Abstract
Background
Cancer is the world's second leading cause of death, but a significant advancement in cancer treatment has been achieved within the last few decades. However, major adverse effects and drug resistance associated with standard chemotherapy have led towards targeted treatment options.
Objectives
Transforming growth factor‐β (TGF‐β) signaling plays a key role in cell proliferation, differentiation, morphogenesis, regeneration, and tissue homeostasis. The prime objective of this review is to decipher the role of TGF‐β in oncogenesis and to evaluate the potential of various natural and synthetic agents to target this dysregulated pathway to confer cancer preventive and anticancer therapeutic effects.
Methods
Various authentic and scholarly databases were explored to search and obtain primary literature for this study. The Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) criteria was followed for the review.
Results
Here we provide a comprehensive and critical review of recent advances on our understanding of the effect of various bioactive natural molecules on the TGF‐β signaling pathway to evaluate their full potential for cancer prevention and therapy.
Conclusion
Based on emerging evidence as presented in this work, TGF‐β‐targeting bioactive compounds from natural sources can serve as potential therapeutic agents for prevention and treatment of various human malignancies.
Keywords: apoptosis, cancer, in vitro, in vivo, natural products, proliferation, TGF‐β signalling, treatment
TGF‐β is a superfamily of evolutionarily conserved and growth‐inhibitory cytokines that control pleiotropic cellular functions.
TGF‐β signalling plays a key role in cell proliferation, differentiation, morphogenesis, regeneration and tissue homeostasis implicated in cancer initiation and progression.
Crosstalk of TGF‐β signalling with other pathways is comprehensively discussed.
Various bioactive natural compounds modulate the TGF‐β signalling pathway to confer potential cancer preventive and therapeutic activities.

1. INTRODUCTION
Hippocrates, the father of modern medicine, coined the word ‘cancer’ and utilised the Greek terms ‘carcinoma’ and ‘karakinos’ to describe tumours. 1 Cancer is a global health problem, frequently observed in both developed and developing nations; however, lately, there has been a remarkable improvement in survival rates of patients due to early detection and improvement in the development of treatment options. 2 Cancer represents a hyperproliferative disease characterised by the transformation of cells, apoptotic dysregulation, proliferation, angiogenesis, invasion and metastasis. 3
Cancer remains a prime reason for mortality, being the second leading cause of death worldwide. About 19.3 million new cases of cancer and around 10.0 million cancer‐related deaths were expected to occur worldwide in 2020. 4 It has been estimated that there were roughly 1 898 160 cases of cancer diagnosis and approximately 608 570 deaths from cancer in 2021 in the United States, 5 which is significantly higher than the earlier prediction of the number of expected cases of cancer in the previous year. 6 Several factors can be attributed to cancer, including physical carcinogens (ultraviolet radiation), chemical carcinogens (asbestos, tobacco and arsenic), and any type of infection from viruses, bacteria, fungi or other parasites. Some other factors include dietary and behavioural problems, low intake of fruits and vegetables, lack of physical exercise and excessive tobacco and alcohol usage. 7
Treatment of cancer has always been a significant challenge, as there is no complete resolution developed yet for cancer treatment, although its risk can be reduced by avoiding exposure to carcinogens. Although chemotherapy has been standardised and is commonly used for cancer treatment along with surgery, radiation therapy and immunotherapy, often these chemotherapeutic agents can lead to unhealthy levels of toxicity. 8 Natural products have been an excellent source for drug discovery, 9 , 10 as they have more ability than bacteria, fungi, insects and climate to produce structurally diverse primary and specialised compounds. 11 Natural products are the reservoir of phytoconstituents possessing substantial chemoprotective and anticancer properties and are components of over 60% of currently available anticancer drugs. 12 It is also noteworthy that ‘combination chemotherapy’, which is widely useful for effective cancer treatment, utilises both natural and synthetic agents. 9 Several potent plant‐based chemotherapeutic agents such as vinca alkaloids, taxols, epothilones and podophyllotoxins are used commercially. 13 , 14 Additionally, there are many different compounds, including curcumin, resveratrol, epigallocatechin gallate (EGCG), genistein, quercetin, lycopene, apigenin, gallic acid, sulforaphane and ursolic acid, which exhibit significant therapeutic effects against cancer. 8 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25
Various phytoconstituents have gained special attention and provided a novel avenue for cancer treatment through targeting different cross‐linked pathways, such as oxidative stress, mutagenesis, inflammation, apoptosis and autophagy, as well as several signalling intermediaries, including cancer metabolism. 26 , 27 , 28 Out of the various other pathways, some prominent therapeutic targets for plant secondary metabolites are the mammalian target of rapamycin (mTOR), phosphatidylinositol 3‐kinases (PI3K), protein kinase B (or Akt), hypoxia‐inducible factor‐1α (HIF‐1α) and extracellular signal‐regulated kinase (ERK), 29 nuclear factor‐κB (NF‐κB), activator protein 1, 30 c‐Jun NH2‐terminal kinases (JNKs), mitogen‐activated protein kinase (MAPK), tumour necrosis factor (TNF) receptor‐associated factor (TRAF), TNF receptor 1‐associated death domain protein, Janus kinase (JAK) and the signal transducer and activator of transcription (STAT). 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35
The transforming growth factor‐β (TGF‐β) signalling pathway plays a crucial role in various cellular responses; misregulation of this pathway often leads to tumour progression. 36 The regulatory cytokine TGF‐β has tumour‐suppressive effects, which can be evaded by cancer cells through malignant evolution. TGF‐β can modify various processes, for example, immune regulation, cell invasion and alteration of microenvironments, which may be exploited by cancer cells for their own advantage. 37
TGF‐β is involved in various biological functions, including embryonic stem cell differentiation and self‐renewal, homeostasis differentiation in cells, immune suppression and the promotion of cancer development. 38 The characterisation of the TGF‐β signalling pathway is well‐defined. The cell membrane is the location where TGF‐β binds to its receptor and thus initiates a signalling cascade by phosphorylating small mothers against decapentaplegic homolog 2 and 3 (Smad2/3). Afterwards, the phosphorylated Smad2/3 attaches to Smad4, and translocation of the cytoplasmic complex occurs towards the nucleus for activation of transcription of end effectors, including p21, p15 and the parathyroid hormone‐related protein. 39 , 40
Several prior reviews are available on different features of TGF‐β signalling. In this line, a review by Lee et al., 41 published in 2013, was based on the pros and cons of affecting the TGF‐β pathway during the progression and development of cancer by various natural products. This work was mainly focused on the alteration of gene‐specific DNA methylation levels by natural products in different target tissues and systemic circulations. Another review by Markov et al. 42 focused on natural triterpenoids and their semi‐ and synthetic‐derivatives on various tumour‐related signalling pathways, including TGF‐β and human epidermal growth factor receptor. A third review published by Farooqi et al. 43 analysed the role of single phytoconstituent EGCG on the regulation of various deregulated signalling pathways, including TGF‐β, in cancer.
Despite the aforementioned reviews, 41 , 42 , 43 an updated and comprehensive evaluation of the information is still lacking due to the effect of the broader categories of natural products on TGF‐β signalling pathways during tumourigenesis. The available information is limited to drawing conclusive insight into the role of natural and synthetic agents targeting TGF‐β in cancer therapeutics. Therefore, we aim to provide for the first time a comprehensive and critical review of the natural products as well as the synthetic agents that act on TGF‐β along with their mechanistic attributes. The prime objective of this review is to decipher the role of TGF‐β in oncogenesis and to evaluate the potential of various natural and synthetic agents to target this dysregulated pathway to confer cancer preventive and anticancer therapeutic effects. In addition, the review provides insight into the omics approaches associated with TGF‐β in oncology as well as comprehensive preclinical and clinical studies pertaining to various natural products against different cancers.
2. TGF‐β RECEPTORS AND SIGNALLING PATHWAY
TGF‐β is a superfamily of evolutionarily conserved and growth‐inhibitory cytokines that control pleiotropic cellular functions. 44 In normal and primary cancer cells, TGF‐β suppresses the tumour by inducing apoptosis over proliferation. 39 However, it also encourages tumour metastasis via epithelial‐mesenchymal transition (EMT) stimulation, invasion, migration, chemoattraction and cell adhesion. 45
2.1. TGF‐ß receptors
There are three receptor types, namely, Type Ⅰ (TβRI), Type Ⅱ (TβRII) and Type III (TβRIII), along with seven human type Ⅰ receptors, and five Type Ⅱ receptors; Type III is the most abundant type. Different members of the TGF‐β family bind to a combination of the Types Ⅰ, Ⅱ and III receptors. The TGF‐β isoforms, that is, TGF‐β1, TGF‐β2 and TGF‐β3, usually bind to the Type Ⅱ receptor. Their role is crucial, as they exert both promoter and terminator properties. In normal cells, they indicate tumour‐suppressive features, and in malignant cells, they display tumour‐promoting roles. 46
TGF‐β Type III is the most abundant type of TGF‐β ligand that is transferred to the signalling receptors TβRI and TβRII, which are occupied with serine/threonine protein kinases at their intracellular domains. 47 Upon binding of TGF‐β ligand to TβRIII, it either binds to TβRII straight away or presents TGF‐β to TβRII. 48 Once the TGF‐β ligand binds to the TβRII receptor, it is auto‐phosphorylated and constitutively transphosphorylases the TβRI GS domain, thus stimulating the activity of protein kinase. Activated receptors work with adaptors or cofactors to induce transcriptional activity. TGF‐β through the non‐Smad pathway binds with different cytokines, such as JNK, p38, ERK1/2 and PI3K, which promotes transcriptional activity. 49
Canonical TGF‐β signalling takes place upon binding of one of the three ligands to TGFBR2, which phosphorylates TGFBR1. Then, this TGFBR1 phosphorylates downstream Smad2 and Smad3 on the carboxy terminus (pSmad2/3C) of a serine residue. Subsequently, it recruits Smad4 and translocates into the nucleus where it controls the transcription of TGF‐β target genes. Upon binding of one of three ligands to TGFBR2, it phosphorylates and recruits TGFBR1, resulting in canonical TGF signalling. Consequently, the downstream Smad2 and Smad3 are phosphorylated by the action of TGFBR1 on a serine residue at its pSmad2/3C (carboxy terminus), which attracts Smad4 and translocates towards the nucleus, where it modulates the transcription of TGF‐targeted genes. 46
A chimeric receptor model was used to gather more insight. TGFBRs are continuously recycled in the absenteeism of ligands, and ligand interaction only causes heteromeric receptors (TGFBR1/TGFBR2) to be degraded, while homomeric receptors (TGFBR1/TGFBR1) are recycled again to the plasma membrane. 50
2.2. Secretion of TGF‐β
Several cells, including macrophages, secrete TGF‐β. It is secreted either through cancer cells or via cells present on the local stroma, and the attribution of TGF‐β‐induced anticancer T‐cell immunity inhibition can be an adverse event for the host. 51 Serum proteinases, for instance, plasmin, accelerate TGF‐β release from the complex. This process takes place on the macrophages (surface) in which the latent TGF‐β complex is bound to CD36 by its ligand, thrombospondin‐1. The active TGF‐β release is enhanced by inflammatory stimuli, which are responsible for the activation of macrophages via plasmin activation. 52 The synthesis of TGF‐β ligands takes place as precursor proteins with a longer N‐terminal pro‐peptide and a shorter mature C‐terminal polypeptide. 53
2.3. Biology and functions of TGF‐β
TGF‐β displays various biological activities, such as chemotaxis, extracellular matrix synthesis, cell differentiation and angiogenesis. It also exhibits growth inhibition of various cells, including endothelial, epithelial and lymphocytic cells. It induces the proliferation of mesenchymal cells, such as fibroblasts. Additionally, it has also been associated with several pathophysiological processes comprising tissue fibrosis, wound repair, morphogenesis and immunosuppression. These bioactivities are usually carried out through specific cell surface receptors, TβR‐I and TβR‐II. 54 , 55 TGF‐β plays dualistic roles in tumourigenesis. 56 In the initial stages, it inhibits cellular growth by blocking tumourigenesis and acts as a tumour suppressor through the regulation of EMT and cell migration. It usually inhibits cell division via cell cycle arrest at the G1 phase. This further enhances the cyclin‐dependent kinase (CDK) inhibitors expression, namely, p15 and p21, with successive c‐Myc suppression, which is a multifunctional oncogene and considered to be present in various human cancers. When it functions as a tumour promoter, it overexpresses itself and leads to invasiveness, cell proliferation and enriched metastatic potential. This is due to regulation in the levels of EMT, angiogenesis and immunosuppression. 57
TGF‐β dysfunctions lead to diseases such as cancer, connective disorders and fibrotic diseases. 58 The development of TGF‐β chimaera has emerged recently as a potential approach due to the presence of high‐affinity TGF‐β ligands for their receptors through rearranging, mixing and mutating their binding epitopes. 58 , 59
2.4. Regulation of TGF‐β
The TGF‐β signalling pathway is largely involved in the cellular process. They have been observed to possess both positive and negative effects by several mechanisms. TGF‐β plays an influential, multifunctional role in cell differentiation and proliferation; however, at different stages, there is a different response of TGF‐β within production, secretion, activation and signalling. In the nucleus, it regulates gene expression by binding with two types of receptors in the cell membrane with intrinsic serine/threonine kinase activity, which is then conducted by intercellular Smad proteins. These are transcription factors that regulate gene expression 60 as shown in Figure 1. The R‐Smad/Co‐Smad complex attaches with other nuclear cofactor proteins in the nucleus, and the process of gene transcription starts. 60 , 61 In the epithelial cells, TGF‐β is believed to function as a regulatory tumour suppressor factor of utmost importance due to its effect of early inhibition of proliferation and apoptosis induction. 62
FIGURE 1.
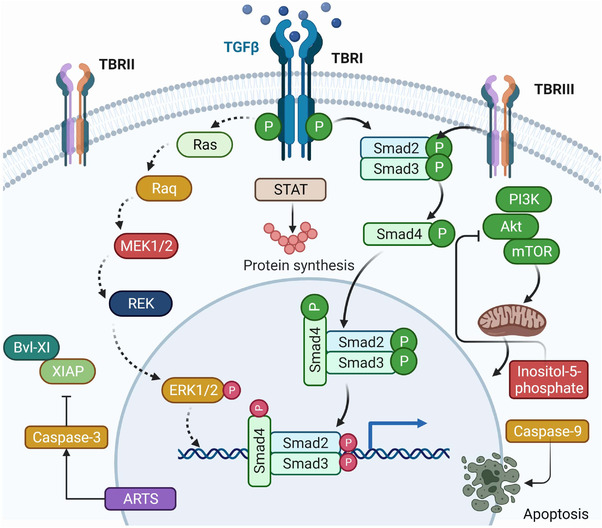
Figure showing molecular pathways involving the transforming growth factor‐β (TGF‐β) role in cancer. The TGF‐β signalling pathway regulates embryogenesis, cell homeostasis, proliferation, differentiation and death. TβRI, TβRII and TβRIII—TGF‐β receptor types—serine/threonine kinases induce heterotetrameric receptor complexes. TGF‐β can carry out apoptosis through small mothers against decapentaplegic (Smad)‐dependent and ‐independent pathways. TGF‐β also follows a non‐Smad pathway where it binds with different cytokine‐like p38, c‐Jun N‐terminal kinases, extracellular signal‐regulated kinase and phosphoinositide 3‐kinase and promotes transcriptional activity. In the nucleus, it regulates gene expression by binding with two types of receptors in the cell membrane with intrinsic serine/threonine kinase activity which is then conducted by intercellular Smad proteins. These are transcription factor that regulates gene expression. Created with BioRender.com
3. ROLE OF TGF‐β IN TUMOURIGENESIS
3.1. Proliferation
TGF‐β is quite efficient in suppressing the proliferative activity of cancer cells in multiple ways. As normal epithelial cells and haematopoietic cells multiply by targeting CDKS, TGF‐β either inhibits CDKS directly by suppressing further proliferation of cells, or it upregulates expression of CDKS inhibitors, such as p15ink4b, p27kip1 and p21cip1. This process inhibits the CDKS complex and causes G1 stage cell cycle arrest. TGF‐β can even inhibit CDK4, which is needed for the progression from the G1 stage to S phase of the cell cycle. Directly inhibiting the cell cycle, it even suppresses the activity of c‐Myc, which inhibits the action of CDKS inhibitors and leads to a reduction in cell proliferation. 49
TGF‐β, or the TGF‐β/Smad4 signalling pathway in particular, regulates the process of signal transduction from cell membrane to the nucleus. it is also accountable for a broad array of cellular processes, such as differentiation, proliferation, migration and apoptosis. In addition, it is also responsible for the onset and progression of cancer. 63 As mentioned previously, TGF‐β protein plays a dual role in tumourigenesis. During the initial stages of tumourigenesis, TGF‐β plays a suppressive role. Afterwards, with the tumour progression, cancer cells slowly become resistant, and finally the TGF‐β protein augments immunosuppression of the tumour and facilitates invasion, tumour angiogenesis and metastasis. 64
3.2. Differentiation
TGF‐β expresses its differentiating property differently depending upon the stage of cancer, type of tumour and changes in the tumour microenvironment. During the initial cancer stages, TGF‐β plays a tumour‐suppressive role by arresting the cell cycle and stimulating apoptosis. It can display this activity through the generation of CDK inhibitor p21Waf1/Cip1, which is considered the controller of the cell cycle. Cyclin A is a substitute for CDK, and upregulation of cyclin A accelerates the entry of the S phase into NIH3T3 and U937 cells. The complex of cyclin A/CDK is involved in the regulation of progression through the S phase, and it pushes the cell to the G2 phase. Thus, decreasing the expression of cyclin A can stop the cell cycle at the S phase. 65 TGF‐β has been reported to regulate the immunological response and differentiation of T and B lymphocytes, which are associated with the inflammatory cascade in cancer progression at organ and tissue levels. 66
3.3. Apoptosis
TGF‐β can carry out apoptosis through Smad‐dependent and Smad‐independent pathways. Through Smad‐dependent pathways, TGF‐β is involved in the release of pro‐apoptotic proteins, mediated by TGF‐β‐inducible early response genes, which lead to cellular oxidative stress. 67 It also activates the death‐associated protein kinase, 68 which causes apoptosis through mitochondrial cytochrome c release. 68 , 69 through inositol‐5‐phosphatase, it downregulates the pro‐survival PI3K‐Akt pathway, which leads to cell death easily. 69 Through the Smad4‐signalling pathway, the stress‐activated protein kinase/JNK pathway is regulated, which controls the expression of pro‐apoptotic genes, including the Bcl‐2 modifying factor, Bcl‐2 interacting mediator of cell death, Bcl‐2‐associated X protein and caspase‐9. However, cells resistant to TGF‐β for growth inhibition can still react to apoptosis by translocating the mitochondrial apoptosis‐related protein in the TGF‐β signalling pathway to the nucleus, which stimulates caspase‐3 activation and downregulation of Bcl‐xL and X‐linked inhibitor of apoptosis protein expression, leading to apoptosis. 49 , 69 , 70 , 71 , 72
3.4. Migration
TGF‐β increases the migratory property of cancer cells by re‐constructing the cytoskeleton structure of cells through releasing focal adhesion kinase signalling, stress fibre formation and smooth muscle actin expression. 49 The changes associated with EMT allow the cells to lose connection with the epithelial cells and easily migrate the cell from the initial position as well as promote cancer metastasis. Like in prostate cancer, by cytoskeleton rearrangement of Cdc42 and RhoA in a dependent fashion and via increasing the stress fibres and lamellipodia, 73 it promotes the migratory property of cancer cells. 49 , 74 , 75
3.5. Cancer initiation and progression
TGF‐β plays a vital dual function in tumour progression by suppressing and promoting it accordingly. The downregulation of TGF‐β is considered vital for the onset of cancer. When cell proliferation is inhibited due to factors, such as CDK inhibitors or by the secretion of anti‐angiogenic factors and through repressing the expression of c‐Myc, it acts as a tumour suppressor. It plays a role as a tumour promoter by activating matrix deposition and agitating immune function, thus stimulating EMT. EMT is an important factor in cancer progression and is described as suppressed epithelial markers and enhanced mesenchymal markers. TGF‐β not only regulates EMT but is also involved in the metastasis of cancer cells. It is considered as a tumour suppressor in malignant cells; however, it acts as a promoter in the metastasis of cancer cells. 76 TGF‐β’s involvement in cancer microenvironment and its dual roles have been extensively reviewed elsewhere. 66 , 77 , 78 , 79
3.6. TGF‐β downregulation and reactive oxygen species (ROS) production
ROS and TGF‐β both affect cancer progression and tumourigenesis; hence, their mutual effects are very complex. Different cells and tissues produce high amount of ROS through various mechanisms; an increased amount of ROS is a characteristic of all types of cancer as it induces tumour initiation, progression and acts as a secondary messenger for regulating different pathways, such as cell proliferation, survival and apoptosis. 80 ROS can lead to cancer cells’ apoptosis at the molecular level and cause damage to the nucleic acids. 74 , 81
4. CROSSTALK OF TGF‐β WITH OTHER PATHWAYS
The TGF‐β is a superfamily of proteins consisting of TGF‐β1, TGF‐β2 and TGF‐β3 isoforms; they are similar in structure but quite distinct in exerting biological response after interacting with different receptors. 82 Along with TGF‐β, other pathways have roles pertaining to cancer, and they interact differently with receptors and cytokines to produce responses accordingly. Some of the prominent pathways that have interactions with TGF‐ β are presented in this section.
4.1. ERK pathway
MAPK is a superfamily of specific protein kinases that transforms extracellular signals to the nucleus and regulates gene transcription; it has an impact largely on the complex cellular process, comprising cell differentiation, proliferation, transformation and apoptosis. MAPK is constituted of subfamilies, such as ERK, JNK and p38/MAPKs. These subfamilies are associated with protein phosphorylation that modifies the cell behaviour and have an ‘on‐and‐off’ mechanism, which is regulated for the treatment of cancer. However, their activation can be triggered by various extracellular molecules, including TGF‐β cytokines. 83
ERK is one of the major pathways under the MAPK pathway. This pathway is mainly involved in numerous cellular processes, including cell proliferation, differentiation, apoptosis, cell growth and survival. These extracellular signals are conducted and end with the activation of the phosphorylation of proteins. This pathway is activated by RAS/RAF/MEK, which is usually initiated with tyrosine kinase. Even TGF‐β may facilitate the activation of ERK rapidly in cancer cells and normal epithelial cells. 84 In vitro studies showed that the ERK pathway activation was induced by TGF‐β. 85
4.2. p38 pathway
p38, a stress‐induced kinase activated through several stress stimuli, is involved in various cellular processes. However, p38 can be stimulated by TGF‐β through TAK1 activation and TβR I‐TRAF6 interaction. TGF‐β‐induced p38 activation can augment Smad4 sumoylation via the PIAS family of E3 ligase, hence enabling Smad4‐dependent transcription. Both p38 and TGF‐β activation is vital for the activation of transcription of Agc expression. 86 , 87
Interestingly, a crosstalk of TGF‐β, RAC1 and RAC1b on the tumour cells of the breast and pancreas has been demonstrated. 88 TGF‐β is considered a paradox as depending upon the cancer stage, it can act as both an enhancer or a suppressor of cancer cells to invade, migrate and metastasise through the Smad pathway. However, RAC1 and RAC1b proteins have a role in tumourigenesis and overexpression in breast cancer and can be activated by TGF‐β. 88
TGF‐β and the agonist of G‐protein coupled receptor proteinase‐activated receptor‐1 (PAR1) and PAR‐2 are involved in fibrosis and cancer, as well as being involved in the regulation of many processes, such as tumour cell proliferation, migration, invasion and metastasis. TGF‐β provides a regulatory effect on PAR1 expression, and upregulation is associated with enhanced expression of integrin αv and β6 subunits. However, platelet activation, along with the agonist of PAR1, stimulates the release of TGF‐β, which brings EMT in cancer cells. Moreover, TGF‐β and the PAR1 or PAR2 ligand and its receptors interact mutually at different levels of transcription and post‐transcriptional stages. More precisely, they modulate both PAR1 and PAR2 at the transcriptional level. This mutual interaction between PAR1/PAR2 and the TGF‐β signalling leads to feed‐forward loops/brutal cycles of matrix deposition and malignant traits, which aggravate fibrosis and oncogenesis. 89 Hence, it has been found that the TGF‐β can have crosstalks with various other pathways, such as MAPK, P38 and ERK. This can also be examined further for the simultaneous modulation of these pathways from various natural and synthetic agents.
Higher TGF‐β1 expression was implicated in prostate tumour progression in spite of its growth‐inhibitory effect on normal prostate epithelial and carcinoma cells. It was also reported that TGF‐β1 stimulated the G1‐to‐S transition of the cell cycle in the TSU‐Pr1 prostate cancer cell line. PD98059, a MAPK inhibitor, caused blockade of the MAPK pathway, and also reinstated the growth‐inhibitory role of TGF‐β1 in TSU‐Pr1 cells. Moreover, it was also reported that there was a complete inhibition of negative growth regulation through TGF‐β1 by Smad2, Smad3 or Smad4, revealing a Smad‐independent action. In conclusion, it has been opined that by producing autocrine TGF‐1, prostate carcinoma cells with activated Ras/MAPK pathway may have a selective growth advantage. 90 The detailed information on the role of TGF‐β and MAPK in the progression of cancer has been described elsewhere. 91
5. TGF‐β INHIBITORS FOR CANCER THERAPY
5.1. TGF‐β inhibitors
As described earlier, TGF‐β can act as a tumour promoter or suppressor. 39 , 82 , 92 , 93 , 94 , 95 , 96 TGF‐β has a prominent role in cancer via the primary phase tumour suppression of neoplasia, and additionally it promotes progression of tumours and metastasis in later stages. The tumour cell growth suppression through TGF‐β is based on the potential of upregulation of cyclin kinase inhibitors. Several malignant tumours can downregulate or mutate the TGF‐βRII receptor or further abnormalities of the TGF‐β signalling pathway. 97 Several tumours generate large amounts of TGF‐β and are resistant towards its growth inhibition. Simultaneously, TGF‐β generated through tumours can depress antitumour immune responses at cytotoxic T lymphocytes, T‐helper cells, dendritic cells, natural killer (NK), B cells and macrophage levels though enhanced Tr cells. 98 Downregulation of TGF‐βRIII (betaglycan) is also associated with breast cancer progression. 99
During late cancer progression phases, TGF‐β acts as a promoter of metastasis through EMT induction. This leads to the enhancement of the invasion of cancerous cells through induction of gene expression, which eases metastatic colonisation of the secondary organ sites, such as bones, lungs, brains and livers. 37 Despite the fact that information about opposing functions of TGF‐β has existed for a long time within both earlier/later stages of cancer, it is still not clear exactly how and when TGF‐β switches from its role as a tumour suppressor to the metastasis promoter. 78 In light of the prominent effect of the TGF‐β signalling pathway in tumourigenesis and other diseases, efforts have been made to target TGF‐β signalling in tumours, as well as its microenvironment for the development of chemotherapeutic agents. 64 , 100 , 101 , 102 , 103 , 104
5.2. Synthetic TGF‐ β inhibitors
Many TGF‐β signalling antagonists, such as antisense molecules, antireceptor monoclonal antibodies and ligands trap for suppression of the interaction of ligand‐receptor. Inhibitors of TGF‐β receptor kinases aptamers are developed and used in clinical practice. 105 Many TGF‐β pathway inhibitors are approved and used clinically, and a few small molecules are also emerging within new research (Table 1). Small molecule inhibitors are fundamentally designed to inhibit TGF‐β receptors and are dependent upon imidazole scaffolds or dihydropyrrolopyrazole scaffold. Other inhibitors are based on pyrazole, pyrazolopyridine, triazole, imidazopyridine, isothiazole and pyridopyrimidine scaffolds. Out of the small molecule inhibitors, one drug candidate, galunisertib (LY2157299 monohydrate), established by Eli Lilly, is among the most advanced and promising outcomes in two Phase II trials. 106 However, the development of this drug was discontinued by Eli Lilly in January 2020 due to financial reasons.
TABLE 1.
Clinical studies conducted on various synthetic and small molecules as transforming growth factor‐β (TGF‐β) inhibitors studied against different cancers
| Drug | Cancer type | Phase | Patient numbers | Outcome | Reference |
|---|---|---|---|---|---|
| Fresolimumab (GC1008) | Malignant melanoma and renal cell carcinoma | I | 29 | Block TGF‐β activation by neutralising TGF‐β1, TGF‐β2 receptors | 106 , 249 |
| LY3022859, an anti‐ TGF‐ß receptor Type II (TβRII) monoclonal antibody | Advanced solid tumours | I | 14 | Inhibit the activation of receptor‐mediated TGF‐β signalling | 250 |
| Galunisertib | Advanced hepatocellular carcinoma | II | 149 | Decrease in TGF‐β1 and circulating serum α‐fetoprotein and associated with longer survival | 251 |
| Galunisertib + gemcitabine | Pancreatic cancer | II | 104 | Attach to adenosine triphosphate (ATP)‐binding domain of TβR kinases and inhibit receptors’ kinase activity improved overall survival | 252 |
| AP12009 (trabedersen) | High‐grade glioma | IIb | 145 | Found superior risk assessment and positive risk‐benefit | 253 |
| Lanreotide | Pancreatic and intestinal neuroendocrine tumours | III | 88 | Found beneficial and showed antitumour effects | 254 |
| Romidepsin | Relapsed peripheral T‐cell lymphoma | II | 131 | Complete and durable responses with manageable toxicity (approved by Food and Drug Administration) | 255 |
| Dovitinib | Castration‐resistant prostate cancer | II | 44 | Modest antitumour activity with controllable toxicities | 256 |
| M7824 | Advanced solid tumours | I | 600 | Manageable study design and showed efficacy | 257 |
An entirely humanised, high‐affinity monoclonal antibody, XOMA089, for the neutralisation of TGF‐β1 and TGF‐β2, has been co‐developed by Novartis and Xoma. Preclinical evidence revealed that XOMA089 exhibited antitumour activity against the growth of squamous cell neck and head carcinoma as well as breast cancer in preclinical models. Additionally, the results also revealed that XOMA089 may have a synergistic effect with programmed cell death protein 1 inhibition. 106 Another Phase IIb trial was conducted on trabedersen (synthetic TGF‐β2 antisence oligodeoxynucleotide), and temozolomide (or procarbazine/vincristine/lomustine) was conducted on 145 patients with refractory, recurrent glioblastoma multiforme or anaplastic astrocytoma and showed positive results. 107
A few antibodies, ligand traps and vaccines have also been developed/are under development as TGF‐ β inhibitors with new levels of understanding of the promising therapeutic potential for the development of TGF‐ β inhibitors in cancer therapy. Fresolimumab (GC1008), LY2382770 and P144 are some examples that target the TGF‐β1, β2, β3, TGF‐β1 and TβRI/II complexes, respectively. 108 However, all these agents are in Phase I or II trials.
6. NATURAL COMPOUNDS AS CANCER PREVENTIVE AND THERAPEUTIC AGENTS VIA TARGETING TGF‐β
Within the history of drug discovery, various compounds derived from plants have delivered promising effects in cancer treatment. 109 Therefore, the inclusion of natural products in cancer treatment in combination therapy (surgery, radiation and chemotherapy) is an active area of interest. 110 In a recent comprehensive analysis by Newmann and Cragg, 9 the authors clearly indicated natural products as the best options to search for novel agents/active templates that offer tremendous potential for drug discovery for a range of human diseases, including cancer. Several studies have reported that a diverse range of dietary and non‐dietary compounds possess an important role in cancer prevention and therapy. 111
6.1. Methodology for literature search and selection
Various authentic and scholarly databases, such as PubMed, Science Direct, Google Scholar and Scopus, were explored to search and obtain primary literature for this study. The Preferred Reporting Items for Systematic Reviews and Meta‐Analysis criteria 112 was followed for the review (Figure 2). Unpublished results, conference abstracts, books and articles written in other languages than English were excluded. For the literature search, major keywords, such as phytochemicals, natural products, extracts, TGF‐β, cancer, apoptosis, proliferation, signalling pathway, treatment, prevention, in vitro, in vivo and clinical studies, were used in various combinations. In addition, bibliographies of primary literature were studied to gather additional appropriate articles. We have also used the Boolean information retrieval method 113 , 114 using TGF with the ‘AND’ operator. The last search was conducted in August 2021.
FIGURE 2.
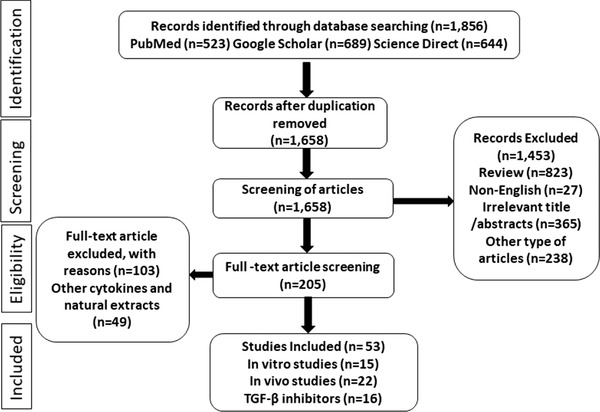
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis diagram showing literature search and selection strategy
6.2. TGF‐β signalling pathway modulation by anticancer phytochemicals
Researchers have performed various experiments to understand the mode of action of several natural products on the TGF‐β signalling pathway in various models of cancer through in vitro and in vivo studies. The following sections summarise the main findings regarding different natural products, which were evaluated for their potential anticancer effects through interference with the TGF‐β pathway.
6.2.1. Arjunolic acid
Arjunolic acid (Figure 3) is a triterpenoid saponin, isolated initially from Terminalia arjuna as well as from other plant species, such as Combretum nelsonii and Leandra chaeton. 115 A study was performed to examine the antitumour effect of 100 and 250 mg/kg of arjunolic acid in mice bearing Ehrlich ascites carcinoma, and it was found that arjunolic acid was able to reduce the volume of tumours as well as decrease their cell count and viability. It was also reported that arjunolic acid increased cellular toxicity. Additionally, arjunolic acid reduced interleukin (IL)‐1β, TNF‐α, TGF‐β and TGF‐β Type I receptors and levels of latency‐linked peptide related with elevated IL‐10. Therefore, arjunolic acid was found to possess antitumour activity against Ehrlich ascites carcinoma cells via enhancement of cytotoxicity and apoptosis. This effect was mediated by the partial blocking of TGF‐βR1 and also by its impact upon the levels of inflammatory cytokine 116 (Table 2). Although the study showed an inhibition of TGF‐β by arjunolic acid, it has not been explored how arjunolic acid caused this effect.
FIGURE 3.
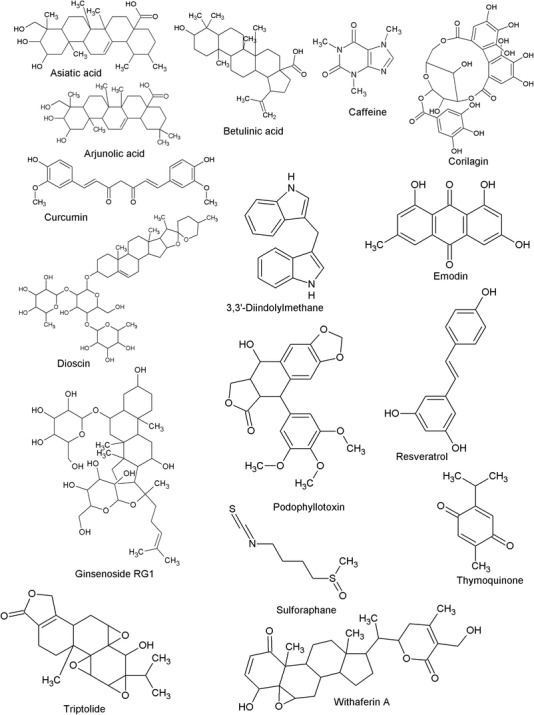
Chemical structures of bioactive phytochemicals affecting TGF‐β signalling in cancer
TABLE 2.
Natural products conferring anticancer effects via regulation of TGF‐β signalling in vitro
| Compound/ extract | Concentration tested | Cell line used (cancer type) | Anticancer effects | Effect on TGF‐β signalling | Reference |
|---|---|---|---|---|---|
| Arjunolic acid | 20–120 μM | Ehrlich ascites carcinoma (breast cancer) | ↑Cytotoxicity, ↑apoptosis | Blocked TGF‐β1R1 | 116 |
| Asiatic acid | 5–80 μM | A549 (lung cancer) | ↓Cell viability | Inhibited TGF‐β1‐induced cell invasion and migration | 119 |
| Betulinic acid | 1–15 μM | RKO and SW480 (colon cancer) | ↓Cell growth, ↑ apoptosis | Downregulated Sp1, Sp3 and Sp4 | 122 |
| Caffeine | .2–.8 mM | MDA‐MB‐231 (breast cancer) | Stabilise active breast stromal fibroblasts and ↓metastatic potential, ↓SDF‐1, and ↓ matrix metalloproteinase 2 (MMP‐2) expression | Suppressed TGF‐β expression | 134 |
| Corilagin | 20–80 μM | SKOv3ip, Hey and HO‐8910PM (ovarian cancer) | Arrested G2/M phase, ↑apoptosis, ↓cytokine, ↓cyclin B1, ↓Myt1, ↓phospho‐cdc 2 | Inhibited secretion of TGF‐β and blocked the TGF‐β‐induced Snail stabilisation | 139 |
| Curcumin | 10 μM | A549 (lung cancer) | ↓Metastasis via miRNA gene network, and ↓ mitogen‐activated protein kinase (MAPK), ↓Wnt signalling pathways. | Downregulated of TGF‐β | 145 |
| Curcumin | 12.5–50 μM | BCPAP cell line (thyroid cancers) | ↑E‐cadherin and ↓vimentin expression and ↓cell attachment, ↓migration, ↓progression, ↓ small mothers against decapentaplegic homolog 2 (Smad2) and Smad3 phosphorylation | Inhibited TGF‐β1 | 147 |
| Curcumin | 10–30 μM/ml | PANC‐1 cell line (pancreatic cancer) | ↓Cell proliferation, ↓cell migration, ↑apoptosis | Inhibited TGF‐β1 signalling pathway, reversed epithelial‐mesenchymal transition (EMT) of TGF‐β1 via Hedgehog signalling pathway | 148 |
| Curcumin + endoxifen + betaestradiol | 8.5–17 μM | MCF‐7 (breast cancer) | ↓Cell viability, ↑vimentin, and ↑E‐cadherin expression. | Endoxifen increased mRNA expressions of TGF‐β1; however, curcumin decreased TGF‐β1 mRNA expressions | 238 |
| Curcumin + emodin | 15–25 μM | SiHa and HeLa (cervical cancer) | ↓Cell migration, ↓EMT markers | Downregulated TGF‐β, ↓TGF‐β receptor II expression; ↓Wnt/β‐catenin signalling in the presence of TGF‐β↓ P‐Smad3, Smad4, ↓cyclin D1, p21 and Pin1 | 146 |
| 3,3′‐Diindolymethane | 10–40 μM | MCF‐7 and HCC38 (breast cancer) | ↓Cell migration, ↓Smad2/3, ↓ extracellular signal‐related kinase 1/2 | Suppressed TGF‐β/ tumour necrosis factor‐α signalling pathway | 159 |
| Dioscin | .5–4 μM | A549 (lung cancer) | ↓Cell growth, and ↓cell proliferation | Suppressed the EMT induced by TGF‐β, ↑ E‐cadherin and N‐cadherins expression induced by TGF‐β | 171 |
| Dioscin (disogenin) | .5–2 μM | HepG2 cells (hepatocellular carcinoma) | ↓Cell division, invasion and migration and ↓MAPK pathway, ↓cell proliferation | Reversed growth‐promoting activity of TGF‐β | 170 |
| Emodin | 15–25 μM | SiHa and HeLa (cervical cancer) | ↓Cell migration, and ↓ EMT markers | Downregulated TGF‐β signalling pathway, ↓TGF‐β receptor II expression; ↓Wnt/β‐catenin signalling pathway in the presence of TGF‐β↓ P‐Smad3, Smad4, ↓cyclin D1, p21 and Pin1 | 146 |
| Ginsenoside Rb2 | 20–40 μM | Ishikawa cell lines and HEC1A (endometrial cancer) | ↓Growth of cells and metastasis, ↑E‐cadherin levels, ↓vimentin | Decreased TGF‐β and Snail levels | 180 |
| Ginsenoside Rb2 | .1 mg/ml | PC3 (prostate cancer) | ↓Cell proliferation and ↓invasion by regulation of cell‐cycle controllers and MMPs | Activated TGFβ receptor signalling | 181 |
| Ginsinoside Rb2 | .1–10 μg/ml | HCT116 and SW620 (colorectal cancer) | ↓EMT, adhesion, growth, metastasis of colorectal cancer cell (CRC) | Inhibited TGF‐β1 expression. Docking simulation showed binding of ginsinoside Rb2 to TGF‐β1 and disrupted TGF‐β1 dimerisation | 179 |
| Ginsinoside Rb2 | 25–100 μg/ml | HNE1 and CNE2 nasopharyngeal carcinoma (NPC) | ↓Invasion and migration ability of NPC cells and the EMT process | Reversed TGF‐β induced morphological conversion and alteration in marker proteins and EMT | 182 |
| Podophyllotoxin | 1.56 μM | Hepatocellular carcinoma cell lines (liver cancer) | ↓Migration and invasion and ↓ MMP. | No effect due to TGF‐β1 but due to p53/ phosphatidylinositol 3‐kinases (PI3K)/ protein kinase B (Akt)/ mammalian target of rapamycin pathway | 186 |
| Resveratrol | 6.25–200 μM | LoVo (colorectal cancer) | ↓EMT | Decreased TGF‐β1; effect produced via TGF‐β1/Smads signalling pathway‐mediated expression of Snail/E‐cadherin | 194 |
| Resveratrol | 5–80 μmol/L | PLA‐802 (rhabdomyo sarcoma) | ↓Cell growth, ↓Smad4 expression at protein and mRNA levels, ↓cells in the S phase, arrested G0/G1 transition | Decreased TGF‐β1 | 195 |
| Resveratrol | 50 μM | Tamoxifen‐resistant MCF‐7 (breast cancer) | Reversed EMT | Suppressed production of endogenous TGF‐β; acts as a chemosensitiser by TGF‐β/Smad signalling, ↓ Smad cascade | 196 |
| Resveratrol | 25 μmol/L | A549, NCI H23 and NCI H460 (lung cancer) | ↓Proliferation by cell cycle arrest, ↑apoptosis, ↓Smad activators, ↓Smad2 and Smad4, ↓mRNA level, ↑Smad7 | Blocked the nuclear signalling of TGF‐ β pathway and altered the intracellular Smad signalling of the TGF‐β | 197 |
| Sulforaphane | 10–80 μM | HepG2 (hepatocellular carcinoma) | ↓Cell invasion, ↓cell migration, ↓cell proliferation | Inhibited TGF‐β induced EMT via ROS‐dependent pathway | 207 |
| Thymoquinone | 5–20 μM | MCF 7 and MDA‐MB‐231 (breast cancer) | ↓Cell proliferation, ↓Bcl2, ↑p53 | Restored the basal level of TGF‐ β | 213 |
| Triptolide | 25–100 nM | HCT116, CRC, HT29 and SW620 (colon cancer) | ↓Cell migration, ↓cell proliferation | Suppressed TGF‐βRI ans II | 219 |
| Withaferin A | .8–1.2 μM | Caski and SK‐Hep1 (metastatic cancer) | ↓Invasive property, ↓migratory ability, ↓MMP‐9 expression via pAkt signalling pathway suppression | Inhibited TGF‐β induced phosphorylation of Akt | 225 |
| Withaferin A | 2 μM | MDA‐MB‐231, MCF‐10A and MCF‐7, cells (breast cancer) | Reversed biochemical features of EMT | Inhibited TGF‐β‐induced EMT and migration | 224 |
| Withania somnifera root extract | 500 nM | MDA‐MB‐231, HCC1806, T47D, MCF‐7, Hs578‐T and MDA‐MB‐468 (breast cancer) | Potential association was found between vimentin expression and cytotoxicity | TGF‐β inhibits vimentin expression at the protein level but not at mRNA level | 228 |
| Andrographis paniculata herb extract | 800 μg/ml | EC‐109 (oesophagal cancer) | ↑Apoptosis, ↓proliferation, ↓metastatic, ↓drug resistance and ↓intercellular adhesion | TGF‐β gave negative feedback | 258 |
| Momordica charantia leaf extract | 25–50 mg/ml | PLS10 cell line (prostate cancer) | ↓Progression by anti‐invasive effects | Induction of TGF‐β has not reported | 259 |
6.2.2. Asiatic acid
Asiatic acid (Figure 3), an ursane‐type pentacyclic triterpenoid molecule, is mainly obtained from Centella asiatica. 117 In a study conducted with asiatic acid in combination with naringenin, a potential inhibition of translation and phosphorylation of Smad3 was shown; however, a restoration of Smad7 expression was also reported. 118 The effect was mediated by the promotion of NK cell differentiation, maturation and cytotoxicity via Id2/IRF2‐associated mechanisms. In addition, naringenin and asiatic acid exhibited an additive‐type effect on TGF‐β1/Smad3 signalling inactivation and reduced lung carcinoma as well as melanoma growth. 118 In another study, asiatic acid increased protein and mRNA expression levels of E‐cadherin, and reduced expression levels of N‐cadherin, snail family transcriptional repressor (Snail) and β‐catenin in A549 cells treated with TGF‐β1, hence inhibiting TGF‐β1‐induced EMT in lung cancer. 119
6.2.3. Betulinic acid
Betulinic acid (Figure 3) is a triterpenoid phytoconstituent reported for the growth inhibition of different tumours. 120 , 121 Betulinic acid was evaluated against prostate cancer using SW480 and RKO colon cancer cells and also by utilising the athymic nude mice xenograft model (Table 3). The study exhibited that betulinic acid has the ability to inhibit tumour growth and induce apoptosis by decreasing the expression of various transcription factors, namely, Sp1, Sp3 and Sp4, as well as decreasing levels of the survivin, p65 subunit of NF‐κB and cyclin D1. 122 It was also reported that betulinic acid did not have the ability to inhibit the production of TGF‐ β but butein did. 117 However, it is notable a substantial amount of studies are not available for understanding the effect of betulinic acid against different cancer models.
TABLE 3.
In vivo anticancer activities of natural products affecting TGF‐β signalling
| Compound/extract | Cancer type | Animal model | Dose (mg/kg) | Anticancer effect | Effect on TGF‐β signalling | Reference |
|---|---|---|---|---|---|---|
| Betulinic acid | Colon cancer | RKO xenograft athymic mice model | 25 | ↓Tumour growth, ↓tumour volume | Reduced expression of Sp1, Sp3 and Sp4 | 122 |
| Ginsenoside Rh2 | Prostate cancer | PC3‐luc glioblastoma imaging by bioluminescence | 1 | ↓Cell growth, ↓cell proliferation and invasion of prostatic cancer | Activated TGFβ signalling | 181 |
| Ginsenoside Rb2 | Colorectal cancer | HCT116 xenograft tumour model | 10 | ↓Tumour volume, ↓tumour growth | Inhibition of EMT and inhibited TGF‐β1, TGFβRI and TGFβRII | 179 |
| Polyphyllin | Gastric cancer | GC7901/DDP xenograft studies | 1 | ↓Tumour growth, ↓tumour weight | Antagonised the facilitative effects of TGF‐β1 | 226 |
| Resveratrol | Colorectal cancer | LoVo cells in vivo imaging in mice by tail vein injection | 150 | Reduced metastatic lesions | Inhibited TGF‐β1 effects on EMT | 194 |
| Resveratrol | Lung cancer | MDA231 xenograft‐bearing mouse model | 40 | ↓Lung metastasis of breast cancer, ↓tumour growth | Inhibited EMT induced by TGF‐β1‐via PI3K/Akt, Smad and MMP regulation | 198 |
| Sulforaphane | Liver cancer | HepG2 xenograft mouse tumour model | 50 | ↓Tumour growth, ↓tumour volume ↓Proliferation, migration and invasion, ↓EMT via reactive oxygen species‐dependent pathway | Suppressed TGF‐β‐induced EMT | 207 |
| Thymoquinone | Hepatocellular carcinoma | Thioacetamide induced liver cancer | 20 | ↓Growth and progression through ↓ oxidative stress, induction of TNF‐related apoptosis‐inducing ligand (TRAIL)‐mediated apoptosis | Decreased hepatic TGF‐β1 mRNA level by 1.8‐fold | 214 |
| Thymoquinone | Renal cell carcinoma | Tumour xenograft model male C57BL/6 on mice | 10 and 20 | Prevents 786‐O‐SI3 cells transfer to lungs in mice,↓invasion, ↓cell movement and production, cell adhesion and cytoskeletal reorganisation | Suppressed TGF‐β1 | 215 |
| Triptolide | Melanoma | Melanoma bearing mice model | .15 | ↓Tumour growth by reduction in proportion of regulatory T cells and Foxp3 level in lymph node and spleen in tumour‐bearing mice | Decreased TGF‐β, IL10 and vascular endothelial growth factor | 221 |
| Withaferin A | Breast cancer | MMTV‐neu transgenic mouse model using female athymic mice | 4 | ↓Growth of cancer cells and migration with ↓vimentin protein expression | Reversed the EMT induced by TGF‐β | 224 |
| Withania somnifera root extract | Breast cancer | Human xenograft and mouse mammary carcinoma models | 4, 8 | ↓Motility, ↓invasion of cancer cells and disturbed vimentin morphology and ↓tumour volume | Inhibited EMT induced by TGF‐β | 228 |
TGF‐receptors are translocated from lipid raft/caveolae microdomains to non‐caveolae microdomains by betulinic acid, with no change in total TGF‐receptor levels. TGF‐receptor translocation mediated by betulinic acid is rapid, and it correlates with TGF‐driven plasminogen activator inhibitor‐1 (PAI‐1) reporter gene activation and growth inhibition in Mv1Lu cells. The betulinic acid‐induced TGF‐β receptor translocation is also rapid and correlates with the growth inhibition and TGF‐β‐induced PAI‐1 reporter gene activation in Mv1Lu cells. 54
Another study on a bioactive subfraction from Mesua ferrea stem bark was performed on HCT 116 colon cancer cells. The betulinic acid fraction was the major active component examined. This study has reported that multiple pathways were involved in the anticancer activity of the standardised fraction. The mechanism behind the effect was attributed to the downregulation of Wnt, epidermal growth factor receptor and HIF‐1α along with a simultaneous upregulation of TGF‐β, p53 and Myc/Max signalling pathways. 123 This shows that betulin shows its anticancer effect by simultaneous modulation of multiple pathways, including TGF‐β signalling.
6.2.4. Caffeine
Caffeine (Figure 3) is naturally found in over 60 different plants as well as various beverages and food items, including tea, chocolate, cocoa beans, kola nuts and guarana berries. 124 Caffeine was found potentially effective in the treatment of many cancers, such as colon, bladder and pancreatic cancers. 125 , 126 , 127 , 128 It is easily available as an over‐the‐counter drug for pain relief and also possesses anti‐inflammatory and analgesic effects. 129 , 130 Caffeine suppresses oxidative stress and inflammation, improves insulin sensitivity, inhibits angiogenesis and has a protective role in liver cancer. 131 Various studies convey that consumption of caffeine reduces the risks of different types of cancer as it affects cell cycle progression, influences apoptosis and inhibits angiogenesis and migration by antagonising adenosine receptors. 132
In recent years, caffeine has emerged as a potentially active compound for its preventive and therapeutic role in cancer. Mechanistically, caffeine affects the progression of the cell cycle at checkpoints, persuades apoptosis and hinders drug efflux from cells. 131 , 132 , 133 Caffeine showed potent anticancer effects through TGF‐β, as it upregulated various tumour suppressor proteins, such as p16, p21, p53 and caveolin‐1in breast stromal fibroblasts. It also inhibited the metastasis of cancer‐associated fibroblast cells (CAF) through phosphatase and tensin homolog‐dependent ERK1/2 and by Akt activation. It inhibited the release of vascular endothelial growth factor (VEGF) A in CAF cells and inhibited the pro‐angiogenic activity of breast cancer cells. 134
Caffeine inhibits TGF‐β‐induced CTGF production in rat hepatocytes via stimulation of TGF‐β effector Smad2 degradation, inhibiting phosphorylation of Smad3, and PPARγ‐receptor upregulation. 135 Caffeine is able to upregulate various tumour suppressor proteins, such as p21, p16, p53 and Cav‐1, and is also able to reduce expressions of IL‐6, SDF‐1, TGF‐β and matrix metalloproteinase 2 (MMP‐2), as well as downregulate α‐SMA. The study has concluded that caffeine may suppress the pro‐carcinogenic actions of active stromal fibroblasts, making it a safe and efficient way to prevent breast tumour growth and recurrence. 134 A similar purine alkaloid to caffeine, theacrine, was reported to attenuate EMT, cell adhesion, invasion and migration induced by TGF‐β in MDA‐MB‐231 cells. The results indicated that theacrine inhibited breast cancer cell metastasis through EMT process reversal. 136
In a clinical study, after 5 months of green tea and chemotherapy administration, the TGF‐β and IL‐10 serum levels decreased in patients and control groups during the green tea intake period. Green tea appears to be able to modify circulating Tregs in chronic lymphocytic leukaemia patients in the early stages of the disease. This can help to keep lymphocytosis under control while also preventing disease development. 137
6.2.5. Corilagin
Corilagin (β‐1‐O‐galloyl‐3,6‐(R)‐hexahydroxydiphenoyl‐d‐glucose, Figure 3) is an ellagitannin compound isolated from Phyllanthus niruri L. as well as other plants, such as Caesalpinia coriaria. Corilagin has been recently examined for several therapeutic activities, including cancer. 138 Corilagin was studied against ovarian cancer cell lines, namely, SKOv3ip, HO‐8910PM and Hey, and also in a xenograft tumour model. 139 The study demonstrated that corilagin has a specific targeting effect on TGF‐β secretion, and thus was able to block non‐canonical ERK/Akt and canonical Smad pathway activation.
When evaluated in ovarian cancer cell lines (OC316, OVCAR5 and HOPMSnail), corilagin treatment was able to downregulate Snail expression. TGFβ‐enhanced Snail was also blocked in HOPM‐Snail cells. Thus, it was revealed that corilagin not only showed its effect by acting via distinct pathways, such as Snail inhibition but also as an apoptosis inducer, such as chemotherapeutic drugs. 140 In another similar study, corilagin inhibited TGF‐β secretion into the culture supernatant of different ovarian cancer cell lines and inhibited TGF‐β‐induced Snail stabilisation. TGF‐β production was not reduced in cancer cells treated with paclitaxel, demonstrating that corilagin specifically targeted TGF secretion. 139
6.2.6. Curcumin
Curcumin ((1E,6E)‐1,7‐bis(4‐hydroxy‐3‐methoxyphenyl)‐1,6‐heptadiene‐3,5‐dione Figure 3) is extracted from the rhizome of Curcuma longa of family Zingiberaceae. Curcumin has aromatic parts, such as an O‐methoxy phenolic and β‐dicarbonyl group. There are various biological properties and therapeutic benefits of curcumin that can be utilised for the treatment of chronic human disease, as it possesses antioxidant, anti‐inflammatory, anticancer, antidiabetic, immune‐regulatory, cardiovascular protective, neuroprotective and hepatoprotective effects. 141 , 142 , 143
Curcumin possesses anticancer effects when studied in pre‐clinical and clinical conditions. 16 , 144 Curcumin delivers its anticancer properties by stimulation of apoptotic pathway in cancer cells along with suppression of tumour microenvironment, such as inflammation, angiogenesis and tumour metastasis. 16 Several studies were performed to evaluate the potential of curcumin to treat various cancers, such as thyroid, lung, cervical, pancreatic and breast cancers, through modulation of the TGF‐β pathway. In lung cancer, curcumin was able to inhibit metastasis and suppress cancer cell proliferation via interference with many other pathways along with TGF‐β, such as MAPK or the Wnt signalling pathway. 145 The TGF‐β signalling pathway was downregulated by curcumin via a decrease in the expression of TGF‐β receptor II, P‐Smad3 and Smad4 and reduced TGF‐β‐induced invasion and migration in the HeLa and SiHa cells lines. Additionally, it inhibited p21, cyclin D1 and Pin1, with Slug and Snail in human cervical cancer cell lines. 146 The study 146 showed that curcumin and emodin exhibited a synergistic effect and inhibited cell migration and TGF‐β activated the Wnt/β‐catenin signalling pathway.
Curcumin increased E‐adherin expression while repressing vimentin expression and also suppressed cell attachment, migration and progression in BCPAP thyroid cancer cells. It was also found to inhibit TGF‐β1‐mediated Smad2 and Smad3 phosphorylation and also inhibited TGF‐β1‐induced EMT via Smad2/3 downregulation. 147 Sun et al. 148 studied the effect of curcumin against pancreatic cancer and showcased that curcumin repressed cell proliferation, decreased cell migration, invasion, induced apoptosis and reversed EMT of TGF‐β1‐stimulated PANC‐1 cells via Hedgehog signalling pathway inhibition. 148
In a notable study, curcumin in combination with doxorubicin reduced the survival and proliferation of neuroblastoma cells by inducing apoptosis via p53 and p21 upregulation. 149 Another study emphasised the importance of time and demonstrated that a combination of curcumin and doxorubicin promotes apoptosis via Bcl‐2 downregulation, Bax and acaspase‐9 overexpression in a concentration‐ and time‐dependent way. 150 In another study, curcumin and emodin synergistically suppressed cell migration and population in HeLa and SiHa cells. TGF‐ β also stimulated the Wnt/β‐catenin signalling pathway in HeLa cells, and emodin and curcumin decreased β‐catenin, hence suppressing the pathway. Therefore, curcumin and emodin can be used synergistically for the treatment of cervical cancer. 146
HCT116/oxaliplatin (OXA), an oxaliplatin‐resistant cell line, was effectively developed, and OXA combined with curcumin lowered OXA resistance in vitro. Furthermore, the combination treatment reduced p‐p65 and Bcl‐2 expression while increasing activated caspase‐3 levels. Curcumin also prevented EMT by regulating the TGF/Smad2/3 signalling pathway. Curcumin could also reduce OXA resistance in colorectal cancer cells in an in vivo investigation. 151 Curcumin blocked the TGF‐β and PI3K/Akt signalling pathways, which are both involved in doxorubicin‐induced EMT. Curcumin also improved doxorubicin's antiproliferative actions in triple‐negative breast cancer cells and showed that doxorubicin in conjunction with curcumin could be a viable treatment option for triple‐negative breast cancer. 152
A quercetin and curcumin combination was evaluated against K562/CCL‐243 chronic myeloid cell lines and found to be effective on various genes attributed to the p53, NF‐κβ and TGF‐α pathways. For instance, the combination exhibited a downregulatory effect on IFN‐ γ AKT1, CDKN1B and an upregulatory effect on CDKN1A, BTG2 and FAS. A multitargeted therapy for chronic myeloid leukaemia cells without impacting healthy cells may be provided by the downregulation of CDKN1B, AKT1, IFN‐ α and upregulation of gene and protein expressions of BTG2, CDKN1A and FAS. 153 The combination of cisplatin and nanocurcumin reduced the volume and weight of ovarian tumours significantly. There was also a decrease in Ki67, TGF‐β, PI3K and Akt phosphorylation expression. JAK expression, STAT3 phosphorylation and IL‐6 concentrations were all lowered when cisplatin and nanocurcumin were combined. Overall, nanocurcumin, when given as a co‐treatment with cisplatin, inhibited proliferation in ovarian cancer models by downregulating the PI3K/Akt and JAK/STAT3 signalling pathways. 154 However, one in vivo study showed no involvement of TGF‐β when curcumin was investigated against tumourigenicity assay using an athymic nude mouse model. This suggests that more confirmatory studies are required to understand the effect of curcumin on TGF‐β. 155
6.2.7. 3,3′‐Diindolymethane (DIM)
DIM (Figure 3) is a dimeric condensation product of indole‐3‐carbinol and a potent anticancer compound obtained from several vegetables of the genus Brassica, including broccoli, cabbage and Brussels sprouts. It is considered to exhibit anticancer properties by affecting cell proliferation, autophagy, endoplasmic reticulum stress, the cell cycle and apoptosis through different signalling mechanisms. 156 DIM selectively kills cancer cells without toxic exposure to normal cells. 157 DIM was examined against human endometrial cancer cells and exhibited a cytostatic effect. The mechanistic investigation revealed that this effect was associated with the enhancement of TGF‐α. 158 Another study has shown that DIM has the potential to inhibit breast cancer migration through inhibition of the TGF‐β and TGF‐α signalling pathways, which was associated with the suppression of EMT. 159 In a recent study, DIM was evaluated against MCF‐7 breast cancer cells, and it was reported that DIM significantly reduced the TNF‐α/TGF‐β‐induced breast cancer cells migration. Furthermore, the results demonstrated that DIM efficiently suppressed EMT processes by the inhibition of TNF‐α/TGF‐β‐associated signalling pathways in breast cancer cells. Hence, it can be stated that DIM has the potential to serve as a candidate for breast cancer therapy. 159
6.2.8. Dioscin
Diosgenin (Figure 3) is a steroid saponin extracted from the tuber of various species of Dioscorea. It is frequently used in the commercial synthesis of estrogen, progesterone, cortisone and steroids. Diosgenin displays many beneficial properties, such as antioxidant, 160 anti‐inflammatory, 161 hypoglycemic, 161 hypolipidemic 162 and antidiabetic 163 effects. Additionally, it is effective against a variety of cancers and has shown a pro‐apoptotic effect. 164 It has been demonstrated that diosgenin induces anticancer activity mainly by its antiproliferative property, induces apoptotic cell death, and avoids the formation of malignant tissue by arresting the cell cycle phases. 164 , 165 , 166 , 167 Dioscin, a natural derivative of diosgenin, is used for the treatment of breast cancer. 168 , 169
Dioscin exhibited inhibition of proliferating HepG2 hepatocellular cancer cells. Low concentrations of dioscin reversed the growth‐promoting effect on HepG2 cells induced by TGF‐β1. Furthermore, it suppressed TGF‐β1‐induced proliferation of HepG2 cells. The compound also inhibited the migrations and invasive property of cells, and treatment with dioscin increased the expression of various proteins, such as claudin‐1 and E‐cadherin. It decreased the expression of N‐cadherin, vimentin and slug. Dioscin inhibited the MAPK pathway, which has a significant role in the growth of cancer cells and metastasis, as well as inhibiting the EMT malignant transformation triggered by TGF‐β1 in HepG2 liver cancer cells. 170 When evaluated against lung cancer cell lines, dioscin inhibited cell growth and suppressed cell proliferation in a concentration‐dependent manner. The compound was also found to suppress the EMT induced by TGF‐β in A549 lung cancer cells. Additionally, it increased the expression of E‐cadherin and N‐cadherin, and decreased cell migration and invasion of TGF‐β1‐induced A549 lung cancer cells. 171
6.2.9. Emodin
Emodin (1,3,8‐trihydroxy‐6‐methylanthraquinone, Figure 3) is a natural anthraquinone derivative, mostly present in several Chinese medicinal herbs. It is isolated from many plant parts, such as roots and barks of plants belonging to the Rubiaceae, Rhamnaceae, Fabaceae and Polygonaceae families. It exerts various pharmacological effects, including antibacterial, antiviral, antiulcerogenic, diuretic vasorelaxant and antitumourigenic properties. 172 Emodin was studied against human cervical cancer cell lines, and it was found that emodin was able to downregulate the TGF‐β signalling pathway. 146
Emodin in combination with 3′‐azido‐3′‐deoxythymidine was found to exhibit a synergistic effect on cell apoptosis and proliferation and downregulated NF‐κB, TGF‐β mRNA, and Bcl‐2 proteins in the concentrated leukaemia stem cells (KG‐la cells). The synergic effect was mechanistically associated with Bcl‐2 activation inhibition and TGF‐β and NF‐κB downregulation. 173
6.2.10. Ginsenosides
Ginsenosides (Figure 3) are triterpene saponins obtained from Panax ginseng, commonly known as Chinese ginseng of the family Araliaceae. Ginsenosides were first isolated from this family in 1963 174 and have shown substantial potential in the treatment of cancer. Furthermore, ginsenosides are well‐known for their cardioprotection, antiobesity, antidiabetic, immunomodulation, neuroprotection, antimicrobial action, sexual potentiating and antitumour activities. 175 They have many beneficial effects, which explains their use as a complementary or an alternative form for the treatment of various cancers, such as breast, colorectal, endometrial and nasopharyngeal carcinoma. 175 , 176 , 177 , 178 They exhibit anticancer effects by inhibiting the proliferation of cancer cells through oxidation prevention and by inducing apoptosis and autophagy. 177
To evaluate the anticancer effect in colorectal cancer through modulation of TGF‐β, a docking study was performed between the ginsenoside Rb2 and TGF‐β complex. The docking score was −9.667 by binding to the hydrophobic pocket of TGF‐β1 and partially overlapping with the TGF‐binding site and finally disrupting the TGF‐β1 dimerisation. Further, western blot analysis also showed inhibition of the expression of TGF‐β1 in HCT116 and SW620 cells. Ginsenoside Rb2 was able to inhibit EMT and upregulate the expression of E‐cadherin as well as downregulate the function of N‐cadherin and vimentin. 179
Ginsenoside Rb2 was evaluated on HEC1A and Ishikawa cell lines for endometrial cancer. It displayed growth inhibitory effects on the cancer cells by reversing EMT‐induced changes in cell mobility and metastasis, and through further western blot analysis, it displayed an improvement in the E‐cadherin levels while the expression level of vimentin along with TGF‐β and Snail declined. The result suggested that ginsenoside can deliver the anticancer effect by inducing apoptosis and by inhibiting EMT. 180 Another study was conducted to examine the effect of ginsenoside Rb2 on PC3 prostate cancer cell lines. The results revealed that the compound activated TGF‐β receptor signalling and inhibited cell proliferation and invasion by regulation of cell cycle controllers and MMPs. 181 A recent study showed the potential suppressive effect of ginsenoside Rg3 against nasopharyngeal carcinoma (NPC) cells, where Rg3 inhibited migration and invasion ability of NPC cells and the EMT process. Additionally, Rg2 altered marker proteins of EMT and affected TGF‐β‐induced morphological transition. 182
6.2.11. Podophyllotoxin
Podophyllotoxin (Figure 3), a non‐alkaloidal antimitotic lignan, is extracted from the roots and rhizomes of podophyllum species. The compound also has different derivatives, such as teniposide and etoposide, which have obtained scientific focus due to their widespread biological properties, including anthelminthic, antiviral and antineoplastic effects. 183 The podophyllotoxin, along with its derivatives, is widely used for the effective treatment of breast, lung, ovary and stomach cancer. 184 , 185 Upon evaluation of liver cancer cells (Bel‐7402, HepG2 and HCCLM3), podophyllotoxin was found to reverse the effect of EMT without having any effect from TGF‐β1. In addition, a significant inhibition in metastasis and invasion on liver carcinoma cells was also recorded. 186
The polyamidoamine dendrimer‐conjugated podophyllotoxin treatment substantially decreased NF‐κB and IL‐6 in mice tissue and serum, respectively. A significant reduction was reported in liver fibrous tissue deposition, which was confirmed by declined levels of mRNA and TGF‐β expressions in the liver. Thus, DPODO treatment suppressed hepatocellular carcinoma progression via modulation of fibrogenic and inflammatory factors. 187
6.2.12. Resveratrol
Resveratrol (3,4,5‐trans‐trihydroxy‐stilbene, Figure 3) is a naturally occurring phytoalexin, belonging to the stilbene family. It is isolated from white hellebore Polygonum cuspidatum roots as well as many other plant families, such as Fabaceae, Gnetaceae and Cyperaceae. Various food products, including grapes, wine, mulberry and peanuts, contain this compound. 188 Resveratrol has the capability to target cancer cells at their initiation, promotion and progressing stages. It displays chemopreventive and chemotherapeutic effects by regulating the signal transduction pathways that manage cell division, initiates apoptosis, reduces inflammation, angiogenesis and inhibition of metastasis and invasion of human tumour cells. 15 , 189
Resveratrol has many medicinal properties, including antioxidant, anticancer, cardioprotective, immunomodulatory, antihypertensive, anti‐inflammatory, antimicrobial, antidiabetic and neuroprotective activities; hence, it can protect against many diverse chronic diseases. 15 , 190 , 191 , 192 , 193 Various in vitro and in vivo studies have established the potential effect of resveratrol against colorectal cancer, and it was found effective in inhibition of EMT by TGF‐β1 and suppressed invasive and metastatic properties. Additionally, resveratrol was able to enhance the expression of E‐cadherin and suppress the expression of vimentin. It also suppressed the invasive and migratory properties and inhibited the TGF‐β1/Smad pathway when examined in the LoVo cell line. 194
Resveratrol was evaluated against rhabdomyosarcoma soft tissue malignant tumour cells and was found to decrease cell growth, reduce cells in the S phase, arrest G0/G1 transition and reduce TGF‐β1and Smad4 expression at the protein and mRNA levels in PLA‐802 human alveolar rhabdomyosarcoma cell. 195 Shi et al. 196 studied the effect of resveratrol on MCF‐7 breast cancer cells and found that resveratrol reversed EMT and has the ability to overcome tamoxifen resistance. The effect of resveratrol was meditated by TGF‐β/Smad signalling, and resveratrol was found to suppress the production of endogenous TGF‐β and downstream Smad cascade. Whyte et al. 197 studied the effect of resveratrol against A549, NCI H23 and NCI H460 lung cancer cell lines and found that resveratrol inhibited A549 cell proliferation via cell cycle arrest, apoptosis induction and alteration of the intracellular Smad signalling of the TGF‐β pathway. Following resveratrol treatment, Smad activators, namely, Smad2 and Smad4, were downregulated at the mRNA level, Smad7 was upregulated and Smad2/Smad3 at the protein levels blocked nuclear signalling of the TGF‐β pathway.
Resveratrol was reported to inhibit the migration of MDA231 cells via TGF‐β1‐induced EMT reversal and also inhibited the lung metastasis in the MDA‐MB‐231 xenograft mouse tumour model. The MMP‐9, MMP‐2, α‐SMA, fibronectin, p‐PI3K, Smad2, Smad3, p‐Akt, p‐Smad2, p‐Smad3, Snail1, vimentin and Slug expressions were also decreased by resveratrol, and E‐cadherin in MDA‐MB‐231 cells was increased. 198 All these results support the hypothesis that resveratrol in combination with nutlin‐3 would be able to induce programmed cell death at about half of the typical concentration of nutlin‐3 for the induction of apoptosis. 199 A combination of resveratrol and cisplatin synergistically inhibited the MDA‐MB‐231 breast cancer cell viability and also inhibited TGF‐β1‐induced migration and invasion of MDA‐MB‐231cells by inhibiting EMT. Resveratrol was also reported to enhance antitumour effects and decrease adverse effects of cisplatin in MDA‐MB‐231 xenografts and involved in the mechanism for the effect of the regulations of the JNK, PI3K/Akt, NF‐κB and ERK expressions. 200 Another study showed that the combination of sitagliptin (an antidiabetic drug) and resveratrol synergistically ameliorated clear cell renal cell carcinoma, and this effect was mediated by the decrease in TGF‐β1 and other inflammatory cytokines, such as TNF‐α, IL‐6 and STAT3. 201 In irradiated mice, HS1793 (a resveratrol analogue) reduced tumour development by activating effector T cells. HS1793 treatment improved the outcome of radiation therapy by enhancing antitumour immunity. Additionally, HS1793 reduced the number of Tregs and lowered IL‐10 and TGF‐β secretion in irradiation tumour‐bearing mice. 202
6.2.13. Sulforaphane
Sulforaphane (Figure 3) is an isothiocyanate isolated from different vegetables of the crucifereae family, prominently from broccoli. Both preclinical and clinical studies underscore the ability of sulforaphane in preventing the development and/or suppressing the progression of various cancers. 203 , 204 , 205 , 206 The compound was studied against HepG2 hepatocellular carcinoma cells and also through a xenograft tumour growth model. 207 This study revealed that sulforaphane has emerged as a safe compound for the treatment of hepatocellular carcinoma. The same study also confirmed that sulforaphane has the potential to inhibit TGF‐β‐induced EMT through the ROS‐dependent pathway. 207
A recent study by Luo et al. 208 was conducted to record the impact of sulforaphane on long non‐coding RNAs in pancreatic ductal adenocarcinoma using several human immortalised pancreatic duct cell lines. This study revealed that sulforaphane exhibited potential activity against pancreatic cancer, which was due to APOBEC3G downregulation that prevented Smad2 phosphorylation, and hence TGF‐driven pancreatic ductal adenocarcinoma progression.
6.2.14. Thymoquinone
Thymoquinone (2‐isopropyl‐5‐methylbenzo‐1,4‐quinone, Figure 3) is mostly found as the biologically active compound in the oils of Nigella sativa seeds. Thymoquinone is considered a potential anticancer agent, 209 and it plays dual roles, depending upon the molecular environment; it can act as an antioxidant or pro‐oxidant (by generating ROS in tumour cells). 210 Thymoquinone inhibited the growth of cancer cells by inducing apoptosis, regulating the cell cycle and inhibiting the proliferation, migration and metastasis of tumour cells. 211 , 212 Its anticancer effect has been observed in various cancer types, such as lung, colorectal, prostate and breast cancer. In a recent study, thymoquinone was investigated as a radiosensitiser in the MCF‐7 and MDA‐MB‐231 breast cancer cells. Ionising radiation is considered to induce metastasis in cancer cells by eliciting TGF‐β, which is also known to regulate radio‐resistance. Thymoquinone downregulated E‐cadherin and cytokeratin 19, while mesenchymal markers, such as integrin aV, MMP‐9 and MMP‐2 were upregulated by irradiation treatment. 213 Interestingly, in an in vivo study, decreased hepatic TGF‐β1 mRNA levels by 1.8‐fold were recorded from thymoquinone in hepatocellular carcinoma. 214 Another in vivo study on renal cell carcinoma showed that TGF‐β1 might promote the metastasis in renal cell carcinoma, and thymoquinone has the ability to inhibit TGF‐β1‐induced metastasis. 215
6.2.15. Triptolide
Triptolide (Figure 3), a diterpenoid triepoxide compound, is extracted from different herbs used in Chinese medicine. Due to its considerable therapeutic effects, it has attracted considerable attention from researchers. It has been found to possess various pharmacological effects, such as antirheumatic, antimicrobial, anti‐inflammatory, immunomodulatory and antitumour activities. 216 , 217 Triptolide can produce cancer cell death by modulating apoptotic and autophagic pathways as well as inhibition of EMT and metastasis. 217 , 218
Triptolide inhibited the growth, migration and invasion of HT29 and HCT116 colon cancer cells with simultaneous reduction in the expression of VEGF and cyclooxygenase‐2. It also inhibited the expression of different TGF‐β receptor subunits (TGFΒRI and TGFΒRII). 219 In another study, triptolide was tested against HT‐29 and SW480 colon cancer cells, where it reduced cell viability, metastatic potential and enhanced the apoptosis rate with simultaneous caspase‐3 and caspase‐9. Furthermore, it neutralised the changes caused by TGF‐β, such as alterations in E‐cadherin, N‐cadherin, vimentin and snail. 220 In vivo studies showed that triptolide was able to decrease TGF‐ β, IL10 and VEGF in B16‐F10 xenograft mouse tumour model. 221
6.2.16. Withaferin A
Withania somnifera, commonly known as ashwagandha, or Indian ginseng, belongs to the family Solanaceae. its main constituents are alkaloids, steroidal lactone (withanolides and withaferins) and saponins. 222 Withaferin A (Figure 3) isolated from W. somnifera was found to exert antineoplastic effects againg various in vitro and in vivo tumour models. 223 This phytochemical was evaluated against MCF‐10A breast cancer cells and reported to partially reverse TGF‐β levels. During invasive breast cancer, EMT is usually characterised by low E‐cadherin and occludin levels and increased levels of the mesenchymal marker protein vimentin and fibronectin. However, after the treatment of withaferin A on MCF‐7 cells, there was an increase in the level of E‐cadherin and occludin, as well as suppression in vimentin. Withaferin A also led to the apoptosis of MDA‐MB‐231 and MCF‐7 by the intrinsic caspases pathway, without affecting vimentin levels. 224
Withaferin A also inhibited the invasive property of human cervical cancer Caski cells and resulted in a reduction of MMP‐9 expression via Akt signalling pathway suppression. It was also found to inhibit TGF‐β‐induced Akt phosphorylation and caused downregulation of MMP‐9. This effect strongly supports the ability of withaferin A to control the invasiveness of the tumours. 225
6.2.17. Miscellaneous natural products and their synergistic effects
Polyphyllin, an active phytoconstituent of Paris polyphylla, exhibited a decrease in the tumour growth in the xenograft tumour model by antagonising facilitative effects of TGF‐β1. 226 Similarly, andrographolide from Andrographis paniculata was also reported to decrease tumour growth and was bound to TGF‐β in an in vivo study for prostate cancer. 227 The root extract of W. somnifera was studied on MDA‐MB‐231, MCF‐7 and T47D breast cancer cell lines and demonstrated a potential association between vimentin expression and cytotoxicity. Vimentin plays an important role in EMT's being an intermediate filament protein to promote metastasis. After treatment with the root extract of W. somnifera, TGF‐β did not inhibit expression of vimentin at the mRNA level but did on the protein level by disrupting its morphology in cells, finally inhibiting EMT. 228
Marine natural products offer huge diversity but have limited explored resources. Marine products are enormous potential source reservoirs to isolate novel bioactive compounds from, and they also possess diverse chemical structures, which are potential sources for the drug discovery process. 229 Marine organisms are valuable sources for novel anticancer agents and can be of prominent importance in the process of drug discovery. 230 , 231 , 232 , 233 , 234 , 235 Out of many marine products, very few have been investigated specifically for their effect on TGF‐β in cancer. For example, MS80 is a sulfated oligosaccharide (8000 Da) isolated from seaweed. This compound was reported to exhibit a potential inhibition of TGF‐β/Smad signalling in breast and lung cancer cell lines, and the compound also inhibited lung metastasis in orthotopic 4T1 xenografts by inhibiting TGF‐β. 236
Natural products are very effective when utilised in association with other natural or synthetic agents. Studies have reported that the combined effect of natural products might improvise chemotherapeutic treatment effects against cancer cell proliferation. 237 In a study on breast cancer, a combination of curcumin, endoxifen (a drug under development for breast cancer) and β‐estradiol (estrogen steroid hormone) was tested on MCF‐7 cell lines. The endoxifen upregulated EMT markers and altered MCF‐7 breast cancer cells’ morphology. However, curcumin addition did not prevent EMT activation. Endoxifen activated EMT and increased mRNA expressions of TGF‐β1; however, curcumin decreased TGF‐β1 mRNA expressions in comparison to endoxifen. 238
Emodin, along with curcumin, was evaluated in cervical cancer cell lines SiHa and HeLa, which induced the EMT by the TGF‐β signalling pathway. The combination effectively downregulated the TGF‐β signalling pathway; hence, there was a decrease in the TGF‐β receptor II, P‐Smad3 and Smad4 expression level. Moreover, it also maintained the tumourigenic effects by inhibiting migration and invasion of cancer cells. Therefore, this combination had a synergistic chemotherapeutic effect against cervical cancer cells. 146
7. TGF‐β‐ASSOCIATED AND ‐DEPENDENT OMICS APPROACHES IN CANCER THERAPEUTICS
To understand and subtype Wnt signalling‐driven malignancies and subsequent determination of relevant Wnt signalling‐targeted treatments, transcriptomic, genome sequencing and/or immunohistochemistry studies are of tremendous importance. These studies are also beneficial for the identification of the subset of patients that can get benefitted from the combination therapies with various signalling inhibitors. 239 A multiomics data‐based study was performed to understand the molecular signatures linked with TGF‐β in gastrointestinal adenocarcinoma and a comprehensive neural network was framed. The results of this study suggested that TGF‐β can be a potential and possible drug target in gastrointestinal adenocarcinoma. 240
An explorative pathway analysis study conducted by Helleman et al. 241 of various gene sets found that TGF‐β was a prominent gene associated with ovarian cancers that were chemotherapy‐resistant. Extra cellular matrix (ECM)/integrin‐mediated pathway induced by TGF‐β was considered as a potential target for ovarian carcinomas. This also showed that TGF‐β could play an important role in chemotherapy‐resistant cancers, and natural products targeting TGF‐β might show significant potential for such neoplasms. Various other proteomic and genomic analyses were conducted to explore the mechanism of TGF‐β in cancer progression. 242 , 243 However, a substantial gap is there, and more exhaustive and exclusive omics studies are needed to unravel the precise role of TGF‐β in tumourigenesis. A schematic representation of TGF‐β‐associated multidimensional omics approaches in cancer medicine is presented in Figure 4.
FIGURE 4.

Schematic representation of various multiomics approaches for the mechanistic investigations of cancer. The image depicts omics‐based mechanistic evaluation of a multiple cancer patient's tumour environment with the help of metabolomics and genomics investigations. The image symbolically represents the utilisation of multiomics approach for the development of TGF‐β neural network
8. CONCLUSION, CURRENT LIMITATION, CHALLENGES AND FUTURE RESEARCH DIRECTIONS
Cancer is one the deadliest diseases known to man, the rate of which is increasing unexpectedly due to urbanisation, environmental issues and collective lifestyle changes. Although there are numerous significant medical and technological developments to combat this disease, many conventional cancer‐targeted therapies have severe side effects and complications, including exposure to severe toxicities and the development of resistance to the medications. Although cancer drugs act through various mechanisms involving different signalling pathways to treat individuals, we have narrowed the scope of our research to the TGF‐β signalling pathway. The TGF‐β signalling pathway plays a critical role in various cellular responses related to cell growth, apoptosis, motility, invasion, angiogenesis and differentiation. Although TGF‐β’s role is extensively studied for its tumour suppressive character during the early stages of cancer, it is also known to be involved in cancer progression, especially within the later stages. The possession of this unique property allows the pathway to act as a double‐edged sword, as it can play the roles of both tumour suppressor and promoter at different stages of cancer.
TGF‐β induces EMT in various epithelial cells, which is able to induce a stem cell‐like phenotype in cancer cells. Inhibiting TGF‐β signalling decreases the expression of protein markers and induces cell differentiation to less aggressive phenotypes. Clinical studies have revealed that drugs, such as galunisertib, show severe side effects in different phases of clinical trials. Several of these prominent side effects include a significant decline in lymphocytes, platelet count and white blood cells along with more common side effects, such as fatigue, nausea, constipation and alopecia. 244 , 245 Similar adverse events were also recorded for other drugs, such as fresolimumab. 246 Due to the adverse effects pertaining to many of the TGF‐β inhibitors, usage of natural products may arise to become a better therapeutic approach for treating cancer due to their lesser amount of side effects in comparison to chemotherapy. In addition, natural compounds, such as curcumin, resveratrol, podophyllotoxin and emodin, can also be used as a bioenhancer for their synergistic effects to promote the anticancer potential. However, substantial clinical studies are required to have a proper understanding of the therapeutic efficacy of various natural products.
The prospective of natural agents in the field of drug discovery is boundless. A minimum of about one‐third of apex 20 drugs on the market are derived from natural products (specifically from plants), and approximately 50% of today's available and marketed drugs are categorised as naturally derived or designed based on natural products. 247 , 248
Therefore, treating cancer through targeting the TGF‐β pathway with various phytoconstituents is a new strategy that is most likely to result in fewer side effects for cancer patients. Despite ongoing research on natural product‐based cancer therapeutics many questions may arise, such as: (i) Do these natural products and their derivatives only kill cancer cells? If there is an effect on other types of cells, do they produce a favourable or harmful effect? (ii) Do these natural compounds and their derivatives have a synergistic effect with the conventional form of cancer treatment, or does their interaction induce unexpected effects? (iii) Does the use of natural products have advantages over the usage of conventional cancer treatment? Our review addresses these questions and provides comprehensive details about natural products and their derivatives, and whether they manage or inhibit cell proliferation with negligible effects on normal cells. To answer the second question, it is clear from multiple studies that natural products and their derivatives have highly synergistic effects and can be used as a bioenhancer to augment the anticancer effects of other chemotherapeutic drugs. Finally, additional evidence is available that shows the beneficial effects of natural products over conventional chemotherapeutic agents, specifically in terms of severe adverse effects of chemotherapy. However, comprehensive clinical studies are required not only to establish the anticancer effects of the natural products and their impact on TGF‐ β but also to evaluate the synergistic anticancer effects with other natural products and synthetic agents.
As demonstrated, cancer treatment with the usage of natural products has a long way to go within the area of preclinical and clinical studies. It is of utmost importance to optimise doses of natural compounds that will not lead to toxicity, but exhibit favourable pharmacokinetics and pharmacodynamics parameters, along with the precise mechanisms of action. As summarised in Figure 5, several bioactive natural compounds act through diverse mechanisms of the TGF‐βsignalling pathway. These include suppression of cell proliferation and inhibition of invasion and metastasis. Various natural compounds either directly inhibit TGF‐β signalling or can possibly act through mitochondria and induce apoptosis or cause cell cycle arrest at different stages.
FIGURE 5.
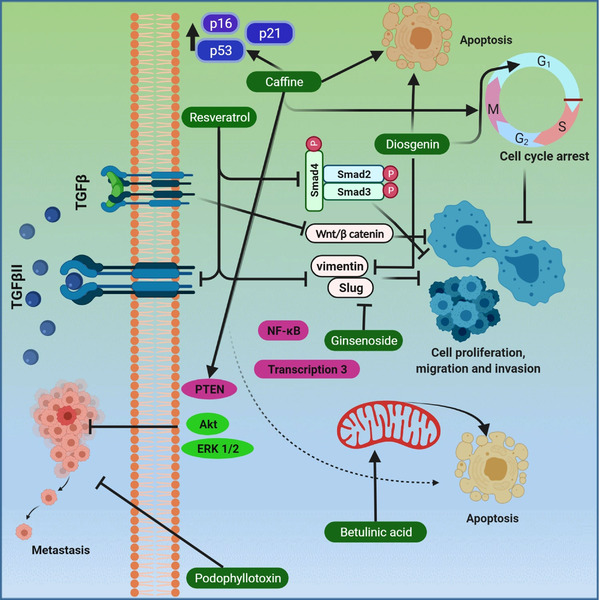
Mechanism of different natural compounds targeting the TGF‐β pathway in cancer. The figure illustrates the effects of various bioactive phytocompounds, such as betulinic acid, caffeine, diosgenin, ginsenoside, podophyllotoxin and resveratrol, on apoptosis, cell cycle, cell proliferation and metastasis linked to interference with the TGF‐β signalling
Various natural products play important roles in the alteration of DNA methylation. Since there is a close association between the profiles of DNA methylation and TGF‐β signalling, a comprehensive investigation to better understand the value of TGF‐β as a target for anticancer natural agents is crucial. Several natural products have the potential of inducing TGF‐β production in the target cells, which might be valuable for cancer prevention. However, it could also be potentially harmful to patients with advanced‐stage cancer. Very few clinical studies pertaining to the effects of various natural products on TGF‐β signalling have been carried out; therefore, additional clinical studies are urgently warranted. We also recommend future studies utilising advanced pharmacological approaches, such as multiomics investigations. There is also a tremendous scope in the understanding of the involvement of TGF‐β at genomic levels. Additionally, the impact of various plant secondary metabolites can also be thoroughly investigated by using approaches, such as metabolomics. Based on emerging evidence as presented in this work, TGF‐β‐targeting bioactive compounds from natural sources can serve as potential therapeutic agents for the prevention and treatment of various human malignancies.
Tewari D, Priya A, Bishayee A, Bishayee A. Targeting transforming growth factor‐β signalling for cancer prevention and intervention: Recent advances in developing small molecules of natural origin. Clin Transl Med. 2022;12:e795. 10.1002/ctm2.795
Devesh Tewari and Anu Priya contributed equally to this study and are first authors.
Contributor Information
Devesh Tewari, Email: devesh.24531@lpu.co.in, Email: dtewari3@gmail.com.
Anupam Bishayee, Email: abishayee@lecom.edu, Email: abishayee@gmail.com.
REFERENCES
- 1. Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv Pharm Bull. 2014;4(1):421‐427. 10.5681/apb.2014.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ohnishi S, Takeda H. Herbal medicines for the treatment of cancer chemotherapy‐induced side effects. Front Pharmacol. 2015;6:14. 10.3389/fphar.2015.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tavakoli J, Miar S, Majid Zadehzare M, Akbari H. Evaluation of effectiveness of herbal medication in cancer care: a review study. Iran J Cancer Prev. 2012;5(3):144‐156. https://pubmed.ncbi.nlm.nih.gov/25628834 [PMC free article] [PubMed] [Google Scholar]
- 4. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7‐34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 7. Whiteman DC, Wilson LF. The fractions of cancer attributable to modifiable factors: a global review. Cancer Epidemiol. 2016;44:203‐221. 10.1016/j.canep.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 8. Tewari D, Rawat P, Singh PK. Adverse drug reactions of anticancer drugs derived from natural sources. Food Chem Toxicol. 2019;123:522‐535. 10.1016/j.fct.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 9. Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83(3):770‐803. 10.1021/acs.jnatprod.9b01285 [DOI] [PubMed] [Google Scholar]
- 10. Atanasov AG, Zotchev SB, Dirsch VM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20:200‐216. 10.1038/s41573-020-00114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demain AL, Vaishnav P. Natural products for cancer chemotherapy. Microb Biotechnol. 2011;4(6):687‐699. 10.1111/j.1751-7915.2010.00221.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rayan A, Raiyn J, Falah M. Nature is the best source of anticancer drugs: indexing natural products for their anticancer bioactivity. PLoS One. 2017;12(11):e0187925‐e0187925. 10.1371/journal.pone.0187925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Nat Prod Rep. 2000;17(3):215‐234. 10.1039/a902202c [DOI] [PubMed] [Google Scholar]
- 14. Cragg GM, Newman DJ. Plants as a source of anti‐cancer agents. J Ethnopharmacol. 2005;100(1‐2):72‐79. 10.1016/j.jep.2005.05.011 [DOI] [PubMed] [Google Scholar]
- 15. Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res (Phila). 2009;2(5):409‐418. 10.1158/1940-6207.CAPR-08-0160 [DOI] [PubMed] [Google Scholar]
- 16. Kasi PD, Tamilselvam R, Skalicka‐Wozniak K, et al. Molecular targets of curcumin for cancer therapy: an updated review. Tumour Biol. 2016;37(10):13017‐13028. 10.1007/s13277-016-5183-y [DOI] [PubMed] [Google Scholar]
- 17. Tuli HS, Tuorkey MJ, Thakral F, et al. Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol. 2019;10:1336. 10.3389/fphar.2019.01336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aggarwal V, Tuli HS, Tania M, et al. Molecular mechanisms of action of epigallocatechin gallate in cancer: recent trends and advancement. Semin Cancer Biol. 2022;80:256‐275. 10.1016/j.semcancer.2020.05.011 [DOI] [PubMed] [Google Scholar]
- 19. Khan F, Niaz K, Maqbool F, et al. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8(9):529. 10.3390/nu8090529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sharoni Y, Linnewiel‐Hermoni K, Zango G, et al. The role of lycopene and its derivatives in the regulation of transcription systems: implications for cancer prevention. Am J Clin Nutr. 2012;96(5):1173S‐8S. 10.3945/ajcn.112.034645. [DOI] [PubMed] [Google Scholar]
- 21. Imran M, Atif M, et al. Apigenin as an anticancer agent. Phytother Res. 2020;34(8):1812‐1828. 10.1002/ptr.6647 [DOI] [PubMed] [Google Scholar]
- 22. Verma S, Singh A, Mishra A. Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol. 2013;35(3):473‐485. 10.1016/j.etap.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 23. Wu G, Yan Y, Zhou Y, et al. Sulforaphane: expected to become a novel antitumor compound. Oncol Res. 2020;28(4):439‐446. 10.3727/096504020X15828892654385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shanmugam MK, Dai X, Kumar AP, Tan BKH, Sethi G, Bishayee A. Ursolic acid in cancer prevention and treatment: molecular targets, pharmacokinetics and clinical studies. Biochem Pharmacol. 2013;85(11):1579‐1587. 10.1016/j.bcp.2013.03.006 [DOI] [PubMed] [Google Scholar]
- 25. Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K‐Akt‐mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 2022;80:1‐1. 10.1016/j.semcancer.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 26. Majnooni MB, Fakhri S, Smeriglio A, et al. Antiangiogenic effects of coumarins against cancer: from chemistry to medicine. Molecules. 2019;24(23):4278. 10.3390/molecules24234278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Block KI, Gyllenhaal C, Lowe L, et al. Designing a broad‐spectrum integrative approach for cancer prevention and treatment. Semin Cancer Biol. 2015;35(Suppl):S276‐S304. 10.1016/j.semcancer.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braicu C, Zanoaga O, Zimta A‐A, et al. Natural compounds modulate the crosstalk between apoptosis‐ and autophagy‐regulated signaling pathways: controlling the uncontrolled expansion of tumor cells. Semin Cancer Biol. 2022;80:218‐236. 10.1016/j.semcancer.2020.05.015 [DOI] [PubMed] [Google Scholar]
- 29. Ma Z, Xiang X, Li S, et al. Targeting hypoxia‐inducible factor‐1, for cancer treatment: recent advances in developing small‐molecule inhibitors from natural compounds. Semin Cancer Biol. 2022;80:379‐390. 10.1016/j.semcancer.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 30. Tewari D, Nabavi SF, Nabavi SM, et al. Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention. Pharmacol Res. 2018;128:366‐375. 10.1016/j.phrs.2017.09.014 [DOI] [PubMed] [Google Scholar]
- 31. Fakhri S, Moradi SZ, Yarmohammadi A, Narimani F, Wallace CE, Bishayee A. Modulation of TLR/NF‐κB/NLRP signaling by bioactive phytocompounds: a promising strategy to augment cancer chemotherapy and immunotherapy. Front Oncol. 2022;12:834072. https://www.frontiersin.org/article/10.3389/fonc.2022.834072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fakhri S, Moradi SZ, Farzaei MH, Bishayee A. Modulation of dysregulated cancer metabolism by plant secondary metabolites: a mechanistic review. Semin Cancer Biol. 2022;80:276‐305. 10.1016/j.semcancer.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 33. Shanmugam MK, Lee JH, Chai EZP, et al. Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin Cancer Biol. 2016;40‐41:35‐47. 10.1016/j.semcancer.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 34. Bose S, Banerjee S, Mondal A, et al. Targeting the JAK/STAT signaling pathway using phytocompounds for cancer prevention and therapy. Cells. 2020;9(6):1451. 10.3390/cells9061451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tewari D, Bawari S, Sharma S, DeLiberto LK, Bishayee A. Targeting the crosstalk between canonical Wnt/β‐catenin and inflammatory signaling cascades: a novel strategy for cancer prevention and therapy. Pharmacol Ther. 2021;227:107876. 10.1016/j.pharmthera.2021.107876 [DOI] [PubMed] [Google Scholar]
- 36. Meng X, Nikolic‐Paterson DJ, Lan HY. TGF‐β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325‐338. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 37. Massagué J. TGFbeta in Cancer. Cell. 2008;134(2):215‐230. 10.1016/j.cell.2008.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616‐630. 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF‐beta in homeostasis and cancer. Nat Rev Cancer. 2003;3(11):807‐821. 10.1038/nrc1208 [DOI] [PubMed] [Google Scholar]
- 40. Yin JJ, Selander K, Chirgwin JM, et al. TGF‐beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103(2):197‐206. 10.1172/JCI3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee C, Zhang Q, Kozlowski J, et al. Natural products and transforming growth factor‐beta (TGF‐β) signaling in cancer development and progression. Curr Cancer Drug Targets. 2013;13(5):500‐505. 10.2174/15680096113139990034 [DOI] [PubMed] [Google Scholar]
- 42. Markov AV, Zenkova MA, Logashenko EB. Modulation of tumour‐related signaling pathways by natural pentacyclic triterpenoids and their semisynthetic derivatives. Curr Med Chem. 2017;24(13):1277‐1320. 10.2174/0929867324666170112115313 [DOI] [PubMed] [Google Scholar]
- 43. Farooqi AA, Pinheiro M, Granja A, et al. EGCG mediated targeting of deregulated signaling pathways and non‐coding RNAs in different cancers: focus on JAK/STAT, Wnt/β‐catenin, TGF/SMAD, NOTCH, SHH/GLI, and TRAIL mediated signaling pathways. Cancers (Basel). 2020;12(4):951. 10.3390/cancers12040951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tominaga K, Suzuki HI. TGF‐beta signaling in cellular senescence and aging‐related pathology. Int J Mol Sci. 2019;20(20):5002. 10.3390/ijms20205002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu J, Lamouille S, Derynck R. TGF‐beta‐induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156‐172. 10.1038/cr.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vander Ark A, Cao J, Li X. TGF‐beta receptors: in and beyond TGF‐beta signaling. Cell Signal. 2018;52:112‐120. 10.1016/j.cellsig.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 47. Derynck R, Zhang YE. Smad‐dependent and Smad‐independent pathways in TGF‐beta family signalling. Nature. 2003;425(6958):577‐584. 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- 48. Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23(9):2078‐2093. 10.1200/JCO.2005.02.047 [DOI] [PubMed] [Google Scholar]
- 49. Huang JJ, Blobe GC. Dichotomous roles of TGF‐beta in human cancer. Biochem Soc Trans. 2016;44(5):1441‐1454. 10.1042/BST20160065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doré JJJ, Yao D, Edens M, Garamszegi N, Sholl EL, Leof EB. Mechanisms of transforming growth factor‐beta receptor endocytosis and intracellular sorting differ between fibroblasts and epithelial cells. Mol Biol Cell. 2001;12(3):675‐684. 10.1091/mbc.12.3.675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Trapani JA. The dual adverse effects of TGF‐beta secretion on tumor progression. Cancer Cell. 2005;8(5):349‐350. 10.1016/j.ccr.2005.10.018 [DOI] [PubMed] [Google Scholar]
- 52. Clark DA, Coker R. Molecules in focus transforming growth factor‐beta (TGF‐β). Int J Biochem Cell Biol. 1998;30(3):293‐298. 10.1016/S1357-2725(97)00128-3 [DOI] [PubMed] [Google Scholar]
- 53. Moustakas A, Heldin C‐H. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699‐3714. 10.1242/dev.030338 [DOI] [PubMed] [Google Scholar]
- 54. Chen CL, Chen CY, Chen YP, et al. Betulinic acid enhances TGF‐β signaling by altering TGF‐β receptors partitioning between lipid‐raft/caveolae and non‐caveolae membrane microdomains in mink lung epithelial cells. J Biomed Sci. 2016;23(1):30. 10.1186/s12929-016-0229-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lichtman MK, Otero‐Vinas M, Falanga V. Transforming growth factor beta (TGF‐β) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24(2):215‐222. 10.1111/wrr.12398 [DOI] [PubMed] [Google Scholar]
- 56. Akhurst RJ, Derynck R. TGF‐beta signaling in cancer–a double‐edged sword. Trends Cell Biol. 2001;11(11):S44‐S51. 10.1016/s0962-8924(01)02130-4 [DOI] [PubMed] [Google Scholar]
- 57. Haque S, Morris JC. Transforming growth factor‐β: a therapeutic target for cancer. Hum Vaccin Immunother. 2017;13(8):1741‐1750. 10.1080/21645515.2017.1327107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zi Z. Molecular engineering of the TGF‐β signaling pathway. J Mol Biol. 2019;431(15):2644‐2654. 10.1016/j.jmb.2019.05.022 [DOI] [PubMed] [Google Scholar]
- 59. Kwiatkowski W, Gray PC, Choe S. Engineering TGF‐β superfamily ligands for clinical applications. Trends Pharmacol Sci. 2014;35(12):648‐657. 10.1016/j.tips.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 60. Poniatowski ŁA, Wojdasiewicz P, Gasik R, Szukiewicz D. Transforming growth factor Beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators Inflamm. 2015;2015:137823. 10.1155/2015/137823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hanyu A, Ishidou Y, Ebisawa T, Shimanuki T, Imamura T, Miyazono K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor‐beta signaling. J Cell Biol. 2001;155(6):1017‐1027. 10.1083/jcb.200106023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fabregat I, Fernando J, Mainez J, Sancho P. TGF‐beta signaling in cancer treatment. Curr Pharm Des. 2014;20(17):2934‐2947. 10.2174/13816128113199990591 [DOI] [PubMed] [Google Scholar]
- 63. Shi Y, Massagué J. Mechanisms of TGF‐β Signaling from Cell Membrane to the Nucleus. Cell. 2003;113(6):685‐700. 10.1016/S0092-8674(03)00432-X [DOI] [PubMed] [Google Scholar]
- 64. Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF‐β targeted cancer therapy. Int J Biol Sci. 2012. 8(7):964‐978. 10.7150/ijbs.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang Z, Auyeung KK‐W, Sze SC‐W, Zhang S, Yung KK‐L, Ko JK‐S. The dual roles of calycosin in growth inhibition and metastatic progression during pancreatic cancer development: a “TGF‐beta paradox.” Phytomedicine. 2020;68:153177. 10.1016/j.phymed.2020.153177 [DOI] [PubMed] [Google Scholar]
- 66. Pickup M, Novitskiy S, Moses HL. The roles of TGFβ in the tumour microenvironment. Nat Rev Cancer. 2013;13(11):788‐799. 10.1038/nrc360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tachibana I, Imoto M, Adjei PN, et al. Overexpression of the TGFbeta‐regulated zinc finger encoding gene, TIEG, induces apoptosis in pancreatic epithelial cells. J Clin Invest. 1997;99(10):2365‐2374. 10.1172/JCI119418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jang C‐W, Chen C‐H, Chen C‐C, Chen J, Su Y‐H, Chen R‐H. TGF‐beta induces apoptosis through Smad‐mediated expression of DAP‐kinase. Nat Cell Biol. 2002;4(1):51‐58. 10.1038/ncb731 [DOI] [PubMed] [Google Scholar]
- 69. Valderrama‐Carvajal H, Cocolakis E, Lacerte A, et al. Activin/TGF‐beta induce apoptosis through Smad‐dependent expression of the lipid phosphatase SHIP. Nat Cell Biol. 2002;4(12):963‐969. 10.1038/ncb885 [DOI] [PubMed] [Google Scholar]
- 70. Atfi A, Buisine M, Mazars A, Gespach C. Induction of apoptosis by DPC4, a transcriptional factor regulated by transforming growth factor‐beta through stress‐activated protein kinase/c‐Jun N‐terminal kinase (SAPK/JNK) signaling pathway. J Biol Chem. 1997;272(40):24731‐24734. 10.1074/jbc.272.40.24731 [DOI] [PubMed] [Google Scholar]
- 71. Wildey GM, Patil S, Howe PH. Smad3 potentiates transforming growth factor beta (TGFbeta)‐induced apoptosis and expression of the BH3‐only protein Bim in WEHI 231 B lymphocytes. J Biol Chem. 2003;278(20):18069‐18077. 10.1074/jbc.M211958200 [DOI] [PubMed] [Google Scholar]
- 72. Ohgushi M, Kuroki S, Fukamachi H, et al. Transforming growth factor beta‐dependent sequential activation of Smad, Bim, and caspase‐9 mediates physiological apoptosis in gastric epithelial cells. Mol Cell Biol. 2005;25(22):10017‐10028. 10.1128/MCB.25.22.10017-10028.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Edlund S, Landström M, Heldin C‐H, Aspenström P. Transforming growth factor‐beta‐induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13(3):902‐914. 10.1091/mbc.01-08-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Krstic J, Trivanovic D, Mojsilovic S, Santibanez JF. Transforming growth factor‐beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. 2015;2015:654594. 10.1155/2015/654594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11(11):1287‐1296. 10.1038/ncb1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu S, Chen S, Zeng J. TGF‑β signaling: a complex role in tumorigenesis (Review). Mol Med Rep. 2018;17(1):699‐704. 10.3892/mmr.2017.7970 [DOI] [PubMed] [Google Scholar]
- 77. Lee B, Oh Y, Jo S, Kim T‐H, Ji JD. A dual role of TGF‐β in human osteoclast differentiation mediated by Smad1 versus Smad3 signaling. Immunol Lett. 2019;206:33‐40. 10.1016/j.imlet.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 78. Sun N, Taguchi A, Hanash S. Switching roles of TGF‐β in cancer development: implications for therapeutic target and biomarker studies. J Clin Med. 2016;5(12):109. 10.3390/jcm5120109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lebrun J‐J. The dual role of TGFβ in human cancer: from tumor suppression to cancer metastasis. ISRN Mol Biol. 2012;2012:381428. 10.5402/2012/381428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kirtonia A, Sethi G, Garg M. The multifaceted role of reactive oxygen species in tumorigenesis. Cell Mol Life Sci. 2020;77(22):4459‐4483. 10.1007/s00018-020-03536-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Han Z, Kang D, Joo Y, et al. TGF‐β downregulation‐induced cancer cell death is finely regulated by the SAPK signaling cascade. Exp Mol Med. 2018;50(12):162. 10.1038/s12276-018-0189-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jakowlew SB. Transforming growth factor‐beta in cancer and metastasis. Cancer Metastasis Rev. 2006;25(3):435‐457. 10.1007/s10555-006-9006-2 [DOI] [PubMed] [Google Scholar]
- 83. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9‐18. 10.1038/sj.cr.7290105 [DOI] [PubMed] [Google Scholar]
- 84. Arkun Y, Yasemi M. Dynamics and control of the ERK signaling pathway: sensitivity, bistability, and oscillations. PLoS One. 2018;13(4):e0195513. 10.1371/journal.pone.0195513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF‐beta1‐induced EMT in vitro. Neoplasia. 2004;6(5):603‐610. 10.1593/neo.04241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhao M, Mishra L, Deng C‐X. The role of TGF‐β/SMAD4 signaling in cancer. Int J Biol Sci. 2018;14(2):111‐123. 10.7150/ijbs.23230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Watanabe H, de Caestecker MP, Yamada Y. Transcriptional cross‐talk between Smad, ERK1/2, and p38 mitogen‐activated protein kinase pathways regulates transforming growth factor‐beta‐induced aggrecan gene expression in chondrogenic ATDC5 cells. J Biol Chem. 2001;276(17):14466‐14473. 10.1074/jbc.M005724200 [DOI] [PubMed] [Google Scholar]
- 88. Melzer C, Hass R, von der Ohe J, Lehnert H, Ungefroren H. The role of TGF‐β and its crosstalk with RAC1/RAC1b signaling in breast and pancreas carcinoma. Cell Commun Signal. 2017;15(1):19. 10.1186/s12964-017-0175-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ungefroren H, Gieseler F, Kaufmann R, Settmacher U, Lehnert H, Rauch BH. Signaling crosstalk of TGF‐β/ALK5 and PAR2/PAR1: a complex regulatory network controlling fibrosis and cancer. Int J Mol Sci. 2018;19(6). 10.3390/ijms19061568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Park B‐J, Park J‐I, Byun D‐S, Park J‐H, Chi S‐G. Mitogenic conversion of transforming growth factor‐β1 effect by oncogenic Ha‐Ras‐induced activation of the mitogen‐activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 2000;60(11):3031LP‐3038. http://cancerres.aacrjournals.org/content/60/11/3031.abstract [PubMed] [Google Scholar]
- 91. Chapnick DA, Warner L, Bernet J, Rao T, Liu X. Partners in crime: the TGFβ and MAPK pathways in cancer progression. Cell Biosci. 2011;1:42. 10.1186/2045-3701-1-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bottinger EP, Jakubczak JL, Haines DC, Bagnall K, Wakefield LM. Transgenic mice overexpressing a dominant‐negative mutant type II transforming growth factor beta receptor show enhanced tumorigenesis in the mammary gland and lung in response to the carcinogen 7,12‐dimethylbenz‐[a]‐anthracene. Cancer Res. 1997;57(24):5564‐5570. [PubMed] [Google Scholar]
- 93. Forrester E, Chytil A, Bierie B, et al. Effect of conditional knockout of the type II TGF‐beta receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen induced tumor formation and metastasis. Cancer Res. 2005;65(6):2296‐2302. 10.1158/0008-5472.CAN-04-3272 [DOI] [PubMed] [Google Scholar]
- 94. Tian F, Byfield SD, Parks WT, et al. Smad‐binding defective mutant of transforming growth factor beta type I receptor enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res. 2004;64(13):4523‐4530. 10.1158/0008-5472.CAN-04-0030 [DOI] [PubMed] [Google Scholar]
- 95. Derynck R, Akhurst RJ, Balmain A. TGF‐beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29(2):117‐129. 10.1038/ng1001-117 [DOI] [PubMed] [Google Scholar]
- 96. Galliher AJ, Neil JR, Schiemann WP. Role of transforming growth factor‐beta in cancer progression. Future Oncol. 2006;2(6):743‐763. 10.2217/14796694.2.6.743 [DOI] [PubMed] [Google Scholar]
- 97. Stover DG, Bierie B, Moses HL. A delicate balance: TGF‐beta and the tumor microenvironment. J Cell Biochem. 2007;101(4):851‐861. 10.1002/jcb.21149 [DOI] [PubMed] [Google Scholar]
- 98. Prud'homme GJ. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Investig. 2007;87(11):1077‐1091. 10.1038/labinvest.3700669 [DOI] [PubMed] [Google Scholar]
- 99. Dong M, How T, Kirkbride KC, et al. The type III TGF‐beta receptor suppresses breast cancer progression. J Clin Invest. 2007;117(1):206‐217. 10.1172/JCI29293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6(7):506‐520. 10.1038/nrc1926 [DOI] [PubMed] [Google Scholar]
- 101. Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor‐beta and the immune response: implications for anticancer therapy. Clin cancer Res an Off J Am Assoc Cancer Res. 2007;13(18):5262‐5270. 10.1158/1078-0432.CCR-07-1157 [DOI] [PubMed] [Google Scholar]
- 102. Neuzillet C, Tijeras‐Raballand A, Cohen R, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22‐31. 10.1016/j.pharmthera.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 103. Lampropoulos P, Zizi‐Sermpetzoglou A, Rizos S, Kostakis A, Nikiteas N, Papavassiliou AG. TGF‐beta signalling in colon carcinogenesis. Cancer Lett. 2012;314(1):1‐7. 10.1016/j.canlet.2011.09.041 [DOI] [PubMed] [Google Scholar]
- 104. Liu S, Ren J, ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):8. 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res. 2009;19(1): 89‐102. 10.1038/cr.2008.316 [DOI] [PubMed] [Google Scholar]
- 106. de Gramont A, Faivre S, Raymond E. Novel TGF‐β inhibitors ready for prime time in onco‐immunology. Oncoimmunology. 2017;6(1):1‐5. 10.1080/2162402X.2016.1257453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high‐grade glioma with the TGF‐β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132‐142. 10.1093/neuonc/noq142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang C‐Y, Chung C‐L, Hu T‐H, Chen J‐J, Liu P‐F, Chen C‐L. Recent progress in TGF‐β inhibitors for cancer therapy. Biomed Pharmacother. 2021;134:111046. 10.1016/j.biopha.2020.111046 [DOI] [PubMed] [Google Scholar]
- 109. Desai AG, Qazi GN, Ganju RK, et al. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008;9(7):581‐591. 10.2174/138920008785821657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Chamberlin SR, Blucher A, Wu G, et al. Natural product target network reveals potential for cancer combination therapies. Front Pharmacol. 2019;10:557. 10.3389/fphar.2019.00557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Banikazemi Z, Haji HA, Mohammadi M, et al. Diet and cancer prevention: dietary compounds, dietary microRNAs, and dietary exosomes. J Cell Biochem. 2018;119(1):185‐196. 10.1002/jcb.26244 [DOI] [PubMed] [Google Scholar]
- 112. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Pohl S, Zobel J, Moffat A, Extended Boolean retrieval for systematic biomedical reviews. In: Mans B, Reynolds M, eds. Proceedings of the Thirty‐Third Australasian Conferenc on Computer Science, Volume 102 . ACSC ’10. Australian Computer Society, Inc.; 2010:117‐126. http://dl.acm.org/citation.cfm?id=1862199.1862212 [Google Scholar]
- 114. Tewari D, Stankiewicz AM, Mocan A, et al. Ethnopharmacological approaches for dementia therapy and significance of natural products and herbal drugs. Front Aging Neurosci. 2018;10(2):3. 10.3389/fnagi.2018.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hemalatha T, Pulavendran S, Balachandran C, Manohar BM, Puvanakrishnan R. Arjunolic acid: a novel phytomedicine with multifunctional therapeutic applications. Indian J Exp Biol. 2010;48(3):238‐247. [PubMed] [Google Scholar]
- 116. Elsherbiny NM, Al‐Gayyar MMH. Anti‐tumor activity of arjunolic acid against Ehrlich Ascites Carcinoma cells in vivo and in vitro through blocking TGF‐β type 1 receptor. Biomed Pharmacother. 2016;82:28‐34. 10.1016/j.biopha.2016.04.046 [DOI] [PubMed] [Google Scholar]
- 117. Nagoor Meeran MF, Goyal SN, Suchal K, Sharma C, Patil CR, Ojha SK. Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: a pentacyclic triterpenoid of therapeutic promise. Front Pharmacol. 2018;9:892. https://www.frontiersin.org/article/10.3389/fphar.2018.00892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lian G‐Y, Wang Q‐M, Tang PM‐K, Zhou S, Huang X‐R, Lan H‐Y. Combination of asiatic acid and naringenin modulates NK cell anti‐cancer immunity by rebalancing Smad3/Smad7 Signaling. Mol Ther. 2018;26(9):2255‐2266. 10.1016/j.ymthe.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cui Q, Ren J, Zhou Q, Yang Q, Li B. Effect of asiatic acid on epithelial‐mesenchymal transition of human alveolar epithelium A549 cells induced by TGF‐β1. Oncol Lett. 2019;17(5):4285‐4292. 10.3892/ol.2019.10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fulda S. Betulinic acid for cancer treatment and prevention. Int J Mol Sci. 2008;9(6):1096‐1107. 10.3390/ijms9061096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Fulda S, Kroemer G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov Today. 2009;14(17‐18):885‐890. 10.1016/j.drudis.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 122. Chintharlapalli S, Papineni S, Lei P, Pathi S, Safe S. Betulinic acid inhibits colon cancer cell and tumor growth and induces proteasome‐dependent and ‐independent downregulation of specificity proteins (Sp) transcription factors. BMC Cancer. 2011;11(1):371. 10.1186/1471-2407-11-371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Asif M, Shafaei A, Abdul Majid AS, et al. Mesua ferrea stem bark extract induces apoptosis and inhibits metastasis in human colorectal carcinoma HCT 116 cells, through modulation of multiple cell signalling pathways. Chin J Nat Med. 2017;15(7):505‐514. 10.1016/S1875-5364(17)30076-6 [DOI] [PubMed] [Google Scholar]
- 124. Heckman MA, Weil J, De Mejia EG. Caffeine (1, 3, 7‐trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci. 2010;75(3):R77‐R87. 10.1111/j.1750-3841.2010.01561.x [DOI] [PubMed] [Google Scholar]
- 125. Dong J, Zou J, Yu X‐F. Coffee drinking and pancreatic cancer risk: a meta‐analysis of cohort studies. World J Gastroenterol. 2011;17(9):1204‐1210. 10.3748/wjg.v17.i9.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Edling CE, Selvaggi F, Ghonaim R, Maffucci T, Falasca M. Caffeine and the analog CGS 15943 inhibit cancer cell growth by targeting the phosphoinositide 3‐kinase/Akt pathway. Cancer Biol Ther. 2014;15(5):524‐532. 10.4161/cbt.28018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Micek A, Gniadek A, Kawalec P, Brzostek T. Coffee consumption and colorectal cancer risk: a dose‐response meta‐analysis on prospective cohort studies. Int J Food Sci Nutr. 2019;70(8):986‐1006. 10.1080/09637486.2019.1591352 [DOI] [PubMed] [Google Scholar]
- 128. Wang A, Wang S, Zhu C, et al. Coffee and cancer risk: a meta‐analysis of prospective observational studies. Sci Rep. 2016;6:33711. 10.1038/srep33711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cappelletti S, Piacentino D, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2015;13(1):71‐88. 10.2174/1570159X13666141210215655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Temple JL, Bernard C, Lipshultz SE, Czachor JD, Westphal JA, Mestre MA. The safety of ingested caffeine: a comprehensive review. Front Psychiatry . 2017;8:80. 10.3389/fpsyt.2017.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Inoue M, Tsugane S. Coffee drinking and reduced risk of liver cancer: update on epidemiological findings and potential mechanisms. Curr Nutr Rep. 2019;8(3):182‐186. 10.1007/s13668-019-0274-1 [DOI] [PubMed] [Google Scholar]
- 132. Tej GNVC, Nayak PK. Mechanistic considerations in chemotherapeutic activity of caffeine. Biomed Pharmacother. 2018;105:312‐319. 10.1016/j.biopha.2018.05.144 [DOI] [PubMed] [Google Scholar]
- 133. Gaascht F, Dicato M, Diederich M. Coffee provides a natural multitarget pharmacopeia against the hallmarks of cancer. Genes Nutr. 2015;10(6):51. 10.1007/s12263-015-0501-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Al‐Ansari MM, Aboussekhra A. Caffeine mediates sustained inactivation of breast cancer‐associated myofibroblasts via up‐regulation of tumor suppressor genes. PLoS One. 2014;9(3):e90907. 10.1371/journal.pone.0090907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM. Pharmacological application of caffeine inhibits TGF‐beta‐stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3‐dependent pathways. J Hepatol. 2008;49(5):758‐767. 10.1016/j.jhep.2008.03.029 [DOI] [PubMed] [Google Scholar]
- 136. Ko J‐H, Yang MH, Baek SH, Nam D, Jung SH, Ahn KS. Theacrine attenuates epithelial mesenchymal transition in human breast cancer MDA‐MB‐231 cells. Phytother Res. 2019;33(7):1934‐1942. 10.1002/ptr.6389 [DOI] [PubMed] [Google Scholar]
- 137. D'Arena G, Simeon V, De Martino L, et al. Regulatory T‐cell modulation by green tea in chronic lymphocytic leukemia. Int J Immunopathol Pharmacol. 2013;26(1):117‐125. 10.1177/039463201302600111 [DOI] [PubMed] [Google Scholar]
- 138. Gupta A, Singh AK, Kumar R, et al. Corilagin in cancer: a critical evaluation of anticancer activities and molecular mechanisms. Mol. 2019;24(18):3399. 10.3390/molecules24183399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jia L, Jin H, Zhou J, et al. A potential anti‐tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF‐beta signaling pathways. BMC Complement Altern Med. 2013;13:33. 10.1186/1472-6882-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jia L, Zhou J, Zhao H, et al. Corilagin sensitizes epithelial ovarian cancer to chemotherapy by inhibiting Snail‑glycolysis pathways. Oncol Rep. 2017;38(4):2464‐2470. 10.3892/or.2017.5886 [DOI] [PubMed] [Google Scholar]
- 141. Xu X‐Y, Meng X, Li S, Gan R‐Y, Li Y, Li H‐B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: current progress, challenges, and perspectives. Nutrients. 2018;10(10):1553. 10.3390/nu10101553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Li H, Sureda A, Devkota HP, et al. Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol Adv. 2020;38:107343. 10.1016/j.biotechadv.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 143. Singh L, Sharma S, Xu S, Tewari D, Fang J. Curcumin as a natural remedy for atherosclerosis: a pharmacological review. Molecules. 2021;26(13):4036. 10.3390/molecules26134036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an “old‐age” disease with an “age‐old” solution. Cancer Lett. 2008;267(1):133‐164. 10.1016/j.canlet.2008.03.025 [DOI] [PubMed] [Google Scholar]
- 145. Zhan J‐W, Jiao D‐M, Wang Y, et al. Integrated microRNA and gene expression profiling reveals the crucial miRNAs in curcumin anti‐lung cancer cell invasion. Thorac Cancer. 2017;8(5):461‐470. 10.1111/1759-7714.12467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Thacker PC, Karunagaran D. Curcumin and emodin down‐regulate TGF‐beta signaling pathway in human cervical cancer cells. PLoS One. 2015;10(3):e0120045. 10.1371/journal.pone.0120045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang L, Cheng X, Gao Y, et al. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down‐regulation of the TGF‐beta/Smad2/3 signaling pathway. Exp Cell Res. 2016;341(2):157‐165. 10.1016/j.yexcr.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 148. Sun X‐D, Liu X‐E, Huang D‐S. Curcumin reverses the epithelial‐mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29(6):2401‐2407. [DOI] [PubMed] [Google Scholar]
- 149. Namkaew J, Jaroonwitchawan T, Rujanapun N, Saelee J, Noisa P. Combined effects of curcumin and doxorubicin on cell death and cell migration of SH‐SY5Y human neuroblastoma cells. In Vitro Cell Dev Biol Anim. 2018;54(9):629‐639. 10.1007/s11626-018-0288-9 [DOI] [PubMed] [Google Scholar]
- 150.Firouzi Amoodizaj F, Baghaeifar S, Taheri E, et al. Enhanced anticancer potency of doxorubicin in combination with curcumin in gastric adenocarcinoma. J Biochem Mol Toxicol. 2020;34(6):e22486. 10.1002/jbt.22486 [DOI] [PubMed] [Google Scholar]
- 151. Yin J, Wang L, Wang Y, Shen H, Wang X, Wu L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF‐β/Smad2/3 signaling pathway. Onco Targets Ther. 2019;12:3893‐3903. 10.2147/OTT.S199601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chen W‐C, Lai Y‐A, Lin Y‐C, et al. Curcumin suppresses doxorubicin‐induced epithelial‐mesenchymal transition via the inhibition of TGF‐β and PI3K/AKT signaling pathways in triple‐negative breast cancer cells. J Agric Food Chem. 2013;61(48):11817‐11824. 10.1021/jf404092f [DOI] [PubMed] [Google Scholar]
- 153. Mutlu Altundağ E, Yılmaz AM, Serdar BS, Jannuzzi AT, Koçtürk S, Yalçın AS. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells: II. signal transduction pathways involved. Nutr Cancer. 2021;73(4):703‐712. 10.1080/01635581.2020.1767167 [DOI] [PubMed] [Google Scholar]
- 154. Sandhiutami NMD, Arozal W, Louisa M, Rahmat D, Wuyung PE. Curcumin nanoparticle enhances the anticancer effect of cisplatin by inhibiting PI3K/AKT and JAK/STAT3 pathway in rat ovarian carcinoma induced by DMBA. Front Pharmacol. 2020;11:603235. 10.3389/fphar.2020.603235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Datta R, Halder SK, Zhang B. Role of TGF‐β signaling in curcumin‐mediated inhibition of tumorigenicity of human lung cancer cells. J Cancer Res Clin Oncol. 2013;139(4):563‐572. 10.1007/s00432-012-1352-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tian X, Liu K, Zu X, et al. 3,3’‐Diindolylmethane inhibits patient‐derived xenograft colon tumor growth by targeting COX1/2 and ERK1/2. Cancer Lett. 2019;448:20‐30. 10.1016/j.canlet.2019.01.031 [DOI] [PubMed] [Google Scholar]
- 157. Kim SM. Cellular and molecular mechanisms of 3,3’‐Diindolylmethane in gastrointestinal cancer. Int J Mol Sci. 2016;17(7):1155. 10.3390/ijms17071155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Leong H, Firestone GL, Bjeldanes LF. Cytostatic effects of 3,3’‐diindolylmethane in human endometrial cancer cells result from an estrogen receptor‐mediated increase in transforming growth factor‐alpha expression. Carcinogenesis. 2001;22(11):1809‐1817. 10.1093/carcin/22.11.1809 [DOI] [PubMed] [Google Scholar]
- 159. Lee J. 3,3’‐Diindolylmethane inhibits TNF‐α‐ and TGF‐β‐induced epithelial‐mesenchymal transition in breast cancer cells. Nutr Cancer. 2019;71(6):992‐1006. 10.1080/01635581.2019.1577979 [DOI] [PubMed] [Google Scholar]
- 160. Cheng S‐M, Ho Y‐J, Yu S‐H, et al. Anti‐apoptotic effects of diosgenin in D‐galactose‐induced aging brain. Am J Chin Med. 2020:48(2):391‐406. 10.1142/S0192415X20500202 [DOI] [PubMed] [Google Scholar]
- 161. Wang L, Ma T, Zheng Y, Lv S, Li Y, Liu S. Diosgenin inhibits IL‐1β‐induced expression of inflammatory mediators in human osteoarthritis chondrocytes. Int J Clin Exp Pathol. 2015;8(5):4830‐4836. [PMC free article] [PubMed] [Google Scholar]
- 162. Son IS, Kim JH, Sohn HY, Son KH, Kim J‐S, Kwon C‐S. Antioxidative and hypolipidemic effects of diosgenin, a steroidal saponin of yam (Dioscorea spp.), on high‐cholesterol fed rats. Biosci Biotechnol Biochem. 2007;71(12):3063‐3071. 10.1271/bbb.70472 [DOI] [PubMed] [Google Scholar]
- 163. Gan Q, Wang J, Hu J, et al. The role of diosgenin in diabetes and diabetic complications. J Steroid Biochem Mol Biol. 2020;198:105575. 10.1016/j.jsbmb.2019.105575. [DOI] [PubMed] [Google Scholar]
- 164. Sethi G, Shanmugam MK, Warrier S, et al. Pro‐apoptotic and anti‐cancer properties of diosgenin: a comprehensive and critical review. Nutrients. 2018;10(5):645. 10.3390/nu10050645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Michalak O, Krzeczynski P, Cieslak M, et al. Synthesis and anti‐tumour, immunomodulating activity of diosgenin and tigogenin conjugates. J Steroid Biochem Mol Biol. 2020;198:105573. 10.1016/j.jsbmb.2019.105573 [DOI] [PubMed] [Google Scholar]
- 166. Raju J, Mehta R. Cancer chemopreventive and therapeutic effects of diosgenin, a food saponin. Nutr Cancer. 2009;61(1):27‐35. 10.1080/01635580802357352 [DOI] [PubMed] [Google Scholar]
- 167. Jesus M, Martins APJ, Gallardo E, Silvestre S. Diosgenin: recent Highlights on Pharmacology and Analytical Methodology. J Anal Methods Chem. 2016;2016:4156293. 10.1155/2016/4156293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Kim E‐A, Jang J‐H, Lee Y‐H, et al. Dioscin induces caspase‐independent apoptosis through activation of apoptosis‐inducing factor in breast cancer cells. Apoptosis. 2014;19(7):1165‐1175. 10.1007/s10495-014-0994-z [DOI] [PubMed] [Google Scholar]
- 169. Aumsuwan P, Khan SI, Khan IA, et al. The anticancer potential of steroidal saponin, dioscin, isolated from wild yam (Dioscorea villosa) root extract in invasive human breast cancer cell line MDA‐MB‐231 in vitro. Arch Biochem Biophys. 2016;591:98‐110. 10.1016/j.abb.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 170. Chen B, Zhou S, Zhan Y, et al. Dioscin inhibits the invasion and migration of hepatocellular carcinoma HepG2 cells by reversing TGF‐beta1‐induced epithelial‐mesenchymal transition. Molecules. 2019;24(12):2222. 10.3390/molecules24122222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Lim W‐C, Kim H, Kim Y‐J, et al. Dioscin suppresses TGF‐β1‐induced epithelial‐mesenchymal transition and suppresses A549 lung cancer migration and invasion. Bioorg Med Chem Lett. 2017;27(15):3342‐3348. 10.1016/j.bmcl.2017.06.014 [DOI] [PubMed] [Google Scholar]
- 172. Shrimali D, Shanmugam MK, Kumar AP, et al. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013;341(2):139‐149. 10.1016/j.canlet.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 173. Wang L‐N, Li Z‐J, Xi Y‐M, et al. Effect of emodin combined with AZT on the proliferation and the expression of BCL‐2, NF‐κB, TGF‐β in the leukemia stem cells‐KG‐1a cells]. Zhongguo shi yan xue ye xue za zhi. 2015;23(5):1265‐1271. 10.7534/j.issn.1009-2137.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 174. Shibata S, Ando T, Tanaka O, Meguro Y, Sôma K, Iida Y. Saponins and sapogenins of Panax ginseng C.A. Meyer and some other Panax spp. Yakugaku Zasshi. 1965;85(8):753‐755. [PubMed] [Google Scholar]
- 175. Sodrul IMD, Wang C, Chen X, Du J, Sun H. Role of ginsenosides in reactive oxygen species‐mediated anticancer therapy. Oncotarget. 2018;9(2):2931‐2950. 10.18632/oncotarget.23407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Guo Y‐H, Kuruganti R, Gao Y. Recent advances in ginsenosides as potential therapeutics against breast cancer. Curr Top Med Chem. 2019;19(25):2334‐2347. 10.2174/1568026619666191018100848 [DOI] [PubMed] [Google Scholar]
- 177. Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3 (Review). Int J Mol Med. 2017;39(3):507‐518. 10.3892/ijmm.2017.2857 [DOI] [PubMed] [Google Scholar]
- 178. Wang C‐Z, Anderson S, DU W, He T‐C, Yuan C‐S. Red ginseng and cancer treatment. Chin J Nat Med. 2016;14(1):7‐16. 10.3724/SP.J.1009.2016.00007 [DOI] [PubMed] [Google Scholar]
- 179. Dai G, Sun B, Gong T, Pan Z, Meng Q, Ju W. Ginsenoside Rb2 inhibits epithelial‐mesenchymal transition of colorectal cancer cells by suppressing TGF‐beta/Smad signaling. Phytomedicine. 2019;56:126‐135. 10.1016/j.phymed.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 180. Kim JH, Kim M, Yun S‐M, et al. Ginsenoside Rh2 induces apoptosis and inhibits epithelial‐mesenchymal transition in HEC1A and Ishikawa endometrial cancer cells. Biomed Pharmacother. 2017;96:871‐876. 10.1016/j.biopha.2017.09.033 [DOI] [PubMed] [Google Scholar]
- 181. Zhang Q, Hong B, Wu S, Niu T. Inhibition of prostatic cancer growth by ginsenoside Rh2. Tumour Biol. 2015;36(4):2377‐2381. 10.1007/s13277-014-2845-5 [DOI] [PubMed] [Google Scholar]
- 182. Wang D, Wu C, Liu D, et al. Ginsenoside Rg3 Inhibits Migration and Invasion of Nasopharyngeal Carcinoma Cells and Suppresses Epithelial Mesenchymal Transition. Wei J‐F. Biomed Res Int. 2019;2019:8407683. 10.1155/2019/8407683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wu G‐R, Xu B, Yang Y‐Q, et al. Synthesis and biological evaluation of podophyllotoxin derivatives as selective antitumor agents. Eur J Med Chem. 2018;155:183‐196. 10.1016/j.ejmech.2018.05.052 [DOI] [PubMed] [Google Scholar]
- 184. Ardalani H, Avan A, Ghayour‐Mobarhan M. Podophyllotoxin: a novel potential natural anticancer agent. Avicenna J phytomedicine. 2017;7(4):285‐294. [PMC free article] [PubMed] [Google Scholar]
- 185. Giri A, Lakshmi Narasu M. Production of podophyllotoxin from Podophyllum hexandrum: a potential natural product for clinically useful anticancer drugs. Cytotechnology. 2000;34(1‐2):17‐26. 10.1023/A:1008138230896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Li Y, Wang T, Sun Y, et al. p53‐mediated PI3K/AKT/mTOR pathway played a role in PtoxDpt‐induced EMT inhibition in liver cancer cell lines. Oxid Med Cell Longev. 2019:2531493. 10.1155/2019/2531493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Sharma S, Mehak Chhimwal J, Patial V, Sk UH. Dendrimer‐conjugated podophyllotoxin suppresses DENA‐induced HCC progression by modulation of inflammatory and fibrogenic factors. Toxicol Res (Camb). 2019;8(4):560‐567. 10.1039/c9tx00103d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Yeung AWK, Aggarwal BB, Orhan IE, et al. Resveratrol, a popular dietary supplement for human and animal health: quantitative research literature analysis‐a review. Anim. Sci. Pap. Rep. 2019;37(2):103‐118.
- 189. Ko J‐H, Sethi G, Um J‐Y, et al. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18(12):2589. 10.3390/ijms18122589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Pangeni R, Sahni JK, Ali J, Sharma S, Baboota S. Resveratrol: review on therapeutic potential and recent advances in drug delivery. Expert Opin Drug Deliv. 2014;11(8):1285‐1298. 10.1517/17425247.2014.919253 [DOI] [PubMed] [Google Scholar]
- 191. Singh AP, Singh R, Verma SS, et al. Health benefits of resveratrol: evidence from clinical studies. Med Res Rev. 2019;39(5):1851‐1891. 10.1002/med.21565 [DOI] [PubMed] [Google Scholar]
- 192. Meng X, Zhou J, Zhao C‐N, Gan R‐Y, Li H‐B. Health benefits and molecular mechanisms of resveratrol: a narrative review. Foods (Basel, Switzerland). 2020;9(3):340. 10.3390/foods9030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Vestergaard M, Ingmer H. Antibacterial and antifungal properties of resveratrol. Int J Antimicrob Agents. 2019;53(6):716‐723. 10.1016/j.ijantimicag.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 194. Ji Q, Liu X, Han Z, et al. Resveratrol suppresses epithelial‐to‐mesenchymal transition in colorectal cancer through TGF‐beta1/Smads signaling pathway mediated Snail/E‐cadherin expression. BMC Cancer. 2015;15:97. 10.1186/s12885-015-1119-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Yang H, Yuan Y, Luo C, He H, Zhou Y. Inhibitory effects of resveratrol on the human alveolar rhabdomyosarcoma cell line PLA‐802 through inhibition of the TGF‐beta1/Smad signaling pathway. Pharmacology. 2016;98(1‐2):35‐41. 10.1159/000443966 [DOI] [PubMed] [Google Scholar]
- 196. Shi X‐P, Miao S, Wu Y, et al. Resveratrol sensitizes tamoxifen in antiestrogen‐resistant breast cancer cells with epithelial‐mesenchymal transition features. Int J Mol Sci. 2013;14(8):15655‐15668. 10.3390/ijms140815655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Whyte L, Huang Y‐Y, Torres K, Mehta RG. Molecular mechanisms of resveratrol action in lung cancer cells using dual protein and microarray analyses. Cancer Res. 2007;67(24):12007‐12017. 10.1158/0008-5472.CAN-07-2464 [DOI] [PubMed] [Google Scholar]
- 198. Sun Y, Zhou Q‐M, Lu Y‐Y, et al. Resveratrol inhibits the migration and metastasis of MDA‐MB‐231 human breast cancer by reversing TGF‐β1‐Induced epithelial‐mesenchymal transition. Molecules. 2019;24(6):1131. 10.3390/molecules24061131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Marimuthu P, Kaur K, Kandalam U, et al. Treatment of ovarian cancer cells with nutlin‐3 and resveratrol combination leads to apoptosis via caspase activation. J Med Food. 2011;14(1‐2):46‐52. 10.1089/jmf.2009.0270 [DOI] [PubMed] [Google Scholar]
- 200. Yang M‐D, Sun Y, Zhou W‐J, et al. Resveratrol enhances inhibition effects of cisplatin on cell migration and invasion and tumor growth in breast cancer MDA‐MB‐231 cell models in vivo and in vitro. Molecules. 2021;26(8):2204. 10.3390/molecules26082204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Kabel AM, Atef A, Estfanous RS. Ameliorative potential of sitagliptin and/or resveratrol on experimentally‐induced clear cell renal cell carcinoma. Biomed Pharmacother. 2018;97:667‐674. 10.1016/j.biopha.2017.10.149 [DOI] [PubMed] [Google Scholar]
- 202. Kim JS, Jeong SK, Oh SJ, et al. The resveratrol analogue, HS‑1793, enhances the effects of radiation therapy through the induction of anti‑tumor immunity in mammary tumor growth. Int J Oncol. 2020;56(6):1405‐1416. 10.3892/ijo.2020.5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Houghton CA. Sulforaphane: its “coming of age” as a clinically relevant nutraceutical in the prevention and treatment of chronic disease. Oxid Med Cell Longev. 2019;2019:2716870. 10.1155/2019/2716870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Hudlikar R, Wang L, Wu R, et al. Epigenetics/epigenomics and prevention of early stages of cancer by isothiocyanates. Cancer Prev Res (Phila). 2021;14(2):151‐164. 10.1158/1940-6207.CAPR-20-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Yagishita Y, Fahey JW, Dinkova‐Kostova AT, Kensler TW. Broccoli or sulforaphane: is it the source or dose that matters? Mol. 2019;24(19):3593. 10.3390/molecules24193593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Kaiser AE, Baniasadi M, Giansiracusa D, et al. Sulforaphane: a broccoli bioactive phytocompound with cancer preventive potential. Cancers. 2021;13(19). 10.3390/cancers13194796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Wu J, Han J, Hou B, Deng C, Wu H, Shen L. Sulforaphane inhibits TGF‐β‐induced epithelial‐mesenchymal transition of hepatocellular carcinoma cells via the reactive oxygen species‐dependent pathway. Oncol Rep. 2016;35(5):2977‐2983. 10.3892/or.2016.4638 [DOI] [PubMed] [Google Scholar]
- 208. Luo Y, Yan B, Liu L, et al. Sulforaphane inhibits the expression of long noncoding RNA H19 and its target APOBEC3G and thereby pancreatic cancer progression. Cancers. 2021;13(4):827. 10.3390/cancers13040827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Asaduzzaman Khan M, Tania M, Fu S, Fu J. Thymoquinone, as an anticancer molecule: from basic research to clinical investigation. Oncotarget. 2017;8(31):51907‐51919. 10.18632/oncotarget.17206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Darakhshan S, Bidmeshki Pour A, Hosseinzadeh Colagar S, Sisakhtnezhad S. Thymoquinone and its therapeutic potentials. Pharmacol Res. 2015;95‐96:138‐158. 10.1016/j.phrs.2015.03.011 [DOI] [PubMed] [Google Scholar]
- 211. Ahmad A, Mishra RK, Vyawahare A, et al. Thymoquinone (2‐Isoprpyl‐5‐methyl‐1, 4‐benzoquinone) as a chemopreventive/anticancer agent: chemistry and biological effects. Saudi Pharm J SPJ Off Publ Saudi Pharm Soc. 2019;27(8):1113‐1126. 10.1016/j.jsps.2019.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Shanmugam MK, Arfuso F, Kumar AP, et al. Modulation of diverse oncogenic transcription factors by thymoquinone, an essential oil compound isolated from the seeds of Nigella sativa Linn. Pharmacol Res. 2018;129:357‐364. 10.1016/j.phrs.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 213. Rajput S, Kumar BNP, Banik P, Parida S, Mandal M. Thymoquinone restores radiation‐induced TGF‐β expression and abrogates EMT in chemoradiotherapy of breast cancer cells. J Cell Physiol. 2015;230(3):620‐629. 10.1002/jcp.24780 [DOI] [PubMed] [Google Scholar]
- 214. Helmy SA, El‐Mesery M, El‐Karef A, Eissa LA, El Gayar AM. Thymoquinone upregulates TRAIL/TRAILR2 expression and attenuates hepatocellular carcinoma in vivo model. Life Sci. 2019;233:116673. 10.1016/j.lfs.2019.116673 [DOI] [PubMed] [Google Scholar]
- 215. Liou Y‐F, Hsieh Y‐S, Hung T‐W, et al. Thymoquinone inhibits metastasis of renal cell carcinoma cell 786‐O‐SI3 associating with downregulation of MMP‐2 and u‐PA and suppression of PI3K/Src signaling. Int J Med Sci. 2019;16(5):686‐695. 10.7150/ijms.32763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chen S‐R, Dai Y, Zhao J, Lin L, Wang Y, Wang Y. A Mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front Pharmacol. 2018;9:104. 10.3389/fphar.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Noel P, Von Hoff DD, Saluja AK, Velagapudi M, Borazanci E, Han H. Triptolide and its derivatives as cancer therapies. Trends Pharmacol Sci. 2019;40(5):327‐341. 10.1016/j.tips.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 218. Ding X, Zhou X, Jiang B, Zhao Q, Zhou G. Triptolide suppresses proliferation, hypoxia‐inducible factor‐1α and c‐Myc expression in pancreatic cancer cells. Mol Med Rep. 2015;12(3):4508‐4513. 10.3892/mmr.2015.3960 [DOI] [PubMed] [Google Scholar]
- 219. Johnson SM, Wang X, Evers BM. Triptolide inhibits proliferation and migration of colon cancer cells by inhibition of cell cycle regulators and cytokine receptors. J Surg Res. 2011;168(2): 97‐205. 10.1016/j.jss.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Qi Y, Li J. Triptolide inhibits the growth and migration of colon carcinoma cells by down‐regulation of miR‐191. Exp Mol Pathol. 2019;107:23‐31. 10.1016/j.yexmp.2019.01.008 [DOI] [PubMed] [Google Scholar]
- 221. Liu B, Zhang H, Li J, et al. Triptolide downregulates Treg cells and the level of IL‐10, TGF‐β, and VEGF in melanoma‐bearing mice. Planta Med. 2013;79(15):1401‐1407. 10.1055/s-0033-1350708 [DOI] [PubMed] [Google Scholar]
- 222. Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: a Rasayana (rejuvenator) of Ayurveda. African J Tradit Complement Altern Med AJTCAM. 2011;8(5):208‐213. 10.4314/ajtcam.v8i5S.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Tewari D, Chander V, Dhyani A, et al. Withania somnifera (L.) Dunal: phytochemistry, structure‐activity relationship, and anticancer potential. Phytomedicine. 2022;98: 153949. 10.1016/j.phymed.2022.153949 [DOI] [PubMed] [Google Scholar]
- 224. Lee J, Hahm E‐R, Marcus AI, Singh SV. Withaferin A inhibits experimental epithelial–mesenchymal transition in MCF‐10A cells and suppresses vimentin protein level in vivo in breast tumors. Mol Carcinog. 2015;54(6):417‐429. 10.1002/mc.22110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Lee DH, Lim I‐H, Sung E‐G, et al. Withaferin A inhibits matrix metalloproteinase‐9 activity by suppressing the Akt signaling pathway. Oncol Rep. 2013;30(2):933‐938. 10.3892/or.2013.2487. [DOI] [PubMed] [Google Scholar]
- 226. Zhang Y, Huang P, Liu X, et al. Polyphyllin I inhibits growth and invasion of cisplatin‐resistant gastric cancer cells by partially inhibiting CIP2A/PP2A/Akt signaling axis. J Pharmacol Sci. 2018;137(3):305‐312. 10.1016/j.jphs.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 227. Forestier‐Román IS, López‐Rivas A, Sánchez‐Vázquez MM, et al. Andrographolide induces DNA damage in prostate cancer cells. Oncotarget. 2019;10(10):1085‐1101. 10.18632/oncotarget.26628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Yang Z, Garcia A, Xu S, et al. Withania somnifera root extract inhibits mammary cancer metastasis and epithelial to mesenchymal transition. PLoS One. 2013;8(9):e75069. 10.1371/journal.pone.0075069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8(1):69‐85. 10.1038/nrd2487 [DOI] [PubMed] [Google Scholar]
- 230. Dayanidhi DL, Thomas BC, Osterberg JS, et al. Exploring the diversity of the marine environment for new anti‐cancer compounds. Front Mar Sci. 2021;7:1184. https://www.frontiersin.org/article/10.3389/fmars.2020.614766 [Google Scholar]
- 231. Simmons TL, Andrianasolo E, McPhail K, Flatt P, Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4(2):333 LP‐342. http://mct.aacrjournals.org/content/4/2/333.abstract [PubMed] [Google Scholar]
- 232. Lichota A, Gwozdzinski K. Anticancer activity of natural compounds from plant and marine environment. Int J Mol Sci. 2018;19(11):3533. 10.3390/ijms19113533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Correia‐da‐Silva M, Sousa E, Pinto MMM, Kijjoa A. Anticancer and cancer preventive compounds from edible marine organisms. Semin Cancer Biol. 2017;46:55‐64. 10.1016/j.semcancer.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 234. Calcabrini C, Catanzaro E, Bishayee A, Turrini E, Fimognari C. Marine sponge natural products with anticancer potential: an updated review. Mar Drugs. 2017;15(10):310. 10.3390/md15100310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Mondal A, Bose S, Banerjee S, et al. Marine cyanobacteria and microalgae metabolites‐a rich source of potential anticancer drugs. Mar Drugs. 2020;18(9):476. 10.3390/md18090476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Zhou J, You W, Sun G, et al. The marine‐derived oligosaccharide sulfate MS80, a novel transforming growth factor β1 inhibitor, reverses epithelial mesenchymal transition induced by transforming growth factor‐β1 and suppresses tumor metastasis. J Pharmacol Exp Ther. 2016;359(1):54‐61. 10.1124/jpet.116.234799 [DOI] [PubMed] [Google Scholar]
- 237. Kojima‐Yuasa A, Huang X, Matsui‐Yuasa I. Synergistic anticancer activities of natural substances in human hepatocellular carcinoma. Dis (Basel, Switzerland). 2015;3(4):260‐281. 10.3390/diseases3040260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Paramita P, Wardhani BWK, Wanandi SI, Louisa M. Curcumin for the prevention of epithelial‐mesenchymal transition in endoxifen‐treated MCF‐7 breast cancer cel. Asian Pac J Cancer Prev. 2018;19(5):1243‐1249. 10.22034/APJCP.2018.19.5.1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Katoh M. Canonical and non‐canonical WNT signaling in cancer stem cells and their niches: cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review). Int J Oncol. 2017;51(5):1357‐1369. 10.3892/ijo.2017.4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Zhang Q, Liu F, Qin L, et al. Characterization of TGFβ‐associated molecular features and drug responses in gastrointestinal adenocarcinoma. BMC Gastroenterol. 2021;21(1):284. 10.1186/s12876-021-01869-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Helleman J, Jansen MPHM, Burger C, van der Burg MEL, Berns EMJJ. Integrated genomics of chemotherapy resistant ovarian cancer: a role for extracellular matrix, TGFbeta and regulating microRNAs. Int J Biochem Cell Biol. 2010;42(1):25‐30. 10.1016/j.biocel.2009.10.016 [DOI] [PubMed] [Google Scholar]
- 242. Guerrero‐Martínez JA, Ceballos‐Chávez M, Koehler F, Peiró S, Reyes JC. TGFβ promotes widespread enhancer chromatin opening and operates on genomic regulatory domains. Nat Commun. 2020;11(1):6196. 10.1038/s41467-020-19877-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Schwörer S, Berisa M, Violante S, et al. Proline biosynthesis is a vent for TGFβ‐induced mitochondrial redox stress. EMBO J. 2020;39(8):e103334. 10.15252/embj.2019103334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Ikeda M, Takahashi H, Kondo S, et al. Phase 1b study of galunisertib in combination with gemcitabine in Japanese patients with metastatic or locally advanced pancreatic cancer. Cancer Chemother Pharmacol. 2017;79(6):1169‐1177. 10.1007/s00280-017-3313-x [DOI] [PubMed] [Google Scholar]
- 245. Wick A, Desjardins A, Suarez C, et al. Phase 1b/2a study of galunisertib, a small molecule inhibitor of transforming growth factor‐beta receptor I, in combination with standard temozolomide‐based radiochemotherapy in patients with newly diagnosed malignant glioma. Invest New Drugs. 2020;38(5):1570‐1579. 10.1007/s10637-020-00910-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Trachtman H, Fervenza FC, Gipson DS, et al. A phase 1, single‐dose study of fresolimumab, an anti‐TGF‐β antibody, in treatment‐resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79(11):1236‐1243. 10.1038/ki.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Howitz KT, Sinclair DA. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133(3):387‐391. 10.1016/j.cell.2008.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the Last 25 Years. J Nat Prod. 2007;70(3):461‐477. [DOI] [PubMed] [Google Scholar]
- 249. Morris JC, Tan AR, Olencki TE, et al. Phase I study of GC1008 (fresolimumab): a human anti‐transforming growth factor‐beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. 2014;9(3):e90353. 10.1371/journal.pone.0090353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Tolcher AW, Berlin JD, Cosaert J, et al. HHS Public Access. 2018;79(4):673‐680. 10.1007/s00280-017-3245-5.A [DOI] [Google Scholar]
- 251. Holmgaard RB, Schaer DA, Li Y, et al. Targeting the TGF β pathway with galunisertib, a TGF β RI small molecule inhibitor, promotes anti‐tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer. 2018:6(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Melisi D, Garcia‐Carbonero R, Macarulla T, et al. Galunisertib plus gemcitabine vs. gemcitabine for first‐line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119(10):1208‐1214. 10.1038/s41416-018-0246-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Huynh LK, Hipolito CJ, Ten DijkeP. A perspective on the development of TGF‐β inhibitors for cancer treatment. Biomolecules. 2019;9(11):743. 10.3390/biom9110743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Caplin ME, Pavel M, Ćwikła JB, et al. Anti‐tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open‐label extension study. Endocr Relat Cancer. 2016;23(3):191‐199. 10.1530/ERC-15-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open‐label, phase II study of romidepsin in relapsed or refractory peripheral T‐cell lymphoma after prior systemic therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(6):631‐636. 10.1200/JCO.2011.37.4223 [DOI] [PubMed] [Google Scholar]
- 256. Choi YJ, Kim HS, Park SH, et al. Phase II study of dovitinib in patients with castration‐resistant prostate cancer (KCSG‐GU11‐05). Cancer Res Treat. 2018;50(4):1252‐1259. 10.4143/crt.2017.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD‐L1 and TGFβ, in advanced solid tumors. Clin Cancer Res. 2018;24(6):1287‐1295. 10.1158/1078-0432.CCR-17-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Li L, Yue GG‐L, Lee JK‐M, et al. Gene expression profiling reveals the plausible mechanisms underlying the antitumor and antimetastasis effects of Andrographis paniculata in esophageal cancer. Phytother Res. 2018;32(7):1388‐1396. 10.1002/ptr.6074 [DOI] [PubMed] [Google Scholar]
- 259. Pitchakarn P, Ogawa K, Suzuki S, et al. Momordica charantia leaf extract suppresses rat prostate cancer progression in vitro and in vivo. Cancer Sci. 2010;101(10):2234‐2240. 10.1111/j.1349-7006.2010.01669.x [DOI] [PMC free article] [PubMed] [Google Scholar]


