Abstract
Background
Pediatric cancer incidence has steadily increased concurrent with rising adult obesity, but associations between maternal obesity and associated comorbidities and pediatric cancer risk remain understudied. We aimed to quantitatively characterize associations of pediatric cancer risk with maternal prepregnancy body mass index (BMI), gestational weight gain, and maternal diabetes.
Methods
We performed a comprehensive and systematic literature search in Ovid and EMBASE from their inception to March 15, 2021. Eligible studies reported risk estimates and sample sizes and provided sufficient description of outcome and exposure ascertainment. Random effects models were used to estimate pooled effects.
Results
Thirty-four studies were included in the analysis. Prepregnancy BMI was positively associated with leukemia risk in offspring (odds ratio [OR] per 5-unit BMI increase =1.07, 95% confidence intervals [CI] = 1.04 to 1.11; I2 = 0.0%). Any maternal diabetes was positively associated with acute lymphoblastic leukemia risk (OR = 1.46, 95% CI = 1.28 to 1.67; I2 = 0.0%), even after restricting to birthweight-adjusted analyses (OR = 1.74, 95% CI = 1.29 to 2.34; I2 = 0.0%), and inversely associated with risk of central nervous system tumors (OR = 0.73, 95% CI = 0.55 to 0.97; I2 = 0.0%). Pregestational diabetes (OR = 1.57, 95% CI = 1.11 to 2.24; I2 = 26.8%) and gestational diabetes (OR = 1.40, 95% CI = 1.12 to 1.75; I2 = 0.0%) were also positively associated with acute lymphoblastic leukemia risk. No statistically significant associations were observed for gestational weight gain.
Conclusions
Maternal obesity and diabetes may be etiologically linked to pediatric cancer, particularly leukemia and central nervous system tumors. Our findings support weight management and glycemic control as important components of maternal and offspring health. Further validation is warranted.
Although pediatric cancers remain rare, their global incidence is increasing (1). In the United States, pediatric cancer incidence is increasing by an average of 0.8% per year (2) and has increased by almost 40% since the mid-1970s (3), making them the second-leading cause of death among US children (4). In addition to the immediate challenges of a pediatric cancer diagnosis, including risk of death, pediatric cancer patients are more likely to experience adverse late effects, including premature aging, cognitive deficits, obesity, infertility, and secondary cancers (5-8), highlighting the need to identify risk factors and opportunities for primary prevention.
A clear etiology for the steady rise in pediatric cancer incidence has not been sufficiently elucidated. Certain perinatal risk factors, including advanced parental age, high birthweight, and Cesarean delivery (9-11), are known to be associated with certain pediatric cancers. However, with the exceptions of congenital anomalies, certain genetic conditions, and ionizing radiation (12), knowledge of strong risk factors remains elusive. Maternal anthropometrics may play a meaningful role in pediatric cancer etiology, yet such characteristics have not been thoroughly studied. Maternal obesity has been particularly understudied. As incidence of pediatric cancers has increased, prevalence of adult obesity has seen a concurrent rise (13,14), with US adult obesity more than tripling from 13% in 1960 to 42% in 2018 (15). Obesity is linked to 13 adulthood cancers and is potentially associated with others (16,17). Given obesity prevalence and its association with adult cancer risk, there is increasing interest in the role of maternal obesity as a risk factor for childhood cancer in offspring.
The objective of this meta-analysis is to characterize the association of childhood cancer with maternal obesity and associated comorbidities, including pregestational diabetes (PGD), gestational diabetes (GD), and gestational weight gain (GWG). As maternal obesity and diabetes are also associated with known pediatric cancer risk factors, including congenital abnormalities and birthweight (18-23), it is critical to understand whether maternal obesity and associated comorbidities play a meaningful, independent role in the etiology of pediatric cancer.
Methods
We conducted this meta-analysis according to Preferred Reporting Items for Systematic reviews and Meta-analyses guidelines (24).
Search Strategy and Eligibility Criteria
A comprehensive and systematic literature search was performed in Ovid and EMBASE databases from their inception (1946 for Ovid, 1947 for EMBASE) to March 15, 2021. Search terms used can be found in the Supplementary Methods (available online). For inclusion in the analyses, eligible studies needed to meet the following criteria: published in English; were nested case-control, case-control, cohort, or case-cohort; analyzed childhood cancer risk as outcome of interest and included prepregnancy body mass index (BMI), maternal diabetes (pregestational and/or gestational), or GWG as exposure variables; reported risk estimates (odds ratio [OR], standardized incidence ratio, hazard ratio, or relative risk); reported sample sizes; described how outcome and exposure variables were ascertained. Studies were excluded if they did not meet these criteria; if they were not complete, published, or peer-reviewed studies; or if they provided incompatible exposure data.
Data Extraction and Quality Assessment
Two reviewers (AM and AD) independently performed the data extraction and quality assessment. Extracted data included first author, study year, study design, study period, country of origin, type of cancer(s), sample, patients, controls, age range, ascertainment of BMI and/or diabetes and/or GWG, number of mothers in various exposure categories, ascertainment of cancer diagnosis, method of analysis, effect sizes and 95% confidence intervals (CIs), and adjusted covariates.
The Newcastle-Ottawa Scale was used to assess the quality of each included study. Quality was judged on study group selection, group comparability, and ascertainment of the exposure or outcome of interest (25). Scores of 7-9 were considered high quality, 4-6 were considered moderate quality, and 1-3 were considered poor quality.
Statistical Analyses
Summary odds ratios and 95% confidence intervals were estimated to assess effects of prepregnancy BMI, maternal diabetes, and GWG on risk of pediatric cancer in offspring. Because pediatric cancer is rare and incidence was low in included cohort studies, we used odds ratios to estimate effect sizes. Data for a given exposure or outcome category were meta-analyzed if the category contained at least 3 effect sizes and confidence intervals extracted from the literature. If an exposure/outcome category contained less than 3 effect sizes, the data were considered insufficient for meta-analysis. Prepregnancy BMI was assessed according to BMI categories defined by the Centers for Disease Control and Prevention (BMI <18.5: underweight; BMI 18.5-24.9: normal weight; BMI 25-29.9: overweight; BMI ≥30: obese) (26). Because not all studies used these cutoffs, we calculated risk per 5-unit increase in prepregnancy BMI using methodology of Il’yasova et al. (27). Maternal diabetes was evaluated as any diabetes, GD, and PGD. If studies provided separate risk estimates for type 1 and type 2 diabetes, we extracted type 2 data as PGD and excluded type 1 data. We assessed GWG as inadequate, appropriate, and excessive according to weight gain guidelines by the Institute of Medicine (28). Because several studies presented GWG as raw weight gain, however, we also provided risk estimates per 5-kilograms of GWG.
Random effects models were used to estimate summary odds ratios and 95% confidence intervals when heterogeneity was greater than 25%; when heterogeneity was less than 25%, fixed effect models were used. I2 was used to assess between-study heterogeneity. Data were grouped together to perform meta-analyses for any cancer and, when data were sufficient, for individual cancers, including acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), any leukemia, neuroblastoma, retinoblastoma, hepatoblastoma, central nervous system (CNS) tumors, Wilms tumor, germ cell tumors, rhabdomyosarcoma, and lymphoma. Where data allowed, subgroup analyses were performed according to study design (case-control and nested case-control vs cohort and case-cohort) and location (North America vs Europe and other). Sensitivity analyses were performed to evaluate result robustness, including restricting to high-quality studies for all statistically significant main analyses. Associations for individual cancer types were considered suggestive if upper- and lower-bound confidence intervals were 1.00 to 1.05 and 0.95 to 1.00, respectively. Funnel plots and Egger tests (P < .05) were used to evaluate publication bias. Stata 16.1 (Stata Corp, College Station, TX) was used for all analyses. Tests were 2-sided, with P values less than .05 indicating statistical significance.
Results
Study Selection and Characteristics
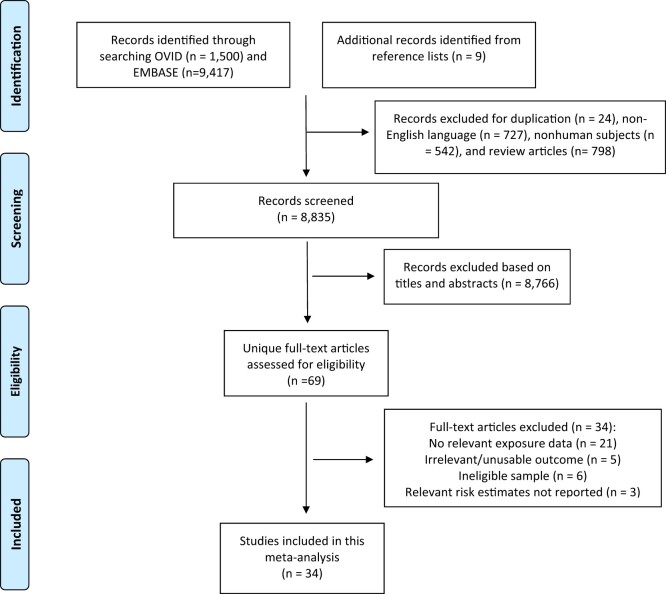
Overall, 10 917 citations were retrieved from the literature search, and 9 additional citations were identified from reference lists. After excluding duplicates, non-English articles, review articles, and nonhuman studies, 8835 citations were screened, with 34 studies included in our meta-analysis (Figure 1).
Figure 1.
Preferred Reporting Items for Systematic reviews and Meta-analyses flow diagram of study selection and study identification.
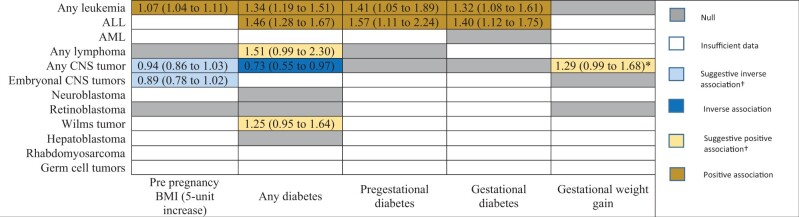
Nine studies provided eligible prepregnancy BMI data (Supplementary Table 1, available online) (29-37). An additional 7 studies with maternal prepregnancy weight data were found but excluded because of vague obesity definitions/lack of ascertainment description (38,39), providing weight rather than BMI (40-42), or expressing BMI in categories that could not be converted into usable data (43,44). Eligible studies covered 3 404 747 participants and 14 706 childhood cancer patients across 5 countries. Of the studies, 25 provided diabetes data (Supplementary Table 2, available online) (29,32,36,38,39,41,42,45-62) and covered 14 748 772 participants and 44 628 childhood cancer patients from 12 countries, and 11 studies provided GWG data (Supplementary Table 3, available online) (29-34,37,40-42,63). Two additional GWG studies were found but excluded because of weight gain expressed in unusable categories (39,55). Eligible studies covered 2 124 647 participants and 15 915 pediatric cancer patients from 4 countries. Of the 34 included studies, 28 were considered high quality, and 6 were considered moderate quality (Supplementary Tables 4 and 5, available online). A heat map was created to summarize meta-analysis results across all exposures and tumors (Figure 2).
Figure 2.
Heat map of the associations between prepregnancy BMI, gestational weight gain, maternal diabetes, and risk of pediatric cancers. Cells depicting statistically significant or suggestive associations display meta-analyzed odds ratios (95% confidence intervals). † Considered suggestive if upper- and lower-bound confidence intervals were 1.00 to 1.05 and 0.95 to 1.00, respectively. *For inadequate gestational weight gain (Institute of Medicine defined guidelines) only. ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; BMI = body mass index; CNS = central nervous system.
Prepregnancy BMI and Childhood Cancer Risk in Offspring
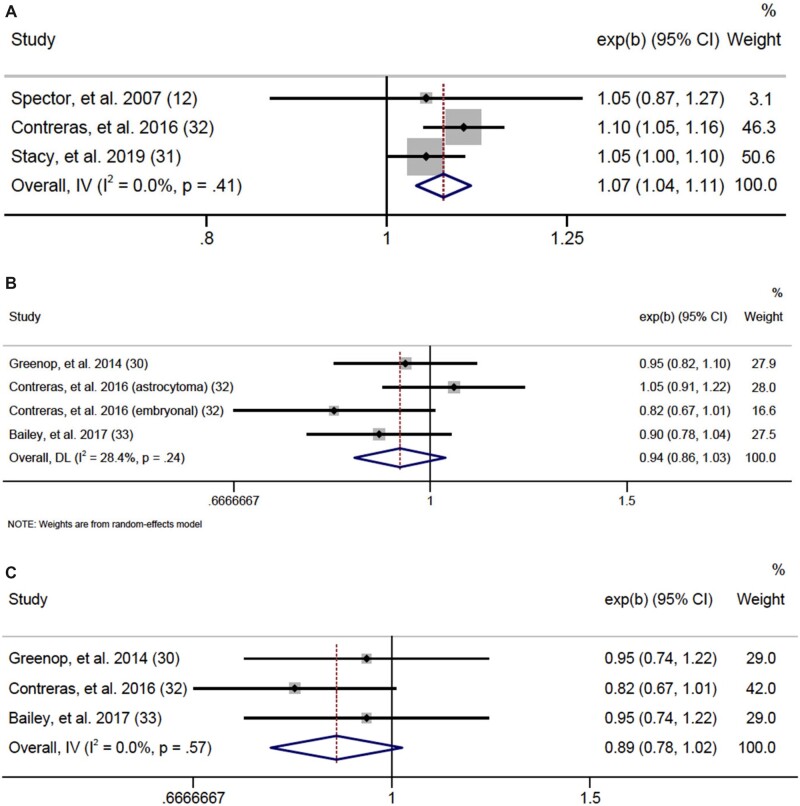
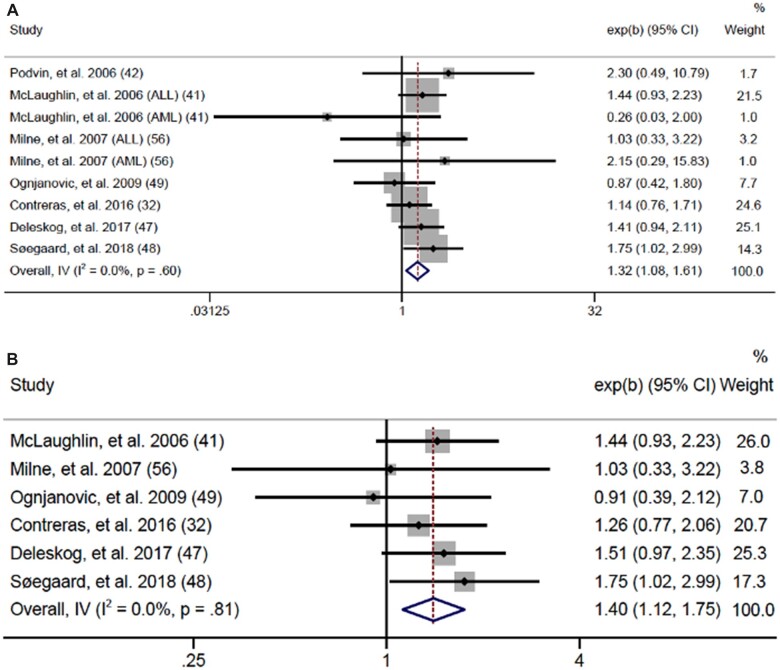
To assess prepregnancy BMI at an equivalent scale and ensure all eligible data were analyzed, we calculated risk per 5-unit increase in BMI (Figure 3). Data were sufficient to meta-analyze associations for any leukemia, any lymphoma, any CNS tumor, embryonal CNS tumors, and retinoblastoma. Although unable to analyze ALL or AML individually, we identified a statistically significant association for any leukemia, finding a 7% increased leukemia risk in offspring for each 5-unit increase in maternal prepregnancy BMI (OR = 1.07, 95% CI = 1.04 to 1.11; I2 = 0.0%, heterogeneity P = .41) with no evidence of publication bias according to Egger test (P = .92). No other statistically significant results were seen, although we found suggestive evidence of an inverse association for CNS tumors (OR = 0.94, 95% CI = 0.86 to 1.03; I2= 28.4%, heterogeneity P = .24), particularly embryonal CNS tumors (OR = 0.89, 95% CI = 0.78 to 1.02; I2 = 0.0%, heterogeneity P = .57). There were insufficient data to meta-analyze neuroblastoma, hepatoblastoma, Wilms tumor, germ cell tumors, and rhabdomyosarcoma.
Figure 3.
Forest plots: meta-analysis of the association between a 5-unit increase in prepregnancy BMI and risk of (A) any leukemia, (B) central nervous system tumors, and (C) embryonal central nervous system tumors. The error bars represent the 95% confidence intervals (CIs). Fixed effects models (inverse variance method) were used for panels A and C, and random effects models (DerSimonian and Laird method) were used for panel B. Tests were 2-sided. DL = DerSimonian-Laird; IV = inverse variance.
Any Maternal Diabetes and Childhood Cancer Risk in Offspring
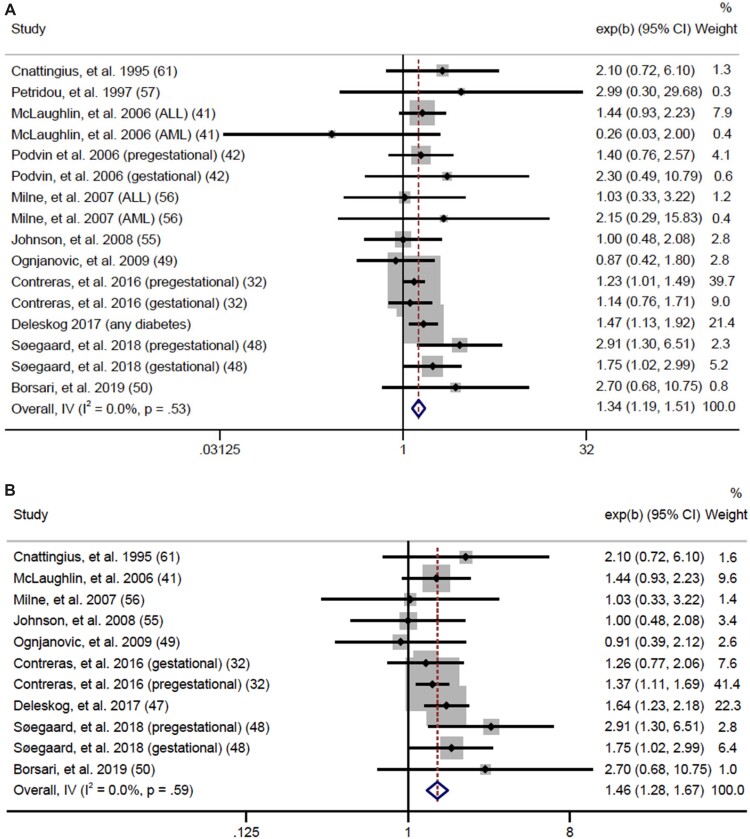
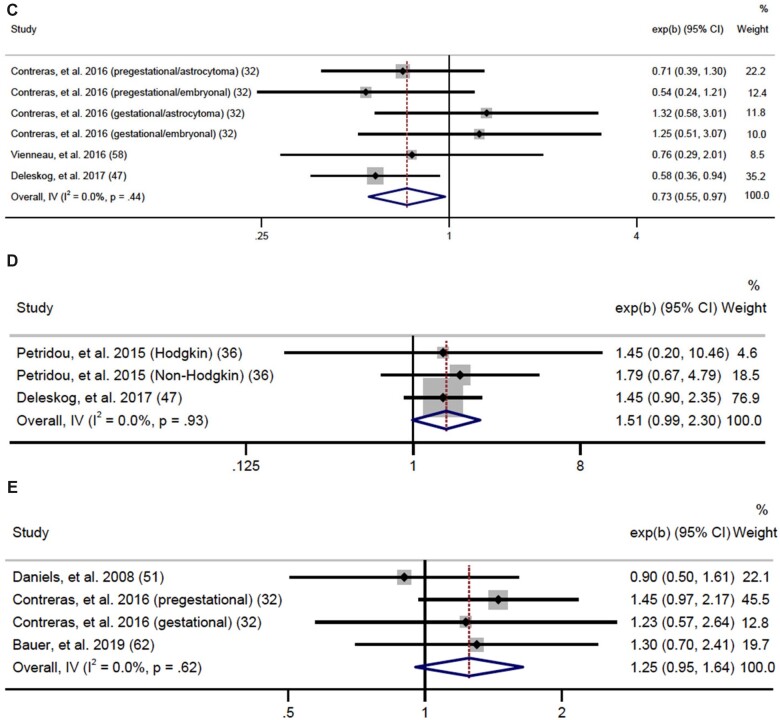
We analyzed maternal diabetes as any diabetes, PGD, and GD. We found a statistically significant association between any diabetes and any cancer (OR = 1.20, 95% CI = 1.12 to 1.29; I2 = 1.7%, heterogeneity P = .44) (Supplementary Figure 1, available online). For individual cancers, we had sufficient data to meta-analyze associations for any leukemia, ALL, any lymphoma, any CNS tumor, neuroblastoma, retinoblastoma, Wilms tumor, and hepatoblastoma. As seen in Figure 4, we found a positive association for any leukemia (OR = 1.34, 95% CI = 1.19 to 1.51; I2 = 0.0%, heterogeneity P = .53, Egger P = .41) and a stronger effect for ALL (OR = 1.46, 95% CI = 1.28 to 1.67; I2 = 0.0%, heterogeneity P = .59, Egger P = .68). Sensitivity analyses were performed to determine whether high birthweight could explain associations between any diabetes and any leukemia or ALL and demonstrated that associations persisted even after restricting to birthweight-adjusted analyses (41,47,48,57,61) (any diabetes OR = 1.56, 95% CI = 1.28 to 1.90; ALL OR = 1.74, 95% CI = 1.29 to 2.34) (Supplementary Figure 2, available online). Additionally, we found any maternal diabetes was inversely associated with risk of CNS tumors (OR = 0.73, 95% CI = 0.55 to 0.97; I2 = 0.0%, heterogeneity P = .44) with no evidence of publication bias (Egger P = .19). Data were not sufficient to analyze PGD and GD separately for this association. There were no other statistically significant associations among individual cancers, although lymphoma (OR = 1.51, 95% CI = 0.99 to 2.30; I2 = 0.0%, heterogeneity P = .93) and Wilms tumor (OR = 1.25, 95% CI = 0.95 to 1.64; I2 = 0.0%, heterogeneity P = .62) provided suggestive evidence of an association (Figure 4). Data for AML, germ cell tumors, and rhabdomyosarcoma were insufficient for meta-analysis.
Figure 4.
Forest plots: meta-analysis of the association between any diabetes and risk of (A) any leukemia, (B) acute lymphoblastic leukemia, (C) central nervous system tumors, (D) lymphoma, and (E) Wilms tumor. The error bars represent the 95% confidence intervals (CIs). Fixed effects models (inverse variance method) were used for statistical analyses. Tests were 2-sided. ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; IV = inverse variance.
Figure 4.
(Continued)
PGD and GD and Childhood Cancer Risk in Offspring
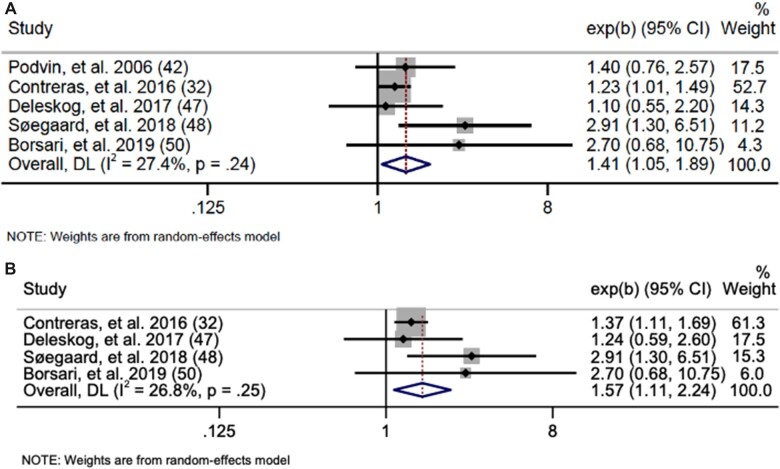
For PGD and GD, we found statistically significant positive associations for any cancer (PGD OR = 1.19, 95% CI = 1.02 to 1.37; I2 = 28.8%, heterogeneity P = .11; GD OR = 1.20, 95% CI = 1.09 to 1.33; I2 = 0.0%, heterogeneity P = .65) (Supplementary Figure 3, available online). We also found positive associations between PGD and GD and risk for ALL and any leukemia. PGD (Figure 5) was associated with a 41% increased risk of any leukemia (OR = 1.41, 95% CI = 1.05 to 1.89; I2 = 27.4%, heterogeneity P = .24, Egger P = .20) and a 57% increased risk for ALL (OR = 1.57, 95% CI = 1.11 to 2.24; I2 = 26.8%, heterogeneity P = .25, Egger P = .31). No statistically significant associations were found for lymphoma or CNS tumors, and data were insufficient to meta-analyze associations for AML, neuroblastoma, retinoblastoma, Wilms, hepatoblastoma, rhabdomyosarcoma, and germ cell tumors. GD (Figure 6) was associated with a 32% increased risk for any leukemia (OR = 1.32, 95% CI = 1.08 to 1.61; I2 = 0.0%, heterogeneity P = .60, Egger P = .56) and a 40% increased risk of ALL (OR = 1.40, 95% CI = 1.12 to 1.75; I2 = 0.0%, heterogeneity P = .81, Egger P = .16). No statistically significant associations were found for AML or CNS tumors, and data for lymphoma, neuroblastoma, retinoblastoma, Wilms tumor, hepatoblastoma, rhabdomyosarcoma, and germ cell tumors were insufficient to meta-analyze.
Figure 5.
Forest plots: meta-analysis of the association between pregestational diabetes and (A) any leukemia and (B) acute lymphoblastic leukemia. The error bars represent the 95% confidence intervals (CIs). Random effects models (DerSimonian and Laird method) were used for statistical analyses. DL = DerSimonian-Laird.
Figure 6.
Forest plots: meta-analysis of the association between gestational diabetes and risk of (A) any leukemia and (B) acute lymphoblastic leukemia. The error bars represent the 95% confidence intervals (CIs). Fixed effects models (inverse variance method) were used for statistical analyses. Tests were 2-sided. ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; IV = inverse variance.
GWG and Childhood Cancer Risk in Offspring
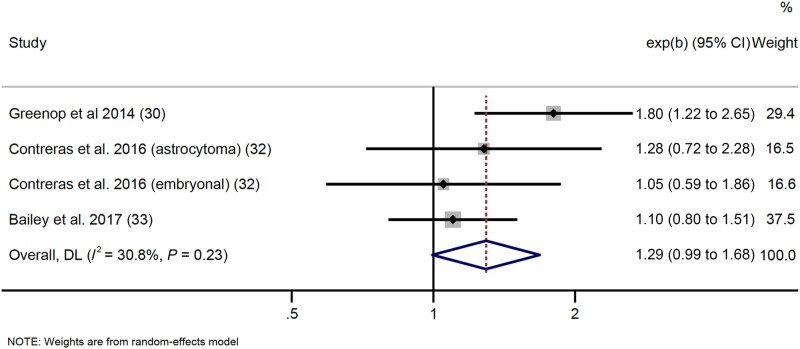
Data were sufficient to meta-analyze GWG data for any leukemia, any CNS tumor, embryonal CNS tumors, and retinoblastoma. Results (any cancer and individual tumors) lacked any statistically significant results; however, we found suggestive evidence of a positive association between inadequate GWG (Institute of Medicine guidelines) and risk of any CNS tumor (OR = 1.29, 95% CI = 0.99 to 1.68; I2 = 30.8%, heterogeneity P = .23) (Figure 7). Data for ALL, AML, lymphoma, neuroblastoma, Wilms tumor, hepatoblastoma, rhabdomyosarcoma, and germ cell tumors were insufficient for meta-analysis. Funnel plots for results presented in Figures 2-7 analysis are displayed in Figure 8.
Figure 7.
Forest plot: meta-analysis of the association between inadequate gestational weight gain and risk of central nervous system tumors. The error bars represent the 95% confidence intervals (CIs). Random effects models (DerSimonian and Laird method) were used for statistical analyses. Tests were 2-sided. DL = DerSimonian-Laird.
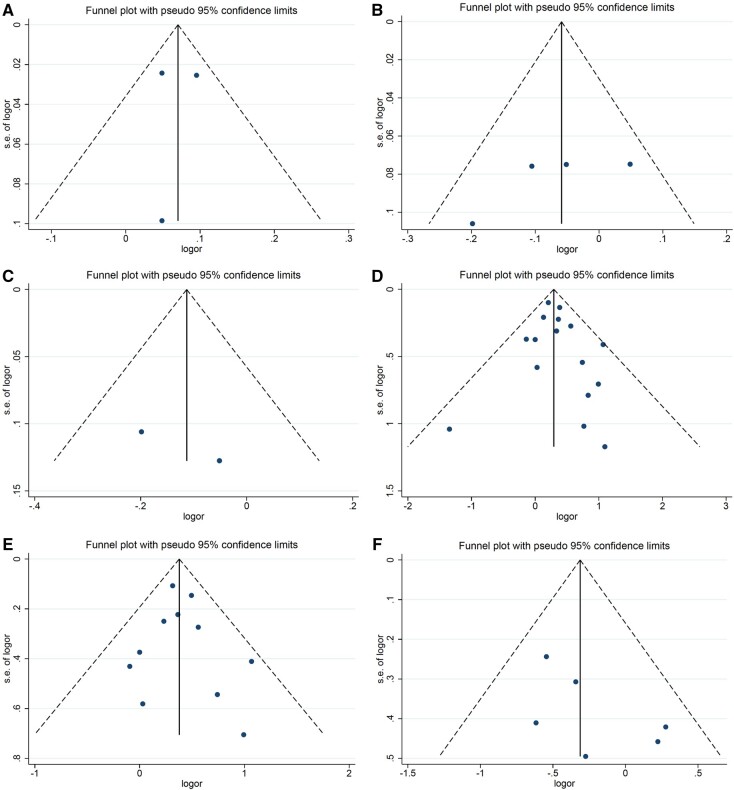
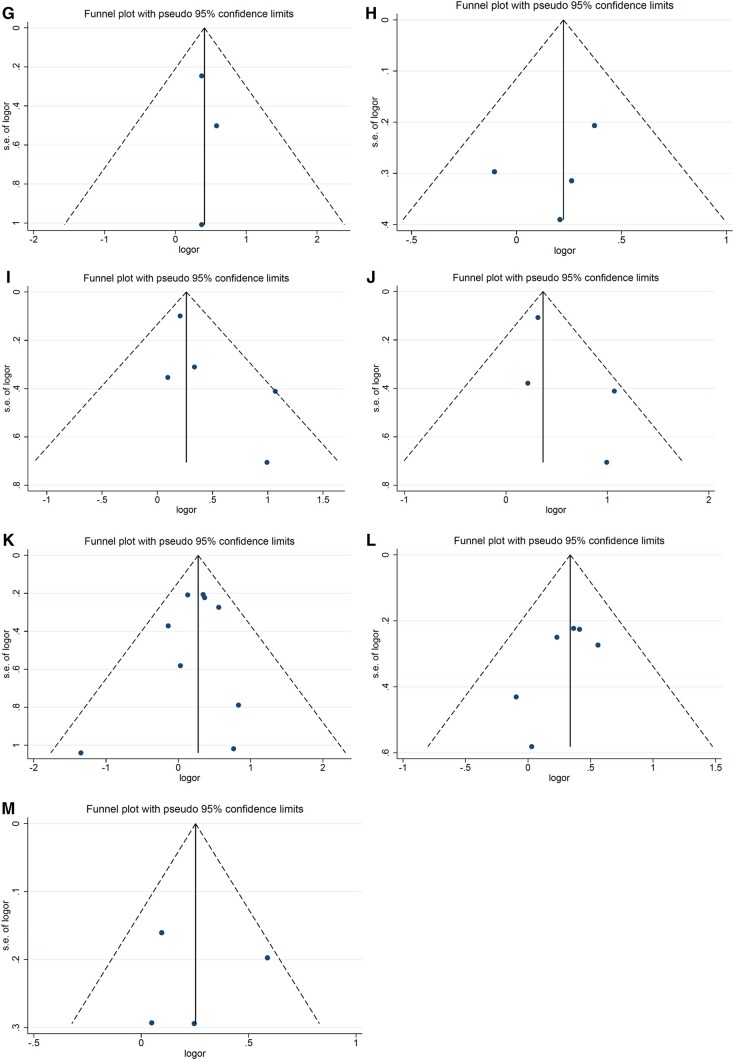
Figure 8.
Funnel plots for meta-analysis results of the associations between prepregnancy BMI, maternal diabetes, and gestational weight gain and risk of pediatric cancers. A) Funnel plot for the association between prepregnancy BMI and risk of any leukemia; (B) funnel plot for the association between prepregnancy BMI and risk of central nervous system tumors; (C) funnel plot for the association between prepregnancy BMI and risk of embryonal central nervous system tumors; (D) funnel plot for the association between any diabetes and risk of any leukemia; (E) funnel plot for the association between any diabetes and risk of acute lymphoblastic leukemia; (F) funnel plot for the association between any diabetes and risk of central nervous system tumors; (G) funnel plot for the association between any diabetes and risk of lymphoma; (H) funnel plot for the association between any diabetes and Wilms tumor risk; (I) funnel plot for the association between pregestational diabetes and risk of any leukemia; (J) funnel plot for the association between pregestational diabetes and risk of acute lymphoblastic leukemia; (K) funnel plot for the association between gestational diabetes and risk of any leukemia; (L) funnel plot for the association between gestational diabetes and risk of acute lymphoblastic leukemia; (M) funnel plot for the association between inadequate gestational weight gain and risk of central nervous system tumors. BMI = body mass index; logor = natural log of the odds ratio; s.e. = standard error.
Figure 8.
(Continued)
Subgroup and Sensitivity Analyses
As data allowed, subgroup analyses were performed according to study geographic region and study design. Subgroup analyses were primarily performed among any cancer, ALL, and any leukemia and among pregestational, gestational, and any diabetes. With some exceptions, effect sizes were larger among studies conducted in other regions vs in North America and among cohort and case-cohort studies vs case-control and nested case-control studies (Supplementary Table 6, available online). Where data allowed, sensitivity analyses restricting to high-quality studies were performed for all statistically significant main results. No appreciable changes were observed.
Discussion
We summarized current evidence evaluating associations between maternal obesity, maternal diabetes, and GWG with risk of pediatric cancers in offspring. We found greater prepregnancy BMI was associated with an increased risk of pediatric leukemia; maternal diabetes was associated with a decreased risk of CNS tumors but increased risk of leukemia, particularly ALL; and inadequate GWG may increase risk for pediatric CNS tumors. To our knowledge, this is the first meta-analysis of prepregnancy BMI and GWG as pediatric cancer risk factors. One prior meta-analysis for maternal diabetes and childhood cancer risk has been recently published (64); however, our meta-analysis includes more studies and greater detail on risks associated with individual tumor types and differing forms of maternal diabetes.
We found a statistically significant 7% increased risk of childhood leukemia for every 5-unit increase in prepregnancy BMI. Obesity has been repeatedly linked to leukemia risk in adult populations (65-67), but recent research has identified links between childhood obesity and childhood leukemia (68-71). Our own research has demonstrated a statistically significant association between childhood obesity and ALL risk among 4726 pediatric leukemia cases (71), suggesting early obesity exposure may propagate pediatric leukemia risk. Obesity may specifically promote leukemogenesis via several mechanisms, including altered adipokine secretion (68), decreased circulating adiponectin (72), and increased leptin bioavailability (73,74). Additionally, as obesity heritability is estimated to be 0.85-0.9 (75,76), obese mothers are more likely to have obese children, thus increasing leukemogenesis risk; however, with our data, there was no way to determine leukemia risk attributable to obesity genomics. These mechanisms may not just apply to obese persons individually, but may confer obesity-associated risk to infants during gestation. Similar mechanisms may be present for other pediatric tumors, however, with limited data available, associations were not detected.
We identified similar, approximately 20% increased risks for any cancer associated with any maternal diabetes and PGD. These results are consistent with a recently published meta-analysis (64), although our summary effect sizes are smaller because of inclusion of several more studies. Our finding for GD was not consistent with the previous meta-analysis, which found GD to be associated with a non-statistically significant 10% increased cancer risk (OR = 1.10, 95% CI = 0.94 to 1.28) (64). This inconsistency is likely due to the more robust nature of our analysis (15 vs 6 studies). We also found that any diabetes, PGD, and GD were specifically associated with risk of any leukemia and ALL, consistent with the previous meta-analysis (64). Although PGD and GD are associated with differing effect sizes in other perinatal and/or pediatric conditions (77), our findings of similar but slightly higher effect sizes for PGD vs GD indicate both conditions influence pediatric cancer risk via similar mechanisms, with earlier fetal exposure to PGD resulting in a slightly higher risk of pediatric cancer. These findings could also reflect the earlier, quicker onset of other perinatal conditions (ie, congenital anomalies) vs the later, longer onset of GD and of leukemogenesis. Mechanistically, these findings may be due to decreased levels of adiponectin and higher levels of insulin-like growth factors (IGF)–1 and leptin associated with gestational and type 2 diabetes (78-80), as well as increased fetal oxidative stress, altered fetal metabolism (80,81), and epigenetic modifications (82) associated with maternal hyperglycemia. Maternal diabetes may also promote leukemogenesis via its impact on birthweight. Maternal hyperglycemia is associated with large for gestational age offspring (83), a well-established leukemia risk factor (84,85). However, when restricting birthweight-adjusted analyses, we found the association between any diabetes and any leukemia persisted, suggesting other biological mechanisms may be relevant.
An association between GWG and risk of pediatric cancer was not identified. Although GWG was hypothesized to impact offspring cancer risk via similar mechanisms as prepregnancy BMI, our lack of statistically significant findings indicates that temporary GWG, even when excessive, does not confer the same risks as longer-term overweight or obesity.
We identified unexpected and interesting results for CNS tumors. We found a statistically significant inverse association between any maternal diabetes and CNS tumor risk (OR = 0.73, 95% CI = 0.55 to 0.97). To assess whether this result may be a function of data quality, we performed a sensitivity analysis and found no appreciable difference when restricting to studies considered high quality (OR = 0.73, 95% CI = 0.54 to 0.98;). Additionally, we found suggestive evidence of inverse associations for CNS tumors, including a decreased CNS tumor risk per 5-unit BMI increase and an increased risk of CNS tumors associated with inadequate GWG. These findings have not been widely researched, thus we can only speculate as to underlying causes. One hypothesis involves adipokines interacting with the blood-brain barrier and exerting CNS effects. Increasing adiposity results in increased secretion of adipokines. Transforming growth factor–β1, an adipokine that can act at the blood-brain barrier and has potent anti-inflammatory properties, may protect against metabolic disturbances (86,87) and therefore protect against CNS tumorigenesis. Another hypothesis speculates that, because diabetes is associated with decreased IGF-1, and IGF-1 has been demonstrated to stimulate glioma cell division (88), diabetes may protect against CNS tumorigenesis via the IGF pathway. Again, these speculative hypotheses need to be further studied and understood.
Our meta-analysis is subject to several limitations. As with all analyses of observational studies, we cannot rule out residual confounding, and as many case-control studies were included in the analyses, results are subject to recall bias; also, included studies adjusted for different covariates. Furthermore, only a few studies adjusted for established risk factors such as congenital anomalies or radiographic exposure. Also notable, none of the maternal diabetes studies adjusted for prepregnancy BMI, and none of the included studies adjusted for child’s weight or BMI at time of diagnosis, allowing for possible confounding. Additionally, lack of available data limited our ability to analyze individual tumors, and therefore some outcomes were analyzed according to cancer grouping (eg, all leukemia, CNS tumors, lymphoma). As various cancers within a grouping can have distinct etiologies, grouped results should be interpreted with caution. Analyses were particularly limited for neuroblastoma, hepatoblastoma, Wilms tumor, and AML, and no analyses were possible for germ cell tumors and rhabdomyosarcoma. Finally, we were limited by differences in GWG categorization, disallowing us from combining these data as we did for prepregnancy BMI.
These findings advance knowledge of pediatric cancer risk factors and have direct implications for clinical practice. Our findings highlight the importance of maintaining health checkups before and throughout pregnancy and emphasize glycemic control as an important part of prenatal and perinatal health. These findings also highlight the importance of weight management for both maternal and offspring health. Lastly, for mothers struggling with excessive weight gain, our GWG findings may offer reassurance that such gain is not associated with increased risk of childhood malignancies.
In conclusion, the current meta-analysis provides evidence that maternal obesity and diabetes may play a role in the development of pediatric malignancies, specifically for leukemias and CNS tumors. Further investigation is needed to support these variables as potential factors in the etiology of pediatric cancers and confirm of our novel findings.
Funding
This work was supported by the National Cancer Institute [T32 CA099936 to LS].
Notes
Role of the funder: The funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author contributions: Conceptualization (TG, LT, LS); data curation (AM, AD); formal analysis (AM), funding acquisition (LS); investigation (AM, AD, TG); methodology (AM, AD, TG); project administration (LS, LT); resources & software (AM, AD); writing—original draft (AM, TG); writing—review & editing (all authors).
Data Availability
Data analyzed for this study are contained within the manuscript and/or its supplementary materials.
Supplementary Material
References
- 1. Hubbard AK, Spector LG, Fortuna G, Marcotte EL, Poynter JN.. Trends in international incidence of pediatric cancers in children under 5 years of age: 1988-2012. JNCI Cancer Spectr. 2019;3(1):pkz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Annual Report to the Nation: Cancer Death Rates Continue to Decline. https://www.cdc.gov/media/releases/2020/p0312-annual-report.html. Accessed March 15, 2022.
- 3. National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975-2017. National Cancer Institute; 2020. https://seer.cancer.gov/archive/csr/1975_2017/. Accessed June 08, 2021.
- 4. Centers for Disease Control and Prevention. Leading Causes of Death and Injury. https://www.cdc.gov/injury/wisqars/LeadingCauses.html. Accessed June 08, 2021.
- 5. Henderson TO, Ness KK, Cohen HJ.. Accelerated aging among cancer survivors: from pediatrics to geriatrics. Am Soc Clin Oncol Educ Book. 2014;34(34):e423–e430. [DOI] [PubMed] [Google Scholar]
- 6. Landier W, Armenian S, Bhatia S.. Late effects of childhood cancer and its treatment. Pediatr Clin North Am. 2015;62(1):275–300. [DOI] [PubMed] [Google Scholar]
- 7. Kopp LM, Gupta P, Pelayo-Katsanis L, Wittman B, Katsanis E.. Late effects in adult survivors of pediatric cancer: a guide for the primary care physician. Am J Med. 2012;125(7):636–641. [DOI] [PubMed] [Google Scholar]
- 8. Choi DK, Helenowski I, Hijiya N.. Secondary malignancies in pediatric cancer survivors: perspectives and review of the literature. Int J Cancer. 2014;135(8):1764–1773. [DOI] [PubMed] [Google Scholar]
- 9. Marcotte EL, Thomopoulos TP, Infante-Rivard C, et al. Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC). Lancet Haematol. 2016;3(4):e176–e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petridou ET, Georgakis MK, Erdmann F, et al. Advanced parental age as risk factor for childhood acute lymphoblastic leukemia: results from studies of the Childhood Leukemia International Consortium. Eur J Epidemiol. 2018;33(10):965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Neill KA, Murphy MF, Bunch KJ, et al. Infant birthweight and risk of childhood cancer: international population-based case control studies of 40 000 cases. Int J Epidemiol. 2015;44(1):153–168. [DOI] [PubMed] [Google Scholar]
- 12. Spector LG, Pankratz N, Marcotte EL.. Genetic and nongenetic risk factors for childhood cancer. Pediatr Clin North Am. 2015;62(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skinner AC, Skelton JA.. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr. 2014;168(6):561–566. [DOI] [PubMed] [Google Scholar]
- 14. Hales CM, Carroll MD, Fryar CD, Ogden CL.. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2017.
- 15. Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960-1962 through 2017-2018. NCHS Health E-Stats; 2020. [Google Scholar]
- 16. Psaltopoulou T, Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Riza E, Dimopoulos MA.. Anthropometric characteristics, physical activity and risk of hematological malignancies: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2019;145(2):347–359. [DOI] [PubMed] [Google Scholar]
- 17. Patel AV, Diver WR, Teras LR, Birmann BM, Gapstur SM.. Body mass index, height and risk of lymphoid neoplasms in a large United States cohort. Leuk Lymphoma. 2013;54(6):1221–1227. [DOI] [PubMed] [Google Scholar]
- 18. Gaudet L, Ferraro ZM, Wen SW, Walker M.. Maternal obesity and occurrence of fetal macrosomia: a systematic review and meta-analysis. Biomed Res Int. 2014;2014:640291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stothard KJ, Tennant PW, Bell R, Rankin J.. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636–650. [DOI] [PubMed] [Google Scholar]
- 20. Leddy MA, Power ML, Schulkin J.. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1(4):170–178. [PMC free article] [PubMed] [Google Scholar]
- 21. Kaul P, Bowker SL, Savu A, Yeung RO, Donovan LE, Ryan EA.. Association between maternal diabetes, being large for gestational age and breast-feeding on being overweight or obese in childhood. Diabetologia. 2019;62(2):249–258. [DOI] [PubMed] [Google Scholar]
- 22. Kong L, Nilsson IA, Gissler M, Lavebratt C.. Associations of maternal diabetes and body mass index with offspring birth weight and prematurity. JAMA Pediatr. 2019;173(4):371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu Y, Liu B, Sun Y, et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020;43(12):2983–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P, for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Oxford: University of Ottawa; 2000. [Google Scholar]
- 26. Centers for Disease Control and Prevention. How to measure and interpret weight status. https://www.cdc.gov/healthyweight/assessing/index.html. Accessed June 21, 2021.
- 27. Il’yasova D, Hertz-Picciotto I, Peters U, Berlin JA, Poole C.. Choice of exposure scores for categorical regression in meta-analysis: a case study of a common problem. Cancer Causes Control. 2005;16(4):383–388. [DOI] [PubMed] [Google Scholar]
- 28. Institute of Medicine. Weight gain during pregnancy: Reexamining the guidelines. The National Academies Collection: Reports funded by National Institutes of Health. Washington, DC: National Academy Press; 2009:2. [PubMed]
- 29. Heck JE, Omidakhsh N, Azary S, et al. A case-control study of sporadic retinoblastoma in relation to maternal health conditions and reproductive factors: a report from the Children’s Oncology group. BMC Cancer. 2015;15(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greenop KR, Blair EM, Bower C, Armstrong BK, Milne E.. Factors relating to pregnancy and birth and the risk of childhood brain tumors: results from an Australian case-control study. Pediatr Blood Cancer. 2014;61(3):493–498. [DOI] [PubMed] [Google Scholar]
- 31. Stacy SL, Buchanich JM, Ma Z-Q, et al. Maternal obesity, birth size, and risk of childhood cancer development. Am J Epidemiol. 2019;188(8):1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Contreras ZA, Ritz B, Virk J, Cockburn M, Heck JE.. Maternal pre-pregnancy and gestational diabetes, obesity, gestational weight gain, and risk of cancer in young children: a population-based study in California. Cancer Causes Control. 2016;27(10):1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bailey HD, Rios P, Lacour B, et al. Factors related to pregnancy and birth and the risk of childhood brain tumours: the ESTELLE and ESCALE studies (SFCE, France). Int J Cancer. 2017;140(8):1757–1769. [DOI] [PubMed] [Google Scholar]
- 34. McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS.. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163(9):818–828. [DOI] [PubMed] [Google Scholar]
- 35. Peckham‐Gregory EC, Danysh HE, Brown AL, et al. Evaluation of maternal and perinatal characteristics on childhood lymphoma risk: a population‐based case‐control study. Pediatr Blood Cancer. 2017;64(5):e26321. [DOI] [PubMed] [Google Scholar]
- 36. Petridou ET, Sergentanis TN, Skalkidou A, et al. Maternal and birth anthropometric characteristics in relation to the risk of childhood lymphomas: a Swedish nationwide cohort study. Eur J Cancer Prev. 2015;24(6):535–541. [DOI] [PubMed] [Google Scholar]
- 37. Spector LG, Davies SM, Robison LL, Hilden JM, Roesler M, Ross JA.. Birth characteristics, maternal reproductive history, and the risk of infant leukemia: a report from the Children’s Oncology Group. Cancer Epidemiol Biomarkers Prev. 2007;16(1):128–134. [DOI] [PubMed] [Google Scholar]
- 38. Kessous R, Wainstock T, Walfisch A, Sheiner E.. The risk for childhood malignancies in the offspring of mothers with previous gestational diabetes mellitus: a population-based cohort study. Eur J Cancer Prev. 2019;28(4):377–381. [DOI] [PubMed] [Google Scholar]
- 39. Musselman JR, Georgieff MK, Ross JA, et al. Maternal pregnancy events and exposures and risk of hepatoblastoma: a Children’s Oncology Group (COG) study. Cancer Epidemiol. 2013;37(3):318–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McLaughlin CC, Baptiste MS, Schymura MJ, Zdeb MS, Nasca PC.. Perinatal risk factors for neuroblastoma. Cancer Causes Control. 2009;20(3):289–301. [DOI] [PubMed] [Google Scholar]
- 41. McLaughlin C, Baptiste M, Schymura M, Nasca P, Zdeb M.. Birth weight, maternal weight and childhood leukaemia. Br J Cancer. 2006;94(11):1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Podvin D, Kuehn CM, Mueller BA, Williams M.. Maternal and birth characteristics in relation to childhood leukaemia. Paediatr Perinat Epidemiol. 2006;20(4):312–322. [DOI] [PubMed] [Google Scholar]
- 43. Kessous R, Wainstock T, Sheiner E.. Pre‐pregnancy obesity and childhood malignancies: a population‐based cohort study. Pediatr Blood Cancer. 2020;67(6):e28269. [DOI] [PubMed] [Google Scholar]
- 44. Stephansson O, Wahnström C, Pettersson A, et al. Perinatal risk factors for childhood testicular germ-cell cancer: a Nordic population-based study. Cancer Epidemiol. 2011;35(6):e100–e104. [DOI] [PubMed] [Google Scholar]
- 45. Hamrick SE, Olshan AF, Neglia JP, Pollock BH.. Association of pregnancy history and birth characteristics with neuroblastoma: a report from the Children’s Cancer Group and the Pediatric Oncology Group. Paediatr Perinat Epidemiol. 2001;15(4):328–337. [DOI] [PubMed] [Google Scholar]
- 46. Chow EJ, Friedman DL, Mueller BA.. Maternal and perinatal characteristics in relation to neuroblastoma. Cancer. 2007;109(5):983–992. [DOI] [PubMed] [Google Scholar]
- 47. Deleskog A, den Hoed M, Tettamanti G, et al. Maternal diabetes and incidence of childhood cancer-a nationwide cohort study and exploratory genetic analysis. Clin Epidemiol. 2017;9:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Søegaard SH, Rostgaard K, Kamper-Jørgensen M, Schmiegelow K, Hjalgrim H.. Maternal diabetes and risk of childhood acute lymphoblastic leukaemia in the offspring. Br J Cancer. 2018;118(1):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ognjanovic S, Puumala S, Spector LG, et al. Maternal health conditions during pregnancy and acute leukemia in children with Down syndrome: a Children’s Oncology Group study. Pediatr Blood Cancer. 2009;52(5):602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borsari L, Malagoli C, Della Torre F, et al. Maternal pregestational diabetes and risk of acute lymphoblastic leukemia in the offspring: a population-based study in Northern Italy. Cancer Epidemiol. 2019;62:101572. [DOI] [PubMed] [Google Scholar]
- 51. Daniels JL, Pan I-J, Olshan AF, Breslow NE, Bunin GR, Ross JA.. Obstetric history and birth characteristics and Wilms tumor: a report from the Children’s Oncology Group. Cancer Causes Control. 2008;19(10):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Seppälä LK, Vettenranta K, Pitkäniemi J, Hirvonen E, Leinonen MK, Madanat‐Harjuoja LM.. Maternal diabetes and risk of childhood cancer in the offspring. Int J Cancer. 2020;147(3):662–668. [DOI] [PubMed] [Google Scholar]
- 53. Åberg A, Westbom L.. Association between maternal pre‐existing or gestational diabetes and health problems in children. Acta Paediatr. 2001;90(7):746–750. [PubMed] [Google Scholar]
- 54. Heck JE, Lombardi CA, Meyers TJ, Cockburn M, Wilhelm M, Ritz B.. Perinatal characteristics and retinoblastoma. Cancer Causes Control. 2012;23(9):1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnson KJ, Soler JT, Puumala SE, Ross JA, Spector LG.. Parental and infant characteristics and childhood leukemia in Minnesota. BMC Pediatr. 2008;8(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Milne E, Laurvick CL, Blair E, Bower C, De Klerk N.. Fetal growth and acute childhood leukemia: looking beyond birth weight. Am J Epidemiol. 2007;166(2):151–159. [DOI] [PubMed] [Google Scholar]
- 57. Petridou E, Trichopoulos D, Kalapothaki V, et al. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br J Cancer. 1997;76(9):1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vienneau D, Infanger D, Feychting M, et al. A multinational case-control study on childhood brain tumours, anthropogenic factors, birth characteristics and prenatal exposures: a validation of interview data. Cancer Epidemiol. 2016;40:52–59. [DOI] [PubMed] [Google Scholar]
- 59. Westbom L, Åberg A, Källén B.. Childhood malignancy and maternal diabetes or other auto-immune disease during pregnancy. Br J Cancer. 2002;86(7):1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu CS, Nohr EA, Bech BH, Vestergaard M, Olsen J.. Long-term health outcomes in children born to mothers with diabetes: a population-based cohort study. PLoS One. 2012;7(5):e36727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cnattingius S, Zack MM, Ekbom A, et al. Prenatal and neonatal risk factors for childhood lymphatic leukemia. J Natl Cancer Inst. 1995;87(12):908–914. [DOI] [PubMed] [Google Scholar]
- 62. Bauer H, Rios P, Schleiermacher G, et al. Maternal and perinatal characteristics, congenital malformations and the risk of Wilms tumor: the ESTELLE study. Cancer Causes Control. 2020;31(5):491–411. [DOI] [PubMed] [Google Scholar]
- 63. Puumala SE, Soler JT, Johnson KJ, Spector LG.. Birth characteristics and Wilms tumor in Minnesota. Int J Cancer. 2008;122(6):1368–1373. [DOI] [PubMed] [Google Scholar]
- 64. Yan P, Wang Y, Yu X, Liu Y, Zhang Z-J.. Maternal diabetes and risk of childhood malignancies in the offspring: a systematic review and meta-analysis of observational studies. Acta Diabetol. 2021;58(2):153–168. [DOI] [PubMed] [Google Scholar]
- 65. Kashanian SM, Li AY, Mustafa Ali M, et al. Increased body mass index is a risk factor for acute promyelocytic leukemia. EJHaem. 2021;2(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Recalde M, Davila-Batista V, Díaz Y, et al. Body mass index and waist circumference in relation to the risk of 26 types of cancer: a prospective cohort study of 3.5 million adults in Spain. BMC Med. 2021;19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Larsson SC, Wolk A.. Overweight and obesity and incidence of leukemia: a meta‐analysis of cohort studies. Int J Cancer. 2008;122(6):1418–1421. [DOI] [PubMed] [Google Scholar]
- 68. Dushnicky MJ, Nazarali S, Mir A, Portwine C, Samaan MC.. Is there a causal relationship between childhood obesity and acute lymphoblastic leukemia? A review. Cancers (Basel). 2020;12(11):3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Orgel E, Tucci J, Alhushki W, et al. Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood. 2014;124(26):3932–3938. [DOI] [PubMed] [Google Scholar]
- 70. Mittelman SD, Kim J, Raca G, Li G, Oberley MJ, Orgel E.. Increased prevalence of CRLF2 rearrangements in obesity-associated acute lymphoblastic leukemia. Blood. 2021;138(2):199–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ghosh T, Richardson M, Gordon PM, Ryder JR, Spector LG, Turcotte LM.. Body mass index associated with childhood and adolescent high‐risk B‐cell acute lymphoblastic leukemia risk: a Children’s Oncology Group report. Cancer Med. 2020;9(18):6825–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. El-Baz HA, Mosa TE, Elabd EM, et al. Serum adiponectin and resistin levels in de novo and relapsed acute lymphoblastic leukemia children patients. Iran J Public Health. 2013;42(5):504. [PMC free article] [PubMed] [Google Scholar]
- 73. Calle EE, Kaaks R.. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579–591. [DOI] [PubMed] [Google Scholar]
- 74. Hino M, Nakao T, Yamane T, Ohta K, Takubo T, Tatsumi N.. Leptin receptor and leukemia. Leuk Lymphoma. 2000;36(5-6):457–461. [DOI] [PubMed] [Google Scholar]
- 75. Elks CE, Den Hoed M, Zhao JH, et al. Variability in the heritability of body mass index: a systematic review and meta-regression. Front Endocrinol (Lausanne). 2012;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Feng R. How much do we know about the heritability of BMI? Am J Clin Nutr. 2016;104(2):243–244. [DOI] [PubMed] [Google Scholar]
- 77. Sharpe PB, Chan A, Haan EA, Hiller JE.. Maternal diabetes and congenital anomalies in South Australia 1986-2000: a population‐based cohort study. Birth Defects Res A Clin Mol Teratol. 2005;73(9):605–611. [DOI] [PubMed] [Google Scholar]
- 78. Lauszus FF, Klebe JG, Flyvbjerg A.. Macrosomia associated with maternal serum insulin-like growth factor-I and-II in diabetic pregnancy. Obstet Gynecol. 2001;97(5):734–741. [DOI] [PubMed] [Google Scholar]
- 79. Jansson N, Nilsfelt A, Gellerstedt M, et al. Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am J Clin Nutr. 2008;87(6):1743–1749. [DOI] [PubMed] [Google Scholar]
- 80. Dimasuay KG, Boeuf P, Powell TL, Jansson T.. Placental responses to changes in the maternal environment determine fetal growth. Front Physiol. 2016;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sarikabadayi YU, Aydemir O, Aydemir C, et al. Umbilical cord oxidative stress in infants of diabetic mothers and its relation to maternal hyperglycemia. J Pediatr Endocrinol Metab. 2011;24(9-10):671–674. [DOI] [PubMed] [Google Scholar]
- 82. Ma RC, Tutino GE, Lillycrop KA, Hanson MA, Tam WH.. Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog Biophys Mol Biol. 2015;118(1-2):55–68. [DOI] [PubMed] [Google Scholar]
- 83. Kerényi Z, Tamás G, Kivimäki M, et al. Maternal glycemia and risk of large-for-gestational-age babies in a population-based screening. Diabetes Care. 2009;32(12):2200–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hjalgrim LL, Westergaard T, Rostgaard K, et al. Birth weight as a risk factor for childhood leukemia: a meta-analysis of 18 epidemiologic studies. Am J Epidemiol. 2003;158(8):724–735. [DOI] [PubMed] [Google Scholar]
- 85. Caughey RW, Michels KB.. Birth weight and childhood leukemia: a meta‐analysis and review of the current evidence. Int J Cancer. 2009;124(11):2658–2670. [DOI] [PubMed] [Google Scholar]
- 86. Qian L, Wei S-J, Zhang D, et al. Potent anti-inflammatory and neuroprotective effects of TGF-β1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol. 2008;181(1):660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Pan W, Kastin AJ.. Adipokines and the blood-brain barrier. Peptides. 2007;28(6):1317–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Trojan J, Cloix J-F, Ardourel M-Y, Chatel M, Anthony D.. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience. 2007;145(3):795–811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data analyzed for this study are contained within the manuscript and/or its supplementary materials.