Abstract
An efficient protocol for the preparation of pyridine skeletons has been successfully developed involving the TMSOTf/HMDS (trifluoromethanesulfonic acid/hexamethyldisilane) system for the intermolecular cyclization of chalcones under MW (microwave) irradiation conditions. This method provides a facile approach to synthesize 2,4,6-triaryl or 3-benzyl-2,4,6-triarylpyridines in good to excellent yields. Interestingly, the 2,6-diazabicyclo[2.2.2]oct-2-ene core was obtained by changing the acid additive to Sn(OTf)2, and the desired product was also confirmed using X-ray single-crystal diffraction analysis.
A Kröhnke pyridine synthetic route towards functionalized TAPs and 3-benzyl TAPs has been established using TMSOTf/HMDS under microwave irradiation conditions.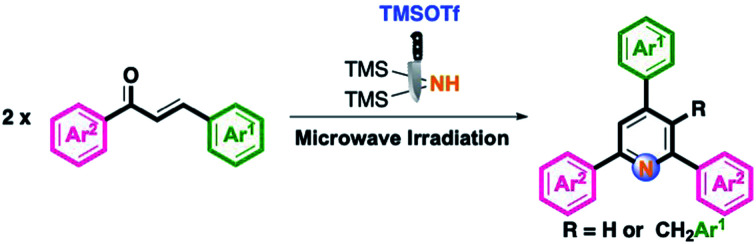
Pyridines serve as an essential framework, and their derivatives have gained considerable interest in organic chemistry owing to their presence in a wide range of natural products such as NAD (nicotinamide adenine dinucleotide) nucleotides, pyridoxine (vitamin B6), pyridine alkaloids,1 pharmaceuticals (as antimalarial, vasodilatory, anaesthetic, anticonvulsant and antiepileptic drugs),2 agrochemicals (as fungicides, pesticides, and herbicides),3 electrochemical,4 functionalized materials,5 organocatalysts,6 and synthetic ligands.7 Among them, 2,4,6-triarylpyridines (TAPs, known as Kröhnke pyridines) are practical intermediates in the synthesis of drugs, herbicides, insecticides, desiccants, and surfactants.8 Due to their excellent π-stacking ability and thermal stability, these substituted pyridines can be used in fluorescent small molecules, polymers, supramolecules, and for asymmetric catalysis and coordination.9 They are also recognized as useful precursors as they are structurally related to symmetrical triaryl-thiopyrylium, triaryl-selenopyrylium, and triaryl-telluropyrylium photosensitizers, and have been investigated for photodynamic cell-specific cancer therapeutic agents.10 These chalcogenopyrylium analogues are also applied in achieving nucleophilic displacement,11C-alkylation of β-diketones via a radical pathway,12 electrophilic aminations,13 and for the preparation of substituted azepines.14 Considering the versatile functions of TAPs in the fields of organic synthesis and medicinal chemistry, the synthetic protocols of TAPs are still of great interest.15
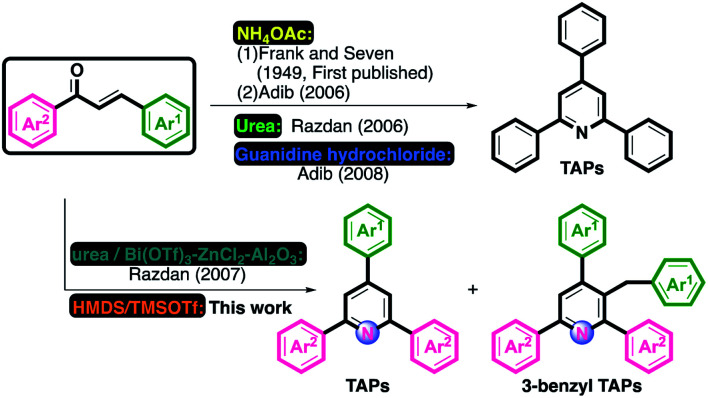
TAPs were first prepared via the thermal condensation of aldehydes with ammonia by the Russian chemist Aleksey Chichibabin in 1906.17 This reaction was then modified as the condensation of aldehydes and enolizable ketones with various nitrogen sources, and has become the most popular protocol for the construction of symmetrical scaffolds, which contain identical substituents at the 2- and 6-positions of the pyridine ring.18
Most Chichibabin-type TAP synthetic approaches start from acetophenones with benzaldehydes in the presence of ammonium acetate and are catalyzed by a variety of catalysts.15,16,19 Among these, the synthetic routes for the synthesis of symmetrical TAPs not only involve the Chichibabin reaction but also methods with fixed nitrogen on starting materials such as benzylamines20 or oxime acetates.21 For the preparation of symmetrical TAPs, chalcones are usually applied as the starting material or paired with condensable synthons from various ammonia sources.22 As shown in Scheme 1, Frank and Seven reported the revised Chichibabin reaction for the preparation of pyridines.18 In 2006, Adib described a revised method involving the solvent-free synthesis of TAPs.23 Meanwhile, Razdan developed a solid-supported synthesis of TAPs using urea as an ammonia source.24 Later, Adib substituted guanidine hydrochloride for urea to prepare TAPs under neat and MW conditions.25 Interestingly, Razdan's group reported a synthetic route for the synthesis of TAPs and 3-benzyl TAPs using immobilized metal catalysts and urea as an ammonia source.26
Scheme 1. Synthesis of 2,4,6-triarylpyridines (TAPs).
In continuation of our efforts in the synthesis of diversified heterocyclic skeletons under microwave (MW) conditions,27 we recently developed a series of synthetic protocols for the synthesis of substituted nitrogen-containing compounds involving the HMDS/TMSOTf system under MW conditions.27a–c On the basis of our previous successful experiments, HMDS serves as a useful N source in this synthetic routes. Only one method for one-pot synthesis of TAPs and 3-benzyl TAPs from chalcones, using urea as the N source, and the immobilized metal catalysts, have been reported.26 Therefore, we applied such metal-free conditions to synthesize substituted TAPs and 3-benzyl TAPs. Although the synthetic protocols for TAPs are well documented, the development of facile methodologies is of great interest. In this work, we describe a facile synthetic route for the synthesis of TAPs and rare 3-benzyl TAPs under MW with modest to good yields.
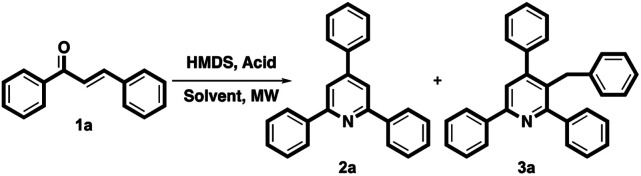
First, the chalcone compound 1a was selected as a model substrate to react with HMDS as a nitrogen source by using acidic additives under MW to optimize this Kröhnke reaction conditions, as shown in Table 1. Based on the basis of our previous work, we believe that TMSOTf is an appropriate acidic additive to catalyze HMDS in heterocyclic compound synthesis.27a–c In entry 1, the reaction was conducted in DCM and HMDS and catalyzed by TMSOTf under MW at 150 °C in 0.5 h, 50% yield of 2a and 44% yield of 3a were obtained. When the reaction solvent was changed to other nonpolar solvents such as toluene and THF, no obvious yield changes were detected (entries 2–3), but MeCN affected the decreasing yield and only a trace amount of 3a was isolated (entry 4). Surprisingly, (CuOTf)2·toluene did not favor the reaction, while AgOTf and Cu(OTf)2 provided modest yields (entries 5–7). Some metal(iii) triflates were also investigated in this synthesis route, including Sc(OTf)3, Bi(OTf)3, Fe(OTf)3, and In(OTf)3; similar yields of 2a and lower yields of 3a were detected (entries 8–11). In(OTf)3 showed a higher tendency for the formation of 2a than for 3a. Other Lewis acids were also chosen to test the reaction, and the results suggest that non-triflate-type Lewis acids give poor results (entries 12–14). In entries, 15–17, Brønsted acids, AcOH, TfOH and p-TsOH were also investigated in this reaction. AcOH and TfOH could successfully promote this reaction and provide modest yields, but p-TsOH did not work. Notably, only one literature report has discussed the preparation of 3-benzyl TAPs. According to the results for entries 1 to 17, we confirmed that entry 1 shows the best conditions for this intermolecular cyclization for the preparation of substituted TAPs and 3-benzyl TAPs in totally high yields.
Optimization of the reaction conditionsa.
| ||||
|---|---|---|---|---|
| Entry | Acid | Solvent | Yieldsb (%) | |
| 2a | 3a | |||
| 1 | TMSOTf | DCM | 50 | 44 |
| 2 | TMSOTf | Toluene | 48 | 43 |
| 3 | TMSOTf | THF | 56 | 33 |
| 4 | TMSOTf | MeCN | 44 | <5 |
| 5 | AgOTf | DCM | 44 | 17 |
| 6c | (CuOTf)2·toluene | DCM | N.R. | N.R. |
| 7 | Cu(OTf)2 | DCM | 58 | 15 |
| 8 | Sc(OTf)3 | DCM | 52 | 24 |
| 9 | Bi(OTf)3 | DCM | 47 | 30 |
| 10 | Fe(OTf)3 | DCM | 52 | 30 |
| 11 | In(OTf)3 | DCM | 62 | 20 |
| 12 | Ac2O | DCM | N.R. | N.R. |
| 13 | AlCl3 | DCM | 23 | __d |
| 14 | BF3·OEt2 | DCM | 31 | 20 |
| 15 | AcOH | DCM | 52 | 20 |
| 16 | TfOH | DCM | 50 | 23 |
| 17c | p-TsOH | DCM | N.R. | N.R. |
Reaction conditions: 1a (1.0 mmol), HMDS (0.5 mL, 2.4 mmol), acid (0.5 mmol), solvent (2 mL), MW (150 °C), 0.5 h.
Isolated yields.
No reaction occurred.
Unknown products were obtained.
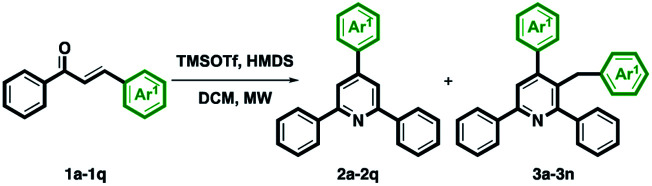
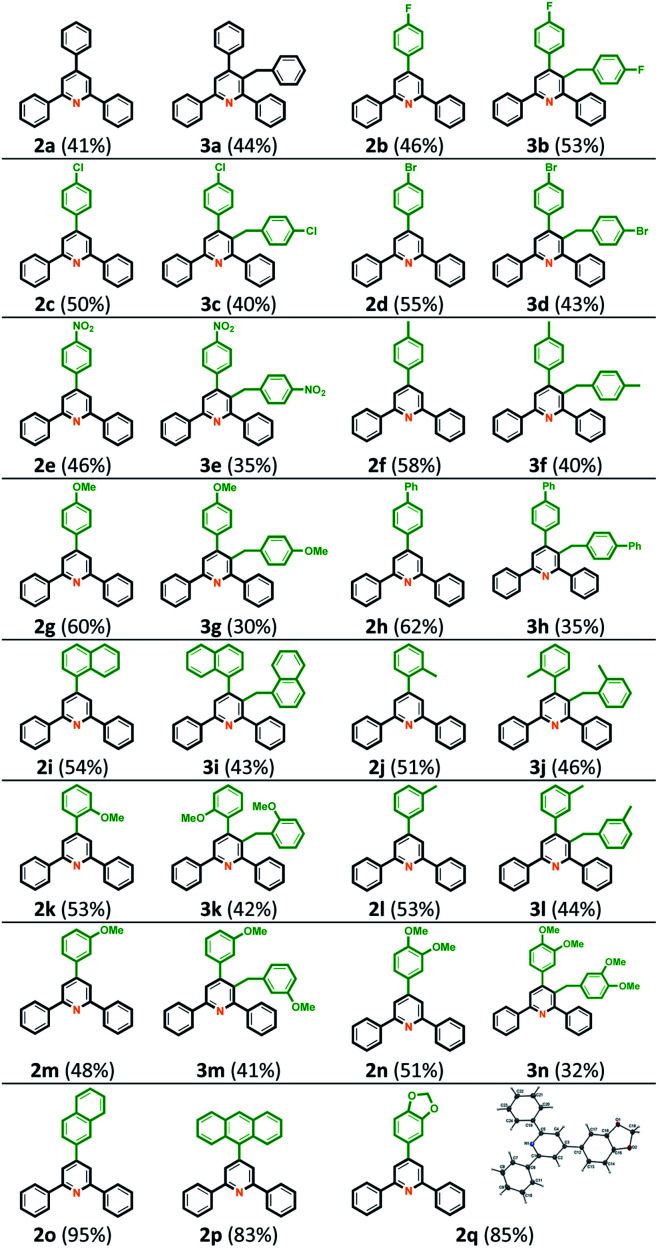
With the optimal conditions in hand, we initially examined the scope and generality of functionalized chalcones 1b–1q for this intermolecular cyclization. As shown in Table 2, we first investigated the electron-withdrawing substituents on the Ar1 ring, including the 4-halogenated substituent 1b–1d and 4-nitro group 1e. The corresponding TAPs 2b–2e and 3-benzyl TAPs 3b–3e were obtained in modest to good yields. Some electron-donating substituents on para-position of the Ar1 ring, such as methyl 1f, methoxy 1g, and phenyl 1h were also examined under optimized conditions and the desired TAPs 2f–2h and 3-benzyl TAPs 3f–3h were isolated in good yields. Other functionalized groups on the Ar1 ring, including 1-naphthalene 1i, 2-methyl 1j, 2-methoxy 1k, 3-methyl 1l, 3-methoxy 1m, 3,4-dimethoxy 1n also reacted smoothly under optimal conditions and provided the relevant TAPs 2i–2n and 3-benzyl TAPs 3i–3n in totally good to excellent yields. Notably, when the Ar1 ring was 2-naphthalene 1o, anthracene 1p, or piperonal 1q, only corresponding TAPs 2o–2q were obtained. This may be because the steric hindrance effect causes the unsuccessful formation of 3-benzyl TAPs 3o–3q. The structure of 2q was also confirmed from X-ray single-crystal crystallography.28
Synthesis of 2a–2q and 3a–3na,b.
|
|---|
|
Reaction conditions: 1a–1q (1.0 mmol), HMDS (0.5 mL, 2.4 mmol), TMSOTf (0.1 mL, 0.5 mmol), DCM (2 mL), MW (150 °C), 0.5 h.
Isolated yields.
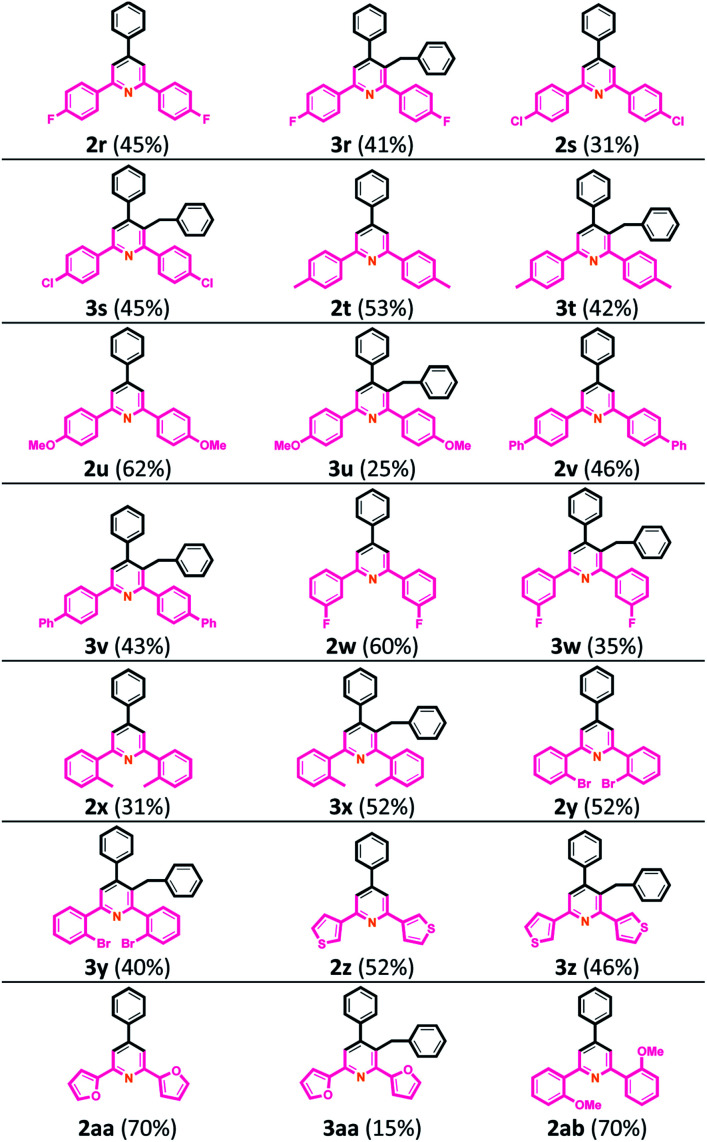
Then, we tested a variety of chalcones with various functional groups on the Ar2 ring to extend the scope of the reaction and generality of this intermolecular cyclization. The results showed high generality and broad functional group tolerance. The corresponding compound structures and related yields are illustrated in Table 3. The para-substituted group of the Ar2 ring was first examined; when the Ar1 ring was benzene, including 1r (Ar2 = 4-FC6H4), 1s (Ar2 = 4-FC6H4), 1t (Ar2 = 4-MeC6H4), 1u (Ar2 = 4-OMeC6H4) and 1v (Ar2 = 4-PhC6H4), total yields of corresponding TAPs 2r–2v and 3-benzyl TAPs 3r–3v were between 76% and 95% yields. Some meta- and ortho-substituents on Ar2 ring, such as 1w (Ar2 = 3-FC6H4), 1x (Ar2 = 2-MeC6H4) and 1y (Ar2 = 2-BrC6H4), and heterocyclic ring 1z (Ar2 = 3-thiophene) and 1aa (Ar2 = 2-furan) provided the desired products 2w–2aa and 3w–3aa in totally good to excellent yields. We assume that the steric hindrance is the reason for the selective formation of product 2 ab from 1 ab at 70% yield.
Synthesis of 2r–2ab and 3r–3aaa,b.
|
|---|
|
Reaction conditions: 1r–1ab (1.0 mmol), HMDS (0.5 mL, 2.4 mmol), TMSOTf (0.1 mL, 0.5 mmol), DCM (2 mL), MW (150 °C), 0.5 h.
Isolated yields.
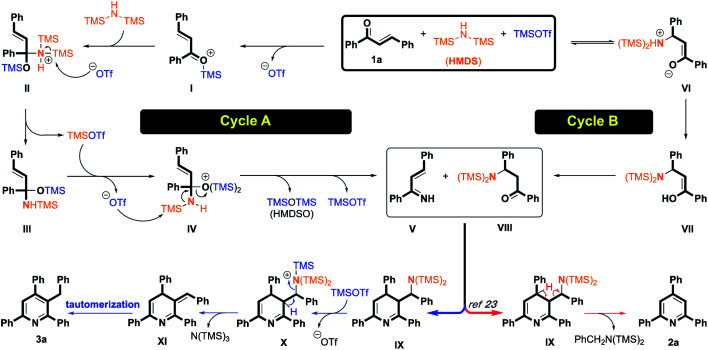
Based on the above-mentioned results and our previous work,27 a plausible pathway for the selective synthesis of TAPs and 3-benzyl TAPs is illustrated as shown in Scheme 2. In cycle A, compound 1a was first activated using TMSOTf to generate intermediate I with the release of the triflate anion. HMDS was added to intermediate I through nucleophilic addition to give intermediate II, which was then attacked by the triflate anion to form intermediate III along with recycling of TMSOTf. TMSOTf activated the oxygen of intermediate III again to give intermediate IV, which was also attacked by the triflate anion to afford intermediate V together with HMDSO (hexamethyldisiloxane) and recycle TMSOTf. In cycle B, the 1,4-addition of HMDS with compound 1a occurred to generate derivative VI, followed by proton exchange to give intermediate VII. Intermediate VIII was formed via keto–enol tautomerization. After the formation of intermediate V from cycle A and the generation of intermediate VIII from cycle B, the intermolecular cyclization occurred smoothly, yielding intermediate IX. For the red arrow, the oxidative aromatization with the release of N,N-di(trimethylsilyl)-benzylamine would yield TAPs product 2a.23 Considering the blue arrow, TMSOTf activated benzylamine fragment to obtain intermediate X, and then aromatization occurred with the removal of tris(trimethylsilyl)amine to form 3-benzylidiene-3,4-dihydropyridine XI, which is spontaneously converted to the corresponding 3- benzyl TAPs 3a by tautomerism.
Scheme 2. Proposed mechanism for the synthesis of TAPs.
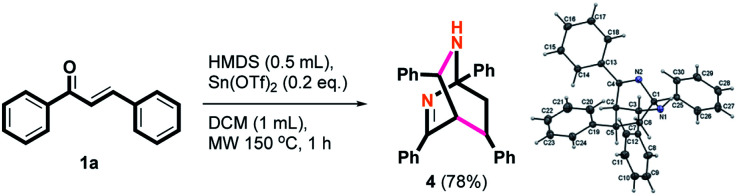
Surprisingly, an unexpected product 4 was obtained in 78% yield when the acidic additive was changed from TMSOTf to Sn(OTf)2 under this condition. Interestingly, one C–C bond and two C–N bonds were established in one step to generate a new diazobicyclo[2.2.2]octene product. This is the first report of the synthesis of 2,6-diazabicyclo[2.2.2]oct-2-ene core. Considering the novelty of the structure, the mechanism will be proposed with the scope on various substrates. Based on these intriguing chemical aspects, further possible applications should also be investigated from a future perspective. As shown in Scheme 3, the structure of 4 was also confirmed using X-ray single-crystal crystallography.28
Scheme 3. Synthesis of product 4.
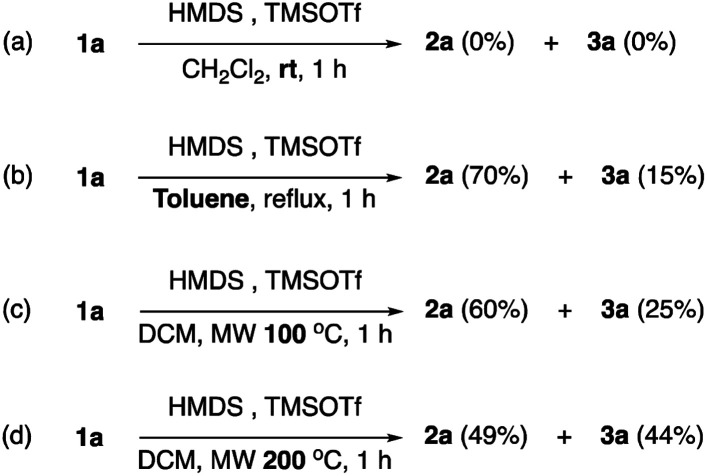
This intermolecular cyclization in the same reagents and equivalents was heated by using a normal hot plate as a heating source that was also studied in some control experiments, as shown in Scheme 4. No reaction occurred when the reaction was conducted at room temperature (eqn (a)). Owing to the low boiling point of dichloromethane, changing evaporable dichloromethane to high boiling point toluene in a hot plate at reflux is necessary for the investigation. In eqn (b) and (c), although the total yields are similar to the optimized condition, a lower yield of 3a was obtained. No obvious yield change was observed while increasing the reaction temperature to 200 °C under the MW system (eqn (d)). According to these control experiments, we think that the MW system is a better heating source than a normal hot plate.
Scheme 4. The control experiments.
Conclusions
In conclusion, we have described an efficient protocol for the synthesis of pyridine skeleton, including TAPs and 3-benzyl TAPs, from simple chalcones with functional group tolerance in good to excellent yields. This intermolecular annulation involved the combination of TMSOTf/HMDS and was conducted in a microwave irradiation system. By changing the acidic additive to Sn(OTf)2 in the reaction, a new 2,6-diazabicyclo[2.2.2]oct-2-ene core was obtained in moderate yield. The structures of some products were confirmed by X-ray single-crystal diffraction analysis. Considering the importance of the pyridine skeleton, biological and medicinal activities will be further examined and published in due course.
Experimental section
General information
All used reagents and solvents were commercially available and used without further purification. Reactions were routinely performed using the Discover SP system (CEM) in the sealed reaction vessels in a standard mode with the temperature monitored using a vertically focused IR sensor. All reactions were monitored using thin-layer chromatography on silica gel 60 F254 (Merck) with detection by UV light. Column chromatography was performed using silica gel (200–300 mesh). Products in organic solvents were dried with anhydrous magnesium sulfate before concentration in vacuo. Melting points were determined using an MP-2D melting apparatus. 1H and 13C NMR spectra were recorded on a Bruker AVIII 500 spectrometer operating at 500 and 125 MHz, respectively. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, br = broad), coupling constants (Hz) and integration. HRMS were obtained on a Waters LCT Premier XE (Waters Corp., Manchester, UK) instrument equipped with an electrospray source. The X-ray intensity data were measured at a low temperature of 100 K using a Mo Kα radiation diffractometer equipped with a kappa geometry goniometer and corrected for absorption effects using the numerical method (SADABS).
General procedure for the synthesis of skeletons 2 and 3
A mixture of chalcone 1 (1.0 mmol), hexamethyldisilane (0.5 mL, 2.4 mmol), trimethylsilyl trifluoromethanesulfonate (0.1 mL, 0.5 mmol) in dichloromethane (2 mL), in a dried 35 mL microwave vial at 25 °C. The mixture was treated in a microwave irradiation instrument and stirred at 150 °C for 0.5 h. The consumption of the starting materials was confirmed by TLC. The reaction was cooled to 25 °C, the mixture of crude products was transferred to a 100 mL round bottom flask, and the solvent was concentrated. The residue was diluted with water (10 mL) and the mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic layers were washed with brine, dried, filtered and evaporated to afford the crude product under reduced pressure. Purification on silica gel (hexanes/EtOAc = 4/1–1/1) afforded compounds 2a–2ab, 3a–3n and 3r–3aa.
2,4,6-Triphenylpyridine (2a)20c
Yield = 41% (63 mg); colorless solid; mp = 138–139 °C; HRMS (ESI, M+ + H) calcd for C23H18N 308.1439, found 308.1447; 1H NMR (500 MHz, CDCl3): δ 8.35–8.31 (m, 4H), 7.96 (s, 2H), 7.82–7.80 (m, 2H), 7.66–7.57 (m, 6H), 7.57–7.51 (m, 3H); 13C NMR (125 MHz, CDCl3): δ 157.32 (2x), 149.99, 139.45 (2x), 138.87, 128.96 (2x), 128.93 (2x), 128.83, 128.58 (4x), 127.03 (6x), 116.93 (2x).
3-Benzyl-2,4,6-triphenylpyridine (3a)26
Yield = 44% (87 mg); white solid; mp = 134–135 °C; HRMS (ESI, M+ + H) calcd for C30H24N 398.1909, found 398.1914; 1H NMR (500 MHz, CDCl3): δ 8.21–8.12 (m, 2H), 7.73–7.67 (m, 1H), 7.61–7.54 (m, 2H), 7.53–7.47 (m, 2H), 7.46–7.35 (m, 7H), 7.33–7.27 (m, 2H), 7.14–7.05 (m, 3H), 6.74–6.66 (m, 2H), 4.16 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 160.28, 154.49, 152.31, 141.27, 140.90, 139.96, 138.99, 129.83, 129.10 (2x), 128.78, 128.58 (2x), 128.48 (2x), 128.17 (2x), 128.13 (2x), 127.94 (2x), 127.85 (2x), 127.72, 127.65, 126.92 (2x), 125.44, 120.59, 35.02.
4-(4-Fluorophenyl)-2,6-diphenylpyridine (2b)20c
Yield = 46% (75 mg); colorless solid; mp = 141–142 °C; HRMS (ESI, M+ + H) calcd for C23H17FN 326.1340, found 326.1337; 1H NMR (500 MHz, CDCl3): δ 8.22 (d, J = 8.0 Hz, 4H), 7.84 (s, 2H), 7.74–7.71 (m, 2H), 7.54 (t, J = 8.0 Hz, 4H), 7.49–7.46 (m, 2H), 7.23 (t, J = 8.5 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 163.31 (d, J = 247.25 Hz), 157.49 (2x), 149.04, 139.36 (2x), 135.03 (d, J = 3.0 Hz), 129.08 (2x), 128.87 (d, J = 8.25 Hz, 2x), 128.68 (4x), 127.06 (4x), 116.83 (2x), 116.06 (d, J = 21.375 Hz, 2x).
3-(4-Fluorobenzyl)-4-(4-fluorophenyl)-2,6-diphenylpyridine (3b)26
Yield = 53% (115 mg); white solid; mp = 166–167 °C; HRMS (ESI, M+ + H) calcd for C30H22F2N 434.1720, found 434.1716; 1H NMR (500 MHz, CDCl3): δ 8.09 (d, J = 9.0 Hz, 2H), 7.60 (s, 1H), 7.54–7.35 (m, 8H), 7.21–7.13 (m, 2H), 7.04 (t, J = 9.0 Hz, 2H), 6.74 (t, J = 8.0 Hz, 2H), 6.61–6.52 (m, 2H), 4.04 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 162.34 (d, J = 245.875 Hz), 160.93 (d, J = 242.375 Hz), 160.26, 154.78, 151.31, 141.00, 138.77, 136.32 (d, J = 2.875 Hz), 135.80 (d, J = 3.25 Hz), 130.11 (d, J = 8.0 Hz, 2x), 129.85, 129.41 (d, J = 7.625 Hz, 2x), 129.05 (2x), 128.97, 128.67 (2x), 128.10 (2x), 127.95, 126.94 (2x), 120.73, 115.24 (d, J = 21.25 Hz, 2x), 114.73 (d, J = 21.0 Hz, 2x), 34.27.
4-(4-Chlorophenyl)-2,6-diphenylpyridine (2c)20c
Yield = 50% (85 mg); yellow solid; mp = 119–120 °C; HRMS (ESI, M+ + H) calcd for C23H17ClN 342.1044, found 342.1048; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 8.0 Hz, 4H), 7.84 (s, 2H), 7.68 (d, J = 8.5 Hz, 2H), 7.56–7.49 (m, 6H), 7.48–7.44 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.66 (2x), 148.92, 139.38 (2x), 137.46, 135.17, 139.31 (2x), 129.15 (2x), 128.72 (4x), 128.43 (2x), 127.10 (4x), 116.79 (2x).
3-(4-Chlorobenzyl)-4-(4-chlorophenyl)-2,6-diphenylpyridine (3c)26
Yield = 40% (92 mg); white solid; mp = 179–180 °C; HRMS (ESI, M+ + H) calcd for C30H22Cl2N 466.1124, found 466.1120; 1H NMR (500 MHz, CDCl3): δ 8.09 (d, J = 7.5 Hz, 2H), 7.59 (s, 1H), 7.52–7.47 (m, 4H), 7.43–7.36 (m, 4H), 7.33 (d, J = 8.5 Hz, 2H), 7.14 (d, J = 8.0 Hz, 2H), 7.04 (d, J = 8.0 Hz, 2H), 6.56 (d, J = 8.0 Hz, 2H), 4.02 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 160.39, 154.95, 151.13, 140.92, 139.15, 138.72, 138.21, 134.04, 131.48, 129.79 (2x), 129.40 (2x), 129.20, 129.03 (2x), 128.69 (2x), 128.51 (2x), 128.12 (4x), 128.01 (2x), 126.96 (2x), 120.48, 34.47.
4-(4-Bromophenyl)-2,6-diphenylpyridine (2d)21a
Yield = 55% (106 mg); white solid; mp = 128–129 °C; HRMS (ESI, M+ + H) calcd for C23H17BrN 386.0539, found 386.0533; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 7.5 Hz, 4H), 7.84 (s, 2H), 7.66 (d, J = 8.5 Hz, 2H), 7.61 (d, J = 8.5 Hz, 2H), 7.53 (t, J = 8.0 Hz, 4H), 7.49–7.43 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.68 (2x), 148.93, 139.37 (2x), 137.95, 132.25 (2x), 129.12 (2x), 128.68 (6x), 127.09 (4x), 123.36, 116.67 (2x).
3-(4-Bromobenzyl)-4-(4-bromophenyl)-2,6-diphenylpyridine (3d)26
Yield = 43% (119 mg); white solid; mp = 180–181 °C; HRMS (ESI, M+ + H) calcd for C30H22BrN 554.0114, found 554.0109; 1H NMR (500 MHz, CDCl3): δ 8.08 (d, J = 7.5 Hz, 2H), 7.58 (s, 1H), 7.52–7.43 (m, 6H), 7.42–7.34 (m, 4H), 7.19 (d, J = 8.0 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 6.51 (d, J = 8.0 Hz, 2H), 4.00 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 160.43, 154.97, 151.11, 140.93, 139.67, 138.71, 131.47 (2x), 131.07 (2x), 130.09 (2x), 129.79 (2x), 129.02 (5x), 128.66 (2x), 128.10 (2x), 127.99, 126.95 (2x), 122.20, 120.32, 119.55, 34.54.
4-(4-Nitrophenyl)-2,6-diphenylpyridine (2e)26
Yield = 46% (81 mg); white solid; mp = 174–175 °C; HRMS (ESI, M+ + H) calcd for C23H17N2O2 353.1290, found 353.1290; 1H NMR (500 MHz, CDCl3): δ 8.39 (d, J = 8.5 Hz, 2H), 8.24–8.18 (m, 4H), 7.90 (d, J = 8.5 Hz, 2H), 7.88 (s, 2H), 7.56–7.51 (m, 4H), 7.51–7.45 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.96 (2x), 148.14, 147.83, 145.44, 139.00 (2x), 129.42 (2x), 128.81 (4x), 128.16 (2x), 127.11 (4x), 124.35 (2x), 116.93 (2x).
3-(4-Nitrobenzyl)-4-(4-nitrophenyl)-2,6-diphenylpyridine (3e)26
Yield = 35% (112 mg); yellow solid; mp = 206–207 °C; HRMS (ESI, M+ + H) calcd for C30H22N3O4 488.1605, found 488.1603; 1H NMR (500 MHz, CDCl3): δ 8.23 (d, J = 8.5 Hz, 2H), 8.13–8.06 (m, 2H), 7.92 (d, J = 8.5 Hz, 2H), 7.62 (s, 1H), 7.51–7.35 (m, 10H), 6.78 (d, J = 8.5 Hz, 2H), 4.17 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 160.80, 155.70, 150.06, 147.77, 147.62, 146.22, 146.10, 140.35, 138.18, 129.50 (2x), 128.88 (2x), 128.83 (2x), 128.73 (2x), 128.42, 128.39 (2x), 127.64, 127.01 (2x), 123.73 (2x), 123.38 (2x), 119.92, 35.15.
2,6-Diphenyl-4-(p-tolyl)pyridine (2f)20c
Yield = 58% (93 mg); white solid; mp = 117–118 °C; HRMS (ESI, M+ + H) calcd for C24H20N 322.1590, found 322.1588; 1H NMR (500 MHz, CDCl3): δ 8.23 (d, J = 8.5 Hz, 4H), 7.90 (s, 2H), 7.68 (d, J = 8.5 Hz, 2H), 7.54 (t, J = 8.0 Hz, 4H), 7.47 (t, J = 7.5 Hz, 2H), 7.36 (d, J = 8.0 Hz, 2H), 2.46 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 157.42 (2x), 150.00, 139.64 (2x), 139.03, 136.03, 129.79 (2x), 128.95 (2x), 128.65 (4x), 127.10 (4x), 126.96 (2x), 116.85 (2x), 21.22.
3-(4-Methylbenzyl)-2,6-diphenyl-4-(p-tolyl)pyridine (3f)26
Yield = 40% (85 mg); white solid; mp = 153–154 °C; HRMS (ESI, M+ + H) calcd for C32H28N 426.2216, found 426.2215; 1H NMR (500 MHz, CDCl3): δ 8.11 (d, J = 8.0 Hz, 2H), 7.64 (s, 1H), 7.54–7.43 (m, 4H), 7.42–7.33 (m, 4H), 7.17 (s, 4H), 6.89 (d, J = 8.0 Hz, 2H), 6.57 (d, J = 8.0 Hz, 2H), 4.06 (s, 2H), 2.39 (s, 3H), 2.25 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.28, 154.39, 152.29, 141.41, 139.16, 138.03, 137.47, 137.15, 134.85, 130.03, 129.14 (2x), 128.86 (2x), 128.72, 128.60 (4x), 128.50 (2x), 128.04 (2x), 127.89 (2x), 127.68, 126.95 (2x), 120.78, 34.55, 21.18, 20.92.
4-(4-Methoxyphenyl)-2,6-diphenylpyridine (2g)21a
Yield = 60% (101 mg); white solid; mp = 101–102 °C; HRMS (ESI, M+ + H) calcd for C24H20NO 338.1545, found 338.1541; 1H NMR (500 MHz, CDCl3): 8.22 (d, J = 7.5 Hz, 4H), 7.87 (s, 2H), 7.72 (d, J = 9.0 Hz, 2H), 7.57–7.50 (m, 4H), 7.49–7.43 (m, 2H), 7.06 (d, J = 9.0 Hz, 2H), 3.89 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.43, 157.41 (2x), 149.59, 139.68 (2x), 131.24, 128.93 (2x), 128.64 (4x), 128.28 (2x), 127.09 (4x), 116.57 (2x), 114.49 (2x), 55.38.
3-(4-Methoxybenzyl)-4-(4-methoxyphenyl)-2,6-diphenylpyridine (3g)26
Yield = 30% (69 mg); yellow solid; mp = 147–148 °C; HRMS (ESI, M+ + H) calcd for C32H28NO2 458.2115, found 458.2112; 1H NMR (500 MHz, CDCl3): δ 8.09 (d, J = 8.0 Hz, 2H), 7.62 (s, 1H), 7.52–7.47 (m, 2H), 7.46–7.42 (m, 2H), 7.41–7.33 (m, 4H), 7.18 (d, J = 9.0 Hz, 2H), 6.89 (d, J = 9.0 Hz, 2H), 6.62 (d, J = 9.0 Hz, 2H), 6.57 (d, J = 9.0 Hz, 2H), 4.02 (s, 2H), 3.83 (s, 3H), 3.73 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.20, 159.15, 157.38, 154.41, 151.90, 141.35, 139.11, 133.25, 132.36, 130.35, 129.77 (2x), 129.11 (2x), 129.07 (2x), 128.72, 128.60 (2x), 127.93 (2x), 127.71, 126.93 (2x), 120.93, 113.59 (2x), 113.31 (2x), 55.28, 55.12, 34.09.
4-([1,1′-Biphenyl]-4-yl)-2,6-diphenylpyridine (2h)20d
Yield = 62% (119 mg); white solid; mp = 136–137 °C; HRMS (ESI, M+ + H) calcd for C29H22N 384.1747, found 384.1744; 1H NMR (500 MHz, CDCl3): δ 8.29–8.19 (m, 4H), 7.95 (s, 2H), 7.84 (d, J = 8.0 Hz, 2H), 7.77 (d, J = 8.0 Hz, 2H), 7.69 (d, J = 7.5 Hz, 2H), 7.59–7.44 (m, 8H), 7.43–7.39 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 157.54 (2x), 149.62, 141.86, 140.28, 139.56 (2x), 137.78, 129.04 (2x), 128.90 (2x), 128.69 (4x), 127.78 (2x), 127.69, 127.54 (2x), 127.12 (4x), 127.10 (2x), 116.89 (2x).
4-([1,1′-Biphenyl]-4-yl)-3-([1,1′-biphenyl]-4-ylmethyl)-2,6-diphenylpyridine (3h)
Yield = 35% (96 mg); yellow solid; mp = 87–88 °C; HRMS (ESI, M+ + H) calcd for C42H32N 550.2529, found 550.2523; 1H NMR (500 MHz, CDCl3): δ 8.16–8.08 (m, 2H), 7.70 (s, 1H), 7.61 (d, J = 8.0 Hz, 2H), 7.58 (d, J = 8.0 Hz, 2H), 7.55–7.49 (m, 4H), 7.46 (t, J = 8.0 Hz, 4H), 7.42–7.28 (m, 12H), 6.73 (d, J = 8.5 Hz, 2H), 4.17 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 160.41, 154.68, 152.03, 141.29, 140.83, 140.65, 140.49, 140.18, 139.04, 138.98, 138.34, 129.90, 129.18 (2x), 129.06 (2x), 128.87, 128.85 (2x), 128.68 (6x), 128.04 (2x), 127.84, 127.52, 127.08 (2x), 127.01 (3x), 126.92 (2x), 126.84 (2x), 126.59, 120.68 (2x), 34.86.
4-(Naphthalen-1-yl)-2,6-diphenylpyridine (2i)19g
Yield = 54% (96 mg); white solid; mp = 251–252 °C; HRMS (ESI, M+ + H) calcd for C27H20N 358.1590, found 358.1587; 1H NMR (500 MHz, CDCl3): δ 8.26–8.21 (m, 4H), 8.00–7.94 (m, 3H), 7.86 (s, 2H), 7.63–7.43 (m, 10H); 13C NMR (125 MHz, CDCl3): δ 156.94 (2x), 150.24, 139.39 (2x), 138.05, 133.79, 130.97, 129.08 (2x), 128.77, 128.71 (4x), 128.50, 127.12 (4x), 126.72, 126.67, 126.17, 125.38 (2x), 120.14 (2x).
4-(Naphthalen-1-yl)-3-(naphthalen-1-ylmethyl)-2,6-diphenylpyridine (3i)
Yield = 43% (107 mg); yellow solid; mp = 208–209 °C; HRMS (ESI, M+ + H) calcd for C38H28N 498.2216, found 498.2209; 1H NMR (500 MHz, CDCl3): δ 8.20–8.13 (m, 2H), 7.77 (d, J = 8.0 Hz, 1H), 7.73 (s, 1H), 7.69–7.61 (m, 4H), 7.53–7.27 (m, 12H), 7.19–7.07 (m, 4H), 6.81 (d, J = 8.0 Hz, 1H), 4.46 (d, J = 16.0 Hz, 1H), 4.18 (d, J = 16.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 160.25, 154.65, 151.05, 141.00, 138.92, 136.86 (2x), 133.30, 133.23, 131.22, 131.02, 130.96, 129.14 (2x), 128.93, 128.67 (2x), 128.24 (2x), 128.03, 128.01 (2x), 127.92, 126.98 (2x), 126.29, 126.25 (2x), 126.10, 125.77, 125.32, 125.23, 125.09, 124.95, 124.78, 123.02, 121.56, 32.76.
2,6-Diphenyl-4-(o-tolyl)pyridine (2j)20c
Yield = 51% (82 mg); white solid; mp = 119–120 °C; HRMS (ESI, M+ + H) calcd for C24H20N 322.1590, found 322.1592; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 8.0 Hz, 4H), 7.68 (s, 2H), 7.52 (t, J = 8.0 Hz, 4H), 7.48–7.42 (m, 2H), 7.35 (d, J = 8.0 Hz, 4H), 2.38 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 156.79 (2x), 151.33, 139.80, 139.47 (2x), 135.14, 130.66, 129.23, 129.01 (2x), 128.69 (4x), 128.34, 127.07 (4x), 126.10, 119.35 (2x), 20.37.
3-(2-Methylbenzyl)-2,6-diphenyl-4-(o-tolyl)pyridine (3j)
Yield = 46% (98 mg); yellow solid; mp = 127–128 °C; HRMS (ESI, M+ + H) calcd for C32H28N 426.2216, found 426.2210; 1H NMR (500 MHz, CDCl3): δ 8.12 (d, J = 7.5 Hz, 2H), 7.57 (dd, J = 1.5, 7.5 Hz, 2H), 7.56 (s, 1H), 7.46 (t, J = 8.0 Hz, 2H), 7.42–7.33 (m, 4H), 7.20 (t, J = 7.5 Hz, 1H), 7.17 (t, J = 7.5 Hz, 1H), 7.06 (t, J = 7.5 Hz, 1H), 6.98–6.91 (m, 3H), 6.89–6.84 (m, 1H), 6.72–6.66 (m, 1H), 3.97 (d, J = 16.0 Hz, 1H), 3.73 (d, J = 16.0 Hz, 1H), 1.97 (s, 3H), 1.71 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.27, 154.48, 152.11, 141.16, 139.09, 139.05, 138.90, 135.83, 135.13, 130.60, 129.94, 129.38, 129.13 (2x), 128.82, 128.67, 128.64 (2x), 128.52, 127.98 (2x), 127.80 (2x), 126.93 (2x), 125.52, 125.47, 125.35, 120.47, 32.17, 19.73, 19.26.
4-(2-Methoxyphenyl)-2,6-diphenylpyridine (2k)19a
Yield = 53% (89 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C24H20NO 338.1545, found 338.1541; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 7.5 Hz, 4H), 7.88 (s, 2H), 7.55–7.49 (m, 4H), 7.48–7.41 (m, 4H), 7.14–7.09 (m, 1H), 7.07 (d, J = 8.5 Hz, 1H), 3.88 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 156.74 (2x), 156.60, 147.87, 139.85 (2x), 130.50, 130.02, 128.78 (2x), 128.60 (4x), 128.46, 127.13 (4x), 121.06, 119.70 (2x), 111.44, 55.67.
3-(2-Methoxybenzyl)-4-(2-methoxyphenyl)-2,6-diphenylpyridine (3k)
Yield = 42% (96 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C32H28NO2 458.2115, found 458.2114; 1H NMR (500 MHz, CDCl3): δ 8.11 (d, J = 8.0 Hz, 2H), 7.59 (s, 1H), 7.57–7.53 (m, 2H), 7.47–7.41 (m, 2H), 7.40–7.30 (m, 4H), 7.28–7.23 (m, 1H), 7.02–6.95 (m, 2H), 6.87–6.81 (m, 2H), 6.66 (s, 1H), 6.65 (s, 1H), 6.51 (d, J = 8.0 Hz, 1H), 4.00 (d, J = 15.0 Hz, 1H), 3.95 (d, J = 15.0 Hz, 1H), 3.67 (s, 3H), 3.49 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 159.94 (2x), 156.50, 156.01, 154.09, 149.44, 141.55, 139.39, 131.65, 130.28, 129.43 (2x), 129.16 (2x), 128.97, 128.76, 128.52 (3x), 127.74 (2x), 127.43, 126.96 (2x), 126.32, 121.24, 120.12, 119.82, 110.40, 109.43, 55.18, 54.96, 28.80.
2,6-Diphenyl-4-(m-tolyl)pyridine (2l)20c
Yield = 53% (85 mg); yellow solid; mp = 84–85 °C; HRMS (ESI, M+ + H) calcd for C24H20N 322.1590, found 322.1588; 1H NMR (500 MHz, CDCl3): δ 8.26–8.20 (m, 4H), 7.90 (s, 2H), 7.5–7.51 (m, 6H), 7.49–7.41 (m, 3H), 7.31 (d, J = 7.5 Hz, 1H), 2.49 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 157.42 (2x), 150.30, 139.61 (2x), 139.02, 138.80, 129.70, 128.99 (2x), 128.67 (5x), 127.86, 127.11 (4x), 124.27, 117.13 (2x), 21.52.
3-(3-Methylbenzyl)-2,6-diphenyl-4-(m-tolyl)pyridine (3l)
Yield = 44% (94 mg); white gum; HRMS (ESI, M+ + H) calcd for C32H28N 426.2216, found 426.2209; 1H NMR (500 MHz, CDCl3): δ 8.13 (d, J = 8.0 Hz, 2H), 7.65 (s, 1H), 7.59–7.51 (m, 2H), 7.51–7.44 (m, 2H), 7.44–7.34 (m, 4H), 7.29–7.23 (m, 1H), 7.18 (d, J = 7.5 Hz, 1H), 7.08 (d, J = 7.5 Hz, 1H), 7.01 (s, 1H), 6.96 (d, J = 8.0 Hz, 1H), 6.87 (d, J = 7.0 Hz, 1H), 6.48 (d, J = 7.5 Hz, 1H), 6.42 (s, 1H), 4.06 (s, 2H), 2.33 (s, 3H), 2.17 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.15, 154.35, 152.44, 141.38, 140.96, 139.96, 139.09, 137.74, 137.25, 130.13, 129.45, 129.20 (4x), 128.74, 128.59 (2x), 128.31, 128.00, 127.94 (2x), 127.70, 126.93 (2x), 126.13, 125.55, 125.32, 120.59, 35.01, 21.30, 21.20.
4-(3-Methoxyphenyl)-2,6-diphenylpyridine (2m)20c
Yield = 48% (81 mg); white solid; mp = 124–125 °C; HRMS (ESI, M+ + H) calcd for C24H20NO 338.1539, found 338.1538; 1H NMR (500 MHz, CDCl3): δ 8.23 (d, J = 7.5 Hz, 4H), 7.90 (s, 2H), 7.54 (t, J = 7.5 Hz, 4H), 7.50–7.43 (m, 3H), 7.35 (d, J = 7.5 Hz, 1H), 7.28 (t, J = 2.0 Hz, 1H), 7.03 (dd, J = 2.0, 8.0 Hz, 1H), 3.92 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.14 157.46 (2x), 150.06, 140.53, 139.53 (2x), 130.14, 129.02 (2x), 128.67 (4x), 127.10 (4x), 119.57, 117.13 (2x), 114.17, 112.99, 55.40.
3-(3-Methoxybenzyl)-4-(3-methoxyphenyl)-2,6-diphenylpyridine (3m)
Yield = 41% (94 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C32H28NO2 458.2115, found 458.2114; 1H NMR (500 MHz, CDCl3): δ 8.11 (d, J = 7.5 Hz, 2H), 7.65 (s, 1H), 7.54 (d, J = 7.0 Hz, 2H), 7.46 (t, J = 7.0 Hz, 2H), 7.43–7.33 (m, 4H), 7.28 (t, J = 8.0 Hz, 1H), 7.00 (t, J = 8.0 Hz, 1H), 6.90 (dd, J = 2.0, 8.0 Hz, 1H), 6.87 (d, J = 7.5 Hz, 1H), 6.74 (s, 1H), 6.61 (dd, J = 1.0, 8.0 Hz, 1H), 6.31 (d, J = 7.5 Hz, 1H), 6.24 (s, 1H), 4.07 (s, 2H), 3.66 (s, 3H), 3.65 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.22, 159.30, 159.23, 154.59, 152.20, 142.83, 141.28, 139.02, 129.56, 129.28, 129.17 (2x), 128.87 (2x), 128.82, 128.62 (2x), 127.99 (2x), 127.79, 126.97 (2x), 120.92, 120.81, 120.52, 113.90 (2x), 113.79, 111.19, 55.06, 55.02, 35.14.
4-(3,4-Dimethoxyphenyl)-2,6-diphenylpyridine (2n)22e
Yield = 51% (92 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C25H22NO2 368.1645, found 368.1641; 1H NMR (500 MHz, CDCl3): δ 8.24–8.17 (m, 4H), 7.85 (s, 2H), 7.56–7.50 (m, 4H), 7.48–7.43 (m, 2H), 7.33 (dd, J = 2.5, 8.5 Hz, 1H), 7.24 (d, J = 2.0 Hz, 1H), 7.02 (d, J = 8.5 Hz, 1H), 4.01 (s, 3H), 3.97 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 157.46 (2x), 149.94 (2x), 149.43, 139.64 (2x), 131.78, 128.98 (2x), 128.66 (4x), 127.10 (4x), 119.78, 116.79 (2x), 111.54, 110.15, 56.10, 56.01.
3-(3,4-Dimethoxybenzyl)-4-(3,4-dimethoxyphenyl)-2,6-diphenylpyridine (3n)26
Yield = 32% (83 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C34H32NO4 518.2326, found 518.2328; 1H NMR (500 MHz, CDCl3): δ 8.10 (d, J = 7.5 Hz, 2H), 7.65 (s, 1H), 7.59–7.50 (m, 2H), 7.45 (t, J = 8.0 Hz, 2H), 7.41–7.33 (m, 4H), 6.88 (d, J = 8.0 Hz, 1H), 6.84 (dd, J = 2.0, 8.0 Hz, 1H), 6.74 (d, J = 8.0 Hz, 1H), 6.63 (d, J = 8.0 Hz, 1H), 6.26 (dd, J = 2.0, 8.0 Hz, 1H), 6.23 (d, J = 2.0 Hz, 1H), 4.03 (s, 2H), 3.91 (s, 3H), 3.79 (s, 3H), 3.67 (s, 3H), 3.65 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.12, 154.57, 152.05, 148.63, 148.51, 148.40, 146.97, 141.36, 139.05, 134.17, 132.65, 130.00, 129.19 (2x), 128.82, 128.63 (2x), 127.99 (2x), 127.81, 126.94 (2x), 121.00, 120.83, 120.31, 111.98, 111.61, 110.86 (2x), 55.90, 55.84, 55.65, 55.61, 34.68.
4-(Naphthalen-2-yl)-2,6-diphenylpyridine (2o)22e
Yield = 95% (170 mg); white solid; mp = 128–129 °C; HRMS (ESI, M+ + H) calcd for C27H20N 358.1590, found 358.1590; 1H NMR (500 MHz, CDCl3): δ 8.30–8.21 (m, 5H), 8.05–7.96 (m, 4H), 7.95–7.90 (m, 1H), 7.87 (dd, J = 1.5, 8.0 Hz, 1H), 7.60–7.52 (m, 6H), 7.51–7.45 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.56 (2x), 150.07, 139.59 (2x), 136.27, 133.49, 133.40, 129.05 (2x), 128.91, 128.70 (4x), 128.42, 127.74, 127.15 (4x), 126.74, 126.68, 126.45, 124.81, 117.28 (2x).
4-(Anthracen-9-yl)-2,6-diphenylpyridine (2p)
Yield = 83% (169 mg); yellow solid; mp = 222–223 °C; HRMS (ESI, M+ + H) calcd for C31H22N 408.1747, found 408.1745; 1H NMR (500 MHz, CDCl3): δ 8.58 (s, 1H), 8.28–8.22 (m, 4H), 8.10 (d, J = 8.5 Hz, 2H), 7.84 (s, 2H), 7.75 (d, J = 9.0 Hz, 2H), 7.54–7.48 (m, 6H), 7.47–7.43 (m, 2H), 7.42–7.37 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.03 (2x), 148.90, 139.23 (2x), 134.30, 131.29 (2x), 129.53 (2x), 129.20 (2x), 128.74 (4x), 128.53 (2x), 127.48, 127.13 (4x), 126.15 (2x), 126.07 (2x), 125.35 (2x), 121.39 (2x).
4-(Benzo[d][1,3]dioxol-5-yl)-2,6-diphenylpyridine (2q)20d
Yield = 85% (149 mg); white solid; mp = 152–153 °C; HRMS (ESI, M+ + H) calcd for C24H18NO2 352.1332, found 352.1333; 1H NMR (500 MHz, CDCl3): δ 8.20–8.18 (m, 4H), 7.82 (s, 2H), 7.55–7.48 (m, 4H), 7.47–7.42 (m, 2H), 7.28–7.21 (m, 2H), 6.96 (d, J = 8.0 Hz, 1H), 6.06 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 157.46 (2x), 149.71, 148.47, 148.44, 139.55 (2x), 133.12, 129.02 (2x), 128.69 (4x), 127.09 (4x), 121.06, 116.76 (2x), 108.86, 107.45, 101.49. Single-crystal X-ray diagram: crystal of 2q was grown by slow diffusion of EtOAc into a solution of 2q in CH2Cl2 to yield colorless prisms. The compound crystallizes in the monoclinic crystal system, space group P21/n, a = 6.4058(2) Å, b = 12.1092(3) Å, c = 21.6453(7) Å, V = 1677.71(9) Å3, Z = 4, dcalcd = 1.391 mg m−3, F(000) = 736, 2θ range 1.927–27.102, R indices (all data) R1 = 0.0412, wR2 = 0.0956. CCDC number is 2085356.
2,6-Bis(4-fluorophenyl)-4-phenylpyridine (2r)20c
Yield = 45% (77 mg); white solid; mp = 175–176 °C; HRMS (ESI, M+ + H) calcd for C23H16F2N 344.1245, found 344.1242; 1H NMR (500 MHz, CDCl3): δ 8.20–8.16 (m, 4H), 7.82 (d, J = 6.0 Hz, 2H), 7.73 (d, J = 8.0 Hz, 2H), 7.57–7.52 (m, 2H), 7.51–7.47 (m, 1H), 7.20 (t, J = 8.5 Hz, 4H); 13C NMR (125 MHz, CDCl3): δ 163.64 (d, J = 247.125 Hz, 2x), 156.46 (2x), 150.44, 138.84 (2x), 135.57 (d, J = 2.75 Hz, 2x), 129.15 (2x), 129.11, 128.89 (d, J = 8.2 Hz, 4x), 127.14 (2x), 116.67, 115.62 (d, J = 21.3625 Hz, 4x).
3-Benzyl-2,6-bis(4-fluorophenyl)-4-phenylpyridine (3r)
Yield = 41% (89 mg); colorless gum; HRMS (ESI, M+ + H) calcd for C30H22F2N 434.1715, found 434.1710; 1H NMR (500 MHz, CDCl3): δ 8.10–8.03 (m, 2H), 7.59 (s, 1H), 7.47–7.40 (m, 2H), 7.38–7.31 (m, 3H), 7.25–7.21 (m, 2H), 7.16–7.10 (m, 2H), 7.08–7.00 (m, 5H), 6.66–6.61 (m, 2H), 4.06 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 163.51 (d, J = 246.75 Hz), 162.54 (d, J = 245.5 Hz), 159.40, 153.59, 152.60, 140.70, 139.75, 137.24 (d, J = 3.125 Hz), 135.07 (d, J = 2.75 Hz), 130.82 (d, J = 8.0 Hz, 2x), 129.88, 128.74 (d, J = 8.25 Hz, 2x), 128.48 (2x), 128.27 (2x), 128.13 (2x), 128.01 (2x), 127.86, 125.65, 120.43, 115.57 (d, J = 21.5 Hz, 2x), 114.92 (d, J = 21.375 Hz, 2x), 35.04.
2,6-Bis(4-chlorophenyl)-4-phenylpyridine (2s)20c
Yield = 31% (58 mg); colorless solid; mp = 186–187 °C; HRMS (ESI, M+ + H) calcd for C23H16Cl2N 376.0654, found 376.0654; 1H NMR (500 MHz, CDCl3): δ 8.12 (d, J = 8.5 Hz, 4H), 7.84 (s, 2H), 7.73–7.71 (m, 2H), 7.56–7.49 (m, 7H); 13C NMR (125 MHz, CDCl3): δ 156.31 (2x), 150.54, 138.67, 137.75 (2x), 135.27 (2x), 129.17 (3x), 128.90 (4x), 128.33 (4x), 127.14 (2x), 117.06 (2x).
3-Benzyl-2,6-bis(4-chlorophenyl)-4-phenylpyridine (3s)
Yield = 45% (47 mg); white solid; mp = 169–170 °C; HRMS (ESI, M+ + H) calcd for C30H22Cl2N 466.1124, found 466.1120; 1H NMR (500 MHz, CDCl3): δ 8.02 (d, J = 8.5 Hz, 2H), 7.61 (s, 1H), 7.41 (d, J = 8.5 Hz, 2H), 7.39 (d, J = 8.5 Hz, 2H), 7.35–7.31 (m, 5H), 7.24–7.21 (m, 2H), 7.08–7.04 (m, 3H), 6.68–6.59 (m, 2H), 4.05 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 159.28, 153.43, 152.70, 140.57, 139.59, 139.54, 137.26, 135.07, 133.99, 130.44 (2x), 130.23, 128.85 (2x), 128.46 (2x), 128.29 (2x), 128.21 (2x), 128.19 (2x), 128.13 (2x), 128.06 (2x), 127.93, 125.71, 120.63, 35.02.
4-Phenyl-2,6-di-p-tolylpyridine (2t)20c
Yield = 53% (89 mg); colorless solid; mp = 159–160 °C; HRMS (ESI, M+ + H) calcd for C25H22N 336.1747, found 336.1744; 1H NMR (500 MHz, CDCl3): δ 8.12 (d, J = 8.0 Hz, 4H), 7.85 (s, 2H), 7.76–7.74 (m, 2H), 7.55–7.52 (m, 2H), 7.49–7.47 (m, 1H), 7.33 (d, J = 8.0 Hz, 4H), 2.45 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 157.38 (2x), 149.99, 139.26, 138.93 (2x), 136.88 (2x), 129.38 (4x), 129.04 (2x), 128.83, 127.16 (2x), 126.97 (4x), 116.49 (2x), 21.30 (2x).
3-Benzyl-4-phenyl-2,6-di-p-tolylpyridine (3t)
Yield = 42% (89 mg); white solid; mp = 149–150 °C; HRMS (ESI, M+ + H) calcd for C32H28N 426.2216, found 426.2219; 1H NMR (500 MHz, CDCl3): δ 7.99 (d, J = 7.5 Hz, 2H), 7.57 (s, 1H), 7.40 (d, J = 7.5 Hz, 2H), 7.34–7.28 (m, 3H), 7.24 (d, J = 7.5 Hz, 2H), 7.21–7.18 (m, 2H), 7.17 (d, J = 7.5 Hz, 2H), 7.07–6.99 (m, 3H), 6.68–6.61 (m, 2H), 4.07 (s, 2H), 2.39 (s, 3H), 2.37 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.17, 154.48, 152.24, 141.17, 140.19, 138.71, 138.54, 137.44, 136.31, 129.43, 129.32 (2x), 129.08 (2x), 128.66 (2x), 128.52 (2x), 128.24 (2x), 128.10 (2x), 127.85 (2x), 127.58, 126.82 (2x), 125.41, 120.16, 35.10, 21.27, 21.24.
2,6-Bis(4-methoxyphenyl)-4-phenylpyridine (2u)21a
Yield = 62% (114 mg); white solid; mp = 131–132 °C; HRMS (ESI, M+ + H) calcd for C25H22NO2 368.1645, found 368.1645; 1H NMR (500 MHz, CDCl3): δ 8.20 (d, J = 9.0 Hz, 4H), 7.79 (s, 2H), 7.75 (d, J = 7.5 Hz, 2H), 7.58.7.52 (m, 2H), 7.51–7.46 (m, 1H), 7.07 (d, J = 8.0 Hz, 4H), 3.89 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 160.40 (2x), 156.81 (2x), 149.84, 139.21, 132.23 (2x), 128.94 (2x), 128.72, 128.27 (4x), 127.05 (2x), 115.53 (2x), 113.93 (4x), 55.24 (2x).
3-Benzyl-2,6-bis(4-methoxyphenyl)-4-phenylpyridine (3u)
Yield = 25% (57 mg); white solid; mp = 117–118 °C; HRMS (ESI, M+ + H) calcd for C32H28NO2 458.2115, found 458.2119; 1H NMR (500 MHz, CDCl3): δ 8.12 (d, J = 8.5 Hz, 2H), 7.60 (s, 1H), 7.53 (d, J = 8.5 Hz, 2H), 7.39–7.33 (m, 3H), 7.30–7.23 (m, 2H), 7.15–7.06 (m, 3H), 7.02 (d, J = 9.0 Hz, 2H), 6.95 (d, J = 9.0 Hz, 2H), 6.77–6.70 (m, 2H), 4.14 (s, 2H), 3.87 (s, 3H), 3.85 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.28, 159.65, 159.22, 154.04, 152.22, 141.15, 140.14, 133.92, 131.68, 130.39 (2x), 128.87, 128.44 (2x), 128.14 (4x), 128.03 (2x), 127.84 (2x), 127.51, 125.39, 119.51, 113.89 (2x), 113.33 (2x), 55.20, 55.18, 35.06.
2,6-Di([1,1′-biphenyl]-4-yl)-4-phenylpyridine (2v)20c
Yield = 46% (106 mg); white solid; mp = 180–181 °C; HRMS (ESI, M+ + H) calcd for C35H26N 460.2060, found 460.2058; 1H NMR (500 MHz, CDCl3): δ 8.32 (d, J = 8.0 Hz, 4H), 7.95 (s, 2H), 7.82–7.75 (m, 6H), 7.70 (d, J = 8.0 Hz, 4H), 7.59–7.53 (m, 2H), 7.53–7.46 (m, 5H), 7.43–7.37 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 157.12 (2x), 150.23 (2x), 141.81 (2x), 140.68 (2x), 139.06, 138.47 (2x), 129.13 (2x), 129.00 (2x), 128.83 (4x), 127.52 (4x), 127.43 (4x), 127.20 (2x), 127.12 (4x), 117.04 (2x).
2,6-Di([1,1′-biphenyl]-4-yl)-3-benzyl-4-phenylpyridine (3v)
Yield = 43% (118 mg); white solid; mp = 182–183 °C; HRMS (ESI, M+ + H) calcd for C42H32N 550.2529, found 550.2537; 1H NMR (500 MHz, CDCl3): δ 8.21 (d, J = 8.5 Hz, 2H), 7.73–7.69 (m, 3H), 7.68–7.62 (m, 4H), 7.61 (s, 4H), 7.49–7.43 (m, 4H), 7.39–7.33 (m, 5H), 7.29–7.23 (m, 2H), 7.10–7.04 (m, 3H), 6.73–0.6.66 (m, 2H), 4.15 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 160.03, 154.19, 152.44, 141.55, 140.99, 140.91, 140.66, 140.62, 140.27, 139.99, 137.94, 130.00, 129.60 (2x), 128.78 (2x), 128.75 (2x), 128.54 (2x), 128.27 (2x), 128.20 (2x), 127.95 (2x), 127.73, 127.44, 127.36 (2x), 127.34 (2x), 127.30, 127.14 (2x), 127.07 (2x), 126.79 (2x), 125.53, 120.58, 35.17.
2,6-Bis(3-fluorophenyl)-4-phenylpyridine (2w)16c
Yield = 60% (103 mg); white solid; mp = 147–148 °C; HRMS (ESI, M+ + H) calcd for C23H16F2N 344.1245, found 344.1254; 1H NMR (500 MHz, CDCl3): δ 7.98–7.92 (m, 4H), 7.89 (s, 2H), 7.77–7.72 (m, 2H), 7.58–7.53 (m, 2H), 7.52–7.45 (m, 3H), 7.18–7.13 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 163.35 (d, J = 243.75 Hz, 2x), 156.19 (d, J = 2.25 Hz, 2x), 150.62, 141.62 (d, J = 7.375 Hz, 2x), 138.59, 130.20 (d, J = 8.125 Hz, 2x), 129.22, 129.20 (2x), 127.15 (2x), 122.56 (d, J = 2.375 Hz, 2x), 117.62 (2x), 116.00 (d, J = 21.125 Hz, 2x), 114.06 (d, J = 22.75 Hz, 2x).
3-Benzyl-2,6-bis(3-fluorophenyl)-4-phenylpyridine (3w)
Yield = 35% (76 mg); white solid; mp = 102–103 °C; HRMS (ESI, M+ + H) calcd for C30H22NF2 434.1715, found 434.1724; 1H NMR (500 MHz, CDCl3): δ 7.86–7.80 (m, 2H), 7.63 (s, 1H), 7.44–7.38 (m, 1H), 7.37–7.33 (m, 3H), 7.32–7.28 (m, 1H), 7.25–7.21 (m, 3H), 7.19–7.15 (m, 1H), 7.11–7.02 (m, 5H), 6.65–6.59 (m, 2H), 4.08 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 163.32 (d, J = 243.875 Hz), 162.48 (d, J = 244.375 Hz), 159.12, 153.29, 152.74, 143.17 (d, J = 7.5 Hz), 141.19 (d, J = 7.375 Hz), 140.49, 139.56, 130.63, 130.15 (d, J = 8.125 Hz), 129.53 (d, J = 8.125 Hz), 128.48 (2x), 128.31 (2x), 128.14 (2x), 128.03 (2x), 127.95, 125.71, 124.78 (d, J = 2.625 Hz), 122.42 (d, J = 2.375 Hz), 121.03, 116.32 (d, J = 22.0 Hz), 115.80 (d, J = 21.375 Hz), 114.84 (d, J = 21.0 Hz), 113.92 (d, J = 22.75 Hz), 35.01.
4-Phenyl-2,6-di-o-tolylpyridine (2x)21a
Yield = 31% (52 mg); white solid; mp = 133–134 °C; HRMS (ESI, M+ + H) calcd for C25H22N 336.1747, found 336.1747; 1H NMR (500 MHz, CDCl3): δ 7.74–7.72 (m, 2H), 7.60 (s, 2H), 7.54–7.49 (m, 4H), 7.48–7.43 (m, 1H), 7.34–7.27 (m, 6H), 2.49 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 160.09 (2x), 148.79, 140.72 (2x), 138.49, 135.90 (2x), 130.68 (2x), 129.83 (2x), 129.13 (2x), 129.02, 128.24 (2x), 127.12 (2x), 125.83 (2x), 120.11 (2x), 20.62 (2x).
3-Benzyl-4-phenyl-2,6-di-o-tolylpyridine (3x)
Yield = 52% (111 mg); colorless gum; HRMS (ESI, M+ + H) calcd for C32H28N 426.2216, found 426.2220; 1H NMR (500 MHz, CDCl3): δ 7.51–7.46 (m, 1H), 7.43–7.32 (m, 6H), 7.29–7.18 (m, 5H), 7.17–7.02 (m, 2H), 7.04–6.97 (m, 3H), 6.56–6.48 (m, 2H), 4.00 (d, J = 15.0 Hz, 1H), 3.97 (d, J = 15.0 Hz, 1H), 2.45 (s, 3H), 1.99 (s, 3H); 13C NMR (125 MHz, CDCl3): δ 160.42, 157.27, 151.27, 140.34, 140.21, 140.19, 139.86, 136.06, 135.93, 30.66, 130.20, 130.06, 129.78, 128.98, 128.76 (2x), 128.38 (2x), 128.33 (2x), 128.08, 127.77 (2x), 127.71 (2x), 125.75, 125.47, 125.32, 124.02, 34.78, 20.60, 19.54.
2,6-Bis(2-bromophenyl)-4-phenylpyridine (2y)19g
Yield = 52% (120 mg); white solid; mp = 150–151 °C; HRMS (ESI, M+ + H) calcd for C23H16Br2N 463.9644, found 463.9644; 1H NMR (500 MHz, CDCl3): δ 7.85 (s, 2H), 7.78–7.75 (m, 2H), 7.74–7.70 (m, 4H), 7.55–7.50 (m, 2H), 7.49–7.46 (m, 1H), 7.45–7.41 (m, 2H), 7.27 (td, J = 2.0, 8.0 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 158.39 (2x), 148.20, 141.18 (2x), 138.11, 133.29 (2x), 131.75 (2x), 129.76 (2x), 129.13 (3x), 127.58 (2x), 127.21 (2x), 121.94 (2x), 121.41 (2x).
3-Benzyl-2,6-bis(2-bromophenyl)-4-phenylpyridine (3y)
Yield = 40% (111 mg); yellow gum; HRMS (ESI, M+ + H) calcd for C30H22Br2N 554.0114, found 554.0108; 1H NMR (500 MHz, CDCl3): δ 7.69 (dd, J = 2.0, 7.5 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 8.0 Hz, 1H), 7.59 (s, 1H), 7.42–7.31 (m, 6H), 7.25–7.13 (m, 4H), 7.05–6.99 (m, 3H), 6.63–6.56 (m, 2H), 4.13 (d, J = 16.0 Hz, 1H), 3.88 (d, J = 16.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 159.31, 155.56, 151.08, 141.27, 140.83, 140.06, 139.41, 133.21, 132.49, 131.78, 131.19, 131.12, 129.61, 129.30, 128.70 (2x), 128.34 (2x), 128.28 (2x), 127.89, 127.84 (2x), 127.53, 127.03, 125.55, 125.26, 122.95, 121.98, 35.03.
4-Phenyl-2,6-di(thiophen-3-yl)pyridine (2z)20c
Yield = 52% (83 mg); yellow solid; mp = 135–136 °C; HRMS (ESI, M+ + H) calcd for C19H14NS2 320.0562, found 320.0561; 1H NMR (500 MHz, CDCl3): δ 8.08 (dd, J = 1.5, 3.0 Hz, 2H), 7.84 (dd, J = 1.5, 5.0 Hz, 2H), 7.76–7.72 (m, 4H), 7.59–7.54 (m, 2H), 7.52–7.48 (m, 1H), 7.45 (dd, J = 3.0, 5.0 Hz, 2H); 13C NMR (125 MHz, CDCl3): δ 153.64 (2x), 150.15, 142.44 (2x), 138.90, 129.09 (2x), 128.98, 127.10 (2x), 126.45 (2x), 126.14 (2x), 123.78 (2x), 116.60 (2x).
3-Benzyl-4-phenyl-2,6-di(thiophen-3-yl)pyridine (3z)
Yield = 46% (94 mg); yellow solid; mp = 138–139 °C; HRMS (ESI, M+ + H) calcd for C26H20NS2 410.1032, found 410.1024; 1H NMR (500 MHz, CDCl3): δ 7.97 (dd, J = 1.5, 3.0 Hz, 1H), 7.73 (dd, J = 1.5, 5.0 Hz, 1H), 7.49 (s, 1H), 7.43–7.36 (m, 3H), 7.35–7.28 (m, 4H), 7.21–7.08 (m, 5H), 6.81 (d, J = 7.0 Hz, 2H), 4.12 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 155.17, 152.65, 150.95, 142.01, 141.77, 141.19, 139.81, 129.47, 129.00, 128.40 (2x), 128.26 (2x), 128.14 (2x), 128.12, 127.77 (2x), 126.35, 126.11, 125.75, 124.83, 124.70, 123.59, 120.34, 35.40.
2,6-Di(furan-2-yl)-4-phenylpyridine (2aa)21a
Yield = 70% (100 mg); brown solid; mp = 118–119 °C; HRMS (ESI, M+ + H) calcd for C19H14NO2 288.1025, found 288.1033; 1H NMR (500 MHz, CDCl3): δ 7.81 (s, 2H), 7.78–7.74 (m, 2H), 7.58–7.55 (m, 2H), 7.54–7.50 (m, 2H), 7.49–7.44 (m, 1H), 7.20 (dd, J = 1.0, 3.0 Hz, 2H), 6.58–6.55 (m, 2H); 13C NMR (125 MHz, CDCl3): δ 153.77 (2x), 149.77, 149.69 (2x), 143.28 (2x), 138.38, 129.11, 129.04 (2x), 127.03 (2x), 114.80 (2x), 112.05 (2x), 109.09 (2x).
3-Benzyl-2,6-di(furan-2-yl)-4-phenylpyridine (3aa)
Yield = 15% (28 mg); brown gum; HRMS (ESI, M+ + H) calcd for C26H20NO2 378.1494, found 378.1489; 1H NMR (500 MHz, CDCl3): δ 7.54 (s, 1H), 7.54–7.52 (m, 1H), 7.49–7.47 (m, 1H), 7.36–7.30 (m, 3H), 7.20–7.16 (m, 2H), 7.16–7.07 (m, 4H), 6.87–6.82 (m, 3H), 6.56–6.53 (m, 1H), 6.44–6.41 (m, 1H), 4.29 (s, 2H); 13C NMR (125 MHz, CDCl3): δ 153.50 (2x), 153.14, 149.22, 147.05, 143.27, 142.98, 140.98, 139.50, 128.50 (2x), 128.38, 128.18 (2x), 128.15 (2x), 128.03 (2x), 127.90, 125.62, 118.78, 111.98, 111.83, 111.40, 108.85, 34.92.
2,6-Bis(2-methoxyphenyl)-4-phenylpyridine (2ab)21c
Yield = 44% (129 mg); white solid; mp = 133–134 °C; HRMS (ESI, M+ + H) calcd for C25H22NO2 368.1651, found 368.1642; 1H NMR (500 MHz, CDCl3): δ 8.00 (s, 2H), 7.96 (dd, J = 1.5, 7.5 Hz, 2H), 7.76–7.72 (m, 2H), 7.54–7.49 (m, 2H), 7.47–7.42 (m, 1H), 7.42–7.36 (m, 2H), 7.14–7.09 (m, 2H), 7.04 (d, J = 8.5 Hz, 2H), 3.90 (s, 6H); 13C NMR (125 MHz, CDCl3): δ 157.08 (2x), 155.97 (2x), 147.66, 139.48, 131.56 (2x), 129.72 (2x), 129.62, 128.91 (2x), 128.51 (2x), 127.34 (2x), 121.41 (2x), 121.05 (2x), 111.41 (2x), 55.72 (2x).
1,3,5,8-Tetraphenyl-2,6-diazabicyclo[2.2.2]oct-2-ene (4)
A mixture of chalcone 1 (1.0 mmol), hexamethyldisilane (0.5 mL, 2.4 mmol), tin(ii) trifluoromethanesulfonate (0.2 mmol) in dichloromethane (2 mL) was taken in a dried 35 mL microwave vial at 25 °C. The mixture was treated in a microwave irradiation instrument and stirred at 150 °C for 1 h. The consumption of starting materials were confirmed by TLC. The reaction was cooled to 25 °C, the mixture of crude product was transferred to a 100 mL round bottom flask, and the solvent was concentrated. The residue was diluted with water (10 mL) and the mixture was extracted with CH2Cl2 (3 × 15 mL). The combined organic layers were washed with brine, dried, filtered and evaporated to afford crude product under reduced pressure. Purification on silica gel (hexanes/EtOAc = 4/1–1/1) afforded compound 4. Yield = 78% (162 mg); colorless solid; mp = 176–177 °C; HRMS (ESI, M+ + Na) calcd for C30H26N2Na 437.1988, found 437.1980; 1H NMR (500 MHz, CDCl3): δ 8.27 (d, J = 7.5 Hz, 2H), 7.81 (d, J = 8.0 Hz, 2H), 7.69 (d, J = 8.0 Hz, 2H), 7.58–7.47 (m, 4H), 7.46–7.32 (m, 5H), 7.16–7.04 (m, 3H), 6.96–6.83 (m, 2H), 4.16 (d, J = 1.5 Hz, 1H), 3.64 (d, J = 1.5 Hz, 1H), 3.29 (dd, J = 5.5, 10.0 Hz, 1H), 2.76 (dd, J = 10.0, 13.0 Hz, 1H), 1.97 (br s, 1H), 1.89 (dd, J = 5.5, 13.0 Hz, 1H); 13C NMR (125 MHz, CDCl3): δ 173.82, 145.40, 144.05, 142.35, 137.50, 130.21, 128.47 (2x), 128.35 (2x), 128.29 (2x), 128.20 (2x), 127.49 (2x), 127.38, 127.07 (3x), 126.87 (2x), 126.54 (2x), 126.29, 75.05, 57.69, 47.42, 42.91, 34.98. Single-crystal X-ray analysis: crystals of 4 were grown by slow diffusion of EtOAc into a solution of 4 in CH2Cl2 to yield colorless prisms. The compound crystallizes in the monoclinic crystal system, space group P1̄, a = 9.8179(3) Å, b = 10.4973(3) Å, c = 11.9206(3) Å, V = 1094.83(5) Å3, Z = 2, dcalcd = 1.257 mg m−3, F(000) = 440, 2θ range 1.761–30.034, R indices (all data) R1 = 0.0529, wR2 = 0.1131. CCDC number is 2085361.
Conflicts of interest
The authors declare no conflicts of interest.
Supplementary Material
Acknowledgments
The authors thank the Academia Sinica (AS-TP-111-M01; AS-SUMMIT-110) and Ministry of Science and Technology (MOST 110-2113-M-001-025-; MOST 110-2811-M-001-644-) for financial support. Chieh-Kai Chan acknowledges Postdoctoral Scholar Program from Academia Sinica.
Electronic supplementary information (ESI) available: Experimental procedures, characterisation data, copies of 1H and 13C NMR spectra, and crystallographic data for 2q and 4. CCDC 2085356 and 2085361. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d2ra00084a
Notes and references
- (a) Balasubramanian M. and Keay J. G., in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. V. F. Scriven, Pergamon Press, London, 1996, chapter 6, vol. 5, pp. 245–300 [Google Scholar]; (b) Watson Z. C. Bampos N. Sanders J. K. M. New J. Chem. 1998:1135. doi: 10.1039/A805504A. [DOI] [Google Scholar]; (c) Michael J. P. Nat. Prod. Rep. 2005;22:627–646. doi: 10.1039/B413750G. [DOI] [PubMed] [Google Scholar]; (d) Abass M. Heterocycles. 2005;65:901–965. doi: 10.3987/REV-04-592. [DOI] [Google Scholar]; (e) Bull J. A. Mousseau J. J. Pelletier G. Charette A. B. Chem. Rev. 2012;112:2642–2713. doi: 10.1021/cr200251d. [DOI] [PubMed] [Google Scholar]; (f) Desimoni G. Faita G. Quadrelli P. Chem. Rev. 2014;114:6081–6129. doi: 10.1021/cr4007208. [DOI] [PubMed] [Google Scholar]
- Px: ; (a) Lanza P. L. Pack M. F. Li Z. Krajewski S. A. Blank M. A. Aliment. Pharmacol. Ther. 2000;14:1663. doi: 10.1046/j.1365-2036.2000.00887.x. [DOI] [PubMed] [Google Scholar]; (b) Carey J. S. Laffan D. Thomson C. Williams M. T. Org. Biomol. Chem. 2006;4:2337–2347. doi: 10.1039/B602413K. [DOI] [PubMed] [Google Scholar]; (c) Fisher D. F. Sarpong R. J. Am. Chem. Soc. 2010;132:5926–5927. doi: 10.1021/ja101893b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yuan C. Chang C.-T. Axelrod A. Siegel D. J. Am. Chem. Soc. 2010;132:5924–5925. doi: 10.1021/ja101956x. [DOI] [PubMed] [Google Scholar]; (e) Roughley S. D. Jordan A. M. J. Med. Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]; (f) Roughley S. D. Jordan A. M. J. Med. Chem. 2011;54:3451–3479. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]; (g) Whitehead T. P. Havel C. Metayer C. Benowitz N. L. Jacob P. Chem. Res. Toxicol. 2015;28:1007–1014. doi: 10.1021/acs.chemrestox.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Prachayasittikul S. Pingaew R. Worachartcheewan A. Sinthupoom N. Prachayasittikul V. Ruchirawat S. Prachayasittikul V. Mini-Rev. Med. Chem. 2017;17:869–901. doi: 10.2174/1389557516666160923125801. [DOI] [PubMed] [Google Scholar]
- Agrochemicals: ; (a) Nauha E. Kolehmainen E. Nissinen M. CrystEngComm. 2011;13:6531–6537. doi: 10.1039/C1CE05730H. [DOI] [Google Scholar]; (b) Guan A. Liu C. Chen W. Yang F. Xie Y. Zhang J. Li Z. Wang M. J. Agric. Food Chem. 2017;65:1272–1280. doi: 10.1021/acs.jafc.6b05580. [DOI] [PubMed] [Google Scholar]; (c) Burriss A. Edmunds A. J. F. Emery D. Hall R. G. Jacob O. Schaetzer J. Pest Manage. Sci. 2018;74:1228–1238. doi: 10.1002/ps.4806. [DOI] [PubMed] [Google Scholar]
- Electrochemicals: ; (a) Momblona C. Etrl C. D. Pertegas A. Junquera-Hernandez J. M. Bolink H. J. Constable E. C. Sessolo M. Orti E. Housecroft C. E. J. Mater. Chem. C. 2018;6:12679–12688. doi: 10.1039/C8TC04727H. [DOI] [Google Scholar]; (b) McMillion N. D. Wilson A. W. Goetz M. K. Chang M.-C. Lin C.-C. Feng W.-J. McCrory C. C. L. Anderson J. S. Inorg. Chem. 2019;58:1391–1397. doi: 10.1021/acs.inorgchem.8b02942. [DOI] [PubMed] [Google Scholar]; (c) Shi H. Wang R. Lou M. Jia D. Guo Y. Wang X. Huang Y. Sun Z. Wang T. Wang L. Electrochim. Acta. 2019;294:93–101. doi: 10.1016/j.electacta.2018.10.061. [DOI] [Google Scholar]
- Materials: ; (a) Su S. J. Chiba T. Takeda T. Kido J. Adv. Mater. 2008;20:2125–2130. doi: 10.1002/adma.200701730. [DOI] [Google Scholar]; (b) Sasabe H. Kido J. Chem. Mater. 2011;23:621–630. doi: 10.1021/cm1024052. [DOI] [Google Scholar]; (c) Hang X.-C. Fleetham T. Turner E. Brooks J. Li J. Angew. Chem., Int. Ed. 2013;52:6753–6756. doi: 10.1002/anie.201302541. [DOI] [PubMed] [Google Scholar]; (d) Hang X.-C. Fleetham T. Turner E. Brooks J. Li J. Angew. Chem., Int. Ed. 2013;52:6753–6756. doi: 10.1002/anie.201302541. [DOI] [PubMed] [Google Scholar]; (e) Cheng G. Weng Y. Yang X. Cui X. Org. Lett. 2015;17:3790–3793. doi: 10.1021/acs.orglett.5b01733. [DOI] [PubMed] [Google Scholar]; (f) Rokesh K. Sakar M. Do T.-O. Mater. Lett. 2019;242:99–102. doi: 10.1016/j.matlet.2019.01.109. [DOI] [Google Scholar]
- Organocatalysts: ; (a) Zhang Y. Zhang Y. Sun Y. L. Du X. Shi J. Y. Wang W. D. Wang W. Chem.–Eur. J. 2012;18:6328–6334. doi: 10.1002/chem.201103028. [DOI] [PubMed] [Google Scholar]; (b) Bertuzzi G. Sinisi A. Caruana L. Mazzanti A. Fochi M. Bernardi L. ACS Catal. 2016;6:6473–6477. doi: 10.1021/acscatal.6b01962. [DOI] [Google Scholar]; (c) Binnani C. Rai R. K. Tyagi D. Mobin S. M. Singh S. K. Eur. J. Inorg. Chem. 2018;2018:1435–1445. doi: 10.1002/ejic.201701446. [DOI] [Google Scholar]; (d) Ramesh R. Arivazhagan M. Malecki J. G. Lalitha A. Synlett. 2018;29:1897–1901. doi: 10.1055/s-0037-1609579. [DOI] [Google Scholar]; (e) Greve E. Porter J. D. Dockendorff C. Synthesis. 2019;51:450–462. doi: 10.1055/s-0037-1610285. [DOI] [Google Scholar]
- Ligands: ; (a) Gunanathan C. Milstein D. Acc. Chem. Res. 2011;44:588–602. doi: 10.1021/ar2000265. [DOI] [PubMed] [Google Scholar]; (b) Sasaki I. Synthesis. 2016;48:1974–1992. doi: 10.1055/s-0035-1561974. [DOI] [Google Scholar]; (c) Guo J. Pan X. Li J. Wu W. Zhang J. Spectrochim. Acta, Part A. 2019;216:179–189. doi: 10.1016/j.saa.2019.03.013. [DOI] [PubMed] [Google Scholar]; (d) Meng X. Bai R. Wang X. Pan F. He L. Dyes Pigm. 2019;165:458–466. doi: 10.1016/j.dyepig.2019.03.003. [DOI] [Google Scholar]; (e) Pearce B. H. Ogutu H. F. O. Saban W. Luckay R. C. Inorg. Chim. Acta. 2019;490:57–67. doi: 10.1016/j.ica.2019.02.020. [DOI] [Google Scholar]; (f) Dai Z. Yu Z. Bai Y. Li J. Peng J. Appl. Organomet. Chem. 2021;35:e6027. [Google Scholar]
- Peter S., Gerhard H., Elisabeth H., Ralf K., Hartmann K., Albercht H., Norbert G., Helmut W., Karl-Otto W. and Uif M., US Pat 5733850, 1998Chem. Abstr., 1996, 125, 167792w
- (a) Constable E. C. Housecroft C. E. Neuburger M. Phillips D. Raithby P. R. Schofield E. Sparr E. Tocher D. A. Zehnder M. Zimmermann Y. J. Chem. Soc., Dalton Trans. 2000:2219–2228. doi: 10.1039/B000940G. [DOI] [Google Scholar]; (b) Cave G. W. V. Hardie M. J. Roberts B. A. Raston C. L. Eur. J. Org. Chem. 2001:3227–3231. doi: 10.1002/1099-0690(200109)2001:17<3227::AID-EJOC3227>3.0.CO;2-V. [DOI] [Google Scholar]; (c) Jetti R. K. R. Nagia A. Xue F. Mak T. C. W. Chem. Commun. 2001;10:919–920. doi: 10.1039/B102150H. [DOI] [Google Scholar]; (d) Liu H.-Y. Chen L.-F. Wang H.-Y. Wan Y. Wu H. RSC Adv. 2016;6:94833–94839. doi: 10.1039/C6RA16408K. [DOI] [Google Scholar]; (e) Ran Q. Ma J. Wang T. Fan S. Yang Y. Qi S. Cheng Y. Song F. New J. Chem. 2016;40:6281–6288. doi: 10.1039/C5NJ03722K. [DOI] [Google Scholar]; (f) Vaithilingam S. Jayanthi K. P. Muthukaruppan A. Polymer. 2017;108:449–461. doi: 10.1016/j.polymer.2016.12.017. [DOI] [Google Scholar]; (g) Zhang Y. Wang D. Sun C. Feng H. Zhao D. Bi Y. Dyes Pigm. 2017;141:202–208. doi: 10.1016/j.dyepig.2017.02.028. [DOI] [Google Scholar]; (h) Chalmardi G. B. Tajbakhsh M. Hasani N. Bekhradnia A. Tetrahedron. 2018;74:2251–2260. doi: 10.1016/j.tet.2018.03.046. [DOI] [Google Scholar]; (i) Wang H. Liu C.-H. Wang K. Wang M. Yu H. Kandapal S. Brzozowski R. Xu B. Wang M. Lu S. Hao X.-Q. Eswara P. Nieh M.-P. Cai J. Li X. J. Am. Chem. Soc. 2019;141:16108–16116. doi: 10.1021/jacs.9b08484. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhang X. Ye Q. Fan Y. Hu X. Shen Y. Chem. Pap. 2020;74:2145–2152. doi: 10.1007/s11696-020-01063-y. [DOI] [Google Scholar]
- (a) Leonard K. A. Nelen M. I. Simard T. P. Davies S. R. Gollnick S. O. Oseroff A. R. Gibson S. L. Hilf R. Chen L. B. Detty M. R. J. Med. Chem. 1999;42:3953–3964. doi: 10.1021/jm990245q. [DOI] [PubMed] [Google Scholar]; (b) Phillips G. Davey D. D. Keith A. E. J. Med. Chem. 1999;42:1749–1756. doi: 10.1021/jm980667k. [DOI] [PubMed] [Google Scholar]; (c) Quiroga J. Portilla J. Insuasty B. Nogueras M. Sortino M. Zacchino S. J. Heterocycl. Chem. 2005;42:61. doi: 10.1002/jhet.5570420108. [DOI] [Google Scholar]
- (a) Katritzky A. R. Tetrahedron. 1980;36:679–699. doi: 10.1016/S0040-4020(01)93679-8. [DOI] [Google Scholar]; (b) Katritzky A. R. Adamson J. Elisseou E. M. Musumarra G. Patel R. C. Sakizadeh K. Yeung W. K. J. Chem. Soc., Perkin Trans. 2. 1982:1041–1048. doi: 10.1039/P29820001041. [DOI] [Google Scholar]
- Marquet J. Moreno-Manas M. Pacheco P. Prat M. Katritzky A. R. Brycki B. Tetrahedron. 1990;46:5333–5346. doi: 10.1016/S0040-4020(01)87840-6. [DOI] [Google Scholar]
- Abramovitch R. A. Beckert J. M. Chinnasamy P. Xiaohua H. Pennington W. Sanjivamurthy A. R. V. Heterocycles. 1989;28:623–628. doi: 10.3987/COM-88-S86. [DOI] [Google Scholar]
- Katritzky A. R. Aurrecoechea J. M. Quian K. K. Anna E. Palenik G. J. Heterocycles. 1987;25:387–389. doi: 10.3987/S-1987-01-0387. [DOI] [Google Scholar]
- Shabalin D. A. Org. Biomol. Chem. 2021;19:8184–8204. doi: 10.1039/D1OB01310F. [DOI] [PubMed] [Google Scholar]
- (a) Han J. Guo X. Liu Y. Fu Y. Yan R. Chen B. Adv. Synth. Catal. 2017;359:2676–2681. doi: 10.1002/adsc.201700053. [DOI] [Google Scholar]; (b) Ding Y. Ma R. Xiao X.-Q. Wang L. Wang Z. Ma Y. J. Org. Chem. 2021;86:3897–3906. doi: 10.1021/acs.joc.0c02764. [DOI] [PubMed] [Google Scholar]; (c) Bai C. Guo H. Liu X. Liu D. Sun Z. Bao A. Baiyin M. Muschin T. Bao Y.-S. J. Org. Chem. 2021;86:12664–12675. doi: 10.1021/acs.joc.1c01194. [DOI] [PubMed] [Google Scholar]
- Wang Z., Organic Name Reactions and Reagents, John Wiley & Sons, 2010, pp. 635–638 [Google Scholar]
- Frank R. L. Seven R. P. J. Am. Chem. Soc. 1949;71:2629–2635. doi: 10.1021/ja01176a008. [DOI] [Google Scholar]
- (a) Huang H. Ji X. Wu W. Huang L. Jiang H. J. Org. Chem. 2013;78:3774–3782. doi: 10.1021/jo400261v. [DOI] [PubMed] [Google Scholar]; (b) Bai Y. Tang L. Huang H. Deng G.-J. Org. Biomol. Chem. 2015;13:4404–4407. doi: 10.1039/C5OB00162E. [DOI] [PubMed] [Google Scholar]; (c) Xiang J.-C. Wang M. Cheng Y. Wu A.-X. Org. Lett. 2015;18:24–27. doi: 10.1021/acs.orglett.5b03037. [DOI] [PubMed] [Google Scholar]; (d) Rohokale R. S. Koenig B. Dhavale D. D. J. Org. Chem. 2016;81:7121–7126. doi: 10.1021/acs.joc.6b00979. [DOI] [PubMed] [Google Scholar]; (e) Zhang X. Wang Z. Xu K. Feng Y. Zhao W. Xu X. Yan Y. Yi W. Green Chem. 2016;18:2313–2316. doi: 10.1039/C5GC02747K. [DOI] [Google Scholar]; (f) Wu X. Zhang J. Liu S. Gao Q. Wu A. Adv. Synth. Catal. 2016;358:218–225. doi: 10.1002/adsc.201500683. [DOI] [Google Scholar]; (g) Wang H. Zhao W. Du J. Wei F. Chen Q. Wang X. RSC Adv. 2019;9:5158–5163. doi: 10.1039/C9RA00653B. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- (a) Zhang X. Wang Z. Xu K. Feng Y. Zhao W. Xu X. Yan Y. Yi W. Green Chem. 2016;18:2313–2316. doi: 10.1039/C5GC02747K. [DOI] [Google Scholar]; (b) Shaabani A. Boroujeni M. B. Laeini M. S. RSC Adv. 2016;6:27706–27713. doi: 10.1039/C6RA00102E. [DOI] [Google Scholar]; (c) Yang Q. Zhang Y. Zeng W. Duan Z.-C. Sanga X. Wang D. Green Chem. 2019;21:5683–5690. doi: 10.1039/C9GC02409C. [DOI] [Google Scholar]; (d) Gopalaiah K. Choudhary R. Tetrahedron. 2021;98:132429. doi: 10.1016/j.tet.2021.132429. [DOI] [Google Scholar]
- (a) -Ki Yi Y. Zhao M.-N. Ren Z.-H. Wang Y.-Y. Guan Z.-H. Green Chem. 2017;19:1023–1027. doi: 10.1039/C6GC03137D. [DOI] [Google Scholar]; (b) Gao Q. Wang Y. Wang Q. Zhu Y. Liu Z. Zhang J. Org. Biomol. Chem. 2018;16:9030–9037. doi: 10.1039/C8OB02230E. [DOI] [PubMed] [Google Scholar]; (c) Le T. N. M. Doan S. H. Pham P. H. Trinh K. H. Huynh T. V. Tran T. T. T. Le M.-V. Nguyen T. T. Phan N. T. S. RSC Adv. 2019;9:23876–23887. doi: 10.1039/C9RA04096J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Varaprasad B. Kumar K. B. Siddaiah V. Shyamala P. Chinnari L. New J. Chem. 2021;45:15205–15209. doi: 10.1039/D1NJ01987B. [DOI] [Google Scholar]
- (a) Borthakur M. Dutta M. Gogoi S. Boruah R. C. Synlett. 2008;20:3125–3128. [Google Scholar]; (b) Lau C. Tsui G. C. Lautens M. Synthesis. 2011;23:3908–3914. [Google Scholar]; (c) Khajuria R. Kannaboina P. Kapoor K. K. Gupta A. Raina G. Jassal A. K. Rana L. K. Hundal M. S. Das P. Org. Biomol. Chem. 2015;13:5944–5954. doi: 10.1039/C5OB00545K. [DOI] [PubMed] [Google Scholar]; (d) Zhu C. Bi B. Ding Y. Zhang T. Chen Q.-Y. Tetrahedron. 2015;71:9251–9257. doi: 10.1016/j.tet.2015.10.040. [DOI] [Google Scholar]; (e) Mao Z.-Y. Liao X.-Y. Wang H.-S. Wang C.-G. Huang K.-B. Pan Y.-M. RSC Adv. 2017;7:13123–13129. doi: 10.1039/C7RA00780A. [DOI] [Google Scholar]
- Adib M. Tahermansouri H. Koloogani S. A. Mohammadi B. Bijanzadeh H. R. Tetrahedron Lett. 2006;47:5957–5960. doi: 10.1016/j.tetlet.2006.01.162. [DOI] [Google Scholar]
- Kumar A. Koul S. Razdan T. K. Kapoor K. K. Tetrahedron Lett. 2006;47:837–842. doi: 10.1016/j.tetlet.2005.11.043. [DOI] [Google Scholar]
- Adib M. Mohammadi B. Rahbari S. Mirzaei P. Chem. Lett. 2008;37:1048–1049. doi: 10.1246/cl.2008.1048. [DOI] [Google Scholar]
- Verma A. K. Koul S. Pannu A. P. S. Razdan T. K. Tetrahedron. 2007;63:8715–8722. doi: 10.1016/j.tet.2007.06.049. [DOI] [Google Scholar]
- (a) Chan C.-K. Lai C.-Y. Lo W.-C. Cheng Y.-T. Chang M.-Y. Wang C.-C. Org. Biomol. Chem. 2020;18:305–315. doi: 10.1039/C9OB02445J. [DOI] [PubMed] [Google Scholar]; (b) Chan C.-K. Lai C.-Y. Wang C.-C. Org. Biomol. Chem. 2020;18:7201–7212. doi: 10.1039/D0OB01507E. [DOI] [PubMed] [Google Scholar]; (c) Asressu K. H. Chan C.-K. Wang C.-C. RSC Adv. 2021;11:28061–28071. doi: 10.1039/D1RA05802A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chan C.-K. Lai C.-Y. Wang C.-C. Synthesis. 2020;18:1779–1794. [Google Scholar]; (e) Chan C.-K. Lai C.-Y. Wang C.-C. Catalyst. 2021;11:877. doi: 10.3390/catal11080877. [DOI] [Google Scholar]
- CCDC 2085356 (2q) and 2085361 (4), contain the supplementary crystallographic data for this paper
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.