Abstract
Lung cancer, with non-small cell lung cancer (NSCLC) being the major type, is the second most common malignancy and the leading cause of cancer-related death globally. Immunotherapy, represented by immune checkpoint inhibitors (ICIs), has been one of the greatest advances in recent years for the treatment of solid tumors including NSCLC. However, not all NSCLC patients experience an effective response to immunotherapy with the established selection criteria of programmed death ligand 1 (PD-L1) and tumor mutational burden (TMB). Furthermore, a considerable proportion of patients experience unconventional responses, including pseudoprogression or hyperprogressive disease (HPD), immune-related toxicities, and primary or acquired resistance during the immunotherapy process. To better understand the immune response in NSCLC and provide reference for clinical decision-making, we herein review the rationale and recent advances in using immunotherapy to treat NSCLC. Moreover, we discuss the current challenges and future strategies of this approach to improve its efficacy and safety in treating NSCLC.
Keywords: non-small cell lung cancer, immunotherapy, immune checkpoint inhibitors, adoptive cell therapy, vaccine
Introduction
Lung cancer, with non-small cell lung cancer (NSCLC) being the major type, is the second most common malignancy and the leading cause of cancer-related death worldwide. According to the latest Global Cancer Statistics 2020, the estimated number of new lung cancer cases in the world was 2.206 million in 2020, accounting for 11.4% of all new malignancies; and the estimated number of lung cancer deaths in the world was 1.796 million in 2020, accounting for 18.0% of all cancer deaths.1 At present, the treatment of NSCLC mainly includes surgery, chemotherapy, radiotherapy, molecular targeted therapy and immunotherapy, depending on the specific stage and condition. Since the majority of patients with lung cancer are diagnosed at an advanced stage, they usually have missed the opportunity of radical surgery treatment.2 For advanced NSCLC, chemotherapy has long been the major treatment, but it seems to have limited effect. Molecular targeted therapy such as EGFR-TKI and ALK-TKI have become the standard first-line therapy for patients with advanced NSCLC with positive driver gene mutations, significantly prolonging the survival period and improving the quality of life of patients. However, the “bottleneck” of molecular targeted therapy lies in that secondary mutations often occur during treatment, resulting in drug resistance.3,4 After chemotherapy and molecular targeted therapy, the treatment of advanced NSCLC has entered a new era of immunotherapy represented by immune checkpoint inhibitors (ICIs).5
Immunotherapy is a relatively new treatment approach for cancers including NSCLC, which is hoped to further improve the prognosis of NSCLC. This review discusses our current knowledge of the immune response in NSCLC, the latest and ongoing immune-based therapies, and the future of immunotherapies in NSCLC.
Tumor immunology and immunotherapy in NSCLC
The wrestling between cancer and immunity
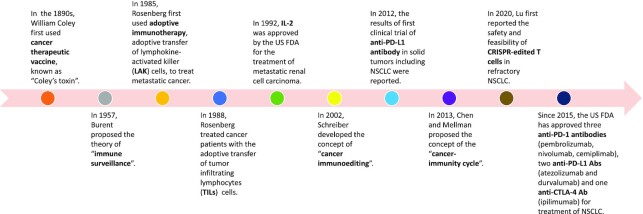
Under normal physiological conditions, the “surveillance function” of human immune system enables it to identify and eliminate foreign components, including invading pathogenic microorganism, allografts, and tumor cells. Though such function of recognizing and killing tumors was first proposed as a hypothesis in as early as 1909,6 it was not until 50 years later that Prehn and Main demonstrated the presence of specific antigens in tumor cells and adaptive immune response of host in experimental animal model.7 In 1957, Burent proposed the theory of “immune surveillance”, holding that nascent transformed cells may arise in our bodies and the immune system can recognize and eradicate these transformed cells before they are clinically manifested.8 In 2002, Schreiber et al. developed the concept of “cancer immunoediting” that there are complex interactions between tumor and immune system in the process of tumor development, mainly consisting of three phases: (1) elimination phase, in which the immune system effectively recognizes and attacks early tumor; (2) equilibrium phase, where the killing of tumor by immune system and the growth of tumor are in a dynamic equilibrium state; and (3) escape stage, in which tumor grows and metastasizes by escaping from the recognition and eradication of immune system through different mechanisms (Fig. 1).9
Figure 1.
History of cancer immunotherapy.
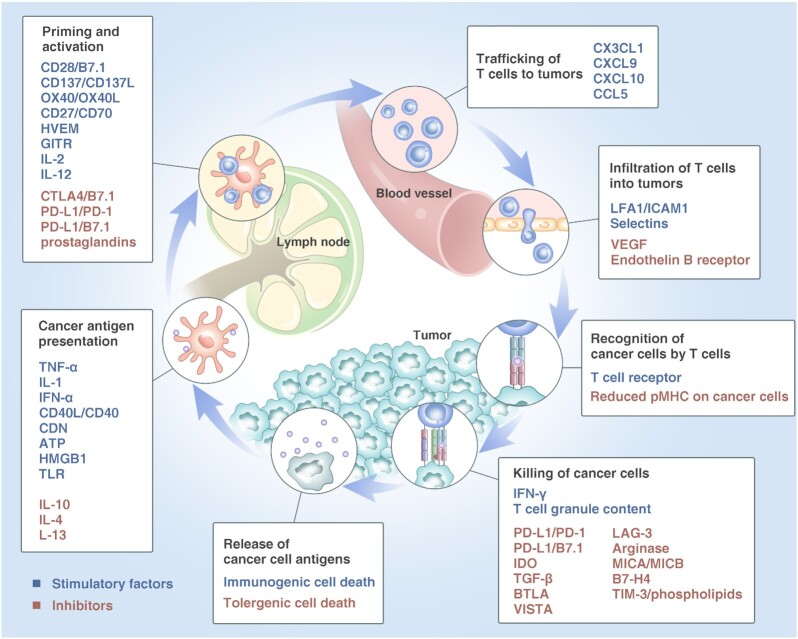
As our understanding of the relationship between tumor and immunity deepens, we now know that for immune system's effective killing of cancer cells, a series of stepwise events must be initiated and allowed to proceed and expand iteratively. The “cancer-immunity cycle” proposed by Chen and Mellman in 2013 reveals the mechanism by which the immune system recognizes and eradicates tumor cells. The cancer-immunity cycle is divided into seven steps (Fig. 2).10,11 (1) Release of cancer cell antigen. Cancer cell antigens, created by cancer cell deaths, genetic alterations and cancer differentiation etc, lead to expression and binding of antigen peptides to major histocompatibility class (MHC) molecules on the surface of cancer cells, distinguishing them from their normal counterparts. (2) Cancer antigen presentation. Antigen presenting cells (APCs), mainly dendritic cells (DCs), capture cancer-specific antigen peptide by binding antigen peptide to MHC molecules on the surface of APCs, and subsequently present them to T cells. (3) Priming and activation. In lymph node, T cell receptor (TCR) recognizing the antigen/MHC complex on the APC surface, as well as the interaction between CD28 molecule on the T cell surface and the B7.1 molecule on the APC surface, prime and activate the T cells. (4) Trafficking of T cells to tumor. The activated effector T cells traffic to the tumor bed through blood circulation. (5) Infiltration of T cells into tumors. Effector T cells migrate from the circulating blood to the tumor bed across the vascular endothelial barrier. (6) Recognition of cancer cells by T cells. Cytotoxic T lymphocytes (CTLs) specifically recognize and bind to cancer cells through the interaction between its TCR and antigen/MHC complex on the cancer cells. (7) Killing of cancer cells. CTLs kill their target cancer cells, lead to releasing additional cancer antigens (step one) and subsequent another circulation of the cycle. Through the mechanism of cancer-immunity cycle above, the host immune system can effectively kill cancer cells.
Figure 2.
The “cancer-immunity cycle”. Figure adapted from Ref. 10. Copyright Elsevier, 2013.
However, such cancer-immunity cycle does not always perform perfectly in cancer patients. Tumors can escape from the host immune system through changing tumor cells themselves or the tumor microenvironment (TME), thus maintaining the continuous proliferation and invasion of tumor cells and eventually leading to the occurrence and development of tumor, which is called “immune escape”.12 The immune escape of tumor is essentially achieved by disrupting certain steps in the cancer-immunity cycle.
Rationale for immunotherapy against NSCLC
At each step of the cancer-immunity cycle, there are positive and negative regulators that keep the activation of the immune system within the normal range.10 Therefore, we can achieve the therapeutic purpose through strengthening the positive regulation signals or suppressing the negative regulation signals.
Directing at the negative immune checkpoints signaling, ICIs are the most developed and widely-used strategy against NSCLC.13 Immune checkpoint is a class of immunosuppressive molecules, which are expressed on immune cells and can regulate the degree of immune activation. Cytotoxic T lymphocyte associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) are the representatives of immune checkpoints in NSCLC, which act as inhibitors in the activation of T cells.14 CTLA-4 is presented on T cells and its interaction with B7 on APCs reduces IL-2 production and T cell proliferation in lymphoid organs. Such reduction can be blocked by CTLA-4 inhibitors.15 Besides, several recent studies found that CTLA-4 inhibitors exhibit antitumor function by selectively depleting regulatory T (Treg) cells in the TME through an Fc-dependent mechanism.16–18 PD-1 is expressed on activated T cells, and its ligand programmed cell death protein ligand-1 (PD-L1) can be expressed on the surface of tumor cells and immune cells. The binding of PD-1 and PD-L1 can inhibit activated T cell proliferation, promote activated T cell apoptosis and reduce cytokine secretion in the TME.15 Apart from CTLA-4 and PD-1, novel immune checkpoint molecules on T cells have been discovered, including TIGIT, LAG-3, TIM-3, VISTA and CD244.19–23 These immune checkpoints can inhibit T cell function by binding to their ligands on tumor cells, APC cell or other cells. For instance, TIGIT is an immune checkpoint mainly expressed on the surface of T cells and natural killer (NK) cells and can inhibit cell function by binding to its ligands CD55 and CD122; LAG-3 is expressed on activated CD4+, CD8+ T cells and NK cells, and can inhibit T cell function by binding to its ligand FGL1. Blocking these immune checkpoints can achieve reactivation of T cells and NK cells to enhance the antitumor activity.
Adoptive cell therapy (ACT) and cancer vaccine are other two promising immunotherapy strategies for patients with NSCLC. ACT, including chimeric antigen receptor (CAR) T-cell therapy, engineered T-cell receptor (TCR) T-cell therapy, tumor-infiltrating lymphocyte (TIL) therapy and so on, aimed at reprograming immune cells to enhance tumor cells’ recognizing and killing.24,25 Cancer vaccines, including tumor antigen associated vaccines, neoantigen associated vaccines and cell vaccines, are designed to amplify tumor-specific T cell responses via active immunization.26,27
Recent advance of immunotherapy in NSCLC
In the past decade, ICIs treatment has achieved significant progress in NSCLC.15,28,29 First, the use of ICIs has expanded from the initially second-line therapy to multiple clinical settings, including neoadjuvant, adjuvant, first, second, and subsequent lines treatment. Second, ICIs treatment has expanded from monotherapy to combination therapy, including combination of different types of ICIs (i.e. PD-1/PD-L1 inhibitors with CTLA-4 inhibitors), as well as ICI treatment with chemotherapy, radiology, and chemotherapy plus anti-VEGF antibody. Third, several novel ICIs targeting LAG-3, TIM-3 and VISTA are undergoing clinical trials to evaluate their efficacy and safety in treatment of solid tumors including NSCLC and exhibit great therapeutic promise.30–33 Currently, three anti-PD-1 monoclonal antibodies (mAbs; pembrolizumab, nivolumab, cemiplimab), two anti-PD-L1 mAbs (atezolizumab and durvalumab) and one anti-CTLA-4 mAb (ipilimumab) have been approved by the US Food and Drug Administration (FDA) for treatment of NSCLC.34,35 Besides, the China National Medical Products Administration (NMPA) has approved four more mAbs that target PD-1 (camrelizumab, sintilimab, tiselizumab and toripalimab) for treatment of NSCLC.36 More recently, novel anti-TIGIT mAb tiragolumab is approved by FDA for treatment of NSCLC (Table 1).
Table 1.
Currently approved immunotherapy in NSCLC.
| Type | Regimen | FDA approval | NMDA approval |
|---|---|---|---|
| PD-1 mAb | Pembrolizumab | First and second line treatment for squamous/non-squamous advanced NSCLC | First line treatment for advanced squamous/non-squamous NSCLC |
| PD-1 mAb | Nivolumab | Second line treatment for squamous/non-squamous advanced NSCLC | Second line treatment for squamous/non-squamous advanced NSCLC |
| PD-1 mAb | Cemiplimab | First line treatment | / |
| PD-L1 mAb | Atezolizumab | First and second line treatment for squamous/non-squamous advanced NSCLC | / |
| PD-L1 mAb | Durvalumab | Unresectable stage III NSCLC | Unresectable stage III NSCLC |
| PD-1 mAb | Camrelizumab | / | First line treatment for advanced non-squamous NSCLC |
| PD-1 mAb | Sintilimab | / | First line treatment for advanced non-squamous NSCLC |
| PD-1 mAb | Tiselizumab | / | First line treatment for squamous advanced NSCLC |
| PD-1 mAb | Toripalimab | / | First line treatment for advanced NSCLC |
| CTLA4 mAb | Ipilimumab | First line treatment | First line treatment |
| TIGIT mAb | Tiragolumab | First line treatment | / |
ICIs monotherapy as first-line treatment for advanced NSCLC
Five phase III trials reported outcomes for first-line ICIs monotherapy in advanced NSCLC. In KEYNOTE-024, KEYNOTE-042, Impower 110, pembrolizumab and atezolizumab showed significantly improved overall survival (OS) compared with chemotherapy in patients with advanced NSCLC.37–42 On the other hand, CheckMate 026 trial reported that nivolumab failed to prolong progression-free survival (PFS) and OS when compared with chemotherapy, indicating not all ICIs monotherapies as first-line therapy can be helpful for advanced NSCLC.43 More recently, EMPOWER-Lung 1 trial reported its efficacy and safety outcomes of cemiplimab monotherapy. Significant improvements in OS (median: not evaluable versus 14.2 months) and PFS (median: 8.2 months versus 5.7 months) were observed in patients who received cemiplimab monontherapy compared to those underwent chemotherapy. Moreover, lower frequency of grade 3–4 immune-related adverse events (irAEs) occurred in patients treated with cemiplimab than in those treated with chemotherapy (28% versus 39%).44,45 Based on results of these trials, pembrolizumab, atezolizumab and cemiplimab monotherapy have been approved for first-line therapy in advanced NSCLC.
ICIs-based combination therapy as the first-line treatment for advanced NSCLC
Multiple completed phase III trials have evaluated the efficacy and safety of ICIs-based combination therapy (including PD-1/PD-L1 inhibitors plus chemotherapy, PD-1/PD-L1 inhibitors plus chemotherapy plus anti-angiogenetic therapy, PD-1/PD-L1 inhibitors plus CTLA-4 inhibitors, and PD-1/PD-L1 inhibitors plus CTLA-4 inhibitors plus chemotherapy) as the first-line treatment in advanced NSCLC. In these trials, pembrolizumab-chemotherapy (KEYNOTE-021, KEYNOTE-189 and KEYNOTE-407),46–49 atezolizumab-chemotherapy (IMpower 130),50 atezolizumab-bevacizumab-chemotherapy (IMpower 150),5152 nivolumab-ipilimumab (CheckMate 227)53 and nivolumab-ipilimumab-chemotherapy (CheckMate 9LA)54 showed significantly improved OS, PFS and objective response rate (ORR) compared with controls in patients with advanced NSCLC; and subsequently are approved for first-line treatment of advanced NSCLC. Notably, FDA approved another ICI tiragolumab in 2021, based on the results of phase II CITYSCAPE trial, demonstrating that combination of TIGIT and PD-L1 inhibitors may enhance antitumor activity by potentially amplifying the immune response. In the CITYSCAPE trial, comparable PFS improvement with tiragolumab plus atezolizumab relative to atezolizumab monotherapy was seen in PD-L1–high NSCLC patients (PFS hazard ratio (HR) 0.23, 95% CI: 0.10–0.53).55
ICIs monotherapy as second-line treatment for advanced NSCLC
ICIs monotherapy pembrolizumab, nivolumab and atezolizumab have been approved by FDA/NMPA for the second-line treatment in advanced NSCLC based on improved survival and safety data from five phase III clinical trials (KEYNOTE-010, OAK, CheckMate 078, CheckMate 017 and CheckMate 057).56–61
ICIs neoadjuvant therapy for early-stage resectable NSCLC
Although ICIs have yet been approved for the neoadjuvant treatment in NSCLC, reported efficacy data from trials has been promising and ICIs will likely play an important role in the treatment of early-stage resectable NSCLC. In the setting of neoadjuvant monotherapy with ICIs, series of trials have demonstrated that ICIs (nivolumab, atezolizumab and sintilimab) have great potentials with higher major pathologic response (MPR) and pathological complete response (pCR) when compared with chemotherapy.62–64 For ICIs-based neoadjuvant combination therapy, two recently completed studies have reported efficacy outcomes in patients treated with neoadjuvant chemoimmunotherapy. In the phase II trial of toripalimab plus chemotherapy as neoadjuvant treatment in resectable stage III NSCLC (NeoTPD01 Study) with 30 out of the total 33 enrolled patients undergoing resection, the MPR rate was 66.7% (20/30), the pCR rate was 50% (15/30), and 96.7% (29/30) patients achieved R0 resection.65 The phase III Checkmate-816 trial, which aimed to evaluate nivolumab plus chemotherapy versus chemotherapy as neoadjuvant therapy for resectable stage IB-IIIA NSCLC, published its latest results at the 2021 AACR congress. NIVO plus chemo significantly improved pCR compared to chemo (24.0% versus 2.2%, P < 0.0001), MPR (36.9% versus 8.9%), as well as ORR (53.6% versus 37.4%). Furthermore, neoadjuvant treatment did not cause death or delay in surgery.66
ICIs adjuvant therapy for early-stage resectable NSCLC
Similar to neoadjuvant immunotherapy, safety and efficacy of adjuvant immunotherapy in patients with early-stage resectable NSCLC are being explored in multiple phase II to III trials. Recently, the primary results were released for the phase III IMpower010 trial, which assessed the safety and efficacy of atezolizumab versus best supportive care (BSC) after adjuvant chemotherapy in resected stage IB-IIIA NSCLC. Atezolizumab showed statistically significant disease-free survival (DFS) benefit versus BSC (36 months: 60.0% versus 48.2%).67 Two main trials of adjuvant therapy with anti-PD-1 agents, ANVIL (nivolumab) and PEARLS (pembrolizumab), are underway and efficacy outcomes have yet to be published.68
ICIs consolidation therapy for unresectable stage III NSCLC
PACIFIC was a phase III trial in patients with unrectable stage III NSCLC treated with consolidative durvalumab or placebo after concurrent chemoradiotherapy. Median PFS were 16.8 months in the duvalizumab group versus 5.6 months in the placebo group (HR = 0.51; 95%CI: 0.41–0.63).69 Based on this result, FDA and NMDA approved duvalizumab as consolidation therapy in unresectable stage III NSCLC. In 2021, PACIFIC reported its latest efficacy outcomes. Estimated 4-year OS rates were 49.6% for durvalumab versus 36.3% for placebo, and 4-year PFS rates were 35.3% (duravlumab) versus 19.5% (placebo).70
Adoptive cell therapy (ACT) and cancer vaccine for advanced NSCLC
So far, TCR-T has gone through four iterations. In recent years, TCR-T therapy worldwide has mainly targeted solid tumors including NSCLC. For instance, ADP-A2M4CD8, a novel TCR-T therapy, which coexpress the CD8 coreceptor with the engineered TCR targeting MAGE-A4, is currently being investigated for the treatment of solid tumors. In the ongoing phase 1 SURPASS trial, which enrolled multiple solid tumors including NSCLC, the majority of evaluable patients (13/15) had evidence of disease control and there were RECIST responses in several types of solid tumor.71 On the other hand, scientists developed several cancer vaccines, L-BLP25, MAGE-A3, TG4010, NY-ESO-1, CIMAvax-EGF and others in the past two decades.72–75 Among them, CIMAvax-EGF, which is built on the induction of a specific immune response, aiming to sequester EGF, showed ideal efficiency in clinical trials. A phase III trial enrolled stage IIIB/IV NSCLC patients and randomly assigned to receive CIMAvax-EGF or placebo, and found significantly increased median survival time in patients in CIMAvax-EGF group, and CIMAvax-EGF was well tolerated.76 More recently, another vaccine OSE2101, which modifies epitopes restricted to HLA-A2+ from five tumor-associated antigens, was demonstrated to have better prognosis (median OS: 11.1 months vs 7.5 months, HR 0.59) and fewer severe adverse events (38% vs 68%, p < 0.001) compared with standard of care in advanced NSCLC.77 Currently, multiple clinical trials assessing ACT and cancer vaccine in NSCLC are still underway.
Challenges and perspectives of immunotherapy in NSCLC
Screening of potential benefit population
Numerous clinical trials and studies have confirmed that only a small fraction of NSCLC patients show objective responses to immunotherapy and get long-term benefit from immunotherapy; nevertheless, there are currently no optimal predictors to identify patients who will likely benefit from immunotherapy.
PD-L1, TMB and dMMR/MSI-H
At present, biomarkers including PD⁃L1, tumor mutational burden (TMB) and mismatch repair deficient (dMMR)/microsatellite instability-high (MSI-H) have shown some predictive value, and are approved by the FDA and/or the NMPA as indicators for predicting the efficacy of immunotherapy in NSCLC or other solid tumors. Among these markers, PD-L1 is the most widely-used in NSCLC. However, even for those advanced NSCLC patients with relatively high PD-L1 expression (≥50% of tumor cells or ≥ 10% of tumor-infiltrating immune cells), NSCLC patients receiving first-line monotherapy with PD-1/PD-L1 inhibitors (pembrolizumab and atezolizumab) had ORR of about 38.3%–46.1%.37,39,41,42 For TMB, the ORR in NSCLC patients with TMB high (TMB-H, defined as TMB ≥ 10 mutations/Mb) treated with nivolumab plus ipilimumab was only around 33%-48%, according to the previous trials.78–80 Although recent studies suggested that dMMR/MSI-H may be a predictor for PD-1 inhibitors therapy regardless of the cancer origin, the incidence of dMMR/MSI-H in NSCLC is very low and their predictive value for NSCLC needs further verification through more clinical trials and studies.81–83 To improve forecasting ability, scholars analyzed the predictive utility of combination of PD-L1 expression and TMB in NSCLC, and found that combined use of PD-L1 expression and TMB is a promising biomarker to evaluate patients’ survival after immunotherapy (1-year PFS AUC 0.826; 3-year PFS AUC 0.948).84
Predictive model
Studies have shown that the small fraction of patients who get improved clinical outcomes tend to have some common features, including male, smoking history, and good general physical condition, with performance status (PS) score of 0–1.85 Thus, predictive models were built based on these features to help screening potential target population. So far the best predictive model for NSCLC immunotherapy is iSEND, which includes gender, PS score, Neutrophil-to-lymphocyte ratio (NLR), and Delta NLR as the variables to categorize patients into different risk groups and significantly discriminates each group's clinical outcome.86,87 Although these data are from small-scale clinical studies, predictive models based on integrative analysis of real-world data should be promising in screening potential benefit populations in today's environment of big data analysis and artificial intelligence.
ctDNA
Detection of circulating tumor DNA (ctDNA) has not only value in precise diagnosis of NSCLC (i.e. driver mutations, TMB and MMR), but also probable potential in predicting the efficacy of immunotherapy in NSCLC.88–90 For instance, Sarah et al. showed that in metastatic NSCLC receiving ICIs, a ctDNA response (defined as a > 50% decrease in mutant allele fraction from baseline) was associated with superior PFS (HR = 0.29, P = 0.03), and superior OS (HR = 0.17, P = 0.007); besides, the decline in ctDNA levels preceded the imaging confirmation of tumor shrinkage (24.5 days versus 72.5 days).91 Thus, monitoring ctDNA levels in NSCLC patients receiving ICIs enables early assessment of immunotherapy response and might avoid the prolonged administration of ineffective treatments.
Immune-related toxicity
Immunotherapy has significantly prolonged the patient survival, but it also brings immune-related toxicity, or irAEs. Notably, more and more studies have suggested that development of irAEs predicts a better response to immunotherapy in NSCLC.92–96 In 2018, scholars first reported that the PFS and OS of patients with irAEs were significantly better than those without adverse reactions after treatment with nivolumab (P = 0.04 and 0.01, respectively); the ORR of patients with irAEs was significantly higher than that of patients without irAEs (52.3% and 27.9%, respectively).93 Further studies found that among the various types of irAEs, endocrine toxicity and dermatological toxicity may be most closely related to the efficacy of immunotherapy.92 However, the mechanism remains not clear at present, though it is speculated to be related to the important role of immune checkpoint in maintaining the process of autoimmune balance.
Apart from the predictors mentioned above, CD8+ T-cell tumor-infiltrating, genetic mutations (RYR1, MGAM and STK11), copy number alteration and HLA class I diversity are also being explored for treating NSCLC and other solid tumors.84,97–99 Taken together, there cannot be a single perfect predictor to screen potential target populations for immunotherapy in NSCLC; predictive models are needed that take into account different parameters affecting tumor-host interactions.
Objective evaluation of response to immunotherapy
Different from chemotherapy, radiotherapy, or molecular targeted therapy, ICIs do not exert direct cytotoxic effects on tumor cells, but restore or enhance the immune system's antitumor response with immune cells as the target. The complexity of the response patterns after immunotherapy warrants special attention.
At present, four unconventional response patterns for immunotherapy have been observed: pseudoprogression, delayed response, mixed response, and hyperprogressive disease (HPD).100,101 Pseudoprogression is an initial increase in the tumor volume or number of tumor lesions followed by a decrease.102 The reported incidence of pseudoprogression is 2.6%–4.7% in NSCLC.102–105 In 2017, the RECIST Working Group officially proposed a modified RECIST 1.1 for immune-based therapeutics (termed iRECIST).106 The iRECIST criteria introduce the concepts of immune unconfirmed progressive disease (iUPD) and immune confirmed progressive disease (iCPD). A progressive disease (PD) previously assessed by traditional RECIST 1.1 is temporarily evaluated as iUPD, and continuation of treatment is determined based on the tumor type, disease stage and clinical situation of the patient; it can only be confirmed as iCPD by re-evaluation at 4–8 weeks. This reassessment approach can identify unconventional responses such as pseudoprogression and delayed responses. Moreover, serological biomarkers, including ctDNA and NLR, might help distinguish pseudoprogression from true progression.107–109
HPD is featured with drastic progression of disease after immunotherapy, but there has been no standard definition. Kato et al. first defined HPD with three criteria: time to treatment failure (TTF) <2 months, > 50% increase in tumor burden and > 2-fold increase in progression rate.110 Russo et al. later required three out of five criteria for being diagnosed with HPD, including TTF < 2 months, ≥ 50% increase in the sum of the diameter of target lesions, appearance of at least two new lesions in an affected organ, dissemination to a new organ or clinical deterioration to PS ≥ 2.111 The reported incidence of HPD ranged from 9.2% to 17.9% in NSCLC due to application of different criteria.105,112,113 The prognosis of patients with HPD is extremely poor with median OS of 1.7–3.4 months.105,114,115 Once HPD is suspected, immunotherapy should be interrupted and a detailed evaluation should be conducted immediately. However, there are currently no reliable predictors for HPD after immunotherapy. Thus, the recognition of HPD warrants further studies.
Management of immune-related toxicity
The immune-related toxicity induced by ICIs not only limits the use of these beneficial drugs, but also threatens the patient's health. The irAEs can occur in all tissues and organs throughout the body, including skin, endocrine system, lung, liver, gastrointestinal system, musculoskeletal system, nervous system, cardiovascular system, eyes, hematologic system, and others.116–119 Although the overall incidence of irAEs is low in NSCLC, some of them can be severe and even life-threatening, requiring early accurate recognition and adequate management.
Searching for predictive biomarkers of irAEs, which is crucial for early diagnosis and timely treatment, represents an aspect of active investigation in immunotherapy. Scientists have explored clinical parameters (gender, preexisting autoimmune disease, etc.) as well as laboratory biomarkers (absolute lymphocyte count, NLR, etc.) that are associated with increased risk of irAEs.120,121 However, many studies present conflicting findings. Recently, some novel biomarkers have been shown promise for clinical application. First, CD8 T cells clonal expansion in the peripheral blood could predict the development of irAEs. Subudhi et al. found that in prostate cancer patients treated with ICIs, expansion of ≥ 55 CD8 T cell clones preceded the development of grade 2–3 irAEs.122 Additionally, detection of autoantibody in the serum is another potential predictor for the occurrence of irAEs. In a study of 92 patients with NSCLC receiving the anti-PD1 mAb nivolumab,123 detection of more than one of autoantibodies (including ANA, ENAs and ASMA) within 30 days of starting therapy was correlated with the risk of irAEs (P = 0.002). Furthermore, several studies indicated that the baseline gut microbiome might predict immune-related colitis in patients treated with ICIs.124,125 Further validation studies are needed for these specific biomarkers in larger-scale cohorts of NSCLC patients receiving immunotherapy.
Exploring approaches to limit irAEs is another area of active investigation in immunotherapy. Changing dose and schedule is the most acceptable and easy to implement. For example, using lower and/or less frequent dosing of ipilimumab could maintain therapeutic benefit but reduce irAEs in the Checkmate 227 trial for NSCLC.78 Besides, early intervention is another feasible approach to prevent some fatal irAEs. For example, in a retrospective study of patients who developed immune-related colitis, patients receiving immunosuppression early (≤10 days) required fewer hospitalizations (P = 0.03), experienced steroid taper failure less frequently (P = 0.03), had a shorter course of steroid treatment (P = 0.09) and a shorter duration of symptoms (P < 0.01) compared with patients receiving immunosuppressive therapy > 10 days after onset of colitis.126 In addition to these two approaches, others including prophylactic use of drugs (such as vedolizumab), repurposed drugs (such as tofacitinib), alternative checkpoints and tumor-targeted ICIs, are also being explored to limit irAEs.127
Dealing with immune resistance
With the gradual widespread clinical application of ICIs in NSCLC, immune resistance is observed in subsets of patients. Some do not respond to the inhibitors at all; for the initial responders, a substantial proportion ultimately relapse with lethal drug-resistant diseases, months or years after administration of the ICIs.
Due to the complex resistance mechanisms of immunotherapy, there is still no standardized solution to this problem. Currently, combination therapy to reverse or slow down immune resistance is the most effective measure, including combination of different types of ICIs (i.e. PD-1 inhibitor plus CTLA-4 inhibitor) or combination of ICIs with other types of therapy (i.e. chemotherapy, radiotherapy, molecular targeted therapy, anti-angiogenesis therapy). For example, combination of CTLA-4 inhibitor ipilimumab with PD-1 inhibitors nivolumab is promising as first-line treatment in advanced NSCLC.78,128 Due to different mechanisms of action, the combination of PD-1 and CTLA-4 inhibitor can play a synergistic effect, which can not only induce the production of a large number of T cells by antagonizing CTLA-4 at the early stage of immune response, but also restore the killing function of T cells to tumor cells by blocking the binding of PD-1 and PD-L1, and reducing T cell depletion. Exploring new therapy strategies is another important way to conquer immune resistance. T cells genetically equipped with TCRs have shown great potential in treating solid tumors including NSCLC. Therapeutic vaccines against cancer have also been explored. However, challenges including weak immunogenicity, systematic toxicity, and off-target effects remain as barriers to their clinical translation.129
Conclusions
The extraordinary clinical outcomes through the application of ICI regimens make us believe that immunotherapy will constitute a more and more widely-used treatment strategy for NSCLC in the near future. The next step is to better screen potential benefit population, objectively evaluate response to immunotherapy, manage immune-related toxicity and deal with immune resistance. The exploration of new biomarkers or models to predict the efficacy, awareness of unconventional response patterns for immunotherapy, and the development of new immunotherapy including ACT therapy and cancer vaccine will improve the application of immunotherapy in clinical practice; and the ongoing trials and studies about new treatment strategies with existing and novel drugs promise to improve the precision, efficacy and safety of immunotherapy in NSCLC.
Acknowledgements
This study was supported by the National Natural Science Foundation of China to Wemin Li (Grants No. 81871890 and 91859203) and the Science and Technology Support Program of Sichuan Province to Yalun Li (Grant No. 2020YFS0572).
Contributor Information
Wenxin Luo, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, China.
Zhoufeng Wang, Precision Medicine Research Center, West China Hospital, Sichuan University, Chengdu 610041, China.
Ting Zhang, Clinical Medical College and the First Affiliated Hospital of Chengdu Medical College, Chengdu 610500, China.
Lan Yang, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, China.
Jinghong Xian, Department of Clinical Research Management, West China Hospital, Sichuan University, Chengdu 610041, China.
Yalun Li, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, China.
Weimin Li, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu 610041, China; Precision Medicine Research Center, West China Hospital, Sichuan University, Chengdu 610041, China.
Author contributions
Conceptualization, W.X.L. and W.M.L.; writing-original draft preparation, W.X.L.; writing-review and editing, Z.F.W., T.Z., L.Y. and J.H.X.; visualization, Z.F.W. and Y.L.L.; supervision, W.M.L.; all the authors read the article and approved the final version.
Conflict of interest
As a Co-EIC of Precision Clinical Medicine, the corresponding author Weimin Li was blinded from reviewing or making decisions on this manuscript.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2. Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75. doi: 10.1007/978-3-319-40389-2_3. [DOI] [PubMed] [Google Scholar]
- 3. Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol. 2018;29:i10–9. doi: 10.1093/annonc/mdx703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCoach CE, Le AT, Gowan K, et al. Resistance mechanisms to targeted therapies in ROS1(+) and ALK(+) non-small cell lung cancer. Clin Cancer Res. 2018;24:3334–47. doi: 10.1158/1078-0432.Ccr-17-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–40. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 6. Ehrlich P. Ueber den jetzigen stand der karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:73–290. doi: 10.1101/833004. [Google Scholar]
- 7. Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–78. [PubMed] [Google Scholar]
- 8. Burnet M. Cancer: a biological approach. BMJ. 1957;1:841–7. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 10. Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 11. Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stankovic B, Bjorhovde HAK, Skarshaug R, et al. Immune cell composition in human non-small cell lung cancer. Front Immunol. 2018;9:3101. doi: 10.3389/fimmu.2018.03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Version 5.2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf(Accessed June 15, 2021). [DOI] [PubMed]
- 14. Lim SM, Hong MH, Kim HR. Immunotherapy for Non-small cell lung cancer: Current landscape and future perspectives. Immune Network. 2020;20:e10. doi: 10.4110/in.2020.20.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raju S, Joseph R, Sehgal S. Review of checkpoint immunotherapy for the management of non-small cell lung cancer. ImmunoTargets and Therapy. 2018;7:63–75. doi: 10.2147/ITT.S125070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tang F, Du X, Liu M, et al. Anti-CTLA-4 antibodies in cancer immunotherapy: selective depletion of intratumoral regulatory T cells or checkpoint blockade?. Cell & Bioscience. 2018;8:30. doi: 10.1186/s13578-018-0229-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ingram JR, Blomberg OS, Rashidian M, et al. Anti–CTLA-4 therapy requires an Fc domain for efficacy. Proc Natl Acad Sci. 2018;115:3912–7. doi: 10.1073/pnas.1801524115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arce Vargas F, Furness AJS, Litchfield K, et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell. 2018;33:649–63..e4. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maruhashi T, Sugiura D, Okazaki IM, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014. doi: 10.1136/jitc-2020-001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8:e000911. doi: 10.1136/jitc-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chocarro L, Blanco E, Zuazo M, et al. Understanding LAG-3 signaling. Int J Mol Sci. 2021;22:5282. doi: 10.3390/ijms22105282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mulati K, Hamanishi J, Matsumura N, et al. VISTA expressed in tumour cells regulates T cell function. Br J Cancer. 2019;120:115–27. doi: 10.1038/s41416-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8:e000957. doi: 10.1136/jitc-2020-000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qu J, Mei Q, Chen L, et al. Chimeric antigen receptor (CAR)-T-cell therapy in non-small-cell lung cancer (NSCLC): current status and future perspectives. Cancer Immunol Immunother. 2021;70:619–31. doi: 10.1007/s00262-020-02735-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–82. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Desrichard A, Snyder A, Chan TA. Cancer neoantigens and applications for immunotherapy. Clin Cancer Res. 2016;22:807–12. doi: 10.1158/1078-0432.Ccr-14-3175. [DOI] [PubMed] [Google Scholar]
- 28. Qiu Z, Chen Z, Zhang C, et al. Achievements and futures of immune checkpoint inhibitors in non-small cell lung cancer. Experimental Hematology & Oncology. 2019;8:19. doi: 10.1186/s40164-019-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Memon H, Patel BM. Immune checkpoint inhibitors in non-small cell lung cancer: A bird's eye view. Life Sci. 2019;233:116713. doi: 10.1016/j.lfs.2019.116713. [DOI] [PubMed] [Google Scholar]
- 30. Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8:e000911. doi: 10.1136/jitc-2020-000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feeney K, Kelly R, Lipton LR, et al. CA224-060: A randomized, open label, phase II trial of relatlimab (anti-LAG-3) and nivolumab with chemotherapy versus nivolumab with chemotherapy as first-line treatment in patients with gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2019;37, doi: 10.1200/JCO.2019.37.15_suppl.TPS4143. [Google Scholar]
- 32. Harding JJ, Patnaik A, Moreno V, et al. A phase Ia/Ib study of an anti-TIM-3 antibody (LY3321367) monotherapy or in combination with an anti-PD-L1 antibody (LY3300054): Interim safety, efficacy, and pharmacokinetic findings in advanced cancers. J Clin Oncol. 2019; 37:12. doi: 10.1200/JCO.2019.37.8_suppl.12.30379624 [Google Scholar]
- 33. Zauderer M, Brody J, Marron T, et al. P2.06-07 phase 1 study of CA-170: first-in-class small molecule targeting VISTA/PD-L1 in patients with malignant pleural mesothelioma. J Thorac Oncol. 2019;14:S757–8. doi: 10.1016/j.jtho.2019.08.1625. [Google Scholar]
- 34. Vafadar S. Immunotherapy for non-small cell lung cancer. Journal of the American Academy of Physician Assistants. 2019;32:37–42. doi: 10.1097/01.JAA.0000569792.99069.e6. [DOI] [PubMed] [Google Scholar]
- 35. Picardo SL, Doi J, Hansen AR. Structure and optimization of checkpoint inhibitors. Cancers. 2019;12:38. doi: 10.3390/cancers12010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhou C, Wang J, Bu H, et al. Chinese experts consensus on immune checkpoint inhibitors for non-small cell lung cancer (2019 version). (Article in Chinese). Chin J Lung Cancer. 2020;23:65–76. doi: 10.3779/j.issn.1009-3419.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 38. Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. LBA51 KEYNOTE-024 5-year OS update: First-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. Ann Oncol. 2020;31:S1181–2. doi: 10.1016/j.annonc.2020.08.2284. [Google Scholar]
- 39. Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med. 2020;383:1328–39. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 40. Herbst R, De Marinis F, Giaccone G, et al. FP13.03 IMpower110: updated OS analysis of atezolizumab vs platinum-based chemotherapy as first-line treatment in PD-L1–selected NSCLC. J Thorac Oncol. 2021;16:S224–5. doi: 10.1016/j.jtho.2021.01.142. [DOI] [PubMed] [Google Scholar]
- 41. Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet North Am Ed. 2019;393:1819–30. doi: 10.1016/s0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 42. Wu YL, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China Study. Int J Cancer. 2021;148:2313–20. doi: 10.1002/ijc.33399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376:2415–26. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sezer A, Kilickap S, Gümüş M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet North Am Ed. 2021;397:592–604. [DOI] [PubMed] [Google Scholar]
- 45. Sezer A, Kilickap S, Gümüş M, et al. LBA52 EMPOWER-Lung 1: Phase III first-line (1L) cemiplimab monotherapy vs platinum-doublet chemotherapy (chemo) in advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) ≥50%. Ann Oncol. 2020;31:S1182–3. doi: 10.1016/j.annonc.2020.08.2285. [Google Scholar]
- 46. Paz-Ares L, Vicente D, Tafreshi A, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol. 2020;15:1657–69. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 47. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38:1505–17. doi: 10.1200/jco.19.03136. [DOI] [PubMed] [Google Scholar]
- 48. Garassino MC, Gadgeel S, Esteban E, et al. Patient-reported outcomes following pembrolizumab or placebo plus pemetrexed and platinum in patients with previously untreated, metastatic, non-squamous non-small-cell lung cancer (KEYNOTE-189): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:387–97. doi: 10.1016/s1470-2045(19)30801-0. [DOI] [PubMed] [Google Scholar]
- 49. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–508. doi: 10.1016/s1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–37. doi: 10.1016/s1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 51. Socinski MA, Mok TS, Nishio M, et al. Abstract CT216: IMpower150 final analysis: Efficacy of atezolizumab (atezo)+ bevacizumab (bev) and chemotherapy in first-line (1L) metastatic nonsquamous (nsq) non-small cell lung cancer (NSCLC) across key subgroups. In AACR Annual Meeting 2020, Philadelphia, 2020. doi: 10.1158/1538-7445.AM2020-CT216. [Google Scholar]
- 52. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 53. Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab+ ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: Three-year update from CheckMate 227 Part 1. J Clin Oncol. 2020;38:9500. doi: 10.1200/JCO.2020.38.15_suppl.9500. [Google Scholar]
- 54. Reck M, Ciuleanu T-E, Dols MC, et al. Nivolumab (NIVO)+ ipilimumab (IPI)+ 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38:9501. doi: 10.1200/JCO.2020.38.15_suppl.9501. [Google Scholar]
- 55. Patil N, Cho BC, Johnson M, et al. P77.02 Efficacy of Tiragolumab + Atezolizumab in PD-L1 IHC and TIGIT Subgroups in the Phase II CITYSCAPE Study in First-Line NSCLC. J Thorac Oncol. 2021;16:S635–6. doi: 10.1016/j.jtho.2021.01.1160. [Google Scholar]
- 56. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet North Am Ed. 2016;387:1540–50. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 57. Herbst RS, Garon EB, Kim DW, et al. Five year survival update from KEYNOTE-010: pembrolizumab versus docetaxel for previously treated, programmed death-ligand 1-positive advanced NSCLC. J Thorac Oncol. 2021;16:1718–32. doi: 10.1016/j.jtho.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 58. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet North Am Ed. 2017;389:255–65. doi: 10.1016/s0140-6736(16)32517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14:867–75. doi: 10.1016/j.jtho.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 60. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shu CA, Gainor JF, Awad MM, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:786–95. doi: 10.1016/S1470-2045(20)30140-6. [DOI] [PubMed] [Google Scholar]
- 63. Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N Engl J Med. 2018;378:1976–86. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gao S, Li N, Gao S, et al. Two-year follow-up of single PD-1 blockade in neoadjuvant resectable NSCLC. J Clin Oncol. 2021;39:8522. doi: 10.1200/JCO.2021.39.15_suppl.8522. [Google Scholar]
- 65. Zhao Z, Chen S, Qi H, et al. Phase II trial of toripalimab plus chemotherapy as neoadjuvant treatment in resectable stage III non-small cell lung cancer (NeoTPD01 Study). J Clin Oncol. 2021;39:8541. doi: 10.1200/JCO.2021.39.15_suppl.8541. [Google Scholar]
- 66. Spicer J, Wang C, Tanaka F, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO)+ platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39:8503. doi: 10.1200/JCO.2021.39.15_suppl.8503 [Google Scholar]
- 67. Wakelee HA, Altorki NK, Zhou C, et al. IMpower010: Primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39:8500. doi: 10.1200/JCO.2021.39.15_suppl.8500. [Google Scholar]
- 68. Pirker R, Filipits M. Adjuvant therapy in patients with completely resected non-small-cell lung cancer: current status and perspectives. Clinlung cancer. 2019;20:1–6. doi: 10.1016/j.cllc.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 69. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III Non-Small-Cell lung cancer. N Engl J Med. 2017;377:1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 70. Faivre-Finn C, Vicente D, Kurata T, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC—an update from the PACIFIC trial. J Thorac Oncol. 2021;16:860–7. doi: 10.1016/j.jtho.2020.12.015. [DOI] [PubMed] [Google Scholar]
- 71. Hong DS, Clarke JM, Asch A, et al. 540P Safety and efficacy from the SURPASS trial with ADP-A2M4CD8, a SPEAR T-cell therapy incorporating a CD8α co-receptor and an affinity optimized TCR targeting MAGE-A4. Ann Oncol. 2021;32:S604–5. doi: 10.1016/j.annonc.2021.08.1062. [Google Scholar]
- 72. Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–35. doi: 10.1016/s1470-2045(16)00099-1. [DOI] [PubMed] [Google Scholar]
- 73. Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/s1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 74. Quoix E, Lena H, Losonczy G, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17:212–23. doi: 10.1016/s1470-2045(15)00483-0. [DOI] [PubMed] [Google Scholar]
- 75. Saavedra D, Neninger E, Rodriguez C, et al. CIMAvax-EGF: Toward long-term survival of advanced NSCLC. Semin Oncol. 2018;45:34–40. doi: 10.1053/j.seminoncol.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 76. Rodriguez PC, Popa X, Martinez O, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22:3782–90. doi: 10.1158/1078-0432.CCR-15-0855. [DOI] [PubMed] [Google Scholar]
- 77. Besse B, Garcia Campelo MR, Cobo Dols MA, et al. LBA47 Activity of OSE-2101 in HLA-A2+ non-small cell lung cancer (NSCLC) patients after failure to immune checkpoint inhibitors (IO): Final results of phase III Atalante-1 randomised trial. Ann Oncol. 2021;32:S1325. doi: 10.1016/j.annonc.2021.08.2126. [Google Scholar]
- 78. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ready N, Hellmann MD, Awad MM, et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol. 2019;37:992–1000. doi: 10.1200/jco.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–95. doi: 10.1016/s1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 81. Takamochi K, Takahashi F, Suehara Y, et al. DNA mismatch repair deficiency in surgically resected lung adenocarcinoma: Microsatellite instability analysis using the Promega panel. Lung Cancer. 2017;110:26–31. doi: 10.1016/j.lungcan.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 82. Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–8. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 83. Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22:1342–50. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 84. Yu Y, Zeng D, Ou Q, et al. Association of survival and immune-related biomarkers with immunotherapy in patients with non-small cell lung cancer: a meta-analysis and individual patient-level analysis. JAMA Network Open. 2019;2:e196879. doi: 10.1001/jamanetworkopen.2019.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. El-Osta H, Jafri S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: a meta-analysis. Immunotherapy. 2019;11:189–99. doi: 10.2217/imt-2018-0086. [DOI] [PubMed] [Google Scholar]
- 86. Park W, Mezquita L, Okabe N, et al. Association of the prognostic model iSEND with PD-1/L1 monotherapy outcome in non-small-cell lung cancer. Br J Cancer. 2020;122:340–7. doi: 10.1038/s41416-019-0643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Park W, Kwon D, Saravia D, et al. Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin Lung Cancer. 2018;19:280–8..e4. e4. doi: 10.1016/j.cllc.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 88. Cabel L, Proudhon C, Romano E, et al. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat Rev Clin Oncol. 2018;15:639–50. doi: 10.1038/s41571-018-0074-3. [DOI] [PubMed] [Google Scholar]
- 89. Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–51. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Iijima Y, Hirotsu Y, Amemiya K, et al. Very early response of circulating tumour-derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Eur J Cancer. 2017;86:349–57. doi: 10.1016/j.ejca.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 91. Goldberg SB, Narayan A, Kole AJ, et al. Early Assessment of lung cancer immunotherapy response via circulating tumor DNA. ClinCancer Res. 2018;24:1872–80. doi: 10.1158/1078-0432.Ccr-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun X, Roudi R, Dai T, et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: a PRISMA systematic review and meta-analysis. BMC Cancer. 2019;19:558. doi: 10.1186/s12885-019-5701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4:374–8. doi: 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71–4. doi: 10.1016/j.lungcan.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 95. Rogado J, Sanchez-Torres JM, Romero-Laorden N, et al. Immune-related adverse events predict the therapeutic efficacy of anti-PD-1 antibodies in cancer patients. Eur J Cancer. 2019;109:21–7. doi: 10.1016/j.ejca.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 96. Ricciuti B, Genova C, De Giglio A, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145:479–85. doi: 10.1007/s00432-018-2805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19:133–50. doi: 10.1038/s41568-019-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rossi G, Russo A, Tagliamento M, et al. Precision medicine for NSCLC in the era of immunotherapy: new biomarkers to select the most suitable treatment or the most suitable patient. Cancers. 2020;12:1125. doi: 10.3390/cancers12051125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Liu L, Bai X, Wang J, et al. Combination of TMB and CNA stratifies prognostic and predictive responses to immunotherapy across metastatic cancer. Clin Cancer Res. 2019;25:7413–23. doi: 10.1158/1078-0432.CCR-19-0558. [DOI] [PubMed] [Google Scholar]
- 100. Frelaut M, du Rusquec P, de Moura A, et al. Pseudoprogression and Hyperprogression as new forms of response to immunotherapy. BioDrugs. 2020;34:463–76. doi: 10.1007/s40259-020-00425-y. [DOI] [PubMed] [Google Scholar]
- 101. Rauwerdink DJW, Molina G, Frederick DT, et al. Mixed response to immunotherapy in patients with metastatic melanoma. Ann Surg Oncol. 2020;27:3488–97. doi: 10.1245/s10434-020-08657-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ferrara R, Caramella C, Besse B, et al. Pseudoprogression in non-small cell lung cancer upon immunotherapy: few drops in the ocean?. J Thorac Oncol. 2019;14:328–31. doi: 10.1016/j.jtho.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 103. Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudoprogression in previously treated patients with non-small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol. 2019;14:468–74. doi: 10.1016/j.jtho.2018.10.167. [DOI] [PubMed] [Google Scholar]
- 104. Won SE, Park HJ, Byun S, et al. Impact of pseudoprogression and treatment beyond progression on outcome in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. Oncoimmunology. 2020;9:1776058. doi: 10.1080/2162402X.2020.1776058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ferrara R, Mezquita L, Texier M, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–52. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52. doi: 10.1016/s1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lee JH, Long GV, Menzies AM, et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 2018;4:717–21. doi: 10.1001/jamaoncol.2017.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang Y, Ding H, Wu Q, et al. Neutrophil-lymphocyte ratio dynamics are useful for distinguishing between recurrence and pseudoprogression in high-grade gliomas. Cancer Management and Research. 2019;11:6003–9. doi: 10.2147/CMAR.S202546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kiriu T, Yamamoto M, Nagano T, et al. Pseudo-progression and the neutrophil-to-lymphocyte ratio in non-small cell lung cancer treated with immune checkpoint inhibitors: a case-control study. OncoTargets and Therapy. 2019;12:10559–68. doi: 10.2147/OTT.S228138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–50. doi: 10.1158/1078-0432.CCR-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lo Russo G, Moro M, Sommariva M, et al. Antibody-Fc/FcR interaction on macrophages as a mechanism for hyperprogressive disease in non-small cell lung cancer subsequent to PD-1/PD-L1 blockade. Clin Cancer Res. 2019;25:989–99. doi: 10.1158/1078-0432.CCR-18-1390. [DOI] [PubMed] [Google Scholar]
- 112. Champiat S, Dercle L, Ammari S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–8. doi: 10.1158/1078-0432.CCR-16-1741. [DOI] [PubMed] [Google Scholar]
- 113. Arasanz H, Zuazo M, Bocanegra A, et al. Early detection of hyperprogressive disease in non-small cell lung cancer by monitoring of systemic t cell dynamics. Cancers. 2020;12:344. doi: 10.3390/cancers12020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kim CG, Kim KH, Pyo KH, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol. 2019;30:1104–13. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
- 115. Kim JY, Lee KH, Kang J, et al. Hyperprogressive disease during anti-PD-1 (PDCD1) /PD-L1 (CD274) therapy: a systematic review and meta-analysis. Cancers. 2019;11:1699. doi: 10.3390/cancers11111699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Thompson JA. New NCCN guidelines: recognition and management of immunotherapy-related toxicity. Journal of the National Comprehensive Cancer Network. 2018;16:594–6. doi: 10.6004/jnccn.2018.0047. [DOI] [PubMed] [Google Scholar]
- 117. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 118. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68. doi: 10.1200/jco.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. China Society of Clinical Oncology Guidelines Working Committee Editor-in-Chief . Guidelines for Toxicity Management Related to Immune Checkpoint Inhibitors. Beijing: People's Medical Publishing House, 2019: 53. doi: 10.3969/j.issn.1006⁃5725.2019.04.001 [Google Scholar]
- 120. Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Frontiers in Medicine. 2019;6:119. doi: 10.3389/fmed.2019.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Xu Y, Fu Y, Zhu B, et al. Predictive biomarkers of immune checkpoint inhibitors-related toxicities. Front Immunol. 2020;11:2023. doi: 10.3389/fimmu.2020.02023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Subudhi SK, Aparicio A, Gao J, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci. 2016;113:11919–24. doi: 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Giannicola R, D'Arrigo G, Botta C, et al. Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol Clin Oncol. 2019;11:81–90. doi: 10.3892/mco.2019.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018;24:1804–8. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 126. Abu-Sbeih H, Ali FS, Wang X, et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J Immunother Cancer. 2019;7:93. doi: 10.1186/s40425-019-0577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discovery. 2021. doi: 10.1038/s41573-021-00259-5. [DOI] [PubMed] [Google Scholar]
- 128. Reck M, Schenker M, Lee KH, et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non–small-cell lung cancer with high tumour mutational burden: patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur J Cancer. 2019;116:137–47. doi: 10.1016/j.ejca.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 129. Zhang R, Billingsley MM, Mitchell MJ. Biomaterials for vaccine-based cancer immunotherapy. J Control Release. 2018;292:256–76. doi: 10.1016/j.jconrel.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]