dermatol 125: 615–628, 2005. [DOI] [PubMed] [Google Scholar]
- 42. Fabbri M, Di Meglio S, Gagliani MC, Consonni E, Molteni R, Bender JR, Tacchetti C, and Pardi R. Dynamic partitioning into lipid rafts controls the endo-exocytic cycle of the αL/β2 integrin, LFA-1, during leukocyte chemotaxis. Mol Biol Cell 16: 5793–5803, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fagerholm SC, MacPherson M, James MJ, Sevier-Guy C, and Lau CS. The CD11b-integrin (ITGAM) and systemic lupus erythematosus. Lupus 22: 657–663, 2013. [DOI] [PubMed] [Google Scholar]
- 44. Feng Q, Bonder EM, Engevik AC, Zhang L, Tyska MJ, Goldenring JR, and Gao N. Disruption of Rab8a and Rab11a causes formation of basolateral microvilli in neonatal enteropathy. J Cell Sci 130: 2491–2505, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fernandez DR, Telarico T, Bonilla E, Li Q, Banerjee S, Middleton FA, Phillips PE, Crow MK, Oess S, Muller-Esterl W, and Perl A. Activation of mammalian target of rapamycin controls the loss of TCRzeta in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol 182: 2063–2073, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferrín G, Guerrero M, Amado V, Rodríguez-Perálvarez M, and De la Mata M. Activation of mTOR signaling pathway in hepatocellular carcinoma. Int J Mol Sci 21: 1266, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frank JE. Diagnosis and management of G6PD deficiency. Am Fam Physician 72: 1277–1282, 2005. [PubMed] [Google Scholar]
- 48. Frossi B, De Carli M, Piemonte M, and Pucillo C. Oxidative microenvironment exerts an opposite regulatory effect on cytokine production by Th1 and Th2 cells. Mol Immunol 45: 58–64, 2008. [DOI] [PubMed] [Google Scholar]
- 49. Gardner H. Integrin α1β1. Adv Exp Med Biol 819: 21–39, 2014. [DOI] [PubMed] [Google Scholar]
- 50. Geiger B, Bershadsky A, Pankov R, and Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol 2: 793–805, 2001. [DOI] [PubMed] [Google Scholar]
- 51. Goitre L, Pergolizzi B, Ferro E, Trabalzini L, and Retta SF. Molecular crosstalk between integrins and cadherins: do reactive oxygen species set the talk? J Signal Transduct 2012: 807682, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gongora MC, Lob HE, Landmesser U, Guzik TJ, Martin WD, Ozumi K, Wall SM, Wilson DS, Murthy N, Gravanis M, Fukai T, and Harrison DG. Loss of extracellular superoxide dismutase leads to acute lung damage in the presence of ambient air: a potential mechanism underlying adult respiratory distress syndrome. Am J Pathol 173: 915–926, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grant AJ, Lalor PF, Salmi M, Jalkanen S, and Adams DH. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 359: 150–157, 2002. [DOI] [PubMed] [Google Scholar]
- 54. Grant BD and Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 10: 597–608, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Greenhalgh SN, Matchett KP, Taylor RS, Huang K, Li JT, Saeteurn K, Donnelly MC, Simpson EEM, Pollack JL, Atakilit A, Simpson KJ, Maher JJ, Iredale JP, Sheppard D, and Henderson NC. Loss of integrin αvβ8 in murine hepatocytes accelerates liver regeneration. Am J Pathol 189: 258–271, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gregg D, de Carvalho DD, and Kovacic H. Integrins and coagulation: a role for ROS/redox signaling? Antioxid Redox Signal 6: 757–764, 2004. [DOI] [PubMed] [Google Scholar]
- 57. Guadagno NA and Progida C. Rab GTPases: switching to human diseases. Cells 8: 909, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guo Q, Furuta K, Lucien F, Gutierrez Sanchez LH, Hirsova P, Krishnan A, Kabashima A, Pavelko KD, Madden B, Alhuwaish H, Gao Y, Revzin A, and Ibrahim SH. Integrin β(1)-enriched extracellular vesicles mediate monocyte adhesion and promote liver inflammation in murine NASH. J Hepatol 71: 1193–1205, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Haberger V, Elgner F, Roos J, Bender D, and Hildt E. Regulation of the transferrin receptor recycling in hepatitis C virus-replicating cells. Front Cell Dev Biol 8: 44, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hautekeete ML and Geerts A. The hepatic stellate (Ito) cell: its role in human liver disease. Virchows Arch 430: 195–207, 1997. [DOI] [PubMed] [Google Scholar]
- 61. Hayyan M, Hashim MA, and AlNashef IM. Superoxide ion: generation and chemical implications. Chem Rev 116: 3029–3085, 2016. [DOI] [PubMed] [Google Scholar]
- 62. He L, He T, Farrar S, Ji L, Liu T, and Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44: 532–553, 2017. [DOI] [PubMed] [Google Scholar]
- 63. Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med 359: 285–292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Heinrich PC, Morris HP, and Weber G. Behavior of transaldolase (EC 2.2.1.2) and transketolase (EC 2.2.1.1) Activities in normal, neoplastic, differentiating, and regenerating liver. Cancer Res 36: 3189–3197, 1976. [PubMed] [Google Scholar]
- 65. Heo J. Redox control of GTPases: from molecular mechanisms to functional significance in health and disease. Antioxid Redox Signal 14: 689–724, 2011. [DOI] [PubMed] [Google Scholar]
- 66. Higuera-de la Tijera F, Servín-Caamaño AI, Serralde-Zúñiga AE, Cruz-Herrera J, Pérez-Torres E, Abdo-Francis JM, Salas-Gordillo F, and Pérez-Hernández JL. Metadoxine improves the three- and six-month survival rates in patients with severe alcoholic hepatitis. World J Gastroenterol 21: 4975–4985, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hintermann E and Christen U. The many roles of cell adhesion molecules in hepatic fibrosis. Cells 8: 1503, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirten R, Sultan K, Thomas A, and Bernstein DE. Hepatic manifestations of non-steroidal inflammatory bowel disease therapy. World J Hepatol 7: 2716–2728, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ho HY, Lin YT, Lin G, Wu PR, and Cheng ML. Nicotinamide nucleotide transhydrogenase (NNT) deficiency dysregulates mitochondrial retrograde signaling and impedes proliferation. Redox Biol 12: 916–928, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoek JB and Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J 254: 1–10, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang Q, Liu Q, and Ouyang D. Sorbinil, an aldose reductase inhibitor, in fighting against diabetic complications. Med Chem 15: 3–7, 2019. [DOI] [PubMed] [Google Scholar]
- 72. Huang W, Zhao F, Huang Y, Li X, Zhu S, Hu Q, and Chen W. Rapamycin enhances HBV production by inducing cellular autophagy. Hepat Mon 14: e20719, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hülsbusch N, Solis GP, Katanaev VL, and Stuermer CA. Reggie-1/Flotillin-2 regulates integrin trafficking and focal adhesion turnover via Rab11a. Eur J Cell Biol 94: 531–545, 2015. [DOI] [PubMed] [Google Scholar]
- 74. Huse M, Lillemeier BF, Kuhns MS, Chen DS, and Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol 7: 247–255, 2006. [DOI] [PubMed] [Google Scholar]
- 75. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. [DOI] [PubMed] [Google Scholar]
- 76. Hynes RO. The extracellular matrix: not just pretty fibrils. Science 326: 1216–1219, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jones DE. Pathogenesis of primary biliary cirrhosis. Gut 56: 1615–1624, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Junutula JR, De Maziére AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, Klumperman J, and Scheller RH. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell 15: 2218–2229, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kaffe ET, Rigopoulou EI, Koukoulis GK, Dalekos GN, and Moulas AN. Oxidative stress and antioxidant status in patients with autoimmune liver diseases. Redox Rep 20: 33–41, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Karasawa R, Ozaki S, Nishioka K, and Kato T. Autoantibodies to peroxiredoxin I and IV in patients with systemic autoimmune diseases. Microbiol Immunol 49: 57–65, 2005. [DOI] [PubMed] [Google Scholar]
- 81. Kazzaz NM, Coit P, Lewis EE, McCune WJ, Sawalha AH, and Knight JS. Systemic lupus erythematosus complicated by diffuse alveolar haemorrhage: risk factors, therapy and survival. Lupus Sci Med 2: e000117, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kerkar N, Dugan C, Rumbo C, Morotti RA, Gondolesi G, Shneider BL, and Emre S. Rapamycin successfully treats post-transplant autoimmune hepatitis. Am J Transplant 5: 1085–1089, 2005. [DOI] [PubMed] [Google Scholar]
- 83. Khan SQ, Khan I, and Gupta V. CD11b activity modulates pathogenesis of lupus nephritis. Front Med (Lausanne) 5: 52, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kinashi T. Overview of integrin signaling in the immune system. In: Integrin and Cell Adhesion Molecules: Methods and Protocols, edited by Shimaoka M. Totowa, NJ: Humana Press, 2012, pp. 261–278. [DOI] [PubMed] [Google Scholar]
- 85. Koundouros N and Poulogiannis G. Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front Oncol 8: 160, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Krawczyk M, Leimgruber E, Seguín-Estévez Q, Dunand-Sauthier I, Barras E, and Reith W. Expression of RAB4B, a protein governing endocytic recycling, is co-regulated with MHC class II genes. Nucleic Acids Res 35: 595–605, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kubes P and Jenne C. Immune responses in the liver. Annu Rev Immunol 36: 247–277, 2018. [DOI] [PubMed] [Google Scholar]
- 88. Kumar PA and Reddy GB. Focus on molecules: aldose reductase. Exp Eye Res 85: 739–740, 2007. [DOI] [PubMed] [Google Scholar]
- 89. Kurkijarvi R, Yegutkin G, Gunson B, Jalkanen S, Salmi M, and Adams D. Circulating soluble vascular adhesion protein 1 accounts for the increased serum monoamine oxidase activity in chronic liver disease. Gastroenterology 119: 1096–1103, 2000. [DOI] [PubMed] [Google Scholar]
- 90. Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, Francis L, Garcia R, Dawood M, Yu J, Ramos I, Coman I, Faraone SV, Phillips PE, and Perl A. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 64: 2937–2946, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, and Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol 169: 983–992, 2002. [DOI] [PubMed] [Google Scholar]
- 92. Lalor PF, Shields P, Grant AJ, and Adams DH. Recruitment of lymphocytes to the human liver. Immunol Cell Biol 80: 52–64, 2002. [DOI] [PubMed] [Google Scholar]
- 93. Lee WM, Hynan LS, Rossaro L, Fontana RJ, Stravitz RT, Larson AM, Davern TJ, 2nd, Murray NG, McCashland T, Reisch JS, and Robuck PR. Intravenous N-acetylcysteine improves transplant-free survival in early stage non-acetaminophen acute liver failure. Gastroenterology 137: 856–864, 864.e1, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li R, Li H, Luo HJ, Lin ZX, Jiang ZW, and Luo WH. SSAO inhibitors suppress hepatocellular tumor growth in mice. Cell Immunol 283: 61–69, 2013. [DOI] [PubMed] [Google Scholar]
- 95. Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, and Yin Y. Quercetin, inflammation and immunity. Nutrients 8: 167, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li Z, Weller SG, Drizyte-Miller K, Chen J, Krueger EW, Mehall B, Stöckli J, Casey CA, Cao H, and McNiven MA. Maturation of lipophagic organelles in hepatocytes is dependent upon a Rab10/Dynamin-2 complex. Hepatology 72: 486–502, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liden J, Rafter I, Truss M, Gustafsson JA, and Okret S. Glucocorticoid effects on NF-kappaB binding in the transcription of the ICAM-1 gene. Biochem Biophys Res Commun 273: 1008–1014, 2000. [DOI] [PubMed] [Google Scholar]
- 98. Liu Y, Yu J, Oaks Z, Marchena-Mendez I, Francis L, Bonilla E, Aleksiejuk P, Patel J, Banki K, Landas SK, and Perl A. Liver injury correlates with biomarkers of autoimmunity and disease activity and represents an organ system involvement in patients with systemic lupus erythematosus. Clin Immunol 160: 319–327, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lob HE, Marvar PJ, Guzik TJ, Sharma S, McCann LA, Weyand C, Gordon FJ, and Harrison DG. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 55: 277–283, 6p following 283, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lorant DE, Topham MK, Whatley RE, McEver RP, McIntyre TM, Prescott SM, and Zimmerman GA. Inflammatory roles of P-selectin. J Clin Invest 92: 559–570, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Losman JA and Kaelin WG Jr. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes Dev 27: 836–852, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Lu D, Wang W, Liu J, Qi L, Zhuang R, Zhuo J, Zhang X, Xu X, and Zheng S. Peroxiredoxins in inflammatory liver diseases and ischemic/reperfusion injury in liver transplantation. Food Chem Toxicol 113: 83–89, 2018. [DOI] [PubMed] [Google Scholar]
- 103. Lu SC. Glutathione synthesis. Biochim Biophys Acta 1830: 3143–3153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lu X, Zhu C, Qian J, Chen X, Ye S, and Gu Y. Intrathecal cytokine and chemokine profiling in neuropsychiatric lupus or lupus complicated with central nervous system infection. Lupus 19: 689–695, 2010. [DOI] [PubMed] [Google Scholar]
- 105. Magadán JG, Barbieri MA, Mesa R, Stahl PD, and Mayorga LS. Rab22a regulates the sorting of transferrin to recycling endosomes. Mol Cell Biol 26: 2595–2614, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mahoney SJ, Narayan S, Molz L, Berstler LA, Kang SA, Vlasuk GP, and Saiah E. A small molecule inhibitor of Rheb selectively targets mTORC1 signaling. Nat Commun 9: 548, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Marrack P, Kappler J, and Kotzin BL. Autoimmune disease: why and where it occurs. Nat Med 7: 899–905, 2001. [DOI] [PubMed] [Google Scholar]
- 108. McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, and Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A 101: 8852–8857, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. McKenney AS and Levine RL. Isocitrate dehydrogenase mutations in leukemia. J Clin Invest 123: 3672–3677, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Meimaridou E, Kowalczyk J, Guasti L, Hughes CR, Wagner F, Frommolt P, Nürnberg P, Mann NP, Banerjee R, Saka HN, Chapple JP, King PJ, Clark AJ, and Metherell LA. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet 44: 740–742, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Miyasaka M and Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 4: 360–370, 2004. [DOI] [PubMed] [Google Scholar]
- 112. Mok CC, Soliman S, Ho LY, Mohamed FA, Mohamed FI, and Mohan C. Urinary angiostatin, CXCL4 and VCAM-1 as biomarkers of lupus nephritis. Arthritis Res Ther 20: 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Molenaar RJ, Radivoyevitch T, Maciejewski JP, van Noorden CJ, and Bleeker FE. The driver and passenger effects of isocitrate dehydrogenase 1 and 2 mutations in oncogenesis and survival prolongation. Biochim Biophys Acta 1846: 326–341, 2014. [DOI] [PubMed] [Google Scholar]
- 114. Moser M, Legate KR, Zent R, and Fässler R. The tail of integrins, talin, and kindlins. Science 324: 895–899, 2009. [DOI] [PubMed] [Google Scholar]
- 115. Moussion C and Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479: 542–546, 2011. [DOI] [PubMed] [Google Scholar]
- 116. Mueller S, Millonig G, and Seitz HK. Alcoholic liver disease and hepatitis C: a frequently underestimated combination. World J Gastroenterol 15: 3462–3471, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mumtaz K, Azam Z, Hamid S, Abid S, Memon S, Ali Shah H, and Jafri W. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int 3: 563–570, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Murayama G, Furusawa N, Chiba A, Yamaji K, Tamura N, and Miyake S. Enhanced IFN-α production is associated with increased TLR7 retention in the lysosomes of palasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther 19: 234, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nabi T, Nabi S, Rafiq N, and Shah A. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: a prospective study. Saudi J Gastroenterol 23: 169–175, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Nagy G, Ward J, Mosser DD, Koncz A, Gergely P, Jr.,Stancato C, Qian Y, Fernandez D, Niland B, Grossman CE, Telarico T, Banki K, and Perl A.. Regulation of CD4 expression via recycling by HRES-1/RAB4 controls susceptibility to HIV infection. J Biol Chem 281: 34574–34591, 2006. [DOI] [PubMed] [Google Scholar]
- 121. Nandi A, Yan L-J, Jana CK, and Das N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid Med Cell Longev 2019: 9613090, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nicolussi A, D'Inzeo S, Capalbo C, Giannini G, and Coppa A. The role of peroxiredoxins in cancer (Review). Mol Clin Oncol 6: 139–153, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Nishikimi A, Ishihara S, Ozawa M, Etoh K, Fukuda M, Kinashi T, and Katagiri K. Rab13 acts downstream of the kinase Mst1 to deliver the integrin LFA-1 to the cell surface for lymphocyte trafficking. Sci Signal 7: ra72, 2014. [DOI] [PubMed] [Google Scholar]
- 124. O'Mahony F, Wroblewski K, O'Byrne SM, Jiang H, Clerkin K, Benhammou J, Blaner WS, and Beaven SW. Liver X receptors balance lipid stores in hepatic stellate cells through Rab18, a retinoid responsive lipid droplet protein. Hepatology 62: 615–626, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Oaks Z, Jimah J, Grossman CC, Beckford M, Kelly R, Banerjee S, Niland B, Miklossy G, Kuloglu Z, Kansu A, Lee W, Szonyi L, Banki K, and Perl A. Transaldolase haploinsufficiency in subjects with acetaminophen-induced liver failure. J Inherit Metab Dis 43: 496–506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Park SC and Jeen YT. Anti-integrin therapy for inflammatory bowel disease. World J Gastroenterol 24: 1868–1880, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Parmar N and Tamanoi F. Rheb G-proteins and the activation of mTORC1. Enzymes 27: 39–56, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Patel A HN, Oaks Z, and Perl A. Rab4A activation predisposes to hepatitis in spontaneous and pristane-induced mouse models of systemic lupus erythematosus. In: ACR Convergence 2020. Virtual: American College of Rheumatology, 2020. [Google Scholar]
- 129. Paul Nikki R, Jacquemet G, and Caswell Patrick T. Endocytic trafficking of integrins in cell migration. Curr Biol 25: R1092–R1105, 2015. [DOI] [PubMed] [Google Scholar]
- 130. Pellinen T and Ivaska J. Integrin traffic. J Cell Sci 119: 3723–3731, 2006. [DOI] [PubMed] [Google Scholar]
- 131. Perl A. Systems biology of lupus: mapping the impact of genomic and environmental factors on gene expression signatures, cellular signaling, metabolic pathways, hormonal and cytokine imbalance, and selecting targets for treatment. Autoimmunity 43: 32–47, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Perl A. Pathogenesis and spectrum of autoimmunity. Methods Mol Biol 900: 1–9, 2012. [DOI] [PubMed] [Google Scholar]
- 133. Perl A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat Rev Rheumatol 9: 674–686, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Perl A. Treatment of Systemic Lupus Erythematosus (SLE) With N-acetylcysteine. 2020. https://ClinicalTrials.gov/show/NCT00775476 (Accessed on June 16, 2021).
- 135. Perl A, Hanczko R, Telarico T, Oaks Z, and Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med 17: 395–403, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, Degott C, Valla D, Erlinger S, and Rueff B. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology 27: 1717–1722, 1998. [DOI] [PubMed] [Google Scholar]
- 137. Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, and Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5: 20–36, 2004. [DOI] [PubMed] [Google Scholar]
- 138. Ramani S, Pathak A, Dalal V, Paul A, and Biswas S. Oxidative stress in autoimmune diseases: an under dealt malice. Curr Protein Pept Sci 21: 611–621, 2020. [DOI] [PubMed] [Google Scholar]
- 139. Rasineni K, McVicker BL, Tuma DJ, McNiven MA, and Casey CA. Rab GTPases associate with isolated lipid droplets (LDs) and show altered content after ethanol administration: potential role in alcohol-impaired LD metabolism. Alcohol Clin Exp Res 38: 327–335, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Redpath GMI, Ecker M, Kapoor-Kaushik N, Vartoukian H, Carnell M, Kempe D, Biro M, Ariotti N, and Rossy J. Flotillins promote T cell receptor sorting through a fast Rab5–Rab11 endocytic recycling axis. Nat Commun 10: 4392, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ren Y, Tang J, Mok MY, Chan AW, Wu A, and Lau CS. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum 48: 2888–2897, 2003. [DOI] [PubMed] [Google Scholar]
- 142. Roberts M, Barry S, Woods A, van der Sluijs P, and Norman J. PDGF-regulated rab4-dependent recycling of alphavbeta3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr Biol 11: 1392–1402, 2001. [DOI] [PubMed] [Google Scholar]
- 143. Roucher-Boulez F, Mallet-Motak D, Samara-Boustani D, Jilani H, Ladjouze A, Souchon P-F, Simon D, Nivot S, Heinrichs C, Ronze M, Bertagna X, Groisne L, Leheup B, Naud-Saudreau C, Blondin G, Lefevre C, Lemarchand L, and Morel Y. NNT mutations: a cause of primary adrenal insufficiency, oxidative stress and extra-adrenal defects. Eur J Endocrinol 175: 73, 2016. [DOI] [PubMed] [Google Scholar]
- 144. Rubio-Ruiz ME, Guarner-Lans V, Cano-Martínez A, Díaz-Díaz E, Manzano-Pech L, Gamas-Magaña A, Castrejón-Tellez V, Tapia-Cortina C, and Pérez-Torres I. Resveratrol and quercetin administration improves antioxidant DEFENSES and reduces fatty liver in metabolic syndrome rats. Molecules 24: 1297, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Rydström J, Da Cruz AT, and Ernster L. Steady-state kinetics of mitochondrial nicotinamide nucleotide transhydrogenase. 2. The energy-linked reaction. Eur J Biochem 23: 212–219, 1971. [DOI] [PubMed] [Google Scholar]
- 146. Sahgal P, Alanko J, Icha J, Paatero I, Hamidi H, Arjonen A, Pietilä M, Rokka A, and Ivaska J. GGA2 and RAB13 promote activity-dependent β1-integrin recycling. J Cell Sci 132: jcs233387, 2019. [DOI] [PubMed] [Google Scholar]
- 147. Salmi M and Jalkanen S. Vascular adhesion protein-1: a cell surface amine oxidase in translation. Antioxid Redox Signal 30: 314–332, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Salmi M, Kalimo K, and Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med 178: 2255–2260, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Samland AK and Sprenger GA. Transaldolase: from biochemistry to human disease. Int J Biochem Cell Biol 41: 1482–1494, 2009. [DOI] [PubMed] [Google Scholar]
- 150. Sancho D, Vicente-Manzanares M, Mittelbrunn M, Montoya MC, Gordón-Alonso M, Serrador JM, and Sánchez-Madrid F. Regulation of microtubule-organizing center orientation and actomyosin cytoskeleton rearrangement during immune interactions. Immunol Rev 189: 84–97, 2002. [DOI] [PubMed] [Google Scholar]
- 151. Sandvig K, Kavaliauskiene S, and Skotland T. Clathrin-independent endocytosis: an increasing degree of complexity. Histochem Cell Biol 150: 107–118, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Saxton RA and Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Schroeder B, Schulze RJ, Weller SG, Sletten AC, Casey CA, and McNiven MA. The small GTPase Rab7 as a central regulator of hepatocellular lipophagy. Hepatology 61: 1896–1907, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Schulz C, Leuschen NV, Fröhlich T, Lorenz M, Pfeiler S, Gleissner CA, Kremmer E, Kessler M, Khandoga AG, Engelmann B, Ley K, Massberg S, and Arnold GJ. Identification of novel downstream targets of platelet glycoprotein VI activation by differential proteome analysis: implications for thrombus formation. Blood 115: 4102–4110, 2010. [DOI] [PubMed] [Google Scholar]
- 155. Scoazec J-Y and Feldmann G. The cell adhesion molecules of hepatic sinusoidal endothelial cells. J Hepatol 20: 296–300, 1994. [DOI] [PubMed] [Google Scholar]
- 156. Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, and Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol 6: 497–506, 2005. [DOI] [PubMed] [Google Scholar]
- 157. Sheeran FL, Rydström J, Shakhparonov MI, Pestov NB, and Pepe S. Diminished NADPH transhydrogenase activity and mitochondrial redox regulation in human failing myocardium. Biochim Biophys Acta 1797: 1138–1148, 2010. [DOI] [PubMed] [Google Scholar]
- 158. Shibanuma M, Mori K, Kim-Kaneyama J-R, and Nose K. Involvement of FAK and PTP-PEST in the regulation of redox-sensitive nuclear-cytoplasmic shuttling of a LIM protein, Hic-5. Antioxid Redox Signal 7: 335–347, 2005. [DOI] [PubMed] [Google Scholar]
- 159. Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, Xuan YT, Srivastava S, and Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res 91: 240–246, 2002. [DOI] [PubMed] [Google Scholar]
- 160. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, and Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Solhi H, Ghahremani R, Kazemifar AM, and Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: a randomized clinical trial. Caspian J Intern Med 5: 9–12, 2014. [PMC free article] [PubMed] [Google Scholar]
- 162. Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, and Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149: 901–914, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Speicher T, Siegenthaler B, Bogorad RL, Ruppert R, Petzold T, Padrissa-Altes S, Bachofner M, Anderson DG, Koteliansky V, Fässler R, and Werner S. Knockdown and knockout of β1-integrin in hepatocytes impairs liver regeneration through inhibition of growth factor signalling. Nat Commun 5: 3862, 2014. [DOI] [PubMed] [Google Scholar]
- 164. Stanley P, Tooze S, and Hogg N. A role for Rap2 in recycling the extended conformation of LFA-1 during T cell migration. Biol Open 1: 1161–1168, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Steinhoff G, Behrend M, Schrader B, Duijvestijn A, and Wonigeit K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2, and LFA-3. Am J Pathol 142: 481, 1993. [PMC free article] [PubMed] [Google Scholar]
- 166. Stotesbury C, Alves-Peixoto P, Montoya B, Ferez M, Nair S, Snyder CM, Zhang S, Knudson CJ, and Sigal LJ. α2β1 Integrin is required for optimal NK cell proliferation during viral infection but not for acquisition of effector functions or NK cell–mediated virus control. J Immunol 204: 1582–1591, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Strick DJ and Elferink LA. Rab15 effector protein: a novel protein for receptor recycling from the endocytic recycling compartment. Mol Biol Cell 16: 5699–5709, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sun P, Li L, and Li Z. RAB9A plays an oncogenic role in human liver cancer cells. Biomed Res Int 2020: 5691671, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Svensson L, Stanley P, Willenbrock F, and Hogg N. The Gαq/11 proteins contribute to T lymphocyte migration by promoting turnover of integrin LFA-1 through recycling. PLoS One 7: e38517, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Svineng G, Ravuri C, Rikardsen O, Huseby NE, and Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res 49: 197–202, 2008. [DOI] [PubMed] [Google Scholar]
- 171. Tainer JA, Getzoff ED, Richardson JS, and Richardson DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature 306: 284–287, 1983. [DOI] [PubMed] [Google Scholar]
- 172. Teh CL, Phui VE, Ling GR, Ngu LS, Wan SA, and Tan CH. Causes and predictors of mortality in biopsy-proven lupus nephritis: the Sarawak experience. Clin Kidney J 11: 56–61, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. ten Broeke T, Wubbolts R, and Stoorvogel W. MHC class II antigen presentation by dendritic cells regulated through endosomal sorting. Cold Spring Harb Perspect Biol 5: a016873, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Tian Z, Chen Y, and Gao B. Natural killer cells in liver disease. Hepatology 57: 1654–1662, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Trial J, Baughn RE, Wygant JN, McIntyre BW, Birdsall HH, Youker KA, Evans A, Entman ML, and Rossen RD. Fibronectin fragments modulate monocyte VLA-5 expression and monocyte migration. J Clin Invest 104: 419–430, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Tsokos GC. Systemic lupus erythematosus. N Engl J Med 365: 2110–2121, 2011. [DOI] [PubMed] [Google Scholar]
- 177. Tu K, Li J, Verma VK, Liu C, Billadeau DD, Lamprecht G, Xiang X, Guo L, Dhanasekaran R, Roberts LR, Shah VH, and Kang N. Vasodilator-stimulated phosphoprotein promotes activation of hepatic stellate cells by regulating Rab11-dependent plasma membrane targeting of transforming growth factor beta receptors. Hepatology 61: 361–374, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Umemura A, Park Eek J, Taniguchi K, Lee Jun H, Shalapour S, Valasek Mark A, Aghajan M, Nakagawa H, Seki E, Hall Michael N, and Karin M. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab 20: 133–144, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. van der Heide D, Weiskirchen R, and Bansal R. Therapeutic targeting of hepatic macrophages for the treatment of liver diseases. Front Immunol 10: 2852, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. van der Sluijs P, Hull M, Webster P, Mâle P, Goud B, and Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell 70: 729–740, 1992. [DOI] [PubMed] [Google Scholar]
- 181. Wang H, Liu Y, Wang D, Xu Y, Dong R, Yang Y, Lv Q, Chen X, and Zhang Z. The upstream pathway of mTOR-mediated autophagy in liver diseases. Cells 8: 1597, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wang M, and Zhang H. The pathogenesis of autoimmune hepatitis. Frontiers in Laboratory Medicine 2: 36–39, 2018. [Google Scholar]
- 183. Weston CJ, Shepherd EL, and Adams DH. Cellular localization and trafficking of vascular adhesion protein-1 as revealed by an N-terminal GFP fusion protein. J Neural Transm (Vienna) 120: 951–961, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Weston CJ, Shepherd EL, Claridge LC, Rantakari P, Curbishley SM, Tomlinson JW, Hubscher SG, Reynolds GM, Aalto K, Anstee QM, Jalkanen S, Salmi M, Smith DJ, Day CP, and Adams DH. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest 125: 501–520, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab 29: 579–585, 2003. [DOI] [PubMed] [Google Scholar]
- 186. Will JS, Snyder CJ, and Westerfield KL. N-acetylcysteine (NAC) for the prevention of liver failure in heat injury-mediated ischemic hepatitis. Mil Med 184: 565–567, 2019. [DOI] [PubMed] [Google Scholar]
- 187. Williams M, Valayannopoulos V, Altassan R, Chung WK, Heijboer AC, Keng WT, Lapatto R, McClean P, Mulder MF, Tylki-Szymańska A, Walenkamp ME, Alfadhel M, Alakeel H, Salomons GS, Eyaid W, and Wamelink MMC. Clinical, biochemical, and molecular overview of transaldolase deficiency and evaluation of the endocrine function: update of 34 patients. J Inherit Metab Dis 42: 147–158, 2019. [DOI] [PubMed] [Google Scholar]
- 188. Wyman B and Perl A. Metabolic pathways mediate pathogenesis and offer targets for treatment in rheumatic diseases. Curr Opin Rheumatol 32: 184–191, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Xu D, Hu MJ, Wang YQ, and Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24: 1123, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Yang J, Liu W, Lu X, Fu Y, Li L, and Luo Y. High expression of small GTPase Rab3D promotes cancer progression and metastasis. Oncotarget 6: 11125–11138, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Yang XZ, Li XX, Zhang YJ, Rodriguez-Rodriguez L, Xiang MQ, Wang HY, and Zheng XF. Rab1 in cell signaling, cancer and other diseases. Oncogene 35: 5699–5704, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yano S, Arroyo N, and Yano N. Catalase binds Grb2 in tumor cells when stimulated with serum or ligands for integrin receptors. Free Radic Biol Med 36: 1542–1554, 2004. [DOI] [PubMed] [Google Scholar]
- 193. Yano S, Arroyo N, and Yano N. SHP2 binds catalase and acquires a hydrogen peroxide-resistant phosphatase activity via integrin-signaling. FEBS Lett 577: 327–332, 2004. [DOI] [PubMed] [Google Scholar]
- 194. Yen K, Travins J, Wang F, David MD, Artin E, Straley K, Padyana A, Gross S, DeLaBarre B, Tobin E, Chen Y, Nagaraja R, Choe S, Jin L, Konteatis Z, Cianchetta G, Saunders JO, Salituro FG, Quivoron C, Opolon P, Bawa O, Saada V, Paci A, Broutin S, Bernard OA, de Botton S, Marteyn BS, Pilichowska M, Xu Y, Fang C, Jiang F, Wei W, Jin S, Silverman L, Liu W, Yang H, Dang L, Dorsch M, Penard-Lacronique V, Biller SA, and Su S-SM. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov 7: 478–493, 2017. [DOI] [PubMed] [Google Scholar]
- 195. Yeoman AD, Westbrook RH, Zen Y, Bernal W, Al-Chalabi T, Wendon JA, O'Grady JG, and Heneghan MA. Prognosis of acute severe autoimmune hepatitis (AS-AIH): the role of corticosteroids in modifying outcome. J Hepatol 61: 876–882, 2014. [DOI] [PubMed] [Google Scholar]
- 196. Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal 10: 179–206, 2008. [DOI] [PubMed] [Google Scholar]
- 197. Yoshida S, Hong S, Suzuki T, Nada S, Mannan AM, Wang J, Okada M, Guan K-L, and Inoki K. Redox regulates mammalian target of rapamycin complex 1 (mTORC1) activity by modulating the TSC1/TSC2-Rheb GTPase pathway. J Biol Chem 286: 32651–32660, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zhang Z, Zhao S, Yao Z, Wang L, Shao J, Chen A, Zhang F, and Zheng S. Autophagy regulates turnover of lipid droplets via ROS-dependent Rab25 activation in hepatic stellate cell. Redox Biol 11: 322–334, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 199. Zhen Y and Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 128: 3171–3176, 2015. [DOI] [PubMed] [Google Scholar]
- 200. Zhuang H, Han S, Lee PY, Khaybullin R, Shumyak S, Lu L, Chatha A, Afaneh A, Zhang Y, Xie C, Nacionales D, Moldawer L, Qi X, Yang LJ, and Reeves WH. Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol 69: 1280–1293, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]