Abstract
Objective
To determine the false-negative rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse-transcription polymerase chain reaction (RT-PCR) test results, and to describe the characteristics of coronavirus disease 2019 (COVID-19) patients with false-negative results.
Methods
We validated SARS-CoV-2-positive RT-PCR test results performed in hospitalized patients between February 1 and August 31, 2020 and classified the patients according to disease severity.
Results
In total, 2038 SARS-CoV-2 RT-PCR tests were performed on 1890 patients. Of these, 145 patients had positive results and were diagnosed with COVID-19. Among the 145 patients with COVID-19, the initial RT-PCR tests were negative in five patients. Of these, three had moderate illness and were initially tested in the early stage of disease, and two had severe illness and were initially tested in the late stage of disease, when RT-PCR testing has lower sensitivity due to viral clearance.
Conclusions
These findings suggest that false-negative results can be caused by both observer errors and by low viral RNA levels in the later stages of disease, after the infection has cleared. Clinicians should be aware that patients with COVID-19 can have negative RT-PCR test results in the later stages of infection.
Keywords: COVID-19, False-negative result, Real-Time Reverse Transcription Polymerase Chain Reaction, SARS-CoV-2, Sensitivity
Highlights
-
•
All SARS-CoV-2 RT-PCR test results of hospitalized COVID-19 patients were reviewed.
-
•
Of 145 confirmed COVID-19 cases, 5 (3.45%) had a negative first RT-PCR test result.
-
•
Of the 5 patients, 3 with moderate disease first tested soon after symptom onset.
-
•
The initial testing was delayed in 2 patients with severe/critical disease.
-
•
False-negative results can occur in deteriorating patients who have delayed testing.
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first reported in December 2019 in Wuhan, China. It rapidly spread worldwide and became a pandemic owing to its high transmissibility. COVID-19 can rapidly progress to hypoxemia, acute respiratory distress syndrome, and death in some cases. Early diagnosis is essential for disease prevention and control, and to enable early treatment [1], [2]. The reverse-transcription polymerase chain reaction (RT-PCR) test, which typically uses upper respiratory tract specimens, is the gold standard test for confirming SARS-CoV-2 infection, and plays an important role in clinical decision-making. However, previous studies suggest that some RT-PCR tests have low sensitivity [3]. Causes of false-negative SARS-CoV-2 results include incorrect use of testing kits, insufficient samples, and low viral loads [4], [5]. To prevent cases with false-negative results from being overlooked, retesting is recommended for patients with clinical findings suggestive of COVID-19 despite negative RT-PCR results [1], [2].
Previous studies have shown that most patients with false-negative results tend to have mild disease [6]; however, individuals with false-negative results occasionally progress and develop critical disease [7], which can be extremely difficult to manage. The underlying mechanisms whereby individuals with false-negative results exhibit this dual severity remain unclear. Therefore, we aimed to evaluate the false-negative rate of SARS-CoV-2 RT-PCR in COVID-19 patients tested at our hospital, and describe our experience of patients who initially had a negative RT-PCR result. To our knowledge, this is the first study to investigate the false-negative rate of RT-PCR among patients with subsequently confirmed SARS-CoV-2 infection in Japan.
Patients and methods
The study was conducted at Showa General Hospital, a medical institution designated for managing type II infectious diseases, located near Tokyo, Japan. We validated SARS-CoV-2-positive RT-PCR test results in patients hospitalized between February 1 and August 31, 2020. Clinical specimens, including nasopharyngeal or oropharyngeal swabs and sputum, were collected by physicians, and stored in accordance with the World Health Organization standardized protocol [8].
The test device and the number of positive results differed according to the date of specimen collection as follows: A total of 831 positive test results were obtained between March 10 and June 23, 2020, using Cobas SARS-CoV-2 8800 (Roche Molecular Systems), which amplifies the SARS-CoV-2 gene envelope (E), and has a limit of detection of 125 copies/mL, with a Ct value of ≤ 36.03–38.78 (variable). A total of 1181 positive test results were obtained between June 24 and August 31, 2020, using Cobas SARS-CoV-2 8800 (Roche Molecular Systems), which targets the open reading frame 1/a (ORF1/a) gene (a nonstructural region specific to SARS-CoV-2) (target 1) and the structural protein envelope (E) gene shared by the Sarbecovirus subgenus (target 2), with a limit of detection of 25 and 32 copies/mL at Ct values of ≤ 35 and ≤ 40, respectively. A total of 26 positive test results were obtained from specimens tested at a health center between February 1 and March 30, 2020, using QuantStudio 12 K (Applied Biosystems, ThermoFisher Scientific) that targets the ORF1/a gene and had a sigmoidal amplification curve with a Ct value of ≤ 40.
Data of patients with positive results were extracted and their disease severity was assessed according to the severity of COVID-19 definition of the United States National Institutes of Health [9]. Then, the records of patients who initially tested negative and subsequently tested positive within 14 days of the first test were reviewed. The patients’ laboratory test results, imaging findings, RT-PCR results (including Ct values), and clinical course were evaluated. In all cases here reported (1–5), the same platform was used for both the initial and subsequent PCR tests.
All study procedures were conducted according to the World Medical Association Declaration of Helsinki. The retrospective case study was approved by the Human Research Ethics Committee of Showa General Hospital (approval no.: REC-242). Cases 1–5 provided written informed consent for publication of their case details. The requirement for informed consent was waived for the rest of the patients whose records were reviewed.
Cases
Case 1
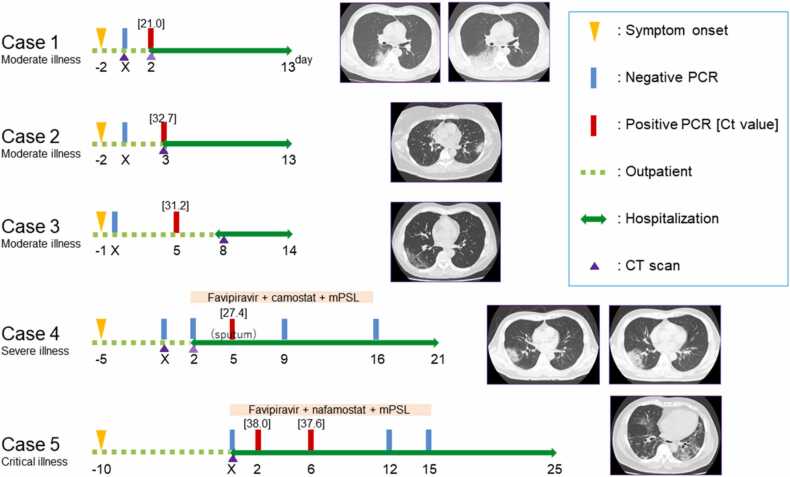
In mid-August 2020, a 39-year-old man with a 17 pack-year smoking history and no known contact with a COVID-19 patient was admitted to our hospital on day X (the day on which the patient’s initial sample with the false-negative PCR test result was collected), with a 2-day history of fever and dry cough. He tested negative for SARS-CoV-2 on RT-PCR using a nasopharyngeal sample on the day of admission. However, he tested positive in a repeat RT-PCR test on day X + 2 (cycle threshold [Ct] value, 21.0).
On admission, computed tomography (CT) showed mild emphysema and extensive consolidation with bronchiectasis in the right lung adjoining the upper and lower lobes (Fig. 1). In this case, as in the following two cases (Cases 2 and 3), the patient did not require treatment with steroids, antiviral drugs, or oxygen administration, and no subsequent RT-PCR tests were performed. The patient was discharged on day X + 13.
Fig. 1.
Clinical course and computed tomography findings of five patients with SARS-CoV-2 infection and an initial false-negative PCR test result. Day X is the day on which the patient’s initial sample with a false-negative PCR test result was collected. Cases 1–3 were initially tested ≤ 2 days after symptom onset, developed moderate disease and had a short hospitalization. Cases 4–5 were initially tested ≥ 5 days after symptom onset, developed severe/critical disease, and required intensive treatment and prolonged hospitalization. In Case 1, CT shows a unilateral shadow in the left lung. In Cases 2–5, CT shows bilateral shadows in the lungs. In Cases 3 and 5, CT shows ground-glass opacities and, in Cases 1, 2, and 4, CT shows ground-glass opacities and consolidation. In Cases 1 and 2 mild emphysema is evident. In Cases 1 and 4, CT shows the increasing extent of opacities on subsequent days. Ct, cycle threshold; CT, computed tomography; mPSL, methylprednisolone; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Case 2
In mid-June 2020, a 35-year-old woman with a 30 pack-year smoking history, and a 2-day history of fever and dry cough, tested negative for SARS-CoV-2 on RT-PCR using a nasopharyngeal sample on day X. She had a medical history of untreated type 2 diabetes mellitus and had engaged in commercial sex work from days X − 9 to X − 2. She tested positive on a repeat RT-PCR test on day X + 3 (Ct value, 32.7) and was admitted to our hospital on the same day. On admission, she had mild oxygen desaturation of 94% on room air, and chest CT revealed mild emphysema and bilateral subpleural patchy ground-glass opacities (Fig. 1). She was hospitalized for 11 days, and was discharged on recovery.
Case 3
In early July 2020, a 55-year-old man, who was living with his sons, who had been diagnosed with COVID-19 on day X − 2, developed fever, headache, and myalgia on day X − 1. A familial cluster of COVID-19 was suspected; however, he tested negative for SARS-CoV-2 in an RT-PCR test performed using a nasopharyngeal sample on day X. His symptoms continued; therefore, he was retested on day X + 5. He tested positive (Ct value, 31.2) on this second test and was admitted to our hospital on day X + 8. On admission, CT revealed bilateral subpleural reticular shadows (Fig. 1). He was discharged on day X + 14 without any specific treatment.
Case 4
In mid-April 2020, A 53-year-old man without any history of smoking or known contact with COVID-19 patients developed persistent fever, dry cough, and shortness of breath (day X − 5). CT performed on days X and X + 2 revealed rapidly increasing multiple bilateral patchy ground-glass opacities, compatible with COVID-19 (Fig. 1). He tested negative for SARS-CoV-2 on an RT-PCR test of a nasopharyngeal swab performed on day X, and a repeat RT-PCR test was performed on day X + 2, owing to the high suspicion of COVID-19. The patient eventually tested positive for SARS-CoV-2 on RT-PCR using sputum samples on day X + 5 (Ct value, 27.4). He was admitted to a negative-pressure isolation room and sequentially treated with favipiravir, camostat, and methylprednisolone from day X + 2, because of his deteriorating respiratory condition. The patient required a maximum oxygen intake of 6 L/min through a non-rebreather mask on day X + 5. As his respiratory condition improved, oxygen administration was tapered. His RT-PCR test result was negative on day X + 9, and he was discharged on day X + 21.
Case 5
In mid-April, 2020, a 51-year-old man without a history of smoking or any known contact with COVID-19 patients visited our hospital with complaints of fever, dyspnea, and diarrhea for 10 days. Physical examination revealed a temperature of 37.9 ℃ and an oxygen saturation of 80% on room air. CT revealed bilateral subpleural reticular shadows, compatible with COVID-19 (Fig. 1). A nasopharyngeal swab was tested using an RT-PCR assay, and was negative for SARS-CoV-2 on day X. The patient was promptly admitted to a negative-pressure isolation room and sequentially treated with favipiravir, nafamostat, and methylprednisolone. Thereafter, he tested positive on a repeat test performed on days X + 2 and X + 6 (Ct values, 38.0 and 37.6, respectively). He underwent intensive care unit monitoring, owing to a worsened respiratory condition, requiring a maximum oxygen intake of 100% through a 40 L/min nasal high-flow cannula. As his respiratory condition improved, oxygen administration was tapered. His RT-PCR test result was negative on day X + 12, and he was discharged on day X + 25.
Discussion
In total, 2038 SARS-CoV-2 RT-PCR tests were performed in 1890 patients, and a total of 145 patients had positive results and were diagnosed with COVID-19, while the rest of the patients tested were determined not to have SARS-CoV-2 infection. There were no false-positive results identified. The positivity rate was 7.67%, which is comparable to the 5.46% positivity rate (16,525 positive test results among a total of 302,554 tests performed) reported in Tokyo during the study period [10]. Among the 145 patients with COVID-19, 112 (77.2%), 27 (18.6%), and 6 (4.1%), had moderate, severe, and critical forms of the disease, respectively. This distribution of disease severity among patients with positive RT-PCR results, was similar to that reported in Japan during the study period [11], suggesting that these results are likely to be broadly representative of the general situation in Japan during the study period.
We conducted an in-depth case review of five patients with initially negative RT-PCR results among the 145 patients with COVID-19 confirmed by RT-PCR testing (Table 1).This false-negative rate of 3.45% is comparable to that reported in a systematic review, which found a false-negative RT-PCR rate of 2–29% [3].
Table 1.
Clinical features and laboratory results of the five patients in the case series.
| Case | Age (years) | Sex | Medical history | Smoking | Symptom onset | Symptoms | SpO2 on admission | WBC/lymp (× 104/L) | LDH (U/L) | CRP (mg/dL) | D-dimer (μg/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | M | Nil of note | 17 PYR | Day Xa − 2 | Fever and cough | 96% RA | 4240/460 | 215 | 6.01 | 1.2 |
| 2 | 35 | F | Diabetes | 30 PYR | Day X − 2 | Fever and cough | 94% RA | 5000/1390 | 353 | 5.49 | < 0.5 |
| 3 | 55 | M | Nil of note | Never | Day X − 1 | Fever, headache, and myalgia | 99% RA | 4400/1300 | 243 | 1.94 | 0.8 |
| 4 | 53 | M | Nil of note | Never | Day X − 5 | Fever, cough, and breathlessness | 95% RA | 3900/390 | 318 | 7.13 | 1.8 |
| 5 | 51 | M | Nil of note | Never | Day X − 10 | Fever, dyspnea, and diarrhea | 80% RA | 8500/1150 | 578 | 11.01 | 2.5 |
CRP, C-reactive protein; F, female; LDH, lactate dehydrogenase; lymp, lymphocytes; M, male; PCR, polymerase chain reaction; PYR, pack-year; RA, on room air; SpO2, oxygen saturation; WBC, white blood cells.
Day X is the day on which the patient’s initial sample with a false-negative PCR test result was collected.
Among the five patients with a false-negative initial RT-PCR result, Cases 1–3 had distinctly different characteristics than Cases 4 and 5 with respect to the PCR test timing, illness severity, and clinical course. Cases 1–3 all had moderate disease, moderate levels of laboratory inflammation markers, needed no specific treatment, and had short hospitalizations. Their initial tests were performed at an appropriate time (a few days after symptom onset), when the false-negative rate is low. The probability of initial false-negative result is< 10% when testing is performed within a few days of symptom onset [12]. Their Ct values of 21.0, 32.7, and 31.2, on retesting were relatively low, which suggests that they were in the early stages of infection at the time of the initial testing. The false-negative initial RT-PCR results in Cases 1–3 are may have been caused by technical errors, insufficient sample collection, or poor storage conditions [4], [13].
In contrast, Cases 4 and 5 had delayed initial testing, severe disease, high levels of laboratory inflammation markers, and required intensive treatment and prolonged hospitalization. The false-negative rate is affected by viral load, reflecting viral clearance; RT-PCR performed using upper respiratory tract samples is often negative if performed more than 9 days after symptom onset [5], [14]. Based on the interval between symptom onset and the initial RT-PCR testing, the probability of an initial false-negative result was approximately 15% and 33% for Cases 4 and 5, respectively [12]. In Case 4, testing sputum specimens was helpful, because they had a significant viral load even though 10 days had passed since symptom onset and the upper respiratory tract samples were negative [15]. In Case 5, The Ct values of 38.0 and 37.6, suggesting that the patient was in the late stage of infection at the time of the initial testing, and probably had almost no viable virus present [16]. We interpreted that the false-negative results in Cases 4 and 5 may have been due to a viral load below the detection threshold due to viral clearance [17]. The worsening of disease severity in Cases 4 and 5 may have been due to a high level of inflammatory cytokine production, rather than due to the presence of the virus [7], [17], [18]. The viral load naturally decreases in the days following symptom onset, making it challenging to diagnose and manage patients with COVID-19 who deteriorate due to cytokine storms, if the diagnosis has been missed during the earlier stages of the disease [1], [7].
We also tried to assess the chest CT features of Cases 1–5. Their features were similar to those reported in a previous study of patients with false-negative RT-PCR results [19]; however, there were no obvious differences between the CT findings in Cases 1–3 and those in Cases 4 and 5.
In this study, the patients with false-negative results had disease of varying severity. The two patients who had a delay in PCR testing following symptom onset had more severe disease than the three patients initially tested soon after symptom onset. We assumed that the false-negative RT-PCR results of two patients with severe disease was due to low viral loads, and that their clinical deterioration was due to high cytokine levels, which made diagnosis and clinical management challenging. Generally, high Ct values, reflecting low viral load levels on RT-PCR testing, predicts disease of mild severity; however, this is not applicable in cases with delayed testing [20]. It is essential for clinicians to confirm the time since symptom onset patients suspected to have COVID-19 with negative RT-PCR results.
This study has some limitations. We were unable to monitor the patient’s viral load and cytokine levels, because testing was not available in our hospital. As an alternative, we used Ct values and inflammation markers to assess the disease stage. Future research on false-negative SARS-CoV-2 RT-PCR results should consider the infection period, viral load, and cytokine measurements [16], to obtain a better understanding of the causes of false-negative results in COVID-19 patients.
Ethical approval
The retrospective case study was approved by the Human Research Ethics Committee of Showa General Hospital (approval no.: REC-242).
Consent
Cases 1–5 provided written informed consent for publication of their case details.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Hidenori Takahashi: Data curation, Formal analysis, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. Naoki Ichinose: Conceptualization, Methodology, Project administration. Yasusei Okada: Resources and Supervision.
Conflicts of interest
None to declare.
Acknowledgments
None.
Declarations of interest
None.
Data statement
All the relevant data pertaining to this study are included in the manuscript.
References
- 1.Chen L.D., Li H., Ye Y.M., et al. A COVID-19 patient with multiple negative results for PCR assays outside Wuhan, China: a case report. BMC Infect Dis. 2020;20:517. doi: 10.1186/s12879-020-05245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavare A.N., Braddy A., Brill S., et al. Managing high clinical suspicion COVID-19 in patients with negative RT-PCR: a pragmatic and limited role for thoracic CT. Thorax. 2020;75:537–538. doi: 10.1136/thoraxjnl-2020-214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woloshin S., Patel N., Kesselheim A.S. False negative tests for SARS-CoV-2 infection – challenges and implications. N Engl J Med. 2020;383 doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G., Simundic A.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin Chem Lab Med. 2020;58:1070–1076. doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
- 5.Kanji J.N., Zelyas N., MacDonald C., et al. False negative rate of COVID‑19 PCR testing: a discordant testing analysis. Virol J. 2021;18:13. doi: 10.1186/s12985-021-01489-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long D.R., Gombar S., Hogan C.A., et al. Occurrence and timing of subsequent severe acute respiratory syndrome coronavirus 2 reverse-transcription polymerase chain reaction positivity among initially negative patients. Clin Infect Dis. 2021;72:323–326. doi: 10.1093/cid/ciaa722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y., Yang M., Shen C., et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. Innovation. 2020;1 doi: 10.1016/j.xinn.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Diagnostic testing for SARS-CoV-2 interim guidance; 2020. 〈https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2/〉, [Accessed 29 January 2021].
- 9.National Institutes of Health. COVID-19 treatment guidelines. Clinical spectrum of SARS-CoV-2 infection; 2021. 〈https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/〉, [Accessed 29 January 2021].
- 10.Tokyo Metropolitan Government. New coronavirus infectious disease test positive rate/number of testers; 2021. 〈https://stopcovid19.metro.tokyo.lg.jp/cards/positive-rate/〉, [Accessed 29 January 2021].
- 11.Saito S., Asai Y., Matsunaga N., et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2021;82:84–123. doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wikramaratna P., Paton R.S., Ghafari M., et al. Estimating false-negative detection rate of SARS-CoV-2 by RT-PCR. Eur Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.50.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchan B.W., Hoff J.S., Gmehlin C.G., et al. Distribution of SARS-CoV-2 PCR cycle threshold values provide practical insight into overall and target-specific sensitivity among symptomatic patients. Am J Clin Pathol. 2020;154:479–485. doi: 10.1093/ajcp/aqaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallett S., Allen A.J., Graziadio S., et al. At what times during infection is SARS-CoV-2 detectable and no longer detectable using RT-PCR-based tests? A systematic review of individual participant data. BMC Med. 2020;18:346. doi: 10.1186/s12916-020-01810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh K.A., Jordan K., Clyne B., et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.La Scola B., Le Bideau M., Andreani J., et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J.J., Cao Y.Y., Dong X., et al. Distinct characteristics of COVID-19 patients with initial rRT-PCR-positive and rRT-PCR-negative results for SARS-CoV-2. Allergy. 2020;75:1809–1812. doi: 10.1111/all.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee C., Kanjilal S., Baker M., et al. Duration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectivity: when is it safe to discontinue isolation? Clin Infect Dis. 2021;72:1467–1474. doi: 10.1093/cid/ciaa1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z.H., Li Y.J., Wang X.J., et al. Chest CT of COVID-19 in patients with a negative first RT-PCR test: comparison with patients with a positive first RT-PCR test. Medicine. 2020;99 doi: 10.1097/MD.0000000000020837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukushima T., Kabata H., Yamamoto R., et al. The real-time reverse transcription-polymerase chain reaction threshold cycle values for severe acute respiratory syndrome coronavirus 2 predict the prognosis of coronavirus disease 2019 pneumonia. Respir Invest. 2021;59:360–363. doi: 10.1016/j.resinv.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data pertaining to this study are included in the manuscript.