Abstract
BACKGROUND
Elexacaftor–tezacaftor–ivacaftor is a small-molecule cystic fibrosis transmembrane conductance regulator (CFTR) modulator regimen shown to be efficacious in patients with at least one Phe508del allele, which indicates that this combination can modulate a single Phe508del allele. In patients whose other CFTR allele contains a gating or residual function mutation that is already effectively treated with previous CFTR modulators (ivacaftor or tezacaftor–ivacaftor), the potential for additional benefit from restoring Phe508del CFTR protein function is unclear.
METHODS
We conducted a phase 3, double-blind, randomized, active-controlled trial involving patients 12 years of age or older with cystic fibrosis and Phe508del–gating or Phe508del–residual function genotypes. After a 4-week run-in period with ivacaftor or tezacaftor–ivacaftor, patients were randomly assigned to receive elexacaftor–tezacaftor–ivacaftor or active control for 8 weeks. The primary end point was the absolute change in the percentage of predicted forced expiratory volume in 1 second (FEV1) from baseline through week 8 in the elexacaftor–tezacaftor–ivacaftor group.
RESULTS
After the run-in period, 132 patients received elexacaftor–tezacaftor–ivacaftor and 126 received active control. Elexacaftor–tezacaftor–ivacaftor resulted in a percentage of predicted FEV1 that was higher by 3.7 percentage points (95% confidence interval [CI], 2.8 to 4.6) relative to baseline and higher by 3.5 percentage points (95% CI, 2.2 to 4.7) relative to active control and a sweat chloride concentration that was lower by 22.3 mmol per liter (95% CI, 20.2 to 24.5) relative to baseline and lower by 23.1 mmol per liter (95% CI, 20.1 to 26.1) relative to active control (P<0.001 for all comparisons). The change from baseline in the Cystic Fibrosis Questionnaire–Revised respiratory domain score (range, 0 to 100, with higher scores indicating better quality of life) with elexacaftor–tezacaftor–ivacaftor was 10.3 points (95% CI, 8.0 to 12.7) and with active control was 1.6 points (95% CI, −0.8 to 4.1). The incidence of adverse events was similar in the two groups; adverse events led to treatment discontinuation in one patient (elevated aminotransferase level) in the elexacaftor–tezacaftor–ivacaftor group and in two patients (anxiety or depression and pulmonary exacerbation) in the active control group.
CONCLUSIONS
Elexacaftor–tezacaftor–ivacaftor was efficacious and safe in patients with Phe508del–gating or Phe508del–residual function genotypes and conferred additional benefit relative to previous CFTR modulators. (Funded by Vertex Pharmaceuticals; VX18-445-104 ClinicalTrials.gov number, NCT04058353.)
CYSTIC FIBROSIS IS A LIFE-SHORTENING autosomal recessive disease that affects more than 80,000 people worldwide.1–3 In cystic fibrosis, deficiencies in the cystic fibrosis transmembrane conductance regulator (CFTR) protein, an epithelial anion channel, manifest as a complex multiorgan disease, including progressive respiratory impairment, exocrine pancreatic insufficiency, hepatobiliary disease, and abnormal sweat composition.1,3 Measurement of sweat chloride concentration is a key diagnostic test for cystic fibrosis, and the concentration is used as an indicator of systemic CFTR function in clinical trials.4
Cystic fibrosis results from biallelic mutations in the CFTR gene.1 More than 1000 pathogenic CFTR mutations have been identified.5,6 Processing and trafficking mutations (e.g., Phe508del, the most common CFTR mutation) reduce the quantity of CFTR on the cell surface,1,6,7 and channel-gating defects (e.g., Gly551Asp and other CFTR gating mutations) limit anion transport. CFTR mutations that result in lesser impairment of CFTR protein activity, collectively defined as residual function mutations, have also been identified.1,8 Most patients with gating or residual function CFTR mutations are heterozygous for the Phe508del mutation.6
Elucidation of the molecular consequences of CFTR mutations has supported the development of small-molecule modulators capable of restoring CFTR protein function.9–15 Ivacaftor, a CFTR potentiator, augments gating of mutant CFTR proteins.9 In patients with gating mutations, ivacaftor improves lung function, nutritional status, and quality of life and decreases pulmonary exacerbations.10,16 Ivacaftor monotherapy is also efficacious and safe in patients with residual function mutations.8
Tezacaftor is a CFTR corrector that ameliorates the defects in CFTR protein processing and cell-surface trafficking intrinsic to Phe508del.11 Because Phe508del CFTR proteins also possess gating defects,9 modulation requires both correction and potentiation. In patients heterozygous for Phe508del and specific residual function mutations, the combination of tezacaftor and ivacaftor improved lung function and sweat chloride concentrations as compared with ivacaftor alone.8
The recently developed CFTR corrector elexacaftor has a mechanism of action that is complementary to that of tezacaftor.13 Pivotal studies showed that elexacaftor–tezacaftor–ivacaftor was efficacious and safe in patients with two Phe508del alleles and also in those with one Phe508del allele and an allele that makes no CFTR protein, which indicates that the presence of a single Phe508del allele is sufficient to confer responsiveness.13,14 These findings suggest that elexacaftor–tezacaftor–ivacaftor would provide additional clinical benefit in patients with Phe508del–gating and Phe508del–residual function genotypes by enhancing CFTR activity from the Phe508del allele. Here, we report results of a trial (VX18–445-104) designed to evaluate the magnitude of benefit of elexacaftor–tezacaftor–ivacaftor as compared with ivacaftor and tezacaftor–ivacaftor in patients 12 years of age or older with these genotypes.
METHODS
PATIENTS, TRIAL DESIGN, AND OVERSIGHT
This phase 3, multicenter, double-blind, parallel-group, randomized, active-controlled trial of elexacaftor–tezacaftor–ivacaftor enrolled patients 12 years of age or older with cystic fibrosis and Phe508del–gating or Phe508del–residual function genotypes. Complete inclusion and exclusion criteria as well as additional details on trial design, dosing, and statistical analysis are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org; qualifying mutations are listed in Table S1.
To establish a reliable on-treatment baseline, patients entered a 4-week run-in period to receive either ivacaftor at a dose of 150 mg every 12 hours (ivacaftor comparator cohort; Phe508del–gating genotypes, including Phe508del–Arg117His) or tezacaftor at a dose of 100 mg once daily combined with ivacaftor (tezacaftor–ivacaftor comparator cohort; Phe508del–residual function genotypes) (Fig. S1). These genotype-defined comparator cohorts were based on approved indications for CFTR modulators in each country where the trial was conducted.
After the run-in period, patients who entered the 8-week treatment period were randomly assigned in a 1:1 ratio to receive either elexacaftor–tezacaftor–ivacaftor (elexacaftor, 200 mg once daily) or the regimen they received during the run-in period (ivacaftor or tezacaftor–ivacaftor). Stratification of randomization was determined during the run-in period on the basis of comparator cohort (ivacaftor vs. tezacaftor–ivacaftor), percentage of predicted forced expiratory volume in 1 second (FEV1; <70 vs. ≥70), and sweat chloride concentration (<30 mmol per liter vs. ≥30 mmol per liter).
The trial was designed by Vertex Pharmaceuticals in collaboration with the authors. Each patient or the patient’s legal guardian provided written informed consent, with assent obtained when age appropriate. Safety was monitored by an independent data monitoring committee. During the trial, the coronavirus disease 2019 pandemic led to the implementation of a global protocol addendum enabling patients to remain in the trial with in-home assessments and trialdrug provision. Data collection and analysis were performed by Vertex Pharmaceuticals in collaboration with the authors and the VX18–445-104 Study Group. The first two authors and last two authors wrote the first manuscript draft. All the authors had full access to the trial data after the final database lock, critically reviewed the manuscript, and approved it for submission. The investigators vouch for the accuracy and complete-ness of data generated at their respective sites, and the investigators and Vertex Pharmaceuticals vouch for the fidelity of the trial to the protocol, available at NEJM.org. Confidentiality agreements were in place between the sponsor and each investigative site during the trial.
END POINTS
The primary end point was the absolute change in the percentage of predicted FEV1 from baseline through week 8 in the elexacaftor–tezacaftor–ivacaftor group. Key secondary end points, in hierarchical order, were the absolute change in sweat chloride concentration from baseline through week 8 in the elexacaftor–tezacaftor–ivacaftor group, the absolute change in the percentage of predicted FEV1 from baseline through week 8 for elexacaftor–tezacaftor–ivacaftor as compared with active control (ivacaftor or tezacaftor–ivacaftor), and the absolute change in sweat chloride concentration from baseline through week 8 for elexacaftor–tezacaftor–ivacaftor as compared with active control. Other secondary end points were the absolute change in the score on the respiratory domain of the Cystic Fibrosis Questionnaire–Revised (CFQ-R; range, 0 to 100, with higher scores indicating a higher patient-reported quality of life with regard to respiratory symptoms) from baseline through week 8 in the elexacaftor–tezacaftor–ivacaftor group and for elexacaftor–tezacaftor–ivacaftor as compared with active control, as well as safety and the side-effect profile. The percentages of patients who reached sweat chloride concentrations below 60 mmol per liter and below 30 mmol per liter were assessed in a post hoc analysis.
STATISTICAL ANALYSIS
Efficacy analyses included all the patients who underwent randomization and received at least one dose during the treatment period. The absolute change from baseline in the percentage of predicted FEV1 through week 8 was analyzed with the use of a mixed-effects model for repeated measures. The model included treatment group, visit, and treatment-group–by–visit interaction as fixed effects as well as continuous baseline percentage of predicted FEV1, continuous baseline sweat chloride concentration, and comparator cohort (ivacaftor vs. tezacaftor–ivacaftor) as covariates, with an unstructured covariance used for within-patient errors. The primary result that was obtained from the model was the estimated within-group change from baseline in the percentage of predicted FEV1 through week 8 for elexacaftor–tezacaftor–ivacaftor. A similar mixed-effects model for repeated measures was applied to each of the key secondary end points. A hierarchical testing procedure was used to control the overall type I error rate at an alpha level of 0.05 for the primary and key secondary end points, the latter of which were prioritized in the testing hierarchy as a within-group analysis of sweat chloride concentration through week 8, a between-group analysis of the percentage of predicted FEV1 through week 8, and a between-group analysis of sweat chloride concentration through week 8. For a hypothesis test to be considered statistically significant, the P value for that test and all the preceding tests in the hierarchy had to be below 0.05. Within- and between-group analyses of the other secondary end point of the absolute change in the CFQ-R respiratory domain score through week 8 were performed in a manner similar to the analyses of the primary and key secondary end points.
Subgroup analyses according to comparator cohort (ivacaftor [Phe508del–gating genotypes] vs. tezacaftor–ivacaftor [Phe508del–residual function genotypes]) were performed in a manner similar to the main analyses, including those for absolute changes in the percentage of predicted FEV1, in sweat chloride concentration, and in the CFQ-R respiratory domain score. Except for those involving the primary end point, these subgroup analyses were post hoc.
RESULTS
POPULATION
The trial was conducted at 96 sites in North America, Europe, and Australia, from August 28, 2019, to June 12, 2020. Overall, 271 patients entered the 4-week run-in period. After the run-in period, 258 patients (95 with Phe508del–gating genotypes and 163 with Phe508del–residual function genotypes) were randomly assigned to either the elexacaftor–tezacaftor–ivacaftor group (132 patients) or the active control group (126 patients) and received at least one dose of trial medication. Additional details on patient recruitment are provided in Figure S2. Treatment groups were well matched at baseline (Table 1 and Table S2). Demographic and clinical characteristics at baseline according to comparator cohort and treatment assignment (Table S3) and individual mutations on the second CFTR allele according to treatment assignment (Table S4) are provided in the Supplementary Appendix.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Elexacaftor–Tezacaftor–Ivacaftor (N = 132) | Active Control: Ivacaftor or Tezacaftor–Ivacaftor (N = 126) |
|---|---|---|
| Sex — no. (%) | ||
| Male | 65 (49.2) | 65 (51.6) |
| Female | 67 (50.8) | 61 (48.4) |
| Age | ||
| Mean — yr | 37.7±14.7 | 37.6±14.3 |
| Distribution — no. (%)† | ||
| 12 to <18 yr | 15 (11.4) | 9 (7.1) |
| ≥18 yr | 117 (88.6) | 117 (92.9) |
| Hispanic or Latino ethnic group — no. (%)‡ | ||
| Hispanic or Latino | 5 (3.8) | 4 (3.2) |
| Not Hispanic or Latino | 117 (88.6) | 114 (90.5) |
| Not collected per local regulations | 10 (7.6) | 8 (6.3) |
| Race or ethnic group — no. (%)‡§ | ||
| White | 122 (92.4) | 111 (88.1) |
| Black | 0 | 2 (1.6) |
| American Indian or Alaska Native | 0 | 1 (0.8) |
| Other | 1 (0.8) | 4 (3.2) |
| Not collected per local regulations | 9 (6.8) | 9 (7.1) |
| Geographic region — no. (%) | ||
| North America | 49 (37.1) | 48 (38.1) |
| Europe | 70 (53.0) | 64 (50.8) |
| Australia | 13 (9.8) | 14 (11.1) |
| Genotype — no. (%)¶ | ||
| Phe508del–gating | 50 (37.9) | 45 (35.7) |
| Phe508del–residual function | 82 (62.1) | 81 (64.3) |
| Percentage of predicted FEV1 | ||
| Mean | 67.1±15.7 | 68.1±16.4 |
| Distribution — no. (%) | ||
| <40% | 2 (1.5) | 2 (1.6) |
| 40 to <70% | 70 (53.0) | 63 (50.0) |
| 70 to ≤90% | 53 (40.2) | 52 (41.3) |
| >90% | 7 (5.3) | 9 (7.1) |
| Sweat chloride concentration — mmol/liter | 59.5±27.0 | 56.4±25.5 |
| CFQ-R respiratory domain score‖ | 76.5±16.6 | 77.3±15.8 |
| Body-mass index** | 24.07±4.72 | 24.05±4.71 |
Plus–minus values are means ±SD. Shown are the demographic and clinical characteristics of the full analysis set, which was defined as all randomly assigned patients who received at least one dose of trial drug in the treatment period. Baseline was defined as the most recent nonmissing measurement before the first dose of trial drug in the treatment period (after the 4-week run-in period). Percentages may not total 100 because of rounding. FEV1 denotes forced expiratory volume in 1 second.
Age distribution was calculated on the basis of age at the time of screening.
Ethnic group and race were reported by the patient.
There were no patients of Asian race or Native Hawaiian or other Pacific Islander ethnic group. If a patient was reported to have multiple races or ethnic groups, then the patient was counted for each race or ethnic group reported.
Patients with Phe508del–gating genotypes who were assigned to the active control group received ivacaftor, and patients with Phe508del–residual function genotypes who were assigned to the active control group received tezacaftor—ivacaftor.
Scores on the respiratory domain of the Cystic Fibrosis Questionnaire–Revised (CFQ-R) range from 0 to 100, with higher scores indicating a higher patient-reported quality of life with regard to respiratory symptoms.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
EFFICACY
Overall
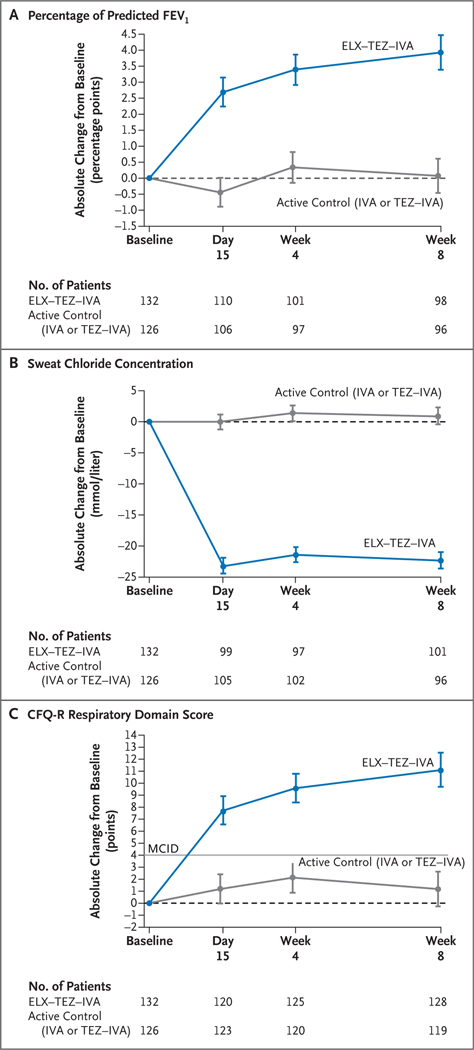
The mean absolute change in the percentage of predicted FEV1 from baseline (measured at the end of the run-in period) through week 8 with elexacaftor–tezacaftor–ivacaftor treatment (the primary end point) was 3.7 percentage points (95% confidence interval [CI], 2.8 to 4.6; P<0.001) (Table 2 and Fig. 1A). In contrast, the mean absolute change with active control (ivacaftor or tezacaftor–ivacaftor) was 0.2 percentage points (95% CI, −0.7 to 1.1), reflecting a between-group difference of 3.5 percentage points (95% CI, 2.2 to 4.7; P<0.001). The mean absolute change in sweat chloride concentration from baseline through week 8 with elexacaftor–tezacaftor–ivacaftor was −22.3 mmol per liter (95% CI, −24.5 to −20.2; P<0.001), as compared with a mean absolute change with active control of 0.7 mmol per liter (95% CI, −1.4 to 2.8), reflecting a between-group difference of −23.1 mmol per liter (95% CI, −26.1 to −20.1; P<0.001) (Table 2 and Fig. 1B). The mean absolute change in the CFQ-R respiratory domain score from baseline through week 8 with elexacaftor–tezacaftor–ivacaftor was 10.3 points (95% CI, 8.0 to 12.7) and with active control was 1.6 points (95% CI, −0.8 to 4.1), reflecting a between-group difference of 8.7 points (95% CI, 5.3 to 12.1) (Table 2 and Fig. 1C).
Table 2.
Efficacy Results.*
| End Point | Elexacaftor–Tezacaftor–Ivacaftor (N = 132) | Active Control: Ivacaftor or Tezacaftor–Ivacaftor (N = 126) |
|---|---|---|
| Percentage of predicted FEV1 | ||
| Value at baseline | 67.1±15.7 | 68.1±16.4 |
| Absolute change from baseline through wk 8 | ||
| Sample size† | 115 | 114 |
| Least-squares mean change (95% CI) | 3.7 (2.8 to 4.6)‡§ | 0.2 (−0.7 to 1.1) |
| Between-group difference (95% CI) | 3.5 (2.2 to 4.7)¶‖ | |
| Sweat chloride concentration | ||
| Value at baseline — mmol/liter | 59.5±27.0 | 56.4±25.5 |
| Absolute change from baseline through wk 8 | ||
| Sample size† | 120 | 119 |
| Least-squares mean change (95% CI) — mmol/liter | −22.3 (−24.5 to −20.2)§¶ | 0.7 (−1.4 to 2.8) |
| Between-group difference (95% CI) — mmol/liter | −23.1 (−26.1 to −20.1)¶ | |
| CFQ-R respiratory domain score** | ||
| Value at baseline | 76.5±16.6 | 77.3±15.8 |
| Absolute change from baseline through wk 8 | ||
| Sample size†† | 130 | 126 |
| Least-squares mean change (95% CI) | 10.3 (8.0 to 12.7) | 1.6 (−0.8 to 4.1) |
| Between-group difference (95% CI) | 8.7 (5.3 to 12.1) | |
Plus–minus values are means ±SD. Baseline, considered to be the end of the run-in period, was defined as the most recent nonmissing measurement before the first dose of trial drug in the treatment period.
The sample size is the number of patients through week 8 with in-clinic data that could be evaluated.
The primary end point was the absolute change in the percentage of predicted FEV1 from baseline through week 8 in the elexacaftor–tezacaftor–ivacaftor group.
P<0.001 for the within-group change from baseline.
The key secondary end points, in hierarchical order, were the absolute change in sweat chloride concentration from baseline through week 8 in the elexacaftor–tezacaftor–ivacaftor group, the absolute change in the percentage of predicted FEV1 from baseline through week 8 for elexacaftor–tezacaftor–ivacaftor as compared with active control, and the absolute change in sweat chloride concentration from baseline through week 8 for elexacaftor–tezacaftor–ivacaftor as compared with active control.
P<0.001 for the between-group difference in the change from baseline.
Pooled CFQ-R data were obtained both in the clinic and in the home and were based on both the “Children Ages 12 and 13” and “Adolescents and Adults” versions. The minimal clinically important difference for the CFQ-R respiratory domain score is 4 points.
The sample size is the number of patients through week 8 with in-clinic or in-home data that could be evaluated.
Figure 1. Efficacy End Points.
Panel A shows the absolute change from baseline at each visit in the percentage of predicted forced expiratory volume in 1 second (FEV1) on the basis of a mixed-effects model for repeated measures. Panel B shows the absolute change from baseline at each visit in the sweat chloride concentration on the basis of a mixed-effects model for repeated measures. Panel C shows the absolute change from baseline at each visit in the score on the respiratory domain of the Cystic Fibrosis Questionnaire–Revised (CFQ-R) on the basis of a mixed-effects model for repeated measures. Respiratory domain scores are normalized to a 100-point range, with higher scores indicating a higher patient-reported quality of life with regard to respiratory symptoms; the minimal clinically important difference (MCID) is 4 points and is indicated in the plot by the straight gray line. In Panels A through C, data are least-squares means, and the I bars indicate standard errors; the dashed line at 0 cor responds to no change from baseline. The sample size shown under each x axis is the number of patients at that time point with data that could be evaluated. ELX–TEZ–IVA denotes elexacaftor–tezacaftor–ivacaftor, IVA ivacaftor, and TEZ–IVA tezacaftor–ivacaftor.
Subgroup Analyses
The results of a prespecified subgroup analysis of the primary end point (within-group absolute change from baseline in the percentage of predicted FEV1) according to age at screening (<18 years vs. ≥18 years), sex, comparator cohort (Phe508del–gating vs. Phe508del–residual function genotypes), percentage of predicted FEV1 at baseline (<70 vs. ≥70), and geographic region (North America vs. Europe and Australia) were consistent with the results of the primary analysis (Fig. S3A). The same held for the results of a post hoc subgroup analysis of the between-group difference in the change from baseline in the percentage of predicted FEV1 (Fig. S3B).
Additional post hoc analyses were performed to assess the role of genotype in treatment response. For patients with Phe508del–gating genotypes, including Phe508del–Arg117His, the mean absolute change in the percentage of predicted FEV1 from baseline through week 8 with elexacaftor–tezacaftor–ivacaftor was 5.8 percentage points (95% CI, 4.2 to 7.4) and with ivacaftor control was 0.1 percentage points (95% CI, −1.6 to 1.7), for a difference of 5.8 percentage points (95% CI, 3.5 to 8.0); the mean absolute change in sweat chloride concentration with elexacaftor–tezacaftor–ivacaftor was −21.8 mmol per liter (95% CI, −25.7 to −17.8) and with ivacaftor was −1.8 mmol per liter (95% CI, −5.7 to 2.2), for a difference of −20.0 mmol per liter (95% CI, −25.4 to −14.6); and the mean absolute change in the CFQ-R respiratory domain score with elexacaftor–tezacaftor–ivacaftor was 10.2 points (95% CI, 6.6 to 13.8) and with ivacaftor was 1.3 points (95% CI, −2.5 to 5.2), for a difference of 8.9 points (95% CI, 3.8 to 14.0) (Table 3). The results of a post hoc analysis of data from patients with the Phe508del–Arg117His genotype were consistent with the results for the overall Phe508del–gating cohort (Table S5).
Table 3.
Subgroup Analysis of Efficacy Results According to Comparator Cohort.*
| Variable | Phe508del–Gating Genotypes | Phe508del–Residual Function Genotypes | ||
|---|---|---|---|---|
| Elexacaftor–Tezacaftor–Ivacaftor (N = 50) | Active Control: Ivacaftor (N = 45) | Elexacaftor–Tezacaftor–Ivacaftor (N = 82) | Active Control: Tezacaftor–Ivacaftor (N = 81) | |
| Percentage of predicted FEV1 | ||||
| Value at baseline | 66.0±14.8 | 68.1±16.6 | 67.8±16.3 | 68.1±16.4 |
| Absolute change from baseline through wk 8 (95% CI) | 5.8 (4.2 to 7.4) | 0.1 (−1.6 to 1.7) | 2.5 (1.4 to 3.5) | 0.5 (−0.5 to 1.5) |
| Between-group difference (95% CI) | 5.8 (3.5 to 8.0) | 2.0 (0.5 to 3.4) | ||
| Sweat chloride concentration — mmol/liter | ||||
| Value at baseline | 50.9±23.3 | 47.6±19.1 | 64.7±27.9 | 61.4±27.3 |
| Absolute change from baseline through wk 8 (95% CI) | −21.8 (−25.7 to −17.8) | −1.8 (−5.7 to 2.2) | −23.1 (−25.6 to −20.6) | 1.7 (−0.9 to 4.3) |
| Between-group difference (95% CI) | −20.0 (−25.4 to −14.6) | −24.8 (−28.4 to −21.2) | ||
| CFQ-R respiratory domain score† | ||||
| Value at baseline | 76.3±16.4 | 75.8±17.6 | 76.7±16.9 | 78.1±14.7 |
| Absolute change from baseline through wk 8 (95% CI) | 10.2 (6.6 to 13.8) | 1.3 (−2.5 to 5.2) | 10.4 (7.2 to 13.7) | 1.9 (−1.4 to 5.1) |
| Between-group difference (95% CI) | 8.9 (3.8 to 14.0) | 8.5 (4.0 to 13.1) | ||
Plus–minus values are means ±SD. Baseline was defined as the most recent nonmissing measurement before the first dose of trial drug in the treatment period. Absolute changes from baseline are least-squares means. A similar mixed-effects model for repeated measures as for the primary analysis was applied to each subgroup category, with treatment group, visit, and treatment-group–by–visit interaction as fixed effects and continuous baseline percentage of predicted FEV1 and continuous baseline sweat chloride concentration as covariates. Model-based estimates for a given category are shown here provided that the analysis converged in that category. All subgroup analyses, except those involving the primary end point of the absolute change in the percentage of predicted FEV1 from baseline through week 8 with elexacaftor–tezacaftor–ivacaftor, were post hoc.
The minimal clinically important difference for the CFQ-R respiratory domain score is 4 points.
For patients with Phe508del–residual function genotypes, the mean absolute change in the percentage of predicted FEV1 from baseline through week 8 with elexacaftor–tezacaftor–ivacaftor was 2.5 percentage points (95% CI, 1.4 to 3.5) and with tezacaftor–ivacaftor control was 0.5 percentage points (95% CI, −0.5 to 1.5), for a difference of 2.0 percentage points (95% CI, 0.5 to 3.4); the mean absolute change in sweat chloride concentration with elexacaftor–tezacaftor–ivacaftor was −23.1 mmol per liter (95% CI, −25.6 to −20.6) and with tezacaftor–ivacaftor was 1.7 mmol per liter (95% CI, −0.9 to 4.3), for a difference of −24.8 mmol per liter (95% CI, −28.4 to −21.2); and the mean absolute change in the CFQ-R respiratory domain score with elexacaftor–tezacaftor–ivacaftor was 10.4 points (95% CI, 7.2 to 13.7) and with tezacaftor–ivacaftor was 1.9 points (95% CI, −1.4 to 5.1), for a difference of 8.5 points (95% CI, 4.0 to 13.1) (Table 3).
After 8 weeks of treatment with elexacaftor–tezacaftor–ivacaftor, the mean (±SD) sweat chloride concentrations were 32.7±23.5 mmol per liter for patients with Phe508del–gating genotypes and 39.9±19.3 mmol per liter for those with Phe508del–residual function genotypes, as compared with 52.0±21.9 mmol per liter with ivacaftor and 63.4±27.3 mmol per liter with tezacaftor–ivacaftor for patients in those active control groups. An analysis of individual sweat chloride concentrations through week 8 for patients who received elexacaftor–tezacaftor–ivacaftor showed that 83.3% had concentrations below 60 mmol per liter and 50.0% had concentrations below 30 mmol per liter; for patients who received active control, the corresponding percentages were 55.5% and 17.6% (Fig. S4 and Table S6). For patients with Phe508del–gating genotypes, 65% who received elexacaftor–tezacaftor–ivacaftor were below 30 mmol per liter through week 8, as compared with 16% of those who received ivacaftor. For patients with Phe508del–residual function genotypes, 42% who received elexacaftor–tezacaftor–ivacaftor were below 30 mmol per liter through week 8, as compared with 19% of those who received tezacaftor–ivacaftor.
SAFETY
Overall, 66.7% of the patients in the elexacaftor–tezacaftor–ivacaftor group and 65.9% of those in the active control group (ivacaftor or tezacaftor–ivacaftor) had one or more adverse events, which for most patients were mild or moderate in severity and resolved during the trial (Table 4). Serious adverse events were reported in 5 patients (3.8%) in the elexacaftor–tezacaftor–ivacaftor group and 11 patients (8.7%) in the active control group, with the difference attributable to a higher incidence of pulmonary exacerbation in the active control group. One patient in the elexacaftor–tezacaftor–ivacaftor group discontinued treatment owing to an adverse event (elevated aminotransferase level), and 2 patients in the active control group discontinued treatment owing to an adverse event (anxiety or depression in 1 patient and pulmonary exacerbation in 1 patient).
Table 4.
Adverse Events.*
| Adverse Event | Elexacaftor–Tezacaftor–Ivacaftor (N = 132) | Active Control: Ivacaftor or Tezacaftor–Ivacaftor (N = 126) |
|---|---|---|
| number of patients (percent) | ||
| Any adverse event | 88 (66.7) | 83 (65.9) |
| Adverse event according to maximum severity | ||
| Mild | 58 (43.9) | 50 (39.7) |
| Moderate | 25 (18.9) | 29 (23.0) |
| Severe | 5 (3.8) | 4 (3.2) |
| Life-threatening | 0 | 0 |
| Serious adverse event | 5 (3.8) | 11 (8.7) |
| Infective pulmonary exacerbation of cystic fibrosis† | 2 (1.5) | 7 (5.6) |
| Adverse event leading to treatment discontinuation | 1 (0.8) | 2 (1.6) |
| Adverse event leading to death | 0 | 0 |
| Most common adverse events‡ | ||
| Headache | 11 (8.3) | 19 (15.1) |
| Alanine aminotransferase increased | 8 (6.1) | 0 |
| Aspartate aminotransferase increased | 8 (6.1) | 0 |
| Abdominal pain | 7 (5.3) | 2 (1.6) |
| Sputum increased | 6 (4.5) | 8 (6.3) |
| Diarrhea | 5 (3.8) | 8 (6.3) |
| Cough | 3 (2.3) | 18 (14.3) |
| Infective pulmonary exacerbation of cystic fibrosis | 3 (2.3) | 13 (10.3) |
| Nausea | 2 (1.5) | 9 (7.1) |
| Any adverse event involving rash§ | 4 (3.0) | 5 (4.0) |
| Any adverse event involving elevated aminotransferase level¶ | 8 (6.1) | 1 (0.8)‖ |
A patient with multiple events within a category was counted only once in that category.
Infective pulmonary exacerbation of cystic fibrosis was the only serious adverse event that occurred in two or more patients in either treatment group.
Shown are adverse events that occurred in at least 5% of the patients in either group. Adverse events are listed according to the preferred term in the Medical Dictionary for Regulatory Activities (MedDRA), version 23.0.
Adverse events involving rash included the MedDRA terms rash, rash erythematous, rash maculopapular, rash papular, skin exfoliation, and urticaria.
Shown are adverse events that included the MedDRA terms alanine aminotransferase increased, aspartate aminotransferase increased, and liver function test increased.
This adverse event was reported as the MedDRA term liver function test increased.
On the basis of previous experience with elexacaftor–tezacaftor–ivacaftor, including the phase 3 trials,14,17 data regarding aminotransferase levels, rash, creatine kinase level, and blood pressure were reviewed. Elevated levels of alanine aminotransferase or aspartate aminotransferase that were greater than three times, greater than five times, and greater than eight times the upper limit of the normal range occurred in 4 of 125 patients for whom data were available (3.2%), 1 patient (0.8%), and 1 patient (0.8%), respectively, in the elexacaftor–tezacaftor–ivacaftor group and in 2 of 123 patients (1.6%), 1 patient (0.8%), and no patients, respectively, in the active control group (Table S7). Eight patients (6.1%) in the elexacaftor–tezacaftor–ivacaftor group and 1 patient (0.8%) in the active control group had adverse events involving elevated aminotransferase levels (Table 4). No patient had a serious adverse event involving elevated aminotransferase levels. Rash was observed in 4 patients (3.0%) in the elexacaftor–tezacaftor–ivacaftor group and in 5 patients (4.0%) in the active control group (Table S8). All cases of rash were mild or moderate in severity. Increased blood creatine kinase levels were reported in 2 patients (1.5%) in the elexacaftor–tezacaftor–ivacaftor group and in no patients in the active control group. Baseline mean systolic and diastolic blood pressures increased by 3.0 mm Hg and 2.5 mm Hg, respectively, in the elexacaftor–tezacaftor–ivacaftor group and by 0.5 mm Hg and 0.3 mm Hg, respectively, in the active control group at week 8 (Table S9). There were no adverse events involving hypertension in the elexacaftor–tezacaftor–ivacaftor group or the active control group. There were no notable safety findings in other clinical or laboratory assessments.
DISCUSSION
Here, we report results of an 8-week trial of elexacaftor–tezacaftor–ivacaftor in patients with cystic fibrosis and either Phe508del–gating or Phe508del–residual function genotypes. Elexacaftor–tezacaftor–ivacaftor treatment improved lung function and sweat chloride concentration relative to an active control (ivacaftor or tezacaftor–ivacaftor). Most patients had adverse events that were mild or moderate in severity and that were consistent with those observed in previous studies.14,17 These results confirm that by enhancing activity from the Phe508del allele, elexacaftor–tezacaftor–ivacaftor can provide additional benefit to patients with a single Phe508del allele plus a gating or residual function allele that is responsive to previous CFTR modulator regimens.
For patients with CFTR gating mutations, the current benchmark for effective treatment is ivacaftor monotherapy, which has been shown to improve the percentage of predicted FEV1 by 10.6 percentage points in patients with the Gly551Asp gating mutation relative to placebo.10 In previous studies, tezacaftor–ivacaftor improved the percentage of predicted FEV1 by 6.8 percentage points in patients with Phe508del–residual function genotypes relative to placebo.8 Despite the clinical heterogeneity of these genotype groups, elexacaftor–tezacaftor–ivacaftor treatment resulted in additional increases in the percentage of predicted FEV1 as compared with either ivacaftor or tezacaftor–ivacaftor treatment; this is largely attributable to enhanced function of CFTR protein arising from the Phe508del allele.14 In a post hoc subgroup analysis of the percentage of predicted FEV1, the treatment differences between elexacaftor–tezacaftor–ivacaftor and active control were 5.8 percentage points (95% CI, 3.5 to 8.0) for patients with Phe508del–gating genotypes and 2.0 percentage points (95% CI, 0.5 to 3.4) for patients with Phe508del–residual function genotypes. Relative to patients with Phe508del–gating genotypes, smaller changes in lung function in response to CFTR modulation among patients with Phe508del–residual function genotypes have been observed previously and probably reflect differences in the progression of lung disease in patients with Phe508del–residual function genotypes, who are generally older than those in other genotype groups.8 (In the current trial, such patients had a mean age of 40.8 years, as compared with 32.2 years for patients with Phe508del–gating genotypes.) Elexacaftor–tezacaftor–ivacaftor treatment also resulted in changes in CFQ-R respiratory domain scores, relative to ivacaftor or tezacaftor–ivacaftor, that exceeded the established minimal clinically important difference (4 points), a finding that shows further abatement of respiratory symptoms in these patients.8,10,16,18
Sweat chloride concentration may differentiate the effectiveness of CFTR modulator regimens at a population level. At baseline, the mean sweat chloride concentration in patients was approximately 60 mmol per liter, the threshold for definitive diagnosis of cystic fibrosis.19 After 8 weeks of elexacaftor–tezacaftor–ivacaftor treatment, 50.0% of the patients had sweat chloride concentrations below 30 mmol per liter, a level that matches those generally seen in the population of asymptomatic carriers with a single mutant CFTR allele and a level below which a diagnosis of cystic fibrosis is unlikely,19,20 whereas only 17.6% of the patients who received active control had sweat chloride concentrations below 30 mmol per liter. This result reflects improved CFTR function with elexacaftor–tezacaftor–ivacaftor treatment.
This phase 3 trial showed the efficacy of elexacaftor–tezacaftor–ivacaftor therapy in patients with Phe508del–gating and Phe508del–residual function genotypes, with clinical benefit exceeding previous CFTR modulators. No new safety findings were noted as compared with previous studies involving patients with cystic fibrosis.14,17 Sweat chloride concentrations after elexacaftor–tezacaftor–ivacaftor treatment reached levels at or near those found in asymptomatic carriers with a single CFTR mutation. These findings confirm the efficacy and safety of elexacaftor–tezacaftor–ivacaftor in patients with at least one Phe508del allele.
Supplementary Material
Acknowledgments
Supported by Vertex Pharmaceuticals. The National Institute for Health Research (NIHR) provided support to the NIHR Manchester Clinical Research Facility. The National Institutes of Health provided support to the University of Alabama (P30DK072482 and UL1TR003096) and the University of Kansas Medical Center Research Institute (P20GM130423).
Disclosure forms provided by the authors are available at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank the patients and their families for participating in this trial and all site investigators and coordinators for their contributions to the trial; the members of the Cystic Fibrosis Foundation Therapeutics Development Network and the European Cystic Fibrosis Society Clinical Trials Network for their support of the trial sites; Emily Poulin, Ph.D., an employee of Vertex Pharmaceuticals, who may own stock or stock options in the company, for providing editorial coordination and support; and Nathan Blow, Ph.D., of Vertex Pharmaceuticals, who may own stock or stock options in the company, and Christopher Edwards, Ph.D., C.M.P.P., of ArticulateScience, for providing editorial assistance under the guidance of the authors and with support from Vertex Pharmaceuticals.
Appendix
The authors’ full names and academic degrees are as follows: Peter J. Barry, M.D., Marcus A. Mall, M.D., Antonio Álvarez, M.D., Carla Colombo, M.D., Karin M. de Winter-de Groot, M.D., Isabelle Fajac, M.D., Ph.D., Kimberly A. McBennett, M.D., Ph.D., Edward F. McKone, M.D., Bonnie W. Ramsey, M.D., Sivagurunathan Sutharsan, M.D., Jennifer L. Taylor-Cousar, M.D., M.S.C.S., Elizabeth Tullis, M.D., Neil Ahluwalia, M.D., Lucy S. Jun, Ph.D., Samuel M. Moskowitz, M.D., Valentin Prieto-Centurion, M.D., Simon Tian, M.D., David Waltz, M.D., Fengjuan Xuan, Ph.D., Yaohua Zhang, Ph.D., Steven M. Rowe, M.D., M.S.P.H., and Deepika Polineni, M.D., M.P.H.
REFERENCES
- 1.Bell SC, Mall MA, Gutierrez H, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020;8:65–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med 2016;4:662–74. [DOI] [PubMed] [Google Scholar]
- 3.Elborn JS. Cystic fibrosis. Lancet 2016; 388:2519–31. [DOI] [PubMed] [Google Scholar]
- 4.Accurso FJ, Van Goor F, Zha J, et al. Sweat chloride as a biomarker of CFTR activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros 2014;13: 139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Mutation Database (CFTR1). 2011. (http://www.genet.sickkids.on.ca/ ).
- 6.The Clinical and Functional TRanslation of CFTR (CFTR2). 2011. (https://cftr2.org/).
- 7.Cystic Fibrosis Foundation patient registry: 2018 annual data report. Bethesda, MD: Cystic Fibrosis Foundation, 2019. [Google Scholar]
- 8.Rowe SM, Daines C, Ringshausen FC, et al. Tezacaftor–ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017;377:2024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Goor F, Hadida S, Grootenhuis PDJ, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 2009;106:18825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey BW, Davies J, McElvaney NG, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Goor F. Nonclinical profile of the CFTR corrector VX-661. Presented at the 30th annual North American Cystic Fibrosis Conference, Orlando, FL, October 27–29, 2016. (Poster.) [Google Scholar]
- 12.Taylor-Cousar JL, Munck A, McKone EF, et al. Tezacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 2017;377:2013–23. [DOI] [PubMed] [Google Scholar]
- 13.Keating D, Marigowda G, Burr L, et al. VX-445–tezacaftor–ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 2018;379: 1612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med 2019;381:1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mall MA, Mayer-Hamblett N, Rowe SM. Cystic fibrosis: emergence of highly effective targeted therapeutics and potential clinical implications. Am J Respir Crit Care Med 2020;201:1193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros 2014;13:674–80. [DOI] [PubMed] [Google Scholar]
- 17.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019;394:1940–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135:1610–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrell PM, White TB, Ren CL, et al. Diagnosis of cystic fibrosis: consensus guidelines from the Cystic Fibrosis Foundation. J Pediatr 2017;181:Suppl:S4-S15.e1. [DOI] [PubMed] [Google Scholar]
- 20.Wilschanski M, Dupuis A, Ellis L, et al. Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials. Am J Respir Crit Care Med 2006;174:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.