Abstract
Neutrophils are considered as complex innate immune cells and play a critical role in maintaining intestinal mucosal homeostasis. They exert robust pro-inflammatory effects and recruit other immune cells in the acute phase of pathogen infection and intestinal inflammation, but paradoxically, they also limit exogenous microbial invasion and facilitate mucosal restoration. Hyperactivation or dysfunction of neutrophils results in abnormal immune responses, leading to multiple autoimmune and inflammatory diseases including systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel diseases (IBD). As a refractory intestinal inflammatory disease, the pathogenesis and progression of IBD are associated with complicated immune response processes in which neutrophils are profoundly involved. However, the consensus on potential roles of neutrophils in modulating pathogenic and repair processes of IBD remains not fully understood. Accumulated infiltrating neutrophils cross the epithelial barrier and contribute to microbial dysbiosis, aggravated intestinal architectural damage, compromised resolution of intestinal inflammation and increased risk of thrombosis during IBD. Paradoxically, activated neutrophils are also associated with effective elimination of invaded microbiota, promoted angiogenesis and tissue restoration of gut mucosa in IBD. Here, we discuss the beneficial and detrimental roles of neutrophils in the onset and resolution of intestinal mucosal inflammation, hoping to provide a precise overview of neutrophil functions in the pathogenesis of IBD.
Keywords: neutrophil, innate immune cell, inflammatory bowel disease, intestinal inflammation, mucosal homeostasis, immune response, microbial dysbiosis
Introduction
Neutrophils are the most abundant innate immune cells in the circulation and are capable of promptly accumulating in large numbers and responding to multiple pathogenic signals at the sites of tissue damage.1 Neutrophils have long been considered as an initiator in acute and chronic inflammation that are associated with excessive immune responses and aggravate tissue injuries. They are armed with effective immunoregulatory and bactericidal properties using manifold intracellular and extracellular mechanisms, including reactive oxygen species (ROS), degranulation, phagocytosis, neutrophil extracellular traps (NETs), recruitment and activation of other immune cells (e.g. macrophages, dendritic cells, natural killer cells, T and B cells).2,3 Despite pro-inflammatory roles, neutrophils also play an essential role in protecting the host from exogenous pathogen invasion and avoiding detrimental effects mediated by dead or injured cells.4
Inflammatory bowel disease (IBD), encompassing Crohn's disease (CD) and ulcerative colitis (UC), is chronic and idiopathic inflammatory diseases of unknown etiology affecting the gastrointestinal tract with increasing global prevalence.5–7 IBD is progressive and destructive, with heterogeneous complications including abscesses, stenoses, fistulas, extraintestinal manifestations and colitis-associated cancer, and induced by interacting genetical, environmental, microbial and immune pathogenic factors.8,9 Several lines of evidence have shown that intestinal mucosal T helper (Th)-related pro-inflammatory cytokines including interferon (IFN)-γ and tumor necrosis factor (TNF)-α, as well as Th17-derived cytokines including interleukin (IL)-17A and IL-23, are associated with the pathogenesis of IBD.10–13 Numerous studies have also demonstrated that neutrophils participate in the pathogenesis and progression of IBD. Exacerbated intestinal inflammation and mucosal damage in the early phase of IBD are associated with activated neutrophil infiltration that further contributes to the recruitment of subsequently activated immune cells.4 However, these accumulated neutrophils in the inflamed mucosa also phagocytose pathogenic bacteria and dysfunctional cells, and promote mucosal restoration and resolution of inflammation. The diversified functions of neutrophils endow them with a fickle role in intestinal inflammation. In this review, we summarize the dichotomous roles of neutrophils in modulating the pathogenic and repair processes of IBD and highlight their potential therapeutic application for the management of IBD.
Physiological functions of neutrophils
Neutrophils are predominant innate immune cells with unique physiological characteristics that furnish effective immunoregulatory properties despite a short life span. As the most abundant circulated leukocytes, neutrophils can quickly migrate and recruit into the inflamed tissue, and collaborate to build the first-line defense against exogenous pathogens.14,15 Under intestinal homeostasis conditions, multiple functions of neutrophils including production of ROS, phagocytosis, degranulation and release of NETs enable them to modulate innate and adaptive immune responses (Table 1). When neutrophils encounter invading pathogens, ROS are formed in respiratory burst by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that effectively destroy pathogens, followed by phagocytosis to minimize deleterious effects mediated by dying or damaged cells.4,16 Another bactericidal weapon of neutrophils is the affluent pool of membrane and intracellular granules that include azurophilic (primary) granules containing myeloperoxidase (MPO) and defensins, specific (secondary) granules containing cathelicidin, gelatinase (tertiary) granules containing matrix metalloproteinase-9 (MMP-9), and other secretory granules containing integrins (Table 2).17,18
Table 1.
The arsenal of neutrophils.
| Site | Item | Components | Functions | References |
|---|---|---|---|---|
| Intracellular | ROS | Hydrogen peroxide (H2O2), hypochlorite ion (OCl−), and superoxide anion (O2−) | Kill the pathogens; promote neutrophil apoptosis | 2 |
| Degranulation | Azurophilic (primary) granules: MPO, elastase, lysozyme, defensins, azurocidin; specific (secondary) granules: lactoferrin, cathelicidin; gelatinase (tertiary) granules: MMP-9; other secretory granules: integrins | Amplify inflammatory responses; recruit neutrophils; eliminate microbiota; facilitate cell adhesion and angiogenesis | 103,104 | |
| Phagocytosis | Receptor-mediated processes: pattern-recognition receptors; FcγRs; complement receptors | Phagocytize pathogens and cell debris | 104,105 | |
| Extracellular | NETs | Chromatin coated with histones, proteases, granular and cytosolic bactericidal proteins | Limit and kill bacterial, fungi, viruses and parasites; promote thrombosis | 3,106 |
| Cytokines | IFN-γ, TNF, L-1β, IL-4, IL-8, IL-10 and IL-12 | Pro- and anti-inflammatory | 22,107 | |
| Chemokines | CXCL-1-6,8-13,16; CCL-2-4,17-20 | Recruit both innate and adaptive immune cells (neutrophils, monocytes, macrophages, DCs, NK cells and T cells); amplify the inflammatory immune responses | 20,108–113 |
ROS, reactive oxygen species; MPO, myeloperoxidase; MMP-9, matrix metalloproteinase-9; FcγRs, Fcγ receptors; NETs, neutrophil extracellular traps.
Table 2.
Granules of neutrophils.
| Granule type | Granules | Functions | References |
|---|---|---|---|
| Primary or azurophilic granules | MPO, elastase, lysozyme, defensins, azurocidin, BPI, cathepsin G, proteinase 3, sialidase, β-glucuronidase | Oxidative burst; antimicrobial effect; proteolytic effect; recruitment and activation of other immune cells | 17,18,114 |
| Secondary or specific granules | Lysozyme, lactoferrin, NGAL, hCAP-18, β2-microglobulin, collagenase, pentraxin 3 | Antimicrobial effect; proteolytic effect; modulation and suppression inflammatory response | 17,18,115 |
| Tertiary or gelatinase granules | Gelatinase, MMP-8, MMP-9, MMP-25, arginase-1, leukolysin | Antimicrobial effect; tissue restoration; mobilization of cytokines | 17,18,93,99 |
| Secretory vesicles | FcγRIII, fMLP receptors, β2 integrins, complement receptor 1, plasma proteins | Neutrophil chemoattraction; cell adhesion | 17,18 |
| Membrane granules | NOX2 (Gp91phox/p22phox), Mac-1 (CD11b/CD18), CD10, CD14, CD35, CD63, CD68, CD177, G-CSFR | Host defense against invaded microbes; neutrophil migration | 16–18,87,116,117 |
| Other proteins | Alkaline phosphatase, histones, calprotectin | Toxicity to invaded pathogens; regulation of cell division; regression of inflammation | 17,18,118,119 |
BPI, bactericidal/permeability-increasing protein; NGAL, neutrophil gelatinase-associated lipocalin; hCAP-18, human 18 kDa cathelicidin antimicrobial protein; NOX2, NADPH oxidases 2; Mac-1, Macrophage 1 antigen; G-CSFR, granulocyte colony-stimulating factor receptor.
Neutrophils are capable of forming and releasing NETs which can degrade and eradicate pathogens, and prevent them from uncontrolled spreading.19 Once neutrophils arrive at the inflamed tissues, they can recruit themselves (through CXCL-1, -2, -5 and -8) and other immune cells including monocytes (through CCL-2, -3 and -4), macrophages (through CCL-2, -3 and -4), NK cells (through CCL-2, -3, -4 and CXCL-10), dendritic cells (through CCL-2, -4, -18, -19 and -20), and T cells (through CXCL-9, -10, -11, -12, CCL-2, -17, -18 and -20).20 IL-8 produced by the intestinal epithelial cells (IECs) also triggers neutrophil recruitment to the intestinal lamina propria, thus further proving the interaction between neutrophils and other immune cells.21 Additionally, neutrophils secrete several pro- and anti-inflammatory cytokines including IFN-γ, TNF, IL-4, and IL-10 in response to inflammatory signals and pathogens associated molecular patterns (PAMPs).22 Taken together, neutrophils not only participate in the occurrence and development of inflammation, but also facilitate resolution of inflammation and healing of damaged tissue, and ultimately maintain intestinal immune homeostasis.
Recruitment and infiltration of neutrophils in inflamed mucosa of IBD
Although the precise etiology and pathology of IBD remain unclear, multifactorial pathological injury (e.g. compromised epithelial barrier integrity) and dysregulated innate and adaptive immune responses are proved to be associated with the pathogenesis of IBD.23,24 Massive recruitment and accumulation of neutrophils are observed in the intestinal mucosa of IBD, accompanied with upregulated levels of protein arginine deiminase 4, elastase, MPO and NETs in the colonic mucosa of UC patients, even in clinical remission.4,25,26
Recruitment and infiltration of neutrophil into inflamed mucosa of IBD are in a multifactor-dependent manner. Increased cytokines (e.g. IL-1β, IL-6, IL-8, IL-17, TNF-α, GM-CSF and G-CSF) and chemokines (e.g. CXCL-1, -8 and -10) produced by neutrophils themselves and other immune cells (e.g. IECs, macrophages and fibroblasts) during intestinal inflammation are important contributors to recruitment and infiltration of neutrophils into the intestinal mucosa.27–30 The complement component C5a is an important chemoattractant of neutrophils, while decreased neutrophil infiltration is found in the colon of C5ar−/− mice and anti-C5a-treated wild-type mice in dextran sulphate sodium (DSS)- or 2,4,6-trinitronitrobenzene sulfonic acid (TNBS)-induced colitis.31,32 Additionally, an increase of leukotriene B4 and hepoxilin A3 also promotes neutrophil infiltration in IBD.33,34 Moreover, neutrophils can be recruited by bacteria-derived metabolites, such as formyl-methionylleucyl-phenylalanine (fMLF) and short-chain fatty acids (e.g. acetate and butyrate).35–37 Upregulated IEC-derived MMPs (e.g. MMP-3 and MMP-7) result in enhanced migration of neutrophils by regulating activity of chemokines (e.g. CXCL-7 and CXCL-8) during intestinal inflammation.38,39 Intriguingly, monocyte chemotactic protein 1-induced protein 1 (MCPIP-1) is found to be highly expressed in neutrophils of IBD patients and profoundly suppresses neutrophil migration and neutrophil-mediated pro-inflammatory responses via a MCPIP-1-mediated negative feed-back loop.40 However, neutrophils are also observed to evolve an intrinsic mechanism that restrains uncontrolled aggregation through G protein-coupled receptor (GPCR)-mediated desensitization, which further allows neutrophils to scan wider tissue areas for pathogens during inflammation.41 Thus, despite many neutrophil chemoattractants released by themselves, neutrophils can also self-limit their migration and infiltration at inflammatory state and maintain the homeostatic regulation in gut mucosa.
During intestinal inflammation, activated neutrophils undergo transendothelial migration (including tethering, rolling, adhesion, crawling and transmigration) and transepithelial migration to reach the inflamed mucosa in the stimulation of multiple chemoattractant gradients within minutes.42,43 Under physiological conditions, apoptotic neutrophils in the intestinal mucosa are phagocytosed by macrophages through efferocytosis which prevents neutrophil lysis and dampens immune responses.44 However, under inflammatory or infectious conditions, the longevity of neutrophils can be extended by a variety of inflammatory mediators such as G-CSF, GM-CSF, component C5a, C-reactive protein (CRP), serum amyloid A (SAA), bacterial components, and pro-inflammatory cytokines (e.g. IL-1β, IL-6, IL-8 and IFN-γ). The apoptosis of neutrophils can then be delayed which allows neutrophils to carry out more durable activities and accumulate in the gut mucosa.45–49 Accordingly, orderly recruitment, activation and apoptosis of neutrophils are indispensable for resolution of intestinal inflammation, while dysfunction in any of these procedures leads to aggravating mucosal inflammation and intestinal damage consequently.
Pathogenic roles of neutrophils in the pathogenesis of IBD
Under conditions of gut inflammation, neutrophils migrate from the circulation toward the intestine mucosa and result in a robust inflammatory response, characterized by destruction of the mucosal epithelial barrier, dysregulated immune responses to commensal bacteria, and defects in the resolution of the intestinal inflammation.
Neutrophils amplify immune responses and exacerbate intestinal mucosal damage
Given that neutrophils are capable of recruiting and activating plentiful immune cells to participate in the intestinal inflammation, conspiracy of these immune cells would bring about inflammatory cascades and epithelial damages that are associated with the development of IBD.50 As a prominent hallmark of IBD, dysregulated immune response to commensal bacteria contributes to the compromised epithelial barrier integrity and chronic relapsing intestinal inflammation.51,52 Neutrophils equipped with manifold antimicrobial weapons exert potent bactericidal functions to limit the dissemination of deleterious bacteria, but inevitably cause tissue damage by excessive production of ROS, MPO, MMPs, pro-inflammatory cytokines and NETs. Indeed, proteases (e.g. proteinase 3, elastase and MMPs) released by transepithelial neutrophils weaken the mucosal barrier through disrupting adherens junctions and guiding neutrophils into a direction of basolateral to apical, as demonstrated in T84 (one of human intestinal epithelial cells) monolayers.53 Additionally, NETs are demonstrated to contribute to the pathogenesis of intestinal inflammation through the impairment of mucosal barrier function in mouse colitis models induced by DSS or TNBS. NETs compromise gut mucosal permeability, induce the apoptosis of IECs, and destroy the integrity of adherens junctions and tight junctions which promotes luminal microbial dissemination and initiation of mucosal inflammation.54 Consistently, experimental colitis models also prove that depletion of neutrophils using neutralizing antibodies has been shown to ameliorate DSS/TNBS-induced colitis in rat and in mice.55,56 In patients with IBD, massive amounts of neutrophils migrate to colonic epithelial crypts and form cryptitis and crypt abscesses, which further cause damage of the physiological architecture of crypt, leading to intestinal mucosa injury.42
Moreover, immunoglobulin G (IgG) receptors expressed by neutrophils also contribute to aggravated immune responses in IBD. IgG-Fc gamma receptors (FcγRs) are associated with the pathogenesis of various IgG-dependent immune diseases, such as IBD, rheumatoid arthritis, systemic lupus erythematosus and systemic anaphylaxis.57,58 Neutrophils constitutively express FcγRs, which mainly encompass FcγR I (CD64), FcγR II (CD32) and FcγR III (CD16), to facilitate the phagocytosis of IgG-opsonized microbiota and drive cell activation via crosslinking of several receptors.59 Neutrophil expression of FcγR I, which is capable of recognizing and phagocytizing immune complexes, executing antibody-dependent cytotoxicity and triggering respiratory burst, is upregulated in adult patients with active IBD.60,61 Soluble FcγR IIIb (sFcγRIIIb) is also increased in both CD and UC patients, with the concentration of sFcγRIIIb reflecting the degree of mucosal inflammation.62 Additionally, monoclonal antibodies against TNF have been found engaged with FcγRs. The Fc-FcγR interaction is required for the therapeutic efficacy of anti-TNF in IBD. Consistently, activated FcγR-deprived mice fail to effectively response to anti-TNF therapy in T-cell transfer colitis model, while administration of anti-TNF monoclonal antibodies with enhanced Fc-binding affinity shows improved efficacy.63
Neutrophils undermine the resolution of intestinal inflammation
Neutrophils are short-lived, spontaneously die in apoptosis after an average circulatory lifespan of approximately 3 hours for mouse cells and 16 hours for human.64 Previous study has reported that nearly 109 peripheral neutrophils per kg of body weight are refurbished daily under physiological conditions.65 The engulfment process of apoptotic neutrophils performed by phagocytes such as macrophages, termed as efferocytosis, prevents secondary lysis and extravasation of noxious neutrophil granules, dampens pro-inflammatory signaling, and ameliorates immune responses, which appears to be crucial for maintenance of intestinal mucosal homeostasis and is a prerequisite for the resolution of intestinal inflammation.46,66,67 Moreover, apoptotic neutrophils exert anti-inflammatory effects and trigger pro-resolving responses in monocytes, macrophages and dendritic cells.68 As described previously, the longevity of neutrophils can be extended by pro-inflammatory mediators during intestinal inflammation of IBD, which allow neutrophils to carry out more durable activities and accumulate in the gut mucosa. Given that efferocytosis of apoptotic neutrophils is a fundamental process required for resolution of intestinal inflammation and maintenance of mucosal homeostasis, its dysregulation leads to over-activated immune responses, excessive inflammation, and uncontrollable infection. Thus, approaches that regulate and enhance efferocytosis can be harnessed to combat intestinal microbial infection and mucosal inflammatory damage.
Moreover, infiltrated neutrophils are able to utilize membrane-derived microparticles to deliver active inflammatory mediators (e.g. MPO, elastase and MMPs) to modulate local immune responses. Specifically, MPO, which is abundantly cumulated in neutrophil azurophilic granules used for pathogen killing, mobilizes to the neutrophil surface and subsequently releases within microparticles to promote neutrophil activation and binds to IECs, leading to potent inhibition of epithelial wound healing.69 Impeding epithelial migration and wound closure are observed in the colonic biopsy of a wound murine experiment model through inhibition of IEC proliferation (shown by Ki67 staining) in vivo. Another cytotoxic granule, neutrophil elastase (NE), released by infiltrating neutrophils in situ during inflammatory responses, is involved in mucosal damage and repairment in patients with UC. NE normally serves in mucosal defense against intruding microbial pathogens, but can also cause impediment of tissue repairment when produced in an excessive manner. In patients with UC, NE is observed to be cumulated in the inflamed mucosa and undermines mucosal restitution through suppressive effects on recombinant hepatocyte growth factor (rHGF)-induced IEC proliferation.70 Additionally, on reaching the intestinal epithelial surface, membrane-derived microparticles released by neutrophils deposit onto the apical epithelium. These microparticles containing miRNAs and MMP-9 elicit destruction of epithelial intercellular adhesions by cleaving desmoglein-2 (instead of E-cadherin) in a MMP-9-dependent manner in vivo, leading to elevated neutrophil infiltration and inflammation in the ligated intestinal loop murine model.71
These data elucidate the nonnegligible roles of neutrophils in amplifying immune responses and preventing resolution of intestinal mucosal inflammations, contributing to a persistent course of IBD.
Neutrophils participate in the thrombosis in IBD
Patients with active IBD have an elevated incidence of microvascular thrombosis and thromboembolism which are associated with enhanced procoagulant phosphatidylserine (PS) exposure on platelets (PLTs) and increased platelet microparticles (MPs).72,73 Despite the pathogen-killing effects of NETs, an increase of NETs in plasma and the deposition in colon tissues are observed in active IBD, followed by exacerbated colon tissue damage and increased thrombotic tendency. NETs are also found to be accumulated in DSS-induced murine colitis model, and inhibition of NETs through DNase administration could attenuate mucosal inflammation as well as thrombus formation.74 Evidenced by experiment results, NETs have been demonstrated to activate PLTs, which then promote coagulation via externalizing PS and releasing procoagulant MPs in vitro. Consistently, active UC-derived NETs are found to convert human umbilical vein cells (HUVECs) to a procoagulant phenotype due to increased PS expression.74
Dysfunctional neutrophils are associated with therapeutic failure in UC
Accumulated neutrophils are found in inflamed colon of UC patients with corticoid-resistance or cyclosporine A-unresponsiveness, accompanied with high levels of ROS, NE and uncontrolled T cell proliferation,75,76 indicating that increased neutrophil infiltration may be associated with the therapeutic failure in UC patients. The enrichment of twist-1 has been found in neutrophils isolated from inflamed colon of UC patients with corticoid-resistance, which interacts with glucocorticoid receptor α to attenuate the effects of steroids on regulating neutrophil activities. Moreover, the inhibition of twist-1 can block neutrophil-mediated corticoid-resistance in colitis mice and restore sensitivity to steroid therapy in the colon.75 Another therapeutic approach in the treatment of UC patients, cyclosporine A, can robustly induce effective clinical remission in severe steroid-refractory UC.77 However, the rate of short-term nonresponse to cyclosporine A is approximately 29%, and neutrophils are proved to be associated with the nullity of cyclosporine A therapy.76,78 Cyclosporine A is observed to suppress the migration and the apoptosis of neutrophils in response group, dampens release of IL-8, ROS and antimicrobial peptides in neutrophils, and transforms the neutrophil from a pro-activated status to a prolonged, pro-glycolytic and quiescent status, thereby driving the resolution of gut inflammation.76
Neutrophil dysfunction contributes to intestinal mucosal inflammation
Although most studies have shown that neutrophils accumulate and overact in the intestinal mucosa of patients with IBD, there is considerable evidence demonstrating the association between defective neutrophil function and intestinal inflammation. For example, defective superoxide generation and phagocytosis are found in neutrophils from patients with IBD,79,80 which thus contribute to reduced clearance of intestinal pathogens and exacerbated lymphocyte-mediated immune response.81 Furthermore, neutrophil dysfunction appears to be associated with compromised macrophage properties including decreased production of pro-inflammatory cytokines (e.g. GCSF, IL-6, IFN-γ and TNF-α) and neutrophil chemoattractants in CD, which further dampen neutrophil functions.82,83 Accumulated neutrophils at the sites of microbial infection show markedly impaired clearance of bacteria in CD. Impaired accumulation of neutrophils was observed in traumatized rectal mucosa from eight patients with CD (79% reduction, n = 8, P = 0.0003) compared to healthy individuals. Consistently, low frequency of IL-8-positive cells was observed in CD patients (63% reduction, n = 8, P = 0.003). Further, IL-8 production by macrophages from CD patients was significantly reduced (38% reduction, n = 50, P < 0.0001), together with decreased production of C5a (48% reduction, n = 41, P = 0.0005) and TNF-α (52% reduction, n = 27, P < 0.0001). Similar defects of neutrophil accumulation were also found in the impairment applied to the ileum (57% reduction, n = 3, P = 0.05 for neutrophils; 63% reduction, n = 3, P = 0.05 for IL-8-positive cells).83 These findings indicate that decreased neutrophil accumulation and IL-8 secretion at the sites of acute inflammation in the intestinal mucosa, indicative of abnormal neutrophil response, are associated with persistent tissue damage in CD. Moreover, intruding bacteria may remain in the mucosal tissues due to suboptimal destruction by neutrophils, and are then phagocytized by macrophages instead.
Taken together, accumulating lines of evidence highlight a pathogenic role of neutrophils in the destruction of epithelial barrier integrity, aberrant immune responses to microbiota, development of the intestinal inflammation, and therapeutic failure of IBD. It also manifests that both hyperactivation and functional deficiency of neutrophils contribute to pathological intestinal inflammation, which emphasizes the multiple roles of neutrophils in intestinal inflammatory diseases.
Restorative roles of neutrophils in tissue repair of IBD
In addition to fueling inflammation response by ROS, cytotoxic intracellular granular contents (e.g. MPO, defensins, lysozyme, elastase, proteases and hydrolases), and NETs, neutrophils also have the healing potential to exert restorative actions including eliminating microbe translocation, facilitating angiogenesis and aiding in the resolution of mucosal wounds.
Neutrophils eliminate microbial invasion
Contrary to previously referred animal experiments,55,56 neutrophil depletion in vivo aggravates both experimental acute colitis in mice induced by dinitrobenzene sulfonic acid (DNBS) or DSS and chronic colitis in severe combined immunodeficient mice reconstituted with CD4+CD45RBhigh T cells, and enhances translocation of microbes in colitic mice, indicative of a beneficial role of neutrophils during the intestinal inflammation.84–86 The characteristics of rapid mobilization and chemoattract endow neutrophils with profound bactericidal capacity after effective recognition and capture of invading microbes. Especially, a unique subset of neutrophils, CD177+ neutrophils, are capable of producing higher levels of IL-22 and transforming growth factor (TGF)-β to promote tissue healing, and dampening the production of pro-inflammatory cytokines (e.g. IL-6, IL-17A and IFN-γ) to restrain the inflammatory responses compared with CD177− neutrophils. Moreover, functionally activated CD177+ neutrophils exhibit increased bactericidal activities (e.g. ROS, antimicrobial peptides and NETs) which demonstrate its protective role in modulating intestinal mucosal inflammation.87 Dysfunctional neutrophils in the intestinal mucosa under massive bacterial invasion promote the susceptibility to disease, suggesting a dominant role of the requirement of neutrophils in preventing bacterial reproduction in gut mucosa.88 The intestinal inflammation in IBD patients is characterized by mucosal damage, increased epithelial permeability, intrusion of commensal microbiota into the subepithelial space or lamina propria and exceeded infiltration of neutrophils. Removal of dead cells and microbiota by neutrophil phagocytic activity contributes to clear the area for mucosal barrier remodeling which is a prerequisite for the resolution of the intestinal inflammation.89 Collectively, these data indicate that neutrophils play a key role in manipulating intestinal bacterial homeostasis and regulating the inflammatory response, which are tightly modulated by limiting excess translocation of commensal microbes and fighting against invasion by bacterial pathogens under conditions of inflammatory diseases.
Neutrophils promote angiogenesis in gut mucosa
Angiogenesis mediated by multiple factors is fundamental to restoration of damaged epithelia in IBD. Vascular endothelial growth factor A (VEGF-A) as one of major proangiogenic factors triggers angiogenic activities of human intestinal microvascular endothelial cells (HIMECs) via VEGF receptor 2 (VEGFR-2) in vitro and facilitates neutrophil adherence to intestinal endothelium through intercellular adhesion molecule-1 (ICAM-1) in vivo.90 Furthermore, it is reported that CD11b+/Gr-1+CXCR4high neutrophils, a proangiogenic circulating neutrophil subset, are recruited by VEGF-A and CXCL-12 and release additional effector protein MMP-9 to augment the initiation of angiogenesis after inflammatory tissue damage.91–93 Noteworthily, only neutrophil-derived MMP-9 at metalloproteinases (TIMP)-free status owns proangiogenic capacity and serves as unique proangiogenic molecule at the sites of inflammation.93 These findings prove that infiltrated neutrophils deliver potent proangiogenic mediators and promote angiogenesis during intestinal inflammation.
Neutrophils promote tissue restoration
One of primary effects of neutrophils is eliminating microbial intrusion at the sites of mucosal injuries, but they also function to restore damaged tissue during wound healing. Specific pathological characteristic of IBD is the immoderate inflammatory responses followed by the persistence of mucosal epithelial injuries. At the sites of injuries, neutrophils initiate healing program via direct effects not only on angiogenesis but also on cell proliferation. During the acute inflammatory phase, neutrophils play a critical role in cleansing damaged tissue by eliminating exogenous bacterial pathogens, and contributing to the production of growth factors, such as VEGF-A,94 and lipid mediators, including resolvins, lipoxins and protectins, which promote wound healing.95–97 Moreover, accumulated neutrophils bind to the apical epithelium through the interaction with increased expression of ICAM-1 under inflammatory conditions, which results in reduced neutrophil apoptosis, increased IEC proliferation and accelerated wound healing via Akt and β-catenin signaling.98
The functions of neutrophils, including bactericidal actions, angiogenesis and tissue restoration, are tightly relevant to healing mucosal wounds secondary to aberrant inflammatory responses and the restoration of intestinal homeostasis.
Regulation of neutrophil functions and the therapeutic prospects in IBD
It is well accepted that neutrophils exert potent effects on recruitment and activation of crucial immune cells involved in intestinal inflammation. On the other hand, several cytokines and chemokines produced by IECs and other immune cell types during intestinal inflammation also tightly regulate neutrophil migration and functions. For example, IL-8, an effective chemoattractant of neutrophils produced by IECs, promotes infiltration of neutrophils from lamina propria to epithelium.21 Moreover, classic neutrophil chemoattractants (e.g. CXCL-1, CXCL-8, CXCL-10, GM-CSF, G-CSF, IL-1β, IL-6 and TNF-α) produced by different cell types, such as macrophages, IECs and fibroblasts, have been shown to contribute to neutrophil recruitment and activation. However, non-classical chemoattractants of neutrophils such as growth-regulated oncogene-α (GRO-α) have also been demonstrated to be essential for neutrophil recruitment during intestinal inflammation.99
As highlighted above, neutrophils comprise a vital component of innate immune system and are equipped with sufficient bactericidal weapons to defend against invading microbiota. However, microbial ingredients such as lipopoproteins, lipopolysaccharide, and various metabolites, also have potent effects on neutrophils and other multiple intestinal cell types (e.g. IECs, Paneth cells, goblet cells, enteroendocrine cells, myofibroblasts, macrophages, monocytes, dendritic cells and T cells) and subsequently trigger a multitude of Toll and Nod-like receptor-mediated responses, participating in both the maintenance of intestinal mucosal homeostasis and development of pathological intestinal inflammation.100,101 Bacteria also produce metabolites capable of directly recruiting and activating neutrophils, such as fMLF and short chain fatty acids (SCFAs). The peptide fMLF owns potent chemoattractant effect on neutrophils, and increased expression of its receptor (i.e. N-formyl peptide receptor) is observed in CD patients.35 Microbial metabolites are capable of modulating intestinal immune responses and homeostasis. Microbial metabolites butyrate belongs to SCFAs and inhibits activated neutrophils isolated from IBD patients to produce pro-inflammatory cytokines, ROS, MPO and calprotectin in a histone deacetylase inhibitors (HDACi)-dependent manner. Butyrate also suppresses the formation of NETs and migration of neutrophils isolated from both CD and UC patients in vitro. Consistently, in vivo experiments also demonstrate that oral administration of butyrate alleviates mucosal inflammation in DSS-induced colitic mice through restricting neutrophil-associated immune responses such as production of pro-inflammatory cytokines and NET formation.102
Taken together, these findings highlight crucial roles for inflammatory mediators from other cell types and microbiota-derived products in manipulating neutrophil infiltration and function during intestinal inflammation through inflammatory mediators such as cytokines and chemokines, indicative of synergistic roles of various cell types in modulating neutrophil properties to further affect the onset and resolution of intestinal inflammation in IBD. Thus, combined with the previously described facts that neutrophil dysfunction is involved in the therapeutic failure of UC, approaches to regulate neutrophil dynamics (e.g. migration and infiltration towards the inflamed mucosa) and functions (e.g. release of ROS, phagocytosis, degranulation and formation of NETs) may provide a novel approach for treatment of IBD.
Conclusions and future perspectives
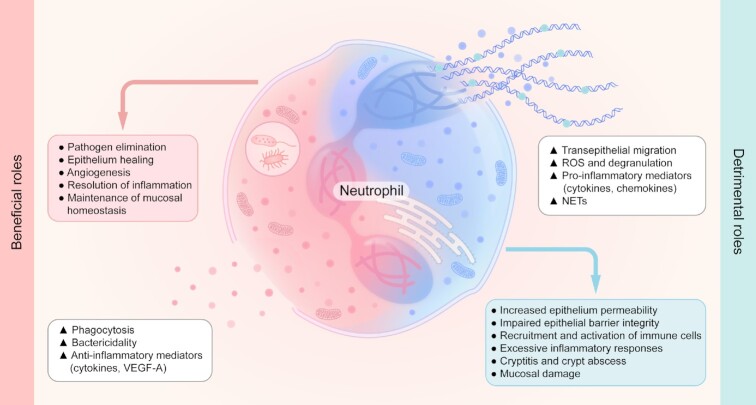
Although the understanding of immune regulation and immune response of neutrophils has gained great improvement during gut inflammation, especially in human IBD, the beneficial and detrimental roles of neutrophils remain controversial. Indeed, neutrophils are critical for the maintenance of mucosal homeostasis. It is also well known that neutrophils act as a double-edged sword by not only contributing to the restoration of mucosal inflammatory damage through the clearance of detrimental pathogens and the promotion of mucosal wound healing, but also participating in the excessive immune response and the extensive intestinal mucosal inflammation, owing to the release of toxic granules and massive transepithelial infiltration (Fig. 1). The contradictory ‘yin’ and ‘yang’ of neutrophil functions in the pathogenesis of IBD are manipulated by complex parameters involved in the migration, activation, immunoregulatory functions and apoptosis processes. Thus, better understanding of neutrophil properties in IBD will be crucial to uncover potential targets exploited in disease etiology and provide new insights for orchestrating neutrophil functions to counterbalance intestinal inflammation.
Figure 1.
The ‘yin’ and ‘yang’ of neutrophil functions in the pathogenesis of IBD. Neutrophils not only participate in the mucosal injury, aberrant responses to icrobiota, extensive intestinal inflammation, and therapeutic failure in IBD, but also play an important role in the elimination of pathogens, angiogenesis and wound healing, which is a prerequisite for the restoration of intestinal inflammation. All this knowledge elucidates the beneficial and deleterious roles of neutrophils in the pathogenesis of IBD.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grants No. 81630017 and 91942312).
Contributor Information
Huimin Chen, Center for Inflammatory Bowel Disease Research, the Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai 200072, China.
Xiaohan Wu, Center for Inflammatory Bowel Disease Research, the Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai 200072, China.
Chunjin Xu, Department of Gastroenterology, the First People's Hospital of Shangqiu City Affiliated to Xinxiang Medical University, Shangqiu 476100, China.
Jian Lin, Department of Gastroenterology, Affiliated Hospital of Putian University, Putian 351106, China.
Zhanju Liu, Center for Inflammatory Bowel Disease Research, the Shanghai Tenth People's Hospital, Tongji University School of Medicine, Shanghai 200072, China.
Conflict of interest
All authors claim no conflict of interests. In addition, as an Editorial Board Member of Precision Clinical Medicine, the corresponding author Zhanju Liu was blinded from reviewing and making decision on this manuscript.
References
- 1. Ng L, Ostuni R, Hidalgo A. Heterogeneity of neutrophils. Nat Rev Immunol. 2019;19:255–65. doi:10.1038/s41577-019-0141-8. [DOI] [PubMed] [Google Scholar]
- 2. Winterbourn C, Kettle A, Hampton M. Reactive Oxygen Species and Neutrophil Function. Annu Rev Biochem. 2016;85:765–92. doi:10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 3. Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18:134–47. doi:10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 4. Fournier B, Parkos C. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–66. doi:10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 5. Lloyd-Price J, Arze C, Ananthakrishnan A, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–62. doi:10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roda G, Chien Ng S, Kotze P, et al. Crohn's disease. Nat Rev Dis Primers. 2020;6:22. doi:10.1038/s41572-020-0156-2. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6:74. doi:10.1038/s41572-020-0205-x. [DOI] [PubMed] [Google Scholar]
- 8. de Souza H, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. 2017;14:739–49. doi:10.1038/nrgastro.2017.110. [DOI] [PubMed] [Google Scholar]
- 9. Neurath M. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14:269–78. doi:10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 10. Liu Z, Yadav P, Xu X, et al. The increased expression of IL-23 in inflammatory bowel disease promotes intraepithelial and lamina propria lymphocyte inflammatory responses and cytotoxicity. J Leukoc Biol. 2011;89:597–606. doi:10.1189/jlb.0810456. [DOI] [PubMed] [Google Scholar]
- 11. Sun M, He C, Cong Y, et al. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015;8:969–78. doi:10.1038/mi.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Su J, Chen T, Ji X, et al. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:720–8. doi:10.1097/MIB.0b013e3182802a76. [DOI] [PubMed] [Google Scholar]
- 13. Ma C, Wu W, Lin R, et al. Critical Role of CD6highCD4+ T Cells in Driving Th1/Th17 Cell Immune Responses and Mucosal Inflammation in IBD. J Crohns Colitis. 2019;13:510–24. doi:10.1093/ecco-jcc/jjy179. [DOI] [PubMed] [Google Scholar]
- 14. Sadik C, Kim N, Luster A. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–60. doi:10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou G, Liu Z. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J Dig Dis. 2017;18:495–503. doi:10.1111/1751-2980.12540. [DOI] [PubMed] [Google Scholar]
- 16. Petry A, Weitnauer M, Görlach A. Receptor activation of NADPH oxidases. Antioxid Redox Sign. 2010;13:467–87. doi:10.1089/ars.2009.3026. [DOI] [PubMed] [Google Scholar]
- 17. Borregaard N, Sørensen O, Theilgaard-Mönch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–5. doi:10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18. Cowland J, Borregaard N. Granulopoiesis and granules of human neutrophils. Immunol Rev. 2016;273:11–28. doi:10.1111/imr.12440. [DOI] [PubMed] [Google Scholar]
- 19. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–5. doi:10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 20. Tecchio C, Cassatella M. Neutrophil-derived chemokines on the road to immunity. Semin Immunol. 2016;28:119–28. doi:10.1016/j.smim.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kucharzik T, Hudson J, Lügering A, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54:1565–72. doi:10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gideon H, Phuah J, Junecko B, et al. Neutrophils express pro- and anti-inflammatory cytokines in granulomas from Mycobacterium tuberculosis-infected cynomolgus macaques. Mucosal Immunol. 2019;12:1370–81. doi:10.1038/s41385-019-0195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maloy K, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi:10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 24. Kaser A, Zeissig S, Blumberg R. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi:10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bennike T, Carlsen T, Ellingsen T, et al. Neutrophil Extracellular Traps in Ulcerative Colitis: A Proteome Analysis of Intestinal Biopsies. Inflamm Bowel Dis. 2015;21:2052–67. doi:10.1097/mib.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dinallo V, Marafini I, Di Fusco D, et al. Neutrophil Extracellular Traps Sustain Inflammatory Signals in Ulcerative Colitis. J Crohns Colitis. 2019;13:772–84. doi:10.1093/ecco-jcc/jjy215. [DOI] [PubMed] [Google Scholar]
- 27. Ina K, Kusugami K, Yamaguchi T, et al. Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1342–6. [PubMed] [Google Scholar]
- 28. Shea-Donohue T, Thomas K, Cody M, et al. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-alpha), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008;14:117–24. doi:10.1177/1753425908088724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Laan M, Cui Z, Hoshino H, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 30. Park H, Li Z, Yang X, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi:10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johswich K, Martin M, Bleich A, et al. Role of the C5a receptor (C5aR) in acute and chronic dextran sulfate-induced models of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1812–23. doi:10.1002/ibd.21012. [DOI] [PubMed] [Google Scholar]
- 32. Chen G, Yang Y, Gao X, et al. Blockade of complement activation product C5a activity using specific antibody attenuates intestinal damage in trinitrobenzene sulfonic acid induced model of colitis. Lab Invest. 2011;91:472–83. doi:10.1038/labinvest.2010.183. [DOI] [PubMed] [Google Scholar]
- 33. Jupp J, Hillier K, Elliott D, et al. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:537–46. doi:10.1002/ibd.20094. [DOI] [PubMed] [Google Scholar]
- 34. Mrsny R, Gewirtz A, Siccardi D, et al. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc Natl Acad Sci U S A. 2004;101:7421–6. doi:10.1073/pnas.0400832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anton P, Targan S, Shanahan F. Increased neutrophil receptors for and response to the proinflammatory bacterial peptide formyl-methionyl-leucyl-phenylalanine in Crohn's disease. Gastroenterology. 1989;97:20–8. doi:10.1016/0016-5085(89)91410-8. [DOI] [PubMed] [Google Scholar]
- 36. Sina C, Gavrilova O, Förster M, et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183:7514–22. doi:10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 37. Le Poul E, Loison C, Struyf S, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–9. doi:10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 38. Kruidenier L, MacDonald T, Collins J, et al. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology. 2006;130:127–36. doi:10.1053/j.gastro.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 39. Swee M, Wilson C, Wang Y, et al. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol. 2008;83:1404–12. doi:10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lin J, Li G, Xu C, et al. Monocyte chemotactic protein 1-induced protein 1 is highly expressed in inflammatory bowel disease and negatively regulates neutrophil activities. Mediators Inflamm. 2020;2020:8812020. doi:10.1155/2020/8812020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kienle K, Glaser K M, Eickhoff S, et al. Neutrophils self-limit swarming to contain bacterial growth in vivo. Science. 2021;372:abe7729. doi:10.1126/science.abe7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brazil J, Louis N, Parkos C. The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1556–65. doi:10.1097/MIB.0b013e318281f54e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi:10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 44. Kourtzelis I, Li X, Mitroulis I, et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nat Immunol. 2019;20:40–9. doi:10.1038/s41590-018-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11:1039–46. doi:10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Greenlee-Wacker M. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev. 2016;273:357–70. doi:10.1111/imr.12453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simon H. Neutrophil apoptosis pathways and their modifications in inflammation. Immunol Rev. 2003;193:101–10. doi:10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 48. Biffl W, Moore E, Moore F, et al. Interleukin-6 stimulates neutrophil production of platelet-activating factor. J Leukoc Biol. 1996;59:569–74. doi:10.1002/jlb.59.4.569. [DOI] [PubMed] [Google Scholar]
- 49. Klebanoff S, Olszowski S, Van Voorhis W, et al. Effects of gamma-interferon on human neutrophils: protection from deterioration on storage. Blood. 1992;80:225–34. [PubMed] [Google Scholar]
- 50. Williams I, Parkos C. Colonic neutrophils in inflammatory bowel disease: double-edged swords of the innate immune system with protective and destructive capacity. Gastroenterology. 2007;133:2049–52. doi:10.1053/j.gastro.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 51. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17:223–37. doi:10.1038/s41575-019-0258-z. [DOI] [PubMed] [Google Scholar]
- 52. Chen H, Li H, Liu Z. Interplay of intestinal microbiota and mucosal immunity in inflammatory bowel disease: a relationship of frenemies. Therap Adv Gastroenterol. 2020;13:1756284820935188. doi:10.1177/1756284820935188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kamekura R, Nava P, Feng M, et al. Inflammation-induced desmoglein-2 ectodomain shedding compromises the mucosal barrier. Mol Biol Cell. 2015;26:3165–77. doi:10.1091/mbc.E15-03-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin E, Lai H, Cheng Y, et al. Neutrophil extracellular traps impair intestinal barrier function during experimental colitis. Biomedicines. 2020;8(8):275. doi:10.3390/biomedicines8080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kankuri E, Vaali K, Knowles R, et al. Suppression of acute experimental colitis by a highly selective inducible nitric-oxide synthase inhibitor, N-[3-(aminomethyl)benzyl]acetamidine. J Pharm Exp Therap. 2001;298:1128–32. [PubMed] [Google Scholar]
- 56. Natsui M, Kawasaki K, Takizawa H, et al. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J Gastroenterol Hepatol. 1997;12:801–8. doi:10.1111/j.1440-1746.1997.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 57. Mancardi D, Albanesi M, Jönsson F, et al. The high-affinity human IgG receptor FcγRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood. 2013;121:1563–73. doi:10.1182/blood-2012-07-442541. [DOI] [PubMed] [Google Scholar]
- 58. Castro-Dopico T, Clatworthy M. IgG and Fcγ receptors in intestinal immunity and inflammation. Front Immunol. 2019;10:805. doi:10.3389/fimmu.2019.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang Y, Jönsson F. Expression, role, and regulation of neutrophil Fcγ receptors. Front Immunol. 2019;10:1958. doi:10.3389/fimmu.2019.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Minar P, Haberman Y, Jurickova I, et al. Utility of neutrophil Fcγ receptor I (CD64) index as a biomarker for mucosal inflammation in pediatric Crohn's disease. Inflamm Bowel Dis. 2014;20:1037–48. doi:10.1097/mib.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tillinger W, Jilch R, Jilma B, et al. Expression of the high-affinity IgG receptor FcRI (CD64) in patients with inflammatory bowel disease: a new biomarker for gastroenterologic diagnostics. Am J Gastroenterol. 2009;104:102–9. doi:10.1038/ajg.2008.6. [DOI] [PubMed] [Google Scholar]
- 62. Hommes D, Meenan J, de Haas M, et al. Soluble Fc gamma receptor III (CD 16) and eicosanoid concentrations in gut lavage fluid from patients with inflammatory bowel disease: reflection of mucosal inflammation. Gut. 1996;38:564–7. doi:10.1136/gut.38.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McRae B, Levin A, Wildenberg M, et al. Fc Receptor-mediated effector function contributes to the therapeutic response of anti-tnf monoclonal antibodies in a mouse model of inflammatory bowel disease. J Crohns Colitis. 2016;10:69–76. doi:10.1093/ecco-jcc/jjv179. [DOI] [PubMed] [Google Scholar]
- 64. Galli S, Borregaard N, Wynn T. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–44. doi:10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Athens J, Haab O, Raab S, et al. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961;40:989–95. doi:10.1172/jci104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stark M, Huo Y, Burcin T, et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi:10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 67. Nathan C. Points of control in inflammation. Nature. 2002;420:846–52. doi:10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 68. Korns D, Frasch S, Fernandez-Boyanapalli R, et al. Modulation of macrophage efferocytosis in inflammation. Front Immunol. 2011;2:57. doi:10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Slater T, Finkielsztein A, Mascarenhas L, et al. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J Immunol. 2017;198:2886–97. doi:10.4049/jimmunol.1601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuno Y, Ina K, Nishiwaki T, et al. Possible involvement of neutrophil elastase in impaired mucosal repair in patients with ulcerative colitis. J Gastroenterol. 2002;22–32. doi:10.1007/bf03326409. [DOI] [PubMed] [Google Scholar]
- 71. Butin-Israeli V, Houser M, Feng M, et al. Deposition of microparticles by neutrophils onto inflamed epithelium: a new mechanism to disrupt epithelial intercellular adhesions and promote transepithelial migration. FASEB J. 2016;30:4007–20. doi:10.1096/fj.201600734R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yan S, Russell J, Harris N, et al. Platelet abnormalities during colonic inflammation. Inflamm Bowel Dis. 2013;19:1245–53. doi:10.1097/MIB.0b013e318281f3df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Novacek G, Weltermann A, Sobala A, et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology. 2010;139:779–87. doi:10.1053/j.gastro.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 74. Li T, Wang C, Liu Y, et al. Neutrophil Extracellular Traps Induce Intestinal Damage and Thrombotic Tendency in Inflammatory Bowel Disease. J Crohns Colitis. 2020;14:240–53. doi:10.1093/ecco-jcc/jjz132. [DOI] [PubMed] [Google Scholar]
- 75. Liu C, Mo L, Feng B, et al. Twist1 contributes to developing and sustaining corticosteroid resistance in ulcerative colitis. Theranostics. 2021;11:7797–812. doi:10.7150/thno.62256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lu H, Lin J, Xu C, et al. Cyclosporine modulates neutrophil functions via the SIRT6-HIF-1α-glycolysis axis to alleviate severe ulcerative colitis. Clin Transl Med. 2021;11:e334. doi:10.1002/ctm2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hindryckx P, Jairath V, D'Haens G. Acute severe ulcerative colitis: from pathophysiology to clinical management. Nat Rev Gastroenterol Hepatol. 2016;13:654–64. doi:10.1038/nrgastro.2016.116. [DOI] [PubMed] [Google Scholar]
- 78. Bamba S, Tsujikawa T, Inatomi O, et al. Factors affecting the efficacy of cyclosporin A therapy for refractory ulcerative colitis. J Gastroenterol Hepatol. 2010;25:494–8. doi:10.1111/j.1440-1746.2009.06119.x. [DOI] [PubMed] [Google Scholar]
- 79. Verspaget H, Peña A, Weterman I, et al. Diminished neutrophil function in Crohn's disease and ulcerative colitis identified by decreased oxidative metabolism and low superoxide dismutase content. Gut. 1988;29:223–8. doi:10.1136/gut.29.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wandall J. Function of exudative neutrophilic granulocytes in patients with Crohn's disease or ulcerative colitis. Scand J Gastroenterol. 1985;20:1151–6. doi:10.3109/00365528509088887. [DOI] [PubMed] [Google Scholar]
- 81. Korzenik J, Dieckgraefe B. Is Crohn's disease an immunodeficiency? A hypothesis suggesting possible early events in the pathogenesis of Crohn's disease. Dig Dis Sci. 2000;45:1121–9. doi:10.1023/a:1005541700805. [DOI] [PubMed] [Google Scholar]
- 82. Smith A, Rahman F, Hayee B, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn's disease. J Exp Med. 2009;206:1883–97. doi:10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Marks D, Harbord M, MacAllister R, et al. Defective acute inflammation in Crohn's disease: a clinical investigation. Lancet. 2006;367:668–78. doi:10.1016/s0140-6736(06)68265-2. [DOI] [PubMed] [Google Scholar]
- 84. Kühl A, Kakirman H, Janotta M, et al. Aggravation of different types of experimental colitis by depletion or adhesion blockade of neutrophils. Gastroenterology. 2007;133:1882–92. doi:10.1053/j.gastro.2007.08.073. [DOI] [PubMed] [Google Scholar]
- 85. Zhang R, Ito S, Nishio N, et al. Dextran sulphate sodium increases splenic Gr1(+)CD11b(+) cells which accelerate recovery from colitis following intravenous transplantation. Clin Exp Immunol. 2011;164:417–27. doi:10.1111/j.1365-2249.2011.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nemoto Y, Kanai T, Tohda S, et al. Negative feedback regulation of colitogenic CD4+ T cells by increased granulopoiesis. Inflamm Bowel Dis. 2008;14:1491–503. doi:10.1002/ibd.20531. [DOI] [PubMed] [Google Scholar]
- 87. Zhou G, Yu L, Fang L, et al. CD177 neutrophils as functionally activated neutrophils negatively regulate IBD. Gut. 2018;67:1052–63. doi:10.1136/gutjnl-2016-313535. [DOI] [PubMed] [Google Scholar]
- 88. Li Y, Karlin A, Loike J, et al. Determination of the critical concentration of neutrophils required to block bacterial growth in tissues. J Exp Med. 2004;200:613–22. doi:10.1084/jem.20040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Phillipson M, Kubes P. The Healing Power of Neutrophils. Trends Immunol. 2019;40:635–47. doi:10.1016/j.it.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 90. Scaldaferri F, Vetrano S, Sans M, et al. VEGF-A links angiogenesis and inflammation in inflammatory bowel disease pathogenesis. Gastroenterology. 2009;136:585–95. doi:10.1053/j.gastro.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 91. Christoffersson G, Lomei J, O'Callaghan P, et al. Vascular sprouts induce local attraction of proangiogenic neutrophils. J Leukoc Biol. 2017;102:741–51. doi:10.1189/jlb.1MA0117-018R. [DOI] [PubMed] [Google Scholar]
- 92. Christoffersson G, Vågesjö E, Vandooren J, et al. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood. 2012;120:4653–62. doi:10.1182/blood-2012-04-421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ardi V, Kupriyanova T, Deryugina E, et al. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2007;104:20262–7. doi:10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Taichman N, Young S, Cruchley A, et al. Human neutrophils secrete vascular endothelial growth factor. J Leukoc Biol. 1997;62:397–400. doi:10.1002/jlb.62.3.397. [DOI] [PubMed] [Google Scholar]
- 95. Sylvia C. The role of neutrophil apoptosis in influencing tissue repair. J Wound Care. 2003;12:13–6. doi:10.12968/jowc.2003.12.1.26458. [DOI] [PubMed] [Google Scholar]
- 96. Serhan C, Brain S, Buckley C, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–32. doi:10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Serhan C, Chiang N, Van Dyke T. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi:10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sumagin R, Brazil J, Nava P, et al. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 2016;9:1151–62. doi:10.1038/mi.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Van den Steen P, Proost P, Wuyts A, et al. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–81. [PubMed] [Google Scholar]
- 100. Cario E. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis. 2010;16:1583–97. doi:10.1002/ibd.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Werts C, Rubino S, Ling A, et al. Nod-like receptors in intestinal homeostasis, inflammation, and cancer. J Leukoc Biol. 2011;90:471–82. doi:10.1189/jlb.0411183. [DOI] [PubMed] [Google Scholar]
- 102. Li G, Lin J, Zhang C, et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes. 2021;13:1968257. doi:10.1080/19490976.2021.1968257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–70. doi:10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 104. Amulic B, Cazalet C, Hayes G, et al. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89. doi:10.1146/annurev-immunol-020711-074942. [DOI] [PubMed] [Google Scholar]
- 105. Branzk N, Lubojemska A, Hardison S, et al. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–25. doi:10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Honda M, Kubes P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat Rev Gastroenterol Hepatol. 2018;15:206–21. doi:10.1038/nrgastro.2017.183. [DOI] [PubMed] [Google Scholar]
- 107. Scapini P, Lapinet-Vera J, Gasperini S, et al. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi:10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 108. Yang D, de la Rosa G, Tewary P, et al. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–7. doi:10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Cailhier J, Partolina M, Vuthoori S, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–42. doi:10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 110. Schmielau J, Finn O. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 111. Soehnlein O, Zernecke A, Eriksson E, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–71. doi:10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Costantini C, Calzetti F, Perbellini O, et al. Human neutrophils interact with both 6-sulfo LacNAc+ DC and NK cells to amplify NK-derived IFN{gamma}: role of CD18, ICAM-1, and ICAM-3. Blood. 2011;117:1677–86. doi:10.1182/blood-2010-06-287243. [DOI] [PubMed] [Google Scholar]
- 113. Müller I, Munder M, Kropf P, et al. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms?. Trends Immunol. 2009;30:522–30. doi:10.1016/j.it.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 114. Lacy P. The role of Rho GTPases and SNAREs in mediator release from granulocytes. Pharmacol Ther. 2005;107:358–76. doi:10.1016/j.pharmthera.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 115. Carney E. Protective role of NGAL in ANCA-induced glomerulonephritis. Nat Rev Nephrol. 2020;16:429. doi:10.1038/s41581-020-0317-2. [DOI] [PubMed] [Google Scholar]
- 116. Belambri S, Rolas L, Raad H, et al. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur J Clin Invest. 2018;48:e12951. doi:10.1111/eci.12951. [DOI] [PubMed] [Google Scholar]
- 117. Hyun Y, Choe Y, Park S, et al. LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) distinctly regulate neutrophil extravasation through hotspots I and II. Exp Mol Med. 2019;51:1–13. doi:10.1038/s12276-019-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Locke M, Francis R, Tsaousi E, et al. Fibrinogen protects neutrophils from the cytotoxic effects of histones and delays neutrophil extracellular trap formation induced by ionomycin. Sci Rep. 2020;10:11694. doi:10.1038/s41598-020-68584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Li H, Zhao Y, Li W, et al. Critical role of neutrophil alkaline phosphatase in the antimicrobial function of neutrophils. Life Sci. 2016;157:152–7. doi:10.1016/j.lfs.2016.06.005. [DOI] [PubMed] [Google Scholar]