Abstract
Background
This open-label, phase II study aimed to investigate the efficacy and safety of recombinant human endostatin (Rh-endostatin) plus irinotecan/cisplatin as second-line treatment in patients with advanced esophageal squamous cell carcinoma (ESCC).
Methods
Eligible patients received 15mg/m2 Rh-endostatin as a continuous intravenous pump infusion (7 continuous days), 60mg/m2 irinotecan (days 1 and 8), and 60mg/m2 cisplatin (day 1) every 3 weeks. The primary endpoint was progression-free survival (PFS).
Results
A total of 50 patients were assessable for efficacy and safety analysis. The median follow-up was 10.97 months (95%CI: 7.03-19.42) as the data cutoff. Median PFS was 4.01 months (95% CI: 3.19-5.49), and median overall survival (OS) was 12.32 months (95% CI: 8.21-17.45); 13 (26%; 95% CI: 15.87-39.55) of 50 patients had an objective response, and 31 (62%; 95% CI: 48.15-74.14) had disease control. Grade 3 or greater treatment-related adverse events (AEs) occurred in 12 (24.0%) patients, and no deaths were reported. The common grade 3 or greater AEs were leucopenia (18.0%) and neutropenia (16.0%). Five (10%) patients discontinued treatment because of AEs.
Conclusion
Rh-endostatin plus irinotecan/cisplatin showed promising anti-tumor activity in advanced ESCC patients with a good safety profile in the second-line setting, which warrants further study in this population. (ClinicalTrials.gov identifier: NCT03797625).
Keywords: recombinant human endostatin (Rh-endostatin), irinotecan, cisplatin, esophageal squamous cell carcinoma, phase II study
Accumulating evidence has shown that combination therapies with anti-angiogenics are a good strategy for advanced esophageal squamous cell carcinoma (ESCC). This prospective, open-label, phase II study provides the first analysis of the anti-tumor activity and safety for Rh-endostatin plus irinotecan/cisplatin (a commonly used regimen in ESCC) in patients with advanced ESCC.
Lessons Learned.
Rh-endostatin plus irinotecan/cisplatin had promising clinical anti-tumor activity and a manageable safety profile in patients with advanced esophageal squamous cell carcinoma.
Discussion
Esophageal squamous cell carcinoma is known to be a highly angiogenic tumor.1,2 Accumulating evidence has shown that combination therapies with anti-angiogenics are an attractive strategy for advanced ESCC. Recombinant human endostatin (Rh-endostatin), a novel artificially synthesized endostatin, has been shown to potently inhibit angiogenesis.3 Here, this prospective, open-label, phase II study provides the first analysis of the anti-tumor activity and safety for Rh-endostatin plus irinotecan/cisplatin (a commonly used regimen in ESCC) in patients with advanced ESCC.
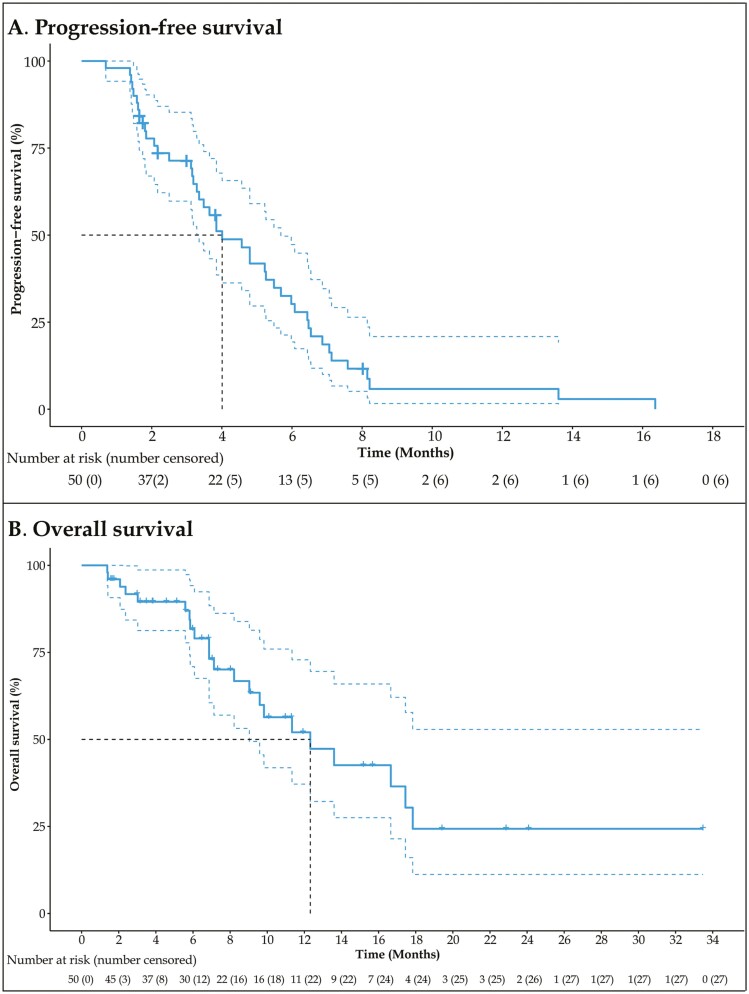
Fifty-two eligible patients were enrolled between May 2017 and June 2020. Fifty patients were assessable for efficacy and safety analysis. The data cutoff for analysis was February 26, 2021, with a median follow-up of 10.97 months (95% CI: 7.03-19.42). Our trial met the primary endpoint, showing promising anti-tumor activity in this patient population in the second-line setting, with a median PFS of 4.01 months (Fig. 1A). At the data cutoff, 22 patients died, and the median OS was 12.32 months (95% CI: 8.21-17.45; Fig. 1B). Target lesion size decreased in 30 patients (60.0%). The objective response rate (ORR) was 26% (95% CI, 15.87-39.55), and the disease control rate was 62% (95% CI, 48.15-74.14).
Figure 1.
Kaplan-Meier analyses of survival. (A) Progression-free survival (PFS). (B) Overall survival (OS).
In terms of safety, Rh-endostatin plus irinotecan/cisplatin had a manageable and acceptable safety profile in this population, which was similar to those previously reported for monotherapies. Rh-endostatin may not increase the risks of toxicity. Common events reported in the study, such as leucopenia, anemia, and neutropenia, were generally manageable with dose reductions, interruptions, or supportive care. Study discontinuation (10.0%) due to adverse events (AEs) was infrequent, and no treatment-related deaths occurred. More importantly, bleeding and gastric perforation, as known AEs of anti-angiogenic therapies, were not reported in our study.
In conclusion, the study suggested that Rh-endostatin plus irinotecan/cisplatin has promising clinical anti-tumor activity and a manageable safety profile in patients with advanced ESCC, which might offer a new potential therapeutic option for this patient population. Furthermore,study is warranted to explore the values of the Rh-endostatin-based regimens with novel therapeutic agents.
Trial Information
| Disease | esophageal cancer |
| Stage of disease/treatment | metastatic/advanced |
| Prior therapy | no designated number of regimens |
| Type of study | phase II, single arm |
| Primary endpoint | progression-free survival |
| Secondary endpoints | overall survival, overall response rate, disease control rate |
| Investigator’s analysis | Active and should be pursued further |
Additional Details of Endpoints or Study Design
Study Design and Participants
In this open-label, single-arm, phase II study (Trial Registration ID: NCT03797625), patients with advanced ESCC were recruited from Fudan University Shanghai Cancer Center (FUSCC) in China. The cutoff date for clinical activity and safety data was February 26, 2021.
Eligible patients were aged from 18 to 75 years with pathologically (cytology or biopsy) confirmed stage IV ESCC according to TNM staging system (AJCC, 2009) and had at least one measurable disease according to Response Evaluation Criteria In Solid Tumors (RECIST, version 1.1) by radiology assessment. Eligible patients were required to have progressive disease after first-line chemotherapy who experienced recurrence after radiation within a year. Inclusion criteria also included an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, a life expectancy of 3 months or longer, the capability to take at least liquid diet, no evidence of esophageal perforation, no unhealed wounds, and no severe allergic reactions to biologics (especially for genetically engineered products made from E. coli). All patients had to have adequate blood coagulation (neutrophil count ≥2×109/L; platelet count ≥100×109/L; hemoglobin ≥90g/L), liver (total bilirubin ≤upper normal limit [UNL]; aspartate aminotransferase and alanine aminotransferase ≤2.5×UNL; alkaline phosphatase ≤5.0×UNL), renal (serum creatinine ≤ULN or creatinine clearance rate ≥60mL/minute), and cardiac function.
Patients who were experiencing uncontrolled severe acute infection, purulent and chronic infection, or had other primary malignancies (except for skin basal cell carcinoma) at screening were ineligible for this study. Patients with complete obstruction of the esophagus, deep esophageal ulceration, perforation, hematemesis, or other complications such as a stomal leak and pulmonary disease were not allowed to participate. Patients were excluded if they had serious comorbidities, such as heart disease (congestive heart failure, high-risk heart failure, myocardial infarction, or refractory hypertension), psychiatric illnesses, bleeding tendency, hereditary bleeding disorder, or evidence of coagulation disorders. Pregnant or lactating patients, as well as patients of childbearing potential and not using contraception if sexually active, were also excluded.
This phase II study was done in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines. The study protocol was reviewed and approved by the institutional review board of Fudan University Shanghai Cancer Center before study initiation (Ethical approval number: 1703170-13; approved on March 27, 2017). Written informed consent was obtained from all patients prior to any study-related procedure in this study.
Procedures
All eligible patients were scheduled to receive 15mg/m2 Rh-endostatin daily as a continuous intravenous pump infusion (7 continuous days), 60mg/m2 intravenous irinotecan (days 1 and 8), and 60mg/m2 intravenous cisplatin (day 1) every 3 weeks. The chemotherapy regimen was administered for 4 to 6 cycles, until disease progression, development of unacceptable toxicity, or withdrawal of consent.
Dose modification was allowed according to the adverse events (AEs) grading. Toxicity was managed with supportive care, prespecified reductions in the doses of irinotecan and cisplatin, and discontinuation of Rh-endostatin dose until AEs became tolerable. The doses of irinotecan and cisplatin could be reduced if patients had unacceptable grade 2 or grade 3 treatment-related AEs, which were determined by investigators. Irinotecan and cisplatin could be resumed after patients recovered, but the dose could not be increased in subsequent cycles. In patients who experienced a cholinergic crisis after irinotecan administration, prophylactic premedication with atropine was permitted for subsequent infusions. If patients experienced irinotecan-induced delayed-onset diarrhea, loperamide was administered at the first evidence of diarrhea and/or abdominal cramps after 24 hours of irinotecan infusion. If patients experienced AEs related to anti-angiogenesis (upper gastrointestinal bleeding, perforation, or thrombosis), the Rh-endostatin dose was discontinued.
Tumor response was assessed by investigators according to RECIST (version 1.1) using central radiology review. Tumor assessments were performed at baseline, every 2 cycles during study treatment, and every 8 weeks after completion of study treatment until disease progression. Laboratory analyses, including hematology, serum biochemistry, and urinalysis, were assessed at baseline, before every treatment cycle, at the end of treatment, and at 8-week follow-up. Throughout the treatment period, all AEs were monitored and recorded. After the last dose of chemotherapy, all patients were followed up every 2 months during the first year, every 3 months during the second year, and every 6 months thereafter to monitor survival.
Outcomes
The primary endpoint was progression-free survival (PFS), defined as the time from the first dose of the study treatment to disease progression or patient death from any cause. Secondary endpoints were overall survival (OS), objective response rate (ORR), and disease control rate (DCR). Overall survival was defined as the time from the first dose of the study treatment to death from any cause. Objective response rate was defined as the percentage of patients who experienced a complete response (CR) or partial response (PR) at the time of data cutoff. DCR was defined as the percentage of patients with CR, PR, or stable disease (SD).
Safety profile was evaluated by AEs through vital signs, laboratory analyses, electrocardiography, chest radiography, and ECOG performance status score. All AEs were assessed by investigators and classified by severity grade using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.0). Serious adverse events were defined as serious if they led to death, life-threatening events, in-patient hospitalization or prolongation of existing hospitalization, a persistent or severe disability or incapacity, congenital anomalies/birth defects, or any other medically important events that required intervention.
Statistical Analysis
The calculation of sample size for this study was based on the analysis of the PFS (primary endpoint). According to historical data4,5, the median PFS was approximately 2.5 months after second-line treatment in patients with ESCC. We hypothesized that the study treatment could improve 1.5 months of PFS and had an expected median PFS of 4 months. A total of 65 patients was required for a power of 93% at a two-sided significance level of 5%, with an expected accrual period of 2 years and a follow-up period of 1 year. Considering a lost-to-follow-up of 16%, 76 patients will be enrolled.
Patient characteristics, safety analysis, and tumor response were summarized descriptively. Progression-free survival and OS were analyzed by the Kaplan-Meier method and expressed as median values with 95% confidence intervals (CI). The 95% CIs of the best overall response were calculated using the Clopper and Pearson method. The best change in target lesions from baseline was calculated as the maximum reduction rate of the sum diameters of target lesions. The last observation carried forward approach was used to impute missing data in the intention-to-treat (ITT) analyses. Efficacy analyses were done in a modified ITT population who received at least one dose of study treatment and underwent any post-treatment assessment. Safety analyses were done in patients who received at least one dose of study treatment and had at least one assessment in safety outcome, regardless of protocol deviation. All statistical analyses were done with R (version 4.0.3, The R Foundation for Statistical Computing, Vienna, Austria).
Drug Information
| Recombinant human endostatin | |
|---|---|
| Generic/working name | Recombinant human endostatin |
| Trade name | Endostar |
| Company name | Nanjing Simcere-Medgenn Bio-Pharmaceuticals Company |
| Drug type | Biological |
| Drug class | Angiogenesis-antivascular |
| Dose | 15mg/m² |
| Route | Continuous intravenous infusion |
| Schedule of administration | Rh-endostatin (15mg/m2) was administered daily as a continuous intravenous pump infusion (7 continuous days) every 3 weeks. |
| Irinotecan | |
| Generic/working name | Irinotecan |
| Trade name | Campto |
| Company name | Pfizer Inc. |
| Drug type | Biological |
| Drug class | Topoisomerase I |
| Dose | 60mg/m² |
| Route | i.v. |
| Schedule of administration | Irinotecan (60mg/m2) was administered intravenously on day 1 and day 8 every 3 weeks. |
| Cisplatin | |
| Generic/working name | Cisplatin |
| Trade name | Nuoxin |
| Company name | HANSOH PHARMA. Inc |
| Drug type | Small molecule |
| Drug class | Platinum compound |
| Dose | 60mg/m² |
| Route | i.v. |
| Schedule of administration | Cisplatin (60mg/m2) was administered intravenously on day 1 every 3 weeks. |
Patient Characteristics
| Number of patients, male | 46 |
| Number of patients, female | 4 |
| Stage | All patients were at stage IV |
| Age | Median (range): 61 (47-74) years |
| Number of prior systemic therapies | Median (range): 3.5 (1-6) |
| Performance Status: ECOG | 0—14
1—36 2—0 3—0 Unknown—0 |
| Other | In this study, 102 patients with advanced ESCC were screened between May 2017 and June 2020. Among these patients, 50 were ineligible. Eventually, a total of 52 patients were enrolled. One patient did not receive study treatment due to withdrawal of consent, and one patient was excluded from the efficacy analysis due to protocol violation that received concurrent radiotherapy. In total, anti-tumor activity and safety outcomes were analyzed in 50 patients (Table 1). The median age of enrolled patients was 61 years (range, 47-74); of these, most patients were male (46/59, 92.0%). Eastern Cooperative Oncology Group performance status score was 0 in 14 (28%) of 50 patients. All patients were at stage IV. Most patients received platinum-based doublet chemotherapy as first-line treatment, with 34.0% of them underwent paclitaxel and carboplatin chemotherapy.
The data cutoff for safety and efficacy analysis was on February 26, 2021, with a median follow-up of 10.97 months (95% CI: 7.03-19.42). At the data cutoff, all patients had discontinued the protocol treatment, including completion of study (n = 25), disease progression (n = 15), AEs (n = 5), and loss to follow up (n = 5). The patients received a median of 3.5 cycles (range, 1-6) of study treatment. |
| Cancer types or histologic subtypes | Esophageal squamous cell carcinoma: 50 |
Primary Assessment Method
| Title | Efficacy |
|---|---|
| Number of patients screened | 102 |
| Number of patients enrolled | 52 |
| Number of patients evaluable for toxicity | 50 |
| Number of patients evaluated for efficacy | 50 |
| Evaluation method | RECIST 1.1 |
| Response assessment CR | n = 0 (0%) |
| Response assessment PR | n = 13 (26%) |
| Response assessment SD | n = 18 (36%) |
| Response assessment PD | n = 15 (30%) |
| Response assessment OTHER | n = 4 (8%) |
| (Median) duration assessments PFS | 4.01 months, CI: 3.19-5.49 |
| (Median) duration assessments OS | 12.32 months, CI: 8.21-17.45 |
Outcome Notes
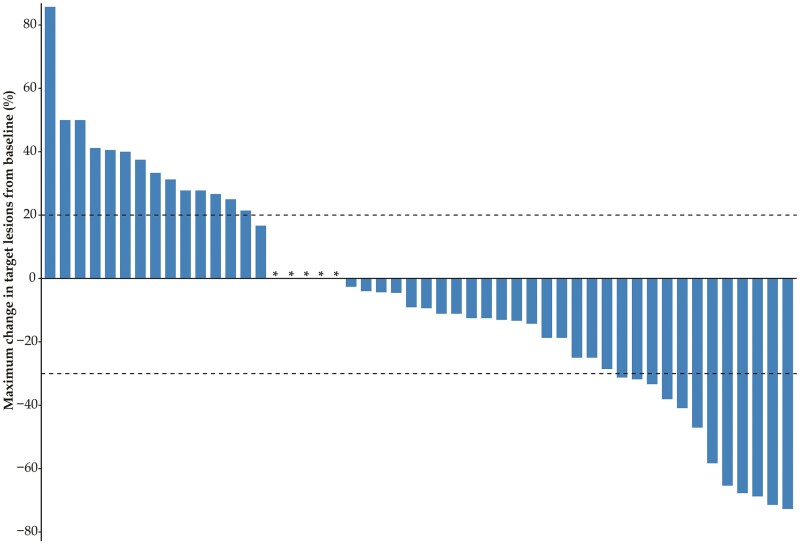
Fourty-four (88.0%) of 50 patients had a PFS event (disease progression or death). The median PFS was 4.01 months (95% CI: 3.19-5.49; Fig. 1A). At the data cutoff, 22 patients died and the median OS was 12.32 months (95% CI: 8.21-17.45; Fig. 1B). Among 50 patients, no patients experienced CR, but 13 (26%; 95% CI, 15.87-39.55) patients had a PR (Table 2). Thirty-one (62%; 48.15-74.14) of 50 patients had disease control (Table 2). Target lesion size decreased in 30 patients (60.0%; Fig. 2).
Table 2.
Tumor response to Rh-endostatin plus irinotecan/cisplatin.
| Response | Patients (N = 50) |
|---|---|
| Best objective response | |
| Complete response | 0 |
| Partial response | 13 (26%; 16-40) |
| Stable disease | 18 (36%; 24-50) |
| Progressive disease | 15 (30%; 19-44) |
| Not evaluablea | 4 (8%; 3-19) |
| Objective responseb | 13 (26%; 16-40) |
| Disease controlc | 31 (62%; 48-74) |
Data were expressed as n (%; 95% CI).
Patients had no valid postbaseline response assessments.
Objective response = complete response plus partial response.
Disease control = complete response plus partial response plus stable disease.
Figure 2.
Change in size of target lesions from baseline. Asterisk indicates no change in target lesion size.
Table 1.
Baseline characteristics of patients n = 50.
| Characteristics | Patients, n (%) |
|---|---|
| Age, years | |
| Median age (range) | 61 (47-74) |
| <65 | 32 (64.0) |
| ≥65 | 18 (36.0) |
| Sex | |
| Male | 46 (92.0) |
| Female | 4 (8.0) |
| ECOG performance status score | |
| 0 | 14 (28.0) |
| 1 | 36 (72.0) |
| TNM staging | |
| IV | 50 (100.0) |
| Treatment cycles | |
| 1 | 7 (14.0) |
| 2 | 16 (32.0) |
| 3 | 2 (4.0) |
| 4 | 11 (22.0) |
| 5 | 1 (2.0) |
| 6 | 13 (26.0) |
| Previous treatment | |
| Paclitaxel and carboplatin | 17 (34.0) |
| Fluorouracil and oxaliplatin | 9 (18.0) |
| Docetaxel and cisplatin | 8 (16.0) |
| Fluorouracil and cisplatin | 5 (10.0) |
| Others | 11 (22.0) |
Data are expressed as n (%) unless otherwise indicated.
Table 3.
Treatment-related adverse events in all treated patients (n = 50).
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any grade | |
|---|---|---|---|---|---|
| Number of patients (%) | 10 (20.0) | 20 (40.0) | 8(16.0) | 4 (8.0) | 42 (84.0) |
| Number of events (%) | 60 | 44 | 18 | 7 | 129 |
| Hematological | |||||
| Neutropenia | 3 (6.0) | 4 (8.0) | 5 (10.0) | 3 (6.0) | 15 (30.0) |
| Leucopenia | 4 (8.0) | 12 (24.0) | 6 (12.0) | 3 (6.0) | 25 (50.0) |
| Anemia | 10 (20.0) | 7 (14.0) | 1 (2.0) | 0 | 18 (36.0) |
| Thrombocytopenia | 4 (8.0) | 5 (10.0) | 0 | 1 (2.0) | 10 (20.0) |
| Non-hematological | |||||
| Appetite decrease | 8 (16.0) | 2 (4.0) | 0 | 0 | 10 (20.0) |
| Nausea and vomiting | 4 (8.0) | 1 (2.0) | 0 | 0 | 5 (10.0) |
| Diarrhea | 3 (6.0) | 1 (2.0) | 5 (10.0) | 0 | 9 (18.0) |
| Abdominal distention | 11 (22.0) | 4 (8.0) | 0 | 0 | 15 (30.0) |
| Neurotoxicity | 3 (6.0) | 0 | 0 | 0 | 3 (6.0) |
| Venous thrombosis | 0 | 1 (2.0) | 0 | 0 | 1 (2.0) |
| Asthenia | 9 (18.0) | 4 (8.0) | 1 (2.0) | 0 | 14 (28.0) |
| Alopecia | 1 (2.0) | 1 (2.0) | 0 | 0 | 2 (4.0) |
| Renal function abnormal | 0 | 2 (4.0) | 0 | 0 | 2 (4.0) |
Adverse Events (All Dose Levels, All Cycles)
| Name | ∗NC/NA | 1 | 2 | 3 | 4 | 5 | All grades |
|---|---|---|---|---|---|---|---|
| Neutrophil count decreased | 70% | 6% | 8% | 10% | 6% | 0% | 30% |
| White blood cell decreased | 50% | 8% | 24% | 12% | 6% | 0% | 50% |
| Anemia | 64% | 20% | 14% | 2% | 0% | 0% | 36% |
| Platelet count decreased | 80% | 8% | 10% | 0% | 2% | 0% | 20% |
| Anorexia | 76% | 16% | 8% | 0% | 0% | 0% | 24% |
| Nausea/Vomiting | 90% | 8% | 2% | 0% | 0% | 0% | 10% |
| Diarrhea | 82% | 6% | 2% | 10% | 0% | 0% | 18% |
| Abdominal distention | 70% | 22% | 8% | 0% | 0% | 0% | 30% |
| Neurotoxicity | 94% | 6% | 0% | 0% | 0% | 0% | 6% |
| Venous thrombosis | 98% | 0% | 2% | 0% | 0% | 0% | 2% |
| Asthenia | 72% | 18% | 8% | 2% | 0% | 0% | 28% |
Abbreviations: NC/NA, no change, no adverse event.
Treatment-related adverse events in all treated patients (n = 50) are shown in Table 3. At the data cutoff, a total of 129 treatment-related AEs of any grade were reported in 42 (84.0%) patients (Table 3), with the common events being leucopenia (50.0%), anemia (36.0%), neutropenia (30.0%), and abdominal distention (30.0%). Grade 3 or 4 treatment-related AEs occurred in 12 (24.0%) patients and included leucopenia (18.0%), neutropenia (16.0%), and anemia (2.0%). However, most grade 3 or 4 treatment-related AEs were reversible by a dose reduction, interruptions, or supportive care. During the study, all deaths (n = 22) occurred due to disease progression; no deaths were deemed to be related to treatment. No treatment-related AEs led to study discontinuation, while 5 (10.0%) patients discontinued the protocol treatment because of AEs, including renal function abnormal (n = 1), diarrhea (n = 2), appetite decrease (n = 1), and neutropenia (n = 1).
Assessment, Analysis, and Discussion
| Completion | Competing agents; study terminated before completion |
| Investigator’s assessment | Active and should be pursued further |
In recent years, combinations of anti-angiogenic drugs and other anti-cancer strategies have appeared as an appealing approach for optimizing outcomes in patients with cancer.6,7 The present prospective, open-label, phase II study provides the first analysis of the anti-tumor activity and safety for Rh-endostatin plus irinotecan/cisplatin in patients with advanced ESCC. Our trial met the primary endpoint, showing promising anti-tumor activity in this patient population in the second-line setting, with a median PFS of 4.01 months. In addition, this regimen had a manageable and acceptable safety profile, which was similar to those previously reported for monotherapies.
Previous studies demonstrated that most patients with advanced ESCC experienced disease progression with a median PFS of 2-4 months and OS of 5.2-9.5 months after second-line therapy.8,9 Although NCCN Guidelines provide recommended options for second-line therapy in advanced or metastatic ESCC, much of the evidence base focused on esophageal adenocarcinoma and did not specifically consider ESCC.10 Therefore, the optimal second-line therapeutic strategy for ESCC remained controversial. To address this issue, the present study aimed to evaluate a promising new second-line therapy option for advanced ESCC patients. As ESCC was a highly aggressive and angiogenic tumor, the anti-tumor activity of Rh-endostatin (an anti-angiogenics) plus irinotecan/cisplatin regimen in advanced ESCC was investigated. In this study, the PFS (4.01 months) and ORR (26%) were comparable with the results of a phase II study on irinotecan/cisplatin.11 However, the OS (12.32 months) compared favorably with previously reported activity data of the study on irinotecan/cisplatin (8.8 months) alone11 or other anticancer drugs, such as docetaxel/capecitabine (8.3 months),8 cetuximab/pemetrexed (9.4 months),9 and apatinib (7.0 months).12 In brief, these results indicated that the outcome of the combination regimen containing Rh-endostatin was more favorable and satisfactory in prolonging the OS than those of other monotherapies. Additionally, our study demonstrated similar anti-tumor activity to a similar phase II study on Rh-endostatin plus nedaplatin/paclitaxel as first-line treatment.13 Interestingly, recent studies revealed a synergistic effect of immune checkpoint blockade and anti-angiogenesis.14 Accordingly, further investigations would be promising in exploring the present regimen and immune checkpoint blockade in the treatment of advanced ESCC.
In terms of safety, Rh-endostatin plus irinotecan/cisplatin were generally well tolerated in this population. Common events reported in the study, such as leucopenia, anemia, neutropenia, and abdominal distention, were generally tolerable and manageable with dose reductions, interruptions, or supportive care. Previously, a meta-analysis has shown that the addition of anti-angiogenic drugs would increase the risks of common AEs, such as pain, hypertension, gastrointestinal symptom, metabolic disorders, and neurology.15 Notably, the safety profile in this study was similar to that of Rh-endostatin or irinotecan/cisplatin monotherapies. These reported AEs were known and also resulted from the nedaplatin/paclitaxel regimen alone,11 suggesting that the Rh-endostatin may not increase the risks of toxicity in this population. Similarly, several previous studies also reported that Rh-endostatin with platinum-based regimens would not increase the safety profile of original regimens.13,16,17 Meanwhile, the study discontinuation (10.0%) due to AEs was infrequent with only a few occurrences, and no treatment-related deaths occurred. More importantly, bleeding and gastric perforation, as known AEs of anti-angiogenic therapies, were not reported in our study. However, the risks of bleeding caused by Rh-endostatin remained to be verified in large-scale studies. In general, based on their single-agent safety profiles, no new safety signals were identified for Rh-endostatin plus irinotecan/cisplatin regimen in patients with advanced ESCC, indicating a manageable and acceptable safety profile.
The study still had some limitations. First, the primary limitation was that the sample size did not reach the pre-specified target owing to early termination. In recent years, new therapeutic approaches, such as immunotherapy, have emerged. Accordingly, the clinical treatment strategy was gradually optimized which led to difficult enrollment. Additionally, the COVID-19 pandemic slowed the enrollment and increased dramatically the rate of lost-to-follow-up, thus we prematurely terminated the study in June 2021. Although the sample size in our study was less than pre-specified, it was still deemed to be sufficient for the primary endpoint because the PFS has provided the statistical power of 86%.
Second, as is typical of early-phase clinical trials, the study had a small sample size recruited from a single institution and was an open-label, non-randomized, single-arm phase II design, precluding comparison of outcomes with irinotecan/cisplatin or other existing therapeutic approaches. Besides, our study only enrolled the selected population with squamous carcinoma, but not adenocarcinoma who usually had a poor outcome to routinely administered therapies. Thus, the results for ESCC patients cannot be extrapolated to wider patient populations with different subtypes of esophageal cancer, which indicated that the benefit of this regimen in patients with esophageal adenocarcinoma needs to be further confirmed.
Conflict of Interest
The authors indicated no financial relationships.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1.Oshima Y, Yajima S, Yamazaki K, et al Angiogenesis-related factors are molecular targets for diagnosis and treatment of patients with esophageal carcinoma. Ann Thorac Cardiovasc Surg. 2010;16(6):389-393. [PubMed] [Google Scholar]
- 2. Noma K, Smalley KS, Lioni M, et al. . The essential role of fibroblasts in esophageal squamous cell carcinoma-induced angiogenesis. Gastroenterology. 2008;134(7):1981-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li N, Zheng D, Wei X, Jin Z, Zhang C, Li K.. Effects of recombinant human endostatin and its synergy with cisplatin on circulating endothelial cells and tumor vascular normalization in A549 xenograft murine model. J Cancer Res Clin Oncol. 2012;138(7):1131-1144. [DOI] [PubMed] [Google Scholar]
- 4. Chu L, Chen Y, Liu Q, et al. . A phase II study of apatinib in patients with chemotherapy-refractory esophageal squamous cell carcinoma (ESO-Shanghai 11). Oncologist. 2021;26(6):e925-e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matsubara Y, Toriyama K, Kadowaki S, et al. . Impact of PD-L1 combined positive score (cps) on clinical response to nivolumab in patients with advanced esophageal squamous cell carcinoma. J Clinic Oncol 2021;39:e16045-e16045. [DOI] [PubMed] [Google Scholar]
- 6. Rajabi M, Mousa SA.. The role of angiogenesis in cancer treatment. Biomedicines 2017;5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Russo M, Giavazzi R.. Anti-angiogenesis for cancer: current status and prospects. Thromb Res. 2018;164(Suppl 1):S3-S6. [DOI] [PubMed] [Google Scholar]
- 8. Li X, Lin W, Wang H, Lin W, Lin S, Lin Y.. Phase II trial of second-line chemotherapy with docetaxel and capecitabine in advanced esophageal squamous cell carcinoma. Med Oncol. 2013;30(4):746. [DOI] [PubMed] [Google Scholar]
- 9. Tian J, Shang M, Shi SB, Han Y, Xu J.. Cetuximab plus pemetrexed as second-line therapy for fluorouracil-based pre-treated metastatic esophageal squamous cell carcinoma. Cancer Chemother Pharmacol. 2015;76(4):829-834. [DOI] [PubMed] [Google Scholar]
- 10. Abraham P, Gricar J, Zhang Y, Shankaran V.. Real-world treatment patterns and outcomes in patients receiving second-line therapy for advanced/metastatic esophageal squamous cell carcinoma. Adv Ther. 2020;37(7):3392-3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim M, Keam B, Kim TM, et al. . Phase II study of irinotecan and cisplatin combination chemotherapy in metastatic, unresectable esophageal cancer. Cancer Res Treat. 2017;49(2):416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Wang L.. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Onco Targets Ther. 2017;10:3965-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang ZQ, Wang DS, Wang FH, Ren C, Tan Q, Li YH.. Recombinant human endostatin plus paclitaxel/nedaplatin for recurrent or metastatic advanced esophageal squamous cell carcinoma: a prospective, single-arm, open-label, phase II study. Invest New Drugs. 2021;39(2):516-523. [DOI] [PubMed] [Google Scholar]
- 14. Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A.. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin H, Li L, Luo S, et al. . Efficacy and safety of angiogenesis inhibitors in small-cell lung cancer. Oncotarget. 2017;8(1):1141-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang W, Liu J, Wu F, et al. . The efficacy and safety of endostar combined with taxane-based regimens for HER-2-negative metastatic breast cancer patients. Oncotarget. 2016;7(21):31501-31507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Han B, Xiu Q, Wang H, et al. . A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with endostar for advanced non-small cell lung cancer. J Thorac Oncol. 2011;6(6):1104-1109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.