Abstract
Background
Dysmenorrhoea is the occurrence of painful menstrual cramps of uterine origin and is a very common gynaecological complaint with negative effect on a sufferer's quality of life. Medical therapy for dysmenorrhoea includes oral contraceptive pills (OCP) and nonsteroidal anti‐inflammatory drugs (NSAIDs) which both act by suppressing prostaglandin levels. While these treatments are very successful there is still a 20 to 25% failure rate and surgery has been an option for such cases. Uterine nerve ablation (UNA) and presacral neurectomy (PSN) are two surgical treatments that have become increasingly utilised in recent years due to advances in laparoscopic procedures. These procedures both interrupt the majority of the cervical sensory pain nerve fibres. Observational studies have supported the use of these procedures for primary dysmenorrhoea. However, both operations only partially interrupt the cervical sensory nerve fibres in the pelvic area and, therefore, this type of surgery may not always benefit women with dysmenorrhoea.
Objectives
To assess the effectiveness of surgical interruption of pelvic nerve pathways as treatment for primary and secondary dysmenorrhoea, and to determine the most effective surgical treatment.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group Trials Register (searched 9 June 2004), CENTRAL (The Cochrane Library Issue 2, 2004), MEDLINE (1966 to Nov 2003), EMBASE (1980 to Nov 2003), and CINAHL (1982 to Oct 2003). Attempts were also made to identify trials from the metaRegister of Controlled Trials and the citation lists of review articles and included trials. In most cases the first or corresponding author of each included trial was contacted for additional information.
Selection criteria
The inclusion criteria were randomised comparisons of surgical techniques of interruption of the pelvic nerve pathways (using both open and laparoscopic procedures) for the treatment of primary and secondary dysmenorrhoea. The main outcome measures were pain relief and adverse effects.
Data collection and analysis
Eleven randomised controlled trials (RCTs) were identified that initially appeared to fulfil the inclusion criteria for this review. Two trials were subsequently excluded (Garcia Leon 2003; Sutton 1991). Of the remaining nine trials, eight were included in the meta‐analysis. The results of one trial were included in the text of the review for discussion because the data were not available in a form that allowed them to be combined in the meta‐analysis. Five trials investigated laparoscopic uterine nerve ablation (LUNA), two trials laparoscopic presacral neurectomy (LPSN) and two open presacral neurectomy (PSN).
Main results
For the treatment of primary dysmenorrhoea there was some evidence of the effectiveness of laparoscopic uterine nerve ablation (LUNA) when compared to a control or no treatment. The comparison between LUNA and laparoscopic presacral neurectomy (LPSN) for primary dysmenorrhoea showed no significant difference in pain relief in the short term; however, long‐term LPSN was shown to be significantly more effective than LUNA. For the treatment of secondary dysmenorrhoea six identified RCTs addressed endometriosis and one included women with uterine myomas. The treatment of LUNA combined with surgical treatment of endometrial implants versus surgical treatment of endometriosis alone showed that the addition of LUNA did not aid pain relief. For PSN combined with endometriosis treatment versus endometriosis treatment alone there was an overall difference in pain relief although the data suggests this may be specific to laparoscopy and for midline abdominal pain only. Adverse events were significantly more common for presacral neurectomy; however, the majority were complications such as constipation, which may spontaneously improve.
Authors' conclusions
There is insufficient evidence to recommend the use of nerve interruption in the management of dysmenorrhoea, regardless of cause. Future methodologically sound and sufficiently powered RCTs should be undertaken.
Plain language summary
Not enough evidence to support the use of surgical nerve interruption for dysmenorrhoea
Dysmenorrhoea (painful menstrual cramps) is a common problem. The contraceptive pill and anti‐inflammatory drugs (NSAIDs) are effective treatments in 80% of women with dysmenorrhoea but for others surgery is a considered option. Uterine nerve ablation (UNA) and presacral neurectomy (PSN) both involve surgical interruption of the sensory nerve fibres near the cervix to block the pain pathway. The review of trials found there was only limited evidence to support the use of surgery for primary dysmenorrhoea and little evidence for its use in women with endometriosis. No adverse effects were found with UNA but PSN was found to cause treatable constipation. More research is needed.
Background
Dysmenorrhoea is the occurrence of painful menstrual cramps of uterine origin. Dysmenorrhoea is a very common gynaecological complaint that can affect up to 50% of women (Sobczyk 1980). As such, it has a significant impact not just on personal health but also economically, through lost working hours (Dawood 1988).
Dysmenorrhoea is commonly defined as two subcategories. When the menstrual pelvic pain is associated with an identifiable pathological condition, such as endometriosis, adenomyosis or pelvic adhesions reflecting previous inflammation, it is considered to be secondary dysmenorrhoea. In contrast menstrual pain without organic pathology is considered to be primary dysmenorrhoea (Lichten 1987). According to standard gynaecological texts primary dysmenorrhoea usually occurs at or shortly after (6 to 12 months) menarche, when ovulatory cycles are established. The pain duration is typically 48 to 72 hours and is associated with menstrual flow. In contrast, secondary dysmenorrhoea is more likely to occur years after the onset of menarche and premenstrually as well as during menstruation. In practice the accuracy of diagnosis depends on the availability and the use of diagnostic tools.
The aetiology of primary dysmenorrhoea has been the source of considerable debate. However, laboratory and clinical research have identified over‐production of uterine prostaglandins as a substantial contributing factor to the painful cramps that are the major symptom of dysmenorrhoea (Rosenwaks 1980). Prostaglandins are also implicated in secondary dysmenorrhoea: however, anatomical mechanisms can also be identified, depending on the type of accompanying pelvic pathology (Dawood 1990).
Medical therapy includes oral contraceptive pills (OCP) and nonsteroidal anti‐inflammatory drugs (NSAIDs) which both act by suppressing prostaglandin levels (Furniss 1982). Although the use of both OCPs and NSAIDs (Marjoribanks 2003; Proctor 2003) has been very successful, there is still a 20 to 25% failure rate (Dawood 1985; Henzl 1985). Pelvic nerve surgery has been a treatment for cases of dysmenorrhoea that fail to respond to medical therapy.
The pelvic viscera (internal organs) receive nerve impulses from both sympathetic (thoracolumbar) and parasympathetic (craniosacral) nervous systems (seeFigure 1). The parasympathetic and sympathetic pelvic nerve pathways are associated with the spinal vertebrae, in particular the second to fourth sacral segments (S2 to 4) and the tenth thoracic (T10) to the first lumbar segment (L1). The corpus, cervix and proximal fallopian tubes transmit pain through sympathetic fibres that arise from T10 to L1. These fibres include neurons that are part of the uterosacral ligaments and eventually merge into the superior hypogastric plexus (presacral nerve). The presacral nerve does not receive fibres from the ovaries or lateral pelvic structures. The lateral pelvis transmits pain via nervi erigentes (pelvic splanchnic nerve) arising from S2 to 4. The presacral nerve divides into the hypogastric nerve that form the inferior hypogastric plexus, and this plexus divides into vesical, middle rectal and uterovaginal (Frankenhauser's) plexuses (Frankenhauser 1864). The transection of the uterosacral ligaments, and the network of nerves it contains, is a simple surgical procedure for treatment of pelvic pain. The original work by Doyle described vaginal and abdominal approaches to divide the attachments of the uterosacral ligaments to the cervix (Doyle 1955).
1.

Sensory afferent nerve supply of the female pelvic organs. C = afferent nerve supply of cervix (illustrated on right side of diagram); O = afferent nerve supply of ovary (illustrated on left side of diagram); U = afferent nerve supply of uterus (illustrated on right side of diagram); P = parasympathetic nerve; S = sympathetic nerve. Copyright approval for reproduction of this figure has been kindly granted by Blackwell Science, publisher of Gynaecological Endoscopy (Johnson 2000).
When diagnostic laparoscopy is indicated, uterine nerve ablation (UNA) and presacral neurectomy (PSN) are two surgical treatments that have become increasingly utilised in recent years. UNA involves the transection of the uterosacral ligaments at their insertion into the cervix, whereas PSN involves the total removal of the presacral nerves lying within the boundaries of the interiliac triangle. These procedures both interrupt the majority of the cervical sensory nerve fibres thus diminishing uterine pain (Sutton 1993). There is wide variation in the techniques and practice of laparoscopic UNA (Latthe 2004b) and it is considered to be a relatively straightforward procedure that is achievable by all competent gynaecologic laparoscopists. Observational studies have supported the use of laparoscopic UNA for both primary and secondary dysmenorrhoea with either complete relief or substantial reduction in menstrual pain in the majority of participants (Donnez 1989; Ewen 1994; Feste 1985; Gurgan 1992; Perez 1990; Sutton 1989; Wiborny 1998). PSN involves the interruption of a greater number of nerve pathways than UNA: therefore, it is a more complex procedure, entails more operative risk and demands a high degree of skill by a surgeon who has specific training in this technique (Daniell 1995). However, despite these drawbacks the use of PSN is also supported by observational studies showing similar results to that of UNA for both primary and secondary dysmenorrhoea (Chen 1997; Nezhat 1992).
There are, however, limitations to the usefulness of UNA and PSN. A long‐term study showed that success rates declined rapidly over time, from 72% in the first year to 39% in the fourth for LUNA (Papasakelariou 1996). Other studies have shown risk of anatomical distortion such as uterine prolapse and bladder dysfunction (Davis 1996; Fitzpatrick 1995). There is also the concern regarding the effects of interruption of the pelvic nerves to the uterine muscles in subsequent pregnancies, such as painless labour. In addition, both operations only partially interrupt some of the cervical sensory nerve fibres in the pelvic area; therefore, dysmenorrhoea associated with additional pelvic pathology may not always benefit from this type of surgery. For this reason these techniques are often combined with additional treatments such as vaporisation or excision of endometrial implants.
Objectives
To determine the effectiveness of surgical interruption of pelvic nerve pathways (by both open and laparoscopic UNA or PSN) when compared to no treatment (where the control group receives either no treatment or a recognised treatment which is also performed in the intervention group) and to explore if the effects vary according to: (1) primary or secondary dysmenorrhoea and (2) UNA or PSN.
Methods
Criteria for considering studies for this review
Types of studies
All prospective randomised controlled trials comparing surgical interruption of pelvic nerve pathways (by both open and laparoscopic UNA and PSN) to no treatment or other treatment, for women with primary or secondary dysmenorrhoea.
Types of participants
Inclusion criteria:
women of reproductive years;
women with primary dysmenorrhoea (no identifiable organic pathology);
women with secondary dysmenorrhoea (identifiable specific pathology).
Exclusion criteria:
women with secondary dysmenorrhoea associated with the use of intrauterine contraceptive devices.
Types of interventions
Specific interventions that were considered.
UNA (laparoscopic or open) versus no treatment (as control) or other treatment for primary dysmenorrhoea.
PSN (laparoscopic or open) versus no treatment (as control) or other treatment for primary dysmenorrhoea.
UNA (laparoscopic or open) versus PSN (laparoscopic or open) for primary dysmenorrhoea.
UNA (laparoscopic or open) versus no treatment (where the control group was either no treatment or a recognised treatment which was also performed in the intervention group) or other treatment for secondary dysmenorrhoea.
PSN (laparoscopic or open) versus no treatment (where the control group was either no treatment or a recognised treatment which was also performed in the intervention group) or other treatment for secondary dysmenorrhoea.
UNA (laparoscopic or open) versus PSN (laparoscopic or open) for secondary dysmenorrhoea.
Laparoscopic and open techniques were combined for these interventions as there is some evidence to suggest they have similar ranges of pain relief for dysmenorrhoea (Nezhat 1992; Perez 1990).
Types of outcome measures
(1) Pain relief (a) Follow up more than six months after treatment (b) Follow up ‐ six months or less after treatment
Use of a visual analogue scale has been shown to be reliable in objective measurement of pain. Measurement with a VAS or other validated pain scales were studied and where these were not used other scales or dichotomous data were also considered. Changes in pain intensity were included.
(2) Adverse effects from the treatment (dichotomous data, number of participants with side effects) (3) Quality of life
Search methods for identification of studies
All reports which described (or might have described) randomised controlled trials of surgical interruption of pelvic nerve pathways (both open and laparoscopic) in the treatment of dysmenorrhoea were obtained using the search strategy developed by the Cochrane Menstrual Disorders and Subfertility Group.
We searched:
(1) the Cochrane Menstrual Disorders and Subfertility Group Trials Register (searched 9 June 2004);
(2) the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library Issue 2, 2004;
(3) electronic databases MEDLINE (1966 to Nov 2003), EMBASE (1980 to Nov 2003), and CINAHL (1982 to Oct 2003) using OVID software. These databases were searched using the following keywords: dysmenorrh$.tw dysmenorrhea/ painful menstruat$.tw pelvic pain/ surgery/ laparoscop$.tw surgical procedures, laparoscopic denervation uterine nerve ablation.tw presacral neurectomy.tw
(4) the metaRegister of Controlled Trials;
(5) citation lists of review articles and all included and excluded trials.
In most cases the first or corresponding author of included trials were contacted for additional information.
Data collection and analysis
Two authors of the review (MP and CF or MP and PL) performed the selection of trials for inclusion after employing the search strategy described previously.
Included trials were analysed for the following quality criteria and methodological details. This information is presented in the Table of included studies and helps provide a context for discussing the reliability of results.
Trial characteristics (1) Method of randomisation (2) Quality of allocation concealment until randomisation (3) Presence or absence of blinding to treatment allocation after randomisation (4) Number of women randomised, excluded or lost to follow up (5) Whether an intention‐to‐treat analysis was done (6) Whether a power calculation was done (7) Duration, timing and location of the study (8) Source of funding
Characteristics of the study participants (1) Age and any other recorded characteristics of women in the study (2) Other inclusion criteria (3) Exclusion criteria (4) Previously administered treatments
Interventions used (1) Type of surgical treatment used (UNA or PSN; either laparoscopic or open) used (2) Type of control, placebo or additional treatment used
Outcomes (1) Methods used to measure pain relief achieved by treatment (2) Methods used to measure adverse effects (3) Methods used to measure quality of life
All assessments of the quality of trials and data extraction were performed independently by two authors of the review (MP and OS or MP and PL) using forms designed according to Cochrane guidelines. A third author (NJ) resolved any discrepancies. Additional information on trial methodology or actual original trial data were sought from the authors of trials which appeared to meet the eligibility criteria but had aspects of methodology that were unclear, or where the data were in a form unsuitable for meta‐analysis. Authors from three trials were contacted to request additional information or data. Dr Vercellini supplied information about his study for the original review. Information and individual patient data were also supplied from the authors of Sutton 2001. John A Rock, a co‐author from the Tjaden 1990 trial also provided additional information on methodology and allocation. Authors for the Candiani 1992 and Chen 1996 trials were contacted but have not replied with additional information.
Statistical analyses were to be performed according to the statistical guidelines of the Cochrane Collaboration (Deeks 2004). Difficulties were encountered with the reporting of pain relief as a continuous outcome. Meta‐analysis with RevMan software offers a weighted mean difference (WMD) option to combine outcomes and requires data to be presented as absolute values of means with their standard deviations.
There is evidence to suggest that dichotomous outcomes can be derived from continuous data when interpreting pain relief data (Moore 1997). Therefore, attempts were made to dichotomise all other reported pain relief data in order to do subgroup analyses. Other pain scales were collapsed into dichotomous outcomes, pain relief or no pain relief. Lichten 1987 used a 5‐point scale where 0 = no pain; 1 = mild pain requiring no medication; 2 = moderate pain responding to mild analgesia; 3 = severe pain necessitating potent pain relievers; 4 = incapacitating pain, unresponsive to potent pain relievers. This scale was dichotomised with pain scores of 0, 1, and 2 labelled as pain relief (baseline pain for all participants was a score of 3 or 4). Candiani 1992 and Chen 1996 had reported dichotomised data measured in a similar way in their articles, so their reported figures were used in the analysis. Tjaden 1990 reported pain relief data as three subgroups midline, lateral and back pain; therefore, these data were described but not included in the meta‐analysis. Sutton 2001 used a 0 to 10 scale for recording information on pain, 0 representing no pain and 10 the worst pain ever experienced. This scale was dichotomised with scores of 4 or less labelled as pain relief. Where data points for six months post‐treatment were missing due to loss to follow up the last value recorded was carried forward (5 out of 26 women in the LUNA group and 5 of 23 in the control group). Zullo 2003 reported the number of women without dysmenorrhoea or dysmenorrhoea mild enough that no medication was required; these figures were combined and labelled as pain relief. Data from intention‐to‐treat analyses were used where available (for example Johnson 2004).
Heterogeneity between the results of different studies was examined by inspecting the scatter in the data points and the overlap in their confidence intervals and more formally by checking the results of the chi squared tests. For the dichotomous data, results of each study were expressed as an odds ratio with 95% confidence intervals and combined for meta‐analysis with RevMan software using the Peto‐modified Mantel‐Haenszel method. The outcome of pain relief is considered a positive consequence of treatment, therefore, a higher proportion of women with pain relief was considered a benefit (odds ratio (OR) greater than 1). The outcome of adverse effects is a negative consequence, therefore, higher numbers were considered to be detrimental (desirable, OR less than 1). This needs to be taken into consideration when the summary graphs are viewed.
Results
Description of studies
Eleven RCTs were identified involving surgical interruption of the pelvic nerve pathways as treatment for dysmenorrhoea.
Trials excluded from the review Two trials were excluded from the review. One excluded trial (Sutton 1994) compared laser laparoscopy (involving LUNA and surgical treatment of endometrial implants) to no treatment (expectant management only). Therefore due to the lack of a control group that had laser vaporisation only, the outcome data as a result of LUNA surgery could not be distinguished from the outcomes resulting from the laser vaporisation. The other excluded trial (Garcia Leon 2003) compared LUNA and LPSN in women with chronic pelvic pain which may or may not have included dysmenorrhoea. Approximately 60 to 80% of women in each treatment group had dysmenorrhoea at baseline however, we were unable to separate out data for women with dysmenorrhoea at baseline to determine which women benefited from treatment.
Trials included in the review Nine RCTs met the criteria for inclusion in the review. All the studies described clear inclusion and exclusion criteria (see Included studies table for more information). An ongoing randomised trial of LUNA for women with chronic pelvic pain without endometriosis was also identified (Birmingham trial; Latthe 2003). Data from this trial will be included when it becomes available. Currently 421 women have been recruited into this trial (October 2004) (http://www.luna.bham.ac.uk/trial/clinicians/recruitment.htm). The expected finishing date for the trial is September 2005 and the expected reporting date is December 2006 (Latthe 2003). Interventions Two trials (Johnson 2004; Lichten 1987) compared laparoscopic uterine nerve ablation (LUNA) with diagnostic laparoscopy only for women with primary dysmenorrhoea. Three trials (Johnson 2004; Sutton 2001; Vercellini 2003) combined LUNA and laser treatment of endometriosis implants as surgical techniques and compared them with laser treatment only, for women with secondary dysmenorrhoea. One trial compared LUNA and laparoscopic bipolar coagulation of uterine vessels with laparoscopic bipolar coagulation of uterine vessels only in women with dysmenorrhoea secondary to uterine myomas (Yen 2001). Three trials compared presacral neurectomy (PSN) combined with surgical treatment of endometriosis versus surgical treatment of endometriosis only as a control for treatment of secondary dysmenorrhoea (Candiani 1992; Tjaden 1990; Zullo 2003). The final included trial compared LUNA and LPSN as treatments for primary dysmenorrhoea (Chen 1996).
The surgery involved was performed using standard techniques. In one trial of LUNA, with diagnostic laparoscopy as a control (Lichten 1987), the participants in the experimental group had bilateral uterosacral ligament electrocautery at their insertion into the cervix. The ligaments were then cut through and an electrical current was reapplied to the depth of the incision. The generator used was set at 5.8 W.
The Vercellini trial (Vercellini 2003) also used mechanical instruments and electro‐surgery. The full thickness of the uterosacral ligaments was coagulated with bipolar forceps. A segment 1 cm or greater in depth and length was excised with scissors close to the uterine junction.
The Guildford, UK trial (Sutton 2001) performed uterine nerve ablation with a CO2 laser using a triple‐puncture technique.
In the New Zealand trial (Johnson 2004) bilateral uterosacral ligament electrocautery was applied (blend current at power 30 units) 0.5 cm from the ligamentous insertion into the cervix until tissue was blanched. Complete transection of the ligaments was then undertaken with laparoscopic scissors and bipolar electrocautery reapplied to the base of the incision to secure haemostasis.
In the Taipei, Taiwan trial ligaments were coagulated and dissected at their insertion into the cervix with unipolar or bipolar electrocoagulation (Yen 2001). After dissection, grasping forceps were used to connect coagulated areas in a U‐like fashion along the base of the cervix and uterosacral ligaments were cut through with mechanical operating scissors. Current was then applied to the depth of this incision.
The Keelung, Taiwan trial (Chen 1996) compared LUNA and LPSN. The technique used for LUNA was comparable to that used by Lichten 1987, and LPSN involved laparoscopic surgery and total removal of the presacral nerves that lie within the boundaries of the interiliac triangle. This was performed using unipolar or bipolar electrocautery, or both, the excised nerve tissue being later confirmed histologically.
The Italian trial (Zullo 2003) compared laparoscopic PSN with ablation or excision of visible endometriotic lesions versus ablation or excision of visible endometriotic lesions only. In women undergoing PSN the presacral area was exposed with blunt dissection and all underlying tissue layers down to the periosteum were cauterised. A semilunar piece of retroperitoneal tissue was dissected and sent for pathologic confirmation of nerve fibre presence.
The last two trials (Candiani 1992; Tjaden 1990) used PSN via laparotomy combined with resection of endometriosis implants for the experimental groups.
Participants All the trials included women between the ages of 18 to 50 years of age and the majority of the studies' participants appeared to have sought medical assistance for dysmenorrhoea. None of the trials gave clear information on the source of the women or how they were recruited into the studies. Two of the studies (Chen 1996; Lichten 1987) looked exclusively at women with primary dysmenorrhoea, excluding all participants with any pelvic pathology with a diagnostic laparoscopy. One study included women with dysmenorrhoea related to uterine myoma (Yen 2001). One study included two patient groups: women with primary dysmenorrhoea and women with secondary dysmenorrhoea associated with endometriosis (Johnson 2004). The other studies included women with secondary dysmenorrhoea associated with endometriosis. Two of the included studies involving women with endometriosis included those with an AFS classification of stage III to IV endometriosis (Candiani 1992; Tjaden 1990), one trial included women with only stage I to III (Sutton 2001) and the other trials included all stages I to IV (Johnson 2004; Vercellini 2003; Zullo 2003). Outcomes The primary outcome in all nine trials was pain relief. This was measured and reported in a variety of ways. Three studies (Chen 1996; Lichten 1987; Yen 2001) used a 5‐point pain scale; Chen and Yen converted scores into either success or failure in pain relief while Lichten reported each participant's raw scores. Visual analogue scales (VAS) were also used in some of the trials: however, the length of the scales varied. Sutton 2001 used a 10 cm VAS as well as a 10‐point pain scale. Candiani 1992 used a 10‐point VAS (but only reported dichotomised data) and a multidimensional scale. This scale comprised three components: limitation of working ability, coexistence of systematic symptoms and need for analgesics. Johnson 2004 used a 10‐point VAS and a dichotomous measure of pain relief. Vercellini 2003 and Zullo 2003 used a 100mm VAS, and Tjaden 1990 simply reported whether pain relief did or did not occur.
Side effects were reported by the majority of studies as the number of women who suffered any specific adverse events for example constipation. Outcomes were assessed at various time periods following surgery. In two trials (Tjaden 1990; Yen 2001) participants were assessed at six months, although Tjaden stated that participants were followed for a minimum of 42 months. One trial assessed participants at three and six months (Sutton 2001). In two trials (Chen 1996; Johnson 2004) participants were assessed at three and 12 months. Two trials (Vercellini 2003; Zullo 2003) assessed participants at six and 12 months, although Vercellini extended follow up of some women for up to 36 months. In the final two trials (Candiani 1992; Lichten 1987) all participants were followed up for at least 12 months.
Risk of bias in included studies
Randomisation and allocation concealment Three of the trials received a quality allocation score of A based on adequate concealment prior to allocation (Johnson 2004; Tjaden 1990; Vercellini 2003). Two studies received an allocation score of C (Lichten 1987; Yen 2001) due to the use of case numbers in the allocation process. Despite poor allocation concealment other aspects of the Lichten study were adequate, such as double blinding and very strict inclusion and exclusion criteria. Other trials received an allocation score of B as there was not enough information in the trial report to determine whether the randomisation sequence was adequately concealed until interventions were assigned.
All nine included trials randomised participants to treatment.
Blinding Double blinding was used in six studies, with blinding of the patient and the investigator (Johnson 2004; Lichten 1987; Sutton 2001; Vercellini 2003; Yen 2001; Zullo 2003); one was single blind (Tjaden 1990); and for the other trials blinding was unclear.
Intention‐to‐treat analysis and power calculations Two trials (Johnson 2004; Vercellini 2003) included an intention‐to‐treat (ITT) analysis.
A power calculation was performed in five studies (Candiani 1992; Johnson 2004; Lichten 1987; Vercellini 2003; Zullo 2003). Tjaden 1990 also included a power calculation but the trial was stopped before the number of women needed was reached. The monitoring committee stopped the study after evaluation of data from the first 26 women as it was considered unethical to deprive women with midline dysmenorrhoea the relief afforded by presacral neurectomy. Only eight of the 26 participants were randomised, while the others chose their group; however the data for each method of allocation was reported separately, so for this review only the randomised data were included.
All the included studies assessed comparability of the treatment and control groups at baseline; no appreciable differences in age, parity, condition or pain scores were reported.
Follow up Follow up and withdrawal rates varied among trials. Two trials reported no withdrawals or losses to follow up (Chen 1996; Tjaden 1990). In five trials less than 15% of randomised participants withdrew or were lost to follow up (Candiani 1992; Johnson 2004; Sutton 2001; Yen 2001; Zullo 2003). In one trial of 180 participants only 116 (64%) were analysed: 29 became pregnant, 14 used OCP, 15 (8%) were lost to follow up and six women withdrew for other reasons (Vercellini 2003). In another trial, 18 of 39 women (46%) were excluded from analysis due to pathology at follow up (Lichten 1987).
Effects of interventions
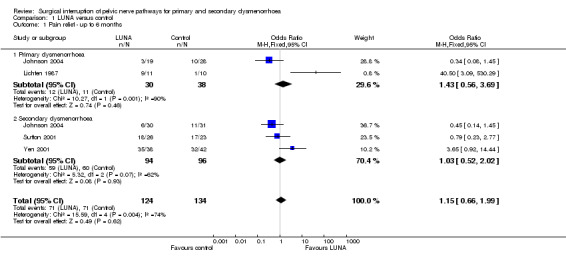
There were two trials with open PSN; all other trials use laparoscopic techniques. UNA (laparoscopic or open) versus control Primary dysmenorrhoea There were two studies compared LUNA versus control for primary dysmenorrhoea (Johnson 2004; Lichten 1987). At six months or less follow up there was no significant difference in pain relief (2 RCTs; N = 68; OR 1.43, 95% CI 0.56 to 3.69). The Chi squared test for heterogeneity showed there was significant heterogeneity between these two trials (10.27, degrees of freedon (df) = 1, P value < 0.001). This heterogeneity was also evident by the scatter in the data points on the meta‐analysis.
However, longer term pain relief (assessed at 12 months) showed a significant difference between the experimental and control groups (2 RCTs; N = 68; OR 6.12, 95% CI 1.78 to 21.03). One trial (Johnson 2004) reported additional outcomes related to quality of life following treatment. Satisfaction rates at 12 months showed no difference between group (LUNA 15 of 18 satisfied; control 22 of 32; P value > 0.05). Information on the need for further surgery (one hysterectomy in the LUNA group and two in the control group), and the need for additional treatment (three women in the no LUNA group were using OCP or Mirena), also indicated no difference between the two groups.
Secondary dysmenorrhoea There were four trials that compared LUNA with surgical treatment of endometriosis versus surgical treatment of endometriosis only (Johnson 2004; Sutton 2001; Vercellini 2003; Yen 2001). At six months or less follow up there was no significant difference in pain relief (3 RCTs; N= 190; OR 1.03, 95% CI 0.52 to 2.02). Longer term pain relief also showed no significant difference between groups (2 RCTs; N= 217; OR 0.77, 95% CI 0.43 to 1.39). Sutton 2001 reported comparable baseline pain scores, and at six months post‐operative showed no significant difference between the experimental and control groups for dysmenorrhoea pain scores for the VAS scale (Mann‐Whitney test, P value = 0.21 respectively). On the 10 cm VAS scale the experimental group pain scores at six months had a median of 4.8 (range 1 to 9.0), while the control pain scores had a median of 3.0 (range 0 to 9.8).
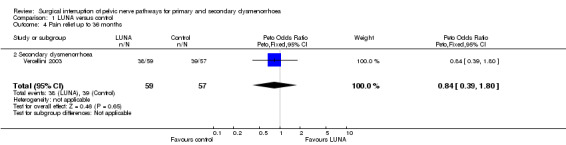
One trial reported data on pain relief up to 36 months following treatment (Vercellini 2003). There was no significant difference in pain relief between the treatment and control group following this extended follow up (1 RCT; N = 116; OR 0.84, 95% CI 0.39 to 1.80).
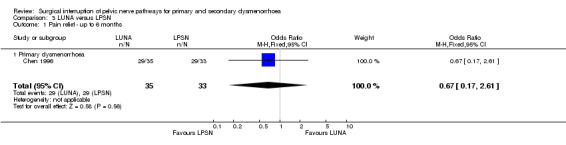
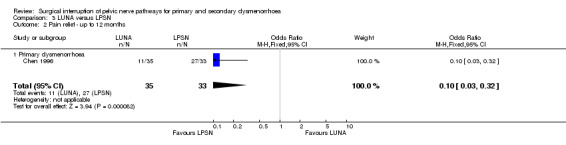
In one trial (Vercellini 2003) the 12 month Kaplan‐Meyer cumulative probability of recurrence of moderate to severe dysmenorrhoea was 33.7% for the experimental group and 27.55% for the control group. An intention‐to‐treat analysis on participant satisfaction showed that 68% of the experimental group and 73% of the control group were very satisfied or satisfied with treatment; while 32% of the experimental group and 27% of the control group were uncertain, dissatisfied, or very dissatisfied with treatment. No adverse effects were reported for either group. Vercellini 2003 also collected additional quality of life data including the Short Form‐36, Hospital Anxiety and Depression Scale, and the Revised Sabbatsberg Sexual Rating Scale. There were significant mean improvements in all scales; however, at one‐year follow up the trial reported that there were no significant differences between groups. PSN (laparoscopic or open) versus control Secondary dysmenorrhoea Three trials compared PSN with surgical treatment of endometriosis versus surgical treatment of endometriosis only (Candiani 1992; Tjaden 1990; Zullo 2003). Two trials used PSN (Candiani 1992; Tjaden 1990) and one trial LPSN (Zullo 2003). At six months or less follow up there was a significant difference in pain relief in favour of PSN (1 RCT; N = 126; OR 4.52, 95% CI 1.84 to 11.09). Pain relief measured up to 12 months following treatment also showed a significant difference between treatment groups (2 RCTs; N = 197; OR 3.14, 95% 1.59 to 6.21). In one trial (Candiani 1992), which reported no difference in treatments, the authors originally collected information on the incidence, site and severity of pain and in analysis split their results into separate areas of pain. They interpreted their findings as showing a significant difference in the recurrence of midline abdominal dysmenorrhoeic pain, with the experimental group reporting what the authors interpreted as a significantly lower recurrence (P value = 0.06). There was a strong significant difference in the proportion of women with adverse effects from the treatment: the control group reported none but the PSN group reported 13 women with constipation, three with urinary urgency and two experienced a painless first stage of labour (OR 14.6, 95% CI 5.0 to 42.2). This trial also evaluated dysmenorrhoea on a multidimensional scoring system that included limitation of working ability, systemic symptoms, and need for analgesics. Using this scoring system there was no significant difference between the treatment and control groups with both groups showed a large reduction in symptoms (absent or mild symptoms ‐ PSN 30 of 35 women, control 29 of 36 women) Tjaden 1990 also originally collected information on incidence and location of pain and split pain relief into areas of pain. They found that pain relief in the experimental and control groups were significantly different for midline abdominal pain (Fisher exact test, P value = 0.028). However, for back pain or lateral pain associated with dysmenorrhoea there were no significant differences between the groups. These results were based on only eight randomised participants. UNA versus PSN (laparoscopic or open) Primary dysmenorrhoea There was only one study in this comparison and both types of surgery were performed laparoscopically (Chen 1996). Results of the meta‐analysis showed that there was no significant difference between the two treatments at six months or less follow up (1 RCT; N = 68; OR 0.67, 95% CI 0.17 to 2.61). However at more than six months follow up the pain relief scores were significantly different with LPSN achieving more effective pain relief (1 RCT; N = 68; OR 0.10, 95% CI 0.03 to 0.32). There was also a strong significant difference in the number of adverse effects reported by the participants 94% of the LPSN group reported constipation with the LUNA group reporting none (1 RCT; OR 0.02, 95% CI 0.01 to 0.06).
Discussion
This review assessed the effectiveness of surgical interruption of pelvic nerve pathways in the treatment of dysmenorrhoea. The techniques specifically investigated were uterine nerve ablation and presacral neurectomy (using both open and laparoscopic procedures).
The use of LUNA is shown by this review to be effective for primary dysmenorrhoea but in the long term (12 months) only. Lichten 1987 (N = 21) reported significant differences in pain scores between the experimental and control groups at both short‐term and long‐term follow up; however, a large trial (Johnson 2004: primary dysmenorrhoea, N = 47) showed no difference in the short term (up to six months) yet a difference in favour of LUNA in the longer term. The results of the Lichten trial alone suggested that the effectiveness of LUNA may decline over time; however, results from the Johnson trial showed this was not the case. It should be noted that the Johnson trial had adequate allocation concealment and randomisation while the Lichten trial used sequential allocation. The drawback of short‐term follow up is that the effect of treatment may overlap with the placebo effect of laparoscopy, thus the placebo effect could diminish differences between groups (Baker 1992). The results of Chen 1996, which also investigated primary dysmenorrhoea, showed a clear decline in effectiveness. Chen reported a dramatic drop in the pain relief afforded by LUNA compared to LPSN at three and 12 months after surgery. These results are consistent with a previous long‐term study which showed that success rates declined rapidly over time, from 72% in the first year to 39% in the fourth year (Papasakelariou 1996). In the long term, the lack of sustained benefit could be either due to regrowth of nerves or pain signals being transferred via alternative routes. Overall the meta‐analysis suggested that the short‐term results for LUNA are still uncertain. The results at 12 months showed that LUNA effectively reduced pain in primary dysmenorrhoea; however, the wide confidence intervals prompt caution in applying the results in practice.
For the comparison between LUNA and LPSN, the results of the meta‐analysis showed that in the short term the two techniques were not significantly different in effectiveness; but long term, LPSN was significantly more effective in reducing pain (Chen 1996). One drawback of LPSN was the significantly higher level of adverse effects; constipation being the commonest, but urinary urgency and painless labour also being reported. Constipation is a manageable but inconvenient side effect and the authors suggested that it may spontaneously improve over time.
In the evaluation of the effectiveness of LUNA in treating secondary dysmenorrhoea, the meta‐analysis shows no difference between LUNA compared to control at any time point. Vercellini 2003 continued to follow the majority of participants for 36 months and the lack of difference continued. The four studies indicated that UNA combined with surgical treatment of endometriosis was no more effective than surgical treatment of the endometriosis alone for treatment of secondary dysmenorrhoea.
The results for PSN as a treatment for secondary dysmenorrhoea associated with endometriosis were mixed. Overall the meta‐analysis showed that PSN with conservative surgery for endometriosis was more effective than conservative surgery alone; however, results from individual trials varied. Candiani 1992 showed no significant difference between the experimental and control groups but midline abdominal dysmenorrhoeic pain was interpreted by the authors as responding better to PSN; however, the difference was not statistically significant (P value = 0.06). The Candiani trial included women with either a laparotomic or laparoscopic diagnosis of endometriosis and no information was reported on how the PSN procedure was performed. The other study, which was not included in the meta‐analysis, reported a statistically significant difference in midline abdominal pain (P value = 0.03) but no difference in pain relief for women with lateral or back pain (Tjaden 1990). This trial included PSN via laparotomy only. A major shortcoming of both these trials was the small sample sizes. Candiani had a sample of 71 participants and, therefore, power to detect differences greater than 40% (Candiani 1992). The difference in pain relief between the two groups was only 5%, therefore, no conclusions could be accurately drawn. The Tjaden study only randomised eight participants so the statistically significant result regarding midline pain was based on the results of the four participants in the experimental group. While the monitoring committee considered this to be a strong enough result to discontinue the trial it is not a large enough sample from which to claim effectiveness. The largest study in the meta‐analysis (N = 126) (Zullo 2003), which demonstrated that LPSN was effective as a treatment, was the only trial in which it was clear that all women had PSN via laparoscopy. This trial also excluded women without a midline component to their dysmenorrhoea; however, it should be noted that over 80% of women in this trial had a lateral component to their dysmenorrhoea.
Quality of life measures were reported in only one study (Candiani 1992) even though improvement of this is the ultimate goal for the patient and the clinician.
Overall, the small number of participants who have been entered into randomised controlled trials on UNA and PSN make it difficult to assess effectiveness in treating dysmenorrhoea. In the trials with null results inadequacy of power to detect a clinically important difference is an issue of concern. Overestimation of the expected clinical difference at the time of power calculation can lead to underestimation of the sample size. This seems to have happened in the Vercellini trial (Vercellini 2003) with the observed effect size showing wide confidence intervals (OR 1.13, 95% CI 0.56 to 2.28), indicating a potential for benefit as well as harm at the extremes of the confidence intervals. Candiani 1992 also reported wide confidence intervals after overestimating the clinical difference in treatments when calculating sample size (it had power to demonstrate a difference of 40%) (OR 1.61, 95% 0.51 to 5.13). The drawbacks of the Zullo study (Zullo 2003) were single (fixed) block randomisation, lack of intention‐to‐treat analysis and limited generalisability of results due to a single centre trial (Latthe 2004a).
Authors' conclusions
Implications for practice.
Laparoscopic presacral neurectomy (PSN) is a surgical procedure that requires a high degree of skill by an experienced pelvic laparoscopic surgeon who is trained specifically in this retroperitineal operation. The presacral region may be highly vascular and the procedure carries major potential hazards for the unwary or inadequately trained surgeon. Conversely, although laparoscopic uterine nerve ablation (UNA) must be performed precisely to avoid complications it should be within the scope of all competent pelvic laparoscopic surgeons.
There is some evidence for continuing to investigate the use of laparoscopic PSN and UNA in primary dysmenorrhoea within clinical trials. There is currently no evidence to recommend the use of UNA to treat endometriosis although there is some evidence for the use of PSN.
Implications for research.
To help resolve the issue of effectiveness of neuroablation, clinicians may initiate good quality and adequately powered trials or participate in ongoing multicentre trials. There is a lack of good quality RCTs in all the comparisons examined in this review. The main issues are sample size and trial methodology. Trial design should include the following aspects to ensure adequate methodological quality: double blinding; secure concealed randomisation and allocation of participants; longer‐term follow up (at least one year); placebo arms; valid and reliable measurement of outcomes (such as the VAS scale for pain and quality of life measures); use of a power calculation to ensure enough participants are included and followed up. Inclusion criteria should also include a full description of any pathology. An individual patient data meta‐analysis may address the uncertainty by combining raw data from various studies included in this review as well as the data from ongoing studies (Birmingham trial; Birmingham trial; Latthe 2003).
Feedback
figures
Reply
:
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2010 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 12 November 2008 | Amended | Converted to new review format. |
| 24 August 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors wish to acknowledge the referees of the previous versions of this review for their helpful comments and the authors, of the included trials, that have supplied extra information or data. We would also like to acknowledge Owen Sinclair who, in the original review; performed the independent data extraction and quality assessment of six included trials, and Kathryn Burns who, in this update, added information from new trials into the appropriate tables.
Data and analyses
Comparison 1. LUNA versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief ‐ up to 6 months | 4 | 258 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.66, 1.99] |
| 1.1 Primary dysmenorrhoea | 2 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.56, 3.69] |
| 1.2 Secondary dysmenorrhoea | 3 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.52, 2.02] |
| 2 Pain relief ‐ up to 12 months | 3 | 285 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.72, 1.99] |
| 2.1 Primary dysmenorrhoea | 2 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.12 [1.78, 21.03] |
| 2.2 Secondary dysmenorrhoea | 2 | 217 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.39] |
| 4 Pain relief up to 36 months | 1 | 116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
| 4.2 Secondary dysmenorrhoea | 1 | 116 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
1.1. Analysis.
Comparison 1 LUNA versus control, Outcome 1 Pain relief ‐ up to 6 months.
1.2. Analysis.

Comparison 1 LUNA versus control, Outcome 2 Pain relief ‐ up to 12 months.
1.4. Analysis.
Comparison 1 LUNA versus control, Outcome 4 Pain relief up to 36 months.
Comparison 2. PSN versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief ‐ up to 6 months | 1 | 126 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [1.84, 11.09] |
| 1.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 1 | 126 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.52 [1.84, 11.09] |
| 2 Pain relief ‐ up to 12 months | 2 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.59, 6.21] |
| 2.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 2 | 197 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.59, 6.21] |
| 3 Adverse effects | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 14.57 [5.04, 42.15] |
| 3.2 PSN with surgical treatment of endometriosis vs surgical treatment of endometriosis only | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 14.57 [5.04, 42.15] |
2.1. Analysis.

Comparison 2 PSN versus control, Outcome 1 Pain relief ‐ up to 6 months.
2.2. Analysis.

Comparison 2 PSN versus control, Outcome 2 Pain relief ‐ up to 12 months.
2.3. Analysis.

Comparison 2 PSN versus control, Outcome 3 Adverse effects.
Comparison 3. LUNA versus LPSN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain relief ‐ up to 6 months | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.61] |
| 1.1 Primary dysmenorrhoea | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.17, 2.61] |
| 2 Pain relief ‐ up to 12 months | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.32] |
| 2.1 Primary dysmenorrhoea | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.03, 0.32] |
| 3 Adverse effects | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.3 Primary dysmenorrhoea | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.02 [0.01, 0.06] |
3.1. Analysis.
Comparison 3 LUNA versus LPSN, Outcome 1 Pain relief ‐ up to 6 months.
3.2. Analysis.
Comparison 3 LUNA versus LPSN, Outcome 2 Pain relief ‐ up to 12 months.
3.3. Analysis.
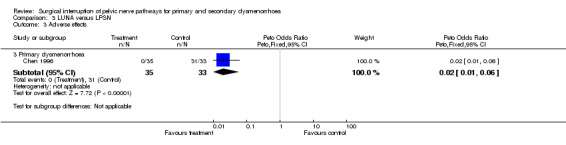
Comparison 3 LUNA versus LPSN, Outcome 3 Adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Candiani 1992.
| Methods | Allocation Concealment: unclear. Randomisation: randomisation list, no other details reported. Blinding: unclear. Power Calculation: sample size based on assumption that dysmenorrhoea would resolve in 50% controls and 80 ‐ 90% PSN group. Number of included patients gives 80% power for a difference of 30% 90% power for a difference of 40%, 0.05 level of significance. Duration of trial: Recruitment from June 1986 to Jan 1990. Follow up of all women for at least one year. | |
| Participants | Number of women randomised: 78. Number of women analysed: 71. Drop‐outs/withdrawals: 7 women did not accept randomisation. Inclusion criteria: women with endometriosis stage III or IV undergoing conservative surgery, moderate or severe midline or midline and lateral menstrual pelvic pain (dysmenorrhoea). Diagnosis: gastro, urologic and orthopaedic evaluation to exclude other causes of pelvic pain, endometriosis confirmed at surgery. Age: Control group mean 31.1, SD 3.6; PSN surgery group mean 32.5, SD 4.2. Location: Milan, Italy. | |
| Interventions | Treatment: presacral neurectomy with conservative surgery for endometriosis. Control: conservative surgery for endometriosis. | |
| Outcomes | Dysmenorrhoea, measured by a 0 ‐10 analog scale and by a multidimensional scale which included limitation of working ability, systemic symptoms, and need for analgesics. Data reported as mild, moderate or severe pain prior to surgery and 12 months following surgery. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Chen 1996.
| Methods | Allocation Concealment: unclear. Randomisation: unclear, stated as randomised no further details given. Blinding: unclear. Power Calculation: none stated. Duration of trial: Recruited from Jan 1992 to July 1993. Patients followed up for 12 months. | |
| Participants | Number of women randomised: 68. Number of women analysed: 68. Drop‐outs/withdrawals: none. Inclusion criteria: women with primary dysmenorrhoea and/or chronic pelvic pain. Exclusion criteria: pelvic pathology (lesions). Diagnosis: at laparoscopy, those without lesions that could be assoc with dysmenorrhoea were randomised. Age: 18 to 40 years. Location: Taiwan. | |
| Interventions | Treatment: laparoscopic uterine nerve ablation. Control: laparoscopic presacral neurectomy. | |
| Outcomes | Pain relief measured on a 5 point scale (0 = no pain to 4 = incapacitating pain unresponsive to potent pain relievers and the inability to function). Pain was measured at baseline, 3 months, 12 months and data was dichotomised into success 100 > 50% pain relief or failure 50 > 0% pain relief. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Johnson 2004.
| Methods | Allocation concealment: stated as maintained securely by storage in sealed, sequentially‐numbered opaque envelopes until the interventions were assigned during the laparoscopic procedure Randomisation: computer‐generated random number sequences Blinding: used; participant and investigator blinded throughout the study Power Calculation: for women with chronic pelvic pain in the absence of endometriosis, in order to have 80% power at the 95% confidence level to detect benefit in 50% of women, assuming 'benefit' in 10% controls, at least 48 participants would be required for analysis following randomisation. For women with endometriosis, in order to have 80% power at 95% confidence level to detect benefit in 90%, assuming benefit in 60% controls undergoing conventional endometriosis surgery, at least 76 participants would be required for analysis following randomisation. Allowing for losses to follow up, it was planned to recruit 50 women with chronic pelvic pain in the absence of endometriosis (achieved) and 80 women with endometriosis (not achieved). Duration of trial: April 1997 to Dec. 2001. Follow up: 24 hours, 3 and 12 months. | |
| Participants | Number of women randomised: 123 (108 with dysmenorrhoea) Number of women analysed: 123 Drop‐outs/withdrawals: 14 were excluded based on laparoscopic findings Loss to follow up: 24 hours: 1 3 months: 3 (2 LUNA and 0 no LUNA in the population with no endometriosis; 0 LUNA and 1 no LUNA in the endometriosis population). 12 months: 17 (4 LUNA and 2 no LUNA in the population with no endometriosis; 6 LUNA and 5 no LUNA in the endometriosis population) Inclusion criteria: Women aged 18 to 45 years inclusive; a history of chronic pelvic pain (either dysmenorrhoea, non‐menstrual pelvic pain, defaecatory pain or deep dyspareunia for more than 6 months); no change in medication for the 3 months prior to trial recruitment. Exclusion criteria: previous hysterectomy or pelvic malignancy; previous LUNA; known ovarian cysts; plan for a pregnancy within 12 months; intention to change other medical treatment which could influence pelvic pain scores within 12 months; laparoscopic findings rendering LUNA impossible (for example frozen pelvis with no access to uterosacral ligaments) or the finding of pelvic adhesions which did not appear to be due to endometriosis. Location: Auckland, New Zealand. | |
| Interventions | 2 groups: Group with endometriosis: Treatment: LUNA with conservative surgery for endometriosis. Control: conservative surgery for endometriosis. Group without endometriosis: Treatment: LUNA at laparoscopy. Control: laparoscopy alone. | |
| Outcomes | Changes in non‐menstrual pelvic pain, dysmenorrhoea, deep dyspareunia and dyschezia were assessed primarily by whether there was a decrease in visual analog score for these types of pain of 50% or more from baseline; additionally whether there was a significantly different change in median visual analog score. The numbers requiring further surgery or starting a new medical treatment for pelvic pain and complications were also measured. Adverse effects: no important intraoperative or postoperative complications occurred (specifically there were no cases of ureteric injury, intraoperative bleeding nor postoperative haematoma formation), other than 2 women who had urinary retention requiring catheterisation within 24 hours of the surgery (both in the endometriosis population not undergoing LUNA). | |
| Notes | 137/200 agreed to participate; 14 excluded at laparoscopy; follow up at 12 months: 106/123 (86.2%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Lichten 1987.
| Methods | Allocation concealment: inadequate. Randomisation: randomised by last digit of medical case number on day of surgery. Blinding: both participant and clinical psychologist, who recorded outcomes, were blinded. Power Calculation: none stated. Duration of trial: 12 mths | |
| Participants | Number of women recruited: 39. 18 were excluded due to pathology (endometriosis, PID). Number of women randomised: 21. Number of women analysed: 21. Drop‐outs/withdrawals: none. Inclusion criteria: women with severe dysmenorrhoea and no improvement with at least 2 NSAIDs and an oral contraceptive (30 and 50 ug of estrogen only) concurrently. Exclusion criteria: history of psychotherapy, major abdominal procedures, drug abuse or demonstrable pelvic pathology. Diagnosis: diagnostic laparoscopy. Age: 18‐34 yrs. Location: USA. | |
| Interventions | Treatment: laparoscopic uterine nerve ablation. Control: diagnostic laparoscopic surgery only. | |
| Outcomes | Pain was measured on a five point scale (0 = no pain to 4 = incapacitating pain unresponsive to potent pain relievers and the inability to function). Pain scores for each patient were reported preoperatively and at 3mths and 12mths. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Sutton 2001.
| Methods | Allocation Concealment: unclear. Randomisation: computer‐generated randomisation sequence. Blinding: double; participant and research nurse. Power Calculation: done; sample for 90% power was 22 women in each group. Duration of trial: women assessed at 3 and 6 months post operative (recruitment occurred over 33 months Feb.1995 to Nov. 1997). | |
| Participants | Number of women randomised: 51. Number of women analysed: 46 at 6 months. Drop‐outs/withdrawals: 5 (1 became pregnant and 4 were lost to follow up). However data points for to 14 women were missing for some analyses. Inclusion criteria: women with history and physical or laparoscopic examination suggestive of endometriosis who had not received medical treatment for endometriosis within the last 6 months, and had not previously undergone surgical treatment of their disease. Exclusion criteria: stage IV disease or any other pathology that may have been responsible in whole, or in part for their symptoms. Diagnosis: at laparoscopy those with stage IV disease or other pathology were excluded. Age: mean 28 (20 to 41). Location: Surrey, UK. | |
| Interventions | Treatment: LUNA with laparoscopic treatment of all visible endometriosis. Control: laparoscopic treatment of all visible endometriosis . | |
| Outcomes | Dysmenorrhoea, measured by linear analogue scale (0 ‐ 10) and pain scoring questionnaire. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Tjaden 1990.
| Methods | Allocation concealment: adequate. Randomisation: centralised randomisation process, with sealed, opaque, sequentially numbered, identical envelopes. Blinding: single; randomised participants were blinded. Power Calculation: none stated. Duration of trial: 6 months post‐operative; however follow‐up continued for a minimum of 42 months. | |
| Participants | Number of women randomised: 8; also 18 women not randomised but followed up. Number of women analysed: 26. Drop‐outs/withdrawals: none. Inclusion criteria: women with moderate to severe dysmenorrhoea scheduled to undergo laparotomy for conservative resection of endometriosis. Diagnosis: initial detailed history of pain and anatomical diagram for localisation of dysmenorrhoea, endometriosis confirmed at laparotomy. Age: mean 30 yrs. Location: USA. | |
| Interventions | Treatment: presacral neurectomy and resection of endometriosis. Control: resection of endometriosis only. | |
| Outcomes | Relief of pain was reported as the number of women with pain relief in 3 locations. Adverse effects. | |
| Notes | Data analysed pooled, and split into protocol (randomised) and non‐protocol (non‐randomised) groups. Study stopped by monitoring committee after 26 participants, as it was considered unethical not to provide those with midline dysmenorrhoea the pain relief that PSN exhibited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vercellini 2003.
| Methods | Allocation concealment: adequate. Randomisation: computer‐generated randomisation in single blocks. Blinding: used; patient and investigator blinded throughout the study. Power Calculation: sample size based on assumption that dysmenorrhoea would reoccur in 30% of the controls and 10% of the treatment group. 72 patients were needed in each group to demonstrate a difference of 20% between control and treatment groups and to define statistical significance between the groups with an alpha of 0.05 and beta of .20. Duration of trial: Sept. 1998 to Oct 2001. Follow up : 6 and 12 months. | |
| Participants | Number of women randomised: 180. Number of women analysed: 116. Drop‐outs/withdrawals: 29 pregnant, 14 used oral contraception, 15 lost to follow‐up, 6 miscellaneous reasons. Inclusion criteria: Aged 18 to 40, undergoing first‐line operative laparoscopy for symptomatic minimal to severe endometriosis who reported pelvic pain of more than 6 months duration. Exclusion criteria: previous diagnosis of endometriosis, other diseases that might cause pelvic pain, treatment for endometriosis other than nonsteroid anti‐inflammatory drugs up to 6 months before entry in the study, presence of vaginal endometriotic lesions, previous diagnosis of gastrointestinal, urologic and orthopedic diseases in which pain may radiate to the pelvic area, known psychiatric disturbances. Age: 18‐40. mean for each group not given. Location: Milan, Italy. | |
| Interventions | Treatment: conservative laparoscopic surgery with the addition of uterosacral ligament resection. Control: conservative laparoscopic surgery. | |
| Outcomes | Dysmenorrhoea, measured by a 100 mm visual analog scale that ranged from "least possible pain" to "worst possible pain". Frequency was expressed as the number of episodes per each cycle for dysmenorrhoea and chronic pelvic pain. Hospital anxiety and depression scale, sexual rating scale, SF 36. Adverse effects: None attributable to pelvic denervation | |
| Notes | Proportion of women satisfied with the treatment were similar. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yen 2001.
| Methods | Allocation concealment: inadequate. Randomisation: Women with even hospital nos.(assigned on the day of surgery) were cases and those with odd hospital nos. were controls. Blinding: double; patient and clinical psychologist. Power Calculation: not stated. Duration of trial: follow up at 1, 3 and 6 months. | |
| Participants | Number of women randomised: 85. Number of women analysed: 80 at 1, 3 and 6 months. Drop‐outs/withdrawals:5 (1 in each group had procedure converted to abdominal hysterectomy due to adhesions, 1 became pregnant and 2 were lost to follow‐up). Inclusion criteria: women with symptoms of uterine myomas, including dysmenorrhoea, menorrhagia, and bulk related symptoms, documented absence from school or work due to dysmenorrhoea and no response to OC or NSAIDS for at least two cycles. Exclusion criteria: history of psychotherapy, major abdominal surgery or drug abuse. Diagnosis: ultrasound. Age: Control group mean 43.1, SD 5.1; Treatment group mean 44.5, SD 4.4. Location: Taipei, Taiwan. | |
| Interventions | Treatment: laparoscopic uterine nerve ablation with laparoscopic bipolar coagulation of uterine vessels (LBCUV). Control: LBCUV. | |
| Outcomes | Dysmenorrhoea, improvement measured by analgesic use, and scale ‐ completely resolved, significantly improved, slightly improved, unchanged or worsened. Pain data was reported the numbers of women slightly, significantly or completely improved. Adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Zullo 2003.
| Methods | Allocation concealment: unclear. Randomisation: computer‐generated randomisation in single blocks. Blinding: double; participant and investigator blinded throughout the study. Power Calculation: sample size based on assumption that dysmenorrhoea would resolve in 50% controls, 58 women were needed in each group to demonstrate a difference of 25% between control and experimental groups and to define statistical significance between the groups with an alpha of 0.05 and beta of 0.20. Duration of trial: Sept. 1998 to Oct 2001. Follow up: 6 and 12 post operative. | |
| Participants | Number of women randomised: 141. Number of women analysed: 126. Drop‐outs/withdrawals: 7 due to the presence of other gynecological diseases, 5 because endometriosis was not confirmed, 3 failed to undergo postoperative subjective evaluation of dysmenorrhea. Inclusion criteria: sexually active women of fertile age with severe dysmenorrhea for more than six months who were unresponsive to medical treatment and had a clinical and/or ultrasonographic diagnosis of endometriosis. Exclusion criteria: pregnancy, women without midline dysmenorrhoea, breastfeeding, use of an intrauterine device, major medical diseases, psychiatric disorders, neurologic alterations of the lumbar‐sacral tract, previous pelvic surgery, history of severe abdominal or pelvic infection, presence of other gynaecologic diseases, body mass index of >30 kg/m2, history of alcohol or other drug abuse. Age: control group mean 31.8, SD 4.9; PSN surgery group mean 30.1, SD 3.7 Location: Catanzaro, Rome and Messina, Italy. | |
| Interventions | Treatment: laparoscopic presacral neurectomy with conservative surgery for endometriosis. Control: conservative surgery for endometriosis. | |
| Outcomes | Dysmenorrhoea, measured by a 100 mm visual analog scale that ranged from "least possible pain" to "worst possible pain". Frequency was expressed as the number of episodes per each cycle for dysmenorrhea and chronic pelvic pain. Adverse effects: Significant bleeding from middle sacral vein in 1 woman in treatment group, initial urinary retention in 2 women in treatment group; significant increase in operating time in treatment group (P value < 0.05). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Garcia Leon 2003 | Randomised controlled trial comparing LPSN and LUNA (Spanish). Patients had chronic pelvic pain which may or may not have included dysmenorrhoea. Approximately 60 to 80% of women in each treatment group had dysmenorrhoea at baseline. Unable to separate out data for women with dysmenorrhoea at baseline or at end of treatment. |
| Sutton 1994 | Double blind, randomised trial, interventions were laparoscopic uterine nerve transection combined with vaporisation of endometriosis implants versus no treatment (expectant management only). Therefore data on pain relief as a result of LUNA could not be distinguished from pain relief as a result of the laser vaporisation. |
Characteristics of ongoing studies [ordered by study ID]
Birmingham trial.
| Trial name or title | A randomised controlled trial to assess the efficacy of laparoscopic uterosacral nerve ablation (LUNA) in the treatment of chronic pelvic pain. A multi‐centre, prospective randomised‐controlled trial with double blind assessment of outcomes |
| Methods | |
| Participants | New patients presenting to the gynaecology outpatient clinic with pelvic pain (cyclical or non‐cyclical) and/or dyspareunia, and requiring diagnostic laparoscopy for evaluation of these conditions, will be invited to participate. Inclusion criteria: pelvic pain for longer than 6 months duration; pain located within the true pelvis or between and below the anterior iliac crests; associated functional disability; lack of response to medical treatment; diagnostic laparoscopy planned. Exclusion criteria: previous LUNA; mild, moderate and severe endometriosis (American Fertility Society Score >5); previous surgery for endometriosis; previous surgery for pelvic inflammatory disease; previous hysterectomy; adnexal pathology |
| Interventions | Diagnostic laparoscopy plus uterosacral nerve ablation (experimental group) or laparoscopy without pelvic denervation (control group). |
| Outcomes | Postal questionnaires including visual analogue scale for pain (primary outcome), an index of sexual satisfaction and the EuroQol 5D‐EQ instrument (secondary outcomes) will be administered at 3, 6, 12, 24 and 36 months and 5 and 10 years. The primary assessment of the effectiveness of LUNA will be from comparison of outcomes at the one‐year follow‐up, although the short‐term and longer‐term risks and benefits of LUNA will also be evaluated. |
| Starting date | Date ISRCTN assigned: Oct 2002 |
| Contact information | The LUNA Trial Collaboration, Dr Pallavi Latthe. Email: pallavi.latthe@bwhct.nhs.uk |
| Notes | ISRCTN41196151 |
Contributions of authors
Michelle Proctor: took the lead in writing the protocol and review, searched for trials, selected studies for inclusion and exclusion, performed independent data extraction and quality assessment of the included trials, and was responsible for statistical analysis and interpretation of the data. Pallavi Latthe: selected studies for inclusion and exclusion, performed independent data extraction and quality assessment of the included trials for the 2004 update of the review and commented on drafts of the review. Cindy Farquhar: initiated and conceptualised the review, and commented on drafts of the protocol and review. Khalid S. Khan: supervised P. Latthe and commented on drafts of the review. Neil Johnson: revised drafts of the protocol and review, and contributed to interpretation of the data.
Sources of support
Internal sources
No sources of support supplied
External sources
Princess of Wales Memorial Trust administered by the Mercia Barnes Fund, New Zealand.
LUNA trial grant CF 371 funded by WellBeing, Royal College of Obstetrics and Gynaecology (RCOG), UK.
Declarations of interest
Dr C Farquhar and Dr N Johnson are investigators in a randomised controlled trial of laparoscopic uterine nerve ablation (LUNA), which was funded by a grant from the Princess of Wales Memorial Trust and administered by the Mercia Barnes Fund of the Royal Australian and New Zealand College of Obstetrics and Gynaecology (RANZCOG) (NZ branch) (Johnson 2004). Dr P Latthe is the Clinical Research Fellow and Mr KS Khan is the principal investigator on a LUNA trial funded by WellBeing, Royal College of Obstetrics and Gynaecology (RCOG), UK (CF/371) (Latthe 2003).
Edited (no change to conclusions)
References
References to studies included in this review
Candiani 1992 {published data only}
- Candiani GB, Fedele L, Vercellini P, Bianchi S, Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. American Journal of Obstetrics and Gynecology 1992;167(1):100‐3. [DOI] [PubMed] [Google Scholar]
Chen 1996 {published data only}
- Chen FP, Chang SD, Chu KK, Soong YK. Comparison of laparoscopic presacral neurectomy and laparoscopic uterine nerve ablation for primary dysmenorrhea. Journal of Reproductive Medicine 1996;41(7):463‐6. [PubMed] [Google Scholar]
Johnson 2004 {published data only}
Lichten 1987 {published data only}
- Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. Journal of Reproductive Medicine 1987;32:37‐41. [PubMed] [Google Scholar]
Sutton 2001 {published data only}
- Sutton C, Pooley AS, Jones KD, Dover RW, Haines P. A prospective, randomized, double‐blind controlled trial of laparoscopic uterine nerve ablation in the treatment of pelvic pain associated with endometriosis. Gynaecological Endoscopy 2001;10(4):217‐22. [Google Scholar]
Tjaden 1990 {published and unpublished data}
- Tjaden B, Schlaff WD, Kimball A, Rock JA. The efficacy of presacral neurectomy for the relief of midline dysmenorrhoea. Obstetrics and Gynecology 1990;76:89‐91. [PubMed] [Google Scholar]
Vercellini 2003 {published data only}
- Vercellini P, Aimi G, Busacca M, Uglietti A, Viganali M, Crosignani PG. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis: Results of a randomized controlled trial (abstract). Fertility and Sterility 1997;October(Suppl):S3. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Frontino G, Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of a levonorgestrel‐releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: A pilot study. Fertility and Sterility 2003;80(2):305‐9. [DOI] [PubMed] [Google Scholar]
Yen 2001 {published data only}
- Yen YK, Liu WM, Yuan CC, Ng HT. Addition of laparoscopic uterine nerve ablation to laparoscopic bipolar coagulation of uterine vessels for women with uterine myomas and dysmenorrhea. Journal of the American Association of Gynecologic Laparoscopists 2001;8(4):573‐8. [DOI] [PubMed] [Google Scholar]
Zullo 2003 {published data only}
- Zullo F, Palomba S, Zupi E, Russo T, Morelli M, Cappiello F, Mastrantonio P. Effectiveness of presacral neurectomy in women with severe dysmenorrhea caused by endometriosis who were treated with laparoscopic conservative surgery: a 1‐year prospective randomized double‐blind controlled trial. American Journal of Obstetrics & Gynecology 2003;189(1):5‐10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Garcia Leon 2003 {published data only}
- Garcia Leon F, Oviedo Ortega G, Reyes Cuervo H, Ibarrola Buenabad E, Meden W. Presacral neurectomy and uterine nerve ablation in chronic pelvic pain. Laparoscopic management. A comparative study. Ginecologia y Obstetricia De Mexico 2003;71:137‐42. [PubMed] [Google Scholar]
Sutton 1994 {published data only}
- Sutton CJ, Ewen S, Whitelaw N, Haines P, Pooley A. Prospective randomized controlled trial of laser laparoscopy against placebo in stages I‐III endometriosis: one year follow‐up data. 27th British Congress of Obstetrics and Gynaecology. 1995:176.
- Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized double‐blind controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild and moderate endometriosis. Fertility and Sterility 1994;62(4):696‐700. [DOI] [PubMed] [Google Scholar]
- Sutton CJ, Pooley AS, Ewen SP, Haines P. Follow‐up report on a randomised controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal to moderate endometriosis. Fertility and Sterility 1997;68(6):1070‐4. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Birmingham trial {published data only (unpublished sought but not used)}
- A randomised controlled trial to assess the efficacy of laparoscopic uterosacral nerve ablation (LUNA) in the treatment of chronic pelvic pain.A multi‐centre, prospective randomised‐controlled trial with double blind assessment of outcomes. Ongoing study Date ISRCTN assigned: Oct 2002.
Additional references
Baker 1992
- Baker PN, Symonds EM. The resolution of chronic pelvic pain after normal laparoscopy findings. American Journal of Obstetrics and Gynecology 1992;166:835‐6. [DOI] [PubMed] [Google Scholar]
Chen 1997
- Chen FP, Soong YK. The efficacy and complications of laparoscopic presacral neurectomy in pelvic pain. Obstetrics and Gynecology 1997;90(6):974‐5. [DOI] [PubMed] [Google Scholar]
Daniell 1995
- Daniell JF, Lalonde CJ. Advanced laparoscopic procedures for pelvic pain and dysmenorrhoea. Balliere's Clinical Obstetrics and Gynaecology 1995;9(4):795‐807. [DOI] [PubMed] [Google Scholar]
Davis 1996
- Davis GD. Uterine prolapse after laparoscopic uterosacral transection in nulliparous airborne trainees: A report of three cases. Journal of Reproductive Medicine 1996;41(4):279‐82. [PubMed] [Google Scholar]
Dawood 1985
- Dawood MY. Dysmenorrhea. Pain and Analgesia 1985;1:20. [Google Scholar]
Dawood 1988
- Dawood MY. Nonsteroidal anti‐inflammatory drugs and changing attitudes towards dysmenorrhea. American Journal of Medicine 1988;84(5A):23‐9. [DOI] [PubMed] [Google Scholar]
Dawood 1990
- Dawood MY. Dysmenorrhea. Clinical Obstetrics and Gynecology 1990;33(1):168‐78. [DOI] [PubMed] [Google Scholar]
Deeks 2004
- Deeks JJ, Higgins, JPT, Altman DG. Analysing and presenting results. In: Alderson P, Green S, Higgins J (Eds). Cochrane Reviewers’ Handbook 4.2.2 [updated December 2003]; Section 8. The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Donnez 1989
- Donnez J, Nisolle E. CO2 laser laparoscopic surgery. Bailliere's Clinical Obstetrics and Gynecology 1989;3:545‐62. [DOI] [PubMed] [Google Scholar]
Doyle 1955
- Doyle JB. Paracervical uterine denervation by transection of the cervical plexus for the relief of dysmenorrhoea. American Journal of Obstetrics and Gynecology 1955;70:1. [DOI] [PubMed] [Google Scholar]
Ewen 1994
- Ewen SP, Sutton CJG. A combined approach to painful heavy periods: Laparoscopic laser uterine nerve ablation and endometrial resection. Gynaecological Endoscopy 1994;3(3):167‐8. [Google Scholar]
Feste 1985
- Feste JR. Laser laparoscopy: A new modality. Journal of Reproductive Medicine 1985;30:413‐7. [PubMed] [Google Scholar]
Fitzpatrick 1995
- Fitzpatrick CC, Flood H, Punch M, Hilgers TW, Elkins TE, McGuire EJ. Bladder dysfunction after repeat laparoscopic uterine nerve ablation (LUNA). International Urogynecological Journal 1995;6(1):31‐3. [Google Scholar]
Frankenhauser 1864
- Frankenhauser G. Die Bewegungenerven der Gebarmutter 1864;1:35. [Google Scholar]
Furniss 1982
- Furniss LD. Nonsteroidal anti‐inflammatory agents in the treatment of primary dysmenorrhea. Clinical Pharmacology 1982;1:327‐33. [PubMed] [Google Scholar]
Gurgan 1992
- Gurgan T, Develioglu O, Bulent U, Hulusi Z, Aksu T, Kisnisci HA. Laparoscopic CO2 laser uterine nerve ablation for treatment of drug resisistant primary dysmenorrhoea. Fertility and Sterility 1992;58:422‐4. [DOI] [PubMed] [Google Scholar]
Henzl 1985
- Henzl MR. Dysmenorrhea: Achievements and challenge. Sexual Medicine Today 1985;9:8‐12. [Google Scholar]
Latthe 2003
- Latthe P, Khan KS, Gupta JK, Daniels J, Hills RK, Gray R, et al. A randomised controlled trial to assess the efficacy of laparoscopic uterosacral nerve ablation (LUNA) in the treatment of chronic pelvic pain: The trial protocol. Biomed Central Women's Health 2003; Vol. 8 Dec:3. [MEDLINE: ; ISSN: 1472‐6874]
Latthe 2004a
- Latthe P, Khan K, Commentary on Zullo F, Palomba S, Zupi E, Russo T, Morelli M, Cappiello F, et al. Presacral neurectomy in addition to surgery for endometriosis increased the cure of severe dysmenorrhea. Evidence‐based Obstetrics and Gynecology 2004; Vol. 6, issue 1:15‐6.
Latthe 2004b
- Latthe PM, Powell RJ, Daniels J, Hills RK, Gray R, Gupta JK, et al. Variation in practice of laparoscopic uterosacral nerve ablation: A European survey. Journal of Obstetrics and Gynaecology 2004;24(5):547‐51. [DOI] [PubMed] [Google Scholar]
Lichten 1987
- Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. Journal of Reproductive Medicine 1987;32:37‐41. [PubMed] [Google Scholar]
Marjoribanks 2003
- Marjoribanks J, Procotor ML, Farquhar C. Non‐steroidal anti‐inflammatory drugs for primary dysmenorrhoea (Cochrane Review). Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI] [PubMed] [Google Scholar]
Moore 1997
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 1997;69:127‐30. [DOI] [PubMed] [Google Scholar]
Nezhat 1992
- Nezhat C, Nezhat F. A simplified method of laparoscopic presacral neurectomy for the treatment of central pelvic pain due to endometriosis. British Journal of Obstetrics and Gynaecology 1992;99(8):659‐63. [DOI] [PubMed] [Google Scholar]
Papasakelariou 1996
- Papasakelariou C. Long‐term results of laparoscopic uterosacral nerve ablation. Gynaecological Endoscopy 1996;5:177‐9. [Google Scholar]
Perez 1990
- Perez JJ. Laparoscopic presacral neurectomy. Results of the first 25 cases. Journal of Reproductive Medicine 1990;35(6):625‐30. [PubMed] [Google Scholar]
Proctor 2003
- Proctor ML, Roberts H, Farquhar C. Combined oral contraceptive pill (OCP) as treatment for primary dysmenorrhoea (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI] [PubMed] [Google Scholar]
Rosenwaks 1980
- Rosenwaks Z, Seegar‐Jones G. Menstrual pain: its origin and pathogensis. Journal of Reproductive Medicine 1980;25(Suppl 4):207‐12. [PubMed] [Google Scholar]
Sobczyk 1980
- Sobczyk R. Dysmenorrhea: The neglected syndrome. Journal of Reproductive Medicine 1980;25:200. [PubMed] [Google Scholar]
Sutton 1989
- Sutton CJG. Laser uterine nerve ablation. In: Donnez J editor(s). An atlas of laser operative laparoscopy and hysteroscopy. Leuven: Nauwelaerts, 1989:43‐52. [Google Scholar]
Sutton 1993
- Sutton C, Whitelaw N. Laparoscopic uterine nerve ablation for intractable dysmenorrhoea. In: Sutton C, Diamond M editor(s). Endoscopic Surgery for Gynecologists. London: WB Saunders, 1993:39‐65. [Google Scholar]
Wiborny 1998
- Wiborny R, Pichler B. Endoscopic dissection of the uterosacral ligaments for the treatment of chronic pelvic pain. Gynaecological Endoscopy 1998;7(1):33‐5. [Google Scholar]
References to other published versions of this review
Johnson 2000
- Johnson N, Proctor M, Farquhar C. Surgical pelvic neuroablation for chronic pelvic pain: A systematic review. Gynaecological Endoscopy 2000;9(6):351‐61. [Google Scholar]


