Abstract
Herein, we disclose an NHC-catalyzed aerobic oxidation of unactivated aldimines for the synthesis of amides via umpolung of imines proceeding through an aza-Breslow intermediate. We have developed an eco-friendly method for the conversion of imines to amides by using molecular oxygen in air as the sole oxidant and dimethyl carbonate (DMC) as a green solvent under mild reaction conditions. Broad substrate scope, high yields and gram scale syntheses expand the practicality of the developed method.
A general NHC-catalyzed conversion of imines to amides proceeding through umpolung–oxidation under aerobic conditions in green solvent is reported.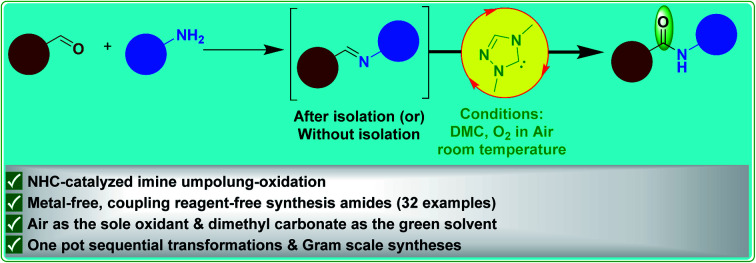
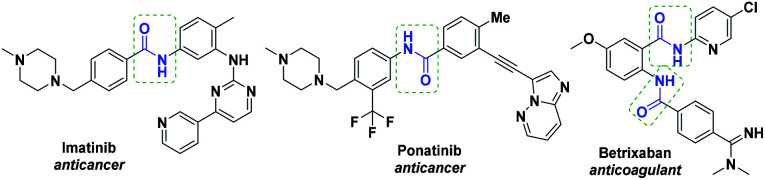
NHCs have emerged as an important class of organocatalysts for unconventional organic transformations due to their unique property of umpolung i.e. essentially reversing the polarity of electrophilic carbon centers. NHC-catalyzed umpolung reactivity has been extensively exploited over the past two decades for the construction of carbon–carbon and carbon–heteroatom bonds.1 NHC-catalytic transformations proceeding through umpolung reactivity, via Breslow intermediate, have been well established using aldehydes.1,2 Despite imines being considered to be potentially important building blocks, the obvious use of imines as reactants for the same is still an under developed area, probably due their lower reactivity. The umpolung of imine came into existence after the isolation of a nitrogen containing Breslow intermediate (known as aza-Breslow intermediate) by the Douthwaite and Rovis groups from the reaction of stoichiometric amounts of NHC and imine or iminium salt, respectively.3 For the first time, our group4 and the Biju group,5 independently, reported the NHC-catalyzed imine umpolung transformations. Recently, a few other groups including our group,6 the Wei–Fu–Huang,7 Biju,8 Lupton9 and Tian–Zhang–Chi10 groups reported NHC-catalyzed imine C–H functionalization or oxidation of imines. In continuation of our ongoing research on NHC-catalyzed umpolung transformations,4,6,11 herein, we present the development of NHC-catalyzed aerobic oxidation of aldimines to amides, via imine umpolung without using any additive. We became interested to access the amide functionality because amide is a very crucial functional group due its ubiquitous presence in life-processes in the form of peptide bond in protein molecules as well as its appearance in several of the drug molecules.12 For example, imatinib13a and ponatinib13a are used in the chemotherapy treatment of chronic myelogenous leukemia (CML) in cancer disease (Fig. 1). Betrixaban containing two amide functional groups is an oral anticoagulant drug (Fig. 1).13b
Fig. 1. Selected amide containing therapeutic molecules.
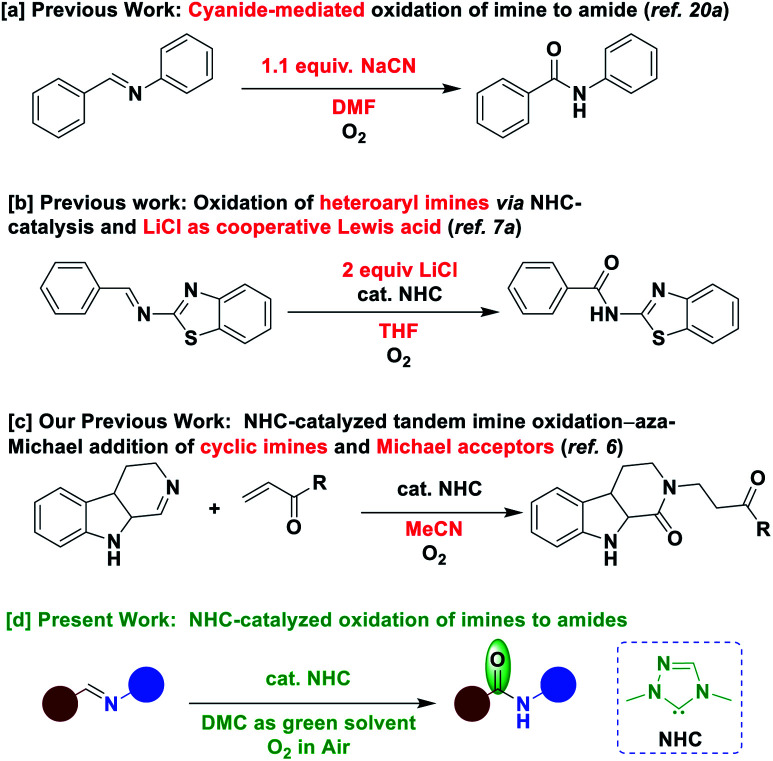
The scientific community has been showing great interest to develop new and efficient methods for the construction of amide bond. Coupling of carboxylic acid with amine is one of the most common methods used for the construction of amide molecule.14 However, this method requires stoichiometric amounts of peptide coupling reagents such as carbodiimides and 1-hydroxy benzotriazoles or activated carboxylic acid derivatives.15 The Schmidt reaction16 and Beckmann rearrangement17 are classical examples for the synthesis of amides. However, there are considerably a few reports available for the oxidation of imine to amide. Palladium-catalyzed oxidation of imines to amides was reported by using excess tert-butyl hydroperoxide (TBHP) as an oxidant.18 Methods for the oxidation of imines to amides were reported by using peroxy acids in the presence of strong Lewis acid and Brønsted acid, which generate stoichiometric amount of by-products.19 Cheon and co-workers reported the oxidation of imines to amides by using sodium cyanide (NaCN) in stoichiometric amounts. High toxic nature of NaCN is the limitation of this methodology (Scheme 1a).20a Recently, Fu and Huang group reported the oxidation of imines, limited to the imines derived from heteroaryl amines, to amides by using NHC catalysis with the assistance of excess lithium chloride as Lewis acid (Scheme 1b).7a Recently, we reported an NHC-catalyzed tandem aza-Michael oxidation of β-carboline cyclic imines in the presence of external Michael acceptors (Scheme 1c).6 Besides the limitations associated with the above transformations, those were performed in non-green solvent media. However, to the best of our knowledge, there are no reports for the conversion of imines to amides under NHC-catalysis without using an external additive/assistance.21 On the other hand, the development of new methods to access amide functionality inclusive of green chemistry principles such as organocatalysis, air as the sole oxidant and use of green solvent medium under ambient conditions is highly desirable. Herein, we report an NHC-catalyzed conversion of aldimines to amides, proceeding through imine umpolung–oxidation, in the presence of air in a green solvent such as DMC22 under mild conditions.
Scheme 1. Prior work and this work.
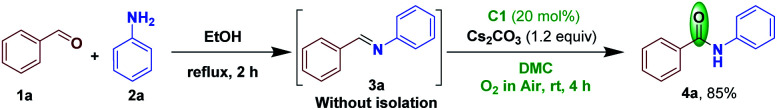
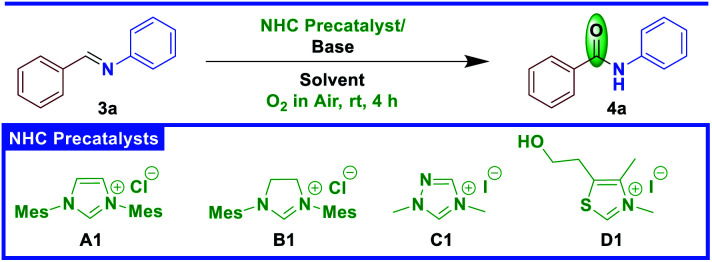
Initially, we began our investigation with the reaction of aldimine 3a, derived from benzaldehyde 1a and aniline 2a, in the presence of NHC A1 catalyst under open air conditions at room temperature in a green solvent such as DMC. Gratifyingly, we observed the formation of the corresponding amide 4a in 53% yield (Table 1, entry 1). Motivated by this initial result, we assayed different NHCs. As shown in Table 1, the imidazolinium NHC B1 was ineffective in this transformation (Table 1, entry 2). Delightfully, with triazolium NHC C1 the amide 4a was isolated in 87% yield (Table 1, entry 3). It was found that thiazolium NHC D1 was not effective for this transformation (Table 1, entry 4). We then moved to the screening of bases with NHC C1 and it was observed that Cs2CO3 proved to be the most effective choice for this transformation (Table 1, entry 3), while bases such as DBU, DABCO, NaH and K2CO3 also provided the desired amide 4a in moderate to good yields (Table 1, entries 5–8). Furthermore, we examined the effect of solvent for this protocol and observed the best yields in DMC (Table 1, entry 3), while other solvents such as THF, EtOAc and DMSO are tolerated and gave moderate to good yields of 4a (Table 1, entry 9–11). However, this reaction did not work in ethanol (Table 1, entry 12). After successfully identifying the optimal NHC/base/solvent, we investigated the loading of the NHC catalyst in this transformation. Accordingly, the catalyst loading of NHC C1 was reduced from 20 mol% to 15 mol% to observe 70% yield of 4a. Similarly, when the base loading was decreased from 120 mol% to 100 mol% the yield of 4a was reduced to 72% (Table 1, entry 13-14). Subsequently, we performed a couple of reactions to know the necessity of NHC and base for this transformation. Accordingly, two experiments were performed in the presence of NHC or base alone, and neither of these reactions gave the product 4a (Table 1, entry 15-16) (see ESI† for an extensive optimization survey). We conducted an experiment in presence of LiCl (1.2 equiv.), and it did not help to improve the yield of the product.
Optimization study.
| ||||
|---|---|---|---|---|
| Entrya | NHC precatalyst | Base | Solvent | Yield of 4ab |
| 1 | A1 | Cs2CO3 | DMC | 53 |
| 2 | B1 | Cs2CO3 | DMC | — |
| 3 | C1 | Cs 2 CO 3 | DMC | 87 |
| 4 | D1 | Cs2CO3 | DMC | — |
| 5 | C1 | DBU | DMC | 71 |
| 6 | C1 | DABCO | DMC | 65 |
| 7 | C1 | NaH | DMC | 72 |
| 8 | C1 | K2CO3 | DMC | 55 |
| 9 | C1 | Cs2CO3 | THF | 75 |
| 10 | C1 | Cs2CO3 | EtOAc | 65 |
| 11 | C1 | Cs2CO3 | DMSO | 63 |
| 12 | C1 | Cs2CO3 | EtOH | — |
| 13 | C1 | Cs2CO3 | DMC | 70c |
| 14 | C1 | Cs2CO3 | DMC | 72d |
| 15 | — | Cs2CO3 | DMC | — |
| 16 | C1 | — | DMC | — |
Reaction conditions: 3a (0.5 mmol), NHC precatalyst (0.1 mmol), base (0.6 mmol), solvent (4 mL).
Yields are of pure compounds after crystallization.
With 0.075 mmol of C1.
With 0.5 mmol of Cs2CO3; Mes: 2,4,6-trimethylphenyl; DBU: 1,8-diazabicyclo[5.4.0]undec-7-ene; DABCO: 1,4-diazabicyclo[2.2.2]octane; DMSO = dimethyl sulfoxide.
By choosing the acceptable optimized conditions from Table 1 (entry 3), we next conducted NHC-catalyzed conversion of imine to amide in a sequential manner-starting from benzaldehyde 1a and aniline 2a. Accordingly, 1a and 2a were reacted to give the corresponding aldimine 3a. Subsequently, without further purification, the crude aldimine 3a was subjected to NHC catalyzed imine umpolung–oxidation to furnish the corresponding benzanilide 4a in a comparable yield of 85% (Scheme 2).
Scheme 2. Sequential imine formation–NHC-catalyzed aerobic oxidation to access amide 4a.
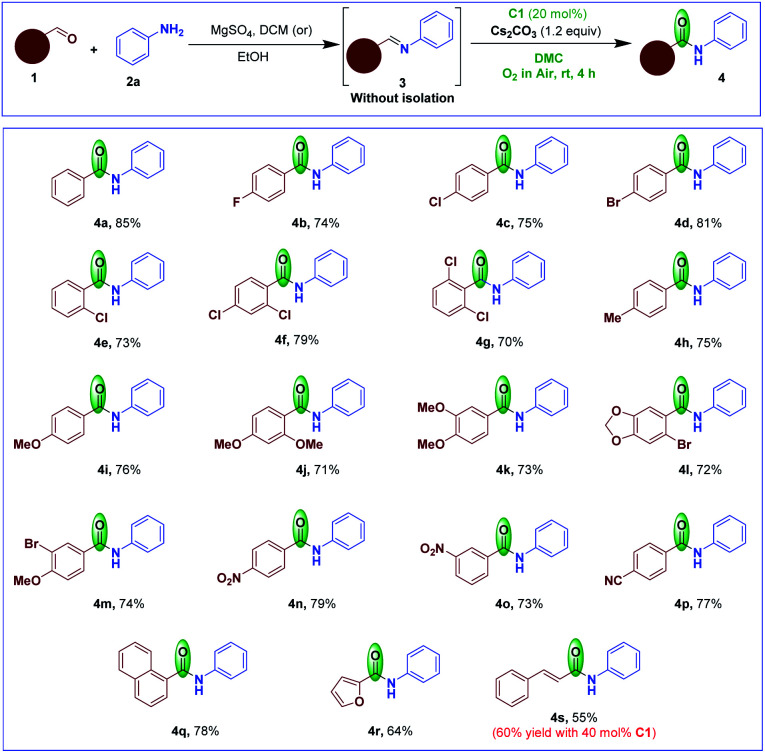
We then examined the scope of the NHC-catalyzed imine umpolung–oxidation to access amides under aerobic conditions in DMC at room temperature. Firstly, the reaction of variously substituted aromatic, heteroaromatic and vinyl aldehydes 1 were converted to the corresponding aldimines 3 with aniline 2a. Subsequently the aldimine 3, without further purification, was subjected to optimized NHC catalysis conditions (Scheme 3). Imines derived from aromatic aldehydes bearing either electron-withdrawing or electron-donating groups smoothly afforded the corresponding substituted amides 4 in high yields. The imines derived from ortho-/para-halo-substituted benzaldehydes provided the amides 4b–f in high yields. It was interesting to note that sterically hindered 2,6-dichlorobenzaldehyde derived imine also provided the corresponding amide 4g in 70% yield. Aldimines bearing mono-/di-substituted electron-donating groups provided the respective amides 4h–k in good yields. The aldimines containing both electron-donating and halogen substituents furnished the corresponding amides 4l and 4m in 72% and 74%, yields, respectively. Imines having electron-withdrawing functional groups such as NO2 or CN also shown tolerance to afford their amides 4n–p in 73–79% yields. The naphthaldehyde imine provided its amide 4q in 78% yield. We also tested the imines derived from heterocyclic aldehyde such as 2-furaldehyde and α,β-unsaturated aldehyde such as cinnamaldehyde in this transformation to produce the corresponding amides 4r and 4s in 64% and 55%, yields, respectively. The yield of 4s was only slightly increased with 40 mol% NHC C1.
Scheme 3. Scope of the reaction starting with different aldehydes.
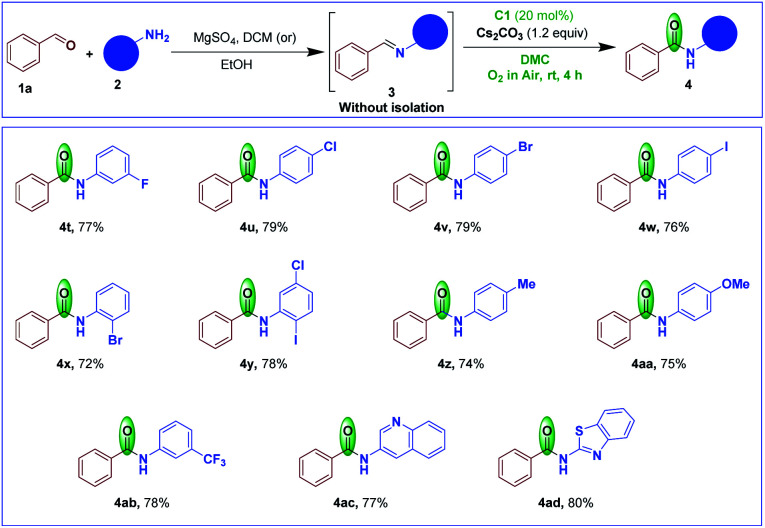
To further study the substrate scope of the NHC-catalyzed imines to amides, benzaldehyde imines derived from variously substituted aromatic/heteroaromatic amines were tested in this transformation (Scheme 4). The imines bearing mono-/di-substituted halogen groups on the aniline side gave the corresponding amides 4t–y in high yields. The imines containing electron-donating and electron-withdrawing groups on the aniline side were also smoothly converted to their respective amides 4z-4ab in good yields. Imines derived from heteroaromatic amines such as 3-aminoquinoline and 2-aminobenzothiazole furnished the corresponding amides 4ac and 4ad in 77% and 80% yields, respectively.
Scheme 4. Scope of the reaction starting with different amines.
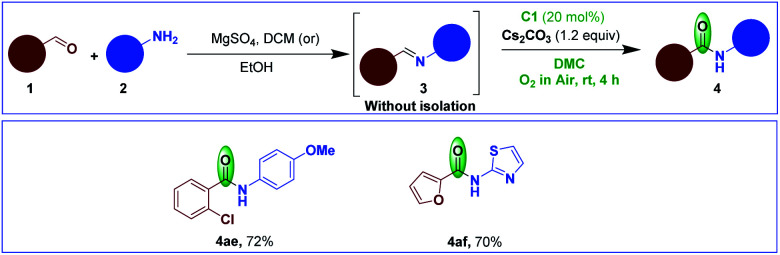
We also tested the imines derived from substituted benzaldehyde and substituted aniline to give the desired amide 4ae in 72% yield (Scheme 5). Later, an imine derived from heteroaromatic aldehyde and heteroaromatic amine was subjected to NHC-catalyzed oxidation to afford the respective amide 4af in 70% yield (Scheme 5). We also conducted the reactions with imines derived from aliphatic aldehyde/aliphatic amine, however, the corresponding amide formation was not observed. The reason may be attributed to the less reactivity of these imines.
Scheme 5. Additional scope of the reaction.
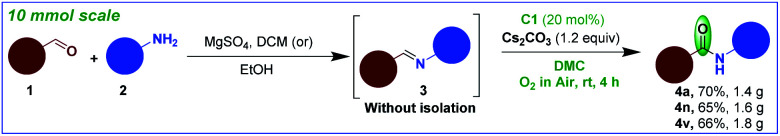
The practicality of this transformation was tested by gram-scale syntheses on 10 mmol scales; the NHC-catalyzed aerobic oxidation of imines 3a, 3n, 3v proceeded smoothly to afford the corresponding amides 4a, 4n, 4v in 70%, 65%, 66% yields, respectively (Scheme 6).
Scheme 6. Gram-scale syntheses of 4a, 4n and 4v.
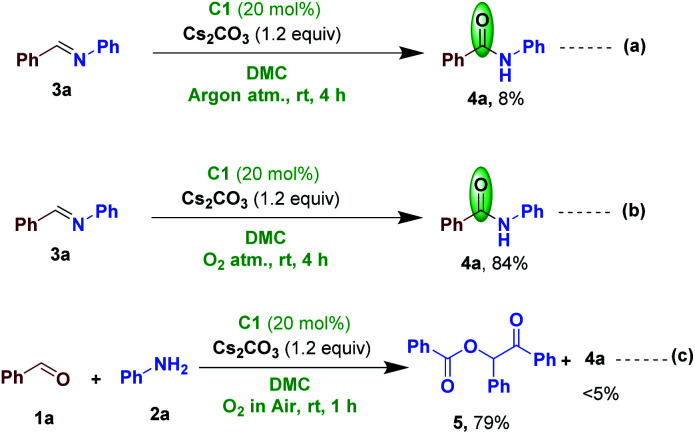
We have performed a few control experiments to know the requirement of molecular oxygen for the NHC-catalyzed oxidation of imine to amide. Accordingly, we conducted a reaction under inert conditions and observed a drastic decrease in the yield of the product (Scheme 7a).6 This result indicate that molecular oxygen in air is responsible and acting as the sole oxidant in this transformation. We also conducted the NHC-catalyzed imine 3a oxidation in the presence of pure oxygen to give the desired amide 4a in 84% yield (Scheme 7b). We further conducted a direct reaction of 1a and 2a under optimized NHC-catalyzed conditions to know whether oxidation of 1a provide NHC-azolium intermediate and add to the amine, akin to the NHC-catalyzed ester formation from the reaction of aldehydes and alcohols.23 However, in this reaction we did not observed the formation of amide but obtained the compound 520b resulted from the benzoin condensation-acylation (Scheme 7c).
Scheme 7. Control experiments.
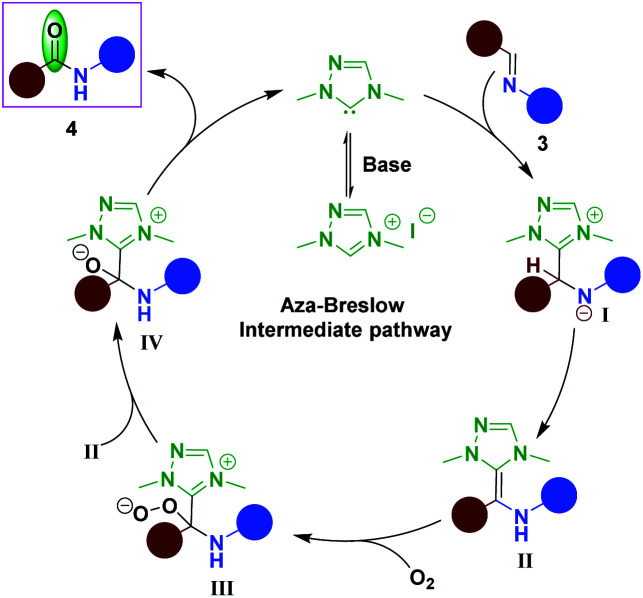
Based on the previous literature reports,6,7,24 a possible mechanism for the NHC-catalyzed aerobic oxidation of imine to amide is depicted in Scheme 8. Initially, the free NHC would add to the imine 3 to form intermediate I. Aza-Breslow intermediate II would generate from intermediate I upon proton shift. The intermediate II would react with molecular oxygen and undergo single electron transfer of the intermediate II with dioxygen followed by radical recombination to give intermediate III.7,24 Thereafter one more molecule of aza-Breslow intermediate II would react with intermediate III to produce two molecules of intermediate IV. Then from intermediate IV NHC would regenerate and produce the amide 4. This mechanism suggests that one molecule of oxygen is sufficient to produce two molecules of the amide 4.
Scheme 8. Plausible mechanism.
Conclusions
In conclusion, we have developed an efficient and environmentally friendly approach for the conversion of imines to amides with NHC-catalyzed aerobic oxidation of imines proceeding through imine umpolung involving aza-Breslow intermediate. In this protocol, we have used a green solvent such as dimethyl carbonate and molecular oxygen in air acts as the sole oxidant under mild conditions without using any additive. Further research work is in progress in our laboratory towards the investigation and application of the NHC-catalyzed imine umpolung for its potential in organic transformations and will be communicated in due course.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank the Science & Engineering Research Board (SERB), Department of Science and Technology (DST), India for an Extra-mural Research grant (EMR/2017/002601). JR thanks UGC, New Delhi, SY and KS thank CSIR, New Delhi, for fellowships. We thank the Director, CSIR-IICT for the support (communication No. IICT/Pubs./2021/370).
Electronic supplementary information (ESI) available: Experimental procedures, spectral data, copies of NMR spectra for products. See DOI: 10.1039/d2ra00897a
Notes and references
- For selected excellent reviews on NHC organocatalysis, see: ; (a) Enders D. Niemeier O. Henseler A. Chem. Rev. 2007;107:5606. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]; (b) Marion N. Díez-González S. Nolan S. P. Angew. Chem., Int. Ed. 2007;46:2988. doi: 10.1002/anie.200603380. [DOI] [PubMed] [Google Scholar]; (c) Izquierdo J. Hutson G. E. Cohen D. T. Scheidt K. A. Angew. Chem., Int. Ed. 2012;51:11686. doi: 10.1002/anie.201203704. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bode J. W. Nat. Chem. 2013;5:813. doi: 10.1038/nchem.1766. [DOI] [PubMed] [Google Scholar]; (e) Ryan S. J. Candish L. Lupton D. W. Chem. Soc. Rev. 2013;42:4906. doi: 10.1039/C3CS35522E. [DOI] [PubMed] [Google Scholar]; (f) Hopkinson M. N. Richter C. Schedler M. Glorius F. Nature. 2014;510:485. doi: 10.1038/nature13384. [DOI] [PubMed] [Google Scholar]; (g) Flanigan D. M. Romanov-Michailidis F. White N. A. Rovis T. Chem. Rev. 2015;115:9307. doi: 10.1021/acs.chemrev.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Wangand M. H. Scheidt K. A. Angew. Chem., Int. Ed. 2016;55:14912. doi: 10.1002/anie.201605319. [DOI] [PubMed] [Google Scholar]; (i) Zhao M. Zhang Y.-T. Chen J. Zhou L. Asian J. Org. Chem. 2018;7:54. doi: 10.1002/ajoc.201700538. [DOI] [Google Scholar]; (j) N-Heterocyclic Carbenes in Organocatalysis, ed. A. T. Biju, John Wiley & Sons, Weinheim, 2019 [Google Scholar]
- For a selected review on the benzoin reaction, see: ; (a) Menon R. S. Biju A. T. Nair V. Beilstein J. Org. Chem. 2016;12:444. doi: 10.3762/bjoc.12.47. [DOI] [PMC free article] [PubMed] [Google Scholar]; ; For a recent review on Stetter reaction, see: ; (b) Heravi M. M. Zadsirjan V. Kafshdarzadeh K. Amiri Z. Asian J. Org. Chem. 2020;9:1999. doi: 10.1002/ajoc.202000378. [DOI] [Google Scholar]; ; For a review on umpolung of α,β-unsaturated aldehydes, see: ; Menon R. S. Biju A. T. Nair V. Chem. Soc. Rev. 2015;44:5040. doi: 10.1039/C5CS00162E. [DOI] [PubMed] [Google Scholar]
- (a) Simonovic S. Frison J.-C. Koyuncu H. Whitwood A. C. Douthwaite R. E. Org. Lett. 2009;11:245. doi: 10.1021/ol802572n. [DOI] [PubMed] [Google Scholar]; (b) DiRocco D. A. Oberg K. M. Rovis T. J. Am. Chem. Soc. 2012;134:6143. doi: 10.1021/ja302031v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish B. Subbireddy M. Suresh S. Chem. Commun. 2017;53:3338. doi: 10.1039/C6CC10292A. [DOI] [PubMed] [Google Scholar]
- Patra A. Mukherjee S. Das T. K. Jain S. Gonnade R. G. Biju A. T. Angew. Chem., Int. Ed. 2017;56:2730. doi: 10.1002/anie.201611268. [DOI] [PubMed] [Google Scholar]
- Satyam K. Harish B. Nanubolu J. B. Suresh S. Chem. Commun. 2020;56:2803. doi: 10.1039/D0CC00321B. [DOI] [PubMed] [Google Scholar]
- (a) Wang G. Fu Z. Huang W. Org. Lett. 2017;19:3362. doi: 10.1021/acs.orglett.7b01195. [DOI] [PubMed] [Google Scholar]; ; For NHC-catalyzed aerobic oxidation of iminium compounds such as isoquinolinium salts, see: ; (b) Wang G. Hu W. Hu Z. Zhang Y. Yao W. Li L. Fu Z. Huang W. Green Chem. 2018;20:3302. doi: 10.1039/C8GC01488D. [DOI] [Google Scholar]; (c) Wang G. Zhang Q.-C. Wei C. Zhang Y. Zhang L. Huang J. Wei D. Fu Z. Huang W. Angew. Chem., Int. Ed. 2021;60:7913. doi: 10.1002/anie.202017017. [DOI] [PubMed] [Google Scholar]
- (a) Patra A. Gelat F. Pannecoucke X. Poisson T. Besset T. Biju A. T. Org. Lett. 2018;20:1086. doi: 10.1021/acs.orglett.7b04055. [DOI] [PubMed] [Google Scholar]; (b) Das T. K. Ghosh A. Balanna K. Behera P. Gonnade R. G. Marelli U. K. Das A. K. Biju A. T. ACS Catal. 2019;9:4065. doi: 10.1021/acscatal.9b00737. [DOI] [Google Scholar]
- Fernando J. E. M. Nakano Y. Zhang C. Lupton D. W. Angew. Chem., Int. Ed. 2019;58:4007. doi: 10.1002/anie.201812585. [DOI] [PubMed] [Google Scholar]
- Yang X. Xie Y. Xu J. Ren S. Mondal B. Zhou L. Tian W. Zhang X. Hao L. Jin Z. Chi Y. R. Angew. Chem., Int. Ed. 2021;60:7906. doi: 10.1002/anie.202016506. [DOI] [PubMed] [Google Scholar]
- (a) Satyam K. Ramarao J. Suresh S. Org. Biomol. Chem. 2021;19:1488. doi: 10.1039/D0OB02606A. [DOI] [PubMed] [Google Scholar]; (b) Yadav S. Suresh S. Asian J. Org. Chem. 2021;10:1406. doi: 10.1002/ajoc.202100158. [DOI] [Google Scholar]
- Kumari S. Carmona A. V. Tiwari A. K. Trippier P. C. J. Med. Chem. 2020;63:12290. doi: 10.1021/acs.jmedchem.0c00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Rossari F. Minutolo F. Orciuolo E. J. Hematol. Oncol. 2018;11:1. doi: 10.1186/s13045-017-0548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Murphy G. Grace Y. Chaudry S. Chamoun R. J. Pharm. Technol. 2018;34:123. doi: 10.1177/8755122518759765. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kompella A. Adibhatla B. R. K. Muddasani P. R. Rachakonda S. Gampa V. K. Dubey P. K. Org. Process Res. Dev. 2012;16:1794. doi: 10.1021/op300212u. [DOI] [Google Scholar]; (d) Larocque E. Chu E. F. Y. Naganna N. Sintim H. O. ACS Omega. 2020;5:2690. doi: 10.1021/acsomega.9b03223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Handbook of Reagents for Organic Synthesis, ed. P. Wipf, Wiley and Sons, New York, 2005 [Google Scholar]; (b) Lundberg H. Tinnis F. Selander N. Adolfsson H. Chem. Soc. Rev. 2014;43:2714. doi: 10.1039/C3CS60345H. [DOI] [PubMed] [Google Scholar]
- (a) Humphrey J. M. Chamberlin A. R. Chem. Rev. 1997;97:2243. doi: 10.1021/cr950005s. [DOI] [PubMed] [Google Scholar]; (b) Teichert A. Jantos K. Harms K. Studer A. Org. Lett. 2004;6:3477. doi: 10.1021/ol048759t. [DOI] [PubMed] [Google Scholar]; Shendage D. M. Froehlich R. Haufe G. Org. Lett. 2004;6:3675. doi: 10.1021/ol048771l. [DOI] [PubMed] [Google Scholar]; (c) Montalbetti C. A. G. N. Falque V. Tetrahedron. 2005;61:10827. doi: 10.1016/j.tet.2005.08.031. [DOI] [Google Scholar]; (d) Black D. A. Arndtsen B. A. Org. Lett. 2006;8:1991. doi: 10.1021/ol060277p. [DOI] [PubMed] [Google Scholar]; (e) Katritzky A. R. Cai C. Singh S. K. J. Org. Chem. 2006;71:3375. doi: 10.1021/jo052443x. [DOI] [PubMed] [Google Scholar]
- (a) Schmidt R. F. Ber. Dtsch. Chem. Ges. 1924;57:704. doi: 10.1002/cber.19240570423. [DOI] [Google Scholar]; (b) Yao L. Aubé J. J. Am. Chem. Soc. 2007;129:2766. doi: 10.1021/ja068919r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nyfeler E. Renaud P. Chimia. 2006;60:276. doi: 10.2533/000942906777674714. [DOI] [Google Scholar]
- (a) Ramalingan C. Park Y.-T. J. Org. Chem. 2007;72:4536. doi: 10.1021/jo070297k. [DOI] [PubMed] [Google Scholar]; (b) Augustine J. K. Kumar R. Bombrun A. Mandal A. B. Tetrahedron Lett. 2011;52:1074. doi: 10.1016/j.tetlet.2010.12.090. [DOI] [Google Scholar]; (c) Kaur K. Srivastava S. New J. Chem. 2020;44:18530. doi: 10.1039/D0NJ02034F. [DOI] [Google Scholar]
- Gao S. Ma Y. Chen W. Luo J. Synlett. 2018;29:2191. doi: 10.1055/s-0037-1610653. [DOI] [Google Scholar]
- For selected examples on the oxidation of imines using super-stoichiometric amounts of oxidants involving probable iminium ion intermediates, see: ; (a) An G.-i. Kim M. Kim J. Y. Rhee H. Tetrahedron Lett. 2003;44:2183. doi: 10.1016/S0040-4039(03)00156-4. [DOI] [Google Scholar]; (b) Mohamed M. A. Yamada K.-i. Tomioka K. Tetrahedron Lett. 2009;50:3436. doi: 10.1016/j.tetlet.2009.02.174. [DOI] [Google Scholar]; (c) Bingham T. W. Hernandez L. W. Olson D. G. Svec R. L. Hergenrother P. J. Sarlah D. J. Am. Chem. Soc. 2019;141:657. doi: 10.1021/jacs.8b12123. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Llopis N. Gisbert P. Baeza A. J. Org. Chem. 2020;85:11072. doi: 10.1021/acs.joc.0c01579. [DOI] [PubMed] [Google Scholar]; ; for selected examples on the oxidation of imines using enzymes as catalysts, see:; (e) Bechi B. Herter S. McKenna S. Riley C. Leimkühler S. Turner N. J. Carnell A. J. Green Chem. 2014;16:4524. doi: 10.1039/C4GC01321B. [DOI] [Google Scholar]
- (a) Seo H.-A. Cho Y.-H. Lee Y.-S. Cheon C.-H. J. Org. Chem. 2015;80:11993. doi: 10.1021/acs.joc.5b01922. [DOI] [PubMed] [Google Scholar]; (b) Kim Y.-J. Kim N. Y. Cheon C.-H. Org. Lett. 2014;16:2514. doi: 10.1021/ol5008845. [DOI] [PubMed] [Google Scholar]
- Very recently, during the preparation of this manuscript, an NHC-mediated (but not catalyzed) oxidation of imines to amides appeared. However, this method requires NHC in more than stoichiometric quantities and use of non-green solvent such as 1,4-dioxane besides generating the by-product such as imidazolidinone in stoichiometric quantities, see: ; Sun S. Guo D. Li F. Wang J. Org. Chem. Front. 2022;9:356. doi: 10.1039/D1QO01661J. [DOI] [Google Scholar]
- According to CHEM21 selection guide “dimethyl carbonate (DMC) is considered to be a potential replacement for several solvents such as MEK, ethyl acetate, MIBK, butyl acetate and most other ketones and glycol ethers, further DMC seems to be the greenest carbonate”, see: ; Prat D. Wells A. Hayler J. Sneddon H. McElroy C. R. Abou-Shehada S. Dunn P. J. Green Chem. 2016;18:288. doi: 10.1039/C5GC01008J. [DOI] [Google Scholar]
- Sarkar S. D. Grimme S. Studer A. J. Am. Chem. Soc. 2010;132:1190. doi: 10.1021/ja910540j. [DOI] [PubMed] [Google Scholar]
- For oxidation of Breslow intermediates, see: ; (a) Bortolini O. Chiappe C. Fogagnolo M. Giovannini P. P. Massi A. Pomelli C. S. Ragno D. Chem. Commun. 2014;50:2008. doi: 10.1039/C3CC48929A. [DOI] [PubMed] [Google Scholar]; (b) Bortolini O. Chiappe C. Fogagnolo M. Massi A. Pomelli C. S. J. Org. Chem. 2017;82:302. doi: 10.1021/acs.joc.6b02414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.