Abstract
The intestinal microbiota promote colorectal cancer, but their role in metastasis is poorly defined. In this issue of Cancer Cell, Bertocchi et al. report that intratumoral bacteria disrupt the gut vascular barrier, causing bacterial dissemination to the liver and the formation of a premetastatic niche, favoring recruitment of metastatic cells.
Intestinal microbiota composition and function influence susceptibility to multiple forms of cancer including colorectal cancer (CRC). Both luminal and mucosal intestinal microbes have been linked to adenoma-carcinoma progression by virtue of bacterial properties that modulate the immune response and host DNA stability (Janney et al., 2020). However, little is known about the impact of the intestinal microbiota on cancer metastasis. The main organ site of primary CRC metastasis is the liver, a location physiologically linked to the gut by the hepatic portal vein, which drains venous blood from the colon and rectum. One of the critical steps in tumor dissemination is the intravasation of primary cancer cells into the circulatory system through blood or lymph node vessels. The vast network of intestinal capillaries that feed back to the portal vein represents a potentially important route for migrating cancer cells. However, under homeostatic conditions, structural barriers prevent luminal bacteria and large molecules from disseminating across the intestinal epithelium and into the vasculature. For example, the intestinal epithelium and associated tight junction proteins form an efficient barrier that chemically and physically separates luminal content from the submucosal immune and vascular systems. Another physical barrier is the gut vascular barrier (GVB), a cellular network composed of interacting endothelial cells glued together by tight junction proteins, glial cells, and pericytes (Spadoni et al., 2017). However, these barriers could be breached upon certain pathological conditions such as enteric infection. For example, during infection with the enteric pathogen Salmonella typhimurium, compromised GVB leads to the translocation of larger molecules and dissemination of the infectious agent (Spadoni et al., 2015). This observation suggests that GVB could represent an important barrier in the dissemination of intestinal bacteria with potentially deleterious consequences for host homeostasis, especially for liver function. In this issue, Bertocchi et al. (Bertocchi et al., 2021) demonstrate that Virf+ tumor-resident Escherichia coli C17 disrupts GVB and translocates to the liver, where it creates a premetastatic niche (PMN) favorable to cancer cell seeding, thereby facilitating the formation of CRC liver metastasis (Figure 1).
Figure 1. Schematic representation of CRC liver metastasis facilitated by VirF+ E. coli.
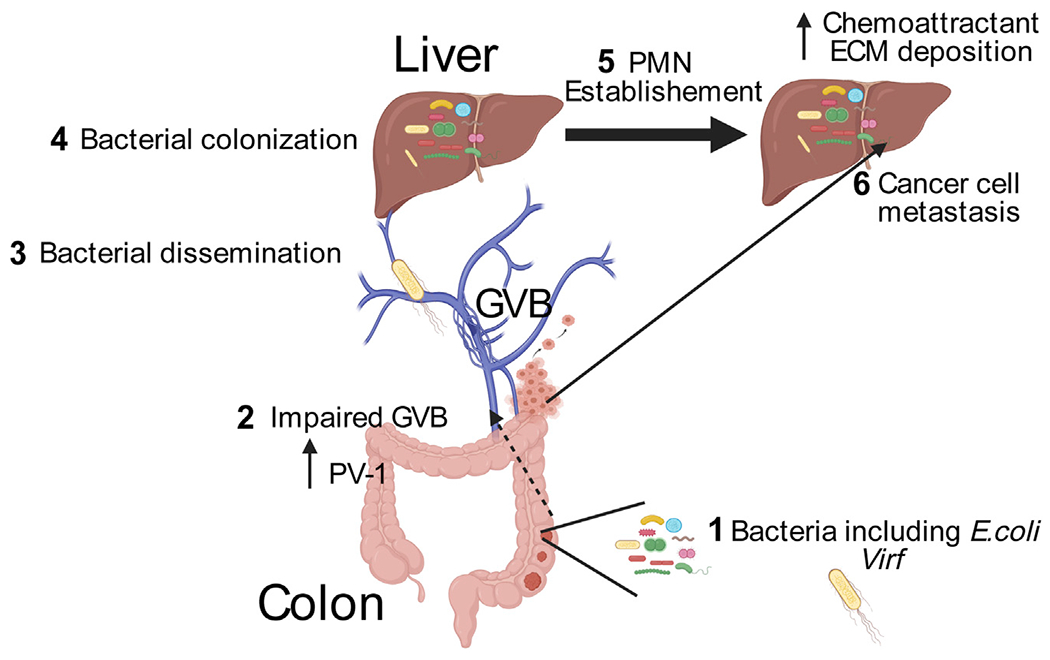
Intratumor Virf+ E. coli (step 1) breaks down the GVB associated with increased expression of PV-1 (step 2), allowing the translocation of gut microbes to the liver (step 3). Disseminated microbes colonize the liver (step 4) and establish a PMN microenvironment that features increased expression of chemotactic factors and ECM deposition (step 5). Primary colorectal cancer cells metastasize to the liver, where implantation is facilitated by PMN (step 6). Created by Biorender.com
To investigate the link between GVB disruption and liver metastasis, Bertocchi et al. performed a retrospective expression analysis of plasmalemma vesicle (PV)-1, a marker of GVB disruption, using formalin-fixed paraffin embedded (FFPE) human colons resected from individuals with CRC. They found an association between GVB disruption as evaluated by PV-1 expression in primary tumors, the presence of distant liver metastasis, and poor patient outcomes. Interestingly, PV-1 expression did not correlate with the number of metastatic regional lymph nodes (LN); this result suggests that disseminating cancer cells following GVB disruption do not travel through the LN. In addition, individuals with CRC with high PV-1 expression in the primary tumor had increased bacterial presence in metastatic liver lesions, which suggests potential microbial dissemination through GVB.
To mechanistically investigate the relationship between GVB and microbial dissemination, Bertocchi et al. utilized ApcMin/+ mice crossed to C3a complement anaphylatoxin receptor (C3ar)−/− mice as a spontaneous CRC model. They observed increased GVB permeability and high bacterial abundance in the livers of ApcMin/+;C3ar−/− mice compared to healthy control mice, in line with observations from patients with metastatic CRC. Genomic analysis of the microbial 16S rRNA gene showed that tissue-resident and fecal microbial composition is similar in cancer-susceptible mice (ApcMin/+;C3ar−/−) but not in control mice (wild type or C3ar−/−), and this suggests bacterial dissemination from the colon to the liver. Interestingly, the most frequent bacterial isolates found in the colons and livers of cancer mice were E. coli strains, including a strain from a primary murine colon tumor they named E. coli C17. Comparative genomic analysis between E. coli C17 and several commensal E. coli strains identified the virulence factor Virf1 in C17, a transcription factor that regulates the expression of genes specifically involved in the formation of type three secretion system (TTSS) machinery. Importantly, the C17 parental strain, but not an isogenic Virf1-deficient strain, was able to translocate to the liver when orally administered to mice. This phenomenon was associated with increased expression of PV-1 in the colonic vascular endothelium.
Transcriptome analysis of liver tissues containing disseminated bacteria showed a molecular profile similar to known PMN signatures, including increased expression of chemoattractant for myeloid cells and extracellular matrix (ECM) deposition. In line with this signature, the authors also observed increased infiltration of innate immune cells (macrophages, neutrophils, and inflammatory monocytes) in the liver; this provides additional support for the idea that bacteria favor cellular conditions that promote PMN. To assess the contribution of bacteria to these host responses, the authors used wide-spectrum antibiotics to ablate the microbiota. They observed that immune cell infiltration was prevented when bacteria were depleted. To further document the functional link between disseminated bacteria and liver metastasis, the authors intra-splenically injected luciferase-labeled MC38 cells into mice with colitis-associated colon cancer. In this model, they observed that liver metastasis was prevented after microbiota depletion by antibiotic treatment, and this result supports the roles of liver bacteria in PMN formation and metastasis.
Importantly, in order to address the clinical relevance of C17 for human CRC metastasis, the authors sequenced bacterial DNA from FFPE sections obtained from patients with PV-1High metastatic CRC and patients with PV-1Low non-metastatic tumors. They found that the abundance of E. coli C17 was statistically higher in metastatic compared to non-metastatic sections, with a concomitant increase in liver metastases. Altogether, these findings bring forward a new concept where bacterial virulence factors target vascular gatekeepers and open a passage for dissemination, thereby orchestrating new environmental immune conditions in the secondary organ, conditions that are supportive of cancer cell growth from extravasated primary tumors (Figure 1).
This study brings exciting and clinically relevant findings, especially regarding the relationship between PV-1 expression and CRC liver metastasis. This link needs to be confirmed using prospective studies before PV-1 could be validated as a liver metastasis biomarker. The study also highlights how complex microbiota-host interaction may influence cancer development. For example, a previous study showed that intestinal microbiota-mediated bile acid metabolism reduces recruitment of anti-tumor natural killer T (NKT) cells, thereby promoting liver metastasis (Ma et al., 2018). In addition to this metabolic link, disruption of the GVB as shown by Bertocchi et al. may represent another means by which microbiota alter metastatic processes. Thus, bacteria could influence metastasis through both distant (metabolite) and local (dissemination) mechanisms by modifying the immune environment (antitumor cells and PMN formation). Although molecular evidence points to a role of E. coli Virf in GVB breakdown, this protein is a transcriptional regulator of T3SS components and not an effector protein. Therefore, identifying T3SS-dependent effector protein(s) responsible for GVB degradation may have clinical implications. With the findings that numerous tumors sites contain microbiota (Nejman et al., 2020) and that circulating bacteria have cancer diagnostic value (Poore et al., 2020), potentially as liquid biopsy tools (Newsome and Jobin, 2021), one should take a hard look at microbiota as a potential marker of metastasis. In addition, the development of microbial-based CRC diagnostic tools continues to progress (Zhou et al., 2020). These markers could be combined with existing host-based biomarkers (Zarour et al., 2017) to more accurately identify patients at high risk for CRC liver metastasis. In summary, Bertocchi et al. contribute to a novel understanding of the role of bacteria in CRC liver metastasis, and future investigations should focus on elucidating the clinical impact of these findings.
ACKNOWLEDGMENTS
C.J. is supported by NIH grant R01DK073338, the University of Florida Health Cancer Center Funds, and the University of Florida Department of Medicine Gatorade Fund.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Bertocchi A, Rescigno M, et al. (2021). Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39. [DOI] [PubMed] [Google Scholar]
- Janney A, Powrie F, and Mann EH (2020). Host-microbiota maladaptation in colorectal cancer. Nature 585, 509–517. [DOI] [PubMed] [Google Scholar]
- Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, Agdashian D, Terabe M, Berzofsky JA, Fako V, et al. (2018). Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, et al. (2020). The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome RC, and Jobin C (2021). Microbiome-Derived Liquid Biopsy: New Hope for Cancer Screening? Clin. Chem 67, 463–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, et al. (2020). Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, et al. (2015). A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350, 830–834. [DOI] [PubMed] [Google Scholar]
- Spadoni I, Fornasa G, and Rescigno M (2017). Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat. Rev. Immunol 17, 761–773. [DOI] [PubMed] [Google Scholar]
- Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF, Coussens LM, Gast CE, Geltzeiler CB, Hansen L, et al. (2017). Colorectal cancer liver metastasis: evolving paradigms and future directions. Cell. Mol. Gastroenterol. Hepatol 3, 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Ge S, Li Y, Ma W, Liu Y, Hu S, Zhang R, Ma Y, Du K, Syed A, and Chen P (2020). Human Gut Microbiome-Based Knowledgebase as a Biomarker Screening Tool to Improve the Predicted Probability for Colorectal Cancer. Front. Microbiol 11, 596027. [DOI] [PMC free article] [PubMed] [Google Scholar]


