Abstract
Prior to infection, phytopathogenic bacteria face a challenging environment on the plant surface, where they are exposed to nutrient starvation and abiotic stresses. Pathways enabling surface adhesion, stress tolerance, and epiphytic survival are important for successful plant pathogenesis. Understanding the roles and regulation of these pathways is therefore crucial to fully understand bacterial plant infections. The phytopathogen Pseudomonas syringae pv. tomato (Pst) encodes multiple polysaccharides that are implicated in biofilm formation, stress survival, and virulence in other microbes. To examine how these polysaccharides impact Pst epiphytic survival and pathogenesis, we analysed mutants in multiple polysaccharide loci to determine their intersecting contributions to epiphytic survival and infection. In parallel, we used qRT–PCR to analyse the regulation of each pathway. Pst polysaccharides are tightly coordinated by multiple environmental signals. Nutrient availability, temperature, and surface association strongly affect the expression of different polysaccharides under the control of the signalling protein genes ladS and cbrB and the second messenger cyclic-di-GMP. Furthermore, functionally redundant, combinatorial phenotypes were observed for several polysaccharides. Exopolysaccharides play a role in mediating leaf adhesion, while α-glucan and alginate together confer desiccation tolerance. Our results suggest that polysaccharides play important roles in overcoming environmental challenges to Pst during plant infection.
Keywords: Biofilm, cyclic-di-GMP, exopolysaccharides, lipopolysaccharide, plant infection Pseudomonas syringae, surface adhesion
Pseudomonas syringaeuses the coordinated deployment of polysaccharides to address environmental challenges during plant colonization. Functional redundancy renders individual polysaccharides dispensable during plant infection, but their combined loss impedes pathogenicity.
Introduction
Pseudomonas syringae is a Gram-negative, plant pathogenic bacterium and a widely used model system to understand plant–microbe interactions (Hirano and Upper, 2000; Xin et al., 2018). Taxonomic studies indicate that P. syringae is in fact a diverse phylogenetic group containing >15 species and >60 pathovars (Young, 2010). Pathovars of P. syringae infect almost all economically important crop plants and are considered to be one of the most common and damaging bacterial plant pathogens that infect the phyllosphere (Xin et al., 2018). Pseudomonas syringae pathogenesis is attributed to a repertoire of virulence factors, including secretion systems, phytotoxins and phytohormones, quorum-sensing pathways, ice nucleation agents, cell wall-degrading enzymes, and exopolysaccharides (EPSs) (Hirano and Upper, 2000; Pfeilmeier et al., 2016a; Xin et al., 2018). Plant infection by P. syringae consists of epiphytic and endophytic phases (Xin and He, 2013). Survival on plant surfaces such as leaves, stems, or fruits is referred as the epiphytic phase, while the endophytic phase describes bacterial entry into the plant tissue and colonization of the intercellular apoplastic space. Bacteria face a challenging environment on the plant surface, where they are routinely exposed to mechanical, temperature, and desiccation stresses, nutrient starvation, and UV irradiation (Andrews and Harris, 2000; Wu et al., 2012; Djonovic et al., 2013; Pfeilmeier et al., 2016a). Thus, adhesion to plant surfaces, stress tolerance, and epiphytic survival are likely to be important for successful pathogenesis.
Extracellular matrix and cell envelope components such as lipopolysaccharide (LPS) and EPS molecules interact directly with the host/plant surface and hence are vital in establishing an interaction during the epiphytic phase (Mhedbi-Hajri et al., 2011; King and Roberts, 2016). Genetic studies have shown that LPS production is required for efficient host colonization and for full virulence in plant pathogenic Pseudomonas spp., Erwinia amylovora, and Burkholderia cenocepacia (Berry et al., 2009; Khodai-Kalaki et al., 2015; Kutschera et al., 2019). Similarly, EPS production has been implicated in epiphytic survival of P. syringae and Xanthomonas spp., and in the wider plant microbiome (Yu et al., 1999; Dunger et al., 2007). It has also been suggested to contribute to bacterial freeze–thaw resistance (Wu et al., 2012). The widely studied model plant pathogen P. syringae pv. tomato str. DC3000 (Pst) contains multiple polysaccharide gene clusters that can potentially contribute to biofilm matrix formation, including alginate, Wss cellulose, Psl, and α-glucan (Winsor et al., 2016).
Alginate is a copolymer of acetylated β-1,4-linked d-mannuronic acid and l-glucuronic acid that contributes to antibiotic and immune system protection in the human pathogen Pseudomonas aeruginosa (Hentzer et al., 2001; Pier et al., 2001). Alginate is not considered essential to Pseudomonas biofilm formation (Wozniak et al., 2003; Laue et al., 2006), but has been implicated in epiphytic fitness and virulence in P. syringae pathovars actinidiae and syringae (Yu et al., 1999; McAtee et al., 2018; Helmann et al., 2019). Cellulose is a homopolymer made of β-d-glucose monomers and is a primary component of the biofilm matrix of Pst and many other bacteria (Serra et al., 2013; Prada-Ramirez et al., 2016; Farias et al., 2019). Cellulose has been suggested to play an important role in the transition between epiphytic and pathogenic phases of leaf association (Arrebola et al., 2015). Similarly, acetylated cellulose (Wss) contributes substantially to root association of the rhizosphere bacterium P. fluorescens (Gal et al., 2003). Psl is a pentasaccharide polymer of d-mannose, d-glucose, and l-rhamnose subunits that plays essential roles in biofilm formation, adhesion, motility, and stress protection in P. aeruginosa (Byrd et al., 2009; Billings et al., 2013; Periasamy et al., 2015). The function of Psl in P. syringae remains relatively unclear, although it has recently been implicated in swarming motility and pathogenicity regulation in the P. syringae mango pathovar UMAF0158 (Heredia-Ponce et al., 2020).
GlgE-derived α-glucan is a glycogen-like homopolymer of glucose monomers with α-1,4 glycosidic links and α-1,6-linked branch points (Edstrom, 1972). α-Glucan is a ubiquitous carbon store and plays important roles in desiccation stress tolerance in P. aeruginosa (Woodcock et al., 2021), and virulence in Mycobacterium tuberculosis (Sambou et al., 2008; Koliwer-Brandl et al., 2016). Recently, α-glucan has been implicated as an EPS in the virulent kiwi pathovar P. actinidiae (Ghods et al., 2015). The ubiquitous compatible solute trehalose is not only a precursor of α-glucan (Woodcock et al., 2021) but also contributes to osmotic stress tolerance during epiphytic survival by P. syringae (Freeman et al., 2010). Finally, levan is a β-2,6 polyfructan with extensive branching through β-2,1 linkages (Li and Ullrich, 2001; Laue et al., 2006). Levan has not been implicated in biofilm formation or epiphytic survival. Instead, it has been suggested to function as a storage molecule that may be produced in the apoplast (Laue et al., 2006; Yu et al., 2013).
LPS molecules are complex glycoconjugate molecules whose biosynthesis, packing, and role in pathogenesis and immune evasion is well understood in animal/human pathosystems but less explored in plant pathogens. The Pst genome encodes at least four LPS biosynthesis operons, including genes for WaaP and Waa proteins alongside wapQ/wapG and wbpL as per annotations in the Pseudomonas genome database pseudomonas.com (Winsor et al., 2016). In Erwinia amylovora, LPS production contributes to virulence and oxidative stress protection (Berry et al., 2009). Similarly, in Burkholderia cenocepacia and Xylella fastidiosa, virulence is compromised in LPS mutants (Khodai-Kalaki et al., 2015; Rapicavoli et al., 2018). Recent studies have shown that Pst cells lacking a wbpL orthologue failed to produce O-polysaccharide and exhibited reduced apoplast colonization and pathogenesis (Kutschera et al., 2019). The LPS core kinase gene waaP can be deleted in several Gram-negative bacteria such as Escherichia coli and Salmonella enterica but not in P. aeruginosa (DeLucia et al., 2011). Finally, PSPTO4998 of Pst encodes a putative WaaP family LPS kinase (wapQ/wapG/inaA) but is poorly characterized in P. syringae.
Pst phytotoxins and effector proteins and their roles in immune suppression have been extensively studied. However, to obtain a comprehensive understanding of plant infection, we also need to unravel the relationship between bacterial survival, stress tolerance, and pathogenesis. In this study, we build on prior research on cellulose and alginate production in Pst (Keith et al., 2003; Farias et al., 2019; Perez-Mendoza et al., 2019) to examine the intersecting roles of multiple Pst polysaccharide molecules in mediating bacterial infection and enabling epiphytic survival. We hypothesize that bacterial polysaccharides are differentially expressed in response to the environmental cues and play important roles in abiotic stress tolerance and epiphytic survival during plant infection. To test this, Pst mutants were constructed in the alginate (alg), psl, cellulose (wss), and α-glucan (glg/tre) pathways, and the putative LPS kinase gene wapQ, and their relative contributions to phenotypes including colony morphology, abiotic stress tolerance, epiphytic survival, and plant infection were defined.
The polysaccharide pathways in Pst are each tightly regulated by external environmental cues. In particular, alg and psl expression was shown to be strongly nutrient dependent, while wss production was activated by low temperatures and overproduction of the second messenger molecule bis-(3ʹ-5ʹ)-cyclic dimeric GMP (or cyclic-di-GMP) (Jenal et al., 2017). Expression of the glg trehalose/α-glucan locus is induced by surface association, under the control of the global regulators ladS and cbrB (Sonnleitner et al., 2009; Grenga et al., 2017). Strikingly, we observed a substantial degree of functional redundancy and phenotypic interaction between the polysaccharide molecules in our study. While disruption of individual polysaccharide loci had little effect on Pst plant infection, mutation of multiple loci led to compromised infection or delayed disease onset for several different combinations. Similarly, we observed combinatorial phenotypic effects for several loci, suggestive of functional redundancy. Alginate and α-glucan combine to confer desiccation stress tolerance, while disruption of EPS genes only impacted leaf surface adhesion when combined with a wapQ deletion. Our data suggest that bacterial polysaccharides play important, intersecting roles in enabling infection by plant pathogens.
Materials and methods
Strains and growth conditions
Unless otherwise stated, all strains were grown at 28 °C with shaking. Bacterial strains and plasmids used in this study are listed in Table 1. King’s B (KB) medium (King et al., 1954), Lennox (L) medium (Sambrook and Russel, 2001), soya flour mannitol medium (SFM) [2% soya flour (SF) and 2% mannitol], and M9 medium [M9 salts (Sambrook and Russel, 2001) supplemented with 0.4% glucose, 0.4% casamino acids, and 50 µM FeCl3] were used for culturing and for in vitro assays. Antibiotics were used at a final concentration of gentamicin at 25 μg ml–1, tetracycline at 12.5 μg ml–1, and kanamycin at 25 μg ml–1 for selection of mutants or during genetic manipulations. Final concentrations of X-gal of 40 μg ml–1 and isopropyl-β-d-thiogalactopyranoside (IPTG) of 1 mM were used for blue–white screening the of reporter strain.
Table 1.
List of strains used in this study
| P. syringae pv tomato DC3000 (Pst) | ||
|---|---|---|
| Strain name | Description | Reference |
| WT | Rifampicin resistant Pst considered as wild type | Buell et al. (2003) |
| ∆alg | Strain with algG (PSPTO1238) and algX (PSPTO1237) deletion | Lammertz et al. (2019) |
| ∆psl | Strain with pslD (PSPTO3531) and pslE (PSPTO3532) deletion | Lammertz et al. (2019) |
| ∆wss | Strain with wssB (PSPTO1027) and wssC (PSPTO1028) deletion | Lammertz et al. (2019) |
| ∆treS | Strain with glgE (PSPTO2760), treS (PSPTO2761), and glgB (PSPTO2762) deletion | This study |
| ∆treY/Z | Strain with glgA (PSPTO3125), treZ (PSPTO3126), malQ (PSPTO3127), treY (PSPTO3128) and (PSPTO3129), and glgX (PSPTO3130) deletion | This study |
| ∆treS ∆treY/Z | A combination of ∆treS and ∆treY/Z | This study |
| ∆alg ∆psl | Strain with algG-X (PSPTO1238, PSPTO1237) and pslD-E (PSPTO3531, PSPTO3532) deletion | Lammertz et al. (2019) |
| ∆alg ∆wss | Strain with algG (PSPTO1238) and algX (PSPTO1237) and wssB-C (PSPTO1027, PSPTO1028) deletion | Lammertz et al. (2019) |
| ∆psl ∆wss | Strain with pslD-E (PSPTO3531, PSPTO3532) and wssB-C (PSPTO1027, PSPTO1028) deletion | Lammertz et al. (2019) |
|
∆alg ∆treS
∆treY/Z |
Strain with algG-X (PSPTO1238, PSPTO1237) deletion combined with ∆treS ∆treY/Z | Lammertz et al. (2019) |
| ∆EPS (∆alg ∆psl ∆wss) | Strain with algG-X, pslD-E, and wssB-C deletion | Lammertz et al. (2019) |
| ∆wapQ (∆PSPTO4998) | Strain with deletion of an orthologue of wapQ (∆PSPTO4998) | This study |
| ∆EPS+∆wapQ | A combination of ∆EPS and ∆wapQ | This study |
| Pst PglgA-lacZ | Reporter strain developed by genome integration of the construct into neutral site using mini Tn7. Reporter construct was generated by cloning lacZ gene under the control of glgA promoter. | This study |
| Tn::cbrB | Pst PglgA-lacZ strain with identified transposon insertion in cbrB gene | This study |
| Tn::ladS | Pst PglgA-lacZ strain with identified transposon insertion in ladS gene | This study |
| Tn::PSPTO1866 | Pst PglgA-lacZ strain with identified transposon insertion in PSPTO1866 gene | This study |
| Plasmids | ||
| pTS1 | TetR, suicide vector; ColE1-replicon, IncP-1, Mob, lacZ | Scott et al. (2017) |
| pBBR-wspR19 | KanR, resistant and di-guanylate cyclase (wspR19)-expressing plasmid | Pfeilmeier et al. (2016b) |
Mutagenesis and genetic manipulation
Gene deletion vectors were constructed by amplifying the upstream and downstream regions flanking the desired gene from the Pst genome using primers nos 1–72, listed in Supplementary Table S1. Amplified up- and downstream flanking regions were then cloned into the multiple cloning site of the suicide vector pTS1 (Scott et al., 2017). Pst cells were transformed using electroporation with the appropriate deletion vectors following the method described in Choi et al. (2006). Single crossover integrations of each plasmid into the chromosome were selected on tetracycline plates and re-streaked before single colonies were grown overnight in KB medium without selection. Double crossovers were counterselected by plating serial dilutions onto L agar containing 10% (w/v) sucrose. Deletion mutants were confirmed by PCR using corresponding primers labelled test-F and test-R in Supplementary Table S1. To produce the Pst-PglgA-lacZ reporter, the promoter region of Pst glgA (PSPTO3125) including several codons of the ORF was amplified using primers nos 73 and 74, given in Supplementary Table S1 and cloned between the BamHI and HindIII sites of pUC18-mini-Tn7T-Gm-LacZ10 (Choi and Schweizer, 2006).
Transposon mutagenesis screening
The plasmid pALMAR3 was introduced into Pst-PglgA-lacZ via biparental mating with E. coli S17-1. Mariner transposon insertion mutants were selected by plating onto L agar containing gentamycin, tetracycline, and X-Gal. Colonies showing changes in LacZ activity were re-streaked and the location of the transposon in each case was determined by arbitrary PCR (O’Toole and Kolter, 1998) using primers 75–78 given in Supplementary Table S1.
Extraction and NMR analysis of metabolites
Overnight Pst cultures were grown in M9 medium, then adjusted to a cell density of 0.5 (OD600) in phosphate-buffered saline (PBS). Mixed cellulose ester filter discs (Merck Millipore) were placed on the surface of M9 agar plates and coated with the diluted cell suspensions. Plates were then incubated at 28 °C for 48 h before cells were harvested from each disc and resuspended in 5 ml of double-distilled water (ddH2O) in a 15 ml plastic tube by vigorous vortexing. Vacuum dried cells were weighed (DW), resuspended in ddH2O, and boiled at 95 °C for 20 min. Boiled cells were then centrifuged (10 000 g, 10 min) and the resulting supernatant was subject to additional boiling and centrifugation steps to remove any remaining insoluble contaminants. The supernatant, now containing the total water-soluble metabolite content of the cell sample, was dried under vacuum for 16 h. The resulting dried pellet was resuspended in 1200 μl of D2O and analysed by 1H-NMR spectroscopy (Bruker AVANCE III 400 spectrometer), at room temperature with water suppression. Metabolite chemical shifts were recorded as parts per million (ppm) relative to 0.5 mM trimethylsilyl propanoic acid (TMSP; 0.00 ppm). Spectra were analysed using Topspin 3.0 (Bruker), and the concentrations of metabolites were established through the manual integration of peaks relative to TMSP. The resonances were assigned based on previously established spectra (Usui et al., 1974; Woodcock et al., 2021)
Plant infection assays and bacterial load estimation
Arabidopsis thaliana Col-0 (Col-0) plants were grown in a controlled-environment room in short-day conditions: 10 h light, 22 °C, 70% relative humidity. Cell cultures were grown overnight in L medium, then resuspended in 10 mM MgCl2 and adjusted to a final density (OD600) of 0.2, equivalent to 1 × 108 colony-forming units (CFU) ml–1. Then 0.04% (w/v) of Silwet® L-77 (phytotech labs) was added to the cell suspension as a surfactant just before spraying on the plants. Four- to five-week-old plants were infected using a hand-held sprayer until all leaves appeared wet. For leaf infiltration experiments, cells at a final OD600 of 0.02 (equivalent to 107 cells ml–1) were gently infiltrated into the leaf apoplast using a 1 ml syringe until leaves appeared wet. For calculating bacterial load, two 7 mm diameter leaf discs (area 0.384 cm2) were collected for each sample and decanted into 200 µl of sterile 10 mM MgCl2. Post-infiltration/spray infection, bacterial suspensions were allowed to absorb/dry onto leaf surfaces for ~1 h before initial (0 D) samples were collected. Subsequent samples were collected after 24 h (1 D), 48 h (2 D), etc., up to 5 d over the course of each infection. Samples were lysed using a Geno/Grinder 2010 high-throughput tissue homogenizer, with two 3 mm glass beads and two cycles of 1700 vibrations min–1 with a 1 min break. Bacterial counts were determined by plating 10-fold serial dilutions on L agar plates with 50 µg ml–1 rifampicin and 20 µg ml–1 nystatin. A minimum of eight plants were used for each condition, and assays were run at least twice independently. Bacterial load was calculated and presented as CFU per unit leaf area (CFU cm–2).
Growth assays
Bacterial growth was measured in a microplate spectrometer using a minimum of five biological replicates and is presented as the mean ±SD. A 150 μl aliquot of the indicated growth medium in each case was added to the wells of clear-bottomed, black-walled 96-well microplates. Growth was initiated by the addition of 5 μl of overnight cell culture (L medium), to obtain a starting OD600 of 0.01. Plates were incubated statically at 28 °C, and agitated (500 rpm, 5 s) prior to each data acquisition step. The OD was measured at 600 nm. Experiments were conducted at least twice independently.
Osmotic stress assays
Overnight Pst cultures were grown in M9 medium, then diluted to an OD600 of 0.01 in M9 medium. A 5 µl aliquot of each diluted sample was used to inoculate 150 µl of M9 medium. To examine osmotic stress conditions, growth media were supplemented with 0.35 M NaCl. Growth was monitored by measuring the OD at 600 nm every hour for 6 d. Assays were conducted in triplicate and repeated at least twice independently, with a representative sample shown in each case.
Desiccation tolerance assays
Desiccation tolerance assays were conducted following the method as previously described (Woodcock et al., 2021). Overnight Pst cultures were grown in M9 medium, then diluted to an OD600 of 0.1 in PBS. A 10 µl aliquot of each culture was spotted onto 15 mm grade 1 Whatman filter discs. After drying for 1 min at room temperature, discs were placed onto M9 agar plates and incubated at 28 °C for 4 h to enable bacteria to recover and begin dividing. After incubation, the filter discs were subjected to controlled desiccation in tightly sealed bell chambers containing either water (100% relative humidity) or a saturated solution of NaCl (75% relative humidity) (de Goffau et al., 2009) for 2 h. Bacteria were then recovered from filter discs in 3 ml of PBS and serially diluted before spreading onto L agar plates. CFU were determined for each strain, then log10(CFU) values were analysed by linear mixed modelling using restricted maximum likelihood (REML) following the methodology described in Woodcock et al. (2021). Assays were conducted in triplicate and repeated at least twice independently.
Assessment of colony morphology
To assess the colony morphology of the Pst mutant strains, overnight cultures were grown in L medium, resuspended in 10 mM MgCl2 solution, and final densities were adjusted to an OD600 of 0.1. Agar plates were prepared and allowed to dry for 45 min in a sterile flow chamber. KB and SFM (Kieser et al., 2000) plates were prepared, with Congo red (CR) dye added as appropriate (30 μg ml–1). A 5 μl aliquot of each culture was spotted onto the agar surface and allowed to dry in a sterile flow chamber. Plates were then incubated at 28 °C for the indicated period before photographing, with a representative image shown in each case. For temperature treatments, plates were incubated at 28 °C for 24 h to enable colony establishment before plates were transferred to the designated temperature for a further incubation period as stated in the text.
Leaf surface adhesion and surface survival assays
Adhesion assays were performed according to Arrebola et al. (2015) with minor modifications. Tomato (cultivar Moneymaker) leaf discs (1 cm diameter) were prepared and placed in 70% ethanol for 30 s with gentle swirling, then washed three times with 200 ml of sterile water. These leaf discs were placed on water agar with the adaxial surface facing upwards and allowed to air-dry in a sterile flow hood. Overnight bacterial cultures were adjusted to 108 CFU ml–1 in 10 mM MgCl2. Drops (10 μl) of each strain were then inoculated on the adaxial surface of the leaf discs and allowed to air-dry. After 5 h, the leaf pieces were gently washed by placing each disc in 1 ml of sterile 0.85% NaCl solution and gently inverting 3–4 times to remove unattached cells. Three washed leaf discs were placed in 1 ml of sterile 0.85% NaCl, vortexed for 30 s to release adhered cells, followed by serial dilution and plating onto L agar with rifampicin (50 µg ml–1) and nystatin (20 µg ml–1) plates for CFU counting. Three such replicates for each strain and at least two independent experiments were performed. For in vitro leaf surface survival assays, tomato (cultivar Moneymaker) leaf discs (1 cm diameter) were prepared and inoculated with bacteria as above. At the respective time points, leaf discs were collected and placed in 1 ml of sterile 10 mM MgCl2, vigorously vortexed for 30 s to release cells, and CFU were counted following serial dilution.
RNA isolation and qRT–PCR
Total RNA was extracted from cells grown on KB agar or SFM agar plates for 24 h at 28 °C. For low temperature treatment, 24-hour-old cultures were shifted from 28 °C to 8 °C and allowed to grow for another 24 h before collection. Cells scraped from plates were resuspended in 1 ml of RNA later and pelleted by centrifugation. RNA was isolated from pelleted cells using column capture (Qiagen RNeasy Mini Kit) following the manufacturer’s instructions. Purified RNA was subjected to additional DNase treatment (Turbo DNase, Ambion). RNA integrity was verified by agarose gel electrophoresis, and the absence of genomic DNA contamination was confirmed by a negative response to 16S rRNA gene amplification in PCRs using isolated RNA as template. cDNA was prepared from isolated RNA using Superscript II reverse transcriptase (Invitrogen) following the manufacturer’s instructions. Gene-specific primers were designed using the IDT primer quest tool, then quantitative reverse transcription–PCR (qRT–PCR) assays were conducted in a Bio-Rad CFX96 Touch RT-PCR machine using a SensiFAST™ SYBR® No-ROX Kit, with the following settings: 3 min at 95 °C and 50 cycles of 5 s at 95 °C, 10 s at 62 °C, and 10 s at 72 °C followed by a melting curve. Two independent RNA extractions and three technical replicates per extraction were assessed. 16S rRNA and gyrA genes were used as internal controls for calculation of relative gene expression. Gene-specific primer sequences (nos 79–92) used in this study are listed in (Supplementary Table S1).
TEM and imaging
Pst cells grown on KB agar for 24 h were harvested and fixed using a method described by (DeLucia et al. (2011). In brief, cells from agar were fixed using a mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer (CB), pH 7.4, for 2 h at room temperature. Chemically fixed samples were washed in 0.1 M CB and post-fixed with 1% osmium tetroxide (OsO4)–1.5% potassium ferricyanide {K3[Fe(CN) 6]} for 1 h, washed in water three times, incubated in 1% aqueous uranyl acetate for 1 h, then dehydrated in ascending grades of ethanol (30, 50, 70, 95, and two changes of 100% ethanol, each for 1 h). Once dehydrated, the samples were gradually infiltrated with LR White resin (London Resin Company, Reading, UK) by successive changes of resin:ethanol mixes at room temperature (1:1 for 1 h, 2:1 for 1 h, 3:1 for 1 h, 100% resin for 1 h, then 100% resin for 16 h and a fresh change again for a further 8 h) then the samples were transferred into gelatine capsules full of fresh LR White and placed at 60 °C for 16 h to polymerize. The material was sectioned with a diamond knife using a Leica UC6 ultramicrotome (Leica, Milton Keynes, UK), and ultrathin sections of ~90 nm were picked up on 200 mesh copper grids which had been coated with pyroxylin and carbon. The sections were stained with 2% (w/v) uranyl acetate for 1 h and 1% (w/v) lead citrate for 1 min, washed in distilled water, and air-dried. The grids were viewed in a FEI Talos 200C transmission electron microscope (FEI UK Ltd, Cambridge, UK) at 200 kV and imaged using a Gatan OneView 4K×4K digital camera (Gatan, Cambridge, UK) to record DM4 files.
Results
Deletion of individual Pst polysaccharide loci has little effect on Arabidopsis infection
The Pst genome contains well-conserved predicted gene clusters for production of the polysaccharides alginate, Wss, Psl, α-glucan, and levan, as well as WapP/WaaP LPS biosynthetic clusters as per annotations in pseudomonas.com (Winsor et al., 2016), summarized in Supplementary Figs S1–S6. Levan has been suggested to function as an apoplastic storage molecule (Laue et al., 2006; Yu et al., 2013) and is not implicated in biofilm or survival on leaf surfaces. Therefore, it was not studied further here. To probe the relationship between the remaining polysaccharide pathways and plant pathogenicity, we produced non-polar deletion mutants in key biosynthetic genes from each operon. Deletions in algX-G (Δalg), wssB-C (Δwss), and pslD-E (Δpsl), alongside a triple mutant of all three clusters (ΔEPS) have been described previously (Lammertz et al., 2019). Pst has a similar arrangement of trehalose and α-glucan biosynthetic genes to P. aeruginosa PA01 (Woodcock et al., 2021), and we predict that the pathway is likely to function similarly in both species (Supplementary Figs S5, S6, S7. Consequently, we produced mutants of the treS (ΔPSPTO2760-62) and treY/Z (ΔPSPTO3125-3130) operons. Finally, we deleted the conserved, putative LPS kinase gene wapQ (PSPTO4998) (Supplementary Figs S1, S8). wapQ can be deleted in P. aeruginosa, where its disruption induces phenotypes consistent with LPS disruption (Walsh et al., 2000), but has relatively minor effects on bacterial fitness, unlike many other LPS biosynthetic genes. Despite this, wapQ is highly conserved between the LPS biosynthetic operons of Pseudomonas spp. (Kutschera et al., 2021).
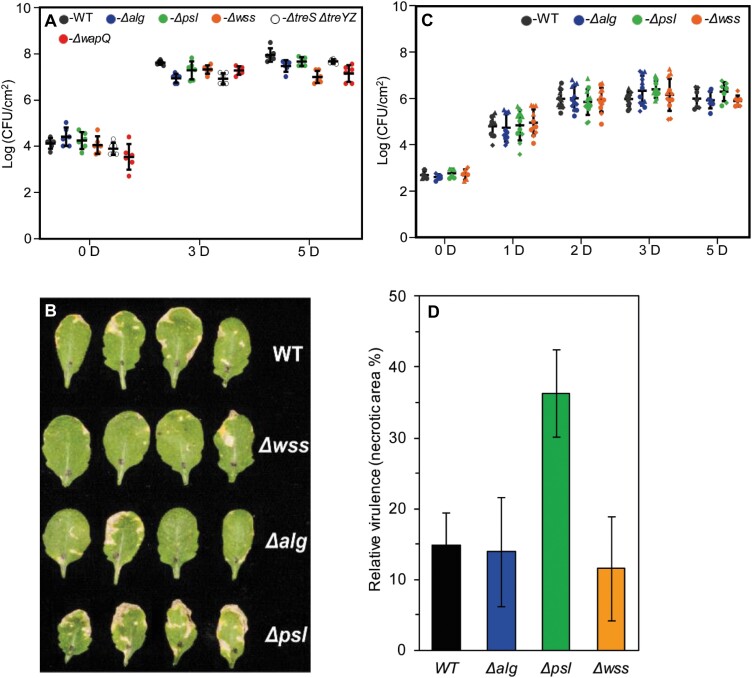
To investigate the role of individual polysaccharides in plant infection, we conducted a series of infection experiments in A. thaliana Col-0 (Col-0) plants. No significant differences were observed between any of the Pst single mutant strains for spray infections (Fig. 1A). Previous research has highlighted the importance of the EPS alginate in apoplastic, but not epiphytic, survival in P. syringae B728a (Helmann et al., 2019). This suggests that bypassing the initial stages of infection may uncover differences between our mutants. To test this, we conducted leaf infiltration assays with our three Pst EPS single mutants. However, while a slight increase in necrosis symptoms was observed for leaves infected with the Δpsl mutant (Fig. 1B), no significant difference in bacterial load versus the wild type was seen for any of the single EPS mutants tested (Fig. 1C).
Fig. 1.
Deletion of individual polysaccharide loci has little effect on Pst Arabidopsis infections (A) Bacterial load following spray infection at different days post-infection [D]. Means ±SD from biological duplicates and technical triplicates are shown. (B) Arabidopsis thaliana Col-0 leaves 5 d post-infiltration with different Pst mutants. Similar results were obtained in three biologically independent experiments and a representative picture is shown. (C) Bacterial load post-infection following infiltration at different days post-infection [D]. Means ±SD from three biologically independent experiments are presented in each case. Values from independent experiments are represented using different shapes. (D) Relative virulence calculated based on necrotic area (from leaves in B) 5 d post-infiltration.
Wss, alginate, Psl, and α-glucan production are regulated by cyclic-di-GMP and nutrient availability
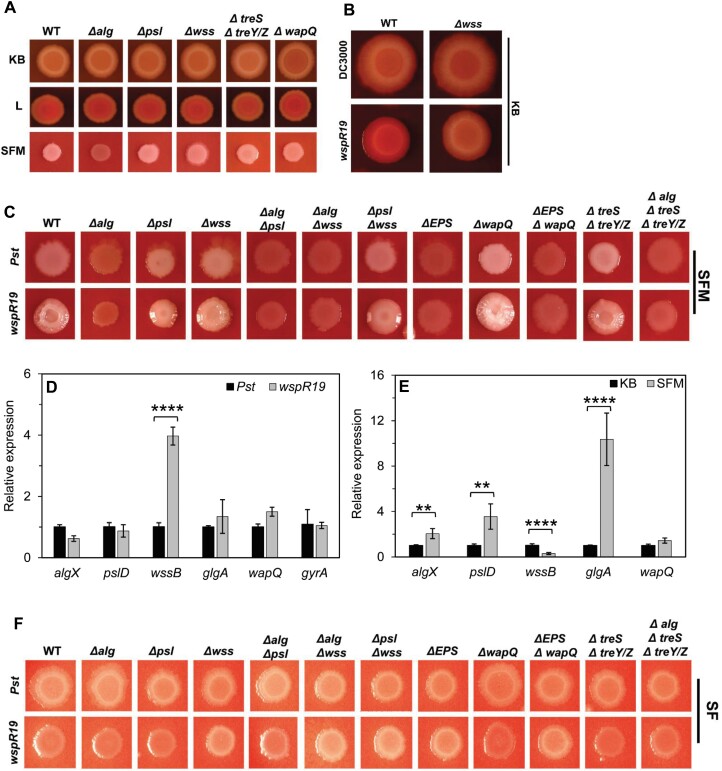
To understand why the Pst polysaccharide loci are apparently dispensable for plant infection, we tested their expression and production in response to a variety of intracellular and external signals. In general, disruption of individual polysaccharide loci did not lead to marked differences in Pst morphology for colonies grown on different nutrient media, with a few exceptions (Fig. 2A). On SFM, the distinctive, alginate-dependent white mucoid colony phenotype was absent in the Δalg mutant. Deletion of ΔtreS ΔtreY/Z produced noticeably thicker, more mucoid white colonies on SFM, suggesting a possible increased production of alginate in the absence of α-glucan. Deletion of wapQ produced a distinctive phenotype on KB CR agar, with a dark halo surrounding the central zone of the Pst colony and higher CR binding at the centre of the colony on L agar with CR (Fig. 2A; Supplementary Fig. S9A). CR is an amyloid dye that is widely used in studying production of EPS molecules due to its strong binding to certain polysaccharides. The transient CR binding in the centre of the colony by ΔwapQ suggests that WapQ plays a role in altering the Pst cell surface, possibly via modification of LPS. However, the absence of WapQ did not result in the visible accumulation of polysaccharides inside the cells, as was observed for the WaaP kinase of P. aeruginosa (DeLucia et al., 2011) (Supplementary Fig. S9B, C).
Fig. 2.
Wss, alginate, Psl, and α-glucan production are stimulated by cyclic-di-GMP and nutrient availability. (A) Phenotypes of mutant strains on CR-supplemented King’s B (KB), L medium (L), and soy flour mannitol medium (SFM) agar plates. (B) Effect of high cyclic-di-GMP on Pst colony morphology at 28 °C on CR-supplemented KB. (C) Colony morphology of Pst strains on CR-supplemented SFM. Strains with natural levels of cyclic-di-GMP (Pst); strains with high c-di-GMP (wspR19). (D) Quantitative real-time PCR analysis of the expression of polysaccharide-producing genes in the wild type (WT; Pst, black) and in the presence of high levels of cyclic-di-GMP (wspR19, grey). (E) Quantitative real-time PCR analysis of the expression of polysaccharide-producing genes for WT Pst grown on KB agar (black) and SFM agar (grey). Error bars represent means ±SD with n=2 (three technical replicates each) for (D) and (E). Significance as determined by Student’s t-test (∗∗P<0.005 and ∗∗∗∗P<0.0001). (F) Colony morphology of Pst strains on CR-supplemented soy flour (SF) medium. Similar results were obtained in two independent experiments, and a representative picture is shown.
The second messenger cyclic-di-GMP stimulates EPS production in many bacterial species including Pst (Jenal et al., 2017; Perez-Mendoza et al., 2019). To examine how cyclic-di-GMP levels affect Pst polysaccharide production, we transformed our mutants with the plasmid pBBR-wspR19, which elevates cyclic-di-GMP levels by ~15-fold in Pst (Pfeilmeier et al., 2016b). Curiously, unlike with many Pseudomonas strains (Malone et al., 2010), we saw little impact of cyclic-di-GMP overproduction on colonies grown on KB agar at 28 °C, besides a slight increase in CR binding that was absent in the Δwss background (Fig. 2B; Supplementary Fig. S10A). Conversely, growth on SFM led to the formation of mucoid, wrinkly colonies upon wspR19 expression (Fig. 2C). Two polysaccharide gene deletions markedly affected this colony morphology. First, wspR19+Δpsl mutants produced smooth, mucoid colonies, supporting a role for Psl in maintaining the architecture of wrinkly colonies on SFM. Second, alg disruption abolished both mucoidy and the wrinkly phenotype in all backgrounds, implicating alginate as the primary structural EPS for Pst on SFM (Fig. 2C).
These results were supported by qRT–PCR of the EPS genes. wssB mRNA abundance increased markedly upon wspR19 expression in Pst grown on KB agar, while the other tested genes were unaffected (Fig. 2D). However, a comparison of wild-type Pst grown on KB and SFM agar showed significantly higher algX, pslD, and glgA mRNA abundance, and reduced levels of wssB mRNA for colonies on SFM, suggesting that expression of these EPS loci is strongly dependent on nutrient cues from the environment (Fig. 2E). The cyclic-di-GMP-dependent, alginate-linked mucoid phenotype disappeared in the absence of mannitol; that is; for strains grown on SF agar, suggesting that mannitol is a key nutrient for alginate production. Curiously, Pst wspR19+ strains lacking the complete Wss produced white slightly mucoid colonies on SF plates. This phenotype was independent of alginate, Psl, or WapQ, suggesting that another, unknown polysaccharide may be up-regulated under these conditions (Fig. 2F).
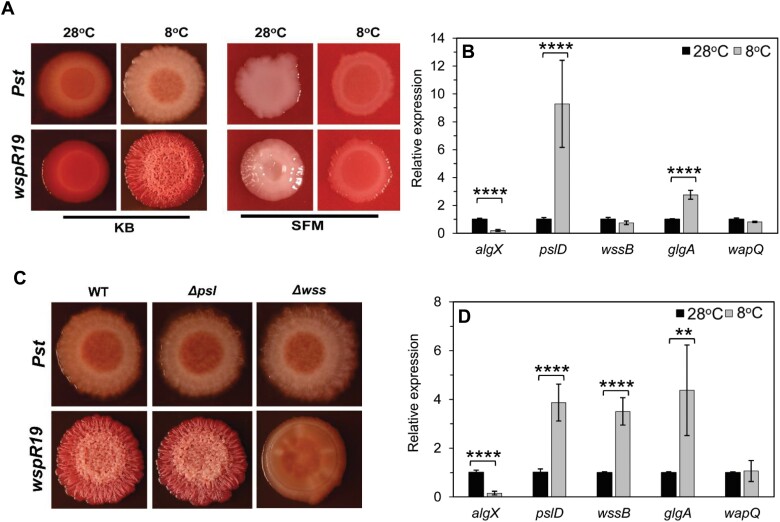
Wss, Psl, and α-glucan production are stimulated, while alginate is suppressed, by low temperature
Next, we examined the impact of temperature on polysaccharide gene expression and the associated changes in colony morphology. Switching the temperature from 28 °C to 8 °C produced relatively subtle changes in wild-type Pst but led to the formation of dry, wrinkled colonies upon wspR19 expression on KB agar (Fig. 3A). The switch from 28 °C to 8 °C led to significant increases in mRNA abundance for pslD and glgA and reduced levels of algX (Fig. 3B). A similar pattern was seen for Pst wspR19+, with the exception of wssB, where a greater increase in mRNA abundance was seen at 8 °C than at 28 °C (compare Figs 2D and 3B–D).
Fig. 3.
Wss, Psl, and α-glucan production are stimulated by low temperature. (A) Effect of temperature on Pst colony morphology on CR-supplemented media. Wild-type (WT) Pst and Pst wspR19 grown on KB and SFM at 28 °C and 8 °C. (B) qRT–PCR analysis of polysaccharide genes in WT Pst grown on KB agar at 28 °C (black) and 8 °C (grey). (C) Colony morphology of the WT, Δpsl, and Δwss on KB agar after 5 d of cold treatment at 8 °C. Strains with high cyclic-di-GMP (wspR19) are shown. (D) qRT–PCR analysis of polysaccharide genes in Pst wspR19 grown on KB agar at 28 °C (black) and 8 °C (grey). Values represented are means ±SD with n=2 (three technical replicates each). Significance was determined by Student’s t-test (∗∗P<0.005 and ∗∗∗∗P<0.0001). (A and C) Similar results were obtained in two independent experiments and a representative image is shown.
The wrinkled colony phenotype on KB was dependent on Wss alone (Fig. 3C). Conversely, growth at 8 °C saw an almost complete abolition of the alginate-dependent, wrinkled mucoid phenotype on SFM plates (Fig. 3A; Supplementary Fig. S10A, B). This was further supported by the decreased levels of algX mRNA seen at low temperature (Fig. 3B–D). The Wss-dependent wrinkled morphology did not manifest on SFM plates at 8 °C, suggesting that Wss production is nutrient dependent in Pst in a similar manner to Psl and alginate (Fig. 3A; Supplementary Fig. S10B).
α-Glucan and alginate production are stimulated by global carbon storage regulators and surface association
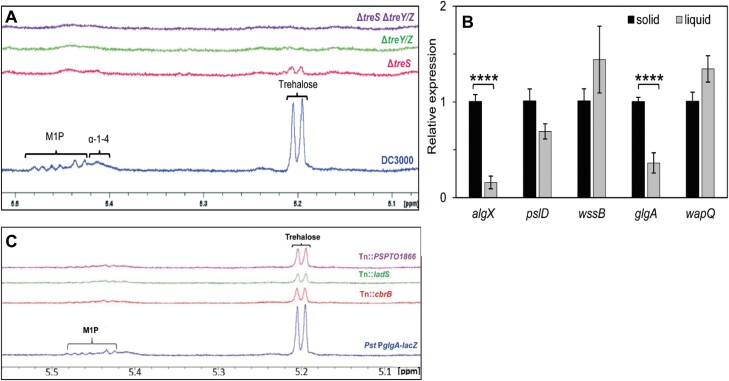
The treS and treY/Z operons have previously been implicated in Pst leaf surface survival (Freeman et al., 2010), suggesting that their expression is likely to be most relevant during epiphytic growth. To investigate this further, we first determined the abundance of key metabolites in Pst by 1H-NMR spectroscopy. Wild-type Pst grown in M9 medium accumulated trehalose and maltose 1-phosphate (M1P) to 0.13 ± 0.03% and 0.30 ± 0.03% of cellular dry weight, respectively (Table 2), alongside a broad NMR peak at 5.41 ppm corresponding to α-glucan (Fig. 4A). Deletion of the treS operon alone (glgE, treS/pep2, and glgB) not only blocked the production of M1P and α-glucan, as expected, but also led to less trehalose being detected (0.04 ± 0.01%) compared with the wild type. Deletion of glgA, treZ, malQ, treY, and glgX (ΔtreY/Z) resulted in no detectable metabolites from these pathways as expected (Fig. 4A; Table 2), whether or not the other operon was deleted.
Table 2.
Concentrations of trehalose and M1P produced by Pst strains
| Strain | Trehalose (%) | M1P (%) | Presence of α-glucan |
|---|---|---|---|
| WT | 1.33 ± 0.14 | 0.43 ± 0.15 | + |
| ΔtreS | 0.04 ± 0.01 | ND | – |
| ΔtreY/Z | ND | ND | – |
| ΔtreS ΔtreY/Z | ND | ND | – |
Metabolites are presented as percentages of cellular DW ±SE (n ≥ 2). ND indicates no measurable metabolite. Presence or absence of α-glucan is indicated by + or –, respectively.
Fig. 4.
α-Glucan and alginate biosynthesis are stimulated by global carbon storage regulators and surface association. (A) 1H-NMR spectra for Pst wild type (WT; blue), ΔtreS (pink), ΔtreY/Z (green), and a double mutant ΔtreS ΔtreY/Z (purple). Peaks corresponding to key metabolites are labelled: maltose 1-phosphate (M1P), α-glucan (α-1,4), α-1,1-probable terminal linkage of maltooligosyltrehalose (trehalose). (B) qRT–PCR analysis of polysaccharide genes in WT Pst grown on KB agar (solid) versus KB liquid (liquid). Values represented are means ±SD with n=2 (three technical replicates each). Significance was determined by Student’s t-test (∗∗∗∗P<0.0001. (C) 1H-NMR spectra for the Pst PglgA-lacZ reporter strain and selected glgA regulatory Tn mutants. Peaks corresponding to key metabolites are labelled as in (A).
Next, we used qRT–PCR to measure mRNA abundance for the different polysaccharide genes for Pst cells grown on a solid agar surface and in liquid media. Normalized to the gyrA internal control, the mRNA level for the α-glucan synthase gene glgA was significantly higher in surface-grown compared with liquid-grown Pst cells (Fig. 4B). Interestingly, the same pattern of increased mRNA abundance in surface-grown cells was also seen for algX, while no differences were seen for the other tested genes.
To further investigate the regulation of Pst trehalose/α-glucan gene expression, the 500 bp region upstream of the glgA start codon was cloned upstream of lacZ and incorporated into the Pst chromosome at the neutral att::Tn7 site. The resulting strain (Pst PglgA-lacZ) produced blue colonies on XGal+IPTG plates and was then used to conduct a transposon mutagenesis screen for regulators of glgA transcription. Once false positives/negatives and likely indirect hits had been discarded, we identified promising transposon insertions in three regulatory loci: the response regulator cbrB, the histidine kinase gene ladS, and a predicted TetR family transcriptional regulator, PSPTO1866. Transposon insertion led to light blue colonies in each case, suggesting that these regulators may function as activators of glgA expression.
To test the effects of gene disruption on trehalose and α-glucan biosynthesis, the Pst water-soluble metabolome was analysed for the three transposon mutants. No significant difference was observed between the levels of analysed metabolites in the soluble metabolomes of wild-type Pst and Pst PglgA-lacZ. Tn::PSPTO1866 showed a small, but not statistically significant (P=0.06) reduction in trehalose and no change in M1P abundance compared with Pst PglgA-lacZ. Conversely, the metabolomes of Tn::cbrB and Tn::ladS showed significant decreases in both trehalose and M1P levels (Fig. 4C; Table 3), supporting roles for the global carbon utilization and chronic/acute lifestyle regulatory proteins LadS (and by extension the Gac/Rsm pathway) and CbrB in stimulating trehalose and α-glucan gene expression (Sonnleitner et al., 2009; Grenga et al., 2017).
Table 3.
Concentrations of trehalose and M1P produced by the Pst PglgA-lacZ strain and mutants generated by transposon random mutagenesis
| Strain | Trehalose (%) | M1P (%) |
|---|---|---|
| Pst-PglgA-lacZ | 1.68 ± 0.19 | 0.20 ± 0.03 |
| Tn::cbrBa | 0.41 | 0.11 |
| Tn::ladS | 0.31 ± 0.07∗ | 0.05 ± 0.02 |
| Tn::PSPTO1866 | 0.82 ± 0.21† | 0.26 ± 0.04 |
Metabolites are presented as percentages of cellular DW ±SE (n≥2).
a Indicates n=1.
Statistical significance compared with parent strain, ∗P<0.05 and †P>0.05 determined by Student’s t-test.
Disruption of trehalose, α-glucan, and alginate production leads to stress sensitivity and compromised plant infection
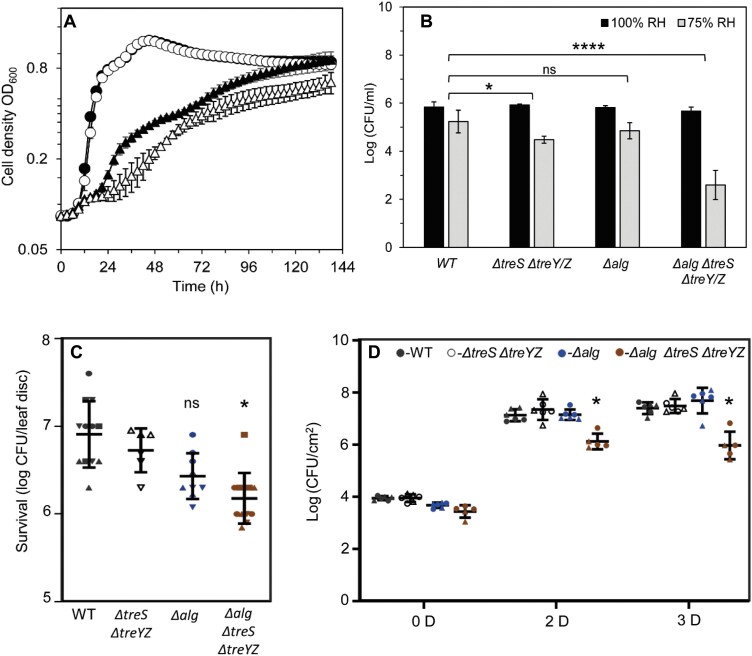
To investigate the roles of trehalose and α-glucan in Pst during osmotic stress, wild-type Pst and ΔtreS ΔtreY/Z were grown in the presence and absence of 0.35 M NaCl in M9, a defined medium. While both strains exhibited a longer lag phase and attenuated growth rate when cultured in 0.35 M NaCl, the ΔtreS ΔtreY/Z mutant showed substantially increased osmotic sensitivity relative to the wild type as expected, with the production of trehalose being blocked (Fig. 5A). Next, we analysed the relative desiccation stress tolerance of ΔtreS ΔtreY/Z and wild-type Pst as described previously (Woodcock et al., 2021). The CFU recovered after exposure to 100% and 75% relative humidity were counted, analysed using linear mixed modelling, and represented as predicted means of log10(CFU ml–1). The sensitivity of a strain to reduced humidity was calculated as the difference between its mean log10(CFU ml–1) at 100% and at 75% relative humidity. A greater response to lower relative humidity of a mutant compared with the wild type translates to a more desiccation-sensitive strain. Following incubation of bacterial spots on filter discs at 100% relative humidity, 5.83 log10(CFU ml–1) wild-type cells were recovered against 5.23 log10(CFU ml–1) at 75% relative humidity, equating to a desiccation response of ~0.60 log10(CFU ml–1) (Fig. 5B). The equivalent values for ΔtreS ΔtreY/Z were 5.92 (100% relative humidity) and 4.47 (75% relative humidity) log10(CFU ml–1), giving a desiccation response of ~1.50 log10(CFU ml–1) (Fig. 5B). Based on our recent analysis of PA01 (Woodcock et al., 2021), it is likely that this desiccation-sensitive phenotype is linked to the loss of α-glucan production.
Fig. 5.
Disruption of trehalose, α-glucan, and alginate production leads to stress sensitivity and compromised plant infection. (A) Growth of the wild type (WT) and ΔtreS ΔtreY/Z in M9 medium (circles), WT (filled), ΔtreS ΔtreY/Z (open), and in M9 medium supplemented with 0.35 M NaCl (triangles), WT (filled), ΔtreS ΔtreY/Z (open). Similar results were obtained for three biologically independent experiments. Data shown are the means ±SD of three biological replicates with two technical replicates each. (B) Desiccation stress survival assays for strains exposed to 100% relative humidity (black) and 75% (grey) for 2 h. Data shown are means ±SD of three biological replicates. (C) Surface survival assay on tomato leaf discs after 24 h. Recovered CFU after 24 h for selected mutant strains are presented. Similar results were obtained in at least two independent experiments. Means ±SD are presented, with values from independent experiments and triplicates of each measurement represented with a different shape in the figure. (D) Bacterial load following spray infection at different days post-infection [D]. WT Pst (black), ΔtreS ΔtreY/Z (white), Δalg (blue), and ΔtreS ΔtreY/Z Δalg (brown). Means ±SD are presented. with values from two independent experiments shown using a different shape. In each case, significance was determined by Student’s t-test (∗P<0.05, ∗∗∗∗P<0.0001, and ns not significant).
Alginate has also previously been associated with bacterial stress tolerance and epiphytic fitness (Yu et al., 1999; Helmann et al., 2019). Based on the apparent link between their colony morphology phenotypes on SFM plates and co-expression on a solid surface (KB-agar), we investigated the role of alginate in protecting Pst during desiccation. As the Δalg strain yielded a desiccation response of 0.96 log10(CFU ml–1), which was not significantly different to the response of wild-type Pst when analysed using linear mixed modelling (Fig. 5B), we tested whether α-glucan and alginate may exhibit functional redundancy in Pst. An alginate+α-glucan mutant (Δalg ΔtreS ΔtreY/Z) was generated and exposed to desiccation stress. This strain yielded a highly significant (P≤0.0001) desiccation response of 3.1 log10(CFU ml–1) (Fig. 5B). Furthermore, the Δalg ΔtreS ΔtreY/Z strain showed reduced epiphytic survival on tomato leaf discs, while no significant difference was observed for other tested strains after 24 h (Fig. 5C). This suggests that alginate and α-glucan functionally complement each other during desiccation stress response on plant leaves.
Next, we investigated whether the interaction of trehalose/α-glucan and alginate is also relevant during plant infection. Col-0 plants were spray infected with the different Δalg and ΔtreS ΔtreY/Z mutants monitored over 3 d growth without watering. In agreement with our previous assay (Fig. 1A), a lack of neither alginate nor trehalose/α-glucan alone affected the course of infection. However, we saw a significant decrease in bacterial cell counts for infections with Δalg ΔtreS ΔtreY/Z both 2 d and 3 d post-infection (Fig. 5D; Supplementary Fig. S11A). Together, our results suggest that alginate and α-glucan work together in Pst to mediate both desiccation stress tolerance and plant infectivity. Interestingly, no significant difference in bacterial colonization was observed either when plants are infiltrated or if plants were watered during the course of infection (Supplementary Figs S12A, S13). This supports the hypothesis that alginate and α-glucan play roles in surviving the water stress during epiphytic survival.
Disrupting wapQ and EPS alters Pst leaf adhesion and compromises plant infection
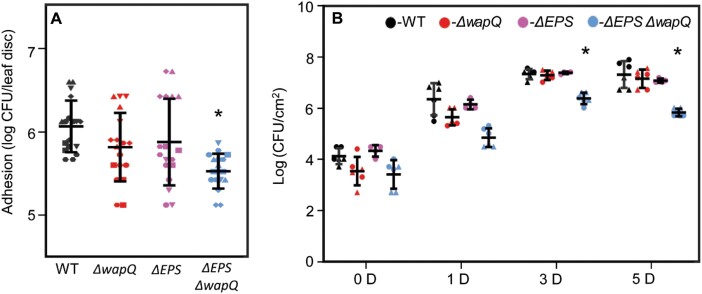
Several studies have suggested links between the LPS and EPS pathways in Pseudomonas spp. (McDonald et al., 2009). Given the lack of impact of single gene deletions on Col-0 infection and the complementarity observed between alginate and α-glucan, we tested the effect of disrupting both wapQ and the EPS biosynthesis loci on leaf surface interaction and plant infection. Following initial trial assays, Arabidopsis leaves proved too small for reliable measurements of surface association, so tomato leaves (cultivar Moneymaker) were used to examine Pst adhesion to and survival on leaf surfaces. In leaf disc adhesion assays (Arrebola et al., 2015), the ΔEPS ΔwapQ mutant showed a statistically significant reduction (P<0.05) in adhesion to tomato leaf discs compared with the other tested strains (Fig. 6A). Consistent with this, the ΔEPS ΔwapQ mutant displayed visibly compromised disease phenotypes on Col-0 plants, alongside significantly lower bacterial counts relative to wild-type Pst, the ΔEPS triple mutant, or ΔwapQ after 3 d and 5 d upon spray infection (Fig. 6B; Supplementary Fig. S11B). However, no significant differences in bacterial counts were observed upon infiltration (Supplementary Fig. S12B). This suggests that the altered surface attachment phenotype seen with this mutant translates into compromised plant infections.
Fig. 6.
Disrupting EPS and the putative LPS kinase WapQ alters Pst leaf adhesion and compromises plant infection. (A) Adhesion to tomato leaf surfaces. The graph denotes the number of cells adhered to leaf surfaces after 5 h. Means ±SD are presented, with values from two independent experiments and triplicates of each measurement represented with a different shape in the figure. (B) Bacterial load following spray infection at different days post-infection [D]. Wild-type (WT) Pst (black), ΔwapQ (red), ΔEPS (purple), and ΔEPS ΔwapQ (blue). Similar results were obtained in two biologically independent experiments and means ±SD are presented, with values from two independent experiments and triplicates of each measurement represented with a different shape in the figure. In each case, ∗P<0.05 determined by Student’s t-test.
Discussion
Phyllosphere-colonizing bacterial pathogens such as P. syringae face an array of environmental challenges on external plant surfaces (Wu et al., 2012; Djonovic et al., 2013; Freeman et al., 2013; Pfeilmeier et al., 2016b). Phytopathogenic bacteria must be able to tolerate rapid shifts in humidity and osmotic pressure, to adhere to leaf surfaces under mechanical disturbance, and to respond effectively to changes in temperature and nutrient availability, among other cues. Systems that facilitate bacterial survival and persistence in the face of these challenges thus play important roles in enabling infection (Pfeilmeier et al., 2016a). Based on gene/protein homologies, EPS pathways and cell envelope LPSs are ubiquitous in P. syringae genomes (Winsor et al., 2016) and are among the first bacterial molecules to interact with the host plant (Mhedbi-Hajri et al., 2011; King and Roberts, 2016). Polysaccharide biosynthesis has been linked to epiphytic survival, stress tolerance, and biofilm formation in various phytopathogenic bacteria (Pfeilmeier et al., 2016a). It has also been shown that alginate might play role in apoplastic survival in P. syringae B728a (Helmann et al., 2019). Furthermore, as major components of the biofilm matrix, polysaccharides play important roles not just in leaf surface survival but also during the transition to more acute stages of pathogenicity (Yu et al., 1999; Dunger et al., 2007; Pfeilmeier et al., 2016b; Xin et al., 2018).
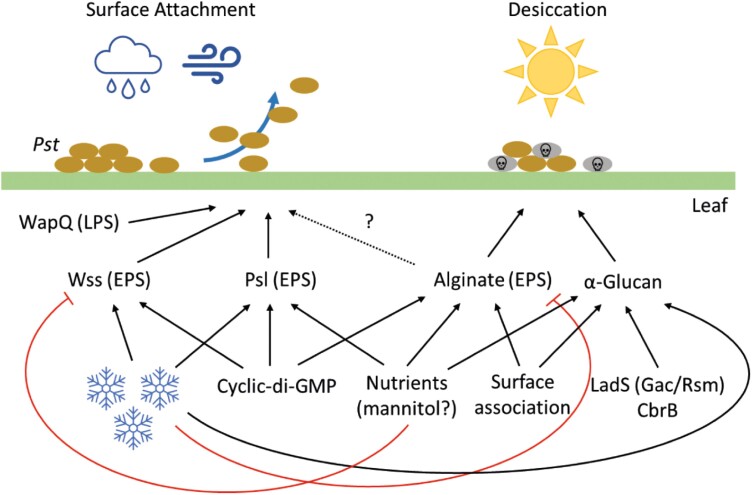
In this study, we examine the regulation of and interactions between five major polysaccharide pathways in Pst and determine their importance to plant infection and epiphytic survival. While EPS and LPS pathways have been implicated in phytopathogen virulence, stress response, and biofilm formation (Pfeilmeier et al., 2016a), previous studies have generally focused on the impact of individual polysaccharides (Dunger et al., 2007; Arrebola et al., 2015; Heredia-Ponce et al., 2020). The importance of interactions between the different polysaccharide pathways for plant infection and epiphytic survival is currently poorly understood. Our results suggest that polysaccharide production is tightly regulated and coordinated in Pst, with deployment of each polysaccharide system dependent on a number of shared environmental cues (Fig. 7). We observed a substantial degree of regulatory and functional redundancy: each external input stimulated multiple polysaccharide pathways, and infection/survival phenotypes were only observed for Pst mutants missing several coordinating pathways.
Fig. 7.
A model for polysaccharide deployment during plant infection by P. syringae Pst. Positive or stimulatory interactions are denoted by black arrows. Negative/suppressive interactions are shown by red arrows. An uncertain interaction is indicated with a dashed arrow. Blue snowflakes denote low temperature, brown ovals indicate Pst cells, and grey ovals indicate dying cells.
Cyclic-di-GMP conditionally stimulates production of all three Pst EPS molecules. However, unlike in other Pseudomonas spp. where increased cyclic-di-GMP levels lead to constitutive EPS production and wrinkled, aggregative colony morphologies (Malone et al., 2007, 2010), cyclic-di-GMP overproduction in Pst does not lead to major changes in colony morphology under standard laboratory conditions. Rather, Pst EPS production is highly dependent on external environmental cues, with production of the two major structural EPS molecules—Wss and alginate—stimulated by low temperature and changes to nutrient availability respectively. In each case, the formation of Wss- or alginate-driven wrinkled colonies requires both increased cyclic-di-GMP production and the correct growth environment. The two phenotypes are mutually exclusive, with Wss linked to growth on KB medium at low temperatures and alginate to SFM and surface association, but with little/no effect seen for gene deletions in the other condition.
Psl, which maintains the architecture of mucoid, wrinkly colonies on SFM plates, is stimulated both by low temperature and a switch to SFM, suggesting that Psl is linked to both the alginate- and Wss-mediated lifestyles. A central role for Psl in mediating the Pst transition from an epiphytic, biofilm lifestyle to more acute pathogenesis was supported by the apparent increase in disease symptoms seen for the Δpsl mutant during Col-0 leaf infections. Similar observations have been reported for a Salmonella enterica cellulose mutant, and upon Psl or cellulose disruption in P. syringae UMAF0158 (Pontes et al., 2015; Heredia-Ponce et al., 2020). The mechanism by which Psl suppresses disease symptoms during infection, and the relevance of this for bacterial fitness in the plant environment remain to be determined.
Expression of glgA, the first gene of the treY/Z operon in Pst, is stimulated by surface association, SFM, and the global regulatory proteins CbrB and LadS. The response regulator CbrB and its cognate signal kinase CbrA control carbon metabolism, biofilm formation, and stress tolerance (Nishijyo et al., 2001; Amador et al., 2010) by inducing expression of RpoN-dependent genes such as the sRNA crcZ (García-Mauriño et al., 2013), which acts to antagonize the carbon catabolite repressor Crc (Moreno et al., 2009). The Gac/Rsm system controls processes including biofilm, motility, virulence, and the stress response at the level of mRNA translation (Brencic et al., 2009; Chambers and Sauer, 2013). In Pseudomonas spp., the Gac/Rsm system is controlled by a series of accessory sensor kinases including LadS, which acts as a positive regulator of GacAS activity (Chambonnier et al., 2016).
Trehalose/α-glucan and alginate production appears to be subject to a shared regulatory hierarchy in Pseudomonas spp. CbrA/CbrB has been implicated in controlling alginate production in P. fluorescens (Ertesvåg et al., 2017), and ChIP-seq analysis of the P. putida genome identified a CbrB-binding site in the algD promoter (Barroso et al., 2018). Similarly, the Gac/Rsm system regulates alginate production in both Azotobacter and Pseudomonas (Manzo et al., 2011; Romero et al., 2018), with the anti-sigma factor gene mucA an mRNA target for Rsm proteins in P. aeruginosa. CbrA/CbrB and LadS are positive regulators of both alginate and trehalose/α-glucan biosynthesis (Ertesvåg et al., 2017; Romero et al., 2018), which makes sense in the context of their coordinated roles in protecting the cell from desiccation stress. Further research is needed to determine the extent to which the well-established alginate regulon (Wozniak and Ohman, 1994) also controls trehalose and α-glucan biosynthesis.
Trehalose/α-glucan, and potentially also alginate, individually contribute to Pst desiccation stress tolerance (Fig. 5B), although the effects on survival or infectivity of disrupting either pathway alone were minimal. However, we saw evidence for a substantial degree of functional redundancy between the two pathways, with trehalose/α-glucan/alginate triple mutants becoming highly sensitive to desiccation stress under laboratory conditions. This in vitro desiccation sensitivity translated to decreased epiphytic surface survival (Fig. 5C) and compromised plant infection when plants are grown in dry conditions (i.e. without watering; Fig. 5D). Although Pst is believed to be a weak epiphyte, our surface survival assays showed that a significant number of viable Pst cells remain on the surface of leaf discs even after 24 h of incubation. Our results therefore show that epiphytic survival is an important virulence determinant even for relatively weak epiphytes such as Pst. We also observed functional redundancy between the WapQ putative LPS kinase and the Pst EPS pathways. Leaf attachment and plant infection were unaffected for ΔwapQ, or upon disruption of the wss, psl, and alg operons. However, disruption of all four operons together led to significantly reduced leaf attachment and compromised Col-0 infection (Fig. 6A, B).
The emerging picture from our research is that Pst uses the coordinated deployment of distinct sets of polysaccharides to address different environmental challenges (Fig. 7). The first of these, desiccation and osmostress response, are addressed by the deployment of alginate, trehalose, and α-glucan in response to surface contact and changes to nutrient availability, under the control of the Gac/Rsm and CbrA/B regulatory pathways. Conversely, when attachment to leaf surfaces is a high priority, EPS pathways and particularly Wss are up-regulated in response to reduced temperatures and under the control of cyclic-di-GMP. These two responses appear to be mutually exclusive, with conditions that stimulate Wss production repressing the production of alginate, and vice versa.
Our data also suggest that in the context of these broad regulatory groups, Pst fine-tunes the deployment of individual polysaccharides to create an optimal response to the environment. For example, glg and alg regulation are closely aligned, except at low temperatures, where alg mRNA is reduced but glgA levels increase. Similarly, Psl appears to contribute to both alginate and Wss biofilm formation and is stimulated by SFM and low temperatures. Finally, cyclic-di-GMP signalling appears to stimulate several Pst polysaccharide pathways, although transcription-level regulation is only apparent for the wss locus. Further research is needed to fully understand the regulatory underpinnings of Pst polysaccharide deployment during plant infection.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. Mutagenesis of EPS-producing genes and a putative wapQ gene.
Fig. S2. Comparison of proteins encoded by alginate gene clusters.
Fig. S3. Comparison of proteins encoded by cellulose/wss gene clusters.
Fig. S4. Comparison of proteins encoded by Psl gene clusters.
Fig. S5. Comparison of proteins encoded by trehalose/α-glucan gene cluster-1.
Fig. S6. Comparison of proteins encoded by trehalose/α-glucan gene cluster-2.
Fig. S7. A scheme representing the pathway for the production of α-glucan in Pst.
Fig. S8. Comparison of WapQ protein sequences in pathovars of P. syringae.
Fig. S9. Effect of the absence of WapQ on Pst morphology.
Fig. S10. Effect of low temperature on colony phenotype of different mutant strains.
Fig. S11. Disease symptoms on Col-0 plants post-spray infection.
Fig. S12. Absence of alginate, α-glucan, EPS, and WapQ has no effect in infection upon infiltration.
Fig. S13. Absence of alginate and α-glucan has no effect on plant infection by Pst when plants are watered.
Table S1. List of primers used in this study.
Glossary
Abbreviations
- CFU
colony-forming units
- CR
Congo red dye
- cyclic-di-GMP
bis-(3ʹ-5ʹ)-cyclic dimeric GMP
- EPS
exopolysaccharide
- KB medium
King’s B medium
- LPS
lipopolysaccharide
- M1P
maltose 1-phosphate
- OD600
optical density at λ 600 nm
- Pst
Pseudomonas syringae pv. tomato DC3000
- SFM
soy flour mannitol medium
Author contributions
JGM, CZ, and SB: conceptualization, project administration, and funding acquisition; PSK, SDW, and SP: investigation, data curation, formal analysis, and methodology; PSK and JGM: writing—original draft; JGM, SDW, SP, PSK, CZ, and SB: writing—review and editing.
Conflict of interest
All authors contributing to this work have no conflicts of interest to disclose.
Funding
Research in the laboratories of JGM, CZ, and SB was supported by UK Research and Innovation- Biotechnology and Biological Sciences Research Council Norwich Research Park (UKRI-BBSRC) Institute Strategic Program Grants BB/J004553/1 (BIO), BB/J004561/1 (MET) and BBS/E/J/000PR9797 (Plant Health) to the John Innes Centre and The Sainsbury Laboratory. PSK was additionally supported by UKRI-BBSRC Grant BB/T004363/1. SDW was funded by a BBSRC Doctoral Training Partnership (BB/J014524/1) PhD studentship. SP was supported by a Norwich Research Park PhD studentship.
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary data published online. The data supporting the findings of this study and raw data used for making the graphs are available from the corresponding author, Jacob Malone, upon request.
References
- Amador CI, Canosa I, Govantes F, Santero E.. 2010. Lack of CbrB in Pseudomonas putida affects not only amino acids metabolism but also different stress responses and biofilm development. Environmental Microbiology 12, 1748–1761. [DOI] [PubMed] [Google Scholar]
- Andrews JH, Harris RF.. 2000. The ecology and biogeography of microorganisms on plant surfaces. Annual Review of Phytopathology 38, 145–180. [DOI] [PubMed] [Google Scholar]
- Arrebola E, Carrion VJ, Gutierrez-Barranquero JA, Perez-Garcia A, Rodriguez-Palenzuela P, Cazorla FM, de Vicent, A.. 2015. Cellulose production in Pseudomonas syringae pv. syringae: a compromise between epiphytic and pathogenic lifestyles. FEMS Microbiology Ecology 91, fiv071. [DOI] [PubMed] [Google Scholar]
- Barroso R, García-Mauriño SM, Tomás-Gallardo L, Andújar E, Pérez-Alegre M, Santero E, Canosa I.. 2018. The CbrB regulon: promoter dissection reveals novel insights into the CbrAB expression network in Pseudomonas putida. PLoS One 13, e0209191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MC, McGhee GC, Zhao Y, Sundin GW.. 2009. Effect of a waaL mutation on lipopolysaccharide composition, oxidative stress survival, and virulence in Erwinia amylovora. FEMS Microbiology Letters 291, 80–87. [DOI] [PubMed] [Google Scholar]
- Billings N, Millan MR, Caldara M, Rusconi R, Tarasova Y, Stocker R, Ribbeck K.. 2013. The extracellular matrix component Psl provides fast-acting antibiotic defense in Pseudomonas aeruginosa biofilms. PLoS Pathogens 9, e1003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S.. 2009. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Molecular Microbiology 73, 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell CR, Joardar V, Lindeberg M, et al. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proceedings of the National Academy of Sciences, USA 100, 10181–10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd MS, Sadovskaya I, Vinogradov E, et al. 2009. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Molecular Microbiology 73, 622–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JR, Sauer K.. 2013. Small RNAs and their role in biofilm formation. Trends in Microbiology 21, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambonnier G, Roux L, Redelberger D, Fadel F, Filloux A, Sivaneson M, de Bentzman, S, Bordi C.. 2016. The hybrid histidine kinase LadS forms a multicomponent signal transduction system with the GacS/GacA two-component system in Pseudomonas aeruginosa. PLoS Genetics 12, e1006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Kumar A, Schweizer HP.. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. Journal of Microbiological Methods 64, 391–397. [DOI] [PubMed] [Google Scholar]
- Choi KH, Schweizer HP.. 2006. mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nature Protocols 1, 153–161. [DOI] [PubMed] [Google Scholar]
- de Goffau MC, Yang XM, van Dijl JM, Harmsen HJM.. 2009. Bacterial pleomorphism and competition in a relative humidity gradient. Environmental Microbiology 11, 809–822. [DOI] [PubMed] [Google Scholar]
- DeLucia AM, Six DA, Caughlan RE, Gee P, Hunt I, Lam JS, Dean CR.. 2011. Lipopolysaccharide (LPS) inner-core phosphates are required for complete LPS synthesis and transport to the outer membrane in Pseudomonas aeruginosa PAO1. Mbio 2, e00142-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djonovic S, Urbach JM, Drenkard E, et al. 2013. Trehalose biosynthesis promotes Pseudomonas aeruginosa pathogenicity in plants. PLoS Pathogens 9, e1003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunger G, Relling VM, Tondo ML, Barreras M, Ielpi L, Orellano EG, Ottado J.. 2007. Xanthan is not essential for pathogenicity in citrus canker but contributes to Xanthomonas epiphytic survival. Archives of Microbiology 188, 127–135. [DOI] [PubMed] [Google Scholar]
- Edstrom RD. 1972. Structure of a low molecular weight form of glycogen isolated from the liver in a case of glycogen storage disease. Journal of Biological Chemistry 247, 1360–1367. [PubMed] [Google Scholar]
- Ertesvåg H, Sletta H, Senneset M, Sun, Q, Klinkenberg G, Konradsen TA, Ellingsen TE, Valla S.. 2017. Identification of genes affecting alginate biosynthesis in Pseudomonas fluorescens by screening a transposon insertion library. BMC Genomics 18, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GA, Olmedilla A, Gallegos MT.. 2019. Visualization and characterization of Pseudomonas syringae pv. tomato DC3000 pellicles. Microbial Biotechnology 12, 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman BC, Chen CL, Beattie GA.. 2010. Identification of the trehalose biosynthetic loci of Pseudomonas syringae and their contribution to fitness in the phyllosphere. Environmental Microbiology 12, 1486–1497. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Chen CL, Yu XL, Nielsen L, Peterson K, Beattie GA.. 2013. Physiological and transcriptional responses to osmotic stress of two Pseudomonas syringae strains that differ in epiphytic fitness and osmotolerance. Journal of Bacteriology 195, 4742–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal M, Preston GM, Massey RC, Spiers AJ, Rainey PB.. 2003. Genes encoding a cellulosic polymer contribute toward the ecological success of Pseudomonas fluorescens SBW25 on plant surfaces. Molecular Ecology 12, 3109–3121. [DOI] [PubMed] [Google Scholar]
- García-Mauriño SM, Pérez-Martínez I, Amador CI, Canosa I, Santero E.. 2013. Transcriptional activation of the CrcZ and CrcY regulatory RNAs by the CbrB response regulator in Pseudomonas putida. Molecular Microbiology 89, 189–205. [DOI] [PubMed] [Google Scholar]
- Ghods S, Sims IM, Moradali MF, Rehm BHA.. 2015. Bactericidal compounds controlling growth of the plant pathogen Pseudomonas syringae pv. actinidiae, which forms biofilms composed of a novel exopolysaccharide. Applied and Environmental Microbiology 81, 4026–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenga L, Little RH, Malone JG.. 2017. Quick change: post-transcriptional regulation in Pseudomonas. FEMS Microbiology Letters 364, fnx125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmann TC, Deutschbauer AM, Lindow SE.. 2019. Genome-wide identification of Pseudomonas syringae genes required for fitness during colonization of the leaf surface and apoplast. Proceedings of the National Academy of Sciences, USA 116, 18900–18910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentzer M, Teitzel GM, Balzer GJ, Heydorn A, Molin S, Givskov M, Parsek MR.. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. Journal of Bacteriology 183, 5395–5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia-Ponce Z, Gutierrez-Barranquero JA, Purtschert-Montenegro G, Eberl L, Cazorla FM, de Vicent, A.. 2020. Biological role of EPS from Pseudomonas syringae pv. syringae UMAF0158 extracellular matrix, focusing on a Psl-like polysaccharide. NPJ Biofilms and Microbiomes 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano SS, Upper CD.. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—a pathogen, ice nucleus, and epiphyte. Microbiology and Molecular Biology Reviews 64, 624–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenal U, Reinders A, Lori C.. 2017. Cyclic di-GMP: second messenger extraordinaire. Nature Reviews Microbiology 15, 271–284. [DOI] [PubMed] [Google Scholar]
- Keith RC, Keith LMW, Hernandez-Guzman G, Uppalapati SR, Bender CL.. 2003. Alginate gene expression by Pseudomonas syringae pv. tomato DC3000 in host and non-host plants. Microbiology (Reading) 149, 1127–1138. [DOI] [PubMed] [Google Scholar]
- Khodai-Kalaki M, Andrade A, Mohamed YF, Valvano MA.. 2015. Burkholderia cenocepacia lipopolysaccharide modification and flagellin glycosylation affect virulence but not innate immune recognition in plants. Mbio 6, e00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieser T, Bibb MJ, Chater KF, Butter MJ, Hopwood DA, Chater KF, Bittner MJ.. 2000. Practical streptomyces genetics. Norwich: John Innes Foundation. [Google Scholar]
- King EO, Ward MK, Raney DE.. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. Journal of Laboratory and Clinical Medicine 44, 301–307. [PubMed] [Google Scholar]
- King JE, Roberts IS.. 2016. Bacterial surfaces: front lines in host–pathogen interaction. Biophysics of Infection 915, 129–156. [DOI] [PubMed] [Google Scholar]
- Koliwer-Brandl H, Syson K, van de Weer R, et al. 2016. Metabolic network for the biosynthesis of intra- and extracellular alpha-glucans required for virulence of Mycobacterium tuberculosis. PLoS Pathogens 12, e1005768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera A, Schombel U, Schwudke D, Ranf S, Gisch N.. 2021. Analysis of the structure and biosynthesis of the lipopolysaccharide core oligosaccharide of Pseudomonas syringae pv. tomato DC3000. International Journal of Molecular Sciences 22, 3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera A, Schombel U, Wrobel M, Gisch N, Ranf S.. 2019. Loss of wbpL disrupts O-polysaccharide synthesis and impairs virulence of plant-associated Pseudomonas strains. Molecular Plant Pathology 20, 1535–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammertz M, Kuhn H, Pfeilmeier S, Malone J, Zipfel C, Kwaaitaal M, Lin NC, Kvitko BH, Panstruga R.. 2019. Widely conserved attenuation of plant MAMP-induced calcium influx by bacteria depends on multiple virulence factors and may involve desensitization of host pattern recognition receptors. Molecular Plant-Microbe Interactions 32, 608–621. [DOI] [PubMed] [Google Scholar]
- Laue H, Schenk A, Li H, Lambertsen L, Neu TR, Molin S, Ullrich MS.. 2006. Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiology (Reading) 152, 2909–2918. [DOI] [PubMed] [Google Scholar]
- Li HQ, Ullrich MS.. 2001. Characterization and mutational analysis of three allelic lsc genes encoding levansucrase in Pseudomonas syringae. Journal of Bacteriology 183, 3282–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JG, Jaeger T, Spangler C, Ritz D, Spang A, Arrieumerlou C, Kaever V, Landmann R, Jenal U.. 2010. YfiBNR mediates cyclic di-GMP dependent small colony variant formation and persistence in Pseudomonas aeruginosa. PLoS Pathogens 6, e1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone JG, Williams R, Christen M, Jenal U, Spiers AJ, Rainey PB.. 2007. The structure–function relationship of WspR, a Pseudomonas fluorescens response regulator with a GGDEF output domain. Microbiology (Reading) 153, 980–994. [DOI] [PubMed] [Google Scholar]
- Manzo J, Cocotl-Yañez M, Tzontecomani T, et al. 2011. Post-transcriptional regulation of the alginate biosynthetic gene algD by the Gac/Rsm system in Azotobacter vinelandii. Journal of Molecular Microbiology and Biotechnology 21, 147–159. [DOI] [PubMed] [Google Scholar]
- McAtee PA, Brian L, Curran B, et al. 2018. Re-programming of Pseudomonas syringae pv. actinidiae gene expression during early stages of infection of kiwifruit. BMC Genomics 19, 822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MJ, Gehrig SM, Meintjes PL, Zhang XX, Rainey PB.. 2009. Adaptive divergence in experimental populations of Pseudomonas fluorescens. IV. Genetic constraints guide evolutionary trajectories in a parallel adaptive radiation. Genetics 183, 1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhedbi-Hajri N, Jacques MA, Koebnik R.. 2011. Adhesion mechanisms of plant-pathogenic Xanthomonadaceae. Advances in Experimental Medicine and Biology 715, 71–89. [DOI] [PubMed] [Google Scholar]
- Moreno R, Marzi S, Romby P, Rojo F.. 2009. The Crc global regulator binds to an unpaired A-rich motif at the Pseudomonas putida alkS mRNA coding sequence and inhibits translation initiation. Nucleic Acids Research 37, 7678–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijyo T, Haas D, Itoh Y.. 2001. The CbrA–CbrB two-component regulatory system controls the utilization of multiple carbon and nitrogen sources in Pseudomonas aeruginosa. Molecular Microbiology 40, 917–931. [DOI] [PubMed] [Google Scholar]
- O’Toole GA, Koleter R.. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Molecular Microbiology 28, 449–461 [DOI] [PubMed] [Google Scholar]
- Perez-Mendoza D, Felipe A, Ferreiro MD, Sanjuan J, Gallegos MT.. 2019. AmrZ and FleQ co-regulate cellulose production in Pseudomonas syringae pv. tomato DC3000. Frontiers in Microbiology 10, 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy S, Nair HA, Lee KW, Ong J, Goh JQ, Kjelleberg S, Rice SA.. 2015. Pseudomonas aeruginosa PAO1 exopolysaccharides are important for mixed species biofilm community development and stress tolerance. Frontiers in Microbiology 6, 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilmeier S, Caly DL, Malone JG.. 2016a. Bacterial pathogenesis of plants: future challenges from a microbial perspective: challenges in bacterial molecular plant pathology. Molecular Plant Pathology 17, 1298–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilmeier S, Saur IM, Rathjen JP, Zipfel C, Malone JG.. 2016b. High levels of cyclic-di-GMP in plant-associated Pseudomonas correlate with evasion of plant immunity. Molecular Plant Pathology 17, 521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier GB, Coleman F, Grout M, Franklin M, Ohman DE.. 2001. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infection and Immunity 69, 1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes MH, Lee EJ, Choi J, Groisman EA.. 2015. Salmonella promotes virulence by repressing cellulose production. Proceedings of the National Academy of Sciences, USA 112, 5183–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada-Ramirez HA, Perez-Mendoza D, Felipe A, Martinez-Granero F, Rivilla R, Sanjuan J, Gallegos MT.. 2016. AmrZ regulates cellulose production in Pseudomonas syringae pv. tomato DC3000. Molecular Microbiology 99, 960–977. [DOI] [PubMed] [Google Scholar]
- Rapicavoli JN, Blanco-Ulate B, Muszynski A, Figueroa-Balderas R, Morales-Cruz A, Azadi P, Dobruchowska JM, Castro C, Cantu D, Roper MC.. 2018. Lipopolysaccharide O-antigen delays plant innate immune recognition of Xylella fastidiosa. Nature Communications 9, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero M, Silistre H, Lovelock L, et al. 2018. Genome-wide mapping of the RNA targets of the Pseudomonas aeruginosa riboregulatory protein RsmN. Nucleic Acids Research 46, 6823–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambou T, Dinadayala P, Stadthagen G, et al. 2008. Capsular glucan and intracellular glycogen of Mycobacterium tuberculosis: biosynthesis and impact on the persistence in mice. Molecular Microbiology 70, 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russel DW.. 2001. Molecular cloning. A laboratory manual.: Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Scott TA, Heine D, Qin Z, Wilkinson B.. 2017. An l-threonine transaldolase is required for l-threo-beta-hydroxy-alpha-amino acid assembly during obafluorin biosynthesis. Nature Communications 8, 390. [Google Scholar]
- Serra DO, Richter AM, Hengge R.. 2013. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. Journal of Bacteriology 195, 5540–5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnleitner E, Abdou L, Haas D.. 2009. Small RNA as global regulator of carbon catabolite repression in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences, USA 106, 21866–21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Yokoyama M, Yamaoka N, Matsuda K, Tuzimura K, Sugiyama H, Shuichi S.. 1974. Proton magnetic resonance spectra of d-gluco-oligosaccharides and d-glucans. Carbohydrate Research 33, 105–116. [Google Scholar]
- Walsh AG, Matewish MJ, Burrows LL, Monteiro MA, Perry MB, Lam JS.. 2000. Lipopolysaccharide core phosphates are required for viability and intrinsic drug resistance in Pseudomonas aeruginosa. Molecular Microbiology 35, 718–727. [DOI] [PubMed] [Google Scholar]
- Winsor GL, Griffiths EJ, Lo R, Dhillon BK, Shay JA, Brinkman FSL.. 2016. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Research 44, D646–D653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock SD, Syson K, Little RH, Ward D, Sifouna D, Brown JKM, Bornemann S, Malone JG.. 2021. Trehalose and alpha-glucan mediate distinct abiotic stress responses in Pseudomonas aeruginosa. PLoS Genetics 17, e1009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DJ, Ohman DE.. 1994. Transcriptional analysis of the Pseudomonas aeruginosa genes algR, algB, and algD reveals a hierarchy of alginate gene expression which is modulated by algT. Journal of Bacteriology 176, 6007–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DJ, Wyckoff TJO, Starkey M, Keyser R, Azadi P, O’Toole GA, Parsek MR.. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proceedings of the National Academy of Sciences, USA 100, 7907–7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Kan FWK, She YM, Walker VK.. 2012. Biofilm, ice recrystallization inhibition and freeze–thaw protection in an epiphyte community. Applied Biochemistry and Microbiology 48, 363–370. [PubMed] [Google Scholar]
- Xin XF, He SY.. 2013. Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annual Review of Phytopathology 51, 473–3498. [DOI] [PubMed] [Google Scholar]
- Xin XF, Kvitko B, He SY.. 2018. Pseudomonas syringae: what it takes to be a pathogen. Nature Reviews. Microbiology 16, 316–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JM. 2010. Taxonomy of Pseudomonas syringae. Journal of Plant Pathology 92, S5–S14. [Google Scholar]
- Yu J, Penaloza-Vazquez A, Chakrabarty AM, Bender CL.. 1999. Involvement of the exopolysaccharide alginate in the virulence and epiphytic fitness of Pseudomonas syringae pv. syringae. Molecular Microbiology 33, 712–720. [DOI] [PubMed] [Google Scholar]
- Yu X, Lund SP, Scott RA, Greenwald JW, Records AH, Nettleton D, Lindow SE, Gross DC, Beattie GA.. 2013. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proceedings of the National Academy of Sciences, USA 110, E425–E434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and within its supplementary data published online. The data supporting the findings of this study and raw data used for making the graphs are available from the corresponding author, Jacob Malone, upon request.