Planteose stored in tissues surrounding the embryo in Orobanche minordry seeds is hydrolysed in the apoplast around the embryo by α-galactosidase OmAGAL2 during germination.
Keywords: Apoplast, α-galactosidase, matrix-assisted laser desorption/ionization–mass spectrometry imaging, Orobanche minor, parasitic plant, planteose, seed germination, storage carbohydrate
Abstract
Root parasitic weeds of the Orobanchaceae, such as witchweeds (Striga spp.) and broomrapes (Orobanche and Phelipanche spp.), cause serious losses in agriculture worldwide, and efforts have been made to control these parasitic weeds. Understanding the characteristic physiological processes in the life cycle of root parasitic weeds is particularly important to identify specific targets for growth modulators. In our previous study, planteose metabolism was revealed to be activated soon after the perception of strigolactones in germinating seeds of O. minor. Nojirimycin inhibited planteose metabolism and impeded seed germination of O. minor, indicating a possible target for root parasitic weed control. In the present study, we investigated the distribution of planteose in dry seeds of O. minor by matrix-assisted laser desorption/ionization–mass spectrometry imaging. Planteose was detected in tissues surrounding—but not within—the embryo, supporting its suggested role as a storage carbohydrate. Biochemical assays and molecular characterization of an α-galactosidase family member, OmAGAL2, indicated that the enzyme is involved in planteose hydrolysis in the apoplast around the embryo after the perception of strigolactones, to provide the embryo with essential hexoses for germination. These results indicate that OmAGAL2 is a potential molecular target for root parasitic weed control.
Introduction
Root parasitic weeds belonging to the Orobanchaceae, such as witchweeds (Striga spp.) and broomrapes (Orobanche and Phelipanche spp.), cause serious losses in agriculture worldwide (Parker, 2013). Many studies seeking to identify an effective solution to control these parasitic weeds have been conducted (Fernández-Aparicio et al., 2020), but it is still difficult to control them practically. One potential method is utilization of growth modulators specific to the root parasitic weeds. Therefore, understanding the characteristic physiological processes in the life cycle of root parasitic weeds is particularly important to identify specific targets of growth modulators.
The life cycle of root parasitic weeds is dependent on their hosts, because the weeds are obligate parasites. Germination of the parasitic weed seeds is host-dependent and requires germination stimulants, in most cases, strigolactones (SLs; Brun et al., 2018; Bouwmeester et al., 2019, 2021; Yoneyama, 2020). Strigolactones are released from plant roots as signals to establish symbiotic relationships with arbuscular mycorrhizal fungi (Akiyama et al., 2005; López-Ráez, 2015; Lanfranco et al., 2018). Strigolactones function in planta as hormones that alter plant architecture in response to the nutritional status (Gomez-Roldan et al., 2008; Umehara et al., 2008; Al-Babili and Bouwmeester, 2015; Waters et al., 2017; Bürger and Chory, 2020). Recent biochemical and genomic studies revealed that the receptors of SLs acting as germination stimulants in root parasitic weeds have diverged from common SL receptors (Tsuchiya et al., 2015, Khosla and Nelson, 2016). In Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa), SLs are perceived by the SL receptor DWARF14/DECREASED APICAL DOMINANCE2 (D14/DAD2; Arite et al., 2009; Hamiaux et al., 2012; Nakamura et al., 2013; Seto et al., 2019). However, in Striga hermonthica, SLs are perceived by a subset of KARRIKIN INSENSITIVE2 (KAI2)/HYPOSENSITIVE TO LIGHT (HTL) receptors (KAI2ds; Conn et al., 2015; Tsuchiya et al., 2015; Conn and Nelson, 2016). KAI2d genes are paralogous to D14 and are highly diversified in the Orobanchaceae (Conn et al., 2015; Tsuchiya et al., 2015; Conn and Nelson, 2016; Yoshida et al., 2019). The diversification might be closely linked to SL-dependent germination and the host specificity of the parasitic species (Khosla and Nelson, 2016). Precise characterization of the binding affinity of KAI2d/HTLs in S. hermonthica to SLs have led to selection and design of a prominent SL agonist, sphynolactone-7 (SPL-7; Uraguchi et al., 2018).
In comparison with SL perception in root parasitic weeds, metabolic processes in germinating seeds after SL perception are poorly understood. Previously, we conducted metabolome analyses on germinating seeds of Orobanche minor and revealed that metabolism of planteose [α-d-galactopyranosyl-(1→6)-β-d-fructofuranosyl-(2→1)-α-d-glucopyranoside] is activated soon after the perception of a synthetic SL, rac-GR24 (Wakabayashi et al., 2015). We also observed that nojirimycin (NJ) inhibits planteose metabolism and impedes the germination of O. minor seeds (Wakabayashi et al., 2015; Harada et al., 2017). Transcriptome analysis of the effect of NJ on O. minor germination suggested that NJ inhibits planteose metabolism through disturbance of sugar signaling (Okazawa et al., 2020). These studies indicate that planteose metabolism and the subsequent change in sugar profile in the seeds are critical processes in the germination of O. minor.
Planteose is accumulated in the seeds of certain plant species, such as tobacco (Nicotiana tabacum;French, 1955), mints (Lamiaceae spp.; French et al., 1959), sesame (Sesamum indicum;Dey, 1980), and chia (Salvia hispanica;Xing et al., 2017). In addition to O. minor, seeds of the Orobanchaceae root parasitic weeds O. crenata, P. aegyptiaca, and S. hermonthica contain planteose, and its hydrolysis is rapidly induced after perception of rac-GR24 (Wakabayashi et al., 2015). We previously revealed that the first step of planteose metabolism is hydrolysis of the α-galactosyl linkage to produce Suc and Gal (Wakabayashi et al., 2015). However, the enzymes involved in the metabolism and biosynthesis of planteose in plants remain to be elucidated.
α-Galactosidases (EC 3.2.1.22; AGALs) in plants are characterized in relation to metabolism of the raffinose family of oligosaccharides (RFOs; Van den Ende, 2013) and galactomannan (Buckeridge et al., 2000) during seed germination. Acid AGALs belong to the plant α-galactosidase sub-family in family 27 of glycoside hydrolases (GH27; Van den Ende, 2013; Imaizumi et al., 2017), whereas alkaline AGALs belong to GH36 (Van den Ende, 2013). Seeds of various plant species contain RFOs (Kuo et al., 1988), which are hydrolysed by AGALs during germination. In pea (Pisum sativum), constant activity of acid AGAL was confirmed during germination, whereas alkaline AGAL was expressed after radicle protrusion (Blöchl et al., 2008). Increase in acid AGAL activity to hydrolyse RFOs or galactomannan has been confirmed in soybean (Glycine max) (Guimarães et al., 2001) and Tachigali multijuga (members of the Fabaceae; Fialho et al., 2008). Acid AGAL activity also increases in the micropylar region of the endosperm of tomato (Solanum lycopersicum; Feurtado et al., 2001) and date palm (Phoenix dactylifera; Sekhar and DeMason, 1990) during germination. Transcripts of a gene encoding AGAL were isolated from aleurone cells in germinating seeds of guar (Cyampsis tetragonaloba), suggesting that AGAL is secreted into the endosperm for degradation of storage galactomannan (Hughes et al., 1988). These results indicate that AGALs are involved in the metabolism of storage carbohydrates in the endosperm to promote seed germination in a number of plant species.
In this study, we investigated AGAL activity during seed germination of O. minor together with planteose distribution in dry seeds to gain insight into the stimulant-induced metabolism driving germination. Cloning and characterization of a member of the acid AGALs sub-family, OmAGAL2, indicated its involvement in planteose hydrolysis. The results suggest that planteose is stored outside the embryo in the dry seeds, and after SL perception, is hydrolysed by OmAGAL2 in the apoplast to supply Suc, and subsequentially release hexoses to the embryo.
Materials and methods
Plant material and germination treatment
Seeds from Orobanche minor plants grown in the wild were collected in Yokohama, Japan in June 2013. Seed germination was induced as described previously (Wakabayashi et al., 2015). The seeds were surface-sterilized with 1% sodium hypochlorite containing 0.1% (w/v) Tween 20 for 2 min at 42 °C with shaking, then rinsed several times with distilled water, and dried under vacuum. The dried seeds were placed on two layers of glass microfiber filters (Whatman GF/D, GE Healthcare, Chicago, IL, USA) moistened with distilled water in a Petri dish in the dark at 23 °C for a week (conditioning). After conditioning, the seeds on the upper layer of the glass filter were transferred to a new Petri dish with a single glass microfiber filter, and strigolactone solution (rac-GR24, final concentration 1 mg l−1) was applied. To visualize α-galactosidase activity in vivo, 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (X-α-Gal; 40 µg ml−1) was applied directly to the germinating seeds. A radicle with embryo was separated from the seed coat with perisperm and endosperm by pushing the germinating seeds gently at opposite sides of the micropyle with forceps, using a digital microscope (DMS1000, Leica, Wetzlar, Germany).
Seeds of Nicotiana benthamiana were surface-sterilized with 1% sodium hypochlorite containing 0.02% (w/v) Tween 20, for 5 min by shaking, rinsed several times with distilled water, then sown in a mixture of commercial potting soil and vermiculite (1:1, v:v). Plants were grown under a photoperiod of 16 h light/8 h dark (100 µmol m−2 s−1) at 25 °C for 4 weeks.
Purification of planteose from chia seeds
Planteose was extracted according to the method reported previously (Xing et al., 2017) with minor modifications. Dry chia (Salvia hispanica) seeds (1 g) were incubated in 20 ml distilled water at 80 °C for 2 h. A viscous fluid was obtained by passing the liquid through a drain net, and was then frozen in liquid nitrogen and freeze-dried. Aqueous ethanol (70%) was added to the dried sample, stirred for 10 min, and centrifuged at 4000 × g for 10 min. Planteose was purified by HPLC, comprising a system controller (SCL-10Avp, Shimadzu, Kyoto, Japan), a column oven (CTO-10A, Shimadzu), a pump (LC-10AT, Shimadzu), and a refractive index detector (RID-10A, Shimadzu) equipped with a COSMOSIL Sugar-D packed column (20 mm ID×250 mm, Nacalai Tesque, Kyoto, Japan) at 35 °C. A mixture of acetonitrile:water (65:35, v/v) was used as the mobile phase at the flow rate of 1 ml min−1 with an isocratic mode.
MALDI–MSI
2,5-Dihydroxybenzoic acid (2,5-DHB) and ultrapure water were purchased from Merck (Darmstadt, Germany). Indium-thin-oxide (ITO) glass slides (SI0100N) for matrix-assisted laser desorption/ionization–mass spectrometry imaging (MALDI–MSI) analysis were purchased from Matsunami Glass (Osaka, Japan). As an embedding medium, 4% carboxymethyl cellulose was purchased from Section Lab (Yokohama, Japan). The 2,5-DHB solution was prepared at a concentration of 50 mg ml−1 using 50% methanol. Planteose solution (0.01, 0.1, or 1.0 mg ml−1 in ultrapure water) was mixed with 2,5-DHB solution of the same concentration, and the mixed solution was spotted onto the ITO glass slide. After crystallization, the spots were analysed to optimize laser power and detector voltage.
Seeds of O. minor (1 mg) and 500 µl of 4% carboxymethyl cellulose were mixed, and the mixture was placed in a disposable base mould (Tissue-Tek, Sakura Finetek, Tokyo, Japan). After freezing at −80 °C, tissue sectioning was performed using a cryomicrotome (CM1950, Leica, Nussloch, Germany). The obtained tissue sections were thaw-mounted onto ITO glass slides. The glass slides were air-dried and then subjected to matrix application. The matrix, 2,5-DHB, was heated at 180 °C and sublimated at a thickness of 1.0 µm using a vacuum deposition system (iMLayer, Shimadzu, Kyoto, Japan). After formation of the matrix layer on the sample surface, the imaging experiment was performed using an iMScope TRIO (Shimadzu). Based on the results of standard sample analysis, the target peak was identified at m/z 527.16. To improve the specificity of the imaging results, MS/MS imaging was performed. The peak at m/z 527.16 was isolated inside the ion trap and dissociation was induced using argon gas. The target fragment peaks were m/z 347.09 and m/z 365.11. To confirm the accuracy of the imaging result, both peak intensity maps were generated. It should be noted that the fragment m/z 347.09 was not detected when raffinose, an isomer of planteose, was measured as a reference (Supplementary Fig. S1). In the imaging experiment, the laser diameter was 5 µm and the interval of data points was 3 µm.
Protein extraction from germinating seeds of O. minor
Protein extraction was conducted as described previously (Wakabayashi et al., 2015) at 4 °C with minor modifications. Germinating seeds (~40 mg) of O. minor were frozen in liquid nitrogen and disrupted with a ball mill (20 Hz, 5 min; MN301, Verder Scientific, Haan, Germany). Extraction buffer (1.5 ml, pH 7.0) composed of 50 mM HEPES, 1 mM DTT, 1 mM EDTA, 20 mg polyvinylpolypyrrolidone (PVPP), and 1% Protease Inhibitor Cocktail (Merck, Kenilworth, NJ, USA) was added and incubated for 5 min. The homogenate was centrifuged at 12 000 ×g for 15 min and 0.8 ml of supernatant was collected. The residue was re-extracted in extraction buffer without PVPP, and 0.7 ml of extract was collected after centrifugation and combined with the first extract. The combined extracts (1.5 ml) were used as a soluble enzyme fraction. The enzyme extract was desalted on a PD-10 column (Cytiva, Marlborough, MA, USA) previously equilibrated with 50 mM HEPES buffer (pH 7.0) and concentrated with an Amicon Ultra-4 10K centrifugal filter (Merck).
α-Galactosidase assay
p-Nitrophenyl-α-d-galactopyranoside (p-NP-α-gal; Tokyo Chemical Industry, Tokyo, Japan) was used as a model substrate to measure α-galactosidase activity. Proteins (5 µg) were incubated with the substrate (final concentration 1.0 mM) in 0.1 M citrate buffer (pH 3.0−6.0) or 0.1 M phosphate buffer (pH 6−8) at 37 °C for 30 min, and 0.5 M Na2CO3 was added to stop the reaction. The amount of released p-nitrophenol was quantified by measuring absorbance at 410 nm with a microplate reader (SH-9000, Corona Electric, Hitachinaka, Japan). When planteose was used as a substrate, the reaction was monitored using an ultra-performance liquid chromatograph equipped with an evaporative light scattering detector (UPLC–ELSD; ACQUITY, Waters, Milford, MA, USA), as reported previously (Wakabayashi et al, 2015). Sugars were separated by an ACQUITY UPLC BEH Amide 1.7 µm column (2.1 mm ID×150 mm, Waters) at 35 °C. The mobile phase was acetonitrile with 0.2% triethylamine (TEA; solvent A) and ultrapure water with 0.2% TEA (solvent B). Separation was performed using a linear gradient program as follows: 20–30% B for 0–2.8 min, 30–50% B for 2.8–4.5 min, 50–80% B for 4.5–5.0 min, 80–20% B for 5.0–6.5 min, and 20% B for 6.5–7.0 min. The flow rate was set as follows: 0.25 ml min−1 for 0–4.5 min, 0.25–0.10 ml min−1 for 4.5–5.0 min, 0.10–0.25 ml min−1 for 5.0–6.5 min, and 0.25 ml min−1 for 6.5–7.0 min. The sample injection volume was 5 µl.
RT–qPCR
Total RNA was isolated from germinating seeds of O. minor using Invitrogen TRIzol Plus RNA Purification System (Thermo Fischer Scientific, Waltham, MA, USA) and treated with PureLink DNase Set (Qiagen, Hilden, Germany) in accordance with the manufacturer’s instructions. Synthesis of cDNA was conducted using the PrimeScript RT Master Mix (Takara Bio, Kusatsu, Japan) with the thermal cycler LifeECO (Hangzhou Bioer Technology, Hangzhou, China). The quality of purified total RNA was evaluated by electrophoresis using Qsep1 Bio-Fragment Analyzer (BiOtic, New Taipei City, Taipei). RT–qPCR was conducted using KAPA SYBR Fast qPCR Kit (Roche, Basel, Switzerland) by Mic qPCR Cycler (Bio Molecular Systems, Upper Coomera QLD, Australia) in accordance with the manufacturers’ instructions. The sequences of gene-specific primers used were as follows: OmAGAL2 Fw, 5ʹ-GGGATGACTGCCGAAGAATA -3ʹ; OmAGAL2 Rv, 5ʹ-TGCTTAGTGTCGCCAATGTC-3ʹ; comp71446_c0_seq1 Fw, 5ʹ-TCGGACCTCACAAAACCCAG-3ʹ; and comp71446_c0_seq1 Rv, 5ʹ-GCAAGGGGATCAAAAGCAGC-3ʹ. The gene comp71446_c0_seq1 was shown to be constitutively expressed in our previous transcriptome analysis (Okazawa et al, 2020), and was selected as a reference gene for normalization using the 2−∆Ct method. This gene is a homolog of MONENSIN SENSITIVITY1 (MON1) in Arabidopsis (At2g28390), which is reported as a stably expressed gene (Czechowski et al, 2005). Constitutive expression of comp71446_c0_seq1 was also confirmed by RT–qPCR using cDNAs synthesized from the same amounts of total RNAs (Ct: 20.03 ± 0.88; mean ±SD, n=15).
Expression of OmAGAL2 in Escherichia coli
Genes encoding α-galactosidases (OmAGALs) were surveyed in the transcriptome in germinating seeds of O. minor (Okazawa et al., 2020). The OmAGAL2 coding sequence without the expected signal peptide (SP) sequence [ΔSP-OmAGAL, amino acids (AA) 47–412] was cloned into the pENTR/D-TOPO vector (Thermo Fisher Scientific, Waltham, MA, USA) in accordance with the manufacturer’s instructions, then sub-cloned into the pGEX-5X-1-GW vector (Okazawa et al., 2014) using the Gateway LR reaction. Escherichia coli BL21 (DE3) was transformed with the constructed expression vector, pGEX-ΔSP-OmAGAL2. The transformed E. coli cells were pre-cultured in LB medium supplemented with 100 µg ml−1 ampicillin for 18 h at 37 °C. A portion of the pre-culture (1/250) was transferred to new LB medium supplemented with 100 µg ml−1 ampicillin and cultured at 37 °C until OD600=0.45 was attained. Protein expression was induced by the addition of isopropyl β-d-thiogalactoside (final concentration 0.1 mM). After incubation at 9 °C for 72 h, the cultured cells were harvested by centrifugation (400 × g, 4 °C, 15 min). The harvested cells were sonicated with an ultrasonic disruptor (UR-20P, Tomy Seiko, Tokyo, Japan) in buffer (10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, and 1 mM dithiothreitol, pH 7.4) for 15 s, for a total of five times. After centrifugation (400 × g, 4 °C, 15 min), the supernatant was collected as the crude enzyme solution. Recombinant GST-∆SP-OmAGAL2 was purified using the GST SpinTrap column (Cytiva, Marlborough, MA, USA) in accordance with the manufacturer’s instructions. The glutathione S-transferase (GST) tag was cleaved using Factor Xa Protease (New England Biolabs, Ipswich, MA, USA) in accordance with the manufacturer’s instructions.
Sub-cellular localization analyses
OmAGAL2, ∆SP-OmAGAL2, and SP (AA 1–46) were cloned separately into the mC121 vector, generated by the GUS in pBI121 binary vector (Takara Bio, Shiga, Japan) being substituted with mCherry, to express proteins fused with mCherry at the C-terminus under the control of the 35S promoter, thus generating mC121-OmAGAL2, mC121-∆SP-OmAGAL2, and mC121-SP, respectively. Transient expression was conducted by co-inoculation of Agrobacterium tumefaciens strain GV3101 cultures carrying each construct with those carrying the vector pMDC-At5g11420:pH-tdGFP, used as an apoplast marker (Stoddard and Rolland, 2019), and pDGB3alph2_35S:P19:Tnos, used as RNA silencing suppressor (GB1203, Addgene, Watertown, MA, USA), into leaves of Nicotiana benthamiana. mCherry and green fluorescent protein (GFP) were excited at 555 nm and 488 nm, respectively, and observed in the range of 570–600 and 490–520 nm, respectively, using a LSM700 laser scanning confocal microscope (Carl Zeiss, Jena, Germany).
Agrobacterium tumefaciens GV3101 cultures carrying mC121-OmAGAL2, mC121-∆SP-OmAGAL2, and mC121-SP, were used to inoculate Arabidopsis plants by floral inoculation, and obtained the T2 generation of transgenic plants. Five- to 8-day-old seedlings were treated with ClearSee solution (Kurihara et al, 2015), stained with Calcofluor White Stain (CWS, Merck) and observed using a LSM700 laser scanning confocal microscope. CWS was excited at 405 nm and observed in the range of 400–520 nm.
Protein expression in tobacco BY-2 cells
Agrobacterium tumefaciens GV3101 cultures carrying mC121-OmAGAL2, mC121-∆SP-OmAGAL2, and mC121-SP were used to infect tobacco BY-2 cells. Transgenic BY-2 cells were cultured in liquid Murashige and Skoog medium in a rotary shaker at 80 rpm at 25 °C. Cells and medium were separately collected by filtration 4 d after sub-culture. The collected BY-2 cells were ground in liquid nitrogen with a mortar and pestle, and soluble proteins were extracted in the extraction buffer. The culture medium was concentrated using Amicon Ultra-15 Centrifugal Filter Units, 10 kDa (Merck, Kenilworth, NJ, USA), desalted with PD 10 desalting columns (Merck) in extraction buffer [50 mM HEPES with 1 mM EDTA, 1 mM of 2-mercaptoethanol 0.1% (w/v) NaN3, and 0.1% (v/v) Protease Inhibitor Cocktail (Promega, Madison, MA, USA), pH 7.0] and used as apoplast protein fractions.
Western-blot analysis
Proteins were separated on 10% (w/v) SDS-PAGE and transferred to a PVDF membrane (Trans-Blot Turbo Mini 0.2 µm PVDF Transfer Packs, Bio-Rad, Hercules, CA, USA) using the Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA, USA; 1.3 A, 25 V, 10 min). The membrane was blocked with 5% (w/v) skimmed milk in TBST buffer [20 mM Tris-HCl, 150 mM NaCl, and 0.2% (w/v) Tween 20, pH 7.6] blocking solution for 1 h with gentle shaking. After washing with TBST buffer, the membrane was incubated with rabbit anti-mCherry polyclonal antibody (Proteintech Group, Rosemont, IL, USA) in blocking solution (1: 6000) for 1.5 h. Multi Capture HRP (Fujifilm Wako Pure Chemical, Osaka, Japan) and ImmunoStar LD (Fujifilm Wako Pure Chemical) were used for detection of the antibody and chemiluminescence reactions, respectively, in accordance with the manufacturer’s instructions. A luminescent image was captured using the Luminograph system (Atto, Tokyo, Japan).
Results
Distribution of planteose in dry seeds of O. minor
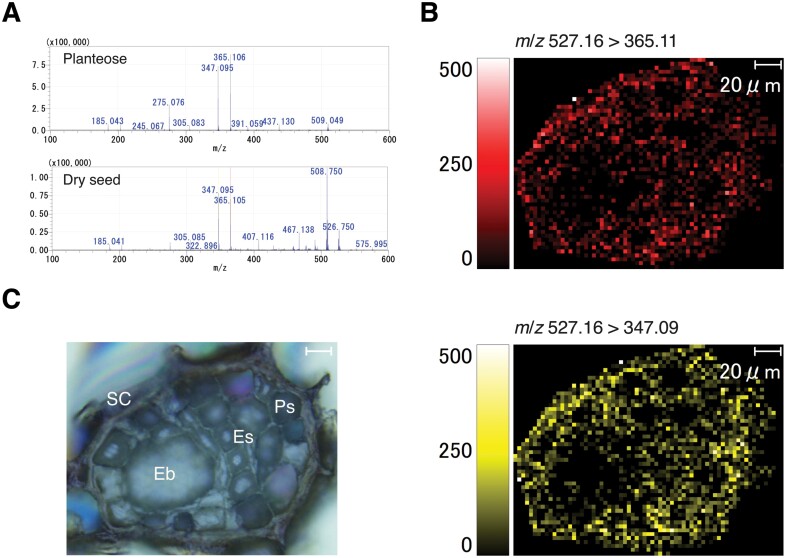
Planteose accumulates in the dry seeds of O. minor as a storage carbohydrate (Wakabayashi et al., 2015). However, no tissue that accumulates planteose has been identified in any plant species. In the present study, MALDI–MSI was conducted to reveal the distribution of planteose in O. minor dry seeds. Two MS/MS fragment ions (m/z 527.16 [M+Na]+>365.11 [M+Na–C6H10O5]+, 347.09 [M+Na–C6H10O5–H2O]+) from planteose (Fig. 1A) were detected at 3 µm resolution (Fig. 1B). Images obtained for both fragment ions were almost identical, indicating that these fragment ions were generated from a single source, planteose. Strong signals were mainly detected from the seed coat, and some areas in the perisperm and endosperm, but not the embryo (Fig. 1B, C). This distribution pattern is in agreement with the role of planteose as a storage carbohydrate.
Fig. 1.
Mass imaging of planteose in dry seeds of O. minor. (A) MALDI–MS/MS spectra of purified planteose (upper panel) and O. minor dry seed (lower panel). 2,5-Dihydroxybenzoic acid was mixed with purified planteose or sprayed on a dry seed section as matrix, and m/z 527.16 [M+Na]+ was selected as a precursor ion. (B) Mass images of fragment ions m/z 365.11 (upper image) and 347.09 (lower image) from a precursor ion m/z 526.17. (C) Bright field image of an O. minor dry seed section. Eb, embryo; Es, endosperm, Ps, perisperm; SC, seed coat. Scale bar=20 µm.
α-Galactosidase activity in germinating seeds of O. minor
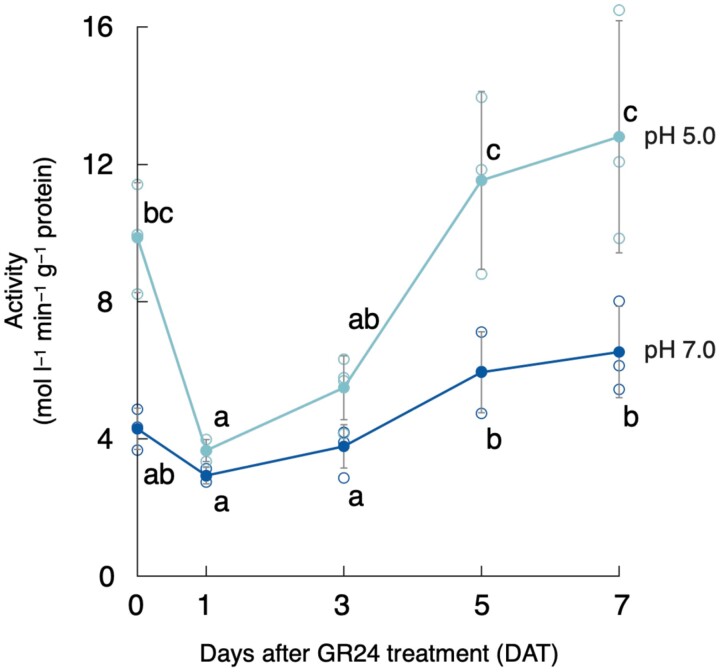
Planteose was almost completely hydrolysed by 5 d after rac-GR24 treatment (DAT) in O. minor (Wakabayashi et al., 2015). Given that the first step of planteose metabolism is hydrolysis of an α-galactosyl linkage, AGAL activities in crude enzyme fractions prepared from germinating seeds of O. minor were measured at pH 5.0 or 7.0 using p-NP-α-Gal as a substrate. The AGAL activity at 1 DAT was lower than that after conditioning, just before 1 mg l−1rac-GR24 treatment (0 DAT), then increased at both pH 5.0 and 7.0, although the activity at pH 5.0 was much higher than that at pH 7.0 (Fig. 2). These results indicated that acid AGAL was activated after perception of SL in germinating seeds of O. minor.
Fig. 2.
Changes in α-galactosidase activity in germinating seeds of O. minor treated with 1 mg l−1rac-GR24. α-Galactosidase activity in the crude enzyme fraction prepared at different time points during germination was measured at pH 5.0 or 7.0 using p-nitrophenyl-α-d-galactopyranoside (1.0 mM) as a substrate. Values are means ±SD (n=3 or 4). Different lowercase letters indicate significant differences under the same pH (P<0.05, Tukey–Kramer test).
In vivo visualization of α-galactosidase activity
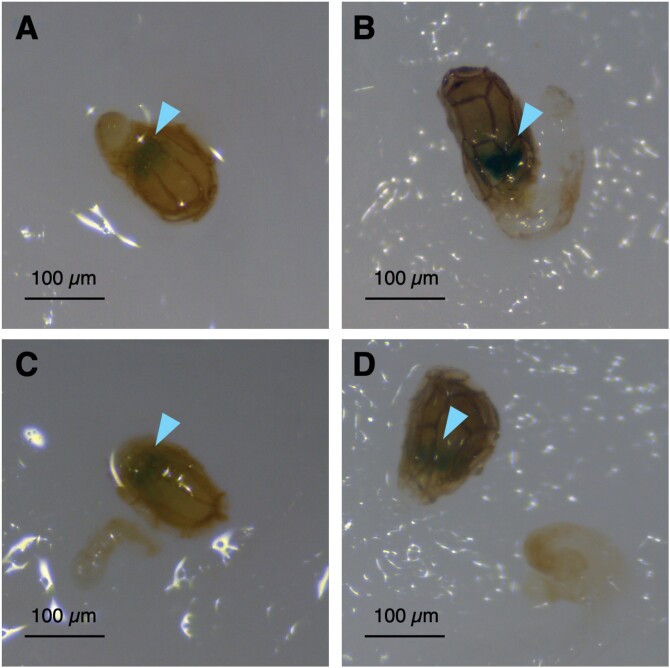
MALD–MSI suggested that planteose accumulated in tissues other than the embryo, such as the endosperm, perisperm, and seed coat. We visualized AGAL activity in germinating seeds of O. minor using X-α-Gal. No pigmentation was observed before radicle emergence with 1 mg l−1rac-GR24 until 2 DAT (Supplementary Fig. S2). After X-α-Gal solution was applied to the germinating seeds, blue colouration, indicating hydrolysis of the α-galactosyl linkage in X-α-Gal, was observed in the seed coats near the micropyle where the radicle emerged at 3 and 5 DAT (Fig. 3A, B). Given that AGAL activity was detected near the embryo, we manually separated the embryo with the radicle from the endosperm and seed coat, and X-α-Gal solution was applied. The AGAL activity was confirmed within the seed coats, and was not detected on or in the embryo and radicle at 3 and 5 DAT (Fig. 3C, D). The localization of detected AGAL activity indicated that AGAL was involved in translocation of the storage carbohydrate into the embryo from the surrounding tissues.
Fig. 3.
In vivo visualization of α-galactosidase activity in germinating seeds of O. minor with 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside (X-α-Gal). The seeds at (A) 3 d after rac-GR24 treatment (DAT) or (B) 5 DAT were treated with X-α-Gal solution (40 µg ml−1). The endosperm and seed coat were separated manually from the embryo with emerging radicles at (C) 3 DAT or (D) 5 DAT, and treated with X-α-Gal solution. Arrowheads indicate the pigmentation of X-α-Gal. Scale bars=100 µm.
Molecular cloning and characterization of α-galactosidase in O. minor
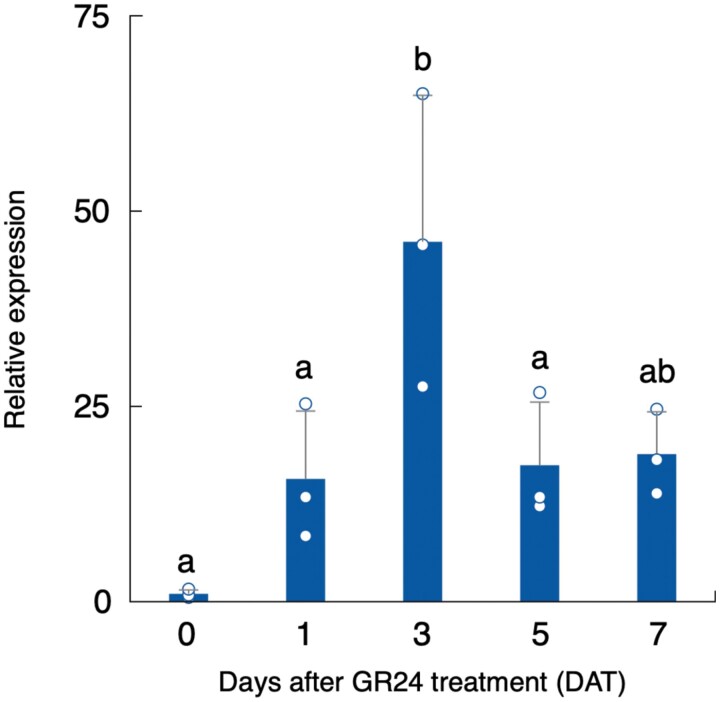
Transcriptomic data (Okazawa et al., 2020) were surveyed for molecular cloning of AGAL genes in O. minor. Three candidate genes, comp64068_c0_seq7, comp35887_c0_seq1, and comp34431_c0_seq1, were annotated as AGAL-homologous genes and were named OmAGAL1, OmAGAL2, and OmAGAL3, respectively, based on the homology with AtAGAL1 (At5g08380), AtAGAL2 (At5g08370), and AtAGAL3 (At3g56310) of Arabidopsis (Imaizumi et al., 2017;Supplementary Figs S3, S4). Because the expression of OmAGAL2 was highest among these genes during germination (Supplementary Fig. S5), OmAGAL2 was chosen for further analysis. A RT–qPCR assay confirmed that expression of OmAGAL2 was induced after perception of 1 mg l−1rac-GR24 and peaked at 3 DAT (Fig. 4).
Fig. 4.
Expression of OmAGAL2 during seed germination by 1 mg l−1rac-GR24. OmAGAL2 expression was quantified by RT–qPCR analysis. A constitutively expressed gene, comp71446_c0_seq1, was used as a reference gene for normalization of gene expression. Values are means ±SD (n=3). Different lowercase letters indicate a significant difference (P<0.05, Tukey–Kramer test).
The coding sequence of OmAGAL2 was isolated from the germinating seeds of O. minor. The open reading frame was 1239 bp and encoded 412 AA residues with a predicted molecular mass of 45 kDa. OmAGAL2 showed high homology with other AGALs in GH27, possessing a SP at its N-terminus, an α-galactosidase motif, and two conserved aspartate residues at active sites (Tapernoux-Lüthi et al., 2004; Imaizumi et al., 2017;Supplementary Fig. S4). Phylogenetic analysis revealed that OmAGAL2 was placed in a clade including AtAGAL2 (Imaizumi et al., 2017) and showed the highest homology with S. asiatica α-galactosidase (SaAGAL; Supplementary Fig. S6). Orobanche minor and S. asiatica are both members of the Orobanchaceae.
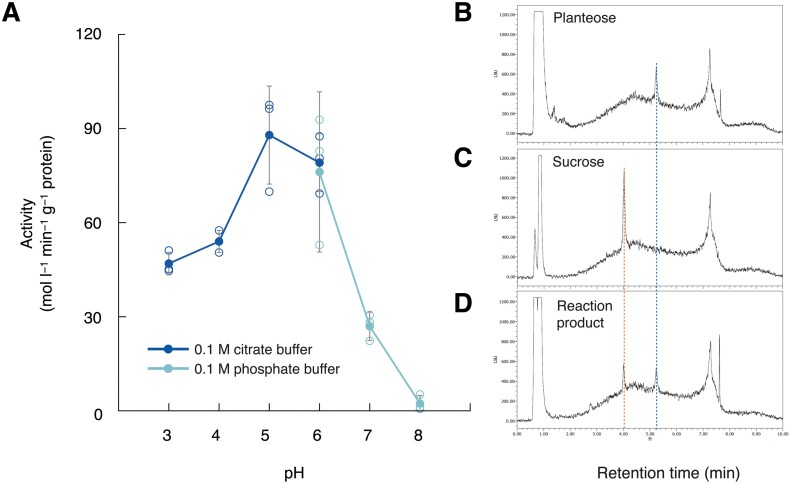
OmAGAL2 lacking the SP (AA 1–46), ΔSP-OmAGAL2, was heterologously expressed in E. coli. ΔSP-OmAGAL2 exhibited AGAL activity with optimal activity detected at pH 5.0−6.0 (Fig. 5A), indicating that OmAGAL2 is an acid AGAL active in acidic compartments. ΔSP-OmAGAL2 hydrolysed planteose to Suc at pH 5.0 (Fig. 5B–D).
Fig. 5.
Enzymatic activity of ΔSP-OmAGAL2 expressed in E. coli. (A) Effect of pH on α-galactosidase activity of ΔSP-OmAGAL2. The activity was measured in 0.1 M citrate buffer (pH 3.0−6.0, dark blue) or 0.1 M phosphate buffer (pH 6.0−8.0, pale blue) at 37 °C for 30 min using p-NP-α-gal (1.0 mM) as a substrate. Values are means ±SD (n=3). (B–D) UPLC-ELSD chromatogram of purified planteose (B), authentic Suc (C), and reaction product after incubation of planteose with ΔSP-OmAGAL2 under pH 5.0 (D). Blue and red dashed lines indicate the peaks of planteose and Suc, respectively.
Sub-cellular localization of OmAGAL2
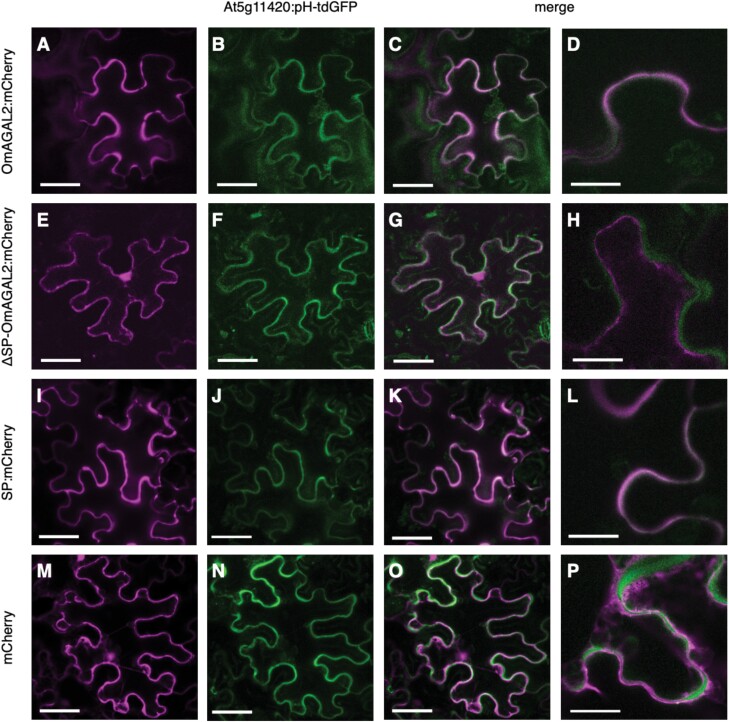
The function of the N-terminal SP was evaluated using mCherry fusion constructs. OmAGAL2, ΔSP-OmAGAL2, and SP fused with mCherry at the C-terminus were transiently expressed in leaves of N. benthamiana together with an apoplast marker, At5g11420:pH-tdGFP (Stoddard and Rolland, 2019) by co-inoculation of A. tumefaciens strain GV3101 cultures carrying each construct with those carrying the vector pMDC-At5g11420:pH-tdGFP. mCherry fluorescence in the cells expressing SP-containing constructs, OmAGAL2:mCherry and SP:mCherry, overlapped with GFP fluorescence from the apoplast marker At5g11420:pH-tdGFP, indicating that OmAGAL2 was secreted into the apoplast as a result of the function of the SP (Fig. 6A–D, I–L). In contrast, mCherry fluorescence from ΔSP-OmAGAL2 that lacks SP, and the negative control mCherry, was localized in the nucleus and cytoplasm, and did not overlap with At5g11420:pH-tdGFP (Fig. 6E–H, M–P).
Fig. 6.
Localization of mCherry fusion proteins transiently expressed in leaves of Nicotiana benthamiana. (A–D) OmAGAL2:mCherry, (E–H) ΔSP-OmAGAL2:mCherry, (I–L) SP:mCherry, and (M–P) mCherry were co-expressed with the apoplast marker At5g11420:pH-tdGFP (B, F, J, N). (C, D, G, H, K, L, O, P) Merged images of fluorescence from mCherry and GFP. Scale bars=50 µm (A–C, E–G, I–K, M–O) and 20 µm (D, H, L, P).
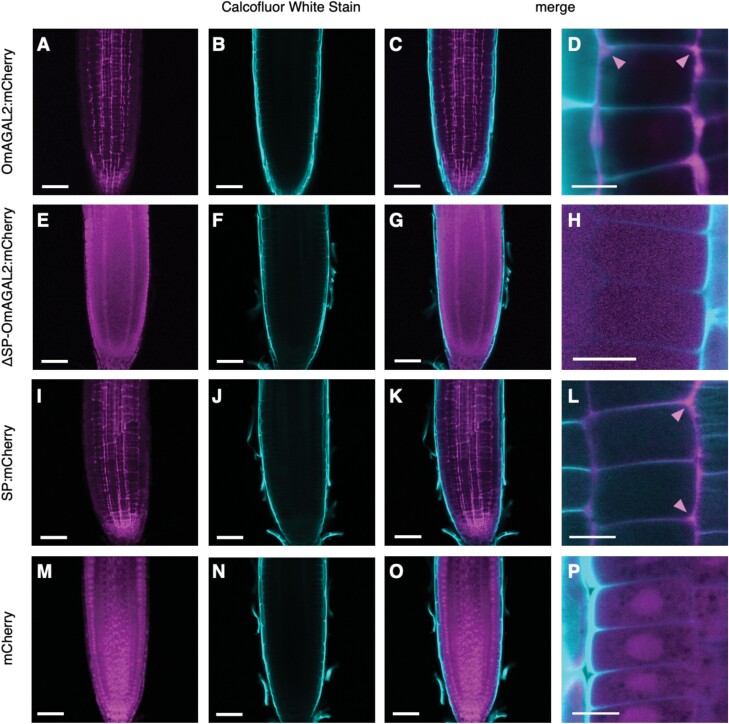
To confirm the transient assay results, Arabidopsis was transformed with A. tumefaciens strain GV3101 carrying the same constructs by floral inoculation, and the T2 generation of transgenic plants was obtained. Five- to 8-day-old seedlings were treated with ClearSee solution (Kurihara et al, 2015), and the cell walls were stained with Calcofluor White Stain. Fluorescence from mCherry generated lattice-like patterns surrounding the root cells, and strong fluorescence was observed in intercellular spaces (indicated by arrowheads in Fig. 7D, L) in the case of SP-containing OmAGAL2:mCherry and SP:mCherry (Fig. 7A–D, I–L). In contrast, fluorescence from ΔSP-OmAGAL2 was observed within the cells (Fig. 7E–H). Fluorescence from mCherry as a negative control was also detected within the cells and especially accumulated in the nucleus (Fig. 7M–P). The results of transient expression of the constructs in N. benthamiana and transgenic Arabidopsis were consistent and indicated that secretion of OmAGAL2 into the apoplast was dependent on SP function.
Fig. 7.
Localization of mCherry fusion proteins expressed in roots of transgenic Arabidopsis. (A–D) OmAGAL2:mCherry, (E–H) ΔSP-OmAGAL2:mCherry, (I–L) SP:mCherry, and (M–P) mCherry. (B, F, J, N) Cell walls were stained with Calcofluor White Stain (CWS). (C, D, G, H, K, L, O, P) Merged images of fluorescence from mCherry and CWS. Scale bars=50 µm (A–C, E–G, I–K, M–O) and 20 µm (D, H, L, P). Arrowheads indicate the fluorescence observed in intercellular spaces.
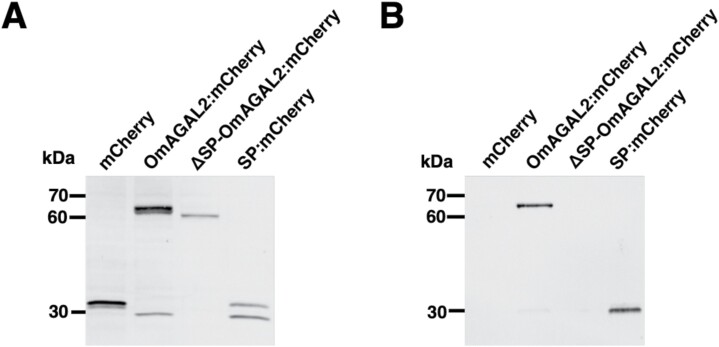
Secretion of the proteins was evaluated by western blot analysis using tobacco BY-2 cells transformed with the same constructs. Anti-mCherry polyclonal antibody detected the corresponding proteins in protein extracts from transformed BY-2 cells (Fig. 8A), whereas secretion of SP-containing proteins was confirmed in the medium in which BY-2 cells were cultured (Fig. 8B). Taken together, the presented results demonstrate that the SP in OmAGAL2 functions as a signal for protein secretion into the apoplast.
Fig. 8.
Secretion of proteins by the signal peptide (SP) from transgenic tobacco BY-2 cells expressing mCherry, OmAGAL2:mCherry, ΔSP-OmAGAL2:mCherry, and SP:mCherry. Western blot analysis of protein extracts from transgenic BY-2 cells (A) and medium in which BY-2 cells were cultured (B) with anti-mCherry polyclonal antibody.
Discussion
Planteose distribution in O. minor dry seeds
Planteose is contained in the seeds of several plant species, although its physiological role remains elusive. During seed germination of root parasitic weeds, planteose is rapidly hydrolysed after perception of SLs, which is indicative of its role as a storage carbohydrate (Wakabayashi et al., 2015). In general, the endosperm stores storage carbohydrates and supplies nutrients such as starch in cereals or lipids in Arabidopsis, to the embryo during germination (Yan et al., 2014). It is also considered that tissues surrounding the embryo, namely the endosperm, perisperm, and seed coat, play roles in nutrient supply in root parasitic weeds (Joel et al., 2012; Joel and Bar, 2013). In the present study, we demonstrated that planteose is distributed in the endosperm, perisperm and seed coat in the dry seeds of O. minor (Fig. 1), which coincides with its role as a storage carbohydrate. Ultrastructural analysis of P. aegyptiaca seeds indicated that an endothelial cell layer beneath the seed coat surrounding the perisperm is filled with mucilage (Joel et al., 2012). In chia seeds, planteose is a major oligosaccharide in the mucilage (Xing et al., 2017). Accordingly, fragment ions detected in the seed coat might be from planteose accumulated in the endothelial mucilage (Fig. 1B). In addition, planteose was widely detected in the endosperm and perisperm, indicating that planteose is contained not only in the mucilage but also in compartments of the endosperm and perisperm. The use of MALDI–MSI to visualize carbohydrates in plant tissues, such as apple fruit (Horikawa et al., 2019) and wheat stems (Robinson et al., 2007), has proved to be a powerful technique to reveal carbohydrate distribution. In the present study, we visualized for the first time, the distribution of the storage carbohydrate planteose in seeds of a root parasitic weed.
α-Galactosidase expression during O. minor seed germination
Metabolic profiling previously revealed that the first step of planteose metabolism is hydrolysis of the α-galactosyl linkage (Wakabayashi et al., 2015), although the corresponding enzymes have not been elucidated in any plant species. An enzymatic assay confirmed that AGAL activity increased with the progression of germination, especially under an acidic pH (Fig. 2). This result indicated that acid AGAL was activated in acidic compartments, i.e. the vacuole or apoplast. There was a decrease in AGAL activity at the onset of germination (1 DAT) compared with that in the conditioned seeds (0 DAT). The slight decrease of acid AGAL activity at the onset of germination has also been reported in pea seeds (Blöchl et al., 2008), although the physiological meaning of the decrease is unclear. Previously, we confirmed that planteose was hydrolysed only after GR24 treatment (Wakabayashi et al., 2015). Therefore, AGALs in the conditioned seeds (0 DAT) might not be involved in planteose hydrolysis. Gene expression of OmAGAL2 also supported that planteose hydrolysis was induced only after rac-GR24 perception (Fig. 4). Elucidation of AGALs in conditioned seeds is required to reveal the physiological role of AGAL activity before SL perception.
After X-α-Gal was applied to the germinating seeds to visualize AGAL activity, a blue colouration indicated that activity was localized near the micropyle (Fig. 3). Interestingly, if X-α-Gal was applied after separation of the embryo with the emerging radicle from the seed coat, blue colouration was confirmed only in the seed coat, suggesting that AGAL was expressed in tissues surrounding, but not within the embryo (Fig. 3). This result was consistent with the MALDI-MSI analysis of planteose, which showed that planteose accumulated mainly outside the embryo (Fig. 1). However, AGAL activity was detected specifically near the micropyle, whereas planteose was distributed in the non-embryonic tissues in the seed. Small perisperm cells surrounding the emerging radicle in the micropylar region are considered to supply nutrients to the embryo (Joel et al., 2012). The present results indicated that AGAL is activated specifically in perisperm cells surrounding the embryo, thus suggesting the involvement of AGAL in nutrient transport. Whether planteose is translocated by a specific route, or moves by passive diffusion to its hydrolytic site remains to be determined.
Characterization of AGALs in some plant species has been conducted in relation to metabolism of RFOs (Van den Ende, 2013) and galactomannan (Buckeridge et al., 2000). Activation of AGAL during germination has been observed in plant species such as lettuce (Lactuca sativa; Leung and Bewley, 1981), soybean (Guimarães et al., 2001), coffee (Coffea arabica; Marraccini et al., 2005), and pea (Blöchl et al., 2008). The AGAL activity in cell walls of the endosperm is increased during seed germination in date palm (Sekhar and DeMason, 1990). In tomato, acid AGAL activity is increased in the lateral and micropylar regions of the endosperm, but not in the embryo during germination (Feurtado et al., 2001). These observations indicate that AGALs are universally expressed during seed germination to mobilize stored carbohydrates to the embryo, although their substrate, i.e. galactomannan, raffinose, or other oligosaccharides, may differ among species. Given that raffinose or galactomannan are not reported to be present in seeds of root parasitic weeds, and planteose is the only trisaccharide detected in our previous study (Wakabayashi et al., 2015), planteose may be the substrate of AGAL in O. minor and other root parasitic weeds, as supported by the high similarity of OmAGAL2 and SaAGAL (Supplementary Fig. S6). Further characterization of polysaccharides in the cell wall of seeds of root parasitic weeds is a future challenge to elucidate the storage carbohydrates present.
Characteristics of OmAGAL2
As a candidate planteose-hydrolytic enzyme, OmAGAL2 was cloned and expressed in E. coli as ΔSP-OmAGAL2. OmAGAL2 belongs to the GH27 protein family, to which plant acid AGALs are assigned (Van den Ende, 2013). A putative SP at its N-terminus (AA 1–32), and an AGAL motif and three aspartate residues required for recognition and hydrolysis of α-galactosyl saccharides (Tapernoux-Lüthi et al., 2004; Imaizumi et al., 2017) are conserved in OmAGAL2 (Supplementary Fig. S4). In comparison with biochemical studies, few molecular investigations have used recombinant AGALs from plants. Characterization of ArGGT1, an ortholog of AtAGAL1 in common bugle (Ajuga reptans), revealed its function as a raffinose oligosaccharide chain elongation enzyme, rather than a hydrolytic enzyme (Tapernoux-Lüthi et al., 2004; Supplementary Fig. S6). AtAGAL2 and AtAGAL3 show activity toward RFOs, but RFOs are not expected to be their main substrate in planta (Imaizumi et al., 2017). Involvement of AtAGAL3 in the hydrolysis of l-arabinopyranose residues in cell wall components has been proposed, because of the β-l-arabinopyranosidase activity of the enzyme (Imaizumi et al., 2017). Rice AGALs also hydrolyse RFOs and galactomannans, but their intrinsic substrates have not been determined to date (Li et al., 2007). As described herein, planteose is a possible substrate of AGALs and OmAGAL2 was demonstrated to hydrolyse planteose at pH 5.0. Moreover, OmAGAL2 was proven to be secreted to the apoplast through the function of the N-terminus SP. Accordingly, planteose is likely to be hydrolysed in the acidic apoplast by OmAGAL2.
Hypothetical model of planteose mobilization in germinating seeds of O. minor
From the present results, we propose a hypothetical model of planteose mobilization during seed germination of O. minor. Planteose is stored outside the embryo in some compartments, such as the endothelial mucilage, endosperm, and perisperm. After perception of SLs, transcription of OmAGAL2 is up-regulated and the expression peaks at 5 DAT when planteose is almost completely hydrolysed (Wakabayashi et al., 2015). The expression of OmAGAL2 might be restricted to tissues surrounding the embryo near the micropyle, as indicated by visualization of AGAL activity using X-α-Gal. Given that nutrients are mobilized from the endosperm and perisperm into the embryo, the localized AGAL activity emphasizes its involvement in the hydrolysis of storage carbohydrates. Sucrose released from planteose by OmAGAL2 may be directly translocated into embryonic cells through Suc transporters (SUTs). However, SUTs are unlikely to be involved in germination, because application of exogenous Suc does not induce the germination of NJ-treated O. minor seeds. However, NJ-treated seeds germinated in response to application of exogenous Glc (Wakabayashi et al., 2015). Therefore, Suc might be further hydrolysed by cell wall invertases. Involvement of invertases in seed germination in O. minor (Wakbayashi et al., 2015) and P. ramosa (Draie et al., 2011) has been documented previously. Hexoses may be incorporated into embryonic cells through hexose transporters.
Previously, we observed that NJ suppressed seed germination of O. minor through inhibition of planteose metabolism (Wakabayashi et al., 2015; Harada et al., 2017; Okazawa et al., 2020). The present data suggest that OmAGAL2 is a potential target for control of root parasitic weeds. The apoplastic localization of OmAGAL2 is a favourable characteristic for inhibitor design, because consideration of the membrane permeability of inhibitors can be omitted. Screening of OmAGAL2 inhibitors is ongoing using recombinant proteins, and evaluation of the effects of the inhibitors on carbohydrate metabolism and germination in O. minor is the focus of an ensuing study.
Supplementary data
The following supplementary data are available at JXB online.
Fig. S1. MALDI–MS/MS spectra of raffinose, a planteose isomer, and purified planteose.
Fig. S2. In vivo visualization of α-galactosidase activity in germinating seeds of O. minor with X-α-Gal after conditioning and 2 DAT.
Fig. S3. Alignments of partial sequences of OmAGAL1 with Arabidopsis α-galactosidase AtAGAL1, and OmAGAL3 with AtAGAL3.
Fig. S4. Multiple sequence alignment of OmGAL2 with AtAGAL1, AtAGAL2, and AtAGAL3.
Fig. S5. Expression of OmAGAL1, OmGAL2, and OmAGAL3 in germinating seeds of O. minor.
Fig. S6. Phylogenetic tree for plant AGALs.
Acknowledgements
The authors would like to thank Dr Vivien Rolland (CSIRO Agriculture & Food, Canberra, Australia) for providing pMDC-At5g11420:pH-tdGFP, and Yumi Mori, Maki Hazama, Tomoko Sakai, and Hiromi Saito for technical assistance.
Author contributions
AO conceived the study, designed and supervised the research, analysed the data, and wrote the article with the support of all authors; AB, TT, HO, MO, and TN performed most of the experiments and analysed the data; SS conducted mass imaging; TO, YS, and DO supervised the research.
Conflict of interest
The authors have no conflict of interest to declare.
Funding
This work was partly supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no. JP20H02924 to AO and DO, JP20KK0131 to AO and YS) and by Japan Science and Technology Agency (JST)/Japan International Cooperation Agency (JICA) Science and Technology Research Partnership for Sustainable Development (SATREPS; grant no. JPMJSA1607 to AO and YS).
Data availability
The following mRNA sequences are deposited in NCBI database: OmAGAL1 (LC636199), OmAGAL2 (LC636200), OmAGAL3 (LC636201), and comp71446_c0_seq1 (LC636330). All other data supporting the findings of this study are available within the paper and within the supplementary data published online.
References
- Akiyama K, Matsuzaki K, Hayashi H.. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435, 824–827. [DOI] [PubMed] [Google Scholar]
- Al-Babili S, Bouwmeester HJ.. 2015. Strigolactones, a novel carotenoid-derived plant hormone. Annual Review of Plant Biology 66, 161–186. [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J.. 2009. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant & Cell Physiology 50, 1416–1424. [DOI] [PubMed] [Google Scholar]
- Blöchl A, Peterbauer T, Hofmann J, Richter A.. 2008. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta 228, 99–110. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Li C, Thiombiano B, Rahimi M, Dong L.. 2021. Adaptation of the parasitic plant lifecycle: germination is controlled by essential host signaling molecules. Plant Physiology 185, 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester HJ, Fonne-Pfister R, Screpanti C, De Mesmaeker A.. 2019. Strigolactones: plant hormones with promising features. Angewante Chemie International Edition 58, 12778–12786. [DOI] [PubMed] [Google Scholar]
- Brun G, Braem L, Thoiron S, Gevaert K, Goormachtig S, Delavault P.. 2018. Seed germination in parasitic plants: what insights can we expect from strigolactone research? Journal of Experimental Botany 69, 2265–2280. [DOI] [PubMed] [Google Scholar]
- Buckeridge M, dos Santos HP, Tiné MAS.. 2000. Mobilisation of storage cell wall polysaccharides in seeds. Plant Physiology and Biochemistry 38, 141–156. [Google Scholar]
- Bürger M, Chory J.. 2020. The many models of strigolactone signaling. Trends in Plant Science 25, 395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn CE, Bythell-Douglas R, Neumann D, Yoshida S, Whittington B, Westwood JH, Shirasu K, Bond CS, Dyer KA, Nelson DC.. 2015. PLANT EVOLUTION. Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 349, 540–543. [DOI] [PubMed] [Google Scholar]
- Conn CE, Nelson DC.. 2016. Evidence that KARRIKIN-INSENSITIVE2 (KAI2) Receptors may perceive an unknown signal that is not karrikin or strigolactone. Frontiers in Plant Science 6, 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR.. 2005. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology 139, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey PM. 1980. Biosynthesis of planteose in Sesamum indicum. FEBS Letters 114, 153–156. [Google Scholar]
- Draie R, Péron T, Pouvreau JB, Véronési C, Jégou S, Delavault P, Thoiron S, Simier P.. 2011. Invertases involved in the development of the parasitic plant Phelipanche ramosa: characterization of the dominant soluble acid isoform, PrSAI1. Molecular Plant Pathology 12, 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Aparicio M, Delavault P, Timko MP.. 2020. Management of infection by parasitic weeds: a review. Plants 9, 1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurtado JA, Banik M, Bewley JD.. 2001. The cloning and characterization of alpha-galactosidase present during and following germination of tomato (Lycopersicon esculentum Mill.) seed. Journal of Experimental Botany 52, 1239–1249. [PubMed] [Google Scholar]
- Fialho Lda S, Guimarães VM, Callegari CM, Reis AP, Barbosa DS, Borges EE, Moreira MA, de Rezende ST.. 2008. Characterization and biotechnological application of an acid alpha-galactosidase from Tachigali multijuga Benth. seeds. Phytochemistry 69, 2579–2585. [DOI] [PubMed] [Google Scholar]
- French D. 1955. Isolation and identification of planteose from tobacco seeds. Journal of American Chemical Society 77, 1024–1025. [Google Scholar]
- French D, Youngquist RW, Lee A.. 1959. Isolation and crystallization of planteose from mint seeds. Archives of Biochemistry and Biophysics 85, 471–473. [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. 2008. Strigolactone inhibition of shoot branching. Nature 455, 189–194. [DOI] [PubMed] [Google Scholar]
- Guimarães VM, de Rezende ST, Moreira MA, de Barros EG, Felix CR.. 2001. Characterization of alpha-galactosidases from germinating soybean seed and their use for hydrolysis of oligosaccharides. Phytochemistry 58, 67–73. [DOI] [PubMed] [Google Scholar]
- Hamiaux C, Drummond RS, Janssen BJ, Ledger SE, Cooney JM, Newcomb RD, Snowden KC.. 2012. DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Current Biology 22, 2032–2036. [DOI] [PubMed] [Google Scholar]
- Harada K, Kurono Y, Nagasawa S, et al. 2017. Enhanced production of nojirimycin via Streptomyces ficellus cultivation using marine broth and inhibitory activity of the culture for seeds of parasitic weeds. Journal of Pesticide Science 42, 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa K, Hirama T, Shimura H, Jitsuyama Y, Suzuki T.. 2019. Visualization of soluble carbohydrate distribution in apple fruit flesh utilizing MALDI-TOF MS imaging. Plant Science 278, 107–112. [DOI] [PubMed] [Google Scholar]
- Hughes SG, Overbeeke N, Robinson S, Pollock K, Smeets FL.. 1988. Messenger RNA from isolated aleurone cells directs the synthesis of an alpha-galactosidase found in the endosperm during germination of guar (Cyamopsis tetragonaloba) seed. Plant Molecular Biology 11, 783–789. [DOI] [PubMed] [Google Scholar]
- Imaizumi C, Tomatsu H, Kitazawa K, Yoshimi Y, Shibano S, Kikuchi K, Yamaguchi M, Kaneko S, Tsumuraya Y, Kotake T.. 2017. Heterologous expression and characterization of an Arabidopsis β-l-arabinopyranosidase and α-d-galactosidases acting on β-l-arabinopyranosyl residues. Journal of Experimental Botany 68, 4651–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel DM, Bar H, Mayer AM, Plakhine D, Ziadne H, Westwood JH, Welbaum GE.. 2012. Seed ultrastructure and water absorption pathway of the root-parasitic plant Phelipanche aegyptiaca (Orobanchaceae). Annals of botany 109, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel DM, Bar H.. 2013. The seed and the seedling. In: D Joel JG, Musselman L, eds, Parasitic Orobanchaceae, Springer, Berlin, Heidelberg, 147–166. [Google Scholar]
- Khosla A, Nelson DC.. 2016. Strigolactones, super hormones in the fight against Striga. Current Opinion in Plant Biology 33, 57–63. [DOI] [PubMed] [Google Scholar]
- Kuo TM, VanMiddlesworth JF, Wolf WJ.. 1988. Content of raffinose oligosaccharides and sucrose in various plant seeds. Journal of Agricultural and Food Chemistry 36, 32–36. [Google Scholar]
- Kurihara D, Mizuta Y, Sato Y, Higashiyama T.. 2015. ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco L, Fiorilli V, Venice F, Bonfante P.. 2018. Strigolactones cross the kingdoms: plants, fungi, and bacteria in the arbuscular mycorrhizal symbiosis. Journal of Experimental Botany 69, 2175–2188. [DOI] [PubMed] [Google Scholar]
- Leung DW, Bewley JD.. 1981. Red-light- and gibberellic-acid-enhanced α-galactosidase activity in germinating lettuce seeds, cv. Grand Rapids: Control by the axis. Planta 152, 436–441. [DOI] [PubMed] [Google Scholar]
- Li S, Kim WD, Kaneko S, Prema PA, Nakajima M, Kobayashi H.. 2007. Expression of rice (Oryza sativa L. var. Nipponbare) alpha-galactosidase genes in Escherichia coli and characterization. Bioscience, Biotechnology, and Biochemistry 71, 520–526. [DOI] [PubMed] [Google Scholar]
- López-Ráez JA. 2015. How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 243, 1375–1385. [DOI] [PubMed] [Google Scholar]
- Marraccini P, Rogers WJ, Caillet V, Deshayes A, Granato D, Lausanne F, Lechat S, Pridmore D, Pétiard V.. 2005. Biochemical and molecular characterization of α-d-galactosidase from coffee beans. Plant Physiology and Biochemistry 43, 909–920. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Xue YL, Miyakawa T, et al. 2013. Molecular mechanism of strigolactone perception by DWARF14. Nature Communications 4, 2613. [DOI] [PubMed] [Google Scholar]
- Okazawa A, Kusunose T, Ono E, Kim HJ, Satake H, Shimizu B, Mizutani M, Seki H, Muranaka T.. 2014. Glucosyltransferase activity of Arabidopsis UGT71C1 towards pinoresinol and lariciresinol. Plant Biotechnology 31, 561–566. [Google Scholar]
- Okazawa A, Wakabayashi T, Muranaka T, Sugimoto Y, Ohta D.. 2020. The effect of nojirimycin on the transcriptome of germinating Orobanche minor seeds. Journal of Pesticide Science 45, 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. 2013. The parasitic weeds of the Orobanchaceae. In: Joel D, Gressel J, Musselman L, eds. Parasitic Orobanchaceae, Berlin, Heidelberg: Springer, 313–344. [Google Scholar]
- Robinson S, Warburton K, Seymour M, Clench M, Thomas-Oates J.. 2007. Localization of water-soluble carbohydrates in wheat stems using imaging matrix-assisted laser desorption ionization mass spectrometry. The New Phytologist 173, 438–444. [DOI] [PubMed] [Google Scholar]
- Sekhar KN, Demason DA.. 1990. Identification and immunocytochemical localization of α-galactosidase in resting and germinated date palm (Phoenix dactylifera L.) seeds. Planta 181, 53–61. [DOI] [PubMed] [Google Scholar]
- Seto Y, Yasui R, Kameoka H, et al. 2019. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nature Communications 10, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard A, Rolland V.. 2019. I see the light! Fluorescent proteins suitable for cell wall/apoplast targeting in Nicotiana benthamiana leaves. Plant Direct 3, e00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapernoux-Lüthi EM, Böhm A, Keller F.. 2004. Cloning, functional expression, and characterization of the raffinose oligosaccharide chain elongation enzyme, galactan: galactan galactosyltransferase, from common bugle leaves. Plant Physiology 134, 1377–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Yoshimura M, Sato Y, et al. 2015. PARASITIC PLANTS. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 349, 864–868. [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, et al. 2008. Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. [DOI] [PubMed] [Google Scholar]
- Uraguchi D, Kuwata K, Hijikata Y, et al. 2018. A femtomolar-range suicide germination stimulant for the parasitic plant Striga hermonthica. Science 362, 1301–1305. [DOI] [PubMed] [Google Scholar]
- Van den Ende W. 2013. Multifunctional fructans and raffinose family oligosaccharides. Frontiers in Plant Science 4, 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T, Joseph B, Yasumoto S, et al. 2015. Planteose as a storage carbohydrate required for early stage of germination of Orobanche minor and its metabolism as a possible target for selective control. Journal of Experimental Botany 66, 3085–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Gutjahr C, Bennett T, Nelson DC.. 2017. Strigolactone signaling and evolution. Annual Review of Plant Biology 68, 291–322. [DOI] [PubMed] [Google Scholar]
- Xing X, Hsieh YSY, Yap K, Ang ME, Lahnstein J, Tucker MR, Burton RA, Bulone V.. 2017. Isolation and structural elucidation by 2D NMR of planteose, a major oligosaccharide in the mucilage of chia (Salvia hispanica L.) seeds. Carbohydrate Polymers 175, 231–240. [DOI] [PubMed] [Google Scholar]
- Yan D, Duermeyer L, Leoveanu C, Nambara E.. 2014. The functions of the endosperm during seed germination. Plant & Cell Physiology 55, 1521–1533. [DOI] [PubMed] [Google Scholar]
- Yoneyama K. 2020. Recent progress in the chemistry and biochemistry of strigolactones. Journal of Pesticide Science 45, 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Kim S, Wafula EK, et al. 2019. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Current Biology 29, 3041–3052.e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following mRNA sequences are deposited in NCBI database: OmAGAL1 (LC636199), OmAGAL2 (LC636200), OmAGAL3 (LC636201), and comp71446_c0_seq1 (LC636330). All other data supporting the findings of this study are available within the paper and within the supplementary data published online.