Abstract
Typically, Suzuki couplings used in polymerizations are performed at raised temperatures in inert atmospheres. As a result, the synthesis of aromatic materials that utilize this chemistry often demands expensive and specialized equipment on an industrial scale. Herein, we describe a bimetallic methodology that exploits the distinct reactivities of palladium and copper to perform high yielding aryl–aryl dimerizations and polymerizations that can be performed on a benchtop under ambient conditions. These couplings are facile and can be performed by simple mixing in the open vessel. To demonstrate the utility of this method in the context of polymer synthesis: polyfluorene, polycarbazole, polysilafluorene, and poly(6,12-dihydro-dithienoindacenodithiophene) were created at ambient temperature and open to air.
Graphical Abstract

Polymers built from aromatic monomers have been used in a variety of applications, from water bottles1 to bullet proof body armor.2 The trigonal pyramidal geometry of sp2 carbons impart rigidity to the materials, while aromaticity enhances thermal stability,3 liquid crystallinity, and unique electronic properties.4 In addition, conjugated polymers used in electronics5 and LEDs6 are commonly built from aromatic systems. Suzuki couplings are often used to produce such materials. However, these reactions can call for specialized equipment to maintain an inert atmosphere, high temperatures, and/or specialized catalysts.7 Previous literature has shown that palladium-catalyzed homocoupling of diboronates is an effective strategy for making small polyfluorenes.8 However, these synthetic strategies required a pure oxygen atmosphere at 80 °C under strictly dry conditions which could trigger combustion on a reactor scale. To enhance the accessibility of these aromatic polymers we sought to create a method that used the oxygen in air as the oxidant, was tolerant to wet solvents, and operated at room temperature.
Reports of oxidative aromatic homocoupling were reported by Davidson and Triggs as early as 1968.9 In their report palladium was used to homocouple aryl boronic acids with sodium perchlorate as an oxidant. Later reports showed that aromatic homocoupling of phenyl boronic acids could be done under oxidative conditions with stoichiometric Cu(II) as an oxidant.10 These reports showed that a bimetallic approach to homocoupling was possible, allowing for wet benchtop coupling conditions and green solvents, such as ethanol. However, in previous reports the catalyst loading for dimerization was high (5–10% Pd).8,11 More recently, coupling of phenyl hydrazines suggested that catalytic amounts of copper(II) could be used instead of stoichiometric amounts.12 However, the yields were generally moderate with a limited substrate scope when performed in water. Therefore, this methodology was not yet ideal for synthesis of functionally rich aromatic polymers. Being inspired by these previous reports we believed that a Pd/Cu system could be optimized to efficiently catalyze benchtop aromatic polymerizations. Herein, we report such an improvement, based upon an anticipated mechanism such as that shown in Figure 1. Our new conditions involve a significantly lower catalyst loading (from 5% to 1% Pd), faster reaction times (from 72 h down to 1 h), and a wider substrate scope—being able to couple unprotected phenols. Finally, we were able to use this improved bimetallic system to form a set of aromatic polymers from aryl diboronic acids by simply stirring the starting materials on a benchtop.
Figure 1.
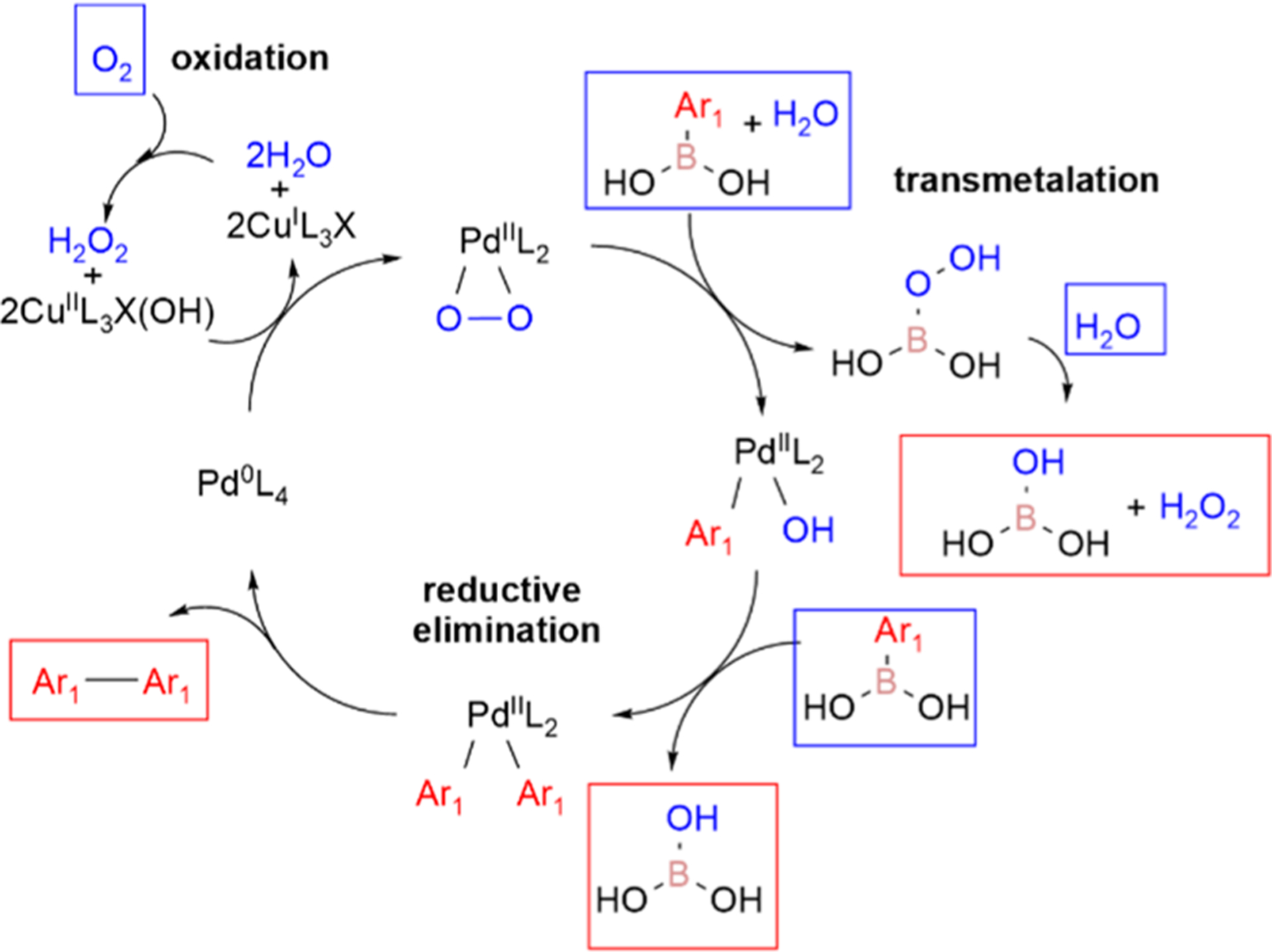
Proposed mechanism of bimetallic reaction where copper is oxidized by oxygen which in turn oxidizes Pd(0) to Pd(II) (adapted from previous literature).13 The aryl group is transmetalated to palladium. Subsequently, the Pd(II) forms the biaryl dimer through reductive elimination. Palladium(0) is oxidized by copper(I) to restart the catalytic cycle. The Cu ligands (L) would be combinations of acetic acid, water, and DMSO.
We started our study by performing a simple Pd(II) homocoupling of arylboronic acids on the benchtop (Figure 2a). As suspected,12 adding Cu(OAc)2 (5 mol %) to the reaction mixture allowed us to lower Pd(OAc)2 loading while significantly enhancing the reaction rate and maintaining a high percent conversion (Figure 2b, Red). The reaction with 2% Pd(II) and 5% Cu(II) proceeds to completion within 1 h, while the reaction with only 2 mol % Pd(II) requires over 24 h. Complete reactions were observed with Pd(II) amounts as low as 1 mol % in the presence of Cu(II) (Figure 2b). These results supported our design hypothesis that the Cu(II) facilitated the redox cycle of Pd(0) to Pd(II) enhancing the turnover rate. In fact, we found that the dimerization reactions do not even occur under an inert atmosphere and deoxygenated solvents. Hence, similar to the Wacker oxidation,13 Pd(0) is generated then reoxidized by Cu(II) to Pd(II), which performs the homocoupling. Cu(II) is quickly regenerated by atmospheric oxygen, resuming the catalytic cycle (Figure 1).
Figure 2.
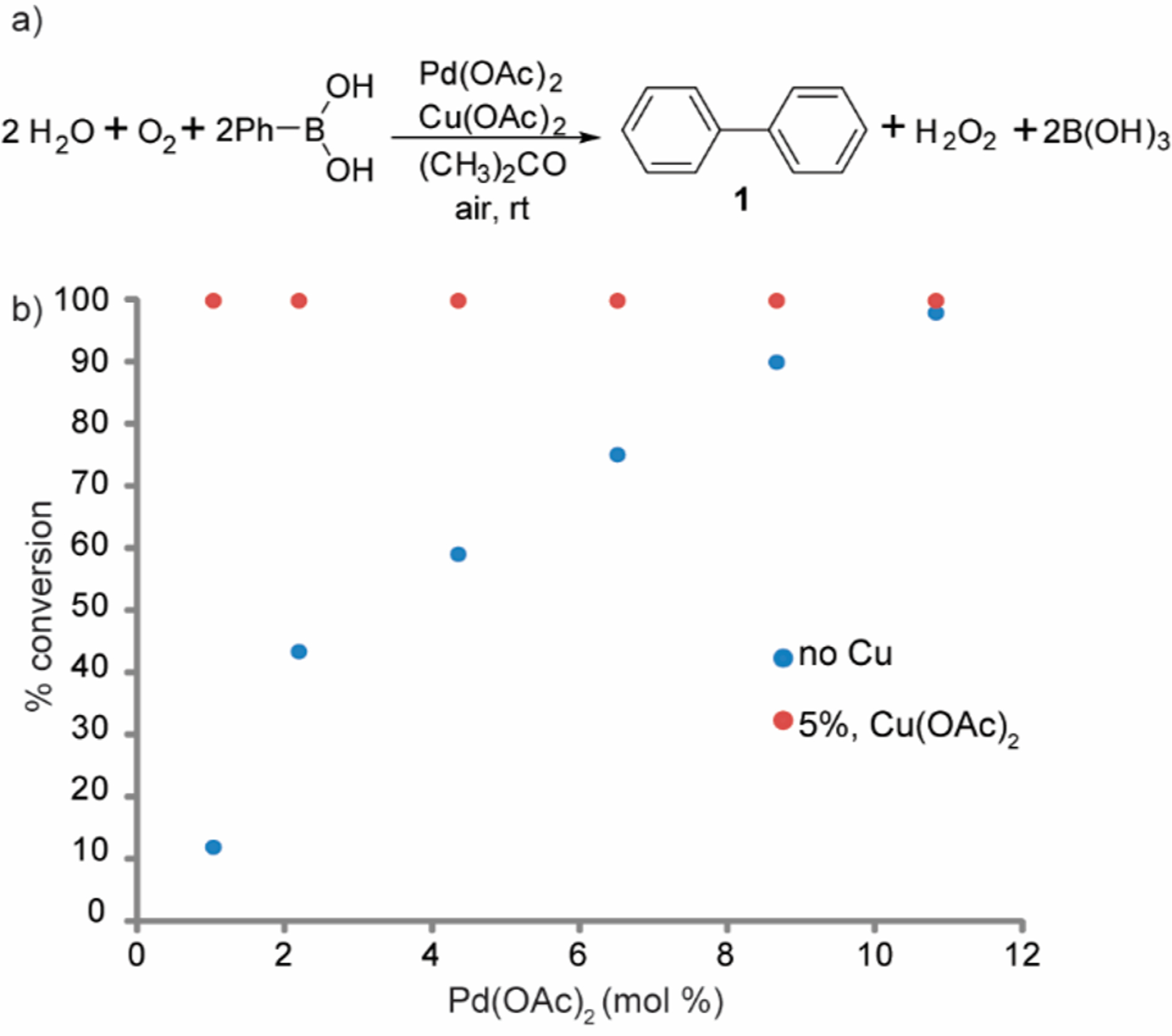
Palladium and copper react with phenyl boronic acid (0.5 M) for 14 h at different catalyst concentrations. (a) Reaction scheme. (b) Ratio of starting material to product as seen by GC-MS in varying concentration of Pd(OAc)2 (x-axis). The reaction conversion without copper (blue) and with 5 mol % Cu(OAc)2 (red) in acetone.
Investigation of Mechanism.
Previous literature reports assisted us in the optimization of the cocatalyst system12 and allowed us to derive a balanced chemical equation. To verify this balanced equation (Figure 2a), we first validated the necessity of water in the reaction. As suspected the reaction stalls in dried solvents. Inversely, the reaction was seemingly insensitive to excess water, coupling well in 1:1 H2O/DMSO. The generation of H2O2 was tested with a commercially available peroxide test kit (Figure S1) which revealed H2O2 being produced as the reaction proceeded. An obvious source of H2O2 is as the product of Cu(I) oxidation by molecular oxygen (Figure 1b upper left). Another source of H2O2 could be the transmetalation from an arylboronate to the Pd(O2) complex,13 followed by hydrolysis (Figure 1b in the cycle).
Broadening the Substrate Scope.
After screening several solvents (Table S2), DMSO was chosen due to its solvating and coordinating properties, and the anticipation it would help to solubilize our ultimate polymer products (see below). Similar to previously reported results,14 electron-withdrawing substituents reacted with yields of 50%–quant. (Scheme 1). However, in stark contrast to previous homocoupling strategies, electron-donating groups were even more successful with yields of 85%–quant. (9–11).14 In addition, most of the ortho-substituted structures coupled with good to moderate yields of 65%–quant. (12–16). Unfortunately, the dimerization did not work with the diortho substituted substrate (17); however, the dimeta substituted substrate coupled in quantitative yield (18).
Scheme 1. Aromatic Dimerization Reactions Were Performed under Ambient Conditionsa.
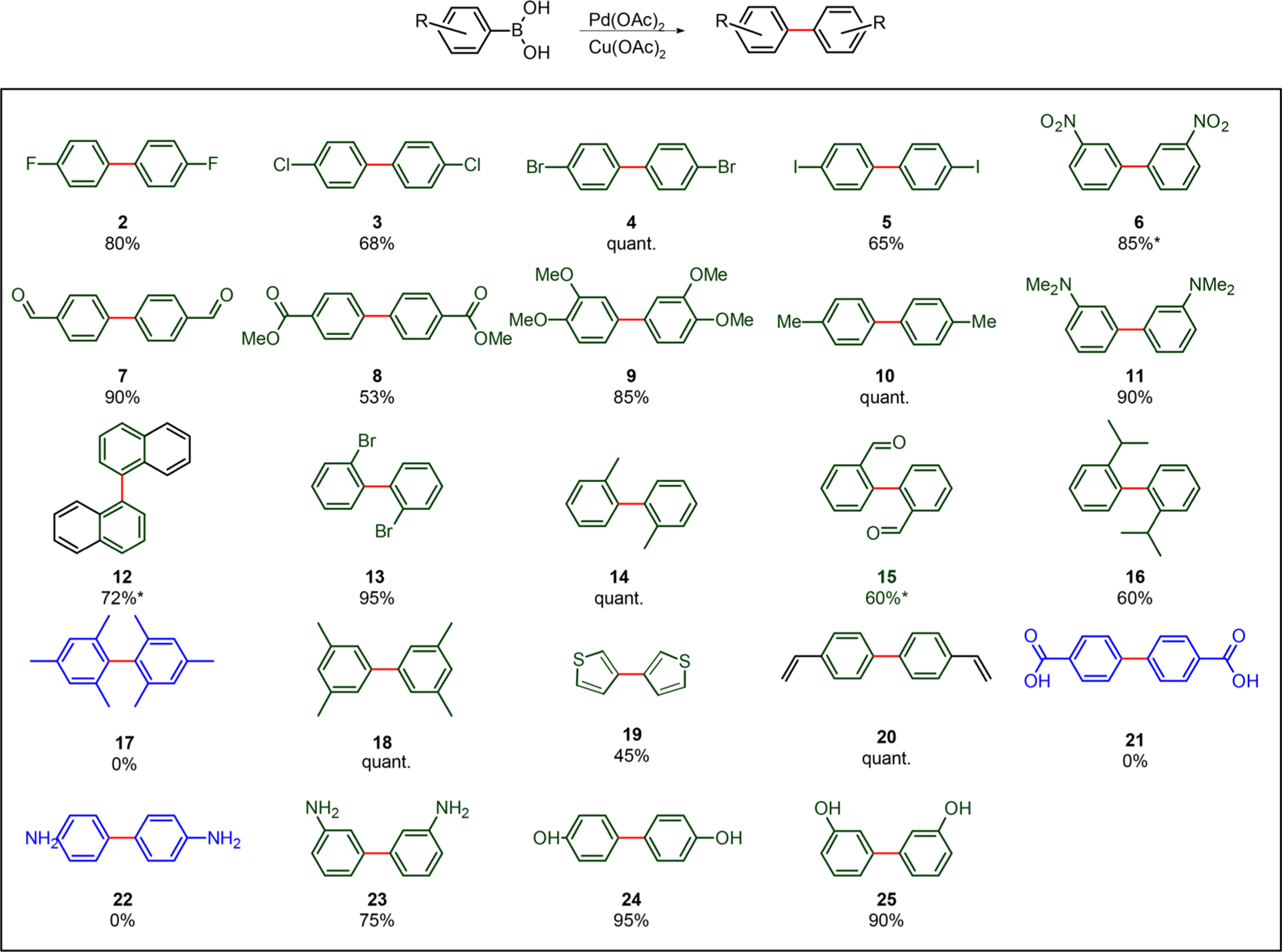
a1 mol % Pd(OAc)2, 3 mol % Cu(OAc)2, 5% (v/v) H2O/DMSO, rt, and air. Reactions (1.7 mL) were run at 0.18 M. Water can be added to anhydrous or benchtop DMSO without affecting yields. * NMR yields.
Next, we examined the tolerance of the method to more challenging functional groups. Both 3- and 4-hydroxyphenylboronic acids coupled with greater than 90% yield (24–25). The anilines behaved differently. While 3-amino phenyl boronic acid coupled well (23), 4-amino phenyl boronic acid was almost completely proteodeborylated, presumably due to the more nucleophilic nature of the aromatic ring (22). The nucleophilic 2-thiophenyl boronic acid also homocoupled with acceptable yields considering its challenging nature (19). However, the carboxylic acid failed to react (21). The presence of the carboxylic acid appears to stop the reaction. To access carboxylic acids, the aldehydes 7 and 15 could be subsequently oxidized post-reaction. Interestingly, aldehyde oxidation to carboxylic acid did not occur in any detectable amounts (7,15) under the oxidative reaction conditions. Next, we investigated the reaction in the presence of metal coordinating π-nucleophiles, such as terminal olefins. Gratifyingly, only aryl–aryl coupling was observed (20). Finally, being inspired by a previous study12 we explored the cross-coupling of boronic acids in our bimetallic system. However, initial attempts resulted in statistical mixtures of the homo- and heterodimers, and this idea was not further pursued.
Benchtop Synthesis of Polymers.
Being satisfied by the broad functional scope and coupling efficiency of this method, we turned our attention to the original goal—benchtop synthesis of conjugated polymers. Our first attempt involved coupling the simple commercially available 1,4-phenyldiboronic acid. Self-reinforced poly paraphenylenes (SRPs) have been commercially useful in hard thermoplastics because of their rigid rod structure and high thermal stability.3 With our approach, the resulting reaction affords 5–8-mers of polyparaphenylene. We were able to observe their mass distribution by MALDI-TOF MS, and their physical appearance and fluorescence matched well with literature reports.3
Encouraged by this result, we decided to broaden the scope of this polymerization method by coupling fluorene monomers. Polyfluorenes are commonly used as conjugated polymers for optoelectronic materials.5 The commercially available 9,9-dioctylfluorene-2,7-diboronic acid bis(1,3-pro-panediol) esters coupled effectively to yield polyfluorenes (PFs) with an Mn of 7.9 kDa and Đ of 2.2 in good yields (Figure 3). Similar to methyl boronic acid, boric acid was used as an additive to facilitate the in situ hydrolysis of the boronate esters.15 To our knowledge, this is the first report of polyfluorenes being created by simply mixing the respective ingredients in an open vessel (Figure 4).
Figure 3.
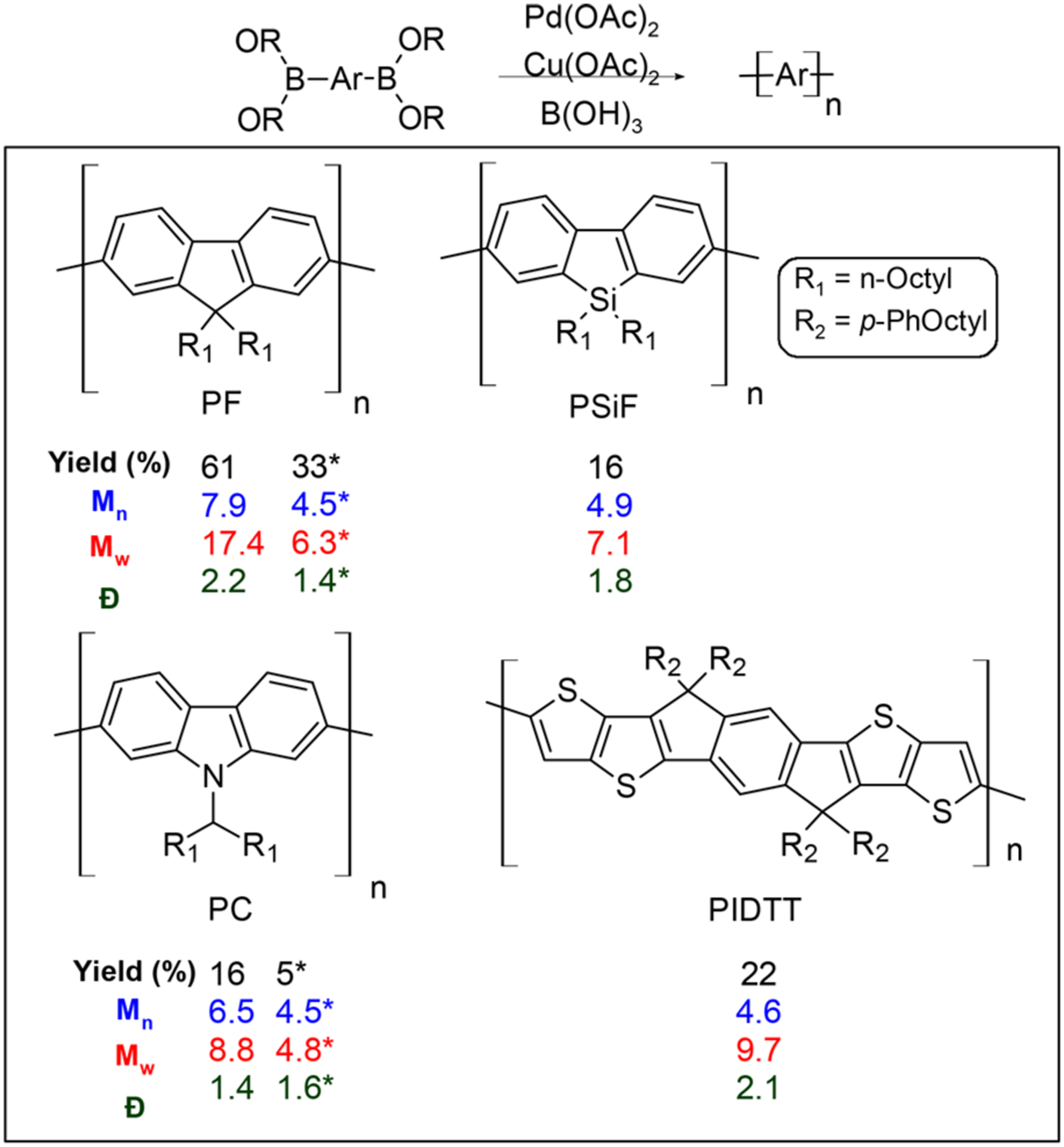
Synthesis of homopolymers via multimetallic homocoupling methods using Pd(OAc)2 (5 mol %) Cu(OAc)2 (10 mol %), B(OH)3 (3 equiv), DMSO, rt, air, 48 h; (inset) polymer structure, molecular weights, and yield. * Synthesis of polymers at Pd(OAc)2 (2 mol %) Cu(OAc)2 (5 mol %), B(OH)3 (3 equiv), DMSO, rt, air, 48 h. Reactions (2 mL) were run at 0.09 M.
Figure 4.

Benchtop polymerizations—wet solvents open to the air at room temperature. Simply mix, stir, and wait.
To further extend the scope, heterocyclic aromatic systems were explored. Polycarbazoles and polysilafluorenes are used as donor materials in photovoltatic devices and light emitting diodes.6 Using our method, polycarbazole (PC) and polysilafluorene (PSiF) were successfully synthesized at room temperature from the analogous diboronate esters. The isolated polymers of PC and PSiF had an Mn of 6.5 and 4.9 kDa and Đ of 1.4 and 1.8, respectively. However, a majority of colored material (70–80%) was insoluble and remained in the Soxhlet thimble after extraction. Therefore, we believe the insoluble materials to be higher molecular weight polymer. In addition, the low molecular weight polymers likely contain trace metals because they were not filtered through silica for fear of losing polymer in the process.
In the case of PC, the yields of soluble polymer have not been previously reported.4 Instead, only the crude yield, a combination of soluble and insoluble PC, has been reported. Furthermore, the soluble portion of PC was previously reported as an Mn of 2 kDa, n = 12, while our method affords a soluble polymer with Mn of 6.5 kDa, n = 16. This confirms that the yields are primarily limited by the solubility of the resulting homopolymers. The successful synthesis of commercially valuable heterocyclic polymers, such as PC and PSiF, on the benchtop speaks for the potential utility of this method with different aromatic polymers.
Polythiophenes are commonly used in materials for their optoelectronic properties.16 However, polythiophenes are challenging to synthesize. The aromatic 6,6,12,12-tetrakis(4-hexylphenyl)-6,12-dihydro-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b]dithiophene (IDTT) is used as electron acceptor materials. Using our method, we were able to create an IDTT polymer with an Mn of 4.6 kDa and Đ of 2.1. There are many examples of copolymers and small molecules that contain IDTT,16 but we could not find any reports of the PIDDT homopolymer in the literature. Therefore, this cocatalyst system enables access to new sulfur-rich materials as well, by simply stirring in a vial on the benchtop at room temperature (Figure 4). Overall, PF, PSiF, PIDTT, and PC could be made in meaningful quantities using this cocatalytic strategy.
In summary, using Pd(II) and Cu(II) cocatalysis, functionally rich biaryl small molecules can be quickly made on a benchtop open to the air with wet solvents. To our knowledge this is the only methodology that also allows for the generation of biaryl conjugated polymers in air, with moisture, at ambient temperature. With this methodology we achieved benchtop access to previously reported conjugated polymers PF, PSiF, and PC with the regioselective aromatic coupling. Then we used this chemistry to create a new polymer, PIDTT. Therefore, we have introduced a bimetallic aerobic method to increase the accessibility of functionally rich aromatic dimers and commercially valuable soft materials.
Supplementary Material
ACKNOWLEDGMENTS
This research was primarily supported by the National Science Foundation through the Center for Dynamics and Control of Materials: an NSF MRSEC under Cooperative Agreement No. DMR-1720595. The IRACDA fellowship program (NIH 1K12GM102745) supported M.B.M. NMR spectra were taken with the Bruker Avance III 500 which was provided by NIH Grant Number OD021508-01. Further, we acknowledge the Welch Regents Chair to E.V.A. (F-0046), a Welch Foundation grant to N.A.L. (F-1904), and a Welch Foundation grant to the PVAMU Department of Chemistry (L-0002).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.orglett.1c00479
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.1c00479.
Additional information concerning preparatory methods, characterization and spectrum (NMR, GC-MS, LC-MS, and MALDI-TOF, GPC) (PDF)
Contributor Information
Matthew B. Minus, Department of Chemistry, Prairie View A&M University, Prairie View, Texas 77446, United States; Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States.
Sarah R. Moor, Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States.
Fathima F. Pary, Department of Chemistry, Oklahoma State University, Stillwater, Oklahoma 74078, United States
L. P. T. Nirmani, Department of Chemistry, Oklahoma State University, Stillwater, Oklahoma 74078, United States
Malgorzata Chwatko, McKetta Department of Chemical Engineering, University of Texas at Austin, Austin, Texas 78712, United States.
Brandon Okeke, Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States.
Josh E. Singleton, Department of Chemistry, Prairie View A&M University, Prairie View, Texas 77446, United States
Toby L. Nelson, Department of Chemistry, Oklahoma State University, Stillwater, Oklahoma 74078, United States.
Nathaniel A. Lynd, McKetta Department of Chemical Engineering, University of Texas at Austin, Austin, Texas 78712, United States.
Eric V. Anslyn, Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States.
REFERENCES
- (1).Bach C; Dauchy X; Chagnon M-C; Etienne S Chemical Compounds and Toxicological Assessments of Drinking Water Stored in Polyethylene Terephthalate (PET) Bottles: A Source of Controversy Reviewed. Water Res. 2012, 46 (3), 571–583. [DOI] [PubMed] [Google Scholar]
- (2).Hazell P Armour: Materials, Theory, and Design, 1st ed.; CRC Press: 2015. [Google Scholar]
- (3).Shacklette LW; Eckhardt H; Chance RR; Miller GG; Ivory DM; Baughman RH Solid-state Synthesis of Highly Conducting Polyphenylene from Crystalline Oligomers. J. Chem. Phys 1980, 73 (8), 4098–4102. [Google Scholar]
- (4).Lévesque I; Bertrand P-O; Blouin N; Leclerc M; Zecchin S; Zotti G; Ratcliffe CI; Klug DD; Gao X; Gao F; Tse JS Synthesis and Thermoelectric Properties of Polycarbazole, Polyindolocarbazole, and Polydiindolocarbazole Derivatives. Chem. Mater 2007, 19 (8), 2128–2138. [Google Scholar]
- (5).Xie L-H; Yin C-R; Lai W-Y; Fan Q-L; Huang W Polyfluorene-Based Semiconductors Combined with Various Periodic Table Elements for Organic Electronics. Prog. Polym. Sci 2012, 37 (9), 1192–1264. [Google Scholar]
- (6).Jin G; Xia L; Liu Z; Lin H; Ling J; Wu H; Hou L; Mo Y Highly Efficient and Stable Blue Polymer Light Emitting Diodes Based on Polysilafluorenes with Pendent Hole Transporting Groups. J. Mater. Chem. C 2016, 4 (5), 905–913. [Google Scholar]
- (7).Martin R; Buchwald SL Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions Employing Dialkylbiaryl Phosphine Ligands. Acc. Chem. Res 2008, 41 (11), 1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Yuan C; Hong J; Liu Y; Lai H; Fang Q Pd(II)-Catalyzed Base-Free Oxidative Homocoupling of Fluorene Diboronic Acid Ester: A New Approach for the Synthesis of π-Conjugated Polymers. J. Polym. Sci., Part A: Polym. Chem 2011, 49 (18), 4098–4101. [Google Scholar]
- (9).Davidson JM; Triggs C Reaction of Metal Ion Complexes with Hydrocarbons. Part I. ‘Palladation’ and Some Other New Electrophilic Substitution Reactions. The Preparation of Palladium(I). J. Chem. Soc. A 1968, No. No. 0, 1324–1330. [Google Scholar]
- (10).Smith KA; Campi EM; Jackson WR; Marcuccio S; Naeslund CGM; Deacon GB High Yields of Symmetrical Biaryls from Palladium Catalysed Homocoupling of Arylboronic Acids under Mild Conditions. Synlett 1997, 1 (1), 131–132. [Google Scholar]
- (11).Wang D; Weinstein AB; White PB; Stahl SS Ligand-Promoted Palladium-Catalyzed Aerobic Oxidation Reactions. Chem. Rev 2018, 118 (5), 2636–2679. [DOI] [PubMed] [Google Scholar]
- (12).Chauhan P; Ravi M; Singh S; Raju KSR; Bajpai V; Kumar B; Wahajuddin; Yadav, P. P. Palladium and Copper-Catalyzed Ligand-Free Coupling of Phenylhydrazines in Water. RSC Adv 2014, 4 (82), 43336–43340. [Google Scholar]
- (13).Adamo C; Amatore C; Ciofini I; Jutand A; Lakmini H Mechanism of the Palladium-Catalyzed Homocoupling of Arylboronic Acids: Key Involvement of a Palladium Peroxo Complex. J. Am. Chem. Soc 2006, 128 (21), 6829–6836. [DOI] [PubMed] [Google Scholar]
- (14).Kirai N; Yamamoto Y Homocoupling of Arylboronic Acids Catalyzed by 1,10-Phenanthroline-Ligated Copper Complexes in Air. Eur. J. Org. Chem 2009, 2009 (12), 1864–1867. [Google Scholar]
- (15).Hinkes SPA; Klein CDP Virtues of Volatility: A Facile Transesterification Approach to Boronic Acids. Org. Lett 2019, 21 (9), 3048–3052. [DOI] [PubMed] [Google Scholar]
- (16).Lin Y; Zhao F; He Q; Huo L; Wu Y; Parker TC; Ma W; Sun Y; Wang C; Zhu D; Heeger AJ; Marder SR; Zhan X High-Performance Electron Acceptor with Thienyl Side Chains for Organic Photovoltaics. J. Am. Chem. Soc 2016, 138 (14), 4955–4961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


